Abstract
Diets that boost ketone production are increasingly used for treating several neurological disorders. Elevation in ketones in most cases is considered favorable, as they provide energy and are efficient in fueling the body’s energy needs. Despite all the benefits from ketones, the above normal elevation in the concentration of ketones in the circulation tend to illicit various pathological complications by activating injurious pathways leading to cellular damage. Recent literature demonstrates a plausible link between elevated levels of circulating ketones and oxidative stress, linking hyperketonemia to innumerable morbid conditions. Ketone bodies are produced by the oxidation of fatty acids in the liver as a source of alternative energy that generally occurs in glucose limiting conditions. Regulation of ketogenesis and ketolysis plays an important role in dictating ketone concentrations in the blood. Hyperketonemia is a condition with elevated blood levels of acetoacetate (AA), 3-β-hydroxybutyrate (BHB), and acetone. Several physiological and pathological triggers, such as fasting, ketogenic diet, and diabetes cause an accumulation and elevation of circulating ketones. Complications of the brain, kidney, liver, and microvasculature were found to be elevated in diabetic patients who had elevated ketones compared to those diabetics with normal ketone levels. This review summarizes the mechanisms by which hyperketonemia and ketoacidosis cause an increase in redox imbalance and thereby increasing the risk of morbidity and mortality in patients.
Keywords: Ketones, hyperketonemia, diabetic ketoacidosis (DKA), ketogenic diet (KD), oxidative stress
1. Introduction
Ketone bodies serve as alternative source of energy for the brain when glucose becomes limiting in the blood. The ketone bodies become important under certain conditions as they can supplement energy to cells when glucose oxidation dramatically decreases. They accumulate in the plasma under conditions of fasting and uncontrolled diabetes. Circulating ketone body levels, which in normal individuals are generally <0.5 mM, can reach up to 6–7.5 mM during prolonged fasting [1, 2], and up to 25 mM in uncontrolled diabetes [3]. Though ketone bodies serve as substrates for energy, several deleterious effects of the accumulation of these moieties have been reported in the literature. Ketoacidosis, a condition with high levels of ketones in the blood is associated with significant mortality and morbidity [4]. The objective of this review is to address and summarize the conditions which lead to increased ketogenesis and the mechanisms involved by which ketones increase oxidative stress and contribute to tissue/cellular injury.
2. Ketone metabolism
Ketone bodies produced are contributed predominantly by the liver and are then transported to extrahepatic tissues for consumption. Conditions that enable insulin suppression leading to a reduction in the circulating insulin levels facilitate the mobilization of fatty acids from the adipose tissue and into the liver favoring ketogenesis (Figure 1).
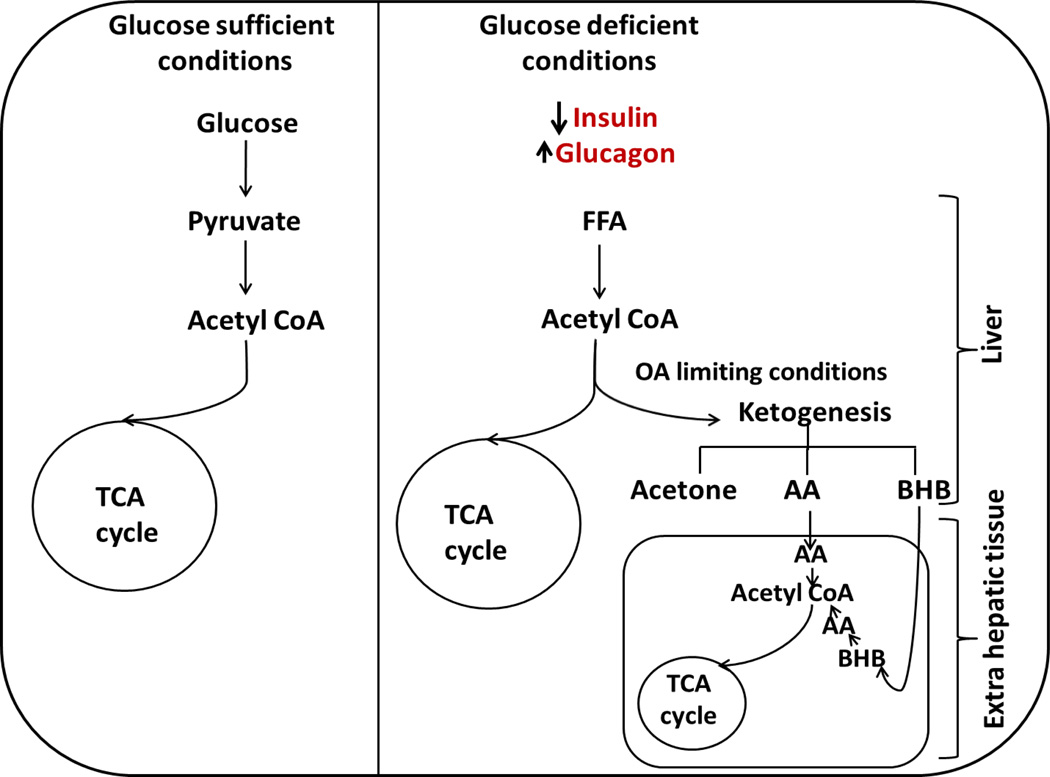
Figure 1.
In normal conditions glucose gets converted to acetyl CoA and enters tricarboxylic acid (TCA) cycle to yield energy. When glucose becomes limiting, glucagon levels rise facilitating free fatty acid (FFA) transport into the liver, fatty acid oxidation is carried out by the liver to generate ketone bodies that can keep up with the brain’s energy demands. Ketogenesis increases as the availability of oxaloacetate (OA) becomes limited forcing the acetyl CoA pools towards ketone production. The ketones get converted to acetyl CoA in the extra hepatic tissues and enter into the TCA cycle providing with energy.
2.1 Ketogenesis
Acetoacetate (AA), 3-β-hydroxybutyrate (BHB), and acetone (least abundant) are the three ketone bodies produced during ketogenesis. AA is the central ketone body, produced and utilized in the course of intermediary metabolism from 3-hydroxy-3-methylglutaryl coenzyme-A (HMG CoA), and the other ketone bodies are derived from it [3, 5]. Acetone is produced by the spontaneous decarboxylation of AA. It is of clinical interest primarily because it imparts a fruity odor by which ketosis can be identified. BHB is formed from the reduction of AA by the action of 3-hydroxybutyrate dehydrogenase (EC 1.1.1.30) [3].
The first trigger that activates the ketogenesis pathway is a change in the molar ratio of glucagon to insulin. The effects of insulin predominate over glucagon function [6]. Insulin inhibits lipolysis, regulating ketone output from the liver, and enhancing ketone utilization by the peripheral tissues [3, 7]. Under low insulin conditions, adipocyte lipolysis occurs and fatty acids are transported to the liver (mediated mostly by glucagon). Hormone-sensitive lipase (EC 3.1.1.79, HSL) plays a key role in the release of fatty acids from the adipocytes into the circulation. The activity of HSL is suppressed in the presence of insulin. In the absence of insulin, lipolysis is stimulated and there is an abundance of high concentrations of free fatty acids in the blood [3]. Glucagon mediates fatty acid release and enables the activation of carnitine acyltransferase 1 (EC 2.3.1.21, CAT-1 or CPT-1). Free fatty acids from the blood move into the liver and CPT-1 transports them across the inner mitochondrial membrane for oxidation. Mitochondrial transport of fatty acids is a rate controlling step for ketogenesis [6]. The production of acetyl coenzyme A (AcCoA) in the liver from the oxidation of fatty acids can exceed cellular energy requirements; ketone bodies are synthesized from this pool of AcCoA. 3-Ketothiolase (EC 2.3.1.16) catalyzes conversion of AcCoA to acetoacetyl CoA. Mitochondrial HMG-CoA synthase (EC 2.3.3.10) mediates HMG CoA formation from acetoacetyl CoA. HMG CoA is then cleaved by HMG-CoA Lyase (EC 4.1.3.4) to give rise to ketone body acetoacetate [3, 8]. HMG-CoA synthase is highly expressed in the liver, and in the presence of glucagon and the absence of insulin, its mRNA levels can rapidly be increased [3]. Thus, in normal individuals the presence of insulin counter regulates the effects of glucagon leading to the inhibition of HSL as well as that of CPT-1; as a result, very little ketogenesis occurs due to limited fatty acid availability. However, in diabetic patients with insulin deficiency, especially as seen in T1D, all of these pathways are upregulated by counter regulatory hormones, in most part by glucagon. In the absence of insulin the inhibitory effect on HSL and CPT-1 are lost leading to the increased influx of FFA and production of ketones in diabetic patients. This is most frequently observed in T1D patients who lack the ability to make insulin. The inherent deficiency of insulin coupled with periods of insulin lack (due to gaps in insulin treatments) in T1D disrupt the balance between ketogenesis and ketolysis, resulting in increased blood ketone levels, that could lead to complications such as frequent episodes of hyperketonemia or even ketoacidosis [9]. Hyperketonemia has also been reported among T2D, but its incidence is very rare [10] as most T2D individuals still have functional β-cell that maintain blood insulin levels. T2D cases where ketosis develops without a precipitating cause are referred to as ketosis-prone diabetes that manifests with impaired insulin sensitivity and impaired β-cell function. The metabolic profile and biochemical parameters of DKA in these patients is similar to that found in T1D patients [10]. Unlike patients with T1D DKA, T2D patients with DKA are more likely to be older, obese, and do not show any autoimmune markers, they also appear to have less complications associated with management of DKA because of their age [11–14].
2.2 Ketolysis
Once formed in the liver, ketones then diffuse into the circulation for use in extrahepatic tissues where ketolysis occurs. In the presence of succinyl-CoA: 3 oxoacid CoA-transferase (EC 2.8.3.18, SCOT), AA is activated to acetoacetyl-CoA, which is then converted to AcCoA by mitochondrial acetoacetyl CoA thiolase (EC 2.3.1.9) [3, 5, 15]. The AcCoA produced can then be used for energy production by oxidative metabolism. The ketolytic capacity of tissues depends on their SCOT activity. The heart is known to have the highest SCOT activity, followed by the kidney. This renders the heart and the kidney to have the greatest capacity for ketone body utilization. Ketones can serve as a major energy source in the brain, with a SCOT activity of ~10% compared to that of the heart, during fasting or starvation. Importantly, neither SCOT protein nor mRNA is detectable in the liver, thereby preventing futile cycling of ketones in the liver. Thus, ketones produced by the liver are transported to extrahepatic tissues to be utilized as fuel, making liver the predominant source of ketogenesis [3, 15, 16]. There is evidence supporting a decline in functional SCOT with diabetes. Research in animal models of diabetes shows that there is an association of diabetes with tyrosine nitration which results in the inactivation of SCOT [17, 18]. This inactivation of SCOT was observed in the heart tissue of the animal models, though not confirmed in humans it can be alluded that diabetics have an impaired capacity to clear ketones from the circulation, owing to a potential impairment in their SCOT activity.
2.3. Acetoacetate and β-hydroxybutyrate homeostasis
Individual ketone body concentration under diabetic conditions varies. BHB is found at concentrations 2 to 3 times greater than that of AA [19]. Depending on the severity of insulin deficiency, the ketone levels, especially the AA-to- BHB ratio, can vary in T1D patients anywhere from 1:1 to 1:4 due to the impaired utilization of BHB as well as the inability of the extra hepatic peripheral tissues to interconvert BHB to AA [7, 19, 20]. Other factors that contribute to the ratio of these circulating ketones are the reduced state of the liver with an increase in NADH levels and the reduction in the activity of β-hydroxybutyrate dehydrogenase [21, 22].
3. DKA, oxidative stress, and diabetic co-morbidities
Although several studies suggest an antioxidant role for ketones and their utilization in the treatment of various disorders [23–26], one study conducted by Beskow et al. evaluated various oxidative stress parameters such as protein and lipid oxidation, levels of enzymatic and non-enzymatic antioxidant defenses, and demonstrated that AA and BHB neither elicit nor prevent oxidative stress in vitro [27].
Nevertheless, it is important to note that when the concentration of ketones reaches much above the normal levels, they tend to interfere with normal cellular functioning. The role of ketones in the pathophysiology of complications in T1D is gaining importance. The severity of elevated ketones parallels that of high glucose levels in diabetic patients. Hyperketonemia with or without acidosis is considered to be an acute complication of poorly controlled or newly diagnosed diabetes mellitus. A growing body of evidence from both in vitro and in vivo research from different laboratories indicates that ketosis and hyperketonemia can have different outcomes based on the metabolic state of the disease. The purpose of increased ketone body production in conditions such as fasting and the administration of ketogenic diet is to either provide an alternative source of energy substrates or to enhance the efficient utilization of fuel for energy purposes. This is very distinct from the increased ketogenesis in diabetics. Diabetic patients not only have increased ketone production but their ketone clearance also significantly goes down [28]. This can be attributed to their inherently low insulin levels as insulin is required for efficient ketone clearance [29]. Additionally, the evidence indicating the decreased SCOT and β-hydroxybutyrate dehydrogenase activities in diabetic conditions also increases the ketone retention in the blood [17, 21]. Thus, the accumulation of ketones in the blood is a consequence of metabolic deregulation and not necessarily to meet energy demands. This enables ketones to turn on signaling pathways that can potentially mediate injurious effects as discussed in the following section.
3.1 Oxidative stress induction
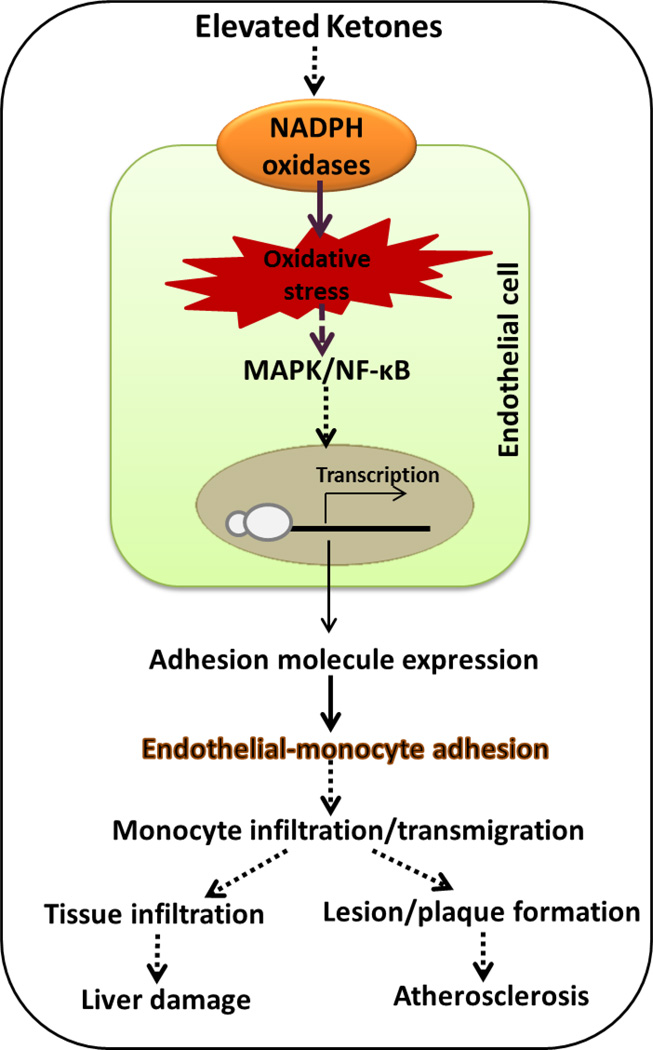
Many recent studies, including our own and by different investigators, have reported that ketone bodies can induce oxidative stress and upregulate several signaling pathways involved in diabetic complications. Ketones were shown to induce oxidative stress in cardiomyocytes, erythrocytes, and endothelial cells [30–32] Cell culture studies demonstrated that AA can activate ERK1/2 and p38MAP kinase in cultured rat hepatocytes [33]. AA has also been shown to promote the myeloperoxidase-catalyzed peroxidation of the unsaturated fatty acid, arachidonic acid [34]. The condition of ketosis is known to increase extra-mitochondrial oxidation of fatty acids and generation of hydrogen peroxide, thereby increasing oxidative stress in hyperketonemic diabetic patients (HKD) [35]. We have reported that the levels of oxidative stress are higher in the RBC and plasma of HKD compared with normoketonemic type 1 diabetic patients [30, 36, 37]. Along with the upregulation of the oxidative stress we have also identified a decrease in the levels of glutathione in HKD patients [30]. A recent study with lean and obese patients with DKA reported that ketoacidosis induced changes in pro-inflammatory cytokines, oxidative stress, and CVD [38]. Induction of oxidative stress by the administration of plasma from DKA mice in a cell culture model was also shown [39]. Studies from our lab demonstrated that elevated levels of ketones upregulate NADPH oxidases, activate MAPK pathway and NF-κB contributing to increased oxidative stress that could be implicated in the develpoment of vascular disease and atherosclerosis [31, 40–42]. Oxidative damage has also been reported in the brains of DKA patients. Markers of oxidative stress such as 8-hydroxyguanosine, 4-hydroxynonenal, and heme oxygenase-1 were increased in DKA patients in comparison to controls [43]. The role of ketones in elevating oxidative stress and leading to endothelial cell damage is dipicted in figure 3.
Figure 3.
Literature has shown that ketones can increase oxidative stress by several mechanisms, and upregulation of NADPH oxidases is one such example. The increase in the production of superoxide radicals, mediated by ketones, can upregulate signaling mechanisms inducing the expression of adhesion molecules. It has been shown in the endothelial cells where the increased adhesion molecule expression can cause the monocytes to adhere that could potentially lead to lesion or plaque initiation or tissue infiltration contributing to tissue damage [31].
3.2 Effects on insulin resistance/secretion
There is evidence that elevated fatty acids can suppress glucose oxidation in various cell types [44]. This phenomenon has been, in part, explained by the glucose-fatty acid cycle, where the elevated levels of plasma fatty acids cause an impairment in the utilization of glucose [45]. It has also been shown that glucose-fatty acid cycle plays an important role in diminishing the responsiveness of β-cells to glucose not only in the presence of elevated fatty acids but also ketones [46]. It has also been reported that ketones play a role in inducing insulin resistance by mediating the downregulation of cell surface insulin receptor and insulin receptor substrate-1 phosphorylation [47]. Impairment in insulin action on the activation of protein kinase B (PKB) was also reported in cardiomyocytes, where insulin-stimulated glucose uptake was reduced after prolonged exposure to ketone body BHB [48]. Similar results were reported by another study suggesting that BHB blocked insulin-mediated phosphorylation of PKB leading to the inhibition of glucose transport in the muscle [49]. It has been shown with in vitro islet studies that the glucose dependent amplification of insulin exocytosis and thereby its secretion was blunted with free fatty acid treatment [50, 51]. It was also shown in cultured islets that reduced GSH levels can significantly impact insulin exocytosis that can be corrected by GSH treatment. We and other investigators have shown in cell culture studies that high levels of ketones as well as glucose deplete cellular GSH pools [30, 31, 52] and their abundance in diabetic conditions might be detrimentally effecting insulin output from the β-cells of the pancreas. It can be speculated that elevated ketone levels coupled with elevated free fatty acids and glucose might impair insulin secretion in T2D patients. This may not apply to other physiological states such as fasting or exercise where the primary function of ketogenesis is to provide with an alternative fuel source unlike in diabetic conditions, where ketones are elevated as a consequence of metabolic deregulation.
3.3 Potentiation of vascular inflammation
DKA-induced inflammation has been a recent focus of investigation. The inflammatory response is mediated by signaling molecules such as cytokines. It has been suggested that ketones are directly involved in promoting pro-inflammatory factors and eliciting systemic inflammation [39, 53]. The role of elevated ketones in macrophage and lymphocyte activation, cytokine release, and perturbation of the endothelial cells lining the capillaries has been reported in T1D patients [54]. Heat shock protein 72 (Hsp72), which is a modulator of cytokine expression that is induced under stress conditions, was shown to be elevated in patients with high ketone levels [55]. Another important point to be considered is the fluctuation in the levels of cytokines before and during the course of DKA treatment and resolution. The rapid cytokine changes over time can also lead to capillary perturbations resulting in the damage and dysfunction of the capillary endothelial cells [56]. The role played by DKA in reactive oxygen species (ROS) production, NF-κB activation, and increased transcription of inflammation-relevant genes has also been reported [39]. It is becoming clear that the elevated levels of oxidative stress experienced by diabetic patients can increase the oxidation of LDL and thereby promote or increase their risk for atherosclerosis and CVD. Studies from our lab have shown that hyperketonemia can bring about an increase in the lipid peroxidation in T1D patients [30, 35]. It has also been reported that DKA can independently be associated with cardiovascular risk in patients in the absence of any history or known susceptibility of cardiovascular pathology [38]. There has been an association reported with ketone levels and congestive heart failure in patients. The level of elevation of ketones was reported to be proportional to the degree of severity of congestive heart failure [57].
3.4 Involvement in liver dysfunction
In diabetic patients, elevated glucose levels that are controlled by the administration of insulin drive large amounts of hepatic glycogen synthesis. In some cases, this can lead to a glycogen overload, resulting in liver damage. Hepatic glycogenosis or hepatomegaly is a glycogen overload disorder [58]. Repeated ketoacidosis episodes accompanied by high blood glucose levels increase the risk of glycogen overload in the liver, as DKA is often treated with insulin administration. A high percentage of cases of hepatomegaly has been reported in patients who presented with DKA [59]. Diabetic patients, both type 1 and type 2, show an elevation in their liver enzymes that in most cases associated with non-alcoholic fatty liver disease (NAFLD) [60, 61]. Liver parameters were found to be deteriorated in diabetic patients with elevated blood ketones [62]. Additionally, ketosis onset diabetic group had higher incidence of NAFLD than that of non-ketotic onset diabetic group [63], suggesting that hyperketonemia could be a possible risk factor in the onset of fatty liver and obesity. In a mouse model, Stadler et al. have shown that acetone administration, a model of ketosis, can increase generation of carbon-centered, lipid derived free radicals in the liver, leading to protein oxidation and lipid peroxidation through a free radical dependent mechanism driven mainly by overexpression of inducible nitric oxide synthase [64]. Hepatic infarction was reported to be associated with hyperketonemia [65]. It was shown that ketogenic diet-fed mice develop nonalcoholic fatty liver disease, systemic glucose intolerance, and that their livers exhibit ER stress, steatosis, cellular injury, and macrophage accumulation [66, 67]. It has been reported that ketosis increases the burden on the liver and kidneys causing deterioration in their function, and that the negative effect of DKA on kidney and liver function was far greater than the effect resulting from a mild infection [62]. Our lab has recently shown using T1D and T2D rat liver and hepatocyte cell culture models that elevated ketone levels when present along with elevated glucose levels can cause macrophage induced liver damage by the upregulation of oxidative stress leading to an increase in adhesion molecules and cytokine expression [68]. These above studies suggest that highly elevated ketones in the form of ketogenic diet or diabetes-induced can interfere with normal liver function.
3.5 DKA and cerebral abnormalities
DKA’s role in cytokine induced capillary perturbation contributing to brain edema has been suggested [56]. Close et al. showed, using both in vivo and in vitro approaches, that DKA is associated with systemic inflammation that may contribute to intracranial microvascular complications [39]. Recent studies using MRI measures show altered brain structure and injury associated with adverse neurocognitive outcomes, including impairment in neuronal function and viability in T1D children [69, 70]. Patients with DKA have been reported to have prothrombotic tendencies due to their higher propensity to develop clots [4, 71]. Cerebral edema, hemorrhagic stroke, or acute ischemia associated with hyperketonemia has been implicated to intracerebral complications and neurological deterioration [72, 73]. A role for increased expression of adhesion molecules like ICAM-1 in the cerebrovascular endothelial cells has been suggested to play a role in the pathogenesis of brain edema which could potentially be fatal [74, 75]. Treatment with ketone body AA was shown to increase the expression of adhesion molecule ICAM-1 in human brain endothelial cells [76] highlighting the role of ketones in cellular activation. The association of ketoacidosis with epilepsy has also been studied in T1D children. There was found to be an increased prevalence of epilepsy in diabetic individuals and those diabetics who experience epileptic seizures have a higher disposition to developing DKA [77]. On the other hand, DKA leading to electroencephalogram abnormalities in T1D children has also been shown to increase the risk of epilepsy [78, 79].
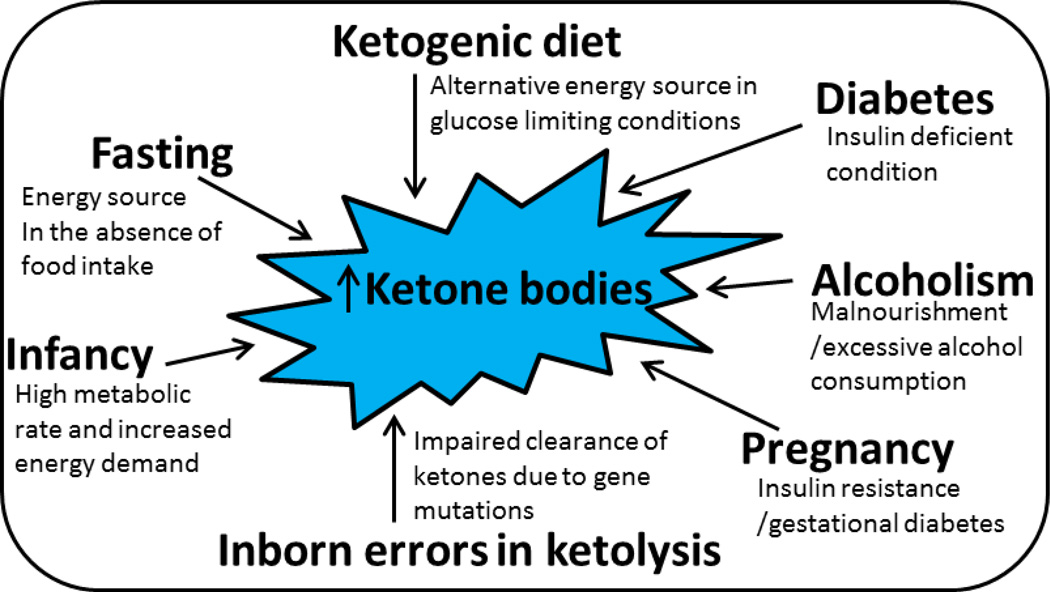
Some of the factors contributing to the elevation of ketones in the blood are shown in figure 2. These could be physiological or pathological in nature. In summary, elevated ketones depending on the nature of the metabolic state might very well have different effects. In normal glycemic conditions or in hyperglycemic states, as observed in diabetic conditions, ketones get accumulated and function in a manner that is detrimental and injurious activating signaling pathways resulting in pro-inflammatory responses. On the other hand, in hypoglycemic states, the elevated ketones are utilized as fuel and are cleared up efficiently due to the lack of glucose availability. Ketone body uptake by tissues for energy purposes is an energy efficient and is deemed beneficial.
Figure 2.
Conditions that induce the upregulation of ketone synthesis or cause the accumulation of ketones in the blood. In individuals during normal physiological conditions such as fasting and pregnancy along with infancy, the concentration of ketones rises in the blood. Other states such as diabetes, also known as an insulin deficient state, alcoholism, and certain mutations in the genes that are required in the breakdown of ketones also increase ketone levels in the blood.
4. Physiological ketosis
In physiological ketosis the blood ketone levels are moderately elevated (~2 mM) [80]. The elevation of ketones achieved here is marginal and does not reach dangerously high levels. Prolonged exercise and fasting are some of the common causes of the normal upregulation of hepatic ketone output.
4.1 Fasting
Physiological response to the absence of food intake is to turn on several pathways that can ultimately provide fuel for tissues, and especially the brain. Fasting or starvation can initiate ketogenesis to regulate and maintain whole body energy homeostasis. The idea that gluconeogenesis from amino acids precedes ketogenesis induction, while the latter is induced for protein sparing purposes, is well accepted [2]. Survival during prolonged starvation is made possible by the adipose fuel stores in the body, thereby sparing the essential proteins required for vital functions. Lack of food (carbohydrate) intake causes a drop in the insulin levels with a concurrent rise in glucagon levels in the blood. Increase in glucagon as discussed above causes the activation of ketogenesis pathway. The ketone body concentrations during this time can reach to 6–8 mM [81]. The ketone body output mainly driven by the liver thus helps to sustain the energy demands of the brain and other vital organs [2, 5, 81]. The ketogenesis that occurs in this context is mainly for sustenance and is deemed beneficial in food deprived conditions.
4.2 Ketogenic diet
Consumption of a high fat diet or ketogenic diet can effectively elevate blood ketone levels. This diet is most commonly prescribed to patients with refractory epilepsy or other neurological conditions [82–84]. Ketogenic diet (KD) is a high fat, low carbohydrate diet that was formulated to replace glucose as main source of fuel and was first developed as a treatment option for children with retractable epilepsy [85]. There is evidence showing the viability of the ketogenic diet in reducing severity and the number of seizures in children with epilepsy [86]. Ketones exhibit a neuroprotective function by reducing glutamate-induced free radical formation and by increasing the NAD+/NADH ratio, restoring normal bioenergetics [26]. BHB, even more so than AA, functions as a ROS scavenger, and this antioxidant property provides neuroprotection [23]. Ketones are a more efficient fuel source compared to glucose, providing more energy per unit oxygen [25]. As many neurological disorders arise from deficient energy production in the brain [87], this energy efficiency of ketones is therefore effective in treating epileptic seizures when ketones are primarily utilized for energy production [88]. Recent evidence points to the applicability of ketogenic diet to various other neurological disorders. Diet induced hyperketonemia was reported to bring about symptomatic improvement in patients with Parkinson’s [89, 90] and Alzheimer’s disease [83].
KD is used to treat glucose transporter 1 (GLUT1) and pyruvate dehydrogenase deficiencies where the patients are unable to use glucose for energy purposes and are in need of an alternative source for their survival [91–94]. GLUT1 deficiency causes impaired glucose transport across the blood brain barrier and the use of KD diet provides ketones as the alternative source of energy for the brain. On the other hand, pyruvate dehydrogenase deficiency impedes the conversion of glucose to energy, and patients with this defect can be helped by the incorporation of ketogenic diets [91–94]. Interestingly, benefits of ketones in the treatment of cancer are also currently being explored. Unlike normal brain cells, brain tumor cells (glioma cells) most often show reduced expression of enzymes in the ketolysis pathway [95, 96]. As a result the tumor cells cannot utilize ketones as they rely heavily on glucose for energy. Recent studies have shown that ketones administered as a KD increased survival in a glioma mouse and rodent models as replacing ketones with glucose hinders the ability of cancer cells to metabolize ketones and thereby, prevents cancer cell growth and survival [97–99].
However, various problems have been encountered in patients adhering to this diet (Table 1). As the duration of diet increases so do the complications. Long term intake of KD has been linked to renal stones, gall stones, elevated liver enzymes and even death in rare cases [100]. Though the diet is well tolerated by infants it poses difficulties among older children. Intricacies such as dehydration, gastrointestinal disturbances, hypercholesterolemia, metabolic acidosis [101], and cardiac complications have been reported in patients following a KD [102]. The complications encountered with the KD have introduced the formulation of different diets that offer similar benefits but can also be well tolerated. The modified Atkin’s diet, medium-chain triglycerides, and low glycemic index treatment have all incorporated greater proportion of protein and carbohydrates into the dietary regimen to provide more food choices, better tolerance and compliance [85].
Table-1.
Table lists the short and long term complications experienced by patients on KD diet.
| Duration of KD intake | Adverse effects |
|---|---|
| Short term | Hypoglycemia [138] |
| Dehydration [139] | |
| Anorexia [138] | |
| Gastroesophageal reflux [140] | |
| Vomiting [139, 141] | |
| Diarrhea [140] | |
| Constipation [141] | |
| Abdominal pain [140] | |
| Long term | Growth alterations [138] |
| Hyperlipidemia [142] | |
| Nephrolithiasis [138] | |
| Vitamin, mineral, & electrolyte deficiency [139] | |
| Hypertriglyceridemia[139, 143] | |
| Cardiac complications [60] | |
4.3 Age and energy demands
Other instances also report elevated ketone production in the body. For example, ingestion of milk which has high fat content can elevate ketone bodies in newborn infants [103]. In young children having very high energy demands, even a day’s fast or infections can increase the production of ketone bodies causing hyperketonemia [104, 105]. Inverse correlation seem to exist between age and blood ketone levels among children [105]. However, in adults, 12–16 h of fasting can cause a moderate increase in ketone levels while fasting for upto 3 days can attain hyperketonemia [106, 107]. An exception to this is pregnancy. Pregnant women tend to have high ketone levels in their blood and their blood ketone levels after fasting can rapidly increase making them more susceptible to ketoacidosis [108].
5. Pathological ketosis
Dysregulated ketone metabolism, that involves defects in ketone clearance or over production of ketones, can increase blood ketone concentrations to levels that can cause changes in the blood pH. This abnormal elevation in ketone levels can be detrimental.
5.1 Impaired ketolysis pathway
Individuals deficient in the enzymes involved in ketone body degradation or ketolysis tend to have high ketone levels in their blood while on the other hand, inborn errors in ketogenesis pathway lead to high FFAs and low ketone levels. Defects in ketolysis pathway include SCOT and acetoacetyl CoA thiolase deficiency, while those in ketogenesis pathway include HMG-CoA synthase and HMG-CoA lyase deficiency [5]. Patients with SCOT deficiency experience continuous hyperketonemia also considered as permanent elevation of blood ketone levels and, after fasting, their ketone levels rise abnormally high and they rapidly develop ketoacidosis [109]. Another condition in which individuals experience abnormally high blood ketone levels is that of acetoacetyl CoA thiolase deficiency which has been linked to recurrent attacks of ketoacidosis [110].
5.2 Alcoholic ketoacidosis
This condition is most often diagnosed in the emergency room in alcoholics. Alcoholic ketoacidosis is an induced state or condition that arises from prolonged intake of ethanol. Alcohol abuse can lead to acidosis mediated by the elevated levels of ketones in the blood [111, 112]. Oxidation of ethanol by hepatic alcohol dehydrogenase results in the increase of NADH and creates an imbalance favoring FFA conversion into ketones [113, 114]. Most commonly this condition is associated with normal blood glucose levels [111] but when accompanied with malnourishment (low carbohydrate intake) the patients become hypoglycemic and are rendered unconscious with potential brain damage [115, 116]. Though the hormonal profile of these patients is similar to those with DKA (low insulin and high counter regulatory hormones), their biochemical and metabolic profile varies quite a bit [117]. Study shows that alcoholic ketoacidosis had a higher BHB to AA ratio and higher lactate levels compared to DKA that can be explained by the altered redox state induced by alcohol metabolism [117].
5.3 Diabetes
Resulting from a relative or absolute lack of insulin increased ketogenesis occurs in diabetic patients, more commonly in individuals with type 1 than type 2.
5.3.1 Type 1 diabetes
Type 1 diabetes (T1D) that accounts for ~10% of the diabetic cases worldwide is most commonly seen in children and during adolescence [118]. T1D is mediated by the autoimmune destruction of pancreatic β-cells leading to absolute insulin deficiency. Exogenous insulin administration is required to achieve control over elevated glucose levels. Any lapse in insulin administration or a missed dose can lead to the activation of the ketogenesis pathway. Ketone levels of 1–2 mM (1–2 µmol/mL) are frequently seen in T1D patients during routine check-up visits at the clinic [119–122]. Ketogenesis is normally favored and deemed beneficial during fasting or starvation conditions as ketones take on the role of energy generation in glucose limiting conditions. This essentially occurs in the absence of any carbohydrate ingestion and is tightly regulated throughout by both glucagon and insulin. In case of diabetes, the absence of insulin coupled with glucagon release drives gluconeogenesis in the liver, lipolysis in the adipose, and hepatic ketogenesis [73]. Although several benefits of ketones have been reported, nevertheless ketones when in excess can cause ill effects. Buildup of ketone concentration in the blood that could potentially result in a drop of blood pH levels can lead to ketoacidosis, a devastating complication [73].
5.3.2 Diabetic ketoacidosis (DKA)
Diabetes plays a prominent role in the development of pathological ketosis. The amounts of ketones produced in diabetic patients are much higher than those observed in fasting subjects, as diabetic patients show decreased insulin levels (impaired insulin secretion or ineffective insulin action), increased levels of counterregulatory hormones such as glucagon that increase free fatty acid levels in the circulation, coupled with impaired ketone clearance. Impaired ketone clearance has been attributed to the decrease in the activities of enzymes involved in ketolysis, namely 3-hydroxybutyrate dehydrogenase and SCOT [21]. The majority of studies have suggested that increased ketogenesis is the major factor responsible for the development of ketoacidosis in diabetics [28, 123, 124]. Insulin deficiency coupled with excess glucagon leads to the unregulated release of ketone bodies from the liver [125]. Ketones are organic acids that are ionized at physiological pH; the hydrogen ions thus released bind to bicarbonate [7]. When the level of ketones in the blood reaches an excess, such that the buffering capacity of the plasma is exhausted due to the depletion of bicarbonates, it can lead to metabolic acidosis with a drop in blood pH, a condition known as ketoacidosis that can occur in many metabolically deranged situations. In diabetic conditions this is termed as diabetic ketoacidosis (DKA) [7, 120]. DKA is a potentially life threatening complication that accounts for the majority of deaths reported in children with T1D [126, 127]. The severity of DKA was reported to be greater in children < 3 years of age [73].
Patients with DKA have blood glucose levels anywhere between 11 mmol/L and 55 mmol/L (normal range 4 mmol/L to 6.1 mmol/L), arterial pH ranging from 7.35 to 7.20 (normal arterial pH = 7.41), and plasma bicarbonate levels ranging from 19 to 10 mmol/L (normal range 18 to 30 mmol/L), a further derangement from the above given values can also be expected in severe cases of DKA [73, 128, 129]. Osmotic diuresis induced by hyperglycemia results in severe fluid loss that is associated with large losses of electrolytes in the urine [125, 126, 129]. Successful treatment of DKA includes the correction of dehydration, hyperglycemia, ketoacidosis, and the deficiency of electrolytes. Insulin, potassium, bicarbonate, and saline are generally administered. Several treatment related complications may also arise in these patients, including cerebral edema, respiratory distress, vascular thrombosis, and hypoglycemia [120, 129]. DKA is considered a medical emergency and the patients are admitted in the hospital where they could be continuously monitored.
5.3.3 Potential triggers of ketoacidosis in diabetic patients
Several factors can potentially trigger the excessive production of ketones leading to ketoacidosis. When an illness or a condition involves the upregulation or high output of hormones that are counter-regulatory to insulin, the ketogenesis pathway will be favored, increasing the synthesis of ketones [130]. During conditions such as infection, trauma, surgery, or heart attack, the usual insulin treatment is inadequate as there is a rise in the levels of hormones counter regulatory to insulin. Epinephrine levels are most commonly elevated under stress conditions. Epinephrine’s function opposes that of insulin and activates lipolysis, leading to increased ketogenesis [131]. These raised blood levels of ketones can drop the plasma pH and cause acidosis as seen in DKA. Pneumonia and urinary tract infections are the most common causes of DKA manifestation [13]. In such cases where there is a very severe insulin deficiency, the serum concentration of these ketone bodies can exceed 25 mM, compared with levels of <0.5 mM in normal individuals [7, 19, 119]. Delay in disease (T1D or T2D) is the major cause of DKA in children and adults while omission of insulin administration is the leading cause of recurrent DKA in diabetics [73]. DKA has also been identified in T2D patients [14, 132]. Infections, new onset diabetes, and other unknown factors contribute to DKA in T2D [7, 11]. Elevated ketone levels in these patients are reported along with severe insulin resistance and worsening of hyperglycemia [63, 133]. There is evidence that due to the advanced age of these patients, DKA in T2D is more severe with worse outcomes compared to those with T1D [11]. Along with DKA a more common disorder that is reported in T2D is hyperglycemic hyperosmolar syndrome (HHS). DKA and HHS are diabetic medical emergencies that require immediate attention. Although they have certain common features, they differ in hyperglycemic status and the presence of ketoacidosis. The underlying difference is that in HHS there is still some residual insulin secretion that prevents ketosis [134]. The presence of minimal insulin in HHS can minimize ketogenesis but fails to control hyperglycemia and as a result these patients show a severe hyperglycemia compared to those with DKA [134, 135]. General understanding is that DKA is most commonly seen in T1D while HHS is more common in T2D, owing to either an absolute or a relative insulin deficiency [134, 135]. Some of the less common triggers of these metabolic derangements include stress, infections, emotional/physical trauma, surgery, and drug abuse [13, 136, 137].
In T1D exogenous insulin administration is required to achieve control over elevated glucose levels. Maintaining insulin timing and dosage not only prevents hyperglycemia but also keeps the ketogenesis pathway regulated. In diabetics the best approach is to prevent ketone accumulation by adhering to the treatment regime. This includes regular monitoring of ketones along with their glucose levels. A rise in the ketone levels is indicative of insulin insufficiency and metabolic deregulation and could potentially be controlled with insulin administration. This monitoring and follow might be especially useful for diabetics during an illness, infection or trauma.
It is still unclear how ketones behave in a hyperglycemic state individually, as well as in combination, since T1D patients frequently experience hyperketonemia in addition to hyperglycemia. Recently we have shown in a cell culture studies using endothelial cells and hepatocytes, and in animal models that ketones when present along with high glucose can cause more damage to the cells compared to that of just ketones or high glucose alone [31]. So far the research that points to the beneficial effects of ketones has only been done using either a calorie restricted diet or a low carbohydrate diet where ketones are utilized as the primary source of energy [23, 87]. It still needs to be explored in vivo how ketones, when elevated to an extent without leading to ketoacidosis, behave in the presence of normal or high glucose conditions. It can be speculated that in normal glucose conditions elevated amounts of ketones would act as signaling molecules and mediate cellular damage, as glucose would still serve as the primary source of energy delaying ketone clearance or ketone uptake by tissues.
6. Conclusion
Evidence from patient, animal, and cell culture studies supports a role of elevated ketones in inducing oxidative damage at cellular and tissue levels. Hence, it needs to be emphasized that measures need to be taken to prevent the elevation of ketone levels, except when deemed necessary clinically. Diabetic patients who most frequently experience hyperketonemia can prevent it by strictly abiding to their insulin treatment regime. As elevated ketones are known to elicit oxidative stress and inflammatory responses which play a prominent role in the development of complications associated with diabetes. Therefore, there is a need to identify them as significant contributors in increasing the risk of vascular inflammation and associated morbidities in diabetes and patients following a long term ketogenic diet.
Highlights.
Hyperketonemia can lead to beneficial or detrimental effects based on the metabolic state of the patient.
Hyperketonemia can cause redox imbalance and activate pro-inflammatory signaling pathways, and is associated with neurological and other complications.
Continuous monitoring of ketonemia is essential in preventing ketone buildup and associated complications.
Acknowledgments
The authors are supported by grants from NIDDK, the Office of Dietary Supplements of the National Institutes of Health RO1 AT007442 and RO1 DK072433, the Malcolm Feist Endowed Chair in Diabetes, and funded by a fellowship from the Malcolm Feist Cardiovascular Research Endowment from LSUHSC, Shreveport.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garber AJ, Menzel PH, Boden G, Owen OE. Hepatic ketogenesis and gluconeogenesis in humans. J Clin Invest. 1974;54:981–989. doi: 10.1172/JCI107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen OE, Felig P, Morgan AP, Wahren J, Cahill GF., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969;48:574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids. 2004;70:243–251. doi: 10.1016/j.plefa.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Bilici M, Tavil B, Dogru O, Davutoglu M, Bosnak M. Diabetic ketoasidosis is associated with prothrombotic tendency in children. Pediatr Hematol Oncol. 2011;28:418–424. doi: 10.3109/08880018.2011.558568. [DOI] [PubMed] [Google Scholar]
- 5.Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Ketone body metabolism and its defects. J Inherit Metab Dis. 2014;37:541–551. doi: 10.1007/s10545-014-9704-9. [DOI] [PubMed] [Google Scholar]
- 6.Foster DW, McGarry JD. The regulation of ketogenesis. Ciba Found Symp. 1982;87:120–131. doi: 10.1002/9780470720691.ch7. [DOI] [PubMed] [Google Scholar]
- 7.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15:412–426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Eledrisi MS, Alshanti MS, Shah MF, Brolosy B, Jaha N. Overview of the diagnosis and management of diabetic ketoacidosis. Am J Med Sci. 2006;331:243–251. doi: 10.1097/00000441-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Sassa M, Yamada Y, Hosokawa M, Fukuda K, Fujimoto S, Toyoda K, Tsukiyama K, Seino Y, Inagaki N. Glycemic instability in type 1 diabetic patients: Possible role of ketosis or ketoacidosis at onset of diabetes. Diabetes Res Clin Pract. 2008;81:190–195. doi: 10.1016/j.diabres.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Smiley D, Chandra P, Umpierrez GE. Update on diagnosis, pathogenesis and management of ketosis-prone Type 2 diabetes mellitus. Diabetes Manag (Lond) 2012;1:589–600. doi: 10.2217/DMT.11.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barski L, Nevzorov R, Harman-Boehm I, Jotkowitz A, Rabaev E, Zektser M, Zeller L, Shleyfer E, Almog Y. Comparison of diabetic ketoacidosis in patients with type-1 and type-2 diabetes mellitus. Am J Med Sci. 2013;345:326–330. doi: 10.1097/MAJ.0b013e31827424ab. [DOI] [PubMed] [Google Scholar]
- 12.Banerji MA, Chaiken RL, Huey H, Tuomi T, Norin AJ, Mackay IR, Rowley MJ, Zimmet PZ, Lebovitz HE. GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4. Flatbush diabetes. Diabetes. 1994;43:741–745. doi: 10.2337/diab.43.6.741. [DOI] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2:95–108. doi: 10.2165/00024677-200302020-00003. [DOI] [PubMed] [Google Scholar]
- 14.Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med. 2004;164:1925–1931. doi: 10.1001/archinte.164.17.1925. [DOI] [PubMed] [Google Scholar]
- 15.Fukao T, Song XQ, Mitchell GA, Yamaguchi S, Sukegawa K, Orii T, Kondo N. Enzymes of ketone body utilization in human tissues: protein and messenger RNA levels of succinyl-coenzyme A (CoA):3-ketoacid CoA transferase and mitochondrial and cytosolic acetoacetyl-CoA thiolases. Pediatr Res. 1997;42:498–502. doi: 10.1203/00006450-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Williamson DH. Ketone body production and metabolism in the fetus and newborn. Philadelphia: W.B.: Saunders; 1992. [Google Scholar]
- 17.Turko IV, Marcondes S, Murad F. Diabetes-associated nitration of tyrosine and inactivation of succinyl-CoA:3-oxoacid CoA-transferase. American journal of physiology. Heart and circulatory physiology. 2001;281:H2289–H2294. doi: 10.1152/ajpheart.2001.281.6.H2289. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Peng F, Tong W, Sun H, Xu N, Liu S. The nitrated proteome in heart mitochondria of the db/db mouse model: characterization of nitrated tyrosine residues in SCOT. Journal of proteome research. 2010;9:4254–4263. doi: 10.1021/pr100349g. [DOI] [PubMed] [Google Scholar]
- 19.Stephens JM, Sulway MJ, Watkins PJ. Relationship of blood acetoacetate and 3-hydroxybutyrate in diabetes. Diabetes. 1971;20:485–489. doi: 10.2337/diab.20.7.485. [DOI] [PubMed] [Google Scholar]
- 20.Nosadini R, Avogaro A, Trevisan R, Duner E, Marescotti C, Iori E, Cobelli C, Toffolo G. Acetoacetate and 3-hydroxybutyrate kinetics in obese and insulin-dependent diabetic humans. Am J Physiol. 1985;248:R611–R620. doi: 10.1152/ajpregu.1985.248.5.R611. [DOI] [PubMed] [Google Scholar]
- 21.Grinblat L, Pacheco Bolanos LF, Stoppani AO. Decreased rate of ketone-body oxidation and decreased activity of D-3-hydroxybutyrate dehydrogenase and succinyl-CoA:3-oxo-acid CoA-transferase in heart mitochondria of diabetic rats. Biochem J. 1986;240:49–56. doi: 10.1042/bj2400049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegardt FG. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J. 1999;338(Pt 3):569–582. [PMC free article] [PubMed] [Google Scholar]
- 23.Haces ML, Hernandez-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol. 2008;211:85–96. doi: 10.1016/j.expneurol.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2012;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF., Jr Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51:241–247. doi: 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]
- 26.Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis. 2007;193:328–334. doi: 10.1016/j.atherosclerosis.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Zitman-Gal T, Golan E, Green J, Bernheim J, Benchetrit S. Vitamin D receptor activation in a diabetic-like environment: potential role in the activity of the endothelial pro-inflammatory and thioredoxin pathways. The Journal of steroid biochemistry and molecular biology. 2012;132:1–7. doi: 10.1016/j.jsbmb.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Hall SE, Wastney ME, Bolton TM, Braaten JT, Berman M. Ketone body kinetics in humans: the effects of insulin-dependent diabetes, obesity, and starvation. J Lipid Res. 1984;25:1184–1194. [PubMed] [Google Scholar]
- 29.Sherwin RS, Hendler RG, Felig P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes. 1976;25:776–784. doi: 10.2337/diab.25.9.776. [DOI] [PubMed] [Google Scholar]
- 30.Jain SK, McVie R. Hyperketonemia can increase lipid peroxidation and lower glutathione levels in human erythrocytes in vitro and in type 1 diabetic patients. Diabetes. 1999;48:1850–1855. doi: 10.2337/diabetes.48.9.1850. [DOI] [PubMed] [Google Scholar]
- 31.Kanikarla-Marie P, Jain SK. Hyperketonemia (acetoacetate) upregulates NADPH oxidase 4 and elevates oxidative stress, icam-1, and monocyte adhesivity in endothelial cells. Cell Physiol Biochem. 2015;35:364–373. doi: 10.1159/000369702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelletier A, Coderre L. Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. Am J Physiol Endocrinol Metab. 2007;292:E1325–E1332. doi: 10.1152/ajpendo.00186.2006. [DOI] [PubMed] [Google Scholar]
- 33.Abdelmegeed MA, Kim SK, Woodcroft KJ, Novak RF. Acetoacetate activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in primary cultured rat hepatocytes: role of oxidative stress. J Pharmacol Exp Ther. 2004;310:728–736. doi: 10.1124/jpet.104.066522. [DOI] [PubMed] [Google Scholar]
- 34.Harrison JE, Saeed FA. Acetoacetate is an electron donor to myeloperoxidase and a promoter of myeloperoxidase-catalyzed fatty acid peroxidation. Biochem Med. 1981;26:339–355. doi: 10.1016/0006-2944(81)90010-7. [DOI] [PubMed] [Google Scholar]
- 35.Jain SK, Kannan K, Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med. 1998;25:1083–1088. doi: 10.1016/s0891-5849(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 36.Jain SK, Kannan K, Lim G, McVie R, Bocchini JA., Jr Hyperketonemia increases tumor necrosis factor-alpha secretion in cultured U937 monocytes and Type 1 diabetic patients and is apparently mediated by oxidative stress and cAMP deficiency. Diabetes. 2002;51:2287–2293. doi: 10.2337/diabetes.51.7.2287. [DOI] [PubMed] [Google Scholar]
- 37.Jain SK, McVie R, Jackson R, Levine SN, Lim G. Effect of hyperketonemia on plasma lipid peroxidation levels in diabetic patients. Diabetes Care. 1999;22:1171–1175. doi: 10.2337/diacare.22.7.1171. [DOI] [PubMed] [Google Scholar]
- 38.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 39.Close TE, Cepinskas G, Omatsu T, Rose KL, Summers K, Patterson EK, Fraser DD. Diabetic ketoacidosis elicits systemic inflammation associated with cerebrovascular endothelial cell dysfunction. Microcirculation. 2013;20:534–543. doi: 10.1111/micc.12053. [DOI] [PubMed] [Google Scholar]
- 40.Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:E298–E306. doi: 10.1152/ajpendo.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rains JL, Jain SK. Effect of hyperketonemia (Acetoacetate) on nuclear factor-kappaB and p38 mitogen-activated protein kinase activation mediated intercellular adhesion molecule 1 upregulation in endothelial cells. Metab Syndr Relat Disord. 2015;13:71–77. doi: 10.1089/met.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rains JL, Kanikarla-Marie P, Jain SK. Hyperketonemia induces upregulation of LFA-1 in monocytes, which is mediated by ROS and P38 MAPK activation. Can J Physiol Pharmacol. 2012;90:1642–1646. doi: 10.1139/y2012-131. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman WH, Siedlak SL, Wang Y, Castellani RJ, Smith MA. Oxidative damage is present in the fatal brain edema of diabetic ketoacidosis. Brain Res. 2011;1369:194–202. doi: 10.1016/j.brainres.2010.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houten SM, Chegary M, Te Brinke H, Wijnen WJ, Glatz JF, Luiken JJ, Wijburg FA, Wanders RJ. Pyruvate dehydrogenase kinase 4 expression is synergistically induced by AMP-activated protein kinase and fatty acids. Cell Mol Life Sci. 2009;66:1283–1294. doi: 10.1007/s00018-009-9066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frayn KN. The glucose-fatty acid cycle: a physiological perspective. Biochem Soc Trans. 2003;31:1115–1119. doi: 10.1042/bst0311115. [DOI] [PubMed] [Google Scholar]
- 46.Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995;80:1584–1590. doi: 10.1210/jcem.80.5.7745004. [DOI] [PubMed] [Google Scholar]
- 47.Yokoo H, Saitoh T, Shiraishi S, Yanagita T, Sugano T, Minami S, Kobayashi H, Wada A. Distinct effects of ketone bodies on down-regulation of cell surface insulin receptor and insulin receptor substrate-1 phosphorylation in adrenal chromaffin cells. J Pharmacol Exp Ther. 2003;304:994–1002. doi: 10.1124/jpet.102.044115. [DOI] [PubMed] [Google Scholar]
- 48.Tardif A, Julien N, Pelletier A, Thibault G, Srivastava AK, Chiasson JL, Coderre L. Chronic exposure to beta-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am J Physiol Endocrinol Metab. 2001;281:E1205–E1212. doi: 10.1152/ajpendo.2001.281.6.E1205. [DOI] [PubMed] [Google Scholar]
- 49.Yamada T, Zhang SJ, Westerblad H, Katz A. {beta}-Hydroxybutyrate inhibits insulin-mediated glucose transport in mouse oxidative muscle. Am J Physiol Endocrinol Metab. 2010;299:E364–E373. doi: 10.1152/ajpendo.00142.2010. [DOI] [PubMed] [Google Scholar]
- 50.Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, Spigelman AF, Wright RC, Plummer G, Suzuki K, Mackay JP, van de Bunt M, Gloyn AL, Ryan TE, Norquay LD, Brosnan MJ, Trimmer JK, Rolph TP, Kibbey RG, Manning Fox JE, Colmers WF, Shirihai OS, Neufer PD, Yeh ET, Newgard CB, MacDonald PE. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest. 2015;125:3847–3860. doi: 10.1172/JCI82498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vernier S, Chiu A, Schober J, Weber T, Nguyen P, Luer M, McPherson T, Wanda PE, Marshall CA, Rohatgi N, McDaniel ML, Greenberg AS, Kwon G. beta-cell metabolic alterations under chronic nutrient overload in rat and human islets. Islets. 2012;4:379–392. doi: 10.4161/isl.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powell LA, Warpeha KM, Xu W, Walker B, Trimble ER. High glucose decreases intracellular glutathione concentrations and upregulates inducible nitric oxide synthase gene expression in intestinal epithelial cells. Journal of molecular endocrinology. 2004;33:797–803. doi: 10.1677/jme.1.01671. [DOI] [PubMed] [Google Scholar]
- 53.Shi X, Li X, Li D, Li Y, Song Y, Deng Q, Wang J, Zhang Y, Ding H, Yin L, Wang Z, Liu G. beta-Hydroxybutyrate activates the NF-kappaB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell Physiol Biochem. 2014;33:920–932. doi: 10.1159/000358664. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman WH, Burek CL, Waller JL, Fisher LE, Jr, Khichi M, Mellick LB. Cytokine response to diabetic ketoacidosis and its treatment. Clinical Immunology. 2003;108:175–181. doi: 10.1016/s1521-6616(03)00144-x. [DOI] [PubMed] [Google Scholar]
- 55.Oglesbee MJ, Herdman AV, Passmore GG, Hoffman WH. Diabetic ketoacidosis increases extracellular levels of the major inducible 70-kDa heat shock protein. Clin Biochem. 2005;38:900–904. doi: 10.1016/j.clinbiochem.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Karavanaki K, Karanika E, Georga S, Bartzeliotou A, Tsouvalas M, Konstantopoulos I, Fotinou A, Papassotiriou I, Karayianni C. Cytokine response to diabetic ketoacidosis (DKA) in children with type 1 diabetes (T1DM) Endocr J. 2011;58:1045–1053. doi: 10.1507/endocrj.ej11-0024. [DOI] [PubMed] [Google Scholar]
- 57.Lommi J, Kupari M, Koskinen P, Naveri H, Leinonen H, Pulkki K, Harkonen M. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol. 1996;28:665–672. doi: 10.1016/0735-1097(96)00214-8. [DOI] [PubMed] [Google Scholar]
- 58.Julian MT, Alonso N, Ojanguren I, Pizarro E, Ballestar E, Puig-Domingo M. Hepatic glycogenosis: An underdiagnosed complication of diabetes mellitus? World J Diabetes. 2015;6:321–325. doi: 10.4239/wjd.v6.i2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giordano S, Martocchia A, Toussan L, Stefanelli M, Pastore F, Devito A, Risicato MG, Ruco L, Falaschi P. Diagnosis of hepatic glycogenosis in poorly controlled type 1 diabetes mellitus. World J Diabetes. 2014;5:882–888. doi: 10.4239/wjd.v5.i6.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bank IM, Shemie SD, Rosenblatt B, Bernard C, Mackie AS. Sudden cardiac death in association with the ketogenic diet. Pediatr Neurol. 2008;39:429–431. doi: 10.1016/j.pediatrneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Regnell SE, Lernmark A. Hepatic steatosis in type 1 diabetes. Rev Diabet Stud. 2011;8:454–467. doi: 10.1900/RDS.2011.8.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai F, Jiang FF, Lu JJ, Ma SG, Peng YG, Jin Y, Xu W, Cheng JP, Wu HF. The impact of hyperglycemic emergencies on the kidney and liver. J Diabetes Res. 2013;2013:967097. doi: 10.1155/2013/967097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu H, Hu F, Zeng Y, Zou L, Luo S, Sun Y, Liu H, Sun L. Ketosis Onset Type 2 Diabetes Had Better Islet beta-Cell Function and More Serious Insulin Resistance. J Diabetes Res. 2014;2014:510643. doi: 10.1155/2014/510643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stadler K, Bonini MG, Dallas S, Duma D, Mason RP, Kadiiska MB. Direct evidence of iNOS-mediated in vivo free radical production and protein oxidation in acetone-induced ketosis. Am J Physiol Endocrinol Metab. 2008;295:E456–E462. doi: 10.1152/ajpendo.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng RC, Sigmund CJ, Jr, Lagos JA, Chernin M. Hepatic infarction and diabetic ketoacidosis. Gastroenterology. 1977;73:804–807. [PubMed] [Google Scholar]
- 66.Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300:G956–G967. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schugar RC, Crawford PA. Low-carbohydrate ketogenic diets, glucose homeostasis, and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:374–380. doi: 10.1097/MCO.0b013e3283547157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanikarla-Marie P, Jain SK. Role of Hyperketonemia in Inducing Oxidative Stress and Cellular Damage in Cultured Hepatocytes and Type 1 Diabetic Rat Liver. Cell Physiol Biochem. 2015;37:2160–2170. doi: 10.1159/000438573. [DOI] [PubMed] [Google Scholar]
- 69.Cameron FJ, Scratch SE, Nadebaum C, Northam EA, Koves I, Jennings J, Finney K, Neil JJ, Wellard RM, Mackay M, Inder TE. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. 2014;37:1554–1562. doi: 10.2337/dc13-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wootton-Gorges SL, Buonocore MH, Kuppermann N, Marcin JP, Barnes PD, Neely EK, DiCarlo J, McCarthy T, Glaser NS. Cerebral proton magnetic resonance spectroscopy in children with diabetic ketoacidosis. AJNR Am J Neuroradiol. 2007;28:895–899. [PMC free article] [PubMed] [Google Scholar]
- 71.Siqueira LF. Cerebrovascular complications of diabetic ketoacidosis in children. Arq Bras Endocrinol Metabol. 2011;55:288–290. doi: 10.1590/s0004-27302011000400009. [DOI] [PubMed] [Google Scholar]
- 72.Foster JR, Morrison G, Fraser DD. Diabetic ketoacidosis-associated stroke in children and youth. Stroke Res Treat. 2011;2011:219706. doi: 10.4061/2011/219706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfsdorf J, Glaser N, Sperling MA. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150–1159. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 74.Stanimirovic DB, Wong J, Shapiro A, Durkin JP. Increase in surface expression of ICAM-1, VCAM-1 and E-selectin in human cerebromicrovascular endothelial cells subjected to ischemia-like insults. Acta Neurochir Suppl. 1997;70:12–16. doi: 10.1007/978-3-7091-6837-0_4. [DOI] [PubMed] [Google Scholar]
- 75.Whalen MJ, Carlos TM, Dixon CE, Robichaud P, Clark RS, Marion DW, Kochanek PM. Reduced brain edema after traumatic brain injury in mice deficient in P-selectin and intercellular adhesion molecule-1. J Leukoc Biol. 2000;67:160–168. doi: 10.1002/jlb.67.2.160. [DOI] [PubMed] [Google Scholar]
- 76.Hoffman WH, Cheng C, Passmore GG, Carroll JE, Hess D. Acetoacetate increases expression of intercellular adhesion molecule-1 (ICAM-1) in human brain microvascular endothelial cells. Neurosci Lett. 2002;334:71–74. doi: 10.1016/s0304-3940(02)00816-9. [DOI] [PubMed] [Google Scholar]
- 77.Schober E, Otto KP, Dost A, Jorch N, Holl R. Association of epilepsy and type 1 diabetes mellitus in children and adolescents: is there an increased risk for diabetic ketoacidosis? J Pediatr. 2012;160:662–666. e661. doi: 10.1016/j.jpeds.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 78.Tsalikian E, Becker DJ, Crumrine PK, Daneman D, Drash AL. Electroencephalographic changes in diabetic ketosis in children with newly and previously diagnosed insulin-dependent diabetes mellitus. J Pediatr. 1981;99:355–359. doi: 10.1016/s0022-3476(81)80317-4. [DOI] [PubMed] [Google Scholar]
- 79.Hauser E, Strohmayer C, Seidl R, Birnbacher R, Lischka A, Schober E. Quantitative EEG in young diabetics. J Child Neurol. 1995;10:330–334. doi: 10.1177/088307389501000419. [DOI] [PubMed] [Google Scholar]
- 80.Comstock JP, G A. Ketonuria. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 81.Owen O. Ketone Bodies as a Fuel for the Brain during Starvation. Biochemistry and molecular biology education. 2005;33:246–251. [Google Scholar]
- 82.Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E. The ketogenic diet: from molecular mechanisms to clinical effects. Epilepsy Res. 2006;68:145–180. doi: 10.1016/j.eplepsyres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Henderson ST. Ketone bodies as a therapeutic for Alzheimer's disease. Neurotherapeutics. 2008;5:470–480. doi: 10.1016/j.nurt.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vamecq J, Vallee L, Lesage F, Gressens P, Stables JP. Antiepileptic popular ketogenic diet: emerging twists in an ancient story. Prog Neurobiol. 2005;75:1–28. doi: 10.1016/j.pneurobio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Halevy A, Peleg-Weiss L, Cohen R, Shuper A. An update on the ketogenic diet, 2012. Rambam Maimonides Med J. 2012;3:e0005. doi: 10.5041/RMMJ.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartman AL, Vining EP. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. doi: 10.1111/j.1528-1167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 87.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behavioural pharmacology. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86:529–537. doi: 10.1046/j.1471-4159.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 89.Cheng B, Yang X, An L, Gao B, Liu X, Liu S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson's disease. Brain Res. 2009;1286:25–31. doi: 10.1016/j.brainres.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 90.Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. 2005;64:728–730. doi: 10.1212/01.WNL.0000152046.11390.45. [DOI] [PubMed] [Google Scholar]
- 91.Klepper J, Leiendecker B, Bredahl R, Athanassopoulos S, Heinen F, Gertsen E, Florcken A, Metz A, Voit T. Introduction of a ketogenic diet in young infants. J Inherit Metab Dis. 2002;25:449–460. doi: 10.1023/a:1021238900470. [DOI] [PubMed] [Google Scholar]
- 92.Klepper J, Voit T. Facilitated glucose transporter protein type 1 (GLUT1) deficiency syndrome: impaired glucose transport into brain-- a review. Eur J Pediatr. 2002;161:295–304. doi: 10.1007/s00431-002-0939-3. [DOI] [PubMed] [Google Scholar]
- 93.Falk RE, Cederbaum SD, Blass JP, Gibson GE, Kark RA, Carrel RE. Ketonic diet in the management of pyruvate dehydrogenase deficiency. Pediatrics. 1976;58:713–721. [PubMed] [Google Scholar]
- 94.Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28:109–121. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- 95.Chang HT, Olson LK, Schwartz KA. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond) 2013;10:47. doi: 10.1186/1743-7075-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Skinner R, Trujillo A, Ma X, Beierle EA. Ketone bodies inhibit the viability of human neuroblastoma cells. Journal of Pediatric Surgery. 2009;44:212–216. doi: 10.1016/j.jpedsurg.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 97.Stafford P, Abdelwahab MG, Kim do Y, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond) 2010;7:74. doi: 10.1186/1743-7075-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, Scheck AC. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7:e36197. doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seyfried TN, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Is the restricted ketogenic diet a viable alternative to the standard of care for managing malignant brain cancer? Epilepsy Res. 2012;100:310–326. doi: 10.1016/j.eplepsyres.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 100.Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol. 2006;35:1–5. doi: 10.1016/j.pediatrneurol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 101.Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45:1116–1123. doi: 10.1111/j.0013-9580.2004.10004.x. [DOI] [PubMed] [Google Scholar]
- 102.Best TH, Franz DN, Gilbert DL, Nelson DP, Epstein MR. Cardiac complications in pediatric patients on the ketogenic diet. Neurology. 2000;54:2328–2330. doi: 10.1212/wnl.54.12.2328. [DOI] [PubMed] [Google Scholar]
- 103.Ward Platt M, Deshpande S. Metabolic adaptation at birth. Seminars in Fetal and Neonatal Medicine. 2005;10:341–350. doi: 10.1016/j.siny.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 104.Bonnefont JP, Specola NB, Vassault A, Lombes A, Ogier H, de Klerk JB, Munnich A, Coude M, Paturneau-Jouas M, Saudubray JM. The fasting test in paediatrics: application to the diagnosis of pathological hypo- and hyperketotic states. Eur J Pediatr. 1990;150:80–85. doi: 10.1007/BF02072043. [DOI] [PubMed] [Google Scholar]
- 105.Saudubray JM, Marsac C, Limal JM, Dumurgier E, Charpentier C, Ogier H, Coude FX. Variation in plasma ketone bodies during a 24-hour fast in normal and in hypoglycemic children: relationship to age. J Pediatr. 1981;98:904–908. doi: 10.1016/s0022-3476(81)80583-5. [DOI] [PubMed] [Google Scholar]
- 106.Cahill GF., Jr Fuel metabolism in starvation. Annual review of nutrition. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 107.Cahill GF, Jr, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Jr, Kipnis DM. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966;45:1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paterson P, Sheath J, Taft P, Wood C. Maternal and foetal ketone concentrations in plasma and urine. Lancet. 1967;1:862–865. doi: 10.1016/s0140-6736(67)91426-2. [DOI] [PubMed] [Google Scholar]
- 109.Mitchell GA, F T. Inborn errors of ketone body metabolism. New York: McGraw-Hill; 2001. [Google Scholar]
- 110.Saudubray JM, Specola N, Middleton B, Lombes A, Bonnefont JP, Jakobs C, Vassault A, Charpentier C, Day R. Hyperketotic states due to inherited defects of ketolysis. Enzyme. 1987;38:80–90. doi: 10.1159/000469194. [DOI] [PubMed] [Google Scholar]
- 111.Duffens K, Marx JA. Alcoholic ketoacidosis--a review. J Emerg Med. 1987;5:399–406. doi: 10.1016/0736-4679(87)90146-6. [DOI] [PubMed] [Google Scholar]
- 112.Soffer A, Hamburger S. Alcoholic ketoacidosis: a review of 30 cases. J Am Med Womens Assoc. 1982;37:106–110. [PubMed] [Google Scholar]
- 113.Halperin ML, Hammeke M, Josse RG, Jungas RL. Metabolic acidosis in the alcoholic: a pathophysiologic approach. Metabolism. 1983;32:308–315. doi: 10.1016/0026-0495(83)90197-x. [DOI] [PubMed] [Google Scholar]
- 114.McGuire LC, Cruickshank AM, Munro PT. Alcoholic ketoacidosis. Emerg Med J. 2006;23:417–420. doi: 10.1136/emj.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jain H, Beriwal S, Singh S. Alcohol induced ketoacidosis, severe hypoglycemia and irreversible encephalopathy. Med Sci Monit. 2002;8:CS77–CS79. [PubMed] [Google Scholar]
- 116.Matsuzaki T, Shiraishi W, Iwanaga Y, Yamamoto A. Case of alcoholic ketoacidosis accompanied with severe hypoglycemia. J UOEH. 2015;37:43–47. doi: 10.7888/juoeh.37.43. [DOI] [PubMed] [Google Scholar]
- 117.Umpierrez GE, DiGirolamo M, Tuvlin JA, Isaacs SD, Bhoola SM, Kokko JP. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. Journal of critical care. 2000;15:52–59. doi: 10.1053/jcrc.2000.7900. [DOI] [PubMed] [Google Scholar]
- 118.American-Diabetes-Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 119.Candiloros H, Muller S, Zeghari N, Donner M, Drouin P, Ziegler O. Decreased erythrocyte membrane fluidity in poorly controlled IDDM. Influence of ketone bodies. Diabetes Care. 1995;18:549–551. doi: 10.2337/diacare.18.4.549. [DOI] [PubMed] [Google Scholar]
- 120.Chiasson JL, Aris-Jilwan N, Belanger R, Bertrand S, Beauregard H, Ekoe JM, Fournier H, Havrankova J. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. 2003;168:859–866. [PMC free article] [PubMed] [Google Scholar]
- 121.Sheikh-Ali M, Karon BS, Basu A, Kudva YC, Muller LA, Xu J, Schwenk WF, Miles JM. Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31:643–647. doi: 10.2337/dc07-1683. [DOI] [PubMed] [Google Scholar]
- 122.Wallace TM, Meston NM, Gardner SG, Matthews DR. The hospital and home use of a 30-second hand-held blood ketone meter: guidelines for clinical practice. Diabet Med. 2001;18:640–645. doi: 10.1046/j.1464-5491.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- 123.Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5:247–270. doi: 10.1002/dmr.5610050304. [DOI] [PubMed] [Google Scholar]
- 124.Fery F, Balasse EO. Ketone body production and disposal in diabetic ketosis. A comparison with fasting ketosis. Diabetes. 1985;34:326–332. doi: 10.2337/diab.34.4.326. [DOI] [PubMed] [Google Scholar]
- 125.Krane EJ. Diabetic ketoacidosis. Biochemistry, physiology, treatment, and prevention. Pediatr Clin North Am. 1987;34:935–960. doi: 10.1016/s0031-3955(16)36296-4. [DOI] [PubMed] [Google Scholar]
- 126.Klingensmith GJ, Tamborlane WV, Wood J, Haller MJ, Silverstein J, Cengiz E, Shanmugham S, Kollman C, Wong-Jacobson S, Beck RW. Diabetic Ketoacidosis at Diabetes Onset: Still an All Too Common Threat in Youth. J Pediatr. 2012;162:330–334. e331. doi: 10.1016/j.jpeds.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 127.Basu A, Close CF, Jenkins D, Krentz AJ, Nattrass M, Wright AD. Persisting mortality in diabetic ketoacidosis. Diabet Med. 1993;10:282–284. doi: 10.1111/j.1464-5491.1993.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 128.Pinkey JH, Bingley PJ, Sawtell PA, Dunger DB, Gale EA. Presentation and progress of childhood diabetes mellitus: a prospective population-based study. The Bart's-Oxford Study Group. Diabetologia. 1994;37:70–74. doi: 10.1007/BF00428780. [DOI] [PubMed] [Google Scholar]
- 129.Lebovitz HE. Diabetic ketoacidosis. Lancet. 1995;345:767–772. doi: 10.1016/s0140-6736(95)90645-2. [DOI] [PubMed] [Google Scholar]
- 130.Johnston DG, Pernet A, McCulloch A, Blesa-Malpica G, Burrin JM, Alberti KG. Some hormonal influences on glucose and ketone body metabolism in normal human subjects. Ciba Found Symp. 1982;87:168–191. doi: 10.1002/9780470720691.ch10. [DOI] [PubMed] [Google Scholar]
- 131.Weiss M, Keller U, Stauffacher W. Effect of epinephrine and somatostatin-induced insulin deficiency on ketone body kinetics and lipolysis in man. Diabetes. 1984;33:738–744. doi: 10.2337/diab.33.8.738. [DOI] [PubMed] [Google Scholar]
- 132.Yared Z, Chiasson JL. Ketoacidosis and the hyperosmolar hyperglycemic state in adult diabetic patients. Diagnosis and treatment. Minerva Med. 2003;94:409–418. [PubMed] [Google Scholar]
- 133.Mahendran Y, Vangipurapu J, Cederberg H, Stancakova A, Pihlajamaki J, Soininen P, Kangas AJ, Paananen J, Civelek M, Saleem NK, Pajukanta P, Lusis AJ, Bonnycastle LL, Morken MA, Collins FS, Mohlke KL, Boehnke M, Ala-Korpela M, Kuusisto J, Laakso M. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 finnish men. Diabetes. 2013;62:3618–3626. doi: 10.2337/db12-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kitabchi AE, Nyenwe EA. Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2006;35:725–751. doi: 10.1016/j.ecl.2006.09.006. viii. [DOI] [PubMed] [Google Scholar]
- 135.Delaney MF, Zisman A, Kettyle WM. Diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Endocrinol Metab Clin North Am. 2000;29:683–705. doi: 10.1016/s0889-8529(05)70159-6. V. [DOI] [PubMed] [Google Scholar]
- 136.Elmas ON, Akinci A, Bilir P. Tuberculous meningitis associated with diabetic ketoacidosis. J Clin Res Pediatr Endocrinol. 2011;3:222–224. doi: 10.4274/jcrpe.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tafakhori A, Tajdini M, Aghamollaii V. Diabetic ketoacidosis as a rare complication of electromyography and nerve conduction velocity examination. Neurol Neurochir Pol. 2012;46:607. doi: 10.5114/ninp.2012.32110. [DOI] [PubMed] [Google Scholar]
- 138.Vaccarezza MM, Silva WH. Dietary therapy is not the best option for refractory nonsurgical epilepsy. Epilepsia. 2015 doi: 10.1111/epi.13074. [DOI] [PubMed] [Google Scholar]
- 139.Ballaban-Gil K, Callahan C, O'Dell C, Pappo M, Moshe S, Shinnar S. Complications of the ketogenic diet. Epilepsia. 1998;39:744–748. doi: 10.1111/j.1528-1157.1998.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 140.Suo C, Liao J, Lu X, Fang K, Hu Y, Chen L, Cao D, Huang T, Li B, Li C. Efficacy and safety of the ketogenic diet in Chinese children. Seizure. 2013;22:174–178. doi: 10.1016/j.seizure.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 141.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 142.Bergqvist AG. Long-term monitoring of the ketogenic diet: Do's and Don'ts. Epilepsy Res. 2012;100:261–266. doi: 10.1016/j.eplepsyres.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 143.Stewart WA, Gordon K, Camfield P. Acute pancreatitis causing death in a child on the ketogenic diet. J Child Neurol. 2001;16:682. doi: 10.1177/088307380101600910. [DOI] [PubMed] [Google Scholar]