Abstract
Innate Defense Regulators (IDRs) are short synthetic peptides that target the host innate immune system via an intracellular adaptor protein which functions at key signaling nodes. In this work, further details of the mechanism of action of IDRs have been discovered.
The studies reported here show that the lead clinical IDR, SGX94, has broad-spectrum activity against Gram-negative and Gram-positive bacterial infections caused by intracellular or extracellular bacteria and also complements the actions of standard of care antibiotics. Based on in vivo and primary cell culture studies, this activity is shown to result from the primary action of SGX94 on tissue-resident cells and subsequent secondary signaling to activate myeloid-derived cells, resulting in enhanced bacterial clearance and increased survival. Data from non-clinical and clinical studies also show that SGX94 treatment modulates pro-inflammatory and anti-inflammatory cytokine levels, thereby mitigating the deleterious inflammatory consequences of innate immune activation. Since they act through host pathways to provide both broad-spectrum anti-infective capability as well as control of inflammation, IDRs are unlikely to be impacted by resistance mechanisms and offer potential clinical advantages in the fight against emerging and antibiotic resistant bacterial infections.
Keywords: Innate, Immune, Antibacterial, Anti-inflammatory, Anti-infective, Broad spectrum
1. Introduction
Innate immunity, with its dual impact on inflammation and infection, plays a significant role in many acute and chronic diseases, ranging from the bacterial, fungal and viral infections, to the inflammatory consequences of gastrointestinal disease (Elia et al. 2015) and to the less intuitive host inflammatory disorders such as oral mucositis (Sonis, 2010). Although the infection-clearing and inflammatory pathways of the innate immune system have been shown to be separable (Foster et al. 2007; Mehta and Jeffrey, 2015), successful therapeutic treatments directed at the innate immune system must balance the impact on these pathways. Therapies focused on innate immunity have primarily targeted either the inputs (e.g., toll-like receptor (TLR) agonists and antagonists (Lin et al. 2009)) or outputs (e.g., TNFα, IL-1 receptor antagonists) of the innate immune system. Such therapies dramatically bias the system towards either increased prophylactic infection-clearing action combined with (potentially harmful) inflammation or towards decreased inflammation with the side effect of increased susceptibility to infection.
The Innate Defense Regulators (IDRs) now offer an alternative approach to infectious diseases and other disorders by targeting a key integrating checkpoint in intracellular innate immune pathways, with the potential to separately modulate infection-clearing and inflammatory pathways to both decrease inflammation and increase infection-clearing responses (Scott et al. 2007; Yu et al. 2009). IDRs are a new class of short, synthetic peptides that were previously shown to interact directly with sequestosome-1 (sqstm; also known as p62) (Yu et al. 2009; Supplemental Figure 1), which is known to function downstream of the TLRs and other innate immune receptors (Into et al. 2010; Park et al. 2013; Kim and Ozato, 2009; Van der Vaart et al. 2014). p62 is an ancient protein, present in all mammals and highly conserved across species with 91% sequence identity between mouse and man and 99% between orangutan and man.
Innate defenses involve numerous cell types distributed throughout the body both in circulation and in tissue-resident compartments and are active at all environmental anatomical barriers (Janeway and Medzhitov, 2002). Given the evolving understanding of the complexity of innate immune interactions and immune surveillance at various anatomical locations, we have chosen to pursue a reductionist approach in the exploration of IDR function. Thus, we initially characterized IDR action in a variety of in vivo models, followed by in vitro and ex vivo evaluations. As previously reported for IDR-1 (Scott et al. 2007), and shown here for a clinical compound from the IDR class (SGX94), the IDRs improve outcome in murine models of both local and systemic infections with a broad array of bacterial pathogens whether administered prophylactically or therapeutically and either as a stand-alone agent or in conjunction with suboptimal antibiotic treatment. Simultaneously the IDRs mitigate deleterious inflammatory consequences of innate immune activation. The clinical compound, SGX94, is an analog of IDR-1, specifically designed to have more drug-like properties, including a lower net charge (and therefore decreased potential for systemic toxicity), smaller size (and therefore lower cost of goods), improved chemical stability and marginally improved biological stability.
Here we report non-clinical and clinical studies indicating that the therapeutic effects of SGX94 depend upon tissue-resident innate immune cells, an under-appreciated therapeutic target in the innate immune system. The data show that IDRs interact primarily with tissue resident cells that use myeloid derived cells (including macrophages) as secondary messengers, resulting in enhanced clearance of bacteria and increased survival after acute bacterial infection.
2. MATERIALS AND METHODS
2.1. Peptides and Endotoxin
SGX94 peptide (acetate salt, USAN: dusquetide) and trifluoroacetic acid (TFA) salts of analog peptides were synthesized by solid phase synthesis (CS-Bio Peptide Synthesizer, Model #CS136) using standard fluorenylmethoxycarbonyl (FMOC) chemistry protocols (Scott et al. 2007) and purified by cold ether precipitation. Purified peptides were analysed by high performance liquid chromatography (HPLC; Agilent Model 1100). Peptide purity was typically greater than 95%. All IDR peptides were formulated in aqueous solution for intravenous (IV) administration. LPS was purchased commercially (E. coli 0111:b4 LPS).
2.2. Cytokine / Chemokine analysis
Chemokine and cytokine concentrations in mouse peritoneal lavage samples were determined using ELISA kits according to the manufacturer’s instructions. ELISA kits for mouse RANTES (CCL5), IP-10 (CXCL10), KC and MIP-1α were purchased from R&D Systems; mouse IL-6 and TNF-α ELISA kits were purchased from BD Biosciences. Mouse TNF-α, IL-1β, and IL-6 levels in bronchiolar lavage fluid were measured using Luminex technology (Progenics) and IL-1ra levels were measured using an ELISA kit (R&D Systems).
Cytokine levels present in mouse cell culture supernatants were examined using Meso Scale Discovery (MSD®) Cytokine Assays with the SECTOR® Imager 2400 (MSD®). Data were analyzed using MSD® DISCOVERY WORKBENCH software (version 3.0.18). For some experiments, IL-10 was measured by ELISA (R&D Systems), according to the manufacturer’s instructions.
IL-1ra levels in the monkey plasma samples were quantified using an ELISA kit (Catalog No. DRA00B, R&D Systems). This human IL-1ra ELISA kit has been shown previously to be useful in quantification of monkey IL-1ra (Reyes and Coe, 1998; Xiao et al. 1999). Human cytokines and chemokines were quantified by Rules Based Medicine, Inc. utilizing their standard protocols (https://rbm.myriad.com/).
2.3. Differential Cell Counting
Cytospin slides were prepared by depositing cells from peritoneal lavage fluid onto microscope slides using a cytofuge (Stat Spin CytoFuge). Cells were then fixed and stained with Hemacolor regular stain set and visualized under a microscope. Macrophages and neutrophils were identified and counted in 3 different views, from which average values for each sample were determined.
2.4. Monocyte -Macrophage differentiation in vitro
Purified human peripheral blood mononuclear cells (PBMCs) were sourced from Stem Cell Technologies. Macrophage differentiation was induced by culturing the CD14+ monocytes with recombinant human GM-CSF (10 ng/ml, rhGM-CSF, R&D Systems) according to the protocol of Verreck et al. (2004). In short, monocytes were incubated for approximately 5 days in RPMI 1640 (ATCC) containing 10% heat-inactivated fetal bovine serum (FBSi) (FBS, Hyclone), 100 U/mL penicillin (Sigma-Aldrich), 100 μg/mL streptomycin (Sigma-Aldrich), 2 mM L-glutamine (Sigma-Aldrich), and GM-CSF. The effect of SGX94 on monocyte to macrophage differentiation was examined by pre-treating the monocytes with SGX94 (200 μM) for 30 minutes (min) prior to addition of growth factor. To determine the effect of pre-incubation with SGX94 on the differentiated macrophage phenotype, differentiated macrophages were then stimulated with various concentrations of LPS (10-1000 ng/ml) for 20 hours (h) and culture supernatants were analyzed for cytokines and chemokines.
2.5. Splenocyte supernatant transfer studies
The impact of SGX94 and peptide analogs on soluble factors released by mouse splenocytes was studied using in vitro supernatant transfer experiments. Spleen cells were isolated from 6-10 week old female C57BL/6 or BALB/c mice by digestion of spleens for 30 min at 37°C after injection of 0.13 Wünsch units/spleen of Liberase TM (Roche) in RPMI (ATCC) containing 10% Fetal Bovine Serum (FBS, Hyclone), 100 U/mL penicillin, 100 μg/mL streptomycin and 50 μM 2-mercaptoethanol (complete RPMI). Splenocytes in complete RPMI medium were cultured at 4×106 cells/mL in 24 well plates for 24 h after addition of 100 ng/mL LPS and 200 μM SGX94. Over the same 24 h period, mouse macrophages were cultured for 24 h in DMEM (ATCC) containing 10% FBS. Splenocyte supernatants were centrifuged and diluted to 20% in DMEM (ATCC) containing 10% FBS. The RAW264.7 culture medium was then aspirated and replaced with splenocyte-conditioned supernatants. After a further 24 h culture, TNFα, IL-6 and IL-10 cytokine levels were measured in the RAW264.7 culture supernatants.
2.6. Animal models
All experimental procedures using animals were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and performed in IACUC-approved research facilities with the approval of the facility’s Animal Care and Use Committee.
2.6.1 Pharmacokinetic (PK) studies in mice and cynomolgus monkeys
Blood samples were collected from mice administered 20, 60 or 90 mg/kg SGX94 daily over 14 days (3 mice/sex/group/time point) on Day 14 at 3, 15, 30 min, 1, 2, 3, 6, and 24 h post-dose. Following IV injection, plasma concentrations of SGX94 declined very rapidly in a multi-exponential manner in both sexes. Relatively high plasma clearance (~ 3 to 5 mL/min/kg) and very short mean residence time (MRT) ranging from 2 to 6 min was noted (Table 1). This suggested that no significant accumulation was occurring upon multiple daily dosing. The PK profile was also characterized in cynomolgus monkeys that had received 20, 80 or 160 mg/kg SGX94 daily for 14 days by slow (2-3 min) IV injection (3M+3F per dose level). Blood samples (approximately 1 mL each) were collected on Day 14 at: pre-dose, 0.05, 0.25, 0.5, 1, 2, 3, 6 and 24 h after SGX94 injection. K3EDTA plasma was prepared and SGX94 concentrations were determined by LC-MS/MS using validated methods (quantitation limit 0.1 ng/mL). PK analysis was performed using non-compartmental methods.
Table 1.
Plasma PK parameters for SGX94 in Mouse (CD-1), NHP (Cynomolgus macaques) and Human
| Species | Dose Level & Infusion Time | Maximum Concentration Cmax (ng/mL) ± SD |
Time of Maximum Concentration Tmax (min) ± SD |
Area under the Curve AUC 0-t (h*ng/mL) ± SD |
Mean Residence Time MRT (min) ± SD |
|---|---|---|---|---|---|
| Mouse (male) Day 14 | 20 mg/kg over ~1 min; N=2 | 23020 | 3 | 4498 | 2.3 |
| 60 mg/kg over ~1 min; N=3 | 41220 | 3 | 9288 | 1.8 | |
| 90 mg/kg over ~1 min; N=4 | 72170 | 3 | 17430 | 1.6 | |
| Mouse (female) Day 14 | 20 mg/kg over ~1 min; N=2 | 8079 | 3 | 1696 | 2.0 |
| 60 mg/kg over ~1 min; N=3 | 27130 | 3 | 6515 | 6.5 | |
| 90 mg/kg over ~1 min; N=4 | 45750 | 3 | 10400 | 1.8 | |
| NHP (male) Day 14 | 20 mg/kg over ~2-3 min; N=3 | 6650 ± 2519 | NR | 1328 ± 438 | 7.8 ± 6.6 |
| 80 mg/kg over ~2-3 min; N=3 | 85860 ± 25830 | NR | 19390 ± 6913 | 7.8 ± 2.4 | |
| 160 mg/kg over ~ 2-3 min; N=3 | 205500 ± 33420 | NR | 45950 ± 9567 | 7.2 ± 1.2 | |
| NHP (female) Day14 | 20 mg/kg over ~2-3 min; N=3 | 12400 ± 6411 | NR | 2412 ± 1231 | 6.6 ± 1.2 |
| 80 mg/kg over ~2-3 min; N=3 | 16870 ± 24750 | NR | 3361 ± 4709 | 55 ± 85 | |
| 160 mg/kg over ~ 2-3 min; N=3 | 178800 ± 132300 | NR | 36340 ± 26430 | 6.6 ± 1.2 | |
| Human (males + females) SAD |
0.15 mg/kg over 4 min; N=6 | 510 ± 222 | 4.0 ± 0.0 | 41 ± 15 | 5.4 ± 1.2 |
| 1.0 mg/kg over 4 min; N=5 | 4326 ± 2829 | 4.8 ± 1.8 | 323 ± 189 | 7.2 ± 0.6 | |
| 2.0 mg/kg over 4 min; N=6 | 5873 ± 2096 | 4.4 ± 0.8 | 491 ± 87 | 4.8 ± 1.2 | |
| 3.0 mg/kg over 4 min; N=6 | 14436 ± 6385 | 4.0 ± 0.0 | 1043 ± 428 | 3.6 ± 0.6 | |
| 5.0 mg/kg over 4 min; N=6 | 13636 ± 7838 | 4.9 ± 1 | 1152 ± 483 | 6.0 ± 2.4 | |
| 8.0 mg/kg over 4 min; N=6 | 31039 ± 19372 | 4.1 ± 0.4 | 2423 ± 1194 | 4.2 ± 0.6 | |
|
Human (males + females) MAD, day 7** |
0.5 mg/kg over 4 min; N=4 | 1164 ± 592 | 4.0 ± 0.0 | 89 ± 14 | 5.4 ± 0.6 |
| 1.5 mg/kg over 4 min; N=4 | 5541 ± 3723 | 4.5 ± 1 | 388 ± 280 | 3.6 ± 1.2 | |
| 3.0 mg/kg over 4 min; N=4 | 13407 ± 4427 | 4.3 ± 0.5 | 1001 ± 321 | 3.6 ± 0.6 | |
| 4.5 mg/kg over 4 min; N=4 | 16583 ± 10218 | 5.0 ± 1.1 | 1473 ± 677 | 3.6 ± 0.6 | |
| 6.5 mg/kg over 4 min; N=4 | 31391 ± 6673 | 4.0 ± 0.0 | 2108 ± 413 | 3.0 ± 0.0 |
Notes: NR = Not Reported; SAD = Single Ascending Dose; MAD = Multiple Ascending Dose; SD = Standard Deviation;
PK was assessed on both days 1 and 7 of the MAD phase, with no significant differences (no evidence of bioaccumulation). Day 1 data not shown.
2.6.2 Quantitative Whole Body Autoradiography (QWBA)
An IV bolus dose of 20 mg/kg [14C]SGX94 was administered to male CD-1 mice (n = 6) and animals were euthanized at 2, 5, 15, 30, 60 and 240 min post-dose. Each carcass was frozen, embedded, sectioned, and mounted for QWBA. Selected sections were exposed to phosphor image screens and tissue radioactivity concentrations were quantified from the whole-body autoradiograms using a validated image analysis system. An additional 3 animals received 20 mg/kg [14C]SGX94 and were euthanized at 5 min post-dose to assess the metabolite distribution in blood.
2.6.3. S. aureus peritoneal infections in mice
Six to 8 week old female CD-1 mice were infected by intraperitoneal (IP) injection with 6 × 107 colony forming units (CFU) S. aureus (Catalog No. 25923, ATCC) in 5% mucin. Control (saline) or SGX94 (TFA form; dissolved in sterile saline; 9.5 mg/kg) was administered IP 4 h after the infection. At 24 h after infection, mice were sacrificed and peritoneal lavage was performed by injecting 5 mL of PBS into the peritoneal cavity and withdrawing the lavage fluid. For enumeration of CFU in the peritoneal lavage fluid, diluted lavage fluid was plated on Mueller Hinton agar plates and incubated at 37°C for 24 h. Bacterial colonies were then counted and the CFU/mL was calculated for each sample.
2.6.4. MRSA peritoneal infections in mice
SGX94 or saline was administered IV to 4 week old female CF-1 mice at 50 mg/kg 48 h prior to bacterial infection (n = 10 per group). Mice were infected IP with 8.2 × 107 CFU of MRSA UC6685. Vancomycin was administered twice subcutaneously (SC) at 3 mg/kg, 1 and 5 h after bacterial infection. Survival was monitored once daily for 5 days.
2.6.5. MRSA bacteremia in mice
Female, BALB/c mice, 7 to 8 weeks old, were challenged IV with MRSA strain USA300 (1.1 – 2 ×107 CFU). Linezolid was prepared for oral dosing in an aqueous dosing vehicle of 0.5% methylcellulose and 5% PEG200. Five separate experiments have been completed with this model using BALB/c mice and one with nu/nu mice, utilising a range of SGX94 (IV) and linezolid (oral) dosing schedules. Representative results are shown in Figures 1d, 1h and 2a, b, c. The primary endpoint used to assess progress of the infection and drug activity was bacteremia-mediated death. Deaths were recorded up to 21 days. Animals were observed at least twice daily during the study period for signs of mortality and morbidity. Kaplan-Meier survival plots were prepared for the resulting data using GraphPad Prism v.4.0.
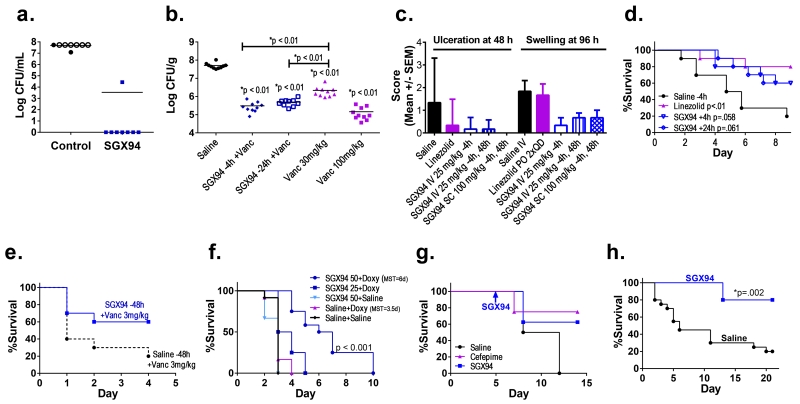
Figure 1. Broad-spectrum activity of SGX94 against bacterial infections.
a) Peritonitis: S. aureus infection in CD-1 mice (n=8/group). Control and SGX94 (9.5 mg/kg) were injected IP 4 h later. Bacterial counts were assessed 24 h after infection. Open symbols indicate dead mice. b) Thigh abscess: SGX94 (50 mg/kg) was administered IV at 24 h or 4 h before infection of neutropenic male CD-1 mice with MRSA (right thigh). Vancomycin (30 or 100 mg/kg) was given SC at 2 h and 14 h after infection. CFU counts were determined 26 h post-infection. c) Skin infection: SGX94 (n=6/gp) was administered IV or SC. Oral linezolid (12.5 mg/kg) was administered daily after infection with MRSA in CD-1 mice. A blinded board-certified pathologist scored the digital images. d) Bacteremia: SGX94 (5 mg/kg) or saline was administered IV 4 h or 24 h after infection of BALB/c mice with MRSA via the tail vein (n=10/group). A single dose of Linezolid (6.25 mg/kg) was administered orally after infection (n=10). e) Peritonitis: SGX94 (50 mg/kg) or saline treatment was administered IV 48 h before MRSA infection of female CF-1 mice (N=10/group), followed by vancomycin (3 mg/kg) was administered to all animals SC, 1 h and 5 h after infection. f) Melioidosis: Female BALB/c mice (N=12/group) were infected intranasally with B. pseudomallei. SGX94 (50 mg/kg) or saline was administered IV 4 h prior to infection and every second day to Day 8. Doxycycline (20 mg/kg) was administered orally upon infection and daily through Day 10. MST = median survival time. N=12/group. The combination of SGX94 + doxycycline was found to be more effective than the additive combination of each treatment alone (p<0.0001). g) Sepsis: Female Sprague-Dawley rats were infected with oral P. aeruginosa on Days 0, 2 and 4 (n=8 SGX94, Saline, n=4 Cefepime). With the onset of fever (Day 5), animals received SGX94 (10 mg/kg IV), Cefepime (40 mg/kg IM on Days 6-8) or saline (IM on Days 6-8). h) Bacteremia: SGX94 (50 mg/kg) or saline was administered IV 4 h prior to infection of BALB/c mice with MRSA via the tail vein (n=10/group). The animals were monitored for 21 days post-infection.
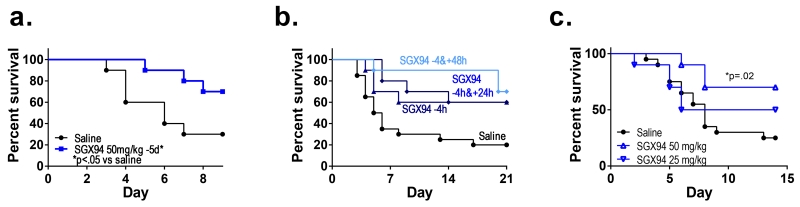
Figure 2. Prophylactic and persistent impacts of SGX94 on MRSA bacteremia.
a) SGX94 (50 mg/kg) or saline (n=10/group) was administered IV to female BALB/c mice 5 days prior to infection via the tail vein with MRSA (USA300, 1.9 × 107 CFU). The statistical significance of differences in survival were assessed using Kaplan Meier analysis. b) SGX94 (5 mg/kg) or saline (n=10/group) was administered IV at the indicated times to female BALB/c mice prior to or after infection via the tail vein with MRSA (USA300, 2 × 107 CFU). Sub-optimal antibiotic treatment (linezolid, 6.25 mg/kg) was administered orally immediately after infection. c) SGX94 (IV) or saline (IV) was administered once 4h prior to infection with MRSA (strain USA300, 1.1 × 107 CFU) via the tail vein into female nu/nu mice. Statistically significant (p≤0.05) increases in survival were found with the 50 mg/kg dose level relative to the saline control, as assessed using Kaplan Meier analysis.
2.6.6. MRSA skin infections in mice
Five groups (n=6/group) of 7-8 week old female, BALB/c mice were challenged by skin inoculation with MRSA strain USA300. On Day -1, each mouse was anesthetized with isoflurane and the lesion site was depilated using Nair®. On Day 0 (at -1 h) each mouse was anesthetized and the lesion site received 7 consecutive applications and removals of surgical tape (3M Nexcare™). At time zero, each animal on the study was administered a topical administration of 10 μl of the bacterial suspension, delivering a total challenge of 3.56×107 CFU per mouse. Group 1 received IV saline 4 h before and 48 h after bacterial challenge. Group 2 received daily oral administration of 12.5 mg/kg Linezolid beginning immediately following the bacterial challenge and continuing for 4 days following challenge. Group 3 received 25 mg/kg SGX94 IV 4 h before bacterial challenge. Group 4 received 25 mg/kg SGX94 IV 4 h before and 48 h after bacterial challenge, while Group 5 received 100 mg/kg SGX94 by SC administration 4 h before and 48 h after bacterial challenge.
Efficacy of test article to protect the skin from scab formation was assessed by digital photography at 48 and 96 h post-infection. A pathologist examined the images in a double-blinded fashion. Pathologic criteria to assess the skin images macroscopically were used as follows: Abrasions; Depressed / Glistening surface; Discoloration; Exfoliation; Scab formation; Swelling; Ulceration. Severity grades ranged from 0 (not present) to 4 (marked/ severe alterations present). At the completion of the evaluation, the data were unblinded and the group total and mean group scores were generated.
2.6.7. P. aeruginosa GI infections in rats
The neutropenic rat model has been described in detail previously (Opal et al. 2001). Specific pathogen-free, female, albino Sprague-Dawley rats (Charles River Laboratories) weighing 150 to 200 g were administered 10 mg/kg Cefamandole intramuscularly (IM) every 2 days between Days -4 and 10 to disrupt their gastrointestinal flora. Severe neutropenia was induced by administration of cyclophosphamide 75 mg/kg IP on Days 0 and 3. P. aeruginosa immunotype 6, strain 12.4.4 (1×106 CFU/ml) was given by orogastric feedings on Days 0, 2 and 4. Due to the breakdown of the gastrointestinal mucosal barrier, rats developed fever by Day 5, and began dying of sepsis by Day 7. SGX94 (10 mg/kg IV) or Saline (IV) was administered once after the appearance of fever. In another group of rats, Cefepime was administered on Days 6, 7 and 8 (25 mg/kg IM). Survival was monitored (N=8 SGX94, Saline, N=4 Cefepime). Differences in survival time between groups were analyzed by Kaplan-Meier survival plots and log-rank testing.
2.6.8. B. pseudomallei lung infection in mice
The efficacy of SGX94 in treating melioidosis was assessed in an acute pneumonic model of infection. Highly susceptible BALB/c mice were challenged intranasally with 3×103 CFU (~3×LD50) of the virulent strain 1026b of B. pseudomallei under BSL3 containment. Intranasal infection of the mice results in an acute disseminated infection with features typical of severe human melioidosis including extensive abscess formation in the lungs and dissemination to other organs such as the spleen and liver (Titball et al. 2008). Terminal illness in the mice is associated with bacterial proliferation in the lungs. SGX94 (50 mg/kg) or saline was administered IV 4 h prior to infection and every second day to Day 8. Sub-optimal Doxycycline (20 mg/kg administered orally upon infection and daily through Day 10) was used as the comparator antibiotic given its characterization in mouse models of melioidosis (Gelhaus et al. 2013) and because a sub-optimal antibiotic treatment enables any additive impact of IDR therapy to be more easily discerned. The log rank Mantel-Cox test was used. Synergy was also assessed, evaluating the treatment of SGX94 plus doxycycline against treatment with either SGX94 or doxycycline alone using one-way ANOVA with planned comparison (contrast/estimate statements). Duration to event was treated as a response variable and treatment with 3 levels as independent variable. The comparison tested Mean(SGX94) + Mean (Doxy)=Mean (Combo).
2.6.9. Pulmonary Inflammation in mice
SGX94 or saline was administered IV to 6-7 week old female BALB/c mice at 50 mg/kg (N=10/group). Pulmonary inflammation was induced by intranasal administration of LPS (Sigma) at 500 ng/mouse 1 h after SGX94 injection. Mice were euthanized 4 h after the LPS administration and bronchoalveolar lavage was performed. The non-cellular portion of the lavage fluid was analyzed for TNF-α, IL-1β, IL-6 and IL-1ra levels. Other than IL-1ra, none of the other parameters were significantly altered by SGX94 treatment at the selected timepoints. Statistical analysis compared the SGX94 treated group and the vehicle treated control groups with a one-tailed Student’s t-test using GraphPad Prism v5.03.
2.6.10. Neutropenic mice: Leukocyte recovery and MRSA thigh infections
CD-1 mice were rendered neutropenic by IP injection of 150 mg/kg and 50 (Supplemental Figure 2) or 100 mg/kg (Fig. 1b) of cyclophosphamide at 96 and 24 h prior to the experiment, respectively. These treatment regimens have been shown to induce severe neutropenia (≤10 neutrophils/mm3 blood) for at least 72 h (Zuluaga et al. 2006). To evaluate the impact of SGX94 on recovery of circulating leukocytes, female mice were used (Supplemental Figure 2). In a separate experiment, neutropenic male mice (n = 10 per group) were treated IV with 50 mg/kg SGX94 or saline 4 or 24 h prior to IM infection with MRSA (1.24 × 105 CFU/mouse) in 1 thigh. In other groups, vancomycin (30 mg/kg or 100 mg/kg, SC) was administered at 2 and 14 h after the infection. For concomitant vancomycin and SGX94 treatment, mice were given 30 mg/kg of vancomycin. Muscle of the infected thigh was removed 26 h after infection and homogenized. Homogenate supernatants were plated for CFU determination (Fig. 1b).
2.6.11. Cynomolgus monkeys
One male and 1 female cynomolgus monkey (Macaca fascicularis) were obtained from Worldwide Primates Inc. At the start of treatment, both animals were young adults (approximately 3 years old weighing 3.2 kg [male] and 2.4 kg [female]). SGX94 was administered to each monkey at dose levels of 3, 10, 30, 90, 180, and then 240 mg/kg as single slow IV injections (over at least 60 seconds) on 6 separate days, with a washout period of at least 3 days between injections. Blood samples were collected on K3EDTA from all animals following the first (3 mg/kg), third (30 mg/kg) and sixth (240 mg/kg) dosing occasions at 8 timepoints (3, 15, 30 min, 1, 2, 6, 10, 24 h post-dose). Samples were centrifuged at 1500g RCF for 10 min to obtain plasma and immediately frozen at −70°C.
2.7. Phase 1 Clinical Study
A Phase 1, single centre, placebo-controlled, randomized, double-blind, single- and multiple-ascending dose (SAD; MAD) study has been completed to evaluate the safety, tolerability and PK of SGX94 injectable solution in normal healthy volunteers. All subjects signed informed consent after a full explanation of the potential risks. A total of 54 subjects (20 females and 34 males), age range 18-55 years, were enrolled into the SAD phase and a total of 30 subjects (5 females and 25 males), age range 18-55 years, were enrolled into the MAD phase. In the SAD phase, a total of 6 dose levels of SGX94 (0.15, 1, 2, 3, 5 and 8 mg/kg) were evaluated. Subjects were randomized within each of Cohorts 1 to 6 to receive a single IV infusion of SGX94 at a single dose level, or placebo. Cohorts were dosed sequentially with ascending doses of SGX94. Cohorts 1 and 2 consisted of 11 subjects (n=8 SGX94, n=3 Placebo) including 3 sentinels who received 60 min infusions (n=2 SGX94, n=1 Placebo) and 8 subjects who received 4 min infusions (n=6 SGX94, n=2 Placebo). Cohorts 3 to 6 consisted of 8 subjects (n=6 SGX94, n=2 Placebo) receiving 4 min infusions.
In the MAD phase, a total of 5 dose levels (0.5, 1.5, 3, 4.5 and 6.5 mg/kg daily) were evaluated, each administered daily over 7 days. Subjects were randomized within each of Cohorts 7 to 11 to receive daily IV 4 min infusions of SGX94 (n=4) or placebo (n=2).
Blood samples were taken at predetermined times from all subjects in both phases of the study for both PK (Table 1) and pharmacodynamic (PD) analysis. In the SAD phase and on days 1 and 7 of the MAD phase, blood samples for PK analyses were collected within 1 h prior to infusion, 4 (immediately after infusion), 6, 9, 15, 20, 30, and 45 min and at 1, 1.5, 2, 3, 4, 6, 8, 12, 16 and 24 h after the start of infusion. Plasma was prepared (K3EDTA) and SGX94 concentrations were determined by LC-MS/MS using GLP validated methods (quantitation limit 0.1 ng/mL). PK analyses were performed using non-compartmental methods.
Blood samples for the PD analyses reported here were drawn at 1 h after SGX94 or saline infusion (4 min infusion only). To determine the PD impact of SGX94, cytokine and chemokine levels were quantified after 4 h of in vitro stimulation of whole blood with LPS (E. coli). Samples were subsequently analyzed by Rules Based Medicine, Inc. utilizing their standard protocols. The following panel of cytokine and chemokine analytes were quantified: IL-1α; IL-1β; IL-6; IL-1ra; TNFα; TNF RII; MCP-1; MIP-1α (CCL3); MIP-1β (CCL4); and RANTES. To increase sample sizes for comparison purposes, data from the SAD and Day 1 of the MAD was combined into 2 dose groups, “low dose” from 0.15 – 2.0 mg/kg (n=26) and a “high dose” from 3.0-8.0 mg/kg (n=30), resulting in roughly equal sample numbers. Similarly, results from SAD and Day 1 MAD subjects receiving placebo were combined (n=22). As expected, given the clinical literature on cytokines and chemokines, initial data analysis revealed a high inter-individual variability in quantitative in vitro responses to LPS. The analyte level at each timepoint for each individual was therefore divided by the pre-dose analyte level for that individual, yielding a self-normalized activity ratio (AR) for each analyte and each individual at every timepoint.
RESULTS
3.1. IDRs enhance clearance of bacterial infection without bactericidal activity and are complementary to antibiotic action
SGX94 enhanced bacterial clearance and / or survival in murine infection models with Gram-positive antibiotic sensitive S. aureus (Fig. 1a) and antibiotic resistant methicillin-resistant Staphylococcus aureus (MRSA; Fig. 1b-e, h) as well as Gram-negative P. aeruginosa (Fig. 1g) and antibiotic resistant B. pseudomallei (Fig. 1f). In addition to this broad spectrum protection, SGX94 is also systemically active, since not only SC administration for skin infections (Fig. 1c), IP administration for peritoneal infection (Fig. 1a) and IM administration for thigh infections (data not shown) but also systemic administration is protective at a variety of anatomical locations including skin (Fig. 1c), tissue (Fig. 1b and 1e), blood (Fig. 1d, h, Fig. 2), gastrointestinal induced septicemia (Fig. 1g), and lung induced septicemia (Fig. 1f). SGX94 treatment, whether administered prophylactically (Fig. 1b-c, 1e, 1h; Fig. 2a) or therapeutically (up to 5 days after infection – Fig. 1a, d, g), is effective when given alone. Dose response studies in the MRSA bacteremia models suggested that efficacy could be demonstrated at doses between 5 and 50 mg/kg, with robust results obtained between 25 and 50 mg/kg IV (Supplemental Table 1). Other infection studies commonly used dose levels between 5 and 50 mg/kg for IV administration, while SC and IM injections generally utilized higher concentrations on the assumption that systemic delivery was required. No dose response studies with non-IV routes of administration have been conducted.
By contrast, when SGX94 was evaluated for direct antimicrobial activity using standard minimum inhibitory concentration (MIC) assays with S. aureus and E. coli it showed no significant activity against either organism. SGX94 was tested in 2 separate experiments for each bacterial strain in serial 1:2 dilutions from 200 μM to 6.25 μM while erythromycin (S. aureus assay) and Polymixin B (E. coli assay) were tested in serial 1:2 dilutions from 200 μg/mL to 6.24 μg/mL. SGX94 showed no activity (MIC > 200 μM = 111 μg/mL); by comparison, erythromycin and polymycin B were both active at < 3.125 μg/ml. Similarly, neither bacteriostatic nor bactericidal activity was detected with SGX94 treatment of either S. aureus or E. coli using a sensitive proliferation assay (Supplemental Table 2). These data indicate that the anti-infective impact of IDRs depends upon interaction with host defense systems. The observed complementarity between SGX94 treatment and antibiotic action (Fig. 1b, 1e, 1f) is consistent with this model.
The broad-spectrum applicability of the IDRs is particularly noteworthy in view of the varying biological niches that each of these bacteria occupy, ranging from primarily extracellular pathogens (S. aureus) to facultative intracellular pathogens (B. pseudomallei, a category B biothreat agent). This broad scope of action suggests that IDRs function as upstream modulators of multiple pathways simultaneously. To explore this concept in more detail, the PK and PD of SGX94 were further investigated.
3.2. IDRs have a short pharmacokinetic half-life and a prolonged pharmacodynamic impact
Studies of SGX94 in cell cultures, plasma and blood indicate a rapid rate of degradation unless stabilized by the application of protease inhibitors and / or rapid cooling. Thus, any SGX94 activity must not be dependent on its enduring extracellular presence.
The PK profile of SGX94 has been characterized in mouse, non-human primate and humans. As would be expected for a 5-amino acid peptide composed entirely of L-amino acids, the PK half-life is very short (minutes), is consistent between species and shows no evidence of bioaccumulation after multiple daily dosing (Table 1). Moreover, since short peptides are primarily degraded by blood and tissue resident proteases and do not depend on liver or kidney metabolism, IDRs would not be expected to interfere with the pharmacokinetics of other drugs, including potentially complementary drugs such as antibiotics. QWBA data showed drug-derived radioactivity to be rapidly and widely distributed, with significant accumulation in stomach, large intestine, small intestine, adrenal gland, kidney, pancreas, liver, lung and salivary glands (data not shown). Additional animals from the same study were analyzed for blood metabolism and by 5 min post-dose the major analyte in blood was a metabolite of SGX94, consistent with the rapid mean residence time (Table 1). Consistent with microsomal stability studies, the major metabolites resulted from the removal of the N-terminal amino acids, suggesting the major degradation pathway was driven by N-terminal peptidases both in vivo and in vitro. Concentrations in all tissues were lower than those in blood at 2 min and in most tissues at 5 min post-dose. Radioactive signals after 5 min post-dose likely represented amino acid recycling of the labelled metabolite, given the metabolite results cited above. Concentrations in the central nervous system (CNS), bone, white adipose and eye lens were the lowest of all tissues and were not observed within 2 min of dosing, indicating low penetration of SGX94 into these tissues.
All studies to date (stability in blood, plasma and cell culture, in vivo pharmacokinetics and metabolite profiling) support a rapid extracellular degradation of SGX94. The in vitro activity of IDRs on selective p62 complex formation (e.g., increased p62:RIP-1 complex formation but not p62:PKCz) has been demonstrated within 30 min (Yu et al. 2009). Thus, while the “on-rate” of SGX94 into the cell and on the target is apparently rapid (within 30 min), the “off-rate” has not been determined and the persistence of binding of SGX94 to the target protein is not known. Competitive assays have demonstrated that SGX94 can be competed off and that the binding is not covalent (Yu et al. 2009) suggesting that some kinetic on-off behavior would be expected and would expose SGX94 to intracellular peptidase action.
The PD profile of SGX94 has been evaluated in a number of contexts. As shown in Fig. 2a, prophylactic administration of SGX94 up to 5 days prior to infection enhances survival. Similar results are seen in peritoneal infection models with administration up to 48 h prior to infection (Fig. 1e). Moreover the impact of SGX94 is enduring (Fig. 1h). The impact of SGX94 on very severe infection was explored by administration of additional doses of SGX94 between 8 and 76 h post-dose (Fig. 2b). Second doses administered within 8 and 28 h of the first dose did not increase survival, while doses administered between 52 and 76 h after the first dose were additively effective. In light of the short half-life of SGX94, these data suggest that a persistent change in cell state is triggered by IDR treatment.
3.3. IDRs alter cellular recruitment and are effective in immune-deficient animals
Early work suggested that IDR action was driven by myeloid derived cells, as it is abrogated by treatment with clodronate (Scott et al. 2007). In contrast, however, SGX94 was shown to be active in animals rendered neutropenic and leukopenic by the administration of cyclophosphamide (Fig. 1b, 1g), with an activity profile similar to that seen in immunocompetent animals (Fig. 1a, 1c-f, Fig 2a-b). SGX94 was also active in protection of T-cell deficient nu/nu mice (Fig. 2c), while Scott et al (2007) reported that IDR-1, a related peptide, was active against S. aureus peritoneal infection in Rag-1−/− knock-out mice. Moreover, SGX94 does not impact the recovery profile of circulating leukocytes following leukopenia induced by cyclophosphamide (Supplemental Figure 2). These results suggest that the main actor in response to IDRs is not a circulating leukocyte; although, a direct study of NK cells has not been undertaken.
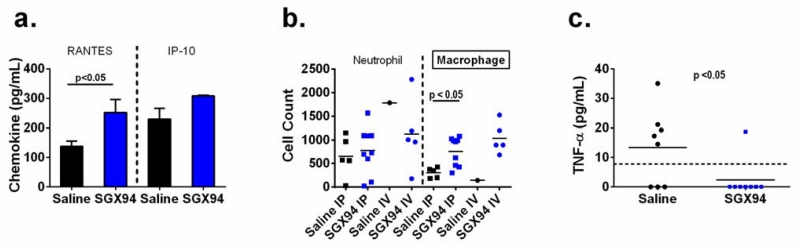
Evaluations of cytokine expression and cellular recruitment to the site of a peritoneal infection revealed that the chemokines RANTES (CCL-5) and IP-10 (CXCL-10) were elevated at 3 h post-infection in SGX94-treated mice (Fig. 3a), consistent with the observed increase in recruitment of macrophages (but not neutrophils) to the site of infection (Fig. 3b). Decreased pro-inflammatory signaling (TNFα) was observed in the same system, most markedly at later timepoints (Fig. 3c). The possibility was therefore considered that IDRs might alter the maturation of monocytes into M1 or M2 macrophages, as has been reported for other cationic peptides such as lactoferrin (van der Does et al. 2010a) and LL-37 (van der Does et al. 2010b). Accordingly, CD14+ mononuclear cells were differentiated to M1- or M2-type macrophages by GM-CSF or M-CSF exposure, respectively, in the presence and absence of 30 min pre-treatment with SGX94. After 5 days of differentiation, the behaviour of the macrophages was assessed by stimulating them with endotoxin (LPS). The ability of the growth factors to drive differentiation was not impacted by SGX94, although the degree of inflammatory response of the M2 macrophages was somewhat modulated with small statistically significant decreases in TNFα and IFNγ (Supplemental Figure 3).
Figure 3. Cytokines and cell migration during S. aureus peritoneal infection.
a) SGX94 (30 mg/kg) was administered IP to female CD-1 mice (n=8/group). 24 h later an inoculum of S. aureus (Catalog No. 25923, ATCC, ~0.5-1 × 108 CFU) with 5% mucin was injected IP. Mice were sacrificed 3 h after infection and peritoneal lavage fluid was assessed for cytokine levels. b) Female CD-1 mice were injected IP with S. aureus (Catalog No. 25923, ATCC, 8.4 × 107 CFU) with 5% mucin. Saline (n=8/group) or SGX94 (15 mg/kg; n=12/group) was administered IP or IV 4h after infection. Mice were sacrificed 24 h after infection and peritoneal lavage fluid was assessed for macrophage and neutrophil numbers. Only 1/8 animals survived in the control saline IV group, whereas 5/12 and 9/12 animals survived in the SGX94-treated IV and IP groups, respectively. Each data point represents a single animal and the bars represent average cell counts for each treatment group. c) SGX94 (30 mg/kg) was administered IP to female CD-1 mice (n=8/group) 24 h prior to an IP inoculation with a low dose of S. aureus (Catalog No. 25923, ATCC, 1.8 × 106 CFU) with 5% mucin. Mice were sacrificed 24 h after infection and peritoneal lavage fluid was assessed for TNFα levels. A t-test was performed comparing the SGX94 and saline groups, assuming that any data point with a value less than the detection limit of the assay (dotted line) was 0.
3.4. IDR effects on signaling by tissue-resident cells
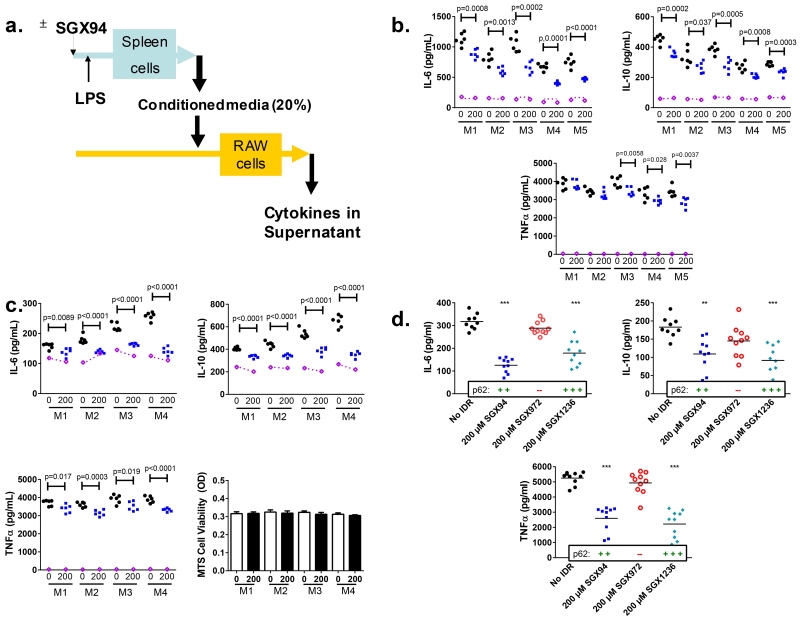
It was postulated that the key responding population may be tissue-resident and studies were undertaken with splenocytes, an immune active and accessible organ containing a heterogeneous population of cell types. Studies to detect any impact of SGX94 exposure on the responses of mouse splenocytes directly stimulated with LPS, LPS ± IFNγ, CpG, Flagellin, PGN, poly(I:C), or TNFα did not reveal any robust or consistent changes in cytokine/chemokine signaling (Fig. 4 – see purple lines). However, since monocytes/macrophages have been shown to be integral to the protective IDR effect (Scott et al. 2007) (Fig. 3b), in vitro supernatant transfer experiments were also undertaken, utilizing cell culture supernatants from mouse splenocytes stimulated with LPS in the presence or absence of SGX94 (Fig. 4a). Conditioned media from these splenocyte cultures were used to culture responder cells from a mouse macrophage cell line (RAW264.7), which lead to statistically significant and reproducible alterations in macrophage responsiveness (Fig. 4). Conditioned medium from each mouse splenocyte preparation was also measured directly for cytokine levels, confirming that the changes after RAW cell incubation were not due to direct differences in the given analytes in the original conditioned media (Supplemental Figure 4a). Similarly, RAW cells are not consistently responsive to SGX94 with LPS stimulation (Supplemental Figure 4b). Viability studies were also undertaken and confirmed that cellular viability did not vary across the treatment conditions (Fig. 4c). Identical results were obtained with splenocytes from BALB/c mice that are Th2 and M2 biased (Locksley et al. 1987; Gessner et al. 1993; Sellers et al. 2012) (Fig. 4b) and C57BL/6 mice that are Th1 and M1 biased (Locksley et al. 1987; Gessner et al. 1993; Sellers et al. 2012) (Fig. 4c), indicating that the IDR response is not dependent on either T-cell or macrophage phenotype bias. This was in agreement with the efficacy observed in various mouse strains (Fig. 1, Fig. 2) (Scott et al. 2007).
Figure 4. Splenocyte supernatant transfer study.
a) Schematic of study design b and c) BALB/c (b) and C57BL/6 (c) splenocytes were pre-incubated with control (black circles) or SGX94 (blue square), 200 μM, as described in the methods and the supernatant medium was applied to the RAW264.7 cells. M1 through M5 indicate splenocyte cultures from individual mice. Purple symbols and lines on the graphs indicate the “input” levels of the cytokines from the splenocyte conditioned media that was applied to the RAW cells. Each symbol represents one well of the RAW cell culture. d) C57BL/6 splenocytes were pre-incubated with each IDR, 200μM, as described in the methods and the pooled supernatant medium was applied to the RAW264.7 cells.
While IDRs have not previously been observed to bind endotoxin directly, it remained possible that inactivation of the negatively charged LPS by the cationic IDRs caused the reductions in cytokine/chemokine levels. The time separation and mixing between endotoxin and SGX94 additions and the elapsed time between SGX94 addition and application of the conditioned media to RAW cells suggested this was not the case. Attempts to remove potential LPS impact by polymyxin B addition were inconclusive because the residual LPS is necessary to stimulate the RAW cell preparation. Therefore, IDR analogs were used as indirect controls to assess the potential impact of direct IDR binding to endotoxin. These included an analogue with a single D-amino acid substitution (SGX972) that does not bind to the protein p62 but maintains the same net charge, and a stronger p62 binder with more structural differences (SGX1236). The expected SGX94 effect was observed, while the stronger binder showed a more significant impact and the non-binder, SGX972, had no impact (Fig. 4d). Given the charge and sequence similarity between SGX94 and the non-binder SGX972, these results support the interpretation that the differences in cytokine responses are not attributable to direct interaction between the IDRs and LPS but correlate with binding to the p62 target protein.
3.5. SGX94 is anti-inflammatory and consistent between species
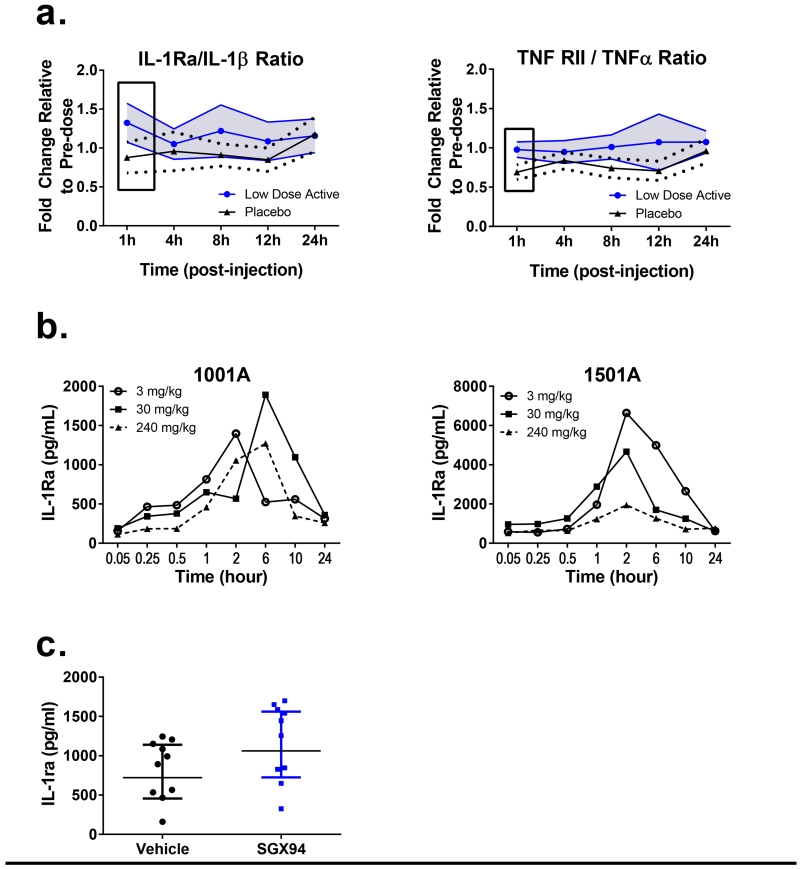
Upon stimulation of whole animals or mixed cell populations in vitro with LPS, SGX94 modulated TNFα, IL-6 and other inflammatory cytokine responses (Fig. 3a, c, Fig. 4a-b). The effect involved multiple cytokines and chemokines, tended to be subtle (i.e., approximately 30-50% suppression) and was associated with coordinated impact on anti-inflammatory cytokines. Consistent with these data, in a Phase 1 human study, where blood taken from humans after SGX94 administration was challenged ex vivo with LPS, TNFα responses were found to decrease while anti-inflammatory TNF RII responses increased, resulting in an increase in the ratio of TNF RII / TNFα (Fig. 5a). Similarly, IL-1β response was found to decrease while IL1-ra increased, resulting in an increase in the IL-1ra/IL-1β ratio (Fig. 5a). While the TNF RII, TNFβ, IL-1ra, IL-1α and IL-1β have minimally overlapping 95% confidence intervals (p-values could not be determined since the analysis method was not defined a priori), other analytes including IL-6, MCP-1, MIP-1α, MIP-1β and RANTES were highly overlapping (Supplemental Figure 5). In addition, while non-overlapping responses were observed for the low-dose group, the high dose group did not demonstrate similar responses and overlapped the placebo population very closely (data not shown), suggesting a suppression of response at higher dose levels. Note that the high dose levels correspond to mouse doses of 40-96 mg/kg, while efficacy testing has generally been limited to no higher than 50 mg/kg in the mouse.
Figure 5. Consistent cytokine profiles across species.
a) In a Phase 1 study in healthy human volunteers blood samples were obtained 1 h post-treatment from the placebo (n=22) and SGX94-treated populations (Low dose: 0.15-2.0 mg/kg, n=26; High dose: 3.0-8 mg/kg, n=30). Blood samples were immediately incubated with LPS (E. coli) for 4 h and analyzed for cytokine/chemokine responses. Placebo group data and combined data from low dose groups of the SAD and MAD data sets are represented in Figure 5a as the ratios of IL-1ra/IL-1β and of TNF RII / TNFα responses, respectively. Solid lines with symbols in Figure 5a indicate the mean responses of the placebo (black triangles) and low dose (blue circles) populations, dashed black lines and solid blue lines without symbols indicate the respective 95% Confidence Intervals (CI) for the placebo and low dose populations, respectively. The 95% CI for the SGX94 treated population is also shaded to emphasize areas of minimal overlap between the 2 populations. Boxes indicate non- or minimally-overlapping 95% CI between the 2 populations. b) SGX94 was administered to one male (1001A) and one female (1501A) cynomolgus monkey at the indicated doses and blood samples were collected up to 24 h post-dose. The levels of IL-1ra in the plasma are shown. c) 50 mg/kg SGX94 or saline was administered IV to female BALB/c mice (n=10/group). One hour later, 500 ng of LPS was administered intranasally in a volume of 30 μL. Four hours after LPS administration, bronchoalveolar lavage was collected. Statistical significance was assessed using a one-sided t-test.
IL-1ra was also found to be responsive in healthy non-human primates (Fig. 5b); note however that the study evaluated increasing dose in the same two individuals. It is unclear if the lack of dose response represents an exhaustion of the IL-1ra response over time or a true reduction in response at higher dose levels. Either explanation would be reasonable given that immunomodulatory compounds are generally known to have non-linear dose response relationships. IL-1ra levels were also observed to change in mice (Figure 5c) after an inflammatory stimulus. Despite changing conditions, IL-1ra levels were found to respond in all species tested, indicating a consistency in the biological response to SGX94 across species with highly conserved innate immune systems.
DISCUSSION
The rise of emerging and antibiotic resistant infectious disease poses increasing challenges in both the developing and developed world. New antibiotic classes are difficult to find and face the same threat of rapidly emergent resistance. A complementary approach to infectious disease would be to further harness the innate immune system. Early attempts to direct innate defenses focused on altering specific inputs or outputs to the system. Compounds like TLR agonists work well as prophylactic treatment for infection, however, their function in also triggering inflammation make them less useful in a therapeutic setting. Similarly, therapies targeted at the inflammatory outputs of the innate immune system also suppress the infection-fighting aspects of the innate immune system (Cantini et al. 2014, 2015; Fleischmann et al. 2003). Modulation of the interplay between infection-fighting and inflammatory signaling may require an alternative approach – i.e., acting on the intracellular signaling pathways that direct the subsequent signaling. IDRs provide a potential tool to accomplish this by targeting the intracellular protein, sequestosome-1 (p62) (Yu et al. 2009), which functions as an adaptor protein downstream of many innate defense receptors (Into et al. 2010; Park et al. 2013; Kim and Ozato, 2009; Van der Vaart et al. 2014).
In studies reported here and those reported previously (Scott et al. 2007), IDRs – while possessing no direct anti-bacterial activity - show broad-spectrum activity against infections with bacteria which occupy differing biological niches – from the primarily extracellular pathogens like S. aureus to the facultative intracellular bacteria like B. pseudomallei. These extensive in vivo evaluations indicate that the IDR SGX94 fulfills the original promise of IDRs – being agnostic to the specific bacterial threat, effective when administered prophylactically or therapeutically, and complementary to antibiotics. Because IDRs are agnostic, they can be administered before the specific causative infectious agent is known. Since IDRs are unlikely to engender resistance, they can also be given to at-risk patients without fear of increasing antibiotic resistance. The use of IDRs may also be antibiotic-sparing, potentially reducing the generation of antibiotic resistance.
In distinct contrast to their rapid degradation and clearance from blood, cells and in cell culture, the pharmacodynamic action of SGX94 is prolonged both in vitro and in vivo, while its therapeutic impact is rapid (Figs. 1, 2). These data suggest that SGX94 triggers an immediate response in the innate immune system which is then sustained in the absence of continued extracellular presence of SGX94. This implies that the IDR effect is mediated on the cellular level by altering the “cell state” of subpopulations of cells (as yet uncharacterised). This interpretation is supported by the exhaustion of the IDR response – such that re-administration of SGX94 within a 24-48 h window has no discernible additive benefit (Fig. 2b). Following this logic, it would appear that the cell state responsive to IDR action is renewed on an approximately 48-72 h cycle.
The data reported here show that SGX94, like IDR-1 (Scott et al 2007), is effective despite immune suppression, including severe leukopenia. The innate immune system is composed of both circulating and tissue-resident cells and is particularly active at all anatomical barriers between the host and its environment – including in the lung, skin and gastrointestinal tract (Hackett, 2003; Janeway, 1989). IDR activity has been shown to be dependent on myeloid cell action (Scott et al, 2007) and it was initially postulated that IDRs were influencing either the recovery of cells from leukopenia or the maturation of monocytes into M1 or M2 phenotypic macrophages. Both of these hypotheses were found to be incorrect. Given the complex interplay of the innate immune system, investigations were then focused on primary mixed-cell cultures, specifically splenocyte cultures. While the impact of IDRs directly on the splenocyte response to innate immune stimuli was minimal and inconsistent within the scope of typical immune responses (i.e., cytokine/chemokine release), the conditioned media from IDR-treated, LPS-stimulated splenocytes had a significant and consistent effect when incubated with a mouse macrophage cell line. This impact was sustained independent of the source of the splenocytes from either BALB/c (Th2 and M2 biased) or C57BL/6 (Th1 and M1 biased) mice. The activity of IDR peptide analogs was correlated with their ability to bind the target protein, p62. These results clearly establish myeloid cells as the secondary mediators of IDR action. The signaling moiety involved in signaling to the macrophages, and the primary responding cell population(s) in the splenocytes, are still under investigation.
The responses of canonical inflammatory cytokines to IDR treatment constitute a tertiary response and are found to be context-dependent, broad-spectrum and fractional, unlike anti-inflammatory agents directed at specific innate immune outputs such as TNFα or IL-1 that obliterate signaling (Cantini et al. 2014, 2015; Fleischmann et al. 2003). Imbalances between IL-1 and IL-1ra have been reported to play an important predisposing role in acute and chronic inflammatory diseases (Arend & Gabay, 2000; Arend & Guthridge, 2000) and, similarly, the relative proportions of TNFα to its soluble receptors TNF RI and TNF RII have proved to be important in a number of clinical conditions (van Dueren, 1994; Feldman et al 1996; Rooney et al 2000). The increase in the ratio of IL-1ra/IL-1β responses and in the ratio of TNF RII/ TNFα responses following SGX94 treatment observed here (Fig. 5a) suggest a beneficial down-modulation of inflammatory signals coupled with an up-modulation of anti-inflammatory signals. Consistent decreases in inflammatory cytokines and coordinated increases in anti-inflammatory cytokines have been demonstrated in mouse, non-human primate and human responses to SGX94 exposure. This modulation of inflammatory responses is also associated with improved tissue healing (Fig. 1c) and suggests that IDRs may also function downstream of not only pathogen associated molecular patterns (PAMPs) (Janeway, 1989) such as LPS but also downstream of damage associated molecular patterns (DAMPs) (Seong and Matzinger, 2004).
IDRs offer clinically important advantages for improved treatment of antibiotic resistant and emerging infectious disease. The evaluation of the mechanism of action of IDRs has also demonstrated the importance of tissue resident cells. The therapeutic ability to target the tissue resident cells in this context has not been previously demonstrated and suggests that therapies directed at these cells of the innate immune system may be particularly fruitful.
Supplementary Material
Highlights.
The Innate Defense Regulator SGX94 acts on tissue-resident host immune cells
Innate Defense Regulators have broad-spectrum activity against bacterial infections
Innate Defense Regulators modulate inflammatory responses
Innate Defense Regulators are not directly antibacterial
Innate Defense Regulators complement the action of antibiotics
ACKNOWLEDGEMENTS
Funding for some of this work was provided by the National Research Council of Canada Industrial Research Assistance Program, agreement #703724 (Inimex Pharmaceuticals Inc) and by the National Institutes of Allergy and Infectious Diseases Small Business Innovation Research grant # 1R43 AI108175-01A1 (Soligenix, Inc).
Abbreviations
- IDR
Innate Defense Regulator
- MAD
Multiple ascending dose study
- SAD
Single ascending dose study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE:
The authors declare that they have competing financial interests in that several of the authors (JRN, ST, AR, AK, CJS, RS, OD) are or have been employees of Inimex Pharmaceuticals, Inc. and/or Soligenix Inc., which were or are developing Innate Defense Regulators as human therapeutics.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi: …. .
REFERENCES
- Arend WP, Gabay C. Physiologic role of interleukin-1 receptor antagonist. Arthritis Res. 2000;2:245–248. doi: 10.1186/ar94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Guthridge CJ. Biological role of interleukin 1 receptor antagonist isoforms. Ann. Rheum. Dis. 2000;59(Suppl 1):i60–64. doi: 10.1136/ard.59.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F, Boccia S, Goletti D, Iannone F, Leoncini E, Panic N, Prignano F, Gaeta GB. HBV reactivation in patients treated with antitumour necrosis factor-alpha (TNF-α) agents for rheumatic and dermatological conditions: a systematic review and meta-analysis. Int J Rheumatol. 2014;2014:926836. doi: 10.1155/2014/926836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F, Nannini C, Niccoli L, Iannone F, Delogu G, Garlaschi G, Sanduzzi A, Matucci A, Prignano F, Conversano M, Goletti D, SAFEBIO Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev. 2015;14(6):503–509. doi: 10.1016/j.autrev.2015.01.011. 2015. [DOI] [PubMed] [Google Scholar]
- Elia PP, Tolentino YF, Bernardazzi C, de Souza HS. The Role of Innate Immunity Receptors in the Pathogenesis of Inflammatory Bowel Disease. Mediators Inflamm. 2015 doi: 10.1155/2015/936193. article ID: 936193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Ann. Rev. Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmester GR, Tesser J, Modafferi D, Poulakos J, Sun G. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis & Rheumatism. 2003;48(4):927–34. doi: 10.1002/art.10870. [DOI] [PubMed] [Google Scholar]
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7417):972–8. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Gelhaus HC, Anderson MS, Fisher DA, Flavin MT, Xu ZQ, Sanford DC. Efficacy of post exposure administration of doxycycline in a murine model of inhalational melioidosis. Sci Rep. 2013;3:1146. doi: 10.1038/srep01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993;189:419–435. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- Hackett CJ. Innate Immune activation as a broad-spectrum biodefense strategy: Prospects and research challenges. J. Allergy Clin. Immunol. 2003;112:686–94. doi: 10.1016/S0091-6749(03)02025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Into T, Inomata M, Niida S, Murakami Y, Shibata K. Regulation of MyD88 Aggregation and the MyD88-dependent Signaling Pathway by Sequestosome-1 and Histone Deacetylase 6. J. Biol. Chem. 2010;285(46):35759–35769. doi: 10.1074/jbc.M110.126904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr., Medzhitov R. Innate immune recognition. Ann. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Kim JY, Ozato K. The sequestosome 1/p62 attentuates cytokine gene expression in activated macrophages by inhibiting IFN regulatory factor 8 and TNF receptor-associated factor 6/NF-kappaB activity. J Immunol. 2009;182(4):2131–40. doi: 10.4049/jimmunol.0802755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Freedman JE, Beaulieu LM. Innate immunity and toll-like receptor antagonists: a potential role in the treatment of cardiovascular diseases. Cardiovasc Ther. 2009;27(2):117–23. doi: 10.1111/j.1755-5922.2009.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Heinzel FP, Sadick MD, Holaday BJ, Gardner KD., Jr. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur Immunol. 1987;138:744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- Mehta S, Jeffrey KL. Beyond receptors and signaling: epigenetic factors in the regulation of innate immunity. Immunol. Cell Biol. 2015;93(3):233–44. doi: 10.1038/icb.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, Sypek JP, Keith JC, Jr., Schaub RG, Palardy JE, Parejo NA. Evaluation of the safety of recombinant P-selectin glycoprotein ligand immunoglobulin G fusion peptide in experimental models of localized and systemic infection. Shock. 2001;15:285–290. doi: 10.1097/00024382-200115040-00006. [DOI] [PubMed] [Google Scholar]
- Park S, Ha SD, Coleman M, Meshkibaf S, Kim SO. P62/SQSTM1 enhances NOD2-mediated signaling and cytokine production through stabilizing NOD2 oligomerization. PLoS One. 2013;8(2):e57138. doi: 10.1371/journal.pone.0057138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Coe CL. The proinflammatory cytoine network: interactions in the CNS and blood of rhesus monkeys. Am. J. Physiol. 1998;274(1 Pt2):R139–44. doi: 10.1152/ajpregu.1998.274.1.R139. [DOI] [PubMed] [Google Scholar]
- Rooney M, Varsani H, Martin K, Lombard PR, Dayer J-M, Woo P. Tumor necrosis factor alpha and its soluble receptors in juvenile chronic arthritis. Rheumatology. 2000;39:432–438. doi: 10.1093/rheumatology/39.4.432. [DOI] [PubMed] [Google Scholar]
- Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, Wang A, Lee K, Doria S, Hamill P, Yu JJ, Li Y, Donini O, Guarna MM, Finlay BB, North JR, Hancock RE. An anti-infective peptide that selectively modulates the innate immune response. Nat. Biotechnol. 2007;25:465–472. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- Sellers RS, Clifford CB, Treuting PM, Brayton C. Immunological Variation between inbred laboratory mouse strains: points to consider in phenotyping genetically immunomodified mice. Veterinary Pathology. 2012;49(1):32–43. doi: 10.1177/0300985811429314. [DOI] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Sonis ST. New thoughts on the initiation of mucositis. Oral Diseases. 2010;16:597–600. doi: 10.1111/j.1601-0825.2010.01681.x. [DOI] [PubMed] [Google Scholar]
- Titball RW, Russell P, Cuccui J, Easton A, Haque A, Atkins T, Sarkar-Tyson M, Harley V, Wren B, Bancroft GJ. Burkholderia pseudomallei: animal models of infection. Trans. R. Soc. Trop. Med. Hyg. 2008;102(Suppl 1):S111–6. doi: 10.1016/S0035-9203(08)70026-9. [DOI] [PubMed] [Google Scholar]
- van der Does AM, Bogaards SJ, Ravensbergen B, Beekhuizen H, van Dissel JT, Nibbering PH. Antimicrobial peptide hLF1-11 directs granulocyte-macrophage colony-stimulating factor-driven monocyte differentiation toward macrophages with enhanced recognition and clearance of pathogens. Antimicrob Agents Chemother. 2010a;54:811–6. doi: 10.1128/AAC.00652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Does AM, Beekhuizen H, Ravensbergen B, Vos T, Ottenhoff TH, van Dissel JT, Drijfhout JW, Hiemstra PS, Nibbering PH. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J Immunol. 2010b;185:1442–9. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
- van der Vaart M, Korbee CJ, Lamers GE, Tengeler AC1, Hosseini R, Haks MC, Ottenhoff TH, Spaink HP, Meijer AH. The DNA damage-regulated autophagy modulator DRAM1 links mycobacterial recognition via TLR-MyD88 to autophagic defense. Cell Host Microbe. 2014;15(6):753–67. doi: 10.1016/j.chom.2014.05.005. [DOI] [PubMed] [Google Scholar]
- van Deuren M. Kinetics of tumour necrosis factor-alpha, soluble tumour necrosis factor receptors, interleukin 1-beta and its receptor antagonist during serious infections. Eur J Clin Microbiol Infect Dis. 1994;13(Suppl 1):S12–6. doi: 10.1007/BF02390680. [DOI] [PubMed] [Google Scholar]
- Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Nat. Acad. Sci. U S A. 2004;101(13):4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao E, Xia L, Ferin M, Wardlaw SL. Intracerebroventricular injection of interleukin-1 stimulates the release of high levels of interleukin-6 and interleukin-1 receptor antagonist into peripheral blood in the primate. J. Neuroimmunol. 1999;97(1-2):70–76. doi: 10.1016/s0165-5728(99)00050-8. [DOI] [PubMed] [Google Scholar]
- Yu HB, Kielczewska A, Rozek A, Takenaka S, Li Y, Thorson L, Hancock RE, Guarna MM, North JR, Foster LJ, Donini O, Finlay BB. Sequestosome-1/p62 is the key intracellular target of innate defense regulator peptide. J. Biol. Chem. 2009;284(52):36007–36011. doi: 10.1074/jbc.C109.073627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis. 2006;6:55. doi: 10.1186/1471-2334-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.