Abstract
Malaria remains common in sub-Saharan Africa, but it is frequently over-diagnosed and over-treated in hospitalized children. HIV is prevalent in many malaria endemic areas and may delay parasite clearance and increase mortality among children with malaria. This prospective cohort study enrolled children with suspected malaria between 3 months and 12 years of age hospitalized at two referral hospitals in Tanzania. Both a thick blood smear (BS) and a malaria rapid diagnostic test (mRDT) were performed. If discordant results were obtained, PCR was performed for P. falciparum. Malaria was confirmed if two out of three tests were positive. Malaria parasite densities were determined for two consecutive days after diagnosis and treatment of malaria. All participants were tested for HIV. Among 1492 hospitalized children, 400 (26.8%) were enrolled with suspected malaria infection. There were 196/400 (49.0%) males, and the median age was 18 [9–36] months. BS was positive in 95/400 (23.8%), and mRDT was positive in 70/400 (17.5%), with moderate agreement (Kappa = 0.598). Concordant results excluded malaria in 291/400 (72.8%) and confirmed malaria in 56/400 (14.0%). PCR performed on 53 discordant results confirmed malaria in 1/39 of the BS-positive/mRDT-negative cases, and 6/14 of the BS-negative/mRDT-positive cases. The prevalence of confirmed malaria was 63/400 (15.8%). In multivariable logistic regression, malaria was associated with HIV (OR 3.45 [1.65–7.20], p=0.001). Current breastfeeding (OR 0.25 [0.11–0.56], p=0.001) and higher hemoglobin (OR 0.70 [0.60–0.81], p<0.001 per 1g/dL) were associated with decreased odds of malaria. Malaria parasite clearance was delayed in HIV-infected participants (p<0.001). Malaria is over-diagnosed even at referral centers in high transmission areas. Hospitalized HIV-infected children are more likely to have malaria and exhibit delayed clearance of parasites. Hospitals should consider using mRDTs as a first step for malaria testing among hospitalized children in sub-Saharan Africa.
Keywords: malaria, children, Tanzania, HIV, diagnosis, clearance
1. Introduction
Malaria remains common worldwide with more than 198 million cases and 584,000 deaths in 2013. Africa carries the brunt of this burden with 82% of all malaria cases and 90% of all deaths occurring on the continent. More than 96% of all deaths from malaria in Africa occur in children < 5 years old (WHO, 2014a). While gains have been made in the worldwide control of malaria, only a slight decrease in the number of years of life lost has been realized, especially in children (GBD 2013 Mortality and Causes of Death Collaborators, 2015). Tanzania is among the 18 countries accounting for 90% of all P. falciparum infections in sub-Saharan Africa (WHO, 2014a). Based on presumptive diagnoses made by clinicians, malaria still ranks as the third most common cause of years of life lost in Tanzania (GBD 2013 Mortality and Causes of Death Collaborators, 2015). Most large surveys focus on rural patients, and little is known about malaria among hospitalized children living in an area of high malaria transmission.
Malaria is frequently over-diagnosed in low resource settings such as sub-Saharan Africa (Mwanziva et al., 2008; Reyburn et al., 2007; Zurovac et al., 2006) even in hospitalized patients (Crump et al., 2011; Nadjm et al., 2012; Reyburn et al., 2004; Strøm et al., 2013). Even clinicians in referral centers vastly overestimate the diagnosis of malaria in areas of low malaria transmission (Crump et al., 2011), perhaps because providers acquiesce to community perception about causes of fever (Hertz et al., 2013). Children with negative malaria test results are often treated as if they have malaria nonetheless and never receive diagnostic workup or treatment for alternative causes of severe febrile illness (Nadjm et al., 2010; Reyburn et al., 2004). Correct diagnosis and treatment of severely ill hospitalized children is an urgent matter. Untreated bacterial sepsis, for example, will lead to higher morbidity and mortality. Inappropriate use of antimalarials could increase hospital costs for families and/or deplete facility drug supplies.
The WHO has recommended that all cases of malaria be confirmed before treatment (WHO, 2012a). Thorough examination of thick and thin blood slides (BS) by experienced technologists using light microscopy remains the gold standard (Bailey et al., 2013; CDC, 2015; WHO, 2011a, 2010). However, several studies from sub-Saharan Africa have shown the real time accuracy of microscopy in basic health facilities is poor (A-Elgayoum et al., 2009; Kahama-Maro et al., 2011; Mwanziva et al., 2008) and that even experienced laboratory technologists at referral centers often report inaccurate BS results (Kilonzo et al., 2014; Strøm et al., 2013).
Malaria rapid diagnostic tests (mRDT) are an alternative diagnostic method with a high degree of sensitivity and specificity in both laboratories (WHO, 2014b) and outpatient settings (Abba et al., 2011; Beadle et al., 1994; Huong et al., 2002; van den Broek et al., 2006). Since 2009, the WHO has recommended the use of mRDTs within national malaria programs (WHO, 2009). mRDTs have several advantages over BS, including no need for continuous electricity and little training needed to perform and interpret. They must be used with some caution as they can remain positive for up to one month after infection and have lower sensitivity at lower levels of parasitemia. Referral hospitals typically rely on BS, assuming that experienced laboratory personnel and reliable electricity will allow for high-quality testing using light microscopy. However, data to support this assumption, particularly among hospitalized children in a malaria-endemic region, are lacking.
Therefore we conducted a prospective cohort study of children hospitalized with suspected malaria at two referral centers in Mwanza, Tanzania. Our objectives were to describe the prevalence of confirmed malaria among hospitalized children suspected to have malaria, to compare the agreement between BS and mRDT, to identify factors associated with confirmed malaria, and to compare the time to malaria parasite clearance and in-hospital mortality between HIV-infected and uninfected children.
2. Materials and Methods
2.1 Study Site
This was a prospective cohort study that included children who were treated for malaria at two referral hospitals in Mwanza, Tanzania: Bugando Medical Centre (BMC) and Sekou Toure Hospital (STH). Mwanza, the 2nd largest urban center in Tanzania, is on the southern shores of Lake Victoria at 1,140 meters above sea level in northwestern Tanzania. The region is located in a highly endemic zone for malaria and has the highest malaria prevalence among children in the country. Among children 6–59 months in the most recent survey, 8.1% were diagnosed with malaria by BS and 14.8% by mRDT (TACAIDS, ZAC, NBS, 2013).
STH is the regional referral hospital for Mwanza Region with a population of approximately 3 million people, and BMC is a consultant and teaching hospital for the larger Lake Zone with a catchment area of approximately 13 million people (National Bureau of Statistics, 2013). STH has 320 total beds and 2,000 pediatric hospitalizations per year. BMC has 1,000 inpatient beds and 3,500 pediatric hospitalizations per year. Approximately 25 % of all pediatric hospitalizations are suspected to have malaria at STH and BMC. All pediatric patients hospitalized at both hospitals routinely undergo provider-initiated testing and counseling (PITC) for HIV, and approximately 10% are HIV-infected.
2.2 Study Population and Inclusion Criteria
All children between 3 months and 12 years of age hospitalized at the STH or BMC pediatric wards and suspected to have malaria by a clinician were eligible for enrollment in the study. If the admitting physician ordered a test for malaria, the child was considered to have suspected malaria. Children were enrolled only after a parent or guardian consented to their involvement in the study and to receiving provider-initiated testing and counseling (PITC) for their child’s HIV testing.
2.3 Study Protocol
Demographic and clinical information including recent fever, use of antimalarials, and presenting symptoms were collected from the parent of each child using a structured questionnaire administered by a trained research assistant. For those who were known to be HIV-infected, information regarding use of antiretroviral medications and cotrimoxazole prophylaxis were also collected, supplemented by information from the medical chart. All children underwent a clinical exam including the measurement of temperature, height, and weight.
A routine thick blood smear for malaria was taken from all participants at the time of hospitalization using the hospitals’ standard operating procedures in accordance with the World Health Organization guidelines (WHO, 2010). The initial BS result was interpreted by on-duty hospital laboratory technicians with a 2 year diploma in laboratory techniques and at least 1 year of experience. At the time of the study, there was no quality assurance program for malaria microscopy in place at our hospitals. For the purposes of this study, 10% of the slides underwent a second reading by another independent, experienced laboratory technician. If these two disagreed, a third experienced technician was asked to provide a tie-breaking interpretation.
If a participant had a positive BS at the time of hospitalization, two more BS were tested at 24 hours and 48 hours after hospitalization to determine parasite clearance. All slides were interpreted by two independent laboratory technicians with a 4 year bachelor’s degree in laboratory techniques and more than 5 years of microscopy experience in parasitology. An average of the two readers was reported. All microscopists were blinded to the HIV status of participants.
An mRDT was also performed on all participants within 24 hours of hospitalization using the SD Bioline Malaria AG Pf/Pan mRDT (Standard Diagnostics, 65, Borahagal-ro, Giheung-gu, Yongin-si, Gyeonggi-do, Republic of Korea). This mRDT detects both the histidine-rich protein-2 (HRP-II) antigen specific to Plasmodium falciparum as well as the plasmodium lactate dehydrogenase (pLDH) enzyme that is common to all Plasmodia species. This mRDT is among the rapid diagnostic tests recommended by the World Health Organization and the Tanzanian Ministry of Health (WHO, 2011b). The mRDT was considered positive if the line for one or both of these antigens was present.
When BS and mRDT results disagreed, a blood sample was frozen at −20°C for later testing with PCR for P. falciparum. PCR testing was performed at the Tanzanian National Institute for Medical Research in Mwanza. Briefly, DNA was isolated from 200μl of blood with QIAamp Blood Kit spin columns using the manufacturer’s recommendations. In each sample, a fixed amount of Phocin Herpes virus 1 (PhHV-1) was added within the isolation lysis buffer to serve as an internal control. Plasmodium-specific PCR primers and detection probe, and primers and detection probe for PhHV-1 were used as described previously (Kaisar et al., 2013). An amplification reaction of each DNA sample was performed in a volume of 25 μl with PCR buffer (HotstarTaq mastermix; Qiagen, Valencia, CA, USA), 5 mM MgCl2, 12.5 pmol of each Plasmodium-specific primer and 15 pmol of each PhHV-1-specific primer, 2.5 pmol of P. falciparum-specific XS- probe and PhHV-1-specific Cy5 double-labeled detection probe (Biolegio, Nijmegen, the Netherlands), and 5 μl of the DNA sample. Amplification consisted of 15 min at 95°C followed by 50 cycles of 15 sec at 95°C, 30 sec at 60°C and 30 sec at 72°C. Amplification, detection and data analysis were performed with the Rotor gene real-time system (Qiagen).
HIV tests were performed on all participants according to the Tanzanian national guidelines for PITC (NACP, 2007). Severe immune suppression was defined as CD4 count < 25% for children ≤ 18 months old, < 15% for those 19–60 months old, and a CD4 count < 350 cells/μL for children > 60 months old. A sample of blood was collected for assessment of anemia on all participants using a Cell Dyn 3700 hematology analyzer (Abbott Diagnostics, Chicago, United States of America).
All participants were managed according to the hospital protocol. All results were promptly provided to the treating clinicians, and all treatment decisions were made by the clinicians supervising the participant’s care, including the decision of when to discontinue malaria treatment. The research team followed each participant to ensure that prescribed medications were issued in a correct and timely manner. Participants were followed until discharge and their discharge diagnoses noted.
2.4 Definitions
Participants were considered breastfed if their mother described their current source of food as primarily breastmilk. Convulsions were defined as any involuntary abnormal movement of the limbs associated with change in consciousness reported by the caregiver during the current illness. Vomiting/diarrhea was defined as two or more episodes of vomiting and/or diarrhea reported by the caregiver in the last 24 hours before hospitalization. Subjective fever was defined as a fever reported by the caregiver in the past 24 hours. Objective fever was defined as an axillary temperature ≥ 37.5° Celsius at the time of hospitalization. Pallor was defined as a clinical assessment of pallor by the admitting clinician. Malnutrition was defined using weight-for-age z-scores and graded as severe (≤ −3 below the median), moderate (>−3 and ≤ −2 from the median), mild (> −2 and ≤ −1 from the median), or none (>−1). Malaria status was considered confirmed positive or confirmed negative in any participant with at least 2 concordant tests (BS, mRDT, or PCR).
2.5 Data Analysis
Data were analyzed using STATA version 13 (College Station, Texas, USA). The primary outcome was the prevalence of confirmed malaria among hospitalized children suspected to have malaria, using BS, mRDT, and malaria PCR.. Secondary outcomes included the agreement between BS and mRDT, factors associated with confirmed malaria, the time to malaria parasite clearance, and the in-hospital mortality rate compared between HIV-infected and HIV-uninfected children. Categorical variables were described as proportions (percentages), and continuous variables were described as medians (interquartile ranges (IQR)). Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using Wilcoxon rank-sum tests. Odds ratios (ORs) with 95% confidence intervals were calculated. Statistical significance was defined as p<0.05. Univariable logistic regression was used to determine the associations of individual variables with the outcomes. A predefined logistic regression model was used to determine the association of multiple variables with the outcomes. The agreement between different diagnostic tests were calculated using a kappa statistic.
2.6 Ethical Considerations
Permission to conduct the study was obtained from the research and publication committee of the Catholic University of Health and Allied Sciences and the institutional review board of Weill Cornell Medical College. Participants were enrolled only after obtaining informed consent from one of their parents. Testing and management of HIV was conducted in accordance with Tanzanian National Guidelines (NACP, 2009).
3. Results
3.1 Enrollment
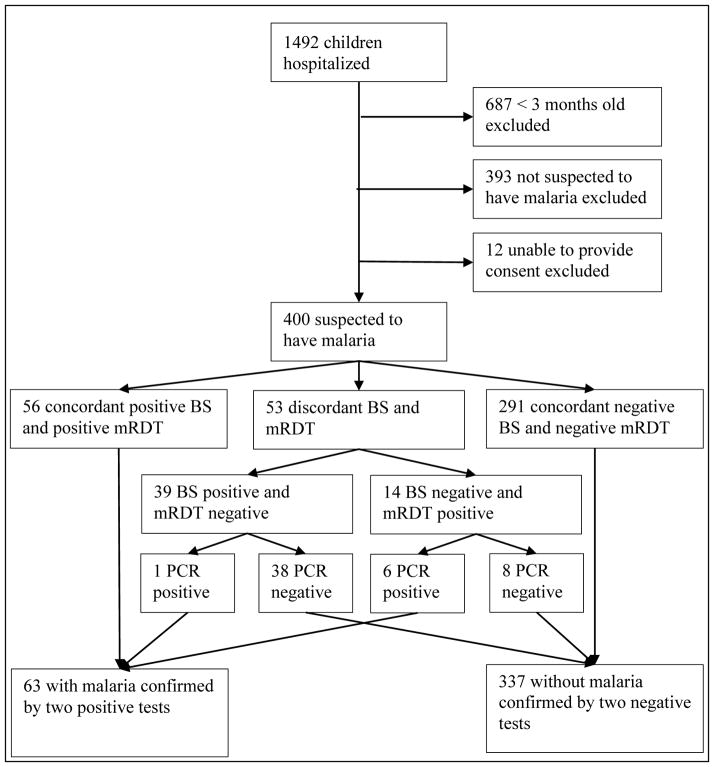
This study was conducted at Bugando Medical Centre and Sekou Toure Hospital from October 2011 to April 2012, during the short and long rainy seasons. During this period, 1492 children younger than 12 years old were hospitalized at both BMC and STH. Of the total 1,492 hospitalizations, 687 were excluded due to an age of less than 3 months, 393 were excluded because they were not suspected to have malaria, and 12 were excluded because consent could not be obtained from their parents. The remaining 400/1492 (26.8%) were suspected to have malaria and subsequently enrolled in the study. Of these 400 participants, 334/400 (83.5%) were hospitalized at BMC, and 66/400 (16.5%) were hospitalized at STH. See Figure 1 for details of enrollment and testing.
Figure 1.
Screening, enrollment and malaria test results for pediatric inpatients in two Tanzanian hospitals. BS: blood slide for malaria; mRDT: malaria rapid diagnostic test; PCR: polymerase chain reaction.
3.2 Baseline Characteristics
Among the participants, 196/400 (49.0%) were male. The median age was 18 months (IQR 9–36), and the median weight was 9 kg (IQR 6.8–13). A total of 237/400 (59.3%) had used antimalarials in the past month. The most common presenting symptoms were fever in 356/400 (89%) with a median duration of five days (IQR 3–7) and diarrhea/vomiting in 211/400 (52.8%). Convulsions were reported in 57/400 (14.3%). The most common sign of illness was malnutrition, with approximately equal numbers of mild (87/400, 21.8%), moderate (98/400, 24.5%), and severe malnutrition (106/400, 26.5%). On physical examination, objective fever was found in 224/400 (56.0%), and pallor in 148/400 (37.0%). On laboratory evaluation, more than 90% had a hemoglobin <10.1 g/dL with 312/400 (78.0%) having an Hb between 5–10 g/dL and 49/400 (12.3%) having an Hb <5 g/dL. Of the 400 participants suspected to have malaria, 52/400 (13%) were HIV-infected, and 38/52 (73.1%) had severe immunosuppression.
3.3 Thick blood smear (BS), rapid diagnostic test (mRDT), and PCR results
Among the 400 participants with suspected malaria, a positive BS was found in 95 of 400 participants (23.8%), and 70 of 400 (17.5%) showed a positive mRDT result. In 56/400 (14.0%) participants, malaria diagnosis was based on concordant positive BS and mRDT; 291 (72.8%) were confirmed not to have malaria based on concordant negative BS and mRDT (Table 1). The remaining 53 of 400 (13.3%) participants with discordant BS and mRDT: 39/53 (72.2%) were BS-positive/mRDT-negative, and 14/53 (26.4%) were BS-negative/mRDT-positive. The agreement between routine BS and mRDT results was moderate (Kappa=0.598). In total, 109/400 (27.3%) participants had at least one positive malaria test.
Table 1.
Routine BS and mRDT results among 400 pediatric inpatients with suspected malaria in two Tanzanian hospitals.
| mRDT positive (%) | mRDT negative (%) | Total (%) | |
|---|---|---|---|
| BS positive | 56 (14.0) | 39 (9.8) | 95 (23.8) |
| BS negative | 14 (3.5) | 291 (72.7) | 305 (76.2) |
| Total (%) | 70 (17.5) | 330 (82.5) | 400 |
| Kappa = 0.598 | |||
mRDT, malaria rapid diagnostic test; BS, blood smear
Among the 53 participants with discordant BS and mRDT results, P. falcifarum-specific PCR was positive in 1/39 of the BS-positive/mRDT-negative tests and in 6/14 of the BS-negative/mRDT-positive tests, yielding seven additional cases of confirmed malaria (Table 2). Therefore the total number of confirmed malaria cases was 63/400 (15.8%). P. falciparum was not identified by PCR in the other 46 cases of discordant results (38/39 of the BS-positive /mRDT-negative tests and 8/14 of the BS-negative/mRDT-positive tests).
Table 2.
Confirmatory P. falciparum PCR results among 53 pediatric inpatients with discordant BS and mRDT.
| PCR positive (%) | PCR negative (%) | Total (%) | |
|---|---|---|---|
| BS +ve/mRDT − ve (%) | 1 (2.6) | 38 (97.4) | 39 (100) |
| BS − ve/mRDT +ve (%) | 6 (42.9) | 8 (57.1) | 14 (100) |
| Total (%) | 7 | 46 | 53 |
BS, blood smear; mRDT, malaria rapid diagnostic test; PCR, polymerase chain reaction
The one BS-positive/mRDT-negative case that was confirmed by PCR was a 36 month old child who was HIV-infected (CD4 15%) with severe anemia (Hb 4.47 g/dL). She presented with a subjective fever for seven days and had used antimalarials in the last 30 days. On hospitalization she had a temperature of 38.5°C and a malaria parasite density of 920 parasites/μL. She received both antimalarials and antibiotics while in the hospital.
The six BS-negative/mRDT-positive cases that were confirmed by PCR had a median age of 72 months (IQR 20–84) and a median weight of 15.5 kg (IQR 8–18). Of the six participants, five had malnutrition: two with mild, one with moderate, and two with severe. None of them were HIV-infected, but all had anemia: five with mild, and one with severe. All six presented with a subjective fever with a median duration of 5.5 days (IQR 3–7), and 5/6 had used antimalarials in the last 30 days. On hospitalization three had diarrhea/vomiting, two had convulsions, and three had an objective fever. All six received both antimalarials and antibiotics while in the hospital.
3.4 Factors associated with confirmed malaria
Table 3 shows factors associated with confirmed malaria by univariable analysis. In the medical history, prior use of antimalarials was associated with increased odds of confirmed malaria (OR 1.89 [1.05–3.40], p = 0.034), and current breastfeeding was associated with a decreased odds of confirmed malaria (OR 0.35 [0.20–0.61], p < 0.001). On physical exam, both mild malnutrition (OR 1.87 [1.03–3.40], p = 0.038) and pallor (OR 1.97 [1.14–3.38], p = 0.015) were associated with increased odds of confirmed malaria. Laboratory results associated with increased odds of confirmed malaria included having HIV infection (OR 4.43 [2.33–8.44], p < 0.001), severe immunosuppression (OR 3.24 [1.56–6.76], p = 0.002), and severe anemia (OR 3.95 [2.04–7.64], p < 0.001). A higher hemoglobin level was associated with a decreased odds of confirmed malaria (OR 0.70 [0.61–0.80] for every 1g/dL increase in Hb, p < 0.001).
Table 3.
Factors associated with confirmed malaria in 400 pediatric Tanzanian inpatients using univariable logistic analysis.
| Characteristic | Confirmed malaria (n=63) number (%) median (IQR) |
Confirmed not malaria (n=337) number (%) median (IQR) |
Odds ratio [95% CI] | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 24 (38.1) | 172 (51.0) | 1.69 [0.98–2.94] | 0.061 |
| Female | 39 (61.9) | 165 (49.0) | ||
| Age in months | 25 (14–48) | 16 (9–36) | 1.00 [0.99–1.01] | 0.316 |
| Hospital | ||||
| Bugando | 52 (82.5) | 282 (83.7) | 1.08 [0.53–2.21] | 0.823 |
| Sekou Toure | 11 (17.5) | 55 (16.3) | ||
| Malnutrition | ||||
| No malnutrition | 15 (23.8) | 85 (25.2) | 0.93 [0.49–1.74] | 0.812 |
| Mild malnutrition | 20 (31.8) | 67 (19.8) | 1.87 [1.03–3.40] | 0.038 |
| Moderate malnutrition | 11 (17.5) | 87 (25.8) | 0.61 [0.30–1.22] | 0.160 |
| Severe malnutrition | 16 (25.4) | 90 (26.7) | 0.93 [0.50–1.73] | 0.829 |
| Fever (subjective) | 59 (93.7) | 297 (88.1) | 1.99 [0.68–5.76] | 0.207 |
| Fever (objective) | 39 (61.9) | 185 (54.9) | 1.34 [0.77–2.32] | 0.305 |
| Fever (duration, days) | 5 (3–7) | 5 (3–7) | 0.99 [0.95–1.03] | 0.701 |
| Temperature (°C) | 37.8 (37.0–38.6) | 37.7 (36.8–38.7) | 1.06 [0.84–1.35] | 0.603 |
| Vomiting/Diarrhea | 36 (57.1) | 175 (51.9) | 1.23 [0.72–2.12] | 0.447 |
| Pallor | 32 (50.8) | 116 (34.4) | 1.97 [1.14–3.38] | 0.015 |
| Convulsions | 11 (17.5) | 46 (13.7) | 1.34 [0.65–2.75] | 0.428 |
| Hemoglobin (g/dL) | 6.5 (4.3–7.4) | 7.6 (6.8–8.9) | 0.70 [0.61–0.80] | <0.001 |
| Severe Anemia (Hb<5) | 18 (28.6) | 31 (9.2) | 3.95 [2.04–7.64] | <0.001 |
| Breastfeeding | 27 (42.9) | 229 (68.0) | 0.35 [0.20–0.61] | <0.001 |
| Prior use of anti-malarials | 45 (71.4) | 192 (57.0) | 1.89 [1.05–3.40] | 0.034 |
| HIV-infected | 20 (31.8) | 32 (9.5) | 4.43 [2.33–8.44] | <0.001 |
| Outcome | ||||
| Died | 16 (25.4) | 65 (19.3) | 1.42 [0.76–2.67] | 0.279 |
| Recovered | 47 (74.6) | 272 (80.7) |
Bold values indicate the statistically significant characteristics.
Association with confirmed malaria was also tested using a predetermined multivariable logistic regression analysis including age, sex, HIV status, hospital of diagnosis, symptoms of convulsion, symptoms of diarrhea and vomiting, presence or absence of malnutrition, hemoglobin level, temperature on hospitalization, breastfeeding status, and prior use of antimalarials. After multivariable logistic regression, HIV infection remained significantly associated with confirmed malaria (OR 3.38 [1.61–7.10], p=0.001). Current breastfeeding (OR 0.24, [0.10–0.55], p=0.001), higher hemoglobin (OR 0.70 per 1 g/dL increase in Hb, [0.60–0.82], p<0.001), and older age (OR 0.98 per month increase in age, [0.97–1.00], p=0.029) were associated with a decreased risk of confirmed malaria.
3.5 Malaria, HIV, and parasite clearance rates
The prevalence of confirmed malaria among the HIV-infected children was 20/52 (38.5%), and the prevalence of confirmed malaria among the HIV-negative children was 43/305 (12.4%). Fifty-seven of the participants with confirmed malaria had a positive BS at diagnosis, and repeat BS were collected 24 and 48 hours later. Malaria parasite clearance rate was significantly diminished in the 20 HIV-infected participants when compared to the 37 HIV-uninfected participants (Table 4). On day 2 (24 hours after hospitalization and the initiation of antimalarials), 4 (20.0%) participants had cleared malaria parasites in the HIV-infected group, while all 37 (100.0%) participants had cleared the infection in the HIV-uninfected group (p < 0.001). This significant difference persisted on day 3 (48 hours after the initiation of antimalarials) when only 17 (85.0%) HIV-infected participants had cleared the infection, compared to all the participants in the HIV-negative group (p = 0.039).
Table 4.
Time to successful parasite clearance among all BS-positive pediatric inpatients (20 HIV-infected and 37 HIV-uninfected) with confirmed malaria.
| Days after the initiation of antimalarial treatment | HIV-infected (N=20) number cleared (%) | HIV-uninfected (N=37) number cleared (%) | p-value |
|---|---|---|---|
| Day 1 | 0 | 0 | – |
| Day 2 | 4 (20) | 37 (100) | <0.001 |
| Day 3 | 17 (85) | 37 (100) | 0.039 |
3.6 Clinical outcomes
Of the 400 participants, 303/400 (75.8%) were prescribed antimalarials at time of hospitalization, and 258/400 (64.5%) were prescribed simultaneous antimalarials and antibiotics. Among the 63 participants with confirmed malaria, all received antimalarials, and 56/63 (88.9%) received concomitant antibiotics. Among the 291 participants who had both a negative BS and a negative mRDT, 194/291 had initially been prescribed antimalarial medication. The clinician stopped antimalarials in only 10/194 (5.2%) cases after receiving the negative malaria test results.
Among the 400 enrolled participants, 81 (20.3%) died during hospitalization. See Table 5 for univariate logistic regression analysis of characteristics associated with death. Confirmed malaria was not associated with an increased odds of death (OR 1.42 [0.76–2.67], p=0.270). HIV-infected participants had a significantly higher odds of death (OR 4.83 [2.61–8.93], p<0.001), as did those with severe malnutrition (OR 4.60 [2.74–7.70], p<0.001). Association with death was also tested using a predetermined multivariable logistic regression analysis including age, sex, HIV status, hospital of diagnosis, symptoms of convulsion, symptoms of diarrhea and vomiting, presence or absence of malnutrition, hemoglobin level, temperature on hospitalization, breastfeeding status, prior use of antimalarials, and confirmed malaria status. After multivariable analysis, HIV infection remained significantly associated with death (OR 4.29 [2.15–8.57], p<0.001). The absence of malnutrition was associated with a decreased risk of death (OR 0.25 [0.11–0.58], p=0.001). HIV-infected children with confirmed malaria who cleared their infection did not have a significant reduction in mortality compared to HIV-infected children with confirmed malaria who had persistent parasitemia either on day 2 (p=0.625) or on day 3 (p=0.074).
Table 5.
Factors associated with death in 400 pediatric Tanzanian inpatients using univariable logistic analysis.
| Characteristic | Dead (n=81) number (%) median (IQR) |
Alive (n=319) number (%) median (IQR) |
Odds ratio [95% CI] | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 36 (44.4) | 160 (50.2) | 1.26 [0.77–2.05] | 0.358 |
| Female | 45 (55.6) | 159 (49.8) | ||
| Age in months | 24 (12–42) | 17 (9–36) | 1.00 [0.99–1.01] | 0.523 |
| Hospital | ||||
| Bugando | 68 (84.0) | 266 (83.4) | 0.96 [0.49–1.86] | 0.902 |
| Sekou Toure | 13 (16.0) | 53 (16.6) | ||
| Malnutrition | ||||
| No malnutrition | 7 (8.6) | 93 (29.2) | 0.23 [0.10–0.52] | <0.001 |
| Mild malnutrition | 11 (13.6) | 76 (23.8) | 0.50 [0.25–1.00] | 0.049 |
| Moderate malnutrition | 19 (23.5) | 79 (24.8) | 0.93 [0.52–1.65] | 0.807 |
| Severe malnutrition | 43 (53.1) | 63 (19.8) | 4.60 [2.74–7.70] | <0.001 |
| Fever (subjective) | 70 (86.4) | 286 (89.7) | 0.73 [0.35–1.52] | 0.407 |
| Fever (objective) | 36 (44.4) | 188 (58.9) | 0.56 [0.34–0.91] | 0.020 |
| Fever (duration, days) | 9.5 (4–14) | 4 (3–7) | 1.09 [1.05–1.12] | <0.001 |
| Temperature (°C) | 37.1 (36.5–38.2) | 37.8 (36.9–38.7) | 0.73 [0.59–0.91] | 0.005 |
| Vomiting/Diarrhea | 36 (44.4) | 175 (54.9) | 0.66 [0.40–1.08] | 0.095 |
| Pallor | 36 (44.4) | 112 (35.1) | 1.48 [0.90–2.43] | 0.121 |
| Convulsions | 14 (17.3) | 43 (13.5) | 1.34 [0.69–2.59] | 0.383 |
| Hemoglobin (g/dL) | 7.3 (6.4–8.7) | 7.4 (6.5–8.9) | 0.97 [0.86–1.09] | 0.581 |
| Severe Anemia (Hb<5) | 7 (8.6) | 42 (13.2) | 0.62 [0.27–1.45] | 0.271 |
| Breastfeeding | 48 (59.3) | 208 (65.2) | 0.77 [0.47–1.28] | 0.320 |
| Prior use of anti-malarials | 55 (67.9) | 182 (57.1) | 1.59 [0.95–2.67] | 0.077 |
| HIV-infected | 25 (30.9) | 27 (8.5) | 4.83 [2.61–8.93] | <0.001 |
| Confirmed malaria | 16 (19.8) | 47 (14.7) | 1.42 [0.76–2.67] | 0.270 |
Bold values indicate the statistically significant characteristics.
4. Discussion
Among 400 children who were hospitalized at two large Tanzanian hospitals with suspected malaria, >75% were empirically started on anti-malarial treatment despite the fact that only 15% had confirmed malaria. Over the past five years the WHO has reported a consistent decrease in worldwide incidence of malaria (WHO, 2014a). As malaria incidence decreases, empiric treatment for malaria becomes increasingly problematic. In 2010 the WHO introduced the “T3: Test. Treat. Track.” initiative and recommended that every suspected malaria case be confirmed by microscopy or mRDT prior to treatment (WHO, 2012a). Despite this initiative only 62% of patients in Africa with suspected malaria were tested before receiving treatment in 2013 (WHO, 2014a). When febrile children are inappropriately treated for malaria, other causes of fever are often ignored and both adverse drug reactions and parasite resistance increase. The present findings support prior studies that have reported the inaccuracy of clinical diagnosis and the high prevalence of malaria over-treatment (Kilonzo et al., 2014; Mwanziva et al., 2008; Nadjm et al., 2012; Zurovac et al., 2006), and show that this remains a problem, even in referral centers.
The present study also demonstrates only moderate agreement between routine BS and malaria mRDT and suggests mRDT is a better initial test in diagnosing malaria among pediatric inpatients with suspected malaria in Tanzania, as we have previously demonstrated in adults (Kilonzo et al., 2014). When testing for malaria at a referral center, BS is widely regarded as the test of choice since there are more experienced laboratory technicians and a large volume of tests. mRDT in this setting is considered an unnecessary expense. We present evidence to the contrary. Among 95 participants with a positive routine BS, only 56 also had a positive mRDT. Only one of 39 participants with a positive BS and a negative mRDT had a positive PCR for P. falciparum. Only 8 of 70 participants with a positive mRDT had a negative PCR, and all 8 of these were PCR-negative, BS-negative, and mRDT-positive, suggesting recent infection with clearance of parasitic DNA, but persistent antigenemia after successful treatment due to prior use of antimalarials.
Despite the potential of mRDT to expedite accurate diagnosis and reduce rates of over diagnosis (Abba et al., 2011; Batwala et al., 2011; D’Acremont et al., 2011; de Oliveira et al., 2009; Harchut et al., 2013; Reyburn et al., 2007; Strøm et al., 2013), mRDT rollout in Tanzania continues to focus on clinics and smaller hospitals even after we reported similar findings in adults from the same large hospitals in 2014 (Kilonzo et al., 2014). mRDT use can yield significant cost savings due to the decreased use of antimalarials (D’Acremont et al., 2011; Yukich et al., 2010). Improved uptake of proper malaria diagnostic algorithms can be challenging (Reyburn et al., 2007), but changes in diagnostic practice can be achieved with a multifaceted approach (Chandler et al., 2014; Masika et al., 2006). This has important implications even for tertiary care centers which already have the infrastructure necessary to use microscopy (i.e. reagents, a microscope, and a dedicated power supply). If mRDT were used on the wards, it could decrease turn-around-time from 6 hours to 20 minutes, providing immediate feedback to physicians. mRDT use would also decrease the work load for an already overstretched laboratory staff. Withholding antimalarials from children who test negative with an mRDT is a safe practice in the outpatient setting (D’Acremont et al., 2010). In hospitalized children with higher risk of mortality withholding antimalarials must be done cautiously. Still mRDT could be valuable as part of the diagnostic algorithm on the wards. For example, instead of reading a BS for every patient with suspected malaria, technicians could focus their time and energy on reading BS carefully for patients with severe and/or persistent symptoms consistent with malaria despite a negative mRDT or other special cases.
Our study demonstrates that HIV-infected Tanzanian children not only were more likely to have confirmed malaria but also had delayed parasite clearance following quinine treatment compared to HIV-uninfected children. The higher prevalence of malaria among HIV-infected children is most likely related to impaired immunity and has been described in other studies (Korenromp et al., 2005; Serghides et al., 2015). To the best of our knowledge this is the first time that delayed clearance of malaria parasites among children has been reported. Studies among HIV-infected adult populations have reported conflicting results depending on the malaria treatment regimen used and the CD4 count (Kamya et al., 2006; Laufer et al., 2007; Müller and Moser, 1990; Patnaik et al., 2005; Shah et al., 2006; Van Geertruyden et al., 2006).
In our previously published study we identified for the first time a decreased clearance of parasites during treatment with quinine compared to HIV-uninfected adults (Kilonzo et al., 2014). In the current study, malaria parasite clearance was delayed in children with HIV at both 24 hours and 48 hours. Our findings contrast with those of several other studies among HIV-infected and uninfected children that reported similar rates of treatment failure among children treated with a variety of regimens including quinine (Byakika-Kibwika et al., 2007; Colebunders et al., 1990; Gasasira et al., 2008; Greenberg et al., 1991; Kamya et al., 2006; Müller and Moser, 1990). Our results provide evidence against the hypothesis that children have not acquired adequate immunity to demonstrate a difference in clearance between HIV-infected and uninfected children (Flateau et al., 2011). Other studies may not have detected this difference because they relied on clinical surrogates for parasite clearance such as resolution of fever rather than checking daily parasite counts. The observed, delayed parasite clearance following quinine treatment could be addressed by using parental artesunate. The study presented has limitations. No data was collected on the 393 children for whom the clinician did not suspect malaria. Due to a limited supply of reagents, PCR could only be performed on specimens that were discordant after BS and mRDT testing. Testing all specimens with PCR in future studies would clarify the accuracy of BS and mRDT methods. Only P. falciparum-specific PCR was performed, and so other Plasmodia species would not have been detected by PCR. The mRDT is also less sensitive for detection of other Plasmodia. Some of the 38 positive BS results that were negative by mRDT and PCR could therefore have been P. ovale or P. malariae cases. However, P. falciparum has consistently been shown to cause > 95% of the cases of severe malaria in our region (Mboera et al., 2006; TACAIDS, ZAC, NBS, 2013; WHO, 2012b). Furthermore, while we instituted a quality control system for reading blood slides in our study, the results of individual readers were not available for comparison. Finally, the specifics antibiotics prescribed and doses used were not recorded during data collection.
5. Conclusion
Our study demonstrates that malaria, although an important cause of hospitalization among children living in an endemic area, is still massively over-diagnosed and over-treated at large referral centers. Only 15% of children diagnosed clinically with malaria actually had malaria. Even experienced referral hospital laboratory technicians over-diagnosed malaria when using BS. mRDT ought to be considered for integration into clinical algorithms in referral hospitals. If properly introduced, this could decrease inappropriate use of antimalarials and result in improved care of hospitalized children. BS could still be used to determine malaria species and confirm clearance, especially among HIV-infected children.
Highlights.
Malaria is over-diagnosed even at referral hospitals using blood slides.
HIV increases the risk of malaria and leads to higher mortality from malaria.
Parasite clearance is delayed in children infected with HIV.
Referral hospitals in Africa should consider using malaria rapid diagnostic tests.
Acknowledgments
The authors thank the BMC and Sekou Toure hospital administrations for their support, NIMR for performing the PCRs, and the Weill Cornell Center for Global Health in New York for funding this study. This study was supported by a grant from the National Institutes of Health Fogarty Center (TW009337). RNP is supported by the National Institutes of Health (NIH K01 TW010281-01).
Abbreviations
- BMC
Bugando Medical Centre
- BS
blood smear
- HRP-II
histidine-rich protein-2
- IQR
interquartile range
- mRDT
rapid diagnostic test
- NPV
negative predictive value
- OR
odds ratio
- PCR
polymerase chain reaction
- PITC
provider-initiated testing and counseling
- pLDH
plasmodium lactate dehydrogenase
- PPV
positive predictive value
- STH
Sekou Toure Hospital
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A-Elgayoum SME, ElFeki AEA, Mahgoub BA, ElRayah E, Giha HA. Malaria overdiagnosis and burden of malaria misdiagnosis in the suburbs of central Sudan: special emphasis on artemisinin-based combination therapy era. Diagn Microbiol Infect Dis. 2009;64:20–6. doi: 10.1016/j.diagmicrobio.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Abba K, Deeks JJ, Olliaro P, Naing C, Jackson SM, Takwoingi Y, Donegan S, Garner P. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev. 2011:CD008122. doi: 10.1002/14651858.CD008122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JW, Williams J, Bain BJ, Parker-Williams J, Chiodini PL. Guideline: the laboratory diagnosis of malaria. General Haematology Task Force of the British Committee for Standards in Haematology. Br J Haematol. 2013;163:573–80. doi: 10.1111/bjh.12572. [DOI] [PubMed] [Google Scholar]
- Batwala V, Magnussen P, Nuwaha F. Comparative feasibility of implementing rapid diagnostic test and microscopy for parasitological diagnosis of malaria in Uganda. Malar J. 2011;10:373. doi: 10.1186/1475-2875-10-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle C, Long GW, Weiss WR, McElroy PD, Maret SM, Oloo AJ, Hoffman SL. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet (London, England) 1994;343:564–8. doi: 10.1016/S0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- Byakika-Kibwika P, Ddumba E, Kamya M. Effect of HIV-1 infection on malaria treatment outcome in Ugandan patients. Afr Health Sci. 2007;7:86–92. doi: 10.5555/afhs.2007.7.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. [accessed 11.18.15];Malaria Diagnosis (US) - Microscopy [WWW Document] 2015 URL http://www.cdc.gov/malaria/diagnosis_treatment/microscopy.html.
- Chandler CIR, Meta J, Ponzo C, Nasuwa F, Kessy J, Mbakilwa H, Haaland A, Reyburn H. The development of effective behaviour change interventions to support the use of malaria rapid diagnostic tests by Tanzanian clinicians. Implement Sci. 2014;9:83. doi: 10.1186/1748-5908-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebunders R, Bahwe Y, Nekwei W, Ryder R, Perriens J, Nsimba K, Turner A, Francis H, Lebughe I, Van der Stuyft P. Incidence of malaria and efficacy of oral quinine in patients recently infected with human immunodeficiency virus in Kinshasa, Zaire. J Infect. 1990;21:167–73. doi: 10.1016/0163-4453(90)91701-e. [DOI] [PubMed] [Google Scholar]
- Crump JA, Ramadhani HO, Morrissey AB, Msuya LJ, Yang LY, Chow SC, Morpeth SC, Reyburn H, Njau BN, Shaw AV, Diefenthal HC, Bartlett JA, Shao JF, Schimana W, Cunningham CK, Kinabo GD. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health. 2011;16:830–7. doi: 10.1111/j.1365-3156.2011.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Acremont V, Kahama-Maro J, Swai N, Mtasiwa D, Genton B, Lengeler C. Reduction of anti-malarial consumption after rapid diagnostic tests implementation in Dar es Salaam: a before-after and cluster randomized controlled study. Malar J. 2011;10:107. doi: 10.1186/1475-2875-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Acremont V, Malila A, Swai N, Tillya R, Kahama-Maro J, Lengeler C, Genton B. Withholding antimalarials in febrile children who have a negative result for a rapid diagnostic test. Clin Infect Dis. 2010;51:506–11. doi: 10.1086/655688. [DOI] [PubMed] [Google Scholar]
- de Oliveira AM, Skarbinski J, Ouma PO, Kariuki S, Barnwell JW, Otieno K, Onyona P, Causer LM, Laserson KF, Akhwale WS, Slutsker L, Hamel M. Performance of malaria rapid diagnostic tests as part of routine malaria case management in Kenya. Am J Trop Med Hyg. 2009;80:470–4. [PubMed] [Google Scholar]
- Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–56. doi: 10.1016/S1473-3099(11)70031-7. [DOI] [PubMed] [Google Scholar]
- Gasasira AF, Kamya MR, Achan J, Mebrahtu T, Kalyango JN, Ruel T, Charlebois E, Staedke SG, Kekitiinwa A, Rosenthal PJ, Havlir D, Dorsey G. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin Infect Dis. 2008;46:985–91. doi: 10.1086/529192. [DOI] [PubMed] [Google Scholar]
- GBD. Mortality and Causes of Death Collaborators, 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2013;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AE, Nsa W, Ryder RW, Medi M, Nzeza M, Kitadi N, Baangi M, Malanda N, Davachi F, Hassig SE. Plasmodium Falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N Engl J Med. 1991;325:105–9. doi: 10.1056/NEJM199107113250206. [DOI] [PubMed] [Google Scholar]
- Harchut K, Standley C, Dobson A, Klaassen B, Rambaud-Althaus C, Althaus F, Nowak K. Over-diagnosis of malaria by microscopy in the Kilombero Valley, Southern Tanzania: an evaluation of the utility and cost-effectiveness of rapid diagnostic tests. Malar J. 2013;12:159. doi: 10.1186/1475-2875-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz JT, Munishi OM, Sharp JP, Reddy Ea, Crump Ja. Comparing actual and perceived causes of fever among community members in a low malaria transmission setting in northern Tanzania. Trop Med Int Health. 2013;18:1406–15. doi: 10.1111/tmi.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huong NM, Davis TME, Hewitt S, Van Huong N, Uyen TT, Nhan DH, Cong LD. Comparison of three antigen detection methods for diagnosis and therapeutic monitoring of malaria: a field study from southern Vietnam. Trop Med Int Health. 2002;7:304–8. doi: 10.1046/j.1365-3156.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Kahama-Maro J, D’Acremont V, Mtasiwa D, Genton B, Lengeler C. Low quality of routine microscopy for malaria at different levels of the health system in Dar es Salaam. Malar J. 2011;10:332. doi: 10.1186/1475-2875-10-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisar MMM, Supali T, Wiria AE, Hamid F, Wammes LJ, Sartono E, Luty AJF, Brienen EAT, Yazdanbakhsh M, van Lieshout L, Verweij JJ. Epidemiology of Plasmodium infections in Flores Island, Indonesia using real-time PCR. Malar J. 2013;12:169. doi: 10.1186/1475-2875-12-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamya MR, Gasasira AF, Yeka A, Bakyaita N, Nsobya SL, Francis D, Rosenthal PJ, Dorsey G, Havlir D. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- Kilonzo SB, Kamugisha E, Downs JA, Kataraihya J, Onesmo R, Mheta K, Jeong JM, Verweij JJ, Fitzgerald DW, Peck RN. Malaria among adult inpatients in two Tanzanian referral hospitals: a prospective study. Acta Trop. 2014;134:95–100. doi: 10.1016/j.actatropica.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Korenromp EL, Williams BG, de Vlas SJ, Gouws E, Gilks CF, Ghys PD, Nahlen BL. Malaria attributable to the HIV-1 epidemic, sub-Saharan Africa. Emerg Infect Dis. 2005;11:1410–9. doi: 10.3201/eid1109.050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer MK, van Oosterhout JJG, Thesing PC, Dzinjalamala FK, Hsi T, Beraho L, Graham SM, Taylor TE, Plowe CV. Malaria treatment efficacy among people living with HIV: the role of host and parasite factors. Am J Trop Med Hyg. 2007;77:627–32. [PubMed] [Google Scholar]
- Masika PM, Semarundu WJ, Urassa R, Mosha J, Chandramohan D, Gosling RD. Over-diagnosis of malaria is not a lost cause. Malar J. 2006;5:120. doi: 10.1186/1475-2875-5-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mboera LEG, Kamugisha ML, Rumisha SF, Msangeni HA, Barongo V, Molteni E, Kitua AY. The relationship between malaria parasitaemia and availability of healthcare facility in Mpwapwa district, central Tanzania. Tanzan Health Res Bull. 2006;8:22–7. doi: 10.4314/thrb.v8i1.14266. [DOI] [PubMed] [Google Scholar]
- Müller O, Moser R. The clinical and parasitological presentation of Plasmodium falciparum malaria in Uganda is unaffected by HIV-1 infection. Trans R Soc Trop Med Hyg. 1990;84:336–8. doi: 10.1016/0035-9203(90)90306-y. [DOI] [PubMed] [Google Scholar]
- Mwanziva C, Shekalaghe S, Ndaro A, Mengerink B, Megiroo S, Mosha F, Sauerwein R, Drakeley C, Gosling R, Bousema T. Overuse of artemisinin-combination therapy in Mto wa Mbu (river of mosquitoes), an area misinterpreted as high endemic for malaria. Malar J. 2008;7:232. doi: 10.1186/1475-2875-7-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACP. National Guidelines for the Management of HIV and AIDS. 3. Government of Tanzania; 2009. [Google Scholar]
- NACP. Guidelines for HIV Testing and Counselling in Clinical Settings. Ministry of Health and Social Welfare; 2007. [Google Scholar]
- Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, Wangai H, Kimera J, Msuya W, Mtei F, Dekker D, Malahiyo R, Olomi R, Crump Ja, Whitty CJM, Reyburn H. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ. 2010;340:c1350. doi: 10.1136/bmj.c1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjm B, Mtove G, Amos B, Walker NF, Diefendal H, Reyburn H, Whitty, et al. Severe febrile illness in adult hospital admissions in Tanzania: A prospective study in an area of high malaria transmission. R Soc Trop Med Hyg. 2012;106:688–695. doi: 10.1016/j.trstmh.2012.08.006. [DOI] [PubMed] [Google Scholar]
- National Bureau of Statistics. Tanzania 2012 Population and Housing Census Population. Dar es Salaam: 2013. [Google Scholar]
- Patnaik P, Jere CS, Miller WC, Hoffman IF, Wirima J, Pendame R, Meshnick SR, Taylor TE, Molyneux ME, Kublin JG. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis. 2005;192:984–991. doi: 10.1086/432730. [DOI] [PubMed] [Google Scholar]
- Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, Whitty CJM. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. BMJ. 2007 doi: 10.1136/bmj.39073.496829.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, Saganda K, Shao J, Kitua A, Olomi R, Greenwood BM, Whitty CJM. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serghides L, Finney CAM, Ayi K, Loutfy M, Kain KC. Chronic HIV infection impairs nonopsonic phagocytosis of malaria parasites. J Acquir Immune Defic Syndr. 2015;68:128–32. doi: 10.1097/QAI.0000000000000427. [DOI] [PubMed] [Google Scholar]
- Shah SN, Smith EE, Obonyo CO, Kain KC, Bloland PB, Slutsker L, Hamel MJ. HIV immunosuppression and antimalarial efficacy: sulfadoxine-pyrimethamine for the treatment of uncomplicated malaria in HIV-infected adults in Siaya, Kenya. J Infect Dis. 2006;194:1519–1528. doi: 10.1086/508892. [DOI] [PubMed] [Google Scholar]
- Strøm GEA, Haanshuus CG, Fataki M, Langeland N, Blomberg B. Challenges in diagnosing paediatric malaria in Dar es Salaam, Tanzania. Malar J. 2013;12:228. doi: 10.1186/1475-2875-12-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACAIDS ZAC, NBS O. Tanzania HIV/AIDS and Malaria Indicator Survey 2011–12. Dar es Salaam: 2013. [Google Scholar]
- van den Broek I, Hill O, Gordillo F, Angarita B, Hamade P, Counihan H, Guthmann JP. Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. Am J Trop Med Hyg. 2006;75:1209–1215. [PubMed] [Google Scholar]
- Van Geertruyden JP, Mulenga M, Mwananyanda L, Chalwe V, Moerman F, Chilengi R, Kasongo W, Van Overmeir C, Dujardin JC, Colebunders R, Kestens L, D’Alessandro U. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J Infect Dis. 2006;194:917–25. doi: 10.1086/507310. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report. World Health Organization; 2014a. [DOI] [Google Scholar]
- WHO. Malaria Rapid Diagnostic Test Performance. World Health Organization; 2014b. [Google Scholar]
- WHO. Scaling up diagnostic testing, treatment and surveillance for malaria: Test, Treat, Track. World Health Organization; 2012a. [Google Scholar]
- WHO. World Malaria Report. World Health Organization; 2012b. [Google Scholar]
- WHO. Universal Access to Malaria Diagnostic Testing: An Operational Manual. World Health Organization; 2011a. [Google Scholar]
- WHO. Good Practices for Selecting and Procuring Rapid Diagnostic Tests for Malaria. World Health Organization; 2011b. [Google Scholar]
- WHO. Basic Malaria Microscopy: Learner’s Guide. 2. World Health Organization; 2010. [Google Scholar]
- WHO. Parasitological confirmation of malaria diagnosis. World Health Organization; 2009. [Google Scholar]
- Yukich J, D’Acremont V, Kahama J, Swai N, Lengeler C. Cost savings with rapid diagnostic tests for malaria in low-transmission areas: evidence from Dar es Salaam, Tanzania. Am J Trop Med Hyg. 2010;83:61–8. doi: 10.4269/ajtmh.2010.09-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurovac D, Midia B, Ochola Sa, English M, Snow RW. Microscopy and outpatient malaria case management among older children and adults in Kenya. Trop Med Int Health. 2006;11:432–40. doi: 10.1111/j.1365-3156.2006.01587.x. [DOI] [PubMed] [Google Scholar]