Abstract
Primary prostate cancer, also known as prostate adenocarcinoma (PCa), is a devastating cancer in men worldwide. Europe and developing countries of Asia have fewer reported cases of prostate cancer compared to increasing cases in the United States with higher incidence in Black men. Risk factors associated with prostate cancer are aging, genetics, lifestyle, high body mass index as well as carcinogenic exposure to carbon-containing fuels, tobacco, and charbroiled meats. Hormone therapy and radical prostatectomy are commonly implemented treatments. The more than 20,000 prostate cancer deaths of 2013 suggest there exists a need for enhanced chemopreventive and therapeutic agents for prostate cancer treatment. Fruits, vegetables, and red wines contain high levels of polyphenolic levels. Consumption of these products may provide chemoprevetion of PCa. Curcumin, the major compound from the turmeric rhizome Curcumin Longa has long been used for medicinal purposes as an antiseptic and wound healing. This review focuses on curcumin’s therapeutic effectiveness in vitro and in vivo in prostate cancer models. The review will highlight the mechanisms of actions of curcumin in the signaling pathways of prostate cancer.
Graphical Abstract
1. Introduction
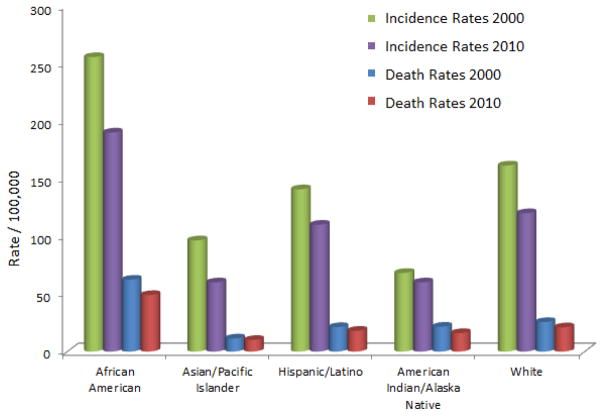
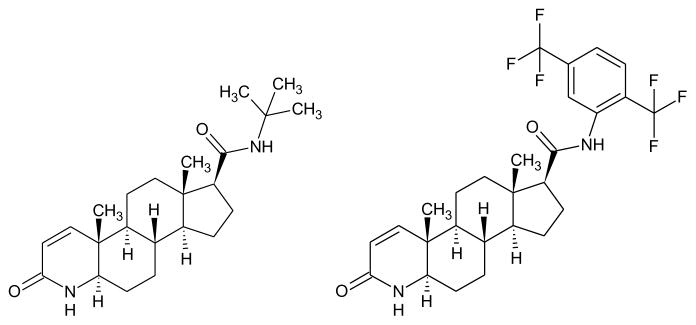
Primary prostate cancer (PCa), prostate adenocarcinoma, is second to skin cancer in its frequency of diagnosis and first in cancer deaths in American men. In 2013, 29,720 deaths were attributed to the malignancy in the United States. There continues to a be a steady decline in the numbers of prostate cancer deaths, however the death rate among Black men as a result of prostate cancer is two times greater [1]. Common PCa risk factors are increased age, elevated BMI and α-linolenic acid levels, and family history. Genetic predisposition contributes to 5% to 10% of prostate cancers. Incident rates of men of African descent are higher worldwide in comparison to men of other races. The annual incidence rate for Native American/Alaska Natives is 77.8; Caucasian is 144.9 and 228.5 per 100,000 for men of African descent (Fig. 1). The incidence and mortality is lower in Asia [2, 3], but predicted to increase because of economic development and western lifestyle influence [4, 5]. In 2010, the United States invested an estimated 11.85 billion dollars in the health care cost of PCa. Limited PCa prevention and treatment modalities are expected to increase future health care expenditures [6]. Prostate cancer is a malignancy with 10% to 20% castration-resistant development within approximately 5 years of survival and 50 % recurrence at 10 years after treatment with prostatectomy or radiation therapy [7–11]. Active surveillance, radical prostatectomy, and radiotherapy are alternative prostate cancer treatment options that are limited in their use when prostate-specific antigen (PSA) levels [12–14] and Gleason score (GS) are elevated [15, 16]. Average PSA levels are lower for members of most Asian countries for men between 60–80 years of age [17, 18]. More than 220,000 new cases of prostate cancer were anticipated to be diagnosed in the United States in 2015 [19]. An effective therapeutic approach to address the malignancy is an active area of research. There are several types of cancer that start in the prostate: sarcoma, small cell carcinoma, neuroendocrine tumors, transitional cell carcinoma, and adenocarcinoma. Prostate adenocarcinoma represents the documented deaths associated with PCa. The molecular mechanisms such as androgen receptor signaling, NF-κB, PI3K, Akt, JAK/STAT, MAPK, TGF-β/SMAD, and Wnt pathways of PCa are the targets of established therapeutic agents implemented in the prevention and treatment of prostate cancer [20]. A study performed by Terlikoswska et al. [21] reported more than twenty signaling pathways mediated by curcumin; PPAR, COX-2, EGFR, and NF-κB. To date, finasteride and dutasteride (Fig. 2) are administered to treat benign prostate enlargement and reduction of male hormones. Clinical trials have demonstrated that two drugs are effective in lowering the risk of PCa by 25%.
Figure 1.
Prostate cancer incidence and death rates
Figure 2.
Finasteride and Dutasteride
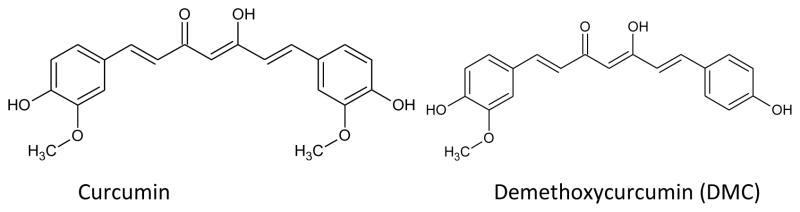
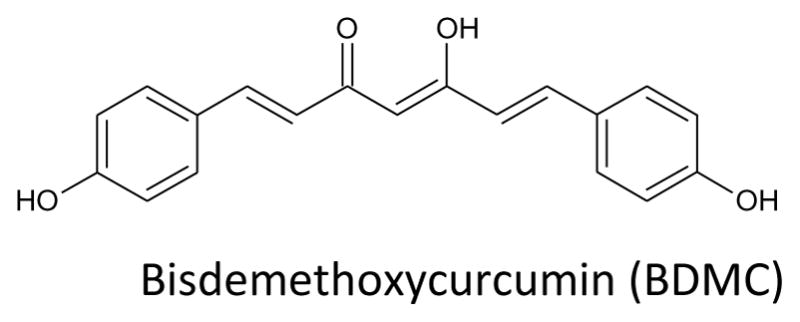
Turmeric, a yellow spice common in India and Southeast Asia, is derived from the plant rhizome Curcuma longa Linn. Its diverse pharmacology action toward various diseased targets attracts great interest from the medicinal chemistry community [22–31]. Turmeric is safely tolerated at a dose of 12 grams a day as a spice, coloring agent, or as a dietary supplement. Natives of Southeast Asia have traditionally used turmeric for medicinal purposes as an antiseptic and wound healing compound. The turmeric powder contains 77% curcumin, 17% demethoxycurcumin and 5% bisdemethoxycurcumin (Fig. 3) [32]. Curcumin, (1,7-bis(4-hydroxy-3 methoxphenyl)-1,6-heptadiene-3,5-dione) has diverse biological activities, such as anti-inflammatory [33–35], antioxidant [36–40], anti-diabetic [41, 42], anti-coagulant [43, 44], antibacterial [45–47], anti-fungal [48–51], and anti-allergic [52–56]. Curcumin has been investigated for its anti-cancer activity in various cancer cell lines such as breast [57–64], prostate [65, 66], lung [67–70], liver [71] and skin [72–74].
Figure 3.
Structures of Natural Curcumins
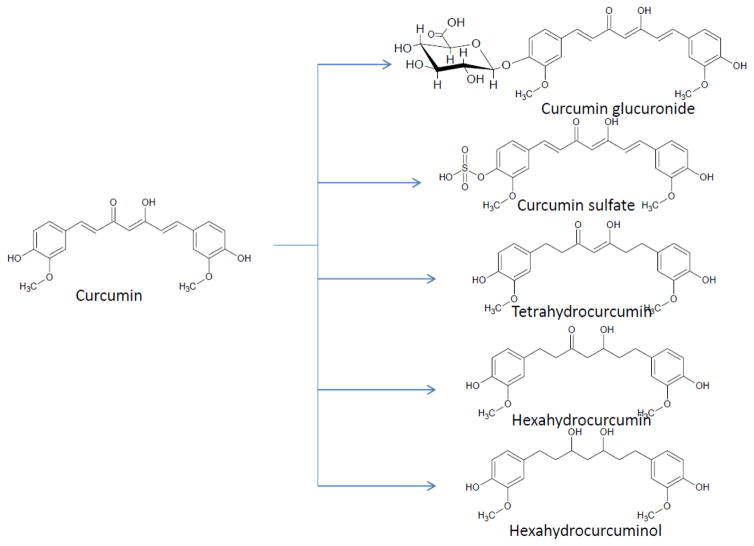
Curcumin’s lack of aqueous solubility, rapid clearance, hepatic and intestinal metabolism explains why the consumption of high doses is required to achieve therapeutic effect. Studies of curcumin have addressed its poor bioavailability, absorption, metabolism and tissue distribution [75–78]. In-vivo studies reveal the oral administration of curcumin undergoes metabolic O-conjugation to curcumin glucuronide and curcumin sulfate and bio-reduction to tetrahydrocurcumin, hexahydrocurcumin, and hexahydrocurcuminol (Fig. 4) [75–78].
Figure 4.
Structures of curcumin metabolites
2. Curcumin and Prostate Cancer: In Vitro Studies
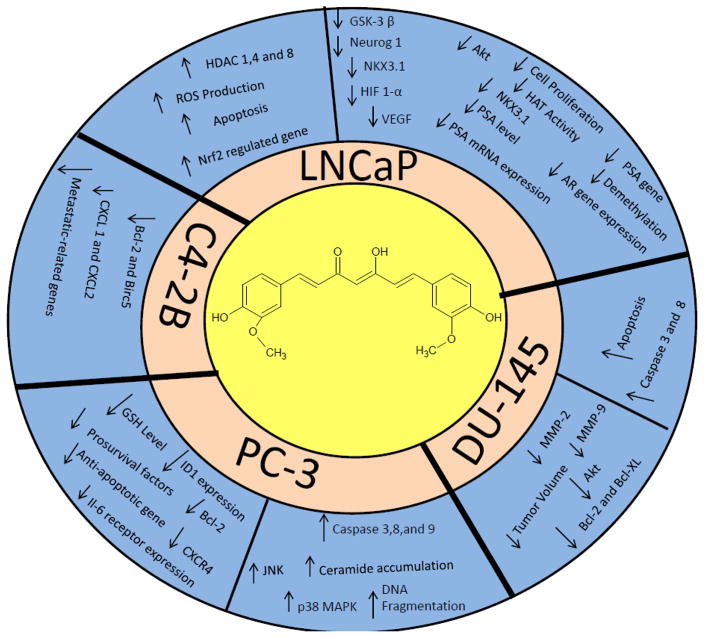
Curcumin’s modulation of various signaling pathways leads to cytotoxicity, antiproliferation, and induced apoptosis in LNCaP (androgen dependent), PC-3 (androgen independent) and DU-145 prostate cancer cell lines. Choi et al. [79] determined curcumin is a cytotoxic agent in prostate cancer cells and inhibitor of androgen receptor (AR) expression and transcription activity of the Wnt/β-catenin signaling pathway. The team determined curcumin suppresses the phosphorylation of downstream targets GSK-β and Akt protein in LNCaP cells when exposed to 20 μM of curcumin for 24 and 48 h. Curcumin has demonstrated the inhibition of the accumulation of β-catenin and down regulation of cyclin D1 and c-myc in LNCaP cells. Chaudhary et al. [80] performed an investigation of curcumin’s cytotoxicity in LNCaP, PC-3, and DU145 cells. The study determined Akt is activated in 5% LNCaP serum and 10% in PC-3 cells when exposed to 35 μM of curcumin. The experiment demonstrated that curcumin performs as an inhibitor of Protein kinase B (PKB/Akt) in a dose-dependent manner and inhibits complete activation of Akt in a time-dependent manner. A DU145 and LNCap study determined curcumin inhibits cell proliferation, induces apoptosis, induces procaspase-3 and procaspase-8 in DU145 and caspase-3 and caspase-8 in LNCaP at 50μM and 10 μM of curcumin respectively [81]. Piantino et al. [82] demonstrated LNCaP and PcBra 1 cells undergo apoptosis at the rates of 90% and 55% respectively when exposed to curcumin. Curcumin has demonstrated anti-androgenic activity through the inhibition of PSA reporter vectors at 20 μM in a dose-dependent manner [83]. Yang et al. [84] performed an LNCaP study to discover the mechanisms responsible for curcumin’s inhibition of cell proliferation and induction of apoptosis. The study revealed curcumin’s ability to target, suppress cell proliferation and improve apoptosis in LNCaP cells when exposed to 40μM of curcumin. Curcumin performs as an inhibitor of prolyl 4-hydroxylase in a dose-dependent manner and modulates hypoxia by downregulation of AR activity and PSA expression in LNCaP cells. At concentration of 10 μM, curcumin blocks HIF1-α overexpression and significantly decreases protein levels of AR by 26% and 31% under normoxia and hypoxia conditions [85]. Du et al. investigated curcumin’s role in cancer associated fibroblasts (CAF) and epithelial to mesenchymal transition (EMT) in PC-3 cells. The study determined curcumin abated CAF invasion and EMT activity and inhibited the production of ROS. The study further identified downregulated receptor expression of CXCR4 and IL-6 by curcumin inhibition of MAOA/mTOR/HIF-α signaling pathway [86]. In an investigation performed by Ling Yu, PC-3 cells treated with 20 μM of curcumin for 30 days displayed more than 50 % of ID1 mRNA reduction, performs as an inhibitor of DNA binding 1 (Id1) in a dose-dependent manner, and modulates the expression of ID1 mRNA and protein in prostate cancer cells [87]. A study of hormone refractory C4-2B prostate cancer cells exposed to curcumin revealed the inhibition of growth factor receptor signaling pathway and activation of NF-kB [88].
In a study performed by Zhang et al. [89] it was observed that NKX3.1, NK-class homeobox gene, and AR expression are significantly down-regulated when exposed to 40μM of curcumin. Shu et al. [90] reported demethylation levels decrease from 37% to 8% when exposed to 5μM of curcumin. The study also observed curcumin, alone or in combination with trichostatin A, demonstrated an increase in histone acetyltransferase activity and inhibition of histone deacetylase. Hong and team [91] performed an investigation of curcumin’s anti-cancer nature in androgen-independent prostate cancer cell line DU-145. The study determined exposure to curcumin concentrations of 25–100 μg resulted in decreased MMP-9 and MMP-2 secretion with significant inhibition of tumor volume and cell proliferation. In PC-3 cells, curcumin has displayed cytotoxicity, apoptotic induced cellular ceramide accumulation (an intermediate in sphingolipid metabolism that leads to apoptosis), activation of MAPK, JNK, cytochrome c, apoptosis-inducing factor (AIF), and caspase-3, 8, and 9 [92]. Chendil [93] demonstrated downregulation of prosurvival factors and anti-apoptotic genes when exposed to 5 μM of curcumin in PC-3 cells and a significant decrease in clonogenic expression when curcumin is administered along with radiation as a therapeutic agent. The administration of curcumin alone resulted in an increase of apoptosis, 7.23% to 11.56%, at 24 and 48h whereas the curcumin-radiation treatment combination yielded apoptotic increase of 21.39 % to 27.57%.
3. In-vivo studies
Curcumin has been remarkably well investigated its anti-prostate cancer activity in in vitro models, but its bioavailability is poor. Several animal models have been employed to investigate the anti-prostate cancer activity of curcumin. Hong et al. [94] investigated the effectiveness of curcumin in a LNCaP xenograft model administered 500 mg/Kg orally three times a day for one month. The study revealed a 27% delay in tumor growth and significant suppression of AR expression. A study with the implementation of a PC-3 cell suspension was placed subcutaneously in male SPF BALBB/c nude mice to generate prostate tumors. 25–100 mg/kg of curcumin was administered by injection every 2 days resulting in decreased PC-3 tumor growth, volume, and weight. The study also revealed efficacy in the induction of apoptosis by significant downregulation of Bcl-2 expression and upregulation of Bax expression [95]. Dorai et al. [96] observed curcumin’s ability to halt cell proliferation and induce apoptosis in LNCaP. Male nude mice inoculated with LNCaP cells were treated with a diet made of curcumin and PICO 5053 for 6 weeks. Curcumin demonstrated significant reduction of tumor growth in a statistically significant manner (P < 0.008). In an interesting study with PC-3 prostate cancer cells, Killian and co-worker [97] demonstrated that exposure to 15 μM of curcumin for 24 h inhibited CXCL1 and CXCL-2 transcription expression to 40 % and 25 % respectively, reduced tumor growth rate by 50 % and inhibited apoptosis genes BCL2 and BIRC5. Induced necrosis rate, apoptosis rate, and down regulation of COX2 by 50 %, SPAC by 25 %, ALDH3A1 by 25 % and EFEMP by 40 % were also observed.
4. Curcumin Delivery and Prostate Cancer
The oral administration of curcumin results in rapid metabolism and undergoes enterohepatic circulation requiring a larger dose of curcumin in order to provide therapeutic effects. Studies of curcumin’s cytotoxic effects on prostate cancer with the implementation of nanoemulsions, nanoparticles, and liposomes have demonstrated increased therapeutic efficacy compared to curcumin administered alone. Thangavel et al. [98] studied Redox nanoparticles (RNP) containing curcumin. Their experiment demonstrated significant increases in cellular uptake, induced apoptosis and cytotoxicity and the suppression of more than 40 % of tumor cell proliferation at 100 μmol/L. RNP-curcumin complex exhibited anti-cancer therapeutic activities in a mouse model displaying significant reduction in tumor volume growth rate. Yallapu et al. [65] formulated a poly (lactic-co-glycolic acid) nanoparticle with curcumin (PLGA-NP) by using a nano-precipitation method that exhibited effective anti-cancer activity in C4-2 (LNCaP cell type) androgen dependent and androgen independent PC-3 and DU-145 cells. The curcumin encapsulated nanoparticle inhibits STAT3 and AKT phosphorylation activity, induces apoptosis, alters expression of miR-21 and miR-205, decreases cell proliferation, and inhibits clonogenic potential in PC-3, DU-145 cells.
The study further identified the delivery systems ability to inhibit tumor growth, enhance recruitment of macrophage, and damage free red blood cell membrane in a C4-2 mouse xenograft model. Curcumin nanolipid carriers (CUR-NLC) fabricated by nanoemulsion were investigated in a comparison study of curcumin versus curcumin-genistein nanolipids, The investigation demonstrated an increase in loading efficiency and cell growth inhibition in PC3 cells, cell viability decrease up to 71% in the presence of curcumin only and 50% inhibition of prostate cancer cells in the presence of a curcumin-genistein combination of 20 and 45μM of respectively [99]. Shukla et al. [100] developed an optimal composition of etoposide and curcumin containing nanoemulsion prepared by ultra-sonication which demonstrated enhanced cytotoxicity in PC-3. A curcumin and resveratrol liposome has shown enhanced anticancer activity in PTEN (phosphatase and tensin homolog) knockout mice. The synergistic relationship induces apoptosis and inhibits cell growth, downregulates p-Akt and cyclin D, and significantly decreases prostatic adenocarcinoma in vivo [101]. Yallapu et al. performed studies on β-cyclodextrin (CD) self-assembled curcumin encapsulated delivery systems. The CD system demonstrated greater anti-proliferative activation in both metastatic and nonmetastatic prostate cancer cells in a time-dose-dependent manner [102, 103].
5. Curcumin Structure Activity Relationship and Prostate Cancer
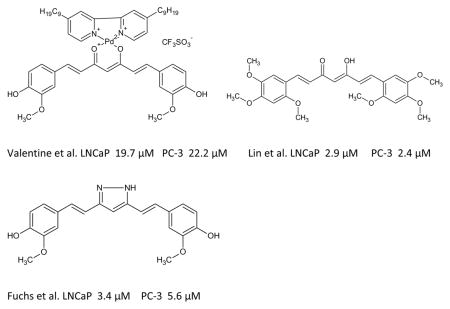
Recent studies have investigated the structural activity relationship of synthesized curcumin analogues. Enhancement of curcumin’s anticancer activity can be achieved through modifications of its chemical structure. The most successful analogues synthesized are pyrazole-curcumin analogue. This section will display the structural activity relationship of curcumin and its semisynthetic analogs. Curcumin-complex, a heteroleptic palladium II complex composed of curcumin and bipyridine has been synthesized by valentine et al. The analogue effectively induces apoptosis and inhibits cell growth in hormone independent prostate cancer cell lines greater than the administration of curcumin alone. In 2005, Lin and team reported the synthesis of more than 40 curcumin analogues, 18 of the synthesized analogues demonstrated cytotoxic behavior in LNCaP and PC-3 prostate cancer cell lines. Fuchs el at. synthesized 13 curcumin analogues with pyrazole ring. The heterocycle modified analogue showed more potency.
6. Synergistic Curcumin combinations
Curcumin administered in vitro and in vivo in synergistic combination with established anti-cancer agents yields enhanced efficacy in prostate cancer cell cytotoxicity and decreased cell viability. Detail information is presented in Table 4.
Table 4.
Synergistic Effects of Curcumin on prostate cancer models
| Biological effect | Mechanism of Action | Dose | Cell line | Ref. |
|---|---|---|---|---|
| Curcumin and nelfinavir co-exposure with docetaxel increase apoptosis, enhance ER-stress transducers and associated death sensors in castration resistant prostate cancer cells (C4-2B) |
 Caspase 3 activity Caspase 3 activity p-eIF2μ p-eIF2μ CHOP/ATF4/TRIB3 CHOP/ATF4/TRIB3 colony forming unit (CFU) colony forming unit (CFU) |
Curcumin (5 μM) Nelfinavir (5 μM) Docetaxel (10 nM) |
CRPC subline (C4-2B) | 107 |
| Curcumin in combination with bicalutamide enhance cell groth inhibition in prostate cancer cells |
 Phosphorylation of ERK1/2 and SAPK/JNK Phosphorylation of ERK1/2 and SAPK/JNK MUC1-C and NF-kβ MUC1-C and NF-kβ |
Curcumin (30μM) Bicalutamide (40 μM) |
PC-3 DU145 |
108 |
| Epigallocatechin gallate (EGCG) in combination with curcumin improve cell cycle arrest in androgen-independent prostate cancer cell lines |
 S and G2/M phase of cell cycle S and G2/M phase of cell cycle |
Curcumin (50 μM) EGCG (50 and 100 μM) |
PC-3 | 109 |
| Curcumin in combination with phenethyl iosthicyante inhibit tumor growth and weight of prostate xenograft mouse |
 Apoptosis Apoptosis Tumor volume Tumor volume |
Curcumin (3 and 6 μM) Phenethyl isothiocyanate (2.5 μM) |
PC-3 xenograft |
110 |
| Curcumin in combination with isoflavones enhance expression of androgen receptor inhibition |
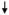 PSA PSA |
Curcumin (25 μM) Isoflavones (10 μg/ml) |
LNCaP | 111 |
| Curcumin, alone or in combination with TRAIL induce apoptosis on mitochondria pathway prostate cancer cells |
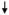 Cell viability Cell viability Apoptosis Apoptosis Mitochondria pathway Mitochondria pathway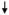 Procaspase 8 and 3 Procaspase 8 and 3
 Cytochrome C Cytochrome C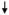 DNA Fragmentation DNA Fragmentation |
Curcumin (10–40 μM) TRAIL (20 ng/ml) |
LNCaP PC-3 Du145 |
112 |
| Curcumin, alone or in combination with TRAIL induce cytotoxic effects on prostate cancer cells |
 Cell viability Cell viability Clonal Growth Clonal Growth |
Curcumin (20–40 μM) TRAIL (20 ng/ml) |
LNCaP | 113 |
7. Clinical Trial Studies
Curcumin’s therapeutic efficacy has been explored and reported in more than 45 completed clinical trials and 39 curcumin trials are scheduled to investigate its efficacy in several disease states. Only one completed clinical study explored curcumin’s activity in prostate cancer and two more are scheduled. A one-year trial conducted by Rastmanesh et al. [114] explored the radioprotective and radiosensitivity of curcumin in patients diagnosed with prostate cancer administered 3 gm daily for up to 8 weeks. A Phase II study lead by Hakim Mamammedi [115], Center Jean Perrin, in the recruiting stage will explore the synergistic relations of Taxotere with curcumin versus Taxotere alone as a first-line treatment of patients with metastatic castration-resistant prostate cancer. Yair Lotan [116], University of Texas Southwestern Medical Center, is in the recruiting stage of a Phase II study to investigate the administration of curcumin and its ability to improve recurrence free survival. Detail information is presented in Table 5.
Table 5.
Curcumin-prostate Cancer Clinical Trials
| Clinical trials gov. identifier no. | Year started | Phase | Patient condition | Curcumin Dose | Purpose |
|---|---|---|---|---|---|
| NCT01917890 | Aug. 2013 | Completed | Prostate cancer/Radiation therapy | 3 gm once daily for 7–8 weeks | Determine curcumin radioprotective and radiosensitizing activity for prostate cancer treatment in |
| NCT02064673 | May 2014 | Phase II/recruiting | Prostate cancer | 500 mg 2x Daily for 6 months | Curcumin vs. placebo for treatment of patient after going radical prostatectomy |
| NCT02095717 | March 2014 | Phase II/recruiting | Metastatic Castration Resistant | Curcumin vs placebo combine with Taxotere for treatment in prostate cancer metastatic castration resistant patients |
8. Conclusion
A steady decline in prostate cancer related deaths since the 1990s may be attributed to early detection by increased implementation of prostate-specific antigen (PSA) screening blood test. The chemopreventive and chemotherapeutic nature of curcumin as a therapeutic agent for the treatment of prostate cancer is promising. Curcumin’s anti-tumor and anti-cancer activity is extensive and identified as potent in liver, breast, ovarian, pancreatic, and prostate cancers. The natural compound’s therapeutic properties are highlighted in its regulation of transduction and inflammatory pathway, cell cycle arrest induction, and apoptotic characteristics. Efficacious in vivo application of curcumin is limited in prostate cancer. The compound is chemically unstable, has poor aqueous solubility, narrowed systemic distribution, and experiences severe biotransformation when administered orally. Curcumin analogs, synergistic therapy, and innovative drug delivery techniques must be implemented to address the pharmaceutical in vivo application hurdles and overcome the aggressive and metastatic nature of cancer. Delivery vessels with encapsulated curcumin have demonstrated alternatives for efficacious oral administration. The chemopreventive and cytoprotective potency character of curcumin is retained in nanoparticle and liposome formulations. The administration of curcumin in a synergistic relationship with established prostate cancer therapeutics such as docetaxel and phenethyl isothiocyanate improves the cytotoxicity and chemotherapeutic characteristics.
Figure 5.
Curcumin on Prostate Cancer Signaling Pathways
Table 1.
Biological Activities of Curcumin Prostate Cancer Signaling Pathways
| Biological Effects | Mechanism of Action | Concentration | Ref. |
|---|---|---|---|
| Curcumin inhibited the phosphorylation of downstream targets of LNCaP cells | ↓Phosphorylation of GSK-3β/Akt ↓AR Expression |
20 μM | 79 |
| Curcumin inhibits protein kinase B activation in DU-145 cells | ↓AKT | 35 μM | 80 |
| Curcumin inhibits NFKb-regulate gene products in DU-145 cells | ↓Bcl-2 and Bcl-XL ↑Caspase 3 and 8, ↑Apoptosis |
50 μM | 81 |
| Curcumin inhibits proliferation of LNCaP and other obstructive prostate cancer cell lines | ↑Apoptosis | 10–50 μM | 82 |
| Curcumin inhibits the activation of androgen and other transcription factors | ↓AR gene expression ↓PSA Level ↓PSA secretion |
20 μM | 83 |
| Curcumin inhibits expression of androgen receptor of LNCaP cells | ↓Cell proliferation ↓PSA promoter gene |
10–40 μM | 84 |
| Curcumin inhibits hypoxia and HIF1 alpha expression on PSA promoter activity | ↓PSA level ↓AR protein level |
10 μM | 85 |
| Curcumin inhibits ROS production and receptor expression of cancer-associated fibroblasts in prostate cancer cells | ↓IL-6 receptor expression ↓CXCR4 |
25 μM | 86 |
| Curcumin inhibits cell viability and induce apoptosis by decrease mRNA and protein expression in prostate cancer cells | ↓ID1 mRNA expression ↓Cell viability |
20μM | 87 |
| Curcumin augments down-regulation of gene expression in prostate cancer cells | ↓NKX3.1 ↓AR expression ↓ARE Binding activity |
10,20,40 μM | 89 |
| Curcumin inhibits methylation through CpG Island of LNCaP | ↓Demethylation, ↑Neurog 1, ↑HDAC 1,4 and 8 ↓HAT activity |
5 μM | 90 |
| Curcumin inhibited proliferation and tumor volume of DU-145 cells | ↑Apoptosis ↓MMP-9 secretion ↓MMP-2 secretion |
10–100 μg/ml | 91 |
| Curcumin augments ceramide accumulation and induce apoptosis in PC-3 cells | ↑Ceramide accumulation ↑p38 MAPK ↑JNK ↑Caspase 3,8, and 9 ↓GSH Level ↑DNA Fragmentation |
25–100 μM | 92 |
| Curcumin, alone or in combination with radiation inhibits regulation of pro-survival factors and anti-apoptotic genes in PC-3 cells | ↓Prosurvival factors ↑Procaspase-9 ↑Caspase 9 and 3 |
5 μM | 93 |
Table 2.
Biological Effects of Curcumin in In Vivo Models of Prostate Cancer
| Biological Effect | Mechanism of Action | Dose/Duration | Ref. |
|---|---|---|---|
| Curcumin inhibited proliferation and tumor volume of DU-145 cells | ↓Tumor Volume ↓MM-9 Secretion ↓MMP-2 Secretion |
5 mg/kg/3x week | 90 |
| Curcumin inhibited AR activity and expression in LNCaP xenograft models | ↓Transcriptional Activtiy of AR mRNA | 500 mg/kg for 4 weeks | 94 |
| Curcumin induce apoptosis and apoptosis related protein in nude mice of prostate cancer cells | ↓Bcl-2 | 25–100 mg/kg for 30 days | 95 |
| Curcumin inhibit LNCaP tumor angiogenesis of in vivo models | ↓Tumor volume ↓Tumor mass ↓Vessel formation ↓Tumor Growth ↓Microvessel |
2%/ for 2 weeks | 96 |
| Curcumin inhibit pro-inflammatory cytokines and increase apoptosis and necrosis rate in prostate cancer cells. | ↓Bcl2 and Birc5 ↓Metastatic-related genes, ↓CXCL1 and CXCL2 |
15 μM | 97 |
Table 3.
Curcumin Drug Delivery Systems on prostate cancer models
| Biological effect | Delivery Vessel | Mechanism of Action | Cell line | Ref. |
|---|---|---|---|---|
| Curcumin in PLGA nano-encapsulation enhance anti-cancer activity in mouse xenograft model of prostate cancer | Poly(lactic-co-glycolic acid) (PLGA) 2.5–40 μM |
 Accumulation and Retention Protrusion of cell membrane Accumulation and Retention Protrusion of cell membrane Shrinkage and Aggregation Shrinkage and Aggregation Disrupt Cytoskeleton Micro and spherical nucleation Disrupt Cytoskeleton Micro and spherical nucleation |
C4-2 xenograft DU145 |
65 |
| Curcumin in oxidative degradable nanoparticle enhance apoptotic cell death, cytotoxicity and inhibits acid ceramidase in prostate cancer cells | Curcumin-loaded pH sensitive redox nanoparticle (Cur-RNP) (10 mg/kg Cur to 20 mg/kg of RNP) |
 Cellular uptake Cellular uptake Tumor volume growth rate Tumor volume growth rate Stability Stability Solubility Solubility |
PC-3 | 98 |
| Curcumin in lipid nanoparticles enhance drug delivery system and cell growth inhibition in prostate cancer cells. | Curcumin co-loaded nanostructured lipid carrier (Cur-NLC) (1200 μg alone) (700 μg/w genistein) |
 Intracellular uptake Solubility to 70% Intracellular uptake Solubility to 70% Stability to 100% for 2 h Stability to 100% for 2 h In vitro drug release to 55% for 8 h In vitro drug release to 55% for 8 h |
PC-3 | 99 |
| Curcumin in nanoemulsion enhance cytotoxicity of etoposide in prostate cancer cells | Nanoemulsion (ETP 5 μM + CUR 25 μM) |
 AUC AUC Bioavailability Bioavailability Cellular uptake Cellular uptake Intracellular availability Intracellular availability |
DU-145 PC-3 |
100 |
| Curcumin and resveratrol combination in liposome encapsulation inhibit prostatic adenocarcinoma and cell viability in PTEN knockout mice | Lipo-curcumin (2.5 mg/kg/bw each) |
 Bioavailability 5 fold increase for rate of apoptosis Bioavailability 5 fold increase for rate of apoptosis |
PTEN-CaP8 | 101 |
| Curcumin loaded β-cyclodextrin inclusion complex enhance anti-proliferative activity in prostate cancer cells | β-cyclodextrin-curcumin (CD-CUR) 5–40 μM |
 Aqueous Solubility Aqueous Solubility Apoptosis Apoptosis Intracellular uptake Intracellular uptake In vitro stability In vitro stability |
C4-2 DU145 |
102 |
| PCD/CUR self-assembly enhance anti-cancer activity in prostate cancer cells | Poly(β-cyclodextrin)/Curcumin self-assembly 20 μM |
 Bioavailability and delivery Bioavailability and delivery Cell growth 3–4 fold increase in cellular uptake Cell growth 3–4 fold increase in cellular uptake Stability and Solubility Stability and Solubility |
C4-2 DU145 PC3 |
103 |
Acknowledgments
This publication was made possible, in part, by RCMI Pilot Project (Dr. Selvam Chelliah) and molecular biology research infrastructure support from grant number 2G12MD007605 from the NIMHD/NIH. Financial support of this research by Texas Southern University and Karpagam Academy of Higher Education are also gratefully acknowledged.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. A Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Akaza H. Asian trends in primary androgen depletion therapy on prostate cancer. Cancer Biol Med. 2013;10:187–191. doi: 10.7497/j.issn.2095-3941.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab. 2013;27:603–616. doi: 10.1016/j.beem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Deng X, He G, Liu J, Luo F, Peng X, Tang S, Gao Z, Lin Q, Keller JM, Yang T. Recent advances in bone-targeted therapies of metastatic prostate cancer. Cancer Treat Rev. 2014;40:730–738. doi: 10.1016/j.ctrv.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ting H, Deep G, Agarwal C, Agarwal R. The strategies to control prostate cancer by chemoprevention approaches. Mutat Res. 2014;760:1–15. doi: 10.1016/j.mrfmmm.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford ED, Stone NN, Yu EY, Koo PJ, Freedland SJ, Slovin SF, Gomella LG, Berger ER, Keane TE, Sieber P. Challenges and recommendations for early identification of metastatic disease in prostate cancer. Urology. 2014;83:664–669. doi: 10.1016/j.urology.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Saman DM, Lemieux AM, Nawal Lutfiyya M, Lipsky MS. A review of the current epidemiology and treatment options for prostate cancer. Dis Mon. 2014;60:150–154. doi: 10.1016/j.disamonth.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Pettaway CA, Lamerato LE, Eaddy MT, Edwards JK, Hogue SL, Crane MM. Benign prostatic hyperplasia: racial differences in treatment patterns and prostate cancer prevalence. BJU Int. 2011;108:1302–1308. doi: 10.1111/j.1464-410X.2010.09991.x. [DOI] [PubMed] [Google Scholar]
- 10.Rabbani F, Yunis LH, Vora K, Eastham JA, Guillonneau B, Scardino PT, Touijer K. Impact of ethnicity on surgical margins at radical prostatectomy. BJU Int. 2009;104:904–908. doi: 10.1111/j.1464-410X.2009.08550.x. [DOI] [PubMed] [Google Scholar]
- 11.Swords K, Wallen EM, Pruthi RS. The impact of race on prostate cancer detection and choice of treatment in men undergoing a contemporary extended biopsy approach. Urol Oncol. 2010;28:280–284. doi: 10.1016/j.urolonc.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Pater LE, Hart KW, Blonigen BJ, Lindsell CJ, Barrett WL. Relationship between prostate-specific antigen, age and body mass index in a prostate cancer screening population. Am J Clin Oncol. 2012;35:490–492. doi: 10.1097/COC.0b013e31821a83be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell IJ, Vigneau FD, Bock CH, Ruterbusch J, Heilbrun LK. Reducing prostate cancer racial disparity: evidence for aggressive early prostate cancer PSA testing of African American men. Cancer Epidemiol Biomarkers Prev. 2014;23:1505–1511. doi: 10.1158/1055-9965.EPI-13-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang P, Du W, Xie K, Fu J, Chen H, Yang W, Moul JW. Characteristics of baseline PSA and PSA velocity in young men without prostate cancer. Racial differences Prostate. 2012;72:173–180. doi: 10.1002/pros.21418. [DOI] [PubMed] [Google Scholar]
- 15.Fraser M, Berlin A, Bristow RG, van der Kwast T. Genomic, pathological and clinical heterogeneity as drivers of personalized medicine in prostate cancer. Urol Oncol. 2015;33:85–94. doi: 10.1016/j.urolonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Espaldon R, Kirby KA, Fung KZ, Hoffman RM, Powell AA, Freedland SJ, Walter LC. Probability of an abnormal screening prostate-specific antigen result based on age, race and prostate-specific antigen threshold. Urology. 2014;83:599–605. doi: 10.1016/j.urology.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroder FH. Prostate cancer around the world. An overview. Urol Oncol. 2010;28:663–667. doi: 10.1016/j.urolonc.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Helfand BT, Catalona WJ. The epidemiology and clinical implications of genetic variation in prostate cancer. Urol Clin North Am. 2014;41:277–297. doi: 10.1016/j.ucl.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jermal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 20.Wallace TJ, Torre T, Grob M, Yu J, Avital I, Brucher B, Stojadinovic A, Man YG. Current approaches, challenges and future directions for monitoring treatment response in prostate cancer. J Cancer. 2014;5:3–24. doi: 10.7150/jca.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed A, Ali S, Sarkar FH. Advances in androgen receptor targeted therapy for prostate cancer. J Cell Physiol. 2014;229:271–276. doi: 10.1002/jcp.24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein J, Sanderson IR, MacDonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 23.Pescosolido N, Giannotti R, Plateroti AM, Pascarella A, Nebbioso M. Curcumin: therapeutical potential in ophthalmology. Planta Med. 2014;80:249–254. doi: 10.1055/s-0033-1351074. [DOI] [PubMed] [Google Scholar]
- 24.Martin RC, Aiyer HS, Malik D, Li Y. Effect on pro-inflammatory and antioxidant genes and bioavailable distribution of whole turmeric vs curcumin: Similar root but different effects. Food Chem Toxicol. 2012;50:227–231. doi: 10.1016/j.fct.2011.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Beevers CS, Huang S. Targets of curcumin. Curr Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furst R, Zundorf I, et al. Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediators Inflamm. 2014:146832. doi: 10.1155/2014/146832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shehzad A, Lee J, Lee YS. Curcumin in various cancers. BioFactors. 2013;39:56–68. doi: 10.1002/biof.1068. [DOI] [PubMed] [Google Scholar]
- 28.Shehzad A, Lee YS, et al. Molecular mechanisms of curcumin action: signal transduction. BioFactors. 2013;39:27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 29.Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest New Drugs. 2008;26:289–297. doi: 10.1007/s10637-007-9099-7. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Li C, Sun H, Luo T, Tan Y, Tian D, Guo Z. Curcumin inhibits monocyte chemoattractant protein-1 expression and enhances cholesterol efflux by suppressing the c-Jun N-terminal kinase pathway in macrophage. Inflamm Res. 2014;63:841–850. doi: 10.1007/s00011-014-0758-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Y, Liu T, Guo Z. Curcumin inhibits ox-LDL-induced MCP-1 expression by suppressing the p38 MAPK and NF-kappaB pathways in rat vascular smooth muscle cells. Inflamm Res. 2012;61:61–67. doi: 10.1007/s00011-011-0389-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H, Beevers CS, Huang S. Targets of curcumin. Curr Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. BioFactors. 2013;39:69–77. doi: 10.1002/biof.1066. [DOI] [PubMed] [Google Scholar]
- 34.Pereira AK, Garcia MT, Pinheiro W, Ejzenberg D, Soares JM, Baracat EC., Jr What is the influence of cyclooxygenase-2 on postmenopausal endometrial polyps? Climacteric. 2015;18:498–502. doi: 10.3109/13697137.2014.966240. [DOI] [PubMed] [Google Scholar]
- 35.Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R. Curcumin as a wound healing agent. Life sci. 2014;116:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 36.El-Bahr SM. Effect of Curcumin on Hepatic Antioxidant Enzymes Activities and Gene Expressions in Rats Intoxicated with Aflatoxin B1. Phytother Res. 2015;29:134–140. doi: 10.1002/ptr.5239. [DOI] [PubMed] [Google Scholar]
- 37.Trujillo J, Chirino YI, Molina-Jijon E, Anderica-Romero AC, Tapia E, Pedraza-Chaverri J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013;1:448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagiroglu T, Kanter M, Yagci MA, Sezer A, Erbogaet M. Protective effct of curcumin on cyclosporin A-induced endothelial dysfunction, antioxidant capacity, and oxidative damage. Toxicol Ind Health. 2014;30:316–327. doi: 10.1177/0748233712456065. [DOI] [PubMed] [Google Scholar]
- 39.Gazal M, Valente MR, Acosta A, Kaufmann FN, Braganhol E, Lencina CL, Stefanello FM, Ghisleni G, Kaster MP. Neuroprotective and antioxidant effects of curcumin in a ketamine-induce model of mania in rats. Eur J Pharmacol. 2014;724:132–139. doi: 10.1016/j.ejphar.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 40.Eshghi N, Asnaashari M, Khodaparast HH, Hosseini F. Evaluating the potential of natural curcumin for oxidative stability of soybean oil. Nat Prod Res. 2014;17:1375–1378. doi: 10.1080/14786419.2014.901319. [DOI] [PubMed] [Google Scholar]
- 41.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naijil G, Anju TR, Jayanarayanan S, Paulose CS. Curcumin pretreatment mediates antidiabetogenesis via functional regulation of adrenergic receptor subtypes in the pancrease of multiple low-dose streptozotocin-induced diabetic rats. Nutr Res. 2015;35:823–833. doi: 10.1016/j.nutres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Kim DC, Ku SK, Bae JS. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012;45:221–226. doi: 10.5483/bmbrep.2012.45.4.221. [DOI] [PubMed] [Google Scholar]
- 44.Prakash P, Misra A, Surin WR, Jain M, Bhatta RS, Pal R, Raj K, Barthwal MK, Dikshit M. Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb Res. 2011;127:111–118. doi: 10.1016/j.thromres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Tajbakhsh S, Mohammadi K, Deilami I, Zandi K, Fouladvand M, Ramedani E, Asayesh G. Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr J Biotechnol. 2008;7:3832–3835. [Google Scholar]
- 46.Mun SH, Joung DK, Kim YS, Kang OK, Kim SB, Seo YS, Kim YC, Lee DS, Shin DW, Kweon KT, Kwon DY. Synergestic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine. 2013;20:714–718. doi: 10.1016/j.phymed.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 47.De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592–1597. doi: 10.1128/AAC.01242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Upendra RS, Khandelwal P, Manjunatha RAH. Turmeric powder (Curcuma longa Linn.) as an antifungal agents in plant tissue culture studies. Int J Eng Sci. 2011;3:1578–1581. [Google Scholar]
- 49.Chowdhury H, Banerje T, Walia S. In vitro screening of Curcuma longa L and its derivatives as antifungal agents against Helminthosporrum oryzae and Fusarium solani. J Pestic Res. 2008;20:6–9. [Google Scholar]
- 50.Khalilj OAK, de Faria Oliveira OMM, Vellosa JCR, Urba de Quadros A, Dalposso LM, Karam TK, Mainardes MR, Khalil NM. Curcumin antifungal and antioxidant activities are increased in the presence of ascorbic acid. Food Chemistry. 2012;133:1001–1005. [Google Scholar]
- 51.Sharma M, Manoharlal R, Puri N, Prasad R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci Rep. 2010;30:391–404. doi: 10.1042/BSR20090151. [DOI] [PubMed] [Google Scholar]
- 52.Chung SH, Choi SH, Choi JA, Chuck RS, Joo CK. Curcumin suppresses ovalbumin-induced allergic conjunctivitis. Mol Vis. 2012;18:1966–1972. [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JH, Kim JW, Ko NY, Mun SH, Her E, Kim BK, Han JW, Lee HY, Beaven MA, Kim YM, Choi WS. Curcumin, a constituent of curry, suppresses IgE-mediated allergic response and mast cell activation at the level of Syk. J Allergy Clin Immunol. 2008;12:1225–1231. doi: 10.1016/j.jaci.2007.12.1160. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki M, Nakamura T, Iyoki S, Fujiwara A, Watanabe Y, Mohri K, Isobe K, Ono K, Yano S. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol Pharm Bull. 2005;28:1438–1443. doi: 10.1248/bpb.28.1438. [DOI] [PubMed] [Google Scholar]
- 55.Subhashini CPS, Kumari S, Dash D, Sing R. Curcumin inhibits compound 48/80 induced systemic anaphylaxis. AJLS. 2013;1:165–170. [Google Scholar]
- 56.Kinney SRM, Carlson L, Ser-Dolansky J, Thompson C, Shah S, Gambrah A, Xing W, Schneider SS, Mathias CB. Curcumin ingestion inhibits mastocytosis and suppresses intestinal anaphylaxis in a murine model of food allergy. PLoS One. 2015;10:1–20. doi: 10.1371/journal.pone.0132467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda S, Okazaki H, Ikeda E, Abe S, Yoshioka Y, Watanabe K, Aramaki H. Down-regulation of cyclooxygenase-2 (COX-2) by cannabidiolic acid in human breast cancer cells. J Toxicol Sci. 2014;39:711–716. doi: 10.2131/jts.39.711. [DOI] [PubMed] [Google Scholar]
- 58.Biomente S, Barbieri A, Palma G, Rea D, Luciano A, D’Aiuto M, Arra C, Izzo F. Dissecting the Role of curcumin in tumour growth and angiogenesis in Mouse model of human breast cancer. Biomed Res Int. 2015:878134. doi: 10.1155/2015/878134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatamie S, Akhavan O, Sadrnezhaad SK, Ahadian MM, Shirolkar MM, Wang HQ. Curcumin-reduced grapheme oxide sheets and their effects on human breast cancer cells. Mater Sci Eng C Mater Biol Appl. 2015;55:482–489. doi: 10.1016/j.msec.2015.05.077. [DOI] [PubMed] [Google Scholar]
- 60.Coleman DT, Soung YH, Surh YS, Cardelli JA, Chung J. Curcumin prevents palmitoylation of integrin β4 in breast cancer cells. PloS One. 2015;10:e0125399. doi: 10.1371/journal.pone.0125399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun K, Duan X, Cai H, Liu X, Yang Y, Li M, Zhang X, Wang J. Curcumin inhibits LPA-induced invasion by attenuating RhoA/ROCK/MMPs pathway in MCF7 breast cancer cells. Clin Exp Med. 2015:1–11. doi: 10.1007/s10238-015-0336-7. [DOI] [PubMed] [Google Scholar]
- 62.Hua WF, Fu YS, Liao YJ, Xia WJ, Chen YC, Zeng YX, Kung HF, Xie D. Curcumin induces down-regulation of EZH2 expression through the MAPK pathwat in MDA-MB-435 human breast cancer cells. Eur J Pharmacol. 2010;637:16–21. doi: 10.1016/j.ejphar.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 63.Liu Q, Wing TYL, Sze SCW, Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation NF-kB, cyclinD and MMP-1 transcription. Phytomedicine. 2009;16:916–922. doi: 10.1016/j.phymed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 64.Chakraborty G, Jain S, Kale S, Raja R, Kumar S, Mishra R, Kundu GC. Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol Med Rep. 2008;1:641–646. doi: 10.3892/mmr_00000005. [DOI] [PubMed] [Google Scholar]
- 65.Yallapu MM, Khan S, Maher DM, Ebeling MC, Sundram V, Chauhan N, Ganju A, Balakrishna S, Gupta BK, Zafar N. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials. 2014;35:8635–8648. doi: 10.1016/j.biomaterials.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandair D, Rossi RE, Pericleous M, Whyand T, Caplin ME. Prostate cancer and the influence of dietary factors and supplements: a systematic review. Nutr Metab (Lond) 2014;11:30. doi: 10.1186/1743-7075-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen HW, Lee JY, Huang JY, Wang CC, Chen WJ, Su SF, Huang CW, Ho CC, Chen JJ, Tsai MF, Sl Y, Yang PC. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428–7438. doi: 10.1158/0008-5472.CAN-07-6734. [DOI] [PubMed] [Google Scholar]
- 68.Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, Lin JP, Ma YS, Wu CC, Chung JG. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF) Cancer Lett. 2009;285:127–133. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 69.Lee J, Im YH, Jung HH, Kim JH, Park JO, Kim K, Kim WS, Ahn JS, Jung CW, Park YS, Kang WK, Park K. Curcumin inhibits interferon-alpha induced NF-kappaB and COX-2 in human A549 non-small cell lung cancer. Biochem Biophys Res Commun. 2005;334:313–318. doi: 10.1016/j.bbrc.2005.06.093. [DOI] [PubMed] [Google Scholar]
- 70.Xu X, Chen D, Ye B, Zhong F, Chen G. Curcumin induces the apoptosis of non-small cell lung cancer cells through a calcium signaling pathway. Int J Mol Med. 2015;35:1610–1616. doi: 10.3892/ijmm.2015.2167. [DOI] [PubMed] [Google Scholar]
- 71.Zhang K, Rui X, Yan X. Curcumin Inhibits the Proliferation and Invasiveness of MHCC97-H Cells via p38 Signaling Pathway. Drug Dev Res. 2014;75:463–468. doi: 10.1002/ddr.21210. [DOI] [PubMed] [Google Scholar]
- 72.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 73.Phillips JM, Clark C, Herman-Ferdinandez L, Moore-Medlin T, Rong X, Roberts GJ, Clifford JL, Abreo F, Nathan CAO. Curcumin inhibits skin squamous cell carcinoma tumor growth in vivo. Otolaryngol Head Neck Surg. 2011;145:58–63. doi: 10.1177/0194599811400711. [DOI] [PubMed] [Google Scholar]
- 74.Phillips J, Moore-Medlin T, Sonavane K, Ekshyyan O, McLarty J, Nathan CA. Curcumin inhibits UV radiation-induced skin cancer in SKH-1 mice. Otolaryngol Head Neck Surg. 2013;148:797–803. doi: 10.1177/0194599813476845. [DOI] [PubMed] [Google Scholar]
- 75.Mohanty C, Sahoo KS. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597–6611. doi: 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 76.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 77.Gordon ON, Luis PB, Sintim HO, Schneider C. Unraveling curcumin degradation: autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J Biol Chem. 2015;290:4817–4828. doi: 10.1074/jbc.M114.618785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343:489–499. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 79.Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/beta-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010;13:343–349. doi: 10.1038/pcan.2010.26. [DOI] [PubMed] [Google Scholar]
- 80.Chaudhary LR, Hruska KA. Inhibition of cell survival signal protein kinase B/Akt by curcumin in human prostate cancer cells. J Cell Biochem. 2003;89:1–5. doi: 10.1002/jcb.10495. [DOI] [PubMed] [Google Scholar]
- 81.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 82.Piantino CB, Salvadori FA, Ayres PP, Kato RR, Srougi V, Leite K, Srougi M. An evaluation of the anti-neouplastic activity of curcumin in prostate cancer cell lines. Int Braz J Urol. 2009;35:354–360. doi: 10.1590/s1677-55382009000300012. [DOI] [PubMed] [Google Scholar]
- 83.Tsui KH, Feng TH, Lin CM, Chang PL, Juang HH. Curcumin blocks the activation of androgen and interlukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells. J Androl. 2008;29:661–668. doi: 10.2164/jandrol.108.004911. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Chen L, Meng B, Suo J, Wang H, Xie H, Jin Q, Yao L, Wang R, Zhang L. The effect of curcumin on proliferation and apoptosis in LNCaP prostate cancer cells. CJCO. 2006;3:55–60. [Google Scholar]
- 85.Chung LC, Tsui KH, Feng TH, Lee SL, Chang PL, Juang HH. Curcumin provides potential protection against the activation of hypoxia and prolyl 4-hydroxylase inhibitors on prostate-specific antigen expression in human prostate carcinoma cells. Mol Nutr Food Res. 2011;55:1666–1676. doi: 10.1002/mnfr.201100328. [DOI] [PubMed] [Google Scholar]
- 86.Du Y, Long Q, Zhang L, Shi Y, Liu X, Li X, Guan B, Tian Y, Wang X, Li L. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAO/mTOR/HIF-1α signaling. Int J Oncol. 2015;47:2064–2072. doi: 10.3892/ijo.2015.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu X, Jing T, Zhao H, Li P, Xu W, Shang F. Curcumin inhibits expression of inhibitor of DNA binding 1 in PC3 Cell and Xenografts. Asian Pac J Cancer Prev. 2014;15:1465–1470. doi: 10.7314/apjcp.2014.15.3.1465. [DOI] [PubMed] [Google Scholar]
- 88.Dorai T, Dutcher JP, Dempster DW, Wiernik PH. Therapeutic potential of curcumin in prostate cancer-IV: Interference with the osteomimetic properties of hormone refractory C4-2B prostate cancer cells. Prostate. 2004;60:1–17. doi: 10.1002/pros.10359. [DOI] [PubMed] [Google Scholar]
- 89.Zhang HN, Yu CX, Zhang PJ, Chen WW, Jiang AL, Kong F, Deng JT, Zhang JY, Young CY. Curcumin downregulates homeobox gene NKX3.1 in prostate cancer cell LNCaP. Acta Pharmacol Sin. 2007;28:423–430. doi: 10.1111/j.1745-7254.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 90.Shu L, Khor TO, Lee JH, Boyanapalli SS, Huang Y, Wu TY, Saw CL, Cheung KL, Kong AN. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. AAPS J. 2011;13:606–614. doi: 10.1208/s12248-011-9300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hong JH, Ahn KS, Bae E, Jeon SS, Choi HY. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006;9:147–152. doi: 10.1038/sj.pcan.4500856. [DOI] [PubMed] [Google Scholar]
- 92.Hilchie AL, Furlong SJ, Sutton K, Richardson A, Robichaud MR, Giacomantonio CA, Ridgway ND, Hoskin DW. Curcumin-induced apoptosis in PC3 prostate carcinoma cells is caspase-independent and involves cellular ceramide accumulation and damage to mitochondria. Nutr Cancer. 2010;62:379–389. doi: 10.1080/01635580903441238. [DOI] [PubMed] [Google Scholar]
- 93.Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–1607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- 94.Hong JH, Lee G, Choi HY. Effect of curcumin on the interaction between androgen receptor and Wnt/β-catenin in LNCaP xenografts. Korean J Urol. 2015;56:656–665. doi: 10.4111/kju.2015.56.9.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J, Ning J, Peng L, He D. Effect of curcumin on Bcl-2 and Bax expression in nude mice prostate cancer. Int J Clin Exp Pathol. 2015;8:9272–9278. [PMC free article] [PubMed] [Google Scholar]
- 96.Dorai T, Cao YC, Dorai D, Buttyan R, Katz AE. Therapuetic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293–303. doi: 10.1002/pros.1074. [DOI] [PubMed] [Google Scholar]
- 97.Killian PH, Kronski E, Michalik KM, Barbieri O, Astigiano S, Sommerhoff CP, Pfeffer U, Nerlich AG, Bachmeier BE. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis. 2012;33:2507–2519. doi: 10.1093/carcin/bgs312. [DOI] [PubMed] [Google Scholar]
- 98.Thangavel S, Yoshitomi T, Sakharkar MK, Nagaski Y. Redox nanoparticles inhibit curcumin oxidative degradation and enhance its therapeutic effet on prostate cancer. J Control Release. 2015;209:110–119. doi: 10.1016/j.jconrel.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 99.Aditya NP, Shim M, Lee I, Lee YJ, Im MH, Ko S. Curcumin and Genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. J Agri Food Chem. 2013;61:1878–1883. doi: 10.1021/jf305143k. [DOI] [PubMed] [Google Scholar]
- 100.Shukla P, Mathur V, Kumar A, Khedgikar V, Teja V, Chaudhary D, Kushwaha P, Bora HK, Konwar R, Trivedi R, Mishra PR. Nanoemulsion based concomitant delivery of curcumin and etoposide: impact on cross talk between prostate cancer cells and osteoblast during metastasis. J Biomed Nanotechnol. 2014;10:1–11. doi: 10.1166/jbn.2014.1912. [DOI] [PubMed] [Google Scholar]
- 101.Narayanan NK, Nargi D, Randolph C, Natayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125:1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 102.Yallapu MM, Jaggi M, Chauhan SC. β-cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B Biointerfaces. 2010;79:113–125. doi: 10.1016/j.colsurfb.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 103.Yallapu MM, Jaggi M, Chauhan SC. Poly(β-cyclodextrin)/curcumin self-assembly: A novel approach to improve curcumin delivery and its therapeutic efficacy in prostate cancer cells. Macromol Biosci. 2010;10:1141–1151. doi: 10.1002/mabi.201000084. [DOI] [PubMed] [Google Scholar]
- 104.Fuchs JR, Pandit B, Bhasin D, Etter JP, Regan N, Abdelhamid D, Li C, Lin J, Li PK. Structure-activity relationship studies of curcumin analogues. Bioorg Med Chem Lett. 2009;19:2065–2069. doi: 10.1016/j.bmcl.2009.01.104. [DOI] [PubMed] [Google Scholar]
- 105.Lin L, Shi Q, Nyarko AK, Bastow KF, Wu CC, Su CY, Shih CC, Lee KH. Antitumor agents. 250. Design and synthesis of new curcumin analogues as potential anti-prostate cancer agents. J Med Chem. 2006;49:3963–3972. doi: 10.1021/jm051043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valentini A, Conforti F, Crispini A, De Martino A, Condello R, Stellitano C, Rostilio G, Ghenini M, Federici G, Bernardini S, Pucci D. Synthesis, oxidant properties, and antitumoral effects of a heteroleptic palladium (II) complex of curcumin on human prostate cancer cells. J Med Chem. 2009;52:484–491. doi: 10.1021/jm801276a. [DOI] [PubMed] [Google Scholar]
- 107.Mathur A, Abd Elmageed ZY, Liu X, Kostochka M, Zhang H, Abdel-Mageed AB, Mondal D. Subverting ER-stress toward apoptosis by nelfinavir and curcumin coexposure augments docetaxel efficacy in castration resistant prostate cancer cells. PLoS One. 9:2014, e103109. doi: 10.1371/journal.pone.0103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li J, Xiang ST, Zhang QH, Wu JJ, Tang Q, Zhou JF, Yang LJ, Chen ZQ, Hann SS. Combination of curcumin and bicalutamide enhanced the growth inhibition of androgen-independent prostate cancer cells through SAPK/JNK and MEK/ERk 1/2-mediated targeting NF-Kb/ p65 and MUC1-C. J Exp Clin Cancer Res. 2015;15:46–57. doi: 10.1186/s13046-015-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eom DW, Lee JH, Kim YJ, Hwang GS, Kim SN, Kwak JH, Cheon GJ, Kim KH, Jang HJ, Ham J, Kang KS, Yamabe N. Synergistic effect of curcumin on epigallocatechin gallate-induce anticancer action in PC3 prostate cancer cells. BMB Rep. 2015;48:461–466. doi: 10.5483/BMBRep.2015.48.8.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khor TO, Keum YS, Lin W. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006;66:613–621. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]
- 111.Ide H, Tokiwa S, Sakamaki K, Nishio K, Isotani S, Muto S, Hama T, Masuda H, Horie S. Combine inhibitory effects of soy isoflavones and cucumin on the production of prostate-specific antigen. Prostate. 2010;70:1127–1133. doi: 10.1002/pros.21147. [DOI] [PubMed] [Google Scholar]
- 112.Deeb D, Jiang H, Gao X, Divine G, Dulchavshy SA, Gautam SC. Chemosensitization of hormone-refractory prostate cancer cells by curcumin to TRAIL-induced apoptosis. J Exp Ther Oncol. 2005;5:81–91. [PubMed] [Google Scholar]
- 113.Deeb D, Xu YX, Jiang H. Curcumin(Diferuloyl-Methane) enchances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in LNCaP prostate cancer cells. Mol Cancer Ther. 2003;2:95–103. [PubMed] [Google Scholar]
- 114.https://clinicaltrials.gov/ct2/show/NCT0191890?term=curcumin+and+prostate&rank=1
- 115.https://clinicaltrials.gov/ct2/show/NCT02064673?term=curcumin+and+prostate&rank=2
- 116.https://clinicaltrials.gov/ct2/show/NCT02095717?tern=curcumin+and+prostate&rank=3