Abstract
Cryotherapy is a therapeutic technique using ice or cold water applied to the skin to reduce bleeding, inflammation, pain, and swelling following soft tissue trauma and injury. While beneficial, there are some side effects such as pronounced vasoconstriction and tissue ischemia that are sustained for hours post-treatment. This study tested the hypothesis that this vasoconstriction is mediated by 1) the Rho-kinase pathway and/or 2) elevated oxidative stress. 9 subjects were fitted with a commercially available cryotherapy unit with a water perfused bladder on the lateral portion of the right calf. Participants were instrumented with three microdialysis probes underneath the bladder. One site received lactated ringers (control site), one received the Rho-Kinase inhibitor Fasudil, and one received Ascorbic Acid. Skin temperature (Tskin) and cutaneous vascular conductance (CVC) was measured at each site. Subjects had 1 °C water perfused through the bladder for 30 min, followed by passive rewarming for 90 min. Tskin fell from ~ 34 °C to ~ 18.0 °C during active cooling across all sites and this response was similar for all sites (P>0.05 for all comparisons). During passive rewarming Tskin rose to a similar degree in all sites (P>0.05 relative to the end of cooling). %CVC was reduced during active cooling in all sites; however, the magnitude of this response was blunted in the Fasudil site relative to control (P<0.001 for all comparisons) and min 25 and 30 of cooling in the Ascorbic Acid site (P<0.05). During passive rewarming %CVC at the control and Ascorbic Acid sites did not change such that values were similar to the end of cooling (P>0.05 for each comparison). %CVC at the Fasudil site remained elevated during passive rewarming such that values were higher compared to the control and Ascorbic Acid sites throughout the 90 min of passive rewarming (P<0.001 main effect of Fasudi). These findings indicate that the Rho-kinase pathway contributes to pronounced vasoconstriction during cryotherapy as well as the sustained vasoconstriction during the subsequent rewarming period post treatment.
Keywords: vasoconstriction, ischemia, skin-surface cooling, cryotherapy, soft tissue injury
Introduction
Localized cooling accomplished via cryotherapy treatment is commonly used after orthopedic surgery and in sports medicine to reduce bleeding, inflammation, metabolism, muscle spasm, pain, and swelling following soft tissue trauma and injury (35). While cryotherapy treatment yields many therapeutic benefits, it is often associated with a number of side effects including, tissue necrosis, and neuropathy (1, 4, 6, 26, 29). These conditions are likely the result of profound reductions in local tissue temperature and the subsequent pronounced tissue ischemia during the period of cryotherapy. Furthermore, we have recently reported that this pronounced vasoconstriction is sustained for up to 2 hr during passive rewarming of the limb despite skin temperature returning back to near baseline (i.e. pre-cooling) values (23, 24). A temporarily reduced blood flow is beneficial in treating soft tissue injuries due to reducing the inflammation cascade and edema formation. However, when a prolonged state of ischemia is maintained over sufficient time, the consequences of a reduced supply of oxygen and cell nutrients in conjunction with the buildup of toxic metabolic byproducts may lead to tissue necrosis and neuropathies (32). In addition, a prolonged state of ischemia can lead to reperfusion injury when flow is reestablished (19). In this regard it is well documented that reduced skin temperatures, even as high as 24°C, causes a profound local decrease in blood perfusion of tissues, which, may be an agent of nonfreezing cold injury (13, 14).
The mechanisms of cutaneous vasoconstriction during localized cooling have been the topic of many previous research studies and reviews (16, 20, 21, 37, 38, 40). These studies indicate that the pronounced vasoconstriction during local cooling is due to adrenergic and nonadrenergic pathways (16, 20, 21, 37, 38, 40). In vivo human studies have reported an attenuated cutaneous vasoconstrictor response during local cooling following intradermal infusion of the antioxidant ascorbic acid (40). Similar findings have been reported following blockade of the Rho kinase pathway and the subsequent translocation of α2c-adrenoreceptors to the smooth muscle plasma membrane using intradermal infusion of fasudil (37, 38).
While these studies are eloquently designed and provide valuable insight into mechanisms of cold-induced vasoconstriction, the application differs from conditions of cryotherapy treatment in a few ways. Many of these studies are performed during cooling of the skin surface to about 24 °C which is approximately 10 °C below baseline values (16, 20, 21, 37, 38, 40). In addition, the skin surface area being cooled, equal to approximately 6.3 cm2, is relatively small (16, 20, 21, 37, 38, 40). In contrast cryotherapy application, using commercially available units, is most commonly performed by circulating ice water through a bladder that covers a much larger surface area such as the shoulder, knee, thigh, or shin region (1, 4). As a result skin temperatures drops to approximately 17 °C or lower over a much larger surface area relative to the aforementioned cold applications (23, 24). Whether or not similar mechanistic pathways involved in cold induced vasoconstriction can be extrapolated to the vasoconstriction that occurs during cryotherapy treatment (23, 24) remains unknown. Furthermore, to our knowledge no studies have investigated mechanisms of sustained vasoconstriction following termination of the cooling stimulus (23, 24) particularly using commercially available cryotherapy units.
This study utilized the technique of intra-dermal administration of vasoactive substances into the cutaneous circulation directly underneath the cryotherapy cooling pad. We hypothesized that cold-induced vasoconstriction during cryotherapy treatment would be attenuated following; 1) local infusion of the global antioxidant Ascorbic Acid and 2) blockade of the Rho kinase pathway using Fasudil. Furthermore, we hypothesized that the sustained vasoconstriction for up to 90 min following cryotherapy treatment (23, 24) would be attenuated following local infusion of Ascorbic Acid and Fasudil.
Methods
Ethical Approval
The Institutional Review Board at The University of Texas at Austin approved all study procedures and the consent process used in the present study. Subjects were given a verbal description of all procedures and informed of the purpose and risks involved in the study before providing their informed, written consent.
Subjects
9 healthy young subjects (10 males) participated in this study. Average (mean ± SD) subject characteristics were: age, 24 ± 1 years; height, 180 ± 1 cm; and weight, 81 ± 3 kg. Subjects were non-smokers, were not taking medications and were free from cardiovascular, neurological, or metabolic diseases. None of the subjects reported a history of knee injury or cryotherapy or other form of cold exposure in the lower extremities for at least a year prior to the experiment. All studies were conducted in the morning following an overnight fast (> 12 hr). Subjects refrained from strenuous exercise and alcoholic beverages for 24 hr and from consuming caffeine and food for 12 hr prior to the experimental trial that was conducted in a temperature controlled laboratory (~24°C and 40% relative humidity).
Instrumentation and Measurements
All data were collected with the subject seated in a semi-recumbent position. Three microdialysis membranes (CMA 31 Linear Microdialysis Probe, 55 KDalton cut-off membrane; Harvard Apparatus, Holliston, MA) with a 10-mm-long semi permeable portion were inserted ~5 cm apart into the dermal layer of the nonglabrous skin on the lateral side of the right calf. Membrane insertion was accomplished by first placing a 25-gauge needle into the dermal layer of the right calf and then threading the membrane through the needle. Once the semi-permeable portion of the membrane was under the skin surface (i.e. in the needle) the needle was completely removed and the membrane was secured to the skin with tape. Following placement each membrane was perfused with lactated Ringer’s solution (Baxter, Deerfield, IL) at a rate of 2 μL/min via a perfusion pump (Harvard Apparatus, Holliston, MA) while insertion trauma associated with membrane placement subsided (minimum 90 min). During this period each membrane site was instrumented with an integrating laser Doppler flow probe (VP7a, Moor Instruments, Wilmington, DE) which provided a continuous index of skin blood flow. A thermocouple (Type T Thermocouple Probe, Physitemp Instruments INC, Cliffton, NJ) was placed immediately adjacent to the Doppler flow probe for continuous assessment of local skin temperature. Following placement of the membranes, Doppler flow probes, and thermocouples a commercially available cryotherapy cooling pad (Arctic Ice Universal Pad; Pain Management Technologies, Akron, OH) was applied overlying the instrumented area, and fixed in place using an Ace bandage. The cooling pad was connected to an Arctic Ice cryotherapy unit (Pain Management Technologies, Akron, OH) which allowed for manipulation of the underlying skin (Tskin) and tissue temperature according to the manufacturer’s recommendation (see below for more detail). A cuff was placed around the left arm for intermittent blood pressure measurements from the brachial artery using electrosphygmomanometry (Tango, SunTech Medical Instruments, Raliegh, NC).
Study Protocol
After the hyperemic response associated with insertion trauma subsided (minimum of 90 min) each site was perfused with its respective vasoactive agent for a 45 min wash in period. One site received lactated Ringer solution (Baxter, Deerfield, IL) which served as the control site, one site received Fasudil (3mM, ToCris Bioscience, Ellisville, MO) to locally block the Rho kinase pathway (37), and the last site received Ascorbic Acid (10mM, Mylan Institutional LLC, Rockford, IL) to supplement antioxidants (40). Fasudil and Ascorbic Acid were dissolved in lactated Ringer solution. Each site was initially perfused at 52 μL/min for a 30 sec priming period after which the rate was reduced to 2 μL/min for the remainder of data collection. After the 45 min wash in period, the cryotherapy unit and cooling pad was perfused with 34 °C water for 15 min of baseline data collection. This was followed by 30 min of active skin-surface cooling which was accomplished by circulating 0 – 2 °C water through the cryotherapy unit and cooling pad. At the end of the cooling phase the cryotherapy unit was turned off for a 90 min period of data collection during passive rewarming.
Data Analysis
Laser-Doppler flux and Tskin data were continuously collected at a sampling rate of 125 Hz via a data-acquisition system (Biopac System, Santa Barbara, CA). One min averages of these data were analyzed at the following time points: the final min of the 34 °C baseline condition, min 5, 10, 15, 20, 25, and 30 of active cooling; and min 30, 60, and 90 of passive rewarming. Mean arterial pressure (MAP), calculated as 1/3 systolic pressure + 2/3 diastolic pressure, was also measured during each of these time points and used for subsequent calculation of cutaneous vascular conductance (CVC) (Doppler-derived flux/MAP). All CVC data throughout active cooling and passive rewarming is reported as a % reduction relative to the value obtained during the final min of 34 °C baseline (%CVC). The Tskin data is reported as the absolute values during the final min of each of the aforementioned time points.
Statistical Analysis
Statistical analyses were performed using a statistical software package (SigmaPlot 12.5; Systat Software, Inc., San Jose, CA). Skin blood flow and skin temperature were both analyzed using a two-way repeated measures ANOVA comparing treatment (Control, Fasudil, and Ascorbic acid) by time effects. When a significant interaction was identified post hoc analyses of multiple comparisons was performed using Bonferroni t tests. All data is shown as mean±SD. For all tests significance was found at P < 0.05.
Results
Tskin during Skin Surface Cooling and Passive Rewarming
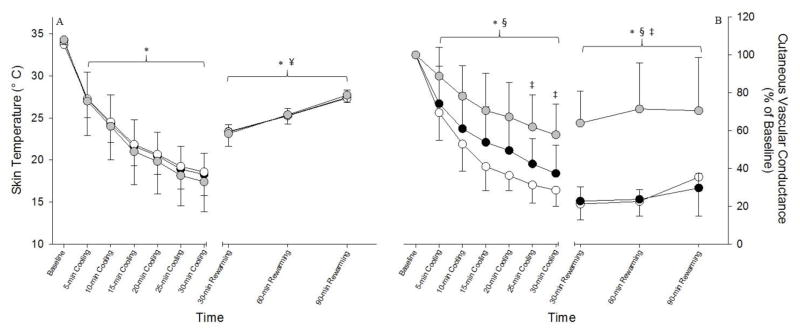
Throughout the active cooling period Tskin decreased at all sites relative to the precooling baseline (main effect of time: P<0.001), and the response was similar at all three sites (Fig 1A). Tskin at pre-cooling baseline was ~34.0 °C for each site (Fig 1A). During active cooling Tskin was immediately reduced to ~ 27.0 °C after the first 5 min (P<0.001 vs. pre-cooling baseline) for each site and continued to reduce throughout active cooling reaching a minimum value of ~ 18.0 °C at the end of 30 min of cooling (Fig 1A; P<0.001 vs. pre-cooling baseline) for each site. Following 30 min of passive rewarming Tskin was elevated to ~ 23.0 °C (P<0.001 vs. end of cooling) for each site and continued to rise throughout passive rewarming reaching a value of ~ 27.5 °C at each site at the end of 90 min of passive rewarming (Fig 1A; P<0.001 vs. end of cooling). Despite this significant elevation during passive rewarming, Tskin remained below pre-cooling baseline values (Fig 1A; P<0.001 vs. pre-cooling baseline). Tskin was similar in each of the measurement sites throughout the active cooling and passive rewarming periods (P>0.05 for all comparisons).
Figure 1. Tskin and CVC during Skin Surface Cooling and Passive Rewarming.
The Tskin response is illustrated in panel A. Skin-surface cooling caused immediate and significant reductions in Tskin and this response was similar between the three sites. Passive rewarming significantly elevated Tskin to a similar degree in all three sites. Throughout the 90 min duration of passive rewarming Tskin was elevated relative to end of cooling; however, Tskin remained reduced relative to pre-cooling baseline. The CVC response with respect to pre-cooling baseline (%CVC) is illustrated in panel B. Skin-surface cooling caused immediate and significant reductions in %CVC in all sites however, this response was blunted with Fasudil compared to the control site as well as min 25 and 30 in the Ascorbic Acid site. 90 min of passive rewarming had no effect on %CVC such that values in each site were similar to the end of cooling. The white, grey, and black circles represent the control, Ascorbic Acid, and Fasudil sites respectively. All data presented as means ± SD. *: P<0.001 for each condition relative to pre-cooling baseline. §: P<0.05 main effect of Ascorbic Acid vs. control.¥: P<0.001 for each condition relative to 30-min of cooling.
CVC during Skin Surface Cooling and Passive Rewarming
Cutaneous vascular conductance, % CVC with respect to the baseline value, throughout the cooling and reheating protocol is illustrated in Figure 1B. Local cold water application decreased %CVC in all sites relative to baseline (Fig 1B; P<0.05 for all comparisons). However, there was a significant treatment x time interaction (P<0.001). Multiple comparison analyses revealed that the magnitude of vasoconstriction was similar in the control and Ascorbic Acid sites (Fig 1B; P>0.05 for all comparisons). The magnitude of vasoconstriction during cooling was blunted with Fasudil relative to control (Fig 1B; P<0.001 for all comparisons) as well as during min 25 and 30 of cooling in the Ascorbic Acid site (Fig 1B; P<0.05). Throughout the duration of passive rewarming, %CVC at the control and Ascorbic Acid sites did not return toward baseline values such that each remained similar to values obtained at the end of skin-surface cooling (Fig 1B; P<0.05 vs baseline for each comparison) despite skin temperatures being elevated towards baseline (i.e. pre-cooling values; Fig 1A). Local blockade of the Rho kinase pathway with Fasudil resulted in a significant elevation in %CVC toward baseline values during passive rewarming such that values were higher compared to the control and Ascorbic Acid sites throughout the 90 min of passive rewarming (Fig 1B; P<0.001 for all comparisons). However, %CVC remained reduced in all sites relative to pre-cooling baseline (Fig 1B; P<0.05 for all comparisons).
Discussion
This study mimicked real life situations in that the cold stimulus was applied using a commercially available cryotherapy unit and the source (i.e. circulating ice water) and duration of the cold stimulus (~ 30 min) was similar to what is commonly prescribed in orthopedic/sports medical settings. To our knowledge, this is the first study to investigate mechanisms of pronounced and sustained cold-induced vasoconstriction during and following cryotherapy treatment. The primary findings are that pronounced cold-induced vasoconstriction that occurs during cryotherapy treatment is attenuated by local infusion of Fasudil which blocks the Rho kinase pathway. These results are in agreement with previous studies which assessed mechanisms of vasoconstriction during a milder cooling stimulus in a much smaller surface area of skin in the arm (37, 40). Additionally, the sustained vasoconstriction that persists during passive rewarming, despite a return of skin temperature towards baseline values, remains attenuated following local infusion of Fasudil.
The general consensus is that cold-induced vasoconstriction is primarily mediated by adrenergic and nonadrenergic pathways (16, 20, 21, 37, 38, 40). At the onset of cooling (i.e. within the first 10 min) vasoconstriction is primarily mediated by norepinephrine released from sympathetic nerve terminals and the subsequent binding to α2-adrenoreceptors (11, 15, 31), whereas prolonged cooling effects are mediated primarily by nonadrenergic mechanisms in part mediated by a reduction in nitric oxide synthase (NOS) activity and thus bioavailability of the potent vasodilator nitric oxide (NO)(16, 22, 31, 41). However, these previous studies were conducted under conditions of much less stressful cooling stimulus (i.e. Tskin reduced 10 °C below pre-cooling baseline) and a much smaller skin surface area (6.3 cm2) than is utilized during cryotherapy treatments. Below we will discuss the aforementioned mechanisms as they pertain to the pronounced and sustained vasoconstriction associated with cryotherapy.
Upon exposure to a local cold stimulus there is an increase in reactive oxygen species (ROS) in the cutaneous circulation originating primarily from the vascular smooth muscle mitochondria (3). ROS reduces NO bioavailability secondary to various pathways that result in NOS uncoupling and an augmentation of cutaneous vasoconstriction. These pathways include increasing available arginase which competes with the essential substrate for endothelial NOS (eNOS) L-arginine (18), oxidation of tetrahydrobiopterin the essential cofactor for eNOS (33), as well as a direct interaction with newly formed NO to produce peroxynitrate (17). Yamazaki demonstrated that skin-surface cooling to 10 °C below baseline resulted in a ~56% reduction in CVC in control site whereas the degree of vasoconstriction in a site pretreated with Ascorbic Acid (~ 31% reduction) was blunted (40). The degree to which Ascorbic Acid alone blunted the vasoconstriction was similar when it was combined with L-NAME to inhibit NOS activity and subsequent NO production (40), suggesting that the effect was not related to an increase in NO bioavailability (40). These findings are in contrast to previous studies showing that a portion of cold induced vasoconstriction is due to an inhibition of NOS activity (16, 22, 31, 41). In the current study, there was no effect of Ascorbic Acid on the magnitude of vasoconstriction compared to the control condition. The reason for this difference is not clear, although it may be related to the cooler skin surfaces temperatures achieved during cryotherapy as well as to the larger skin surface area that is being cooled.
The ROS generated during local cooling also enhances adrenergic mediated vasoconstriction (3, 8, 11, 12, 39), relating in part to an increased affinity of α2-adrenoreceptors for norepinephrine during cooling (3, 8, 11, 12, 39). ROS also activates the Rho kinase pathway which enhances vascular tone by 1) stimulating translocation of α2c-adrenoreceptors from the intracellular golgi apparatus to the smooth muscle cell surface (2, 3, 8, 37), 2) by increasing smooth muscle sensitivity to intracellular Ca2+ secondary to inhibition of myosin light chain phosphatase (2, 38), and 3) by downregulating the eNOS pathway thus reducing NO bioavailability (5, 28, 36). The role of the Rho kinase pathway in cold-induced vasoconstriction has been confirmed in vitro (2, 3) and in vivo in humans (37). The current findings are in agreement with these previous reports.
Following cessation of the cooling stimulus there was a sustained vasoconstriction that persisted for at least 90 min in the control and Ascorbic Acid sites; however, was not present in the Fasudil site. Importantly, this sustained vasoconstriction was present despite a return of Tskin towards baseline (i.e. toward precooling values) during passive rewarming. This non-linear relationship between Tskin and CVC results in a hysteresis loop (23) in the control and Ascorbic Acid sites that was not present in the Fasudil site. This outcome is in agreement with prior work from our group, using a similar cryotherapy cooling protocol, that has already been described in greater detail (23).
To our knowledge, there are limited studies that have monitored vasoconstriction following the cessation of a cooling stimulus. Yamazaki assessed CVC following a local cooling protocol to 24 °C (~10 °C below baseline) in the arm and reported a similar return of CVC in a control site and one treated with Ascorbic Acid (40). Potential differences in the magnitude of CVC recovery (and thus lack of hysteresis) between the studies include: 1) the use of different anatomical locations, 2) different magnitude of cooling, 3) differences in skin surface area being cooled, and 4) the sites were actively warmed to restore local Tskin (40) as opposed to the present study where only passive rewarming was used. Our group has also used active rewarming following cryotherapy cooling to restabilize CVC (25)
Another possible mechanistic explanation for the sustained postcooling vasoconstriction is release of the potent vasoconstrictor neuropeptide Y (NPY) that is co-released from the sympathetic nerve terminals during cold exposure (20, 34). NPY concentration is increased by 300% following 10 min of cold exposure and remains elevated following removal of the stimulus (20, 34). Additionally, NPY increases Rho-kinase activity (7) which could contribute to the present findings.
Methodological Considerations
Specific aspects of the experimental methodology may have contributed to the outcome. The Tskin measurements were obtained at the surface of the skin, whereas the cutaneous perfusion measures were assessed within an interrogation volume focused at an average tissue depth of 0.5 mm (9). Furthermore, due to technical limitations we were not able to assess either temperature or perfusion at an intramuscular level. However, our prior computer modeling simulations show that the differential monitoring depths for temperature and perfusion do not influence the overall findings (23). This conclusion agrees with other studies of direct or simulated temperature measurements during both cooling and passive rewarming (10, 27, 30). While the sample size was sufficient to detect an effect of Fasudil during cooling and passive rewarming it is possible that the lack of effect of Asrocbic Acid relative to the control site was related to a small sample size.
Clinical/Practical Significance
Localized cooling is a common therapeutic practice to treat soft tissue injury. While there are many benefits to this practice there are numerous reports of detrimental side effects driven by exposure to prolonged ischemia. Despite the widespread therapeutic use of localized cooling and cryotherapy, until recently the effect of cryotherapy on alteration of local blood flow and its time course, particularly during the post cooling phase, had not been elucidated. The findings of the current study provide mechanistic insights into the control of vascular perfusion during cryotherapy that may be of direct application in the rational design of cryotherapy protocols and/or therapeutic approaches for effective healing of soft tissue injuries with minimal risk of ischemic side effects.
Conclusion
The findings indicate that the pronounced vasoconstriction that occurs during cooling and the sustained vasoconstriction that persists during passive rewarming can be attenuated by blockade of the Rho kinase pathway.
Highlights.
Cryotherapy treatment is used in treatment after surgery and in sports medicine.
Cryotherapy causes pronounced and sustained vasoconstriction in the cooled area.
This ischemia can cause side effects such as tissue necrosis and neuropathy.
Rho-Kinase blockade blunted the vasoconstriction during and following cryotherapy.
Ascorbic Acid did not impact vasoconstriction during and following cryotherapy.
Acknowledgments
These authors would like to express our appreciation to all of our subjects for their participation in this study.
Grants
This research was sponsored by the National Institute of Health, Grant No. RO1 EB015522 (to K. R. Diller and R. M. Brothers).
Footnotes
Conflict of Interest
Patent applications for some aspects of this technology as applied to cryotherapy have been submitted by Dr. Khoshnevis and Dr. Diller to the United States Patent and Trademark Office. Ownership rights to this IP reside with The University of Texas System. Dr. Diller has served as an expert witness for both plaintiff and defendant counsel since 2000 in numerous legal cases regarding the safety and design of existing cryotherapy devices.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Babwah T. Common peroneal neuropathy related to cryotherapy and compression in a footballer. Res Sports Med. 2011;19:66–71. doi: 10.1080/15438627.2011.536043. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circulation research. 2004;94:1367–1374. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive Oxygen Species from Smooth Muscle Mitochondria Initiate Cold-Induced Constriction of Cutaneous Arteries. Am J Physiol Heart Circ Physiol. 2005 doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
- 4.Bassett FH, 3rd, Kirkpatrick JS, Engelhardt DL, Malone TR. Cryotherapy-induced nerve injury. Am J Sports Med. 1992;20:516–518. doi: 10.1177/036354659202000505. [DOI] [PubMed] [Google Scholar]
- 5.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown WC, Hahn DB. Frostbite of the feet after cryotherapy: a report of two cases. J Foot Ankle Surg. 2009;48:577–580. doi: 10.1053/j.jfas.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Cheng PW, Wu AT, Lu PJ, Yang YC, Ho WY, Lin HC, Hsiao M, Tseng CJ. Central hypotensive effects of neuropeptide Y are modulated by endothelial nitric oxide synthase after activation by ribosomal protein S6 kinase. Br J Pharmacol. 2012;167:1148–1160. doi: 10.1111/j.1476-5381.2012.02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000;278:H1075–1083. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- 9.Clough G, Chipperfield A, Byrne C, de Mul F, Gush R. Evaluation of a new high power, wide separation laser Doppler probe: potential measurement of deeper tissue blood flow. Microvasc Res. 2009;78:155–161. doi: 10.1016/j.mvr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Diller KR. Heat Transfer in Health and Healing. J Heat Transfer. 2015;137:1030011–10300112. doi: 10.1115/1.4030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekenvall L, Lindblad LE, Norbeck O, Etzall MB. α-Adrenoceptors and cold-induced vasoconstriction in humans finger skin. Am J Physiol. 1988;255:H1000–H1003. doi: 10.1152/ajpheart.1988.255.5.H1000. (Heart Circ. Physiol. 24) [DOI] [PubMed] [Google Scholar]
- 12.Flavahan NA, Lindblad LE, Verbeuren TJ, Shepherd JT, Vanhoutte PM. Cooling and alpha 1- and alpha 2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol. 1985;249:H950–955. doi: 10.1152/ajpheart.1985.249.5.H950. [DOI] [PubMed] [Google Scholar]
- 13.Francis TJ. Non freezing cold injury: a historical review. J R Nav Med Serv. 1984;70:134–139. [PubMed] [Google Scholar]
- 14.Francis TJ, Golden FS. Non-freezing cold injury: the pathogenesis. J R Nav Med Serv. 1985;71:3–8. [PubMed] [Google Scholar]
- 15.Freedman RR, Sabharwal SC, Moten M, Migaly P. Local temperature modulates a1- and a2-adrenergic vasoconstriction in men. Am J Physiol. 1992;263:H11970–H11200. doi: 10.1152/ajpheart.1992.263.4.H1197. (Heart Circ. Physiol. 32) [DOI] [PubMed] [Google Scholar]
- 16.Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol. 2006;574:849–857. doi: 10.1113/jphysiol.2006.109884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00295.2007. [DOI] [PubMed] [Google Scholar]
- 18.Holowatz LA, Thompson CS, Kenney WL. L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol. 2006;574:573–581. doi: 10.1113/jphysiol.2006.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia J, Pollock M. Cold nerve injury is enhanced by intermittent cooling. Muscle & nerve. 1999;22:1644–1652. doi: 10.1002/(sici)1097-4598(199912)22:12<1644::aid-mus5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JM. Mechanisms of vasoconstriction with direct skin cooling in humans. Am J Physiol Heart Circ Physiol. 2007;292:H1690–1691. doi: 10.1152/ajpheart.00048.2007. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JM, Kellogg DL., Jr Local thermal control of the human cutaneous circulation. Journal of applied physiology. 2010;109:1229–1238. doi: 10.1152/japplphysiol.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol. 2005;288:H1573–1579. doi: 10.1152/ajpheart.00849.2004. [DOI] [PubMed] [Google Scholar]
- 23.Khoshnevis S, Craik NK, Brothers RM, Diller KR. Cryotherapy-Induced Persistent Vasoconstriction after Cutaneous Cooling: Hysteresis Between Skin Temperature and Blood Perfusion. J Biomech Eng. 2015 doi: 10.1115/1.4032126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoshnevis S, Craik NK, Diller KR. Cold-induced vasoconstriction may persist long after cooling ends: an evaluation of multiple cryotherapy units. Knee Surg Sports Traumatol Arthrosc. 2015;23:2475–2483. doi: 10.1007/s00167-014-2911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoshnevis S, Craik NK, Diller KR. Experimental characterization of the domains of coupling and uncoupling between surface temperature and skin blood flow. International Journal of Transport Phenomena. 2014;13:277–301. [Google Scholar]
- 26.Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30:63–64. doi: 10.3928/01477447-20070101-14. [DOI] [PubMed] [Google Scholar]
- 27.Merrick MA, Knight KL, Ingersoll CD, Potteiger JA. The effects of ice and compression wraps on intramuscular temperatures at various depths. J Athl Train. 1993;28:236–245. [PMC free article] [PubMed] [Google Scholar]
- 28.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moeller JL, Monroe J, McKeag DB. Cryotherapy-induced common peroneal nerve palsy. Clin J Sport Med. 1997;7:212–216. doi: 10.1097/00042752-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Myrer JW, Measom G, Fellingham GW. Temperature changes in the human leg during and after two methods of cryotherapy. J Athl Train. 1998;33:25–29. [PMC free article] [PubMed] [Google Scholar]
- 31.Pérgola PE, Kellogg DL, Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol. 1993;265:H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. (Heart Circ. Physiol. 34) [DOI] [PubMed] [Google Scholar]
- 32.Santilli JD, Santilli SM. Chronic critical limb ischemia: diagnosis, treatment and prognosis. Am Fam Physician. 1999;59:1899–1908. [PubMed] [Google Scholar]
- 33.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. Journal of applied physiology. 2012;112:791–797. doi: 10.1152/japplphysiol.01257.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol. 2004;287:H1404–1409. doi: 10.1152/ajpheart.00061.2004. [DOI] [PubMed] [Google Scholar]
- 35.Swenson C, Sward L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports. 1996;6:193–200. doi: 10.1111/j.1600-0838.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 36.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 37.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol. 2007;292:H1700–1705. doi: 10.1152/ajpheart.01078.2006. [DOI] [PubMed] [Google Scholar]
- 38.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Rho kinase-mediated local cold-induced cutaneous vasoconstriction is augmented in aged human skin. Am J Physiol Heart Circ Physiol. 2007;293:H30–36. doi: 10.1152/ajpheart.00152.2007. [DOI] [PubMed] [Google Scholar]
- 39.Vanhoutte PM, Cooke JP, Lindblad LE, Shepherd JT, Flavahan NA. Modulation of postjunctional alpha-adrenergic responsiveness by local changes in temperature. Clin Sci (Lond) 1985;68(Suppl 10):121s–123s. doi: 10.1042/cs068s121. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki F. Local ascorbate administration inhibits the adrenergic vasoconstrictor response to local cooling in the human skin. J Appl Physiol (1985) 2010;108:328–333. doi: 10.1152/japplphysiol.00814.2009. [DOI] [PubMed] [Google Scholar]
- 41.Yamazaki F, Sone R, Zhao K, Alvarez GE, Kosiba WA, Johnson JM. Rate dependency and role of nitric oxide in the vascular response to direct cooling in human skin. Journal of applied physiology. 2006;100:42–50. doi: 10.1152/japplphysiol.00139.2005. [DOI] [PubMed] [Google Scholar]