Abstract
Background & Aims
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with a 5-year survival rate <7% and is ultimately refractory to most treatments. To date, an assessment of immunologic factors relevant to disease has not been comprehensively performed for treatment naïve patients. We hypothesized that systemic immunologic biomarkers could predict overall survival (OS) in treatment naïve PDAC patients.
Methods
Peripheral blood was collected from 73 patients presenting with previously untreated metastatic PDAC. Extensive immunologic profiling was conducted to assess relationships between OS and the level of soluble plasma biomarkers or detailed immune cell phenotypes as measured by flow cytometry.
Results
Higher baseline levels of the immunosuppressive cytokines IL-6 and IL-10 were strongly associated with poorer OS (p=0.008 and 0.026, respectively; HR=1.16 and 1.28, respectively), while higher levels of the monocyte chemoattractant MCP-1 were associated with significantly longer OS (p=0.045; HR=0.69). Patients with a greater proportion of antigen-experienced T cells (CD45RO+) had longer OS (CD4 p=0.032; CD8 p=0.036; HR=0.36 and 0.61, respectively). While greater expression of the T cell checkpoint molecule CTLA-4 on CD8+ T cells was associated with significantly shorter OS (p=0.020; HR=1.53), the TIM3 molecule had a positive association with survival when expressed on CD4+ T cells (p=0.046; HR=0.62).
Conclusions
These data support the hypothesis that baseline immune status predicts PDAC disease course and overall patient survival. To our knowledge, this work represents the largest cohort and most comprehensive immune profiling of treatment-naïve metastatic PDAC patients to date.
Keywords: pancreatic cancer, immunosuppression, immune profile, immune biomarkers, immune signature, pancreas
Introduction
Pancreatic ductal adenocarcinoma (PDAC), an aggressive malignancy presenting at advanced stages, is the fourth leading cause of cancer-related mortality in the United States with estimated five-year survival < 7% (1) and is projected to become the second leading cause of cancer related death by the year 2030 (2). Despite incremental treatment advances (Gemcitabine/nab-paclitaxel, FOLFIRINOX (3–6)), median survival for metastatic PDAC patients remains < 1 year (7). As evidenced by the poor survival rate, existing treatments for PDAC are largely ineffective and there is an acute need to develop novel treatments for this disease, which is almost uniformly lethal in the metastatic setting.
While surgery is an option for patients with early stage disease, those with metastatic PDAC are limited to any of a number of chemotherapy regimens or clinical trial enrollment (3). While these chemotherapies act in large part as conventional cytotoxic agents, some also exert immunomodulatory activity through a variety of mechanisms (8). Moreover, it is increasingly clear that the immune system contributes to the antitumor activity of several classes of cytotoxic chemotherapy (9). Thus, the status of the patient’s immune system may in large part determine the clinical efficacy of chemotherapy.
There is increasing interest in immunotherapy for the treatment of PDAC. Approaches such as immune checkpoint blockade have produced dramatic clinical responses in other malignancies, most recently non-small cell lung carcinoma (10). These clinical observations have led to a concerted effort to adapt immunotherapy to treat other refractory cancers, including PDAC. Though pancreatic tumors are poorly infiltrated by effector T cells (11, 12), tumor-reactive T cells are found in the peripheral blood and bone marrow of pancreatic cancer patients (13), and may localize to the tumor following chemotherapy (11, 14). This suggests that immunotherapy has the potential to induce clinically beneficial antitumor activity. Indeed, recent clinical trials demonstrate that vaccine-based approaches or CD40 agonists can prolong stable disease or elicit partial responses in a subset of PDAC patients (15–18). However, even in response to these highly immunogenic treatment regimens, most patients eventually progressed, underscoring the need for further improvement in therapy.
These early trial results suggest there are many barriers to harnessing the full potential of immunotherapy, including general immune dysfunction and active immunosuppression in patients with advanced disease. T cells from patients with advanced PDAC are functionally suppressed (19), while we and others have documented elevated levels and negative prognostic significance of myeloid derived suppressor cells (MDSC) in PDAC patients (20–22). We have also reported that stromal elements of pancreatic tumors secrete numerous soluble factors that suppress the immune system (23), particularly IL-6. This cytokine is a key factor in PDAC development, progression, and immune suppression and is frequently elevated during malignancy (21). It mediates immunosuppressive activity through a number of mechanisms, including impairing dendritic cell differentiation and inducing MDSC accumulation (24, 25). IL-6 also directly supports tumor cell proliferation, invasiveness, and tumor progression (26), separate from its immunomodulatory activity, and recent reports argue that IL-6 is critically required for the growth, maintenance, and progression of mutant KRAS-driven pancreatic tumors (27, 28).
In this report, we investigated whether a patient’s baseline immune status was predictive of their OS. Plasma and peripheral blood mononuclear cells (PBMCs) from a large cohort of treatment naïve patients with un-resectable metastatic PDAC were analyzed in the context of OS to identify clinically relevant biomarkers of disease and identify potential immune-related therapeutic targets.
Materials and Methods
Patients
Peripheral blood was obtained from 73 patients with histologically confirmed, inoperable/metastatic PDAC who were treatment naive. Blood samples were obtained prior to treatment and following informed consent. Treatment consisted of carboplatin and paclitaxel with or without an oncolytic virus (Reolysin®) as part of a national Phase II clinical trial NCT01280058/OSU-10045 (Table 1). This work was carried out under a protocol approved by the National Cancer Institute’s (NCI) Cancer Therapy Evaluation Program (CTEP) and the central and local institutional review boards (IRBs). All patients were enrolled between February 2011 and February 2014. PBMCs and plasma were isolated from whole blood via density gradient centrifugation using Ficoll-Paque (Amersham Pharmacia Biotech; Uppsala, Sweeden) as previously described (29). PBMCs were cryopreserved in liquid nitrogen and plasma was stored at −80° until batched analysis.
Table 1.
Characteristics of patients involved in this study.
| Patient Characteristics | n = 73 |
|---|---|
| Gender | |
| Female | 32 |
| Male | 41 |
|
| |
| Age (years) | |
| Median | 64 |
| Range | 39–84 |
|
| |
| ECOG PS | |
| 0 | 37 |
| 1 | 36 |
|
| |
| Race | |
| American Indian | 1 |
| Asian | 1 |
| African American | 2 |
| Caucasian | 67 |
| Other/unknown | 2 |
|
| |
| Metastatic Sites | |
| Liver | 57 |
| Lung | 29 |
| Peritoneum | 19 |
| Other | 41 |
|
| |
| # of Metastatic Sites | |
| 1 | 18 |
| 2 | 16 |
| 3 | 19 |
| >3 | 20 |
|
| |
| CA 19-9 | |
| ≥ 59x ULN | 37 |
| ULN to < 59x ULX | 19 |
| Normal | 11 |
|
| |
| KRAS Status (% mutated) | 74 |
Cytokine, chemokine, and growth factor analysis
A panel of 32 cytokines, chemokines, and growth factors was analyzed in plasma isolated from patient peripheral blood using Luminex Multiplex Cytokine Kits (Procarta Cytokine Assay, eBioscience; San Diego, CA). Samples were analyzed in duplicate, run in batches, and quantified using analyte specific standard curves for each batch.
Flow cytometry
Phenotypic analysis of circulating immune cells was conducted by fluorescence activated cell sorting (FACS). The antibodies used are detailed in Supplemental Table S1. Briefly, cryopreserved PBMCs were thawed at 37°C, washed, centrifuged, and resuspended in FACS buffer (PBS + 5% FBS). Cells were stained with appropriate antibodies in the dark for 45 minutes at 4°C. Cells were then washed, fixed in phosphate buffered saline (PBS) + 1% formalin, and analyzed with an LSRII flow cytometer or FACS calibur (BD Biosciences; San Jose, CA). Compensation controls were generated using ABC capture beads beads (Life Technologies; Eugene, OR). Data were analyzed using FlowJo software version 7.6.4 (FlowJo; Ashland, OR).
Statistical analyses
All markers were summarized and initially evaluated using descriptive and graphical analyses, and were log-transformed for evaluation and modeling in relation to OS in the univariate setting. OS was defined as the time from baseline blood draw to death due to any cause, where living patients were censored at the time of their last evaluation. Baseline marker levels were assessed in relation to OS. Time-to-event OS outcome was evaluated in relation to each of the markers in the univariate and multivariable settings using log-rank statistics and Cox regression models. OS hazard rates and ratios and corresponding 95% confidence intervals were evaluated by continuous measure distribution for these markers in the univariate and multivariable setting. Markers identified as being at least borderline significant (p<0.10) in the univariate setting were evaluated as dichotomized outcomes, categorized as high vs. low expression. Cutpoints for these markers were identified using recursive partitioning algorithms in relation to OS. OS was also evaluated here as a dichotomized outcome, where patients were categorized based on overall survival time above or below the estimated median OS (~8 months). Comparison of continuous measure distributions between groups was done using Wilcoxon rank sum tests and side-by-side boxplots. Comparisons of distributions of categorical variables between groups was done using chi-square tests (or Fisher exact tests in the setting of small subsets). Statistical significance was determined as p<0.05. To ameliorate the limitations of the sample size and lack of a separate independent cohort for validation, we performed modified Monte Carlo cross-validation (MCCV) approaches to assess the robustness of our findings. Specifically, 90% of subjects were randomly selected using stratified random sampling (i.e. random sampling of subjects within each treatment arm to ensure equal representation by arm in the analysis cohort) and we assessed whether or not the markers of interest were statistically significant in this subset cohort. This process was repeated 2000 times and we evaluated how many of those models were still significant with 10% of subjects randomly excluded, maintaining equal representation of each treatment arm. This was done for both the continuous as well as dichotomized versions of markers identified as promising based on the full cohort analysis. All analyses were done using R statistical program v.3.1.2 for Windows (30).
Results
Plasma IL-6 and IL-10 are poor prognostic factors in patients with metastatic PDAC
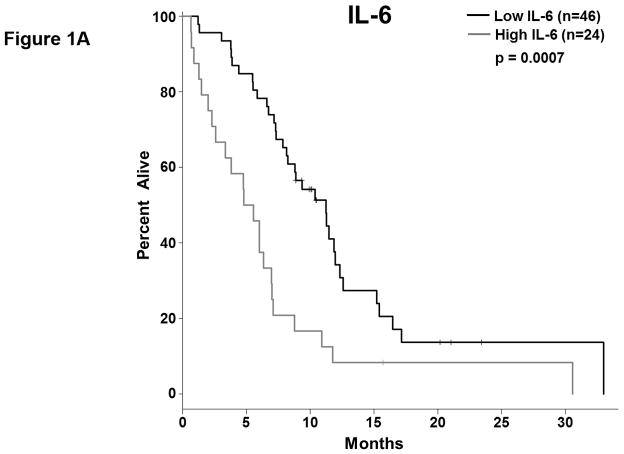
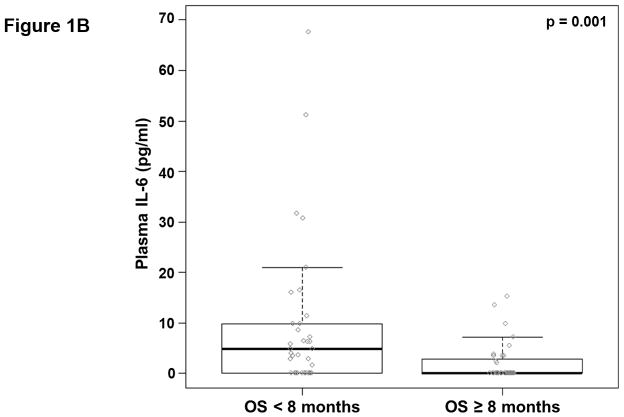
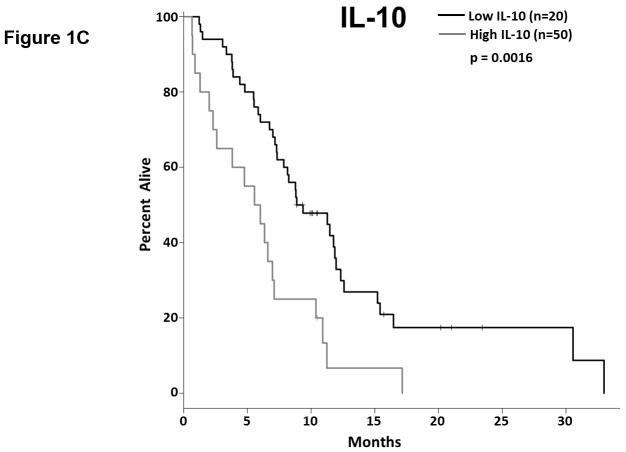
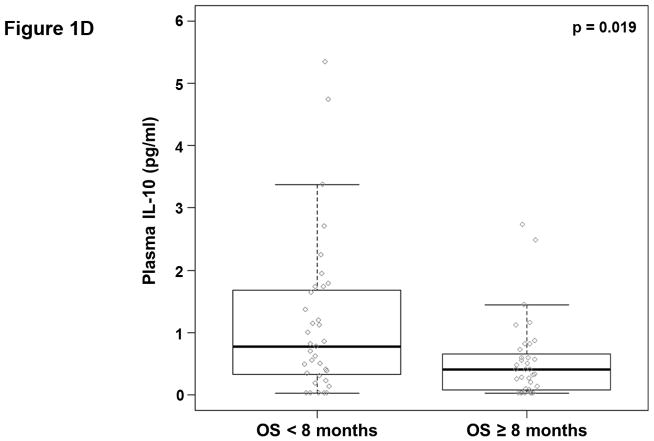
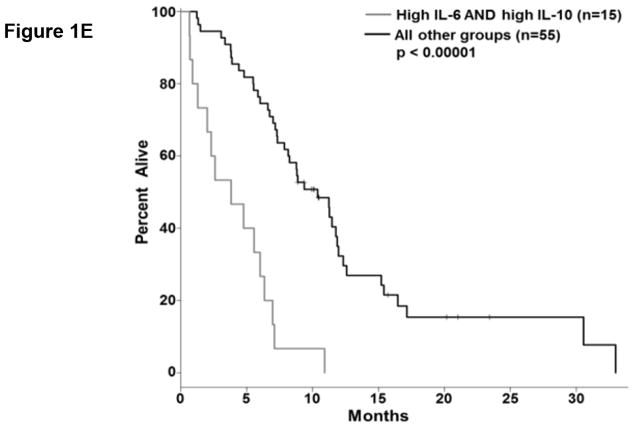
We analyzed the abundance of 32 soluble factors in the plasma of treatment-naïve patients presenting with metastatic PDAC (Table 2). Cytokines associated with differentiation and function of Th1, Th2, and Th17 helper T cell subsets were included on this panel. We did not observe any definitive evidence of helper T cell subsets skewing or dominance based on circulating cytokine levels. Most of these cytokines were expressed at low pg/ml levels in the majority of patients, though with a high degree of variability. Only two of these continuous cytokine measures, IL-6 and IL-10, were significantly associated with OS (p=0.008 and p=0.026, respectively). In our subsequent MCCV analyses, both IL-6 and IL-10 levels remained significantly associated with OS as a continuous measure in 1997/2000 and 1210/2000 simulations, respectively (Supplemental Table S2; IL-6: median p-value = 0.0056, median HR = 1.18; IL-10: median p-value = 0.0415, median HR = 1.27). On this study, patients had average IL-6 plasma levels of 5.9±11.4 pg/ml (Table 2). When dichotomized as high vs. low expressers, patients with higher baseline IL-6 plasma levels had significantly shorter OS than did patients with lower levels (p=0.0007; Figure 1.a), and this observation was further supported by the cross-validation modeling (Supplemental Table S3; 2000/2000 simulations, median p-value = 0.00041, median HR = 3.06). Moreover, when patients are dichotomized based on the duration of their OS, those with OS>8 months had significantly less circulating IL-6 than did patients with shorter survival (p=0.001; Figure 1.b). The other immune suppressive cytokine associated with OS was IL-10. On average, patients had 0.85±1.04 pg/ml IL-10 in their plasma at baseline (Table 2). When dichotomized based on IL-10 plasma level, patients with higher IL-10 levels had significantly shorter OS than did patients with lower levels (p=0.0016; Figure 1.c). Again, this observation was further supported by the subsequent cross-validation modeling (Supplemental Table S3; 1991/2000 simulations, median p-value = 0.005, median HR = 2.5). Similar to data with IL-6, dichotomization of patients by duration of OS revealed that patients with OS>8 months had significantly lower plasma levels of IL-10 than patients with OS<8 months (p=0.019; Figure 1.d). Furthermore, evaluation of both IL-6 and IL-10 expression groups in the multivariable setting demonstrated a significant interaction between the two cytokines (p=0.04). Here, survival was significantly worse in patients with high levels of both cytokines vs. not (i.e. high expression in only one or none of these cytokines; p<0.00001; Fig. 1.e). Of the two cytokines, the effect of IL-6 appeared to be dominant. Namely, patients with higher IL-6 levels had worse survival than those with low IL-6, regardless of their IL-10 status (data not shown). Thus, higher levels of two immunosuppressive cytokines remain predictive of OS even in late stage PDAC.
Table 2. Pancreatic cancer patient plasma chemokines/cytokines.
The concentration of 32 cytokines, chemokines, and growth factors in patient plasma was determined by bioplex assay.
| Soluble Factor | Mean ± S.D. | Median | Range | Association with OS (p-value) |
|---|---|---|---|---|
| Th1 Cytokines | ||||
| IL-2 | 5.0 ± 9.9 | 0.0 | 0.0 – 55.7 | 0.698 |
| IL-12p40 | 43.2 ± 57.4 | 20.3 | 0.0 – 335.3 | 0.969 |
| IFNγ | 1.2 ± 2.1 | 0.2 | 0.0 – 10.8 | 0.627 |
| TNFα | 0.9 ± 2.0 | 0.0 | 0.0 – 9.7 | 0.434 |
| Th2 Cytokines | ||||
| IL-4 | 1.2 ± 5.7 | 0.0 | 0.0 – 44.0 | 0.807 |
| IL-10 | 0.8 ± 1.0 | 0.5 | 0.0 – 5.3 | 0.026 |
| IL-13 | 0.6 ± 1.2 | 0.0 | 0.0 – 7.7 | 0.958 |
| Th17 Cytokines | ||||
| IL-6 | 5.9 ± 11.4 | 1.6 | 0.0 – 67.6 | 0.008 |
| IL-17A | 1.0 ± 2.0 | 0.0 | 0.0 – 11.5 | 0.155 |
| IL-17F | 2.1 ± 13.2 | 0.1 | 0.0 – 112.2 | 0.872 |
| Chemokines | ||||
| CCL2 (MCP-1) | 121.3 ± 85.5 | 98.3 | 8.6 – 391.9 | 0.045 |
| CCL3 (MIP-1α) | 27.2 ± 42.3 | 10.8 | 0.0 – 191.4 | 0.663 |
| CCL4 (MIP-1β) | 508.0 ± 350.2 | 646.6 | 0.0 – 1034.3 | 0.759 |
| CCL5 (RANTES) | 163.8 ± 79.5 | 155.6 | 55.7 – 426.7 | 0.354 |
| CCL7 (MCP-3) | 11.7 ± 17.8 | 0.0 | 0.0 – 62.8 | 0.352 |
| CCL11 (Eotaxin) | 34.0 ± 22.1 | 28.9 | 1.0 – 111.5 | 0.130 |
| CXCL1 (GROα) | 8.1 ± 6.4 | 9.6 | 0.0 – 31.5 | 0.561 |
| CXCL8 (IL-8) | 138.3 ± 173.7 | 77.2 | 2.5 – 705.8 | 0.790 |
| CXCL9 (MIG) | 266.7 ± 292.4 | 163.4 | 10.3 – 1432.4 | 0.847 |
| CXCL10 (IP-10) | 19.2 ± 15.2 | 13.8 | 4.7 – 84.5 | 0.864 |
| CXCL12 (SDF-1) | 575.2 ± 274.1 | 631.9 | 126.3 – 1464.3 | 0.712 |
| CX3CL1 (Fractalkine) | 9.2 ± 9.7 | 6.9 | 0.0 – 75.9 | 0.393 |
| Other Cytokines and Growth Factors | ||||
| CD40L | 1424.5 ± 1304.8 | 1219.5 | 26.2 – 4455.4 | 0.957 |
| GM-CSF | 7.1 ± 28.9 | 0.0 | 0.0 – 238.9 | 0.285 |
| IFNα | 0.5 ± 0.8 | 0.4 | 0.0 – 5.5 | 0.682 |
| IFNβ | 25.1 ± 166.1 | 0.0 | 0.0 – 1410.0 | 0.552 |
| IL-1β | 1.9 ± 3.0 | 1.0 | 0.0 – 16.3 | 0.549 |
| VEGF-A | 778.5 ± 819.4 | 480.4 | 105.2 – 5213.1 | 0.181 |
Not detected/below limit of quantification: IL-5, IL-12p70, M-CSF, CCL23 (MIP-3)
All values pg/ml; p-values based on continuous measure distribution
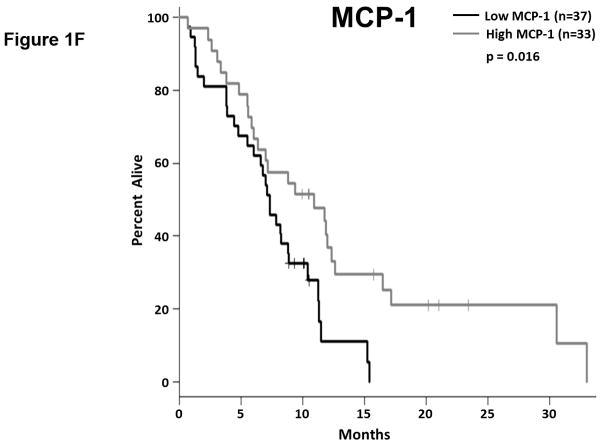
Figure 1. Plasma levels of MCP-1, IL-6, and IL-10 predict the duration of overall survival in treatment naïve metastatic pancreatic cancer.
(a–b) IL-6. (a) Patients were stratified based on IL-6 levels. (b) IL-6 levels in patients stratified around the median OS. (c–d) IL-10. (c) Patients were stratified based on IL-10 levels. (d) IL-10 levels in patients stratified around the median OS. (e) Patients were stratified based on IL-6 and IL-10 levels (cutpoints as in (a) and (c)) (f) Patients were stratified based on MCP-1 levels. P-values based on dichotomized analysis.
A number of immunomodulatory cytokines (IFNα, IFNβ, IL-1β, CD40L) were also included on this panel. While some of these cytokines were detected at high levels (maximum expression of CD40L was 4455.4 pg/ml), none of them were strongly associated with OS (Table 2). Likewise, two growth factors associated with immune suppression in cancer (GM-CSF, VEGF-A) were present at readily detectable levels but were not strongly associated with OS (Table 2).
Plasma levels of MCP-1 are predictive of survival in patients with metastatic PDAC
Among the 32 soluble factors analyzed in patient plasma were 13 chemokines: 7 CC chemokines, 5 CXC chemokines, and 1 CX3C chemokine. The expression of these chemokines was highly variable and most were readily detectable in patient plasma (Table 2). Only the monocyte chemoattractant protein-1 (MCP-1; CCL2) was associated with OS as a continuous measure (p=0.045); however, under subsequent cross-validation analyses MCP-1 only remained significantly associated with OS as a continuous marker in 648/2000 simulations (Supplemental Table S2; median p-value = 0.066, median HR = 0.69), though it met our criteria for subsequent dichotomized analysis (p<0.10) in 1515/2000 simulations. The average concentration of MCP-1 in patient plasma was 121.3±85.5 pg/ml (Table 2) and when dichotomized as high vs. low MCP-1 plasma levels, patients with more MCP-1 had significantly longer OS than did patients with lower levels (p=0.016; Figure 1.f). Unlike its use as a continuous measure, subsequent cross-validation analyses found that MCP-1 as a dichotomous marker was significantly associated with OS in 1734/2000 simulations (Supplemental Table S3; median p-value = 0.023, median HR = 0.49). Patients whose survival exceeded 8 months had more circulating MCP-1 than did patients with OS<8 months, although this difference was not significant (p=0.37; Figure S1).
Higher frequencies of antigen-experienced T cells are associated with greater overall survival
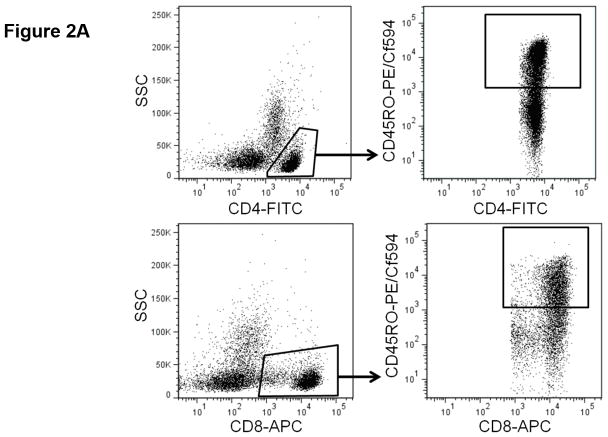
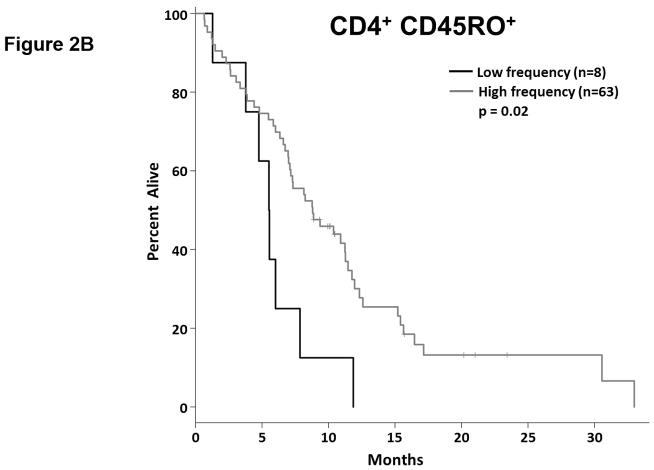
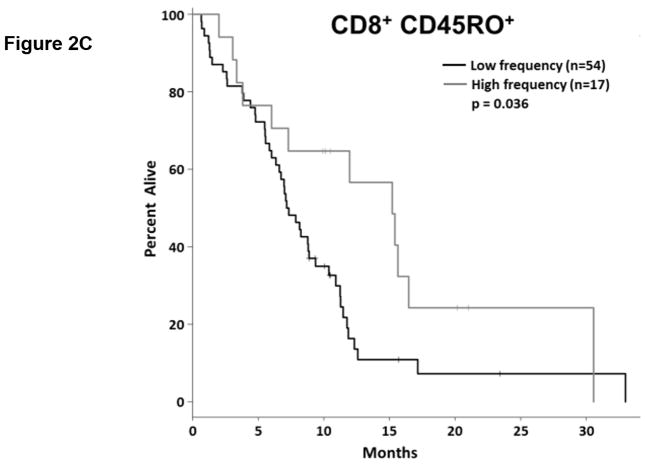
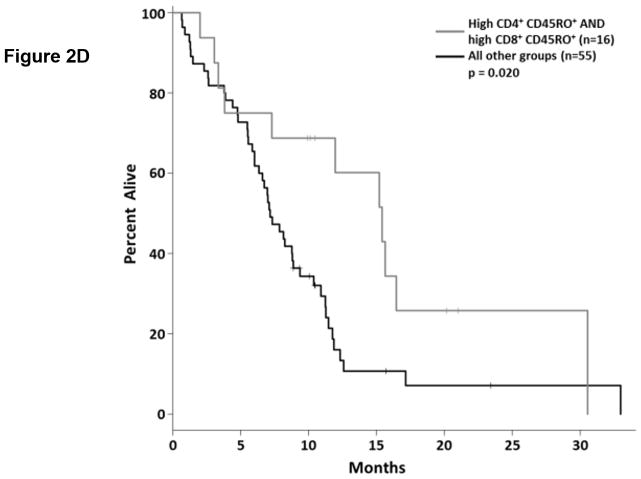
Tumor reactive T cells are present in the blood of pancreatic cancer patients; however, these tumors are generally not well infiltrated by effector T cells (11). One way to characterize circulating T cells is based on their maturation status. In humans, the CD45RO marker is frequently used to denote antigen experienced T cells (i.e. non-naïve T cells). We determined the proportion of circulating CD4+ and CD8+ T cells expressing CD45RO via flow cytometry (Fig. 2.a, Supplemental Table S4). As a continuous marker, CD45RO expression on CD4+ T cells was significantly associated with OS (p = 0.032), though CD45RO expression on CD8+ T cells was not (p = 0.060). On subsequent internal-validation modeling, CD45RO expression on CD4+ T cells remained significant in 1501/2000 simulations (Supplemental Table S2; median p-value = 0.032, median HR = 0.34). When dichotomized as having high vs. low proportions of either CD4+ CD45RO+ or CD8+ CD45RO+ T cells, patients in the “high” groups were associated with significantly longer OS based on univariate analysis (Fig. 2.b,c; p=0.02 and 0.036, respectively). In our validation modeling, dichotomized CD4+ CD45RO+ T cells were significantly associated with OS in 1640/2000 simulations (Supplemental Table S3; median p-value = 0.027, median HR = 0.518) while CD8+ CD45RO+ T cells were only significant in 503/2000 simulations (Supplemental Table S3; median p-value = 0.08, median HR = 0.27). When analyzed by multivariable Cox regression, patients with high proportions of both CD4+ and CD8+ T cells co-expressing CD45RO had significantly longer OS than did patients with lower proportions of antigen experienced CD4+ or CD8+ T cells (p=0.02; Fig. 2.d). These results demonstrate that having a greater frequency of antigen experienced T cells is a potential positive prognostic factor in metastatic PDAC and suggest the presence of ongoing anti-tumor immune reactions.
Figure 2. T cell maturation status predicts the duration of overall survival in treatment naïve metastatic pancreatic cancer.
(a) Analysis of T cell CD45RO expression. The proportion of CD4+ (top) and CD8+ T cells (bottom) that express CD45RO was determined by gating on cells staining positive for CD4+ or CD8+ (left) and then gating on the population staining positive for CD45RO (right). (b–c) Association between CD45RO+ T cells and OS. Patients were dichotomized based on the proportion of CD4+ T cells (b) or CD8 T cells (c) expressing CD45RO. (d) Association between CD45RO on both CD4+ and CD8+ T cells is a predictor of survival. Patients were stratified based on the proportion of both CD4+ and CD8+ T cells that expressed CD45RO (cutpoints as used in (b) and (c)). P-values based on dichotomized analysis.
Prognostic significance of T cell checkpoint and alternative co-stimulatory molecules
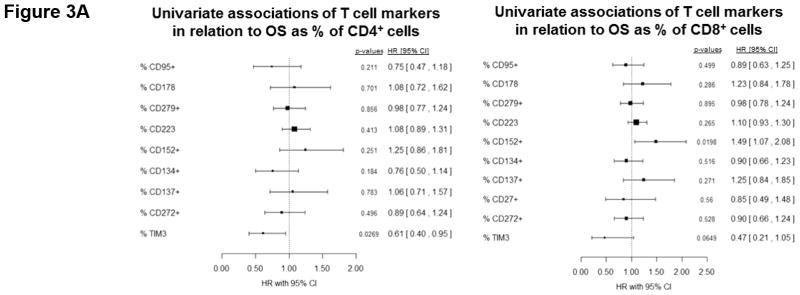
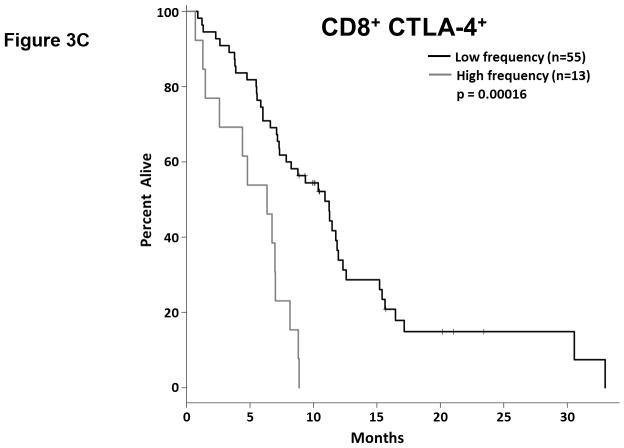
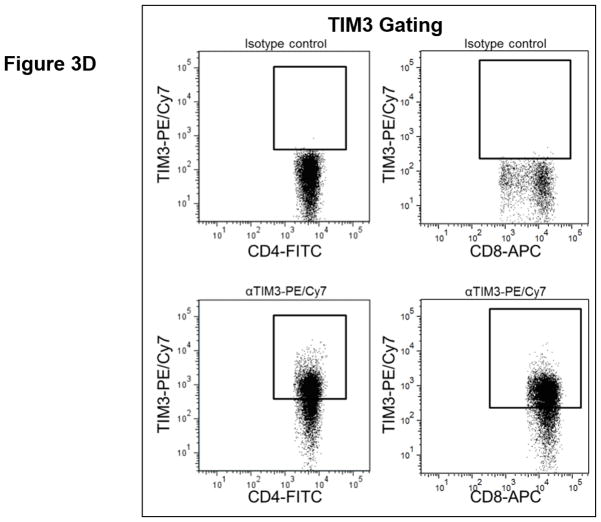
T cell checkpoint molecules (including CD95/FAS, CD152/CTLA-4, CD178/FAS-ligand, CD223/LAG-3, CD279/PD-1, and TIM-3) and alternative co-stimulatory molecules (including CD27, CD134/OX40, CD137/4-1BB, and CD272/BTLA) are powerful modulators of the immune system (31, 32). These receptors have become the subject of great interest as therapeutic targets for the treatment of malignancy. As part of this study, we determined the proportion of T cells expressing the T cell checkpoint molecules CD95, CD178, CD279, CD223, CD152, and TIM3 (Fig. 3, Table S4). There was no significant relationship between expression of CD95, CD178, CD279, or CD223 and the duration of OS by univariate analysis (Fig. 3.a). Two of the T cell checkpoint molecules, however, were strongly associated with survival. CTLA-4, a member of the B7-CD28 superfamily, is a negative regulator of T cell activity. Patients with greater proportions of CD8+ T cells expressing CTLA-4 had significantly shorter OS (p= 0.0198 as a continuous marker, p = 0.00016 when dichotomized into high vs. low groups, Fig. 3.a–c). Validation analysis supports these observations (Supplemental Table S2 and S3). The other molecule, TIM3, was originally identified as a negative regulator of T cell immunity, though more recent reports indicate that it has context-dependent immunostimulatory activity. To our knowledge, the prognostic significance of this molecule has not been studied in PDAC. There was a significant positive correlation with the proportion of CD4+ T cells expressing TIM3 and the duration of OS in this patient cohort (p = 0.046 as a continuous marker, p=0.0024 when dichotomized as high vs. low proportions; Fig. 3.a,d–e). These observations were further supported by subsequent cross-validation modeling, wherein TIM3 expression by CD4+ T cells was significant in 1702/2000 simulations (continuous marker) and 2000/2000 simulations (dichotomized marker)(Supplemental Table S2 and S3). We also determined the frequency with which the alternative co-stimulatory molecules OX40, 4-1BB, CD27, and BTLA appeared on CD4+ and CD8+ T cells in treatment naïve metastatic PDAC patients (Fig. 3.a, Table S2). Though these molecules were frequently expressed on T cells from PDAC patients, none of them were significantly associated OS in this patient cohort. Together, these data suggest that differences in the expression of checkpoint molecules on circulating T cells may impact anti-tumor immune activity and clinical outcome in metastatic PDAC patients.
Figure 3. T cell expression of CTLA4 (CD152) and TIM3 is associated with overall survival.
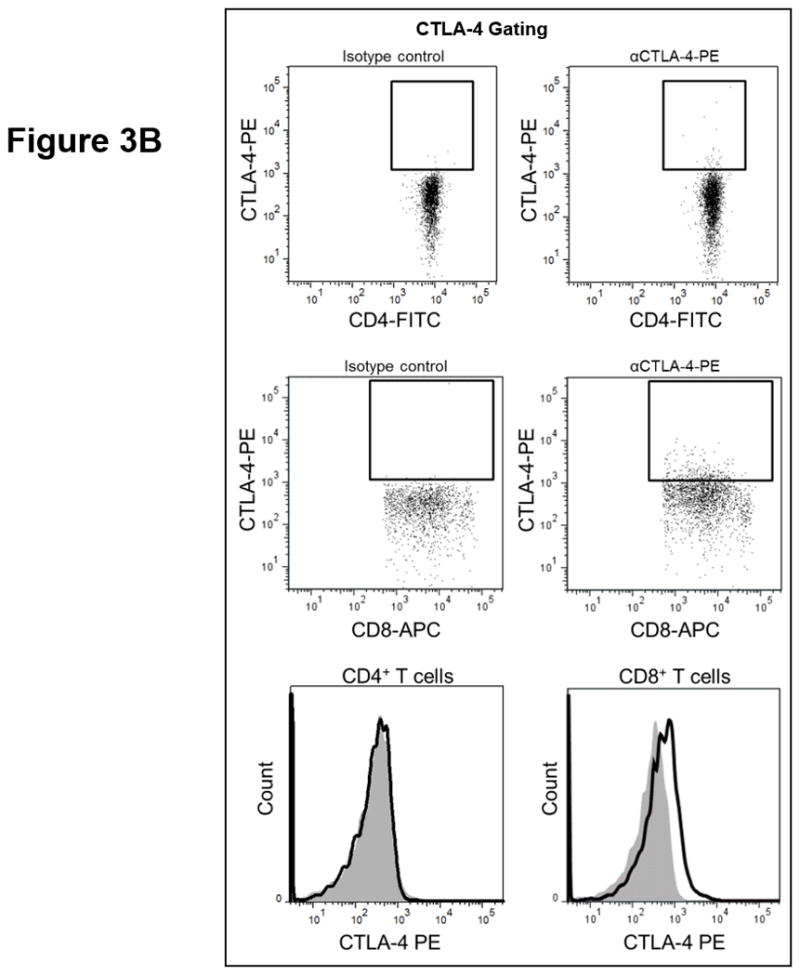
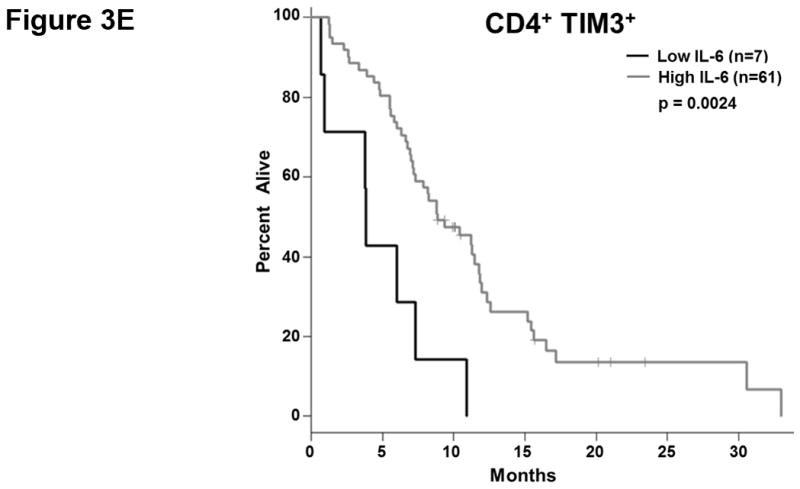
(a) Association between T cell expression of alternative co-stimulatory molecules, checkpoint molecules, and OS. The proportion of CD4+ (left) or CD8+ (right) T cells that express the indicated co-stimulatory or checkpoint molecule was analyzed versus OS. p-values based on continuous measure distributions (b) Analysis of T cell CTLA-4 expression. The proportion of CD4+ (top) and CD8+ (middle) T cells that express CTLA-4 was determined by gating on CD4 or CD8 expressing cells and then gating on the population staining positive for CTLA-4, based on the fluorescence of isotype control antibody stained cells. (bottom) Typical expression of CTLA-4 on CD4+ (left) and CD8+ (right) T cells. Grey histogram – isotype control; black line – CTLA-4 staining. (c) Negative association between CD8+ CTLA-4+ T cells and OS. Patients were dichotomized based on the proportion of CD8+ T cells expressing CTLA-4. (d) Analysis of T cell TIM3 expression. The proportion of CD4+ (left) and CD8+ (right) T cells that express TIM3 was determined by gating on CD4 or CD8 expressing cells and then gating on the population staining positive for TIM3 based on the fluorescence of isotype control antibody stained cells. (e) Positive association between TIM3 expression on CD4+ T cells and OS. Patients were dichotomized based on the proportion of CD4+ T cells expressing TIM3. P-values based on continuous measure distributions (a) or dichotomized analysis (c, e).
Discussion
This study comprehensively examined immune biomarkers from the peripheral blood of metastatic PDAC patients. The goal was to test the hypothesis that patient immune status at baseline, prior to any subsequent treatment, predicts overall survival. The immunosuppressive cytokines IL-6 and IL-10 were strong, independent, negative predictors of OS. Examination of T cell phenotypes found that the proportions of antigen experienced T cells (both CD4+ and CD8+) were strong positive independent predictors of patient survival, and that the frequency of CD8+ T cells expressing the T cell checkpoint molecule CTLA-4 was negatively associated with survival. Paradoxically, CD4+ T cell expression of TIM3, another immunosuppressive molecule, was positively associated with PDAC survival.
To our knowledge, the present report is the largest and most comprehensive analysis of systemic immune status in PDAC to date. Focusing on treatment naïve patients enabled us to identify immune factors as they relate to tumor growth or control, avoiding the potentially powerful confounding influence of chemotherapy on the phenotype of the immune cells (8). The median overall survival (8.2 months; 95% CI = 7.0 – 10.9) for the 73 patients included in this study was consistent with published data from modern studies (5, 6). As we have previously shown, Reolysin® added to chemotherapy was found to be safe but had no appreciable effect on patient outcome (33). The same was true for follow up therapy. Thus overall we believe our observed findings accurately reflect indicators of baseline immune status, rather than a treatment effect resulting from subsequent therapy.
This suggests that the balance between ongoing immune reactions and immunosuppression may influence clinical outcome in treatment naïve metastatic PDAC patients, arguing that this ongoing immune activity continues to have an important impact on disease progression. Thus, these factors may help identify patients with more active, or more immunosuppressed, immune systems, who might experience relatively greater or lesser benefit from vaccine or checkpoint-blockade based immunotherapies.
To our knowledge, the present study is the first to examine the correlation between plasma IL-6 levels and OS strictly in patients with unresectable PDAC in the post single-agent gemcitabine era. IL-6 has previously been linked with worse OS in several solid malignancies, including breast (34) and prostate cancer (35). This cytokine plays a key role in the early growth, maintenance, and metastasis of PDAC tumors (27, 28). Previous studies in either mixed cohorts of patients with resectable and unresectable disease (36, 37) or in those undergoing gemcitabine based therapy (38) have found a negative correlation between IL-6 and clinical outcome. In agreement with these reports, our study found that IL-6 plasma levels in treatment naïve patients were significantly negatively associated OS. While single agent IL-6 blockade recently proved ineffective in a phase I/II trial in patients with solid tumors, including PDAC (39), interest in combination therapies that include IL-6 blockade remains high.
IL-6 mediates systemic detrimental effects on the immune system, namely altering the Th17/T regulatory cell (Treg) balance (40), inhibiting dendritic cell (DC) differentiation (24), and promoting or sustaining MDSC accumulation. Both MDSC and tolerogenic DCs produce IL-10, the other immunosuppressive cytokine found to be negatively correlated with OS in this study (41–43). Previous studies reported significantly elevated IL-10 levels in the peripheral blood of PDAC patients (21, 44), however this is the first study to report a significant negative correlation association between circulating IL-10 levels and OS in PDAC. These cytokines likely work in an immunosuppressive manner to weaken anti-tumor immune responses, and may complement or synergize with each other to profoundly compromise anti-tumor immunity, though this is yet to be tested directly. While complicated as a therapeutic strategy, it suggests that targeting both cytokines may yield superior clinical efficacy in metastatic PDAC in combination with traditional or experimental therapies.
In addition to IL-6 and IL-10, we observed a weaker but statistically significant positive correlation between higher levels of MCP-1 in the circulation and OS in this study. MCP-1 is a chemokine that alters monocyte and MDSC function and migration, though its prognostic significance in this disease remains unclear (45, 46). Monti et al argued that MCP-1 would lead to monocyte-mediated control of pancreatic tumor growth via increased secretion of cytokines such as IL-1β and IFN-γ (45) while Sanford et al. instead suggested that MCP-1 would increase the recruitment of MDSC and inflammatory monocytes to the tumor, and that these would serve an immunosuppressive role (46). Though our results do indicate a beneficial association between systemic MCP-1 and OS, a relatively large proportion of our cross-validation simulations (1352/2000, Supplemental table S2) found no significant effect of MCP-1 on OS. This could be an indication that MCP-1 is associated with OS in an influential subset of patients rather than in metastatic PDAC patients at large. This question will be the subject of future investigations.
An examination of phenotypic markers present on T cells revealed that CD45RO, CTLA-4, and TIM3 are significantly correlated with OS in PDAC. A link between the proportion of circulating T cells that are CD45RO+ (denoting antigen experienced or non-naïve T cells) and OS in PDAC has not been previously reported. Due to limited samples, we were unable to determine the specificity of these mature T cells. However, it is tempting to speculate that they are tumor reactive and it will be important to consider how the frequency of antigen-experienced and/or tumor reactive T cells influences treatment efficacy in future PDAC trials. This is bolstered by the observation that CTLA-4 expression on CD8+ T cells was negatively associated with OS, suggesting that in its absence cytotoxic T cells may exert some level of disease control in PDAC. Unfortunately two recent clinical trials investigating ipilumamab (anti-CTLA-4) in PDAC found very modest efficacy (no objective responses and a small number of disease stabilizations) (17, 47). A third T cell checkpoint molecule, TIM3, was to our surprise positively correlated with overall survival. Interestingly, TIM3 is sometimes considered a marker of T cell exhaustion (24), suggesting that high circulating levels of CD4+ TIM3+ T cells may reflect ongoing beneficial intratumoral immune reactions. Ultimately, more work is needed to determine if TIM3 blockade would enhance anti-tumor activity in PDAC or curtail it. No significant correlation was observed between PD-1/CD279 expression and overall survival; however, this molecule was abundantly expressed on T cells and we were unable to assess whether or not it was being stimulated in these patients. Thus, the potential of this molecule and its ligand (PD-L1) as therapeutic targets in PDAC warrant further investigation.
In this study, we were unfortunately not able to compare the systemic levels of immune biomarkers to their levels within tumors. Since these patients were not considered candidates for surgical resection, only a small initial biopsy was collected and insufficient material was available to generate intratumoral immune profiles. In contrast to past studies (20–22), there was no correlation between the frequency of MDSCs and OS in these patients. Unlike these prior studies, which included PDAC patients of all stages, the patient cohort studied here consisted entirely of patients with metastatic disease (median 3 metastatic sites, Table 1). Since the frequency of MDSCs are known to be positively correlated with stage or disease burden (48, 49), any potential link between MDSC and OS may have been masked by the advanced disease that these patients shared. It is also possible that the immunosuppressive and pro-tumorigenic effects of MDSCs are of relatively little importance for survival probability at this advanced disease state. It is noteworthy that two of the previous studies linking MDSC frequency to prognosis in PDAC included patients with prior (21) and/or ongoing (22) chemotherapy. Rebound hematopoiesis following chemotherapy could certainly influence the overall percentage of circulating myeloid cells, further confounding a direct comparison between these prior studies and the present one. Finally, PBMCs were enriched by Ficoll-Paque density gradient centrifugation and cryopreserved prior to analysis in this study. This has been previously reported to alter MDSC phenotype and reduce their relative frequency in subsequent analysis (50), suggesting that analyzing MDSC immediately following blood collection could lead to a different conclusion on their relationship to OS.
Altogether, this report identifies several immune-based biomarkers from the peripheral blood that constitute strong independent predictors of outcome in patients with metastatic PDAC. While it is not clear from this analysis whether these biomarkers were active mediators of outcome or merely reflected a patient’s underlying status, it does suggest several potential therapeutic targets. Many of these are currently being targeted in PDAC or other malignancies (e.g. IL-6, IL-10, or CTLA-4 blockade or pathway inhibition). Finally, this work suggests that individual PDAC patients could benefit from different therapies tailored to their initial immune profile.
Supplementary Material
Translational Relevance Statement.
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with poor survival outcomes. To date, an assessment of immune factors relevant to disease has not been comprehensively performed for treatment naïve patients. We hypothesized that systemic immunologic biomarkers could predict overall survival in treatment naïve PDAC patients. Several soluble and cellular biomarkers were identified as significant predictors of overall survival. Among these are numerous cytokines that represent actionable drug targets which could be translated clinically to reverse systemic immune suppression and complement immunotherapy for this disease. These data support the hypothesis that baseline immune status predicts PDAC disease course and overall patient survival. To our knowledge, this work represents the largest cohort and most comprehensive immune profiling of treatment-naïve metastatic PDAC patients to date.
Acknowledgments
Financial Support: This study was funded in part by the US National Cancer Institute’s Cancer Therapy Evaluation Program (Trial number: NCT01280058) and associated Phase II N01 program grant (HHSN261201100070C, PI: MVC), the William Hall Fund for Liver and Pancreatic Cancer Research (PI: TBS), an independent grant support from Oncolytics Inc. (PI: TBS and GBL). MRF received salary support from the Pelotonia Research Foundation (Postdoctoral Fellowship) and from an NIH postdoctoral training grant (5 T32 CA 90223-12, PI: William Carson).
The study was funded in part by the US National Cancer Institute’s Cancer Therapy Evaluation Program, NCT01280058 and associated Phase II N01 program grant, HHSN261201100070C. Funding for this work was also provided by the William Hall Fund for Liver and Pancreatic Cancer Research and by Oncolytics, Inc. We acknowledge the OSU CCC Analytical Cytometry Shared Resource and Clinical Trials Office for assistance with these studies. This work was also supported by the Pelotonia Fellowship Program and an NIH postdoctoral training grant, 5 T32 CA 90223-12. Any opinions, findings and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the Pelotonia Fellowship Program. We are especially grateful to all patients who participated in this study.
Footnotes
Conflicts of Interest: GBL and TBS received independent grant support from Oncolytics Inc.
Works Cited
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.NCCN. Pancreatic Adenocarcinoma (Version 2.2015) 2015 cited; Available from: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
- 4.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015:107. doi: 10.1093/jnci/dju413. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–44. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 8.Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2009;38:283–90. doi: 10.1016/j.ejps.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. The lancet oncology. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, et al. Exclusion of T Cells From Pancreatic Carcinomas in Mice is Regulated by Ly6C F4/80 Extra-tumor Macrophages. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer immunology research. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz-Winnenthal FH, Volk C, Z’Graggen K, Galindo L, Nummer D, Ziouta Y, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–87. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 14.Shibuya KC, Goel VK, Xiong W, Sham JG, Pollack SM, Leahy AM, et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PloS one. 2014;9:e96565. doi: 10.1371/journal.pone.0096565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–95. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Annals of surgery. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Xianjun, SJ, Xu Jin, Yao Wantong, Qu Bin, Zhu Wenwei, Xu Wenyan, Zhang Bo, Xu Yongfeng. CD8+ T Cells are compromised in human pancreatic cancer. Translational Medicine. 2012:2. [Google Scholar]
- 20.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, et al. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4(+) T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–79. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz J, Brooks TR, Duggan MC, Paul BK, Pan X, Wei L, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother. 2015;64:149–59. doi: 10.1007/s00262-014-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–18. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farren MR, Carlson LM, Netherby CS, Lindner I, Li PK, Gabrilovich DI, et al. Tumor-induced STAT3 signaling in myeloid cells impairs dendritic cell generation by decreasing PKCbetaII abundance. Science signaling. 2014;7:ra16. doi: 10.1126/scisignal.2004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer cell. 2011;19:456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, et al. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359–74. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goumas FA, Holmer R, Egberts JH, Gontarewicz A, Heneweer C, Geisen U, et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int J Cancer. 2015 doi: 10.1002/ijc.29445. [DOI] [PubMed] [Google Scholar]
- 29.Lesinski GB, Kondadasula SV, Crespin T, Shen L, Kendra K, Walker M, et al. Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon Alfa immunotherapy. J Natl Cancer Inst. 2004;96:1331–42. doi: 10.1093/jnci/djh252. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. http://www.R-project.org/ [Google Scholar]
- 31.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter ME, Hadji A, Murmann AE, Brockway S, Putzbach W, Pattanayak A, et al. The role of CD95 and CD95 ligand in cancer. Cell death and differentiation. 2015;22:549–59. doi: 10.1038/cdd.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bekaii-Saab T, Noonan AM, Lesinski G, Mikhail S, Ciombor K, Pant S, et al. LBA19A MULTI-INSTITUTIONAL RANDOMIZED PHASE 2 TRIAL OF THE ONCOLYTIC VIRUS REOLYSIN IN THE FIRST LINE TREATMENT METASTATIC ADENOCARCINOMA OF THE PANCREAS (MAP) Annals of Oncology. 2014:25. [Google Scholar]
- 34.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 35.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. British journal of cancer. 2004;90:2312–6. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebrahimi B, Tucker SL, Li D, Abbruzzese JL, Kurzrock R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–36. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 37.Schultz NA, Christensen IJ, Werner J, Giese N, Jensen BV, Larsen O, et al. Diagnostic and Prognostic Impact of Circulating YKL-40, IL-6, and CA 19.9 in Patients with Pancreatic Cancer. PloS one. 2013;8:e67059. doi: 10.1371/journal.pone.0067059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Furuse J, Inagaki M, et al. Serum levels of IL-6 and IL-1beta can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. British journal of cancer. 2013;108:2063–9. doi: 10.1038/bjc.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angevin E, Tabernero J, Elez E, Cohen SJ, Bahleda R, van Laethem JL, et al. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2192–204. doi: 10.1158/1078-0432.CCR-13-2200. [DOI] [PubMed] [Google Scholar]
- 40.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 41.Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. The Journal of clinical investigation. 2014;124:4678–80. doi: 10.1172/JCI78891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, et al. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol. 2013;190:2464–71. doi: 10.4049/jimmunol.1202781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiokawa A, Tanabe K, Tsuji NM, Sato R, Hachimura S. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol Lett. 2009;125:7–14. doi: 10.1016/j.imlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, Tibaudi D, et al. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. The American journal of pathology. 1999;155:537–47. doi: 10.1016/s0002-9440(10)65149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–61. [PubMed] [Google Scholar]
- 46.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–15. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 50.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. Journal of immunological methods. 2012;381:14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.