Abstract
PURPOSE
The application of pan-cancer next generation sequencing panels in the clinical setting has facilitated the identification of low frequency somatic mutations and the testing of new therapies in solid tumors using the ‘basket trial’ scheme. However, little consideration has been given to the relevance of non-synonymous germline variants which are likely to be uncovered in tumors and germline and which may be relevant to prognostication and prediction -of treatment response.
EXPERIMENTAL DESIGN
We analyzed matched tumor and normal DNA from 34 melanoma patients using an Ion Torrent cancer-associated gene panel. We elected to study the germline variant Q472H in the kinase insert domain receptor (KDR), which was identified in 35% of melanoma patients in both a pilot and an independent 1,223 patient cohort. Using patient-derived melanoma cell lines and human samples, we assessed proliferation, invasion, VEGF levels and angiogenesis by analyzing tumor microvessel density using anti-CD34 antibody.
RESULTS
Serum VEGF levels and tumor microvessel density were significantly higher in Q472H versus KDR wild-type patients. Primary cultures derived from melanomas harboring the KDR variant were more proliferative and invasive than KDR wild-type. Finally, using a VEGFR2 antibody, we showed that KDR Q472H cells were sensitive to targeted inhibition of VEGFR2, an effect that was not observed in KDR WT cells.
CONCLUSION
Our data support the integration of germline analysis into personalized treatment decision-making and suggest that patients with germline KDR variant might benefit from anti-angiogenesis treatment.
INTRODUCTION
Recently identified melanoma driver mutations have paved the way for rational development of effective targeted inhibitors that have increased survival rates for the metastatic patients whose tumors carry these specific mutations (1–4). Inhibition of the MAPK pathway in BRAF-mutated melanoma patients with BRAF and/or MEK inhibitors produces high response rates (1, 2, 5). However, approximately 30% of melanoma tumors lack activating mutations in BRAF or other somatic mutation drivers NRAS and KIT (6–8), and are designated as wild-type (WT) melanoma. While response rates to combination immune checkpoint blockade in BRAF WT tumors have been reported as high as 61% (9), few effective treatment options are available for WT patients who do not respond to immunotherapy and/or experience unacceptable toxicity, eventually leading to the necessity to stop treatment. As next generation sequencing (NGS) technologies be-come increasingly economical (10, 11), low frequency somatic driver mutations are being identified in multiple tumor types (12–14), facilitating new clinical trial schemes, such as the “basket trial” in which patients are accrued for therapy according to a specific, targetable genetic alteration, independent of tumor histotype (15).
When tumor-normal pairs are compared as a control to identify tumor-specific somatic mutations in NGS, non-synonymous germline variants can be identified through tumor-normal subtractive analysis (16, 17). Recently, the Cancer Genome Atlas (TCGA) published a multilayer profiling of 333 cutaneous melanomas, revealing a catalog of potentially actionable somatic alterations. However, common germline variants were excluded based on their presence in the “normal” sample (18). In melanoma, germline variants have been described in high- (CDKN2A and CDK4), moderate- (MC1R), and low-penetrance susceptibility genes (19). There are around 50 low-penetrance melanoma susceptibility genes that are relevant to pigmentation/nevus count, the anti-tumor immune response, DNA-repair, metabolism and vitamin D receptor polymorphisms. Data from a recent melanoma GWAS (genome-wide association study) (20, 21) and evidence from our group (22, 23) demonstrate their impact on tumor progression and clinical outcome. However, to our knowledge, no therapy targeting germline variants exists in melanoma.
In this study, we sought to examine the clinical utility of a targeted NGS approach to identify actionable somatic and germline variants with immediate relevance to the treatment of melanoma. We analyzed tumor and germline DNA from melanoma patients with a cancer-associated gene panel covering 2,800 COSMIC mutations within 50 known oncogenes and tumor suppressor genes. We identified potentially actionable somatic mutations in melanoma in WT tumors and characterized for the first time a pathogenic KDR germline variant (Q472H) in melanoma. Our data provide support for the concept of integrating germline DNA analyses to improve the personalized treatment of cancer patients.
MATERIAL AND METHODS
Patients
We studied a pilot cohort of 34 Stage III–IV and an independent cohort of 1,223 Stage I–IV melanoma patients. All patients were enrolled in the New-York University Interdisciplinary Melanoma Cooperative Group (IMCG) biorepository database from May 2002 to May 2012, and were prospectively followed according to an NYU IRB-approved protocol and in accordance with the Federal Wide Assurance (FWA) for the Protection of Human Subjects approved by the Department of Health and Human Services. Clinical variables assessed at time of enrollment include age, gender, stage, histological subtype, anatomic site and treatment. Response to treatment, melanoma status and survival information were prospectively obtained via active follow-up every 6 months.
Biospecimens and cell lines
Tumor genomic DNA was extracted from macrodissected, formalin-fixed, paraffin-embedded (FFPE) melanoma tissue samples from the pilot cohort with the Qiagen QIAamp DNA FFPE Tissue Kit.
Germline genomic DNA was extracted from whole blood samples from melanoma patients with the Qiagen QIAamp DNA Blood Kit. Serum samples used for VEGF ELISA were collected from Stage III/IV melanoma patients before metastasectomy. Formalin-fixed paraffin-embedded (FFPE) tissues used for CD34 immunohistochemistry staining were obtained from metastatic melanoma samples. Primary established cell lines (WM 1575 and WM 3248) were purchased commercially from the Wistar Institute (Philadelphia, PA) where they were authenticated by short tandem repeat (STR) profiling (http://www.wistar.org/lab/meenhard-herlyn-dvm-dsc/page/melanoma-cell-str-profiles) and used in our laboratory for no longer than six months following resuscitation. Primary melanoma patient-derived cell cultures were developed previously in our laboratory and were tested and authenticated against the original tumor specimens from which they were derived (24).
Targeted Next-Generation Sequencing
Targeted sequencing: The AmpliSeq Cancer Hotspot Panel v2 assay (Life Technologies) was used to amplify DNA from melanoma tumors and matched germline (peripheral blood), according to manufacturer’s instructions. The assay consists of a single ultraplex PCR reaction with primer sets for 207 amplicons. We used AmpliSeq v.2 chemistry, Ion Express barcode adapters, the Ion PGM (Personal Genome Machine; Life Technologies), 200 sequencing kit, and the Ion 318 Chip.
Targeted next-generation sequencing data analysis
The sequencing data underwent a primary analysis using the Torrent Suite server. Optimized signal processing, base calling and sequence alignment was reviewed to assess the quality and accuracy of the sequencing runs. Detection of SNPs and indels from Ion-sequencing data was performed using Torrent Variant Caller software within the Torrent Suite. Secondary analysis (annotation) was performed by uploading VCF files into Ion-Reporter using the ion reporter uploader plugin. All variants identified were validated by Sanger sequencing.
Genotyping of KDR Q472H variant in validation cohort
1,223 germline DNA samples from the validation cohort were genotyped for the KDR Q472H variant using the MassARRAY iPLEX platform (Agena Bioscience, San Diego, CA, USA). The variant was in Hardy-Weinberg equilibrium (p=0.526).
Immunohistochemistry (IHC) and analysis of microvessel density (MVD)
IHC was performed on 26 formalin-fixed paraffin-embedded (FFPE) metastatic melanoma tumor samples. Tissues were evaluated for CD34 expression using a CD34-specific rabbit monoclonal antibody (1:500; Abcam, #ab81289). For each sample, the intra-tumoral region showing the highest MVD was selected and the density of microvessels were scored in a blinded-fashion in each sample at 20× magnification by an attending pathologist (Dr. Farbod Darvishian).
Quantification of serum VEGF levels
VEGF in cell line supernatants and patient sera was assessed by ELISA according to manufacturer’s instructions (R&D Systems). All assays were performed in duplicate.
Proliferation assays
Four primary melanoma patient-derived cell cultures (10-230, 09-085, 09-241, 11-161) and two established melanoma cell lines (WM 1575 and WM 3248) were treated with vehicle (PBS) or 10 μg/ml of VEGFR2 blocking antibody (R&D Systems, #MAB3572), loaded with 2 μM CFSE (Carboxyfluorescein succinimidyl ester) and cultured in complete media. After 3 days of culture, the CFSE concentration was assessed by flow cytometry as a measure of cell proliferation. Each experiment was performed in duplicate and repeated three times.
Invasion assays
2.5 × 104 cultured melanoma cells were seeded in serum-free medium containing vehicle (PBS) or 10 μg/ml of VEGFR2 blocking antibody (R&D Systems, #MAB3572) in the upper chamber of a porous insert coated with Matrigel (Becton Dickinson) within a 24-well plate containing complete medium (chemoattractant). After 22 h, non-invading cells that remained in the upper chamber were carefully removed using a cotton swab. Cells adhering to the bottom of the filter were fixed with cold 4% PFA and stained with 0.1% crystal violet and 20% methanol. Inserts were scanned and four quarters were photographed for each insert and counted using an Axiovert 10 inverted microscope (Carl Zeiss).
Statistical methods
The association analysis of germline variants with melanoma survival was performed using univariate and multivariate Cox proportional hazards models under co-dominant model (2 degree freedom test) for both pilot and validation cohorts. Multivariate analyses were stratified by tumor stage and adjusted by clinicopathological covariates: age and thickness as continuous covariates; gender, ulceration status (present/absent), and anatomic site (axial/extremity) as dichotomous covariates; and histological type as categorical covariates. Kaplan-Meier plots were generated in R, and the statistical significance of the difference of KM curves was calculated by log-rank test in dominant model: comparing wild type homo-zygotes (Q472) versus carriers of H472 allele (pooling Q472H heterozygotes and H472 homozygotes).
Contingency tables and Chi-square test were used to compare different types of metastases (skin/subcutaneous, lymph nodes, lung, liver and brain) between patients with and without the KDR Q472H variant.
Two tailed unpaired t-test was used to compare KDR Q472H positive vs. negative tumors according to: (i) microvessels density through CD34 immunohistochemistry staining in FFPE metastatic melanoma tumor samples, (ii) VEGF expression in melanoma cell line supernatants and sera from melanoma patients.
Two tailed paired t-test was used to compare melanoma cells treated vs. untreated with VEGFR2 blocking antibody according to: (i) cell proliferation, and (ii) cell invasion. This comparison was made between melanoma cells that were KDR Q472H positive and negative.
RESULTS
WT melanomas exhibit clinically-relevant, actionable mutations
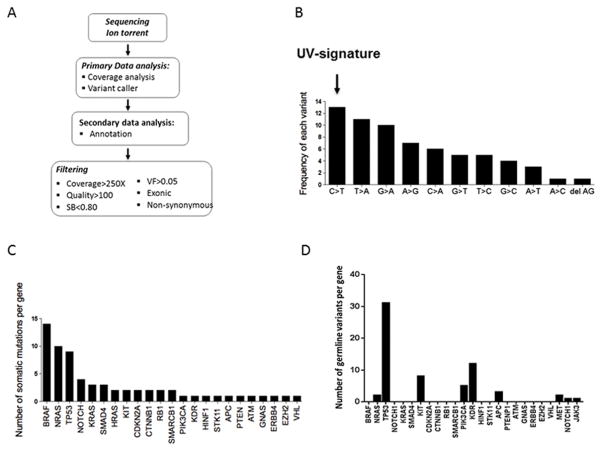
We used Ion Torrent NGS using a targeted panel of hotspot mutations from 50 genes implicated in different cancer types to (i) detect known actionable mutations in melanoma (eg. BRAF V600) and (ii) identify infrequent somatic mutations that occur in other tumor types and could be actionable in melanoma. Tumor and matched germline DNA from a pilot cohort of 34 patients were sequenced by Ion Torrent (Table 1). The data were filtered to exclude all synonymous and intronic variants. A quality score cutoff of 100 and an allele frequency of >5 (with a sequencing coverage > 250X) were also applied to eliminate low frequency artifacts (Figures 1A and Supplementary Figure S1), identifying a total of 66 somatic mutations in our pilot cohort (n=34) that were absent in matched germline DNA. The majority of the somatic mutations were missense mutations (60/66, 91%), 5/66 were nonsense mutations (8%), and only one frameshift deletion mutation was identified. The characteristic C>T transversions associated with the UV-signature of DNA damage were the most frequent changes observed (25) (Figure 1B).
Table 1.
Patient characteristics of the pilot melanoma cohort analyzed by the Ion Torrent cancer-associated gene panel (n=34) and the independent validation melanoma cohort (n=1,223).
| PILOT COHORT (N =34)
| |
|---|---|
| Age (mean) | 63 |
| Gender | |
|
| |
| Female | 12 |
| Male | 23 |
|
| |
| Stage | |
|
| |
| III | 21 |
| IV | 14 |
|
| |
| 36 samples | |
|
| |
| 1 sample/patient | 33 |
| 2 samples/patient | 2 primary and met LN and brain met |
|
| |
| Type of sample | |
|
| |
| Metastases | 35 |
| Lymph Nodes | 20 |
| Skin/Subcutnaneous | 9 |
| Brain | 6 |
| Primary | 2 |
|
| |
| VALIDATION COHORT (N = 1,223) | |
|
| |
| Age (mean) | 58 |
| Gender | |
|
| |
| Female | 527 |
| Male | 694 |
|
| |
| Stage | |
|
| |
| 0 (in situ) | 58 |
| I | 776 |
| II | 206 |
| III | 172 |
| IV | 7 |
Figure 1.
Ion Torrent analysis of the pilot melanoma cohort (n=34) and identification of somatic and germline mutations in melanoma. A) Ion Torrent sequencing analysis workflow. The AmpliSeq Cancer Hotspot Panel v2 assay was used to amplify DNA from tumor and matched germline controls from melanoma patients. Sequencing data underwent a primary analysis using the Torrent Suite server. Secondary analysis (annotation) was performed by uploading VCF files into Ion Reporter using the ion reporter uploader plugin. B) A UV-induced signature is the most frequent somatic variation identified in our cohort. C) The frequencies of somatic mutations identified in BRAF, NRAS and KIT genes in our cohort are consistent with previously reported distributions in melanoma. D) Ion Torrent sequencing identified 9 germline variants in our pilot melanoma patient cohort (NRAS, TP53, KIT, PIK3A, KDR, APC, MET, NOTCH1, JAK3).
In the 36 tumors we analyzed from 34 patients (pilot cohort), we identified somatic mutations in BRAF, NRAS and KIT genes at frequencies that were consistent with those described previously (6–8) BRAF was mutated in 14/36 samples (39%), NRAS was mutated in 10/36 samples (28%), and KIT was mutated in 2/36 samples (6%) (Figure 1C). The majority of BRAF mutations were V600E (7/14; 50%) and V600K (3/14; 21%). Less common but previously characterized BRAF (26–28), NRAS, and KIT mutations are summarized in Supplementary Figure S2. Paired primary/metastatic tumor samples demonstrated several instances of molecular heterogeneity (Supplementary Figure S3).Nine of 36 tumor samples (26%) were negative for BRAF, NRAS or KIT mutations, and hence were designated as WT melanomas. Our results show that these melanomas harbored mutations in known oncogenes and tumor suppressors, including TP53, ERBB4, PIK3CA, NOTCH, EZH2, KRAS, HRAS and RB1 (Figure 1C, Table 2 and Supplementary Figure S4). While these molecules have been implicated in the development and progression of other tumor types, their specific roles in melanoma are less clear. We analyzed genes that were recurrently mutated in at least two samples in our cohort (Supplementary Figure S2).
Table 2.
Wild-type melanomas (BRAF/NRAS/KIT WT) patients harbor mutations in known oncogenes and tumor suppressors.
| WT melanoma patients | Somatic mutations |
|---|---|
|
| |
| 07-249 | KRAS |
| 06-004 | KRAS, TP53, RB1 |
| 03-173 | HRAS |
| 10-071 | TP53 |
| 06-050 | ERBB4 |
| 07-021 | PIK3CA |
| 09-241 | NOTCH, EZH2 |
| 06-092 | - |
| 11-047 | - |
The germline KDR Q472H variant is found in one-third of melanoma patients
In the second phase of our analysis we examined 9 germline coding variants identified through normal-tumor subtractive analysis of the Ion Torrent sequencing of the 34 melanoma patients (Figure 1D). We elected to study a KDR (kinase insert domain receptor; VEGFR2) germline variant Q472H further as it has previously been shown to play a role in multiple cancer types, including lung adenocarcinoma (29) and demonstrated a high frequency in our patient cohort (Figure 1D) but remained unexamined in the context of melanoma. In an independent validation cohort of 1,223 germline DNA samples from melanoma patients (Table 1), the minor allele frequency (MAF) of KDR variant was comparable (36.9%) to that in our pilot cohort. By comparison, the overall MAF of the KDR Q472H variant (rs1870377) is 21% in the general population (1000 Genomes Project database; http://www.1000genomes.org/), ranging from 9% in the African-American population to 47% of the Asian population, with a MAF of 13% in the Caucasian-American population. No significant associations were observed between the variant and clinicopathological variables (Supplementary Figure S5).
The germline KDR Q472H variant is associated with a higher tumor microvessel density in melanoma
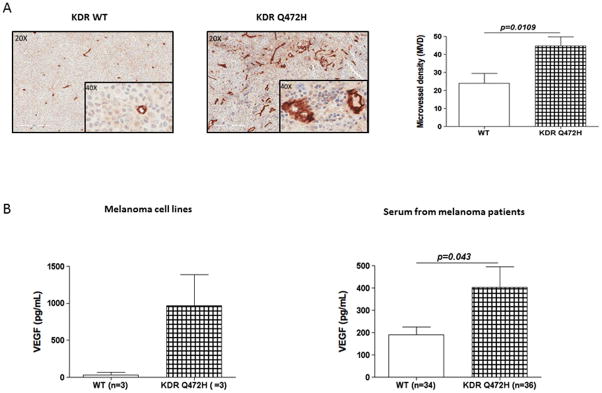
While we did not find a significant correlation between KDR Q472H and patient survival, other studies suggest that the impact of KDR on clinical outcome might depend on other KDR SNPs or the treatments patients received (30–33). Therefore, we sought to determine if KDR Q472H could impact the melanoma phenotype. KDR plays a crucial role in mediating angiogenic endothelial cell responses via the vascular endothelial growth factor (VEGF) pathway, and VEGF levels have been reported to correlate with tumor growth rate and microvessel density (MVD) (29, 34). We performed immunohistochemistry (IHC) using a CD34 antibody to quantitate MVD within 26 metastatic melanoma FFPE samples (from 13 KDR WT homozygous patients and 13 heterozygous KDR Q472H patients, including patients from our pilot cohort). Our results showed nearly two-fold higher MVD in tumor samples from patients with KDR Q472H variant compared to KDR WT patients, where the mean MVD was 44.8 microvessels in tumors from patients carrying Q472H variant compared to 23.9 microvessels in tumors from KDR WT patients (Figure 2A; p=0.01).
Figure 2.
KDR Q472H variant is associated with an angiogenic melanoma phenotype. A) Higher microvessel density (MVD) in KDR Q472H melanoma compared with KDR WT melanoma. MVD was measured by IHC analysis of CD34 expression: KDR WT melanoma (left photomicrograph); KDR Q472H melanoma (right photomicrograph); and plot summarizing MVD in KDR WT (N=13) and KDR Q472H (N=13) melanoma lymph node metastases (right). B) Increased VEGF secretion by KDR Q472H melanomas; cell line supernatants (left panel) and patient sera (right panel).
The germline KDR Q472H variant is associated with increased levels of serum VEGF in melanoma patients
The KDR Q472H variant is an alteration leading to increased VEGFR2 phosphorylation. In addition, the existence of a positive autocrine loop between VEGFR-2 and VEGF has been proposed to lead to higher levels of VEGF secretion that ultimately sustains enhanced angiogenesis (35, 36). Therefore, we hypothesized that the KDR Q472H variant might stimulate an increase in VEGF secretion in melanoma patients and thus drive tumor angiogenesis. We first used a panel of four primary, melanoma patient-derived cell cultures (09-085, 09-241, 10-230, 11-161 (24)), and two established (WM 1575, WM 3248) melanoma cell lines, heterozygous carriers of the H472 variant or WT (Q472) KDR. All primary cultures were derived from patient tumors that were sequenced in our pilot cohort study. Analysis of VEGF levels by ELISA revealed a 29.7-fold increase in VEGF levels in tissue culture supernatants from KDR Q472H cell lines (09-085, 09-241 and 10-230; mean VEGF expression=968.5 pg/ml) compared to the supernatants from KDR WT cell lines (11-161, WM 1575 and WM 3248; mean VEGF expression=32.5 pg/ml) (Figure 2B). We then compared VEGF levels in sera (collected prior to surgical tumor resection) between KDR Q472H melanoma patients (n=36) and KDR WT patients (n=34) using ELISA. Our results confirmed that VEGF levels are significantly higher in sera from KDR Q472H patients (mean VEGF level=401.8 pg/ml) compared to sera from KDR WT patients (mean VEGF level=192.35 pg/ml) (Figure 2B; p=0.04).
Inhibition of KDR significantly decreases proliferation and invasion of melanoma cells carrying the KDR Q472H variant
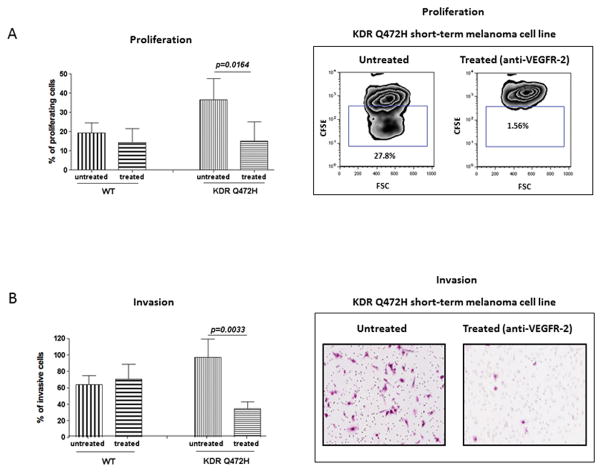
We next assessed the proliferation of melanoma cells carrying KDR Q472H variant compared with KDR WT melanoma cells. While our results showed that proliferation rates were increased in melanoma cells with the KDR Q472H variant, this effect did not reach statistical significance (Figure 3A; p>0.05). We reasoned that KDR inhibition might impact the proliferation and invasion of melanoma cell lines carrying the KDR Q472H variant. To test this, we treated KDR Q472H and KDR WT melanoma cell lines with a VEGFR2 blocking antibody, that neutralizes VEGFR2 activity by antagonizing the binding of VEGF, and assessed cell proliferation. We found that KDR blockade selectively inhibits the proliferation of melanoma cells with the KDR Q472H variant. Our results showed that KDR inhibition abrogated cell proliferation by approximately 50% in melanoma cells with the KDR Q472H allele (p=0.02), whereas no significant growth inhibition was seen in KDR WT melanoma cells (Figure 3A). Finally, pre-treatment of KDR Q472H and KDR WT melanoma cells lines with VEGFR2 blocking antibody resulted in a 64% reduction in cell invasion as compared to KDR WT cells, in which no significant effect on cell invasion upon treatment was observed (Figure 3B; p=0.003). Together, these results suggest an increased dependence of KDR Q472H melanoma cells on VEGFR2 signaling.
Figure 3.
KDR Q472H determines sensitivity of melanoma to anti-VEGFR2 treatment. A) Proliferation of KDR Q472H melanoma cells is sensitive to VEGFR-2 inhibition. The percentage of proliferating cells (KDR WT vs KDR Q472H, 11-161,WM 1575, WM 3248 and 09-085, 09-241,10-230 respectively) is shown without or with treatment with a VEGFR-2 blocking antibody, and in the right panel representative data from flow cytometry analysis of proliferation is shown B) VEGFR-2 inhibition decreases invasion of KDR Q472H melanoma cells. The percentage of invading cells (KDR WT vs KDR Q472H, 11-161, WM 1575, WM 3248 and 09-085, 09-241, 10-230 respectively) is shown without or with treatment with a VEGFR-2 blocking antibody, and in the right panel representative data from flow cytometry analysis of proliferation is shown.
DISCUSSION
In this study we performed targeted NGS to analyze both somatic and germline variants in 50 cancer-associated genes across 36 melanoma samples from 34 patients. We detected several low-frequency novel actionable somatic mutations that warrant consideration for targeting through a “basket” trial scheme. Our data also identified a germline KDR Q472H variant in one third of melanoma patients that is associated with an angiogenic phenotype and which is selectively sensitive to targeted VEGFR2 inhibition.
We demonstrated that WT melanomas harbor somatic mutations that have been implicated in the progression of other cancer types and are potentially actionable, since inhibitors targeted against these mutations are in clinical development or have already been approved for treatment of other cancers (37, 38). Our study identified nine such genes that were mutated in at least two different samples: TP53, NOTCH, KRAS, SMAD4, HRAS, CDKN2A, CTNNB1, RB1 and SMARCB1. Hence, we confirm that the targeted sequencing approach allows cancer-associated somatic mutations to be identified in melanoma tumor specimens, building on large-scale sequencing efforts in this and other tumor types (25, 39). Observed at low frequencies that make individual melanoma specific clinical trials unfeasible, these data support the further development and application of the ‘basket trial’ concept: clinical trials of therapies targeted against a tumor’s mutational status independent of the tumor histotype (eg. ClinicalTrials.gov Identifier: NCT01219699 targeting PI3K).
Clinical sequencing of tumors to identify low frequency somatic driver mutations is likely to uncover incidental germline variants that might be relevant to cancer risk, prognostication, or treatment response. There have been extensive discussions of the utility and ethical considerations of reporting these results (40). However, these discussions have mostly focused on findings that have clear medical value but that are secondary to the indication for ordering sequencing. Although the clinical sequencing practice currently limits the use of germline patient samples as a control to identify tumor-specific somatic mutations, the sequencing of “normal”-tumor pairs will have important clinical implications, by increasing the potential to identify impactful germline variants (22, 23). First, the potential to unravel deleterious germline mutations known to be associated with a high risk of cancer (e.g. BRCA1/2, APC, p53) support the necessity to address the ethical and clinical impact of such incidental findings. Notably, in our study we detected 9 rare germline variants including 2 high-risk genes associated with cancer predisposition (TP53 and APC). The potential utility of common genetic variants in personalized medicine is emerging with the wealth of accumulated data from large population-based whole-exome sequencing efforts (41, 42). Common germline variants have the potential for routine utilization and transformative clinical impact because of their distribution in a large proportion of patients. Second, the impact of germline variants in patients may affect the tumor microenvironment, which is considered essential for tumor development and progression, and may also impact the response to anti-tumor therapies and ultimately patient survival (43, 44). We have recently shown that an interleukin-10 (IL-10) variant (rs3024493) has been significantly associated with melanoma overall survival and IL-10 secretion from CD4+ T cells, a finding that may have implications for the anti-tumor immune response (23). In turn, this could modify determinants of tumor progression and therapy resistance, including angiogenesis, immunity and remodeling of the extracellular matrix. As such, far from being merely incidental findings, the germline variants captured on targeted sequencing panels are, in addition to somatic mutations, plausible candidates for better clinical and molecular stratification of melanoma patients, which would enable more effective personalized prognostication and treatment.
We demonstrated that the KDR Q472H germline variant is associated with an angiogenic melanoma phenotype, characterized by significantly increased tumor vascular density and VEGF secretion. KDR is a VEGF receptor (VEGFR2) critical for physiological and tumor angiogenesis (45), and multiple KDR genetic variants have been implicated in multiple tumor types (29, 30, 46). Consistent with our findings, KDR has previously been correlated with increased endothelial cell angiogenesis via VEGF stimulation (47), and KDR Q472H has been shown to mediate VEGFR2 phosphorylation and enhanced angiogenesis (29). Our study extends these observations by showing that melanoma cells carrying the KDR Q472H variant not only secrete more VEGF but have a higher proliferative and invasive capacity. We demonstrate a dependence of KDR Q472H melanoma cell proliferation and invasion on KDR signaling, as melanoma cells that harbor KDR Q472H were sensitive to targeted inhibition of VEGFR2, an effect that was not observed in KDR WT cell lines. Anti-angiogenic approaches to tumor therapy are used clinically, such as bevacizumab, a VEGF inhibitor that is FDA-approved for colorectal cancer and more recently for platinum-resistant epithelial ovarian cancer in combination with chemotherapy. However, to date no anti-angiogenic treatment has been approved for melanoma, although ongoing clinical trials are investigating VEGFR and VEGF inhibitors in the adjuvant and metastatic melanoma settings (48, 49) (ClinicalTrials.gov Identifier: NCT00139360). Our preclinical data suggest that the sub-set of melanoma patients whose tumors carry the KDR Q472H variant may respond better to VEGFR-2 inhibition. Furthermore, our observations might in part explain the poor clinical responses of a general unselected population of melanoma patients to VEGF/VEGFR inhibition. For example, the addition of bevacizumab to carboplatin plus paclitaxel (BEAM trial) (50) did not significantly improve the PFS of melanoma patients. It is interesting to speculate that the clinical response to anti-angiogenic therapy could be analyzed separately in melanoma patients carrying the KDR Q472H germline variant versus those patients who are KDR WT.
Our observations suggest that KDR Q472H does not influence prognosis in melanoma. Previous studies have shown mixed results on the impact of KDR SNPs on prognosis in other cancers including colorectal and non-small-cell lung and hepatocellular carcinomas. These results suggested that the impact of KDR on clinical outcome might depend on other KDR SNPs or the treatments patients received (30, 33, 46). These conflicting reports indicate that the precise clinical significance of individual KDR variants is likely to be specific to individual tumor types and to depend on the treatment regimen. Importantly, the size of the cohort being studied may play a crucial role in determining the relevance of KDR variants to clinical outcome, as small patient subsets used in many of these prior studies might significantly account for the variability of the observed associations with survival.
In summary, we have used a clinically-validated NGS assay to identify a novel germline variant, KDR Q472H, which promotes an angiogenic phenotype in melanoma. Further, we identified several actionable somatic mutations in BRAF/NRAS/KIT WT melanoma, a finding that has implications for the molecular stratification and treatment of these tumors. We propose that targeted sequencing approaches to identify clinically-relevant gene variants should not focus solely on somatic mutations, but instead should also include germline variants that might inter-act with somatic driver mutations to further promote tumor development and progression and could also represent novel therapeutic targets be-longing to the tumor microenvironment.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
The emerging application of next-generation sequencing in clinical care has facilitated the identification of low frequency somatic mutations and the testing of new therapies in solid tumors. However, little attention has been given to the relevance of non-synonymous germline variants which may be relevant to both prognostication and prediction of treatment response. The findings from our study highlight the utility of clinical sequencing panels across different cancer types for discovery of actionable somatic mutations and germline variants. Using this strategy we identified and functionally validated a KDR (VEGFR-2) germline variant (Q472H) that is present in one-third of melanoma patients. Our study addresses a critical area of translational oncology research: the identification of biomarkers that will allow development of personalized, genetic-based strategies to optimize patient selection and treatment outcomes.
Acknowledgments
Financial Support: This work was supported by the NYU Cancer Institute NCI Cancer Center Support Grant (5 P30 CA 016087-27), the National Institutes of Health (1R01CA155234 to IO) (1R21CA184924-01 to TK), The Chemotherapy Foundation (IO), the Gulbenkian Programme for Advanced Medical Education (IPS), and the Marc Jacobs Campaign for Melanoma Research.
We thank Dr. Farbod Darvishian (New York University School of Medicine) for technical assistance in performing the scoring of the formalin-fixed, paraffin-embedded immunohistochemical staining.
Footnotes
Disclaimers: The authors have no conflicts to disclose.
References
- 1.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine. 2012;367:1694–703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2904–9. doi: 10.1200/JCO.2010.33.9275. [DOI] [PubMed] [Google Scholar]
- 4.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 5.Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, DeMarini DJ, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. The Lancet Oncology. 2012;13:782–9. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakob JA, Bassett RL, Jr, Ng CS, Curry JL, Joseph RW, Alvarado GC, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer. 2012;118:4014–23. doi: 10.1002/cncr.26724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1239–46. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 8.Wilson MA, Zhao F, Letrero R, D’Andrea K, Rimm DL, Kirkwood JM, et al. Correlation of somatic mutations and clinical outcome in melanoma patients treated with Carboplatin, Paclitaxel, and sorafenib. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:3328–37. doi: 10.1158/1078-0432.CCR-14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. The New England journal of medicine. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolaev SI, Rimoldi D, Iseli C, Valsesia A, Robyr D, Gehrig C, et al. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nature genetics. 2012;44:133–9. doi: 10.1038/ng.1026. [DOI] [PubMed] [Google Scholar]
- 11.Van Allen EM, Wagle N, Stojanov P, Perrin DL, Cibulskis K, Marlow S, et al. Whole-exome sequencing and clinical interpretation of formalin-fixed, paraffin-embedded tumor samples to guide precision cancer medicine. Nature medicine. 2014;20:682–8. doi: 10.1038/nm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson DB, Dahlman KH, Knol J, Gilbert J, Puzanov I, Means-Powell J, et al. Enabling a genetically informed approach to cancer medicine: a retrospective evaluation of the impact of comprehensive tumor profiling using a targeted next-generation sequencing panel. The oncologist. 2014;19:616–22. doi: 10.1634/theoncologist.2014-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shitara M, Okuda K, Suzuki A, Tatematsu T, Hikosaka Y, Moriyama S, et al. Genetic profiling of thymic carcinoma using targeted next-generation sequencing. Lung cancer. 2014;86:174–9. doi: 10.1016/j.lungcan.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, Huo X, Ye H, Tang C, Nandakumar V, Lou F, et al. Genetic mutation analysis of human gastric adenocarcinomas using ion torrent sequencing platform. PloS one. 2014;9:e100442. doi: 10.1371/journal.pone.0100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redig AJ, Janne PA. Basket trials and the evolution of clinical trial design in an era of genomic medicine. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:975–7. doi: 10.1200/JCO.2014.59.8433. [DOI] [PubMed] [Google Scholar]
- 16.Amendola LM, Dorschner MO, Robertson PD, Salama JS, Hart R, Shirts BH, et al. Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome research. 2015;25:305–15. doi: 10.1101/gr.183483.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S, Anagnostou V, Lytle K, Parpart-Li S, Nesselbush M, Riley DR, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Science translational medicine. 2015;7:283ra53. doi: 10.1126/scitranslmed.aaa7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas N. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–96. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053–62. doi: 10.1038/sj.onc.1206445. [DOI] [PubMed] [Google Scholar]
- 20.Barrett JH, Iles MM, Harland M, Taylor JC, Aitken JF, Andresen PA, et al. Genome-wide association study identifies three new melanoma susceptibility loci. Nature genetics. 2011;43:1108–13. doi: 10.1038/ng.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macgregor S, Montgomery GW, Liu JZ, Zhao ZZ, Henders AK, Stark M, et al. Genome-wide association study identifies a new melanoma susceptibility locus at 1q21.3. Nature genetics. 2011;43:1114–8. doi: 10.1038/ng.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rendleman J, Shang S, Dominianni C, Shields JF, Scanlon P, Adaniel C, et al. Melanoma risk loci as determinants of melanoma recurrence and survival. Journal of translational medicine. 2013;11:279. doi: 10.1186/1479-5876-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rendleman J, Vogelsang M, Bapodra A, Adaniel C, Silva I, Moogk D, et al. Genetic associations of the interleukin locus at 1q32.1 with clinical outcomes of cutaneous melanoma. Journal of medical genetics. 2015;52:231–9. doi: 10.1136/jmedgenet-2014-102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Miera EV, Friedman EB, Greenwald HS, Perle MA, Osman I. Development of five new melanoma low passage cell lines representing the clinical and genetic profile of their tumors of origin. Pigment cell & melanoma research. 2012;25:395–7. doi: 10.1111/j.1755-148X.2012.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nature genetics. 2012;44:1006–14. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guedes JG, Veiga I, Rocha P, Pinto P, Pinto C, Pinheiro M, et al. High resolution melting analysis of KRAS, BRAF and PIK3CA in KRAS exon 2 wild-type metastatic colorectal cancer. BMC cancer. 2013;13:169. doi: 10.1186/1471-2407-13-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Human pathology. 2005;36:486–93. doi: 10.1016/j.humpath.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Glubb DM, Cerri E, Giese A, Zhang W, Mirza O, Thompson EE, et al. Novel functional germline variants in the VEGF receptor 2 gene and their effect on gene expression and microvessel density in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5257–67. doi: 10.1158/1078-0432.CCR-11-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong G, Guo X, Fu X, Wan S, Zhou F, Myers RE, et al. Potentially functional genetic variants in KDR gene as prognostic markers in patients with resected colorectal cancer. Cancer science. 2012;103:561–8. doi: 10.1111/j.1349-7006.2011.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loupakis F, Cremolini C, Fioravanti A, Orlandi P, Salvatore L, Masi G, et al. Pharmacodynamic and pharmacogenetic angiogenesis-related markers of first-line FOLFOXIRI plus bevacizumab schedule in metastatic colorectal cancer. British journal of cancer. 2011;104:1262–9. doi: 10.1038/bjc.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang F, Tang X, Riquelme E, Behrens C, Nilsson MB, Giri U, et al. Increased VEGFR-2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer research. 2011;71:5512–21. doi: 10.1158/0008-5472.CAN-10-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng YB, Huang JW, Zhan MX, Zhao W, Liu B, He X, et al. Genetic variants in the KDR gene is associated with the prognosis of transarterial chemoembolization treated hepatocellular carcinoma. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:11473–81. doi: 10.1007/s13277-014-2478-8. [DOI] [PubMed] [Google Scholar]
- 34.Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary & pancreatic diseases international : HBPD INT. 2005;4:220–6. [PubMed] [Google Scholar]
- 35.Adamcic U, Skowronski K, Peters C, Morrison J, Coomber BL. The effect of bevacizumab on human malignant melanoma cells with functional VEGF/VEGFR2 autocrine and intracrine signaling loops. Neoplasia. 2012;14:612–23. doi: 10.1593/neo.11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee S, Heukamp LC, Siobal M, Schottle J, Wieczorek C, Peifer M, et al. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. The Journal of clinical investigation. 2013;123:1732–40. doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huynh C, Poliseno L, Segura MF, Medicherla R, Haimovic A, Menendez S, et al. The novel gamma secretase inhibitor RO4929097 reduces the tumor initiating potential of melanoma. PloS one. 2011;6:e25264. doi: 10.1371/journal.pone.0025264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Molecular cancer therapeutics. 2011;10:558–65. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in medicine : official journal of the American College of Medical Genetics. 2013;15:565–74. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu W, O’Connor TD, Jun G, Kang HM, Abecasis G, Leal SM, et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493:216–20. doi: 10.1038/nature11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tennessen JA, Bigham AW, O’Connor TD, Fu W, Kenny EE, Gravel S, et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science. 2012;337:64–9. doi: 10.1126/science.1219240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, et al. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nature genetics. 2010;42:410–4. doi: 10.1038/ng.569. [DOI] [PubMed] [Google Scholar]
- 44.Moes-Sosnowska J, Szafron L, Nowakowska D, Dansonka-Mieszkowska A, Budzilowska A, Konopka B, et al. Germline SMARCA4 mutations in patients with ovarian small cell carcinoma of hypercalcemic type. Orphanet journal of rare diseases. 2015;10:32. doi: 10.1186/s13023-015-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 46.Hansen TF, Sorensen FB, Spindler KL, Olsen DA, Andersen RF, Lindebjerg J, et al. Microvessel density and the association with single nucleotide polymorphisms of the vascular endothelial growth factor receptor 2 in patients with colorectal cancer. Virchows Archiv : an international journal of pathology. 2010;456:251–60. doi: 10.1007/s00428-009-0878-8. [DOI] [PubMed] [Google Scholar]
- 47.Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 48.Corrie PG, Marshall A, Dunn JA, Middleton MR, Nathan PD, Gore M, et al. Adjuvant bevacizumab in patients with melanoma at high risk of recurrence (AVAST-M): preplanned interim results from a multicentre, open-label, randomised controlled phase 3 study. The Lancet Oncology. 2014;15:620–30. doi: 10.1016/S1470-2045(14)70110-X. [DOI] [PubMed] [Google Scholar]
- 49.Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer immunology research. 2014;2:632–42. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KB, Sosman JA, Fruehauf JP, Linette GP, Markovic SN, McDermott DF, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.