Abstract
Background
Chronic pain and tobacco smoking are both highly prevalent and comorbid conditions, and chronic pain may pose a barrier to smoking cessation.
Purpose
The objective of this study was to test associations between chronic pain status and several smoking-related factors that have previously been shown to predict cessation outcomes.
Method
Daily smokers (N = 205) were recruited from the general population to complete an online survey of pain and tobacco smoking.
Results
Results indicated that smokers with chronic pain (vs. no chronic pain) consumed more cigarettes per day, scored higher on an established measure of tobacco dependence, reported having less confidence in their ability to quit, and endorsed expectations for experiencing greater difficulty and more severe nicotine withdrawal during future cessation attempts. Mediation analyses further indicated that the inverse association between chronic pain and abstinence self-efficacy was indirectly influenced by past cessation failures.
Conclusions
These findings suggest that individuals with chronic pain may constitute an important subgroup of tobacco smokers who tend to experience lower confidence and greater difficulty when attempting to quit. Future research would benefit from replicating these findings among older and more diverse samples of heavier tobacco smokers, and extending this work to the study of prospective relations between chronic pain status and cessation-relevant processes/outcomes over the course of a quit attempt.
Keywords: tobacco, pain, chronic pain, expectancies, withdrawal, cessation
Tobacco addiction and chronic pain are both highly prevalent and comorbid disorders that together account for greater than $300 billion in annual heath care expenses and lost productivity (1, 2). The prevalence of tobacco smoking among persons with chronic pain (~42-68%) may be greater than twice the rate (19%) observed in the general population (3-5), and a recently proposed reciprocal model of pain and smoking suggests that these conditions may interact in the manner of a positive feedback loop, resulting in greater pain and the maintenance of tobacco dependence (6). Consistent with this perspective, tobacco smoking has been identified as a unique risk factor in the onset and progression of chronic pain (7, 8), situational pain has been shown to act as a potent motivator of smoking urge and behavior (9, 10), and chronic pain patients have reliably endorsed smoking as a means of pain-coping (11, 12).
A nationally-representative survey of greater than 9,000 U.S. adults revealed that the odds of meeting diagnostic criteria for current nicotine dependence were two-fold greater among smokers with chronic pain (relative to those without chronic pain), even after accounting for a range of sociodemographic and psychiatric factors (5). There is also evidence of covariation between pain intensity and cigarette consumption among smokers in the general population and treatment seeking pain patients (13-15), smoking dependence motives have been positively associated with maladaptive pain-related cognitions (16, 17), and there is initial data to suggest that smokers with recurring pain may be less successful when attempting to quit (18). Taken together, these findings suggest that smokers in pain may be at risk for greater nicotine dependence, increased cigarette consumption, and failed cessation attempts. Additional research is needed to determine whether smoking dependence motives vary across smokers with and without chronic pain, and to identify factors that may influence cessation outcomes among smokers in pain.
In addition to greater nicotine dependence (19) and stronger smoking dependence motives (20, 21), several smoking-related factors consistently predict poorer cessation outcomes including lower abstinence self-efficacy (19, 22), greater withdrawal severity (23), and stronger expectancies for negative outcomes associated with quitting (22). Initial evidence suggests that smokers with recent pain (compared to no recent pain) report lower abstinence self-efficacy (24), and qualitative interviews with chronic pain patients suggest poor confidence in their ability to cope with the additional stress of quitting (12). However, there is a significant lack of empirical knowledge regarding how persons with chronic pain compare to their pain-free counterparts with regard to abstinence self-efficacy, and we are not aware of any research that has examined variations in withdrawal severity and smoking-related outcome expectancies as a function of chronic pain status. Finally, we were interested in examining whether past cessation/withdrawal experiences may help to clarify the nature of anticipated relations between chronic pain and expectancies for smoking cessation. There is long-standing evidence that self-efficacy for smoking cessation can be undermined by past quit failures (25, 26), and there is preliminary data to suggest that past difficulty quitting may mediate associations between current pain status and confidence to remain abstinent during future quit attempts (24).
The main goals of this study were to extend previous work by testing associations between chronic pain status and indices of nicotine/tobacco dependence, recent withdrawal experiences, and several cognitive constructs shown to predict cessation outcomes (i.e., abstinence self-efficacy, expected difficulty quitting, and expected withdrawal severity when quitting). Given evidence of covariation between pain and smoking intensity, we hypothesized that smokers with chronic pain (vs. no chronic pain) would: (1) score higher on measures of nicotine and tobacco dependence and (2) report more severe nicotine withdrawal during their most recent quit attempt. Consistent with previous data obtained among smokers who endorsed recent pain, we also hypothesized that chronic pain status would be associated with: (3) lower abstinence self-efficacy; and (4) expectancies for greater difficulty and withdrawal severity during future quit attempts. We also sought to test whether withdrawal severity during past cessation experiences would mediate hypothesized associations between chronic pain status and current cessation-related self-efficacy/outcome expectancies.
METHOD
Participants and Survey Procedures
Participants completed an online survey that was developed for the purpose of examining relations between pain and tobacco smoking. Survey measures were administered through socialsci.com, a web-based service that connects researchers with adult residents of the United States who agree to participate in IRB-approved research studies in exchange for small points-based rewards. Respondents who expressed an interest in completing the survey were screened for lifetime tobacco smoking and current pain. Participants were required to be at least 18 years of age, United States residents, able to read and write English, and willing to provide electronic informed consent. Of 706 survey respondents, 238 reported never smoking, 209 reported former smoking, and 54 reported currently smoking less than one cigarette per day. The current analyses were restricted to the remaining subsample of respondents who reported currently smoking one or more cigarettes per day (N = 205).
Measures
Chronic pain
Chronic pain status was classified using a single yes/no item adapted from the Kansas Behavioral Risk Factor Surveillance System (27) and the National Health Interview Survey (28), which asked: “Do you currently suffer from any type of chronic pain, that is, pain that occurs constantly or flares up frequently? Do not report aches and pain that are fleeting or minor.” The widely-used Graded Chronic Pain Scale (GCPS) (29) was subsequently administered to characterize chronic pain status and index the severity of pain intensity and degree of functional interference. The GCPS yields a measure of characteristic pain intensity, with a cut-off score of 15 out of 30 representing high pain intensity. The GCPS also provides a categorical measure of chronic pain grade, which reflects both pain intensity and disability severity (Grade I = low intensity–low disability; Grade II = high intensity–low disability; Grade III = high disability–moderately limiting; and Grade IV = high disability-severely limiting). We did not exclude participants based on their responses to the GCPS.
Nicotine/Tobacco Dependence
Two measures were administered to assess nicotine/tobacco dependence, including the Heaviness of Smoking Index (HSI) (30), and the Wisconsin Inventory of Smoking Dependence Motives (WISDM) (31). The HSI consists of two items from the Fagerstrom Test for Nicotine Dependence (FTND) that measure number of cigarettes smoked per day and time to first cigarette of the day (30). Both items are rated on a 0-3 scale, and the HSI yields a total score from 0-6 that has been shown to predict smoking cessation outcomes (32-34). The WISDM is a reliable and valid 68-item multidimensional measure that assesses motives for tobacco use and dependence across 13 domains. The WISDM yields a total dependence score and two composite scores. The Primary Dependence Motives (PDM) composite is the average score across four subscales that represent central features of tobacco dependence (e.g., craving, tolerance, automaticity, loss of control), and the Secondary Dependence Motives (SDM) composite is the average score across the remaining nine subscales that index situational motives for tobacco use. The WISDM has been used to examine dependence motives among lighter smokers (35), and demonstrated excellent internal consistency in the current sample (Chronbach's α = .99).
Abstinence Self-Efficacy
Abstinence self-efficacy was assessed by asking participants to rate their confidence in remaining abstinent from smoking for 1 week, 1 month, and 1 year, on a scale that ranged from 0 (no confidence) to 4 (extremely confident). Consistent with previous research, responses were averaged to create a composite score of abstinence self-efficacy (24, 36).
Expected Difficulty of Smoking Abstinence
A single item from the Thoughts About Abstinence Scale (TAA) was used to evaluate the expected difficulty of quitting smoking (i.e., “Circle a number that best describes how difficult you think it would be for you to quit and remain smoke free?”). Responses ranged from 0 (no difficulty) to 9 (greatest difficulty) (37).
Expectancies for Withdrawal during Smoking Cessation
The withdrawal subscale of the Smoking Abstinence Questionnaire (SAQ) was administered to assess expectancies for withdrawal severity during a future quit attempt (22). Responses for the 7-item SAQ withdrawal subscale (e.g., “I would really crave a cigarette”) ranged from 0 (not likely at all) to 6 (extremely likely), and were summed to generate the total score (Cronbach's alpha = .93).
Withdrawal Experienced During Most Recent Quit Attempt
The Minnesota Nicotine Withdrawal Scale (MNWS) was used to assess the severity of eight prototypical nicotine withdrawal symptoms that participants experienced “during the last time” they tried to quit smoking (38). Individual items were rated from 0 (none) to 6 (severe), and responses were summed to generate the total score (Cronbach's alpha = .93).
Depression and Anxiety Symptoms
Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale (CES-D) which consists of 20-items that are rated on a 4-point Likert scale and summed to generate a total score ranging from 0 to 60 (Cronbach's alpha =.81) (39). Anxiety symptoms were assessed using the Generalized Anxiety Disorder-7 scale (GAD-7) which consists of 7-items (e.g., “Feeling nervous, anxious, or on edge,” “Worrying too much about different things”) that are rated on a 4-point Likert scale and summed to generate a total score ranging from 0 to 21 (Cronbach's alpha = .91) (40).
Data Analytic Approach
First, we calculated all sample characteristics (see Table 1). We then examined bivariate associations between all study variables (see Table 2), and those shown to be correlated with our primary outcomes (age, gender, anxiety, depression) were retained as covariates in subsequent analyses. ANCOVA (controlling for age, gender, anxiety, and depression) was used to test differences in nicotine/tobacco dependence as a function of chronic pain status. Models that tested associations between chronic pains status and expectancies for smoking cessation, abstinence self-efficacy, and past withdrawal severity further controlled for HSI scores to covary for individual differences in physical dependence on nicotine. Analyses that included past withdrawal severity were limited to individuals who reported a past quit attempt (n = 142). We utilized three separate conditional process models to test whether past withdrawal severity mediated hypothesized associations between chronic pain status and abstinence self-efficacy, expected difficulty quitting, and expected withdrawal severity. We conducted mediation analyses using model number 4 of the PROCESS macro for SPSS, which generates bias-corrected 99% confidence intervals and path coefficients via an established bootstrapping approach (41, 42). Bootstrapping was set at 10,000 re-samples and all models were tested in reverse to support the ordering of variables and statistical specificity of the theoretically driven models.
Table 1.
Demographic, Smoking , and Psychological Characteristics by Chronic Pain Status (N = 205)
| Chronic Pain (n = 81) | No Chronic Pain (n = 124) | |
|---|---|---|
| N (%) | N (%) | |
| Gender** | ||
| Female | 49 (55.7) | 39 (44.3) |
| Male | 32 (27.4) | 85 (72.6) |
| Ethnicity | ||
| Hispanic/Latino | 9 (11.1) | 9 (7.3) |
| Race | ||
| White | 68 (84.0) | 105 (84.7) |
| Black/African American | 5 (6.2) | 4 (3.2) |
| Asian | 2 (2.5) | 9 (7.3) |
| American Indian/Alaskan Native | 3 (3.7) | 0 (0.0) |
| Other | 3 (3.7) | 6 (4.8) |
| Education | ||
| Did not graduate high school | 1 (1.2) | 0 (0.0) |
| High school or GED | 7 (8.6) | 5 (4.0) |
| Some college completed | 29 (35.8) | 55 (44.4) |
| Technical school or Associates | 9 (11.1) | 9 (7.3) |
| Four-year college | 21 (25.9) | 35 (28.2) |
| Some school beyond college | 12 (14.8) | 15 (12.1) |
| Professional degree | 2 (2.5) | 5 (4.0) |
| Income | ||
| < 10,000/year* | 6 (7.4) | 23 (18.5) |
| 10,000-25,000/year | 22 (27.2) | 30 (24.2) |
| 25,000-50,000/year | 26 (32.1) | 25 (20.2) |
| 50,000-75,000/year | 9 (11.1) | 25 (20.2) |
| 75,000-100,000/year | 13 (16.0) | 11 (8.9) |
| > 100,000/year | 5 (6.2) | 10 (8.1) |
| M (SD) | M (SD) | |
|---|---|---|
| Age* | 29.06 (8.47) | 26.54 (6.90) |
| CES-D1** | 29.1 (11.5) | 22.0 (12.3) |
| GAD-72** | 10.6 (5.6) | 6.9 (5.2) |
| Cigarettes Per Day (CPD) | 11.20 (12.37) | 7.23 (7.03) |
| WISDM3** | 42.54 (17.73) | 30.63 (17.32) |
| MNWS4 | 26.5 (13.7) | 18.4 (12.9) |
| TAA-Difficulty5 | 6.6 (2.6) | 4.7 (3.0) |
| SAQ-Withdrawal6 | 3.7 (1.0) | 3.0 (1.3) |
| Quit Self-Efficacy | 1.5 (1.2) | 2.2 (1.2) |
Note.
Center for Epidemiological Studies - Depression Scale
Generalized Anxiety Disorder 7-item
Wisconsin Inventory of Smoking Dependence Motives
Minnesota Nicotine Withdrawal Scale
Thoughts About Abstinence Scale
Smoking Abstinence Questionnaire. .
p < .05
p < .001.
Table 2.
Bivariate associations between chronic pain status and other study variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | M(SD) | Range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Age | – | .06 | .07 | .16* | .20* | .04 | −.01 | .30** | .24** | .20** | .20** | −.14 | .13 | 27.5 (7.6) | 18-65 |
| 2 Gender | – | −.03 | .29** | .22* | .17* | .17* | .01 | .07 | .01 | .16* | −.08 | .06 | – | – | |
| 3 Education | – | −.02 | −.04 | −.07 | −.12 | −.06 | −.02 | .01 | −.09 | .07 | −.08 | – | – | ||
| 4 Chronic Pain Status | – | .55* | .32** | .28** | .19** | .29** | .32** | .29** | −.27** | .32** | – | – | |||
| 5 GCPS1-Pain Grade | – | .50** | .51** | .17 | .34** | .29** | .19* | −.24* | .41** | 2.2 (1.2) | 1-4 | ||||
| 6 GAD-72 | – | .75** | .17* | .28** | .19** | .24** | −.16* | .34** | 8.3 (5.6) | 0-21 | |||||
| 7 CES-D3 | – | .18* | .30** | .10 | .15* | −.14* | .34** | 24.8 (12.5) | 0-52 | ||||||
| 8 HSI4 | – | .46** | .28** | .46** | −.47** | .57** | 1.3 (1.4) | 0-5 | |||||||
| 9 MNWS5 | – | .51** | .66** | −.47** | .69** | 21.6 (13.8) | 0-48 | ||||||||
| 10 TAA-Difficulty6 | – | .68** | −.61** | .52** | 5.4 (3.0) | 0-9 | |||||||||
| 11 SAQ-Withdrawal7 | – | −.63** | .70** | 3.3 (1.3) | 0-5 | ||||||||||
| 12 Quit Self-Efficacy | – | −.57** | 1.9 (1.3) | 0-4 | |||||||||||
| 13 WISDM8 | – | 35.3 (18.4) | 0-74 |
Note. Gender: 0 = Male, 1 = Female
Graded Chronic Pain Scale (N = 114)
Generalized Anxiety Disorder 7-item
Center for Epidemiological Studies - Depression Scale
Heaviness of Smoking Index
Minnesota Nicotine Withdrawal Scale
Thoughts About Abstinence Scale
Smoking Abstinence Questionnaire
Wisconsin Inventory of Smoking Dependence Motives.
p < .05
p < .01.
RESULTS
Sample Characteristics
The participants were 205 current daily tobacco smokers (43% female; Mage = 27.5, SD = 7.6), who reported smoking approximately 9 cigarettes per day (M = 8.80; SD = 9.7) for an average of 8 years (M = 8.01; SD = 7.9). The sample was predominantly white (84%) and fairly well educated (44% completed four years of college or more), with a mean HSI score of 1.3 (SD = 1.40), indicating a relatively low level of nicotine dependence (43). Approximately 40% of the sample endorsed current chronic pain, with a fairly even distribution across GCPS chronic pain grades (22.2% Grade I; 23.5% Grade II; 25.9% Grade III; and 28.4% Grade IV). The mean GCPS characteristic pain intensity score was 17.89 (SD = 5.08), indicating high pain intensity. Complete sample characteristics are presented in Table 1.
Chronic Pain Status and Nicotine/Tobacco Dependence
Chronic pain status was positively associated with multiple indices of nicotine/tobacco dependence, after controlling for age, gender, anxiety, and depression. First, smokers with chronic pain reported greater levels of smoking dependence motives, as indexed by the WISDM total score (M = 40.47, SE = 1.98), than did smokers without chronic pain (M = 31.98, SE = 1.57), F(1, 199) = 10.29; p < .01, ηp2 =.049. WISDM composite analyses further revealed that smokers with chronic pain (vs. no chronic pain) scored higher on both Primary (M = 3.03, SE = 0.18 vs. M = 2.21, SE = 0.14), F(1, 199) = 12.39, p < .01, ηp2 = .059 and Secondary Dependence Motives (M = 3.15, SE = 0.15 vs. M = 2.47, SE = 0.12), F(1, 199), p < .01, ηp2 = .039. Second, although HSI scores did not differ as a function of chronic pain status (p = .13), smokers with chronic pain (vs. no chronic pain) did report smoking a greater number of cigarettes per day (M = 10.73, SE = 1.11 vs. M = 7.55, SE = 0.88), F(1, 199) = 4.58; p < .05, ηp2 = .022.
Chronic Pain Status and Expectancies for Smoking Cessation
Expectancies for smoking cessation varied as a function of chronic pain status, even after accounting for age, gender, anxiety, depression, and nicotine dependence scores. Specifically, chronic pain status was positively associated with greater expected difficulty quitting, F(1, 198) = 15.06; p < .001, ηp2 = .071, and expectancies for greater withdrawal severity, F(1, 198) = 4.63; p < .05, ηp2 =.023, such that smokers who endorsed chronic pain also endorsed greater levels of expected difficulty quitting (M = 6.47, SE = 0.33) and expected withdrawal severity (M = 3.48, SE = 0.13), compared to smokers without chronic pain (M = 4.77, SE = 0.26; M = 3.10, SE = 0.10, respectively). Smokers with chronic pain also reported lower self-efficacy for smoking abstinence (M = 1.64, SE = 0.13), relative to smokers without chronic pain (M = 2.10, SE = 0.10), F(1, 198) = 6.94; p = .009, ηp2 =.034.
Chronic Pain Status and Past Experience of Nicotine Withdrawal Severity
Among participants who endorsed a past quit attempt, chronic pain status was positively associated with past nicotine withdrawal severity, F(1,135) = 10.39; p = .002, ηp2 = .071, after controlling for age, gender, anxiety, depression, and nicotine dependence. Specifically, smokers with chronic pain reported experiencing more severe nicotine withdrawal during their most recent quit attempt (M = 27.96, SE = 1.477), relative to smokers without chronic pain (M = 21.61, SE = 1.118). MNWS item-level analyses further revealed that smokers with chronic pain endorsed greater levels of anger, irritability and frustration (p < .01), anxiety and nervousness (p < .05), difficulty concentrating (p < .05) impatience and restlessness (p < .05), hunger (p < .05), and awakening at night (p < .01).
Past Nicotine Withdrawal as a Mediator of Expectancies for Smoking Cessation
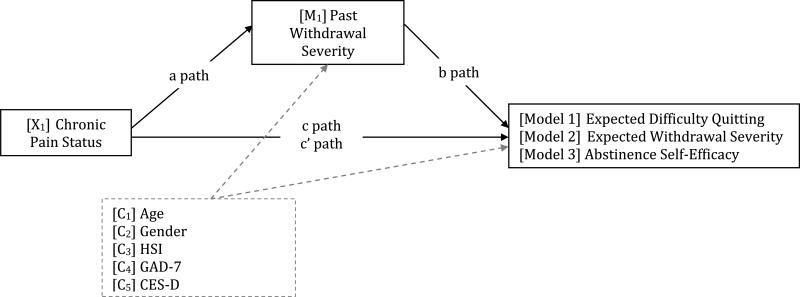
As depicted conceptually in Figure 1 and detailed in Table 3, bootstrapping mediation analyses revealed that severity of nicotine withdrawal experienced during the most recent quit attempt mediated observed associations between chronic pain status and expected difficulty quitting [Model 1: b = .706; SE = .262; 99% CI (.143, 1.518)], expected severity of nicotine withdrawal during future quit attempts [Model 2: b = .401 (SE = .145); 99% CI (.070, .824)], and self-efficacy for smoking cessation [Model 3: b = −.281; SE = .109; 99% CI (−.640, −.055)]. In sum, chronic pain status was associated with increased withdrawal severity, which in turn was associated with greater expected difficulty quitting (Model 1), greater expected withdrawal severity (Model 2), and reduced self-efficacy for cessation (Model 3). Models were no longer significant when run in reverse (all 99% confidence intervals crossed zero) thus providing additional support for our theoretically driven ordering of the variables.
Figure 1.
Conceptual model of indirect associations between chronic pain status and expectancies for smoking cessation.
Table 3.
Path Coefficients and Bootstrapped 99% Confidence Intervals for Three Mediation Models.
| Path | Path Description | b | SE | T | p | CI (l) | CI (u) |
|---|---|---|---|---|---|---|---|
| Model 1 (Y1): Nicotine Withdrawal as a Mediator of Associations between Chronic Pain Status and Expected Difficulty Quitting | |||||||
| a | CP → MNWS | 6.354 | 1.971 | 3.223 | .002** | 1.203 | 11.506 |
| b | MNWS → TAA-D | .111 | .020 | 5.675 | <.001** | .060 | .162 |
| c | CP → TAA-D | 1.654 | .498 | 2.322 | .001** | .353 | 2.955 |
| c | CP → TAA-D | .948 | .466 | 2.035 | .044* | −.269 | 2.165 |
|
Model 2 (Y2) Nicotine Withdrawal as a Mediator of Associations between Chronic Pain Status and Expected Withdrawal Severity | |||||||
| a | CP → MNWS | 6.354 | 1.971 | 3.223 | .002** | 1.203 | 11.506 |
| b | MNWS → SAQ-W | .063 | .007 | 8.582 | <.001** | .044 | .082 |
| c | CP → SAQ-W | .515 | .209 | 2.467 | .015* | −.030 | 1.061 |
| c’ | CP → SAQ-W | .114 | .175 | .654 | .514 | −.342 | .571 |
|
Model (Y3) Nicotine Withdrawal as a Mediator of Associations between Chronic Pain Status and Abstinence Self-Efficacy | |||||||
| a | CP → MNWS | 6.354 | 1.971 | 3.223 | .002** | 1.203 | 11.506 |
| b | MNWS → AS-E | −.044 | .008 | −5.200 | <.001** | −.066 | −.022 |
| c | CP → AS-E | −.318 | .213 | −1.491 | .138 | −.874 | .239 |
| c’ | CP → AS-E | −.036 | .202 | −.179 | .858 | −.565 | −.055 |
Note. In all models, path a represents the association between the independent variable and the mediator, path b represents the associations between the mediator and the dependent variable, controlling for the independent variable, path c represents the total effect of the model (direct effect + indirect effect), and path c’ represents the direct effect of the independent variable on the dependent variable. Model 1 tested nicotine withdrawal severity (MNWS) as a statistical mediator of the association between chronic pain status (CP) and expected difficulty quitting (TAA-D). Model 2 tested nicotine withdrawal severity (MNWS) as a statistical mediator of the association between chronic pain status (CP) and expected tobacco withdrawal severity (SAQ-W). Model 3 tested nicotine withdrawal severity as a statistical mediator of the association between chronic pain status (CP) and abstinence self-efficacy (ASE). P = chronic pain status; MNWS = Minnesota Nicotine Withdrawal Scale; TAA-D = Thoughts About Abstinence Scale – expected difficulty with cessation; SAQ-W = Smoking Abstinence Questionnaire – Withdrawal Subscale; AS-E = Abstinence Self-Efficacy
p < .05
p < .01.
DISCUSSION
This is the first study to test associations between chronic pain status and several constructs that have been shown to predict the initiation and maintenance of smoking cessation. As hypothesized, smokers with chronic pain scored higher on an established measure of tobacco dependence (including central dependence features and situational motives for smoking), endorsed expectations for greater difficulty quitting and more severe nicotine withdrawal, and reported having less confidence in their ability to abstain from smoking. Importantly, these associations remained significant even after accounting for the influence of age, gender, and the presence of comorbid anxiety/depression. Given that our sample was comprised of relatively lighter smokers, it is perhaps not surprising that chronic pain status was not associated with HSI dependence scores. However, post-hoc tests did reveal that smokers with chronic pain reported smoking more cigarettes per day than smokers with no chronic pain.
Also as hypothesized, mediation analyses indicated that smokers with chronic pain endorsed having experienced more severe nicotine withdrawal during previous quit attempts, which in turn was associated with lower abstinence self-efficacy and expectations for greater difficulty and more severe withdrawal during future quit attempts. These results are consistent with long-standing evidence that self-efficacy for smoking abstinence may be diminished by past quit failures (25, 26), and more recent evidence that associations between pain and abstinence self-efficacy may be indirectly influenced by previous cessation experiences (24). Collectively, these findings suggest that smokers with chronic pain may experience more severe nicotine withdrawal when attempting to quit, which in turn may increase expectations for deleterious cessation outcomes and erode self-efficacy for future quit attempts.
The current results contribute to an emerging empirical literature that suggests persons with chronic pain constitute an important subgroup of smokers who are “at-risk” for greater difficulty quitting (6). Consistent with evidence that situational pain can be a potent motivator of smoking (9, 10), chronic pain patients readily endorse using tobacco to cope with pain (11, 12), and it is possible that smokers with comorbid pain disorders may experience greater difficulty quitting in the absence of more adaptive approaches to pain management (44). There is also reason to suspect that recurring pain may deplete resources needed to cope with the early stages of nicotine withdrawal (45), and that the act of abstaining from smoking may increase pain sensitivity (46, 47), thereby placing smokers with chronic pain at greater risk for relapse. Taken together, the current findings are consistent with a reciprocal model of pain and smoking (6), which posits that pain may contribute to the maintenance of tobacco dependence, perhaps via greater nicotine dependence or increased risk of relapse.
Strengths of the current study include the recruitment of daily smokers with and without chronic pain (such that the sample was not limited to treatment-seeking pain patients), and the utilization of valid and reliable measures of chronic pain, nicotine/tobacco dependence, and cessation-related outcome expectancies. In addition, all analyses statistically controlled for the influence of highly relevant third variables. Despite these strengths, several limitations warrant noting.
First, the use of cross-sectional data precludes causal interpretations and any conclusions regarding temporal precedence in our mediation models. Prospective studies are needed to determine the extent to which chronic pain may be associated with decreased success in quitting, and to identify pain-related mechanisms (e.g., pain-related anxiety) that may hamper cessation efforts during early abstinence and over the course of a quit attempt (16, 17). Second, these findings may not generalize to all tobacco smokers with chronic pain, those who may be actively trying to quit smoking, or treatment-seeking pain patients. For example, the current sample was predominantly Caucasian, well-educated (44% completed four years of college or more), and relatively young (average age under 30 years). Our respondents also endorsed relatively low levels of nicotine dependence (smoking an average of 9 cigarettes per day), and the web-based survey required access to the Internet and familiarity with points-based reward systems. Thus, the extent to which chronic pain may be associated with nicotine dependence and expectations for smoking cessation among older and more diverse samples of heavier tobacco smokers remains unclear. Third, chronic pain status was assessed with a single item that did not query pain duration. Although it is possible that associations between pain and tobacco smoking may vary as a function of chronic pain location, these data did not allow for such granular comparisons. Finally, although participants rated the severity of withdrawal experienced during their most recent quit attempt, duration since the most recent quit attempt was not assessed, thus precluding analysis of potential recall bias. Future research would benefit from examining whether expectancies for smoking cessation vary as a function of chronic pain condition/location, pain treatment, heaviness of smoking, or duration of time since the most recent quit attempt. Future research should also seek to biochemically verify smoking status and corroborate chronic pain status via multi-item assessment and medical record chart review.
In conclusion, the present study represents an initial, yet important, step toward a better understanding of how chronic pain may be related to smoking cessation expectations and outcomes. Taken together, these findings suggest that smokers with chronic pain experience more severe nicotine withdrawal when attempting to quit, which in turn, may reduce their confidence to quit and remain smoke free. Considering that nicotine has been shown to reduce pain sensitivity in the short-term (48), and given evidence that smokers with chronic pain are amenable to using pharmacotherapy for smoking cessation (49), future research should examine whether smokers with chronic pain who are attempting to quit would benefit from high-dose or combination nicotine replacement therapy (50, 51). Future work is also needed to determine whether smokers with chronic pain would benefit from tailored interventions that explicitly address the role of pain in the context of smoking cessation and aim to bolster abstinence self-efficacy.
Acknowledgements
This research was supported by NIH Grant Nos. R21DA034285 and R21DA038204 awarded to Joseph W. Ditre, NIDA Grant No. F31DA039628 awarded to Emily L. Zale, and NIH Grant No. 2K05AA16928 awarded to Stephen A. Maisto.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.DHHS . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [Google Scholar]
- 2.Gaskin DJ, Richard P. The economic costs of pain in the United States. J. Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Jamison RN, Stetson BA, Parris WC. The relationship between cigarette smoking and chronic low back pain. Addict. Behav. 1991;16:103–110. doi: 10.1016/0306-4603(91)90002-y. [DOI] [PubMed] [Google Scholar]
- 4.Michna E, Ross EL, Hynes WL, et al. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J. Pain Symptom Manage. 2004;28:250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine Tob Res. 2009;11:1407–1414. doi: 10.1093/ntr/ntp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychol. Bull. 2011;137:1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. Am. J. Med. 2010;123(87):e87–35. doi: 10.1016/j.amjmed.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama D, Nishimura K, Tamaki K, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann. Rheum. Dis. 2010;69:70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 9.Ditre JW, Brandon TH. Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. J. Abnorm. Psychol. 2008;117:467–472. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. J. Abnorm. Psychol. 2010;119:524–533. doi: 10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson AL, Gritzner S, Resnick MP, et al. Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. J. Pain. 2012 doi: 10.1016/j.jpain.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooten WM, Vickers KS, Shi Y, et al. Smoking cessation and chronic pain: patient and pain medicine physician attitudes. Pain Pract. 2011;11:552–563. doi: 10.1111/j.1533-2500.2011.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne TJ, Stetson B, Stevens VM, et al. The impact of cigarette smoking on headache activity in headache patients. Headache. 1991;31:329–332. doi: 10.1111/j.1526-4610.1991.hed3105329.x. [DOI] [PubMed] [Google Scholar]
- 14.Hahn EJ, Rayens MK, Kirsh KL, Passik SD. Brief report: Pain and readiness to quit smoking cigarettes. Nicotine Tob Res. 2006;8:473–480. doi: 10.1080/14622200600670355. [DOI] [PubMed] [Google Scholar]
- 15.Weingarten TN, Moeschler SM, Ptaszynski AE, et al. An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician. 2008;11:643–653. [PubMed] [Google Scholar]
- 16.Ditre JW, Langdon KJ, Kosiba JD, Zale EL, Zvolensky MJ. Relations between pain-related anxiety, tobacco dependence, and barriers to quitting among a community-based sample of daily smokers. Addict. Behav. 2015;42C:130–135. doi: 10.1016/j.addbeh.2014.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ditre JW, Zale EL, Kosiba JD, Zvolensky MJ. A pilot study of pain-related anxiety and smoking-dependence motives among persons with chronic pain. Exp Clinical Psychopharmacol. 2013;21:443–449. doi: 10.1037/a0034174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldie KE, McGee R, Reeder AI, Poulton R. Associations between frequent headaches, persistent smoking, and attempts to quit. Headache. 2008;48:545–552. doi: 10.1111/j.1526-4610.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 19.Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addiction. 2011;106:2110–2121. doi: 10.1111/j.1360-0443.2011.03565.x. [DOI] [PubMed] [Google Scholar]
- 20.Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacio GA, Guzman IY, Shapiro JR, Ray LA. Differences in quit attempts between non-Hispanic Black and White daily smokers: the role of smoking motives. Addict. Behav. 2014;39:1769–1772. doi: 10.1016/j.addbeh.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendricks PS, Wood SB, Baker MR, Delucchi KL, Hall SM. The Smoking Abstinence Questionnaire: measurement of smokers' abstinence-related expectancies. Addiction. 2011;106:716–728. doi: 10.1111/j.1360-0443.2010.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piper ME, Schlam TR, Cook JW, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berl.) 2011;216:569–578. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zale EL, Ditre JW, Dorfman ML, Heckman BW, Brandon TH. Smokers in pain report lower confidence and greater difficulty quitting. Nicotine Tob Res. 2014;16:1272–1276. doi: 10.1093/ntr/ntu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchner TR, Shiffman S, Wileyto EP. Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. J. Abnorm. Psychol. 2012;121:187–197. doi: 10.1037/a0024451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey KB, Carey MP. Changes in self-efficacy resulting from unaided attempts to quit smoking. Psychol of Addict Behav. 1993;7:219–224. [Google Scholar]
- 27.Toblin RL, Mack KA, Perveen G, Paulozzi LJ. A population-based survey of chronic pain and its treatment with prescription drugs. Pain. 2011;152:1249–1255. doi: 10.1016/j.pain.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control H, MD N. C. f. H. Statistics, editor. National Health Interview Survey Questionnaire. 2011 [Google Scholar]
- 29.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 31.Piper ME, Bolt DM, Kim SY, et al. Refining the tobacco dependence phenotype using the Wisconsin Inventory of Smoking Dependence Motives. J. Abnorm. Psychol. 2008;117:747–761. doi: 10.1037/a0013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borland R, Yong HH, O'Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12(Suppl):S45–50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fagerstrom K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerstrom Test for Nicotine Dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14:1467–1473. doi: 10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- 34.Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 35.Scheuermann TS, Nollen NL, Cox LS, et al. Smoking dependence across the levels of cigarette smoking in a multiethnic sample. Addict. Behav. 2015;43:1–6. doi: 10.1016/j.addbeh.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandura A, Cioffi D, Taylor CB, Brouillard ME. Perceived self-efficacy in coping with cognitive stressors and opioid activation. J. Pers. Soc. Psychol. 1988;55:479–488. doi: 10.1037//0022-3514.55.3.479. [DOI] [PubMed] [Google Scholar]
- 37.Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- 38.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of general psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D Scale. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- 40.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 41.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; New York, NY: 2013. [Google Scholar]
- 42.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 43.Chaiton MO, Cohen JE, McDonald PW, Bondy SJ. The Heaviness of Smoking Index as a predictor of smoking cessation in Canada. Addict. Behav. 2007;32:1031–1042. doi: 10.1016/j.addbeh.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: a test of social learning formulations. J. Abnorm. Psychol. 1988;97:218–230. doi: 10.1037//0021-843x.97.2.218. [DOI] [PubMed] [Google Scholar]
- 45.Heckman BW, Ditre JW, Brandon TH. The restorative effects of smoking upon self-control resources: a negative reinforcement pathway. J. Abnorm. Psychol. 2012;121:244–249. doi: 10.1037/a0023032. [DOI] [PubMed] [Google Scholar]
- 46.Cosgrove KP, Esterlis I, McKee S, et al. Beta2* nicotinic acetylcholine receptors modulate pain sensitivity in acutely abstinent tobacco smokers. Nicotine Tob Res. 2010;12:535–539. doi: 10.1093/ntr/ntq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perkins KA, Grobe JE, Stiller RL, et al. Effects of Nicotine on Thermal Pain Detection in Humans. Exp. Clin. Psychopharmacol. 1994;2:95–106. [Google Scholar]
- 48.Ditre JW, Heckman BW, Zale EL, Kosiba JD, Maisto SA. Acute Analgesic Effects of Nicotine and Tobacco in Humans: A Meta-Analysis. doi: 10.1097/j.pain.0000000000000572. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zale EL, Ditre JW. Associations Between Chronic Pain Status, Attempts to Quit Smoking, and Use of Pharmacotherapy for Smoking Cessation. Psychol. Addict. Behav. 2013 doi: 10.1037/a0032515. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatsukami D, Mooney M, Murphy S, et al. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacol. Biochem. Behav. 2007;86:132–139. doi: 10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mills EJ, Wu P, Lockhart I, et al. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann. Med. 2012;44:588–597. doi: 10.3109/07853890.2012.705016. [DOI] [PubMed] [Google Scholar]