Abstract
Background
Stereotactic radiosurgery (SRS) and stereotactic radiotherapy (SRT) are highly conformal, high‐dose radiation treatment techniques used to treat people and dogs with brain tumors.
Objectives
To evaluate the response to SRS‐ and SRT‐treated tumors using volume and perfusion variables and to measure the survival times of affected dogs.
Animals
Prospective study of 34 dogs with evidence of brain tumors undergoing stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT).
Methods
Computed tomography and MRI imaging were used to calculate tumor volume and perfusion at baseline, and at 3 months and 6 months after treatment. Survival analysis was performed to evaluate treatment efficacy.
Results
Mean tumor volume significantly declined from baseline to the first recheck by −0.826 cm3 (95% CI: −1.165, −0.487) (P < .001); this reduction was maintained at the second recheck. Blood flow and blood volume declined significantly in the tumor after treatment. Median survival was 324 days (95% CI: 292.8, 419.4), and 4 dogs survived longer than 650 days. Neither actual tumor volume (hazard ratio = 1.21, P = .19) nor the change in tumor volume from the baseline (hazard ratio = 1.38, P = .12) significantly affected the hazard of death because of the tumor.
Conclusions and Clinical Importance
Stereotactic radiosurgery and SRT are effective treatments for reducing tumor volume, blood flow, and blood volume. Treated dogs surviving for more than 1 year are more likely to die from other causes than of their primary brain tumor. SRS and SRT should be considered for noninvasive treatment of intracranial brain tumors.
Keywords: Dog, Pituitary, Radiation, Blood flow
Abbreviations
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- CT
computed tomography
- MRI
magnetic resonance imaging
- PS
permeability surface
- SRS
stereotactic radiosurgery
- SRT
sereotactic radiotherapy
Stereotactic radiosurgery (SRS) and stereotactic radiotherapy (SRT) are highly conformal, high‐dose radiation treatment techniques used to treat certain cancers in people and dogs with naturally occurring brain tumors.1 Stereotactic radiosurgery is often used to treat small, noninvasive low‐grade malignancies or benign lesion, and SRT is used for multiple brain metastasis in a palliative setting. These modalities are also used for malignant tumors that are nonoperable such as certain types of gliomas. In veterinary medicine a single (SRS) or short series (SRT) of treatments provides a high dose to tumor tissue while maintaining acceptable safety for surrounding normal brain tissue.
The effects of brain tumors2, 3, 4, 5, 6 include mass effect and brain compression, brain edema, brain herniation, and ventricular obstruction. Pituitary tumors can be functional, causing additional effects of hyperadrenocorticism and contributing to morbidity. These effects lead to significant neurologic dysfunction and eventually death in affected animals. Surgical removal of tumors is dependent on location, and residual tumor and morbidity are considerations along with owner's desires in choosing treatment options. Stereotactic radiosurgery and SRT offer noninvasive treatment options for primary and residual disease.
The goal of SRS and SRT is to kill tumor cells directly through radiation effects, as well as to cause damage to tumor vasculature. The expected outcome is a decrease in the size of the tumor and potentially complete regression of the mass over a period of time, which can be up to 4 years in pituitary adenomas in people.7 Perfusion variables, such as permeability surface, blood flow, and blood volume, can be decreased in treated tumor tissue and can serve as an additional outcome measure of treatment efficacy.8 Quantifying the alterations in tumor perfusion can help us to understand the effect radiotherapy has on the tissue and to predict tumor response to treatment before a change in tumor volume. Computed tomography (CT) and magnetic resonance imaging (MRI) can be used to assess these variables noninvasively.
The hypotheses of the study were that SRS and SRT (1) cause a decrease in volume of canine brain tumors, (2) cause altered perfusion variables of tumors that differ between responders and nonresponders, and (3) are effective treatments for canine brain tumors. This prospective study was designed to evaluate the response to SRS‐ and SRT‐treated tumors using volume and perfusion variables, and to measure the survival times of affected dogs. These outcome measures can be used to evaluate the effectiveness of SRS and SRT in companion animals.
Methods
Ethics, Consent, and Permissions
The study was approved by the UC Davis Veterinary Medical Teaching Hospital Clinical Trials Review Board, and informed consent was obtained from the animal owners.
Animals
Dogs with an imaging diagnosis of an intracranial tumor involving the brain, pituitary gland, and cranial nerves whose owners chose to undergo treatment with SRS or SRT were eligible for enrollment into the study. A tissue biopsy was not required for enrollment into the study because of the inaccessibility of many tumors. Patients with two or more imaging examinations, pretreatment and post‐treatment, were included for assessment of perfusion and volume measurement. Computed tomography examinations were performed on all animals. Magnetic resonance imaging examinations were performed on nonpituitary tumors and on animals with clinical indications. Recheck imaging studies were scheduled for 3 months and 6 months post treatment. Follow‐up information on survival and cause of death was obtained from the medical record or from telephone calls to owners and referring veterinarians.
Radiation Treatment
Animals were treated with either 15 Gy in 1 fraction (SRS) or 24 Gy in 3 fractions (SRT), according to tumor type. Treatment was performed using individually designed and tested radiation treatment plans. All treatment plans were created using computerized treatment planning software.1 Treatments were administered using a cone‐based linear accelerator2 with a frameless system (Brainlab, Westchester, IL).9
CT and MR Image Acquisition
Animals were anesthetized and positioned in sternal recumbency in a modified commercially available frameless stereotactic positioning device.3 A precontrast CT4 with 120 kV and 150 mA with 1‐mm collimation was performed through the entire brain. A dynamic CT scan was then performed in the central area of each tumor as identified from the precontrast CT scan, previously performed postcontrast CT or MRI, or both. The dynamic CT was performed with a bolus of nonionic iodinated contrast medium5 at a dose of 740 mg I/kg. Contrast medium was administered IV using a power injector6 at a rate of 5 mL/s. Animals with a calculated dose of less than 10 mL had saline added to the contrast medium for a total injected volume of 10 mL. Images were obtained at 120 kV, 100 mA, 2.5–10 mm collimation, every 2 seconds for 90 seconds. A postcontrast series was then acquired through the entire brain with 1‐mm collimation.
Magentic resonance imaging was subsequently performed on selected animals as outlined above with a 1.5 T system.7 Sequences included precontrast transverse T1 (TR 433, TE 10), T2 (TR 2966, TE 79), PD (TR 2966, TE 26), and FLAIR (TR 8800, TE 131) and postcontrast gadolinium8 T1 transverse and sagittal images with slice thickness of 3–4.5 mm.
Volume
Regions of interest9 were drawn around the tumor in contiguous slices to calculate tumor volumes. Contrast‐enhanced MR images were used preferentially over CT when available because of improved tissue contrast. On poorly enhancing intra‐axial tumors, the FLAIR sequence was used for volume measurements. Volume at reimaging time points was expressed as percent decrease compared to the pretreatment volume. Tumors were assessed post treatment for evidence of control of growth. Tumor volume changes at 3 and 6 months were classified as shrinkage (>20% decrease in volume), stasis (volume change of <20%), or growth. Radiologic control was defined as shrinkage or stasis.10
Perfusion variables
Perfusion maps were generated using a dedicated workstation.10 The blood flow, blood volume, and permeability surface were calculated using a deconvolution algorithm. Regions of interest were drawn around the entire tumor, a peripheral region, a central region, and normal brain (including gray and white matter at the same level as the tumor) on pretreatment and available post‐treatment studies. Regions of interest were drawn with a consistent size and location by one author (AZ). Peripheral and central regions of the tumor were chosen to include variations in tumor vascularization.
Statistical Analysis
Tumor Volume and Perfusion Changes
Two methods were used to assess whether SRS significantly reduced tumor volume and changed perfusion variables (blood flow, blood volume, and permeability). First, paired sample t‐tests were used to compare baseline to values at the first recheck and values at first recheck to the second recheck. Secondly, because of differences among dogs in the timing of the rechecks a longitudinal, linear mixed effects model was used and to evaluate changes over time. For the perfusion variables a random intercept was included for each dog, but for tumor volume a random slope variable was included for each dog because model fit improved. For all analyses, an unstructured correlation structure was used to account for the correlation between measurements on the same dog. Blood flow and volume were normalized by dividing by values from normal brain tissue.11 To normalize permeability, the normal brain tissue value was subtracted from the tumor value because some normal brain tissue values were 0 thus precluding use of a ratio. Changes in perfusion variables were analyzed separately for the whole tumor, the center of the tumor, and peripheral areas of the tumor. P values were adjusted for multiple testing by calculating the false discovery rate (FDR).
Effect of Tumor Volume and SRS on Survival
Several analyses were conducted to characterize and evaluate survival of dogs with brain tumors treated with SRT. First, survival was estimated using Kaplan–Meier curves. Cox's proportional hazard model was then used to assess the effect of tumor volume and change in volume from baseline on survival, incorporating these as time‐varying covariates. In these two analyses, death attributable to the tumor was the event of interest; all other outcomes were considered to be censored. Finally, a simple competing risk analysis was conducted and the cumulative incidence functions (CIFs) were estimated for death attributable to the tumor and death from other causes. The CIFs estimate the probabilities of death from the tumor and other causes over time after the SRS.
Effect of Perfusion Variables on Survival
Cox's proportional hazard model was used to assess the effect of the three perfusion variables (blood flow, blood volume, and permeability) on survival. Perfusion variables from each tumor region were modeled separately. These analyses were conducted using (1) the observed normalized values and (2) the change from baseline. In both cases, the perfusion variables were modeled as time‐varying predictors. P values were adjusted for multiple testing by calculating the FDR.
Perfusion Variable Differences by Survival Status
To determine if perfusion variables differed between dogs that died from their tumor and those that did not, t‐tests were used to compare mean values of normalized perfusion variables between the two groups. These comparisons were done at baseline, first recheck, and second recheck. In addition, the change from baseline to first recheck and second recheck was compared between the two groups.
Perfusion Variable Differences by Tumor Response
The comparison of mean perfusion variable was repeated, but instead of comparing dogs that died of the tumor to those that did not, dogs were split into groups based on whether the tumor increased in size. Specifically, the groups were (1) tumor volume increased from baseline to first recheck and (2) tumor volume decreased or remained the same between baseline and first recheck.
The survival analyses and t‐tests were performed using R Statistical Computing Environment11 ; linear mixed effect analyses were conducted using Proc Mixed in SAS Version 9.3.12
Results
Thirty‐four dogs were enrolled in the imaging study. The mean age was 9.5 years (SD: 3.1), and mean weight was 22.8 kg (SD: 12.4). Thirteen of 34 dogs had a histopathologic diagnosis of tumor type either at surgical resection or necropsy. The remainder of the tumors were classified by imaging characteristics.1 The tumor types included pituitary (3 confirmed, 7 presumed), meningioma (5 confirmed, 7 presumed), trigeminal nerve tumor (1 confirmed, 4 presumed), and glioma (4 confirmed, 3 presumed). Four dogs had surgery to remove their brain tumors (2 meningioma, 2 glioma) before radiation treatment and had residual gross disease. Pituitary tumors and meningiomas were treated with SRS, and gliomas and trigeminal nerve tumors were treated with 3‐fraction SRT.
All dogs had a first recheck imaging study, which was scheduled for approximately 3 months post surgery. The timing of the first recheck was close to 3 months for most of the dogs (median = 96 days, IQR = [90, 110]). Twenty‐three of 34 dogs had a second recheck. This second recheck was scheduled for 6 months post surgery. The timing of the second recheck tended to be more variable with a median of 197 days [IQR = 174, 219].
Tumor Volume Changes
At the first recheck, 21/34 dogs (62%) had shrinkage of the tumor, 11/34 dogs (32%) had stasis of the tumor, and 2/34 dogs (6%) had tumor growth. There was radiologic control of all pituitary (N = 10), meningioma (N = 12), and glioma (N = 7) tumors, with lack of radiologic control of 2/5 trigeminal nerve tumors. At the second recheck, 14/23 dogs (61%) had shrinkage of the tumor, 7/23 (30%) had stasis of the tumor, and 2/23 dogs (9%) had tumor growth. There was radiologic control of all pituitary (N = 6) tumors, gliomas (N = 4) and meningiomas (N = 8) with lack of control of 2/3 trigeminal nerve tumors. Radiologic control was 94% at the first recheck and 91% at the second recheck.
Tumor volumes were plotted over time for each dog (Fig 1). For most dogs, tumor volumes declined considerably at the first recheck and decreased or stabilized at the second recheck. A notable exception was for dogs with trigeminal nerve tumors. Of the five dogs with trigeminal nerve tumors, tumor volumes increased for four of them, which was in contrast to the generally favorable responses of dogs with tumors in other locations.
Figure 1.
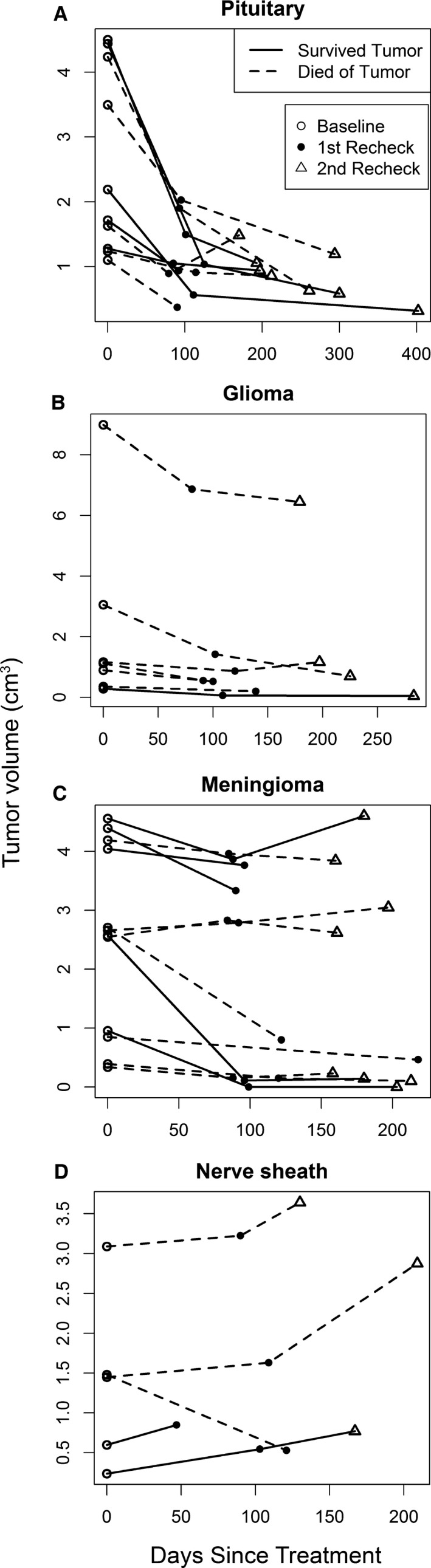
Volume changes in brain tumors over time. Pituitary tumors (A) had a strong initial response followed by a gradual decline in volume. Meningiomas and gliomas (B, C) had more variable responses. There was tumor progression in the majority of the nerve sheath tumors (D). Survived tumor (straight line), died of tumor (dashed line), baseline volume (open circle), first recheck tumor volume (closed circle), second recheck tumor volume (triangle).
Mean tumor volume significantly declined from baseline to the first recheck (P < .001); this reduction was maintained at the second recheck (P = .68). At the first recheck, mean tumor volume was reduced by −0.826 cm3 (95% CI: −1.165, −0.487). Between the first and second rechecks, the mean change in tumor volume was −0.046 cm3 (95% CI: −0.278, 0.186). The mixed effect analysis also showed that mean tumor volume declined significantly (P < .001) over time (Fig 2), shrinking 0.0045 cm3 on average for every day after treatment. However, as shown in Fig 1 and reflected in the paired sample t‐test analysis, often tumor volume declined substantially from baseline at the first recheck followed by maintenance at that level at the second check, rather than a continuous decline over time.
Figure 2.
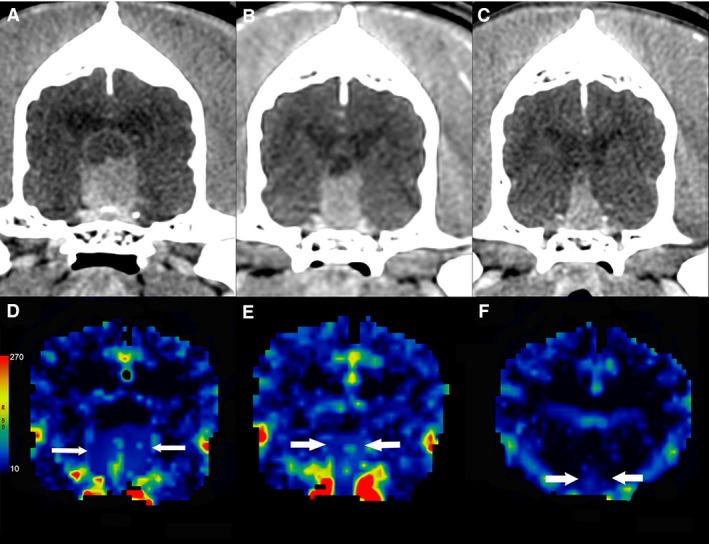
Contrast‐enhanced transverse CT images of a dog with a pituitary tumor treated with stereotactic radiosurgery. The mass decreases in size from the baseline images (A) through the first (B) and second (C) rechecks, from left to right with corresponding blood volume maps (mL/100 g) (D, E, F).
Eleven dogs did not have a second recheck. However, for one of these dogs, its first recheck was more than 200 days after treatment, which was in the time frame of the second rechecks. Of the remaining 10 dogs without a second recheck, tumor volume declined at the first recheck in nine of them, suggesting that the lack of a second recheck was not because of the failure of the tumor to respond to the treatment. Twenty‐one dogs were reported to have died as a result of the tumor. Seven of these dogs did not have a second recheck. The mean reduction in tumor volume at the first recheck, did not differ significantly between dogs that died from the tumor and did not have a second recheck and dogs that did not die from the tumor (P = .37). The change in tumor volume from baseline to the last measurement between dogs that died of the tumor and those that did not die from the tumor was not significantly different (P = .39). Collectively, these results indicate that loss to follow‐up did not qualitatively change the findings.
Tumor Perfusion Changes
Mean blood flow and volume declined significantly (P < .05) between baseline and the first recheck for the whole tumor and was marginally significant for the tumor center (P < .1) based on raw P value although none were significant after adjusting for multiple testing (FDR <0.05) (Table 1). The reductions in blood flow and volume between baseline and the first recheck were maintained or further reduced at the second recheck. Mean permeability did not differ significantly between baseline and first recheck or between the first and second rechecks (Table 1). The mixed effect analysis showed that blood flow and blood volume declined significantly in the whole tumor and tumor center after treatment. In the tumor periphery, however, only blood volume decreased significantly (Table 2).
Table 1.
Mean and standard deviation of normalized perfusion measures relative to brain. Significance (P values) are the results of paired t‐tests between the initial value and first recheck, and first and second rechecks. Blood flow in the whole tumor declined significantly after the first recheck, and in the tumor center after the second recheck. Significant differences were not detected after adjusting for multiple comparisons
| Tissues | Time | Normalized Perfusion Values | |||||
|---|---|---|---|---|---|---|---|
| Blood Flow (mL/100 g/min) | FDR (P) | Blood Volume (mL/100 g) | FDR (P) | Permeability (mL/100 g/min) | FDR (P) | ||
| Whole tumor | Initial | 3.22 ± 1.95 | 3.97 ± 2.63 | 35.45 ± 25.92 | |||
| First check | 2.24 ± 1.71 | 0.15 (0.017) | 2.97 ± 2.37 | 0.054 (0.003) | 29.98 ± 21.90 | 0.20 (0.057) | |
| Second check | 1.72 ± 1.20 | 0.29 (0.21) | 2.13 ± 1.19 | 0.293 (0.202) | 19.74 ± 18.78 | 0.91 (0.91) | |
| Tumor center | Initial | 3.02 ± 2.14 | 3.86 ± 3.48 | 43.84 ± 32.29 | |||
| First check | 1.70 ± 1.77 | 0.21 (0.086) | 2.49 ± 2.19 | 0.21 (0.079) | 40.17 ± 32.33 | 0.22 (0.11) | |
| Second check | 1.20 ± 0.93 | 0.21 (0.041) | 1.65 ± 1.25 | 0.21 (0.093) | 24.94 ± 29.50 | 0.50 (0.47) | |
| Tumor periphery | Initial | 2.98 ± 2.04 | 3.73 ± 2.44 | 38.55 ± 34.08 | |||
| First check | 1.96 ± 2.14 | 0.23 (0.13) | 2.69 ± 2.28 | 0.50 (0.45) | 31.79 ± 30.30 | 0.34 (0.26) | |
| Second check | 2.25 ± 2.69 | 0.24 (0.15) | 2.19 ± 1.63 | 0.21 (0.057) | 25.72 ± 25.00 | 0.46 (0.39) | |
Table 2.
Changes in normalized blood flow, blood volume, and permeability. Estimated change per day significance for changes in normalized values of blood flow, blood volume, and permeability in whole tumors, tumor center, tumor periphery, and normal brain tissue based on linear mixed effect models. Blood flow in the whole tumor and tumor center, blood volume, and permeability surface of the whole tumor declined over time
| Tissue | Normalized Perfusion Values | |||||
|---|---|---|---|---|---|---|
| Blood Flow (mL/100 g/min) | FDR (P) | Blood Volume (mL/100 g) | FDR (P) | Permeability (mL/100 g/min) | FDR (P) | |
| Whole tumor | −0.006 | *0.009 (.004) | −0.009 | *0.005 (.001) | −0.058 | *0.009 (.004) |
| Tumor center | −0.008 | *0.005 (.001) | −0.010 | *0.009 (.005) | −0.069 | 0.104 (.081) |
| Tumor periphery | −0.004 | 0.20 (.20) | −0.007 | 0.027 (.018) | −0.056 | 0.13 (.11) |
A significant difference is noted by an asterisk.
FDR, false discovery rate.
Survival Analysis
Median survival was approximately 1 year (median: 324 days with 95% confidence interval [292.8, 419.4]), but 4 dogs survived longer than 650 days (Fig 3). Neither actual tumor volume (hazard ratio = 1.21, P = .19) nor the change in tumor volume from the baseline (hazard ratio = 1.38, P = .12) significantly affected the hazard of death because of the tumor. The CIFs showed that the probability of death because of the effect of the tumor was considerably greater than the probability of death because of other causes with the difference increasing over time (Fig 4). About 50% of dogs died from the tumor within 1 year of treatment, while death from other causes within 1 year was less than 10%. In fact, most of the mortality resulting from the tumor occurred within 1 year. After 1 year, dogs died from causes unrelated to the tumor with greater frequency.
Figure 3.
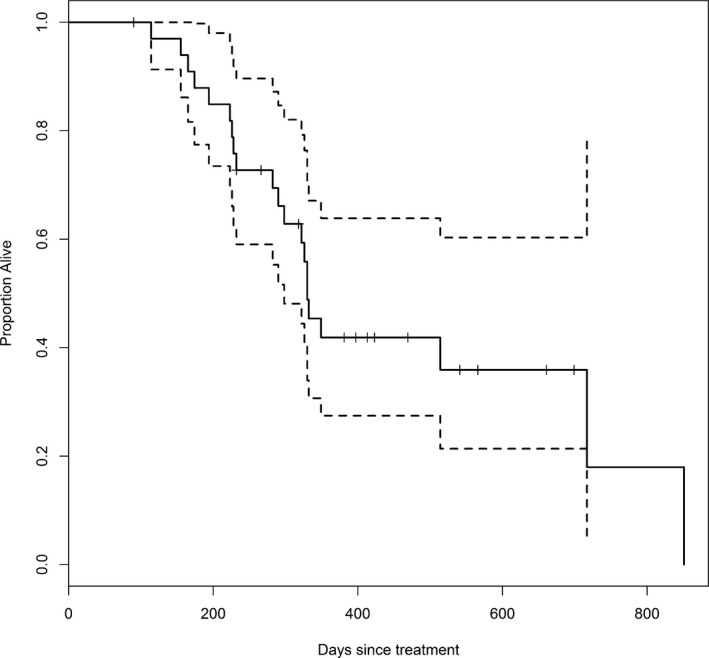
Kaplan–Meier estimate of survival in days for dogs treated with stereotactic radiosurgery or stereotactic radiotherapy. The dotted lines represent 95% confidence bounds. Tick marks indicate censored cases. Approximately half of the dogs survived for more than 1 year after treatment.
Figure 4.
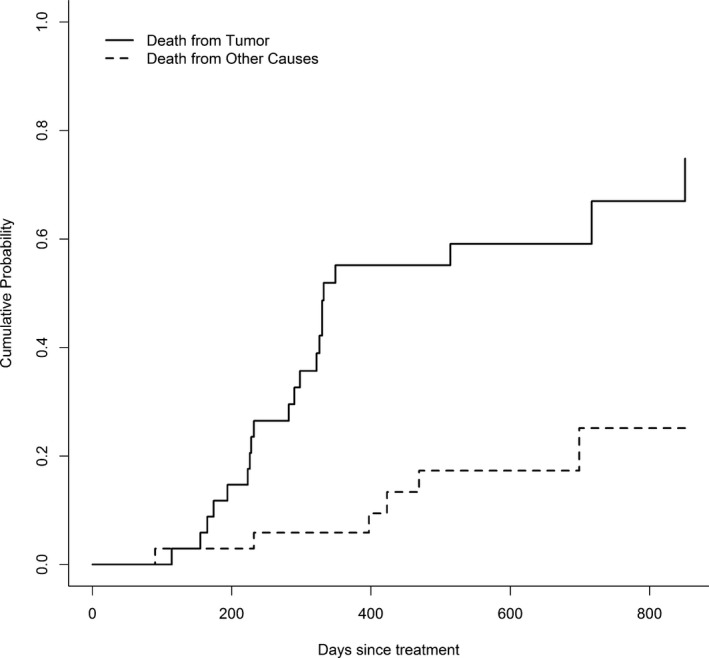
Cumulative probability of death of dogs treated with stereotactic radiosurgery or radiotherapy from brain disease or from other causes over time. After 1 year, the majority of treated dogs died from causes unrelated to the brain tumor.
The hazard of death caused by tumor did not differ significantly with changes in perfusion variables from baseline (Table 3, FDR >0.05). The perfusion variables also did not differ significantly between dogs who died of the tumor and those who died of other causes. There was a significant decrease in blood flow in the tumor center at the second recheck in dogs whose tumors increased in size between the baseline and first recheck (Table 4, FDR = 0.009).
Table 3.
Cox's propotional hazard modeling of change in brain tumor perfusion
| Tumor Region | Variable | Estimate | SE | P value | FDR | Hazard Ratio |
|---|---|---|---|---|---|---|
| Whole tumor | Blood flow (mL/100 g/min) | −0.142 | 0.133 | .29 | 0.52 | 0.868 |
| Blood volume (mL/100 g) | −0.166 | 0.121 | .17 | 0.52 | 0.847 | |
| Permeability (mL/100 g/min) | 0.007 | 0.015 | .62 | 0.70 | 1.008 | |
| Tumor Center | Blood flow (mL/100 g/min) | −0.120 | 0.136 | .38 | 0.57 | 0.887 |
| Blood volume (mL/100 g) | −0.100 | 0.082 | .22 | 0.52 | 0.905 | |
| Permeability (mL/100 g/min) | 0.011 | 0.009 | .26 | 0.52 | 1.011 | |
| Periphery | Blood flow (mL/100 g/min) | −0.078 | 0.111 | .48 | 0.62 | 0.925 |
| Blood volume (mL/100 g) | −0.114 | 0.104 | .27 | 0.52 | 0.892 | |
| Permeability (mL/100 g/min) | −0.003 | 0.010 | .75 | 0.75 | 0.997 |
Results of Cox's proportional hazard modeling using a change in perfusion variables from baseline.
Table 4.
Change in perfusion of brain tumors by tumor volume change Mean ± SD of change in perfusion variables from baseline for each tumor region at first recheck and second recheck for dogs with increased tumor size at first recheck versus those with decreased or stable tumor size
| Tumor Region | Variable | Change from Baseline | First Recheck Tumor Volume Increased from Baseline | FDR | ||
|---|---|---|---|---|---|---|
| No | Yes | P | ||||
| Whole tumor | Blood flow (mL/100 g/min) | First recheck | −0.91 ± 2.10 | −1.36 ± 1.91 | .70 | 0.90 |
| Second recheck | −0.98 ± 1.82 | −4.94 ± 0.41 | .012 | 0.10 | ||
| Blood volume (mL/100 g) | First recheck | −1.05 ± 3.00 | −0.69 ± 3.19 | .83 | 0.90 | |
| Second recheck | −2.27 ± 1.68 | −1.85 ± 3.59 | .78 | 0.90 | ||
| Permeability (mL/100 g/min) | First recheck | −5.96 ± 18.35 | −2.50 ± 21.17 | .73 | 0.90 | |
| Second recheck | −10.58 ± 25.22 | −17.00 ± 4.67 | .73 | 0.90 | ||
| Tumor Center | Blood flow (mL/100 g/min) | First recheck | −1.18 ± 1.92 | −1.99 ± 2.61 | .51 | 0.90 |
| Second recheck | −1.20 ± 1.34 | −6.16 ± 0.43 | <.001 | 0.009* | ||
| Blood volume (mL/100 g) | First recheck | −1.18 ± 1.92 | −1.99 ± 2.61 | 1.0 | 1.0 | |
| Second recheck | −2.22 ± 2.01 | −1.39 ± 1.78 | .60 | 0.890 | ||
| Permeability (mL/100 g/min) | First recheck | −7.91 ± 29.29 | 18.92 ± 46.98 | .17 | 0.78 | |
| Second recheck | −19.12 ± 34.38 | −14.33 ± 42.83 | .85 | 0.90 | ||
| Periphery | Blood flow (mL/100 g/min) | First recheck | −1.16 ± 1.94 | 0.97 ± 4.63 | .51 | 0.90 |
| Second recheck | 0.28 ± 2.98 | −5.35 ± 1.19 | .029 | 0.17 | ||
| Blood volume (mL/100 g) | First recheck | −1.14 ± 3.16 | −0.39 ± 4.54 | .72 | 0.90 | |
| Second recheck | −1.72 ± 3.18 | −2.71 ± 3.91 | .70 | 0.90 | ||
| Permeability (mL/100 g/min) | First recheck | −11.74 ± 29.67 | 22.41 ± 60.99 | .26 | 0.90 | |
| Second recheck | −7.00 ± 30.58 | −12.91 ± 15.35 | .80 | 0.90 | ||
Significant changes are indicated with an asterisk.
Discussion
Stereotactic radiosurgery and SRT were effective in decreasing tumor volume in the majority of animals, which was an effect that lasted until the second recheck at approximately 6 months after treatment. A decrease in volume is expected to reduce intracranial pressure and edema associated with the presence of a large mass and, therefore, decreases the clinical signs associated with the tumor. The proportion of dogs in which radiologic control of the tumor was achieved is similar to a small group of dogs treated with SRS, although the time of imaging after treatment was not reported in that study.1 In people treated after surgery for pituitary adenoma, tumor control was similar to the dogs in this study with 96.5% control rate.12
Trigeminal nerve tumors had lack of radiologic control at initial or second recheck. In contrast, a previous study including three trigeminal nerve tumors reported a decrease in size at an unknown time point after treatment.1 Trigeminal nerve tumors are variable in shape, exiting the skull along the path of a nerve root and extending into the surrounding soft tissues and the mandibular canal of the mandible. These are likely more difficult to contour for effective SRT, which can have contributed to the lack of response of these tumors. Little data are available regarding the efficacy of radiation on these tumor types, and more information is needed to assess response to primary radiation therapy.
Intracranial tumors have been shown to have higher cerebral blood flow, blood volume, and permeability surface compared to surrounding brain, which was similar to our findings.13, 14 Cerebral blood flow, CBV and PS within the tumor decreased significantly after treatment with SRS using the linear mixed effects model. This suggests that the treatment had a measurable effect on the vascularity of the tumor, and the decrease in perfusion variables was paralleled by a decrease in tumor volume. Perfusion has been shown to be increased compared to normal brain in canine and feline meningiomas and high‐grade gliomas.14 Blood volume correlates with the degree of vascular density in human gliomas.15 Decreasing blood flow and blood volume in the tumor supports a slowing of neovascularization, damage to the vasculature of the tumor, or both. High‐dose fractions of radiation >10 Gy have effects of both DNA damage leading to mitotic cell death and apoptosis as well as vascular and stromal damage that causes further cell death.16, 17 Although it cannot be determined whether the cause of the decreased perfusion variables was secondary to tumor cell death or a primary effect on tumor stroma and vasculature, this finding can be supportive of using high fractions to gain added radiation effects on vasculature.
Permeability surface in brain tumors was not altered by SRS or SRT. Tumor vasculature can have variable structure from sinusoids to more mature vessels, often with sluggish blood flow and multiple dead ends. Permeability can be increased in immature neovasculature with large gaps between endothelial cells or decreased, as in primary brain tumors that retain features of the blood–brain barrier. Neoangiogenesis within tumors results in immature, tortuous vessels with increased permeability to macromolecules.18, 19 Measurement of CT perfusion variables was hypothesized to provide insight into the effect of radiation on neovasculature in the tumor reflecting both of these processes; however, effects were not detected in this group of tumors.
Perfusion in tumor tissue can differ from the periphery to the center, with the periphery often being more radiosensitive because of the increased blood flow and oxygenation. There were differences between the center and periphery in this group of tumors, with the periphery less affected by treatment than the tumor center. Blood flow and blood volume declined significantly in the whole tumor and tumor center after treatment. In the tumor periphery, however, only blood volume decreased. Although the tumor periphery is expected to be more radiosensitive, the long‐time intervals between imaging studies can allow for tumor revascularization and maintenance of peripheral blood flow. The decrease in blood volume can relate to a decreased density of tumor vasculature; however, microscopic evaluation would be necessary for correlation.15
There was decreased blood flow in the tumor center at second recheck in cases with lack of radiologic control between baseline and first recheck, which can be correlated with necrosis or hypoxia secondary to the increasing size of the tumor, the radiation effects, or both. Although blood volume and blood flow decreased after treatment in this group of tumors, perfusion variables were not predictive of death because of the tumor. A larger population with less heterogeneity is needed to discover additional correlations in tumor perfusion and physiology in responders versus nonresponders.
Around 50% of dogs survived for 1 year or more with their tumors, indicating possible efficacy of the radiation treatment. Those that survived longer than 1 year were likely to die from other causes expected of older dogs, rather than of their primary brain disease. Longer term follow‐up can provide additional information on the likelihood of recurrence of brain tumors; however, in this population of older dogs, life expectancy is likely limited to a few years.
Radiosurgery is used in people with gliomas to treat a small volume recurrence or to enhance the effect of external beam radiotherapy.20 Although some of the dogs in this study were treated postoperatively, there is limited information on using SRS as a primary treatment for comparison to our results. Low‐grade gliomas are poorly enhancing on postcontrast T1 MR images, which can cause underestimation of both the measurement of tumor volume as an outcome measure and tumor targeting for SRS potentially reducing treatment efficacy.20, 21 However, the results of this small sample are encouraging in treating gliomas, especially those that are not surgically accessible.
Pseudoprogression of tumors on MR images has been documented in people with gliomas, resulting in an increase in the size of enhancing tumor tissue shortly after treatment. This subsequently resolves, and is likely a result of inflammation, edema, and altered vascular permeability secondary to treatment.22 The tumors in this study did not demonstrate this pattern, however, additional time points and a larger number of dogs can be necessary to detect this imaging finding. Magentic resonance perfusion imaging has shown that CBV can differentiate between pseudoprogression and true progression.23 Our results of declining CBV parallel the findings of pseudoprogression in a study measuring the effect of chemotherapy on gliomas, however, a concomitant increase in tumor volume in most dogs was not found. Further investigation can be of interest in using perfusion imaging to differentiate changes in tumor progression and pseudoprogression.
Not all of the tumors in the study had histologic diagnoses because of the noninvasive nature of SRS and inaccessibility of some tumor locations for biopsy. There is risk associated with the biopsy of tumors that are in close association with the vasculature or located in the caudal fossa limiting the likelihood of obtaining tissue in dogs with spontaneously occurring tumors. Every effort was made to obtain tissue at necropsy in dogs who died of any cause. The imaging characteristics of brain tumors can be reasonably accurate in determining tumor type and have been reported as 70–93%.24 There is potential for some error in classification of these tumors, however, it is expected to be low.
Stereotactic radiosurgery and SRT are effective treatments for reducing tumor volume, blood flow, and blood volume in tumor tissue. Treated dogs surviving for more than 1 year are more likely to die from other causes than of their primary brain tumor. Stereotactic radiosurgery and SRT should be considered for noninvasive treatment of intracranial brain tumors, although peripheral nerve sheath tumors can be less successfully treated.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and a grant from the Meadowview Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
A portion of this work was presented at the meeting of the American College of Veterinary Radiology 2011.
Conflict of Interest Declaration: Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at the William R. Pritchard Veterinary Teaching Hospital, School of Veterinary Medicine, University of California, Davis CA.
Footnotes
iPlan RT Dose 4.1, BrainLab Westchester, IL
Clinac 2100C, Varian Medical Systems, Palo Alto, CA
Brainlab AG, Feldkirchen, Germany
Lightspeed 16 General Electric Co, Milwaukee, WI
Iopamidol, 370 mg I/mL, Bracco Diagnostics Inc, Princeton, NJ
Vistron CT, Medrad Inc, Warrendale, CA
MR Signa LX, General Electric Co, Milwaukee, WI
0.2 mL/kg, Magnevist, Bayer HealthCare Pharmaceuticals Inc, Wayne, NJ
Osirix v. 3.7.2, Pixmeo, Bernex, Switzerland
CT Perfusion 4, GE Advantage 4.4, Milwaukee, WI
R Core Team 2013
SAS Institute, Cary NC
References
- 1. Mariani CL, Schubert TA, House RA, et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol 2015;13:409–423. [DOI] [PubMed] [Google Scholar]
- 2. Hu H, Barker A, Harcourt‐Brown T, Jeffery N. Systematic review of brain tumor treatment in dogs. J Vet Intern Med 2015;29:1456–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz M, Lamb CR, Brodbelt DC, Volk HA. Canine intracranial neoplasia: Clinical risk factors for development of epileptic seizures. J Small Anim Prac 2011;52:632–637. [DOI] [PubMed] [Google Scholar]
- 4. Song RB, Vite CH, Bradley CW, Cross JR. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J Vet Intern Med 2013;27:1143–1152. [DOI] [PubMed] [Google Scholar]
- 5. Dickinson PJ. Advances in diagnostic and treatment modalities for intracranial tumors. J Vet Intern Med 2014;28:1165–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bagley RS, Gavin PR, Moore MP, et al. Clinical signs associated with brain tumors in dogs: 97 cases (1992‐1997). J Am Vet Med Assoc 1999;215:818–819. [PubMed] [Google Scholar]
- 7. Tung GA, Noren G, Rogg JM, Jackson IM. MR imaging of pituitary adenomas after gamma knife stereotactic radiosurgery. Am J Roentgenol 2001;177:919–924. [DOI] [PubMed] [Google Scholar]
- 8. Kamiryo T, Lopes MB, Kassell NF, et al. Radiosurgery‐induced microvascular alterations precede necrosis of the brain neuropil. Neurosurgery 2001;49:409–414. [DOI] [PubMed] [Google Scholar]
- 9. Dieterich S, Zwingenberger AL, Hansen K, et al. Inter‐ and intrafraction motion for stereotactic radiosurgery in dogs and cats using a modified Brainlab frameless stereotactic mask system. Vet Radiology 2015;56:563–569. [DOI] [PubMed] [Google Scholar]
- 10. Chang JH, Chang JW, Choi JY, et al. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatr 2003;74:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jain R, Scarpace L, Ellika S, et al. First‐pass perfusion computed tomography: Initial experience in differentiating recurrent brain tumors from radiation effects and radiation necrosis. Neurosurgery 2007;61:778–786; discussion 786–7. [DOI] [PubMed] [Google Scholar]
- 12. Voges J, Kocher M, Runge M, et al. Linear accelerator radiosurgery for pituitary macroadenomas: A 7‐year follow‐up study. Cancer 2006;107:1355–1364. [DOI] [PubMed] [Google Scholar]
- 13. Cenic A, Nabavi DG, Craen RA, et al. A CT method to measure hemodynamics in brain tumors: Validation and application of cerebral blood flow maps. Am J Neuroradiol 2000;21:462–470. [PMC free article] [PubMed] [Google Scholar]
- 14. MacLeod AG, Dickinson PJ, LeCouteur RA, et al. Quantitative assessment of blood volume and permeability in cerebral mass lesions using dynamic contrast‐enhanced computed tomography in the dog. Acad Radiol 2009;16:1187–1195. [DOI] [PubMed] [Google Scholar]
- 15. Shin JH, Lee HK, Kwun BD, et al. Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: Preliminary results. Am J Roentgenol 2012;179:783–789. [DOI] [PubMed] [Google Scholar]
- 16. Kirkpatrick JP, Meyer JJ, Marks LB. The linear‐quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 2008;18:240–243. [DOI] [PubMed] [Google Scholar]
- 17. Kocher M, Treuer H, Voges J, et al. Computer simulation of cytotoxic and vascular effects of radiosurgery in solid and necrotic brain metastases. Radiother Oncol 2000;54:149–156. [DOI] [PubMed] [Google Scholar]
- 18. Jain R, Ellika SK, Scarpace L, et al. Quantitative estimation of permeability surface‐area product in astroglial brain tumors using perfusion CT and correlation with histopathologic grade. Am J Neuroradiol 2008;29:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park HJ, Griffin RJ, Hui S, et al. Radiation‐induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012;177:311–327. [DOI] [PubMed] [Google Scholar]
- 20. Sheehan J. Stereotactic radiosurgery for high‐grade gliomas: Available evidence and the need for further investigation. World Neurosurg 2013;80:814–816. [DOI] [PubMed] [Google Scholar]
- 21. Bentley RT, Daniel GB, Ober CP, et al. Canine intracranial gliomas: Relationship between magnetic resonance imaging criteria and tumor type and grade. Vet J 2013;198:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hygino da Cruz LC, Rodriguez I, Domingues RC, et al. Pseudoprogression and pseudoresponse: Imaging challenges in the assessment of posttreatment glioma. Am J Neuroradiol 2011;32:1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mangla R, Singh G, Ziegelitz D, et al. Changes in relative cerebral blood volume 1 month after radiation‐temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology 2010;256:575–584. [DOI] [PubMed] [Google Scholar]
- 24. Ródenas S, Pumarola M, Gaitero L, et al. Magnetic resonance imaging findings in 40 dogs with histologically confirmed intracranial tumours. Vet J 2011;187:85–91. [DOI] [PubMed] [Google Scholar]


