Abstract
The transcription factor AP-1 is downstream of growth factor (GF) receptors (GFRs) and stress-related kinases, both of which are implicated in breast cancer endocrine-resistance. Previously, we have suggested that acquired endocrine-resistance is associated with increased activity of AP-1 in an in vivo model. In the current report, we provide direct evidence for the role of AP-1 in endocrine-resistance. First, significant overlap was found between genes modulated in tamoxifen (Tam) resistance and a gene-signature associated with GF-induced estrogen receptor (ER) cistrome. Interestingly, these overlapping genes were enriched for key signaling components of GFRs and stress-related kinases and had AP-1 motifs in their promoters/enhancers. Second, to determine a more definitive role of AP-1 in endocrine resistance, AP-1 was inhibited using an inducible dominant-negative (DN) cJun expressed in MCF7 breast cancer cells in vitro and in vivo. AP-1 blockade enhanced the anti-proliferative effect of endocrine treatments in vitro, accelerated xenograft tumor response to Tam and estrogen deprivation in vivo, promoted complete regression of tumors, and delayed the onset of Tam resistance. Induction of DN-cJun after development of Tam resistance resulted in dramatic tumor shrinkage accompanied by reduced proliferation and increased apoptosis. These data suggests that AP-1 is a key determinant of endocrine-resistance by mediating a global shift in the ER transcriptional program.
Implications
AP-1 represents a viable therapeutic target to overcome endocrine-resistance.
Introduction
Over 70–80% of breast cancers express estrogen receptor alpha (ER), which has been implicated in the etiology and progression of this disease. Endocrine therapy using different agents that block the estrogen (E2)/ER pathway has proven highly successful in the clinical setting. Tamoxifen (Tam) is a selective modulator of ER that is widely used for the treatment of pre and postmenopausal patients with all stages of ER-positive breast cancer [1]. Aromatase inhibitors (AI) in postmenopausal women block the synthesis of E2 and have become the treatment of choice for many of these patients [2, 3]. Despite their documented benefits, however, resistance is common in many women treated with both Tam and AIs, either early (de novo or intrinsic resistance) or after prolonged therapeutic intervention (acquired resistance). Understanding the mechanisms of ER signaling and resistance to endocrine therapy remain critical in order to improve outcomes of breast cancer patients.
ER is a member of the greater family of nuclear receptor transcription factors (TFs), which upon activation by E2, will then bind to DNA and regulate gene transcription by recruiting a complex of co-regulator proteins. Importantly, these co-regulators, as well as ER itself, undergo post-translational modifications in response to diverse cellular signals coming from tumor cells, the microenvironment, growth factor receptors (GFR), and stress-related kinases, with potential impact on signaling activity as a consequence [4–6]. These modifications can modulate ER transcriptional activity and result in ligand-independent or Tam-mediated activation of the receptor [7, 8]. ER can modulate gene transcription either by directly binding to DNA on sites that contain E2 response elements (ERE), or by tethering to gene promoters/enhancers via interaction with other TFs, such as AP-1 [9]. Interestingly, Tam can stimulate the ER/AP-1 complex rather than inhibit it [10]. Furthermore, the interaction of ER with AP-1 and other factors can be due to ligand-independent activation and, therefore, may not be susceptible to the estrogen lowering effects of aromatase inhibition [11].
The AP-1 transcription complex is a collection of dimeric proteins that belong to the Jun (cJun, JunB, JunD), Fos (FosB, Fra-1, Fra-2), Maf, and ATF subfamilies. AP-1 complexes, including those of cJun, regulate the transcription of genes involved in cancer cell proliferation, survival, and invasiveness [12, 13]. Levels and activity of the various members of the AP-1 complex are also regulated by multiple signals, including microenvironment stimuli, mitogenic GFRs, and stress-related kinases associated with tumor progression [14]. We have previously shown in pre-clinical models that development of endocrine-resistance is associated with oxidative stress and upregulation of EGFR and HER2 with activation of downstream proliferation and survival pathways [15, 16]. Resistance is only partially overcome by targeting EGFR in these tumors, with similar observations in patients [17, 18], suggesting that other survival pathways also contribute to resistance. In addition, we also demonstrated that endocrine-resistance is associated with increased levels of phosphorylated cJun N-terminal kinase (JNK), a major regulator of cJun activity and phospho-cJun itself, resulting in augmented AP-1 transcriptional activity [16]. Similarly, increased levels of phospho-JNK, phospho-cJun, and enhanced GFR signaling have been observed in patients with Tam-resistant tumors [19, 20]. Additional in vitro data show that Tam-stimulated cell lines display higher levels of AP-1 DNA binding and transcriptional activity [21, 22] and that high expression of AP-1 dependent genes such as VEGF, Cyclin D1 and uPA predicts poor Tam response [22].
Interestingly, recent genome-wide profiling studies have demonstrated that hyperactive GFR signaling under E2-independent conditions can induce a global shift in the ER-DNA binding sites (cistrome), and in the ER transcriptional program from sites containing the ERE-motif, towards those enriched for AP-1 [11].
Based on this and the preliminary data summarized above, we hypothesized that the observed critical role of AP-1 in endocrine resistance is due to the reprogramming of the ER-cistrome under GFR hyperactivation. To test this and to provide additional evidence for the engagement of AP-1 within our model system of endocrine resistance, we performed in silico analyses that indeed suggested AP-1 as a major node integrating diverse signaling pathways that could be responsible for endocrine-resistance. These observations strengthened our hypothesis that inhibition of AP-1 may overcome endocrine-resistance. To test this hypothesis, we used an inducible DN-cJun to inhibit AP-1 activity in vitro and in an in vivo model of endocrine-resistance to both tamoxifen and estrogen deprivation mimicking aromatase inhibition. We demonstrate that AP-1 blockade increases tumor sensitivity to endocrine therapy, delays the onset of resistance, and causes dramatic tumor shrinkage, even after the full development of endocrine-resistance, through inhibition of both proliferative and survival signals.
Materials and methods
Reagents, Hormones, and Antibodies
E2 pellets (0.36-mg 60-day release) for in vivo studies were purchased from Innovative Research (Sarasota, FL). Tamoxifen citrate (Tam) for in vivo studies and 4-hydroxy tamoxifen (Tam) and 17-beta estradiol (E2) for in vitro studies were purchased from Sigma (St Louis, MO). Doxycycline was purchased from Sigma (St Louis, MO). Antibodies against phosphorylated (p)H3, Ki67, and Cleaved Caspase 3–7 were obtained from Millipore (Billerica, MA), Dako (Carpinteria, CA), and Cell Signaling (Danvers, MA) respectively. Antibody against cJun was from Oncogene Research Products (La Jolla, CA). Anti- Flag Tag antibody was from Sigma (St Louis, MO).
Cells and Cell Culture Conditions
Stable MCF7 clones expressing the inducible TAM 67 DN-cJun (MCF7 Tet-off-TAM 67 clones 62 and 67) or the control vector alone (EV 1 and 3) were obtained from the laboratory of Dr. Powel H. Brown in March 2007. MCF7 Tet-off-TAM 67 clones 62 and 67 were phenotypically validated for inducible DN-cJun expression as previously described [25] and reported below. Similarly, MCF7 control vector alone (EV 1 and 3) clones were phenotypically validated for lack of inducible expression of DN-cJun. These cells were maintained in high-glucose Dulbecco’s modified Eagle medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), penicillin (100 IU/mL), streptomycin (100 μg/mL), Geneticin (G418; Gibco) (400 μg/mL), hygromycin (500 μg/mL) and doxycycline (2 μg/mL) (+Dox) to inhibit the expression of the DN-cJun under a humidified atmosphere of 5% CO2/95% air and at 37 °C, as described previously [32, 33]. For full induction of the DN-cJun, Dox was removed (−Dox) and cells were cultured in the presence of Tet System approved FBS (Clontech, Mountain View, CA). Low expression of the DN-cJun was obtained culturing cells in low Dox-containing media (+0.1 ng/ml). For experiments employing endocrine treatment, cells were cultured in in phenol red free medium containing 5% charcoal-stripped FBS (starvation medium) for 12 hours followed by endocrine therapy for 6 days.
Proliferation assays
To induce low or high levels of the DN-cJun, cells were first cultured for 4 days in medium containing Tet system approved FBS +/− Dox. Next, 3,000 cells/well were seeded in 96-well plates in starvation media with continuous exposure to Dox as before. Endocrine treatment was started 12 hours later (day 0) using 10−9 M E2, 10−7 M Tam, or 0.01% ethanol (to mimic ED). Cell growth was assessed at day 0 and 6 days post endocrine treatment as previously described [34]. Briefly, cell cultures were fixed with 4% glutaraldehyde and stained with 0.05% methylene blue. The dye was subsequently extracted with 3% HCl and absorbance measured at 655 nm. Growth fold change was determined by (O.D. 655 nm at six days/O.D. 655 nm at zero days) for each treatment. Experiments were executed in quadruplicate and were repeated at least two times. Results from a representative experiment are shown.
Immunohistochemistry
Tumor tissue was processed and immunohistochemical staining was performed as previously described [35]. Briefly, antigen retrieval was performed with 10 mM sodium citrate pH 6 (for Flag antibody) or with Tris-HCl pH 9 (for Ki67, cleaved caspase 3, and phospho-H3) in a pressure cooker at full pressure for 10 minutes. Mouse IgGs were blocked using the M.O.M kit (Vector Labs, Burlingame, CA) according to the manufacturer’s recommendation. Anti-Flag antibody (1:100 dilution) was incubated overnight in a humidified chamber at 4°C while Ki67 (1:200), cleaved caspase 3 (1:50), and phospho-H3 (1:400) were incubated for 1h at room temperature. Slides were then incubated with the secondary, biotinylated antibody for 30 min. Sections were then incubated with streptavidin-peroxidase for 30 min and the enzyme was visualized after 15 min of incubation with diaminobenzidine. Nuclei were counterstained with hematoxylin before mounting. Markers were scored by counting positive and negative cells in four randomly selected high power fields, and results were expressed as percentage of positive cells.
Protein Extracts and Immunoblots
Protein extracts and immunoblots were performed as previously described [34]. Briefly, cell cultures were harvested in lysis buffer (Cell Signaling Technology) supplemented with 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 1x protease inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN). Twenty-five μg of protein from each sample were separated under denaturing conditions by electrophoresis on polyacrylamide gels containing sodium dodecyl sulfate (SDS-PAGE) and transferred onto nitrocellulose membranes (Invitrogen, Carlsbad, CA, USA). Blots were blocked with appropriate blocking buffer and then reacted at 4°C with primary antibodies at dilutions as per the manufacturer’s directions overnight. Immunoblots were run in duplicate at a minimum, to confirm results.
Xenograft Studies
Xenografts were established by injecting 15–20 × 106 cells subcutaneously into ovariectomized 5- to 6-week–old athymic female mice (Harlan Sprague Dawley, Madison, WI) that had been supplemented with 0.36-mg 60-day–release E2 pellets (Innovative Research) and with Dox (200 μg/ml in the drinking water), as described [33]. When xenografts of the MCF7 Tet-off derivatives reached a size of 150–200 mm3 (2–4 weeks), mice were randomly allocated to continued E2 treatment or to E2 withdrawal (by removal of the E2 pellets) alone or in combination with Tam treatment (500 μg of Tam citrate, administered subcutaneously in peanut oil for 5 days/wk); all in the presence or in the absence of Dox (n= 11–18 mice per treatment group for Clones 62 and 67; n=7–10 mice per treatment group for clone EV3). When tumors reached a volume of 1000 mm3, mice were sacrificed by cervical dislocation under general anesthesia and tumors were harvested unless Dox removal was applied (see below). Each mouse carried a single tumor; a portion of the tumor tissue was fixed and embedded in paraffin for immunohistochemical analyses. In a few cases, DN-cJun was induced later by Dox removal at time when tumor progressed on long-term Tam treatment. These mice were followed for additional 4–6 weeks for tumor response. Since no tumor progression on Tam treatment in normal AP-1 conditions (+Dox) was seen in clone 62 in a first experiment, a second experiment was launched to allow for “late Dox removal” at the time of resistance to Tam for this clone. Animal care was in accordance with institutional guidelines.
Computing growth factor-induced ER/AP-1 dependent gene signature associated with Tam-resistance
Our previously described gene signature of MCF7 xenografts that acquired Tam resistance [15] was intersected with a list of genes putatively associated with growth factor (EGF)-induced ER binding sites (EGF-induced ER cistrome) recently identified with genome-wide chromatin immunoprecipitation using ER antibody followed by DNA microarray analysis (ChIP on-Chip) [11]. Genes putatively associated with sites of EGF-induced ER binding were defined as genes from the RefSeq database that have a functional ER binding site within 20 kilobases upstream or downstream of their transcription start site. ER binding sites were defined as high-stringency EGF-induced sites (FDR 1%) that fail to recruit ER following E2 treatment based on the low-stringency E2-induced ER cistrome as previously described (FDR 20%) [11]. To identify the list of genes putatively associated with EGF-induced ER binding sites that harbour an AP-1 motif, we restricted the previous analysis to sites containing the AP-1 motif (TRANSFAC: M00926.AP-1; PSSM score>5). Enrichment of the genes in our MCF7 xenograft Tam-resistant signature within the list of genes putatively associated with EGF-induced ER binding sites was tested using a one-sided Fisher’s exact test. Ingenuity pathway analysis software (Ingenuity Systems Inc., Mountain View, CA, USA) was further used to identify the top biological networks represented by the Tam-resistant upregulated ER/AP-1 gene signature. In brief, the list of the upregulated Tam-resistant genes overlapping with the list of genes putatively associated with the EGF-induced ER cistrome (n=93 genes) was uploaded into the web application www.ingenuity.com and an analysis was run. The two top-scoring networks were merged into a single network and the visualization tool was used to display graphically both the relationship among the genes in the list and also the relastionship between the same genes and other molecules in the Ingenuity database.
Statistical Analysis
On the basis of our previous studies in similar xenograft models [36], time to tumor doubling (TTD) was defined a priori as the time when tumor volume had increased 2 times from the value measured at the time of randomization for each mouse; time to response (TTR) was defined as the time when tumor volume had decreased to half from randomization. Complete tumor regression was defined as complete tumor disappearance (no palpable nodule or no measurable disease) for at least 2 consecutive measurements, and time to complete response (TCR) was defined as the time when complete tumor regression was observed. The Kaplan–Meier method was used to determine the median TTD, median TTR and median TCR. All P values for the xenograft studies were based on comparisons of variables among groups by use of the generalized Wilcoxon test. Complete tumor regression rates at day 100 and 95% confidence intervals were calculated for each group of animals. The two-sample t test or Wilcoxon rank sum test was used for two-group comparisons of tumor proliferation and other immunohistochemically-assessed biomarkers. All statistical tests were two-sided.
Results
A gene signature of ER/AP-1 cooperation is enriched in signaling pathways known to be involved in endocrine resistance
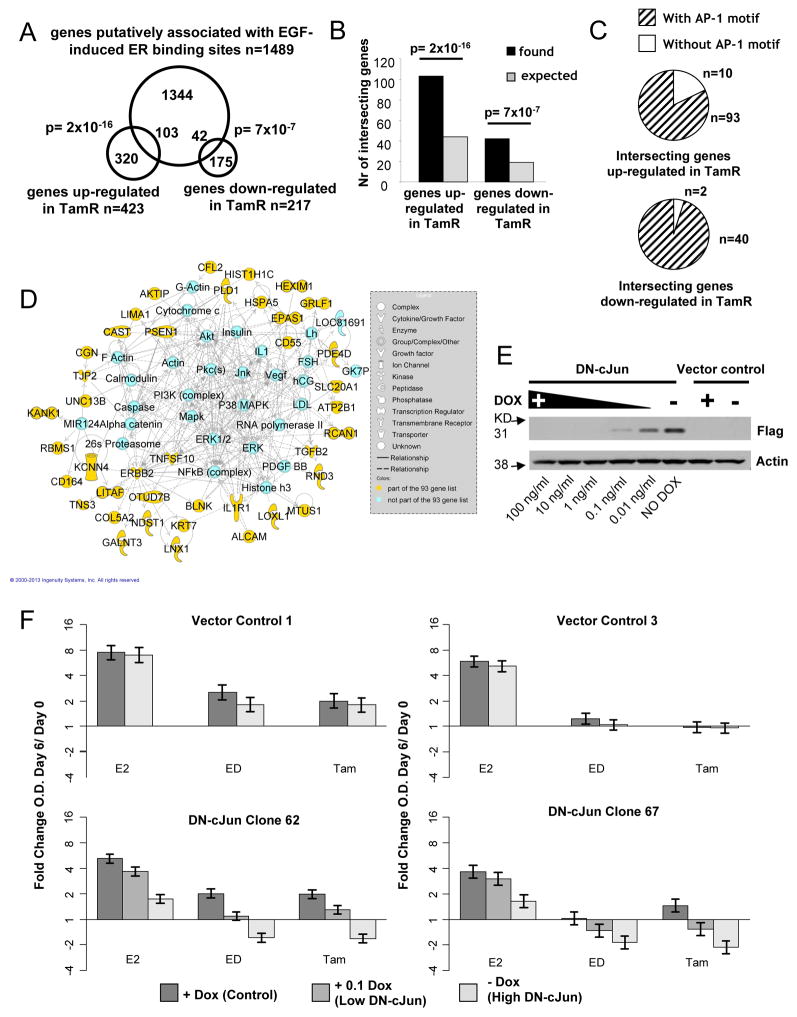
We have previously shown in our MCF7 xenograft model that acquired Tam resistance is driven in part by GFR signaling with repression of classic ER genomic activity [15]. Indeed, GFRs, including Epidermal Growth Factor Receptor (EGFR), and their downstream kinases are implicated in the development of endocrine resistance [15, 23, 24]. In addition, our work as well that of others have provided evidence that AP-1 transcriptional activity is augmented in endocrine-resistant breast cancer [16, 19, 21]. Recent studies mapping ER cistromes using the ChIP-on-chip technology, showed that the ER-DNA binding sites in the presence of EGF and in the absence of E2 were completely distinct from those induced by E2. This altered genomic activity of ER depended on AP-1 DNA-binding sites and on AP-1 transcriptional activity [11]. We therefore hypothesized that the observed role of AP-1 in endocrine-resistance may be related to the reprogramming of the ER cistrome under GFR activation. To this end, we intersected our previously developed Tam-resistant MCF7 xenograft gene signature [15] and the list of genes putatively associated with the EGF-induced ER cistrome (Figure 1A). We found a significant enrichment of the genes associated with the EGF-unique ER DNA-binding sites within our Tam-resistant (Tam-R) signature [p=2E-16 (upregulated) p=7E-7 (downregulated), one-sided Fisher’s exact test] (Figure 1A and B). Remarkably, 92% of these DNA-binding sites harbored an AP-1 motif (Figure 1C) (gene lists are provided in Supplementary Tables 1 and 2). To explore the potential biology underlying this gene list, we next analyzed the list of the 93 Tam-R upregulated overlapping genes by network analysis, which enables visualizing relationships among molecules. Interestingly, merging of the three most represented networks in this analysis showed a network comprising signaling molecules, GFRs, ligands, microenvironment and stress-related kinase pathways thought to be important in breast cancer endocrine resistance, including TGFB1, VEGF, PDGF, ERBB2, IL1R, PI3K, AKT, PKC, ERK1/2, JNK, p38 MAPK, FOS, NFkB, among others (Figure 1D).
Figure 1.
A) Venn diagram depicting the significant overlap between our Tam resistance gene signature and a list of genes putatively associated with EGF-induced ER binding sites (ER-cistrome). B) Bar chart showing that the number of intersecting genes is significantly higher than what would be expected by chance alone. C) Pie chart showing that the vast majority of overlapping genes (93/103 of the genes upregulated in TamR and 40/42 of the genes downregulated in TamR cells) harbored an AP-1 motif within 20Kb of their transcription start site. D) Network analysis of the overlapping genes shows key components of GFR, microenvironment and stress signaling pathways. Molecules shaded in yellow were part of the dataset and molecules in light blue were added from the Ingenuity Knowledge Base, a database containing information extracted from the scientific literature about interactions among molecules. Dashed or solid lines indicate indirect or direct interactions, respectively. Molecules are pictured in different shapes according to their protein function, if applicable, as detailed in the gray box. E) Western blot (WB) analysis of protein lysates from clone 62 cells following either four days of treatment with different concentration of doxycycline (Dox) or cultured in parental media without Dox. The expression of flag-tagged DN-cJun is induced by Dox withdrawal (full length blots are presented in Supplementary Figure 2). F) EV control clones (upper panel), and Clone 62 and 67 cells (lower panel) were treated and assayed for proliferation as described in methods. Bars in the graphs show back-transformed means of log-transformed ratios of optical density (O.D.) measured at day 6/O.D. at day 0; whiskers represent 95% confidence intervals.
DN-cJun expression inhibits MCF7 cell proliferation and potentiates the effect of endocrine treatment in vitro
To more directly study the functional role of AP-1 in endocrine-resistant breast cancer, we adopted a genetic approach using an inducible dominant negative-cJun (DN-cJun). The DN-cJun mutant is a deletion form of the human cJun lacking the transactivation domain (named Trans Activation Mutant-TAM-67) [25]. The DN-cJun is expressed in this cellular system upon doxycycline (Dox) removal, leading to inhibition of AP-1 activity by dimerizing with wild-type AP-1 protein to produce low-activity dimers containing only one transactivation domain. Two clones stably expressing the inducible flag-tagged DN-cJun (MCF7 Tet-off TAM-67, clone 67 and 62) were previously described and used for these studies [25]. The effect of AP-1 blockade on endocrine treatment was assessed under the induction of low and high levels of DN-cJun using various Dox concentrations in the cell growth media. Figure 1E demonstrates a gradual induction of DN-cJun in cell lysates of clone 62 upon titering down the Dox concentration for four days. Decreasing Dox concentrations in the culture medium to 1 ng/ml were still able to fully inhibit the expression of DN-cJun. Low levels of the DN-cJun were first detected in the presence of 0.1 ng/ml Dox and were fully induced upon complete Dox withdrawal (−Dox).
Clone 67 and 62 grown in the presence of 1 ng/ml of Dox (normal AP-1), 0.1 ng/ml Dox (partially impaired AP-1), or absence of Dox (maximally impaired AP-1), were treated for six days with E2, E2 deprivation with ethanol (ED), or ED with Tam, and cell proliferation was then assayed. Two vector-alone stably transfected clones (EV) were used as controls. In the presence of normal AP-1, all clones showed significant and substantial growth inhibition by both ED and Tam treatments, though the degree of sensitivity, as expected, varied somewhat among the clones (Figure 1F). Dox withdrawal resulted in no significant changes in EV control clones. In contrast, in the DN-cJun clones, a gradual decrease in proliferation was observed upon increasing expression of DN-cJun in cells treated with E2 or with endocrine therapy (Figure 1F). Most importantly, maximal impairment of AP-1 (−Dox) resulted in the induction of cell death only in conjunction with endocrine treatment (ED and Tam), as shown by the substantial decrease in cell numbers at the completion of the treatment (day 6) compared to baseline (day 0). These data show that AP-1 inhibition significantly adds to the inhibitory effects of endocrine therapy in vitro, and that this effect on tumor inhibition was most pronounced in the presence of antiestrogen treatment.
Expression of DN-cJun augments the response to endocrine treatment in vivo
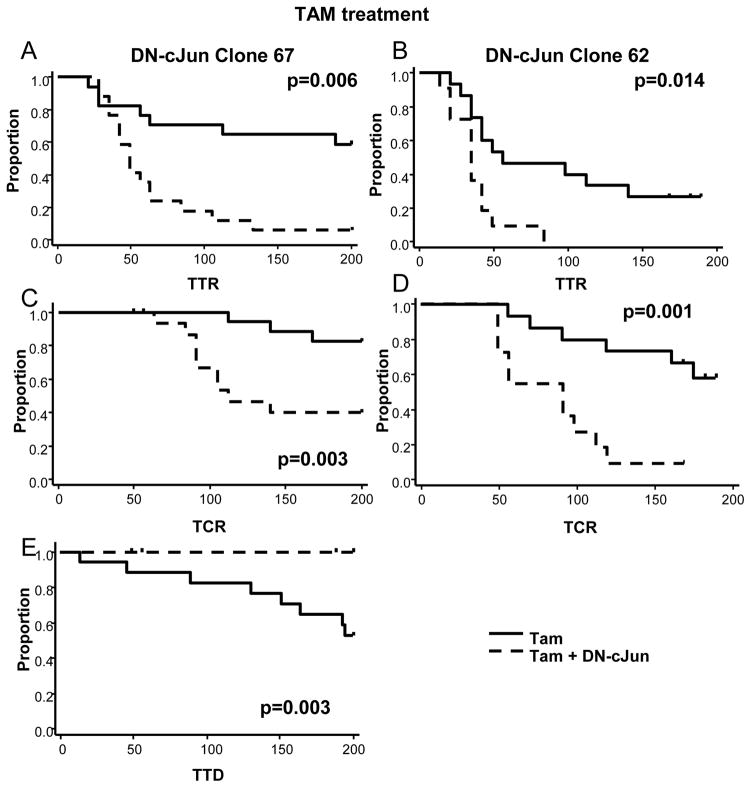
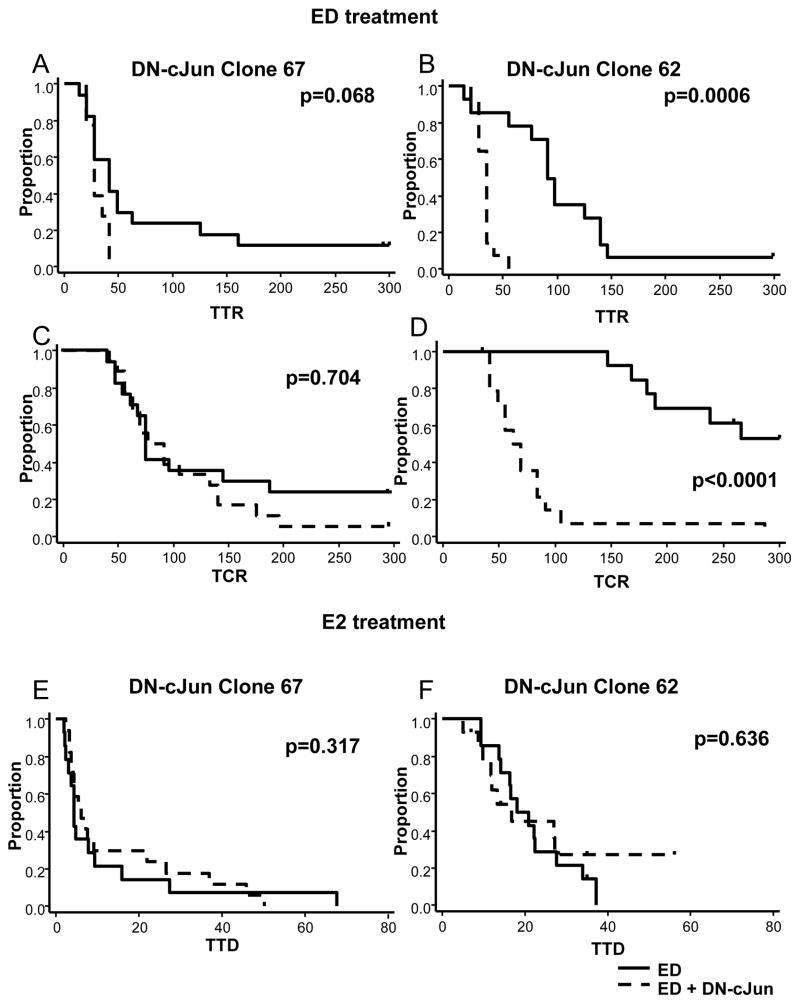
To determine whether the effects of AP-1 blockade on endocrine sensitivity observed in vitro were also observed in the in vivo setting, we next studied xenografts of the MCF7 Tet-off DN-cJun, clone 67 and 62 cells in mice treated with endocrine therapy. Xenograft tumors of clone 67 and clone 62 cells were established in the presence of E2 and Dox supplementation. When tumors reached 150–200 mm3 in size, mice were randomized to control (continued E2 supplementation), or endocrine treatment with ED alone or ED plus Tam, either in the presence (normal AP-1) or in the absence (impaired AP-1) of Dox. Inhibition of AP-1 improved response to endocrine therapy by significantly shortening time to tumor response (TTR), defined as time to tumor size halving from randomization, in the Tam-treatment arms for both clones (Figure 2A, B) and the ED arm for Clone 62, with a trend towards significance for Clone 67 (Figure 3A, B) (Table 1) (Clone 67: Tam p= 0.006 and ED p= 0.068; Clone 62: Tam p= 0.014 and ED p= 0.0006).
Figure 2.
Mice bearing xenograft tumors of either clone 67 (A and C) or clone 62 (B and D) cells were randomly allocated to continued E2 supplementation, ED, or ED in combination with Tam, either in the presence or in the absence of Dox to induce DN-cJun expression causing AP-1 blockade. In the Tam-treated group, inhibition of AP-1 significantly reduced time to tumor response (TTR) (A and B), and time to complete response (TCR) (C and D). Tam-treated Clone 67 tumors also showed significant prolongation in time to tumor doubling (TTD) (E), when AP-1 function was impaired.
Figure 3.
Inhibition of AP-1 in ED-treated mice significantly reduced time to tumor response (TTR) (A and B) and time to complete response (TCR) (C and D) for Clone 62 but not Clone 67. However, in E2-supplemented mice AP-1 inhibition did not delay tumor growth, as measured by TTD, in either clone 67 (E) or clone 62 (F) xenografts.
Table 1.
Time to response, time to complete response, and rate of complete responses to endocrine treatment +/− AP-1 inhibition in xenografts of MCF7 Tet-off DN-cJun clones 62 and 67.
| n | Median TTRA (days) | 95% CI | P value | Median TCRB (days) | 95% CI | P value | % CR at day 100C | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TamD | Clone 62 | Control (+DOX) | 15 | 56 | 35, 140 | 0.014 | NA | 91, NA | 0.001 | 20 | 7, 50 |
| DN-cJun (−DOX) | 11 | 35 | 21, 42 | 91 | 49, 112 | 72.7 | 46.1, 93.7 | ||||
| Clone 67 | Control (+DOX) | 17 | NA | 56, NA | 0.006 | NA | NA, NA | 0.003 | 0 | 0, 0 | |
| DN-cJun (−DOX) | 17 | 49 | 35, 63 | 112 | 91, NA | 33.3 | 15.4, 62.5 | ||||
| EDE | Clone 62 | Control (+DOX) | 14 | 94 | 56, 140 | 0.0006 | NA | 182, NA | <0.0001 | 0 | 0, 0 |
| DN-cJun (−DOX) | 14 | 35 | 28, 35 | 66.5 | 42, 84 | 85.7 | 63.4, 97.7 | ||||
| Clone 67 | Control (+DOX) | 17 | 42 | 28, 63 | 0.068 | 77 | 56, 189 | 0.704 | 64.7 | 43, 85.5 | |
| DN-cJun (−DOX) | 18 | 28 | 28,35 | 84 | 63, 133 | 61.1 | 40, 82.5 |
TTR: time to response (time elapsed from randomization to tumor size halving)
TCR: time to complete response (time elapsed from randomization to tumor disappearance)
% CR: percent of mice with complete response at day 100 (no palpable tumor for two consecutive measurements)
Tam: Tamoxifen
ED: Estrogen deprivation
More importantly, AP-1 inhibition together with endocrine therapy caused complete disappearance of many tumors and also significantly shortened the time to complete response (TCR), defined as the time from randomization to complete regression of the tumors in the Tam-treated mice for both clones (Figure 2C, D) and the ED-treated mice for Clone 62, but not Clone 67 (Figure 3C, D) (Table 1) (Clone 67: Tam p= 0.0034 and ED p= 0.7; Clone 62: Tam p= 0.001 and ED p< 0.0001). About 33% of Clone 67 and 73% of Clone 62 tumors in mice treated with Tam plus DN-cJun were undetectable after 100 days of treatment compared to 0% and 20%, respectively, of tumors in mice treated with Tam alone. Similar data were observed in Clone 62 mice treated with ED (percentage of complete response [%CR] at day 100: 85.7% vs. 0% in ED+ DN-cJun vs. ED alone, respectively) (Table 1). Finally, in clone 67 in which acquired resistance to Tam and tumor progression were detected within 200 days, DN-cJun (−Dox) in combination with Tam significantly delayed the onset of Tam resistance by prolonging the time to tumor doubling (TTD), (p= 0.0028) (Figure 2E) (Table 2). At day 200, no tumors had developed resistance and progressed with Tam plus the DN-cJun compared to half of those treated with Tam alone.
Table 2.
Time to progression to endocrine treatment +/− AP-1 inhibition in xenografts of MCF7 Tet-off DN-cJun clones 62 and 67.
| n | Median TTDA (days) | 95% CI | P value | |||
|---|---|---|---|---|---|---|
| E2 | Clone 62 | Control (+DOX) | 14 | 19.5 | 13.8, 27.7 | 0.636 |
| DN-cJun (−DOX) | 15 | 16.8 | 10, NA | |||
| Clone 67 | Control (+DOX) | 14 | 4.5 | 2.4, 9.5 | 0.317 | |
| DN-cJun (−DOX) | 17 | 6.1 | 3.9, 22.1 | |||
| Tam | Clone 67 | Control (+DOX) | 17 | NA | 131, NA | 0.003 |
| DN-cJun (−DOX) | 17 | NA | NA, NA |
TTD: time to tumor doubling (time elapsed from randomization to tumor size doubling)
In contrast to the in vitro data, inhibition of AP-1 in E2-treated mice had no effect on tumor growth as measured by TTD (Figure 3E, F) (Clone 67 p= 0.3 and Clone 62 p= 0.6) (Table 2), suggesting that increased AP-1 activity becomes relevant only in tumors from mice treated with endocrine therapy where AP-1 may function as an escape pathway to circumvent ER blockade.
To ensure that the observed growth delay was not due to Dox treatment itself, we inoculated mice with an MCF7 subclone that is stably transfected with vector alone (EV3) and then randomized them to E2-treated control or endocrine treatment either in the presence or the absence of Dox. As expected, no significant differences in growth were observed with or without Dox (suppl. Figure 1A, 1B, 1C) (suppl. Table 3 and 4) (Tam+Dox vs. Tam−Dox: TTR p= 0.144; TCR p= 0.196; TTD p= 0.5; ED+Dox vs. ED−Dox: TTR p= 0.771; TCR p= 0.597; E2+Dox vs. E2−Dox: TTD p= 0.871)
DN-cJun expression overcomes the growth of endocrine resistant tumors in vivo by eliciting a cytotoxic effect
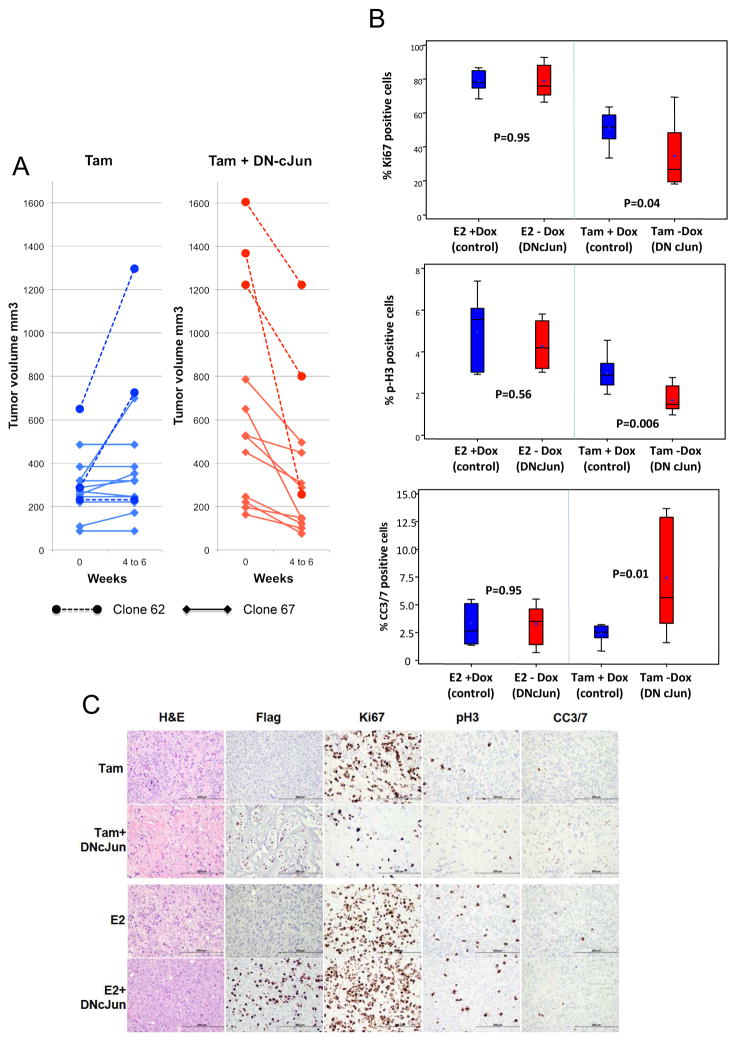
In order to study the effects of AP-1 inhibition in tumors that had already developed resistance to endocrine therapy, we induced the expression of the DN-cJun at a later time point when tumors were growing on endocrine therapy. To this end, we identified tumor pairs of either Clone 67 or Clone 62 with similar size and/or growth characteristics that were either slowly growing or more rapidly progressing after long-term (>10 months) Tam treatment in the presence of Dox (normal AP-1). Mice from different experiments bearing these “paired tumors” were allocated to either continued Dox or Dox withdrawal to induce the expression of the DN-cJun, all in the presence of continued Tam treatment. Although these tumors displayed diverse and heterogeneous growth characteristics under Tam, all the tumors in the Dox-withdrawal group showed shrinkage within 4–6 weeks after DN-cJun induction (Figure 4). In contrast, tumors in mice in the control group (+Dox) remained stable in size or continued to grow over the same time frame, confirming that the effect observed in the Dox-withdrawal group was due to AP-1 inhibition.
Figure 4.
A) Mice bearing clone 67 or clone 62 tumors, which were either stable or progressing under long-term Tam treatment and normal AP-1 conditions, were allocated to either keep (left panel) or withdraw Dox (right panel) to induce DN-cJun expression causing AP-1 blockade in the presence of continued Tam and were observed for 4 to 6 weeks. B) Box and whisker plots of quantification of IHC analysis and C) representative IHC images of proliferation (Ki67 and pH3) and apoptosis (CC3/7) markers in Tam-treated and E2 control tumors in the presence or absence of DN-cJun expression.
To understand the mechanisms by which AP-1 blockade inhibits Tam-resistant growth, tumor samples from mice in the Tam+/− Dox groups (7–10 tumors per treatment group) were assessed by immunohistochemistry (IHC) for apoptosis (cleaved caspase 3–7: CC3-7) and proliferation (Ki67 and phospho-histone 3: pH3). Expression of the DN-cJun was assessed using IHC with an anti-Flag antibody. Importantly, control tumors from the E2-supplemented mice +/− Dox were also assayed in parallel. As expected, expression of the DN-cJun was detected only in −Dox conditions as shown by IHC staining with an anti-Flag antibody (Figure 4C) [and further confirmed by Western blotting using a cJun antibody (Supplementary Figure 1D)]. AP-1 inhibition reduced proliferation and increased apoptosis in association with Tam compared to Tam alone (average Ki67 expression: 51% vs. 34.7%, p= 0.04; average pH3 expression: 2.9% vs. 1.6% p= 0.006; average CC3-7 expression: 2.4% vs. 7.4% in Tam+Dox vs. Tam −Dox, p= 0.01). No significant differences in apoptosis or proliferation rates were detected in E2-treated mice +/− Dox (Figure 4B and C).
Taken together, these findings strongly suggest that AP-1 activity functions as a compensatory pathway when ER signaling is blocked and can thereby mediate resistance to Tam and ED in this model. Furthermore, AP-1 inhibition can reverse the endocrine-resistant phenotype in vivo both by inhibiting tumor cell proliferation and by eliciting a cytotoxic effect.
Discussion
Multiple pathways, including GF, stress, and those originating from microenvironmental stimuli have been proposed to mediate intrinsic or acquired resistance to endocrine therapy. Previous work from our group and others has provided evidence for increased JNK/AP-1 pathway activity and GFR signaling in endocrine resistance [16, 19–22]. Our data support the concept that AP-1 may integrate signals from multiple pathways that cause endocrine resistance and that inhibition of AP-1 function is a very effective strategy for improving sensitivity and overcoming resistance to endocrine therapies. We show that there is a significant overlap between the gene signature of Tam-resistant MCF7 xenografts [15] and the gene signature associated with the EGF-induced ER cistrome [11]. Most of these overlapping genes have an AP-1 motif in their promoter/enhancer regions. Interestingly, the upregulated overlapping genes belong to a network of GFRs, stress-related kinases, and signaling molecules activated by the microenvironment, all of which have been implicated in endocrine resistance [26]. These data suggest that AP-1, by engaging altered ER-dependent genomic networks, may act as a major node integrating diverse signaling pathways mediating endocrine resistance. Indeed, in our in vivo breast cancer model of endocrine resistance, blockade of AP-1 function by DN-cJun leads to accelerated response to endocrine treatment, a higher response rate, a remarkably higher complete tumor disappearance rate, and a marked increase in the time to treatment resistance in endocrine-sensitive tumors. Furthermore, blocking this pathway after the development of endocrine resistance restores Tam sensitivity by inducing apoptosis and reducing cell proliferation. Our in vivo data from the DN-cJun model suggests that AP-1 is indeed crucial for the development of endocrine resistance since AP-1 blockade does not allow tumors to progress whilst undergoing endocrine treatment. Moreover, our study suggests that AP-1 is fundamental for sustaining the resistant phenotype, since AP-1 blockade completely reverses the progression and growth of long-term Tam-treated tumors, without any significant effects on E2-stimulated growth.
Other evidence suggests that AP-1 plays an important role in breast cancer biology and ER function. cJun overexpression in breast cancer cells induces changes correlating with an aggressive and invasive phenotype, promotes tumor formation in mice, and leads to reduced sensitivity to Tam [27]. AP-1 DNA-binding activity is significantly elevated in sub-lines of hormone-independent MCF7 tumors stimulated in vivo by Tam [16, 21], as well as in Tam-resistant patient samples [19]. In ER-positive cells, continuous exposure to Tam can induce ER agonistic activity at AP-1-regulated promoter sites [28]. Indeed, it is well known that ER has non-classical genomic functions where it does not make direct contact with DNA on ERE sites, but rather is tethered to gene promoters/enhancers via interaction with other transcription factors such as AP-1, NFκB and others [9]. This ER function regulates the expression of genes that are critical for proliferation, survival, and growth. In this form of gene regulation, ER is known to modulate the expression of a distinct set of AP-1-dependent genes [29]. Indeed, AP-1 is a transcription factor downstream of diverse mitogenic, survival, and stress stimuli, including GFR and stress-related kinase signaling, implicated in breast cancer endocrine resistance. Interestingly, recent systematic analysis of ER cistromes under growth factor stimulus (EGF) in the absence of E2 showed that EGF induces the modulation of a distinct set of ER-dependent genes which are also dependent on AP-1 [11]. In our analysis, a large fraction of genes modulated in our in vivo Tam-resistant signature have an AP-1 binding site in their regulatory promoter/enhancer regions, thus suggesting that reprogramming of ER nuclear genomic function through its binding to AP-1 sites might be a feature of endocrine therapy resistance. Of note, gene network analysis of the 93 ER/AP-1-dependent genes that are upregulated in our Tam-resistant signature implicated several different signaling pathways, which have been previously shown to be important in endocrine resistance. The multiplicity and diversity of these pathways may explain why inhibition of just EGFR has only a partial effect in overcoming Tam resistance [15] while inhibition of AP-1, in the present study, results in a more dramatic effect in this model including complete tumor regression in mice.
It has previously been shown that DN-cJun induces growth arrest in E2-treated tumors in vitro and in vivo [30]. Although we confirm these results in vitro, we did not observe the same results in vivo. The most likely explanation for the discrepancy is different experimental conditions under which the in vivo studies were conducted. In our experiments, AP-1 blockade was induced when tumors were larger in size. Therefore, established xenograft tumors in the presence of E2 may be less dependent on AP-1. In contrast, Tam-resistant tumors responded well to AP-1 blockade, suggesting that AP-1 may play a role that is relatively specific to endocrine-resistant growth, consistent with our prior observation that AP-1 transcriptional activity is much greater after Tam resistance has developed.
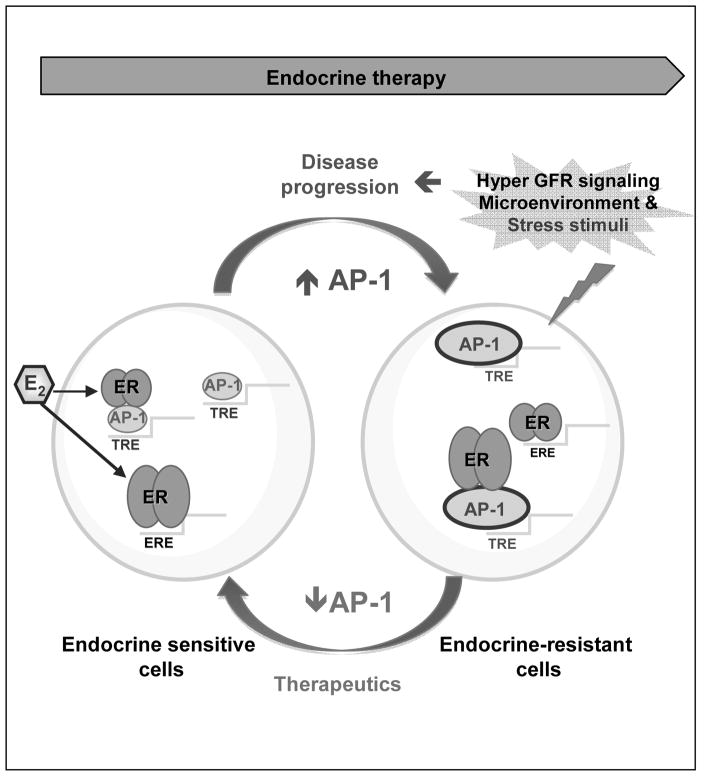
Based on the data presented here, we propose the following working model (Figure 5). In endocrine-sensitive cells, E2 regulation of gene transcription is mediated predominantly by ER interaction with ERE DNA-binding sites (classic nuclear/genomic activity). In response to endocrine therapy, AP-1 becomes a major determinant of transcription for diverse adaptive pathways responsible for resistance. These multiple pathways, including ER itself,, converge on AP-1 to enhance its activity, which in turn activates a molecular shift in the ER genomic network to facilitate an AP-1/ER-dependent transcriptional program. This reprogrammed AP-1-dependent ER genomic activity is at least partly responsible for the crucial role of the AP-1 transcriptional complex in mediating endocrine resistance.
Figure 5.
Working model: in endocrine-sensitive cells grown in the presence of E2 (left side), ER acts predominantly through classical genomic functions where it binds to DNA at E2 responsive elements (ERE) to modulate gene expression and to convey mitogenic and survival signals. At this stage, non-classical ER genomic function (i.e. where ER is tethered to other DNA responsive elements by binding transcription factors like AP-1) or AP-1 activity by itself may also partially contribute to tumor growth. Upon endocrine therapy, activation of adaptive responses or diverse escape pathways (such as GFR, or microenvironment and stress stimuli) contributes to disease progression and the development of endocrine resistance (right side). These multiple pathways converge on AP-1, which becomes a major determinant of global transcription and resulting in a molecular shift from the ER genomic network to an AP-1-dependent transcriptional program. Targeting AP-1 or its key downstream signaling components represent a new therapeutic strategy to enhance endocrine sensitivity and overcome resistance.
Furthermore, these data support the need to develop new drugs to enhance endocrine treatment or to overcome resistance in breast cancer. Compounds with a “DN-cJun-like” activity are potentially promising novel drugs to be tested in combination with endocrine treatment. Alternative treatment strategies to target AP-1 might rely on direct inhibition of AP-1 function by the recently described cJun DNA-zyme [31]. Finally, these data also suggest the need for the development of new biomarkers of endocrine resistance that would indicate activation of the AP-1 pathway. These AP-1-specific candidate genes might also provide novel targets to prevent or reverse endocrine resistance.
Future comprehensive studies including the establishment and integration of ER and AP-1 cistromes in cell line and tumor models are warranted in order to gain a more mechanistic insight into AP-1 role in endocrine resistant breast cancer.
Supplementary Material
Acknowledgments
Funding: This study was partly supported by the American Italian Cancer Foundation, Susan G. Komen for the cure grants (for L.M. and R.S. and Promise grant PG12221410 to C.K.O. and R.S.), Stand Up 2 Cancer Breast Cancer Program, NIH SPORE Grants P50 CA058183 and CA186784-01, and Cancer Center Grant P30CA125123, the EIF/Lee Jeans Breast Cancer Research Program, Breast Cancer Research Foundation, Cancer Prevention Research Institute of Texas (CPRIT RP140102), and Baylor College of Medicine Comprehensive Cancer Training Program (to M.G. and C.D.)
The authors thank Rena Mao and the Smith Breast Center Pathology Core, Tamika Mitchell and Maria Fernanda Prigge for technical assistance, and Dr. Gary Chamness for help with manuscript writing.
This study was partly supported by the American Italian Cancer Foundation, Susan G. Komen for the cure grants (for L.M. and R.S. and Promise grant PG12221410 to C.K.O. and R.S.), a Stand Up 2 Cancer Dream Team Translational Research Grant (Grant Number SU2C-AACR-DT0409), NIH SPORE Grants P50 CA058183 and CA186784-01, and Cancer Center Grant P30CA125123, the EIF/Lee Jeans Breast Cancer Research Program, Breast Cancer Research Foundation, Cancer Prevention Research Institute of Texas (CPRIT RP140102), and Baylor College of Medicine Comprehensive Cancer Training Program (to M.G. and C.D.) Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Schiff R, Osborne CK. Endocrinology and hormone therapy in breast cancer: new insight into estrogen receptor-alpha function and its implication for endocrine therapy resistance in breast cancer. Breast Cancer Res. 2005;7(5):205–11. doi: 10.1186/bcr1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson L, Lawrence D, Dawson C, Bliss J. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2009;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiff R, Massarweh S, Shou J, Osborne CK. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9(1 Pt 2):447S–54S. [PubMed] [Google Scholar]
- 5.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270(5241):1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 6.Balasenthil S, Barnes CJ, Rayala SK, Kumar R. Estrogen receptor activation at serine 305 is sufficient to upregulate cyclin D1 in breast cancer cells. FEBS Lett. 2004;567(2–3):243–7. doi: 10.1016/j.febslet.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 7.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276(13):9817–24. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 8.Weigel NL, Zhang Y. Ligand-independent activation of steroid hormone receptors. J Mol Med. 1998;76(7):469–79. doi: 10.1007/s001090050241. [DOI] [PubMed] [Google Scholar]
- 9.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, et al. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74(5):311–7. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 10.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277(5331):1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 11.Lupien M, Meyer CA, Bailey ST, Eeckhoute J, Cook J, Westerling T, et al. Growth factor stimulation induces a distinct ER(alpha) cistrome underlying breast cancer endocrine resistance. Genes Dev. 2010;24(19):2219–27. doi: 10.1101/gad.1944810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4(5):E131–6. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 13.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20(19):2390–400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Bergami P, Lau E, Ronai Z. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat Rev Cancer. 2010;10(1):65–76. doi: 10.1038/nrc2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. cancer res. 2008;68(3):826–33. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 16.Schiff R, Reddy P, Ahotupa M, Coronado-Heinsohn E, Grim M, Hilsenbeck SG, et al. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J Natl Cancer Inst. 2000;92(23):1926–34. doi: 10.1093/jnci/92.23.1926. [DOI] [PubMed] [Google Scholar]
- 17.Cristofanilli M, Valero V, Mangalik A, Royce M, Rabinowitz I, Arena FP, et al. Phase II randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16(6):1904–14. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 18.Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17(5):1147–59. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston SR, Lu B, Scott GK, Kushner PJ, Smith IE, Dowsett M, et al. Increased activator protein-1 DNA binding and c-Jun NH2-terminal kinase activity in human breast tumors with acquired tamoxifen resistance. Clin Cancer Res. 1999;5(2):251–6. [PubMed] [Google Scholar]
- 20.Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23(11):2469–76. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 21.Dumont JA, Bitonti AJ, Wallace CD, Baumann RJ, Cashman EA, Cross-Doersen DE. Progression of MCF-7 breast cancer cells to antiestrogen-resistant phenotype is accompanied by elevated levels of AP-1 DNA-binding activity. Cell Growth Differ. 1996;7(3):351–9. [PubMed] [Google Scholar]
- 22.Zhou Y, Yau C, Gray JW, Chew K, Dairkee SH, Moore DH, et al. Enhanced NF kappa B and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creighton CJ, Massarweh S, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, et al. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. cancer res. 2008;68(18):7493–501. doi: 10.1158/0008-5472.CAN-08-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144(3):1032–44. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 25.Ludes-Meyers JH, Liu Y, Munoz-Medellin D, Hilsenbeck SG, Brown PH. AP-1 blockade inhibits the growth of normal and malignant breast cells. Oncogene. 2001;20(22):2771–80. doi: 10.1038/sj.onc.1204377. [DOI] [PubMed] [Google Scholar]
- 26.Giuliano M, Trivedi MV, Schiff R. Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications. Breast Care (Basel) 2013;8(4):256–62. doi: 10.1159/000354253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith LM, Wise SC, Hendricks DT, Sabichi AL, Bos T, Reddy P, et al. cJun overexpression in MCF-7 breast cancer cells produces a tumorigenic invasive and hormone resistant phenotype. Oncogene. 1999;18(44):6063–70. doi: 10.1038/sj.onc.1202989. [DOI] [PubMed] [Google Scholar]
- 28.Astruc ME, Chabret C, Bali P, Gagne D, Pons M. Prolonged treatment of breast cancer cells with antiestrogens increases the activating protein-1-mediated response: involvement of the estrogen receptor. Endocrinology. 1995;136(3):824–32. doi: 10.1210/endo.136.3.7867590. [DOI] [PubMed] [Google Scholar]
- 29.DeNardo DG, Kim HT, Hilsenbeck S, Cuba V, Tsimelzon A, Brown PH. Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: identification of estrogen-induced/activator protein-1-dependent genes. Mol Endocrinol. 2005;19(2):362–78. doi: 10.1210/me.2004-0267. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Ludes-Meyers J, Zhang Y, Munoz-Medellin D, Kim HT, Lu C, et al. Inhibition of AP-1 transcription factor causes blockade of multiple signal transduction pathways and inhibits breast cancer growth. Oncogene. 2002;21(50):7680–9. doi: 10.1038/sj.onc.1205883. [DOI] [PubMed] [Google Scholar]
- 31.Cai H, Santiago FS, Prado-Lourenco L, Wang B, Patrikakis M, Davenport MP, et al. DNAzyme targeting c-jun suppresses skin cancer growth. Sci Transl Med. 2012;4(139):139ra82. doi: 10.1126/scitranslmed.3003960. [DOI] [PubMed] [Google Scholar]
- 32.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 33.Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA, et al. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87(10):746–50. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- 34.Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers--role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011;13(6):R121. doi: 10.1186/bcr3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99(9):694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 36.Massarweh S, Osborne CK, Jiang S, Wakeling AE, Rimawi M, Mohsin SK, et al. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. cancer res. 2006;66(16):8266–73. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.