Abstract
Schizophrenia and bipolar disorder are complex psychiatric disorders that present unique challenges in the study of disease biology. There are no objective biological phenotypes for these disorders, which are characterized by complex genetics and prominent roles for gene-environment interactions. The study of the neurobiology underlying these severe psychiatric disorders has been hindered by the lack of access to the tissue of interest – neurons from patients. The advent of reprogramming methods that enable generation of induced pluripotent stem cells (iPSCs) from patient fibroblasts and peripheral blood mononuclear cells has opened possibilities for new approaches to study relevant disease biology using iPSC-derived neurons. While early studies with patient iPSCs have led to promising and intriguing leads, significant hurdles remain in our attempts to capture the complexity of these disorders in vitro. We present here an overview of studies to date of schizophrenia and bipolar disorder using iPSC-derived neuronal cells and discuss potential future directions that can result in the identification of robust and valid cellular phenotypes that in turn can lay the groundwork for meaningful clinical advances.
Keywords: schizophrenia, bipolar disorder, stem cells, iPSC, microglia, gene-environment, neuronal differentiation
Graphical abstract
Introduction
Schizophrenia (SCZ) is a chronic and debilitating psychiatric disorder that is characterized by hallucinations, paranoid delusions, disordered thought processes, and cognitive deficits (Lewis and Lieberman, 2000). Bipolar disorder (BPD), also known as manic-depressive illness, is characterized by debilitating episodes of mania and depression that are often accompanied by psychotic symptoms (Keck et al., 2003, Goodwin and Ghaemi, 2003). SCZ and BPD both afflict ~1% of the population (Kessler et al., 2005, Merikangas et al., 2007). These illnesses manifest themselves in late adolescence or early adulthood, and follow a chronic course requiring treatment for the rest of a patient’s life (Fenton and McGlashan, 1991a, Fenton and McGlashan, 1991b, Salvatore et al., 2007). Patients with SCZ and BPD have a very high risk of suicide compared to the general population (Palmer et al., 2005, Pompili et al., 2013). The diagnosis and treatment of SCZ and BPD are based on clinical symptomatology and there are no biomarkers to aid in diagnosis, in guiding treatment decisions, or in monitoring treatment response (Pillai and Buckley, 2012, Frey et al., 2013).
SCZ and BPD are highly heritable but their genetic architecture is very complex (Kendler and Diehl, 1993, McGuffin et al., 2003). These disorders have monozygotic concordance rates of ~50% and dizygotic concordance rates of ~10% (Davis et al., 1995, Kieseppä et al., 2004, Kety et al., 1971). Despite the strong genetic component to these severe psychiatric disorders, genetic studies are only beginning to identify risk variants (Consortium, 2014, Group, 2011). Recent genome wide association studies have also found recurrent microdeletions and copy number variants that are associated with SCZ and BPD (Georgieva et al., 2014). Genetic studies point to some shared genetic susceptibility to SCZ and BPD (Lichtenstein et al., 2009, Consortium, 2013).
There is a marked dearth of medications that target the underlying disease biology in SCZ and BPD (Insel and Scolnick, 2006). Current antipsychotics were derived from the serendipitous discovery of chlorpromazine in the 1950s when scientists were trying to develop drugs with sedative and anti-histaminergic properties to use during surgeries (López-Muñoz et al., 2005, Ban, 2007). Similarly, lithium, the most efficacious treatment for BPD, was discovered in 1949 (CADE, 1949). The last major clinical advance in the treatment of SCZ was the development of clozapine in 1970s (Rodová et al., 1973) which had better efficacy compared to other antipsychotic medications (Baldessarini and Frankenburg, 1991). Current treatment of SCZ is based on the hypothesis that psychosis results from increased level of dopaminergic activity in the brain (Seeman, 2013). Antipsychotic medications are believed to work by acutely blocking the binding of dopamine to the D2 receptor (Kapur and Mamo, 2003, Seeman, 2006). However, there is significant variability in efficacy in different patients and there is often a lag time in therapeutic response that creates significant challenges in properly and effectively treating these patients (Pouget and Müller, 2014, Case et al., 2011, Takeuchi et al., 2012).
The development of novel therapeutics for SCZ and BPD has been hindered by the lack of our understanding of the neurobiology underlying these disorders. Most biological studies to date of small molecules with potential roles in the treatment of psychiatric disorders have been carried out in animal models (Nestler and Hyman, 2010). Studies in rodents have yielded a wealth of knowledge on basic biology and pathophysiology, including in our understanding of neurobiology. Animal models have been routinely used to identify new therapeutic leads for various human diseases, including for psychiatric disorders (Woodcock and Woosley, 2008). While these studies have led to a better understanding of the biology, there has been a lack of compounds that have translated successfully from animal models to humans (Pound et al., 2004, Hackam and Redelmeier, 2006, Medicine, 2013). Recent studies have found that genomic responses in humans to specific pathophysiological processes often have poor correlation with such responses in rodent models (Seok et al., 2013). In addition, studies in induced human neurons and in cortical neurons from knockout mice showed species-specific differences in the effects of NRXN1 mutations on synaptic biology (Pak et al., 2015). These considerations have led to a note of caution about focusing exclusively on animal studies for preclinical studies and spurred efforts to study the biology relevant to neuropsychiatric diseases in human neuronal cells (van der Worp et al., 2010, Rice, 2012, Haggarty and Perlis, 2014).
Until recently, a significant hurdle to the development of novel therapeutics has been the inability to study live human neurons in the laboratory. Cellular reprogramming methods now enable generation of human iPSCs from patient fibroblasts, which can be differentiated to neurons (Okita and Yamanaka, 2011, Takahashi et al., 2007, Takahashi and Yamanaka, 2006, Brennand et al., 2014a, Brennand et al., 2014c, Brennand et al., 2011, Yu et al., 2014, Pedrosa et al., 2011, Yoon et al., 2014, Wen et al., 2014, Vaccarino et al., 2011, Chen et al., 2014). Given the complexity of brain development and diversity of neuronal subtypes, generation and identification of specific neuronal subtypes may seem daunting. However, there have been recent methodological advances in human iPSC differentiation along specific neuronal lineages (Shi et al., 2012a, Shi et al., 2012c, Yu et al., 2014, Mariani et al., 2012). These advances enable the study of disease-related features in specific neuronal subtypes derived from patient iPSCs. We review here the current status of the field in studying patient iPSC-derived neurons in SCZ and BPD, with a discussion focused on approaches that incorporate environmental factors and clinical information in order to discover disease signatures that can lead to novel therapeutics.
Stem cell models in schizophrenia
There have been a number of promising studies of iPSC-derived neurons in SCZ (Brennand et al., 2014c). In the initial study of iPSC-derived neurons in patients diagnosed with SCZ, an experimental approach that involved transmission of modified rabies virus in differentiated neuronal cultures was used to show that neurons from SCZ patients had decreased neural connectivity, decreased neurites and decreased levels of the synaptic protein PSD95, even though they showed normal physiological properties by whole-cell patch recordings and calcium imaging (Brennand et al., 2011). Gene expression patterns of the SCZ neurons revealed altered expression of genes involved in Wnt signaling, cAMP signaling and glutamate receptors (Brennand et al., 2011). Another study with neural progenitor cells (NPCs) that focused on gene expression and proteomics found abnormalities in cytoskeletal remodeling and oxidative stress in NPCs from SCZ patients (Brennand et al., 2014a).
A number of studies have also been done with SCZ patients that carry specific disease-related genetic abnormalities. iPSCs from a SCZ patient with 22q11.2 del (velocardiofacial syndrome) showed deficits in the down regulation of pluripotency-related genes during neuronal differentiation (Pedrosa et al., 2011). Further studies with iPSC-derived neurons from SCZ 22q11.2 del patients showed altered miRNA expression profiles that recapitulated previously described patterns in postmortem brains and peripheral cells (Zhao et al., 2015). 15q11.2 CNVs have been reported as risk factors for SCZ (Stefansson et al., 2008, Consortium, 2008) and iPSCs from subjects with 15q11.2 del have also been studied. These studies showed that the NPCs derived from these iPSCs had abnormalities in adherens junctions and apical polarity (Yoon et al., 2014). Disrupted in Schizophrenia 1 (DISC1) is another gene associated with SCZ, as well as with other psychiatric disorders (Chubb et al., 2008, Mackie et al., 2007). iPSCs from family members carrying a frame-shift DISC1 mutation were differentiated along the forebrain lineage and were found to have synaptic deficits and dysregulation of many synaptic genes (Wen et al., 2014). Isogenic iPSC lines generated by gene editing showed that mutant DISC1 depleted wild-type DISC1 protein and led to abnormalities in synaptic vesicle release (Wen et al., 2014). While these studies focuses on NPCs and cortical neurons, another study examined iPSC-derived hippocampal neurons from SCZ patients, and showed that hippocampal NPCs had reduced neuronal activity and resulted in deficits in generation of DG granule neurons (Yu et al., 2014).
Stem cell models in bipolar disorder
In the first study of iPSC-derived neurons in BPD, NPCs and neurons from three patients and three subjects were studied (Chen et al., 2014). This study found that while the iPSC transcriptomes were not different between BPD and controls, there were significant differences in the neuronal transcriptomes, with increased expression of ion channels and membrane-bound receptors in BPD neurons. Neurons from control iPSCs were found to express genes involved in dorsal telencephalic fate specification while BPD neurons expressed transcripts for ventral fate specification. This study also found that the calcium transients and wave amplitudes in BPD neurons were significantly decreased by exposure to lithium compared to control neurons (Chen et al., 2014). Another study of NPCs from two BPD patients and their unaffected parents found significant differences in neurogenesis and in expression of genes involved in the WNT signaling and ion channel subunits (Madison et al., 2015). In addition, overexpression of miR-34a, which targets multiples genes implicated in BPD, was shown to result in abnormalities in neuronal differentiation and morphology as well as in the expression of synaptic proteins (Bavamian et al., 2015).
A recent study examined hippocampal dentate gyrus (DG) granule cell-like neurons differentiated from BPD patients and controls(Mertens et al., 2015). Gene expression studies of the patient-derived neurons suggested mitochondrial abnormalities in young neurons from BPD subjects while functional studies revealed hyperexcitability in these BPD neurons. Moreover, they compared the hyperexcitability phenotypes in neurons from patients who were lithium responders and non-responders and found that lithium selectively decreased the hyperexcitable phenotype only in neurons from the responders, and not in neurons from the non-responders (Mertens et al., 2015).
Searching for disease phenotypes – the relevance of cell type
Brain imaging studies of patients consistently show that patients with SCZ and BPD have enlarged ventricles, indicating loss of cortical volume (Steen et al., 2006, Arnone et al., 2009). Patients with SCZ and BPD both show gray matter loss in the cortex, though in different areas of the brain (Sheline, 2003). BPD patients treated with lithium show greater gray matter density compared to untreated patients (Bearden et al., 2007). In addition to findings in the cortex, abnormalities have also been reported in hippocampal volumes in SCZ and BPD (Heckers and Konradi, 2010), specifically in dentate gyrus (DG) and cornu ammonis 3 (CA3) (Mathew et al., 2014, Tamminga et al., 2012, Tamminga et al., 2010). In studies of patients with BPD, treatment with lithium was associated with larger hippocampal subfield volumes (Giakoumatos et al., 2015, Yucel et al., 2007, Yucel et al., 2008).
Postmortem studies in SCZ and BPD do not show any gross pathological abnormalities in the brain. In SCZ, postmortem studies show decreased neural stem cell proliferation in the dentate gyrus (Reif et al., 2006) and indicate deficits in GABAergic neurons (Lewis et al., 2005, Benes and Berretta, 2001). Postmortem SCZ and BPD brains show well replicated but subtle differences in the brain – pyramidal neurons in cortical layer III, but not in other cortical layers, show decreased dendritic spine density and fewer synapses (Glantz and Lewis, 2000, Glausier and Lewis, 2013, Rosoklija et al., 2000, Konopaske et al., 2014). In BPD, postmortem brains also show decreased glial cells in the subgenual prefrontal cortex (Ongür et al., 1998). In postmortem studies of hippocampal tissue, CA3 neurons from SCZ subjects showed increased levels of PSD95 and GluN2B–containing NMDA receptors, as well as a higher number of thorny excrescences and increased dendritic spine density in CA3 neurons, suggesting increased excitatory signaling in CA3 neurons in SCZ (Li et al., 2015).
Studies to date in SCZ and BPD have included investigations in iPSCs, NPCs, differentiated cortical cultures, and hippocampal DG granule-cell like neurons. The postmortem studies can guide decisions on neuronal subtypes that are most likely to reveal disease-related differences. Recent advances in neuronal differentiation of human iPSCs enable the generation of many of the neuronal subtypes of interest in the study of SCZ and BPD, including cortical neurons with dual-SMAD inhibition (Figure 1) (Shi et al., 2012c, Shi et al., 2012a, Mariani et al., 2012) and with Neurogenin-2 overexpression (Zhang et al., 2013), hippocampal dentate gyrus (DG) granule neurons (Figure 2) (Mertens et al., 2015, Yu et al., 2014) and cortical interneurons (Nicholas et al., 2013, Maroof et al., 2013). Human iPSC-derived neurons have been shown to exhibit appropriate functional properties in Ca2+ imaging experiments and in patch clamp studies (Kim et al., 2011, Prè et al., 2014, Paşca et al., 2011, Shcheglovitov et al., 2013). Ca2+ imaging of the iPSC-derived cortical neurons display spontaneous oscillations and bursts of Ca2+ fluorescence at baseline and respond robustly and reliably to a depolarizing stimulus with KCl (Figure 3). Studies in SCZ and BPD that have used specific neuronal subtypes derived from iPSCs have focused on cortical and hippocampal neurons (Wen et al., 2014, Mertens et al., 2015, Yu et al., 2014). Investigations focused on disease biology often aim to study pure populations of specific neuronal subtypes. However, it can also be argued that studying different neuronal subtypes in a more heterogeneous neuronal culture may better reflect the natural physiological surroundings of such neurons in vivo. The optimal nature of the cell populations to be studied will depend on the type of experiments planned, i.e. while homogeneous cell populations may be suitable for gene-expression studies and assay development for high-throughput screens, heterogeneous cultures with glial cells may be more suitable for detailed studies of neuronal morphological features.
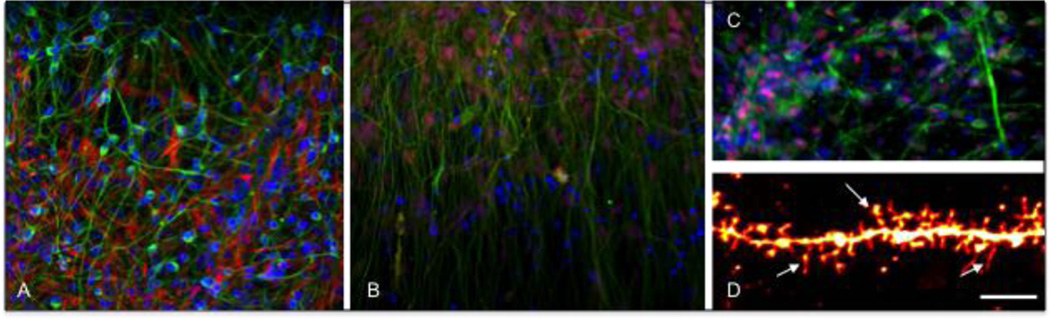
Figure 1.
Human iPSC-derived human cortical neurons. A. Differentiated cultures with neurons and glial cell: β-III tubulin (green), GFAP (red), DAPI (blue) B. Upper-layer cortical neurons : β-III tubulin (green), layer II-IV marker Brn2 (red), DAPI (blue). C. Deep-layer cortical neurons : β-III tubulin (green), layer VI marker Tbr1 (red), DAPI (blue) D. High-magnification image of a dendrite stained with DiI, arrows pointing to dendritic spines. Scale bar: 10 µm.
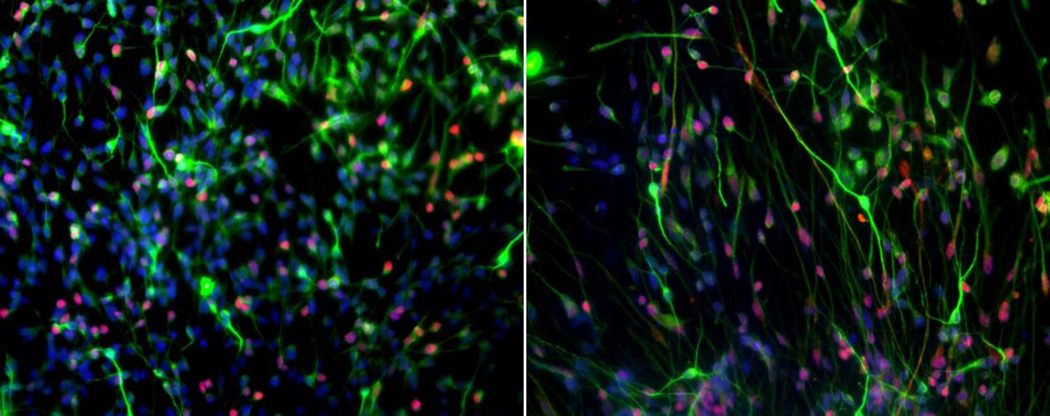
Figure 2.
Human iPSC-derived human hippocampal neurons. : MAP2 (green), PROX1 (red), DAPI (blue).
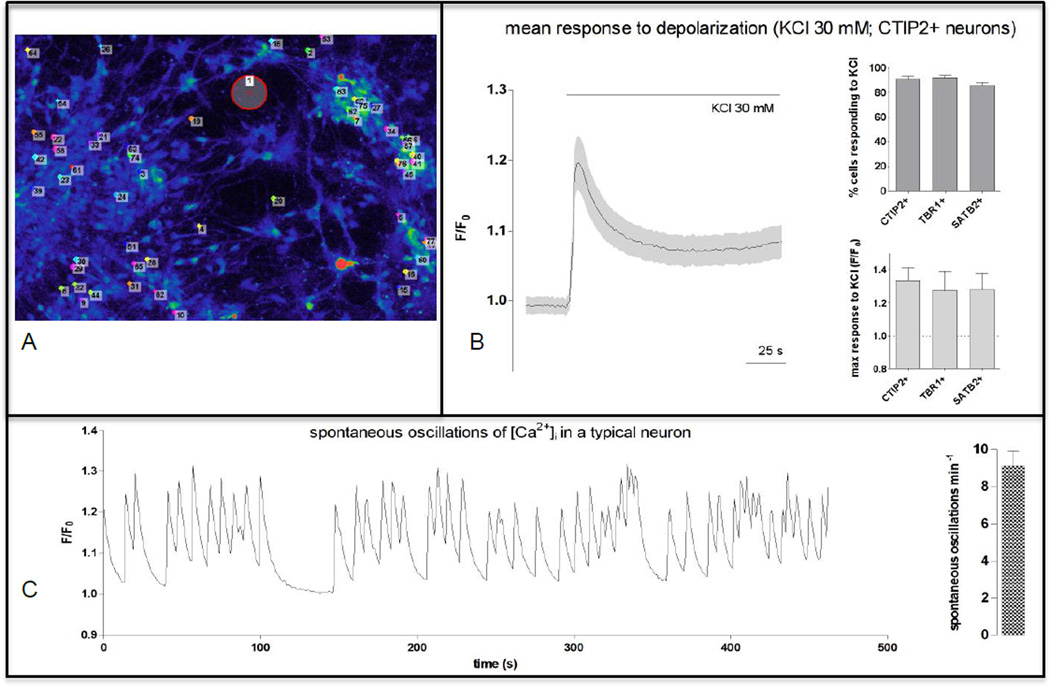
Figure 3.
Ca2+ imaging of human iPSC-derived cortical neurons shows characteristics of functional neurons. A. Neurons incubated with Ca2+ indicator Fluo-4 AM, for measurement of intra-neuronal Ca2+ flux. Immunocytochemistry performed to enable identification of responses belonging to neurons that express cortical markers SATB2, TBR1, and CTIP2. B. >90% of neurons responded to depolarizing stimulus (KCl 30mM). C. Neurons displayed spontaneous oscillations and bursts of Ca2+ fluorescence at rest.
In addition to neuronal differentiation of human iPSCs, there have also been methodological advances in direct induction of somatic cells into neurons, using forced expression of the neurogenic transcription factors Brn2, Ascl1 and Myt1l (Pang et al., 2011) as well as with the microRNAs miR-9/9* and miR-124 (Yoo et al., 2011). While direct reprogramming of human fibroblasts have resulted in the generation of excitatory neurons (Pang et al., 2011, Yoo et al., 2011, Qiang et al., 2011) dopaminergic neurons (Pfisterer et al., 2011, Caiazzo et al., 2011) striatal medium spiny neurons (Victor et al., 2014) and spinal motor neurons (Son et al., 2011), differentiation protocols for human iPSCs lend themselves to the generation of a diverse array of neuronal subtypes from different niches in the human brain, including specific cortical and hippocampal populations that are of special interest in the disease biology of schizophrenia and bipolar disorder (Brennand et al., 2015).
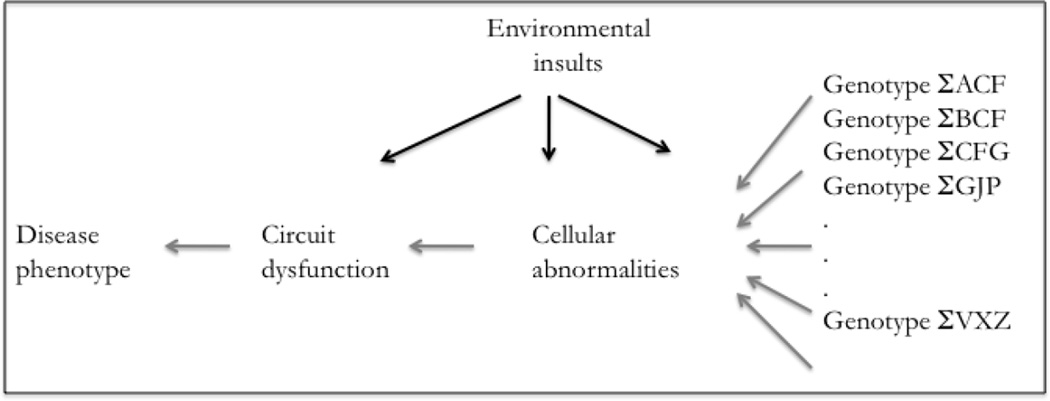
The studies to date in SCZ and BPD iPSC-derived neurons have been carried out in adherent monolayer cultures and focused on abnormalities at the cellular level. This approach assumes that the effects of the underlying complex genetic vulnerabilities converge at the cellular level (Figure 4). Recent developments in the generation of three-dimensional culture systems and organoids present the opportunity to study disease biology in more complex environments and to possibly study the biology at the circuit level. Cerebral organoids can generate three-dimensional neuronal structures that mimic the organizational aspects of the human cortex (Mariani et al., 2012, Lancaster and Knoblich, 2014, Lancaster et al., 2013). Methods have also been developed to generate iPSC-derived human cortical spheroids that recapitulate aspects of human cortical development and organizations (Paşca et al., 2015). Another approach to the generation of three-dimensional networks of stem cell derived neurons has included the use of a matrigel-based support matrix that allow NPCs to differentiate into complex networks in vitro (Choi et al., 2014, Kim et al., 2015). While these three-dimensional approaches have been used to study the disease biology of Alzheimer’s disease and microcephaly, they have yet to be used in the study of SCZ and BPD. Given significant evidence for circuit-level dysfunction in SCZ and BPD (Baker et al., 2014), three-dimensional approaches may lend themselves to models where rudimentary circuits can be formed and interrogated for dysfunctions at the circuit level. Alternatively, microelectrode arrays can also be utilized in conjunction with adherent monolayer cultures to study network function/dysfunction in vitro in neurons from patients and controls (Obien et al., 2014).
Figure 4.
Schematic model for interactions of genetic predisposition with environmental factors that result in cellular and circuit abnormalities leading to disease phenotype.
Uncovering disease phenotypes – the role for perturbations
Complex psychiatric disorders that have strong gene-environment interactions are difficult to model in vitro. Environmental factors impinge on the underlying genetic vulnerability of SCZ and BPD, which results from small contributions from many different genes, for manifestation of the disease. The fact that monozygotic twin concordance in SCZ and BPD is ~ 50%, and not 100% (Davis et al., 1995, Kieseppä et al., 2004, Kety et al., 1971), suggests that additional environmental factors are involved in SCZ pathogenesis (Brown, 2011). While multiple environmental factors have been implicated in SCZ and BPD (van Os et al., 2008, Brown, 2011), there is no easy way to translate such environmental factors into specific cellular perturbations. Cellular stress has been used to uncover underlying disease-related vulnerabilities in cellular systems. In a study mitochondrial electron transport gene expression in lymphocytes from BPD cases and healthy controls, there were no differences when cells were cultured under normal conditions. However, when lymphocytes were cultured in low-glucose conditions, the BPD lymphocytes had a markedly aberrant response compared to cells from healthy controls (Naydenov et al., 2007). Cellular perturbation can be to undertaken in a larger scale in an unbiased way using a range of small molecules that interfere uniquely with different signaling pathways (Seashore-Ludlow et al., 2015). Cellular pathways involved in disease biology can potentially be identified systematically by perturbing specific pathways and identifying perturbations that lead to differential responses in patient cells but not in control cells (Basu et al., 2013).
Co-culture models
Immunological mechanisms have also been implicated in the pathophysiology of SCZ and BPD (Khandaker et al., 2015, Khandaker and Dantzer, 2015, Barbosa et al., 2014). A major neurodevelopmental process that takes place in the adolescent brain is that of synaptic pruning, a process by which superfluous excitatory synaptic connections are eliminated (Petanjek et al., 2011, Rakic et al., 1986, Zecevic et al., 1989, Huttenlocher and Dabholkar, 1997, Giedd et al., 1999). Synaptic pruning during this critical period is hypothesized to be aberrant in SCZ, the same time frame when most patients have their first psychotic break (Feinberg, 1982, Lieberman, 2006, Selemon and Zecevic, 2015). The excessive elimination of synaptic connections during the pruning process in SCZ is believed to result in greater loss of cortical gray matter volume during adolescence and result in decreased dendritic spines in the pyramidal neurons in specific cortical layers (Lewis and Sweet, 2009, Garey et al., 1998, Glantz and Lewis, 2000, Konopaske et al., 2014, Rosoklija et al., 2000, Rapoport et al., 1999, McGlashan and Hoffman, 2000, Selemon and Zecevic, 2015). Microglia, the resident macrophages in the brain, play a central role in synaptic pruning (Kettenmann et al., 2013, Bilimoria and Stevens, 2015). Postmortem studies in SCZ show microglial activation and infiltration in the cortex (Fillman et al., 2013, Radewicz et al., 2000, Steiner et al., 2008, Steiner et al., 2006). In addition, in vivo positron emission tomography (PET) studies in patients show strong evidence for increased microglial activity in the brain in SCZ (van Berckel et al., 2008, Bloomfield et al., 2015, Doorduin et al., 2009). Recent developments in directed differentiation methods now enable the generation of microglial cells from human monocytes (Ohgidani et al., 2015, Ohgidani et al., 2014). This method provides new approaches for co-culturing human iPSC-derived cortical neurons with microglial cells derived from monocytes isolated from the same patient.
Harnessing clinical information – modeling medication response in vitro
The heterogeneity of clinical presentations and genetic makeup often create a hurdle in research efforts, especially when large number of samples cannot be studied (Dacquino et al., 2015). A clinical feature that can be used to validate cellular phenotypes is the pattern of treatment response in patients. An example of such an approach was recently described in the study of iPSC-derived hippocampal DG granule cell-like neurons in BPD. The study found that young neurons in this hippocampal lineage showed a hyperexcitable phenotype, with increased numbers of spontaneous and evoked action potentials (Mertens et al., 2015). They further studied these neurons in two groups of BPD patients – patients that had good therapeutic response to lithium, and patients that did not respond well. When such young neurons were studied in the presence of lithium, only neurons from the lithium responders showed decreased hyperexcitability in the presence of lithium, while neurons from lithium non-responders showed no such change (Mertens et al., 2015). This is an intriguing example of supporting the validity of cellular phenotypes by correlating effects of small molecules in vitro with patterns of medication response in patients.
Conclusion
SCZ and BPD are complex psychiatric disorders that have their symptomatic onset in late adolescence or early adulthood. The disorders become manifest in brains that have been developing over two decades, with concomitant interactions with various environmental factors that impinge on their underlying genetic backgrounds. We are attempting to use iPSC-derived neurons from patients, often in two-dimensional neuronal cultures differentiated over a few weeks, to capture the crux of the disease biology that develop in the most complex organ of the human body over many years. While this prospect seems daunting, there have been many important advances in the last few years that provide a compelling rationale to pursue this quest for cellular disease signatures. New approaches that incorporate environmental perturbations as well as co-cultures with other relevant cell types are potential avenues that can aid in modeling the complexity in these disorders. In addition to a providing us with a better understanding of the cellular-molecular features of SCZ and BPD, identification of robust, reliable and valid cellular disease signatures will enable us to develop assays that can be used in high-throughput screens to discover promising small-molecule leads for therapeutic development.
Acknowledgments
Our research has been supported by funding from the National Institute of Mental Health, Doris Duke Charitable Foundation, Harvard Stem Cell Institute, Ryan Licht Sang Bipolar Foundation, Phyllis & Jerome Lyle Rappaport Foundation and from Steve Willis and Elissa Freud.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, Mcintosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, ÖNGÜR D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini RJ, Frankenburg FR. Clozapine. A novel antipsychotic agent. N Engl J Med. 1991;324:746–754. doi: 10.1056/NEJM199103143241107. [DOI] [PubMed] [Google Scholar]
- Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3:495–500. [PMC free article] [PubMed] [Google Scholar]
- Barbosa IG, Machado-Vieira R, Soares JC, Teixeira AL. The immunology of bipolar disorder. Neuroimmunomodulation. 2014;21:117–122. doi: 10.1159/000356539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Bodycombe NE, Cheah JH, Price EV, Liu K, Schaefer GI, Ebright RY, Stewart ML, Ito D, Wang S, Bracha AL, Liefeld T, Wawer M, Gilbert JC, Wilson AJ, Stransky N, Kryukov GV, Dancik V, Barretina J, Garraway LA, Hon CS, Munoz B, Bittker JA, Stockwell BR, Khabele D, Stern AM, Clemons PA, SHAMJI AF, SCHREIBER SL. An interactive resource to identify cancer genetic and lineage dependencies targeted by small molecules. Cell. 2013;154:1151–1161. doi: 10.1016/j.cell.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, Madison JM, Zhou F, Rueckert EH, Barker D, Perlis RH, Sur M, Haggarty SJ. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol Psychiatry. 2015;20:573–584. doi: 10.1038/mp.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, Trakhtenbroit M, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bilimoria PM, Stevens B. Microglia function during brain development: New insights from animal models. Brain Res. 2015;1617:7–17. doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, Mcguire P, De Paola V, Howes OD. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.14101358. appiajp201514101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, Beaumont KG, Kim HJ, Topol A, Ladran I, Abdelrahim M, Matikainen-Ankney B, Chao SH, Mrksich M, Rakic P, Fang G, Zhang B, Yates JR, 3rd, Gage FH. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2014a doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Landek-Salgado MA, Sawa A. Modeling Heterogeneous Patients With a Clinical Diagnosis of Schizophrenia With Induced Pluripotent Stem Cells. Biol Psychiatry. 2014c;75:936–944. doi: 10.1016/j.biopsych.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Marchetto MC, Benvenisty N, BrÜStle O, Ebert A, Izpisua Belmonte JC, Kaykas A, Lancaster MA, Livesey FJ, Mcconnell MJ, Mckay RD, Morrow EM, Muotri AR, Panchision DM, Rubin LL, Sawa A, Soldner F, Song H, Studer L, Temple S, Vaccarino FM, WU J, Vanderhaeghen P, Gage FH, Jaenisch R. Creating Patient-Specific Neural Cells for the In Vitro Study of Brain Disorders. Stem Cell Reports. 2015;5:933–945. doi: 10.1016/j.stemcr.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, Mccarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2:349–352. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Case M, Stauffer VL, Ascher-Svanum H, Conley R, Kapur S, Kane JM, Kollack-Walker S, Jacob J, Kinon BJ. The heterogeneity of antipsychotic response in the treatment of schizophrenia. Psychol Med. 2011;41:1291–1300. doi: 10.1017/S0033291710001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HM, Delong CJ, Bame M, Rajapakse I, Herron TJ, Mcinnis MG, O’Shea KS. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Translational Psychiatry. 2014;4:e375. doi: 10.1038/tp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Consortium C-DGOTPG. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium IS. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium SWGOTPG. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacquino C, De Rossi P, Spalletta G. Schizophrenia and bipolar disorder: The road from similarities and clinical heterogeneity to neurobiological types. Clin Chim Acta. 2015;449:49–59. doi: 10.1016/j.cca.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Davis JO, Phelps JA, Bracha HS. Prenatal development of monozygotic twins and concordance for schizophrenia. Schizophr Bull. 1995;21:357–366. doi: 10.1093/schbul/21.3.357. [DOI] [PubMed] [Google Scholar]
- Doorduin J, De Vries EF, Willemsen AT, De Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Mcglashan TH. Natural history of schizophrenia subtypes. I. Longitudinal study of paranoid, hebephrenic, and undifferentiated schizophrenia. Arch Gen Psychiatry. 1991a;48:969–977. doi: 10.1001/archpsyc.1991.01810350009002. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Mcglashan TH. Natural history of schizophrenia subtypes. II. Positive and negative symptoms and long-term course. Arch Gen Psychiatry. 1991b;48:978–986. doi: 10.1001/archpsyc.1991.01810350018003. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, Mccrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Houenou J, Jamain S, Goldstein BI, Frye MA, Leboyer M, Berk M, Malhi GS, Lopez-Jaramillo C, Taylor VH, Dodd S, Frangou S, Hall GB, Fernandes BS, Kauer-Sant’Anna M, Yatham LN, Kapczinski F, Young LT. Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust N Z J Psychiatry. 2013;47:321–332. doi: 10.1177/0004867413478217. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva L, Rees E, Moran JL, Chambert KD, Milanova V, Craddock N, Purcell S, Sklar P, Mccarroll S, Holmans P, O’Donovan MC, Owen MJ, Kirov G. De novo CNVs in bipolar affective disorder and schizophrenia. Hum Mol Genet. 2014;23:6677–6683. doi: 10.1093/hmg/ddu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakoumatos CI, Nanda P, Mathew IT, Tandon N, Shah J, Bishop JR, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA, Keshavan MS. Effects of lithium on cortical thickness and hippocampal subfield volumes in psychotic bipolar disorder. J Psychiatr Res. 2015;61:180–187. doi: 10.1016/j.jpsychires.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FK, Ghaemi SN. The course of bipolar disorder and the nature of agitated depression. Am J Psychiatry. 2003;160:2077–2079. doi: 10.1176/appi.ajp.160.12.2077. [DOI] [PubMed] [Google Scholar]
- Group PGCBDW. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. doi: 10.1001/jama.296.14.1731. [DOI] [PubMed] [Google Scholar]
- Haggarty SJ, Perlis RH. Translation: Screening for Novel Therapeutics With Disease-Relevant Cell Types Derived from Human Stem Cell Models. Biol Psychiatry. 2014;75:952–960. doi: 10.1016/j.biopsych.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Keck PE, Mcelroy SL, Havens JR, Altshuler LL, Nolen WA, Frye MA, Suppes T, Denicoff KD, Kupka R, Leverich GS, Rush AJ, Post RM. Psychosis in bipolar disorder: phenomenology and impact on morbidity and course of illness. Compr Psychiatry. 2003;44:263–269. doi: 10.1016/S0010-440X(03)00089-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19:261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum H, Demler O, Falloon IR, Gagnon E, Guyer M, Howes MJ, Kendler KS, Shi L, Walters E, Wu EQ. The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R) Biol Psychiatry. 2005;58:668–676. doi: 10.1016/j.biopsych.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Kety SS, Rosenthal D, Wender PH, Schulsinger F. Mental illness in the biological and adoptive families of adpoted schizophrenics. Am J Psychiatry. 1971;128:302–306. doi: 10.1176/ajp.128.3.302. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Dantzer R. Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KieseppÄ T, Partonen T, Haukka J, Kaprio J, LÖNnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–1821. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- Kim JE, O’Sullivan ML, Sanchez CA, Hwang M, Israel MA, Brennand K, Deerinck TJ, Goldstein LS, Gage FH, Ellisman MH, Ghosh A. Investigating synapse formation and function using human pluripotent stem cell-derived neurons. Proc Natl Acad Sci U S A. 2011;108:3005–3010. doi: 10.1073/pnas.1007753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Choi SH, D’Avanzo C, Hebisch M, Sliwinski C, Bylykbashi E, Washicosky KJ, Klee JB, BrÜStle O, Tanzi RE, Kim DY. A 3D human neural cell culture system for modeling Alzheimer’s disease. Nat Protoc. 2015;10:985–1006. doi: 10.1038/nprot.2015.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal Cortical Dendritic Spine Pathology in Schizophrenia and Bipolar Disorder. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ghose S, Gleason K, Begovic A, Perez J, Bartko J, Russo S, Wagner AD, Selemon L, Tamminga CA. Synaptic proteins in the hippocampus indicative of increased neuronal activity in CA3 in schizophrenia. Am J Psychiatry. 2015;172:373–382. doi: 10.1176/appi.ajp.2014.14010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, BjÖRk C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA. Neurobiology and the natural history of schizophrenia. J Clin Psychiatry. 2006;67:e14. [PubMed] [Google Scholar]
- LÓPez-MuÑOz F, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G. History of the discovery and clinical introduction of chlorpromazine. Ann Clin Psychiatry. 2005;17:113–135. doi: 10.1080/10401230591002002. [DOI] [PubMed] [Google Scholar]
- Mackie S, Millar JK, Porteous DJ. Role of DISC1 in neural development and schizophrenia. Curr Opin Neurobiol. 2007;17:95–102. doi: 10.1016/j.conb.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Madison JM, Zhou F, Nigam A, Hussain A, Barker DD, Nehme R, Van Der Ven K, Hsu J, Wolf P, Fleishman M, O’Dushlaine C, Rose S, Chambert K, Lau FH, Ahfeldt T, Rueckert EH, Sheridan SD, Fass DM, Nemesh J, Mullen TE, Daheron L, Mccarroll S, Sklar P, Perlis RH, Haggarty SJ. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol Psychiatry. 2015;20:703–717. doi: 10.1038/mp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL, Vaccarino FM. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, Widmer HR, Eggan K, Goldstein PA, Anderson SA, Studer L. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ, Clementz B, Pearlson GD, Sweeney JA, Tamminga CA, Keshavan MS. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–777. doi: 10.1001/jamapsychiatry.2014.453. [DOI] [PubMed] [Google Scholar]
- Mcglashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Mcguffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Medicine IO. Improving the Utility and Translation of Animal Models for Nervous System Disorders: Workshop Summary. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J, Wang QW, Kim Y, Yu DX, Pham S, Yang B, Zheng Y, Diffenderfer KE, Zhang J, Soltani S, Eames T, Schafer ST, Boyer L, Marchetto MC, Nurnberger JI, Calabrese JR, ØDegaard KJ, Mccarthy MJ, Zandi PP, Alba M, Nievergelt CM, Mi S, Brennand KJ, Kelsoe JR, Gage FH, Yao J Study P. O. B. D. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527:95–99. doi: 10.1038/nature15526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naydenov AV, Macdonald ML, Ongur D, Konradi C. Differences in lymphocyte electron transport gene expression levels between subjects with bipolar disorder and normal controls in response to glucose deprivation stress. Arch Gen Psychiatry. 2007;64:555–564. doi: 10.1001/archpsyc.64.5.555. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, Sasai Y, Alvarez-Buylla A, Rubenstein JL, Kriegstein AR. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obien ME, Deligkaris K, Bullmann T, Bakkum DJ, Frey U. Revealing neuronal function through microelectrode array recordings. Front Neurosci. 2014;8:423. doi: 10.3389/fnins.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgidani M, Kato TA, Kanba S. Introducing directly induced microglia-like (iMG) cells from fresh human monocytes: a novel translational research tool for psychiatric disorders. Front Cell Neurosci. 2015;9:184. doi: 10.3389/fncel.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgidani M, Kato TA, Setoyama D, Sagata N, Hashimoto R, Shigenobu K, Yoshida T, Hayakawa K, Shimokawa N, Miura D, Utsumi H, Kanba S. Direct induction of ramified microglia-like cells from human monocytes: dynamic microglial dysfunction in Nasu-Hakola disease. Sci Rep. 2014;4:4957. doi: 10.1038/srep04957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Yamanaka S. Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci. 2011;366:2198–2207. doi: 10.1098/rstb.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OngÜR D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak C, Danko T, Zhang Y, Aoto J, Anderson G, Maxeiner S, Yi F, Wernig M, SÜDhof TC. Human Neuropsychiatric Disease Modeling using Conditional Deletion Reveals Synaptic Transmission Defects Caused By Heterozygous Mutations in NRXN1. Cell Stem Cell. 2015 doi: 10.1016/j.stem.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a reexamination. Arch Gen Psychiatry. 2005;62:247–253. doi: 10.1001/archpsyc.62.3.247. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, SÜDhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PaŞCa AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O’Rourke NA, Nguyen KD, Smith SJ, Huguenard JR, Geschwind DH, Barres BA, PaŞCa SP. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PaŞCa SP, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, PaŞCa AM, Cord B, Palmer TD, Chikahisa S, Nishino S, Bernstein JA, Hallmayer J, Geschwind DH, Dolmetsch RE. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa E, Sandler V, Shah A, Carroll R, Chang C, Rockowitz S, Guo X, Zheng D, Lachman HM. Development of patient-specific neurons in schizophrenia using induced pluripotent stem cells. J Neurogenet. 2011;25:88–103. doi: 10.3109/01677063.2011.597908. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, JudaŠ M, ŠImic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, BjÖRklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Buckley PF. Reliable biomarkers and predictors of schizophrenia and its treatment. Psychiatr Clin North Am. 2012;35:645–659. doi: 10.1016/j.psc.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Pompili M, Gonda X, Serafini G, Innamorati M, Sher L, Amore M, Rihmer Z, Girardi P. Epidemiology of suicide in bipolar disorders: a systematic review of the literature. Bipolar Disord. 2013;15:457–490. doi: 10.1111/bdi.12087. [DOI] [PubMed] [Google Scholar]
- Pouget JG, MÜLler DJ. Pharmacogenetics of antipsychotic treatment in schizophrenia. Methods Mol Biol. 2014;1175:557–587. doi: 10.1007/978-1-4939-0956-8_14. [DOI] [PubMed] [Google Scholar]
- pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I, Group RATSR. Where is the evidence that animal research benefits humans? BMJ. 2004;328:514–517. doi: 10.1136/bmj.328.7438.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrÈ D, Nestor MW, Sproul AA, Jacob S, Koppensteiner P, Chinchalongporn V, Zimmer M, Yamamoto A, Noggle SA, Arancio O. A time course analysis of the electrophysiological properties of neurons differentiated from human induced pluripotent stem cells (iPSCs) PLoS One. 2014;9:e103418. doi: 10.1371/journal.pone.0103418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, Moreno H, Abeliovich A. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, Nicolson R, Bedwell J, Lenane M, Zijdenbos A, Paus T, Evans A. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Rice J. Animal models: Not close enough. Nature. 2012;484:S9. doi: 10.1038/nature11102. [DOI] [PubMed] [Google Scholar]
- RodovÁ A, Svestka J, NÁHunek K, CeskovÁ E. A blind comparison of clozapine and perphenazine in schizophrenics. Act Nerv Super (Praha) 1973;15:94–95. [PubMed] [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Salvatore P, Tohen M, Khalsa HM, Baethge C, Tondo L, Baldessarini RJ. Longitudinal research on bipolar disorders. Epidemiol Psichiatr Soc. 2007;16:109–117. doi: 10.1017/s1121189x00004711. [DOI] [PubMed] [Google Scholar]
- Seashore-Ludlow B, Rees MG, Cheah JH, Cokol M, Price EV, Coletti ME, Jones V, Bodycombe NE, Soule CK, Gould J, Alexander B, Li A, Montgomery P, Wawer MJ, Kuru N, Kotz JD, Hon CS, Munoz B, Liefeld T, DanČÍK V, Bittker JA, Palmer M, Bradner JE, Shamji AF, Clemons PA, Schreiber SL. Harnessing Connectivity in a Large-Scale Small-Molecule Sensitivity Dataset. Cancer Discov. 2015;5:1210–1223. doi: 10.1158/2159-8290.CD-15-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10:515–531. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- Seeman P. Schizophrenia and dopamine receptors. Eur Neuropsychopharmacol. 2013;23:999–1009. doi: 10.1016/j.euroneuro.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. doi: 10.1038/tp.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, Mcdonald-Smith GP, Gao H, Hennessy L, Finnerty CC, LÓPez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG Inflammation and host response to injury L. R. S. C. R. P. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheglovitov A, Shcheglovitova O, Yazawa M, Portmann T, Shu R, Sebastiano V, Krawisz A, Froehlich W, Bernstein JA, Hallmayer JF, Dolmetsch RE. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature. 2013;503:267–271. doi: 10.1038/nature12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012a;7:1836–1846. doi: 10.1038/nprot.2012.116. [DOI] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012c;15:477–486. doi: 10.1038/nn.3041. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, Mcclure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, PietilÄInen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, MÖLler HJ, Hartmann A, Shianna KV, Ge D, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, MÜHleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, NÖThen MM, Peltonen L, Collier DA, St Clair D, Stefansson K& group. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG, Bogerts B. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Suzuki T, Uchida H, Watanabe K, Mimura M. Antipsychotic treatment for schizophrenia in the maintenance phase: a systematic review of the guidelines and algorithms. Schizophr Res. 2012;134:219–225. doi: 10.1016/j.schres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Southcott S, Sacco C, Wagner AD, Ghose S. Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophr Bull. 2012;38:927–935. doi: 10.1093/schbul/sbs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Stevens HE, Kocabas A, Palejev D, Szekely A, Grigorenko EL, Weissman S. Induced pluripotent stem cells: a new tool to confront the challenge of neuropsychiatric disorders. Neuropharmacology. 2011;60:1355–1363. doi: 10.1016/j.neuropharm.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Van Der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor MB, Richner M, Hermanstyne TO, Ransdell JL, Sobieski C, Deng PY, Klyachko VA, Nerbonne JM, Yoo AS. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron. 2014;84:311–323. doi: 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, Kim NS, Yoon KJ, Shin J, Zhang C, Makri G, Nauen D, Yu H, Guzman E, Chiang CH, Yoritomo N, Kaibuchi K, Zou J, Christian KM, Cheng L, Ross CA, Margolis RL, Chen G, Kosik KS, Song H, Ming GL. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014 doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Nguyen HN, Ursini G, Zhang F, Kim NS, Wen Z, Makri G, Nauen D, Shin JH, Park Y, Chung R, Pekle E, Zhang C, Towe M, Hussaini SM, Lee Y, Rujescu D, St Clair D, Kleinman JE, Hyde TM, Krauss G, Christian KM, Rapoport JL, Weinberger DR, Song H, Ming GL. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell. 2014;15:79–91. doi: 10.1016/j.stem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, Mei A, Mchenry L, Lisuk D, Grasmick JM, Silberman P, Silberman G, Jappelli R, Gage FH. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Reports. 2014;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel K, Mckinnon MC, Taylor VH, Macdonald K, Alda M, Young LT, Macqueen GM. Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Psychopharmacology (Berl) 2007;195:357–367. doi: 10.1007/s00213-007-0906-9. [DOI] [PubMed] [Google Scholar]
- Yucel K, Taylor VH, Mckinnon MC, Macdonald K, Alda M, Young LT, Macqueen GM. Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology. 2008;33:361–367. doi: 10.1038/sj.npp.1301405. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989;50:11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, SÜDhof TC. Rapid sIngle-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Lin M, Chen J, Pedrosa E, Hrabovsky A, Fourcade HM, Zheng D, Lachman HM. MicroRNA Profiling of Neurons Generated Using Induced Pluripotent Stem Cells Derived from Patients with Schizophrenia and Schizoaffective Disorder, and 22q11.2 Del. PLoS One. 2015;10:e0132387. doi: 10.1371/journal.pone.0132387. [DOI] [PMC free article] [PubMed] [Google Scholar]