Abstract
Antimicrobial photocatalysis involves the UVA excitation of titanium dioxide (TiO2) nanoparticles (particularly the anatase form) to produce reactive oxygen species (ROS) that kill microbial cells. For the first time we report that the addition of sodium bromide to photoactivated TiO2 (P25) potentiates the killing of Gram-positive, Gram-negative bacteria and fungi by up to three logs. The potentiation increased with increasing bromide concentration in the range of 0–10 mM. The mechanism of potentiation is probably due to generation of both short and long-lived oxidized bromine species including hypobromite as shown by the following observations. There is some antimicrobial activity remaining in solution after switching off the light, that lasts for 30 min but not 2 hours, and oxidizes 3,3′,5,5′-tetramethylbenzidine. N-acetyl tyrosine ethyl ester was brominated in a light dose-dependent manner, however no bromine or tribromide ion could be detected by spectrophotometry or LC-MS. The mechanism appears to have elements in common with the antimicrobial system (myeloperoxidase + hydrogen peroxide + bromide).
Keywords: Antimicrobial photocatalysis, bacteria, titanium dioxide, sodium bromide, ultraviolet A, reactive oxygen species, hypobromiteAbstract
Graphical abstract
Introduction
Photocatalysis is a rapidly growing technology that is being investigated for applications in solar energy conversion [1], and for environmental remediation and disinfection [2, 3]. Antimicrobial photocatalysis describes a process in which semiconductor nanoparticles such as titanium dioxide (TiO2) are irradiated with UVA light to generate reactive oxygen species (ROS) in order to kill various types of microorganisms [4–8]. The procedure of antimicrobial photocatalysis has most often been suggested to be used in water purification [9], but other applications in medical science (orthopedics [10], dentistry [11] and surgery [12]) have also been proposed. Photoactivated TiO2 is also beginning to be studied as a possible photodynamic treatment for cancer [13], and could also be used for precise sub-cellular inactivation at a molecular level [14]. The energy needed to excite an electron from the valence band to the conduction band in TiO2 is 3.35 eV, which is equivalent to the energy of a photon with a wavelength of 360 nm. The released electrons in the conduction band can reduce oxygen to superoxide anion, while the positive holes left in the valence band can capture electrons from water, and oxidize water to hydroxyl radicals [15]. Hydrogen peroxide [16] and singlet oxygen [17] are also produced during photocatalysis. Together these oxidants can damage many biomolecules, and efficiently kill all the different classes of microorganisms, such as Gram-positive and Gram-negative bacteria, fungi, viruses and parasites, etc [18].
The advantages of antimicrobial photocatalysis (as compared to other kinds of photodynamic inactivation) are that: (1) it is a heterogeneous system so the solid TiO2 could be removed from the reaction after use; (2) the TiO2 is not easily photobleached in the same way that organic photosensitizers are photobleached by light delivery; and (3) TiO2 can be activated by UVA wavelengths present in natural sunlight making the process suitable for remediation of contaminated wastes in outdoor settings. However there is still a lot of room to improve the efficiency of TiO2 antimicrobial catalysis. Researchers are attempting to dope the TiO2 with platinum or nitrogen or other materials in order to shift the activation wavelength way from the UV to the visible range [19], and to fabricate different types of titania nanostructures such as TiO2 nanotubes [20]. Our approach is to potentiate antimicrobial photocatalysis by the addition of simple non-toxic inorganic salts. In the present study we used P25 TiO2 nanoparticles for our studies as they were commercially available and have been reported to be efficient at mediating photocatalysis. P25 has been reported to be composed of about 75% of the anatase crystalline isoform [21].
We recently discovered that antimicrobial photodynamic inactivation mediated by the phenothiazinium dye, methylene blue [22], and also by cationic functionalized fullerenes [23, 24] could be significantly potentiated by addition of the non-toxic salt, potassium iodide. We then went on to show that antimicrobial TiO2 photocatalysis could be potentiated by addition of iodide anion (manuscript in preparation). We now report for the first time that antimicrobial TiO2 photocatalysis can be significantly potentiated by addition of the non-toxic salt, sodium bromide.
Materials and methods
Chemicals
Titanium(IV) oxide (TiO2) anatase P25, sodium bromide (NaBr), myeloperoxidase from human leukocytes (MPO), 3,3′,5,5′-tetramethylbenzidine (TMB), 30% hydrogen peroxide (H2O2), and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. Hydroxyphenyl fluorescein (HPF) and Singlet Oxygen Sensor Green (SOSG) were purchased from (Molecular Probes, Invitrogen, Bedford, MA). TiO2 and NaBr stock solutions were prepared in distilled H2O (dH2O) prior to use. We have presented the concentration of TiO2 as mM (10mM = 800 μg/mL) to allow comparison with NaBr although the material is actually nanoparticles. We used two different buffers that were composed of 50 mM sodium phosphate at pH 7.4 and pH 5.5. All the photocatalysis experiments were carried out in 24-well plate under magnetic stirring except ROS-specific probe experiments.
Light source
We used a 360 nm UVA light-emitting diode (LED) light source (Larson Electronics LLC, Kemp, TX). The emission spectrum is shown in supplementary figure S1. The irradiance for all experiments was fixed at 16 mW/cm2 (I J/cm2 delivered in 1 min), measured by a model IL-1700 research radiometer-photometer (International Light, Inc., Newburyport, MA). The emission spectrum was measured by a spectroradiometer (SPR-01; Luzchem Research, Inc., Ottawa, ON, Canada) and showed a peak emission at 365±5 nm.
Fluorescence probe assay for generation of specific reactive oxygen species (ROS)
96-well clear-bottom black plates were used for fluorescence probe experiments. SOSG or HPF (final concentration of 5 μM) was added to 10 mM TiO2 with and without addition of 10 mM NaBr in a final volume of 200 μL PBS per well. The fluorescence was detected after each aliquot of 0.5–1 J/cm2 (dose of light), using a fluorescence spectrometer (SpectraMax M5 plate reader, Molecular Devices, Sunnyvale, CA) The excitation and emission settings were used as recommended by the manufacturer of the probes: excitation 504 nm and emission 525 nm for SOSG and excitation 490 nm and emission 515 nm for HPF, respectively.
3,3′,5,5′-Tetramethylbenzidine (TMB) test for hypobromite
Stock solutions of TMB were prepared in DMSO at 1 mg/ml and kept at −20°C. Working solutions of TMB were freshly prepared by diluting 1 mL of stock solution in 9 mL 50 mM sodium acetate buffer, pH 5.5. A suspension of 10mM TiO2 containing 10mM NaBr was exposed with stirring to different fluences of UVA light (0, 5, 10 and 40 J/cm2) and after each fluence a 50 μL aliquot was withdrawn and added into 1 mL 0.1mg/ml TMB solution. After 180 minutes incubation time, 200 μL of the TMB reaction mixture was transferred to 96-well plate and absorbance at 655 nm was measured using a spectrometer (SpectraMax M5 plate reader, Molecular Devices, Sunnyvale, CA).
Bromination of N-acetyl tyrosine ethyl ester
Sample solutions (total volume 400 μL) contained TiO2 (10mM), KI (100mM) and N-acetyl-L-tyrosine ethyl ester (10 mM) in PB buffer (pH 7.4, containing 10% methanol) were irradiated by UVA LED light (360nm) with magnetic stirring. An aliquot of solution (50 μL) was removed at different time point (30mins, 60mins, 120mins and 240 mins) and centrifuged at 4000 rpm. The supernatants were collected for the LCMS analysis. The LCMS analyses were performed on an Agilent 1260 LC system equipped with a triple-quad mass spectrometer. The LC conditions were: column: C18, 2.1 × 50 mm, 1.8 μm; elution gradient: solution A = acetonitrile, solution B = 10 mM ammonium acetate in water, 2% -> 100% of A over 6 min with a flow rate of 0.2 mL/min; ionization mode: negative; injection volume: 5 uL.
Tribromide generation assays
In order to detect any production of bromine we attempted to detect the presence of tribromide ion (Br3−) which is quantitatively formed from the reaction between Br2 and Br− [25]. TiO2 (10 mM) plus NaBr (10 mM) was irradiated with fluences as high as 120 J/cm2 of UVA (2 hours) with stirring. The mixture was centrifuged and the UV-vis absorption spectrum measured. There was no detectable peak at 267 nm (Br3− has a molar absorption coefficient of 40,900 at 267 nm) [25]. Moreover we used HPLC to try to identify Br3− as described by Weinberg and Yamada [25]. These authors used an anion exchange column (AS12) eluted by a carbonate/bicarbonate gradient, but we were unable to detect any peak corresponding to tribromide, while we were able to detect tribromide produced by the positive control reaction of bromine and bromide.
Bacterial strains and culture conditions
We used a methicillin-resistant Staphylococcus aureus (MRSA), CA-MRSA strain USA300 LAC (Los Angeles County clone), Escherichia coli K12 (ATCC, Manassass VA) and Candida albicans DAY286 reference strain (a gift from Aaron Mitchell, Department of Microbiology, Columbia University, New York, NY). Bacterial cells were grown in brain-heart infusion (BHI) medium at 37°C and Candida cells were grown in yeast extract peptone dextrose (YPD) media at 30°C. Cells were grown overnight to stationary phase and refreshed for 2 hours for bacteria and 4 hours for Candida the next day to mid-log phase. Cells were collected by centrifugation at 3500 pm for 10 minutes and resuspended in phosphate buffer at a density of 10(8) cells/mL for bacteria and 10(7) cells/mL for Candida for further experiments. Cell numbers were estimated by measuring the optical density [OD] at 600 nm (OD of 0.5=10(8) cells/mL). To enumerate CFU/mL a 10 μl aliquot of cells was serially diluted 10-fold in PBS to give dilutions of 10−1 to 10−5 times in addition to the original concentration, and 10μL aliquots of each dilution were streaked horizontally on square BHI (bacteria) or YPD (Candida) agar plates. Plates were streaked in triplicate and incubated for 18 hours at 30°C (Candida) or 37°C in the dark to allow colony formation.
Antimicrobial photocatalysis
A cell suspension consisting of 10(8) cells/mL for bacteria or 10(7) cells/mL for Candida was mixed with 10mM TiO2 in the presence of various different concentrations of NaBr. Then 500 μL of this mixture was transferred to a 24-well plate and illuminated at room temperature using UVA light under magnetic stirring The irradiance was fixed at 16 mW/cm2 (I J/cm2 delivered in 1 min). No elevation in temperature (< 1°C) was found. Cells in control group were incubated in the dark for the same time as the treatment groups (30 minutes). After each dose of UVA light had been delivered 10 μL aliquots were withdrawn and serially diluted and streaked on BHI agar plates according to the method of Jett et al [26]. CFU were counted after overnight incubation at 37°C or 30°C for Candida.
Addition of bacteria after light activation of TiO2/NaBr
To investigate the killing effect of the solution produced after light activation, we added aliquots of illuminated TiO2/NaBr solution to the bacterial cells. The bacterial pellet was collected by centrifuging 400 μL of 10(8) cells/mL MRSA or E.coli cells in BHI at 4000rpm for 5mins. 500 μL of 10mM TiO2 with addition of 10mM NaBr was illuminated with different doses of UVA light with stirring. At the completion of each illumination, aliquots (400 μL) of the suspension were added to the bacterial pellet and gently resuspended. After 30 minutes incubation time, 10 μL aliquots were taken from each group to determine colony-forming units (CFU).
Myeloperoxidase (MPO/H2O2) Antimicrobial Studies
A stock solution (0.25U/mL) of MPO was prepared by adding 5U of MPO in 10mL 50/50 glycerol/H2O and kept at −20°C. Suspensions of bacteria (10(8) CFU/mL) were incubated at room temperature with MPO 10 mU/mL and H2O2 100μM with and without added NaBr (ranging from 10 nM to 100 mM) in either 50 mM phosphate buffer pH 5.5 or pH 7.4 for 60 minutes (final volume 1 mL). At the completion of the incubation, aliquots, (100 μL) were taken from each tube to determine CFU as described above.
Statistics
Data are presented as mean ± SD. We used one-way ANOVA for comparisons and the Tukey post-hoc test was used for pairwise comparisons. Significance was defined as p<0.05. SPSS statistics V17.0 (IBM, Armonk, NY) was used for analysis.
Results
Addition of sodium bromide potentiates TiO2 antimicrobial photocatalysis
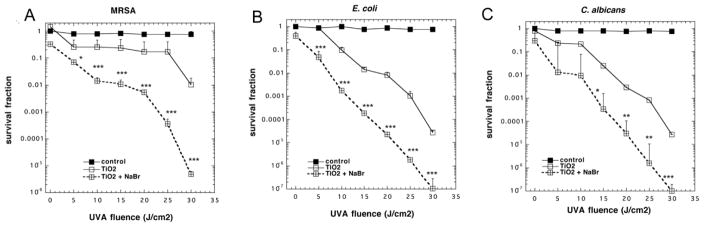
When MRSA cells (10(8) CFU/mL; Gram-positive bacteria) were irradiated with UVA light in the presence of TiO2 (10 mM) there was a light dose-dependent loss of viability reaching almost 2 logs of killing at 30 J/cm2, However when 10 mM NaBr was added to the mixture the antimicrobial effect was potentiated by 1–3 logs of extra killing (Figure 1A). When the experiment was repeated with E. coli (10(8) CFU/mL; Gram-negative bacteria) there was a light dose-dependent killing, with TiO2 and UVA light alone giving over 4 logs of killing at 30 J/cm2. However when 10 mM NaBr was added, there was an extra 1–3 logs of bacterial killing on top of that seen with photocatalysis alone (Figure 1B). When the experiment was repeated with 10(7) CFU/mL of C. albicans (fungal yeast) we found similar results. With TiO2 and UVA light alone there was up to 4 logs of killing, but this was increased by 1–2 logs of additional killing by the addition of 10 mM NaBr to the mixture.
Figure 1. Antimicrobial TiO2 photocatalysis of microbial cells is potentiated by addition of NaBr.
Cells were stirred in the presence of TiO2 (0 or 10 mM) and NaBr (0 or 10 mM) while being exposed to increasing fluences of UVA light. (A) MRSA (10(8) cells/mL); (B) E. coli (10(8) cells/mL); (C) C. albicans (10(7) cells/mL). Values are means of 3 repetitions and bars are SD. * p < 0.05; ** p < 0.01; *** p < 0.001 for TiO2 + NaBr vs TiO2 alone.
Because of comparisons with the antimicrobial effects of the myeloperoxidase + H2O2 + bromide system (see later) we investigated the effects of reducing the pH to 5.5 on the efficiency of the antimicrobial photocatalysis. There were no significant differences between the killing of MRSA and E. coli with UVA irradiated TiO2 (with and without NaBr) when carried out in phosphate buffer at pH 7.4 and at pH 5.5 (data not shown)
Bromide concentration response
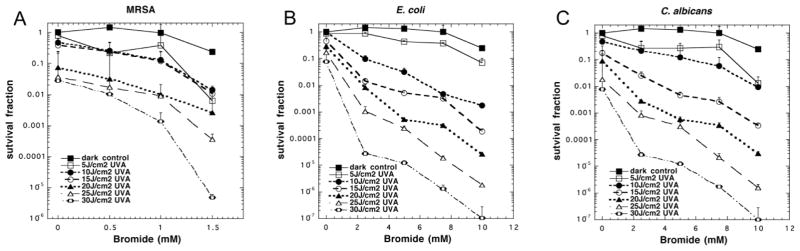
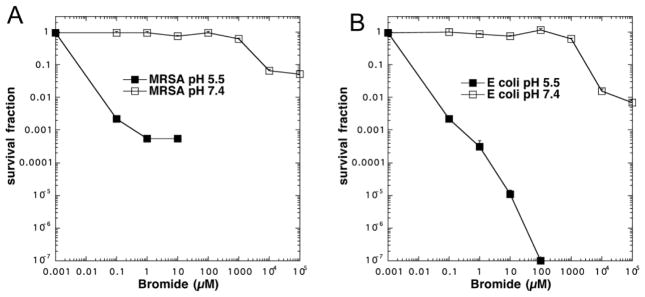
In order to find the most effective concentration of bromide for potentiating the killing we carried out an experiment where we used a series of light doses delivered to bacteria stirred with TiO2 (10 mM) and increasing concentrations of bromide. Figure 2A shows that for MRSA 1.5 mM bromide was sufficient to give 2–3 logs of extra killing, while for E. coli 10 mM bromide gave an impressive 4 logs of extra killing (Figure 2B), and for C. albicans 10 mM bromide also gave 4 logs of extra killing (Figure 2C),
Figure 2. Effect of bromide concentration on antimicrobial TiO2 photocatalysis.
Cells were stirred in the presence of 10 mM TiO2 with addition of a range of concentrations of NaBr while being exposed to increasing fluences of UVA light. (A) MRSA (0–1.5 mM NaBr); (B) E. coli (0–10 mM NaBr); (C) C. albicans (0–10 mM NaBr). Values are means of 3 repetitions and bars are SD.
Killing after light
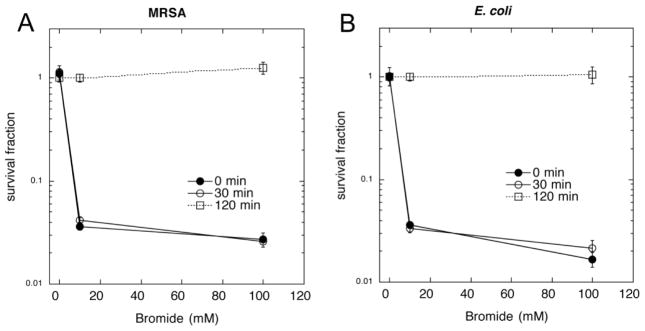
We wished to investigate how much of the synergistic killing was due to production of a relatively long-lived stable antimicrobial species, so we added microbial cells at different times after completion of light delivery. Figure 3A shows that when MRSA cells were added to a suspension of TiO2 that had been treated with 40 J/cm2 of UVA light in the presence of 10 mM or 100 mM bromide there was about 1.5 logs of killing, but no killing at all without bromide. When the bacteria were added to the irradiated suspension 30 min after the end of the illumination period there was the same degree of killing, but when 2 hours was allowed to elapse after the light there was no killing at all. Very similar results were obtained when the experiment was repeated with E. coli (Figure 3B).
Figure 3. Killing of bacteria added to photoactivated TiO2 after light.
TiO2 (10 mM) was stirred with NaBr (0, 10, or 100 mM) under UVA light (40 J/cm2). MRSA (A) or E. coli (B) cells (10(8) cells/mL) were then added at 0, 30 or 120 minutes after the end of the illumination and incubated for 1 hour. Values are means of 3 repetitions and bars are SD.
Chemical assays
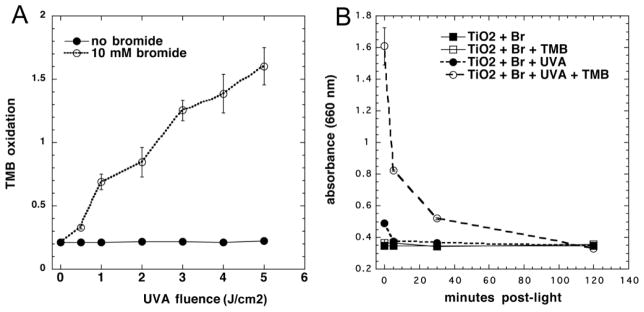
In order to gain some information on the identity of the long-lived antimicrobial chemical species that was produced in an irradiated suspension of TiO2 and bromide, we added 3,3′,5,5′-tetramethylbenzidine (TMB) to the suspension after the end of the illumination. TMB is a widely used chromogenic substrate for detecting oxidizing species, which is oxidized by hypobromite and hypoiodite [27]. Figure 4A shows that when 10 mM bromide was added to irradiated TiO2 there was a light dose-dependent increase in oxidized TMB with as little as 5J/cm2 not seen in the absence of bromide. As we wished to determine how stable this TMB-oxidizing species was (considering the loss of the antimicrobial activity over 2 hours) we added TMB at different times after the end of the illumination period. Figure 4B shows that after 5 min over 50% of the TMB oxidizing species had decayed, with over 80% gone after 30 min and 100% gone after 2 hours.
Figure 4. Oxidation of TMB by photoactivated TiO2 after light.
(A) TiO2 (10 mM) was stirred with NaBr (0 or 10 mM) under UVA light (1–5 J/cm2). A solution of TMB (0.1 mg/mL) was then added and incubated for 3 hours. (B) TiO2 (10 mM) was stirred with NaBr (10 mM) under UVA light (0–5 J/cm2). At different times after the end of the illumination, 50 μl aliquots of the mixture was added to 1 mL TMB (0.1 mg/mL). Values are means of 6 wells and bars are SD.
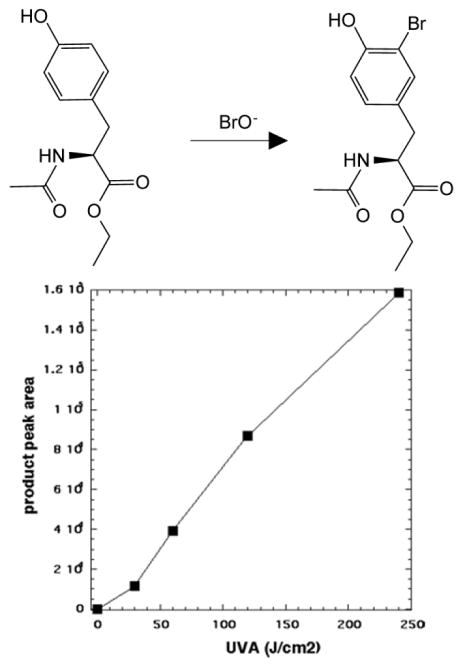
We carried out bromination of N-acetyl tyrosine ethyl ester to provide chemical proof of the formation of a reactive bromine species that was produced in the presence of TiO2, NaBr, and UVA. By analogy with studies that have been carried out to demonstrate nitration of tyrosine derivatives [28], we employed N-acetyl tyrosine ethyl ester as the substrate for the reaction, and this substrate was brominated to produce the product N-acetyl-3-bromotyrosine ethyl ester. We used LC-MS to identify the product (C13H15NBrO4− m/z = 328.02 and 330.02) and were able to construct a linear light-dose response curve shown in Figure 5.
Figure 5. Light-dose dependent bromination of N-acetyl tyrosine ethyl ester by TiO2 + NaBr irradiated with UVA.
3-bromo N-acetyltyrosine ethyl ester was quantified after increasing fluences of UVA light had been delivered to a mixture of TiO2, NaBr and N-acetyltyrosine ethyl ester using LC-MS.
In principle the long-lived antimicrobial reactive species could have been bromine or hypobromite or a mixture of both. In order to try and distinguish between hypobromite and bromine (which would be present as tribromide anion in the presence of a high concentration of bromide) we used spectrophotometry at 267 nm (Br3− has a molar absorption coefficient of 40,900 at 267 nm) [25] and HPLC using an anion exchange column [25]. Despite numerous attempts we were unable to detect any formation of tribromide by spectrophotometry or by HPLC.
ROS-specific fluorescence probes
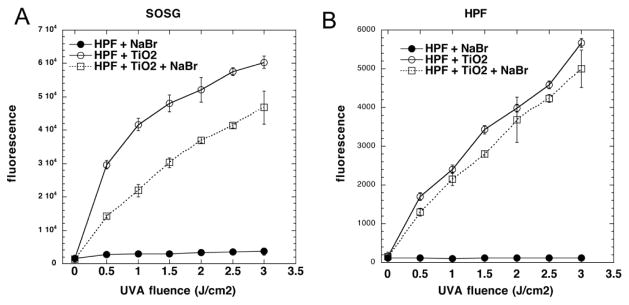
In order to gain some information on whether a specific reactive oxygen species (ROS) produced by the illuminated TiO2 was responsible for oxidizing the bromide, we used two fluorescence probes for specific ROS that we had previously used in photodynamic therapy studies [29, 30] and asked whether their activation would be quenched by addition of bromide. SOSG is relatively specific for singlet oxygen and HPF is relatively specific for detecting hydroxyl radicals. Figure 6A shows that there was modest but significant quenching of light-activated SOSG fluorescence by addition of 10 mM bromide. Figure 6B shows that in contrast there was no significant quenching of the photoactivation of the HPF probe.
Figure 6. Activation of ROS-specific fluorescence probes by photoactivated TiO2.
Probe was illuminated with UVA light (TiO2 (10 mM) was illuminated with UVA light (0–3 J/cm2) in the presence of NaBr (10 mM), TiO2 (10 mM) or both (10 mM NaBr + 10 mM TiO2). Fluorescence was measured in plate reader after each aliquot of light. (A) SOSG; (B) HPF. Values are means of 6 wells and bars are SD.
Comparison with myeloperoxidase/H2O2/bromide system
The closest comparison to bromide potentiation of TiO2 antimicrobial photocatalysis, appeared to be the antimicrobial peroxidase/H2O2/bromide system [31]. Although several different peroxidase enzymes will mediate this antimicrobial system (some with different specificities for bromide compared to chloride and iodide), we chose to test myeloperoxidase (MPO) as this has been widely studied. The antimicrobial effects of the MPO/H2O2/Br− system have been reported to be sensitive to pH, so we tested it in phosphate buffer at pH 7.4 and at pH 5.5. Figure 7A shows the killing of MRSA incubated with 10 mU of MPO and 100 μM H2O2 for one hour in the presence of a wide range of bromide concentrations ranging from 10 nM to 100 mM (8 orders of magnitude) in the 2 different pH buffers. It can be seen that the killing was dramatically better at pH 5.5 where as low a bromide concentration as 100 nM showed some bacterial killing, while at pH 7.4 it was necessary to increase the bromide concentration to 10 mM to get any killing. Figure 7B shows the situation was even more pronounced with E. coli with killing at pH 5.5 starting at 100 nM bromide and with eradication (zero CFU remaining) at 100 μM, while 10 mM bromide was needed for killing at pH 7.4.
Figure 7. Antimicrobial effects of MPO/H2O2/bromide system.
Bacteria (10(8) cells/mL) were incubated for 1 hour with MPO (10 mU), H2O2 (100 μM) and a large range of bromide concentrations (10 nM to 100 mM) in two different 50 mM phosphate buffers (pH 5.5 and pH 7.4). (A) MRSA; (B) E. coli. Values are means of 3 repetitions and bars are SD.
Discussion
We have shown for the first time, that addition of the simple non-toxic inorganic salt, sodium bromide to the antimicrobial TiO2 photocatalysis system potentiates killing of a broad-spectrum of microorganisms by up to 3 logs. The mechanism appears to be based on a 2-electron oxidation of bromide to hypobromite. The order of susceptibility of the different classes of microbial cells was somewhat surprising. We found that Gram-negative E. coli was most susceptible, C. albicans was intermediate, and Gram-positive MRSA was least susceptible. It is well known in the field of antimicrobial photodynamic inactivation (aPDI) that Gram-positive bacteria are most susceptible, fungal yeasts such as C. albicans are intermediate, while Gram-negative bacteria are least susceptible [32]. The pronounced susceptibility difference in the case of aPDI is attributed to differences in permeability of the cell wall, that restricts penetration of the photosensitizer into sensitive parts of the cell especially in Gram-negative bacteria [33, 34].
Many other laboratories have investigated in detail many innovative approaches to improving antimicrobial TiO2 photocatalysis. Many of these approaches have been directed towards shifting the wavelength of the activating light away from the UVA range required by P25, and towards the visible range (blue, green, red, or even NIR). There have been a wide range of “doping” procedures using various impurities reported [35] with elements such as nitrogen [36], platinum [19], molybdenum [37], iron and aluminum [38], various organic compounds [17] etc.
We demonstrated the existence of a reactive brominating species, by brominating the tyrosine derivative, N-acetyl tyrosine ethyl ester, to form the product, N-acetyl 3-bromotyrosine ethyl ester, detected by LC-MS. This method was chosen as a similar reaction scheme had been used to detect the nitration of tyrosine produced by a photocontrollable peroxynitrite generator based on N-methyl-N-nitrosoaminophenol [28].
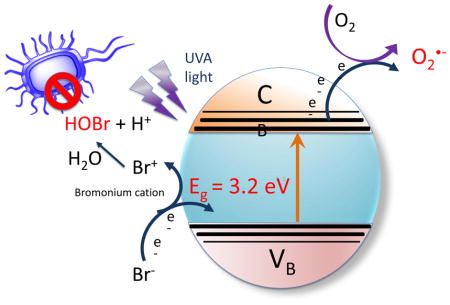
To study the mechanism we first asked whether the oxidation of bromide could be caused by one of the reactive oxygen species formed during TiO2 photocalysis, namely hydroxyl radicals or singlet oxygen [17]. We used the fluorescent ROS probes HPF (detects HO•) and SOSG (detects 1O2). The data (Figure 5) showed no quenching of HPF and only minor quenching of SOSG. Although hydroxyl radical has a redox potential (+ 2.31V) that is more than enough to oxidize bromide (−0.78V), singlet oxygen does not (+ 0.64V) [18]. The minor inhibition of the TiO2-mediated photoactivation of SOSG by bromide is probably caused by the physical quenching of 1O2 caused by its collision with bromide ions in a similar manner to that shown for iodide ions [39]. Therefore we assumed that bromide oxidation was caused by a direct oxidation arising from the photoactivated TiO2 rather than oxidation by an intermediate oxygen-containing oxidizing species.
When TiO2 is irradiated by UVA light it acts as an oxidizing agent, due to the positive holes generated in the valence band by excitation of electrons into the valence band. Water can be oxidized to oxygen
| (1) |
showing that the oxidizing potential is at least +1.23V. The redox potential of bromide to hypobromite is lower (−0.76V) than the redox potential for bromide to bromine (−1.07V) showing that bromide may be preferentially oxidized to hypobromite rather than bromine.
| (2) |
| (3) |
However there is another difference. The oxidation to hypobromite is a true 2-electron oxidation, while the oxidation to bromine is two separate 1-electron oxidations that proceed via the short-lived intermediate bromine radical
| (4) |
By contrast the first step in the 2-electron oxidation to hypobromite is
| (5) |
Bromide initially reacts to form the bromonium cation that subsequently reacts with water to form hypobromite
| (6) |
The additional microbial killing in our studies could not be completely explained by generation of hypobromite. Only about 1 log of killing could be obtained when the microbial cells were added immediately after the light (see Figure 3) while up to 3 additional logs of killing could be obtained when the microbial cells were present during the illumination. The additional killing could be attributed to a short-lived reactive intermediate formed during the photoexcitation. The proposed intermediate in the oxidation of bromide to hypobromite, is bromonium cation, which is a highly reactive brominating electrophile [40] and may be responsible for the short-lived antimicrobial species that is only present during the illumination period.
It is not immediately apparent why photoactivated TiO2 should preferentially carry out 2-electron oxidations rather than 1-electron oxidations. In reality it may be the case that the lower redox potential of the hypobromite reaction is the prinicipal determining factor
Hypobromite is an unstable species and disproportionates to form bromate and bromide
| (7) |
This reaction is probably responsible for the observed time-dependent loss of the antimicrobial activity of the irradiated TiO2 suspension containing added bromide.
There has been a report of the use organic molecules to study the interaction of of photoactivated TiO2 with bromide anion [41]. Using cyclohexene as a probe they proposed a mechanism in which the photoexcited TiO2 carried out a one-electron oxidation of adsorbed bromide, producing surface-bound bromine atoms. These potentially could abstract hydrogen from cyclohexene to initiate autoxidation or could migrate along the semiconductor surface, producing bromine. The difference in the results could be explained by the fact that their experiments were conducted in an organic solvent (anhydrous acetonitrile) while we used water. Another study looked at the effect of chloride on TiO2 photocatalysis (measuring dye decolorization) [42]. They found that in aqueous solution a low concentration of Cl− (< 0.01 M) showed little influence but a high concentration of Cl (> 0.1 M) had a very different influence on the decolorization of dyes: a significant inhibition for methylene blue but a great promotion for orange II.
Peroxidases are a class of enzyme that use a peroxide (most often hydrogen peroxide) to oxidize an organic or inorganic substrate. Many peroxidase enzymes (including a class known as “haloperoxidases”) oxidize a halide ion to the respective hypohalite ion. Heme peroxidases operate by a well-established mechanism involving an intermediate (called “compound I”) (formed by a 2-electron oxidation) that has oxygen attached to the heme-iron (Fe(IV) and a radical cation present on the porphyrin ring [43]. Myeloperoxidase, which occurs in the granules of neutrophils [44], is a good example of a peroxidase with a pronounced antimicrobial activity. MPO can oxidize chloride to hypochlorite and this species is believed to be a major weapon in the arsenal of neutrophils to kill bacteria and other pathogenic microorganisms [45]. MPO plus hydrogen peroxide also forms hypoiodite from iodide and hypobromite from bromide [31]. In agreement with previous studies using MPO/H2O2/I− [46], we found that the antimicrobial activity of MPO/H2O2/Br− system was strongly dependent on the pH. The antibacterial activity against bacteria (and especially the Gram-negative E. coli) was several orders of magnitude more pronounced at pH 5.5 compared to pH 7.4. We have been unable to find a convincing explanation of this pronounced difference between pH values. Interestingly we did not find a difference between the antimicrobial activity of the TiO2/UVA/Br− system at pH 5.5 and 7.4. Moreover at pH 5.5 the MPO/H2O2/Br− system was active with bromide concentrations that were very low in comparison with the TiO2/UVA/Br− system.
A recent publication suggested that hypobromite was the “most powerful endogenous electrophile” [47]. Although hypobromite is a less powerful oxidizing agent than hypochlorite, it is a more powerful halogenating agent. It is at present uncertain how much of the microbicidal effects of hypohalites are due to their oxidizing ability and how much is due to their halogenating ability. There is an enzyme that is widely distributed in marine life-forms called vanadium bromoperoxidase (non heme-containing) whose purpose is to introduce bromine atoms into a variety of brominated secondary metabolites that are common in marine organisms [40]. It is at present uncertain how effective vanadium bromoperoxidase/H2O2/Br− would be in killing microorganisms.
It is at present uncertain whether our discovery will have any practical applications in the real world. The requirement for millimolar concentrations of bromide for significant potentiation of the antimicrobial activity may prove a limiting factor. Could TiO2 be doped with an immobilized form of bromide anion to form an improved photoactivated antimicrobial surface? If so would it still be effective? Are there any situations when it would be realistic to add a solution of bromide to an aqueous solution that was required to be photodisinfected? Clearly further research would be needed to answer these provocative questions.
Supplementary Material
Emission spectrum of the UVA light source measured by a spectroradiometer.
Highlights.
Antimicrobial killing mediated by photocatalysis using titanium dioxide and UVA light is potentiated by addition of sodium bromide.
Broad-spectrum effect active against Gram-positive and Gram-negative bacteria and fungi.
Mechanism involves oxidation of bromide to produce a brominating agent likely to be hypobromite.
Has elements in common with the antimicrobial system (myeloperoxidase + hydrogen peroxide + bromide)
Acknowledgments
This work was supported by US NIH grant R01 AI050875. Yu Kushida was supported by the Research Experience for Undergraduates (REU) Program of the National Science Foundation under Award Number EEC-1358296. We are grateful to Dr Brijesh Bhayana for help with analytical chemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li Puma G, Rodriguez-Gonzalez V, Perez-Larios A. Photocatalysis: from the treatment of emerging contaminants to energy conversion. J Hazard Mater. 2013;263(Pt 1):1. doi: 10.1016/j.jhazmat.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira HG, Ferreira LH, Bertazzoli R, Longo C. Remediation of 17-alpha-ethinylestradiol aqueous solution by photocatalysis and electrochemically-assisted photocatalysis using TiO2 and TiO2/WO3 electrodes irradiated by a solar simulator. Water Res. 2015;72:305–314. doi: 10.1016/j.watres.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Saggioro EM, Oliveira AS, Pavesi T, Maia CG, Ferreira LF, Moreira JC. Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules. 2011;16:10370–10386. doi: 10.3390/molecules161210370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne JA, Dunlop PS, Hamilton JW, Fernandez-Ibanez P, Polo-Lopez I, Sharma PK, Vennard AS. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules. 2015;20:5574–5615. doi: 10.3390/molecules20045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Ghafoor K, Lee J, Feng M, Hong J, Lee DU, Park J. Bacterial inactivation in water, DNA strand breaking, and membrane damage induced by ultraviolet-assisted titanium dioxide photocatalysis. Water Res. 2013;47:4403–4411. doi: 10.1016/j.watres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo L, Della Sala A, Fiorentino A, Li Puma G. Disinfection of urban wastewater by solar driven and UV lamp - TiO(2) photocatalysis: effect on a multi drug resistant Escherichia coli strain. Water Res. 2014;53:145–152. doi: 10.1016/j.watres.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Robertson PK, Robertson JM, Bahnemann DW. Removal of microorganisms and their chemical metabolites from water using semiconductor photocatalysis. J Hazard Mater. 2012;211–212:161–171. doi: 10.1016/j.jhazmat.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 8.Yamada N, Suzumura M, Koiwa F, Negishi N. Differences in elimination efficiencies of Escherichia coli in freshwater and seawater as a result of TiO2 photocatalysis. Water Res. 2013;47:2770–2776. doi: 10.1016/j.watres.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Vidal A. Developments in solar photocatalysis for water purification. Chemosphere. 1998;36:2593–2606. doi: 10.1016/s0045-6535(97)10221-1. [DOI] [PubMed] [Google Scholar]
- 10.Lilja M, Forsgren J, Welch K, Astrand M, Engqvist H, Stromme M. Photocatalytic and antimicrobial properties of surgical implant coatings of titanium dioxide deposited though cathodic arc evaporation. Biotechnol Lett. 2012;34:2299–2305. doi: 10.1007/s10529-012-1040-2. [DOI] [PubMed] [Google Scholar]
- 11.Suketa N, Sawase T, Kitaura H, Naito M, Baba K, Nakayama K, Wennerberg A, Atsuta M. An antibacterial surface on dental implants, based on the photocatalytic bactericidal effect. Clin Implant Dent Relat Res. 2005;7:105–111. doi: 10.1111/j.1708-8208.2005.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura H, Tanaka M, Shinohara S, Gotoh M, Karube I. Development of a self-sterilizing lancet coated with a titanium dioxide photocatalytic nano-layer for self-monitoring of blood glucose. Biosens Bioelectron. 2007;22:1920–1925. doi: 10.1016/j.bios.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Yin ZF, Wu L, Yang HG, Su YH. Recent progress in biomedical applications of titanium dioxide. Phys Chem Chem Phys. 2013;15:4844–4858. doi: 10.1039/c3cp43938k. [DOI] [PubMed] [Google Scholar]
- 14.Brown EM, Allen LL, Pyles H, Solis J, Wileman TA, Willadsen GB. Advancements in using TiO2 bionanoconjugates for precision degradation of intracellular biological structures. J Biomed Nanotechnol. 2013;9:539–550. doi: 10.1166/jbn.2013.1564. [DOI] [PubMed] [Google Scholar]
- 15.Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Li J, Quan X, Wu Y, Li G, Wang F. Formation of hydrogen peroxide and degradation of phenol in synergistic system of pulsed corona discharge combined with TiO2 photocatalysis. J Hazard Mater. 2007;141:336–343. doi: 10.1016/j.jhazmat.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Buchalska M, Labuz P, Bujak L, Szewczyk G, Sarna T, Mackowski S, Macyk W. New insight into singlet oxygen generation at surface modified nanocrystalline TiO2--the effect of near-infrared irradiation. Dalton Trans. 2013;42:9468–9475. doi: 10.1039/c3dt50399b. [DOI] [PubMed] [Google Scholar]
- 18.Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R, Tegos GP, Hamblin MR. Antimicrobial strategies centered around reactive oxygen species - bactericidal antibiotics, photodynamic therapy and beyond. FEMS Microbiol Rev. 2013 doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitoraj D, Janczyk A, Strus M, Kisch H, Stochel G, Heczko PB, Macyk W. Visible light inactivation of bacteria and fungi by modified titanium dioxide. Photochem Photobiol Sci. 2007;6:642–648. doi: 10.1039/b617043a. [DOI] [PubMed] [Google Scholar]
- 20.Wong CL, Tan YN, Mohamed AR. A review on the formation of titania nanotube photocatalysts by hydrothermal treatment. J Environ Manage. 2011;92:1669–1680. doi: 10.1016/j.jenvman.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Ohtani B, Prieto-Mahaney OO, Li D, Abe R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J Photochem Photobiol A : Chemistry. 2010;216:179–182. [Google Scholar]
- 22.Vecchio D, Gupta A, Huang L, Landi G, Avci P, Rodas A, Hamblin MR. Bacterial photodynamic inactivation mediated by methylene blue and red light is enhanced by synergistic effect of potassium iodide. Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin R, Wang M, Huang YY, Landi G, Vecchio D, Chiang LY, Hamblin MR. Antimicrobial photodynamic inactivation with decacationic functionalized fullerenes: oxygen independent photokilling in presence of azide and new mechanistic insights. Free radical biology & medicine. 2015;79:14–27. doi: 10.1016/j.freeradbiomed.2014.10.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Dai T, Wang M, Vecchio D, Chiang LY, Hamblin MR. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: in vitro and in vivo studies. Nanomedicine. 2015;10:603–614. doi: 10.2217/nnm.14.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg HS, Yamada H. Post-ion-chromatography derivatization for the determination of oxyhalides at sub-PPB levels in drinking water. Anal Chem. 1998;70:1–6. doi: 10.1021/ac970651m. [DOI] [PubMed] [Google Scholar]
- 26.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Cao Z, Zhang G, Thannickal VJ, Cheng G. Vascular peroxidase 1 catalyzes the formation of hypohalous acids: characterization of its substrate specificity and enzymatic properties. Free radical biology & medicine. 2012;53:1954–1959. doi: 10.1016/j.freeradbiomed.2012.08.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ieda N, Nakagawa H, Peng T, Yang D, Suzuki T, Miyata N. Photocontrollable peroxynitrite generator based on N-methyl-N-nitrosoaminophenol for cellular application. J Am Chem Soc. 2012;134:2563–2568. doi: 10.1021/ja206744z. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, St Denis TG, Xuan Y, Huang YY, Tanaka M, Zadlo A, Sarna T, Hamblin MR. Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: role of ambient oxygen and azide radicals. Free Radic Biol Med. 2012;53:2062–2071. doi: 10.1016/j.freeradbiomed.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M, Hamblin MR. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg Med. 2012;44:490–499. doi: 10.1002/lsm.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klebanoff SJ. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968;95:2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merchat M, Spikes JD, Bertoloni G, Jori G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J Photochem Photobiol B. 1996;35:149–157. doi: 10.1016/s1011-1344(96)07321-6. [DOI] [PubMed] [Google Scholar]
- 34.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44:522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liou JW, Chang HH. Bactericidal effects and mechanisms of visible light-responsive titanium dioxide photocatalysts on pathogenic bacteria. Arch Immunol Ther Exp (Warsz) 2012;60:267–275. doi: 10.1007/s00005-012-0178-x. [DOI] [PubMed] [Google Scholar]
- 36.Yu B, Lau WM, Yang J. Preparation and characterization of N-TiO2 photocatalyst with high crystallinity and enhanced photocatalytic inactivation of bacteria. Nanotechnology. 2013;24:335705. doi: 10.1088/0957-4484/24/33/335705. [DOI] [PubMed] [Google Scholar]
- 37.Fisher L, Ostovapour S, Kelly P, Whitehead KA, Cooke K, Storgards E, Verran J. Molybdenum doped titanium dioxide photocatalytic coatings for use as hygienic surfaces: the effect of soiling on antimicrobial activity. Biofouling. 2014;30:911–919. doi: 10.1080/08927014.2014.939959. [DOI] [PubMed] [Google Scholar]
- 38.Schlur L, Begin-Colin S, Gilliot P, Gallart M, Carre G, Zafeiratos S, Keller N, Keller V, Andre P, Greneche JM, Hezard B, Desmonts MH, Pourroy G. Effect of ball-milling and Fe-/Al-doping on the structural aspect and visible light photocatalytic activity of TiO2 towards Escherichia coli bacteria abatement. Mater Sci Eng C Mater Biol Appl. 2014;38:11–19. doi: 10.1016/j.msec.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 39.Rosenthal I, Frimer A. The quenching effect of iodide ion on singlet oxygen. Photochem Photobiol. 1976;23:209–211. doi: 10.1111/j.1751-1097.1976.tb07244.x. [DOI] [PubMed] [Google Scholar]
- 40.Butler A, Carter-Franklin JN. The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Nat Prod Rep. 2004;21:180–188. doi: 10.1039/b302337k. [DOI] [PubMed] [Google Scholar]
- 41.Fox MA, Pettit TL. Use of Organic Molecules as Mechanistic Probes for Semiconductor-Mediated Photoelectrochemical Oxidations: Bromide Oxidation. J Org Chem. 1885;50:5013–5015. [Google Scholar]
- 42.Yang SY, Chen YX, Lou LP, Wu XN. Involvement of chloride anion in photocatalytic process. J Environ Sci (China) 2005;17:761–765. [PubMed] [Google Scholar]
- 43.Hiner AN, Raven EL, Thorneley RN, Garcia-Canovas F, Rodriguez-Lopez JN. Mechanisms of compound I formation in heme peroxidases. J Inorg Biochem. 2002;91:27–34. doi: 10.1016/s0162-0134(02)00390-2. [DOI] [PubMed] [Google Scholar]
- 44.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nauseef WM. Myeloperoxidase in human neutrophil host defence. Cell Microbiol. 2014;16:1146–1155. doi: 10.1111/cmi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klebanoff SJ. The iron-H2O2-iodide cytotoxic system. J Exp Med. 1982;156:1262–1267. doi: 10.1084/jem.156.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ximenes VF, Morgon NH, de Souza AR. Hypobromous acid, a powerful endogenous electrophile: Experimental and theoretical studies. J Inorg Biochem. 2015;146:61–68. doi: 10.1016/j.jinorgbio.2015.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Emission spectrum of the UVA light source measured by a spectroradiometer.