Abstract
Although historically viewed as merely anti-microbial effectors in acute infection or injury, neutrophils are now appreciated to be functionally versatile with critical roles also in chronic inflammation. Periodontitis, a chronic inflammatory disease that destroys the tooth-supporting gums and bone, is particularly affected by alterations in neutrophil numbers or function, as revealed by observations in monogenic disorders and relevant mouse models. Besides being a significant debilitating disease and health burden in its own right, periodontitis is thus an attractive model to dissect uncharted neutrophil-associated (patho)physiological pathways. Here, we summarize recent evidence that neutrophils can contribute to inflammatory bone loss not only through the typical bystander injury dogma but intriguingly also through their absence from the affected tissue, where they normally perform important immunomodulatory functions. Moreover, we discuss recent advances in the interactions of neutrophils with the vascular endothelium and – upon extravasation – with bacteria, and how the dysregulation of these interactions leads to inflammatory tissue damage. Overall, neutrophils have both protective and destructive roles in periodontitis, as they are involved in both the maintenance of periodontal tissue homeostasis and the induction of inflammatory bone loss. This highlights the importance of developing approaches that promote or sustain a fine balance between homeostatic immunity and inflammatory pathology.
Keywords: Neutrophils, inflammation, bone loss, periodontitis, leukocyte-adhesion deficiency, Del-1
1. Introduction
Neutrophils are relatively short-lived, terminally differentiated white blood cells that are endowed with capacity for swift recruitment to sites of infection or tissue injury and potent microbicidal mechanisms [1–3]. Huge numbers of neutrophils ([4]) are produced in and released from the bone marrow (BM) into the circulation at a rate of 1011 neutrophils per day under steady-state conditions [5]. Circulating neutrophils migrate to extravascular sites (e.g., in the skin, gut, lungs, or gingiva) in response to tissue infection or injury via the leukocyte adhesion cascade, a sequence of low- and high-affinity adhesive interactions between the neutrophils and the endothelium [6–8]. The first step involves transient rolling interactions, mediated by the binding of endothelial cell surface molecules (P- or E-selectin) to their glycoprotein ligands on neutrophils. This rolling-dependent braking down of neutrophils is followed by their firm adhesion on the endothelium and subsequent intraluminal crawling to identify appropriate sites for transendothelial migration. Firm adhesion and crawling are primarily mediated by β2-integrins (heterodimers with a unique CD11 subunit and a common CD18 subunit) through interactions with their endothelial counter-receptors, such as ICAM-1 and ICAM-2. Specifically, lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) and macrophage-1 antigen (Mac-1; CD11b/CD18) mediate, respectively, firm adhesion and crawling [6,7,9].
As there is a fine balance between the protective and potentially destructive effects of neutrophils, their production, trafficking, and clearance are tightly regulated by several homeostatic mechanisms operating in the BM, intravascularly, or peripheral tissues [10–12]. In mice and humans, the granulocyte colony-stimulating factor (G-CSF) is the major regulator of both granulopoiesis and neutrophil release from the BM [13,14]. Interleukin (IL)-17 (also known as IL-17A) promotes granulopoiesis and orchestrates neutrophil recruitment by up-regulating G-CSF and chemokines [10,15] and/or down-regulating endogenous inhibitors of the leukocyte adhesion cascade [16,17].
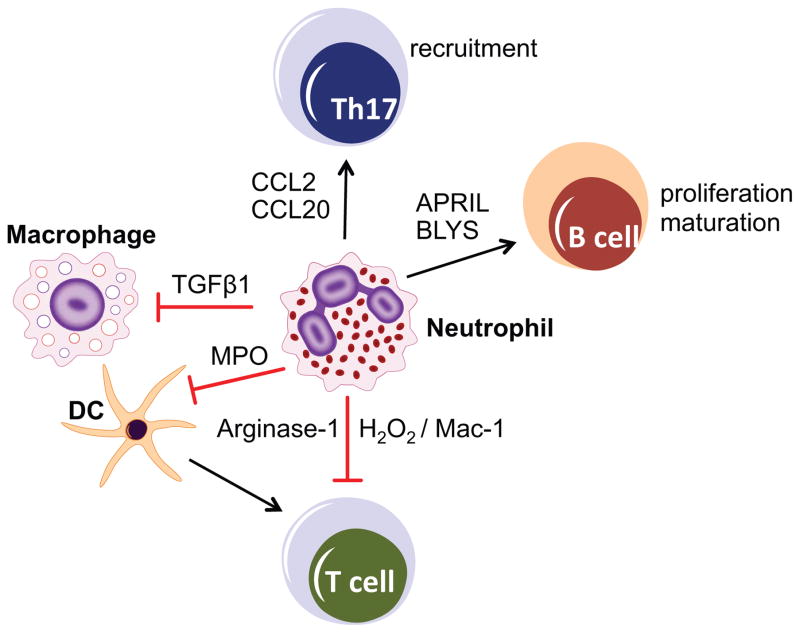
Although neutrophils have been traditionally viewed as merely anti-microbial effectors in the context of acute infection and inflammation, accumulating evidence suggests that neutrophils are functionally versatile and perform hitherto unanticipated functions, including engagement in regulatory crosstalk with both innate and adaptive immune leukocytes [7,18–20] (Figure 1). In addition to their classical antimicrobial and cytotoxic mechanisms (release of reactive oxygen species, antimicrobial peptides such as α-defensins and cathelicidin, and proteases such as elastase, cathepsin G or matrix metalloproteinases), neutrophils are now appreciated for a notable de novo biosynthetic capacity for C-X-C and C-C chemokines, cytokines with proinflammatory, anti-inflammatory, or immunoregulatory properties, as well as angiogenic and fibrogenic factors [18,21]. This previously underappreciated biosynthetic ability of neutrophils contributes to their crosstalk with tissue resident cells and other leukocytes. For instance, by releasing CCL2 and CCL20, neutrophils can recruit IL-17–producing CD4+ T helper cells (Th17) to sites of inflammation [22], whereas by secreting B-lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL), neutrophils can enhance survival, proliferation, and maturation of B cells into plasma cells [23,24]. Moreover, neutrophil-derived transforming growth factor β1 (TGFβ1) downregulates inflammatory responses by macrophages [25]. Evidence for subsets of neutrophils that perform regulatory functions [26] and for the polarization of tumor-associated neutrophils to phenotypes with antitumor or protumorigenic activity [27] suggests that the neutrophil population may not be as homogeneous as traditionally thought, but may display substantial diversity and functional plasticity (reviewed in refs. [3,18,27–31]).
Figure 1. Neutrophil cross-talk with other leukocyte types.
Whereas the recruitment and activation of neutrophils has long been known to be regulated by chemokines and cytokines secreted by both tissue stromal and resident leukocytes, neutrophils are now appreciated for their de novo biosynthetic capacity. They can produce and release immunoregulatory molecules that can activate or suppress other leukocyte types, thereby exacerbating or controlling inflammation depending on context. A few examples with potential relevance to periodontal disease are given. Neutrophils release CCL2 and CCL20 and recruit Th17 cells to sites of inflammation. By secreting BLyS and APRIL, neutrophils promote the proliferation and maturation of B cells into plasma cells. Neutrophils can potentially suppress T cell activation by releasing arginase-1 (depletes arginine required for T cell activation) or by delivering H2O2 into the immunological synapse in a Mac-1 integrin–dependent manner. Neutrophils can also indirectly suppress T cell activation through a myeloperoxidase-dependent mechanism that inhibits dendritic cell (DC) function. Neutrophil-derived TGFβ1 can promote resolution of inflammation by suppressing macrophage inflammatory responses.
Therefore, besides hallmarking acute inflammation, neutrophils are now increasingly acknowledged as important players in chronic inflammatory or aging-related disorders, including psoriasis, rheumatoid arthritis, atherosclerosis, inflammatory bowel disease, cancer, diabetes, and periodontitis [18,32–36]. This review discusses emerging new concepts on the role of neutrophils in infection and inflammation using periodontitis as a paradigm. Periodontitis is an oral inflammatory disease that leads to the destruction of the alveolar bone and other tissues that surround and support the teeth, such as periodontal ligament and gingiva, collectively known as the periodontium [37]. Moreover, periodontitis is associated with increased risk of certain systemic disorders, such as atherosclerosis and rheumatoid arthritis [38]. Periodontitis is an attractive study model of neutrophil-microbe interactions for two important reasons. First, the disease is readily accessible for obtaining host cells and tissue as well as microbial samples directly from specific microenvironments in the mouth, thus facilitating the conduct of longitudinal studies. For instance, the microorganisms studied are those that can be recovered directly from diseased periodontal pockets rather than those that have flowed through the ecosystem [39]. Second, periodontal tissue homeostasis and health is particularly sensitive to neutrophil defects affecting their numbers or function, as shown by clinical studies in patients with monogenic neutrophil disorders and experiments in relevant mouse models, discussed below [16,40–48]. Neutrophil infiltration and immunopathology is a hallmark also in common forms of periodontal disease [36,49,50].
2. Concepts derived from the study of rare monogenic diseases
Rare diseases (defined as affecting ≤1 in 1250 individuals) represent an important medical and social issue, cumulatively affecting 25 million patients in North America alone [51]. Importantly, the investigation of rare monogenic diseases is not relevant only to the treatment of patients with these specific disorders. These rare maladies constitute in fact real-life models to understand human biological pathways and obtain critical mechanistic insights into common diseases [46,52–54]. For instance, the study of leukocyte adhesion deficiency (LAD) has led to a better understanding of neutrophil biology and of mechanisms that control periodontal immunity and tissue homeostasis [46,47,55].
2.1. Leukocyte adhesion deficiency Type I
LAD constitutes a group of inherited disorders that impede the normal extravasation of circulating neutrophils and hence their recruitment to peripheral tissues [42,56]. The underlying genetic defects involve defective expression or function of β2 integrins or related adhesion molecules. LAD type I (LAD-I) is caused by deficiency in β2 integrins, LAD-II involves defective glycosylation of selectin ligands, and LAD-III is due to dysfunction of signaling molecules required for integrin activation [55]. The most common LAD type is LAD-I, an autosomal recessive immunodeficiency arising from mutations in the ITGB2 gene that encodes for the common β2-integrin subunit CD18 [42,56]. Neutrophils are absent or only scarcely found in extravascular sites in LAD-I patients, who have increased blood neutrophil counts (neutrophilia), suffer from frequent infections at mucosal or skin surfaces, and develop aggressive periodontitis in childhood [41,47,56,57].
Periodontitis associated with LAD-I has been historically attributed to impaired neutrophil surveillance of the bacterial infection in the periodontium [41,57–62]. However, recent work has challenged this dogma and linked impaired neutrophil transmigration to disruption of periodontal tissue homeostasis [47,63]. Indeed, clinical and laboratory studies using tissues and mucosal immune cells from LAD-I patients combined with mechanistic experiments in mouse models of LAD-I have shown that defective neutrophil recruitment to the gingiva leads to dysregulated overproduction of IL-17 [47], a pro-inflammatory and bone-resorptive cytokine [64]. Local antibody-mediated neutralization of IL-17 in mice mimicking the LAD-I phenotype (due to LFA-1 [CD11a] deficiency) blocked periodontal inflammation and bone loss as well as diminished the microbial burden [47], suggesting that exaggerated IL-17-driven inflammation fuels microbial overgrowth [65]. In this regard, periodontitis-associated ‘inflammophilic’ bacteria thrive on tissue breakdown products, such as degraded collagen peptides and heme-containing compounds [66,67]. The finding that microbial overgrowth in LAD-I periodontitis can be controlled by controlling inflammation – despite the absence of the presumed protective effects of neutrophils – has important implications for the etiology of the disease. It clearly questions the assumption that the absence of neutrophils promotes disease due to inadequate surveillance of the periodontal microbiota.
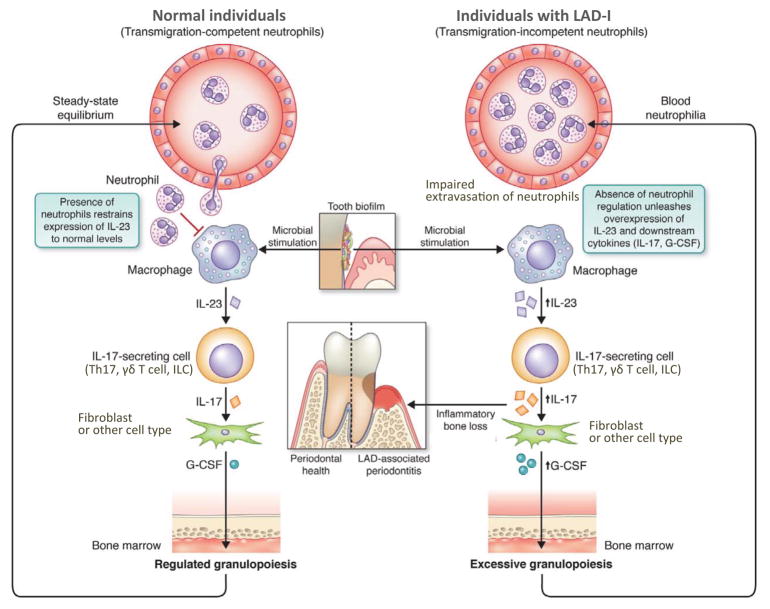
The dysregulated overproduction of IL-17 in the periodontium of LAD-I patients and relevant mouse models [47] may be explained by the disruption of a neutrophil homeostatic mechanism, known as the neutrophil rheostat (‘neutrostat’) [10,68]. Specifically, the phagocytosis of apoptotic neutrophils by tissue phagocytes regulates neutrophil production in and release from the BM through a granulopoietic G-CSF–dependent feedback loop that is mediated by the control of tissue expression of IL-23 and the downstream cytokine IL-17 [10,68]. In this regard, the phagocytosis of apoptotic neutrophils (‘efferocytosis’) by tissue macrophages reprograms the transcriptional activity of the efferocytic macrophage, at least in part, through induction of immunomodulatory signals that include downregulation of IL-23 [68–70].
Therefore, in a mucosal environment exposed to bacterial stimuli such as LPS, which can readily induce IL-23, efferocytic macrophages function to ameliorate IL-23 levels and thereby IL-23–dependent IL-17. In contrast, the absence of recruited neutrophils to the periodontal tissue of LAD-I patients affects this homeostatic pathway and diminishes the regulatory signals normally deriving from efferocytosis. Indeed, the majority of apoptotic cells in gingival tissue sections of normal individuals are neutrophils [71], which – under inflammatory conditions – occupy a large area (≥60%) of the junctional epithelium [72]. The notion that the efferocytosis-associated signals do not properly operate in LAD-I patients is supported by findings of overexpression of IL-23 (and the downstream cytokines IL-17 and G-CSF) in the LAD-I periodontium [47] (Figure 2). Whereas the elevated G-CSF has long been linked to increased granulopoiesis and blood neutrophilia in LAD-I individuals, the LAD-I–associated overproduction of IL-17 unequivocally contributes to the rapid inflammatory periodontal bone loss in these patients, given the well-established potent osteoclastogenic properties of IL-17 [64] (Figure 2). In support of this notion, anti-IL-17 blocks inflammation and bone loss in mice that mimic the LAD-I phenotype [47].
Figure 2. Neutrophil involvement in the pathogenesis of LAD-I periodontitis.
In LAD-I, the neutrostat circuit, and hence the regulation of the granulopoietic IL-23–IL-17–G-CSF cascade, is disrupted because the CD18-deficient neutrophils fail to extravasate. The absence of recruited neutrophils to the periodontal tissue of LAD-I patients leads to unrestrained local production of IL-23 and thus of IL-17 and G-CSF. Whereas increased G-CSF leads to excessive granulopoiesis and blood neutrophilia, elevated IL-17 (produced by Th17, γδ T cells, or innate lymphoid cells [ILC] [47]) leads to inflammatory bone loss. In normal individuals, on the other hand, the recruitment of neutrophils regulates the expression of the same cytokine cascade maintaining homeostasis in terms of periodontal health and granulopoiesis.
The concept that the absence of neutrophils causes LAD-I periodontitis through dysregulation of the host response rather than through defective immune surveillance of the local bacteria, by no means implies that the bacteria do not participate in the pathogenesis of this aggressive form of periodontitis. Indeed, experimental evidence suggests that the tooth-associated bacteria serve as an initial stimulus for local immunopathology through translocation of bacterial products (LPS) into the underlying gingival tissues where they unleash the already dysregulated IL-23–IL-17 axis [65,73]. An equivalent bacterial challenge in normal individuals, however, would likely be tolerated through the aforementioned efferocytosis-associated regulatory signals.
In both LAD-I patients and relevant mouse models, the elevated gingival IL-17 is predominantly derived from different T cell subpopulations, which are abundantly found in the inflammatory infiltrates of the LAD-I periodontitis lesions together with B cells and other cells of hematopoietic origin, whereas neutrophils in LAD-I patients are confined in blood vessels [47]. In this regard, neutrophils appear to be more heavily dependent on CD18 for transmigration than other leukocyte types that can transmigrate via CD18-indepenent mechanisms (e.g., by using very late antigen-4; VLA-4; α4β1) [74–77]. It should be noted, however, that although β2 (CD18) integrins are critical for neutrophil transmigration to several tissues (e.g., gingiva, skin, and intestine) [47,68,78,79], CD18-independent migration of neutrophils has been described in humans and animal models, such as VLA-3 (α3β1)– or VLA-4–mediated neutrophil recruitment to the peritoneal cavity and lungs of septic mice [78,80–83]. An additional reason why lymphocytes can be found in high numbers in peripheral tissues of LAD-I patients is because they can proliferate upon their recruitment (in contrast to the relatively short-lived neutrophils that undergo apoptosis). In fact, the remarkably increased numbers of plasma cells in the lesions of LAD-I periodontitis might, in part, be linked to the overexpression of IL-17, which was shown to enhance the survival, proliferation, and differentiation of B cells [84,85]. Consistently, blocking IL-17 in murine LAD-I periodontitis resulted in reduced expression of the plasma cell marker CD138 [47]. Because B and plasma cells are a major source of RANKL in the bone-resorptive lesions of chronic periodontitis [86], the abundance of B-lineage cells in LAD-I periodontitis might further contribute to inflammatory bone loss, secondarily to the dysregulated IL-17 response. Other secondary causes for bone loss in LAD-I periodontitis might be the deficiency of CD18 in osteoclasts, since Mac-1 (CD11b/CD18) is a negative regulator of osteoclastogenesis [87,88]. However, it should be noted that periodontal bone loss is arrested once teeth are removed or lost in LAD-I patients, who moreover have not been reported to have skeletal bone deficit. This suggests that CD18 deficiency per se is unlikely to cause bone loss in the absence of inflammatory stimuli, which – in LAD-I – are dependent on the disinhibited IL-23–IL-17 axis and the presence of periodontal bacteria that can unleash it.
2.2. Other types of leukocyte adhesion deficiency
Similarly to LFA-1–deficient mice, mice with combined P- and E-selectin deficiency (a model, which displays some resemblance to LAD-II) revealed severe bone loss early in life [40], reproducing the severe periodontal phenotype of LAD-II patients [89]. Although the expression of IL-17 in the periodontium of P/E-deficient mice was not analyzed [40], these double knockout mice have elevated IL-17 mRNA expression in several other peripheral tissues examined, including the spleen and jejunum, also attributed to the lack of neutrophil recruitment to these tissues [68].
The genetic basis for LAD III was shown to involve mutations in kindlin-3 [90], which interacts with the cytoplasmic tail of β2-integrins and regulates integrin-activation and therefore integrin-dependent neutrophil adhesion and transendothelial migration as well as integrin-dependent platelet functions [90,91]. LAD-III patients have severe bleeding disorder and recurrent infections and their prognosis is poor without transplantation [90]. Currently, there is no available information on their periodontal condition.
The GTPase Rac2 regulates neutrophil chemotaxis and margination of neutrophils [92]. In humans, mutations in Rac2 cause severe phagocytic immunodeficiency characterized by life-threatening infections in infancy [93]. These patients also display neutrophilia and impaired neutrophil migration, features that are shared with LAD patients [92,93]. Interestingly, Rac2-deficient mice are significantly more susceptible to experimental periodontitis as compared with wild-type controls, although the periodontal IL-17 response was not assessed [94]. Nevertheless, similar to β2-integrin deficiency, Rac2-deficient mice have only a few neutrophils in the junctional epithelium and gingival connective tissue but exhibit a heavy mononuclear cellular infiltrate in the same regions [94].
2.3. Chronic granulomatous disease
Chronic granulomatous disease (CGD) is a rare disease caused by mutations in the genes encoding for components of the nicotinamide adenine dinucleotide [NADPH] oxidase [95]. Accordingly, CGD neutrophils have defective respiratory burst and fail to kill a number of pathogens [96,97]. As expected, CGD patients are particularly susceptible to recurrent bacterial and fungal infections (e.g., pneumonia and abscesses of the skin), and the disease phenotype is replicated in mice deficient in the 47-kDa cytosolic subunit of the NADPH oxidase [60,98,99]. However, CGD patients do not exhibit increased susceptibility to periodontitis compared to the general population [60,100]. Although this may have appeared as a puzzle, it is now consistent with the findings from LAD-I patients and relevant mouse models discussed above [47,63], as neutrophil recruitment is intact in CGD. In other words, normal neutrophil recruitment by itself may be more crucial for periodontal tissue homeostasis than is the ability of neutrophils to kill pathogens. It is thus possible that periodontal immune surveillance by neutrophils could be compensated for by other cell types (e.g., macrophages). In contrast, the clearance of dead neutrophils by macrophages seems essential for neutrophil homeostasis [10,101]. The observation that neutrophils do migrate to the periodontium in the absence of bacterial colonization (in germ-free mice) [102] further suggests that neutrophil recruitment and clearance serves homeostatic function(s) that are not necessarily related to infection control. Moreover, the lack of correlation between susceptibility to systemic infections and susceptibility to periodontitis is consistent with the recent understanding that periodontitis is not an infection in the classic sense (i.e., caused by specific pathogens) but rather a dysbiotic disease [39,103].
More recently, it became apparent that CGD neutrophils have additional defects with regard to pathogen killing. The formation of neutrophil extracellular traps (NETs), which can entrap bacteria, fungi, and viruses, depends heavily on the generation of reactive oxygen species (ROS) by NADPH. Therefore, neutrophils from CGD patients cannot readily form NETs and have defective antimicrobial activity against certain pathogens [104,105]. Conversely, the reconstitution of NET formation by gene therapy in a GCD patient restored the ability of the patient’s neutrophils to kill Aspergillus nidulans, which is frequently associated with disseminated disease and mortality in CGD patients [105]. However, the fact that CGD patients are not susceptible to periodontitis [60,100] suggests that NETs have, at best, a minor protective role in periodontitis. In fact, the formation of NETs, which has been demonstrated in periodontal pockets of patients [106,107], might contribute to periodontal inflammation and tissue destruction, as is the case with certain other chronic inflammatory and autoimmune diseases [108].
2.4. Papillon-Lefèvre syndrome
The Papillon-Lefèvre syndrome is caused by deficiency in cathepsin C (dipeptidyl peptidase-I), a lysosomal exo-cysteine protease involved in the activation of pro-enzymes of neutrophil-derived serine proteases, such as cathepsin G, elastase, and proteinase 3 [109,110]. Papillon-Lefèvre patients are particularly susceptible to periodontitis affecting both the primary and permanent dentitions [111,112]. Interestingly, neutrophils from Papillon-Lefèvre patients maintain their capacity to kill pathogens [113,114]. These studies also imply that defective control of periodontal bacteria may not be the central factor underlying the periodontal disease susceptibility of Papillon-Lefèvre patients, although another study has shown that serine proteases can neutralize bacterial toxins [115].
Additional evidence suggests that severe periodontitis associated with the Papillon-Lefèvre syndrome could be attributed, at least in part, to a dysregulated inflammatory response. Specifically, in these patients, the failure to activate neutrophil-derived serine proteases due to cathepsin C deficiency results in impaired proteolytic degradation of proinflammatory chemokines and cytokines (e.g., macrophage inflammatory protein-1α) [116], a mechanism important for inflammation resolution and tissue homeostasis.
Because they cannot activate proteinase-3 either, Papillon-Lefèvre neutrophils are also unable to process the cathelicidin precursor protein hCAP18 into the antibacterial peptide LL-37 [113,117]. Given the notion that neutrophil serine proteases (and hence the products dependent on their activity, such as LL-37) are dispensable for immune protection [113], the association of LL-37 with periodontal tissue homeostasis [117] could possibly be explained by the immunoregulatory properties attributed to LL-37, such as downregulation of Toll-like receptor-mediated inflammatory responses and osteoclastogenesis [118–120].
3. Endogenous regulators of neutrophil recruitment to the periodontium: consequences of dysregulation
Neutrophils are the most common leukocyte recruited to the subgingival crevice, which when pathologically deepened (due to periodontitis) is referred to as periodontal pocket [38]. Supernumerary, hyperactive, or dysregulated neutrophils can cause bystander tissue damage via the release of inflammatory and toxic molecules or tissue-degrading enzymes [16,50,100,121]. It has also been proposed that neutrophils can directly induce osteoclastic bone resorption by expressing membrane-bound receptor activator of nuclear factor-κB ligand (RANKL) [122] a key osteoclastogenic cytokine [123,124]. In this context, neutrophils can release collagenase, which contributes to the initiation of bone resorption [125]. Furthermore, neutrophils secrete BLyS and APRIL [23,126] and thereby can enhance the survival of RANKL-expressing B and plasma cells that have been causally linked to periodontal bone loss in an APRIL- and BlyS-dependent manner in mice [127] (Figure 1). Through the release of CCL2 and CCL20 chemokines, neutrophils can recruit CCR2- and CCR6-expressing Th17 cells [22], which are implicated in autoimmune and inflammatory conditions including periodontitis [47,64,128,129]. Regardless of the exact mechanisms, ample clinical evidence indicates that neutrophils mediate a substantial part of periodontal tissue destruction [125,130]. Moreover, the local neutrophil numbers correlate positively with the severity of chronic periodontitis [131,132]. This section focuses on the regulation of neutrophil recruitment to the periodontium and conditions that can result in supernumerary neutrophils leading to inflammatory bone loss in periodontitis.
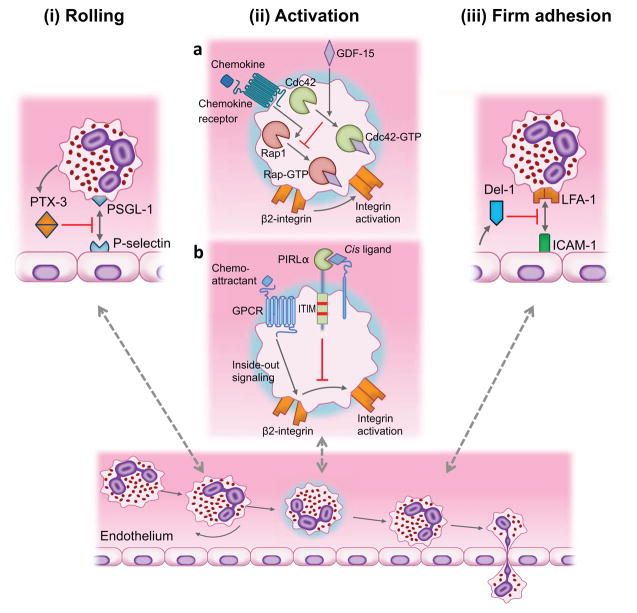
Although the study of the leukocyte adhesion cascade has traditionally focused on positive regulators of the cascade leading to the discovery of multiple chemokine and adhesion receptors, more recently, there has been intense interest to identify endogenous mechanisms that homeostatically control this inflammatory process. Newly identified mechanisms involve local tissue production of infiltration inhibitors or local release of such endogenous inhibitors by the recruited leukocytes upon their interaction with the endothelium [8]. These inhibitors can block distinct steps of the leukocyte adhesion cascade, such as (i) rolling, (ii) activation, and (iii) firm adhesion (Figure 3). For instance, pentraxin-3 (PTX3) inhibits rolling by binding P-selectin and blocking its interaction with PSGL-1 [133]. Growth differentiation factor-15 (GDF-15) antagonizes the activity of Rap1-GTPase and thereby β2 integrin activation [134]. In a related context, the paired immunoglobulin-like type 2 receptor-α on neutrophils mediates immunoreceptor tyrosine-based inhibitory motif (ITIM) signaling, which suppresses chemoattractant-induced β2 integrin activation [135]. Endothelial cell-secreted developmental endothelial locus-1 (Del-1) binds LFA-1 and antagonizes its interaction with endothelial ICAM-1 required for firm leukocyte adhesion to the endothelium [136]. Moreover, specialized pro-resolving lipid mediators act through multiple mechanisms regulating leukocyte infiltration (more below) [137]. Here we discuss in greater detail those inhibitors that have been investigated in the context of periodontal disease.
Figure 3. Inhibition of distinct steps of the leukocyte adhesion cascade by endogenous modulators.
(i) Neutrophil-secreted PTX3 binds to endothelial P-selectin thereby inhibiting its interaction with PSGL-1 required for rolling. (iia) GDF-15 inhibits chemokine-induced activation of β2 integrins. The inhibitory action of GDF-15 on integrin activation is mediated via upregulation of Cdc42-GTPase activity that antagonizes the activity of Rap1-GTPase. (iib) The paired immunoglobulin-like type 2 receptor-α (PILRα) on neutrophils interacts with as yet unidentified cis ligands and induces immunoreceptor tyrosine-based inhibitory motif (ITIM) signaling, which inhibits chemoattractant-induced β2 integrin activation. (iii) Endothelial cell-derived Del-1 inhibits LFA-1-dependent leukocyte adhesion. The interaction of leukocyte LFA-1 with ICAM-1 on the endothelium is a major adhesive mechanism for firm leukocyte arrest on the endothelium and subsequent extravasation. Updated from Hajishengallis and Chavakis (ref. [8]).
3.1 Developmental endothelial locus-1 (Del-1)
Del-1 was originally described as an endothelial cell-secreted 52-kDa protein interacting with the αvβ3 integrin [138]. More recently, Del-1 was shown to bind also β2 integrins (LFA-1 and Mac-1) and to antagonize β2 integrin-dependent neutrophil adhesion to the endothelium, thereby suppressing neutrophil extravasation [16,136,139]. Del-1 likely also inhibits Mac-1-dependent intraluminal crawling of neutrophils, although this was not specifically addressed. Importantly, Del-1-deficient mice develop spontaneous periodontitis typified by excessive neutrophil infiltration and IL-17–driven inflammatory bone loss [16], indicating that normal Del-1 expression is crucial for appropriate regulation of the local host inflammatory response. Disease is abrogated in mice with double deficiency in Del-1 and IL-17 receptor [16]. Interestingly, Del-1 deficiency is also linked to enhanced neutrophil infiltration and IL-17–dependent destructive inflammation in experimental autoimmune encephalomyelitis, a murine model of multiple sclerosis [140].
As discussed in the previous section, mice deficient in the LFA-1 integrin serve as a model mimicking LAD-I and show defective neutrophil recruitment to the periodontium [47,63]. Therefore, with respect to neutrophil recruitment, LFA-1-deficient mice and Del-1-deficient mice exhibit opposite phenotypes. Yet, both LFA-1–deficient mice and Del-1–deficient mice develop spontaneous periodontitis, whereas their wild-type littermates or even their corresponding doubly deficient mice remain relatively healthy [16,47]. Therefore, the presence of ‘normal’ numbers of neutrophils is strictly required for periodontal tissue homeostasis. In a similar context, excessive infiltration or activity of neutrophils has been linked to increased severity of colitis in animal models [141–143]; conversely, depletion or adhesion-blockade of neutrophils still exacerbates experimental colitis [144]. In this regard, the protective function of neutrophils involved their contribution to mucosal epithelial cell homeostasis through the secretion of IL-22 [145].
Interestingly, Del-1 mRNA and protein expression is diminished in the gingival tissue of aged mice (≥ 18 months old), correlating with excessive neutrophil recruitment and IL-17–dependent inflammatory bone loss [16,146]. Accordingly, local periodontal treatment with Del-1 in old mice inhibits IL-17 production, LFA-1-dependent neutrophil infiltration, and bone loss [16] and similar results were obtained by treating non-human primates with human Del-1 [87]. Not only do neutrophils infiltrate the periodontium at increased numbers in old age [16], but recruited neutrophils might be more likely to cause collateral tissue damage owing to age-related cell-intrinsic defects. In this regard, it was shown that neutrophils isolated from elderly individuals (60 years of age or older) exhibit inaccurate (unfocused) chemotaxis during which they release excessive amounts of proteases [147]. This defect was attributed to elevated constitutive PI3K activity, as compared to neutrophils from young controls, since inhibition of PI3K signaling restored chemotactic accuracy in the elderly-derived neutrophils [147]. In a related context, neutrophils from chronic periodontitis patients display reduced velocity and chemotactic accuracy compared to healthy controls [148]. In summary, increased numbers of extravasated neutrophils could exacerbate neutrophil-mediated bystander tissue damage.
3.2. Resolvins in periodontal inflammation; connection with Del-1
The resolution of inflammation is a well-coordinated active process aiming to reinstate tissue integrity and function and is mediated in great part by inhibition of further leukocyte recruitment and by clearance of apoptotic neutrophils by macrophages. Specialized pro-resolving lipid mediators acting via specific receptors are central in resolution of inflammation [69,137]. Such pro-resolving agonists include arachidonic acid-derived lipoxins and omega-3 polyunsaturated fatty acid-derived resolvins and protectins [49,69,149]. In contrast to Del-1, which is released by endothelial cells at the endothelial-leukocyte interface [16,136], pro-resolving lipid mediators can be produced through transcellular biosynthesis involving both endothelial cells and leukocytes [150]. When released within the vascular lumen, lipoxins and resolvins (derived from docosahexaenoic acid [D series] or eicosapentaenoic acid (E series]) can inhibit neutrophil extravasation into the tissues through multiple mechanisms involving modulation of adhesion receptor expression in both neutrophils and endothelial cells. For instance, lipoxin A4 inhibits leukotriene- or peptidoleukotriene-induced neutrophil adhesion by suppressing the expression of P-selectin on endothelial cells and of Mac-1 (CD11b/CD18) on neutrophils [151,152]. Other anti-neutrophil recruitment effects by lipoxins or resolvins include inhibition of L-selectin shedding and of ICAM-1 expression, and upregulation of endothelium-expressed nitric oxide, an inhibitor of leukocyte adhesion to vascular endothelium [153,154]. Multiple studies have established the ability of lipoxins and resolvins to inhibit periodontal inflammation and bone loss, in part by restricting the influx of neutrophils to the periodontal issue [49,155].
A novel connection between Del-1 and D-series resolvins was recently identified with regard to IL-17-dependent inflammatory bone loss [17]. Earlier studies have shown an inverse relationship between the expression levels of Del-1 and IL-17 in both mouse and human gingiva, where Del-1 and IL-17 dominate in healthy and inflamed tissue, respectively [16,146]. IL-17 can directly block Del-1 expression, ostensibly to facilitate neutrophil extravasation (i.e., by reversing the antagonistic effect of Del-1 on neutrophil adhesion to the endothelium) [16,140]; this function of IL-17 may be beneficial in acute bacterial infections or in oropharyngeal candidiasis [156]. Mechanistically, IL-17 inhibits Del-1 expression by inducing GSK-3β-dependent inhibitory phosphorylation of C/EBPβ, leading to diminished binding of this critical transcription factor to the Del-1 promoter, hence suppressing Del-1 expression [17]. Intriguingly, the inhibitory action of IL-17 on Del-1 can be reversed at the GSK-3β level by Akt signaling induced by D-resolvins in vitro and in vivo [17]. Moreover, the ability of resolvin D1 (RvD1) to inhibit periodontal inflammation (e.g., IL-17 and IL-17-dependent neutrophil-specific chemokines such as CXCL-1, -2, -3, and -5) and bone loss depended heavily on the presence of Del-1, as the protective effect of RvD1 was abrogated in Del-1-deficient mice [17].
Although Del-1 contributes crucially to the ability of the periodontium to self-regulate neutrophil-mediated inflammation, this homeostatic mechanism breaks down in aged mice as alluded to above [16,146]. Given that RvD1 positively regulates Del-1 expression [17] and that the levels of D-series resolvins, including RvD1, are decreased in aged mice compared to their young counterparts [157], it is tempting to speculate that the low abundance of RvD1 in old age underlies, in part, the age-related decline of Del-1 expression.
3.3. Pentraxin-3
Pentraxins represent a family of soluble pattern-recognition molecules typified by a radial pentameric structure. Serum amyloid P and C-reactive protein belong to the short pentraxin subfamily, whereas PTX3 is a prototypic member of the long pentraxins and is expressed in various cell types including myeloid dendritic cells and phagocytes [158]. PTX3 participates in distinct aspects of the innate immune response, such as pathogen recognition and opsonization, and regulation of complement and inflammation [158]. In neutrophils, PTX3 is constitutively stored in specific granules and can be released locally at the neutrophil-endothelial cell interface in response to pro-inflammatory cytokines or triggered by Toll-like receptor agonists [159]. Toll-like receptor activation may therefore not only promote integrin activation and neutrophil recruitment [160], but through the secretion of PTX3 may also initiate a negative feedback loop. In particular, PTX-3 was shown to bind P-selectin on activated endothelial cells, thereby blocking P-selectin interaction with its leukocyte ligand PSGL-1, hence blocking neutrophil rolling on the endothelium under shear stress [133]. Accordingly, PTX3-deficient mice display enhanced neutrophil recruitment, as compared to wild-type controls, in models of pleural cavity inflammation and acid-induced acute lung injury [133]. In BM chimera experiments, hematopoietic-specific deficiency of PTX3 was associated with increased neutrophil recruitment, thus confirming involvement of leukocyte-derived PTX3 in counteracting neutrophil rolling [133]. It should be noted that PSGL-1 binding to P-selectin mediates also leukocyte-platelet interactions [161,162]. PSGL-1/P-selectin- and Mac-1/glycoprotein Ib-dependent leukocyte-platelet interactions are implicated in several inflammatory processes, including autoimmunity, acid-induced acute lung injury or the inflammatory response to islet transplantation [163–165]. Interestingly, blocking P-selectin-dependent neutrophil-platelet aggregation reverses lung injury [164]. Therefore, by acting as an inhibitor of the PSGL-1/P-selectin interaction, the ability of PTX3 to inhibit acid-induced acute lung injury may include suppression both of neutrophil rolling (and hence transmigration) and of formation of leukocyte-platelet aggregates.
Clinical studies have shown that periodontitis is associated with increased levels of PTX3 in the gingival tissue, the gingival crevicular fluid, and in saliva [166–169]. Moreover, an experimental periodontitis study in rats confirmed that PTX3 levels are elevated in inflamed gingiva as compared to healthy gingiva [170]. The authors of these studies concurred that PTX3 seems to be associated with periodontal tissue destruction and might be useful as a diagnostic marker for periodontitis. While this is plausible, these studies should not be interpreted to mean that PTX3 necessarily promotes periodontal tissue destruction, as PTX3 levels may be elevated as a compensatory mechanism to mitigate destructive periodontal inflammation. In this regard, useful insights could be obtained by experimental studies investigating the periodontal disease phenotype of PTX3-deficient mice. In vivo animal studies in other inflammatory disease models have shown that, overall, PTX3 mediates host-protective effects, in part by reducing neutrophil infiltration (e.g., in acute myocardial infarction, LPS-induced acute lung injury, and acute kidney injury) although it may also play a detrimental role in certain other pathophysiologic conditions (e.g., intestinal ischemia and reperfusion where PTX3 was associated with increased neutrophil influx) [171].
4. Neutrophils and microbial immune subversion
As discussed above, patients with chronic periodontitis have a greater number and longer-lived neutrophils in the oral tissues compared with healthy individuals [172]. The persistent recruitment and sustained presence of neutrophils in inflamed periodontal pockets is likely due to – in part – their inability to control the subgingival microbial challenge, even though they are viable and can elicit immune responses [37,121]. This suggests that the periodontitis-associated bacteria can evade neutrophil-mediated killing while in an inflammatory environment, that is, without resorting to generalized immune suppression. In fact, this would not be a viable option since periodontitis-associated bacteria rely on inflammation to procure nutrients in the form of inflammatory tissue breakdown products, such as degraded collagen and heme-containing compounds [66].
Recent human microbiome studies and animal model-based mechanistic studies suggest that periodontitis does not conform to a bacterial infection model in the classical sense (i.e., not caused by a single or a select few pathogens) [173–185]. Rather, periodontitis represents a polymicrobial community-induced perturbation of host homeostasis that leads to destructive inflammation in susceptible individuals [39]. According to the polymicrobial synergy and dysbiosis model of periodontitis, dysbiotic communities exhibit synergistic interactions that can enhance colonization, persistence, or virulence. Bacteria known as keystone pathogens are involved in immune subversion and disruption of tissue homeostasis, whereas other, known as pathobionts, can trigger destructive inflammation once tissue homeostasis breaks down [39,186].
Porphyromonas gingivalis is a documented keystone pathogen in periodontitis that can manipulate innate immunity in ways that promote the conversion of a symbiotic community into a dysbiotic one, at least in the mouse model [38,176]. Remarkably, P. gingivalis can uncouple bacterial clearance from inflammation and thereby contribute to the persistence of inflammophilic microbial communities that drive periodontitis [183]. This subversive effect depends on the ability of P. gingivalis to induce signaling crosstalk in neutrophils involving TLR2 and the complement anaphylatoxin receptor C5aR1, which is triggered by C5a generated by the enzymatic action of P. gingivalis Arg-specific gingipains [183,187,188]. The C5aR1-TLR2 crosstalk induces proteasomal degradation of the TLR2 signaling adaptor MyD88 [183], which can otherwise mediate immune clearance of P. gingivalis [189]. The dissection of the underlying signaling pathway in both mouse and human neutrophils revealed that the proteasomal degradation of MyD88 is mediated via ubiquitination by the E3 ligase Smurf1 [183].
Although the MyD88 pathway is both an antimicrobial and proinflammatory pathway, its degradation by P. gingivalis does not abrogate the neutrophil inflammatory response, which as noted above contributes to a nutritionally favourable environment for the bacteria. Indeed, P. gingivalis activates an alternative TLR2 inflammatory pathway involving the adaptor Mal (MyD88 adaptor-like) and phosphoinositide 3-kinase (PI3K) [183]. In this regard, genetic or pharmacological ablation of Mal or PI3K abrogates the production of pro-inflammatory cytokines such as TNF, IL-1β, and IL-6. Intriguingly, P. gingivalis-induced Mal-PI3K activation inhibits bacterial phagocytosis by suppressing GTPase RhoA-dependent actin polymerization [183]. This evasive tactic of P. gingivalis moreover protects ‘bystander’ bacteria that are otherwise susceptible to killing by neutrophils [183]. Consistent with this action, pharmacological inhibition of C5aR1, TLR2, or PI3K in mice previously colonized with P. gingivalis led not only to the elimination of P. gingivalis itself from the periodontium but also reversed uncontrolled bacterial growth and inflammation, which occurred earlier owing to P. gingivalis colonization [183]. Therefore, P. gingivalis manipulates neutrophils via two distinct but interconnected mechanisms that together promote dysbiosis and the perpetuation of periodontal inflammation.
5. Hyper-reactive and suppressor neutrophil populations in chronic periodontitis
Even when periodontitis is considered in the general population (i.e., excluding monogenic defects, such as ITGB2 mutations, that have an obvious impact on the disease), a susceptible host is required for the development of this oral disease. In this respect, there are individuals who do not develop periodontitis despite lack of oral hygiene resulting in massive biofilm accumulation at dentogingival sites [190,191]. In general, the susceptibility to periodontitis may involve a variety of factors, including but not limited to congenital or acquired host immunodeficiencies or immunoregulatory defects, systemic diseases (e.g., obesity and diabetes), environmental factors (e.g., diet, smoking, and stress) and epigenetic modifications in response to environmental changes, which might act alone or in combination [190,192–196]. Although a genetic basis for periodontal disease pathogenesis is suggested by twin studies and familial aggregation of severe forms of the disease [197], the implication of specific genes (e.g., IL1, IL6, TNF, FCGR2A, C5, CD14, WNT5A) is generally weak and open to debate [190,193,195]. This is not surprising given the polygenic nature of adult-type chronic periodontitis, where multiple genes contribute cumulatively to the overall disease risk (or protection) through effects on the host immune response and the microbiome [195,198].
Although of uncertain mechanistic or genetic basis, a hyper-inflammatory neutrophil phenotype was proposed as having an important role in the pathogenesis of chronic periodontitis [50,199]. A hyper-inflammatory neutrophil phenotype is also linked to systemic diseases associated with periodontitis, such as diabetes and cardiovascular disease [200,201], suggesting that common susceptibility (shared hyper-inflammatory neutrophil phenotype) might contribute to comorbidity, i.e., to the epidemiological association of periodontitis with these systemic disorders. Interestingly, whereas peripheral blood neutrophils from patients with chronic periodontitis release increased levels of pro-inflammatory cytokines (e.g., TNF, IL-1β, and IL-8) in response to several stimuli compared to neutrophils from healthy controls, this hyper-inflammatory phenotype persists even after successful periodontal therapy (improvement in all clinical measures of the disease resulting in comparable periodontal status with control subjects) [202]. These findings suggest that this hyper-reactivity is spontaneous rather than being secondary to local inflammation in the periodontium, although long-lasting epigenetic effects on the phenotype cannot be ruled out. Peripheral blood neutrophils from chronic periodontitis patients also show elevated release of reactive oxygen species compared to those from healthy controls, even in the absence of exogenous stimulation; this unstimulated hyper-activity is not reversed by successful periodontal therapy [203]. Ostensibly, the recruitment of hyper-active neutrophils to the periodontium could contribute to periodontitis by causing enhanced oxidative tissue damage [50].
A recent study has shown that human neutrophils produce IL-10 upon direct contact with LPS-stimulated regulatory T cells (Tregs), mediated by Mac-1 and ICAM-1 on neutrophils and Tregs, respectively [204]. Consistently, such suppressor neutrophils could be identified at sites of gram-negative bacteria-induced inflammation, such as the periodontal pockets of chronic periodontitis patients, but not at sites of aseptic inflammation (cerebrospinal fluid of patients with neuromyelitis optica) [204]. The notion that neutrophils can transform into IL-10–producing cells is in line with the emerging concept that neutrophils do not represent a homogeneous population but exhibit functional plasticity that allows them to also act as regulatory cells under certain conditions [18,205]. However, the same notion seemingly deviates from findings of another study showing that the IL10 genomic locus in human neutrophils (stimulated with different pro-inflammatory molecules including LPS) remains in an inactive state, in contrast to autologous monocytes (or mouse neutrophils) that are readily induced to express IL-10 [206]. The authors of this study, nevertheless, did not rule out as-yet-undefined conditions capable of causing major reorganization of the IL-10 genomic locus of human neutrophils, thereby rendering it permissive to activation [206]. In this context, the authors of the study on IL-10–producing neutrophils suggested that, unlike LPS stimulation that promotes a pro-inflammatory neutrophil phenotype and blocks the chromatin modification at the IL-10 locus, LPS-stimulated Tregs induce chromatin modifications (H3K4me3, H3AcLys4) specific for transcriptionally active IL-10 gene in human neutrophils [204]. What also remains uncertain is the biological role of the subpopulation of IL-10–producing neutrophils in the periodontal pockets. Is this neutrophil subset the result of an immune evasion strategy whereby gram-negative bacteria program Tregs to curb the activity of neutrophils, or does this mechanism reflect a regulatory mechanism to ameliorate destructive inflammation and promote resolution thereof?
Additional mechanisms have been described whereby neutrophils can suppress immune responses although their relevance to periodontitis has not yet been addressed. For instance, neutrophils were shown to suppress T cell activation by releasing several factors: arginase-1 that depletes arginine required for T cell activation [207,208]; myeloperoxidase, which inhibits dendritic cell function [209]; and reactive oxygen species (ROS) [26] (Figure 1). In the latter regard, a subset of human mature neutrophils (CD16bright/CD62Ldim) was recently shown to suppress T cell activation by delivering H2O2 into the immunological synapse in a Mac-1 integrin (CD11b/CD18)–dependent manner [26]. It is conceivable, therefore, that in certain settings the presence of neutrophils may be required for restraining excessive and potentially harmful T cell activation.
6. Conclusion and therapeutic implications
Besides the studies discussed above, systems-level investigations of the transcriptome, proteome, and metabolome of neutrophils are also contributing to a comprehensive appreciation of the different roles of neutrophils that reach beyond phagocytosis and killing of bacteria, encompassing host response regulation and resolution of inflammation [1,69,210–213]. The lessons learned from the study of rare monogenic diseases suggest that neutrophil deficiencies may cause disease not necessarily due to lack of neutrophil immune surveillance but might – alternatively or additionally – involve breakdown of neutrophil-associated homeostatic mechanisms [46]. The concepts presented in this review have important therapeutic implications for periodontitis and potentially other neutrophil-mediated inflammatory disorders. Dissecting the precise molecular mechanisms involved in neutrophil disorders affecting their migration or function may help develop sophisticated rational approaches that can complement existing, but often largely ineffective, therapies such as antibiotic treatment. In this regard, the realization that the aggressive form of periodontitis associated with LAD-I is not due to a raging infection but rather due to host response dysregulation explains why this condition has proven unresponsive to antibiotics and/or mechanical removal of the tooth-associated biofilm [41,47]. The new findings call for a host-modulation therapy targeting the IL-23-IL-17 axis, as performed in mouse models of the disease [47]. In disorders associated with excessive neutrophil infiltration, inflammation could be controlled by administering endogenous regulators of the leukocyte adhesion cascade (Figure 3), such as Del-1, which has been shown to be protective in preclinical models of periodontitis and multiple sclerosis [16,140].
Highlights.
Neutrophils are functionally versatile and perform hitherto unanticipated functions
Neutrophil-associated pathology involves more than the bystander injury dogma
Neutrophils have regulatory functions that contribute to tissue homeostasis
Neutrophils have both protective and destructive roles in periodontitis
Acknowledgments
The authors are supported by the following sources: The Intramural Research Program of the NIH, NIDCR (N.M.M.); the European Community’s Seventh Framework Programme under grant agreement n602699 (DIREKT) and the Else-Kröner-Fresenius-Stiftung (2014_A137) (TC); and by U.S. Public Health Service grants AI068730 (E.H. and G.H.) and DE015254, DE017138, DE021685, and DE024716 (GH). We thank Debbie Maizels (Zoobotanica Scientific Illustration) for drawing figure 2 and Toshiharu Abe for help with the drawing of figure 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kobayashi SD, DeLeo FR. Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip Rev Syst Biol Med. 2009;1:309–333. doi: 10.1002/wsbm.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 4.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125:281–288. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 7.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajishengallis G, Chavakis T. Endogenous modulators of inflammatory cell recruitment. Trends Immunol. 2013;34:1–6. doi: 10.1016/j.it.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitroulis I, Alexaki VI, Kourtzelis I, Ziogas A, Hajishengallis G, Chavakis T. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 2015;147:123–135. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugl S, Wirths S, Radsak MP, Schild H, Stein P, Andre MC, et al. Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. Blood. 2013;121:723–733. doi: 10.1182/blood-2012-05-429589. [DOI] [PubMed] [Google Scholar]
- 13.Druhan LJ, Ai J, Massullo P, Kindwall-Keller T, Ranalli MA, Avalos BR. Novel mechanism of G-CSF refractoriness in patients with severe congenital neutropenia. Blood. 2005;105:584–591. doi: 10.1182/blood-2004-07-2613. [DOI] [PubMed] [Google Scholar]
- 14.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 15.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maekawa T, Hosur K, Abe T, Kantarci A, Ziogas A, Wang B, et al. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun. 2015;6:8272. doi: 10.1038/ncomms9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 19.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leliefeld PH, Koenderman L, Pillay J. How neutrophils shape adaptive immune responses. Front Immunol. 2015;6:471. doi: 10.3389/fimmu.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Economopoulou M, Bdeir K, Cines DB, Fogt F, Bdeir Y, Lubkowski J, et al. Inhibition of pathologic retinal neovascularization by alpha-defensins. Blood. 2005;106:3831–3838. doi: 10.1182/blood-2005-03-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2009;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 23.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 26.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christoffersson G, Vagesjo E, Vandooren J, Liden M, Massena S, Reinert RB, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–4662. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother. 2012;61:1155–1167. doi: 10.1007/s00262-012-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 33.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 35.Tseng CW, Liu GY. Expanding roles of neutrophils in aging hosts. Curr Opin Immunol. 2014;29:43–48. doi: 10.1016/j.coi.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Biol. 2015;98:539–548. doi: 10.1189/jlb.3VMR1014-468R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niederman R, Westernoff T, Lee C, Mark LL, Kawashima N, Ullman-Culler M, et al. Infection-mediated early-onset periodontal disease in P/E-selectin-deficient mice. J Clin Periodontol. 2001;28:569–575. doi: 10.1034/j.1600-051x.2001.028006569.x. [DOI] [PubMed] [Google Scholar]
- 41.Deas DE, Mackey SA, McDonnell HT. Systemic disease and periodontitis: manifestations of neutrophil dysfunction. Periodontol 2000. 2003;32:82–104. doi: 10.1046/j.0906-6713.2003.03207.x. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2012;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 44.Matthews JB, Wright HJ, Roberts A, Cooper PR, Chapple IL. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin Exp Immunol. 2007;147:255–264. doi: 10.1111/j.1365-2249.2006.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moutsopoulos NM, Lionakis MS, Hajishengallis G. Inborn errors in immunity: unique natural models to dissect oral immunity. J Dent Res. 2015;94:753–758. doi: 10.1177/0022034515583533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17–driven inflammatory bone loss. Sci Transl Med. 2014;6:229ra240. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J Dent Res. 2014;93:231–237. doi: 10.1177/0022034513507956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasturk H, Kantarci A, Van Dyke TE. Paradigm shift in the pharmacological management of periodontal diseases. Front Oral Biol. 2012;15:160–176. doi: 10.1159/000329678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 51.Schieppati A, Henter JI, Daina E, Aperia A. Why rare diseases are an important medical and social issue. Lancet. 2008;371:2039–2041. doi: 10.1016/S0140-6736(08)60872-7. [DOI] [PubMed] [Google Scholar]
- 52.Dodge JA, Chigladze T, Donadieu J, Grossman Z, Ramos F, Serlicorni A, et al. The importance of rare diseases: from the gene to society. Arch Dis Child. 2011;96:791–792. doi: 10.1136/adc.2010.193664. [DOI] [PubMed] [Google Scholar]
- 53.Peltonen L, Perola M, Naukkarinen J, Palotie A. Lessons from studying monogenic disease for common disease. Hum Mol Genet. 2006;15(Spec No 1):R67–74. doi: 10.1093/hmg/ddl060. [DOI] [PubMed] [Google Scholar]
- 54.Fischer A. Human primary immunodeficiency diseases. Immunity. 2007;27:835–845. doi: 10.1016/j.immuni.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Harris ES, Weyrich AS, Zimmerman GA. Lessons from rare maladies: leukocyte adhesion deficiency syndromes. Curr Opin Hematol. 2013;20:16–25. doi: 10.1097/MOH.0b013e32835a0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci. 2012;1250:50–55. doi: 10.1111/j.1749-6632.2011.06389.x. [DOI] [PubMed] [Google Scholar]
- 57.Waldrop TC, Anderson DC, Hallmon WW, Schmalstieg FC, Jacobs RL. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. J Periodontol. 1987;58:400–416. doi: 10.1902/jop.1987.58.6.400. [DOI] [PubMed] [Google Scholar]
- 58.Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430–449. doi: 10.1177/154411130301400605. [DOI] [PubMed] [Google Scholar]
- 59.Dababneh R, Al-Wahadneh AM, Hamadneh S, Khouri A, Bissada NF. Periodontal manifestation of leukocyte adhesion deficiency type I. J Periodontol. 2008;79:764–768. doi: 10.1902/jop.2008.070323. [DOI] [PubMed] [Google Scholar]
- 60.Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol. 2011;38:49–59. doi: 10.1111/j.1600-051X.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 61.Sollecito TP, Sullivan KE, Pinto A, Stewart J, Korostoff J. Systemic conditions associated with periodontitis in childhood and adolescence. A review of diagnostic possibilities. Med Oral Patol Oral Cir Bucal. 2005;10:142–150. [PubMed] [Google Scholar]
- 62.Larjava H, Koivisto L, Heino J, Hakkinen L. Integrins in periodontal disease. Exp Cell Res. 2014;325:104–110. doi: 10.1016/j.yexcr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Hajishengallis G, Moutsopoulos NM. Etiology of leukocyte adhesion deficiency-associated periodontitis revisited: not a raging infection but a raging inflammatory response. Expert Rev Clin Immunol. 2014;10:973–975. doi: 10.1586/1744666X.2014.929944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000. 2015;69:142–159. doi: 10.1111/prd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hajishengallis G, Moutsopoulos NM. Role of bacteria in leukocyte adhesion deficiency-associated periodontitis. Microb Pathog. 2015 doi: 10.1016/j.micpath.2015.09.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 68.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 2011;32:350–357. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gamonal J, Bascones A, Acevedo A, Blanco E, Silva A. Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J Periodontol. 2001;72:517–525. doi: 10.1902/jop.2001.72.4.517. [DOI] [PubMed] [Google Scholar]
- 72.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 73.Moutsopoulos NM, Chalmers NI, Barb JJ, Abusleme L, Greenwell-Wild T, Dutzan N, et al. Subgingival microbial communities in leukocyte adhesion deficiency and their relationship with local immunopathology. PLoS Pathog. 2015;11:e1004698. doi: 10.1371/journal.ppat.1004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chuluyan HE, Issekutz AC. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest. 1993;92:2768–2777. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz BR, Wayner EA, Carlos TM, Ochs HD, Harlan JM. Identification of surface proteins mediating adherence of CD11/CD18-deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest. 1990;85:2019–2022. doi: 10.1172/JCI114668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol. 1995;154:4099–4112. [PubMed] [Google Scholar]
- 77.Hyun YM, Chung HL, McGrath JL, Waugh RE, Kim M. Activated integrin VLA-4 localizes to the lamellipodia and mediates T cell migration on VCAM-1. J Immunol. 2009;183:359–369. doi: 10.4049/jimmunol.0803388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, et al. Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J Exp Med. 1997;186:1357–1364. doi: 10.1084/jem.186.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FA, Pruijn GJ, Marijnissen RJ, et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol. 2014;192:4103–4111. doi: 10.4049/jimmunol.1301970. [DOI] [PubMed] [Google Scholar]
- 80.Lerman YV, Lim K, Hyun YM, Falkner KL, Yang H, Pietropaoli AP, et al. Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin alpha3beta1-dependent. Blood. 2014;124:3515–3523. doi: 10.1182/blood-2014-01-552943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Subramanian P, Mitroulis I, Hajishengallis G, Chavakis T. Regulation of tissue infiltration by neutrophils: role of integrin alpha3beta1 and other factors. Curr Opin Hematol. 2015 doi: 10.1097/MOH.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hawkins HK, Heffelfinger SC, Anderson DC. Leukocyte adhesion deficiency: clinical and postmortem observations. Pediatr Pathol. 1992;12:119–130. doi: 10.3109/15513819209023288. [DOI] [PubMed] [Google Scholar]
- 83.Kadioglu A, De Filippo K, Bangert M, Fernandes VE, Richards L, Jones K, et al. The integrins Mac-1 and alpha4beta1 perform crucial roles in neutrophil and T cell recruitment to lungs during Streptococcus pneumoniae infection. J Immunol. 2011;186:5907–5915. doi: 10.4049/jimmunol.1001533. [DOI] [PubMed] [Google Scholar]
- 84.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 86.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shin J, Maekawa T, Abe T, Hajishengallis E, Hosur K, Pyaram K, et al. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Science Translational Medicine. 2015;7:307ra155. doi: 10.1126/scitranslmed.aac5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park-Min KH, Lee EY, Moskowitz NK, Lim E, Lee SK, Lorenzo JA, et al. Negative regulation of osteoclast precursor differentiation by CD11b and beta2 integrin-B-cell lymphoma 6 signaling. J Bone Miner Res. 2013;28:135–149. doi: 10.1002/jbmr.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Etzioni A, Tonetti M. Leukocyte adhesion deficiency II-from A to almost Z. Immunol Rev. 2000;178:138–147. doi: 10.1034/j.1600-065x.2000.17805.x. [DOI] [PubMed] [Google Scholar]
- 90.Svensson L, Howarth K, McDowall A, Patzak I, Evans R, Ussar S, et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15:306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Z, Cai J, Gao J, White GC, 2nd, Chen F, Ma YQ. Interaction of kindlin-3 and beta2-integrins differentially regulates neutrophil recruitment and NET release in mice. Blood. 2015;126:373–377. doi: 10.1182/blood-2015-03-636720. [DOI] [PubMed] [Google Scholar]
- 92.Pai SY, Kim C, Williams DA. Rac GTPases in human diseases. Dis Markers. 2010;29:177–187. doi: 10.3233/DMA-2010-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gu Y, Jia B, Yang FC, D’Souza M, Harris CE, Derrow CW, et al. Biochemical and biological characterization of a human Rac2 GTPase mutant associated with phagocytic immunodeficiency. J Biol Chem. 2001;276:15929–15938. doi: 10.1074/jbc.M010445200. [DOI] [PubMed] [Google Scholar]
- 94.Sima C, Gastfreund S, Sun C, Glogauer M. Rac-null leukocytes are associated with increased inflammation-mediated alveolar bone loss. Am J Pathol. 2014;184:472–482. doi: 10.1016/j.ajpath.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Dinauer MC, Orkin SH. Chronic granulomatous disease. Annu Rev Med. 1992;43:117–124. doi: 10.1146/annurev.me.43.020192.001001. [DOI] [PubMed] [Google Scholar]
- 96.Odell EW, Segal AW. Killing of pathogens associated with chronic granulomatous disease by the non-oxidative microbicidal mechanisms of human neutrophils. J Med Microbiol. 1991;34:129–135. doi: 10.1099/00222615-34-3-129. [DOI] [PubMed] [Google Scholar]
- 97.Stroobant J, Harris MC, Cody CS, Polin RA, Douglas SD. Diminished bactericidal capacity for group B streptococci of neutrophils from children with chronic granulomatous disease. Infect Immun. 1983;39:966–969. doi: 10.1128/iai.39.2.966-969.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soler-Palacin P, Margareto C, Llobet P, Asensio O, Hernandez M, Caragol I, et al. Chronic granulomatous disease in pediatric patients: 25 years of experience. Allergol Immunopathol (Madr) 2007;35:83–89. doi: 10.1157/13106774. [DOI] [PubMed] [Google Scholar]
- 99.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sima C, Glogauer M. Neutrophil dysfunction and host susceptibility to periodontal inflammation: Current state of knowledge. Curr Oral Health Rep. 2014;1:95–103. [Google Scholar]
- 101.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 102.Zenobia C, Luo XL, Hashim A, Abe T, Jin L, Chang Y, et al. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol. 2013;15:1419–1426. doi: 10.1111/cmi.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosier BT, de Jager M, Zaura E, Krom BP. Historical and contemporary hypotheses on the development of oral diseases: are we there yet? Front Cell Infect Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Neutrophil fate in gingival crevicular fluid. Ultrastruct Pathol. 2010;34:25–30. doi: 10.3109/01913120903419989. [DOI] [PubMed] [Google Scholar]
- 107.Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Extracellular neutrophil traps in periodontitis. J Periodontal Res. 2009;44:664–672. doi: 10.1111/j.1600-0765.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 108.Pinegin B, Vorobjeva N, Pinegin V. Neutrophil extracellular traps and their role in the development of chronic inflammation and autoimmunity. Autoimmun Rev. 2015;14:633–640. doi: 10.1016/j.autrev.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 109.Dickinson DP. Cysteine peptidases of mammals: their biological roles and potential effects in the oral cavity and other tissues in health and disease. Crit Rev Oral Biol Med. 2002;13:238–275. doi: 10.1177/154411130201300304. [DOI] [PubMed] [Google Scholar]
- 110.Kobayashi T, Sugiura K, Takeichi T, Akiyama M. The novel CTSC homozygous nonsense mutation p.Lys106X in a patient with Papillon-Lefevre syndrome with all permanent teeth remaining at over 40 years of age. Br J Dermatol. 2013 doi: 10.1111/bjd.12429. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 111.Nualart Grollmus ZC, Morales Chavez MC, Silvestre Donat FJ. Periodontal disease associated to systemic genetic disorders. Med Oral Patol Oral Cir Bucal. 2007;12:E211–215. [PubMed] [Google Scholar]
- 112.Hart TC, Atkinson JC. Mendelian forms of periodontitis. Periodontol 2000. 2007;45:95–112. doi: 10.1111/j.1600-0757.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 113.Sorensen OE, Clemmensen SN, Dahl SL, Ostergaard O, Heegaard NH, Glenthoj A, et al. Papillon-Lefevre syndrome patient reveals species-dependent requirements for neutrophil defenses. J Clin Invest. 2014;124:4539–4548. doi: 10.1172/JCI76009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pham CT, Ivanovich JL, Raptis SZ, Zehnbauer B, Ley TJ. Papillon-Lefevre syndrome: correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J Immunol. 2004;173:7277–7281. doi: 10.4049/jimmunol.173.12.7277. [DOI] [PubMed] [Google Scholar]
- 115.de Haar SF, Hiemstra PS, van Steenbergen MT, Everts V, Beertsen W. Role of polymorphonuclear leukocyte-derived serine proteinases in defense against Actinobacillus actinomycetemcomitans. Infect Immun. 2006;74:5284–5291. doi: 10.1128/IAI.02016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ryu OH, Choi SJ, Firatli E, Choi SW, Hart PS, Shen RF, et al. Proteolysis of macrophage inflammatory protein-1α isoforms LD78β and LD78α by neutrophil-derived serine proteases. J Biol Chem. 2005;280:17415–17421. doi: 10.1074/jbc.M500340200. [DOI] [PubMed] [Google Scholar]
- 117.Eick S, Puklo M, Adamowicz K, Kantyka T, Hiemstra P, Stennicke H, et al. Lack of cathelicidin processing in Papillon-Lefevre syndrome patients reveals essential role of LL-37 in periodontal homeostasis. Orphanet J Rare Dis. 2014;9:148. doi: 10.1186/s13023-014-0148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakamichi Y, Horibe K, Takahashi N, Udagawa N. Roles of cathelicidins in inflammation and bone loss. Odontology. 2014;102:137–146. doi: 10.1007/s10266-014-0167-0. [DOI] [PubMed] [Google Scholar]
- 119.Supanchart C, Thawanaphong S, Makeudom A, Bolscher JG, Nazmi K, Kornak U, et al. The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J Dent Res. 2012;91:1071–1077. doi: 10.1177/0022034512460402. [DOI] [PubMed] [Google Scholar]
- 120.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 121.Ryder MI. Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontol 2000. 2010;53:124–137. doi: 10.1111/j.1600-0757.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- 122.Chakravarti A, Raquil MA, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;114:1633–1644. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- 123.Nakashima T, Hayashi M, Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab. 2012;23:582–590. doi: 10.1016/j.tem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 124.Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 125.Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodontal Res. 1995;30:23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 126.Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F, et al. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. 2005;105:830–837. doi: 10.1182/blood-2004-02-0564. [DOI] [PubMed] [Google Scholar]
- 127.Abe T, AlSarhan M, Benakanakere MR, Maekawa T, Kinane DF, Cancro MP, et al. The B Cell-Stimulatory Cytokines BLyS and APRIL Are Elevated in Human Periodontitis and Are Required for B Cell-Dependent Bone Loss in Experimental Murine Periodontitis. J Immunol. 2015;195:1427–1435. doi: 10.4049/jimmunol.1500496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 129.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hernandez M, Gamonal J, Tervahartiala T, Mantyla P, Rivera O, Dezerega A, et al. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: a longitudinal study. J Periodontol. 2010;81:1644–1652. doi: 10.1902/jop.2010.100196. [DOI] [PubMed] [Google Scholar]
- 131.Landzberg M, Doering H, Aboodi GM, Tenenbaum HC, Glogauer M. Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease. J Periodontal Res. 2014 doi: 10.1111/jre.12211. [DOI] [PubMed] [Google Scholar]
- 132.Dutzan Mucosal Immunology. 2015 [Google Scholar]
- 133.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 134.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 135.Wang J, Shiratori I, Uehori J, Ikawa M, Arase H. Neutrophil infiltration during inflammation is regulated by PILRalpha via modulation of integrin activation. Nat Immunol. 2013;14:34–40. doi: 10.1038/ni.2456. [DOI] [PubMed] [Google Scholar]
- 136.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hidai C, Zupancic T, Penta K, Mikhail A, Kawana M, Quertermous EE, et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev. 1998;12:21–33. doi: 10.1101/gad.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mitroulis I, Kang YY, Gahmberg CG, Siegert G, Hajishengallis G, Chavakis T, et al. Developmental endothelial locus-1 attenuates complement-dependent phagocytosis through inhibition of Mac-1-integrin. Thromb Haemost. 2014;111:781–1006. doi: 10.1160/TH13-09-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Choi EY, Lim JH, Neuwirth A, Economopoulou M, Chatzigeorgiou A, Chung KJ, et al. Developmental endothelial locus-1 is a homeostatic factor in the central nervous system limiting neuroinflammation and demyelination. Mol Psychiatry. 2015;20:880–888. doi: 10.1038/mp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yasukawa K, Tokuda H, Tun X, Utsumi H, Yamada K. The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis. Free Radic Res. 2012;46:1427–1436. doi: 10.3109/10715762.2012.732698. [DOI] [PubMed] [Google Scholar]
- 142.Shang K, Bai YP, Wang C, Wang Z, Gu HY, Du X, et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One. 2012;7:e51848. doi: 10.1371/journal.pone.0051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ostanin DV, Kurmaeva E, Furr K, Bao R, Hoffman J, Berney S, et al. Acquisition of antigen-presenting functions by neutrophils isolated from mice with chronic colitis. J Immunol. 2012;188:1491–1502. doi: 10.4049/jimmunol.1102296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuhl AA, Kakirman H, Janotta M, Dreher S, Cremer P, Pawlowski NN, et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133:1882–1892. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 145.Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci U S A. 2013;110:12768–12773. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shin J, Hosur KB, Pyaram K, Jotwani R, Liang S, Chavakis T, et al. Expression and function of the homeostatic molecule Del-1 in endothelial cells and the periodontal tissue. Clin Dev Immunol. 2013:Article ID 617809. doi: 10.1155/2013/617809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Roberts HM, Ling MR, Insall R, Kalna G, Spengler J, Grant MM, et al. Impaired neutrophil directional chemotactic accuracy in chronic periodontitis patients. J Clin Periodontol. 2015;42:1–11. doi: 10.1111/jcpe.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol. 1996;156:2264–2272. [PubMed] [Google Scholar]
- 152.Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4-lipoxin A4 receptor interaction. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- 153.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huppler AR, Conti HR, Hernandez-Santos N, Darville T, Biswas PS, Gaffen SL. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol. 2014;192:1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arnardottir HH, Dalli J, Colas RA, Shinohara M, Serhan CN. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol. 2014;193:4235–4244. doi: 10.4049/jimmunol.1401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jaillon S, Bonavita E, Gentile S, Rubino M, Laface I, Garlanda C, et al. The long pentraxin PTX3 as a key component of humoral innate immunity and a candidate diagnostic for inflammatory diseases. Int Arch Allergy Immunol. 2014;165:165–178. doi: 10.1159/000368778. [DOI] [PubMed] [Google Scholar]
- 159.Maina V, Cotena A, Doni A, Nebuloni M, Pasqualini F, Milner CM, et al. Coregulation in human leukocytes of the long pentraxin PTX3 and TSG-6. J Leukoc Biol. 2009;86:123–132. doi: 10.1189/jlb.0608345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chung KJ, Mitroulis I, Wiessner JR, Zheng YY, Siegert G, Sperandio M, et al. A novel pathway of rapid TLR-triggered activation of integrin-dependent leukocyte adhesion that requires Rap1 GTPase. Mol Biol Cell. 2014;25:2948–2955. doi: 10.1091/mbc.E14-04-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. J Cell Mol Med. 2009;13:1211–1220. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Langer HF, Choi EY, Zhou H, Schleicher R, Chung KJ, Tang Z, et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res. 2012;110:1202–1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kourtzelis I. Thrombosis and Haemostasis. 2015 [Google Scholar]
- 166.Pradeep AR, Kathariya R, Raghavendra NM, Sharma A. Levels of pentraxin-3 in gingival crevicular fluid and plasma in periodontal health and disease. J Periodontol. 2011;82:734–741. doi: 10.1902/jop.2010.100526. [DOI] [PubMed] [Google Scholar]
- 167.Fujita Y, Ito H, Sekino S, Numabe Y. Correlations between pentraxin 3 or cytokine levels in gingival crevicular fluid and clinical parameters of chronic periodontitis. Odontology. 2012;100:215–221. doi: 10.1007/s10266-011-0042-1. [DOI] [PubMed] [Google Scholar]
- 168.Gumus P, Nizam N, Nalbantsoy A, Ozcaka O, Buduneli N. Saliva and serum levels of pentraxin-3 and interleukin-1beta in generalized aggressive or chronic periodontitis. J Periodontol. 2014;85:e40–46. doi: 10.1902/jop.2013.130281. [DOI] [PubMed] [Google Scholar]
- 169.Lakshmanan R, Jayakumar ND, Sankari M, Padmalatha O, Varghese S. Estimation of pentraxin-3 levels in the gingival tissues of chronic and aggressive periodontitis participants: an in vivo study. J Periodontol. 2014;85:290–297. doi: 10.1902/jop.2013.120718. [DOI] [PubMed] [Google Scholar]
- 170.Keles GC, Balli U, Cetinkaya BO, Ayas B, Findik A, Keles ZP, et al. Biochemical analysis of pentraxin 3 and fibrinogen levels in experimental periodontitis model. Mediators Inflamm. 2012;2012:809801. doi: 10.1155/2012/809801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Daigo K, Mantovani A, Bottazzi B. The yin-yang of long pentraxin PTX3 in inflammation and immunity. Immunol Lett. 2014;161:38–43. doi: 10.1016/j.imlet.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lakschevitz FS, Aboodi GM, Glogauer M. Oral neutrophil transcriptome changes result in a pro-survival phenotype in periodontal diseases. PLoS One. 2013;8:e68983. doi: 10.1371/journal.pone.0068983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Orth RK, O’Brien-Simpson NM, Dashper SG, Reynolds EC. Synergistic virulence of Porphyromonas gingivalis and Treponema denticola in a murine periodontitis model. Mol Oral Microbiol. 2011;26:229–240. doi: 10.1111/j.2041-1014.2011.00612.x. [DOI] [PubMed] [Google Scholar]
- 174.Ramsey MM, Rumbaugh KP, Whiteley M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 2011;7:e1002012. doi: 10.1371/journal.ppat.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Settem RP, El-Hassan AT, Honma K, Stafford GP, Sharma A. Fusobacterium nucleatum and Tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect Immun. 2012;80:2436–2443. doi: 10.1128/IAI.06276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jiao Y, Darzi Y, Tawaratsumida K, Marchesan JT, Hasegawa M, Moon H, et al. Induction of bone loss by pathobiont-mediated nod1 signaling in the oral cavity. Cell Host Microbe. 2013;13:595–601. doi: 10.1016/j.chom.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, et al. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. 2014;8:1659–1672. doi: 10.1038/ismej.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jorth P, Turner KH, Gumus P, Nizam N, Buduneli N, Whiteley M. Metatranscriptomics of the human oral microbiome during health and disease. MBio. 2014;5:e01012–01014. doi: 10.1128/mBio.01012-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bao K, Belibasakis GN, Thurnheer T, Aduse-Opoku J, Curtis MA, Bostanci N. Role of Porphyromonas gingivalis gingipains in multi-species biofilm formation. BMC Microbiol. 2014;14:258. doi: 10.1186/s12866-014-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: The Polymicrobial Synergy and Dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 189.Burns E, Eliyahu T, Uematsu S, Akira S, Nussbaum G. TLR2-dependent inflammatory response to Porphyromonas gingivalis is MyD88 independent, whereas MyD88 is required to clear infection. J Immunol. 2010;184:1455–1462. doi: 10.4049/jimmunol.0900378. [DOI] [PubMed] [Google Scholar]
- 190.Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontol 2000. 2012;58:37–68. doi: 10.1111/j.1600-0757.2011.00415.x. [DOI] [PubMed] [Google Scholar]
- 191.Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000. 1994;5:7–25. doi: 10.1111/j.1600-0757.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 192.Kumar PS. Smoking and the subgingival ecosystem: a pathogen-enriched community. Future Microbiol. 2012;7:917–919. doi: 10.2217/fmb.12.71. [DOI] [PubMed] [Google Scholar]
- 193.Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2010;53:138–153. doi: 10.1111/j.1600-0757.2010.00340.x. [DOI] [PubMed] [Google Scholar]
- 194.Zelkha SA, Freilich RW, Amar S. Periodontal innate immune mechanisms relevant to atherosclerosis and obesity. Periodontol 2000. 2010;54:207–221. doi: 10.1111/j.1600-0757.2010.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Divaris K, Monda KL, North KE, Olshan AF, Reynolds LM, Hsueh WC, et al. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet. 2013;22:2312–2324. doi: 10.1093/hmg/ddt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bagaitkar J, Daep CA, Patel CK, Renaud DE, Demuth DR, Scott DA. Tobacco smoke augments Porphyromonas gingivalis-Streptococcus gordonii biofilm formation. PLoS One. 2011;6:e27386. doi: 10.1371/journal.pone.0027386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hart TC, Kornman KS. Genetic factors in the pathogenesis of periodontitis. Periodontol 2000. 1997;14:202–215. doi: 10.1111/j.1600-0757.1997.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 198.Divaris K, Monda KL, North KE, Olshan AF, Lange EM, Moss K, et al. Genome-wide association study of periodontal pathogen colonization. J Dent Res. 2012;91:21S–28S. doi: 10.1177/0022034512447951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cooper PR, Palmer LJ, Chapple IL. Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe? Periodontol 2000. 2013;63:165–197. doi: 10.1111/prd.12025. [DOI] [PubMed] [Google Scholar]
- 200.Hatanaka E, Monteagudo PT, Marrocos MS, Campa A. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol. 2006;146:443–447. doi: 10.1111/j.1365-2249.2006.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yasunari K, Watanabe T, Nakamura M. Reactive oxygen species formation by polymorphonuclear cells and mononuclear cells as a risk factor of cardiovascular diseases. Curr Pharm Biotechnol. 2006;7:73–80. doi: 10.2174/138920106776597612. [DOI] [PubMed] [Google Scholar]
- 202.Ling MR, Chapple IL, Matthews JB. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 2015 doi: 10.1177/1753425915589387. [DOI] [PubMed] [Google Scholar]
- 203.Matthews JB, Wright HJ, Roberts A, Ling-Mountford N, Cooper PR, Chapple IL. Neutrophil hyper-responsiveness in periodontitis. J Dent Res. 2007;86:718–722. doi: 10.1177/154405910708600806. [DOI] [PubMed] [Google Scholar]
- 204.Lewkowicz N, Mycko MP, Przygodzka P, Cwiklinska H, Cichalewska M, Matysiak M, et al. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.66. [DOI] [PubMed] [Google Scholar]
- 205.Granot Z, Fridlender ZG. Plasticity beyond Cancer Cells and the “Immunosuppressive Switch”. Cancer Res. 2015;75:4441–4445. doi: 10.1158/0008-5472.CAN-15-1502. [DOI] [PubMed] [Google Scholar]
- 206.Tamassia N, Zimmermann M, Castellucci M, Ostuni R, Bruderek K, Schilling B, et al. Cutting edge: An inactive chromatin configuration at the IL-10 locus in human neutrophils. J Immunol. 2013;190:1921–1925. doi: 10.4049/jimmunol.1203022. [DOI] [PubMed] [Google Scholar]
- 207.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 208.Rotondo R, Bertolotto M, Barisione G, Astigiano S, Mandruzzato S, Ottonello L, et al. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011;89:721–727. doi: 10.1189/jlb.1109737. [DOI] [PubMed] [Google Scholar]
- 209.Odobasic D, Kitching AR, Yang Y, O’Sullivan KM, Muljadi RC, Edgtton KL, et al. Neutrophil myeloperoxidase regulates T-cell-driven tissue inflammation in mice by inhibiting dendritic cell function. Blood. 2013;121:4195–4204. doi: 10.1182/blood-2012-09-456483. [DOI] [PubMed] [Google Scholar]
- 210.Kobayashi SD, Sturdevant DE, Deleo FR. Genome-scale transcript analyses with human neutrophils. Methods Mol Biol. 2014;1124:437–450. doi: 10.1007/978-1-62703-845-4_26. [DOI] [PubMed] [Google Scholar]
- 211.Neubauer O, Sabapathy S, Lazarus R, Jowett JB, Desbrow B, Peake JM, et al. Transcriptome analysis of neutrophils after endurance exercise reveals novel signaling mechanisms in the immune response to physiological stress. J Appl Physiol (1985) 2013;114:1677–1688. doi: 10.1152/japplphysiol.00143.2013. [DOI] [PubMed] [Google Scholar]
- 212.Trusch M, Tillack K, Kwiatkowski M, Bertsch A, Ahrends R, Kohlbacher O, et al. Displacement chromatography as first separating step in online two-dimensional liquid chromatography coupled to mass spectrometry analysis of a complex protein sample--the proteome of neutrophils. J Chromatogr A. 2012;1232:288–294. doi: 10.1016/j.chroma.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 213.Singh SK, Sethi S, Aravamudhan S, Kruger M, Grabher C. Proteome mapping of adult zebrafish marrow neutrophils reveals partial cross species conservation to human peripheral neutrophils. PLoS One. 2013;8:e73998. doi: 10.1371/journal.pone.0073998. [DOI] [PMC free article] [PubMed] [Google Scholar]