Abstract
While brain connectivity analyses have been demonstrated to identify ill patients for a number of diseases, their ability to predict cognitive impairment after brain injury is not well established. Traditional post brain injury models, such as stroke, are limited for this evaluation because pre‐injury brain connectivity patterns are infrequently available. Patients with severe carotid stenosis, in contrast, often undergo non‐emergent revascularization surgery, allowing the collection of pre and post‐operative imaging, may experience brain insult due to perioperative thrombotic/embolic infarcts or hypoperfusion, and can suffer post‐operative cognitive decline. We hypothesized that a distributed function such as memory would be more resilient in patients with brains demonstrating higher degrees of modularity. To test this hypothesis, we analyzed preoperative structural connectivity graphs (using T1 and DWI MRI) for 34 patients that underwent carotid intervention, and evaluated differences in graph metrics using the Brain Connectivity Toolbox. We found that patients with lower binary component number, binary community number and weighted community number prior to surgery were at greater risk for developing cognitive decline. These findings highlight the promise of brain connectivity analyses to predict cognitive decline following brain injury and serve as a clinical decision support tool. Hum Brain Mapp 37:2185–2194, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: biological markers, brain, brain injuries, cognition disorders, decision making, magnetic resonance imaging, connectome, neuroimaging, carotid stenosis, risk factors
Abbreviations
- BCT
Brain connectivity toolbox;
- CAS
Carotid artery stenting;
- CEA
Carotid endarterectomy;
- DWI
Diffusion weighted imaging;
- FDR
False discovery rate;
- FMRIB
Functional MRI of the Brain;
- MMSE
Mini‐mental status exam;
- RAVLT
Rey auditory verbal learning test
BACKGROUND
Anatomic and physiologic brain organization, referred to as the connectome [DeFelipe, 2010; Sporns et al., 2005] have been used to distinguish healthy controls from patients with schizophrenia, traumatic brain injury, Alzheimer's disease or depression [Peng et al., 2014; Prasad et al., 2015; van den Heuvel et al., 2013; Venkatesan et al., 2015]. However, it is not known if brain connectivity patterns present prior to injury can predict post‐injury pathological clinical outcomes. Complex cognitive functions are known to be influenced by the relative integration and segregation of brain networks as characterized by modules [Chen et al., 2006]. The topology of these networks can be modeled and systematically examined using large‐scale brain network graphs or connectomes. Analysis of these connectomes have been shown to provide insights into both abnormal brain development and neuropsychological conditions [Alexander‐Bloch et al., 2014; Peng et al., 2014; Xu et al., 2013]. Thus, it is reasonable to hypothesize that the organizational information represented in a connectome may be able to predict post‐injury brain function recovery.
A challenge for testing the predictive ability of pre brain injury connectomes is the fact that in many clinical populations, such as patients with stroke, obtaining preinjury information is difficult due to variability in the timing and etiology of injury. An exception is carotid intervention patients, who often undergo non‐emergent carotid vessel manipulation under controlled conditions for the purpose of restoring impaired brain blood flow via the carotid arteries. While the overall benefits of carotid interventions for stroke prevention are well established [North American Symptomatic Carotid Endarterectomy Trial, 1991], these procedures can be associated with intraoperative episodes of decreased blood flow, embolism of atherosclerotic plaques and thrombus formation, all of which can be associated with brain injury [Gossetti et al., 2007]. Patients can also experience postoperative memory or cognitive decline [Zhou et al., 2012]. The combination of planned procedures with potential peri‐procedure brain injury and possible long term cognitive impairment make carotid intervention patients a strong model for testing the ability of pre‐operative connectomes to predict post‐procedure cognitive impairment. Additionally, as no definite pre or peri‐procedure factors have been able to definitely identify patients more likely to develop post‐operative cognitive impairment [Paraskevas et al., 2014], a validated metric of increased pre‐operative risk for post‐operative cognitive impairment could help guide patients earlier to targeted treatments, such as cognitive rehabilitation, enable consideration of alternative treatment regimens as they become available, and anticipate post‐procedure health care needs.
Neuroimaging methods, like structural and diffusion weighted imaging (DWI) MRI, have shown degenerative changes in both the grey and white matter in patients with chronic vascular disease [Cardenas et al., 2012]. Analysis of structural and DWI MRI data combined into connectomes can be performed using tools from the well‐developed field of graph theory. These graph metrics describe complex networks as interrelationships between objects (e.g., connectomes) from which dynamic brain functions emerge [Sporns et al., 2005], and can quantify the integrity of functionally specialized regions as well as connections between those regions across the entire brain. The derived graph analysis can then be used to evaluate the efficiency of how information flows across subunits working together to produce cognitive processes [Albert et al., 2000] (Fig. 1). Such analyses have been shown to discriminate healthy elderly controls from patients with mild cognitive impairment (MCI) [Prasad et al., 2015]. As cortical lesions and subcortical axonal damage have been shown to disrupt brain modular network organization [Crofts et al., 2011], we hypothesized that intrinsic differences in connectomes, either the result of anatomic variation or pre‐operative brain injury, could be characterized in structural connectivity analysis as markers for understanding risk for cognitive impairment. For the first time, we investigate whether features underlying the organization of the brain, as reflected by its structural connectivity connectome, can provide new insights into which patients might have increased vulnerability for cognitive decline after carotid intervention.
Figure 1.
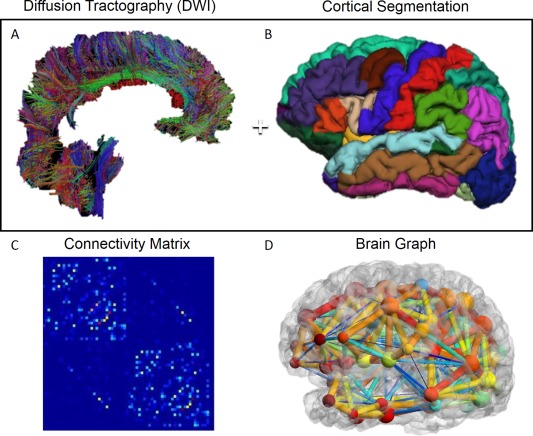
Generation of brain connectivity networks graphs. (A) Whole brain DWI fiber tractography, (B) whole brain FreeSurfer cortical segmentation, (C) connectivity matrix generated combining A & B, and (D) graph representation of brain structural connectivity generated using BrainNet (Xia et al., 2013). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
MATERIALS AND METHODS
Subjects
Under an IRB approved and HIPPA compliant protocol, veterans scheduled to undergo carotid interventions at the Palo Alto Veterans Affairs hospital were prospectively enrolled into the study. Indications for surgical procedures included severe asymptomatic stenosis (> 80%) of carotid arteries identified on carotid duplex ultrasound or moderate to severe stenosis (>60%) with focal neurological symptoms.
Neuropsychological Testing
Subjects underwent a neuropsychological battery prior to and at one month after carotid intervention. The battery included the mini‐mental status exam (MMSE) [Folstein et al., 1975] and the Rey Auditory Verbal Learning Test (RAVLT) [Bean, 2011]. The RAVLT is a verbal memory test that involves reading a series of words repeatedly and asking for patients to recall the word lists. The key subscore used to divide patients who declined from those who did not was the sum of the immediate recall trials. Alternative forms of the RAVLT were used between testing sessions to avoid practice effects. Pre‐procedure memory tests were administered (1) within two weeks prior to the revascularization procedure, and (2) at least one month after the procedure to avoid residual procedure or medication effects. The subjects were separated into two groups based on raw change scores of the sum of immediate recall of list learning (Fig. 2) with patients whose score declined between neuropsychological testing sessions classified as having cognitive decline.
Figure 2.
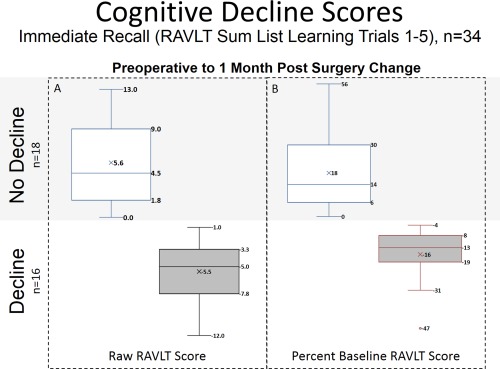
Distribution of post‐operative cognitive decline. Box and Whisker plots of pre to post‐operative change in RAVLT sum of trials 1‐5, a verbal memory test, as (A) raw score and (B) percent of baseline RAVLT score. Cognitive decline was defined as post‐operative decrease in performance on the RAVLT sum of trials 1–5 raw scores. Testing was administered within two weeks prior to, and approximately 1‐month post carotid surgery. Alternative forms of the RAVLT were used to minimize practice effects. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Imaging Acquisition
Subjects received neuroimaging under a clinical protocol using a GE Discovery MR750 3.0 T MRI scanner (G.E., Waukesha, WI). The protocol included a volumetric 3D fast spoiled gradient echo (FSPGR) (TE Min Full; Flip angle11 degrees; voxel size 1.2mm isotropic; TI 400; FOV 27 cm; NEX 1; Matrix 256 × 256), and a whole brain DWI sequence (30 directions; 5 B0 images; B 1,000 s/mm2; FOV 24 cm; slice thickness 2.5 mm; Matrix 96 × 96; interpolated voxel size: 0.96 × 0.96 × 2.5mm using zero filling; TR 6,600; TE Minimum).
Image Preprocessing
FSPGR images were processed using FreeSurfer 5.3 (FS) [Dale et al., 1999], and the resulting segmentations were reviewed and edited as needed under the supervision of a neuroradiologist (SS). The DWI scans underwent motion and eddy current correction using the Functional MRI of the Brain (FMRIB) Software Library (FSL) [Jenkinson et al., 2012]. Whole brain tractography was subsequently performed using the Hough processing stream [Aganj et al., 2011; Prasad et al., 2013], and the results were inspected using TrackVis [Wang et al., 2007].Whole brain cortical segmentations were then registered to the whole brain tractography using FSL fslorient2std [Jenkinson et al., 2012] and FLIRT [Jenkinson et al., 2002].
Connectivity Matrix and Network Measures
Brain connectivity graphs were created for all subjects (Fig. 1D). Connectivity networks were represented as a set of nodes corresponding to the 68 cortical regions (34 per hemisphere) [Desikan et al., 2006] from the FS based cortical parcellation. The presence of edges connecting nodes reflected the presence of any (>0) DWI fiber pathways computed, using tractography as described in the image preprocessing section, between two nodes. Edge weights reflected the number of DWI fibers present between two nodes. The DWI fibers were interpreted to reflect the presence of brain white matter bundles [Basser et al., 1994]. These networks were organized as connectivity matrices of size 68x68 (reflecting each of the cortical regions); their values represented the relative number of DWI fibers connecting each pair of brain regions and were normalized by the total number of DWI fibers computed in the brain [Prasad et al., 2015] (Fig. 1). Graph metrics that summarize modularity characteristics of the networks (binary and weighted forms of community number, and binary component number), were then computed using the brain connectivity toolbox (BCT) [Rubinov and Sporns, 2010]. The binary community number metric is the number of groups achieved when the nodes in a connectome are partitioned in a way that maximizes the edges between the nodes within each group while minimizing the number of edges between each of the groups, while not accounting for edge weights (Fig. 3A). The weighted community number metric, unlike the binary community number metric, takes into account the weights of edges, and reflects the partitioning of nodes that maximize the total weight of the edges within each group of nodes, while minimizing the total weight of edges between each group of nodes (Fig. 3B). The binary component size metric reflects the number of groups in a connectome where all of the nodes in each group are connected by 1 or more edges, with the weight of edges not taken into account (Fig. 3C). To evaluate influence of potential noise in the DWI data, the collection of graph metrics was computed from each network at 10 different thresholds, ranging from 0.1 to 1.0 in increments of 0.1, which retained a proportion of the highest weighted edges [Prasad et al., 2015]. All statistics reflect the number of groups that meet that specific criteria.
Figure 3.
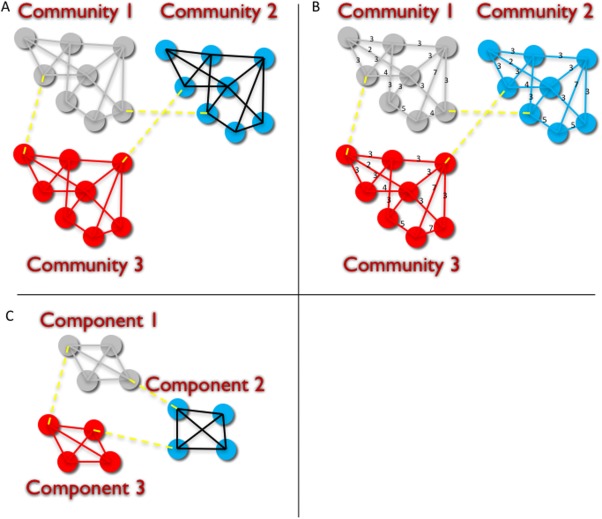
Graph features measuring modularity: A: Binary Communities, B: Weighted Communities, and C: Components. Binary communities (A) reflects a partition of nodes such that edges between nodes in each community are maximized and edges between communities are minimized, and the number of edges between nodes is not taken into account, only the presence of 1 or more edges. Weighted communities (B) reflects a partition of nodes such that the number of edges between nodes in each community are maximized and edges between communities are minimized, and the number of edges between nodes (reflected as weights for each edge) are taken into account. Binary components (C) reflects a partition of nodes such that all nodes in a component are connected by one or more edges, and that the number of connections between nodes is not considered. In this study brain cortical regions were represented as nodes and DWI fibers between two cortical regions was depicted as an edge between the corresponding nodes. Patients who demonstrated cognitive decline as reflected as decreased RAVLT scores at 1 month post‐surgery showed fewer binary or weighted number of communities, and fewer number of components on pre‐operative imaging than patients who did not show declined performance on the 1 month RAVLT testing. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Subject Risk Connectivity Characterization
To assess differences in the graph metrics between decliner and non‐decliner groups we computed two‐sample Student t‐tests between the decliner and non‐decliner group for each brain connectivity metric, for each of 10 nodal degree thresholds (Table 1). We corrected for multiple comparisons using the false discovery rate (FDR) [Benjamini and Hochberg, 1995] at q = 0.05.
Table 1.
P value t‐test results between decliner and non‐decliner groups for brain connectivity toolbox modularity metrics (TOP) and difference between mean value for graph metric for non‐decliners—mean value for graph metrics for decliners (BOTTOM) across 0.1 increment threshold levels
| Threshold Level | Weighted number of communities | Binary number of communities | Binary number of components |
|---|---|---|---|
| A. P Value for t‐test of Difference Between Decliners and Non‐Decliners | |||
| 0.1 | 0.8255 | 0.1812 | 0.2126 |
| 0.2 | 0.1558 | 0.2589 | 0.6495 |
| 0.3 | 0.0035a | 0.0358 | 0.0477 |
| 0.4 | 0.0034a | 0.2026 | 0.0477 |
| 0.5 | 0.0063a | 0.0941 | 0.0477 |
| 0.6 | 0.0064a | 0.0352 | 0.0477 |
| 0.7 | 0.0046a | 0.0953 | 0.0477 |
| 0.8 | 0.0034a | 0.0235 | 0.0477 |
| 0.9 | 0.0123 | 0.1244 | 0.0477 |
| 1 | 0.0708 | 0.0820 | 0.0477 |
| B. Mean Value (Non‐Decliners)—Mean Value (Decliners) | |||
| 0.1 | −0.0625 | −0.7778 | −0.6319 |
| 0.2 | 0.4722 | 0.3333 | 0.1042 |
| 0.3 | 0.9583 | 0.5347 | 0.4167 |
| 0.4 | 1.0208 | 0.3611 | 0.4167 |
| 0.5 | 0.8958 | 0.4236 | 0.4167 |
| 0.6 | 0.8958 | 0.5347 | 0.4167 |
| 0.7 | 0.9583 | 0.4792 | 0.4167 |
| 0.8 | 1.0208 | 0.7639 | 0.4167 |
| 0.9 | 0.7847 | 0.4236 | 0.4167 |
| 1 | 0.5417 | 0.4722 | 0.4167 |
Significant with False Discover Rate (FDR) Threshold at 0.05 level at 6.450000e‐03.
RESULTS
Subjects
Thirty‐four male subjects were recruited with the features listed in Table 2. The Decliner group had more carotid endarterectomy (CEA) patients (69%), while the Non‐decliner group had more carotid artery stenting (CAS) patients (67%). Similarly, the Decliner group had more current smokers (37%) compared to the non‐decliner group (6%). For both of these variables, however, paired separate comparisons of the no and yes groups did not differ, suggesting the significant difference reflected an interaction. Otherwise, no significant difference was noted between groups using Student's t‐test, or χ2 test for demographics or risk factors as listed in Table 2. Enrolled subjects underwent neuropsychological testing which included the RAVLT at least 1 month after surgery (mean 36.7, median 31.5, range 19‐128 days). Almost half (n = 16) of the patients declined to varying degrees soon after carotid procedures (Fig. 1 ). The study cohort demonstrated a mean change from baseline of 5.6 points (18% of baseline) in non‐decliners and −5.5 points (−16% of baseline) in decliners.
Table 2.
Demographic information for subjects, with differences between the decliner and non‐decliner groups using t‐tests (t) or Chi‐squared statistic (χ2) as appropriate. Note that for current smoker and surgery type (CEA or CAS), while the χ2 statistic was significant, the number in each group did not differ, suggesting the significant test reflected an interaction
| Non‐Decliner (N=18), Mean(SD) or N(%) | Decliner (N=16), Mean(SD) or N(%) | t | X 2 | t‐ or chi‐squared test P‐value | |
|---|---|---|---|---|---|
| Age | 69.83 (7.91) | 68.44 (8.11) | 0.498 | 0.622 | |
| Education | 14.17 (1.62) | 13.75 (1.39) | 0.8 | 0.43 | |
| MMSE | 28.28 (1.67) | 27.25 (1.57) | 1.84 | 0.075 | |
| RAVLT Sum | 34.78 (11.68) | 37.31 (8.55) | 0.481 | ||
| Procedure | 4.25 | 0.039a | |||
| CEA | 6 (33.33) | 11 (68.75) | |||
| CAS | 12 (66.67) | 5 (31.25) | |||
| Surgery | 0.083 | 0.774 | |||
| Right | 7 (38.89) | 7 (43.75) | |||
| Left | 11 (61.11) | 9 (56.25) | |||
| Diabetes | 0.105 | 0.746 | |||
| No | 10 (55.56) | 8 (50) | |||
| Yes | 8 (44.44) | 8 (50) | |||
| Current Smoker | 5.287 | 0.021a | |||
| No | 17 (94.44) | 10 (62.5) | |||
| Yes | 1 (5.56) | 6 (37.5) | |||
| Tobacco History | 0.394 | 0.53 | |||
| No | 2 (11.11) | 3 (18.75) | |||
| Yes | 16 (88.89) | 13 (81.25) | |||
| HTN | 0.508 | 0.476 | |||
| No | 1 (5.56) | 2 (12.5) | |||
| Yes | 17 (94.44) | 14 (87.5) | |||
| Hypercholesteremia | 0.007 | 0.932 | |||
| No | 1 (5.56) | 1 (6.25) | |||
| Yes | 17 (94.44) | 15 (93.75) | |||
| Contralateral Occlusion | 18 (100) | 16 (100) | |||
| Contralateral Stenosis | 0.394 | 0.53 | |||
| No | 16 (88.89) | 13 (81.25) | |||
| Yes | 2 (11.11) | 3 (18.75) | |||
| Prior Stroke | 0.394 | 0.53 | |||
| No | 16 (88.89) | 13 (81.25) | |||
| Yes | 2 (11.11) | 3 (18.75) | |||
| Post‐operative Infarct | 0.472 | 0.492 | |||
| No | 10 (55.56) | 7 (43.75) | |||
| Yes | 8 (44.44) | 9 (56.25) | |||
| Symptomatic | 0.007 | 0.934 | |||
| No | 11 (61.11) | 10 (62.5) | |||
| Yes | 7 (38.89) | 6 (37.5) |
Paired, separate, comparisons of the no and yes groups did not differ.
Baseline Measurements of Brain Connectivity
Of the BCT toolbox metrics we computed, all three modularity metrics (weighted number of communities, binary number of communities, binary number of components) were greater in the non‐decliner group at all threshold levels except threshold 0.1. (Table 1). The difference between groups was significant between decliner and non‐decliners for weighted community number at thresholds 0.3 through 0.9, for binary community number at thresholds 0.3 through 0.8, and binary component number at 0.2 through 1.0 (Table 1). Significant difference that met the FDR 0.00645 threshold were noted only for the weighted community number metric at thresholds 0.3 through 0.8 (Table 1).
DISCUSSION
Evidence supporting the dependence of cognitive processes on interactions among distributed neuronal populations and brain regions [Bressler and Menon, 2010; McIntosh, 1999; Mesulam, 1990] led us to suspect that some distributions of brain networks may be likely to manifest cognitive impairment after even subtle brain injury. Work demonstrating the brain recruiting additional regions to compensate for dysfunctional areas after injury [Calautti and Baron, 2003; Kokotilo et al., 2009] and the disruption of brain modular networks by cortical lesions and subcortical axonal damage [Crofts et al., 2011] led us to hypothesize that patients with greater brain modularity may be less likely to exhibit cognitive impairment after brain injury. Leveraging the model of possible subtle brain injury and memory dysfunction experienced during carotid intervention [Zhou et al., 2012], we have shown that patients exhibiting memory decline one month after carotid intervention demonstrated lower binary component number and weighted community number in the structural connectivity arrangement of their brains prior to surgery. These findings suggest that patients whose brains are organized into larger subunits (specifically communities or components) may be more vulnerable to functional decline after subtle brain injury, as is implicated during carotid interventions. A possible mechanism could be based on disruption of functional units secondary to brain injury from thrombotic or embolic infarcts or hypoperfusion. This would imply that having a greater number of modules may facilitate the recruitment of additional brain areas in the non‐decliners after injury results in disruption of brain modular network organization.
This collection of graph metrics was computed from each network at 10 different thresholds, ranging from 0.1 to 1.0 in increments of 0.1, which retained a proportion of the highest weighted edges. This was done to help provide a spectrum of networks that may better characterize the topology while being relatively robust to noisy or false positive connections and links [Rubinov and Sporns, 2010], though other techniques such as those that rely on a single data‐driven threshold [Achard and Bullmore, 2007] or a summary measure based on the area under the curve from a range of thresholds [Zhang et al., 2011] could have been used. Evaluating multiple threshold levels of connectivity allowed us to examine if potential noise in the imaging data influenced our findings. The fact that no graph metrics were significant at threshold 0.1 and few metrics were significant at threshold 0.9 or 1.0 suggest that at these extremes, noise may be distorting the calculated connectivity patterns. The fact that only weighted community number at threshold 0.3 through 0.8 was significant after multiple comparisons correction using FDR suggests that incorporating not only the presence, but the extent of connections may be important in characterizing brain connectivity patterns that are more likely to result in cognitive impairment after injury. This may suggest that among subjects whose brains are organized into similar numbers of communities, those who have fewer interconnections between the nodes in each of those communities may be more vulnerable to loss of function after an injury to a node in that community. These results also suggest that when considering the relative number of connections between nodes in some network measures, there may be a point up to which a moderate threshold of connections is necessary and reduces noise, but then beyond that point, too much information is discarded. One potential way to address this problem in the future may be to choose the threshold level that best models the degree of connectivity thought to be present between specific anatomic regions from functional connectivity (e.g., resting state) or anatomic studies. However, unless this single threshold technique is validated in a variety of datasets, it may still benefit researchers to sample a range of thresholds to guarantee that the full spectrum of graph theoretic information contained within a connectivity network is captured, even if at the cost of decreasing the power of statistical tests to identify these measures.
The literature is inconclusive with regard to cardiovascular risk factors or surgical technique (CEA or CAS) clearly identifying patients who are at increased risk for post‐operative cognitive impairment [Paraskevas et al., 2014]. The fact that differing percentages in surgery type (non‐decliner CAS 12/18, 67% vs. decliner CEA 11/16, 69%), and current smokers (non‐decliner 1/17, 6% vs. decliners 6/16, 37%), resulted in significant χ2 with paired comparisons of the number in each group not differing significantly suggest the significant test reflected an interaction and not a truly explanatory variable. No other variables were noted to be significantly different between groups using χ2 or t‐test.
Whereas executive functioning is often the focus of patients at vascular risk [Hachinski et al., 2006], memory function is a sensitive cognitive measure that declines in patients with even mild cognitive impairment and is shown to be vulnerable to the effects of surgery, such as changes in blood flow. In our previous work [Zhou et al., 2012] we focused on memory decline using a list learning task, the RAVLT, which has also been used in multiple studies looking at cognitive function after carotid intervention [Paraskevas et al., 2014]. The sum of immediate recall of list learning is one of the most sensitive measure of episodic memory impairment in non‐demented older adults [Rabin et al., 2009]. The RAVLT is particularly valuable because, unlike the California Verbal Learning Test (another commonly used list learning measure), it has four parallel forms which enables multiple longitudinal follow‐up assessments with less vulnerability to practice effects (i.e. seeing the same words over repeated test sessions leads to more learning). While normative data suggest minimal practice effects from parallel forms [Strauss et al., 2006], there are also reports of improved cognition following endovascular procedures [Picchetto et al., 2013]. Our results reflect both improvements and decrements post carotid intervention. The relatively small sample size limited our ability to follow the clinical psychology convention to consider a statistically significant decline as 1.5 SD. We instead considered change as a continuous variable but dichotomized based on whether the change was negative and positive. We thought this approach appropriate given there are typically few patients who satisfy the 1.5 SD criteria, despite the clinical distinctions between decliners and non‐decliners not being subtle. Future work should consider larger cohorts and connectome differences using the more rigorous 1.5 SD difference criteria.
While all pre‐operative imaging was reviewed clinically by a radiologist, the radiologist interpretation was not incorporated into the analysis for this study to minimize the influence of inter‐reader variability for interpreting the examinations. We hypothesized that the extent of DWI fibers between regions, as reflected by the presence and weights of edges, could represent the extent of damage or anatomic variation among subjects, reflecting vulnerability to injury during carotid intervention. However, it is also possible that for the subjects demonstrating cognitive decline, that this process may have been part of brain injury resulting in decline that began prior to surgery and which continued postoperatively. Future work analyzing pre to post‐operative connectivity graph changes may be able to distinguish patients whose post‐operative decline is related to perioperative events from patients whose decline may be related to pre‐operative brain injury.
This study contributes a new method for evaluating risk factors influencing the impact of brain injury on cognition by evaluating an important individual difference among patients: variations in brain connectivity. This initial validation work shows the feasibility of applying graph theory in a clinical setting as a first step in integrating this technique into clinical decision making. Future large‐scale cross validation studies would strengthen our conclusions. Translation of these research techniques into clinically available tools will allow increasingly fine grained information like individual subject brain connectivity to help personalize risk stratification and postoperative management for a given patient. There is also a host of clinical phenomena aside from carotid interventions where the brain is exposed to challenges with unclear effects, and where connectivity analyses may be able to identify groups at increased risk for cognitive decline. Future work may seek to combine the structural connectivity analysis method performed in this study with additional connectivity information from resting state fMRI, which has been suggested to yield informative and complementary information to structural connectivity analyses [Hagmann et al., 2008].
This initial validation work is limited by the need to obtain relatively short structural and DWI sequences as part of a routine clinical MRI session. Future studies may be able to apply more nuanced graph features analysis by obtained imaging with more diffusion weighted directions, multi b‐shell DWI or functional MRI, obtained in shorter scan times using acceleration methods such as simultaneous multislice imaging. Additionally, the all‐male cohort for this study limits generalizability, with results only able to be applied to males. Another limitation of the examination is solely relying on DWI based metrics for brain connectivity. Further work could leverage the complimentary information provided by functional connectivity studies [Hagmann et al., 2008].
CONCLUSION
This study provides evidence that connectomes obtained prior to episodes of brain injury based on structural connectivity brain imaging may help clinicians identify patients with increased vulnerability to cognitive decline after injury, with implications for brain resiliency against vascular insults in general. This ability to predict post injury impairment is noteworthy in that the information can guide risk stratification, post‐operative therapeutic choices, and patient caregiver counseling regarding expected outcomes after brain injury. This approach shows promise for assisting clinicians in planning interventions that may be associated with brain injury, and may represent a novel means for incorporating individual patient neuroimaging based biomarkers into their clinical management.
Conflicts of Interest and Financial Disclosures: The authors have no conflicts of interest or financial disclosures.
REFERENCES
- Achard S, Bullmore E (2007): Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aganj I, Lenglet C, Jahanshad N, Yacoub E, Harel N, Thompson PM, Sapiro G (2011): A Hough transform global probabilistic approach to multiple‐subject diffusion MRI tractography. Med Image Anal 15:414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R, Jeong H, Barabasi AL (2000): Error and attack tolerance of complex networks. Nature 406:378–382. [DOI] [PubMed] [Google Scholar]
- Alexander‐Bloch AF, Reiss PT, Rapoport J, McAdams H, Giedd JN, Bullmore ET, Gogtay N (2014): Abnormal cortical growth in schizophrenia targets normative modules of synchronized development. Biol Psychiatry 76:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254. [DOI] [PubMed] [Google Scholar]
- Bean J. (2011): Rey auditory verbal learning test, Rey AVLT In: Kreutzer J, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York: Springer; pp 2174–2175. [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soci Ser B 57:289–300. [Google Scholar]
- Bressler SL, Menon V (2010): Large‐scale brain networks in cognition: Emerging methods and principles. Trend Cogn Sci 14:277–290. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC (2003): Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke 34:1553–1566. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Reed B, Chao LL, Chui H, Sanossian N, DeCarli CC, Mack W, Kramer J, Hodis HN, Yan M, Buonocore MH, Carmichael O, Jagust WJ, Weiner MW (2012): Associations among vascular risk factors, carotid atherosclerosis, and cortical volume and thickness in older adults. Stroke 43:2865–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AJ, Abrams GM, D'Esposito M (2006): Functional reintegration of prefrontal neural networks for enhancing recovery after brain injury. J Head Trauma Rehabil 21:107–118. [DOI] [PubMed] [Google Scholar]
- Crofts JJ, Higham DJ, Bosnell R, Jbabdi S, Matthews PM, Behrens TE, Johansen‐Berg H (2011): Network analysis detects changes in the contralesional hemisphere following stroke. NeuroImage 54:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194. [DOI] [PubMed] [Google Scholar]
- DeFelipe J (2010): From the connectome to the synaptome: An epic love story. Science 330:1198–1201. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Gossetti B, Gattuso R, Irace L, Faccenna F, Venosi S, Bozzao L, Fiorelli M, Andreoli R, Gossetti C (2007): Embolism to the brain during carotid stenting and surgery. Acta Chir Belg 107:151–154. [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG (2006): National Institute of Neurological Disorders and Stroke‐Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37:2220–2241. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. NeuroImage 62:782–790. http://www.ncbi.nlm.nih.gov/pubmed/21979382. [DOI] [PubMed] [Google Scholar]
- Kokotilo KJ, Eng JJ, Boyd LA (2009): Reorganization of brain function during force production after stroke: A systematic review of the literature. J Neurol Phys Ther 33:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR (1999): Mapping cognition to the brain through neural interactions. Memory 7:523–548. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1990): Large‐scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28:597–613. [DOI] [PubMed] [Google Scholar]
- North American Symptomatic Carotid Endarterectomy Trial, C. (1991): Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis. N Engl J Med 325:445–453. [DOI] [PubMed] [Google Scholar]
- Paraskevas KI, Lazaridis C, Andrews CM, Veith FJ, Giannoukas AD (2014): Comparison of cognitive function after carotid artery stenting versus carotid endarterectomy. Eur J Vasc Endovasc Surg 47:221–231. [DOI] [PubMed] [Google Scholar]
- Peng D, Shi F, Shen T, Peng Z, Zhang C, Liu X, Qiu M, Liu J, Jiang K, Fang Y, Shen D (2014): Altered brain network modules induce helplessness in major depressive disorder. J Affect Disord 168:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchetto L, Spalletta G, Casolla B, Cacciari C, Cavallari M, Fantozzi C, Ciuffoli A, Rasura M, Imperiale F, Sette G, Caltagirone C, Taurino M, Orzi F (2013): Cognitive performance following carotid endarterectomy or stenting in asymptomatic patients with severe ICA stenosis. Cardiovasc Psychiatry Neurol 2013:342571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad G, Nir TM, Toga AW, Thompson PM (2013): Tractography density and network measures in Alzheimer's disease. Proc IEEE Int Symp Biomed Imaging 2013:692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad G, Joshi SH, Nir TM, Toga AW, Thompson PM, Alzheimer's Disease Neuroimaging Initiative (2015): Brain connectivity and novel network measures for Alzheimer's disease classification. Neurobiol Aging 36:S121–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Pare N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, Santulli RB (2009): Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer's disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 16:357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Kotter R (2005): The human connectome: A structural description of the human brain. PLoS Comput Biol 1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O, Spreen O. (2006): A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford, New York: Oxford University Press; xvii, 1216 p. [Google Scholar]
- van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, Goni J, Hulshoff Pol HE, Kahn RS (2013): Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry 70:783–792. [DOI] [PubMed] [Google Scholar]
- Venkatesan UM, Dennis NA, Hillary FG (2015): Chronology and chronicity of altered resting‐state functional connectivity after traumatic brain injury. J Neurotrauma 32:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Wedeen VJ (2007): Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. In; 2007; Berlin, Germany. International Society for MRI in Medicine (ISMRM). http://trackvis.org/faq/2007_ISMRM_diffusion_toolkit.pdf.
- Xia M, Wang J, He Y (2013): BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CP, Zhang SW, Fang T, Manxiu M, Chencan Q, Huafu C, Zhu HW, Li YJ, Zuxiang L (2013): Altered functional connectivity within and between brain modules in absence epilepsy: A resting‐state functional magnetic resonance imaging study. BioMed Res Int 2013:734893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, Gong Q (2011): Disrupted brain connectivity networks in drug‐naive, first‐episode major depressive disorder. Biol Psychiatry 70:334–342. [DOI] [PubMed] [Google Scholar]
- Zhou W, Hitchner E, Gillis K, Sun L, Floyd R, Lane B, Rosen A (2012): Prospective neurocognitive evaluation of patients undergoing carotid interventions. J Vasc Surg 56:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]


