Abstract
Neutrophils have traditionally been thought to play only a peripheral role in the genesis of many autoimmune and inflammatory diseases. However, recent studies in a variety of animal models suggest that these cells are central to the initiation and propagation of autoimmunity. The use of mouse models, which allow either deletion of neutrophils or the targeting of specific neutrophil functions, has revealed the many complex ways these cells contribute to autoimmune/inflammatory processes. This includes generation of self antigens through the process of NETosis, regulation of T-cell and dendritic cell activation, production of cytokines such as BAFF that stimulate self-reactive B-cells, as well as indirect effects on epithelial cell stability. In comparing the many different autoimmune models in which neutrophils have been examined, a number of common underlying themes emerge – such as a role for neutrophils in stimulating vascular permeability in arthritis, encephalitis and colitis. The use of animal models has also stimulated the development of new therapeutics that target neutrophil functions, such as NETosis, that may prove beneficial in human disease. This review will summarize neutrophil contributions in a number of murine autoimmune/inflammatory disease models.
Keywords: Neutrophils, Inflammation, Autoimmunity, NETosis, Cytokines, Immune complexes
1. Introduction
Neutrophils are the most abundant cell in the immune system. Between 1011 to 1012 neutrophils are made each day in the human bone marrow, which comprises ~50% of the total cellular content of the marrow, including a large pool of fully mature cells that is poised for release into the circulation in response to immune challenge [1]. The role of neutrophils in host defense against pathogen infection has been extensively studied in many disease contexts. However, current research indicates that neutrophils are also involved in regulating other aspects of immunity. Thus it is now clear that neutrophils are both effectors and modulators of host immune responses [2, 3]. It is also evident that dysfunction of either of these neutrophil roles can lead to immune-based diseases, including a variety of autoimmune and autoinflammatory conditions.
That neutrophils contribute at all to autoimmune disease pathogenesis is somewhat of a novel concept. Clearly, as effector cells, their contribution to tissue injury in inflammatory diseases, for example in immune complex-mediated diseases such as rheumatoid arthritis or glomerulonephritis, has long been appreciated. But the concept that neutrophil dysfunction, in either effector or regulatory properties, could initiate autoimmune disease is a new idea [4, 5]. Indeed, the entire autoimmunity field is undergoing a conceptual shift with the realization that dysregulation of any one of a number of innate immune cell types (especially dendritic cells) can be a primary driver of autoimmunity [6, 7]. Hence, determination of the mechanisms by which neutrophils affect adaptive immune cells is among the most dynamic areas of research in autoimmunity [8].
As effector cells, neutrophils respond to infectious pathogens through a myriad of molecular receptors that recognize pathogen-associated molecules. Activation of neutrophils through FcRs (that recognize Ig opsonized pathogens), integrins or C-type lectin receptors induces phagocytosis of bacteria or fungi, which in concert with stimulation of Toll-like receptors (TLRs), G-protein coupled receptors (such as the formyl peptide receptors) or various intracellular pathogen sensing molecules (such as NOD receptors), leads to stimulation of superoxide production as well as release of granules containing antimicrobial proteases and peptides [9]. Additionally, during host defense reactions neutrophils undergo a distinct form of cell death, referred to as NETosis, which leads to extracellular release of chromatin (that is often decorated with anti-microbial peptides released from granules) that forms a meshwork to trap extracellular pathogens [10]. Defects in any one of these effector functions, such as lack of integrin signaling or impairment of superoxide formation, leads to various forms of immunodeficiency. Importantly, these same defects in effector function can also contribute to autoimmune disease [11]. For example, patients with chronic granulomatous disease (CGD), caused by mutations in genes encoding subunits of the NADPH oxidase resulting in reduced or absent superoxide production, often develop autoimmune disease (colitis), which may be due to changes in the intestinal microbiome, which favor outgrowth of proinflammatory organisms. Similarly, the process of NETosis is now recognized as a major source of self (or auto) antigens that drive autoimmunity in diseases such as systemic lupus erythematous or rheumatoid arthritis [12]. Thus the antimicrobial role of neutrophils underlies aspects of their contribution to autoimmune disease.
As regulatory cells, neutrophils have been found to modulate the function of T-cells, B-cells and dendritic cells, which in turn directly affects autoimmune disease pathogenesis. The regulatory mechanisms utilized by neutrophils includes direct effects, via the production of cytokines such as IL-1, IL-6, IL-10 (in murine neutrophils only), TNF and BAFF that affect other immune cells, as well as indirect effects, through production of superoxides or consumption of nutrients (amino acids or even oxygen) that limit function of neighboring immune cells [13, 14]. Both stimulatory and inhibitory roles for neutrophils, based on their ability to produce various cytokines or indirectly affect other immune cells, have been described in a variety of autoimmune or autoinflammatory processes. Thus, the most productive way to summarize the contributions of neutrophils to any given autoimmune disease is to review the current evidence for each disease individually.
This review will focus on the current evidence linking murine neutrophils to a wide variety of murine autoimmune and autoinflammatory disease models. We will focus on mechanisms by which neutrophils contribute to disease pathogenesis beyond just induction of tissue injury through effector mechanisms (superoxide or protease release) normally operative in host defense reactions. The majority of evidence will involve neutrophil depletion or use of genetic knockout mice in any given disease model. As the reader will see, there are many complex ways neutrophils are involved in autoimmune diseases that were previously just thought to arise from defects in T or B-cell tolerance mechanisms.
2. Roles for Neutrophils in Classical Autoimmune Disease Models
2.1. Neutrophils in Systemic Lupus Erythematosus
There is a wealth of literature demonstrating a pathogenic role for neutrophils in various rheumatologic diseases, in particular systemic lupus erythematosus (SLE). Most of this data is based on human observations, which paints a picture of abnormal neutrophils contributing to both inflammatory states in SLE (through production of disease inducing cytokines such as IL-1β or BAFF) as well as being the source of many auto-antigens in the disease (mainly through NETosis). However, the picture is a bit more complicated when these mechanisms are tested in animal models.
Observational and in vitro experiments suggest that neutrophils contribute to human SLE development [15, 16]. Many SLE patients develop neutropenia during flares of active disease and their remaining cells show a number of functional abnormalities, such as poor phagocytosis and reduced superoxide production [17, 18]. A number of studies report that SLE patients develop an abnormal type of neutrophil referred to as a low-density granulocyte (LDG), which is primed in vivo to undergo NETosis. There are abundant reports linking NETosis to auto antigen formation and disease activity in SLE [19]. NETosis is a specialized form of neutrophil cell death that results in the extrusion of dense fibrillary networks of intact chromatin/DNA complexes that are often coated with granule proteins (such as myeloperoxidase (MPO), elastase or cathepsin G) and anti-microbial peptides (such as LL-37 and others). NETosis occurs following exposure of neutrophils to pathogen-associated molecules (lipopolysaccharide as an example) in the setting of other inflammatory stimuli (such as cytokines, chemokines or immune complexes) and is believed to have evolved to promote host defense against pathogens by physically trapping them in the chromatin meshwork [20]. NETosis requires production of superoxides and H2O2 (and is reduced in patients with defects in the NADPH oxidase) to mobilize MPO and other granule contents to the nucleus, which contribute to rapid breakdown of the nuclear membrane [21]. Decondensation of the nuclear chromatin is assisted by the enzyme peptidyl arginine deaminase (PAD) 4, which converts arginine residues on histones to citrulline to reduce electrostatic interactions between the histone and DNA [22]. The released chromatin complexes also undergo a number of additional modifications including deacetylation of lysine residues on histones. It is believed that this abnormal presentation of citrullinated and/or deacetylated chromatin in the setting of robust inflammation is the source of the neoantigens leading to formation of anti-dsDNA, anti-histone/chromatin and anti-citrullinated proteins (anti-CCP) that characterizes SLE and seropositive rheumatoid arthritis. Besides providing a source of neoantigens, NET contents can directly stimulate IFNα production by plasmacytoid dendritic cells (pDCs) [23]. Increased IFNα acts on a variety of immune cells to promote their activation, including feeding back on the neutrophils themselves to further prime additional NETosis, in the fashion of a feed forward amplification loop. Indeed, neutrophils from SLE patients show the same “interferon signature” of increased expression of IFNα-stimulated genes as well as hypomethylation (and hence activation) of IFNα responsive genes, which is seen in other immune cells in these patients [24]. NETs can also stimulate macrophages and other cells, through the NLRP3 inflammasome or the P2X7 purinergic receptor, resulting in the release of IL-1 and IL-18, further exacerbating the inflammatory state in SLE patients [25]. Finally, neutrophils from SLE patients with active disease also produce high amounts of BAFF, which directly acts on autoreactive B-cells to support their survival and proliferation and hence contribute to autoimmune antibody production [26].
Direct validation of these mechanisms in mouse models, however, is not completely supportive and tends to give a mixed picture of neutrophils in SLE pathogenesis. The most direct experimental evidence of a role for neutrophils in driving systemic autoimmunity comes from chronic neutrophil depletion experiments [13, 14]. Coquery et al. found that neutrophil depletion, achieved by every other day injection of the anti-Ly6G depleting mAb for four weeks, led to a reduction in auto-antibody titers, serum IFNγ, serum BAFF, T cell activation as well as the number of splenic germinal center B cells and plasma cells in the autoimmune prone B6.Faslpr/JTnfrsf17−/− strain. In this strain, high production of BAFF by neutrophils may help drive the selection and survival of autoimmune B cell clones that produce self-reactive antibodies, such as anti-double stranded DNA antibodies. The interplay of BAFF, T cells and IFNγ has also been suggested in the lyn−/− model of autoimmunity [27]. While chronic depletion of neutrophils is fraught with potential complications and is technically challenging, this is one of the few direct methods to show that neutrophils are involved in the development of self-reactive lymphocytes.
Less direct approaches have mainly involved modulation of neutrophil NETosis. In the New Zealand Black/New Zealand White (NZB/W) mouse model of systemic autoimmunity, deposition of NETs in kidneys and skin build up with disease progression, and in turn drive IFNα production [28]. Similar accumulation of tissue deposits of NETs and formation of autoantibodies that recognize NET components such as anti-microbial peptides (CRAMP, the murine analog of human LL-37) have been reported in the related New Zealand mixed 2328 (NZM) model of murine lupus [29]. Like humans, neutrophils from NZM mice undergo increased NETosis in vitro. Neutrophils from a third animal model of SLE, the MRL/lpr model which results from a mutation in the Fas gene leading to reduced immune cell apoptosis, also have been reported to undergo increased spontaneous NETosis [30]. In both the NZM and the MRL/lpr, blocking NET formation by treatment of mice with PAD4 inhibitors reduced systemic disease development [29, 30]. Treatment with the PAD inhibitors Cl-amidine or BB-Cl-amidine leads to reduced tissue deposition of NETs, reduced autoantibody levels, reduced nephritis and reversal of IFNα signature in both models. These inhibitors also reduced lipid oxidation in NZM mice, which correlated with improved endothelial and cardiovascular function in this SLE model [31].
These seemingly positive results are contradicted by the observation that blocking NETosis in MRL/lpr mice by inhibition of NADPH oxidase function (by crossing MRL/lpr mice to Nox2 deficient animals) in fact leads to exaggerated systemic autoimmunity and aggravated kidney injury [32]. Indeed, Schauer et al. have suggested that NETs may provide an anti-inflammatory function through degradation of cytokines and chemokines within inflammatory sites [33]. This study confirms that blockade of NETosis by genetic deficiency in one of the subunits of the NADPH oxidase (in this case the p47phox protein) leads to hyper-inflammatory responses which were reversed by direct transfer of pre-formed NETs into the inflammatory site. Part of this apparent paradox may be explained by other important functions of the neutrophil NADPH oxidase in systemic autoimmunity, besides just stimulating NETosis. Huang et al. recently confirmed that both neutrophil depletion and blockade of neutrophil NADPH oxidation function leads to exacerbated systemic autoimmunity in the NZB/W model [13]. These authors correlated the reduced levels of superoxide production, using in vitro approaches, with increased production of IFNα and IFNβ from pDCs, as well as increased IFNγ from NK cells, that together drove inflammation and promoted autoantibody production. In contrast, blockade of mitochondrial ROS production (which is obviously much less robust than ROS production through the NADPH oxidase) has recently been reported to be sufficient to block NETosis in vitro as well as reduce disease severity and IFNα responses in MRL/lpr mice [34]. Hence, using NADPH ROS blockade to test the role of NETosis in mouse models may be complicated by the fact that both T-cells and NK cells will over produce IFNγ and hence drive inflammation and autoimmunity. Instead, perhaps only blockade of mitochondrial production of ROS is sufficient to validate the role of NETosis in murine SLE models.
A third approach to modulate NETosis effects in animal models has involved alterations in histone modifications during disease development. Various studies have investigated the significance of histone acetylation in SLE neutrophils. Hypoacetylation of histones H3 and H4 has been found in splenocytes from MRL/lpr mice [35]. Treatment of MRL/lpr mice with HDAC inhibitor trichostatin A reverses the hypoacetylation of histones H3 and H4, which correlates with an improved disease phenotype. In addition, mice deficient in histone acetyl transferase p300, resulting in hypoacetylation of H3 and H3 develop a systemic autoimmune disease similar to SLE [36]. These studies suggest that modulation of histone acetylation may ameliorate the pathogenic potential of neutrophil NETs.
The role of neutrophil myeloperoxidase (MPO) in systemic autoimmunity has also been somewhat controversial. The proinflammatory effect of MPO, through production of oxidants and in promotion of NETosis, would seemingly be a major contributor to disease pathogenesis [37]. Yet MPO-knockout mice develop increased autoimmunity and end organ damage in the pristane model of lupus [38]. The mechanism for this apparent paradox is unclear, but since the pristane treated MPO-deficient mice developed high levels of IFNγ producing CD4+ T-cells, it is possible that the loss of MPO releases a brake on T-cell expansion during inflammatory states. Similar to the effect of ROS, it has been suggested that neutrophil-derived MPO limits T-cell mediated inflammation through an effect on DC priming [39]. This is another example of how animal models of SLE can produce somewhat unexpected findings that illuminate complex regulatory loops involving neutrophil effector functions in autoimmunity.
2.2. Models of vasculitis
Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) is a group of inflammatory vasculitides characterized by the development of autoantibodies that recognize neutrophil granule enzymes MPO and proteinase-3 (PR3). ANCAs bind to cytokine-primed neutrophils, leading to neutrophil activation (in part through simultaneous binding to both MPO/PR3 and Ig Fc receptors), degranulation, cytokine release and ROS production [40, 41]. In particular, ANCA-stimulated neutrophils release large amounts of BAFF, which may support further expansion of autoreactive B cells in this disease [42]. ANCA-stimulated neutrophils also undergo NETosis, which may exacerbate tissue injury; indeed, both free circulating and kidney deposits of MPO-DNA complexes have been reported in patients with inflammatory vasculitis [43]. The production of NETs by ANCA-stimulated neutrophils can also prime DCs to present additional MPO and PR3 peptides to further drive autoimmunity in this disease. In mice, transfer of myeloid DCs treated with NET components into naïve animals leads to the development of ANCA antibodies and systemic vasculitis [44]. A mouse model of ANCA-associated vasculitis has been established by sensitizing MPO-deficient mice with MPO then transferring their splenocytes or purified IgG to healthy animals, which results in systemic vasculitis and necrotizing glomerulonephritis [45]. Using both chimeric mice and neutrophil-depletion approaches, it has been demonstrated that the cellular target for the transferred ANCA is indeed the neutrophils in the recipient mouse [46]. The role of neutrophil Fc receptors has been tested in this model through transfer of anti-MPO antibodies into FcR deficient mice. As expected, mice lacking the inhibitory FcγRIIB receptor develop much more fulminant disease following transfer of anti-MPO antibodies [47]. A similar, though less pathogenic model of anti-PR3 ANCA has also been described, again by immunizing PR3/neutrophil elastase deficient mice with recombinant murine PR3 [48]. A monoclonal anti-PR3 ANCA antibody model has been used in isolated rat lungs to show that activation of the neutrophil NADPH oxidase is a major mediator of tissue injury [49]. However, in the anti-MPO antibody-mediated disease model, Schreiber et al. have recently reported just the opposite – mice genetically deficient in NADPH oxidase activity (either Nox2 or p47phox deficient animals) actually developed more severe vasculitis and glomerulonephritis following challenge with anti-MPO serum [50]. These authors found that the NADPH oxidase-deficient neutrophils over produced IL-1β following anti-MPO antibody challenge and that IL-1β-receptor blockade protected recipient mice from severe glomerulonephritis following anti-MPO challenge. Doubly deficient Nox2 and caspase-1 mutant mice were also resistant to anti-MPO mediated disease. Again, these somewhat surprising results in mouse models illustrate the complex interactions between different neutrophil effector functions in autoimmune diseases.
2.3. Autoimmune arthritis models
The contribution of neutrophils to autoimmune arthritis has been extensively studied in a variety of mouse models. In these models, it has been found that neutrophils contribute to both the initiation (or establishment) of disease as well as to the effector (or tissue injury) phase of disease. Various mouse models mimic these different stages of autoimmune arthritis pathogenesis.
The most widely used model for studying the complete disease process is the collagen-immunization model [51]. This model includes both the priming or initiation phase of the disease, which requires T- and B-cell responses leading to production of pathogenic anti-collagen and other antibodies, as well as the effector phases, which involve recruitment of neutrophils to the inflamed joint, leading to cellular activation with cartilage and bone injury. In human rheumatoid arthritis, many of the autoantibody specificities are directed against citrullinated antigens (such as citrullinated collagen, fibrinogen, vimentin or anti-histone antibodies), which are thought to be induced by release of PAD4 during neutrophil NETosis [52]. Indeed, blocking PAD4 activity with Cl-amidine decreases clinical disease in the collagen-induced model by ~50% [53]. Disease reduction in the Cl-amidine treated mice is also associated with reduced production of anti-citrullinated antibodies, directly suggesting that neutrophil NETosis is contributing to the antigenic stimulation in this model. This is supported by previous studies showing that depletion of neutrophils or reduction in neutrophil production through genetic deficiency of G-CSF (or antibody blockade of G-CSF) also blocks disease development in the collagen-induced arthritis model [54]. Besides providing a source of antigens in the priming phase of arthritis, neutrophils are also required for deposition of the preformed antibodies along the articular surfaces of the joints. In mice, deposition of anti-collagen antibodies in the joint, following direct injection into naïve mice, requires neutrophils and neutrophil activation by immune complexes [55]. Similar results have been reported in the passive arthritis KxB/N model (see below) suggesting that neutrophil activation directly influences vascular permeability which allows deposition of immune complexes, such as anti-collagen antibodies, in the earliest stages of disease [56].
The other widely used model of autoimmune arthritis in mice is the passive serum transfer model referred to as the K/BxN model. This model reflects only the effector phase of the disease as it works by transferring pathogenic IgG-containing serum into naïve mice, which results in severe, but transient arthritis due to immune complex deposition in the joint [57]. The pathogenic serum is formed in transgenic K/B mice crossed onto the non obese diabetic (NOD) background; the resulting mice develop progressive erosive arthritis due to formation of IgGs that recognize glucose-6-phosphate isomerase (anti-GPI) along the cartilaginous surface of the large joints in the feet. Transfer of the serum from K/BxN mice to any other mouse produces joint inflammation. Disease in recipient mice is dependent on FcR signaling pathways in neutrophils, suggesting that recognition of joint immune complexes occurs by circulating cells [56, 58]. Neutrophils amplify their own recruitment to the inflamed joint through the coordinated production of chemoattractants, such as LTB4 as well as cytokines such as IL-1β, the latter of which acts on synovial cells to stimulate additional neutrophil recruitment [59, 60]. One of the synovial-derived cytokines involved is G-CSF, which is also required for neutrophil recruitment in the K/BxN serum transfer arthritis model [61]. Neutrophil production of IL-17 has also been implicated as an amplifier of arthritis in this model [62]. As mentioned above, neutrophil activation following recognition of early immune complexes in the joint may also lead to changes in vascular permeability, which further promotes IgG deposition [63]. As expected, PAD4 deficiency does not affect the K/BxN serum transfer arthritis model, since the model reflects only the effector phase of the disease [64]. Interestingly, mice lacking the p47phox subunit of the neutrophil NADPH oxidase develop much more severe arthritis in the K/BxN serum transfer model (as well as in a passive anti-collagen antibody transfer model) [65, 66]. The mechanism of this somewhat surprising observation is unclear, but may involve ROS-mediated suppression of other signaling pathways or chemoattractant (LTB4) release by neutrophils, or as suggested in systemic inflammatory disease, the ability of neutrophil-derived ROS to affect other immune cells, such as Tregs [67]. Hence, even in the effector phases of inflammatory arthritis, neutrophils are critical for regulating the activity of other immune and synovial cells, which act in concert to amplify the inflammatory cascade.
3. Neutrophils in Neuroinflammatory Disease Models
3.1. Experimental autoimmune encephalomyelitis (EAE)
The mouse EAE model has been used extensively to replicate the autoimmune pathogenesis in human multiple sclerosis [68]. EAE is initiated by immunization of mice with myelin protein or peptides, which leads to development of self-reactive CD4+ Th17 cells that infiltrate the CNS leading to demyelination and neuronal injury. Myeloid cell infiltration into the CNS is a major component of the EAE model and is also seen in human multiple sclerosis lesions [69]. Indeed, early reports demonstrated that antibody-mediated depletion of neutrophils results in a dramatic reduction in EAE pathology in both the brain and spinal cord [70], consistent with the view that activation of neutrophil effector function is a major source of tissue injury in this model. Using adoptive transfer methods, Carlson and colleagues found that CXCR2 expression on neutrophils is required for their recruitment into the CNS in the EAE model, suggesting that disease was initiated by other cells (such as CNS macrophages or Th17 cells) which produced CXC chemokines (mainly CXCL1 and CXCL2 in mice, the analogues of CXCL8 or IL-8 in humans) that allowed neutrophil recruitment and subsequent tissue injury [71]. As in other autoimmune models, production of GM-CSF by T-cells and stromal cells in the CNS has also been implicated as a major factor in neutrophil recruitment in EAE [72]. Similar results have been reported in mice lacking the G-CSF receptor, which mount poor neutrophil responses during EAE [73]. Most of these results support the traditional notion that neutrophils act in the effector phase of EAE to mediate tissue injury. Interestingly, neither MPO or neutrophil elastase seem to be involved in the development of neural injury, since EAE proceeds normally in MPO knockout mice and inhibitors of neutrophil elastase do not affect disease progression [74]. However, hyper-activation of neutrophils, through myeloid-specific deletion of the inhibitory factor SOCS3, results in a severe form of EAE in myelin-immunized mice that is characterized by high levels of neutrophil recruitment to the CNS and overproduction of chemokines (mainly CXCL2) and inducible NO [75]. A potential suppressive effect of neutrophil-derived nitric oxide on T-cell proliferation in response to myelin protein has also been described in EAE, similar to in vitro findings in systemic autoimmunity [76]. These observations point to a complicated interaction between neutrophils and other immune cells during the effector phases of EAE.
There is growing recognition that neutrophils may also contribute to the early phases of neuroinflammation in the EAE model [77]. Careful observation of these early events in EAE using two photon imaging methods has shown that neutrophils can directly affect the blood/brain barrier and promote early vascular permeability [78]. Using lysM-eGFP marked cells, Aube and colleagues could visualize the very early recruitment of neutrophils to the neurovasculature within one day following disease initiation. This rapid influx of neutrophils correlates with increases in vascular permeability (determined by dye leakage) in the CNS but precedes the onset of clinical neurologic symptoms in the mice. Neutrophil depletion prevented the early disruption of the blood/brain barrier in immunized mice, correlating with reduced clinical disease development. This early role for neutrophils in promoting vascular permeability during neuroinflammation is reminiscent of a similar role proposed for these cells during autoantibody-mediated arthritis [56]. The mechanisms and mediators by which neutrophils promote early changes in vascular permeability in inflammatory responses remain to be defined.
Neutrophils have also been found to promote the maturation of microglia and monocytes/macrophages into fully mature, MHC class II-expressing antigen presenting cells during EAE [74]. Careful examination of the CNS inflammatory cell infiltrate in EAE mice following neutrophil depletion suggests that the major cell type affected appears to be inflammatory monocytes and CD11c+ dendritic cells, which are reduced in number and manifest reduced expression of co-stimulatory molecules needed for efficient antigen presentation. Hence, progressive diminution of myelin-reactive T-cells within the CNS could also contribute to reduction of disease in neutrophil-depleted mice. Again, this neutrophil-dendritic cell crosstalk in EAE is similar to that seen in other autoimmune inflammatory diseases.
4. Neutrophils in Autoimmune Uveitis
The uvea is the middle layer of the eye, consisting of the iris, the ciliary body and the choroidea. Inflammation of this layer is associated with several autoimmune diseases (e.g. ankylosing spondylitis, Behcet’s disease, etc.) and is the leading cause of blindness in Western societies [79]. Experimental autoimmune uveoretinitis (EAU) can be triggered in mice by immunization with the interphotoreceptor retinoid-binding protein (IRBP). Wild type mice develop leukocytosis with marked neutrophilia in EAU that correlates with increased G-CSF levels in the serum and the eye [79]. In the absence of G-CSF, inflammation and neutrophil accumulation is decreased. In line with this finding, anti-G-CSF antibody treatment also suppresses the intraocular inflammation [79]. As in other autoimmune models, the potential roles of G-CSF in EAU include mobilization of neutrophils from the bone marrow, triggering neutrophil-attracting chemokines from tissue resident cells and upregulating the chemokine receptor CXCR2 on the cell surface of neutrophils [79].
In a second model of autoimmune uveitis, inflammation is triggered by the intraocular injection of ovalbumin into DO11.10 transgenic mice whose T cells are engineered to recognize ovalbumin [80]. Here, intraocular anti-IL-17 treatment decreases neutrophil influx, probably by suppressing the production of neutrophil-attracting chemokines like CXCL2 [80]. The involvement of neutrophil-derived ROS and/or nitric oxide in the early phases of autoimmune uveitis remains to be investigated.
5. Neutrophils in Dermatologic Disease Models
5.1. Models of autoimmune bullous diseases
Two major autoimmune bullous skin diseases have been modeled in mice: bullous pemphigoid (BP) and epidermolysis bullosa acquisita (EBA). BP results from development of pathogenic autoantibodies recognizing hemidesmosomal proteins BP180 or BP320, while EBA is caused by development of autoantibodies recognizing collagen VII [81]. Deposition of these autoantibodies along the dermal/epidermal border produces a neutrophil-dominant inflammatory reaction that leads to separation of the epidermis from the dermis, and blister formation. These diseases can be mimicked in mice by passive transfer of anti-BP180 or anti-collagen VII antibodies, raised in rabbits. Liu and colleagues have carefully dissected the role of neutrophils in the BP model, showing that recognition of skin immune complexes by neutrophil FcRs is required for release of proteases (in particular, neutrophil elastase, but also matrix metallopeptidase-9) that in turn degrade BP180, generating peptides that attract additional neutrophils to amplify the inflammatory process [82]. In contrast to immune-complex arthritis models, C5a receptor signaling is not needed on neutrophils, but instead is required on tissue resident mast cells to initiate the skin disease [83]. Hence, the chemokine receptors involved in neutrophil recruitment/activation in this model remain unclear. In the EBA model, activation of the neutrophil NADPH oxidase leading to ROS production has been implicated as critical mediator of tissue injury [84]. To model both the initiation phase and the effector phase of these diseases, models in adult animals relying on extensive immunization with BP180 or collagen VII have been established. In the immunization models, neutrophil depletion is partially effective in reducing disease [85]. Interestingly, in the immunization-induced EBA model, chronic depletion of neutrophils (or use of GM-CSF knockout mice) leads to reduced autoantibody titers, with reduced skin inflammation [86]. This suggests that neutrophils may also contribute to the initiation of autoantibody production as well as participating in the effector phase of the disease. Clearly, the roles of NETosis and various neutrophil-produced chemokines/cytokines need to be determined in these blistering skin diseases.
5.2. Psoriasis
Psoriasis is a persistent inflammatory skin disease that is driven in large part by T-cell activation leading to chronic IL-17 production. Neutrophilic inflammation is also a major aspect of active psoriatic lesions resulting in accumulation of neutrophil chemokines and chemoattractants. Accumulation of pDCs, which secrete large amounts of IFNα, has also been reported in xenograft models of psoriasis [87]. Psoriasis in animal models has typically been modeled by topical application of imiquimod (a TLR7 and adenosine receptor agonist) that induces chronic skin inflammation, which histologically mimics human psoriasis, including infiltration of T-cells, expression of IL-17 and IFNα along with the development of neutrophil microabscesses in the epidermis [88–90]. However the model is complicated by the fact that some preparations of imiquimod may stimulate inflammasome activation independently of TLR7 signaling [91]. Neutrophil depletion in mice reduces the inflammatory response to topical imiquimod application [92]. Neutrophil depletion also reduces skin inflammation in the fsn/fsn flaky-skin mouse model of psoriasis, which contain a homozygous recessive mutation in the Ttc7 gene that broadly affects immune cells [93, 94]. Mice deficient in the neutrophil chemoattractant receptor BLT1, which recognizes LTB4, are also strongly protected from imiquimod-induced inflammation [92]. This same study implicated neutrophil production of LTB4 and IL-1β as major amplifiers of the skin inflammatory response, similar to the role these neutrophil products play in autoimmune arthritis models. Indeed, a number of neutrophil-derived products have been implicated in amplifying inflammation in the imiquimod psoriasis model. Shao et al have found that lipocalin-2, which is an antimicrobial peptide, is strongly expressed is psoriatic lesions and its neutralization by mAb treatment alleviates inflammation in the imiquimod model [95]. Lipocalin-2 is highly produced by neutrophils in psoriatic skin and can activate neutrophils as well as act as a neutrophil chemoattractant. Recently, Henry et al. reported that neutrophil-derived proteases (cathepsin G, elastase and PR3) promote the cleavage of the IL-1 related cytokine IL-36 in a xenograft model of psoriasis [96]. This finding is particularly intriguing because one of the most virulent forms of human pustular psoriasis is known to be caused by mutations in the IL-36 receptor antagonist, suggesting that dysregulated IL-36 signaling is particularly proinflammatory in the skin [97]. Similarly, mice deficient in IL-36 receptor antagonist are hyper-responsive to imiquimod treatment, while IL-36 receptor knockout mice are protected [98]. Additionally, neutrophil-derived cytokines can act on skin keratinocytes to promote further production of IL-36, which in turn drives more neutrophil recruitment in a self-amplifying loop of inflammation [99]. Such neutrophil-keratinocyte crosstalk is yet another example of the regulatory role of neutrophils in autoimmune/inflammatory diseases.
6. Neutrophils in autoimmune endocrine conditions
6.1. Type 1 diabetes mellitus
Type 1 diabetes mellitus is a T cell-mediated autoimmune disorder that requires insulin replacement therapy for the entire life of patients suffering from the disease. The major mouse model for this disease is the non obese diabetic (NOD) model; these mice develop an inflammatory autoimmunity against their pancreatic islet (insulin producing) β-cells [100]. Early in the disease process the pancreatic islets of NOD mice develop a transient influx of neutrophils [101]. This influx is reduced by pharmacological blockade of the chemokine receptor CXCR2 [102]. The main chemokines that mediate neutrophil arrival seem to be CXCL1 and CXCL2, produced by pancreatic macrophages and insulin-secreting β-cells [102]. Macrophages can trigger CXCR2-ligand production by β-cells through release of IL-1β [102]. Additionally, pancreatic neutrophils can cooperate with CD5-positive B-1a cells to trigger IFNα production by pDCs, as observed in other autoimmune models [101]. The B-1a cells produce anti-DNA autoantibodies that trigger neutrophil activation and NET formation through activating Fcγ receptors [101]. This leads to the release of the antimicrobial peptide CRAMP, which in turn binds to the Ig-bound DNA to form complexes that further potentiate pDC activation through TLR9/Fcγ signaling pathways [101]. Importantly, early blockade of neutrophil accumulation attenuates the onset of diabetes mellitus in mice by decreasing the number of effector CD8-positive T cells in the pancreas [101]. These results suggest that interactions between neutrophils, B-lymphocytes and pDCs play an important role in initiating the destructive anti-islet cell autoimmunity that leads to diabetes in the NOD mouse model.
6.2. Autoimmune thyroiditis
Autoimmune thyroiditis is one of the most prevalent autoimmune diseases in the world. Granulomatous experimental autoimmune thyroiditis (G-EAT) can be triggered by repeated intravenous injection of thyroglobulin in DBA/1 mice [103]. Following immunization neutrophils migrate in huge numbers to the thyroid glands, in response to upregulation of CXCL1 and CXCR2 in the inflamed tissue [104]. As in the NOD mouse model, this early arrival of neutrophils may help prime development of both T-cell and B-cell autoimmunity in the G-EAT model.
7. Roles for Neutrophils in Non-Classical Autoinflammatory-like Disease Models
Autoinflammation and autoimmunity share many common features such as chronicity or self-destruction by immune cells; however, autoinflammatory conditions lack autoantibodies, autoreactive lymphocytes and MHC allele-correlations. According to the Immunological disease continuum view, there is a smooth transition from classical autoinflammatory diseases like the monogenic Familial Mediterranean Fever (FMF) to classical autoimmune diseases like systemic lupus erythematosus (SLE) with intermediate conditions sharing several aspects with autoinflammatory syndromes [105]. Such polygenic diseases include gout and inflammatory bowel diseases (Crohn’s disease and Ulcerative colitis), all of which are known to have dysregulated NOD-like receptor (NLR) signaling as a central mechanism for their pathogenesis [105, 106].
7.1. Neutrophils in gout
Gout is a relatively common autoinflammatory-like arthritis that is characterized by recurrent painful attacks often associated with extra-articular manifestations such as kidney stones and nephropathy. The initial molecular event is the deposition of monosodium urate (MSU) crystals in the joints that triggers neutrophil influx [107]. In the air-pouch model of gouty arthritis, subcutaneous pouches are made by the injection of sterile air that leads to a synovial-like barrier after seven days [108]. Upon MSU crystal injection, resident cells are activated, releasing chemokines such as CXCL1, resulting in rapid recruitment of neutrophils to the air pouch. Mice lacking the CXCL1 receptor (CXCR2) manifest a significant block in neutrophil recruitment in the MSU air-pouch model [108]. Furthermore, S100A8 (MRP8) and S100A9 (MRP14), two important neutrophil-derived factors have also been shown to mediate neutrophil accumulation in the MSU crystal-injected air-pouch, since direct injection of these proteins will stimulate neutrophil influx, whilst their neutralization using anti-S100A8/9 blocking antibodies prevents neutrophil accumulation in the MSU air pouch [109, 110]. The MSU crystals initiate inflammation by activating the NALP3 inflammasome in tissue resident macrophages, leading to robust IL-1β production [111]. Mice deficient in key inflammasome components (ASC, an inflammasome adaptor protein, or Caspase-1, that cleaves pro-IL-1 to mature IL-1β) show a significantly impaired neutrophil influx in MSU-induced peritonitis [111]. Similarly, IL-1 receptor (IL-1R)-deficient mice also fail to accumulate neutrophils in the peritoneal cavity following MSU injection [111]. Moreover, antibody-mediated blocking of IL-1R or use of the IL-1R antagonist Anakinra also markedly impairs MSU-mediated neutrophil influx [112]. In line with previous findings, NLRP3, ASC, Caspase-1, IL-1β, IL-1R are also important in MSU crystal-induced articular inflammation and hyper-nociception [113]. In this model, neutrophil accumulation is also dependent on CXCR2 and the release of CXCL1 is dependent on the NALP3/NLRP3 inflammasome.
It is an intriguing question how the attacks of gouty arthritis resolve spontaneously as self-limiting inflammatory reactions. One theory suggests that uptake of apoptotic debris by neutrophils stimulates their production of TGFβ1, which has the potential to downregulate proinflammatory neutrophil functions like superoxide production in the MSU crystal-induced peritonitis model [114]. On the other hand, MSU crystals have been shown to trigger NET release and the aggregation of NETs; mice with a loss of function mutation in p47phox, which blocks NETosis, develop chronic and aggravated inflammation in the MSU model [33]. These authors suggest that robust neutrophil recruitment induced by MSU crystal injection leads to the formation of densely packed NETs, which are laden with proteases that degrade proinflammatory chemokines and cytokines, thus actually limiting further inflammatory cell recruitment [33]. In line with this finding, ‘NETosis-deficient’ p47phox mutant mice show elevated cytokine levels in the MSU-containing air pouches, which is decreased by the injection of aggregated NETs, isolated from wild type mice, in the pouch. An additional limiting mechanism could be that C5a triggers the release of neutrophil microvesicles that in turn decrease the priming effect of C5a on inflammasome activation and IL-1β production, resulting in a suppressed neutrophil accumulation [115].
7.2. Neutrophils in experimental colitis
Inflammatory bowel diseases (IBD) cause tremendous burden to patients. The role of neutrophils in experimental colitis models is controversial as some data show their contribution to mucosal damage, while others report beneficial effects [116]. In the dextran sulfate sodium (DSS) induced colitis model, the lack of the chemokine receptor CXCR2 suppresses neutrophil infiltration to the gastrointestinal tract thus reducing mucosal injury [117]. In line with this finding, the blockade of CXCR2 also results in an attenuated inflammation in DSS experimental colitis [118]. In the trinitrobenzene sulfonic acid (TNBS) colitis model (which tends to produce a more chronic inflammatory process than DSS), treatment with a blocking anti-CXCL1 antibody or a pharmacologic CXCR2 antagonist also significantly reduces inflammation, neutrophil influx, intestinal myeloperoxidase activity, IL-1β, CXCL1 and CXCL2 levels [119]. It is intriguing that while the early phases of the TNBS-induced acute colitis depend on the CXCR2 receptor, later phases seem to be CXCR2-independent, suggesting, as in other inflammatory diseases, that neutrophils affect early phases of the inflammatory response potentially by altering vascular permeability [120]. Blocking integrin β2 (CD18) also results in a significant decrease of myeloperoxidase activity and the concomitant mucosal permeability in the TNBS-induced acute colitis model, pointing again at a pathological role for neutrophils in experimental colitis [121, 122].
In contrast, treatment with antibodies against L-selectin or neutrophil-depleting antibodies, actually aggravates disease severity in TNBS-induced colitis, indicating a potential protective role for neutrophils during pathogenesis [123]. Campbell et al. recently hypothesized an interesting mechanism by which neutrophils may be protective in intestinal inflammation [124]. As in other disease models, these authors noted that Nox2−/−mice displayed dramatically enhanced inflammatory responses in the TNBS colitis model. Genetic deficiency of the p47phox subunit of the NADPH oxidase also results in increased inflammation in the DSS colitis model [125]. In parallel with these observations, humans suffering from Chronic granulomatous disease are more susceptible to inflammatory bowel diseases [126]. Campbell et al. noted that the intestinal epithelial cells in these models upregulate a number of hypoxia-dependent protective genes, which are transcriptional targets of the hypoxia-inducible factor (HIF) transcription factor [124]. Stable expression of HIF can be visualized in the intestinal epithelia during colitis using a reporter mouse model. These authors postulated that during epithelial transmigration, activation of their NADPH oxidase causes neutrophils to consume large amounts of oxygen leading to a localized hypoxia that affects the transcriptional response of neighboring epithelial cells leading to stable expression of HIF. Indeed, this can be directly demonstrated in co-culture experiments with neutrophils and epithelial cells – induction of superoxide production in neutrophils leads to upregulation of HIF in the epithelial cells. Depletion of neutrophils reduces HIF gene expression in epithelial cells during TNBS colitis. Moreover, pharmacological stabilization of HIF with AKB-4924 [127] dramatically increases HIF expression in the epithelia of Nox2−/− mice and significantly improves inflammatory resolution during TNBS colitis. This neutrophil-epithelial cell crosstalk, through localized consumption of oxygen, points to yet another mechanism by which neutrophil functions indirectly affect immune responses in disease models.
7.3. Alzheimer’s disease
Though not strictly an autoimmune process, it has long been recognized that neuroinflammation is a major component of neurodegenerative diseases such as Alzheimer’s disease. The major causative factor of Alzheimer’s disease is the accumulation of beta-amyloid in plaques and aggregates associated with neurodegeneration. These beta-amyloid protein aggregates have been shown to activate monocytes and microglia to promote neuroinflammation [128]. Indeed, migration of neutrophils into beta-amyloid plaques has been observed in mouse models of Alzheimer’s disease using two photon microscopy techniques. In the 5XFAD mouse (which overexpresses mutated forms of human amyloid precursor protein (APP) and presenilin 1 (PS1)), Baik et al. were able to visualize migration of adoptively transferred neutrophils into amyloid plaques [129]. These results were expanded in a recent study by Zenaro et al., who reported that in both the 5XFAD and the 3XTg AD mouse (which expresses mutated forms of PS1, APP and tau protein), neutrophil adhesion and intraluminal crawling is observed along neurovascular structures adjacent to amyloid plaque deposits [130]. The adhesion of neutrophils to the neurovasculature in these Alzheimer’s mouse models is dependent on expression of the leukocyte integrin LFA-1 by neutrophils, which recognize increased ICAM-1 present on vascular endothelium, similar to neutrophil recruitment in virtually all other inflammatory responses [131]. Most importantly, in these Alzheimer’s models, neutrophil infiltration of the brain begins before the onset of cognitive decline and peaked around the time that memory loss is first observed. Indeed, depletion of neutrophils from the blood of 3XTg AD mice restored cognitive function in two behavioral tests of learning and memory. Similarly, blocking neutrophil recruitment into the brain in these models by using blocking anti-LFA1 antibodies also reduced cognitive deficits and neuropathology in the mice. These exciting and unexpected results suggest that neutrophils may play additional, unanticipated roles in neurodegenerative disease (perhaps regulating vascular permeability as in EAE models). Clearly, this is an area of exciting research [132].
7.4. Neutrophilic dermatosis
The neutrophilic dermatoses are a spectrum of skin inflammatory disorders, characterized by robust neutrophilic inflammation of the skin, in the absence of obvious infection. Often these diseases are associated with other autoimmune or inflammatory processes, such as inflammatory bowel disease [133, 134]. A variety of neutrophil functional defects, in chemotaxis, phagocytosis and other functions have been linked to various neutrophilic dermatoses, but as yet there is little molecular understanding of these processes. Some monogenic forms of neutrophil-mediated skin inflammation have been described, such as the PAPA syndrome, which results from mutations in the PSTPIP-1 gene leading to dysregulated IL-1β and IL-18 production that drive the inflammatory response [135, 136]. A number of mouse models of various autoinflammatory disorders, mainly resulting from inflammasome dysregulation leading to excessive IL-1 production, have features of neutrophilic dermatoses [137], however, none of these are particularly neutrophil specific. Recently, Abram et al. reported the serendipitous generation of a mouse model of sterile neutrophilic skin inflammation in a mouse model with hyperactive β2 integrin signaling restricted to neutrophils [7]. These mice lack the inhibitory tyrosine phosphatase SHP-1 specifically in neutrophils, which renders these cells hyperadhesive and hyper-responsive to ligation of β2 integrins. The only major sequela of this dysregulated neutrophil function is the development of chronic skin inflammation, which is prevented by neutrophil-specific genetic blockade of integrin signaling. Alterations in the gene encoding the SHP-1 phosphatase (PTPN6) have been reported in cases of human neutrophilic dermatoses [138]. Clearly, further work in this area is warranted.
8. Novel Therapeutic Approaches Targeting Neutrophils
Given the wealth of new information revealing novel functions for neutrophils in various autoimmune and inflammatory diseases, the potential for new therapeutics is obvious. Perhaps the most interesting therapeutic target is neutrophil NETosis. Indeed, as described above, blockade of NETosis using PAD inhibitors has been successful in mouse models of lupus and arthritis. The PAD inhibitors may also be useful to modulate neutrophil-mediated inflammation in other diseases such as atherosclerosis, by reducing NETosis [139]. Other pharmacologic approaches to inhibiting NETosis could include use of antimalarials; chloroquine has recently been shown to reduce NET formation in vitro [31], while blockade of calcium flux, through various channel blockers may also modulate NETosis [140]. As mentioned previously, stabilization of histone acetylation may also help reduce NETosis in various inflammatory diseases. Reduction of ROS by treatment with anti-oxidants such as N-acteyl cysteine could also be effective therapeutics [141]. Studies in mouse models suggest that inhibition of mitochondrial ROS production may be sufficient to block NETosis, while avoiding all the other sequela of NADPH oxidase inhibition that have been observed [34]. DNAse treatment to physically degrade NETs is another approach as is blockade of one of the major immune activators that is induced by NETs, namely IFNα. Indeed, therapeutic targeting of NETosis for treatment of autoimmunity, while avoiding substantial risks of treatment-related infections, is a very active area of clinical research [142].
Other neutrophil-specific approaches can be envisioned such as specific targeting of chemokine/chemoattractant receptors on neutrophils, including BLT-1 (the receptor for LTB4) or CXCR1/2 (the receptors for IL-8-like chemokines) using mAb approaches. Second or third generation of bi-specific antibodies could be used to ensure neutrophil-specific targeting of these agents [143, 144]. A similar approach could be used to target cytokines made specifically by neutrophils, such as BAFF. Though anti-BAFF blockade using mAbs recognizing the soluble cytokine have had only moderate efficacy in human clinical trials, targeting the neutrophil site of production, may be much more effective [145, 146]. Combining such therapies with other antibodies, such as Rituximab that targets B-cell CD20, has been proposed [147]. Similar approaches could be envisioned for other neutrophil-derived cytokines, such as IL-1β, which has been implicated in multiple autoimmune models, or the S100A8/9 proteins.
Obviously, most therapeutics are based on small molecule inhibitors of specific enzymes. To achieve neutrophil-specific targeting with small molecules, one needs to focus on target enzymes that are predominately found in neutrophils. Though not restricted to neutrophils, there are a number of potential tyrosine kinase enzymes that are relatively immune cell-specific, whose therapeutic targeting has been validated in a number of murine autoimmune models. This includes Src-family kinases, Syk and Btk [56, 148, 149]. This is only a partial list; indeed, many of the inhibitors against signaling molecules which were developed to treat various malignancies may be “re-purposed” to treat autoimmune/inflammatory diseases through their action on neutrophil functions. Careful clinical testing will be required to identify novel disease indications for currently available small molecule inhibitors.
9. Conclusions
A number of the mechanisms by which neutrophils contribute to autoimmune and inflammatory disease pathology are summarized in Figure 1. These mechanisms emphasize that neutrophils are involved in disease pathogenesis in many ways other than simply inducing tissue injury through release of proteases and ROS. Indeed, as summarized in Table 1 and Table 2, there are examples where neutrophil-derived mechanisms both exacerbate autoimmune disease as well as examples of neutrophils protecting the host from inflammatory tissue injury. Clearly, neutrophils are regulatory players in the overall immune response, both through direct production of cytokines/chemoattractants as well as indirect effects on other immune cells through agents such as MPO and ROS. It is also clear that neutrophils are the source of self-antigens in many autoimmune diseases. Much of this complexity has been revealed in studies using animal models, which will continue to improve as neutrophil-specific gene targeting and interventional approaches improve. In the past, drug development efforts have always shied away from targeting neutrophils, mainly for fear of severe infectious complications. Given what we know now about neutrophil contributions to autoimmune pathogenesis, it is possible to envision the development of agents that can block aspects of neutrophil function while only modestly impairing host-defense. The use of existing animal models, and the development of new ones, will be central to improving our ability to control autoimmune diseases by modulating neutrophil function.
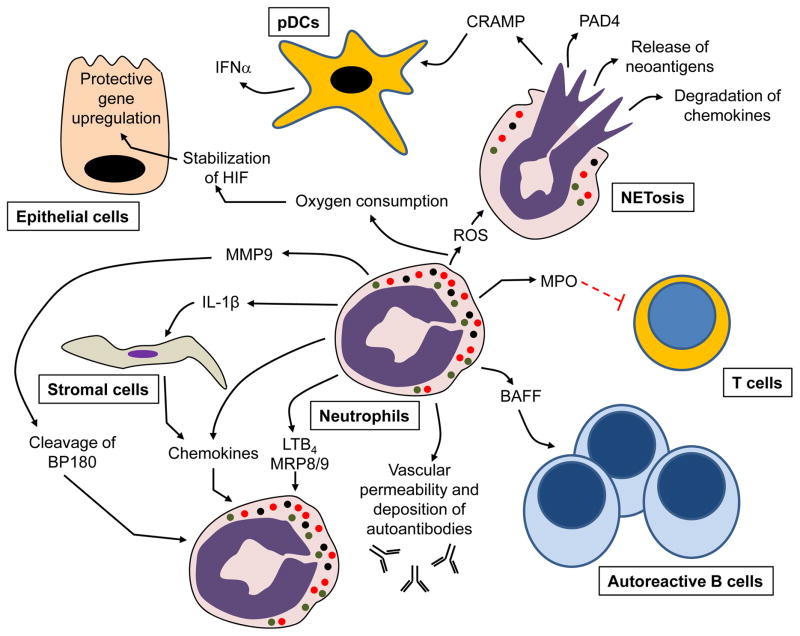
Figure 1.
Cellular mechanisms by which neutrophils can drive autoimmune/inflammatory diseases, as validated in various animal models. For abbreviations see the main text.
Table 1.
Proinflammatory roles of neutrophils in autoimmune-mediated tissue injury
| Animal model | Corresponding human disease | Role for neutrophils | Potential therapy |
|---|---|---|---|
| NZW/B, NZM, MRL/lpr | SLE | ROS-mediated NETosis, promoting IFNα production of DCs, CRAMP and PAD4 release | Targeting ROS production and NET formation, PAD4 inhibition |
| Transferring MPO-sensitized MPO-deficient splenocytes to healthy donors | ANCA-associated vasculitides | NETosis, autoantigen release, Fc receptor-mediated tissue damage | Targeting NET formation and Fc receptor signaling |
| CIA, K/BxN STA | Autoimmune arthritis | Increasing vascular permeability, NETosis, autoantigen and PAD4 release LTB4 and cytokine production | Blocking NET-formation, PAD4, BLT1, cytokine receptor inhibition, targeting Fc receptor signaling |
| EAE | Sclerosis multiplex | Increasing vascular permeability, disruption of the blood-brain barrier, promoting the maturation of resident microglia and macrophages, tissue damage | Blockade of neutrophil recruitment |
| EAU | Autoimmune uveitis | Tissue damage | Targeting G-CSF |
| BP | Bullous pemphigoid | Fc receptor-dependent release of proteases, BP180 degradation | Targeting Fc receptor signaling |
| EBA | Epidermolysis bullosa acquisita | Contribution to the immunization phase; Fc receptor-mediated, ROS-dependent tissue injury | Targeting Fc receptor signaling |
| Imiquimod skin inflammation, fsn/fsn mutants | Psoriasis | Tissue damage, self amplification of inflammation, proteolytic processing of inflammatory cytokines | Blocking BLT1, IL-1β, IL-36 or use of IL-36R antagonist |
| MSU-related air-pouch model, MSU-induced peritonitis/arthritis | Gout | MSU-triggered neutrophil activation | Inflammasome blockade |
| DSS- and TNBS-induced colitis | Inflammatory bowel disease | Tissue damage | Targeting chemokines or chemokine receptors |
Table 2.
Protective roles of neutrophils in limiting autoimmune-mediated tissue injury
| Animal model | Corresponding human disease | Role for neutrophils |
|---|---|---|
| Pristane-induced lupus model | SLE | MPO-mediated inhibition of T cell expansion through the alteration of dendritic cell priming |
| Transferring MPO-sensitized MPO-deficient splenocytes to healthy donors | ANCA-associated vasculitides | NADPH oxidase-dependent downregulation of IL-1β production |
| MSU-induced peritonitis/arthritis | Gout | NET-dependent trapping and protease-mediated degradation of chemokines; Apoptotic neutrophil debris- uptake triggered downregulation of neutrophil function through TGFβ1 release |
| DSS- and TNBS-induced colitis | Inflammatory bowel disease | NADPH oxidase-mediated local mucosal hypoxia and the subsequent HIF- dependent protective gene upregulation in epithelial cells |
Highlights.
Studies in animal models have revealed that neutrophils play complex roles in all phases of autoimmune diseases
Neutrophils modulate autoimmune responses through interactions with other cells
Neutrophil functions such as NETosis, cytokine production, superoxide production and release of antimicrobial peptides have distinct roles in modulating autoimmune diseases
Acknowledgments
The authors thank Clare Abram for careful reading of the manuscript. This work is supported by grants from the National Institutes of Health (RO1 AI068150, AI065495 and AI113272) to C.A.L., and the János Bolyai Research Scholarship of the Hungarian Academy of Sciences to T. N.
Footnotes
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan MJ. Role of neutrophils in systemic autoimmune diseases. Arthritis Res Ther. 2013;15:219. doi: 10.1186/ar4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth T, Mocsai A. The role of neutrophils in autoimmune diseases. Immunol Lett. 2012;143:9–19. doi: 10.1016/j.imlet.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Lamagna C, Scapini P, van Ziffle JA, DeFranco AL, Lowell CA. Hyperactivated MyD88 signaling in dendritic cells, through specific deletion of Lyn kinase, causes severe autoimmunity and inflammation. Proc Natl Acad Sci U S A. 2013;110:E3311–3320. doi: 10.1073/pnas.1300617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity. 2013;38:489–501. doi: 10.1016/j.immuni.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 9.Futosi K, Fodor S, Mocsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayson PC, Kaplan MJ. At the Bench: Neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J Leukoc Biol. 2016;99:253–264. doi: 10.1189/jlb.5BT0615-247R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carneiro-Sampaio M, Coutinho A. Early-onset autoimmune disease as a manifestation of primary immunodeficiency. Front Immunol. 2015;6:185. doi: 10.3389/fimmu.2015.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwivedi N, Radic M. Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Ann Rheum Dis. 2014;73:483–491. doi: 10.1136/annrheumdis-2013-203844. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Li J, Dorta-Estremera S, Di Domizio J, Anthony SM, Watowich SS, et al. Neutrophils regulate humoral autoimmunity by restricting interferon-gamma production via the generation of reactive oxygen species. Cell Rep. 2015;12:1120–1132. doi: 10.1016/j.celrep.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coquery CM, Wade NS, Loo WM, Kinchen JM, Cox KM, Jiang C, et al. Neutrophils contribute to excess serum BAFF levels and promote CD4+ T cell and B cell responses in lupus-prone mice. PLoS One. 2014;9:e102284. doi: 10.1371/journal.pone.0102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol. 2015;27:448–453. doi: 10.1097/BOR.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Su K. Neutrophil extracellular traps and systemic lupus erythematosus. J Clin Cell Immunol. 2013;4 doi: 10.4172/2155-9899.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alves CM, Marzocchi-Machado CM, Louzada-Junior P, Azzolini AE, Polizello AC, de Carvalho IF, et al. Superoxide anion production by neutrophils is associated with prevalent clinical manifestations in systemic lupus erythematosus. Clin Rheumatol. 2008;27:701–708. doi: 10.1007/s10067-007-0768-x. [DOI] [PubMed] [Google Scholar]
- 18.Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, et al. Clearance deficiency and systemic lupus erythematosus (SLE) J Autoimmun. 2007;28:114–121. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Pieterse E, van der Vlag J. Breaking immunological tolerance in systemic lupus erythematosus. Front Immunol. 2014;5:164. doi: 10.3389/fimmu.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 21.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8:883–896. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coit P, Yalavarthi S, Ognenovski M, Zhao W, Hasni S, Wren JD, et al. Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J Autoimmun. 2015;58:59–66. doi: 10.1016/j.jaut.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190:1217–1226. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palanichamy A, Bauer JW, Yalavarthi S, Meednu N, Barnard J, Owen T, et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol. 2014;192:906–918. doi: 10.4049/jimmunol.1302112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, et al. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight JS, Zhao W, Luo W, Subramanian V, O'Dell AA, Yalavarthi S, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123:2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight JS, Subramanian V, O'Dell AA, Yalavarthi S, Zhao W, Smith CK, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015;74:2199–2206. doi: 10.1136/annrheumdis-2014-205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 34.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia BA, Busby SA, Shabanowitz J, Hunt DF, Mishra N. Resetting the epigenetic histone code in the MRL-lpr/lpr mouse model of lupus by histone deacetylase inhibition. J Proteome Res. 2005;4:2032–2042. doi: 10.1021/pr050188r. [DOI] [PubMed] [Google Scholar]
- 36.Forster N, Gallinat S, Jablonska J, Weiss S, Elsasser HP, Lutz W. p300 protein acetyltransferase activity suppresses systemic lupus erythematosus-like autoimmune disease in mice. J Immunol. 2007;178:6941–6948. doi: 10.4049/jimmunol.178.11.6941. [DOI] [PubMed] [Google Scholar]
- 37.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odobasic D, Muljadi RC, O'Sullivan KM, Kettle AJ, Dickerhof N, Summers SA, et al. Suppression of autoimmunity and renal disease in pristane-induced lupus by myeloperoxidase. Arthritis Rheumatol. 2015;67:1868–1880. doi: 10.1002/art.39109. [DOI] [PubMed] [Google Scholar]
- 39.Odobasic D, Kitching AR, Yang Y, O'Sullivan KM, Muljadi RC, Edgtton KL, et al. Neutrophil myeloperoxidase regulates T-cell-driven tissue inflammation in mice by inhibiting dendritic cell function. Blood. 2013;121:4195–4204. doi: 10.1182/blood-2012-09-456483. [DOI] [PubMed] [Google Scholar]
- 40.Kallenberg CG. Pathogenesis and treatment of ANCA-associated vasculitides. Clin Exp Rheumatol. 2015;33:S11–14. [PubMed] [Google Scholar]
- 41.Schreiber A. L10. Animal models of ANCA-associated vasculitis: effector mechanisms and experimental therapies. Presse Med. 2013;42:520–523. doi: 10.1016/j.lpm.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Holden NJ, Williams JM, Morgan MD, Challa A, Gordon J, Pepper RJ, et al. ANCA-stimulated neutrophils release BLyS and promote B cell survival: a clinically relevant cellular process. Ann Rheum Dis. 2011;70:2229–2233. doi: 10.1136/ard.2011.153890. [DOI] [PubMed] [Google Scholar]
- 43.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120:3007–3018. doi: 10.1182/blood-2012-03-416156. [DOI] [PubMed] [Google Scholar]
- 45.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiber A, Xiao H, Falk RJ, Jennette JC. Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J Am Soc Nephrol. 2006;17:3355–3364. doi: 10.1681/ASN.2006070718. [DOI] [PubMed] [Google Scholar]
- 47.Ooi JD, Gan PY, Chen T, Eggenhuizen PJ, Chang J, Alikhan MA, et al. FcgammaRIIB regulates T-cell autoreactivity, ANCA production, and neutrophil activation to suppress anti-myeloperoxidase glomerulonephritis. Kidney Int. 2014;86:1140–1149. doi: 10.1038/ki.2014.189. [DOI] [PubMed] [Google Scholar]
- 48.Pfister H, Ollert M, Frohlich LF, Quintanilla-Martinez L, Colby TV, Specks U, et al. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood. 2004;104:1411–1418. doi: 10.1182/blood-2004-01-0267. [DOI] [PubMed] [Google Scholar]
- 49.Hattar K, Oppermann S, Ankele C, Weissmann N, Schermuly RT, Bohle RM, et al. c-ANCA-induced neutrophil-mediated lung injury: a model of acute Wegener's granulomatosis. Eur Respir J. 2010;36:187–195. doi: 10.1183/09031936.00143308. [DOI] [PubMed] [Google Scholar]
- 50.Schreiber A, Luft FC, Kettritz R. Phagocyte NADPH oxidase restrains the inflammasome in ANCA-induced GN. J Am Soc Nephrol. 2015;26:411–424. doi: 10.1681/ASN.2013111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pietrosimone KM, Jin M, Poston B, Liu P. Collagen-Induced Arthritis: A model for murine autoimmune arthritis. Bio Protoc. 2015;5 doi: 10.21769/bioprotoc.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spengler J, Lugonja B, Jimmy Ytterberg A, Zubarev RA, Creese AJ, Pearson MJ, et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 2015;67:3135–3145. doi: 10.1002/art.39313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, et al. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eyles JL, Hickey MJ, Norman MU, Croker BA, Roberts AW, Drake SF, et al. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112:5193–5201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- 55.Wipke BT, Wang Z, Nagengast W, Reichert DE, Allen PM. Staging the initiation of autoantibody-induced arthritis: a critical role for immune complexes. J Immunol. 2004;172:7694–7702. doi: 10.4049/jimmunol.172.12.7694. [DOI] [PubMed] [Google Scholar]
- 56.Elliott ER, Van Ziffle JA, Scapini P, Sullivan BM, Locksley RM, Lowell CA. Deletion of Syk in neutrophils prevents immune complex arthritis. J Immunol. 2011;187:4319–4330. doi: 10.4049/jimmunol.1100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ditzel HJ. The K/BxN mouse: a model of human inflammatory arthritis. Trends Mol Med. 2004;10:40–45. doi: 10.1016/j.molmed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Mancardi DA, Jonsson F, Iannascoli B, Khun H, Van Rooijen N, Huerre M, et al. Cutting Edge: The murine high-affinity IgG receptor FcgRIV is sufficient for autoantibody-induced arthritis. J Immunol. 2011;186:1899–1903. doi: 10.4049/jimmunol.1003642. [DOI] [PubMed] [Google Scholar]
- 59.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcgammaR signaling. Proc Natl Acad Sci U S A. 2012;109:E3177–3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christensen AD, Haase C, Cook AD, Hamilton JA. Granulocyte colony-stimulating factor (G-CSF) plays an important role in immune complex-mediated arthritis. Eur J Immunol. 2016 doi: 10.1002/eji.201546185. [DOI] [PubMed] [Google Scholar]
- 62.Katayama M, Ohmura K, Yukawa N, Terao C, Hashimoto M, Yoshifuji H, et al. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One. 2013;8:e62231. doi: 10.1371/journal.pone.0062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7:284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 64.Rohrbach AS, Hemmers S, Arandjelovic S, Corr M, Mowen KA. PAD4 is not essential for disease in the K/BxN murine autoantibody-mediated model of arthritis. Arthritis Res Ther. 2012;14:R104. doi: 10.1186/ar3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maicas N, Ferrandiz ML, Brines R, Ibanez L, Cuadrado A, Koenders MI, et al. Deficiency of Nrf2 accelerates the effector phase of arthritis and aggravates joint disease. Antioxid Redox Signal. 2011;15:889–901. doi: 10.1089/ars.2010.3835. [DOI] [PubMed] [Google Scholar]
- 66.Kelkka T, Hultqvist M, Nandakumar KS, Holmdahl R. Enhancement of antibody-induced arthritis via Toll-like receptor 2 stimulation is regulated by granulocyte reactive oxygen species. Am J Pathol. 2012;181:141–150. doi: 10.1016/j.ajpath.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 67.Kim HR, Lee A, Choi EJ, Hong MP, Kie JH, Lim W, et al. Reactive oxygen species prevent imiquimod-induced psoriatic dermatitis through enhancing regulatory T cell function. PLoS One. 2014;9:e91146. doi: 10.1371/journal.pone.0091146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerreiro-Cacais AO, Laaksonen H, Flytzani S, N'Diaye M, Olsson T, Jagodic M. Translational utility of experimental autoimmune encephalomyelitis: recent developments. J Inflamm Res. 2015;8:211–225. doi: 10.2147/JIR.S76707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moline-Velazquez V, Vila-Del Sol V, de Castro F, Clemente D. Myeloid cell distribution and activity in multiple sclerosis. Histol Histopathol. 2016;31:357–370. doi: 10.14670/HH-11-699. [DOI] [PubMed] [Google Scholar]
- 70.McColl SR, Staykova MA, Wozniak A, Fordham S, Bruce J, Willenborg DO. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6421–6426. [PubMed] [Google Scholar]
- 71.Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Croxford AL, Spath S, Becher B. GM-CSF in neuroinflammation: licensing myeloid cells for tissue damage. Trends Immunol. 2015;36:651–662. doi: 10.1016/j.it.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Rumble JM, Huber AK, Krishnamoorthy G, Srinivasan A, Giles DA, Zhang X, et al. Neutrophil-related factors as biomarkers in EAE and MS. J Exp Med. 2015;212:23–35. doi: 10.1084/jem.20141015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinbach K, Piedavent M, Bauer S, Neumann JT, Friese MA. Neutrophils amplify autoimmune central nervous system infiltrates by maturing local APCs. J Immunol. 2013;191:4531–4539. doi: 10.4049/jimmunol.1202613. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y, Holdbrooks AT, Meares GP, Buckley JA, Benveniste EN, Qin H. Preferential recruitment of neutrophils into the cerebellum and brainstem contributes to the atypical experimental autoimmune encephalomyelitis phenotype. J Immunol. 2015;195:841–852. doi: 10.4049/jimmunol.1403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zehntner SP, Brickman C, Bourbonniere L, Remington L, Caruso M, Owens T. Neutrophils that infiltrate the central nervous system regulate T cell responses. J Immunol. 2005;174:5124–5131. doi: 10.4049/jimmunol.174.8.5124. [DOI] [PubMed] [Google Scholar]
- 77.Miller NM, Wang J, Tan Y, Dittel BN. Anti-inflammatory mechanisms of IFN-gamma studied in experimental autoimmune encephalomyelitis reveal neutrophils as a potential target in multiple sclerosis. Front Neurosci. 2015;9:287. doi: 10.3389/fnins.2015.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aube B, Levesque SA, Pare A, Chamma E, Kebir H, Gorina R, et al. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- 79.Goldberg GL, Cornish AL, Murphy J, Pang ES, Lim LL, Campbell IK, et al. G-CSF and Neutrophils Are Nonredundant Mediators of Murine Experimental Autoimmune Uveoretinitis. Am J Pathol. 2016;186:172–184. doi: 10.1016/j.ajpath.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Z, Zhong W, Spencer D, Chen H, Lu H, Kawaguchi T, et al. Interleukin-17 causes neutrophil mediated inflammation in ovalbumin-induced uveitis in DO11.10 mice. Cytokine. 2009;46:79–91. doi: 10.1016/j.cyto.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84–89. doi: 10.1016/j.autrev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Lin L, Betsuyaku T, Heimbach L, Li N, Rubenstein D, Shapiro SD, et al. Neutrophil elastase cleaves the murine hemidesmosomal protein BP180/type XVII collagen and generates degradation products that modulate experimental bullous pemphigoid. Matrix Biol. 2012;31:38–44. doi: 10.1016/j.matbio.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heimbach L, Li Z, Berkowitz P, Zhao M, Li N, Rubenstein DS, et al. The C5a receptor on mast cells is critical for the autoimmune skin-blistering disease bullous pemphigoid. J Biol Chem. 2011;286:15003–15009. doi: 10.1074/jbc.M111.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chiriac MT, Roesler J, Sindrilaru A, Scharffetter-Kochanek K, Zillikens D, Sitaru C. NADPH oxidase is required for neutrophil-dependent autoantibody-induced tissue damage. J Pathol. 2007;212:56–65. doi: 10.1002/path.2157. [DOI] [PubMed] [Google Scholar]
- 85.Oswald E, Sesarman A, Franzke CW, Wolfle U, Bruckner-Tuderman L, Jakob T, et al. The flavonoid luteolin inhibits Fcgamma-dependent respiratory burst in granulocytes, but not skin blistering in a new model of pemphigoid in adult mice. PLoS One. 2012;7:e31066. doi: 10.1371/journal.pone.0031066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samavedam UK, Iwata H, Muller S, Schulze FS, Recke A, Schmidt E, et al. GM-CSF modulates autoantibody production and skin blistering in experimental epidermolysis bullosa acquisita. J Immunol. 2014;192:559–571. doi: 10.4049/jimmunol.1301556. [DOI] [PubMed] [Google Scholar]
- 87.Albanesi C, Scarponi C, Bosisio D, Sozzani S, Girolomoni G. Immune functions and recruitment of plasmacytoid dendritic cells in psoriasis. Autoimmunity. 2010;43:215–219. doi: 10.3109/08916930903510906. [DOI] [PubMed] [Google Scholar]
- 88.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 89.Ueyama A, Yamamoto M, Tsujii K, Furue Y, Imura C, Shichijo M, et al. Mechanism of pathogenesis of imiquimod-induced skin inflammation in the mouse: a role for interferon-alpha in dendritic cell activation by imiquimod. J Dermatol. 2014;41:135–143. doi: 10.1111/1346-8138.12367. [DOI] [PubMed] [Google Scholar]
- 90.Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 91.Walter A, Schafer M, Cecconi V, Matter C, Urosevic-Maiwald M, Belloni B, et al. Aldara activates TLR7-independent immune defence. Nat Commun. 2013;4:1560. doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- 92.Sumida H, Yanagida K, Kita Y, Abe J, Matsushima K, Nakamura M, et al. Interplay between CXCR2 and BLT1 facilitates neutrophil infiltration and resultant keratinocyte activation in a murine model of imiquimod-induced psoriasis. J Immunol. 2014;192:4361–4369. doi: 10.4049/jimmunol.1302959. [DOI] [PubMed] [Google Scholar]
- 93.Schon M, Denzer D, Kubitza RC, Ruzicka T, Schon MP. Critical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin mice. J Invest Dermatol. 2000;114:976–983. doi: 10.1046/j.1523-1747.2000.00953.x. [DOI] [PubMed] [Google Scholar]
- 94.Helms C, Pelsue S, Cao L, Lamb E, Loffredo B, Taillon-Miller P, et al. The Tetratricopeptide repeat domain 7 gene is mutated in flaky skin mice: a model for psoriasis, autoimmunity, and anemia. Exp Biol Med (Maywood) 2005;230:659–667. doi: 10.1177/153537020523000908. [DOI] [PubMed] [Google Scholar]
- 95.Shao S, Cao T, Jin L, Li B, Fang H, Zhang J, et al. Increased lipocalin-2 contributes to the pathogenesis of psoriasis by modulating neutrophil chemotaxis and cytokine secretion. J Invest Dermatol. 2016 doi: 10.1016/j.jid.2016.03.002. in press. [DOI] [PubMed] [Google Scholar]
- 96.Henry CM, Sullivan GP, Clancy DM, Afonina IS, Kulms D, Martin SJ. Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Rep. 2016;14:708–722. doi: 10.1016/j.celrep.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 97.Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 98.Tortola L, Rosenwald E, Abel B, Blumberg H, Schafer M, Coyle AJ, et al. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J Clin Invest. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Milora KA, Fu H, Dubaz O, Jensen LE. Unprocessed interleukin-36alpha regulates psoriasis-like skin inflammation in cooperation with interleukin-1. J Invest Dermatol. 2015;135:2992–3000. doi: 10.1038/jid.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Askenasy N. Mechanisms of autoimmunity in the NOD mouse: effector/regulatory cell equilibrium during peak inflammation. Immunology. 2016 doi: 10.1111/imm.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 102.Diana J, Lehuen A. Macrophages and beta-cells are responsible for CXCR2-mediated neutrophil infiltration of the pancreas during autoimmune diabetes. EMBO Mol Med. 2014;6:1090–1104. doi: 10.15252/emmm.201404144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen K, Wei Y, Sharp GC, Braley-Mullen H. Mechanisms of spontaneous resolution versus fibrosis in granulomatous experimental autoimmune thyroiditis. J Immunol. 2003;171:6236–6243. doi: 10.4049/jimmunol.171.11.6236. [DOI] [PubMed] [Google Scholar]
- 104.Chen K, Wei Y, Alter A, Sharp GC, Braley-Mullen H. Chemokine expression during development of fibrosis versus resolution in a murine model of granulomatous experimental autoimmune thyroiditis. J Leukoc Biol. 2005;78:716–724. doi: 10.1189/jlb.0205102. [DOI] [PubMed] [Google Scholar]
- 105.McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3:e297. doi: 10.1371/journal.pmed.0030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGonagle D, Savic S, McDermott MF. The NLR network and the immunological disease continuum of adaptive and innate immune-mediated inflammation against self. Semin Immunopathol. 2007;29:303–313. doi: 10.1007/s00281-007-0084-1. [DOI] [PubMed] [Google Scholar]
- 107.Cronstein BN, Terkeltaub R. The inflammatory process of gout and its treatment. Arthritis Res Ther. 2006;8(Suppl 1):S3. doi: 10.1186/ar1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Terkeltaub R, Baird S, Sears P, Santiago R, Boisvert W. The murine homolog of the interleukin-8 receptor CXCR-2 is essential for the occurrence of neutrophilic inflammation in the air pouch model of acute urate crystal-induced gouty synovitis. Arthritis Rheum. 1998;41:900–909. doi: 10.1002/1529-0131(199805)41:5<900::AID-ART18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 109.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 110.Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171:2602–2609. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 111.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 112.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, et al. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 2012;64:474–484. doi: 10.1002/art.33355. [DOI] [PubMed] [Google Scholar]
- 114.Steiger S, Harper JL. Neutrophil cannibalism triggers transforming growth factor beta1 production and self regulation of neutrophil inflammatory function in monosodium urate monohydrate crystal-induced inflammation in mice. Arthritis Rheum. 2013;65:815–823. doi: 10.1002/art.37822. [DOI] [PubMed] [Google Scholar]
- 115.Cumpelik A, Ankli B, Zecher D, Schifferli JA. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2015-207338. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 117.Buanne P, Di Carlo E, Caputi L, Brandolini L, Mosca M, Cattani F, et al. Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol. 2007;82:1239–1246. doi: 10.1189/jlb.0207118. [DOI] [PubMed] [Google Scholar]
- 118.Kishida K, Kohyama M, Kurashima Y, Kogure Y, Wang J, Hirayasu K, et al. Negative regulation of DSS-induced experimental colitis by PILRalpha. Int Immunol. 2015;27:307–314. doi: 10.1093/intimm/dxv004. [DOI] [PubMed] [Google Scholar]
- 119.Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol. 2008;84:1213–1221. doi: 10.1189/jlb.0408231. [DOI] [PubMed] [Google Scholar]
- 120.Ajuebor MN, Zagorski J, Kunkel SL, Strieter RM, Hogaboam CM. Contrasting roles for CXCR2 during experimental colitis. Exp Mol Pathol. 2004;76:1–8. doi: 10.1016/j.yexmp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Wallace JL, Higa A, McKnight GW, MacIntyre DE. Prevention and reversal of experimental colitis by a monoclonal antibody which inhibits leukocyte adherence. Inflammation. 1992;16:343–354. doi: 10.1007/BF00917626. [DOI] [PubMed] [Google Scholar]
- 122.Palmen MJ, Dijkstra CD, van der Ende MB, Pena AS, van Rees EP. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin Exp Immunol. 1995;101:351–356. doi: 10.1111/j.1365-2249.1995.tb08363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuhl AA, Kakirman H, Janotta M, Dreher S, Cremer P, Pawlowski NN, et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133:1882–1892. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 124.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rodrigues-Sousa T, Ladeirinha AF, Santiago AR, Carvalheiro H, Raposo B, Alarcao A, et al. Deficient production of reactive oxygen species leads to severe chronic DSS-induced colitis in Ncf1/p47phox-mutant mice. PLoS One. 2014;9:e97532. doi: 10.1371/journal.pone.0097532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, Anaya-O'Brien S, et al. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462–468. doi: 10.1542/peds.114.2.462. [DOI] [PubMed] [Google Scholar]
- 127.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, von Kockritz-Blickwede M, et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med (Berl) 2012;90:1079–1089. doi: 10.1007/s00109-012-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hickman SE, El Khoury J. The neuroimmune system in Alzheimer's disease: the glass is half full. J Alzheimers Dis. 2013;33(Suppl 1):S295–302. doi: 10.3233/JAD-2012-129027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baik SH, Cha MY, Hyun YM, Cho H, Hamza B, Kim DK, et al. Migration of neutrophils targeting amyloid plaques in Alzheimer's disease mouse model. Neurobiol Aging. 2014;35:1286–1292. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 131.Mocsai A, Walzog B, Lowell CA. Intracellular signalling during neutrophil recruitment. Cardiovasc Res. 2015;107:373–385. doi: 10.1093/cvr/cvv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pietronigro E, Zenaro E, Constantin G. Imaging of Leukocyte Trafficking in Alzheimer's Disease. Front Immunol. 2016;7:33. doi: 10.3389/fimmu.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691–698. doi: 10.1016/j.jaad.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 134.Maalouf D, Battistella M, Bouaziz JD. Neutrophilic dermatosis: disease mechanism and treatment. Curr Opin Hematol. 2015;22:23–29. doi: 10.1097/MOH.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 135.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002;11:961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 136.Lindwall E, Singla S, Davis WE, Quinet RJ. Novel PSTPIP1 gene mutation in a patient with pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome. Semin Arthritis Rheum. 2015;45:91–93. doi: 10.1016/j.semarthrit.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 137.Heymann MC, Rosen-Wolff A. Contribution of the inflammasomes to autoinflammatory diseases and recent mouse models as research tools. Clin Immunol. 2013;147:175–184. doi: 10.1016/j.clim.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 138.Nesterovitch AB, Gyorfy Z, Hoffman MD, Moore EC, Elbuluk N, Tryniszewska B, et al. Alteration in the gene encoding protein tyrosine phosphatase nonreceptor type 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. Am J Pathol. 2011;178:1434–1441. doi: 10.1016/j.ajpath.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114:947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One. 2014;9:e97088. doi: 10.1371/journal.pone.0097088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, et al. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide. 2010;22:226–234. doi: 10.1016/j.niox.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 142.Barnado A, Crofford LJ, Oates JC. At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J Leukoc Biol. 2016;99:265–278. doi: 10.1189/jlb.5BT0615-234R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 144.O'Shea JJ, Kanno Y, Chan AC. In search of magic bullets: the golden age of immunotherapeutics. Cell. 2014;157:227–240. doi: 10.1016/j.cell.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Merrill JT, van Vollenhoven RF, Buyon JP, Furie RA, Stohl W, Morgan-Cox M, et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75:332–340. doi: 10.1136/annrheumdis-2015-207654. [DOI] [PubMed] [Google Scholar]
- 146.Zhang H, Chambers W, Sciascia S, Cuadrado MJ. Emerging therapies in systemic lupus erythematous: from clinical trial to the real life. Expert Rev Clin Pharmacol. 2016;9:1–14. doi: 10.1586/17512433.2016.1155446. [DOI] [PubMed] [Google Scholar]
- 147.Ehrenstein MR, Wing C. The BAFFling effects of rituximab in lupus: danger ahead? Nat Rev Rheumatol. 2016 doi: 10.1038/nrrheum.2016.18. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 148.Kovacs M, Nemeth T, Jakus Z, Sitaru C, Simon E, Futosi K, et al. The Src family kinases Hck, Fgr, and Lyn are critical for the generation of the in vivo inflammatory environment without a direct role in leukocyte recruitment. J Exp Med. 2014;211:1993–2011. doi: 10.1084/jem.20132496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Volmering S, Block H, Boras M, Lowell CA, Zarbock A. The Neutrophil Btk Signalosome Regulates Integrin Activation during Sterile Inflammation. Immunity. 2016;44:73–87. doi: 10.1016/j.immuni.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]