Abstract
Ketamine is a popular choice for young drug abusers. Ketamine abuse causes lower urinary tract symptoms, with the underlying pathophysiology poorly understood. Disruption of urothelial barrier function has been hypothesized to be a major mechanism for ketamine cystitis, yet the direct evidence of impaired urothelial barrier function is still lacking. To address this question, 8-wk-old female C57BL/6J mice were injected intraperitoneally with 30 mg·kg−1·day−1 ketamine for 12 wk to induce ketamine cystitis. A spontaneous voiding spot assay showed that ketamine-treated mice had increased primary voiding spot numbers and smaller primary voiding spot sizes than control mice (P < 0.05), indicating a contracted bladder and bladder overactivity. Consistently, significantly increased voiding frequency was observed in ketamine-treated mice on cystometrograms. These functional experiments indicate that ketamine induces voiding dysfunction in mice. Surprisingly, urothelial permeability in ketamine-treated mice was not changed when measured using an Ussing chamber system with isotopic urea and water. Mouse urothelial structure was also not altered, and intact umbrella cell structure was observed by both transmission and scanning electron microscopy. Furthermore, immunostaining and confocal microscopy confirmed the presence of a well-defined distribution of zonula occuldens-1 in tight junctions and uroplakin in umbrella cells. In conclusion, these data indicate that ketamine injection induces voiding dysfunction in mice but does not necessarily disrupt mouse bladder barrier function. Disruption of urothelial barrier function may not be the major mechanism in ketamine cystitis.
Keywords: urinary bladder, ketamine cystitis, voiding spot assay, umbrella cell, permeability
ketamine is an anesthetic that is being increasingly used as a recreational drug due to its hallucinatory effects. Ketamine abuse causes chronic toxicity to many organs, including cardiovascular, hepatobiliary, respiratory, gastrointestinal, and lower urinary systems (2, 3, 11, 15, 21, 26, 27, 29). At least ∼30% of ketamine abusers reported the presence of lower urinary tract symptoms, such as severe dysuria, painful hematuria, urinary frequency, urgency, and urge urinary incontinence. Clinical examination often indicates a contracted bladder with chronic inflammation, ulceration, or necrotic mucosa, but with sterile pyuria, giving rise to the term ketamine cystitis. Vesicoureteric reflux and hydronephrosis with abnormal kidney function are common features in severe conditions (4, 6, 9, 20, 23, 27, 28).
Despite these well-recognized clinical symptoms, the underlying mechanism is still unknown. Several possible mechanisms have been proposed. Ketamine may 1) have direct microvasculature toxicity, causing ischemia and fibrosis; 2) cause neural toxicity and induce nerve fiber degeneration; 3) cause an autoimmune reaction, resulting in bladder inflammation; or 4) the high concentration of ketamine and its metabolite norketamine may have direct toxicity to the mucosa, disrupting urothelial barrier function and causing leakage of urine constituents into the bladder interstitial space and chronic inflammation (6, 27).
Bladder muscosal inflammation with edema, neovascularization, and even denuded urothelium and hemorrhages have often been reported in severe ketamine cystitis patients, suggesting disruption of the urothelial barrier (4, 6, 9, 20, 27). Whether this disrupted barrier is the cause of the ketamine cystitis or is the consequence of severe ketamine cystitis remains an open question. Recent reports have indicated that ketamine-treated rats exhibit increased expression of occludin and decreased expression of cadherin and the tight junction protein zonula occludens (ZO)-1, suggesting a defect in the urothelial barrier (8, 18). Changes in keratin family genes were also observed in urothelia of ketamine-treated mice (22). Moreover, inflammatory molecules such as cyclooxygenase-2 and apoptotic signals were observed in urothelia, confirming the urothelial pathology in ketamine cystitis (10, 17). In contrast to this, other studies have indicated that bladder ulceration or denuded urothelia were not observed in ketamine cystitis, including both human patients or ketamine-induced animal models, suggesting that the disruption of urothelial barrier function may not be the major mechanism of ketamine cystitis (5–7, 17, 24).
The impermeability of the urothelium plays a crucial role in storing of urine and its noxious constituents, yet its role in the pathogenesis of ketamine cystitis has not been directly evaluated. To address this question, we injected mice daily with ketamine for 12 wk. We observed bladder overactivity, which mimicked ketamine cystitis. However, altered urothelial barrier function was not observed, favoring the hypothesis that urothelial pathology is a consequence of ketamine cystitis but not the cause of ketamine cystitis.
MATERIALS AND METHODS
Materials.
Unless otherwise specified, all chemicals were obtained from Sigma (St. Louis, MO) and were of reagent grade or better.
Animals.
Female C57BL/6J mice (8 wk old, Jackson Laboratory, Bar Harbor, ME) were used in this study with the approval of the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center (protocol no. 085-2014).
Study plan.
To induce voiding dysfunction, mice (n = 60) were injected intraperitoneally with ketamine (Patterson Veterinary, Devens, MA) at 30 mg·kg−1·day−1 for 12 wk. Control mice (n = 60) were injected daily with PBS in parallel. Intraperitoneal injection of ketamine has been established to be a repeatable and reliable method to induce ketamine cystitis in animal models in many laboratories. A subanesthetic dosage of 30 mg·kg−1·day−1 to induce mouse ketamine cystitis has been carefully validated in a previous report (24). Both control mice (n = 10) and ketamine-injected mice (n = 10) were subjected to weekly void spotting assays (VSAs) on filter paper to assess voiding function. After 12 wk of injection, mice were further assessed for urodynamic function by cystometrograms (CMGs; n = 5 for control mice and n = 7 for ketamine-injected mice). At this time point, additional mice were also euthanized for permeability measurements (n = 4 for control mice and n = 4 for ketamine-injected mice), scanning electron microscopy (SEM) imaging (n = 2 for control mice and n = 2 for ketamine-injected mice), transmission electron microscopy (TEM) imaging (n = 2 for control mice and n = 2 for ketamine-injected mice), and immunofluorescence (IF) staining and confocal miscroscopy imaging (n = 2 for control mice and n = 2 for ketamine-injected mice). To address whether voiding dysfunction could be recovered after ketamine injection, remaining mice were withdrawn from ketamine for a further 4 wk after 12 wk of injection. At the 16-wk time point, CMGs (n = 7 for control mice and n = 4 for ketamine-injected mice), permeability measurements, SEM, TEM, and IF were all performed again.
Spontaneous VSAs.
VSAs were performed weekly, as previously described (32). Individual mice were gently placed in a standard polycarbonate mouse cage with Blicks Cosmos Blotting Paper (catalog no. 10422-1005) placed in the bottom for 4 h. Mice were given standard dry mouse chow for the duration of the assay. Water was withheld, however, due to problems created by water dripping onto the void spot filter paper. After 4 h, mice were returned to their home cages, and the filter paper was allowed to dry and retrieved. Filters were imaged under ultraviolet light at 365 nm in a UVP Chromato-Vue C-75 system (UVP, Upland, CA) that incorporates an onboard Canon digital single lens reflex camera (EOS Rebel T3, 12 megapixels). Images were analyzed using ImageJ software (http://fiji.sc/wiki/index.php/Fiji). Overlapping voiding spots were visually examined and manually separated by outlining and copying and then pasting to a nearby empty space in ImageJ software. The results, which contained the area of each individual voiding spot and total number of spots in a table, were imported into Excel software for further statistical processing. A volume-area standard curve on this paper determined that 1 mm2 was equal to 0.283 μl urine.
CMGs.
CMGs were performed as previously described (32). Mice were anesthetized by a subcutaneous injection of urethane (1.4 g/kg). Once the pedal reflex was absent, a 1-cm midline abdominal incision was performed, and flame-flanged polyethylene-50 tubing was implanted through the dome of the bladder, which was secured in place with 8-0 silk surgical suture. The mouse was placed into a restrainer, and the catheter was connected to a pressure transducer (and syringe pump by side arm) coupled to data-acquisition devices (Transbridge, World Precision Instruments, Sarasota, FL, and Powerlab 4/35, AD Instruments, Colorado Springs, CO) and a computerized recording system (LabChart software, AD Instruments).
IF staining and confocal microscopy.
Both control and ketamine-treated mice were euthanized for IF staining (2 mice/group) at 12- and 16-wk time points. As previously described (33), the excised bladder was fixed in 4% (wt/vol) paraformaldehyde for 2 h at room temperature. Fixed tissue was cryoprotected, frozen, sectioned, and incubated with rabbit polyclonal antibodies directed against ZO-1 (Invitrogen) or uroplakin (NYU744 and S3045, a kind gift from Dr. T. T. Sun of New York University, 1:100 dilution) overnight at 4°C (16, 25, 30, 31, 34). Sections were then incubated with a mixture of Alexa 488-conjugated secondary antibody (diluted 1:100), rhodamine-phalloidin (1:50), and Topro-3 (1:1,000). Imaging was performed on a Zeiss LSM-510 confocal microscope equipped with argon and green and red helium-neon lasers (Thornwood, NY). Images were acquired by sequential scanning with a ×63 (1.4 numerical aperture) planapochromat oil objective. Images (512 × 512 pixels) were saved as TIFF files and imported into Adobe Illustrator CS3. In the present study, each bladder was sectioned to obtain two slides with four to five sections of tissue on each slide. Each tissue section was examined under the microscopy to ensure the consistency of the staining result, and representative images were taken. The same IF staining and imaging experiments were performed at least twice to ensure their repeatability.
Electron microscopy.
Both control and ketamine-treated mice were euthanized for SEM and TEM (2 mice/group) at 12- and 16-wk time points. For TEM, excised bladders were fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) on ice overnight. The tissue was then processed and imaged in the BIDMC Electron Microscopy Core facility on a JEOL 1400 TEM equipped with a side-mount Gatan Orius SC1000 digital camera as previously described (12). For SEM, excised bladders were fixed for 24–48 h in half-strength Karnovsky's fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.2 M Na2PO4 buffer, pH 7.4) overnight on a shaker at 4°C. Samples were then processed and imaged by a Schepens Eye Research Institute, Harvard University specialist on a JEOL Field Emission Scanning Electron Microscope FESEM 7401F as previously described (12). In the present study, bladder samples were numerically labeled randomly. A large number of bladder urothelial images were taken by the TEM and SEM facilities; the staff that interpreted the images were blinded by the random numbering of samples.
Measurements of permeability and transepithelial resistance.
Opened bladders were mounted in modified Ussing chambers as previously described (13). Briefly, the bladder was mounted on a custom-made Teflon tissue ring with sharp pins, and the tissue-mounted ring was then clamped between two halves of custom-made hemichambers. Transepithelial resistance was measured by placing voltage-measuring Ag/AgCl electrodes in the mucosal and serosal chambers, which were connected to an EC825 current/voltage clamp (Warner Instruments, Hamden, CT). For permeability measurement, [14C]urea (0.25 μCi/ml) and 3H2O (1 μCi/ml; American Radiolabeled Chemicals, St. Louis, MO) were added to the apical (luminal) side of the membrane, and both hemichambers were then sampled (2 samples of 100 μl per hemichamber) at 15-min intervals throughout the experiment. Samples were placed into vials containing scintillation fluid (Scintisafe, Fisher, Pittsburgh, PA) for liquid scintillation counting (Tri-Carb 2500 TR, Packard, Downers Grove, IL). To determine the contribution of unstirred layers to the measured permeability, the apical membrane was destroyed by the addition of 20% (vol/vol) Triton (65 μl) or 10 μM gramicidin (35 μl) 1 h after baseline measurements were taken. In all experiments, the addition of Triton or gramicidin would have abolished transepithelial resistance. Corrections were made for removing the sample volume by the addition of replacement fresh Krebs solution, and the calculated fluxes were corrected for these modest dilution effects. Diffusive permeability coefficients were calculated using the following flux equation: diffusive permeability = Φ/(A)(ΔC), where Φ is the flux of the tracer across the membrane and is calculated from the net increase of the tracer in the basolateral side, A is the area of the apical membrane, and ΔC is the concentration gradient for the isotope across the membrane and was calculated from the mean concentration of the isotope in each chamber for the sampling period.
Statistical analyses.
Data were analyzed using Student's t-test for paired groups or one-way ANOVA for comparison among groups. Bonferroni's multiple-comparison post hoc tests were used where necessary, and P values of <0.05 were considered to be significant.
RESULTS
Behavioral response to ketamine injection.
Mice treated with ketamine exhibit altered behavior ∼1 min after the ketamine injection, and this behavior can last 30–60 min. We observed accelerated movement with unsteady gait and erected tail, which occurred in all ketamine-injected mice on every occasion. The body weights of ketamine treated mice tended to be lighter than those of control mice and showed statistical significance from weeks 3 to 10 (Fig. 1A). The loss of this trend from weeks 11 to 13 was due more to a reduction in average weight in control mice, as reflected by a dip in the curve through this period. This control result is unexplained as we have generally observed steady weight gain over time in cohorts of control animals in other studies. Although body weight did not decrease significantly at week 12, the bladder weight of ketamine-treated mice was significantly lower than that of control mice (Fig. 1B). Visually, bladders in ketamine-treated mice were contracted, but no obvious edema or other abnormalities were observed. Ureters and kidney also appeared normal with no obvious vesicoureteric reflux and hydronephrosis.
Fig. 1.
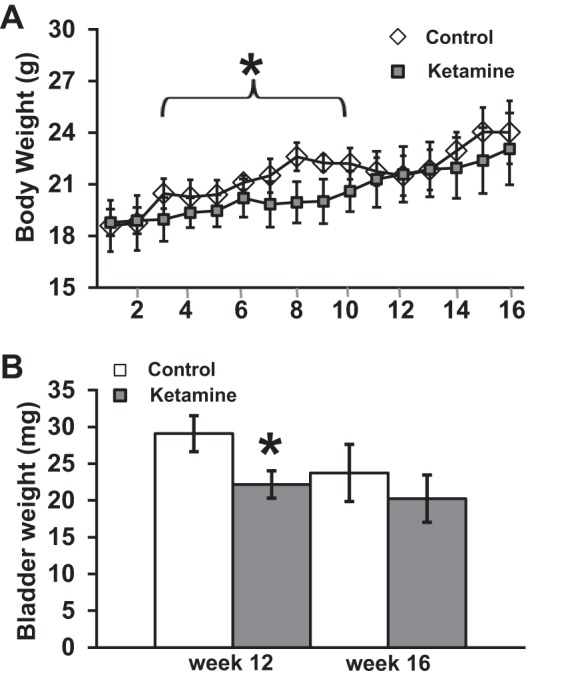
Effect of ketamine treatment on mouse body weight. Mice were injected with PBS or ketamine (30 mg/kg) daily, and their body weights were measured weekly (n = 10 mice/group every week). Ketamine-injected mice tended to have lower body weight, which had statistical significance between 3 and 10 wk (A). Mice were measured (n = 7 mice/group) for their bladder weight at weeks 12 and 16 (after 4 wk of withdrawal from ketamine injection), and the data indicated that ketamine decreased bladder weight at week 12 (B). Error bars are SDs. *P<0.05.
Ketamine treatment induces altered spontaneous voiding patterns.
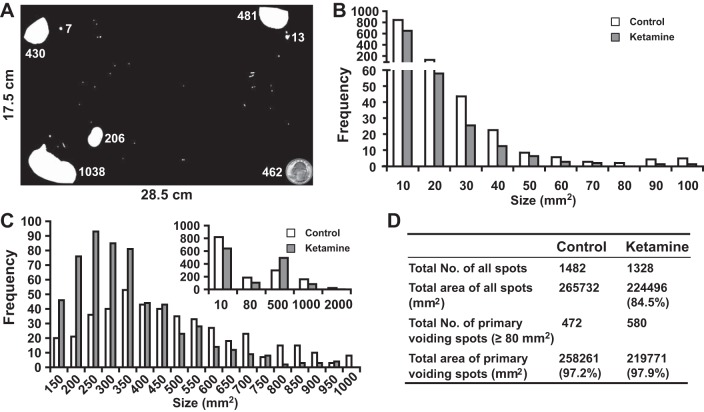
To assess the effect of ketamine on bladder voiding function, spontaneous VSAs were performed weekly. As shown in Fig. 2A, different sizes of urine spots were seen on the filter paper. To extract information from these voiding spots, we grouped all the spots (>1.5 mm2) together from both control and ketamine-treated mice, resulting in 1,482 spots in the control group and 1,328 spots in the ketamine-treated group (Fig. 2D). These spots were then grouped into different sizes, with their frequency distribution showing a distinct pattern (Fig. 2, B and C). As shown in Fig. 2B, the spot number (frequency) decreased as the spot size increased until 80 mm2 was reached in both the control and ketamine-treated groups, and more than half of the spot sizes were <10 mm2. Interestingly, the size frequency chart of voiding spots of >80 mm2 (Fig. 2C and inset) showed a distribution pattern similar to a bell curve (normal distribution), with control group peaks at ∼350 mm2 and ketamine-treated group peaks at ∼250 mm2. We thus labeled the spots having a size of >80 mm2 as primary voiding spots (PVSs) and spots having a size of <80 mm2 as noise (see the discussion for justification). Although there were a large number of spots of <80 mm2, their combined surface area only occupied 2–3% of the total voiding area (Fig. 2D). When we compared the two groups, we observed that the size of PVSs in the ketamine-treated group ranged between 100 and 1,000 mm2, but the control group PVS size range was broader and could reach up to 2,000 mm2 (Fig. 2C), suggesting an altered voiding pattern in ketamine-treated mice.
Fig. 2.
Voiding spot assay. All voiding spots from control (n = 10) and ketamine (n = 10)-treated groups taken between weeks 2 and 12 were imaged and measured for their spot size/area (in mm2). Spot areas ranged from 1.5 to 2,250 mm2. Spots having an area of <1.5 mm2 were discarded. A: representative filter paper with dimensions of 28.5 × 17.5 cm. The white areas on the filter paper are voiding spots, and the adjacent number indicates the spot area (in mm2). The quarter dollar coin at the bottom right corner has a surface area of 462 mm2, which provides a comparison with the actual size of the voiding spots. All spots were then sorted according to their size. B: frequency distribution chart of all voiding spots with a size of <100 mm2. The x-axis indicates the size of the voiding spots, and the number below the axis indicates the spot size range. For example, 10 indicates a spot size range from 1.5 (low threshold) to 10 mm2 and 20 indicates a spot size range from 10 to 20 mm2 in this chart. We noticed that many spots were <10 mm2, and the frequency of spot size decayed gradually with the increase of size until it reached 0 or near 0 at a spot size of 70–80 mm2. At a spot size of larger than 80 mm2, the frequency increased. C: frequency distribution chart of all voiding spots with a size ranging from 100 to 1,000 mm2. The x-axis indicates the size of the voiding spots, and the number below the axis indicates the spot size range. For example, 150 indicates a spot size range from 101 to 150 mm2 and 200 indicates a spot size range from 151 to 200 mm2. Please note that the x-axis of the inset chart has a different scale, for example, 500 indicates a spot size ranging from 81 to 500 mm2. Voiding spots from the ketamine-treated group were more clustered around the size of 201–350 mm2, whereas the range of voiding spots from the control group was broader. The inset in C shows an overall frequency distribution chart of all voiding spots (from 1.5 to 2,250 mm2). According to these frequency distribution charts, we attributed voiding spot sizes of >80 mm2 as primary voiding spot (PVSs), as shown in D, which accounted for ∼98% of the total urine area. It was also clear that ketamine-treated group had more PVS numbers, indicating voiding frequency.
PVS were further compared between control and ketamine-treated groups (Fig. 3). Overall, the ketamine-treated group had significantly more PVS numbers per filter paper than the control group (Fig. 3A), indicating that ketamine-treated mice void more frequently. When we compared the number of PVSs along the time course, the ketamine-treated group generally had consistently more PVS numbers than control mice, although these differences did not reach statistical significance (Fig. 3C). The size of PVSs in the ketamine-treated group was significantly smaller than the control group (Fig. 3B), and this difference was maintained throughout the time course. Ketamine-treated mice showed significantly smaller PVSs even at the second week of treatment, indicating a rapid onset of voiding dysfunction (Fig. 3D).
Fig. 3.
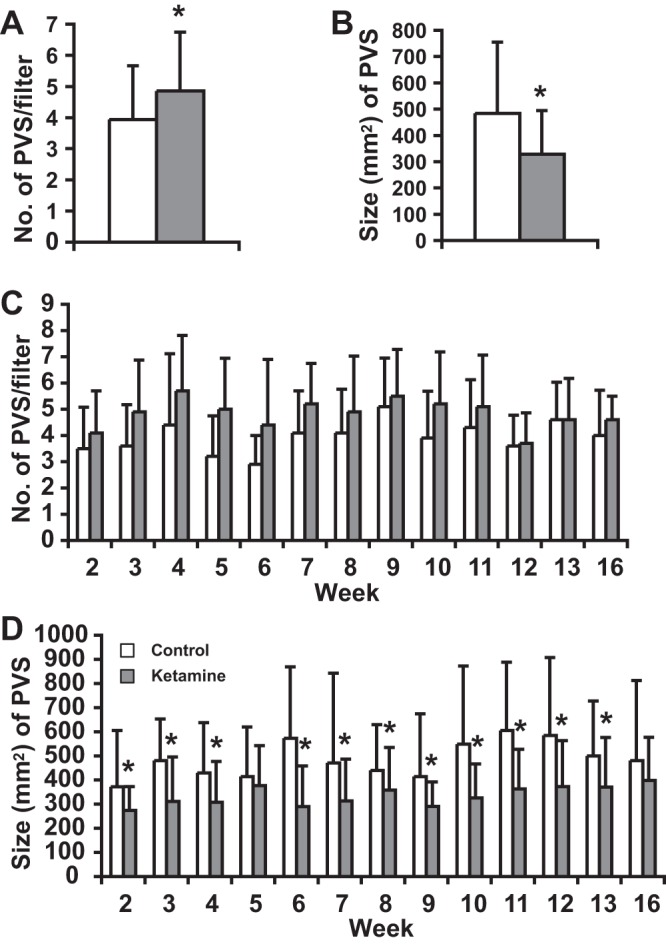
Ketamine treatment induces an altered voiding pattern. Overall, the ketamine-treated group (combined week 2–12 data) had significantly more PVS numbers per filter paper than the control group (A) but had significant smaller sizes of PVSs than the control group (B). Along the weekly time course, the ketamine-treated group consistently yielded a higher PVS number but lacked statistical significance, as shown in C. Ketamine induced a significantly smaller PVS size, even at week 2, as shown in D. The number of filter papers for A and B was 110 papers; the filter paper number for C and D was 10 papers/wk except for weeks 13 and 16, for which the number was 5 papers/wk. Error bars are SDs. *P < 0.05.
Ketamine treatment induces altered voiding dynamics.
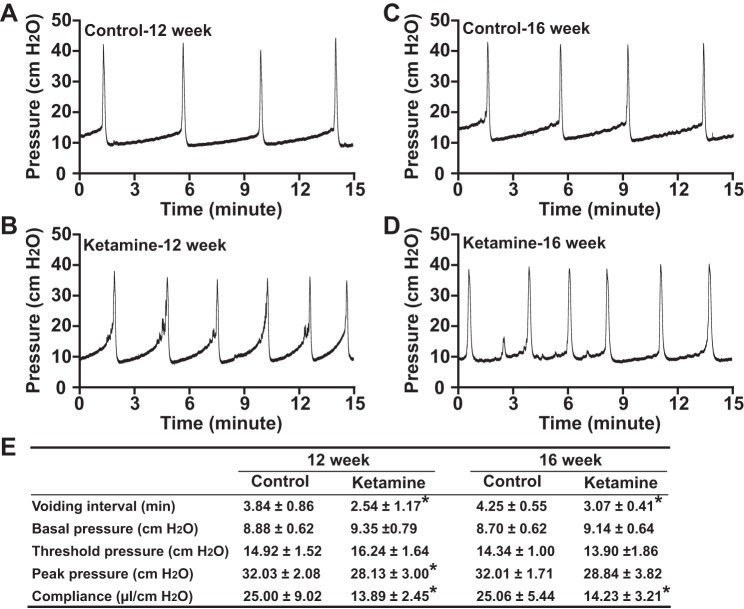
To further confirm that ketamine-induced voiding dysfunction is due to altered bladder function but not due to reduced urine generation, we performed CMGs on anesthetized mice. The results indicated that ketamine treatment increased bladder hyperactivity with increased voiding frequency. As shown in Fig. 4, control mice had voiding intervals of ∼4 min, which was an ∼100 μl urine volume/void (4 min × 25 μl/min infusion rate = 100 μl) and was consistent with the PVS size peak at ∼350 mm2 (350 mm2 × 0.283 μl/mm2 = 99 μl). In ketamine-treated mice, voiding intervals decreased to <3 min (3 min × 25 μl/min infusion rate = 75 μl), which was significantly shorter than the control group and consistent with the PVS peak at ∼250 mm2 (250 mm2 × 0.283 μl/mm2 = 71 μl; Fig. 4, A, B, and E). Even after 4 wk of withdrawal from ketamine, the voiding interval was still significantly shorter than that of control mice, indicating that injection of ketamine for 12 wk induced substantial injuries to the bladder, which cannot be fully recovered after 4 wk of ketamine withdrawal (Fig. 4, C–E). In addition to the shortened voiding interval, ketamine-treated mouse bladders also exhibited significantly lower compliance (Fig. 4E), indicating an overactive bladder. This phenomenon is consistent with human ketamine cystitis patients for whom contracted bladders were the typical symptoms. Interestingly, ketamine-treated mouse bladders had lower peak pressures (Fig. 4E), which might be due to a smaller voiding volume in these mice.
Fig. 4.
Cystometrograms for control and ketamine-treated mice. Representative cystometrograms from control (A and C) and ketamine-treated (B and D) groups are shown. Control mice: n = 5 at 12 wk and n = 7 at 16 wk; ketamine-treated mice: n = 7 at 12 wk and n = 4 at 16 wk. Repeatable voiding cycles were analyzed, and the change of voiding interval (time between peak pressure), basal pressure (minimum pressure after voiding), threshold pressure (pressure before voiding contraction), peak pressure (maximum voiding pressure minus basal pressure), compliance [volume (in μl) required for per unit cmH2O pressure increase] are shown in E. Ketamine decreased the voiding interval significantly at both 12- and 16-wk (ketamine withdrawal for 4 wk) time points; ketamine-treated mouse bladders also had significant less compliance and peak pressure. *P < 0.05.
Altered urothelial permeability is not observed in ketamine-treated bladders.
We next examined whether the altered voiding patterns and urodynamic responses were due to impaired urothelial function. Therefore, we measured transepithelial resistance, water permeability, and urea permeability in Ussing chambers. Our results indicated that 12 wk of ketamine treatment did not change urothelial barrier function, and no significant changes in urothelial transepithelial resistance, water permeability, and urea permeability were detected (Table 1).
Table 1.
Altered urothelial permeability was not observed in ketamine-treated mouse bladders
| Water Permeability, cm/s × 10−5 |
Urea Permeability, cm/s × 10−6 |
||||||
|---|---|---|---|---|---|---|---|
| Transepithelial resistance, Ω/cm2 | Without Triton/gramacidin | With Triton/gramacidin | Fold | Without Triton/gramacidin | With Triton/gramacidin | Fold | |
| 12 wk | |||||||
| Control group | 4,437 ± 665 | 2.5 ± 0.8 | 4.1 ± 3.4 | 1.5 | 11.2 ± 4.7 | 20.2 ± 21. 1 | 1.5 |
| Ketamine-treated group | 4,006 ± 220 | 3.2 ± 1.6 | 4.3 ± 1.8 | 1.4 | 14.3 ± 6.2 | 23.0 ± 13.9 | 1.7 |
| 16 wk | |||||||
| Control group | 3,712 ± 327 | 2.6 ± 1.1 | 5.1 ± 4.1 | 1.8 | 9.9 ± 7.2 | 22.8 ± 18.3 | 2.5 |
| Ketamine-treated group | 3,100 ± 216 | 3.9 ± 1.4 | 5.8 ± 1.8 | 1.6 | 18.6 ± 8.7 | 38.7 ± 13.3 | 2.2 |
Values are means ± SD; n = 4 mice/group.
The urothelial ultrastructure is not altered with ketamine treatment.
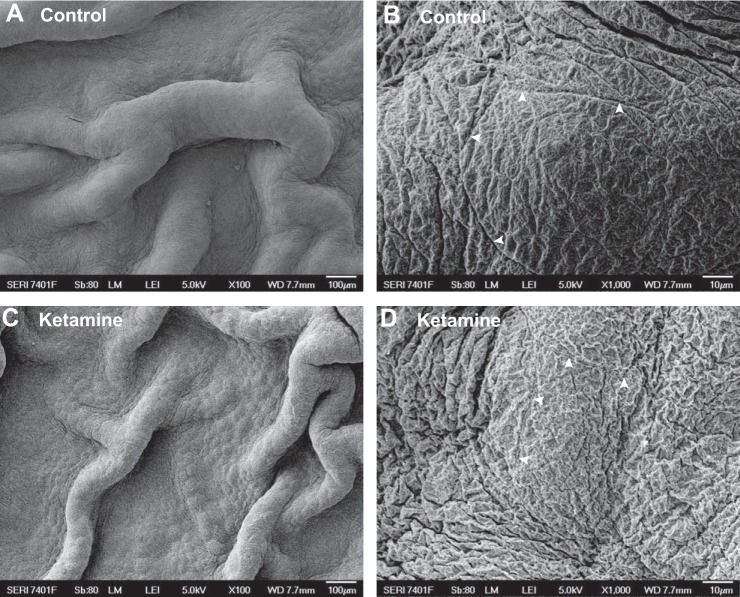
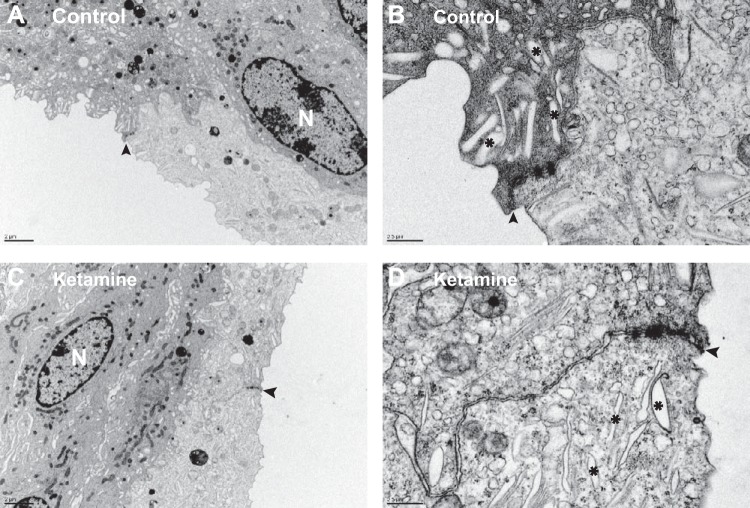
Umbrella cell apical membranes, tight junctions, and intracellular vesicles play crucial roles in urothelial barrier function. To visualize whether ketamine treatment damages these structures, SEM, TEM, immunostaining, and confocal microscopy imaging were performed. SEM images showed that an intact mucosal surface was observed in both control and ketamine-treated bladders, and no denuded surface or detached urothelium was seen (Fig. 5, A and C). At high magnification, a distinct umbrella apical membrane with a tight junction ring could be identified in both control and ketamine-treated bladders (Fig. 5, B and D). Furthermore, TEM experiments found large flat umbrella cells with scalloped apical membranes (Fig. 6, A and C). Comparable numbers of intracellular vesicles and well-defined tight junctions were also preserved in ketamine-treated umbrella cells (Fig. 6, B and D). These data are consistent with our permeability evaluation and indicate that urothelial barrier function is not impaired in ketamine-treated mice bladders.
Fig. 5.
Scanning electron microscopy imaging of the mouse bladder mucosal surface appeared normal in both control (A and B) and ketamine-treated (C and D) groups. Arrowheads indicate tight junction structures between umbrella cells.
Fig. 6.
Transmission electron microscopy imaging of mouse bladder umbrella cells appeared normal in both control (A and B) and ketamine-treated (C and D) groups. N indicates nuclei. Arrowheads indicate tight junction structures between umbrella cells. *Intracellular vesicles.
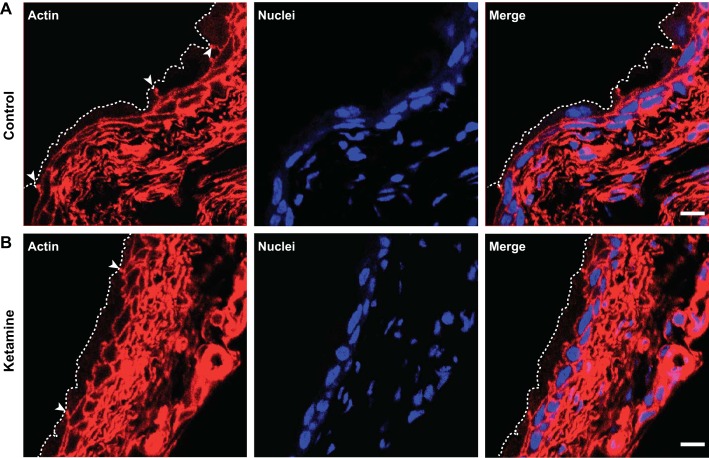
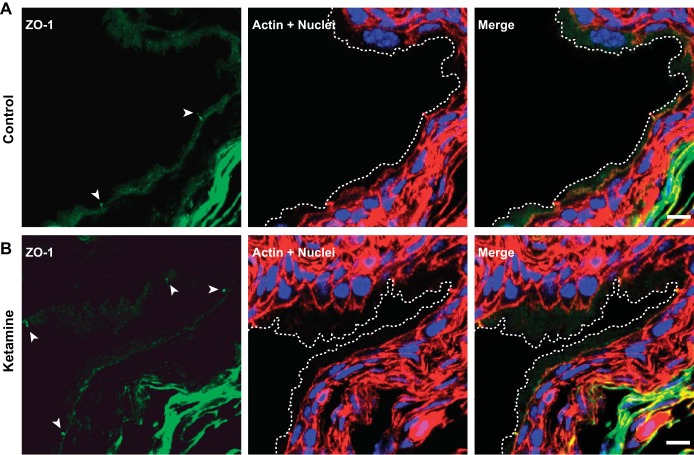
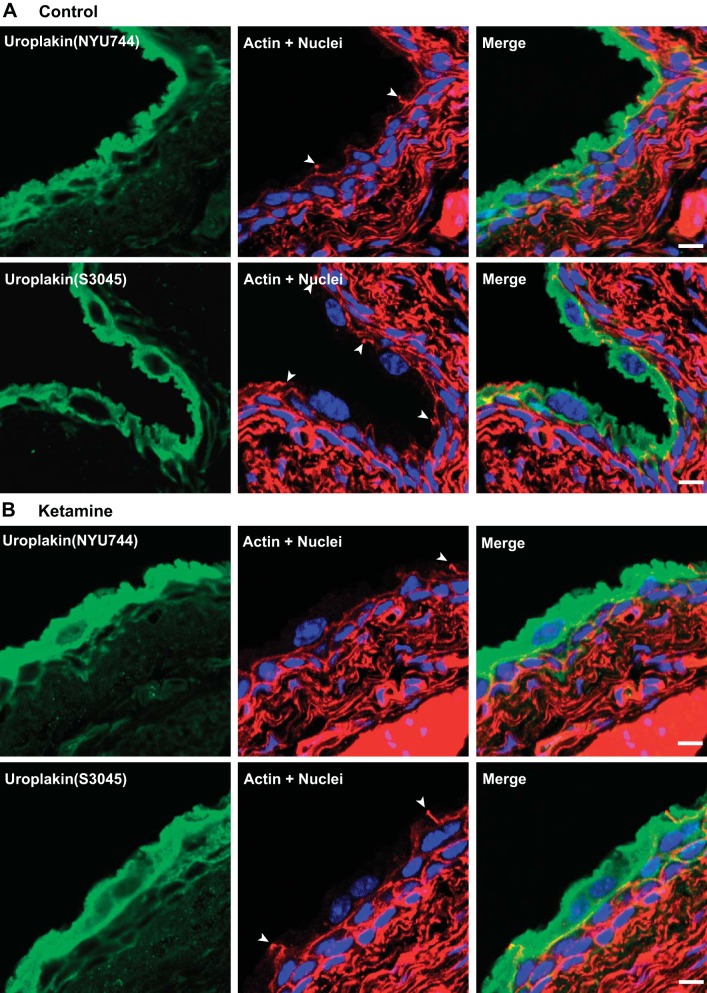
Immunostaining and confocal microscopy were also performed to visualize possible effects of ketamine treatment on umbrella cells. Actin staining confirmed the presence of intact and large flat umbrella cells located on top of small intermediate urothelial cells in both control and ketamine-treated groups (Fig. 7). ZO-1, a major structural protein of tight junctions, was also appropriately expressed in umbrella cells of ketamine-treated bladders (Fig. 8). Uroplakin, a urothelium-specific protein that has been denoted as a marker for the differentiation of mature umbrella cells, was highly expressed in umbrella cells in ketamine-treated bladders (Fig. 9). In summary, these data explicitly indicate that fully differentiated umbrella cells and intact tight junctions were maintained in ketamine-treated bladders and that barrier function was unaffected.
Fig. 7.
Intact umbrella cells are present in both control and ketamine-treated mouse bladders. Cryosections of bladders from control and ketamine-treated groups were labeled with rhodamine phalloidin for the actin cytoskeleton (red) and Topro-3 for nuclei (blue). Images show large mature umbrella cells in both groups. Arrowheads indicate tight junctions of umbrella cells. Dashed lines indicate the umbrella cell apical membrane. Scale bars = 10 μm.
Fig. 8.
Zonula occludens (ZO)-1 is expressed in umbrella cell tight junction in both control and ketamine-treated mouse bladders. Cryosections of bladders from control and ketamine-treated groups were labeled with anti-ZO-1 antibody (green) and rhodamine phalloidin for the actin cytoskeleton (red) and Topro-3 for nuclei (blue). Images show ZO-1 antibody-labeled tight junction structures (arrowheads), which colocalized with actin staining. Dashed lines indicate the umbrella cell apical membrane. Scale bars = 10 μm.
Fig. 9.
Uroplakin is strongly expressed in umbrella cells in both control and ketamine-treated mouse bladders. Cryosections of bladders from control and ketamine-treated groups were labeled with anti-uroplakin antibodies (green) and rhodamine phalloidin for the actin cytoskeleton (red) and Topro-3 for nuclei (blue). Images show strong uroplakin expression in umbrella cells. Arrowheads indicate tight junctions of umbrella cells. Scale bars = 10 μm.
DISCUSSION
Decreased body weight is a common symptom in ketamine abusers (5). In animal models, decreased body weight has also been often reported (8), although in some cases unchanged body weight has also been reported (10, 18). These previous reports are consistent with our observation that ketamine induced decreased body weight, which was significant between 3 and 10 wk in the experiment but not later (Fig. 1A). Consistent with previous reports (10, 18) on the effect of ketamine on bladder weight, we noticed decreased bladder weight with ketamine injection (Fig. 1B). Furthermore, ketamine-injected mice produced less urine volume (∼85% of control mice; Fig. 2D), and this could be due to decreased body weight, reduced water intake (10, 18), or possibly impaired kidney function (6, 10, 18, 23). These general indexes suggest that ketamine induces pathogenesis in the mouse lower urinary tract.
The VSA was used to further define ketamine-induced bladder dysfunction. A frequency distribution chart of voiding spot size was generated (Fig. 2). Our VSA results showed a large number of spots below 80 mm2, with the majority 10 mm2 or below, but these comprised a small percentage of the overall area. We believe that these are not genuine voids but rather the result of urine deposition from fur or claws. Above 80 mm2, the number of spots increased and followed a somewhat normal distribution. We thus attribute spots smaller than 80 mm2 as inappropriate for this analysis and regard those above 80 mm2 as PVSs.
Theoretically, one drop of water (or urine) has a volume of ∼50 μl. In our case, 80 mm2 of surface area equals ∼25 μl of urine volume (80/3.5) according to our calculation. In all likelihood, when urine leaves the mouse urethra outlet, the hair around the urethra will inevitably absorb part of the urine, which later on can stain the filter paper when mice move around the cage. Thus, it is reasonable to consider 80 mm2 or 25 μl is the lower limit for a valid voiding behavior. Interestingly, the area and therefore volume percentage of spots of <80 mm2 was relatively constant in both our control and ketamine-treated groups and only occupied 2–3% of the total area (Fig. 2D).
Using this definition of PVS allowed us to quantitatively differentiate the voiding pattern changes between control and ketamine-injected mice, where ketamine-treated mice gave consistently more PVS numbers but smaller PVS sizes (Fig. 3, A and B). This pattern likely indicates bladder hyperactivity and lower capacity, mimicking a characteristic feature of ketamine-induced bladder symptoms in human patients (6, 23). These changes were confirmed by urodynamic data from CMGs (Fig. 4). More interestingly, our time-course data indicate that this voiding dysfunction appeared as early as the second week of ketamine injection (Fig. 3D), an unanticipated finding that broadens our understanding on ketamine cystitis. Previously, ketamine abuse was deemed to be a chronic consequence of long-term use (8). Our results indicate that ketamine causes voiding abnormalities in a relative short period of time. Comparable conclusions have been recently reported in rats. In these studies, ketamine induced a reduced voiding volume after 2 wk (10, 18).
Urothelial barrier function appeared to be intact according to our permeability assays (Table 1) and morphological experiments (Figs. 5–9). The urothelial barrier plays crucial roles in normal bladder function, and recent rat models reported ketamine-impaired urothelial barrier with urothelial inflammation and apoptosis (10, 14, 18, 22, 24). Intriguingly, only ∼50% of human ketamine cystitis patients have denuded urothelial mucosa and ulceration (17). In another mouse study (24), a normal mucosa was also present after ketamine exposure, which is consistent with our findings. Combining the observation that voiding dysfunction could happen as early as the second week of ketamine treatment with no obvious disruption of the urothelial barrier by week 12, these findings strongly argue that ketamine-induced voiding dysfunction is not a direct consequence of damaged barrier function. On the contrary, these data imply that disrupted urothelial barrier function may not be the cause of ketamine cystitis but the consequence of long-term stress caused by ketamine-induced voiding dysfunction, through an as-yet-unknown mechanism. Indeed, we observed that in the ketamine-treated group, week 16 bladders had relatively higher water and urea permeabilities (Table 1), which, although not reaching statistical significance, suggests that an adverse effect on urothelial barrier function might eventuate.
Since our data indicate an early onset of voiding dysfunction, the possibility of an earlier urothelial injury that was then repaired could not formally be ruled out. However, this seems unlikely, because the voiding dysfunction continues with uninterrupted ketamine injection, and ketamine cystitis generally exacerbates with long-term ketamine abuse/injection. Further study will resolve this possibility.
In rat models, ketamine cystitis shows hematuria with long-term ketamine exposure (8); however, we did not observed hematuria in our mouse model, and the bladder dysfunction did not worsen over our time course (Fig. 3, C and D). These differences may be due to both dose and species. In our study, the dose of 30 mg·kg−1·day−1 has been previously shown to induce mouse ketamine cystitis (24). This dose did induce voiding dysfunction, as shown in this and another study (22), yet a higher ketamine concentration and/or longer exposure time might be needed to induce more severe lower urinary tract damage, like hematuria and hydronephrosis, in mice. It has been reported in a rat model that 50 mg·kg−1·day−1 ketamine injection induced hematuria at a 16-wk time point (8). The species differences may also be due to the higher metabolic rate in the mouse over rat (19). Nevertheless, the voiding dysfunction observed in this study, without any functional and morphological changes in the urothelial barrier, suggests that an alternative mechanism is needed to explain the pathogenesis of ketamine cystitis.
Multiple possible mechanisms exist for ketamine cystitis, such as direct microvasculature toxicity, neuronal toxicity, and an autoimmune response. As an anesthetic, ketamine might have a direct effect on nerve fibers, thereby causing voiding dysfunction. For example, nerve hyperplasia has been reported in ketamine cystitis patients (1). The prevalence of inflammation and apoptosis in the ketamine-treated bladder wall also favors the autoimmune response theory. Recently, the urothelium was found to have a mechanosensory function; therefore, another possible pathway is the effect of accumulated ketamine and its metabolite norketamine in the urine on mechanotransduction, which causes bladder overactivity. Further research is required to fully elucidate the underlying mechanisms.
GRANTS
The authors acknowledge funding received from University of Malaya Research Grant RU21/2014 (to T. A. Ong) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-095922 (to W. Yu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.R., T.A.O., A.H.A.R., M.Z., and W.Y. conception and design of research; R.R., B.M., and W.Y. performed experiments; R.R., B.M., and W.Y. analyzed data; R.R., T.A.O., A.H.A.R., B.M., M.Z., and W.Y. interpreted results of experiments; R.R. and W.Y. prepared figures; R.R., T.A.O., A.H.A.R., B.M., M.Z., and W.Y. drafted manuscript; R.R., T.A.O., A.H.A.R., B.M., M.Z., and W.Y. edited and revised manuscript; R.R., T.A.O., A.H.A.R., B.M., M.Z., and W.Y. approved final version of manuscript.
REFERENCES
- 1.Baker SC, Stahlschmidt J, Oxley J, Hinley J, Eardley I, Marsh F, Gillatt D, Fulford S, Southgate J. Nerve hyperplasia: a unique feature of ketamine cystitis. Acta Neuropathol Commun 1: 64, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bokor G, Anderson PD. Ketamine: an update on its abuse. J Pharm Pract 27: 582–586, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Cheung TT, Poon RT, Chan AC, Lo CM. Education and Imaging. Hepatobiliary and pancreatic: cholangiopathy in ketamine user–an emerging new condition. J Gastroenterol Hepatol 29: 1663, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Chiew YW, Yang CS. Disabling frequent urination in a young adult. Ketamine-associated ulcerative cystitis. Kidney Int 76: 123–124, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Chu PS, Kwok SC, Lam KM, Chu TY, Chan SW, Man CW, Ma WK, Chui KL, Yiu MK, Chan YC, Tse ML, Lau FL. “Street ketamine”-associated bladder dysfunction: a report of ten cases. Hong Kong Med J 13: 311–313, 2007. [PubMed] [Google Scholar]
- 6.Chu PS, Ma WK, Wong SC, Chu RW, Cheng CH, Wong S, Tse JM, Lau FL, Yiu MK, Man CW. The destruction of the lower urinary tract by ketamine abuse: a new syndrome? BJU Int 102: 1616–1622, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Colebunders B, Van Erps P. Cystitis due to the use of ketamine as a recreational drug: a case report. J Med Case Rep 2: 219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu D, Huang J, Yin Y, Shan Z, Zheng S, Wu P. Long-term ketamine abuse induces cystitis in rats by impairing the bladder epithelial barrier. Mol Biol Rep 41: 7313–7322, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Ho CC, Pezhman H, Praveen S, Goh EH, Lee BC, Zulkifli MZ, Isa MR. Ketamine-associated ulcerative cystitis: a case report and literature review. Malays J Med Sci 17: 61–65, 2010. [PMC free article] [PubMed] [Google Scholar]
- 10.Juan YS, Lee YL, Long CY, Wong JH, Jang MY, Lu JH, Wu WJ, Huang YS, Chang WC, Chuang SM. Translocation of NF-κB and expression of cyclooxygenase-2 are enhanced by ketamine-induced ulcerative cystitis in rat bladder. Am J Pathol 185: 2269–2285, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Kalsi SS, Wood DM, Dargan PI. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J 4: 7107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanasaki K, Yu W, von Bodungen M, Larigakis JD, Kanasaki M, Ayala de la Pena F, Kalluri R, Hill WG. Loss of β1-integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. FASEB J 27: 1950–1961, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol 283: F242–F253, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Lee CL, Jiang YH, Kuo HC. Increased apoptosis and suburothelial inflammation in patients with ketamine-related cystitis: a comparison with non-ulcerative interstitial cystitis and controls. BJU Int 112: 1156–1162, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Shi J, Yang BF, Liu L, Han CL, Li WM, Dong DL, Pan ZW, Liu GZ, Geng JQ, Sheng L, Tan XY, Sun DH, Gong ZH, Gong YT. Ketamine-induced ventricular structural, sympathetic and electrophysiological remodelling: pathological consequences and protective effects of metoprolol. Br J Pharmacol 165: 1748–1756, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang FX, Riedel I, Deng FM, Zhou G, Xu C, Wu XR, Kong XP, Moll R, Sun TT. Organization of uroplakin subunits: transmembrane topology, pair formation and plaque composition. Biochem J 355: 13–18, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin HC, Lee HS, Chiueh TS, Lin YC, Lin HA, Lin YC, Cha TL, Meng E. Histopathological assessment of inflammation and expression of inflammatory markers in patients with ketamine-induced cystitis. Mol Med Rep 11: 2421–2428, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu KM, Chuang SM, Long CY, Lee YL, Wang CC, Lu MC, Lin RJ, Lu JH, Jang MY, Wu WJ, Ho WT, Juan YS. Ketamine-induced ulcerative cystitis and bladder apoptosis involve oxidative stress mediated by mitochondria and the endoplasmic reticulum. Am J Physiol Renal Physiol 309: F318–F331, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Martignoni M, Groothuis G, de Kanter R. Comparison of mouse and rat cytochrome P450-mediated metabolism in liver and intestine. Drug Metab Dispos 34: 1047–1054, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Ng SH, Tse ML, Ng HW, Lau FL. Emergency department presentation of ketamine abusers in Hong Kong: a review of 233 cases. Hong Kong Med J 16: 6–11, 2010. [PubMed] [Google Scholar]
- 21.Pappachan JM, Raj B, Thomas S, Hanna FW. Multiorgan dysfunction related to chronic ketamine abuse. Proc (Bayl Univ Med Cent) 27: 223–225, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen CH, Wang ST, Lee YR, Liu SY, Li YZ, Wu JD, Chen YJ, Liu YW. Biological effect of ketamine in urothelial cell lines and global gene expression analysis in the bladders of ketamineinjected mice. Mol Med Rep 11: 887–895, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam YH, Ng CF, Pang KK, Yee CH, Chu WC, Leung VY, Wong GL, Wong VW, Chan HL, Lai PB. One-stop clinic for ketamine-associated uropathy: report on service delivery model, patients' characteristics and non-invasive investigations at baseline by a cross-sectional study in a prospective cohort of 318 teenagers and young adults. BJU Int 114: 754–760, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Tan S, Chan WM, Wai MS, Hui LK, Hui VW, James AE, Yeung LY, Yew DT. Ketamine effects on the urogenital system–changes in the urinary bladder and sperm motility. Microsc Res Tech 74: 1192–1198, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Vieira N, Deng FM, Liang FX, Liao Y, Chang J, Zhou G, Zheng W, Simon JP, Ding M, Wu XR, Romih R, Kreibich G, Sun TT. SNX31: a novel sorting nexin associated with the uroplakin-degrading multivesicular bodies in terminally differentiated urothelial cells. PLoS One 9: e99644, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JW, Kivovich V, Gordon L. Ketamine abuse syndrome: hepatobiliary and urinary pathology among adolescents in Flushing, NY. Pediatr Emerg Care. In press. [DOI] [PubMed] [Google Scholar]
- 27.Wei YB, Yang JR, Yin Z, Guo Q, Liang BL, Zhou KQ. Genitourinary toxicity of ketamine. Hong Kong Med J 19: 341–348, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Winstock AR, Mitcheson L, Gillatt DA, Cottrell AM. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int 110: 1762–1766, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Wong SW, Lee KF, Wong J, Ng WW, Cheung YS, Lai PB. Dilated common bile ducts mimicking choledochal cysts in ketamine abusers. Hong Kong Med J 15: 53–56, 2009. [PubMed] [Google Scholar]
- 30.Wu XR, Lin JH, Walz T, Haner M, Yu J, Aebi U, Sun Mammalian uroplakins TT. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem 269: 13716–13724, 1994. [PubMed] [Google Scholar]
- 31.Wu XR, Manabe M, Yu J, Sun TT. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem 265: 19170–19179, 1990. [PubMed] [Google Scholar]
- 32.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol 306: F1296–F1307, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu W, Hill WG, Apodaca G, Zeidel ML. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am J Physiol Renal Physiol 300: F49–F59, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou G, Liang FX, Romih R, Wang Z, Liao Y, Ghiso J, Luque-Garcia JL, Neubert TA, Kreibich G, Alonso MA, Schaeren-Wiemers N, Sun TT. MAL facilitates the incorporation of exocytic uroplakin-delivering vesicles into the apical membrane of urothelial umbrella cells. Mol Biol Cell 23: 1354–1366, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]