Abstract
Patients and animals with chronic kidney disease (CKD) exhibit profound alterations in the gut environment including shifts in microbial composition, increased fecal pH, and increased blood levels of gut microbe-derived metabolites (xenometabolites). The fermentable dietary fiber high amylose maize-resistant starch type 2 (HAMRS2) has been shown to alter the gut milieu and in CKD rat models leads to markedly improved kidney function. The aim of the present study was to identify specific cecal bacteria and cecal, blood, and urinary metabolites that associate with changes in kidney function to identify potential mechanisms involved with CKD amelioration in response to dietary resistant starch. Male Sprague-Dawley rats with adenine-induced CKD were fed a semipurified low-fiber diet or a high-fiber diet [59% (wt/wt) HAMRS2] for 3 wk (n = 9 rats/group). The cecal microbiome was characterized, and cecal contents, serum, and urine metabolites were analyzed. HAMRS2-fed rats displayed decreased cecal pH, decreased microbial diversity, and an increased Bacteroidetes-to-Firmicutes ratio. Several uremic retention solutes were altered in the cecal contents, serum, and urine, many of which had strong correlations with specific gut bacteria abundances, i.e., serum and urine indoxyl sulfate were reduced by 36% and 66%, respectively, in HAMRS2-fed rats and urine p-cresol was reduced by 47% in HAMRS2-fed rats. Outcomes from this study were coincident with improvements in kidney function indexes and amelioration of CKD outcomes previously reported for these rats, suggesting an important role for microbial-derived factors and gut microbe metabolism in regulating host kidney function.
Keywords: dietary fiber, resistant starch, chronic kidney disease, uremic retention solutes, gut microbiota
patients and animal models with chronic kidney disease (CKD) exhibit an altered gut microbiota and increased gut permeability (52, 75, 78, 79). These changes in gut milieu may occur for several reasons, including diet (i.e., decreased dietary fiber intake), prolongation of intestinal transit time, and influx of uremic retention solutes (URS) (30) from the systemic circulation into the colonic lumen. URS are metabolites that accumulate in the blood and tissues of CKD patients and animal models due to the decline in the number of functioning nephrons (68, 72). In CKD patients, the concentration of urea in the intestinal fluids is similar to its serum concentration. Urea, at concentrations found in stable end-stage renal disease (ESRD) patients, has been shown to significantly reduce epithelial tight junction proteins in human colonocytes. The damaging effect of urea was greatly amplified in the presence of urease, an enzyme that is abundantly expressed by numerous gut microbial species (77). A previous study by Vaziri et al. (75) has shown marked alterations of the gut microbiome in rats and humans with advanced CKD. The CKD-induced changes in the gut microbiome were characterized by expansion of microbial families possessing genes encoding urease, uricase, indole, and p-cresol metabolizing enzymes and depletion of microbes expressing short-chain fatty acid-forming enzymes (84).
Patients with CKD are often advised to limit their consumption of fiber-rich foods that are high in potassium and phosphorus to prevent cardiac arrhythmias and bone mineral disorders, respectively (47a, 56). This is, however, of potential concern since decreased dietary fiber intake can also increase gut permeability (21, 23, 24), which, in theory, could increase blood exposure to gut-derived URS or other factors that impact kidney function and inflammation. Low-fiber diets promote the growth of bacteria that consume host glycans, leading to degradation of the protective mucus barrier lining of the intestinal epithelia and thereby facilitate translocation of luminal contents, such as bacteria and their noxious products/components, into the intestinal wall and systemic circulation (17, 63, 64, 79). Indeed, patients with CKD often display elevated blood levels of the microbial-modified amino acids indoxyl sulfate and p-cresol sulfate. The rise in these URS is attributed to reduced renal elimination (55); however, it is not known at this time to what extent, if any, other factors such as increased gut permeability, changes in intestinal transport, and/or increased microbial production may also contribute to increased circulating levels. These URS metabolites are known to promote inflammation and increase the risk of cardiovascular disease (6, 41, 73). Interestingly, dietary fibers have been shown to improve kidney function and decrease levels of these potentially harmful metabolites (47, 60).
One dietary fiber shown to decrease nitrogenous and microbial-derived URS is high-amylose maize-resistant starch type 2 (HAMRS2) (60, 74). HAMRS2 is derived from corn that has been naturally selected to contain a higher amylose-to-amylopectin ratio. The linear amylose molecules form granules that partially resist digestion by mammalian enzymes in the small intestine. The remaining ∼60% of undigested HAMRS2 passes into the large intestine, where it can be fermented by microbes (1). In animal models, HAMRS2 increased fecal nitrogen excretion, lowered plasma urea, and altered gut bacterial communities (16, 32, 87). Using the adenine-induced CKD rat model, we recently reported that 3 wk of dietary HAMRS2 (59% by weight of the diet) led to significant improvements in kidney histology and kidney function (74). Based on these observations, we set out to identify specific cecal bacteria and cecal, blood, and urinary metabolites that associate with changes in kidney function to identify potential mechanisms involved with CKD amelioration in response to dietary resistant starch.
MATERIALS AND METHODS
Animals and diets.
Animals, housing conditions, and diets have been previously described (74). Briefly, 10-wk-old male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were fed powdered chow (no. 2020X, Harlan Laboratories) containing 0.7% adenine for 2 wk to induce a CKD-like phenotype. Rats were then randomized to receive semipurified pelleted diets supplemented (59% by weight) with either the rapidly digestible starch amylopectin (low fiber) or HAMRS2 (Hi-Maize 260, Ingredion, Westchester, IL) for 3 wk (n = 9 rats/group). Isocaloric diets were prepared by Harlan Laboratories and had an energy content of 14.5%-66.9%-18.6% protein-carbohydrate-fat, respectively. All animals were provided ad libitium access to food and water. Rats were placed in metabolic cages for 24-h urine collection. On the day of tissue harvest, ad libitum-fed rats were anesthetized [ketamine (50 mg/kg) plus xylazine (4 mg/kg ip)] and euthanized via cardiac exsanguination between ∼08:00–11:00. Blood was collected and left at room temperature for 30 min to clot, and serum was collected, frozen on dry ice, and stored at −70°C until processed. Cecal contents were removed, frozen on dry ice, and stored at −70°C until processed. Hydration of cecal contents was determined by weighing a frozen aliquot and then oven drying overnight at 100°C; the sample was allowed to cool and weighed twice to ensure a constant weight was obtained. All experiments were approved by the Institutional Committee for the Use and Care of Experimental Animals of the University of California (Irvine, CA).
pH of cecal contents.
Cecal contents were thawed on ice, and ∼400 mg of cecal contents were transferred to a clean tube. HPLC-grade water was added at a 10:1 ratio, and contents were homogenized for 2 min at 1,200 rpm on a Geno/Grinder and then centrifuged for 10 min at 4°C at 3,509 g (Sorvall Legend X1R). A Corning 320 pH meter was used to determine pH of the cecal water.
Metabolomics.
Details of this procedure have been previously published for serum or plasma (19). Untargeted primary metabolite analysis (i.e., sugars, amino acids, nucleotides, and their derivatives) of cecal contents, serum, and urine was performed on all nine rats in each group at the West Coast Metabolomics Center (http://metabolomics.ucdavis.edu/) using gas chromotography (GC)-time of flight (TOF)-mass spectroscopy (MS). For serum and urine, 15 μl were added to 1 ml of ice-chilled extraction solution (acetonitrile-isopropanol-water: 3:3:2) and vortexed for 10 s. This same procedure was conducted using an ∼10-mg sample of frozen cecal contents. Samples were centrifuged for 2 min at 14,000 g (no. 5415D, Eppendorf), and 500 μl of supernatant were evaporated (Labconco Centrivap) to complete dryness. For derivitization, 10 μl of methoxyamine hydrochloride (Aldrich) were added to the dried samples. Samples were left on a shaker for 90 min at 30°C, and 91 μl of 100:1 N-methyl-N-(trimethylsilyl)-trifluoroacetamide (Aldrich)-fatty acid methyl ester mixture were then added. Samples were left on shaker for 30 min at 37°C.
Analyses were performed using an Agilent 6890 GC equipped with a Gerstel automatic liner exchange system that includes a multipurpose sample (MPS2) dual rail and a Gerstel CIS cold injection system (Gerstel, Muehlheim, Germany) with a temperature ramp of 50–275°C final temperature at a rate of 12°C/s, which was held for 3 min. Injection volume was 0.5 μl with 10 μl/s injection speed on a splitless injector with purge time of 25 s. The liner (no. 011711-010-00, Gerstel) was changed after every 10 samples. Before and after each injection, the 10-μl injection syringe was washed three times with 10 μl ethyl acetate. GC conditions were as follows: a 30-m-long, 0.25-mm inner diameter Rtx-5Sil MS column (0.25-μm 95% dimethyl-5% diphenyl polysiloxane film) with an additional 10-m integrated guard column was used (Restek, Bellefonte PA). Helium (99.9999% purity) with a builtin purifier (Airgas, Radnor PA) was set at constant flow of 1 ml/min. The oven temperature was held constant at 50°C for 1 min and then ramped at 20°C/min to 330°C, at which time it was held constant for 5 min.
Mass spectrometer settings and data acquisition was as follows: a Leco Pegasus IV time of flight mass spectrometer was used, controlled by Leco ChromaTOF software (version 2.32, St. Joseph, MI). The transfer line temperature between the gas chromatograph and mass spectrometer was set to 280°C. Electron impact ionization at 70 V was used with an ion source temperature of 250°C. The acquisition rate was 17 spectra/s, with a scan mass range of 85–500 Da. The result files were exported to servers and processed by the Fiehn laboratory metabolomics database known as BinBase (20). Database entries in BinBase were matched against the Fiehn mass spectral library of 1,200 authentic metabolite spectra using retention index and mass spectrum information or the NIST05 commercial library. Identified metabolites were reported if present in at least 50% of the samples, regardless of treatment group (as defined in the SetupX database) (58). Each metabolite was normalized by the sum of identified metabolite quantifier ion peak heights present in each individual sample. These relative abundances were used for subsequent statistical analysis. Cecal-derived metabolite abundances were corrected for dry weight by dividing the metabolite abundance by percent dry matter obtained by oven drying as described above.
Cecal microbiota.
Total cecal DNA was extracted by bead beating with 0.1 mm zirconia/silica beads (BioSpec) followed by DNA purification using the QIAamp DNA stool mini kit (Qiagen, Valencia, CA). DNA (20 ng/μl) was used to amplify the V4 region of the 16S rRNA gene with 30 PCR cycles at 94°C for 45 s, 54°C for 60 s, and 72°C for 30 s using barcoded 515 forward (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806 reverse (5′-GGACTACHVGGGTWTCTAAT-3′) primers (12). Equal molar amounts of PCR amplicons were then pooled and gel purified using Wizard SV Gel and PCR clean-up system (Promega, Madison, WI). Sequencing of pooled 250-bp paired-end amplicons was performed with an Illumina MiSeq (San Diego, CA) at the University of California-Davis Genome Center (http://dnatech.genomecenter.ucdavis.edu). Raw Illumina FASTQ files were demultiplexed and quality filtered with Quantitative Insights Into Microbial Ecology (QIIME) software (version 1.8.0) (11). Assembled reads were used for operational taxonomic unit (OTU) picking. OTUs sharing at least 97% nucleotide identity were identified using an open-reference OTU picking process according to a 16S rRNA sequence database (Greengenes, version 13_8) and further analyzed in QIIME.
Statistical analysis.
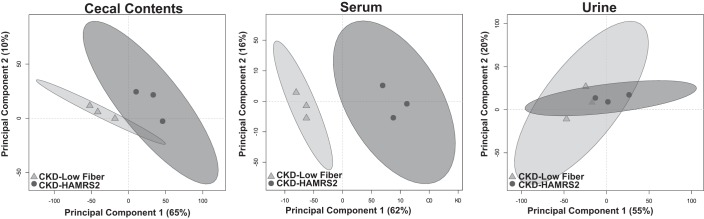
Statistical analyses were performed using GraphPad Prism (version 5.04 for Windows, GraphPad Software, San Diego, CA) or R (version 3.1.2) (51). Diet-related differences in cecal hydration, cecal tissue weight, cecal pH, and number of cecal microbial species were assessed with a two-tailed unpaired Student's t-test. Bacterial percent abundance data expressed as mean percent abundance and group differences were assessed with Mann-Whitney U-tests. Group comparisons of all metabolomics data were assessed by Mann-Whitney U-tests. Results from univariate comparisons were adjusted to account for the false discovery rate using Benjamini and Hochberg (8) and statistical significance was considered at α = 0.05. Multivariate analysis of metabolomics data was performed using partial least squares-discriminate analysis (PLS-DA) from the R package “pls” (45). PLS-DA was used due to its ability to reduce the dimensionality of the data while maximizing the variance between the dependent and exploratory variables. Data used in PLS-DA models were assessed for univariate outliers using Grubb's test with the R package “outliers” (34). Outliers were removed if determined to be significant at α = 0.01. In total, 95 outliers were removed, which accounted for 0.4% of the entire metabolomic data. Removed outliers were imputed using k nearest neighbors from the Bioconducter “impute” package (27). PLS-DA model accuracy was assessed with a cross-validation scheme where the data were randomly partitioned into training and test data sets encompassing 2/3 (n = 6 rats/group) and 1/3 (n = 3 rats/group) of all animals, respectively. Training data were scaled and centered to unit variance before model development, whereas test data were scaled and centered using the means and SDs from the training data. Metabolites of interest were identified with training data using variable importance in projection (VIP) measurements from bootstrapped PLS-DA models. VIP is a weighted measure of the contribution of each metabolite to discriminate the classification groups (low fiber vs. HAMRS2). A VIP score of ≥1 has been argued as an adequate threshold to determine discriminant variables in the PLS-DA model (40, 83); therefore, we used this criterion as a cutoff to assess which metabolites provide discriminate information in the models. Metabolites that had a bootstrapped VIP of ≥1 and an false discovery rate-corrected Mann-Whitney U-test (MWU) P value of ≤0.05 were chosen for inclusion in final PLS-DA models. Model performance was assessed based on the model's ability to accurately predict the classification of the test set animals using data from the training set. Final models were able to predict the classification of the test set animals with 100% accuracy. Variance explaining group classification related to dietary differences were visualized in scores plots using scores from the first two dimensions in PLS-DA models. In these score plots, each symbol represents a single rat and group membership are represented by shape and color. Group confidence regions are represented by ellipses based on 95% confidence intervals determined by Hotelling's T2. Rats whose symbols are closer to one another have a more similar metabolite profile, whereas rats with larger distances from each other have dissimilar metabolite profiles. To illustrate, principle component analysis scores plots from validation test sets are shown in Fig. 1. Metabolites used to generate these plots were the same that were used to generate the PLS-DA scores plots for serum, urine, and cecal metabolites. Principal component analysis is an unsupervised multivariate analysis, meaning that the model is generated without information regarding treatment groups.
Fig. 1.
Principal coordinate analysis score plots of cecal contents, serum, and urine metabolites from male rats with chronic kidney disease (CKD) in the model validation group fed a low fiber diet or high amylose maize-resistant starch type 2 (HAMRS2). Ellipses represent 95% confidence intervals based on Hotelling's T2 statistic, and each symbol represents a rat. Metabolites that contributed to these plots are shown in Tables 2–4. Metabolomic analysis was performed on 9 rats/group, the model was developed using 6 rats/group, and model validation was performed using 3 rats/group.
RESULTS
Our previous publication (74) described kidney function, renal histopathology, renal expression of inflammatory, oxidative, and fibrosis pathway proteins and colonic tight junction protein levels in this cohort of CKD rats. Briefly, supplementation with HAMRS2 markedly improved kidney function and gut permeability indexes, i.e., decreased serum creatinine, increased creatinine clearance, improved tubulointerstitial injury scores, decreased kidney protein levels of inflammatory proteins (i.e., NF-κB, monocyte chemoattractant protein-1, cyclooxygenase-1, and transforming growth factor-β), increased kidney protein levels of endogenous antioxidants (i.e., CuZn SOD, catalase, and glutathione peroxidase), and restored colonic tight junction proteins (occludin and claudin-1). No changes in body weight were observed. The focus of the present research was to explore the effect of HAMRS2 on metabolite and microbiome patterns and their possible association with the observed improvements in kidney function in rats with CKD.
HAMRS2 significantly altered cecal milieu and microbiota.
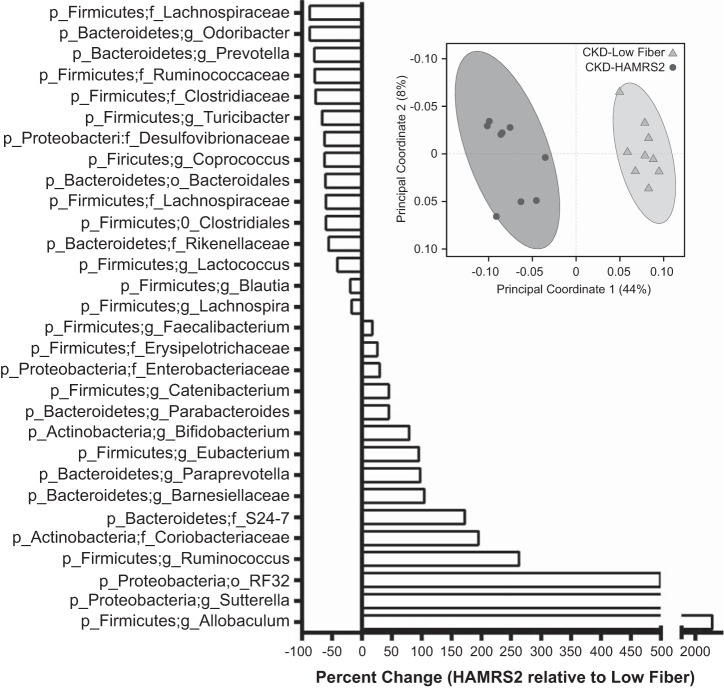
Cecal characteristics and a bacterial phyla summary are shown in Table 1. The cecal contents of HAMRS2-supplemented rats were significantly more hydrated than low fiber-fed rats; this is consistent with fecal hydration data previously reported for these animals (74). Cecal tissue weight was significantly greater and cecal content pH was significantly reduced in HAMRS2-fed rats. Rats that consumed HAMRS2 harbored a distinct cecal microbiota compared with those fed the low-fiber diet, as indicated by principal coordinate analysis of the unweighted UniFrac metric (42), for which 44% of the variation in bacterial composition could be explained by the inclusion of HAMRS2 in the diet (see separation along the PC1 dimension; Fig. 2A). There were, on average, 15% fewer observed bacterial species in the cecum of the HAMRS2-fed group. Detailed bacterial abundance data can be found in Supplemental Table S1.1 The relative abundances of Actinobacteria and Proteobacteria were significantly greater and that of Firmicutes was significantly reduced in HAMRS2-fed rats compared with low fiber-fed rats. HAMRS2-fed rat ceca contained a 66% higher ratio of Bacteroidetes to Firmicutes compared with the low fiber-fed rats despite no significant difference in the proportions of Bacteroidetes between groups (Table 1).
Table 1.
Cecal characteristics and bacterial phyla distribution in male CKD rats fed a low fiber diet or HAMRS2
| Variable | CKD Low Fiber | CKD HAMRS2 | P Value |
|---|---|---|---|
| Percent dry matter of cecal contents | 23.1% ± 0.75 | 19.7% ± 0.95 | 0.011 |
| Cecal weight (g) | 0.7343 ± 0.053 | 1.937 ± 0.098 | <0.0001 |
| Cecal pH | 8.09 ± 0.05 | 6.75 ± 0.16 | <0.0001 |
| Number of observed microbial species in cecum | 791 ± 4 | 673 ± 12 | <0.0001 |
| Phylum | CKD Low Fiber | CKD HAMRS2 | FDR P Value |
|---|---|---|---|
| Unassigned | 0.43% ± 0.06 | 0.66% ± 0.35 | 0.6261 |
| Actinobacteria | 0.73% ± 0.05 | 1.34% ± 0.18 | 0.0086 |
| Bacteroidetes | 19.36% ± 2.82 | 27.49% ± 1.98 | 0.1094 |
| Firmicutes | 77.32% ± 2.72 | 64.77% ± 2.58 | 0.0181 |
| Proteobacteria | 1.51% ± 0.07 | 4.73% ± 0.61 | 0.0027 |
| Tenericutes | 0.58% ± 0.16 | 0.98% ± 0.65 | 0.6831 |
| Verrumicrobia | 0.07% ± 0.04 | 0.04% ± 0.004 | 0.6831 |
| Bacteroidetes-to-Firmicutes ratio | 0.26 ± 0.05 | 0.44 ± 0.05 | 0.02 |
Values are means ± SE; n = 9 rats/group. CKD, chronic kidney disease; HAMRS2, high amylose maize-resistant starch type 2; FDR, false discovery rate.
Fig. 2.
A: unweighted UniFrac beta-diversity principal coordinate analysis plot displays separation between treatment groups based on the cecal microbiota of male CKD rats fed a low-fiber diet or HAMRS2. Axes represent percentage of the variance that can be accounted for based on the cecal microbiota profile. Ellipses represent 95% confidence intervals based on Hotelling's T2 statistic, and each symbol represents a rat. n = 9 rats/group. B: percent change of select cecal bacteria in CKD HAMRS2-fed rats relative to CKD low fiber-fed rats. Bacteria included had a minimum of 0.05% mean abundance in each group and P values of ≤0.05. Bacteria are listed to the lowest level of classification (i.e., if the last taxon assignment is f_, family is the lowest level of classification; p_ is phylum, o_ is order, and g_ is genus). n = 9 rats/group
Percent changes of select bacteria in HAMRS2-fed CKD rats relative to low fiber-fed CKD control rats are shown in Fig. 2B. Within the Actinobacteria phylum, proportions of the genus Bifidobacterium were significantly greater in HAMRS2-fed rats, which had an average relative abundance of 1.11% compared with low fiber-fed rats, which had 0.62% (P = 0.002). Within the Bacteroidetes phylum, proportions of the family Barnesiellaceae were greater in HAMRS2-fed rats compared with low fiber-fed rats, at 0.30% versus 0.15%, respectively (P = 0.0003). HAMRS2-fed rats also had greater proportions of the Bacteroidetes family S24-7 compared with the low fiber-fed group, with 9.75% and 3.58% abundance, respectively (P = 0.0003). Conversely, the Bacteroidetes genus Prevotella was reduced in HAMRS2-fed rats compared with low fiber-fed rats, with 0.11% and 0.05%, respectively (P = 0.0004). Although the total numbers of bacteria within the Firmicutes phylum declined with HAMRS2 consumption, there were strikingly higher proportions of bacteria within the genus Allobaculum, with 0.56% relative abundance in low fiber-fed rats and 15.43% in HAMRS2-fed rats (P = 0.003). Similarly, Faecalibacterium also had greater proportions in the HAMRS2-fed group with 0.84% compared with 0.71% in the low fiber-fed group (P < 0.05). Ruminococcus, another genus within Firmicutes, was higher in the HAMRS2-fed group with 21.27% compared with 5.86% in the low fiber-fed group (P = 0.0003). The following Proteobacteria proportions were significantly enriched in rats fed HAMRS2: order RF32 (Alphaproteobacteria), genus Sutterella (Betaproteobacteria), family Enterobacteraceae (Gammaproteobacteria). There was a significant reduction in the family Desulfovibrionaceae (Deltaproteobacteria) in HAMRS2-fed rats compared with low fiber-fed rats.
HAMRS2 significantly alters cecal, serum, and urine metabolite profiles.
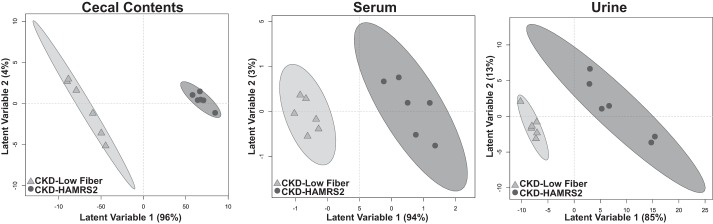
PLS-DA score plots for metabolomics results in the cecal contents, serum, and urine of rats used for statistical model generation are shown in Fig. 3. In these plots, it is readily apparent that variance in selected metabolites can discriminate rats in the low fiber-fed group compared with the HAMRS2-fed group. Annotated metabolites that were featured in PLS-DA models are shown in Tables 2–4 (note that nonannotated metabolites are not listed, for brevity). Percent changes in URS in cecal contents, serum, and urine in male CKD rats fed HAMRS2 relative to low fiber-fed CKD control rats are shown in Table 5. A general pattern of metabolite changes in the cecal contents, serum, and urine is shown in Table 6. All of the metabolomic data, including nonannotated and annotated metabolites identified in cecal contents, serum, and urine, can be found in Supplemental Table S2.
Fig. 3.
Partial least squares-discriminant analysis scores plots based on serum, urine, and cecal metabolites of male CKD rats fed a low fiber diet or HAMRS2. Ellipses represent 95% confidence intervals based on Hotelling's T2 statistic, and each symbol represents a rat. Metabolites that contributed to these plots are shown in Tables 2–4. Metabolomic analysis was performed on 9 rats/group, the model was developed using 6 rats/group, and model validation was performed using 3 rats/group. QIPH, quantifier ion peak height.
Table 2.
Cecal metabolite characteristics of male rats with CKD fed a low-fiber or HAMRS2-supplemented diet
| Metabolite | CKD Low Fiber | CKD HAMRS2 | Percent Change | P Value | VIP |
|---|---|---|---|---|---|
| Amino acids | |||||
| Serine | 43,444.3 ± 2,742.1 | 33,057.7 ± 6,846.4 | −24 | 0.02 | 1.16 |
| Valine | 225,602.4 ± 12,889.2 | 152,212.7 ± 33,535.6 | −33 | 0.02 | 1.09 |
| Lysine | 149,830.3 ± 19,852.5 | 100,913 ± 27,205.2 | −33 | 0.02 | 1.01 |
| Methionine | 23,834.4 ± 2,557.4 | 12,149.7 ± 3,021.5 | −49 | 0.012 | 1.14 |
| Aspartic acid | 264,657.2 ± 48,166.6 | 128,401.8 ± 27,873.9 | −51 | 0.012 | 1.04 |
| Isoleucine | 111,782.2 ± 9,227.6 | 49,476.5 ± 8,454.1 | −56 | <0.0001 | 1.19 |
| Phenylalanine | 50,456.3 ± 5,242.2 | 20,266.7 ± 5,402.5 | −60 | 0.002 | 1.00 |
| Leucine | 206,390.4 ± 21,887 | 46,656.4 ± 11,976.8 | −77 | <0.0001 | 1.15 |
| Other nitrogenous metabolites | |||||
| Glycylproline | 4,677.5 ± 444 | 3,800.3 ± 2,659.7 | −19 | 0.01 | 1.26 |
| Oxoproline | 241,076.5 ± 27,920.3 | 114,907.7 ± 16,551.6 | −52 | 0.002 | 1.08 |
| Creatinine | 2,290.5 ± 363.9 | 1,087.2 ± 228.4 | −53 | 0.007 | 1.08 |
| Trans-4-hydroxy-l-proline | 2,060.6 ± 368 | 855.7 ± 122.6 | −58 | 0.002 | 1.01 |
| Methionine sulfoxide | 13,339.5 ± 1,135.3 | 5,522.4 ± 686.9 | −59 | <0.0001 | 1.23 |
| Maleimide | 3,121.1 ± 220.5 | 1,167.6 ± 153.6 | −63 | <0.0001 | 1.26 |
| Adenosine-5-monophosphate | 2,765.8 ± 414.1 | 1,011.3 ± 309.9 | −63 | 0.002 | 1.09 |
| Ethanolamine | 31,963.1 ± 4,555.3 | 11,460.5 ± 1,778.1 | −64 | 0.002 | 1.12 |
| Pyrrole-2-carboxylic acid | 2,322.4 ± 265.7 | 823.5 ± 63.4 | −65 | <0.0001 | 1.11 |
| N-acetyl-d-hexosamine | 7,645.4 ± 717.3 | 2,535.4 ± 557.8 | −67 | <0.0001 | 1.18 |
| N-acetylglutamate | 2,451.4 ± 492.3 | 807.3 ± 266.3 | −67 | 0.005 | 1.06 |
| 5-Deoxy-5-methylthioadenosine | 2,646.7 ± 361.5 | 843.5 ± 88.5 | −68 | <0.0001 | 1.19 |
| Nicotinic acid | 48,218.4 ± 5,940.8 | 1,5342.4 ± 3685.3 | −68 | <0.0001 | 1.03 |
| Phosphoethanolamine | 1,148.8 ± 147.8 | 362.9 ± 77.6 | −68 | 0.002 | 1.11 |
| Hydroxycarbamate | 1,743.1 ± 161.5 | 529 ± 78.7 | −70 | <0.0001 | 1.21 |
| 4-Pyridoxic acid | 1,281.1 ± 261 | 387.9 ± 58.4 | −70 | <0.0001 | 1.11 |
| 5-Methoxytryptamine | 13,583 ± 1,989.7 | 3,921.6 ± 552 | −71 | <0.0001 | 1.12 |
| Indole-3-lactate | 5,236.1 ± 768.7 | 1,482.7 ± 408.7 | −72 | <0.0001 | 1.05 |
| ϵ-Caprolactam | 7,419.9 ± 836.4 | 1,919.6 ± 267.9 | −74 | <0.0001 | 1.22 |
| UDP-N-acetylglucosamine | 1,528.6 ± 459.7 | 395.2 ± 86.1 | −74 | 0.002 | 1.08 |
| Hydroxylamine | 11,307 ± 1,514.3 | 2,871 ± 352 | −75 | <0.0001 | 1.19 |
| Urea | 6,541.4 ± 795.6 | 1,373.9 ± 138 | −79 | <0.0001 | 1.21 |
| Uracil | 45,220.5 ± 4,289.8 | 8,969.8 ± 1,785.4 | −80 | <0.0001 | 1.19 |
| Xanthine | 10,992.1 ± 1,241.4 | 2,177.1 ± 4,61.3 | −80 | <0.0001 | 1.17 |
| N-acetylgalactosamine | 12,823 ± 1,604.4 | 2,448.7 ± 420.7 | −81 | <0.0001 | 1.22 |
| 5-Hydroxy-3-indoleacetic acid | 3,188.8 ± 672.8 | 5,38.7 ± 49.5 | −83 | <0.0001 | 1.19 |
| Uridine | 14,453.6 ± 2,591.4 | 2,220.9 ± 785.9 | −85 | <0.0001 | 1.19 |
| N-acetylmannosamine | 3,326.3 ± 1,196.1 | 508.8 ± 79.3 | −85 | <0.0001 | 1.16 |
| Uric acid | 2,105.1 ± 310.4 | 313.2 ± 70.2 | −85 | <0.0001 | 1.22 |
| Pseudo-uridine | 5,208.8 ± 844.3 | 754.2 ± 204.9 | −86 | <0.0001 | 1.15 |
| Thymidine | 5,173.3 ± 955.2 | 705.7 ± 309.7 | −86 | <0.0001 | 1.22 |
| N-acetylaspartic acid | 14,061.1 ± 1,755.9 | 1,590.1 ± 350.6 | −89 | <0.0001 | 1.23 |
| Inosine | 46,887 ± 1,0785.8 | 3,185.4 ± 1,122.9 | −93 | <0.0001 | 1.13 |
| Lipids | |||||
| Nonadecanoic acid | 3,610.6 ± 358.3 | 1,626.2 ± 169.3 | −55 | 0.002 | 1.13 |
| Cholesterol | 3,972.7 ± 3735.8 | 14,822.3 ± 2,475.1 | −62 | <0.0001 | 1.06 |
| Myristic acid | 32,541.3 ± 4,157.9 | 11,530.2 ± 1,072.1 | −65 | <0.0001 | 1.21 |
| Heptadecanoic acid | 26,909.5 ± 3,680.6 | 9,442 ± 869.3 | −65 | <0.0001 | 1.20 |
| β-Sitosterol | 110,165 ± 15,754.3 | 38,054.2 ± 3,766.6 | −65 | <0.0001 | 1.09 |
| Octadecanol | 5,076 ± 703.4 | 1,745.5 ± 486 | −66 | 0.005 | 1.09 |
| Isoheptadecanoic acid | 16,336 ± 1,949 | 4,922 ± 1,491.5 | −70 | 0.002 | 1.21 |
| Capric acid | 1,806.5 ± 195.4 | 515.8 ± 83.9 | −71 | <0.0001 | 1.24 |
| Stearic acid | 1,196,670.9 ± 174,737.5 | 340,323.5 ± 23,564.8 | −72 | <0.0001 | 1.25 |
| Cerotinic acid | 2,312.2 ± 368.4 | 643.1 ± 75.6 | −72 | <0.0001 | 1.16 |
| Palmitic acid | 167,954.1 ± 27,149 | 46,428 ± 3,412.1 | −72 | <0.0001 | 1.23 |
| 2,4-Hexadienedioic acid | 1,316.5 ± 169.4 | 322.3 ± 53.8 | −76 | <0.0001 | 1.22 |
| Arachidic acid | 60,200.6 ± 10,426.6 | 14,142.7 ± 1,374.3 | −77 | <0.0001 | 1.18 |
| Cholestan-3-ol | 24,019.1 ± 3,967.6 | 5,422.7 ± 539 | −77 | <0.0001 | 1.15 |
| Linoleic acid | 13,161 ± 3,851.3 | 2,791.5 ± 543.5 | −79 | 0.002 | 1.01 |
| Stigmasterol | 11,333.5 ± 1,508 | 2,304.3 ± 219.8 | −80 | <0.0001 | 1.20 |
| Lauric acid | 20,617.6 ± 2,496.1 | 3,380.4 ± 635.3 | −84 | <0.0001 | 1.22 |
| Phytol | 3,508.9 ± 539.8 | 4,79.7 ± 71.1 | −86 | <0.0001 | 1.15 |
| Carbohydrates | |||||
| Maltose | 564,929.4 ± 86,191.8 | 3,231,747.1 ± 192,336 | 472 | <0.0001 | 1.20 |
| Lactose | 12,348.6 ± 3,868.6 | 60,782.5 ± 11,649.2 | 392 | <0.0001 | 1.00 |
| Ribose | 161,964.7 ± 22,717.2 | 60,156.3 ± 11,073.9 | −63 | <0.0001 | 1.01 |
| 6-Deoxyglucose | 35,299.7 ± 4,108.1 | 11,454.5 ± 3,485.9 | −68 | 0.002 | 1.06 |
| 3,6-Anhydro-d-galactose | 3,058.4 ± 441.9 | 983.5 ± 65.7 | −68 | <0.0001 | 1.08 |
| 1,5-Anhydroglucitol | 2,702.2 ± 416 | 732.8 ± 79.6 | −73 | <0.0001 | 1.19 |
| Xylitol | 2,318.3 ± 223.2 | 610.1 ± 133.5 | −74 | <0.0001 | 1.07 |
| Xylulose | 8,667.4 ± 959.9 | 2,206.6 ± 397.3 | −75 | <0.0001 | 1.16 |
| Pinitol | 1,042.6 ± 159.5 | 239.3 ± 25.4 | −77 | <0.0001 | 1.14 |
| Threonic acid | 2,039.1 ± 366.8 | 443.7 ± 81 | −78 | <0.0001 | 1.09 |
| Myo-inositol | 12,685.8 ± 2,773.1 | 2,123.8 ± 379.7 | −83 | <0.0001 | 1.14 |
| Fucose | 111,501.7 ± 15,245.8 | 18,341.1 ± 3,357.4 | −84 | <0.0001 | 1.20 |
| Galactinol | 9,455.7 ± 1,485.3 | 746 ± 199.5 | −92 | <0.0001 | 1.20 |
| Lyxose | 56,930.9 ± 14,883.6 | 1,328.6 ± 335.8 | −98 | <0.0001 | 1.22 |
| Xylose | 504,305.2 ± 67,733.7 | 4,899.4 ± 596.5 | −99 | <0.0001 | 1.28 |
| Other metabolites | |||||
| Succinic acid | 502,829.7 ± 462,586.3 | 2,659,290.5 ± 226,139 | 429 | 0.005 | 1.27 |
| Lactic acid | 66,885 ± 7178.2 | 39,474.2 ± 17,482.1 | −41 | 0.007 | 1.13 |
| 2-Hydroxybutanoic acid | 6,727.5 ± 640 | 2,915.1 ± 1,076 | −57 | 0.01 | 1.26 |
| Dihydroxyacetone | 6,276.1 ± 820.4 | 1,986.4 ± 530.2 | −68 | 0.002 | 1.13 |
| Propane-1,3-diol | 54,707.3 ± 4,623.7 | 17,201.4 ± 1,686.8 | −69 | <0.0001 | 1.25 |
| Glyceric acid | 52,044.2 ± 6,060.3 | 16,186.2 ± 6,607.7 | −69 | 0.007 | 1.16 |
| Adipic acid | 3,097.4 ± 446.4 | 958.6 ± 124.7 | −69 | <0.0001 | 1.12 |
| Ribonic acid | 1,165.3 ± 132.6 | 350.9 ± 44.7 | −70 | <0.0001 | 1.21 |
| 3-Hydroxyphenylacetic acid | 3,661.5 ± 608.4 | 1,077.4 ± 111 | −71 | <0.0001 | 1.02 |
| Glycerol-α-phosphate | 5,536.2 ± 764.1 | 1,494.4 ± 156.2 | −73 | <0.0001 | 1.20 |
| Glycerol-3-galactoside | 1,888.2 ± 210.5 | 505.5 ± 32.2 | −73 | <0.0001 | 1.24 |
| Dehydroabietic acid | 2,119 ± 252.6 | 549.9 ± 52.5 | −74 | <0.0001 | 1.18 |
| Phenol | 5,565 ± 846.1 | 1,441.9 ± 124.1 | −74 | <0.0001 | 1.17 |
| Shikimic acid | 6,306.6 ± 654.7 | 1,535.5 ± 296.1 | −76 | <0.0001 | 1.22 |
| γ-Tocopherol | 18,386.5 ± 3,081.9 | 4,197.8 ± 196.9 | −77 | <0.0001 | 1.18 |
| Hexuronic acid | 12,872.1 ± 2,298.7 | 2,862.6 ± 723.7 | −78 | <0.0001 | 1.05 |
| Gluconic acid | 2,267.3 ± 287.2 | 477.9 ± 87.7 | −79 | <0.0001 | 1.12 |
| Ethylsuccinate | 2,665.6 ± 378.4 | 552.9 ± 97.6 | −79 | <0.0001 | 1.21 |
| α-Tocopherol | 45,003 ± 6,800.2 | 7,962.4 ± 491.4 | −82 | <0.0001 | 1.19 |
| Pantothenic acid | 2,542.9 ± 353.9 | 415.9 ± 97.6 | −84 | <0.0001 | 1.21 |
| Behenic acid | 52,647.5 ± 8,780 | 8,593.8 ± 881.2 | −84 | <0.0001 | 1.22 |
| Benzoic acid | 12,287.6 ± 1,546 | 1,978.5 ± 332.4 | −84 | <0.0001 | 1.26 |
| Azelaic acid | 2,848.2 ± 423.4 | 428.4 ± 48.8 | −85 | <0.0001 | 1.13 |
| Pelargonic acid | 22,382.1 ± 3,025.4 | 3,253.7 ± 891.9 | −85 | <0.0001 | 1.25 |
| Glycolic acid | 50,511.1 ± 8,802.1 | 6,922.8 ± 709.6 | −86 | <0.0001 | 1.16 |
| 2-Hydroxyglutaric acid | 7,370.1 ± 1,423.2 | 996.8 ± 250.2 | −86 | <0.0001 | 1.22 |
| Sulfuric acid | 2,176.6 ± 447.9 | 285.2 ± 46 | −87 | <0.0001 | 1.14 |
| Methanolphosphate | 1,676.1 ± 282.6 | 218.4 ± 28.6 | −87 | <0.0001 | 1.23 |
| Glycerol | 118,868.5 ± 29,459.2 | 13,476.8 ± 2,562.8 | −89 | <0.0001 | 1.20 |
Values are quantifier peak ion height means ± SE; n = 9 rats/group. Selected metabolites are annotated metabolites that had mean bootstrapped variable importance in projection (VIP) measurements of ≥1 and significant group differeneces after FDR correction. For the sake of brevity, nonannotated metabolites are not presented but are provided in Supplementary Table S2. Group comparisons were assessed by Mann-Whitney U-tests. P values were adjusted for FDR correction. Statistical significance set at adjusted P values of ≤0.05. VIP was calculated from bootstrapped partial least squares-discriminant analysis (PLS-DA) models derived from training data (n = 6 animals rats/group).
Table 4.
Urine metabolite characteristics of male rats with CKD fed a low-fiber or HAMRS2-supplemented diet
| Metabolite | CKD Low Fiber | CKD HAMRS2 | Percent Change | P Value | VIP |
|---|---|---|---|---|---|
| Amino acids | |||||
| Taurine | 11,165.6 ± 2015.9 | 44,428.1 ± 10,799.1 | 298 | 0.002 | 1.22 |
| Tyrosine | 4,618.8 ± 357.4 | 7,810.4 ± 879.4 | 69 | 0.011 | 1.20 |
| Isoleucine | 955.7 ± 63.6 | 1,566.3 ± 156.9 | 64 | 0.027 | 1.00 |
| Alanine | 8,575.8 ± 827.1 | 13,386.2 ± 1,104.6 | 56 | 0.009 | 1.18 |
| Other nitrogenous metabolites | |||||
| Pantothenic acid | 359.7 ± 58.6 | 933.4 ± 95.4 | 159 | 0.002 | 1.33 |
| Furoylglycine | 1,208 ± 169.7 | 2,377.6 ± 270.4 | 97 | 0.022 | 1.19 |
| Creatinine | 19,434.2 ± 2,430 | 35,627 ± 3,013.7 | 83 | 0.011 | 1.41 |
| Isothreonic acid | 3,736.1 ± 330.1 | 6,228.2 ± 420.1 | 67 | 0.004 | 1.44 |
| Pseudouridine | 25,851.9 ± 2,184.4 | 42,166 ± 3,476.7 | 63 | 0.008 | 1.35 |
| Oxoproline | 15,061.7 ± 1,181 | 21,692.2 ± 1,730 | 44 | 0.014 | 1.13 |
| p-Cresol | 182,951.3 ± 13,963.6 | 96,566.9 ± 21,254 | −47 | 0.017 | 1.08 |
| 4-Pyridoxic acid | 406.9 ± 23.3 | 214.2 ± 15.4 | −47 | 0.001 | 1.46 |
| Indole-3-lactate | 1,045 ± 113.1 | 473.6 ± 52.7 | −55 | 0.005 | 1.25 |
| Methionine sulfoxide | 29,84.9 ± 220.4 | 1,270.1 ± 133.6 | −57 | 0.001 | 1.43 |
| Indoxylsulfate | 3,955 ± 517.4 | 1,357.9 ± 116 | −66 | 0.002 | 1.23 |
| Hydroxylamine | 13,658.7 ± 1,759.4 | 4,623.3 ± 1614 | −66 | 0.009 | 1.27 |
| Hippuric acid | 37,300.9 ± 5,689.2 | 9,868.6 ± 1,130.4 | −74 | 0.001 | 1.43 |
| 2,8-Dihydroxyquinoline | 3,870.6 ± 466.5 | 619.7 ± 85.4 | −84 | 0.001 | 1.48 |
| Lipids | |||||
| Heptadecanoic acid | 2625.7 ± 349 | 1,494.4 ± 296.2 | −43 | 0.027 | 1.03 |
| Palmitic acid | 27,796.3 ± 3,393.8 | 14,969.3 ± 3,104.1 | −46 | 0.017 | 1.02 |
| Myristic acid | 6,624.2 ± 720.8 | 3,521 ± 735.1 | −47 | 0.014 | 1.03 |
| Caprylic acid | 2,837.8 ± 189.4 | 1,454.4 ± 300.2 | −49 | 0.009 | 1.07 |
| Pelargonic acid | 8,742.8 ± 1,092.4 | 4334.6 ± 1250.2 | −50 | 0.027 | 1.09 |
| Capric acid | 1,062.1 ± 131.9 | 301.1 ± 71.8 | −72 | 0.002 | 1.25 |
| Carbohydrates | |||||
| Sucrose | 2,818 ± 490.3 | 12,824.6 ± 1,849.4 | 355 | 0.001 | 1.41 |
| Inulotriose | 509.1 ± 55.9 | 1,875.4 ± 307.6 | 268 | 0.001 | 1.38 |
| Levoglucosan | 661 ± 44.5 | 2,208.6 ± 159 | 234 | 0.001 | 1.53 |
| Maltose | 76,813.1 ± 18,249.1 | 196,750.6 ± 33,320 | 156 | 0.014 | 1.23 |
| Xylitol | 2,024 ± 200.5 | 4,067.7 ± 649.4 | 101 | 0.009 | 1.25 |
| Fucose | 9,950.4 ± 700 | 17,077.6 ± 1,361.1 | 72 | 0.004 | 1.39 |
| Erythritol | 10,914 ± 944.7 | 18,329.7 ± 1,329.5 | 68 | 0.006 | 1.45 |
| Ribose | 906.2 ± 43.4 | 1,253.4 ± 107.7 | 38 | 0.032 | 1.01 |
| Threitol | 1,411.7 ± 126.1 | 1,872 ± 109.1 | 33 | 0.04 | 1.19 |
| Digalacturonic acid | 516.8 ± 37.4 | 265.4 ± 46 | −49 | 0.009 | 1.14 |
| Other metabolites | |||||
| Tartaric acid | 9,574.2 ± 2,039.6 | 92,107.6 ± 11,429.4 | 862 | 0.001 | 1.53 |
| Isocitric acid | 4,551 ± 364.7 | 7,746.3 ± 838.8 | 70 | 0.017 | 1.30 |
| Ribonic acid | 1,392.2 ± 166.1 | 2,211 ± 226.8 | 59 | 0.022 | 1.14 |
| Aconitic acid | 2,150.7 ± 174.7 | 3,162.9 ± 350.7 | 47 | 0.032 | 1.14 |
| Pyruvic acid | 443.6 ± 47.4 | 286.8 ± 35.5 | −35 | 0.04 | 1.01 |
| Glycolic acid | 1,799.8 ± 160.7 | 1,119.6 ± 134.1 | −38 | 0.014 | 1.08 |
| Azelaic acid | 408.1 ± 57.2 | 204.3 ± 54.2 | −50 | 0.032 | 1.16 |
| Benzoic acid | 7,514.4 ± 679.2 | 3,506.1 ± 797 | −53 | 0.011 | 1.11 |
| Ferulic acid | 457.4 ± 57 | 200.9 ± 36.7 | −56 | 0.004 | 1.08 |
| 3-Hydroxyphenylacetic acid | 583.4 ± 41.1 | 210.8 ± 36.4 | −64 | 0.001 | 1.46 |
| Phenol | 5,549.9 ± 779.2 | 1,445.2 ± 239.8 | −74 | 0.001 | 1.32 |
| 3-(3-Hydroxyphenyl)propionic acid | 3,547.2 ± 870.4 | 560.4 ± 63.6 | −84 | 0.004 | 1.36 |
| Glycerol-3-galactoside | 7,002.1 ± 557.6 | 913.3 ± 88.7 | −87 | 0.001 | 1.51 |
Values are quantifier peak ion means ± SE; n = 9 rats/group. Selected metabolites are annotated metabolites that had mean bootstrapped VIP measurements of ≥1 and significant group differeneces after FDR correction. For the sake of brevity, nonannotated metabolites are not presented but are provided in Supplementary Table S2. Group comparisons were assessed by Mann-Whitney U-tests. P values were adjusted for FDR correction. Statistical significance set at adjusted P values of ≤0.05. VIP was calculated from bootstrapped PLS-DA models derived from training data (n = 6 animals rats/group).
Table 5.
Percent changes in uremic retention solutes in the cecal contents, serum, and urine in male CKD rats fed HAMRS2 relative to low fiber-fed CKD control rats
| Metabolite | Cecal Contents | Serum | Urine |
|---|---|---|---|
| Nitrogenous metabolites | |||
| Creatinine | −52.5* | −13.4 | 83.3* |
| Hippuric acid | NR | NR | −73.5* |
| Indole-3-acetate | −12.1 | 614.7* | −2.6 |
| Indole-3-lactate | −71.7* | 135.2* | −57.4* |
| Indoxyl sulfate | NR | −36.0 | −65.7* |
| p-Cresol | NR | NR | −47.2* |
| Putrescine | 57.9 | NR | −20.4 |
| Spermidine | −70.7 | NR | −32.6† |
| Urea | −79.0* | −0.3 | −15.4 |
| Uric acid | −85.1* | −36.8* | 51.1† |
| Polyols | |||
| Erythritol | 26.9 | 122.2* | 67.9* |
| Mannitol | 245.5 | −27.9† | −1.3 |
| Myo-inositol | −83.3* | 5.50 | 17.8 |
| Other | |||
| Phenol | −74.1* | NR | −74.0* |
Values are expressed as percentages. NR, metabolite not reported by gas chromotography-time of flight-mass spectroscopy.
Metabolites that were featured in PLA-DA models.
Metabolites that were significantly different (Mann-Whitney U-test) before FDR correction.
Table 6.
Summary of metabolite changes comparing male HAMRS2-fed to control diet-fed CKD rats
| Cecum | Serum | Urine |
|---|---|---|
| 465 identified metabolites | 300 identified metabolites | 276 identified metabolites |
256 metabolites significantly different
|
20 metabolites significantly different
|
114 metabolites significantly different
|
| Main findings | ||
Nitrogenous metabolites reduced in the HAMRS2-fed group:
|
Nitrogenous metabolites reduced in the HAMRS2-fed group:
|
Nitrogenous metabolites reduced in the HAMRS2-fed group:
|
Lipid metabolites reduced in the HAMRS2-fed group:
|
No differences in measured lipid metabolites between groups | Lipid metabolites reduced in the HAMRS2-fed group:
|
Carbohydrate metabolites greater in the HAMRS2-fed group:
|
Carbohydrate metabolites greater in the HAMRS2-fed group
|
Carbohydrate metabolites greater in the HAMRS2-fed group:
|
Carbohydrate metabolites reduced in the HAMRS2-fed group:
|
Carbohydrate metabolites reduced in the HAMRS2-fed group
|
|
Other metabolites greater in the HAMRS-fed group:
|
Other metabolites reduced in the HAMRS2-fed group:
|
|
Cecal metabolites.
A total of 465 cecal metabolites were detected using the GC-TOF analytical platform. Of these, 202 metabolites were annotated in the metabolite database; the remaining metabolites were nonannotated and labeled with a numerical BinBase ID (Supplemental Table S2A). A total of 256 metabolites had an adjusted P value of ≤0.05 and a mean bootstrapped VIP of ≥1 in the PLS-DA model; of these, 109 metabolites were annotated (Table 2). The majority of cecal metabolites were markedly reduced in the HAMRS2-fed group compared with the low fiber-fed group (column F in Supplemental Table 2A). Three metabolites that were higher in HAMRS2-fed rats were lactose, succinic acid, and the resistant starch breakdown product maltose. The following cecal amino acids were significantly reduced in the HAMRS2-fed group: aspartic acid, isoleucine, leucine, lysine, methionine, phenylalanine, serine, and valine. Other nitrogenous metabolites that were significantly reduced in HAMRS2-fed rats included adenine, creatinine, indole-3-lactate, inosine, methionine sulfoxide, thymidine, uracil, urea, uric acid, uridine, and xanthine. The sugar alcohols 1,5-anhydroglucitol, galactinol, myo-inositol, and xylitol were also reduced in HAMRS2-fed rats.
Serum metabolites.
A total of 300 serum metabolites were detected, 145 of which were annotated (Supplemental Table S2B). The abundances of 20 metabolites were found to be significantly different between treatment groups; 12 of which were annotated (Table 3). Two xenometabolites derived from microbial metabolism of tryptophan, indole-3-acetate and indole-3-lactate, were higher in HAMRS2-fed rats. Two DNA bases, cytosine and thymidine, were reduced in the serum of HAMRS-fed rats. Another nitrogen-containing metabolite, uric acid, was reduced in HAMRS2-fed rats compared with low fiber-fed rats. Two polyols, erythritol and xylitol, were greater in HAMRS2-fed rats compared with low fiber-fed rats, respectively. Another polyol, 1,5-anhydroglucitol, was reduced in HAMRS2-fed rats. The organic acids fumaric acid and malic acid were greater in HAMRS2-fed rats. The ketone body β-hydroxybutyric acid was also greater in HAMRS2-fed rats.
Table 3.
Serum metabolite characteristics of male rats with CKD fed a low-fiber or HAMRS2-supplemented diet
| Metabolite | CKD Low Fiber | CKD HAMRS2 | Percent Change | P Value | VIP |
|---|---|---|---|---|---|
| Nitrogenous Metabolites | |||||
| Indole-3-acetate | 185.4 ± 12.1 | 1,325 ± 122.5 | 615 | <0.0001 | 1.98 |
| Indole-3-lactate | 320.1 ± 41.2 | 752.9 ± 105.1 | 135 | 0.045 | 1.52 |
| Thymidine | 3,468 ± 80.1 | 2,797.7 ± 86.6 | −19 | <0.0001 | 1.67 |
| Cytosine | 1,106 ± 47.1 | 800.6 ± 65.1 | −28 | 0.02 | 1.37 |
| Uric acid | 1,389.8 ± 81 | 878.7 ± 50.7 | −37 | <0.0001 | 1.65 |
| Carbohydrates | |||||
| Erythritol | 1,029.8 ± 101 | 2,288.2 ± 309.8 | 122 | <0.0001 | 1.69 |
| Xylitol | 593.7 ± 58.9 | 1,157.2 ± 139.1 | 95 | 0.045 | 1.52 |
| Threonic acid | 2,234.3 ± 122.8 | 3,060.1 ± 191.3 | 37 | 0.045 | 1.30 |
| 1,5-Anhydroglucitol | 16,524.3 ± 1,147.7 | 11,231.1 ± 704.1 | −32 | 0.035 | 1.61 |
| Other metabolites | |||||
| Malic acid | 414.7 ± 26.6 | 777.1 ± 53.7 | 87 | <0.0001 | 1.68 |
| β-Hydroxybutyric acid | 8,938.1 ± 879.2 | 14,936.8 ± 1,466.2 | 67 | 0.02 | 1.44 |
| Fumaric acid | 265 ± 15.3 | 401.2 ± 29.8 | 51 | 0.02 | 1.40 |
Values are quantifier peak ion height means ± SE; n = 9 rats/group. Selected metabolites are annotated metabolites that had mean bootstrapped VIP measurements of ≥1 and significant group differeneces after FDR correction. For the sake of brevity, nonannotated metabolites are not presented but are provided in Supplementary Table S2. Group comparisons were assessed by Mann-Whitney U-tests. P values were adjusted for FDR correction. Statistical significance set at adjusted P values of ≤0.05. VIP was calculated from bootstrapped PLS-DA models derived from training data (n = 6 animals rats/group).
Urine metabolites.
In urine, 276 metabolites were detected, and of these, 143 metabolites were annotated (Supplemental Table S2C). The abundances of 114 metabolites were significantly different between treatment groups; 47 of which were annotated (Table 4). There were 23 annotated metabolites for which concentrations were significantly increased in HAMRS2-fed rats. Tartaric acid was particularly noteworthy in HAMRS2-fed rats; this metabolite had the highest VIP and greatest change of any urine metabolite. Three xenometabolites resulting from microbial metabolism were lower in the HAMRS2-fed group: 2,8-dihydroxyquinoline, 3-(3-hydroxyphenol)propionic acid, and 3-hydroxyphenolacetic acid. Concentrations of several amino acids were also higher in HAMRS2-fed rat urine compared with low fiber-fed rats, i.e., alanine, isoleucine, taurine, and tyrosine. The following sugars were higher in urine of the HAMRS2-fed group compared with the low fiber-fed group: erythritol, fucose, inulotriose, maltose, ribose, sucrose, and xylitol. Several fatty acids were reduced in the HAMRS2-fed group compared with the low fiber-fed group: capric, caprylic, heptadecanoic, myristic, and palmitic. The urine metabolomics data set is also shown as estimated 24-h excretion for each metabolite in Supplemental Table S2D.
HAMRS2 alters uremic retention solutes.
Trends in URS metabolite concentrations in the cecal contents, serum, and urine are shown in Table 5. Overall, most URS were reduced in cecal contents with the exceptions of putrescine, erythritol, and mannitol. Serum metabolite trends did not always track cecum concentration patterns, perhaps due to the dynamics of gut absorption coupled to kidney excretion, both of which would impact the net accumulation in the blood pool. For instance, cecal creatinine was 52% lower in HAMRS2-fed rats, but this marked reduction was less apparent in serum. Urine creatinine concentration, on the other hand, was robustly increased. This may be due to improved creatinine clearance or reduced CKD-induced muscle wasting in fiber-fed rats. Notably, the metabolomic results for urinary creatinine were consistent with results from our previous report (74) that used a more traditional enzyme-based creatinine assay; the values from the two types of analyses had a Spearman's correlation coefficient of 0.77 (P < 0.0001). Urea was reduced by 79.0% in cecal contents, yet there was no change in serum; the latter is consistent with previous findings from these rats in which an enzyme-based urea assay was used (74). Levels of uric acid were significantly lower in the serum and cecal contents and ∼50% higher in the urine of rats fed HAMRS2. HAMRS2-fed rats had 65.7% lower indoxyl sulfate in the urine and 36% lower abundance in the serum. Indoxyl sulfate was not reported as detectable in the cecal contents, consistent with the idea that the sulfate metabolite is produced by the host from microbe-derived indole (15). There were several other examples in which the various metabolite pools differed in the directionality of concentration between the treatment groups. For instance, there was no significant difference in urine and cecal indole-3-acetate between treatment groups, yet its serum level was 615% greater in HAMRS2-fed rats. Indole-3-lactate abundance was reduced by ∼70% in the cecal contents and >50% in urine of HAMRS2-fed rats, yet its serum abundance was 135% higher compared with low fiber-fed rats. p-Cresol, derived from microbial transformation of tyrosine, was reduced by 47% in the urine of the HAMRS2-fed group but was not reported as detectable in the serum or cecal contents. Hippuric acid was reduced by >70% in the urine of HAMRS2-fed rats and not reported as detectable in the serum or cecal contents. Erythritol concentration was greater in the serum and urine of HAMRS2-fed rats but was not different in cecal contents. Mannitol was reduced in the serum but increased by >200% in the cecal contents of HAMRS2-fed rats; there was no change in urine abundance. No differences were observed in serum or urine abundances of myo-inositol; however, cecal content abundance was reduced in the HAMRS2-fed group. Phenol was not reported as detectable in the serum and was reduced by >70% in the urine and cecal contents of HAMRS2-fed rats.
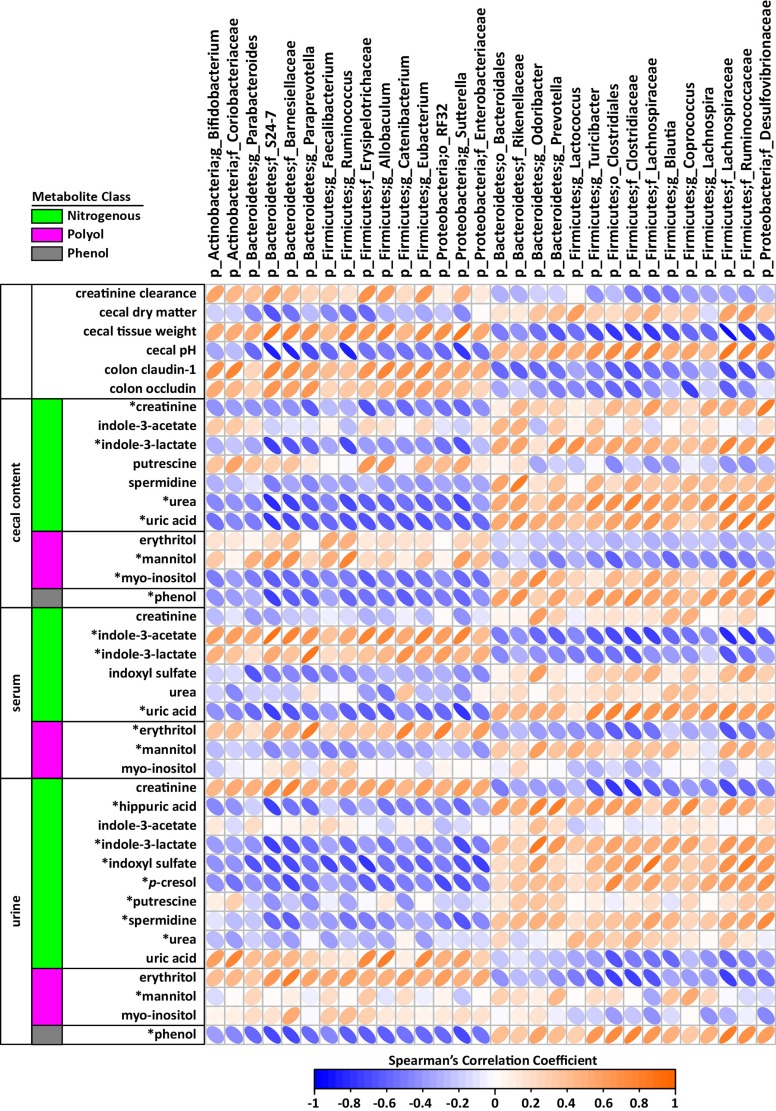
Spearman's correlations between kidney function-relevant phenotype data, URS metabolites, and cecal bacteria for all rats are shown in Fig. 4. Cecal bacteria were included based on having >0.05% mean relative abundance present in each group and an adjusted P value of ≤0.05 when microbiome differences between treatment groups were compared. Several bacteria groups were associated with improved kidney function as measured by creatinine clearance, which covaried with cecal changes reflective of the HAMRS2 diet (i.e., cecal tissue weight, reduced pH, cecal hydration). These included, for instance, Bacteroidetes families S24-7 and Barnesiellaceae, the Firmicutes genus Ruminococcus, and the Proteobacteria genus Sutterella. The suite of bacteria that correlated with improved kidney function also had negative correlations with cecal URS compounds such as creatinine, indole-3-lactate, spermidine, urea, uric acid, phenol, and myo-inositol.
Fig. 4.
Spearman's correlation matrix of cecal bacteria versus metadata and uremic retention solutes in the cecal contents, serum, and urine of male CKD rats fed a low fiber diet or HAMRS2 (n = 18 total rats combined). Bacteria included had a minimum of 0.05% mean abundance in each group and an adjusted Mann-Whitney U-test P value of ≤0.05. Bacteria are listed to the lowest level of classification. Metabolites were selected based on being identified as uremic retention solutes. *Metabolites that had a mean bootstrapped variable importance in projection of ≥1. Colonic tight junction data were imputed for 2 rats/group using k-nearest neighbors, as described in materials and methods. The direction of ellipses represent positive or negative correlation, and the width of ellipse represents strength of correlation (narrow ellipse = stronger correlation).
DISCUSSION
Resistant starch may improve CKD outcomes by altering the gut environment and hence systemic exposure to certain metabolites or other gut-derived factors that impinge upon inflammation and kidney function. Relevant to this concept, a microarray study (33) of cecal tissue gene expression in healthy rats fed HAMRS2 revealed that genes involved in cell growth, proliferation, differentiation, mucin production, and tissue structure were differentially expressed compared with low fiber-fed rats; these changes may aid in reducing translocation of harmful substances into the systemic circulation. In the present study, HAMRS2 increased cecal tissue growth and increased hydration of cecal contents. Resistant starch has been shown to increase fecal output in both humans and animal models (32, 49); increased fecal output may indicate decreased fecal transit time. A recent report by Vandeputte et al. (71) described how decreased fecal transit time and increased fecal hydration can alter the gut microbiota to favor the presence of rapidly-growing bacteria. Furthermore, increased loss of water, and the accompanying electrolytes through feces might help limit the systemic water load on the kidneys.
Significant reductions in several cecal content amino acids were observed, which may be due to an increased need for nitrogen to maintain protein synthesis if bacteria are reproducing more rapidly (or if there are more bacteria) and gut tissue growth is enhanced; thus, under HAMRS2-fed conditions, the gut may be acting as a “nitrogen sink” (86). This would serve to sequester nitrogen in the gut, reducing the amount that enters the portal circulation and hence lowering the nitrogen load on the liver and kidneys. A recent study found that conventional mice had lower amino acids entering the hepatic portal vein compared with germ-free mice. The authors attributed the lower level of amino acids to increased synthesis of microbial biomass (44).
Another interesting consideration is that HAMRS2 feeding might elicit increases in gut urea transporters; this would facilitate bacterial protein synthesis under conditions of resistant starch feeding, considering the microbial conversion of urea to amino acid backbones. This could partially explain why resistant starch reduced blood urea in non-CKD animal models (32, 87). That said, in our CKD rats, metabolomic analysis revealed no diet-associated differences in serum urea. This may be due to reduced distal tubular urea reabsorption, which is a major component of the urine concentrating process and is disturbed in chronic interstitial nephropathy (25). It should be noted that HAMRS2, unlike many cereal fibers, is completely devoid of protein and therefore may be better suited at reducing the nitrogenous load on the host liver and kidneys as well as reduce microbial fermentation of amino acids in the colon (4, 14).
To further explore relationships between metabolite abundances, cecal characteristics, and specific microbe populations, a cross-correlation plot was created, which revealed a variety of significant associations (Fig. 4). This analysis is hypothesis generating; i.e., one may ask if HAMRS2-driven reductions in cecal urea are the result of increasing urea utilization by microbes that display a negative correlation with urea concentration (e.g., Allobaculum). Other possibilities for decreased cecal urea include trapping of urea and its degradation products by HAMRS2 or HAMRS2 breakdown products, urea dilution in larger volume of the cecal content, or rapid elimination of urea occasioned by shortened fecal transit time. These events can simultaneously lower the detectable urea in cecal fluid and minimize enterohepatic recycling of urea. Increased amounts of urea and its breakdown products, such as ammonia, have been shown to increase gut permeability (9, 77). Ammonia is generated by the microbial metabolism of urea by the enzyme urease, which can be further converted to ammonium hydroxide. Ammonium hydroxide increases intestinal pH and can lead to disruption and loss of intestinal tight junction proteins and thereby increase gut permeability (9, 78).
A recent study (84) of the fecal microbiota in patients with end-stage renal disease has shown a marked expansion of bacteria that possess the urease enzyme as well those expressing enzymes that metabolize aromatic amino acids and produce indoles and p-cresol. Microbial aromatic amino acid fermentation that results in the production of metabolites, such as phenol, and microbial proteases that can act as virulence factors and target the host epithelium are more active at a neutral or basic pH (43, 61, 62). Phenol, which was reduced in the cecal contents and urine of HAMRS2-fed rats, has been shown to have toxic effects on human colonic epithelial cells in vitro and contribute to increased intestinal permeability in CKD (36). The net effect of HAMRS2-associated cecal metabolite changes may be a reduction in the systemic exposure to potentially harmful URS metabolites in CKD, such as microbial-derived indole (converted to indoxyl sulfate by the host) and p-cresol. Metabolomic analysis revealed reductions in urinary levels of these URS metabolites, and both were important in discriminating dietary treatment groups in PLS-DA models. Recently, it has been shown that supplementing patients on hemodialysis with 15 g/day of resistant starch for 6 wk reduced plasma levels of unbound indoxyl sulfate (60), consistent with the results described here for CKD rats. It should be noted that indole itself may not be harmful, and beneficial effects of indole and indole-3-acetate have been reported (5, 31). Some of the most robustly increased serum metabolites in HAMRS2-fed CKD rats were indole-3-acetate and indole-3-lactate, despite unchanged or reduced levels in the cecum and urine. The basis for this is not known, but it is speculated that resistant starch feeding increases microbial production of metabolites and/or effects changes to transporters or kidney reabsorption, which, in turn, drives relative accumulation in the blood pool. Another metabolite altered by HAMRS is uric acid. Uric acid and oxalic acid are normally excreted in the urine; however, in chronic renal failure, the colon replaces the kidney as the primary site of their excretion (28, 29). This adaptive response may account for the minor rise in serum oxalic acid observed in HAMRS2-fed rats compared with low fiber-fed rats (Supplemental Table S2).
Many other non-URS metabolites were also altered by HAMRS2 feeding. The metabolite with the greatest change was urinary tartaric acid, which was >800% greater in the HAMRS2-fed group. Tartaric acid is generally associated with grape and wine consumption, so the etiology behind this striking increase remains to be elucidated. It likely involves HAMRS2-associated changes in gut microbe ecology and metabolism of chow components. Two other urinary metabolites that were significantly elevated in the HAMRS2-fed group were furoylglycine and levoglucosan. These metabolites have been reported to form due to heating foods at high temperatures and may have occurred during the manufacturing process of the rodent diets (10, 48). Increased levels in the urine may reflect enhanced urinary excretion in the HAMRS2-fed group due to improved kidney function. Other metabolites that have been reported to be modulated by the gut microbiota were also changed in the HAMRS2-fed group. Urinary hippuric acid was significantly reduced in HAMRS2-fed rats. This metabolite can be formed in a few ways, one of which is by microbial metabolism of aromatic amino acids to form benzoic acid, which the host can then conjugate with glycine to form hippuric acid (39, 82). Interestingly, the hippuric acid precursor benzoic acid was significantly reduced in the cecal contents and urine; there was no change in serum levels. Another microbial-derived metabolite, 3-(3-hydroxyphenyl)propionic acid, was reduced by ∼85% in the urine and ∼100% greater in the cecal contents of HAMRS2-fed rats. Elevated urinary 3-(3-hydroxyphenyl)propionic acid levels have been found in individuals with autism, and these levels decreased after antibiotic treatment, with the latter speaking to the microbial origins of this xenometabolite (59). Several metabolites that have been reported to have antimicrobial activity were also changed in the HAMRS2-fed group, including 4-hydroxybenzoic acid, 3,4-dihydroxybenzoic acid, phenylacetic acid, 3-hydroxyphenylacetic acid, 4-hydroxyphenylacetic acid, and 3,4-dihydroxyphenylacetic acid (13). In summary, changes in xenometabolites appear to reflect the dramatic shifts that occur in the gut microbiota with both HAMRS2 consumption and CKD.
HAMRS2-fed rats exhibited less microbial diversity than low fiber-fed rats. More microbial diversity is generally associated with a healthier phenotype (29a). However, the decrease in diversity observed in HAMRS2-fed rats is likely due to the homogeneous composition and configuration of HAMRS2. Decreased microbial diversity upon feeding of resistant starch has been previously reported (70). HAMRS2 is composed solely of glucose with α-1,4 bonds (1). Fibers with a variety of monosaccharides and bond types likely select for a more diverse microbial population simply by providing a wider array of substrates. A significant increase in the Bacteroidetes-to-Firmicutes ratio was observed in HAMRS2-fed rats. A starch-related increase in the Bacteroidetes-to-Firmicutes ratio has been reported in previous studies (67, 69) and has generally been associated with a healthy gut microbial community. Despite an overall reduction in Firmicutes, there was a bloom in the specific Firmicutes genera Ruminococcus. Ruminococcus bromii has been described as a primary starch degrader that provides substrates to other bacteria (89). We found that the relative abundance of Ruminococcus and several other bacteria had negative correlations with cecal content pH. The decrease in pH is likely due to increased microbial fermentation of HAMRS2, resulting in increased production of short-chain fatty acids such as acetate, propionate, and butyrate (7, 46). A decreased intestinal pH is thought to be beneficial to the host by altering gut bacteria (i.e., changes in metabolite production/utilization, growth, and virulence factors) and maintaining the integrity of the intestinal epithelium (57, 66). Another Firmicutes genera that was substantially higher in HAMRS2-fed rats was the butyrate producer Allobaculum, which has been associated with a lean phenotype and found to be increased in aged mice fed HAMRS2 (54, 67). However, Allobaculum has also been negatively correlated with colonic tight junction and anti-inflammatory gene expression in the colon (37), making its putative role in host health less certain.
The HAMRS2-fed group had an increase in the phylum Proteobacteria, with a bloom in order RF32 (Alphaproteobacteria) and the genus Sutterella (Betaproteobacteria). There is scant information related to Sutterella; this genus has been found to be elevated in the feces and intestinal biopsies of children with autism (80, 81) and in feces from dogs with acute hemorrhagic diarrhea (65). We did observe decreased levels of the family Desulfovibrionaceae (Deltaproteobacteria) in HAMRS2-fed rats. Bacteria from this family are capable of producing sulfide, which may disrupt disulfide bridges in the protective mucus layer, leading to increased intestinal permeability (38, 50). This bacteria has also been found to be increased in mice fed a high-fat diet and reduced by prebiotic supplementation (18).
The intent of this study was to determine if HAMRS2-associated alterations in specific gut microbes as well as cecal, serum, and urine metabolites are correlated with one another and associate with the positive gut and renal outcomes previously reported for these rats (74); this has identified potential mechanisms by which resistant starch impacts kidney function and ameliorates CKD. To demonstrate this, the diet was supplemented 59% by weight with HAMRS2, which is a high amount relative to dietary supplementation in humans but is a level certain to elicit marked alterations in gut microbiota in this proof-of-principle experiment. Approximately 40% of HAMRS2 is absorbed in the small intestine, and the remaining portion reaches the colon, where it can be degraded by microbes. Therefore, the amount of starch reaching the colon is ∼36% by weight of the diet. Future experiments should be conducted to determine dose response, changes in gut permeability, and if the phenotype can be replicated via cecal/fecal transplantation. A limitation of the present study that was not controlled for was coprophagy. Coprophagy may impact results from this, and other related rodent studies, by potentially influencing the recycling of microbes and metabolites. Therefore, extrapolation of these data, especially the serum and urine metabolites, to the human condition should be considered in this light. The impact of coprophagia on microbiota and metabolomics results warrants further investigation.
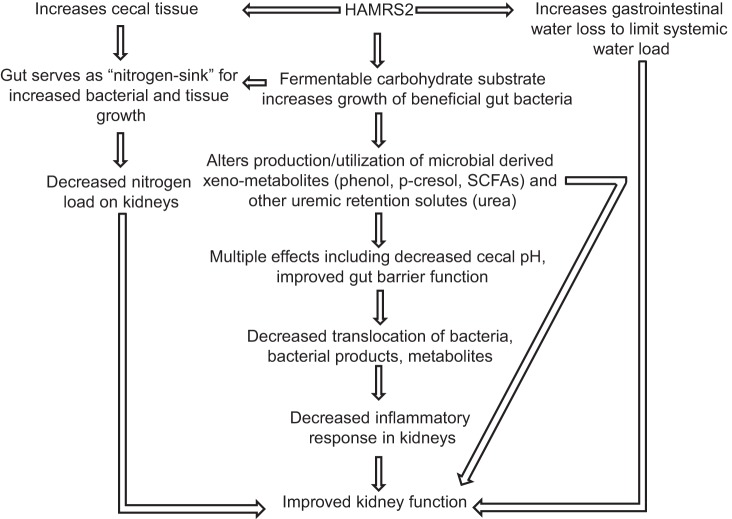
In summary, dietary resistant starch has a protective effect on kidney function in CKD rats that takes place concurrent with alterations in gut microbe ecology and shifts in specific groupings of gut bacteria. Yet, the complete sets of mechanisms linking the microbiome and kidney function remain to be elucidated. Our results support the idea that resistant starch-associated phenotypes stem in part from change in the gut microbiome that alter URS dynamics, nitrogen and water balance, gut pH, and by minimizing inflammation via preservation of the gut epithelial barrier. A working model of the effects of HAMRS2 based on findings from this study and our previous results with the same rats is shown in Fig. 5. Should the protective actions of HAMRS2 observed in the rat model recapitulate in human patients with developing or fulminant kidney disease, dietary resistant starch or other means to modify the microbiome could provide a new approach to complement existing medical therapies. Relevant in this regard, individuals consuming up to 45 g HAMRS2/day reported little to no gastrointestinal discomfort or gas (26), and HAMRS2 is inexpensive and readily incorporated into common foods such as yogurt, orange juice, and baked goods (22). Several epidemiological and clinical studies have shown that increased fiber intake can improve or delay the progression of CKD (35, 53, 88). Thus, HAMRS2 could provide a useful dietary adjunct to existing clinical strategies to retard CKD progression and its associated systemic inflammation and cardiovascular complications. Finally, our discussion of metabolites focused largely on URS and nitrogen-containing molecules. Yet, the survey of metabolism enabled by metabolomic analysis of cecal contents highlighted that modification of dietary resistant starch intake, and concomitant shifts in the gut microbiome, lead to dramatic alterations in gut lumen xenometabolite profiles. Elucidating how these metabolites contribute to the systemic metabolome, host health, intestinal function, and the gut microbial ecology presents an exciting frontier for research.
Fig. 5.
Working model of how HAMRS2 may improve CKD. SCFAs, short-chain fatty acids.
GRANTS
The project was supported in part by a T32 training award (to D. A. Kieffer) funded by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award TL1 TR000133. Additional funding was provided by the Danish Council for Strategic Research Project 10-093526, USDA-ARS Projects 2032-51530-022-00D and 6026-51000-010-05S, and in part by the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. The USDA is an equal opportunity provider and employer.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.A.K., N.D.V., R.J.M., and S.H.A. conception and design of research; D.A.K., S.L., W.L.L., M.K., and S.N. performed experiments; D.A.K., B.D.P., S.L., W.L.L., M.K., S.N., and M.E.M. analyzed data; D.A.K., R.J.M., and S.H.A. interpreted results of experiments; D.A.K. and B.D.P. prepared figures; D.A.K. drafted manuscript; D.A.K., B.D.P., N.D.V., M.L.M., R.J.M., and S.H.A. edited and revised manuscript; D.A.K., B.D.P., N.D.V., S.L., W.L.L., M.K., S.N., M.E.M., M.L.M., R.J.M., and S.H.A. approved final version of manuscript.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Renal Physiology website.
REFERENCES
- 1.Annison G, Topping DL. Nutritional role of resistant starch: chemical structure vs physiological function. Annu Rev Nutr 14: 297–320, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Baker D. Determing fiber in cereals. Cereal Chem 54: 360–365, 1977. [Google Scholar]
- 5.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA 107: 228–233, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 1551–1558, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belobrajdic DP, King RA, Christophersen CT, Bird AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr Metab 9: 93–93, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Series B 57: 289–300, 1995. [Google Scholar]
- 9.Bourke E, Milne MD, Stokes GS. Caecal pH and ammonia in experimental uraemia. Gut 7: 558–561, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell C, Grapov D, Fiehn O, Chandler CJ, Burnett DJ, Souza EC, Casazza GA, Gustafson MB, Keim NL, Newman JW. Improved metabolic health alters host metabolism in parallel with changes in systemic xeno-metabolites of gut origin. PLos One 9: e84260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108, Suppl 1: 4516–4522, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cueva C, Moreno-Arribas MV, Martín-Álvarez PJ, Bills G, Vicente MF, Basilio A, Rivas CL, Requena T, Rodríguez JM, Bartolomé B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res Microbiol 161: 372–382, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Cummings J, Hill M, Bone E, Branch W, Jenkins D. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 32: 2094–2101, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Cummings JH. Fermentation in the human large intestine: evidence and implications for health. Lancet 1: 1206–1209, 1983. [DOI] [PubMed] [Google Scholar]
- 16.De Schrijver R, Vanhoof K, Vande Ginste J. Nutrient utilization in rats and pigs fed enzyme resistant starch. Nutr Res 19: 1349–1361. [Google Scholar]
- 17.Earle KA, Billings G, Sigal M, Lichtman JS, Hansson GC, Elias JE, Amieva MR, Huang KC, Sonnenburg JL. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 18: 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, Delzenne NM, Schrenzel J, Cani PD. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60: 2775–2786, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiehn O, Kind T. Metabolite profiling in blood plasma. Methods Mol Biol 358: 3–17, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Fiehn O, Wohlgemuth G, Scholz M. Setup and annotation of metabolomic experiments by integrating biological and mass spectrometric metadata. In: Data Integration in the Life Sciences, edited by Ludäscher B, Raschid L. Berlin: Springer, 2005, p. 224–239. [Google Scholar]
- 21.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9: 577–589, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Fuentes-Zaragoza E, Riquelme-Navarrete MJ, Sánchez-Zapata E, Pérez-Álvarez JA. Resistant starch as functional ingredient: a review. Food Res Int 43: 931–942, 2010. [Google Scholar]
- 23.Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Beneficial Microbes 5: 3–17, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Gibson GR. Dietary modulation of the human gut microflora using prebiotics. Br J Nutr 80: S209–S212, 1998. [PubMed] [Google Scholar]
- 25.Gilbert RM, Weber H, Turchin L, Fine LG, Bourgoignie JJ, Bricker NS. A study of the intrarenal recycling of urea in the rat with chronic experimental pyelonephritis. J Clin Invest 58: 1348–1357, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabitske HA, Slavin JL. Gastrointestinal effects of low-digestible carbohydrates. Crit Rev Food Sci Nutr 49: 327–360, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Hastie T, Tibshirani R, Narasimhan B, Chu G. impute: Imputation for Microarray Data (online). http://rpackages.ianhowson.com/bioc/impute/ [17 August 2015].
- 28.Hatch M, Freel RW, Vaziri N. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol 5: 1339–1343, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Hatch M, Vaziri N. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin Sci 86: 511–516, 1994. [DOI] [PubMed] [Google Scholar]
- 29a.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen-Urstad AP, Semenkovich CF. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta 1821: 747–753, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 85: 777–788, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalmokoff M, Zwicker B, O'Hara M, Matias F, Green J, Shastri P, Green-Johnson J, Brooks SP. Temporal change in the gut community of rats fed high amylose cornstarch is driven by endogenous urea rather than strictly on carbohydrate availability. J Appl Microbiol 114: 1516–1528, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Keenan MJ, Martin RJ, Raggio AM, McCutcheon KL, Brown IL, Birkett A, Newman SS, Skaf J, Hegsted M, Tulley RT, Blair E, Zhou J. A microarray study indicates high-amylose resistant starch increases hormones and improves structure and function of the GI tract. J Nutrigen Nutrigenomics 5: 26–44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komsta L. Package ‘outliers’ (online). https://cran.r-project.org/web/packages/outliers/outliers.pdf [17 August 2015].
- 35.Krishnamurthy VMR, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, Greene T, Beddhu S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81: 300–306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P, Windey K, Tremaroli V, Bäckhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA 111: E4485–E4493, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SM, Han HW, Yim SY. Beneficial effects of soy milk and fiber on high cholesterol diet-induced alteration of gut microbiota and inflammatory gene expression in rats. Food Funct 6: 492–500, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Lennon G, Balfe A, Bambury N, Lavelle A, Maguire A, Docherty NG, Coffey JC, Winter DC, Sheahan K, O'Connell PR. Correlations between colonic crypt mucin chemotype, inflammatory grade and Desulfovibrio species in ulcerative colitis. Colorectal Dis 16: O161–O169, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Lewis HB. Studies in the synthesis of hippuric acid in the animal organism. II. The synthesis and rate of elimination of hippuric acid after benzoate ingestion in man. J Biol Chem 18: 225–231, 1914. [Google Scholar]
- 40.Li H, Ma ML, Luo S, Zhang RM, Han P, Hu W. Metabolic responses to ethanol in Saccharomyces cerevisiae using a gas chromatography tandem mass spectrometry-based metabolomics approach. Int J Biochem Cell Biol 44: 1087–1096, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Lin CJ, Chuang CK, Jayakumar T, Liu HL, Pan CF, Wang TJ, Chen HH, Wu CJ. Serum p-cresyl sulfate predicts cardiovascular disease and mortality in elderly hemodialysis patients. Arch Med Sci 9: 662–668, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71: 8228–8235, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macfarlane GT, Allison C, Gibson SA, Cummings JH. Contribution of the microflora to proteolysis in the human large intestine. J Appl Bacteriol 64: 37–46, 1988. [DOI] [PubMed] [Google Scholar]
- 44.Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E, Backhed F, Nielsen J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol 11: 834, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mevik B, Wehrens R, Liland KH. Package ‘pls’ (online) https://cran.r-project.org/web/packages/pls/pls.pdf [17 August 2015].
- 46.Morita T, Kasaoka S, Ohhashi A, Ikai M, Numasaki Y, Kiriyama S. Resistant proteins alter cecal short-chain fatty acid profiles in rats fed high amylose cornstarch. J Nutr 128: 1156–1164, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Nasir O, Umbach AT, Rexhepaj R, Ackermann TF, Bhandaru M, Ebrahim A, Artunc F, Kempe DS, Puchchakayala G, Siraskar B, Foller M, Saeed A, Lang F. Effects of gum arabic (Acacia senegal) on renal function in diabetic mice. Kidney Blood Press Res 35: 365–372, 2012. [DOI] [PubMed] [Google Scholar]
- 47a.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003. [PubMed] [Google Scholar]
- 48.Pettersen JE, Jellum E. The identification and metabolic origin of 2-furoylglycine and 2,5-furandicarboxylic acid in human urine. Clin Chim Acta 41: 199–207, 1972. [DOI] [PubMed] [Google Scholar]
- 49.Phillips J, Muir JG, Birkett A, Lu ZX, Jones GP, O'Dea K, Young GP. Effect of resistant starch on fecal bulk and fermentation-dependent events in humans. Am J Clin Nutr 62: 121–130, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Pitcher MC, Cummings JH. Hydrogen sulphide: a bacterial toxin in ulcerative colitis? Gut 39: 1–4, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 52.Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657–670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rampton D, Cohen S, Crammond V, Gibbons J, Lilburn M, Rabet J, Vince A, Wager J, Wrong O. Treatment of chronic renal failure with dietary fiber. Clin Nephrol 21: 159–163, 1984. [PubMed] [Google Scholar]
- 54.Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 20: 738–747, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robert P. Structural and functional adaptation in renal failure-II. Br Med J 1: 1372–1377, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanghavi S, Whiting S, Uribarri J. Potassium balance in dialysis patients. Semin Dial 26: 597–603, 2013. [DOI] [PubMed] [Google Scholar]
- 57.Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut 35: S35–S38, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scholz M, Fiehn O. SetupX–a public study design database for metabolomic projects. Pac Sympos Biocomput 2007: 169–180, 2007. [PubMed] [Google Scholar]
- 59.Shaw W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci 13: 135–143, 2010. [DOI] [PubMed] [Google Scholar]
- 60.Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 9: 1603–1610, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol 81: 288–302, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Smith EA, Macfarlane GT. Formation of phenolic and indolic compounds by anaerobic bacteria in the human large intestine. Microbial Ecol 33: 180–188, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 20: 779–786, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307: 1955–1959, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Suchodolski JS, Markel ME, Garcia-Mazcorro JF, Unterer S, Heilmann RM, Dowd SE, Kachroo P, Ivanov I, Minamoto Y, Dillman EM, Steiner JM, Cook AK, Toresson L. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLos One 7: e51907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y, O'Riordan MX. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv Appl Microbiol 85: 93–118, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tachon S, Zhou J, Keenan M, Martin R, Marco ML. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol Ecol 83: 299–309, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Toyohara T, Akiyama Y, Suzuki T, Takeuchi Y, Mishima E, Tanemoto M, Momose A, Toki N, Sato H, Nakayama M, Hozawa A, Tsuji I, Ito S, Soga T, Abe T. Metabolomic profiling of uremic solutes in CKD patients. Hypertens Res 33: 944–952, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1131, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Umu OC, Frank JA, Fangel JU, Oostindjer M, da Silva CS, Bolhuis EJ, Bosch G, Willats WG, Pope PB, Diep DB. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome 3: 16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65: 57–62, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vanholder R, De Smet R. Pathophysiologic effects of uremic retention solutes. J Am Soc Nephrol 10: 1815–1823, 1999. [DOI] [PubMed] [Google Scholar]
- 73.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 25: 1897–1907, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, Kieffer DA, Adams SH, Martin RJ. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLos One 9: e114881, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013. [DOI] [PubMed] [Google Scholar]
- 77.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in CKD. Am J Nephrol 37: 1–6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant 27: 2686–2693, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton) 17: 733–738, 2012. [DOI] [PubMed] [Google Scholar]
- 80.Wang L, Christophersen C, Sorich M, Gerber J, Angley M, Conlon M. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism 4: 42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio 3: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams RE, Eyton-Jones HW, Farnworth MJ, Gallagher R, Provan WM. Effect of intestinal microflora on the urinary metabolic profile of rats: a 1H-nuclear magnetic resonance spectroscopy study. Xenobiotica 32: 783–794, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometrics Intelligent Lab Syst 58: 109–130, 2001. [Google Scholar]
- 84.Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39: 230–237, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Younes H, Alphonse JC, Behr SR, Demigné C, Rémésy C. Role of fermentable carbohydrate supplements with a low-protein diet in the course of chronic renal failure: experimental bases. Am J Kidney Dis 33: 633–646, 1999. [DOI] [PubMed] [Google Scholar]
- 87.Younes H, Demigné C, Behr S, Rémésy C. Resistant starch exerts a lowering effect on plasma urea by enhancing urea N transfer into the large intestine. Nutr Res 15: 1199–1210, 1995. [Google Scholar]
- 88.Younes H, Egret N, Hadj-Abdelkader M, Rémésy C, Demigné C, Gueret C, Deteix P, Alphonse JC. Fermentable carbohydrate supplementation alters nitrogen excretion in chronic renal failure. J Renal Nutr 16: 67–74, 2006. [DOI] [PubMed] [Google Scholar]
- 89.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 6: 1535–1543, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]