Abstract
The majority of patients with obesity, insulin resistance, and metabolic syndrome have hypertension, but the mechanisms of hypertension are poorly understood. In these patients, impaired sodium excretion is critical for the genesis of Na+-sensitive hypertension, and prior studies have proposed a role for the epithelial Na+ channel (ENaC) in this syndrome. We characterized high fat-fed mice as a model in which to study the contribution of ENaC-mediated Na+ reabsorption in obesity and insulin resistance. High fat-fed mice demonstrated impaired Na+ excretion and elevated blood pressure, which was significantly higher on a high-Na+ diet compared with low fat-fed control mice. However, high fat-fed mice had no increase in ENaC activity as measured by Na+ transport across microperfused cortical collecting ducts, electrolyte excretion, or blood pressure. In addition, we found no difference in endogenous urinary aldosterone excretion between groups on a normal or high-Na+ diet. High fat-fed mice provide a model of metabolic syndrome, recapitulating obesity, insulin resistance, impaired natriuresis, and a Na+-sensitive elevation in blood pressure. Surprisingly, in contrast to previous studies, our data demonstrate that high fat feeding of mice impairs natriuresis and produces elevated blood pressure that is independent of ENaC activity and likely caused by increased Na+ reabsorption upstream of the aldosterone-sensitive distal nephron.
Keywords: epithelial sodium channel, sodium homeostasis, metabolic syndrome, obesity, insulin resistance
metabolic syndrome affects 50 million Americans (10) and is associated with obesity and insulin resistance. These metabolic derangements are associated with Na+-sensitive hypertension (18, 61), which has a significant impact on morbidity and mortality (11). Based on Guyton's theory, Na+-sensitive hypertension is initiated by impaired natriuresis, which can be caused by enhanced renal Na+ reabsorption (25). However, the mechanisms underlying enhanced Na+ reabsorption in metabolic syndrome are poorly understood.
A compelling case has been made that insulin resistance promotes hypertension in metabolic syndrome. Insulin increases renal Na+ reabsorption in humans (14), dogs (42), and rats (8), and weight loss improves insulin sensitivity and reduces blood pressure (BP) in humans (60a). A major unanswered question is the role of the epithelial Na+ channel (ENaC) in promoting Na+-sensitive hypertension in metabolic syndrome. In humans, increased ENaC activity is sufficient to cause hypertension (55). ENaC is expressed in principal cells along the aldosterone-sensitive distal nephron, and aldosterone is elevated in patients with metabolic syndrome (22, 58). Acute insulin infusion increases ENaC expression and activity in mice (11, 57, 60), and rats with genetic forms of insulin resistance have higher renal expression of ENaC channel subunits (8, 57). Additionally, mice lacking the insulin receptor in principal cells of the distal nephron have decreased ENaC subunit expression and activity (12, 49). However, ENaC activity has not been measured in diet-induced models of insulin resistance that faithfully recapitulate metabolic syndrome.
A diet-induced mouse model of metabolic syndrome provides a valuable tool to investigate the mechanisms underlying renal Na+ handling and hypertension (26) and can be applied in transgenic mice to test the role of candidate genes in this common form of hypertension. In the present study, we asked whether increased ENaC activity mediates the hypertension observed with diet-induced obesity and insulin resistance. We first characterized changes in body weight, plasma insulin, plasma lipid profile, and urinary Na+ (UNa) excretion in C57BL/6 mice fed high-fat and high-fructose diets previously associated with obesity and insulin resistance (33, 47). We then studied BP and the contribution of ENaC-mediated Na+ transport to BP by measuring 1) net transepithelial Na+ flux (JNa) in ENaC-expressing cortical collecting ducts ex vivo, 2) urinary aldosterone excretion; 3) Na+ excretion in response to the ENaC antagonist benzamil, 4) the BP response to benzamil, and 5) the BP response to the aldosterone analog fludrocortisone. Our data suggest that 60% high fat feeding of C57BL/6 mice induces obesity and insulin resistance and stimulates a Na+-sensitive rise in BP due to impaired natriuresis and that this response is independent of aldosterone or ENaC activation.
METHODS
Comparison of diet-induced mouse models of obesity and insulin resistance.
We maintained 6-wk-old C57BL/6 male mice (Jackson Laboratories, Bar Harbor, ME) for 12 wk on a 10% kcal/fat diet [low-fat diet (LFD), D12450B, Research Diets, New Brunswick, NJ], 45% kcal/fat diet [45% high-fat diet (HFD), D12451, Research Diets], or 60% kcal/fat diet (60% HFD, D12492 Research Diets). Additional experiments were done to compare the effect of adding 30% fructose in drinking water to the LFD. Compared with the LFD-fed mice, HFD-fed mice had a lower percentage of calories from carbohydrates but an equivalent percentage of calories from protein. For mice fed the 60% HFD, we replaced food twice weekly to avoid spoiling. We maintained mice on a 7 AM:7 PM light-dark cycle at 22°C. For all experiments, we manipulated mice and/or provided food and water at most once daily in a private room to minimize stress. The Institutional Animal Care and Use Committees of Stanford University and Icahn School of Medicine at Mount Sinai approved the experiments, and we euthanized mice in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
We obtained plasma from the retroorbital plexus in both fasting and nonfasting states and stored it at −80°C. We measured insulin levels by ELISA (EMD Millipore, Billerica, MA). We measured glucose and lipid concentrations on a Siemens Dimension Xpand chemistry analyzer (Siemens Medical Solutions, Malvern, PA). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as fasting serum glucose (in mmol) × fasting serum insulin (in μIU/ml) ÷ 22.5.
Effect of high fat feeding on Na+ excretion and ENaC activity.
We randomized 6- to 7-wk-old male mice to a LFD or 60% HFD for 12 wk (N = 8 mice/group). We acclimated mice to gel food (1% agarose solution with 0.52 ml water/kcal) and individual metabolic cages (Tecniplast) for 6 days on their respective diets containing 0.1% Na+ (11 μmol Na+/kcal, normal Na+ diet). After measuring ENaC activity (see below), we measured food and water intake and collected urine for 24 h. We then transitioned mice to diets with an added 40 g NaCl/4,057 kcal (180 μmol Na+/kcal in both groups, high-Na+ diet) and collected urine at defined intervals. Dosing Na+ by calorie resulted in a higher Na+ content in HFDs by mass but similar Na+ intake between LFD- and HFD-fed mice. To minimize evaporation, we collected urine under mineral oil. To minimize contamination, we washed cages daily with deionized water. We measured urine Na+ and K+ by flame photometry (BWB-XP, BWB Technologies) and averaged urine Na+ excretion within each group.
We measured ENaC activity in these mice by measuring UNa and urinary K+ (UK) excretion after administration of the ENaC antagonist benzamil on both a normal or high-sodium diet. Mice received intraperitoneal vehicle and the following day received 1.4 mg/kg body wt ip benzamil hydrochloride (Sigma-Aldrich, St. Louis, MO) in 5 ml/kg body wt saline. We collected urine for 4 h and compared UNa and UK excretion as well as the UNa-to-UK ratio after benzamil to assess ENaC activity (60). We established the efficacy of this intraperitoneal dose by measuring a natriuretic response in low fat-fed mice on a 0.01% Na+ diet (UNa/UK: vehicle 0.6 ± 0.1 and benzamil 6.9 ± 0.4, N = 4, P < 0.05).
To determine if high fat feeding increases aldosterone or metanephrine secretion, we compared 24-h urine hormone excretion by ELISA on a normal Na+ diet and again after 72 h on a high-Na+ diet (Queen Margaret University, Edinburgh; Metanephrines, Eagle Biosciences, Nashua, NH).
Effect of high fat feeding on Na+ sensitivity and BP.
To compare the dependence of BP on Na+ intake and ENaC in LFD- and HFD-fed mice, we randomized another cohort (N = 7 mice/group) of 6- to 7-wk-old mice to the LFD or 60% HFD for 12 wk before carotid artery catheter implantation (TA11-PAC10, Data Sciences, New Brighton, MN). To maintain the difference in body weight for the duration of this experiment, we increased the palatability of these diets using gel food. Mice were allowed 1 wk to recover from surgery, and we then sampled BP waveforms at 500 Hz in 60-s bursts every 10 min and recorded systolic and diastolic BP, heart rate, and locomotor activity (week 13 of low vs. high fat feeding). Locomotor activity was measured by recording radiotelemeter movement during sampling. Mice were then transitioned to the same high-Na+ diet used in experiments measuring natriuresis. After 2 wk on the high-Na+ diet, we continued BP sampling for another week (week 15 of low vs. high fat feeding). We then added 1.4 mg benzamil·kg body wt−1·day−1 to the gel food. We established the efficacy of this oral dose by measuring a natriuretic response in low fat-fed mice on a 0.01% Na+ diet (UNa/UK: vehicle 0.06 ± 0.02 and benzamil 2.18 ± 0.68, N = 4, P < 0.05). The delivered dose was slightly higher in 60% HFD mice (LFD: 1.61 ± 0.03 mg·kg−1·day−1 and 60% HFD: 1.75 ± 0.02 mg·kg−1·day−1, P < 0.05). BP sampling was continued for 1 wk while mice received benzamil daily (week 16 of low vs. high fat feeding). To assess the dependence of BP on mineralocorticoid activity, we administered 3.6 mg/kg fludrocortisone in gel food (46) (actual dose: LFD 3.79 ± 0.1 mg/kg and 60% HFD 3.55 ± 0.1 mg/kg). To assess the effect of locomotor activity on BP, we defined activity at a score > 5 and rest < 5. Frequency of activity was similar to previous reports (62, 63) in singly housed C57BL/6 mice. Mean arterial pressure (MAP) was calculated from systolic and diastolic BP measurements. Daily MAP is reported, and the overall effect of each condition (high-Na+ diet, benzamil, and fludrocortisone) was defined as the daily average MAP for the duration of the sample period.
Effect of high fat feeding on JNa across ENaC-expressing isolated cortical collecting ducts.
To more directly measure ENaC-mediated reabsorption, we randomized C57BL/6 mice (Charles River) to a LFD or 60% HFD normal-Na+ diet at 25–30 days of age for 5–6 wk. Longer high fat feeding was not possible due to technical constraints of isolation and microdissection of single tubules in older animals. We confirmed that these HFD-fed mice had significantly higher body weight and plasma insulin (data not shown). We then removed the left kidney, prepared coronal slices, and dissected single cortical collecting ducts in cold (4°C) MHSS. We preperfused kidneys to facilitate duct isolation as previously described (67).
We immediately transferred each isolated tubule (mean length: 0.50 ± 0.02 mm, n = 12 tubules) to a temperature- and O2/CO2-controlled specimen chamber, mounted it on concentric glass pipettes, and perfused and bathed it at 37°C with Burg's perfusate. We performed transport measurements in the absence of transepithelial osmotic gradients and thus assumed water transport to be zero. We collected three samples of tubular fluid under water-saturated light mineral oil by timed filling of a calibrated ∼12-nl volumetric constriction pipette at low (∼1.3 nl·min−1·mm−1) and high (∼5 nl·min−1·mm−1) flow rates, as indicated. To determine the concentrations of Na+ delivered to the tubular lumen, we added ouabain (1 mM) to the bath at the conclusion of each experiment to inhibit all active transport and obtained an additional three samples. We determined Na+ concentrations of perfusate and collected tubular fluid by helium glow photometry and calculated the rates of net transport (JNa; in pmol·min−1·mm−1 tubular length) using standard flux equations (19). We averaged calculated ion fluxes to obtain a single mean rate of ion transport for the cortical collecting duct at each flow rate under each condition.
Statistical analysis.
For analysis of multiple groups to a control group (i.e., low fat-fed mice), we used one-way ANOVA. For post hoc analysis, we used Dunnett's test to compare each group with the control group (54). For experiments with only two groups, we used a two-tailed paired or unpaired Student's t-tests. We provided results as means ± SE; n is the number of tubules and N is number of mice. We defined statistical significance at P values of <0.05. For BP experiments, hemodynamic values were averaged for each mouse on each day, and we used each daily average value per mouse to test for significance.
RESULTS
High fat feeding induces obesity and insulin resistance.
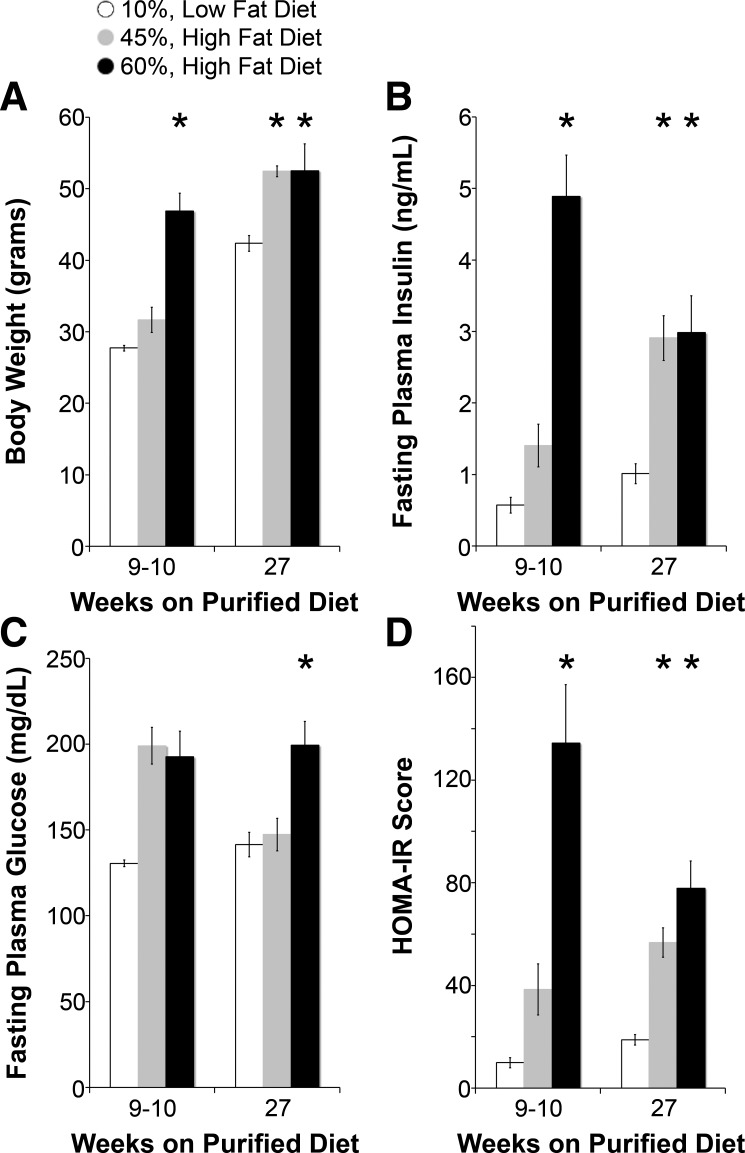
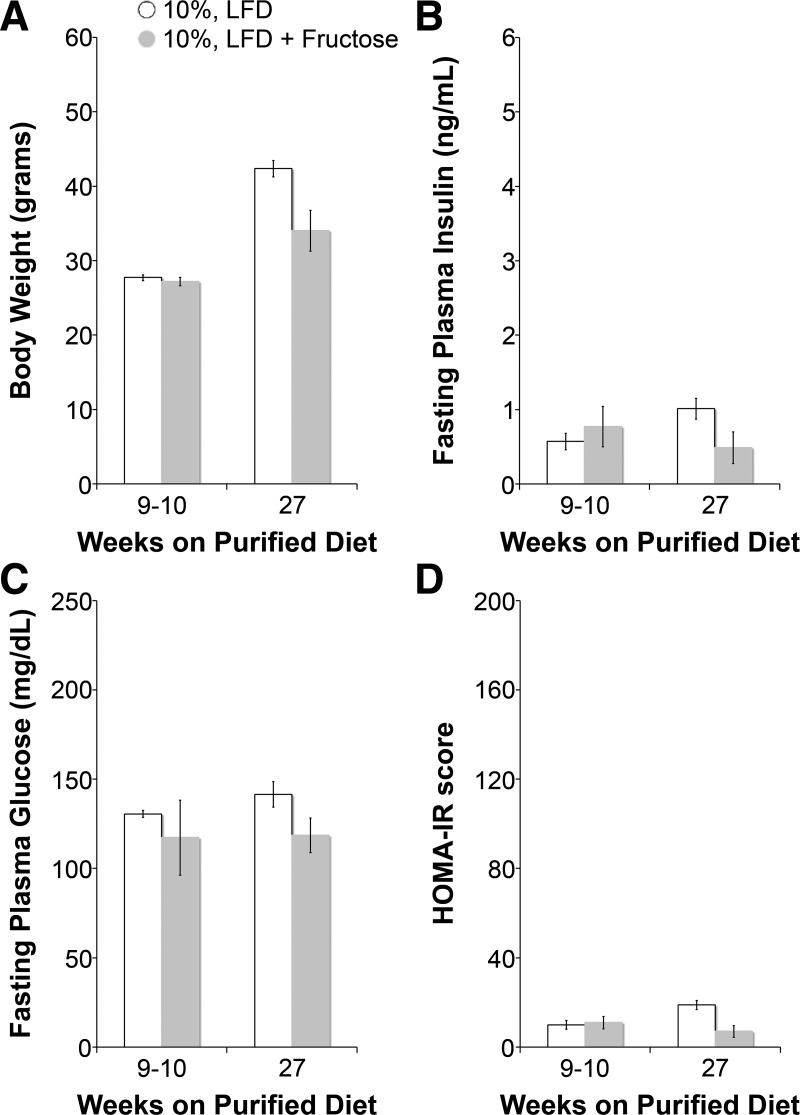
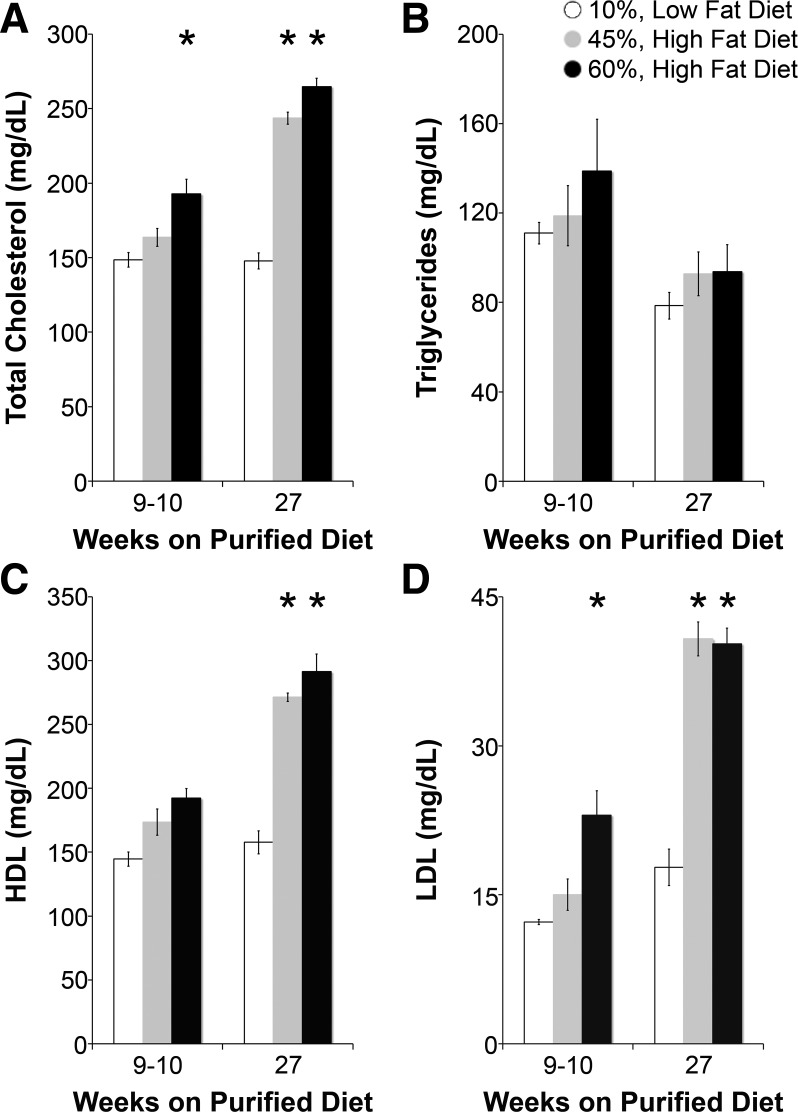
As shown in Fig. 1, after 10 wk on their respective diets, 60% HFD-fed mice but not 45% HFD-fed mice had significantly higher body weights and fasting plasma insulin levels than LFD-fed mice. HOMA-IR, an index of insulin resistance (45), was also higher in 45% HFD-fed mice and significantly higher in 60% HFD-fed mice at 10 wk. Fructose did not contribute to obesity, fasting plasma glucose, or insulin resistance (Fig. 2). As shown in Fig. 3, lipid profiles at 10 wk on specialized diets were no different in 45% HFD-fed mice compared with LFD-fed mice, but total cholesterol and low-density lipoproteins were significantly higher in 60% HFD-fed mice. Given a more severe metabolic phenotype, we measured Na+ reabsorption, BP, and ENaC activity in 60% HFD-fed mice as a model of obesity and insulin resistance.
Fig. 1.
High fat-fed mice develop obesity and hyperinsulinemia. A–D: body weight (A), fasting plasma insulin (B), fasting plasma glucose (C), and homeostatic model assessment of insulin resistance (HOMA-IR; D) measurements of diet-induced models at 9–10 and 27 wk of feeding. HFD, high-fat diet. N = 4–5 mice/group. *P < 0.05 compared with low-fat diet (LFD)-fed mice.
Fig. 2.
Fructose feeding does not cause obesity or insulin resistance. A–D: body weight (A), fasting plasma insulin (B), fasting plasma glucose (C), and HOMA-IR (D) measurements of LFD versus. LFD + fructose at 9–10 and 27 wk of feeding. N = 4 mice/group. All P values were >0.05 compared with LFD-fed mice.
Fig. 3.
High fat-fed mice do not develop atherogenic dyslipidemia. A–D: fasting total plasma cholesterol (A), triglycerides (B), high-density lipoprotein (HDL; C), and low-density lipoprotein (LDL; D) of diet-induced models at 9–10 and 27 wk of feeding. N = 4–5 mice/group. *P < 0.05 compared with LFD-fed mice.
High fat feeding impairs Na+ excretion.
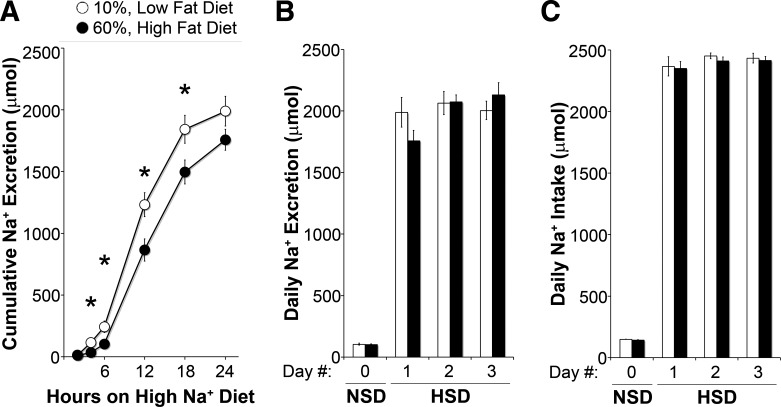
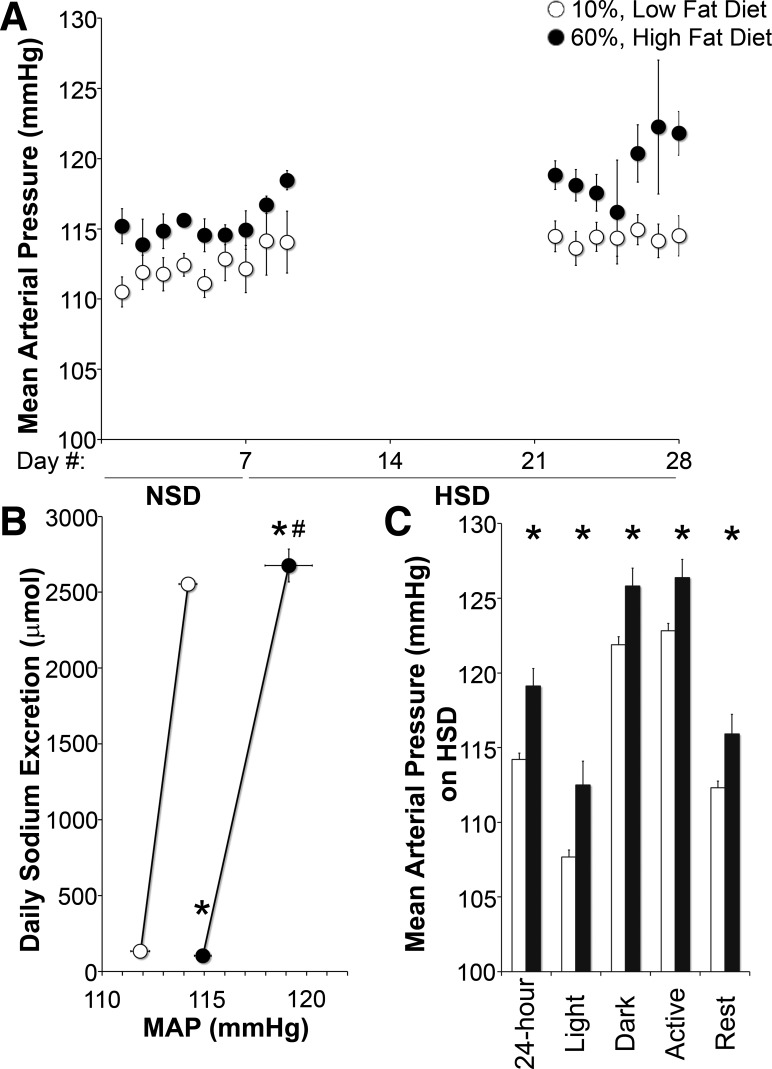
Impaired natriuresis is a defining feature of Na+-sensitive hypertension (25) and has been measured in animals, including humans, in models of obesity and during acute and chronic insulin infusion (14, 21, 42). To assess Na+ reabsorption in the setting of obesity and insulin resistance, we measured Na+ excretion after an acute increase in Na+ intake in LFD- and HFD-fed mice. As shown in Fig. 4A, after 4 h, HFD-fed mice excreted significantly less Na+ than LFD-fed control mice, and mice came into balance by 24 h. As shown in Fig. 4B, Na+ excretion was also similar on days 2 and 3 of the high- Na+ diet. As shown in Fig. 4C, Na+ intake on both a normal or high-Na+ diet was similar between groups for the duration of the experiment.
Fig. 4.
Impaired natriuresis in high fat-fed mice. A: cumulative Na+ excretion for the first 24 h on a high-Na+ diet (HSD). B: daily urine Na+ excretion. C: Na+ intake on a normal Na+ diet (NSD) and the first 3 days on a HSD. N = 8 mice/group. *P < 0.05 compared with LFD-fed mice.
High fat feeding induces Na+-sensitive elevated BP.
To determine the effect of high fat feeding on the Na+ sensitivity of BP, we measured BP while controlling Na+ intake. As shown in Fig. 5B, Na+ intake on both normal or high-Na+ diets was very similar between groups. Figure 5A shows that on a normal Na+ diet, MAP over 7 days was higher in HFD-fed mice (LFD: 111.8 ± 0.5 mmHg vs. HFD: 114.8 ± 0.4 mmHg, P < 0.05). On a high-Na+ diet, MAP increased further: 2.4 ± 0.3 and 4.5 ± 1.2 mmHg in LFD- and HFD-fed mice, respectively, resulting in a +5.1 mmHg higher MAP in HFD-fed mice (P < 0.05). Figure 5B shows that HFD-fed mice had a significant rightward shift and decrease in the slope of the pressure-natriuresis curve compared with LFD-fed mice. Changes in systolic and diastolic BP followed similar patterns to MAP in LFD- and HFD-fed mice on a normal and high-Na+ diet (Table 1).
Fig. 5.
Increased Na+-sensitive blood pressure (BP) in high fat-fed mice. A: daily group-averaged mean arterial pressure (MAP) on a NSD followed by HSD. B: pressure-natriuresis curve. C: group-averaged MAP over different intervals in mice fed a HSD. N = 4–6 mice/group. *P < 0.05 of MAP compared with LFD-fed mice; #P < 0.05 of the change in MAP from the NSD to HSD compared with LFD-fed mice.
Table 1.
Group-averaged systolic and diastolic blood pressures over different intervals in mice fed a normal or high-Na+ diet
| 24 Hour | Light | Dark | Active | Rest | |
|---|---|---|---|---|---|
| Normal Na+ diet (LFD: N = 6 mice and HFD: N = 4 mice) | |||||
| Systolic blood pressure | |||||
| LFD | 124.4 ± 0.7 | 117.9 ± 0.6 | 132.8 ± 0.9 | 134.5 ± 1.0 | 118.2 ± 1.0 |
| HFD | 129.4 ± 1.0* | 122.0 ± 1.1* | 136.7 ± 1.1* | 136.2 ± 1.3 | 124.8 ± 1.0* |
| Diastolic blood pressure | |||||
| LFD | 97.1 ± 0.5 | 91.0 ± 0.6 | 103.0 ± 0.6 | 104.4 ± 0.7 | 91.0 ± 0.6 |
| HFD | 99.0 ± 0.7* | 93.1 ± 0.6* | 104.9 ± 1.0 | 104.6 ± 1.0 | 95.0 ± 0.6* |
| High Na+ diet (LFD: N = 6 mice and HFD N = 4 mice) | |||||
| Systolic blood pressure | |||||
| LFD | 128.6 ± 0.6 | 121.0 ± 0.5 | 136.8 ± 0.7 | 137.8 ± 0.6 | 126.2 ± 0.6 |
| HFD | 133.9 ± 1.0* | 127.1 ± 1.3* | 140.5 ± 1.2* | 141.4 ± 1.2* | 130.5 ± 1.3* |
| Diastolic blood pressure | |||||
| LFD | 99.0 ± 0.4 | 93.0 ± 0.6 | 105.9 ± 0.5 | 106.9 ± 0.6 | 97.1 ± 0.5 |
| HFD | 103.6 ± 1.6* | 97.7 ± 1.7* | 109.5 ± 1.7* | 110.1 ± 1.7* | 100.7 ± 1.6* |
Values are means ± SE.
LFD, low-fat diet; HFD, high-fat diet.
P < 0.05 compared with LFD-fed mice.
HFD-induced elevated BP is independent of circadian rhythm or activity.
Figure 5C shows the Na+-sensitive increase in MAP regardless of dark/light period or activity. The higher MAP in HFD-fed mice was not due to differences in activity, as neither dietary fat nor Na+ content affected locomotor frequency or magnitude (mean activity magnitude: LFD 0.64 ± 0.02 and HFD 0.66 ± 0.03; activity frequency: LFD 38 ± 2% of a day and HFD 39 ± 2% of a day).
HFD-fed mice have impaired natriuresis and elevated BP independent of ENaC activity.
To measure the contribution of ENaC-mediated Na+ transport to the impaired Na+ excretion and elevated Na+-sensitive BP in HFD-fed mice, we measured 1) JNa across ENaC-expressing cortical collecting ducts, 2) stimuli for ENaC activity, and 3) Na+ excretion and BP in the setting of ENaC inhibition.
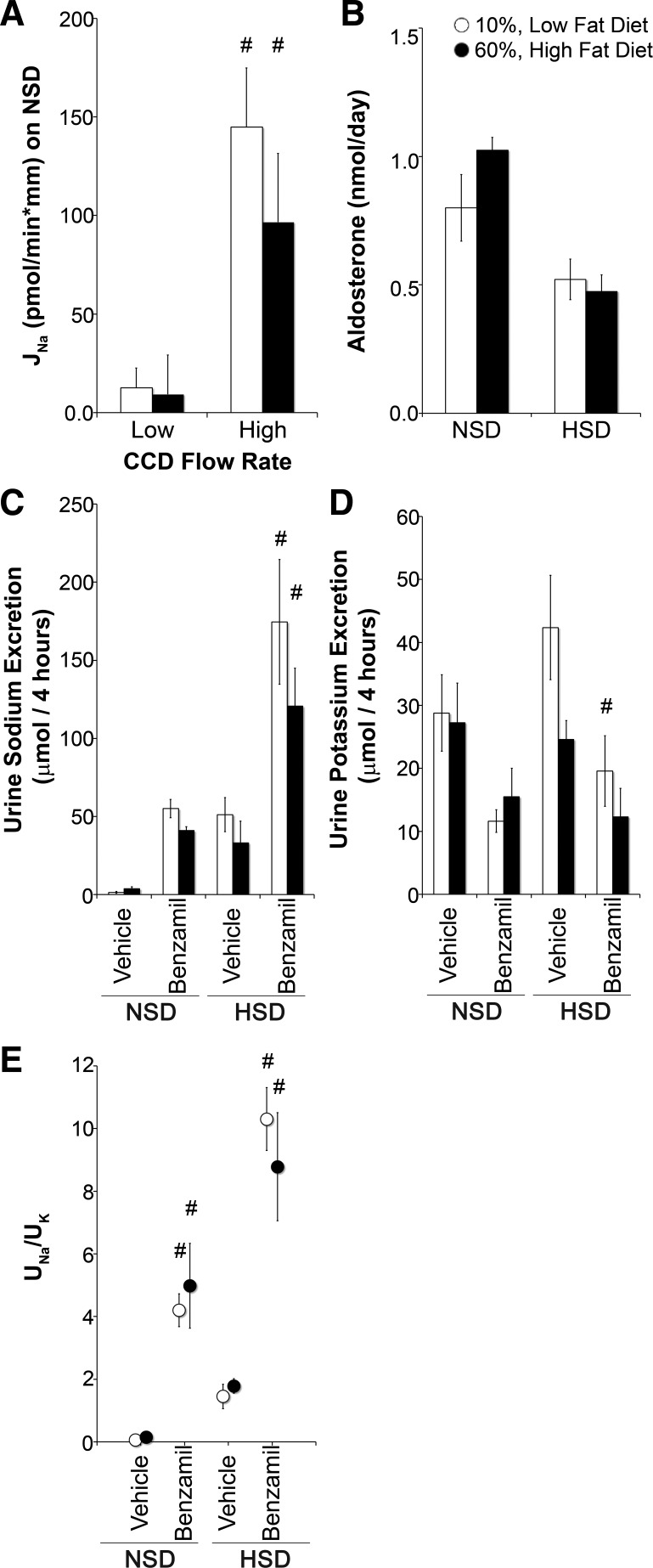
Figure 6A shows that when ENaC-expressing cortical collecting ducts were perfused at a low flow rate, there was no significant difference in net transepithelial JNa between tubules from LFD- and HFD-fed mice on a normal Na+ diet. To activate quiescent ENaC channels, we perfused tubules at high flow rates (53), and JNa was similarly enhanced in tubules from LFD- and HFD-fed mice.
Fig. 6.
Comparable epithelial Na+ channel (ENaC) activity and aldosterone in low fat- and high fat-fed mice. A: net transepithelial Na+ transport (JNa) under low or high flow conditions of isolated cortical collecting duct segments from mice on a NSD. Positive deflection indicates Na+ absorption. n = 6 tubules/group. B: 24-h urinary aldosterone excretion on a NSD or HSD. C and D: urinary Na+ (UNa; C) and urinary K+ (UK; D) excretion. E: ratio of UNa to UK in mice after 4 h of vehicle and intraperitoneal benzamil on a NSD or HSD. N = 8 mice/group. *P < 0.05 compared with LFD-fed mice; #P < 0.05 compared with low flow (A) or vehicle (C–E).
As aldosterone levels directly correlate with Quételet's index (body mass index) in humans with metabolic syndrome (6, 35) and is a primary regulator of ENaC activity (17), we measured aldosterone excretion as a surrogate for production (50). Figure 6B shows that there was no difference between LFD- and HFD-fed mice on either a normal or high-Na+ diet.
We also measured ENaC activity by measuring electrolyte excretion induced by channel inhibition with benzamil on both a normal or high-Na+ diet. As shown in Fig. 6, C and D, neither Na+ nor K+ excretion was significantly different between LFD- and HFD-fed groups after vehicle or benzamil administration. As shown in Fig. 6E, UNa-to-UK concentration ratio after benzamil, a surrogate of ENaC inhibition, increased compared with vehicle but was not significantly different between LFD- and HFD-fed mice on a normal or high-Na+ diet.
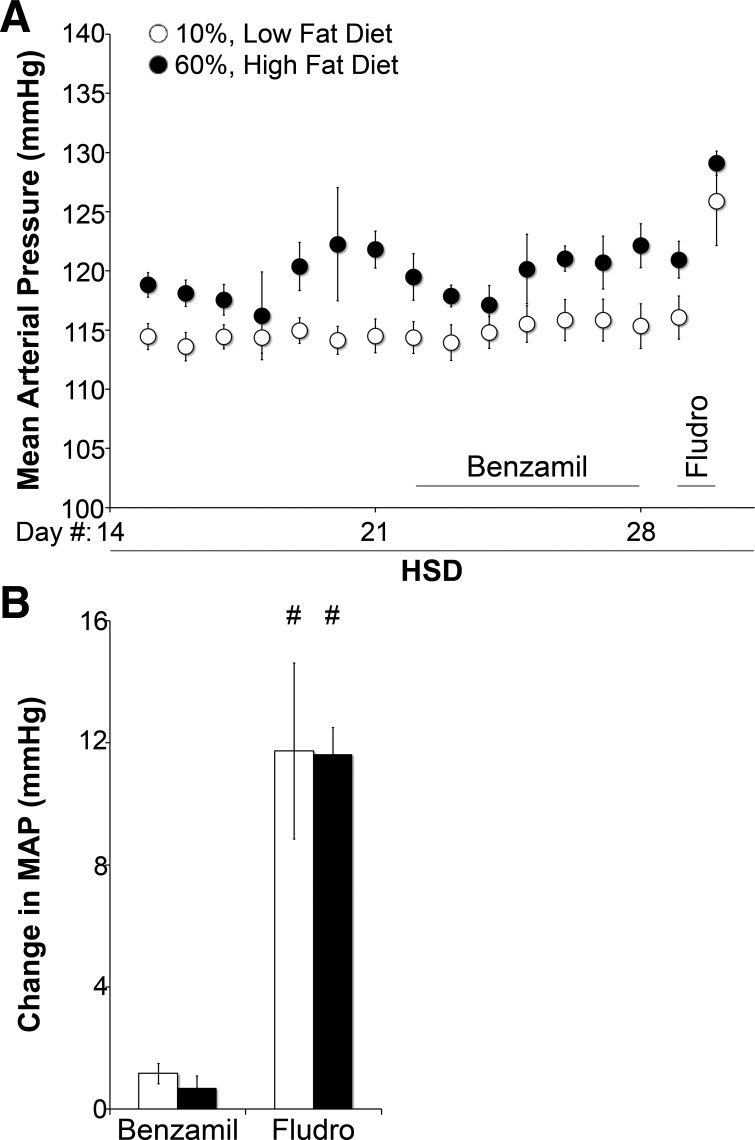
To directly evaluate the contribution of ENaC-mediated Na+ reabsorption or ENaC activity to BP in HFD-fed mice, we compared the effect of chronic ENaC inhibition with benzamil or ENaC stimulation with the aldosterone analog fludrocortisone. Figure 7 shows that HFD-fed mice exhibited higher MAP than LFD-fed mice in the presence or absence of benzamil. Benzamil did not significantly lower MAP in either group (+1.2 mmHg in LFD-fed mice, P = 0.21; +0.7 mmHg in HFD-fed mice, P = 0.44). After washout of benzamil, mice were given fludrocortisone for 1 day. As shown in Fig. 7B, fludrocortisone increased MAP similarly in both groups on a high-Na+ diet but did not significantly increase MAP more in HFD- versus LFD-fed mice.
Fig. 7.
Elevated BP in high fat-fed mice is independent of changes in ENaC activity. A: daily group-averaged MAP on a HSD alone, with benzamil, and with fludrocortisone. B: mean change in MAP with benzamil or fludrocortisone administration. N = 4–6 mice/group. *P < 0.05 compared with LFD-fed mice; #P < 0.05 compared with vehicle treatment.
DISCUSSION
We compared three potential diet-induced forms of metabolic syndrome and found that 60% high fat feeding best recapitulates the cardinal features of metabolic syndrome: obesity, insulin resistance, impaired renal Na+ homeostasis, and Na+-sensitive elevated BP. We then used this model to demonstrate that a Na+-sensitive rise in BP is independent of ENaC-mediated Na+ reabsorption.
Impaired natriuresis with high fat feeding.
A primary increase in Na+ reabsorption is necessary for the Na+-sensitive hypertension observed in rodents and humans with obesity and insulin resistance (14, 18, 21, 42, 61). One possible mechanism for the impaired natriuresis is enhancement of insulin-induced Na+ reabsorption. While humans with chronic insulin resistance demonstrate impaired natriuresis during acute insulin infusion (56), it is unknown whether this is due to a direct effect of insulin, an indirect effect of insulin-mediated vasodilation causing decreased renal perfusion, or an alternative mechanism. Here, we increased only Na+ intake and demonstrate impaired natriuresis in chronically hyperinsulinemic mice. While these data do not prove that hyperinsulinemia is responsible for impaired natriuresis in high fat-fed mice, they do provide a model to test the mechanisms of renal Na+ handling and elevated BP in the setting of insulin resistance.
The magnitude of Na+-sensitive BP with high fat feeding.
The reported changes in BP with diet-induced obesity in C57BL/6 mice range from −13 to +37 mmHg(5, 7, 15, 16, 32, 36, 47, 52, 59, 65). These studies used different methodologies that could explain this variability. Tail-cuff measurement is unlikely to detect modest differences in BP between groups. Standard mouse chow is frequently used as a control diet, and higher phytoestrogen (contained in soybean or alfalfa meal) and fiber content in chow may lower BP and alter insulin sensitivity (4, 64). HFDs are more calorie dense, so they must contain a higher Na+ content (by weight) to provide a similar Na+ intake to LFD-fed mice. Experiments that compare diets with the same percentage of Na+ content result in lower Na+ intake in HFD-fed mice. Furthermore, high-Na+ diets can be unpalatable to mice, resulting in weight loss and loss of the phenotype in HFD-fed mice. Here, we used purified LFDs and HFDs formulated as a gel, with Na+ content normalized to calorie content, resulting in comparable Na+ and calorie intake, and radiotelemetry to detect a modest Na+-sensitive increase in BP in HFD-fed mice. For paired experiments, BP was sampled on a high-Na+ diet 2 wk later than on a normal Na+ diet. Thus, we cannot excluded an effect of additional time on purified diet, but HFD-fed mice only gained an additional 1.6 g (3.5% of mean body weight) during this interval.
These small changes in BP in high fat-fed mice may translate to a clinically significant difference in humans. For example, patients with hypertension due to Liddle's syndrome, i.e., gain-of-function mutations in ENaC subunits, have an average MAP increase of 43 mmHg (44). Transgenic mice carrying similar mutations have an increase in MAP of only ∼10 mmHg on a high-Na+ diet (51), similar to the degree of elevated BP we observed. Similarly, some patients with Gordon's syndrome, another form of Na+-sensitive hypertension, have a systolic BP of >200 mmHg (3), but transgenic mice with mutant, inactive with no lysine kinase (WNK)4, recapitulating this condition, have a systolic BP of <10 mmHg higher than wild-type mice and only 13 mmHg higher than mice that overexpress wild-type WNK4 (38).
Metabolic syndrome, aldosterone, and ENaC activity.
We examined the role of aldosterone, aldosterone sensitivity, and activation of the aldosterone-dependent ENaC pathway in the impaired natriuresis and Na+-sensitive elevated BP of HFD-fed mice. Unlike in humans with metabolic syndrome (22, 58), we found that aldosterone excretion, as an index of production, was no different between LFD- and HFD-fed mice on a normal or high-Na+ diet. The similar increase in MAP with mineralocorticoid agonist administration demonstrates that aldosterone sensitivity is unchanged with high fat feeding. We speculate that an altered lipid profile in HFD-fed mice may be responsible for the normal aldosterone level. In humans, low high-density lipoprotein is specifically correlated with high aldosterone (24, 41), yet we and others have observed an increase in high-density lipoprotein in HFD-fed mice(29).
We also directly studied ENaC activity both ex vivo and in vivo on a normal or high- Na+ diet and found no differences in Na+ transport, urine Na+ excretion, or BP. To our knowledge, these are the first measurements of ENaC channel activity or cortical collecting duct perfusion in obese or insulin-resistant rodents. These data were surprising because acute insulin infusion enhances ENaC activity, and rat models of inherited insulin resistance have increased ENaC subunit expression (8). However, insulin concentrations are significantly lower in high fat-fed mice compared with prior reports of acute insulin infusion, and insulin was not present in the perfusate. The magnitude of hyperinsulinemia measured in our HFD-fed mice is similar to that in humans with metabolic syndrome (28). This distinction was highlighted by Frindt and Palmer (21), who showed that concentrations of insulin measured in humans and animals with insulin resistance did not influence ENaC activity in split-open rat cortical collecting ducts (12, 49). Similarly, in cell culture, insulin stimulates ENaC-mediated Na+ current only at supraphysiological doses of insulin (9, 23). Mice lacking the insulin receptor in ENaC-expressing principal cells (40) have diminished ENaC activity. Although these knockout studies would imply that hyperinsulinemia should enhance ENaC activity, our data demonstrate that, in the setting of diet-induced metabolic syndrome and chronic insulin resistance, enhanced ENaC activity in the distal nephron is not responsible for impaired natriuresis or Na+-sensitive elevated BP in high fat-fed mice. We cannot exclude a role for acute, low doses of insulin to stimulate ENaC in vivo. We also cannot exclude a role for ENaC in the central nervous system (1) as benzamil may not cross the blood-brain barrier. Importantly, these results do not exclude a role for ENaC in models of metabolic syndrome with elevated aldosterone but indicate that high fat feeding is sufficient to increase Na+ reabsorption and BP independent of aldosterone or ENaC-mediated transport.
ENaC-independent mechanisms of impaired natriuresis in diet-induced obesity and insulin resistance.
Whether other mouse models of metabolic syndrome develop hypertension independent of ENaC remains unknown. Among mouse models of metabolic syndrome, NZBWF1 and KKAy/a strains develop obesity, insulin resistance, and elevated BP, but the mechanisms are unknown. Leptin receptor-deficient db/db mice develop obesity and insulin resistance, but BP measurements are variable (43). Of note, this latter model can develop diabetes with glucosuria (48) that can stimulate compensatory Na+ reabsorption, thereby confounding the assessment of impaired natriuresis as a stimulus for hypertension.
Several renal tubular transporters are potential mediators of the observed impaired natriuresis. Na+-Cl− cotransporter (NCC) and STE20/SPS1-related proline/alanine-rich kinase expression are increased in obese Zucker rats compared with lean rats (36). NCC activity is also increased by chronic insulin infusion in Sprague-Dawley rats (57). Huang et al. (31) demonstrated that deletion of serum/glucocorticoid-regulated kinase 1, a kinase that activates ENaC, prevented HFD-induced hypertension (31), but this kinase can also modulate Na+ transporters proximal to the aldosterone-sensitive distal nephron (20, 39, 66). Recently, Davies et al. showed that high fat feeding of mice increases Na+-K+-2Cl−1 cotransporter 2 phosphorylation and decreases AMP-activated kinase activity (13). Using microperfusion, we examined Na+ transport in isolated cortical collecting ducts and found no difference between LFD- and HFD-fed mice, excluding ENaC the thiazide-sensitive Na+-dependent dicarboxylate cotransporter. Thus, high fat feeding likely increases Na+ transport in upstream segments of the nephron. These findings provide a basis for future experiments using genetic knockouts or pharmacotherapies to measure the contribution of upstream transporters in mediating the effect of HFD on the kidney tubule.
Hall and others (27, 30, 34) have also demonstrated that increased renal sympathetic nerve activity results in impaired natriuresis and increased BP in obese dogs. Insulin and catecholamines have also been shown to activate the sympathetic nervous system (37). Urine catecholamine excretion, and hence production, was not different between LFD- and HFD-fed mice (data not shown). These data are consistent with data in humans and dogs that demonstrate organ-specific changes in sympathetic activity rather than overall sympathetic tone. The mechanisms by which a HFD may increase renal sympathetic nerve activity in mice will be an interesting area for future research.
After comparing several potential diet-induced models of metabolic syndrome, we found that 60% HFD-fed C57BL/6 mice develop obesity, insulin resistance, impaired natriuresis, and modest Na+-sensitive elevated BP. We demonstrate that this impaired natriuresis is independent of ENaC activity, highlighting the importance of a mouse model with peripheral insulin resistance and physiological levels of chronic hyperinsulinemia. This model provides a valuable tool to investigate mechanisms of Na+ handling and hypertension, including insulin-dependent or insulin-independent signaling, in the setting of obesity and insulin resistance.
GRANTS
J. M. Nizar was supported by the Tashia and John Morgridge Endowed Postdoctoral Fellowship, Child Health Research Institute at Stanford University, and Stanford Clinical and Translational Science Award [National Institutes of Health (NIH) Grant UL1-TR-001085]. J. M. Nizar is currently supported by the American Heart Association through an award from Blake R. Grossman and Family in honor of Richard and Dixie Grossman. R. B. McClellan also received funding from the Child Health Research Institute. D. G. Goens was supported by NIH Grant 1-RO1-DK-091565-02S1. Y. Zhou and L. M. Satlin receive support from the NIH George O'Brien Kidney Research Core Center (Grant P30-DK-079307). V. Bhalla has received support from NIH Grants 5-R03-DK-083613 and 1-R01-DK-091565 and the American Society of Nephrology (Carl W. Gottschalk Research Grant).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.N., W.D., M.P., L.M.S., and V.B. conception and design of research; J.M.N., W.D., R.B.M., M.L., Y.Z., J.W., D.G.G., M.Z., and D.B. performed experiments; J.M.N., R.B.M., Y.Z., N.V., L.M.S., and V.B. analyzed data; J.M.N., L.M.S., and V.B. interpreted results of experiments; J.M.N., L.M.S., and V.B. prepared figures; J.M.N. drafted manuscript; J.M.N. and V.B. edited and revised manuscript; J.M.N., W.D., R.B.M., M.L., Y.Z., J.W., D.G.G., M.Z., N.V., D.B., M.P., L.M.S., and V.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Drs. Glenn Chertow, Timothy Meyer, and Alan Pao for scientific discussion and critical review of the manuscript.
REFERENCES
- 1.Abrams JM, Osborn JW. A role for benzamil-sensitive proteins of the central nervous system in the pathogenesis of salt-dependent hypertension. Clin Exp Pharmacol Physiol 35: 687–694, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achard JM, Disse-Nicodeme S, Fiquet-Kempf B, Jeunemaitre X. Phenotypic and genetic heterogeneity of familial hyperkalaemic hypertension (Gordon syndrome). Clin Exp Pharmacol Physiol 28: 1048–1052, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Al-Nakkash L, Martin JB, Petty D, Lynch SM, Hamrick C, Lucy D, Robinson J, Peterson A, Rubin LJ, Broderick TL. Dietary genistein induces sex-dependent effects on murine body weight, serum profiles, and vascular function of thoracic aortae. Gend Med 9: 295–308, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension 58: 271–279, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, Garg R. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab 92: 4472–4475, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergaya S, Faure S, Baudrie V, Rio M, Escoubet B, Bonnin P, Henrion D, Loirand G, Achard JM, Jeunemaitre X, Hadchouel J. WNK1 regulates vasoconstriction and blood pressure response to α1-adrenergic stimulation in mice. Hypertension 58: 439–445, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Bickel CA, Verbalis JG, Knepper MA, Ecelbarger CA. Increased renal Na-K-ATPase, NCC, and β-ENaC abundance in obese Zucker rats. Am J Physiol Renal Physiol 281: F639–F648, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Blazer-Yost BL, Liu X, Helman SI. Hormonal regulation of ENaCs: insulin and aldosterone. Am J Physiol Cell Physiol 274: C1373–C1379, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, Hoefler M, Unger T, Sharma AM. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens 17: 904–910, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Byrd JB, Brook RD. A critical review of the evidence supporting aldosterone in the etiology and its blockade in the treatment of obesity-associated hypertension. J Hum Hypertens 28: 3–9, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Carnethon MR, Palaniappan LP, Burchfiel CM, Brancati FL, Fortmann SP. Serum insulin, obesity, and the incidence of type 2 diabetes in black and white adults: the atherosclerosis risk in communities study: 1987–1998. Diabetes Care 25: 1358–1364, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies M, Fraser SA, Galic S, Choy SW, Katerelos M, Gleich K, Kemp BE, Mount PF, Power DA. Novel mechanisms of Na+ retention in obesity: phosphorylation of NKCC2 and regulation of SPAK/OSR1 by AMPK. Am J Physiol Renal Physiol 307: F96–F106, 2014. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 55: 845–855, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deji N, Kume S, Araki S, Isshiki K, Araki H, Chin-Kanasaki M, Tanaka Y, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Maegawa H, Uzu T. Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem Biophys Res Commun 418: 559–564, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Deji N, Kume S, Araki S, Soumura M, Sugimoto T, Isshiki K, Chin-Kanasaki M, Sakaguchi M, Koya D, Haneda M, Kashiwagi A, Uzu T. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol 296: F118–F126, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC. Cell-specific expression of epithelial sodium channel α, β, and γ subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol 127: 1907–1921, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyer AR, Elliott P, Shipley M, Stamler R, Stamler J. Body mass index and associations of sodium and potassium with blood pressure in INTERSALT. Hypertension 23: 729–736, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faresse N, Lagnaz D, Debonneville A, Ismailji A, Maillard M, Fejes-Toth G, Naray-Fejes-Toth A, Staub O. Inducible kidney-specific Sgk1 knockout mice show a salt-losing phenotype. Am J Physiol Renal Physiol 302: F977–F985, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Frindt G, Palmer LG. Effects of insulin on Na and K transporters in the rat CCD. Am J Physiol Renal Physiol 302: F1227–F1233, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab 95: 1986–1990, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Rodriguez E, Gaeggeler HP, Rossier BC. IGF-1 vs. insulin: respective roles in modulating sodium transport via the PI-3 kinase/Sgk1 pathway in a cortical collecting duct cell line. Kidney Int 71: 116–125, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension 25: 30–36, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Guyton AC. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension 19: I2–18, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Hall JE. The kidney, hypertension, and obesity. Hypertension 41: 625–633, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauache OM, Vieira JG. Fasting insulin concentration is highly correlated with quantitative insulin sensitivity check index. Endocrine 21: 137–138, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Hayek T, Chajek-Shaul T, Walsh A, Agellon LB, Moulin P, Tall AR, Breslow JL. An interaction between the human cholesteryl ester transfer protein (CETP) and apolipoprotein A-I genes in transgenic mice results in a profound CETP-mediated depression of high density lipoprotein cholesterol levels. J Clin Invest 90: 505–510, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Healy V, Thompson C, Johns EJ. The adrenergic regulation of proximal tubular Na/H exchanger 3 in the rat. Acta Physiol (Oxf) 210: 678–689, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Huang DY, Boini KM, Osswald H, Friedrich B, Artunc F, Ullrich S, Rajamanickam J, Palmada M, Wulff P, Kuhl D, Vallon V, Lang F. Resistance of mice lacking the serum- and glucocorticoid-inducible kinase SGK1 against salt-sensitive hypertension induced by a high-fat diet. Am J Physiol Renal Physiol 291: F1264–F1273, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Inoue E, Ichiki T, Takeda K, Matsuura H, Hashimoto T, Ikeda J, Kamiharaguchi A, Sunagawa K. Beraprost sodium, a stable prostacyclin analogue, improves insulin resistance in high-fat diet-induced obese mice. J Endocrinol 213: 285–291, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Kanarek RB, Orthen-Gambill N. Differential effects of sucrose, fructose and glucose on carbohydrate-induced obesity in rats. J Nutr 112: 1546–1554, 1982. [DOI] [PubMed] [Google Scholar]
- 34.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 25: 893–897, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Kidambi S, Kotchen JM, Grim CE, Raff H, Mao J, Singh RJ, Kotchen TA. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension 49: 704–711, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Komers R, Rogers S, Oyama TT, Xu B, Yang CL, McCormick J, Ellison DH. Enhanced phosphorylation of Na+-Cl− co-transporter in experimental metabolic syndrome: role of insulin. Clin Sci (Lond) 123: 635–647, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krieger DR, Landsberg L. Mechanisms in obesity-related hypertension: role of insulin and catecholamines. Am J Hypertens 1: 84–90, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86: 1151–1178, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Garikepati RM, Tsukerman S, Kohan D, Wade JB, Tiwari S, Ecelbarger CM. Reduced ENaC activity and blood pressure in mice with genetic knockout of the insulin receptor in the renal collecting duct. Am J Physiol Renal Physiol 304: F279–F288, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lind L, Lithell H. Hypertension, hyperlipidemia, insulin resistance and obesity: parts of a metabolic syndrome. Blood Press Suppl 4: 49–54, 1992. [PubMed] [Google Scholar]
- 42.Manhiani MM, Cormican MT, Brands MW. Chronic sodium-retaining action of insulin in diabetic dogs. Am J Physiol Renal Physiol 300: F957–F965, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens 17: 1949–1953, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Matsushita T, Miyahara Y, Matsushita M, Yakabe K, Yamaguchi K, Furukawa K, Iwasaki T, Naito T, Ikeda S, Miyazaki M, Ogata H, Ohzono Y, Harada T, Kohno S. Liddle's syndrome in an elderly woman. Intern Med 37: 391–395, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 46.McCormick JA, Nelson JH, Yang CL, Curry JN, Ellison DH. Overexpression of the sodium chloride cotransporter is not sufficient to cause familial hyperkalemic hypertension. Hypertension 58: 888–894, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills E, Kuhn CM, Feinglos MN, Surwit R. Hypertension in CB57BL/6J mouse model of non-insulin-dependent diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 264: R73–R78, 1993. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffari MS, Abdelsayed R, Liu JY, Zakhary I, Baban B. Renal distal tubule proliferation and increased aquaporin 2 level but decreased urine osmolality in db/db mouse: treatment with chromium picolinate. Exp Mol Pathol 92: 54–58, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niskanen LK, Uusitupa MI, Sarlund H, Siitonen O, Pyorala K. Five-year follow-up study on plasma insulin levels in newly diagnosed NIDDM patients and nondiabetic subjects. Diabetes Care 13: 41–48, 1990. [DOI] [PubMed] [Google Scholar]
- 50.Pimenta E, Calhoun DA. Resistant hypertension and aldosteronism. Curr Hypertens Rep 9: 353–359, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger JD, Hummler E, Rossier BC. A mouse model for Liddle's syndrome. J Am Soc Nephrol 10: 2527–2533, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med 17: 883–887, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satlin L.M, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Shaffer JP. Multiple comparisons emphasizing selected contrasts: an extension and generalization of Dunnett's procedure. Biometrics 33: 293–303, 1977. [PubMed] [Google Scholar]
- 55.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Skott P, Vaag A, Bruun NE, Hother-Nielsen O, Gall MA, Beck-Nielsen H, Parving HH. Effect of insulin on renal sodium handling in hyperinsulinaemic type 2 (non-insulin-dependent) diabetic patients with peripheral insulin resistance. Diabetologia 34: 275–281, 1991. [DOI] [PubMed] [Google Scholar]
- 57.Song J, Hu X, Riazi S, Tiwari S, Wade JB, Ecelbarger CA. Regulation of blood pressure, the epithelial sodium channel (ENaC), and other key renal sodium transporters by chronic insulin infusion in rats. Am J Physiol Renal Physiol 290: F1055–F1064, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med 150: 776–783, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104: 1085–1094, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiwari S, Nordquist L, Halagappa VK, Ecelbarger CA. Trafficking of ENaC subunits in response to acute insulin in mouse kidney. Am J Physiol Renal Physiol 293: F178–F185, 2007. [DOI] [PubMed] [Google Scholar]
- 60a.Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med 157: 657–667, 1997. [PubMed] [Google Scholar]
- 61.Uzu T, Kimura G, Yamauchi A, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Nishio Y, Maegawa H, Koya D, Haneda M, Kashiwagi A. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. J Hypertens 24: 1627–1632, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol 549: 313–325, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Vliet BN, McGuire J, Chafe L, Leonard A, Joshi A, Montani JP. Phenotyping the level of blood pressure by telemetry in mice. Clin Exp Pharmacol Physiol 33: 1007–1015, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Warden CH, Fisler JS. Comparisons of diets used in animal models of high-fat feeding. Cell Metab 7: 277, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol 30: 769–778, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Yun CC. Concerted roles of SGK1 and the Na+/H+ exchanger regulatory factor 2 (NHERF2) in regulation of NHE3. Cell Physiol Biochem 13: 29–40, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Y, Bouyer P, Boron WF. Role of the AT1A receptor in the CO2-induced stimulation of HCO3− reabsorption by renal proximal tubules. Am J Physiol Renal Physiol 293: F110–F120, 2007. [DOI] [PubMed] [Google Scholar]