Nonalcoholic fatty liver disease (NAFLD) is the most common liver-related disease. Exercise is widely prescribed as treatment, but the mechanism is unknown. We examined the effects of exercise on gut peptides [peptide tyrosine tyrosine (PYY) and glucagon-like peptide-1 (GLP-1)] in patients with clinically diagnosed NAFLD. Prior to training, those with NAFLD exhibited elevated fasting plasma PYY and GLP-1, and the response to glucose ingestion was abnormal. Exercise reduced fasting GLP-1 and normalized the glucose-stimulated response of both hormones.
Keywords: fatty liver disease, obesity, insulin resistance, nonalcoholic steatohepatitis, physical activity
Abstract
Obesity-related nonalcoholic fatty liver disease (NAFLD) is now the most common chronic liver disease. Exercise and diet are uniformly prescribed treatments for NAFLD; however, there are limited empirical data on the effects of exercise training on metabolic function in these patients. The purpose of this study was to investigate the fasting and glucose-stimulated adaptation of gut peptides to short-term aerobic exercise training in patients with NAFLD. Twenty-two obese subjects, 16 with NAFLD [body mass index (BMI), 33.2 ± 1.1 (SE) kg/m2] and 6 obese controls (BMI, 31.3 ± 1.2 kg/m2), were enrolled in a supervised aerobic exercise program (60 min/day, 85% of their heart rate maximum, for 7 days). Fasting and glucose-stimulated glucagon-like peptide-1 (GLP-17-36) and peptide tyrosine tyrosine (PYYTotal) concentrations in plasma were assessed before and after the exercise program. Initially, the NAFLD group had higher fasting PYY (NAFLD = 117 ± 18.6, control = 47.2 ± 6.4 pg/ml, P < 0.05) and GLP-1 (NAFLD = 12.4 ± 2.2, control = 6.2 ± 0.2 pg/ml, P < 0.05) and did not significantly increase GLP-1 or PYY in response to glucose ingestion. After the exercise program, fasting GLP-1 was reduced in the NAFLD group (10.7 ± 2.0 pg/ml, P < 0.05). Furthermore, exercise training led to significant increase in the acute (0–30 min) PYY and GLP-1 responses to glucose in the NAFLD group, while the total area under the glucose-stimulated GLP-1 response curve was reduced in both NAFLD and controls (P < 0.05). In summary, 7 days of vigorous aerobic exercise normalized the dynamic PYY and GLP-1 responses to nutrient stimulation and reduced the GLP-1 response in NAFLD, suggesting that exercise positively modulates gut hormone regulation in obese adults with NAFLD.
NEW & NOTEWORTHY
Nonalcoholic fatty liver disease (NAFLD) is the most common liver-related disease. Exercise is widely prescribed as treatment, but the mechanism is unknown. We examined the effects of exercise on gut peptides [peptide tyrosine tyrosine (PYY) and glucagon-like peptide-1 (GLP-1)] in patients with clinically diagnosed NAFLD. Prior to training, those with NAFLD exhibited elevated fasting plasma PYY and GLP-1, and the response to glucose ingestion was abnormal. Exercise reduced fasting GLP-1 and normalized the glucose-stimulated response of both hormones.
nonalcoholic fatty liver disease (NAFLD) is a major contributor to the progression of a multitude of lifestyle-related diseases, including metabolic syndrome, type 2 diabetes mellitus, and cardiovascular disease (35). It is estimated that ∼30% of the U.S. adult population have NAFLD (33), and this is closely linked to obesity, physical inactivity, and poor diet (42). Recently, in an attempt to develop targeted strategies to address obesity and related disease, much attention has been directed toward two gut-derived hormones that are known to modulate nutrient intake and metabolism. Peptide tyrosine tyrosine (PYY) and glucagon-like peptide-1 (GLP-1) are primarily secreted from the intestinal L cells of the distal gut in response to glucose or meal ingestion. It is well established that PYY targets higher brain centers to induce satiety (16) and also plays a role in the regulation of glucose metabolism (23). Likewise, GLP-1 regulates satiety, but it is also an insulin secretagogue and a potential regulator of lipid metabolism (2).
Previous studies have shown that obesity is associated with abnormalities in PYY and GLP-1 secretion and regulation (4, 13, 40), and this may be linked to eating behaviors, as well as the metabolic disorders that subsequently arise. Metabolic conditions such as insulin resistance and type 2 diabetes coexist with NAFLD and are tied to further dysregulation of these hormones (7, 23, 38). However, the influence of NAFLD on the regulation of GLP-1 and PYY is currently unknown.
Aerobic exercise training is an effective therapy for preventing progression of NAFLD-associated disease and reversing related negative health consequences (8, 10–12, 29, 42), particularly when patients perform >250 min/wk of moderate- to high-intensity aerobic exercise (28). Mechanisms of metabolic improvement include increased insulin sensitivity (5), decreased hyperlipidemia, decreased delivery of fatty acids to the liver (11), enhanced hepatic fatty acid oxidation (32), and reduced visceral adipose tissue (VAT) (17). Additionally, aerobic exercise can have anorexigenic effects in both lean and obese individuals that are mediated through increases in GLP-1 and PYY (3, 24, 34, 36). Over time this may contribute to reduced energy intake and successive weight loss, although evidence suggests that such a response may be blunted in obese individuals (1). Thus the purpose of this study was to investigate fasting and glucose-stimulated plasma GLP-1 and PYY responses in NAFLD and determine if a short-term aerobic exercise intervention could improve the regulation of these peptides in this high-risk patient group.
We hypothesized that individuals with NAFLD would exhibit altered gut peptide responses in both the fasting and glucose-stimulated state because of NAFLD-related hepatic dysfunction and that short-term exercise would ameliorate these effects. We further hypothesized that obese individuals without NAFLD would show beneficial gut peptide changes after short-term exercise.
MATERIALS AND METHODS
Participants.
Twenty-two obese sedentary adults [age 53 ± 3 (SE) yr; 32.7 ± 0.9 kg/m2] were recruited to participate in this study. Sixteen of the participants had NAFLD (8 male, 8 female) based on a measured intrahepatic fat content greater than 5%. Six obese participants (2 male, 4 female) without NAFLD and similar body mass index (BMI) served as obesity-matched controls. All subjects underwent a medical history and physical examination, a complete blood profile (lipid profile and hepatic/renal/hematological function tests), a resting 12-lead ECG and submaximal exercise stress test, and an oral glucose tolerance test (OGTT). Subjects were asked to keep a 24-h food diary prior to the preintervention OGTT and were asked to replicate their diet prior to the post-exercise-training OGTT. Subjects were instructed to maintain their typical diet throughout the study. Volunteers were excluded if they were taking any medications or supplements known to affect outcome variables, if they presented any contraindications to physical activity, or if they participated in 20 min or more of exercise at least two times per week. All participants provided signed informed consent in accordance with the guidelines for the protection of human subjects. This study was approved by the Cleveland Clinic Institutional Review Board.
Intrahepatic and visceral fat content.
Intrahepatic fat content was determined by 1H magnetic resonance spectroscopy as previously described (10). Individuals with intrahepatic fat content higher than 5% were categorized as having NAFLD based on the diagnostic criteria for hepatic steatosis (37). Visceral fat content was quantified using cross-sectional images of 5-mm slices obtained during computed tomography scans (Somotom Sensation 16 scanner; Siemens Medical Solutions, Malvern, PA). The subjects were scanned in the supine position, images were obtained at the fourth lumber vertebra (L4), and visceral fat content was measured (ImageJ; National Institutes of Health).
Glucose metabolism.
Glucose metabolism was assessed during a 75-g OGTT performed prior to and 24–48 h following the 7 days of aerobic exercise training. Participants arrived for testing at ∼7:00 AM after an overnight fast. Following baseline blood samples, subjects ingested a glucose solution and blood was subsequently drawn at 30, 60, 90, and 120 min. Blood was collected in EDTA tubes containing aprotinin and dipeptidyl peptidase 4 (DPP-IV) inhibitor and was immediately placed on ice. Blood was centrifuged (15 min, 3,000 rpm) and plasma was aliquoted and stored at −80°C until analyzed for GLP-1, PYY, glucose, insulin, and C-peptide. The Matsuda index (25) was used to assess insulin sensitivity during the OGTT (ISIOGTT).
Exercise intervention.
All subjects completed 7 consecutive days of supervised aerobic exercise (treadmill/cycle ergometer) for 60 min/day performed at 85% of their heart rate maximum. Each exercise session was preceded with a 5-min warm-up and followed by a 5-min cool-down. Heart rate was measured continuously during exercise using heart rate monitors (Polar Electro, Woodbury, NY).
Aerobic capacity (V̇o2 max).
Aerobic capacity was measured within 1 day prior to the start of the exercise intervention and 1 day after completion of the 7-day program and the OGTT. Maximal oxygen consumption was determined using an incremental treadmill exercise test as previously described (18). Expired air was monitored throughout the protocol using an automated system (Jaeger Oxycon Pro; Viasys, Yorba Linda, CA).
Blood analysis.
At each time point plasma glucose was determined using a YSI 2300 STAT Plus analyzer (Yellow Springs, OH), and plasma insulin and C-peptide were determined via radioimmunoassay (Millipore, Billerica, MA). GLP-17–36 and PYYTotal were also measured through the 120-min time point of the OGTT using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Millipore, St. Charles, MO). All samples were batch analyzed and measured in duplicate to reduce interassay variability, and the coefficient of variation between duplicates was 6.1 ± 0.4 and 3.3 ± 1.1% for PYY and GLP-1, respectively. Baseline preintervention aspartate transaminase (AST) and alanine transaminase (ALT) were measured to assess liver function using the Cobra Integra Aspartate Aminotransferase (ASTL) test and the Alanine Aminotransferase (ALTL) test (Roche Diagnostics, Indianapolis, IN), respectively.
Statistical analysis.
Statistical analysis was carried out using StatView Version 5.0.1 (SAS Institute, Cary, NC). In general, data are expressed as means ± SE. Primary dependent variables were analyzed by repeated-measures ANOVA. The ANOVA models first tested the interaction between groups (NAFLD vs. control) and trial (pre- vs. postexercise) by including the two main effect terms and their interactions. If the interaction was not statistically significant, it was dropped from the model, and the model proceeded with two main effect terms (this was true for all variables) in combination with Bonferroni post hoc analysis. Variables with a high degree of skew (GLP-1 and PYY) were log transformed. Pearson product-moment correlations were used to explore relationships between selected outcomes. Statistical significance was set at two-sided P values <0.05.
RESULTS
Subject characteristics are presented in Table 1. There were no differences in age, weight, or BMI between NAFLD and controls. However, the NAFLD group had higher intrahepatic fat content (IHC) (22.6 ± 3.1% vs. control 3.5 ± 0.7%; P < 0.01) and elevated liver enzymes (AST, 41.1 ± 6.4 U/l vs. control 27.2 ± 4.6 U/l; ALT, 57.9 ± 10.7 U/l vs. control 33.3 ± 10.1 U/l), which confirms the presence of NAFLD. There were no significant changes in weight, BMI, or IHC in either group following 7 days of exercise. Furthermore, there were no interactions between group responses after 7 days of exercise, indicating that the NAFLD group had similar responses to exercise training compared with the obese control group.
Table 1.
Subject characteristics and responses to exercise training
| Baseline |
Posttraining |
||||
|---|---|---|---|---|---|
| Control | NAFLD | Control | NAFLD | P for interaction | |
| Age, yr | 46.8 ± 6.3 | 55.8 ± 2.9 | — | — | |
| Weight, kg | 92.6 ± 6.0 | 94.8 ± 3.5 | 92.4 ± 5.8 | 94.6 ± 3.4 | 0.999 |
| BMI, kg/m2 | 31.3 ± 1.2 | 33.2 ± 1.1 | 31.2 ± 1.2 | 33.1 ± 1.1 | 0.993 |
| HTGC, % | 3.5 ± 0.7 | 22.6 ± 3.1† | 5.0 ± 1.2 | 21.5 ± 3.0† | 0.725 |
| VAT, cm2 | 51.7 ± 10.3 | 124.7 ± 16.4† | 52.9 ± 11.1 | 125.7 ± 15.5† | 0.995 |
| FPG, mg/dl | 93 ± 2 | 113 ± 5† | 92 ± 3 | 106 ± 3*† | 0.56 |
| FPI, μU/ml | 18.1 ± 3.5 | 25.4 ± 2.5 | 14.8 ± 2.7 | 19.9 ± 1.4* | 0.805 |
| IAUC, μU/ml | 11,837 ± 2,104 | 15,955 ± 1,744 | 9,852 ± 1,056 | 12,287 ± 1,347* | 0.670 |
| FCPEP, ng/ml | 2.2 ± 0.4 | 3.5 ± 0.3† | 1.9 ± 0.4 | 3.0 ± 0.2†* | 0.677 |
| CPEPAUC, ng/ml | 793 ± 66 | 1,069 ± 115 | 746 ± 54 | 937 ± 112* | 0.733 |
| FGLP-1, pg/ml | 6.2 ± 0.2 | 12.4 ± 2.2† | 6.2 ± 0.2 | 10.7 ± 2.0* | 0.685 |
| FPYY, pg/ml | 47.2 ± 6.4 | 117 ± 18.6† | 56.6 ± 8.8 | 125.3 ± 22.0 | 0.749 |
Data are presented as means ± SE.
BMI, body mass index; HTGC, hepatic triglyceride content; VAT, visceral adipose tissue; FPG, fasting plasma glucose; FPI, fasting plasma insulin; IAUC, insulin tAUC 120; FCPEP, fasting C-peptide; CPEPAUC, C-peptide tAUC 120; FGLP-1, fasting GLP-1; FPYY, fasting PYY.
P for interaction represents statistical significance between group responses after exercise training.
P < 0.05 from pre- to posttraining,
P < 0.05 from control.
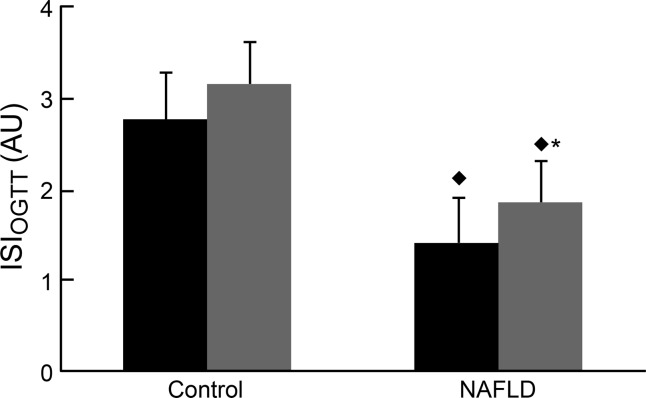
Baseline fasting and OGTT glucose concentrations were significantly higher (P = 0.02) in NAFLD compared with controls. The NAFLD group had higher fasting C-peptide (3.5 ± 0.3 vs. 2.2 ± 0.4 ng/ml) compared with controls (P = 0.02). After exercise training, fasting glucose was significantly decreased (P = 0.03) in the NAFLD group, but still remained higher (P = 0.02) than controls. Exercise training lowered both fasting insulin (P = 0.02) and C-peptide (P = 0.04) and decreased insulin (P < 0.001) and C-peptide total area under the curve 0–120 min (tAUC120; P < 0.01) in NAFLD, but not in control. Insulin sensitivity (ISIOGTT) was significantly lower (P < 0.01) in the NAFLD group compared with control both pre- and postexercise, but significantly increased within the NAFLD group in response to exercise training (P < 0.001, Fig. 1).
Fig. 1.
Insulin sensitivity in the NAFLD and control groups as determined by the Matsuda index at baseline and after the 7-day exercise-training program. *P < 0.05, significantly different from pre-exercise training. ⧫P < 0.05, significantly lower than the control group. AU, arbitrary units.
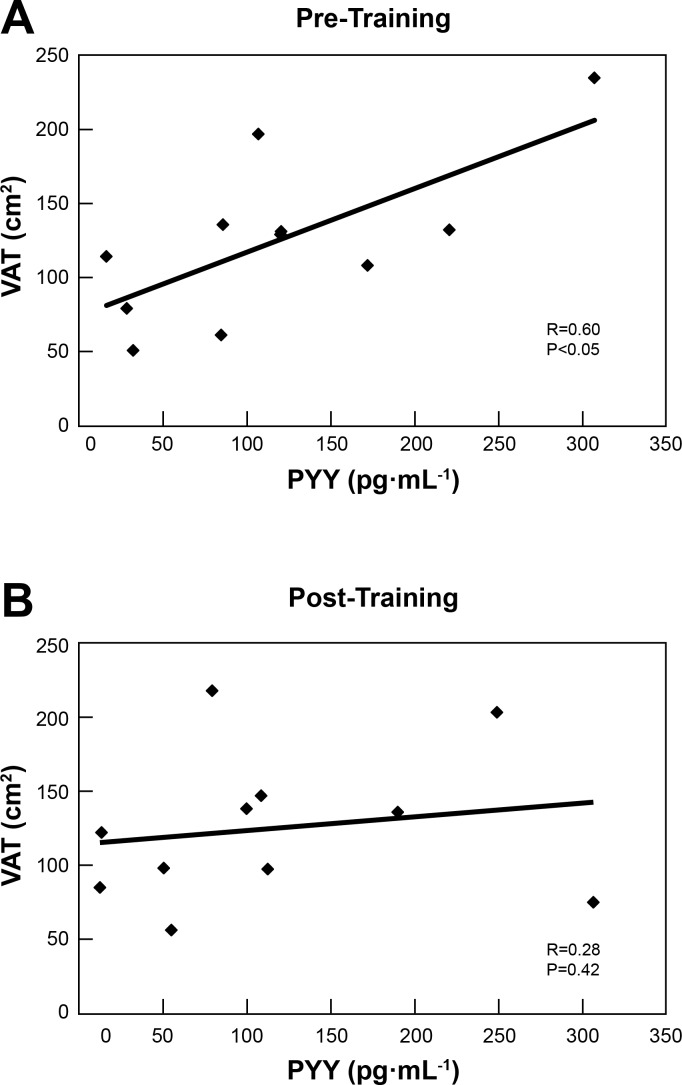
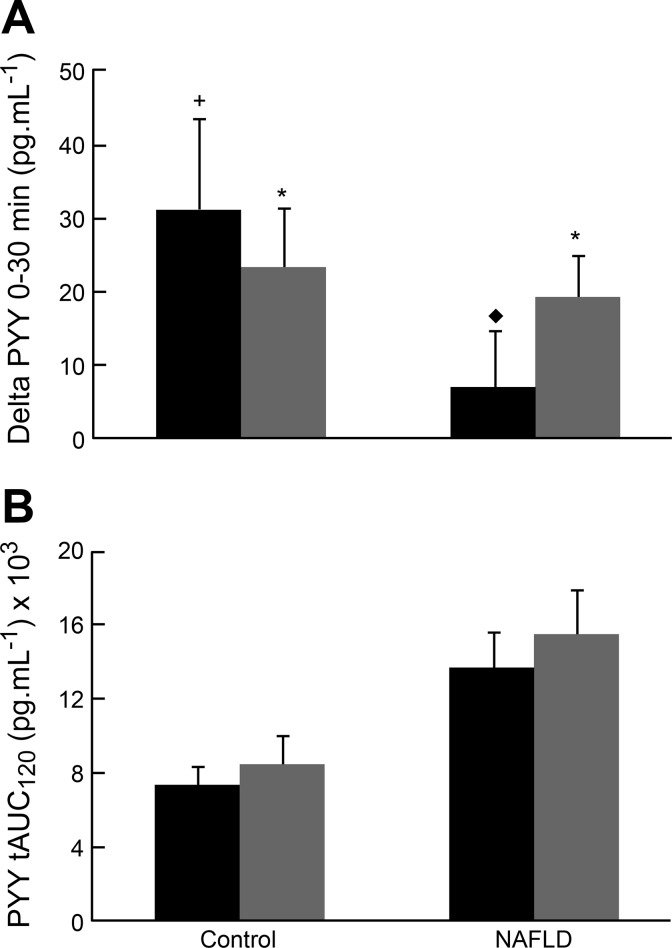
Fasting PYY was significantly higher in NAFLD (P = 0.04) than controls at baseline (Table 1), and this was directly correlated with VAT (r = 0.60, P = 0.049, Fig. 2A). Furthermore, subjects with NAFLD exhibited a blunted PYY response to oral glucose ingestion (Fig. 3A), while the response in the control group trended toward an increase (P = 0.06). Posttraining, the relationship between fasting PYY and VAT disappeared (r = 0.28, P = 0.421, Fig. 2B). Notably, a dynamic PYY response, as reflected in secretion 30 min following glucose ingestion, was significantly increased (P = 0.04) in the NAFLD group after exercise training, suggesting a restoration of the acute PYY response to nutrient stimulation. The control group also significantly increased the dynamic PYY response after exercise training (P = 0.02). While the dynamic component of the PYY response is likely to contribute to satiety regulation immediately after a meal, it should be noted that the total PYY response, as assessed by the PYY tAUC120, was not significantly increased in either group after glucose ingestion, nor was it altered by this short exercise-training stimulus (Fig. 3B).
Fig. 2.
A: significant correlation (P = 0.049, r = 0.60) between visceral adipose tissue (VAT) and fasting PYY for NAFLD subjects before exercise training. B: nonsignificant correlation (P = 0.421, r = 0.277) between visceral adipose tissue (VAT) and fasting PYY for NAFLD subjects after exercise training.
Fig. 3.
A: dynamic plasma PYY response to glucose (0–30 min) in NAFLD and control subjects at baseline and after the 7-day exercise-training program. *P < 0.05, significant increase from fasting. +Trend (P = 0.06) for an increase from fasting. ⧫P < 0.05, significantly different from the control group. B: PYY total area under the curve (tAUC, 0–120 min) after glucose ingestion in NAFLD and control subjects at baseline and after exercise training. There were no significant differences between groups, nor were there any significant changes resulting from exercise training.
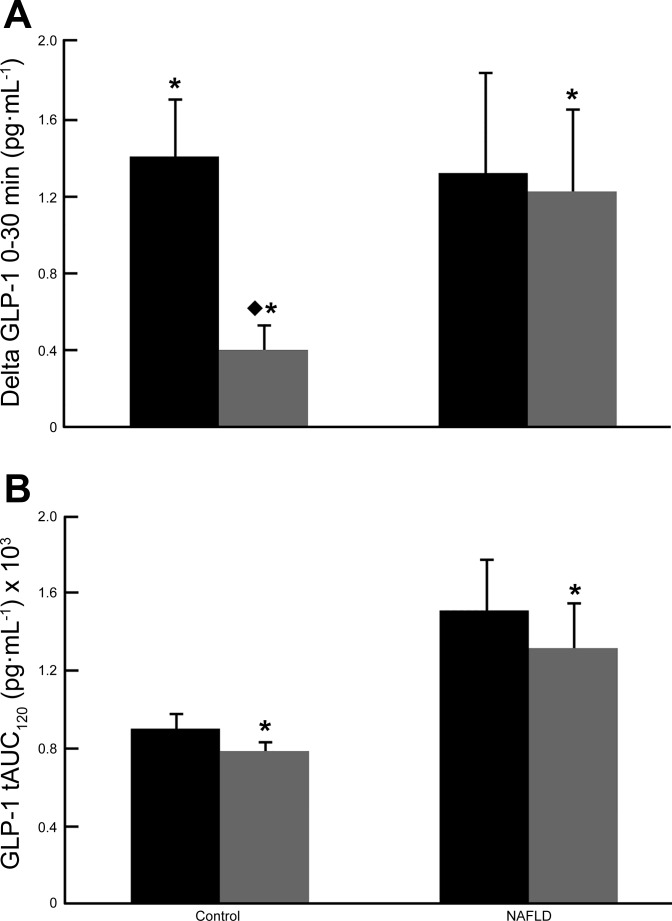
At baseline, fasting GLP-1 was significantly higher (P < 0.05) in NAFLD compared with controls (Table 1), and those with NAFLD did not show the significant GLP-1 response (P = 0.06) 30-min post-glucose ingestion that was evident in the control group (P < 0.05). However, this may have been driven by a high degree of variability in response among NAFLD patients, with 5 of 16 NAFLD subjects exhibiting a decrease in their 30-min GLP-1 response. The abnormal 30-min GLP-1 response was mitigated in the NAFLD group following exercise training with a statistically significant increase (P = 0.002) in GLP-1 at 30 min after glucose ingestion. Among obese control subjects, there was still a dynamic response to glucose after exercise training (P = 0.05), although it was significantly lower than the pre-exercise response (P = 0.0001, Fig. 4A). There were no group differences in the GLP-1 tAUC120 responses to glucose intake either before or after exercise training. Further, exercise training reduced fasting GLP-1 (P < 0.001) and GLP-1 tAUC120 (P = 0.01) in both NAFLD and controls (Fig. 4B).
Fig. 4.
A: dynamic plasma GLP-1 response to glucose (0–30 min) in NAFLD and control subjects at baseline and after the 7-day exercise-training program. *P < 0.05, significant increase from fasting. ⧫P < 0.05, significantly lower than pre-exercise training. B: GLP-1 total area under the curve (tAUC, 0–120 min) after glucose ingestion in NAFLD and control subjects at baseline and after exercise training. *P < 0.05, significantly lower than pretraining.
DISCUSSION
Our data reveal important differences in gut hormones between obese individuals with and without NAFLD, most notably higher fasting PYY and GLP-1 concentrations and a blunted 30-min PYY response to glucose ingestion in patients with NAFLD. Importantly, following short-term exercise, NAFLD patients showed a marked improvement in PYY and GLP-1 responses to glucose stimulation. These data suggest that compromised liver and metabolic function in NAFLD coexists with gastrointestinal dysfunction that may perpetuate the cycle of disease. However, short-term vigorous aerobic exercise training alleviated this effect, even in the absence of weight loss.
PYY regulates satiety, and circulating levels substantially rise in healthy individuals shortly after meal ingestion (16). It was previously shown that fasting PYY is directly associated with the degree of metabolic disease and that individuals with type 2 diabetes exhibit the highest fasting PYY levels (38). Although our analysis did not reveal any correlations between PYY and ISI, glucose or insulin measurements, it is difficult to say that NAFLD independently causes elevations in fasting levels of PYY since all of our subjects had some level of insulin resistance. However, it is noteworthy that we observed a nearly 2.5-fold higher fasting PYY concentration among NAFLD patients, which, interestingly, was directly correlated with a visceral adiposity that was also 2.5 times higher than non-NAFLD controls. This was evident despite the fact that subjects had similar anthropometric measurements, which suggests that signals specific to visceral adipose tissue, such as adipokines, may in part regulate PYY concentrations in this patient group. Exercise training did not change fasting PYY concentrations in either the NAFLD or the control groups, and others have made similar observations in non-NAFLD populations (15, 22, 24). However, after exercise training, the correlation between visceral fat and fasting PYY was no longer evident, suggesting that exercise had a beneficial effect on PYY regulation independent of visceral fat, considering the latter did not change.
In addition to altered fasting PYY, several studies have shown a blunted acute PYY response to food stimulation in subjects who are obese (7, 9), and this is now considered to be a contributing factor to the underlying pathophysiology of obesity. The rapid increase in PYY observed after food ingestion is an important satiety signal that controls the overall intake of food during a meal. In the present study, the diminished acute 0–30-min PYY response to glucose, which was most evident in the NAFLD group, may lead to excess energy consumption during a meal, perpetuating the downward spiral of obesity-related comorbidities in these patients. Exercise training can have an anorexigenic effect, which is mediated in part by increased PYY secretion (3). In the current study, exercise training resulted in an improvement in the 30-min PYY response to glucose ingestion in the NAFLD group. Previously, we observed improved glucose-induced PYY secretion after 12 wk of exercise training in older insulin-resistant obese adults (19). Our finding that 7 days of aerobic exercise can produce a similar result indicates that exercise elicits a very rapid and potent response, even in the presence of severe metabolic disease.
Striking differences between fasting and glucose-induced GLP-1 levels were also observed between the NAFLD and control groups, with fasting GLP-1 being 1.7 times higher in the NAFLD group. Multiple factors could have contributed to the elevated fasting GLP-1 levels, including a loss of GLP-1 sensitivity due to excessive ectopic fat and/or visceral fat (20, 31, 39), systemic chronic inflammation (6) that is associated with NAFLD (30) and excess visceral fat (31), and/or increased GLP-1 secretion from hepatic progenitor cells (HPC) (27). In the context of NAFLD, HPCs are of particular interest. NAFLD causes damage to hepatocytes, which leads to increased HPC activity as a compensatory mechanism to maintain liver function (14). It was recently reported that adolescents with NAFLD had activated HPCs that overexpress GLP-1 (27). Subjects with NAFLD in our study had elevated markers of liver damage and apoptosis (8), and therefore it is possible that increased HPC activity contributed to higher circulating GLP-1 levels, suggesting an independent role of NAFLD-associated liver dysfunction in altered gut hormone concentrations.
Exercise training reduced fasting GLP-1 in the subjects with NAFLD, which is supported by a recent cross-sectional study reporting lower fasting GLP-1 levels in aerobically trained healthy individuals (21). Exercise training was shown to reduce markers of hepatocyte apoptosis among NAFLD patients (8), which may decrease the number of activated HPCs and related expression of GLP-1. While this mechanism is speculative at this point, it is certainly worth further investigation, as it suggests a direct link between NAFLD-associated liver dysfunction and hyperinsulinemia via the incretin effect of GLP-1. Furthermore, this mechanism may explain how exercise lowers fasting GLP-1 while at the same time improving liver function.
Prior to exercise training, the dynamic 0–30-min GLP-1 response to glucose was insignificantly increased in NAFLD patients compared with a significant increase among obese controls. The lack of statistical significance is likely due to the high degree of variability in the GLP-1 responses among the NAFLD subjects. On the basis of the data presented herein and previously (1, 4, 24, 26, 40), we suggest that NAFLD-associated metabolic disruption, in addition to obesity, disturbs signals along the hepatic-gastroenteropancreatic pathway that affect GLP-1 secretion leading to an atypical and more variable response to glucose ingestion.
Few studies have examined GLP-1 changes after exercise training. We found that exercise training reduced the GLP-1 tAUC120 in NAFLD patients. This change mirrored improvements in insulin secretion, indicative of an improved incretin effect (26). Our results are supported by those of Chanoine et al. (4), who observed an improved GLP-1 response to a meal in obese adolescents after 5 days of exercise training. Conversely, others have reported no change in fasting GLP-1 (1, 24) or glucose-stimulated GLP-1 secretion in obese individuals after exercise training, or when comparing exercise trained vs. untrained groups (21, 41). Our results suggest that short-term exercise training improves GLP-1 sensitivity in people with NAFLD.
In conclusion, fasting GLP-1 and PYY, as well as the responses to glucose ingestion, indicate that NAFLD interferes with normal gut hormone regulation. However, short-term aerobic exercise training results in improved metabolic regulation, glucose sensitivity, and PYY and GLP-1 responses to nutrient ingestion, as well as decreased fasting PYY and GLP-1 concentrations in individuals with NAFLD. These findings support the use of aerobic exercise training as an effective intervention for normalizing gut hormone disturbances in patients with NAFLD and provide direction for future mechanistic studies that would reveal the underlying pathophysiology of this metabolic disease.
GRANTS
This research was supported by National Institutes of Health (NIH) Grant R01-AG-12834 (J. P. Kirwan) and was supported in part by the NIH National Center for Research Resources, CTSA-1UL1-RR-024989, and the Case Center for Imaging Research, Case Western Reserve University, Cleveland, Ohio. M. R. Pagadala, E. L. Kullman, and K. R. Kelly were supported by NIH training Grants T32 DK061917 and T32 DK007319 to J. P. Kirwan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.L.K., K.R.K., J.M.H., C.E.F., A.R.S., M.R.P., C.A.F., and J.P.K. performed experiments; E.L.K., K.R.K., J.M.H., C.E.F., A.R.S., M.R.P., C.A.F., and J.P.K. analyzed data; E.L.K., K.R.K., J.M.H., C.E.F., C.A.F., and J.P.K. interpreted results of experiments; E.L.K. and J.P.K. prepared figures; E.L.K. drafted manuscript; E.L.K., K.R.K., J.M.H., C.E.F., and J.P.K. edited and revised manuscript; E.L.K. and J.P.K. approved final version of manuscript; K.R.K., J.M.H., C.A.F., A.J.M., and J.P.K. conception and design of research.
ACKNOWLEDGMENTS
Present address of E. L. Kullman: Department of Health and Human Performance, Cleveland State University, Cleveland, Ohio.
We thank the research volunteers for their outstanding dedication and effort and the staff of the Clinical Research Unit and the technical staff and students who helped with the implementation of the study and assisted with data collection. We acknowledge our clinical research coordinator, Julianne Filion, for her excellent nursing and organizational assistance. We also extend thanks to Dr. Bo Hu for his support with the statistical analysis of the data.
REFERENCES
- 1.Adam TC, Westerterp-Plantenga MS. Activity-induced GLP-1 release in lean and obese subjects. Physiol Behav 83: 459–466, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol 296: R29–R35, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Chanoine JP, Mackelvie KJ, Barr SI, Wong AC, Meneilly GS, Elahi DH. GLP-1 and appetite responses to a meal in lean and overweight adolescents following exercise. Obesity (Silver Spring) 16: 202–204, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Dela F, Prats C, Helge JW. Exercise interventions to prevent and manage type 2 diabetes: physiological mechanisms. Med Sport Sci 60: 36–47, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17: 1481–1489, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English PJ, Ashcroft A, Patterson M, Dovey TM, Halford JC, Harrison J, Eccleston D, Bloom SR, Ghatei MA, Wilding JP. Fasting plasma peptide-YY concentrations are elevated but do not rise postprandially in type 2 diabetes. Diabetologia 49: 2219–2221, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Fealy CE, Haus JM, Solomon TP, Pagadala M, Flask CA, McCullough AJ, Kirwan JP. Short-term exercise reduces markers of hepatocyte apoptosis in non-alcoholic fatty liver disease. J Appl Physiol (1985) 113: 1–6, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatta-Cherifi B, Matias I, Vallee M, Tabarin A, Marsicano G, Piazza PV, Cota D. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. Int J Obes (Lond) 36: 880–885, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR, Lu L, Pagadala MR, McCullough AJ, Flask CA, Kirwan JP. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 98: E1181–E1188, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson NA, George J. Fitness versus fatness: moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology 52: 370–381, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Johnson NA, Keating SE, George J. Exercise and the liver: implications for therapy in fatty liver disorders. Semin Liver Dis 32: 65–79, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Jones TE, Basilio JL, Brophy PM, McCammon MR, Hickner RC. Long-term exercise training in overweight adolescents improves plasma peptide YY and resistin. Obesity (Silver Spring) 17: 1189–1195, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 28: 370–379, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Kanaley JA, Heden TD, Liu Y, Whaley-Connell AT, Chockalingam A, Dellsperger KC, Fairchild TJ. Short-term aerobic exercise training increases postprandial pancreatic polypeptide but not peptide YY concentrations in obese individuals. Int J Obes (Lond) 2013. [DOI] [PMC free article] [PubMed]
- 16.Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol 587: 19–25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keating SE, Hackett DA, Parker HM, O'Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, Johnson NA. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 63: 174–182, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Kelly KR, Blaszczak A, Haus JM, Patrick-Melin A, Fealy CE, Solomon TP, Kalinski MI, Kirwan JP. A 7-d exercise program increases high-molecular weight adiponectin in obese adults. Med Sci Sports Exerc 44: 69–74, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O'Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab 296: E1269–E1274, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjems LL, Holst JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52: 380–386, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Lund MT, Dalby S, Hartmann B, Helge J, Holst JJ, Dela F. The incretin effect does not differ in trained and untrained, young, healthy men. Acta Physiol (Oxf) 210: 565–572, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Lund MT, Taudorf L, Hartmann B, Helge JW, Holst JJ, Dela F. Meal induced gut hormone secretion is altered in aerobically trained compared to sedentary young healthy males. Eur J Appl Physiol 113: 2737–2747, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Manning S, Batterham RL. The role of gut hormone peptide YY in energy and glucose homeostasis: twelve years on. Annu Rev Physiol 76: 585–608, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab 95: 1609–1616, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 57: 1340–1348, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R, Onori P, Alvaro D, Gaudio E. Hepatic progenitor cells activation, fibrosis and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology 56: 2142–2153, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Oh S, Shida T, Yamagishi K, Tanaka K, So R, Tsujimoto T, Shoda J. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology 61: 1205–1215, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Ordonez R, Carbajo-Pescador S, Mauriz JL, Gonzalez-Gallego J. Understanding nutritional interventions and physical exercise in non-alcoholic fatty liver disease. Curr Mol Med 15: 3–26, 2015. [DOI] [PubMed] [Google Scholar]
- 30.Patrick-Melin AJ, Kalinski MI, Kelly KR, Haus JM, Solomon TP, Kirwan JP. Nonalcoholic fatty liver disease: biochemical and therapeutic considerations. Ukr Biokhim Zh (1999) 81: 16–25, 2009. [PubMed] [Google Scholar]
- 31.Pedersen BK. The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol 587: 5559–5568, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 313: 2263–2273, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Russel RR, Willis KS, Ravussin E, Larson-Meyer ED. Effects of endurance running and dietary fat on circulating ghrelin and peptide YY. J Sports Sci Med 8: 574–583, 2009. [PMC free article] [PubMed] [Google Scholar]
- 35.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of fatty liver. Endocr Rev 29: 939–960, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab 57, Suppl 2: 36–42, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288: E462–E468, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Ukkola OH, Puurunen VP, Piira OP, Niva JT, Lepojarvi ES, Tulppo MP, Huikuri HV. High serum fasting peptide YY (3–36) is associated with obesity-associated insulin resistance and type 2 diabetes. Regul Pept 170: 38–42, 2011. [DOI] [PubMed] [Google Scholar]
- 39.van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas 39: 1185–1190, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Vendrell J, El Bekay R, Peral B, Garcia-Fuentes E, Megia A, Macias-Gonzalez M, Fernandez Real J, Jimenez-Gomez Y, Escote X, Pachon G, Simo R, Selva DM, Malagon MM, Tinahones FJ. Study of the potential association of adipose tissue GLP-1 receptor with obesity and insulin resistance. Endocrinology 152: 4072–4079, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Weiss EP, Royer NK, Fisher JS, Holloszy JO, Fontana L. Postprandial plasma incretin hormones in exercise-trained versus untrained subjects. Med Sci Sports Exerc 46: 1098–1103, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol 17: 3377–3389, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]