Abstract
Absence of peristalsis and impaired relaxation of lower esophageal sphincter are the hallmarks of achalasia esophagus. Based on the pressurization patterns, achalasia has been subdivided into three subtypes. The goal of our study was to evaluate the esophageal contraction pattern and bolus clearance in type 3 achalasia esophagus. High-resolution manometry (HRM) recordings of all patients diagnosed with achalasia esophagus in our center between the years 2011 and 2013 were reviewed. Recordings of 36 patients with type 3 achalasia were analyzed for the characteristics of swallow-induced “simultaneous esophageal contraction.” The HRM impedance recordings of 14 additional patients with type 3 achalasia were analyzed for bolus clearance from the impedance recording. Finally, the HRM impedance along with intraluminal ultrasound imaging was conducted in six patients to further characterize the simultaneous esophageal contractions. Among 187 achalasia patients, 30 were type 1, 121 type 2, and 36 type 3. A total of 434 swallows evaluated in type 3 achalasia patients revealed that 95% of the swallow-induced contractions met criteria for simultaneous esophageal contraction, based on the onset of contraction. Interestingly, the peak and termination of the majority of simultaneous esophageal contractions were sequential. The HRM impedance revealed that 94% of the “simultaneous contractions” were associated with complete bolus clearance. Ultrasound image analysis revealed that baseline muscle thickness of patients in type 3 achalasia is larger than normal but the pattern of axial shortening is similar to that in normal subjects. The majority of esophageal contractions in type 3 achalasia are not true simultaneous contractions because the peak and termination of contraction are sequential and they are associated with complete bolus clearance.
Keywords: achalasia esophagus, type 3 achalasia, esophageal peristalsis, multiple intraluminal esophageal impedance, ultrasound imaging of the esophagus
achalasia esophagus is the most well-defined and characterized motility disorder of the esophagus. Esophageal manometry is the gold-standard to diagnose achalasia esophagus; the two major manometric criteria for diagnosis are 1) impaired relaxation of the lower esophageal sphincter (LES) and 2) complete loss of esophageal peristalsis (aperistalsis) or simultaneous contractions of the esophagus (12). Based on the swallow-induced esophageal pressurization patterns on high-resolution manometry (HRM), achalasia patients may be divided into three subtypes: type 1 achalasia, low esophageal pressurization (<20–30 mmHg) with swallows; type 2, esophageal pressurization of >30 mmHg; and type 3, the so-called “spastic achalasia,” simultaneous onset of esophageal pressure waves with distal contractile integral of >450 mmHg (23). Some investigators believe that spastic achalasia may have been previously classified as vigorous achalasia or possibly diffuse esophageal spasm (DES). The definition of DES is high prevalence (>20%) of swallow-induced “simultaneous” esophageal contractions with partial preservation of peristalsis. The definition of “simultaneous contraction” on manometry is based on the onset of the pressure wave being simultaneous (velocity of peristalsis >8–9 cm/s and latency of distal esophageal contraction of <4.5 s) (25). Richter and colleagues (7, 10) found that simultaneous contractions were associated with tertiary contraction and segmental contraction, with impaired bolus transit on the barium swallow X-ray fluoroscopy study. Identification of the onset of true circular muscle contraction wave on manometry may be difficult because intraluminal manometry can't distinguish between contact pressure and bolus or common cavity pressure (16, 19).
The goal of our study was to reassess the nature of “simultaneous esophageal contractions” in type 3 achalasia esophagus. One of the reasons for this ongoing effort in our laboratory is to understand why patients with type 3 achalasia don't respond well to medical and surgical therapies (5, 23, 30).
METHODS AND EXPERIMENTAL DESIGN
Record review.
Protocol for the studies was approved by the University of California San Diego Institutional Review Board for the Protection of Humans, and patients who underwent simultaneous HRM impedance (HRMZ) and ultrasound measurements signed an informed consent prior to participation in the study. The GI function laboratory at the UCSD Medical Center is a referral center for the greater San Diego area; patients are referred for assessment of suspected “esophageal symptoms.” The clinical reports of all esophageal HRM studies conducted during January 2011 through December 2013 were reviewed for manometric diagnosis. The HRM studies with manometric diagnosis of achalasia esophagus were examined to determine three achalasia subtypes, according to the published criteria (23). The manoscan software 3.01 was used for the HRM waveform analysis. Briefly, the criteria for diagnosis were that, for all types of achalasia, the patient was required to have impaired LES relaxation (integrated relaxation LES pressure of >15 mmHg) and complete loss of peristalsis. The distinction between three types of achalasia esophagus was based on the characteristics of swallow-induced esophageal pressure waveforms. For type 1 achalasia, esophageal pressurization of ≥80% swallows was <20 mmHg. The type 2 achalasia was diagnosed when the swallow-induced esophageal pressurization was >20 mmHg with ≥80% of the swallows. For type 3 achalasia, the criteria for “simultaneous pressure” waveform in the distal 10 cm of the esophagus were 1) simultaneous onset of isocontour of 30 mmHg (velocity of peristalsis of >8 cm/s), 2) mean latency of contraction in the distal esophagus of <4.5 s, and 3) distal contractile integral of >450 mmHg of ≥20% contractions. In patients with type 3 achalasia, in addition to the onset of pressure waveform, the peak and the end of esophageal contractions were analyzed to determine whether they were sequential. For the peak of pressure wave, the line tracing was evaluated between 3 and 11 cm above the LES and the first peak was evaluated (in case of multipeaked contraction) by criteria similar to the onset of pressure waveform, i.e., velocity of peristalsis of <8 cm/s for peristaltic contraction and >8 cm/s for simultaneous contraction. The termination of contraction was identified by using isocontour of 30 mmHg and was marked as simultaneous or peristaltic by criteria similar to the one for the onset of contraction.
HRMZ recordings of another 14 patients diagnosed with type 3 achalasia esophagus (different from those described in the previous paragraph) were analyzed to determine the progression of bolus with simultaneous contractions in the distal 10 cm of the esophagus. Progression of bolus was defined as orderly, i.e., “in the aboral direction,” based on the analysis of impedance waveform. For the bolus movement to be defined orderly, the impedance waveform had to meet the following two criteria: 1) the nadir of impedance waves in the distal esophagus had to be sequential, and 2) the bolus clearance, defined as return of esophageal impedance to 50% of its baseline value, had to be sequential and progressing in the aboral direction. Six patients from this group of 14 patients with HRMZ recordings were invited to participate in a simultaneous HRMZ and ultrasound (US) imaging study. The 36-channel pressure and 18-channel impedance (HRMZ) catheter (GIVEN, Duluth, GA) was taped to a high-frequency intraluminal ultrasound imaging (HFIUS) catheter (3.5 F, 30 MHz; CVIS, Sunnyvale, CA). The US catheter was interfaced to the HP Sonos 100 ultrasound machine (Hewlett Packard Sonos Intravascular, Andover, MA). The US images were acquired on a DVD recorder.
Liquid lidocaine spray (2% lidocaine topical solution, USP) and viscous lidocaine (1% lidocaine hydrochloride topical solution, USP) were administered orally and nasally for local anesthesia followed by placement of the HRMZ and US catheter assembly through nose. Swallows were performed using 5 ml of 0.5 N saline, at least 30 s apart. Eight to 10 swallows were recorded with the US transducer located at 5 cm and then at 10 cm above the LES.
Ultrasound image analysis.
US images were captured from the DVD files with Adobe Premier 6.0 (Adobe Systems, Mountain View, CA) and then converted to BMP tomographic or B-mode US image. The latter was converted to 16, equally spaced, M-mode US images (every 22.5° apart) by use of custom-built software, as described previously (1). An M-mode US image, orthogonal to the esophageal wall, in which both circular and longitudinal muscle layers were clearly visualized was selected for data analysis. Borders representing the inner edge of circular muscle, the outer edge of longitudinal muscle layer and intermuscular septum between the two layers were manually drawn with Sigma Scan Pro 5 (Jandel Scientific, San Rafael, CA). Distance between the outer longitudinal muscle edge and septum represents the longitudinal muscle thickness and between the septum and the inner circular muscle edge the circular muscle thickness. Muscle thicknesses were corrected by the magnification factor to estimate the thickness in millimeters. The thickness of circular muscle layer was considered as the radius of the circle, and, by using the equation πr2, the circular muscle cross-sectional area (CSA) was calculated. The CSA based on the total muscle thickness of circular and longitudinal muscle thickness was subtracted from the circular muscle CSA to determine the longitudinal muscle CSA. Temporal plots of pressure, impedance wave at the location of the US transducer, circular muscle CSA, and longitudinal muscle CSA were constructed for five to seven swallows in each subject. From these temporal plots the CSA and muscle thickness measurements were selected, before and at the peak of swallow-induced contractions.
Statistical analysis.
Data are presented as means ± SE except where stated otherwise. Mean values for each subject were calculated and used to estimate the overall mean. Unpaired Student's t-test with unequal variance was used to estimate statistical significance between the groups. P values less than 0.05 were considered statistically significant.
RESULTS
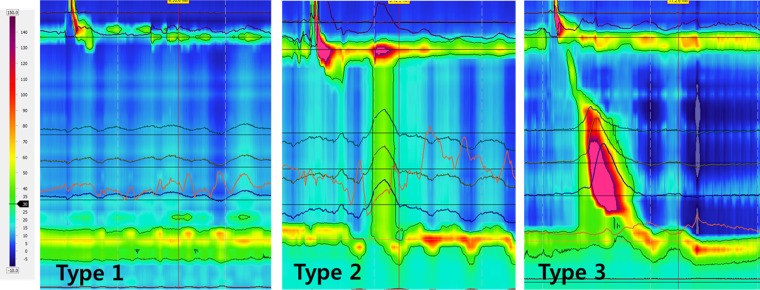
During the 2-yr period, 187 patients were diagnosed with achalasia esophagus; 30 met criteria for type 1 achalasia, 121 for type 2, and 36 for type 3 achalasia (Fig. 1A). Of the 36 type 3 achalasia patients, 20 were male (mean age = 61) and 16 female (mean age = 58). Dysphagia was the predominant symptom (n = 31) in these patients; some also had regurgitation (n = 6), chest pain (n = 5), food impaction (n = 5), epigastric pain (n = 3), and heartburn (n = 3).
Fig. 1.
Three types of achalasia: type 1, type 2, and type 3. Pressure line tracings at multiple locations in the esophagus are superimposed on the high-resolution manometry (HRM) plot with a 30-mm isocontour plot.
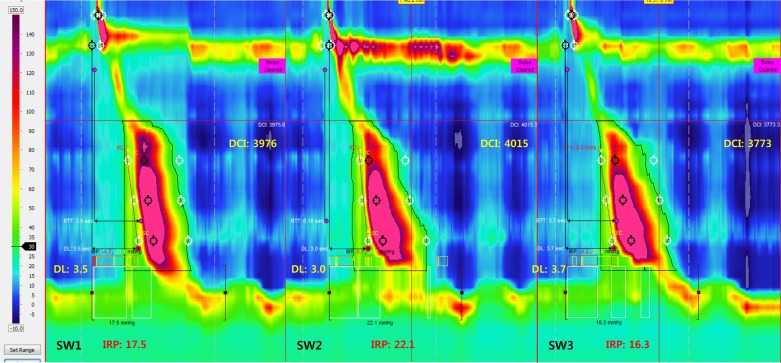
Patients with type 3 achalasia were assessed for the characteristics of pressure waveform in the distal esophagus (the onset, the peak, and the end of contraction) by using the line tracing (Fig. 2 and Table 1). The onset of pressure wave was simultaneous by the criteria described in methods and experimental design, with almost all contractions (420/434). However, in contrast to the onset, the first peak of contraction was sequential with 70% of the 434 contraction. In the remainder 30%, peaks were either simultaneous or retrograde. Twenty-nine of the 36 subjects had more than two types of peak contraction (sequential, simultaneous, and retrograde). Median number of contractions with sequential peaks per subjects was 73% (range 28–100%), simultaneous 13% (range 0–67%), and retrograde 0% (range 0–45%). The termination or end of contraction wave was also sequential with 80% of the 434 swallow-induced contractions. In the remainder 20%, it was either simultaneous or retrograde, and 25 of 36 patients had a mix of peristaltic, simultaneous, and retrograde end of contraction [median for sequential 80% (range 33–100%), simultaneous 13% (range 0–53%), and retrograde 0% (range 0–20%)].
Fig. 2.
HRM plot with isocontour 30 mmHg lines of a patient with type 3 achalasia esophagus. IRP, integrated relaxation pressure; DL, distal latency; DCI, distal contractile integral. Note that each swallow fulfills the criteria for simultaneous contraction, which are simultaneous onset of 30 mmHg isocontour and DL < 4.5 s.
Table 1.
Analysis of achalasia type 3 contractions: analysis of pressure waveforms
| Onset of Peristalsis |
Peak of Peristalsis |
End of Peristalsis |
||||||
|---|---|---|---|---|---|---|---|---|
| Peristaltic | Simultaneous | Retrograde | Peristaltic | Simultaneous | Retrograde | Peristaltic | Simultaneous | Retrograde |
| 14 (3%) | 420 (97%) | 0 (0%) | 304 (70%) | 105 (24%) | 25 (6%) | 346 (80%) | 70 (16%) | 18 (4%) |
N = 36 subjects, number of contractions analyzed = 434.
Impedance HRM analysis.
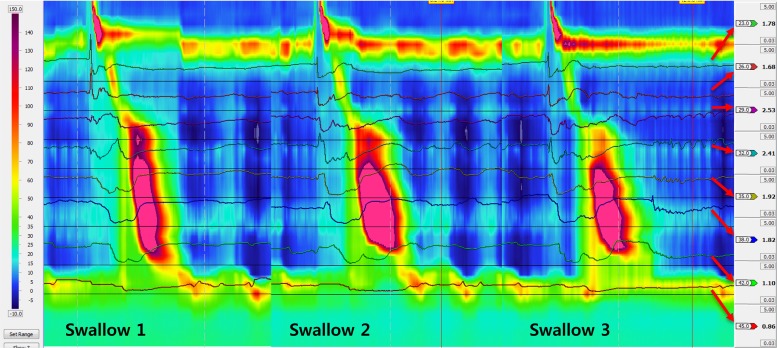
Fourteen patients with type 3 achalasia esophagus, different from the group of 36 described in the previous paragraphs, were studied for bolus clearance with esophageal contraction. In total 136 swallows were analyzed. One hundred nineteen (88%) of the 136 contractions met the criteria for type 3 contractions; the remainder were either type 2 or type 1 contractions. There was either incomplete or no bolus clearance with type 1 and type 2 achalasia contractions. On the other hand, the bolus clearance was complete with 94% of type 3 achalasia contractions. In 112 type 3 achalasia contractions that resulted in complete bolus clearance, sequential peak and sequential termination of contraction were seen in 92% (103/112) and 97% of instances, respectively. In seven type 3 achalasia contractions that met criteria for incomplete bolus clearance, sequential peaks and sequential ends were seen in four of seven and four of seven instances, respectively. Definition of complete clearance included sequential nadir impedance and sequential return of impedance to 50% of the baseline value and both traveling in the aboral direction in the last 10 cm of the esophagus (Fig. 3).
Fig. 3.
HRM plot with superimposed impedance waveforms. Note that each swallow is associated with orderly progression and complete clearance of bolus in the esophagus.
Ultrasound and HRM analysis.
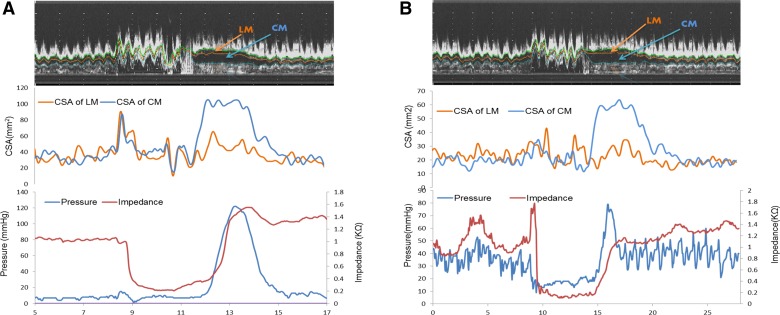
US data from six patients in this study were compared with data from 16 normal subjects reported in a recently published study (26) (Figs. 4 and 5). The baseline muscle thicknesses in these normal subjects is identical to those reported in our earlier studies (9, 27, 35) and by Miller and colleagues (17, 22). The baseline muscle thickness in patients was significantly larger at 5 cm as well as at 10 cm above the LES compared with normal subjects, which was true for both circular and longitudinal muscle. The baseline muscle thickness in patients was significantly greater at 5 cm compared with the 10-cm level (P < 0.05). With esophageal contraction there is an increase in the thickness of circular and longitudinal muscle in normal subjects as well as patients. Axial shortening of the circular muscle during contraction (ratio of peak CSA to baseline CSA) was much greater at 5 cm compared with 10 cm above the LES in normal subjects as well as in patients (P < 0.05). Axial shortening of the two layers was significantly smaller in patients compared with normal at 5 cm above the LES (Table 2).
Fig. 4.
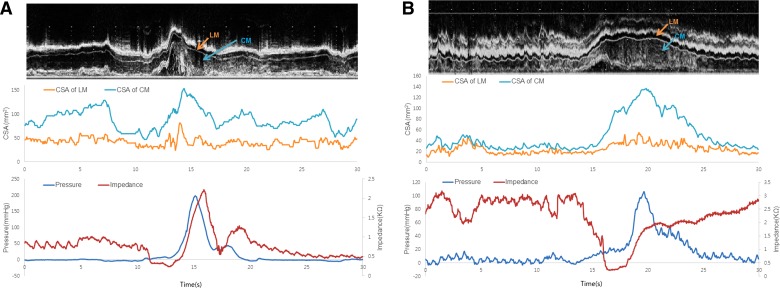
A and B: relationship between changes in ultrasound (US) images, pressure, impedance, and cross-sectional area (CSA) of the circular muscle (CM) and longitudinal muscle (LM) of the esophagus at 5 cm above the lower esophageal sphincter (LES) in a normal subject. Note, the onset of luminal distension is associated with drop in impedance and luminal closure is associated with increase in thickness and CSA of circular and longitudinal muscle thickness.
Fig. 5.
A and B: relationship between changes in US images, pressure, impedance, and CSA of the circular and longitudinal muscle of the esophagus at 5 cm above the LES in 2 patients with type 3 achalasia esophagus. Note, the onset of luminal distension is associated with drop in impedance, and luminal closure is associated with increase in thickness of circular and longitudinal muscle thickness (CSA). Also note that the muscle thickness is significantly greater in patients compared with normal subjects (Fig. 4, A and B).
Table 2.
Analysis of achalasia type 3 contractions: circular and longitudinal muscle thickness in normal subjects and patients with type 3 achalasia esophagus at baseline and peak of swallow- induced contractions
| Normal Healthy Person (N = 16) |
Achalasia Type 3 Patient (N = 6) |
|||
|---|---|---|---|---|
| Muscle Thickness | CM | LM | CM | LM |
| 10 cm | ||||
| Baseline (mm) | 0.68 | 0.66 | 1.71* | 0.85* |
| Peak/Baseline ratio | 2.19 | 1.23 | 2.01 | 1.37 |
| 5 cm | ||||
| Baseline (cm) | 0.68 | 0.65 | 2.33* | 0.93* |
| Peak/Baseline ratio | 2.94† | 1.38† | 1.76‡ | 1.17‡ |
P < 0.05 baseline muscle thickness between normal subjects and patients;
P < 0.05, peak-to-baseline thickness (marker of axial shortening) is bigger at 5 cm compared with 10 cm in normal subjects;
P < 0.05 peak-to-baseline thickness (axial shortening of circular and longitudinal muscle) is significantly smaller in patients compared with normal subjects at 5-cm level. CM, circular muscle; LM, longitudinal muscle.
DISCUSSION
The criteria for defining simultaneous contraction have varied over the years. Prior to manometry, tertiary contractions and segmentation seen on X-ray fluoroscopy were used to define diffuse esophageal spasm. Manometric definition of simultaneous contraction is based on the onset of contraction (pressure wave) in the distal esophagus. Velocity of peristalsis of greater than 8–9 cm/s in the distal esophagus is currently considered simultaneous contraction. Behar and Biancani (4) found that the latency of swallow-induced distal esophageal contraction was shorter in patients with simultaneous esophageal contractions compared with normal subjects. The shorter latency is consistent with impaired deglutitive inhibition in patients. The authors suggested that the short latency in the distal esophagus represents defective nitrinergic innervations of the esophagus because nitric oxide antagonism causes decrease in the latency of distal esophageal contraction and increase in the velocity of peristalsis (20, 33, 36). Using a novel distal esophageal landmark, the contraction deceleration point (CDP) in the HRM recording (15, 24), Pandolfino et al. (25) found that short distal esophageal latency (<4.5 cm) was a better criterion to diagnose simultaneous esophageal contraction than the velocity of peristalsis.
Many investigators have struggled to define what actually represent simultaneous esophageal contraction on manometry (2). The difficulty stems from the fact that identifying the onset of esophageal contraction on manometry recordings is challenging for several reasons: 1) presence of bolus in the esophagus, distal to the contraction contributes to the esophagus pressure, the so-called bolus/common cavity pressure (28). For the above reason Behar and Biancani (4) assessed dry swallow- rather than the wet swallow-induced esophageal contractions. However, dry swallows may not completely eliminate bolus pressure because infusion manometry was used to study esophageal motor activity. The common cavity pressure resulting from a trapped bolus between peristaltic esophageal contraction and partially closed/relaxed LES can contribute significantly to the intraluminal esophageal pressure. The pressure waveform at any location in the esophagus is a blend of bolus pressure and contact pressure. Some investigators have arbitrarily assumed that the esophageal pressure due to bolus has a slow rate of pressure increase and argued that the rapid onset of pressure wave reflects the true contact pressure (10, 29), which may be true in normal subjects and under some circumstance. Others have argued for using isobaric pressure of 30 mmHg to recognize the onset of contact pressure. It is also suggested that cavity pressure can't be greater than the residual LES pressure with swallow-induced LES relaxation (25), which may not be necessarily true, as one can see in Figs. 2 and 3. 2) In normal subjects, with swallow-induced axial shortening of the esophagus, a relaxed LES slides easily in the cranial direction over the manometry catheter. On the other hand, with swallow-related axial esophageal shortening, a partially relaxed LES in achalasia esophagus may grab the catheter rather than slide over it and cause the catheter to bow in the esophagus and in turn press against the esophageal wall.
During peristalsis, at any given time point, a length, rather than a focal point of the esophagus is contracted and this segment moves sequentially along the length of the esophagus (3, 6, 8). The pressure in the contracted segment is bell shaped with the peak pressure in the middle of the bell. The peak and tail end of the pressure are located several centimeters orad to the point of the onset of contraction. The latter on manometry correlates with the tail end of bolus on X-ray fluoroscopy images (8). We found that the peak and termination of contraction in majority of type 3 achalasia contractions are actually sequential, which has never been reported. Our observation that the onset of contraction in type 3 achalasia is simultaneous and the peak and end of contractions are sequential raises two possibilities: either the neuromyogenic factors that control sequential nature of contraction are different for the onset, the peak, and the end of esophageal contraction, or that the manometry technique can't correctly record the onset of contraction, i.e., “true onset of circular muscle contraction” under all circumstances. We suspect latter to be the case; our reasoning is based on the complete bolus clearance in association with majority of type 3 achalasia contractions, which would not be expected if esophageal contractions were truly simultaneous. In an earlier study also we found bolus clearance to be relatively normal in type 3 achalasia, albeit the number of subjects studied was small (n = 4) (11).
Clearance of bolus in the esophagus has been traditionally recorded by X-ray fluoroscopy using a barium swallow. Patients with DES have tertiary and segmental contractions that may or may not be associated with complete bolus clearance. Multiple intraluminal impedance (MII) is a sensitive technique to monitor bolus clearance and has been validated by a number of investigators (21, 31, 32, 34). Return of baseline impedance to 50% of the baseline value corresponds with the bolus clearance. Using the above criteria, we found that a majority of type 3 achalasia contractions are associated with orderly progression of bolus in an aboral direction, which suggests that the so-called simultaneous contractions of achalasia type 3 esophagus are not true simultaneous contractions. One may argue that MII, even though accurate for recording bolus clearance in normal subjects, may not be so in patients if a previous contraction did not clear the bolus completely, which would cause a low baseline impedance value. Our recent studies show that even a minute amount of bolus (normal saline) in the esophagus causes baseline impedance of <400 Ω (13, 37). The baseline impedance after bolus clearance with esophageal contractions was more than 1,000 Ω during majority of contraction arguing against retained bolus prior to the onset of contraction. Interestingly, Tutuian et al. (34) found marked heterogeneity in bolus clearance in patients with DES and recognized difficulty in identifying the simultaneous esophageal contraction. They also felt difficulty in distinguishing bolus pressure from the contact pressure in esophageal pressure waveform.
We do not imply that true simultaneous contractions do not exist but our contention is that majority of the so-called simultaneous contractions in type 3 achalasia are not truly simultaneous. Type 3 achalasia esophagus is an intriguing entity for several reasons: 1) Some investigators believe that these patients were previously classified as either DES or vigorous achalasia. 2) If peristalsis is indeed intact in patients with achalasia esophagus type 3 then why do these patients have dysphagia to begin with? 3) Several studies show that patients with achalasia type 3 do not respond as well to medical and surgical treatments as patients with type 2 and type 1 achalasia. We did not measure muscle thickness of normal subjects and achalasia patients in a blinded fashion, which could be considered as the weakness of our study. However, the difference in thickness values between type 3 achalasia and normal subjects is not subtle, it is severalfold (2 to 3 times or even more), a finding also observed by Krishnan et al. (14). Muscle thickness in achalasia esophagus type 3 > type 2 achalasia > type 1 achalasia (18). We speculate that the thicker muscle in type 3 achalasia esophagus leads to poor distensibility of the esophagus, which may allow liquid bolus to pass through but not a solid bolus. The functional luminal imaging probe (FLIP) can easily measure distensibility of the esophagus. Future studies may investigate whether reduced distensibility of the esophagus is the reason for poor response to treatment in patients with type 3 achalasia esophagus.
In summary, our findings show that majority of esophageal contractions in type 3 achalasia esophagus are sequential and are associated with adequate clearance of liquid bolus. Type 3 achalasia contractions, similar to normal subjects, are associated with an increase in the circular and longitudinal muscle thickness as well as axial shortening of the esophagus. We propose that the mechanism of dysphagia in achalasia type 3 is not the lack of peristalsis; rather it is related to hypertrophy of the muscularis propria, which results in poor distensibility of the esophagus. The latter may also be the reason for poor response to medical and surgical therapy because pneumatic dilation of the LES and surgical myotomy may not address poor distensibility of the esophagus.
GRANTS
This work was supported by a National Institute for Diabetes and Digestive and Kidney Diseases Grant DK060733.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.H.K., M.L.-L., and R.K.M. performed experiments; T.H.K., N.P., and R.K.M. analyzed data; T.H.K. and R.K.M. interpreted results of experiments; T.H.K. prepared figures; T.H.K., N.P., and R.K.M. drafted manuscript; T.H.K. and R.K.M. edited and revised manuscript; T.H.K. and R.K.M. approved final version of manuscript; M.L.-L. and R.K.M. conception and design of research.
REFERENCES
- 1.Abrahao L Jr, Bhargava V, Babaei A, Ho A, Mittal RK. Swallow induces a peristaltic wave of distension that marches in front of the peristaltic wave of contraction. Neurogastroenterol Motil 23: 201–207, e110, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Achem SR. Diffuse esophageal spasm in the era of high-resolution manometry. Gastroenterol Hepatol (N Y) 10: 130–133, 2014. [PMC free article] [PubMed] [Google Scholar]
- 3.Babaei A, Bhargava V, Korsapati H, Zheng WH, Mittal RK. A unique longitudinal muscle contraction pattern associated with transient lower esophageal sphincter relaxation. Gastroenterology 134: 1322–1331, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology 105: 111–118, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Boeckxstaens GE, Annese V, des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, Smout AJ, Tack J, Zwinderman AH, Zaninotto G, Busch OR. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med 364: 1807–1816, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Brasseur JG. Mechanical studies of the esophageal function. Dysphagia 8: 384–386, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Chen YM, Ott DJ, Hewson EG, Richter JE, Wu WC, Gelfand DW, Castell DO. Diffuse esophageal spasm: radiographic and manometric correlation. Radiology 170: 807–810, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Dodds WJ, Stewart ET, Hodges D, Zboralske FF. Movement of the feline esophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest 52: 1–13, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dogan I, Puckett JL, Padda BS, Mittal RK. Prevalence of increased esophageal muscle thickness in patients with esophageal symptoms. Am J Gastroenterol 102: 137–145, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Hewson EG, Ott DJ, Dalton CB, Chen YM, Wu WC, Richter JE. Manometry and radiology. Complementary studies in the assessment of esophageal motility disorders. Gastroenterology 98: 626–632, 1990. [PubMed] [Google Scholar]
- 11.Hong SJ, Bhargava V, Jiang Y, Denboer D, Mittal RK. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology 139: 102–111, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 145: 954–965, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Mittal RK, Patel N, Ledgerwood M, Bhargava V. Esophageal distension during bolus transport: can it be detected by intraluminal impedance recordings? Neurogastroenterol Motil 26: 1122–1130, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan K, Lin CY, Keswani R, Pandolfino JE, Kahrilas PJ, Komanduri S. Endoscopic ultrasound as an adjunctive evaluation in patients with esophageal motor disorders subtyped by high-resolution manometry. Neurogastroenterol Motil 26: 1172–1178, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Z, Pandolfino JE, Xiao Y, Carlson D, Bidari K, Escobar G, Kahrilas PJ. Localizing the contractile deceleration point (CDP) in patients with abnormal esophageal pressure topography. Neurogastroenterol Motil 24: 972–975, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massey BT, Dodds WJ, Hogan WJ, Brasseur JG, Helm JF. Abnormal esophageal motility. An analysis of concurrent radiographic and manometric findings. Gastroenterology 101: 344–354, 1991. [PubMed] [Google Scholar]
- 17.Miller LS, Liu JB, Colizzo FP, Ter H, Marzano J, Barbarevech C, Helwig K, Leung L, Goldberg BB, Hedwig K. Correlation of high-frequency esophageal ultrasonography and manometry in the study of esophageal motility. Gastroenterology 109: 832–837, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Mittal RK, Hong SJ, Bhargava V. Longitudinal muscle dysfunction in achalasia esophagus and its relevance. J Neurogastroenterol Motil 19: 126–136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal RK, Ren J, McCallum RW, Shaffer HA Jr, Sluss J. Modulation of feline esophageal contractions by bolus volume and outflow obstruction. Am J Physiol Gastrointest Liver Physiol 258: G208–G215, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Murray JA, Ledlow A, Launspach J, Evans D, Loveday M, Conklin JL. The effects of recombinant human hemoglobin on esophageal motor functions in humans. Gastroenterology 109: 1241–1248, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen HN, Silny J, Albers D, Roeb E, Gartung C, Rau G, Matern S. Dynamics of esophageal bolus transport in healthy subjects studied using multiple intraluminal impedancometry. Am J Physiol Gastrointest Liver Physiol 273: G958–G964, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Nicosia MA, Brasseur JG, Liu JB, Miller LS. Local longitudinal muscle shortening of the human esophagus from high-frequency ultrasonography. Am J Physiol Gastrointest Liver Physiol 281: G1022–G1033, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 135: 1526–1533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandolfino JE, Leslie E, Luger D, Mitchell B, Kwiatek MA, Kahrilas PJ. The contractile deceleration point: an important physiologic landmark on oesophageal pressure topography. Neurogastroenterol Motil 22: 395–400, e90, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandolfino JE, Roman S, Carlson D, Luger D, Bidari K, Boris L, Kwiatek MA, Kahrilas PJ. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology 141: 469–475, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel N, Jiang Y, Mittal RK, Kim TH, Ledgerwood M, Bhargava V. Circular and longitudinal muscles shortening indicates sliding patterns during peristalsis and transient lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 309: G360–G367, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puckett JL, Bhalla V, Liu J, Kassab G, Mittal RK. Oesophageal wall stress and muscle hypertrophy in high amplitude oesophageal contractions. Neurogastroenterol Motil 17: 791–799, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Ren J, Massey BT, Dodds WJ, Kern MK, Brasseur JG, Shaker R, Harrington SS, Hogan WJ, Arndorfer RC. Determinants of intrabolus pressure during esophageal peristaltic bolus transport. Am J Physiol Gastrointest Liver Physiol 264: G407–G413, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Richter JE, Wu WC, Johns DN, Blackwell JN, Nelson JL 3rd, Castell JA, Castell DO. Esophageal manometry in 95 healthy adult volunteers. Variability of pressures with age and frequency of “abnormal” contractions. Dig Dis Sci 32: 583–592, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Rohof WO, Salvador R, Annese V, Bruley des Varannes S, Chaussade S, Costantini M, Elizalde JI, Gaudric M, Smout AJ, Tack J, Busch OR, Zaninotto G, Boeckxstaens GE. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 144: 718–725; quiz e13–e14, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Sifrim D, Silny J, Holloway RH, Janssens JJ. Patterns of gas and liquid reflux during transient lower oesophageal sphincter relaxation: a study using intraluminal electrical impedance. Gut 44: 47–54, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sifrim D, Tutuian R. Oesophageal intraluminal impedance can identify subtle bolus transit abnormalities in patients with mild oesophagitis. Eur J Gastroenterol Hepatol 17: 303–305, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Tottrup A, Svane D, Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 260: G385–G389, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Tutuian R, Mainie I, Agrawal A, Gideon RM, Katz PO, Castell DO. Symptom and function heterogenicity among patients with distal esophageal spasm: studies using combined impedance-manometry. Am J Gastroenterol 101: 464–469, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Liu J, Smith TK, Mittal RK. Distension-related responses in circular and longitudinal muscle of the human esophagus: an ultrasonographic study. Am J Physiol Gastrointest Liver Physiol 275: G805–G811, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Yamato S, Spechler SJ, Goyal RK. Role of nitric oxide in esophageal peristalsis in the opossum. Gastroenterology 103: 197–204, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Zifan A, Ledgerwood-Lee M, Mittal RK. Measurement of peak esophageal luminal cross-sectional area utilizing nadir intraluminal impedance. Neurogastroenterol Motil 27: 971–980, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]