Abstract
The pathogenesis of necrotizing enterocolitis (NEC), a common gastrointestinal disease affecting premature infants, remains poorly understood. We previously found that intestinal VEGF-A expression is decreased in human NEC samples and in a neonatal mouse NEC model prior to detectable histological injury. Therefore, we hypothesized that lack of VEGF receptor 2 (VEGFR2) signaling facilitates neonatal intestinal injury by impairing intestinal microvasculature development. Here, we found that intestinal VEGF-A and its receptor, VEGFR2, were highly expressed at the end of fetal life and significantly decreased after birth in mice. Furthermore, selective inhibition of VEGFR2 kinase activity and exposure to a neonatal NEC protocol significantly decreased the density of the intestinal microvascular network, which was further reduced when both interventions were provided together. Furthermore, VEGFR2 inhibition resulted in greater mortality and incidence of severe injury in pups submitted to the NEC model. The percentage of lamina propria endothelial cells was decreased during NEC induction, and further decreased when VEGFR2 signaling was inhibited. This was associated with decreased endothelial cell proliferation rather than apoptosis. In conclusion, we found that VEGF-A and VEGFR2 proteins are highly expressed in the intestine before birth, and are significantly downregulated in the immediate neonatal period. Furthermore, VEGFR2 signaling is necessary to maintain the integrity of the intestinal mucosal microvasculature during the postnatal period and lack of VEGFR2 signaling predisposes to NEC in neonatal mice.
Keywords: necrotizing enterocolitis, angiogenesis, vasculature development, VEGF-A/VEGFR2
necrotizing enterocolitis (NEC) is a leading cause of morbidity and mortality among premature neonates. Multiple contributing factors have been identified such as perinatal asphyxia, formula feeding, intestinal microbial imbalance, and immaturity of the immune system (6, 25). NEC is characterized by variable degrees of tissue damage to the intestine ranging from superficial mucosal injury to full-thickness ischemic coagulative necrosis and perforation (18). In animal studies, decreased blood flow in mesenteric vessels was observed prior to NEC (8, 19). However, whether the intestinal microvasculature development plays a role in the pathogenesis of NEC remains unknown.
Angiogenesis is the physiological process that involves the growth of new blood vessels from existing ones, which is driven by VEGFs and other growth factors. Among multiple VEGF family members (VEGF-A, -B, -C, -D, and -E), VEGF-A is considered a key angiogenic factor. The loss of one VEGF-A allele in mice significantly impairs vessel development, causing severe growth delay and mortality at midgestation (4, 9, 13, 16). VEGF-A promotes endothelial cell proliferation, migration, sprouting and tube formation, and facilitates the recruitment of bone marrow-derived accessory cells such as macrophages to assist in vessel formation (1, 14).
VEGF-A binds to tyrosine kinase receptors VEGF receptor-1 (VEGFR1) and VEGF receptor-2 (VEGFR2). VEGFR2 is the primary receptor relaying the VEGF-A signal in endothelial cells and plays a major role in angiogenesis (10). VEGFR1 inhibits angiogenesis and vascular overgrowth by competing with VEGFR2 (11). VEGF-A also binds to neuropilin-1/2 and has been shown to be important for axonal guidance during neuronal development (15, 32) together with VEGF-C and VEGF-D, which are also involved in lymphoangiogenesis via binding to VEGFR3.
We previously showed that VEGF-A expression is decreased in intestinal surgical samples of patients with NEC compared with control tissue samples obtained from patients with other diseases such as ileal atresia, intestinal reanastomosis, duplication cyst, or Hirschsprung disease (36). Furthermore, we found that VEGF-A protein is decreased in a neonatal mouse NEC model (36). Therefore, we hypothesize that VEGF-A/VEGFR2 signaling promotes perinatal intestinal mucosal microvascular development and plays a protective role in neonatal NEC.
In this study, we first examined the perinatal expression of VEGF-A and VEGFR2 in the small intestine. Second, using a neonatal mouse NEC model developed in the laboratory, we examined whether exposure to the NEC protocol affects VEGFR2 protein expression, mucosal vascular density, and endothelial cell proliferation. Third, we investigated the effect of blocking VEGFR2 signaling on the incidence of NEC and on mucosal microvascular density, and determined whether endothelial cell proliferation and apoptosis are involved in the process.
MATERIALS AND METHODS
Materials.
C57BL/6 and CX3CR1-GFP mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Experimental pups were generated by overnight breeding. All animal breeding and procedures were approved by the Institutional Animal Care and Use Committee of Stanley Manne Children's Research Institute. A fluorescein-conjugated in situ cell death detection kit was purchased from Roche Diagnostics (Mannheim, Germany). Rabbit derived anti-CD31 (ab28364), anti-VEGF-A (ab52917 for immunofluorescence), rat anti-5-bromo-2′-deoxyuridine (BrdU) (ab6326), chicken anti-green fluorescence protein (GFP) (ab13970), and donkey anti-goat (ab150129) antibodies were purchased from Abcam (Cambridge, UK). Anti-chromogranin A (sc-1488) and anti-VEGF-A (sc-7269, for Western blot) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Dye-conjugated anti-EpCAM (CD326, 12–5791-81), CD45 (17-0451-82), and CD31 (13-0311-82) antibodies and streptavidin (15–4317-82) for flow cytometry were purchased from eBioscience (San Diego, CA). Goat anti-rabbit (A11037, A11034), goat anti-rat (A11007), and goat anti-chicken (A11039) antibodies; Alexa Fluor 647-conjugated wheat germ agglutinin, and a live/dead cell kit were obtained from Life Technologies (Grand Island, NY). Anti-VEGFR2 (2479) antibodies were purchased from Cell Signaling Technology (Danvers, MA). SU5416 and Ki8751 were obtained from Tocris Bioscience (Bristol, UK).
Animal experiments.
To study perinatal expression of VEGF-A and VEGFR2 proteins, mouse fetuses were delivered via abdominal incision of time-pregnant C57BL/6 mice at embryonic day 16 (E16), E18, E20, or allowed to deliver naturally and left with the dams for different time periods. Small intestinal tissues from these animals were collected for VEGF-A and VEGFR2 protein expression analysis. To study the role of VEGFR2 signaling, litters of 1-day-old mouse pups were separated into four groups: group 1 was left with the dams, allowed to be dam fed (DF) and injected with a VEGFR2 kinase inhibitor once a day (diluted in 2 μl of DMSO then 28 μl of 0.9% saline/1% carboxymethycellulose); group 2 was DF and injected with vehicle only (DMSO-NaCl/carboxymethylcellulose, ip); group 3 was subjected to our NEC protocol (41) and injected with vehicle only; group 4 was subjected to our NEC protocol and injected with SU5416 (20 mg/kg ip) or Ki8751 (1 mg/kg ip) once a day. Daily drug injection were started on day 1 of life and continued throughout the NEC protocol. The NEC protocol (41) includes orogastric inoculation with 108 colony-forming units of a standardized adult mouse commensal bacteria preparation to perturb the normal intestinal colonization process, formula-feeding every 3 h (Esbilac, 200 ml·kg−1·day−1) and exposure to brief episodes of hypoxia (60 s in 100% N2) followed immediately by cold stress (10 min at 4°C) twice daily. We found this protocol to induce a wide range of intestinal lesions from epithelial to transmural intestinal injury resembling human NEC (41). Animal mortality was closely monitored. Whole intestinal tissues were collected and slides stained with hematoxylin and eosin were evaluated and scored by an investigator unaware of the group assignment. Severe NEC was defined as a histological score ≥2. Pups that died before tissue collection were excluded from intestinal injury assessment.
Cell isolation and flow cytometry.
Pups were euthanized, and small intestinal tissues were collected and cut open under microscope. Pieces (1–2 cm in length) were washed in PBS/1 mM DTT three times, then incubated in dissociation buffer [PBS/2% fetal bovine serum (FBS)/1 mM EDTA] for 30 min at room temperature before being digested at 37°C for 45 min in a 1 mg/ml collagenase IV solution (PBS/collagenase/2% FBS). Recovered cells were washed in PBS twice before being stained with live/dead marker and EpCAM1, CD45, and CD31 antibodies for flow cytometry analysis. Endothelial cells were quantified as a percentage of CD31-positive cells in the live, EpCAM− CD45− cell populations.
Microvasculature imaging.
Neonatal pups subjected to the NEC protocol for 48 h, and DF controls were anesthetized with 65 mg/kg of pentobarbital, and 500 μl of 40 μg/ml Alexa Fluor 647-conjugated wheat germ agglutinin was injected by intracardiac infusion. The procedure was performed under microscope and successful perfusion was assessed by blood vessel blanching and appropriate filling with dye solution. Five minutes later, whole intestinal tissues were collected and fixed in formalin. Small intestinal tissues resected 2.0 cm away from the stomach were collected, cut open, washed in PBS twice, and covered with DAPI mounting media. Vascular image Z-stacks at a thickness of 5.81 μm were captured by a Zeiss 510 META Confocal Laser Scanning Microscope within 48 h after perfusion. Z-stacks were then projected by Zen 2009 software. Vessel area percentage was determined as an index of vascular density using Photoshop software. Specifically, images were reopened in Photoshop CS4-extended, and the magic wand tool was used to measure the vessel area by setting up the tolerance threshold around 190 to pick up all the vessel area pixels but not the background. One to three randomly chosen fields (×10) were studied in each sample with a total of 7–11 fields per group, and the mean value was calculated.
Immunofluorescence staining.
Whole intestinal tissues were collected, fixed in formalin, and subsequently embedded in paraffin. Five-micrometer-thick sections were cut and subjected to an antigen retrieval procedure by incubation with 10 mM citrate buffer for 20 min. After treatment with Triton X-100, sections were blocked with 10% normal goat serum or PBS/5% BSA for 1 h at room temperature. Sections were then incubated with primary antibodies or buffer alone at 4°C overnight followed by incubation with fluorescent dye-conjugated secondary antibody for 1 h at room temperature. The specific dilution for each antibody was as follows: anti-VEGF-A (1:50), anti-VEGFR2 (1:200), anti-chromogranin A (1:50), anti-CD31 (1:50), anti-BrdU (1:50), anti-GFP (1:1,000), goat anti-rabbit secondary antibody (1:500), goat anti-rat secondary antibody (1:1,000), and goat anti-chicken secondary antibody (1:500). Images were captured by a Leica DMR-HC upright microscope. For endothelial cell proliferation analysis, 0.3 mg of BrdU was injected ip into the pups 4 h before euthanasia, and intestinal tissues were collected and fixed in 10% formalin. Intestinal tissues were stained with BrdU and CD31 antibodies. Cells were considered proliferating endothelial cells when costained for both CD31 and BrdU. For each group, proliferating endothelial cells were assessed in 3 fields/section, 1 section per sample × 3 samples. Negative controls in which the primary antibody had been omitted were run concomitantly and did not show any staining.
TUNEL staining.
Following antigen retrieval of deparaffinized formalin-fixed intestinal tissues, sections were first stained with rabbit anti-CD31 antibody (1:50) and Alexa Fluor 594-conjugated goat anti-rabbit secondary antibody (1:500). Sections were then pretreated with proteinase K and incubated with TUNEL reaction mixture, including label solution and terminal transferase for 1 h at 37°C. Stained sections were covered with mounting medium that includes DAPI prior to imaging.
Intestinal permeability study.
DF pups or pups subjected to the NEC protocol for 24 h were fasted for 2 h. FITC-conjugated (800 mg/kg) dextran (4 kDa, FD4; Sigma-Aldrich, St. Louis, MO) was administered by gavage. Four hours later, pups were euthanized and whole blood was collected. Serum fluorescence was measured on a fluorescent plate reader and results were compared with serial dilutions of known FITC-dextran concentrations.
Western blotting and immunoprecipitation.
Pup small intestines were snap-frozen prior to homogenization on ice in lysis buffer (10 mM Tris·HCl pH 7.6, 150 mM NaCl, 5 mM EDTA, 1 mM PMSF, 1 mM DTT, 0.25% Nonidet P-40, and Complete Mini Tablet Protease Inhibitor Cocktail from Roche). The protein concentration of the tissue lysates was determined with the Bradford method after separation from tissue debris by centrifugation. For Western blot analysis, samples containing 50–150 μg of protein were run on 10 or 15% SDS-PAGE gels and transferred onto nitrocellulose or polyvinylidene fluoride (PVDF) membranes. Membranes were blocked in 5% milk/Tween 20 (TBST), and incubated at 4°C overnight with primary antibodies against targeted proteins. This was followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. Membrane-bound secondary antibodies were detected using the standard Pierce enhanced chemiluminescence method. For β-actin detection, the membranes listed above were washed in Tris-buffered saline/0.05% TBST twice before being subjected to protein detection as explained above. For immunoprecipitation, ProteaseArrest (786-108; G-Biosciences) and phosphatase inhibitor cocktail 2 (p5726; Sigma-Aldrich) were added to tissue lysates during sample preparation, 1 mg of protein was incubated with VEGFR2 antibody (2479, 1:100 dilution; Cell Signaling) at 4°C overnight. VEGFR2 was immunoprecipitated by adding 100 μl of protein G Surebeads (Bio-Rad) to the protein-antibody solution. The mixture was incubated at 4°C for 60 min. Eluted protein suspended in Laemmli buffer was submitted to a SDS-PAGE gel before transfer to a membrane, which was probed with anti-phosphotyrosine (05–1050 4G10 Platinum, 1:1,000; Millipore) antibody. Subsequent incubation with secondary antibody and development was performed as described above.
Statistical analysis.
A two-sided Student's t-test was used for comparison between two groups, and ANOVA was used for comparison of three or more groups. Results were expressed as means ± SE. Animal survival data were analyzed by log-rank test. To evaluate differences in the incidence of severe NEC (score ≥2), χ2 analysis was used. P < 0.05 was considered statistical significant.
RESULTS
VEGF-A and its receptor VEGFR2 are strongly expressed in the intestine during late fetal life and are decreased in a neonatal mouse model of NEC.
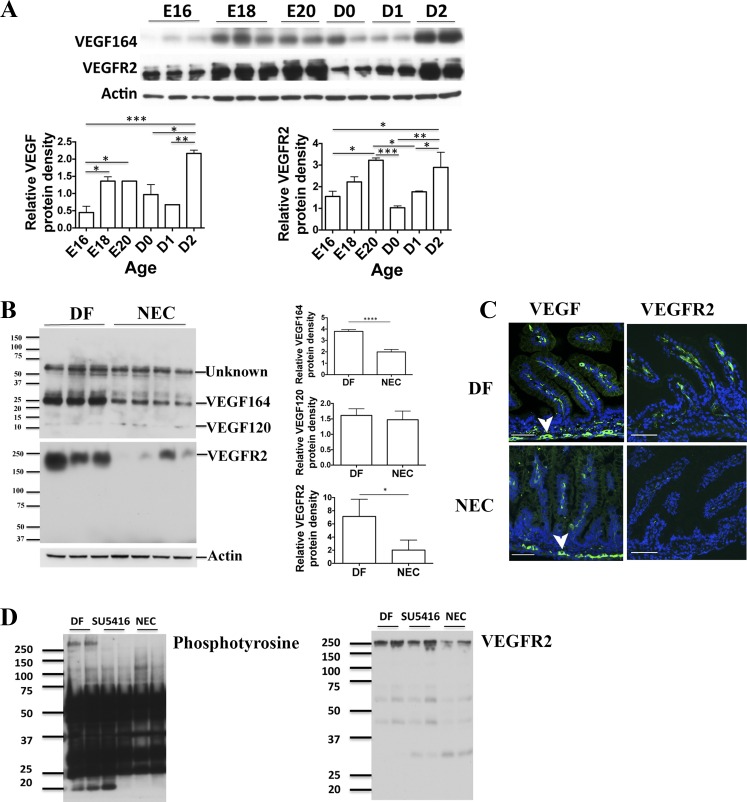
The developmental expression of VEGF-A164 and VEGFR2 in the intestine during the perinatal period was assessed by Western blot analysis. VEGF-A and VEGFR2 proteins are dramatically increased during late intrauterine life (Fig. 1A). After birth, both VEGF-A and VEGFR2 decreased sharply (D0) and remained low until day 2 of life, when they are significantly upregulated (Fig. 1A).
Fig. 1.
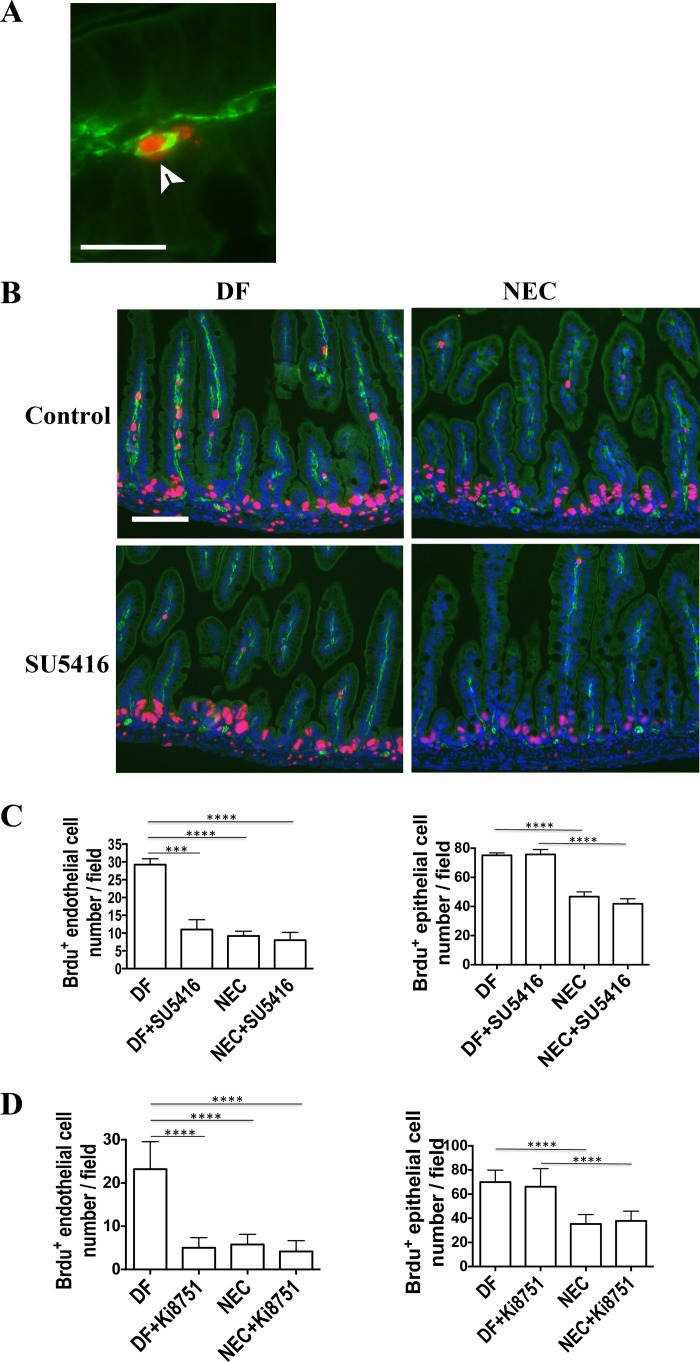
VEGF-A and its receptor VEGFR2 are highly expressed in the mouse intestine before birth. Intestinal VEGF-A and VEGFR2 are decreased in mouse pups subjected to experimental necrotizing enterocolitis (NEC). Intestinal tissues were obtained from mouse pups at embryonic day 16 (E16) to day 2 of life (A) and from pups subjected to the NEC protocol for 24 h or dam-fed (DF) littermate controls (B). VEGF-A and VEGFR2 proteins were analyzed by Western blot of small intestinal tissue lysates (A and B) and their cellular localization examined by immunofluorescence of formalin-fixed, paraffin-embedded tissues, using anti-VEGF-A or anti-VEGFR2 antibodies (green) and DAPI to counterstain nuclei (blue) (C, scale bar = 100 μm). Similar results were obtained in 2 separate experiments using different litters. Band densitometry data normalized to β-actin are presented in (A, bottom) and (B, right). For VEGF120, the densitometry analysis was carried out following prolonged exposure of the membrane. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Arrowhead in C points to myenteric plexus. Intestinal phosphotyrosine level was detected in DF, SU5416-treated pups or pups exposed to NEC after VEGFR2 immunoprecipitation (D, left). Total level of VEGFR2 from the same samples is shown in D, right.
We found that both VEGF164 and VEGFR2 proteins are decreased in the intestine of neonatal mice subjected to the NEC protocol for 24 h (Fig. 1, B and C). In some animals this change occurs in a patchy pattern, whereas in others it affects the entire small intestine. Furthermore, we found that exposure to the NEC protocol for 24 h decreased phosphorylation of VEGFR2 in the small intestine. Indeed, while the level of phosphorylated tyrosine following VEGFR2 immunoprecipitation was strong in DF pups, it was undetectable in pups submitted to the NEC protocol for 24 h, similar to DF pups treated with SU5416. SU5416 treatment did not affect the total level of VEGFR2 protein. In summary, our results suggest that exposure to the NEC protocol not only decreases the level of VEGFR2 protein but also decreases VEGFR2 phosphorylation.
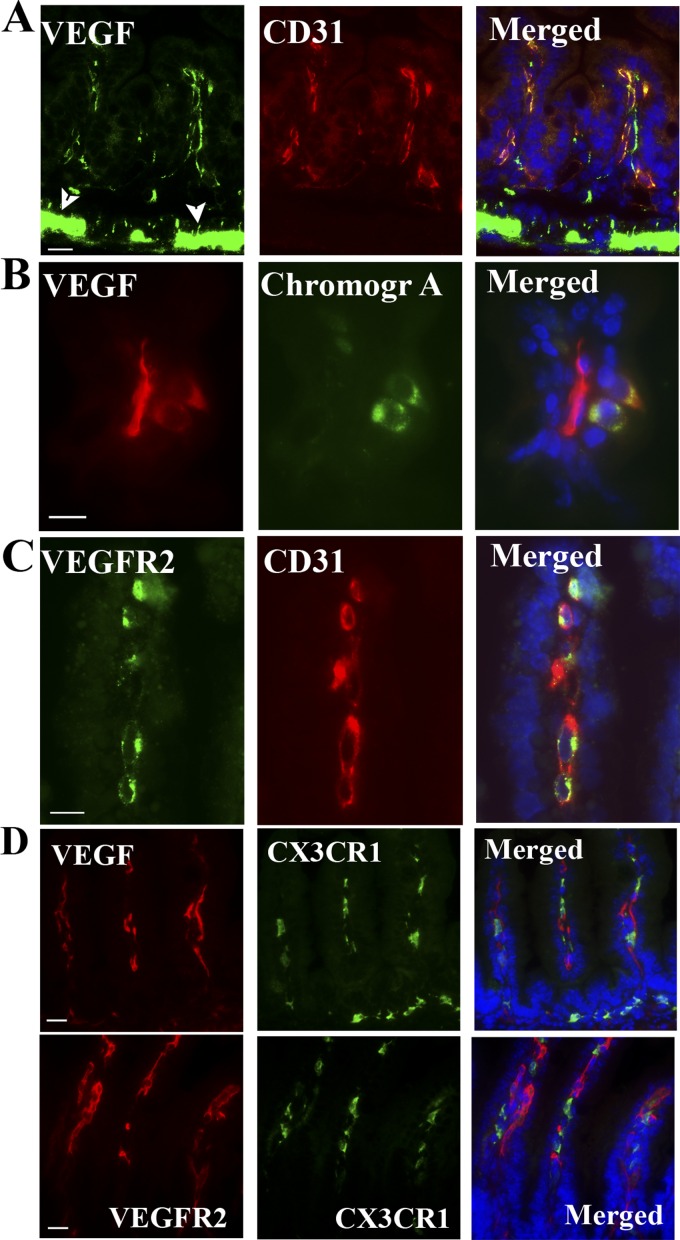
To determine the cell type or types that express VEGF-A and VEGFR2, these proteins were analyzed in small intestinal tissue sections costained with specific cell markers (Fig. 2, A–D). Although VEGFR2 was detected only on endothelial cells (CD31+ cells) (Fig. 2C), VEGF-A was found to be strongly expressed in enteroendocrine cells (chromogranin A+ cells) (Fig. 2B), myenteric neural plexus cells (arrowheads in Fig. 1C and Fig. 2A), and endothelial cells (CD31+) (Fig. 2A). Neither VEGF-A nor VEGFR2 were detected in CX3CR1+ myeloid cells (Fig. 2D).
Fig. 2.
In the intestinal villi, VEGF-A localizes to endothelial cells and enteroendocrine cells, and its receptor, VEGFR2, to endothelial cells. To determine which cells express VEGF-A and VEGFR2, intestinal tissue sections obtained from 48-h-old DF pups were costained with anti-VEGF-A antibody and with antibody against CD31, an endothelial cell marker (A) and against chromagranin A, an enteroendocrine cell marker (B). Other sections were costained with anti-VEGFR2 and anti-CD31 antibodies (C). D: small intestinal tissue sections of 48-h-old DF CX3CR1-GFP transgenic pups were stained for VEGF-A (top) or VEGFR2 (bottom) and costained with anti-GFP antibody to determine whether CX3CR1-myeloid cells produce VEGF-A/VEGFR2. Scale bars = 20 μm in A–D. Arrowhead in A points to the myenteric plexus.
Blocking VEGFR2 kinase activity increases the incidence and severity of NEC.
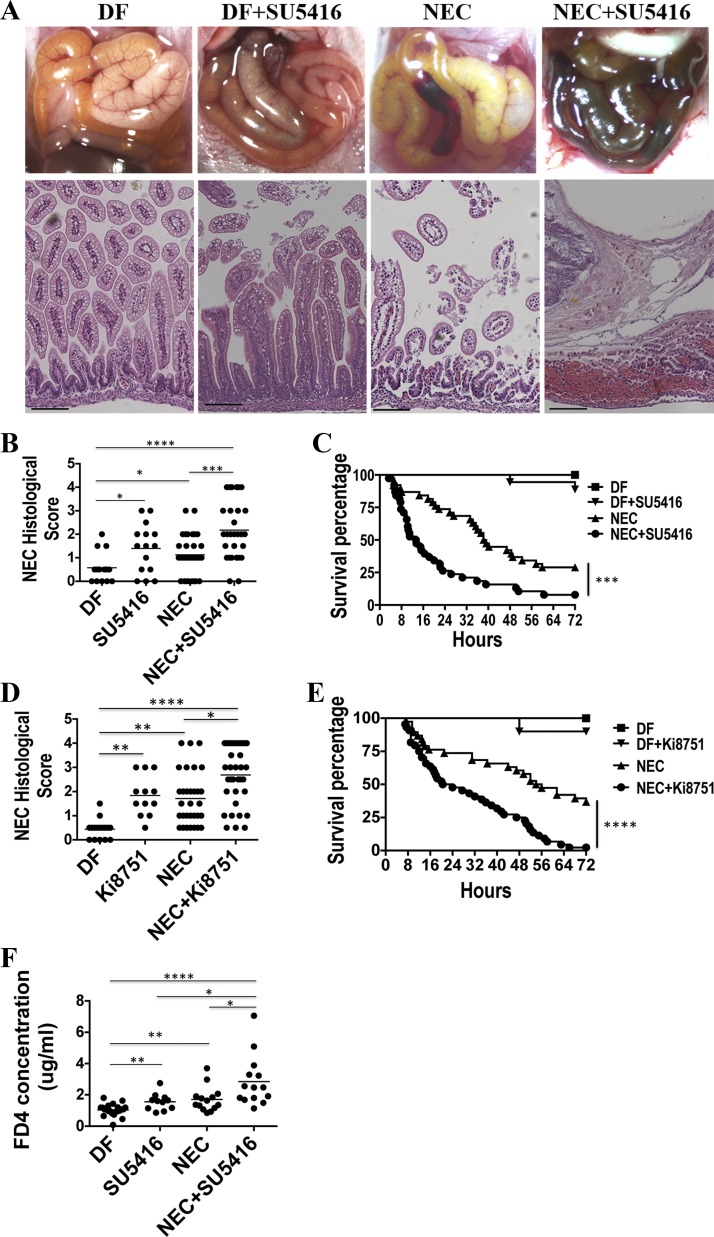
To determine whether the VEGF-A/VEGFR2 pathway is involved in NEC, pups were injected with SU5416 (20 mg/kg ip) or Ki8751 (1 mg/kg ip), two potent VEGFR2 kinase inhibitors (12, 28, 29), or vehicle only starting 12 h before NEC induction. The dose of 20 mg/kg of SU5416 was chosen on the basis of several in vivo studies conducted by other investigators in which 25 mg/kg was administrated to inhibit angiogenesis and hinder tumor growth without measurable toxicity (12, 42). Also, we found 20 mg/kg to be associated with minimal pup mortality in our pilot experiment. Similarly, the dose of 1 mg/kg of Ki8751 was chosen because it was the highest dose that caused minimal mortality in our neonatal mice over the 3-day experimental period. When pups were injected with SU5416 or Ki8751 while they were exposed to the NEC protocol, the incidence of severe NEC (≥2) was increased 2.48- or 1.71-fold, respectively, compared with those injected with vehicle only (SU5416: 18/29 vs. 8/32, χ2 = 7.1, P < 0.0005, Fig. 3, A and B; Ki8751: 27/35 vs. 14/31, χ2 = 5.9, P < 0.05, Fig. 3D). Also, when exposed to the NEC protocol, higher mortality rates were observed in the SU5416 and Ki8751 groups compared with the vehicle-only group as evidenced by a significantly shortened median survival (13.4 vs. 38.8 h for SU5416 injection or 22.3 vs. 55.0 h for Ki8751 injection) and a lower 72-h survival rate (SU5416: 7.90 vs. 28.9%, P < 0.0005, Fig. 3C; Ki8751: 2.80% vs. 36.8%, P < 0.0001, Fig. 3E). The intestinal permeability measured by serum dextran-FITC (FD4) concentration 4 h after FD4 gavage that we previously found to be increased preceding intestinal injury in our NEC model (2) was significantly increased in stressed pups (i.e., subjected to the NEC protocol) for 24 h (1.71 ± 0.21 μg/ml) compared with DF controls (1.04 ± 0.08 μg/ml, P < 0.005), and was further increased when stressed pups were injected with SU5416 (NEC+SU5416, 2.95 ± 0.43 μg/ml, P < 0.0001 vs. DF and P < 0.05 vs. NEC group, Fig. 3F). When DF pups were administered SU5416 or Ki8751 without being subjected to the NEC protocol, 2/19 and 1/13 pups, respectively, died over the 72-h experimental period, whereas all pups in the DF control group survived (Fig. 3, C and E). Also, increased histological injury scores were found in unstressed DF pups that received SU5416 or Ki8751. Also, the intestinal permeability was significantly higher in SU5416-injected DF pups compared with DF controls (1.56 ± 0.16 vs. 1.04 ± 0.08 μg/ml, P < 0.005, Fig. 3E).
Fig. 3.
Blocking VEGFR2 kinase activity increases the incidence and severity of NEC. DF pups or those subjected to the experimental NEC protocol were injected (ip) with 20 mg/kg of SU5416 (A–C and F) or 1 mg/kg of Ki8751 (D and E) or vehicle control daily. Representative photographic images (top) and histological pictures (bottom) from each group (SU5416 experiments) are shown in A. Scale bar = 100 µm. NEC histological scores are shown in B (SU5416) and D (Ki8751). Pup survival curves are shown in C (SU5416) and E (Ki8751). B, n = 14 in DF, 15 in SU5416 alone, 32 in NEC and 29 in NEC+SU5416 group; C, n = 20 in DF, 19 in SU5416 alone, 38 in each of NEC and NEC+SU5416 group; D, n = 16 in DF, 12 in SU5416 alone, 31 in NEC and 35 in NEC+SU5416 group; E, n = 16 in DF, 13 in SU5416 alone, 38 in NEC and 44 in NEC+SU5416 group. ***P < 0.001. ****P < 0.0001. These data were the combined results of 2 to 3 separated experiments. F: intestinal permeability was measured by serum FITC-dextran (FD4) concentration 4 h following enteral administration of 800 mg/kg of FD4 in DF (n = 22), SU5416 (n = 11), NEC (n = 14), and NEC + SU5416 pups (n = 14). Data represent 3 independent experiments combined. *P < 0.05, **P < 0.005, ****P < 0.0001.
Intestinal vascular network density is reduced in stressed pups and in DF pups injected with VEGFR2 inhibitor and further decreased in stressed pups injected with VEGFR2 inhibitor.
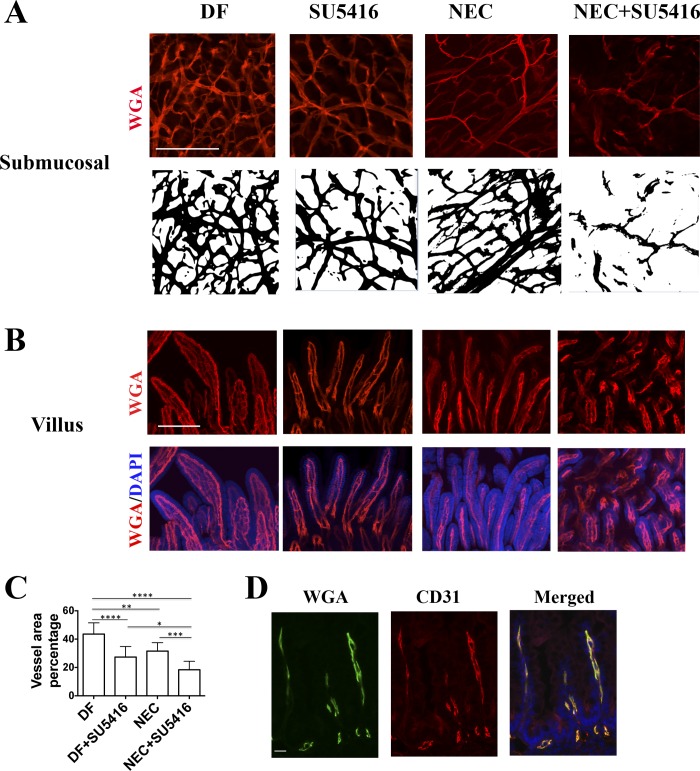
To determine whether vascular network changes are associated with NEC development, fluorescently labeled wheat germ agglutinin was administered to pups by intracardiac perfusion. In DF pups, we found that the intestinal submucosal microvasculature is organized as a lattice-like network with abundant capillary-like spouting and branching interconnected vessels. However, in DF pups injected with SU5416 and stressed vehicle-treated pups, the complexity of the intestinal vascular network and the vascular density were significantly decreased (Fig. 4A). Further reduction was observed in stressed pups injected with SU5416 (Fig. 4A). Indeed, the vascular area average percentage was 40.60 ± 2.65 in DF compared with 27.39 ± 2.50 in SU5416-injected alone, and 31.59 ± 2.00 in stressed pups and 18.39 ± 2.00 in stressed pups injected with SU5416 group (Fig. 4C, P < 0.05). In addition, examination by microscopy further revealed vessel appearance to be distorted in both the submucosa and villi in the stressed pups injected with SU5416 compared with pups in the other three groups (Fig. 4, A and B). To confirm that lectin perfusion would identify all vessels during NEC induction when mesenteric perfusion may be decreased, we costained NEC tissue sections with CD31 and found that all CD31+ vessels were also positive for lectin (Fig. 4D).
Fig. 4.
VEGFR2 inhibition and exposure to a NEC protocol decrease intestinal vascular network density, and these interventions are synergistic. Pups were injected (ip) with SU5416 or vehicle only, and/or subjected to a NEC protocol. Forty-eight hours later, Alexa Fluor 647-conjugated wheat germ agglutinin (WGA) was administered to the pups by intracardiac perfusion and the intestinal microvasculature was imaged at ×10 magnification (A and B, scale bar = 200 μm). C: vessel area percentage was determined as an index of vascular density. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; numbers of random fields counted in C were as follows: DF, 11; DF+SU5416, 7; NEC, 9; and NEC+SU5416, 7. Data are combined from 2 separate experiments using a total of 3 litters. D: lectin WGA-perfused small intestinal tissues of pups subjected to 48 h NEC were formalin-fixed and paraffin-embedded. Sections were stained with anti-CD31 antibody and DAPI (scale bar = 20 μm).
Endothelial cell proliferation is decreased in stressed pups and in DF pups injected with VEGFR2 inhibitor and further impaired when stressed pups are injected with VEGFR2 inhibitor.
To determine whether VEGFR2 kinase inhibition and NEC protocol exposure affect endothelial cell proliferation, BrdU was injected 4 h prior to euthanasia into DF pups, DF pups injected with SU5416, stressed pups, and stressed pups injected with SU5416, at 48 h after NEC protocol initiation. Intestinal tissue sections were stained for antibodies against both BrdU and the endothelial cell marker CD31, and cells costained with both antibodies were considered proliferating endothelial cells (Fig. 5, A and B). The number of proliferating endothelial cells per field (×20 magnification) was decreased in the villi of stressed pups (9.17 ± 1.35) and in stressed pups injected with SU5416 (8.00 ± 2.12) compared with DF controls (29.25 ± 1.65, P < 0.0001, Fig. 5, B and C). Also, SU5416 alone was found to inhibit endothelial cell proliferation in the villi of DF pups (11.00 ± 2.74 vs. 29.25 ± 1.65, P < 0.005, Fig. 5, B and C). A similar decrease in endothelial cell proliferation in stressed pups vs. DF pups was also observed when costaining with Ki67 and CD31 (data not shown). However, although intestinal epithelial cell proliferation was reduced in stressed pups (46.79 ± 3.23 vs. 75.17 ± 1.67 in DF pups, P < 0.0001), it was not affected by injection with SU5416 (41.99 ± 3.42 in stressed+SU5416 and 75.78 ± 3.41 in DF+SU5416 pups, not significant compared with vehicle-treated-only groups, Fig. 5, B and C), indicating that VEGFR2 inhibition specifically targets endothelial cell proliferation without affecting epithelial cell proliferation. Similar results were observed when SU5416 was substituted with Ki8751 (Fig. 5D).
Fig. 5.
VEGFR2 inhibition and exposure to an NEC protocol decrease endothelial cell proliferation, and these interventions are synergistic. Pups were injected (ip) with 5-Bromo-2′-deoxyuridine (BrdU) 4 h before euthanasia and intestine tissue collection. Intestine tissue sections were costained with antibodies against BrdU (red-purple) and CD31 (green) to localize proliferating endothelial cells. A: colocalization of CD31 (green) and BrdU (red) (×63 magnification, scale bar = 20 μm). B: enlarged portion of ×20 magnification fields taken from DF or NEC pups with or without SU5416 injection (scale bar = 80 μm). C: proliferating endothelial (left) and epithelial (right) cells of the villi were counted at ×20 magnification in 3 fields per sample and the average number of BrdU+ endothelial cells per field are presented. DF, n = 4; DF+SU5416, n = 4; NEC, n = 6; and NEC+SU5416, n = 4. Data were obtained from 3 litters in 2 separate experiments. D: bar graphs showing proliferating endothelial cells (left) and epithelial cells (right) of the villi per field in tissues of DF pups (n = 9), DF+Ki8751 (n = 8), NEC (n = 8), and NEC+Ki8751 (n = 6). Data were obtained from 5 litters in 2 separated experiments. ***P < 0.001, ****P < 0.0001.
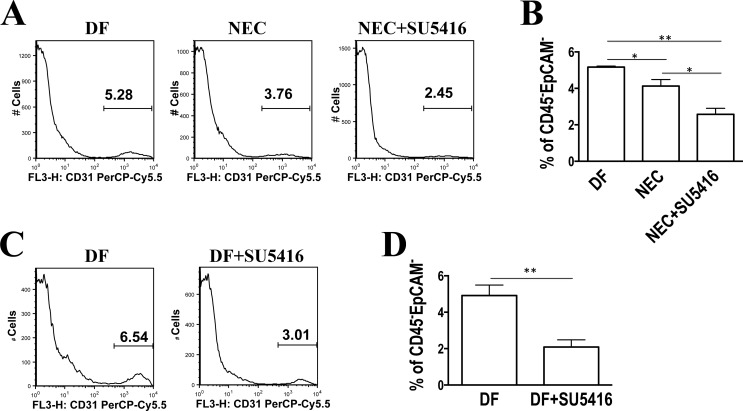
To estimate the number of endothelial cells present in the intestinal mucosa, small intestinal tissue cells were isolated from lamina propria preparations, stained for CD31, and the relative number of endothelial cells was analyzed by flow cytometry. The percentage of CD31+ cells in the live nonepithelial- and nonleukocyte-derived cell populations was found to be decreased in the small intestine of pups stressed for 24 h (4.13 ± 0.36%) compared with DF controls (5.12 ± 0.06%, P < 0.05), and further decreased in stressed pups when the VEGFR2 pathway was inhibited (2.58 ± 0.33%, P < 0.005 vs. DF and P < 0.05 vs. NEC group, Fig. 6, A and B). Compared with DF control pups, the percentage of CD31+ cells was also decreased in the intestinal tissues of pups that received SU5416 without being stressed (2.10 ± 0.40% vs. DF 4.92 ± 0.58%, P < 0.005) (Fig. 6, C and D).
Fig. 6.
The number of endothelial cells is decreased in NEC intestine tissues and further decreased when the VEGFR2 pathway is inhibited. Intestinal tissue cells were dissociated with EDTA followed by collagenase digestion. Single-cell suspensions were generated, stained with indicated markers, and analyzed by flow cytometry. The percentage of CD31+ cells in the live, and nonepithelial- and nonleukocyte-derived cell populations is shown. A: representative histograms showing decreased percentage of CD31+ cells in the NEC (middle) and NEC/SU5416 (right) groups compared with DF controls (left). B: bar graph showing average values of multiple samples including A (n = 3 in each group; *P < 0.05, **P < 0.005). Results were similar in 2 separate experiments. C: representative histogram showing a decrease in percentage of CD31+ cells in DF pups injected with SU5416 (right) compared with DF controls (left). D: bar graph showing average values of multiple samples including C (n = 6 in each group; **P < 0.01). Results were similar in 2 separate experiments.
Exposure to the NEC protocol does not induce endothelial cell apoptosis, but it does increase the apoptosis of epithelial cells, which was not affected by VEGFR2 signaling inhibition.
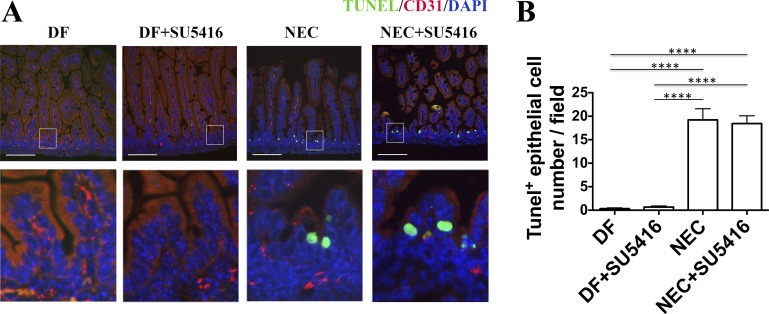
To determine whether endothelial cell apoptosis may contribute to the alteration of intestinal microvascular integrity induced by exposure to the NEC protocol and/or injection with SU5416, TUNEL staining of intestinal tissue sections was performed after CD31 staining. Although exposure to the NEC protocol does induce apoptosis in epithelial cells at 24 h (19.22 ± 2.38 vs. DF 0.33 ± 0.17, P < 0.0001), this was not the case for endothelial cells. When identified by TUNEL staining, the number of apoptotic endothelial cells was extremely low, requiring multiple field analysis to detect one apoptotic cell, and was not different between DF pups and those subjected to the NEC protocol. Furthermore, pretreatment with VEGFR2 kinase inhibitor did not increase intestinal epithelial cell apoptosis in stressed pups (18.44 ± 1.65, not significant, Fig. 7) nor triggered epithelial cell apoptosis in DF pups, which suggests that the effect of SU5416 is specific on endothelial cell proliferation.
Fig. 7.
Exposure to the NEC protocol increases epithelial cell apoptosis, which is not affected by VEGFR2 inhibition. TUNEL staining was performed on intestinal tissue sections from pups of DF, SU5416-treated, NEC, and NEC+SU5416 groups after CD31 cell surface immunofluorescence staining. A: although many apoptotic epithelial cells were found both in the NEC and NEC+SU5416 groups, apoptotic endothelial cells were only rarely observed in the intestinal villi in these groups (scale bar = 100 μm). Bottom: enlarged fields of view. Top: images were taken at ×20 magnification. B: average number of TUNEL-positive epithelial cells counted per field (****P < 0.0001, n = 3 animals per group). Results represent 2 separate experiments.
DISCUSSION
The pathophysiology of NEC remains elusive. Several mechanisms have been implicated, such as an inappropriate inflammatory response and an impaired intestinal perfusion (6, 33). Risk factors for NEC include congenital heart disease, intrauterine growth restriction, birth asphyxia, maternal preeclampsia, and blood transfusion (26, 31), which highly suggest that oxygen transport to intestinal cells plays a critical role in NEC. To date, the role played by the intestinal microvascular networks in NEC development has not been well studied. Here, we found that the development of the microvasculature of the intestinal villi and submucosa was altered in pups subjected to the NEC protocol. Indeed, we found that it consists of a regular lattice network with ample interconnected branches and sprouts in DF pups, although it was reduced to a more rudimentary framework with decreased vascular density and more vessel-free areas in pups subjected to the NEC protocol. Whether this corresponds to a lack of vascular expansion, to a vascular regression, or to both is unclear.
Angiogenesis is a complex process in which multiple factors have been shown to be involved. Recently, we found that VEGF-A is decreased in the intestinal samples of human NEC (36). Furthermore, using a mouse NEC model, we show here that VEGF-A (36) and VEGFR2 proteins are decreased in the intestine in the early stages of NEC development, before evidence of tissue necrosis, compared with DF controls. Interestingly, VEGF-A has been found to regulate the expression of VEGFR2 (23). In this study, we also found that both VEGF-A and VEGFR2 are developmentally regulated, being strongly expressed in the normal intestine during late intrauterine life. At birth, both VEGF-A and VEGFR2 decrease sharply and transiently until day 2 of life. In premature birth, a drop in VEGF-A/VEGFR2 expression at a detrimental time may deprive the intestine of VEGF-A/VEGFR2 signaling during a crucial time for intestinal angiogenesis.
However, the role of VEGF-A/VEGFR2 signaling in NEC remains unknown. In this study, we found that blocking VEGFR2 signaling by administering the VEGFR2 tyrosine kinase inhibitors SU5416 or Ki8751 to pups prior to exposure to the NEC protocol decreased endothelial cell proliferation, increased the incidence of severe intestinal injury, and significantly shortened survival. Also, VEGFR2 inhibition caused a significant decrease in mucosa vascular density in DF pups, which was even more dramatic when pups were concurrently subjected to the NEC protocol. This suggests that the VEGFR2 pathway promotes microvasculature development in the intestinal mucosa, of which alteration may contribute to NEC. Although SU5416 had been previously considered a specific VEGFR2 inhibitor (12, 28, 29) and was used in association with chemotherapy in phase III clinical trials to inhibit angiogenesis for advanced colorectal cancer (7), it has also been reported to inhibit other kinases such as RET, FLT-3 (45), and VEGFR1 (20). Therefore, we used Ki8751, a more potent and selective VEGFR2 inhibitor than SU5416 (IC50 of 0.9 nM vs. 1.4 μM), and further confirmed the worsening effect of VEGFR2 inhibition on NEC protocol-induced intestinal injury, mortality, and endothelial cell proliferation. Ki8751 was found to be >40-fold selective for VEGFR2 than c-Kit, PDGFRα, and FGFR-2, and to have little activity on EGFR, HGFR, and insulin receptor (24). Although VEGF-A is the main ligand of VEGFR2, other ligands such as VEGF-C have been shown to bind VEGFR2, thus increasing angiogenesis and permeability (34). Therefore, we cannot exclude that some of the effects observed by inhibiting VEGFR2 in the neonatal intestinal microvasculature could be via other mechanisms such as VEGF-C. However, VEGF-C binds mainly to VEGFR3, which it does with higher affinity than VEGF-A (Kd = 135 pM vs. Kd = 410 pM) to promote lymphangiogenesis (22).
We previously found that VEGF-A is localized mainly to a few single intestinal epithelial cells and to some cells of the lamina propria and myenteric plexus (36). In this study, we further characterize the cells producing VEGF-A in the intestinal mucosa. Not surprisingly, we found that endothelial cells in the villi highly express VEGF-A and its receptor. Although mesenchymal/stromal cells and pericytes have also been reported to express VEGF-A (5), we were not able to detect VEGF-A in these cell types. However, we cannot rule out the presence of low levels of expression in these cells, or the possibility that its expression is limited to certain stages of differentiation during angiogenesis. We also found that VEGF-A is strongly expressed in the intestinal myenteric neural plexus. Indeed, VEGF-A is known to bind to neuropilin receptors expressed on neurons and to be involved in neuronal development (27). Our study is the first to report that enteroendocrine cells strongly express VEGF-A.
It is possible that the different sites of VEGF production may differentially affect the angiogenesis process during the perinatal development of the intestinal mucosal microvasculature. Besides the importance of VEGF expression in endothelial cells for its direct role in angiogenesis, studies have shown that cross-talk between neuroepithelium and embryonic blood vessels is critical for neurovascular development (17, 21). At midgestation the neural tube has been shown to provide vascular patterning signal(s) that direct the formation of the perineural vascular plexus that encompasses the neural tube, which is mediated by VEGF-A/VEGFR2 signaling pathway (17, 21). Interestingly, we found out that during intestinal development at embryonic stage 16, VEGF-A is already strongly expressed on the myenteric plexus before individual villi have developed and before VEGF-A can be detected in the primitive villi-like structures (data not shown). It is reasonable to speculate that VEGF-A, which is strongly expressed in these neuronal structures, may play an important role in guiding the villi development and the ingression of microvessels into the lamina propria area. In addition, enteroendocrine cells have physical connection with the myenteric plexus (3). Therefore, VEGF-A in enteroendocrine cells may play a role in maintaining the normal structure of microvessels in the villi. Thus VEGF-A signaling at these different sites may play a protective role against intestinal mucosal microvasculature impairment, hypoxia, inflammation, and intestinal injury in an autocrine or paracrine fashion. More work is needed to determine the significance of specific sites of VEGF-A in the intestinal mucosa.
Murine VEGF-A is produced as three major isoforms due to alternative splicing: VEGF-A188, VEGF-A164, and VEGF-A120, corresponding to human VEGF-A189, VEGF-A165, and VEGF-A121 (35, 43). Although we found VEGF-A164 and VEGF-A120 to be present in the neonatal mouse intestine, only VEGF-A164 was found to be downregulated during NEC development. Because the antibody against VEGF-A used for immunofluorescence staining recognizes the three isoforms, we were unable to determine the cellular localization of the specific isoforms. In fact, other studies showed that the relative expression of these isoforms varies in different tissues to facilitate local vascular development. The extreme example is the heart, where only VEGF-A188 isoform is expressed (39). Interestingly, transgenic mice that express only VEGF-A188 displayed higher vascular density, but half of these mice died before birth. Expression of VEGF-A120 alone resulted in decreased vascular density, and leaky and fragile blood vessels due to defective pericytes (39). Alternatively, mice expressing the VEGF-A164 isoform alone exhibited vascularization similar to those of wild-type mice, with all pups viable (39). Thus the decreased level of the VEGF-A164 isoform in the gut of pups subjected to the NEC protocol indicates that this isoform might be responsible for the perinatal development of the intestinal mucosal microvasculature in our model.
To determine the mechanism by which VEGFR2 regulates the intestinal microvasculature, we assessed intestinal mucosal endothelial cell proliferation and apoptosis. We found that the number of endothelial cells undergoing proliferation was significantly decreased, as was the total number of endothelial cells in pups subjected to the NEC protocol for 24 h and in those injected with SU5416 or Ki8751, compared with DF controls.
It is unclear whether microvascular structural changes alone are sufficient to induce NEC, even though congenital heart disease has been recognized as a serious risk factor for NEC (30, 31). In this study, VEGFR2 kinase inhibitor administration to DF pups was able to induce intestinal injury and increase intestinal permeability, but it was not able to induce mortality comparable to exposure to the NEC protocol. This suggests that external stresses such as formula feeding are necessary for induction of mortality during the 3-day experimental period in NEC-affected pups.
The intestinal microbiota has been shown to play an important role in the development of the intestinal mucosal microvasculature (40). Indeed, adult germ-free mice were found to have arrested intestinal villi capillary network formation, which was restored 10 days after colonization with a complete microbiota (40). Furthermore, microbial products have been shown to directly activate mucosal endothelial cells and to increase endothelial cell tube formation in vitro (37). However, we found that when neonatal mice were inoculated with adult commensal bacteria together with administration of other stresses including hypoxia, cold stress, and formula feeding, endothelial cell proliferation in the intestinal villi was decreased and intestinal mucosal microvasculature development was impaired.
We believe that physiological hypoxia and exposure of intestinal mucosa to amniotic fluid VEGF-A before birth are two essential factors for the normal development of the intestinal microvasculature. When infants are born before their intestinal microvasculature is fully developed, exposure of intestinal cells to high partial pressures of O2 at a detrimental time downregulates intestinal endothelial cell VEGF-A and VEGFR2 expression, and abrupt withdrawal of amniotic fluid VEGF-A disrupts the ongoing mucosal angiogenesis process. Furthermore, lack of exposure of the intestine to breast milk, which has been found to contain high levels of VEGF-A (38, 44), further affects the development of the intestinal mucosal microvasculature. Thus, although the intestinal microvasculature is initially sufficient to sustain the metabolic needs of a “sterile” nonfed (or partially fed) intestine, it becomes insufficient to meet the metabolic demand of the intestine when submitted to enteral nutrition and bacterial and viral challenges. This leads to intestinal epithelial cell apoptosis, autophagy, and necrosis, which cause epithelial barrier breakdown, inflammation, further tissue injury, and NEC. This hypothesis explains why infants around 32 wk of age are most susceptible to NEC, when typically their feeds have been fully advanced and when other stresses associated with care in a neonatal intensive care use are generally present. However, the pathogenesis of NEC is complex, resulting from an imbalance between protective and injurious factors. Our study suggests an important role for VEGFR2 and the microvasculature in NEC development; thus inadequate VEGF-A may be a pivotal factor tipping this precarious balance when the premature intestine is subjected to multiple insults. Future investigations examining the role of VEGF-A on gut integrity are needed to discern the precise role of this important growth factor.
In conclusion, our study provides evidence of a critical role for VEGF-A/VEGFR2 in neonatal gut homeostasis. It suggests that lack of VEGF-A/VEGFR2 signaling could be one of the initial events predisposing neonates to NEC via impaired angiogenesis. It also provides a solid basis for testing therapeutic strategies that can increase or maintain local VEGF-A/VEGFR2 production to prevent NEC development.
GRANTS
Support for this study was provided by Friend of Prentice and National Institutes of Health Grants R01 DK-064240 to XD Tan and R01 HD-060876 to I. G. De Plaen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.G.D.P. conception and design of research; X.Y., E.M., C.M., and S.X.L. performed experiments; X.Y. and X.W. analyzed data; X.Y. and I.G.D.P. interpreted results of experiments; X.Y. prepared figures; X.Y. drafted manuscript; E.M., X.-D.T., and I.G.D.P. edited and revised manuscript; I.G.D.P. approved final version of manuscript.
REFERENCES
- 1.Avraham-Davidi I, Yona S, Grunewald M, Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M, Strauss-Ayali D, Mack M, Jung S, Keshet E. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med 210: 2611–2625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol 182: 1595–1606, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohorquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PloS One 9: e89881, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol 264: 275–288, 2003. [DOI] [PubMed] [Google Scholar]
- 6.De Plaen IG. Inflammatory signaling in necrotizing enterocolitis. Clin Perinatol 40: 109–124, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DePrimo SE, Wong LM, Khatry DB, Nicholas SL, Manning WC, Smolich BD, O'Farrell AM, Cherrington JM. Expression profiling of blood samples from an SU5416 phase III metastatic colorectal cancer clinical trial: a novel strategy for biomarker identification. BMC Cancer 3: 3, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downard CD, Grant SN, Matheson PJ, Guillaume AW, Debski R, Fallat ME, Garrison RN. Altered intestinal microcirculation is the critical event in the development of necrotizing enterocolitis. J Pediatr Surg 46: 1023–1028, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439–442, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59: 99–106, 1999. [PubMed] [Google Scholar]
- 13.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124: 175–189, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell 22: 2766–2776, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiratsuka S, Kataoka Y, Nakao K, Nakamura K, Morikawa S, Tanaka S, Katsuki M, Maru Y, Shibuya M. Vascular endothelial growth factor A (VEGF-A) is involved in guidance of VEGF receptor-positive cells to the anterior portion of early embryos. Mol Cell Biol 25: 355–363, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development 131: 1503–1513, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins GB, Gould VE, Stevenson JK, Oliver TK Jr. Necrotizing enterocolitis in premature infants. A clinical and pathologic evaluation of autopsy material. Am J Dis Child 120: 229–232, 1970. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Doelle SM, Clark JA, Halpern MD, McCuskey RS, Dvorak B. Intestinal microcirculatory dysfunction during the development of experimental necrotizing enterocolitis. Pediatr Res 61: 180–184, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Itokawa T, Nokihara H, Nishioka Y, Sone S, Iwamoto Y, Yamada Y, Cherrington J, McMahon G, Shibuya M, Kuwano M, Ono M. Antiangiogenic effect by SU5416 is partly attributable to inhibition of Flt-1 receptor signaling. Mol Cancer Ther 1: 295–302, 2002. [PubMed] [Google Scholar]
- 21.James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development 136: 833–841, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 16: 3898–3911, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kremer C, Breier G, Risau W, Plate KH. Up-regulation of flk-1/vascular endothelial growth factor receptor 2 by its ligand in a cerebral slice culture system. Cancer Res 57: 3852–3859, 1997. [PubMed] [Google Scholar]
- 24.Kubo K, Shimizu T, Ohyama S, Murooka H, Iwai A, Nakamura K, Hasegawa K, Kobayashi Y, Takahashi N, Takahashi K, Kato S, Izawa T, Isoe T. Novel potent orally active selective VEGFR-2 tyrosine kinase inhibitors: synthesis, structure-activity relationships, and antitumor activities of N-phenyl-N′-{4-(4-quinolyloxy)phenyl} ureas. J Med Chem 48: 1359–1366, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis: recent scientific advances in pathophysiology and prevention. Semin Perinatol 32: 70–82, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 368: 1271–1283, 2006.17027734 [Google Scholar]
- 27.Mackenzie F, Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development 139: 1371–1380, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Mendel DB, Laird AD, Smolich BD, Blake RA, Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK, Cherrington JM. Development of SU5416, a selective small molecule inhibitor of VEGF receptor tyrosine kinase activity, as an anti-angiogenesis agent. Anticancer Drug Des 15: 29–41, 2000. [PubMed] [Google Scholar]
- 29.Mendel DB, Schreck RE, West DC, Li G, Strawn LM, Tanciongco SS, Vasile S, Shawver LK, Cherrington JM. The angiogenesis inhibitor SU5416 has long-lasting effects on vascular endothelial growth factor receptor phosphorylation and function. Clin Cancer Res 6: 4848–4858, 2000. [PubMed] [Google Scholar]
- 30.Motta C, Scott W, Mahony L, Koch J, Wyckoff M, Reisch J, Burchfield PJ, Brion LP. The association of congenital heart disease with necrotizing enterocolitis in preterm infants: a birth cohort study. J Perinatol 35: 949–953, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol 32: 83–91, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Neufeld G, Kessler O, Herzog Y. The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol 515: 81–90, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Nowicki PT, Caniano DA, Hammond S, Giannone PJ, Besner GE, Reber KM, Nankervis CA. Endothelial nitric oxide synthase in human intestine resected for necrotizing enterocolitis. J Pediatr 150: 40–45, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7: 359–371, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Ruhrberg C. Growing and shaping the vascular tree: multiple roles for VEGF. Bioessays 25: 1052–1060, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Sabnis A, Carrasco R, Liu SX, Yan X, Managlia E, Chou PM, Tan XD, De Plaen IG. Intestinal vascular endothelial growth factor is decreased in necrotizing enterocolitis. Neonatology 107: 191–198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schirbel A, Kessler S, Rieder F, West G, Rebert N, Asosingh K, McDonald C, Fiocchi C. Pro-angiogenic activity of TLRs and NLRs: a novel link between gut microbiota and intestinal angiogenesis. Gastroenterology 144: 613–623.e9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siafakas CG, Anatolitou F, Fusunyan RD, Walker WA, Sanderson IR. Vascular endothelial growth factor (VEGF) is present in human breast milk and its receptor is present on intestinal epithelial cells. Pediatr Res 45: 652–657, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D'Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest 109: 327–336, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA 99: 15451–15455, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian R, Liu SX, Williams C, Soltau TD, Dimmitt R, Zheng X, De Plaen IG. Characterization of a necrotizing enterocolitis model in newborn mice. Int J Clin Exp Med 3: 293–302, 2010. [PMC free article] [PubMed] [Google Scholar]
- 42.Vajkoczy P, Menger MD, Vollmar B, Schilling L, Schmiedek P, Hirth KP, Ullrich A, Fong TA. Inhibition of tumor growth, angiogenesis, and microcirculation by the novel Flk-1 inhibitor SU5416 as assessed by intravital multi-fluorescence videomicroscopy. Neoplasia 1: 31–41, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vempati P, Popel AS, Mac Gabhann F. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev 25: 1–19, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuorela P, Andersson S, Carpen O, Ylikorkala O, Halmesmaki E. Unbound vascular endothelial growth factor and its receptors in breast, human milk, and newborn intestine. Am J Clin Nutr 72: 1196–1201, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Yee KW, O'Farrell AM, Smolich BD, Cherrington JM, McMahon G, Wait CL, McGreevey LS, Griffith DJ, Heinrich MC. SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood 100: 2941–2949, 2002. [DOI] [PubMed] [Google Scholar]