Abstract
Hepatic inflammation and fibrosis are key elements in the pathogenesis of nonalcoholic steatohepatitis (NASH), a progressive liver disease initiated by excess hepatic lipid accumulation. Lipid droplet protein Perilipin 2 (Plin2) alleviates dietary-induced hepatic steatosis when globally ablated; however, its role in the progression of NASH remains unknown. To investigate this further, we challenged Plin2 liver-specific knockout mice (designated L-KO) and their respective wild-type (WT) controls with a methionine-choline-deficient (MCD) diet for 15 days to induce a NASH phenotype of increased hepatic triglyceride levels through impaired phosphatidylcholine (PC) synthesis and very-low-density lipoprotein (VLDL) secretion. Results on liver weights, body weights, fat tissue mass, and histology in WT and L-KO mice fed the MCD diet revealed signs of hepatic steatosis, fibrosis, and inflammation; however, these effects were blunted in L-KO mice. In addition, levels of PC and VLDL were unchanged, and hepatic steatosis was reduced in L-KO mice fed the MCD diet, due in part to an increase in remodeling of PE to PC via the enzyme phosphatidylethanolamine N-methyltransferase (PEMT). These mice also exhibited decreased hepatic expression of proinflammatory markers cyclooxygenase 2, IL-6, TNF-α, IL-1β, and reduced expression of endoplasmic reticulum (ER) stress proteins C/EBP homologous protein and cleaved caspase-1. Taken together, these results suggest that Plin2 liver-specific ablation alleviates diet-induced hepatic steatosis and inflammation via a PEMT-mediated mechanism that involves compensatory changes in proteins involved in phospholipid remodeling, inflammation, and ER stress that work to alleviate diet-induced NASH. Overall, these findings support a role for Plin2 as a target for NASH therapy.
Keywords: Perilipin 2, lipid droplets, nonalcoholic steatohepatitis, methionine-choline-deficient diet
in parallel to obesity trends, nonalcoholic fatty liver disease (NAFLD) is on the rise, with one person in three potentially affected in the United States. NAFLD covers a spectrum of conditions, including benign steatosis in which excess triglycerides are stored in hepatic lipid droplets, to the more serious nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. Approximately 35% of individuals who have hepatic steatosis will progress to NASH (2, 29, 54), yet factors driving the process forward remain unclear. A “two-hit” model by Day and James (4, 19) proposes that a first hit of excess hepatic lipids leaves the liver more vulnerable to multiple second hits of oxidative stress, mitochondrial dysfunction, and lipid peroxidation that increase production of proinflammatory cytokines, influx of inflammatory cells, and collagen deposition leading to fibrosis, hallmarks of the NASH phenotype (20, 52, 69). However, although numerous studies have defined the molecular and physiological changes that occur in the progression of NASH, it remains unclear what role hepatic lipid droplets and associated proteins play in promoting the disease process. Notably, several proinflammatory metabolites and enzymes that initiate inflammation reside on the surface of lipid droplets (1, 51, 66). More importantly, the most abundant lipid droplet protein in the liver, Perilipin 2 (Plin2), is associated with promoting two key features of NASH, i.e., lipid accumulation and inflammation (17, 18, 23, 25, 38, 48), yet the role of Plin2 in the progression of NASH remains unclear.
Plin2 is part of the perilipin family of proteins that are closely related through sequence homology and affinity for lipid droplets. Members include Plin1 (formerly known as perilipin), Plin2 (ADRP, adipophilin), Plin3 (TIP-47), Plin4 (S3-12), and Plin5 (OXPAT) (28). Plin1 and Plin2 are constitutively located on the lipid droplet surface, whereas Plin3, Plin4, and Plin5 can be found in both cytosolic and lipid droplet compartments (12, 43, 67, 68). Plin1 is the most widely studied lipid droplet protein, but hepatic expression is low. When NASH is present, however, both Plin1 and Plin2 are upregulated and differentially targeted; Plin1 was found on larger lipid droplets in the liver, whereas Plin2 targeted inflamed ballooned hepatocytes (23). This alternate targeting is significant because Plin1 acts to repress lipolysis by inhibiting the lipolytic activities of hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) (13, 62). In contrast, Plin2 has no lipolytic function but can block access of lipases to lipid droplets to limit triglyceride hydrolysis (9, 41). In addition, Plin2 binds and sequesters lipids at the lipid droplet surface (49, 53) and enhances lipid transport (26), suggesting it may act as a regulatory protein, governing the release and deposition of lipids, including eicosanoids that are stored in lipid droplets (1, 10, 65, 66). Consistent with this, Plin2 has been shown to preferentially bind proinflammatory lipids (53) and significantly increase neutral lipid and phospholipid levels when overexpressed in L cells (48). Moreover, knockdown of Plin2 in THP-1 macrophages resulted in decreased cellular lipids and lipid droplet size and number and decreased expression of proinflammatory markers TNF-α, IL-6, and cyclooxygenase 2 (COX2) when inflammation was induced by LPS (17). Conversely, Plin2 overexpression in LPS-stimulated C2C12 cells resulted in increased expression of IL-1β and caspase-1 (18). In mice, global ablation of Plin2 decreased hepatic triglyceride levels and protected against diet-induced obesity, adipose inflammation, and liver steatosis (14–16, 32, 50, 55), whereas reduction of Plin2 by antisense oligonucleotide treatment resulted in decreased hepatic lipid accumulation and enhanced insulin sensitivity (32, 63). However, although these data support a role for Plin2 in promoting hepatic steatosis, relatively little is known whether the protein contributes to the pathogenesis of inflammation-related NASH.
To investigate this further, we challenged Plin2 liver-specific knockout (L-KO) mice with a methionine and choline-deficient (MCD) diet to produce the NASH phenotype of increased hepatic lipid accumulation, inflammation, and fibrosis. A tissue-specific conditional KO model was employed to bypass the limitations of traditional constitutive KO models in terms of compensatory mechanisms and complex phenotypes. Our results suggest that hepatic ablation of Plin2 activates a mechanism that involves phospholipid remodeling enzymes, proinflammatory proteins, and endoplasmic reticulum (ER) stress markers. Taken together, the data herein provide novel insights into the physiological role of Plin2 and suggest that Plin2 plays an important role in hepatic function.
MATERIALS AND METHODS
Mice.
Plin2 liver-specific KO mice were created using a LoxP-Cre approach (36) to allow conditional targeting of the Plin2 allele. Briefly, Plin2 global KO mice (Plin2−/−) carrying the Plin2tm1a(EUCOMM)Wtsi in a C57BL/6NTac background were generated by the European Conditional Mouse Mutagenesis Program (EUCOMM). Heterozygous mice (Plin2+/−) carrying the Plin2 targeted allele were purchased from Welcome Trust Sanger Institute and were bred together to obtain mice globally null in Plin2 (Plin2−/− mice). To derive Plin2 liver-specific KO mice (Plin2L−/−), the floxed mouse (Plin2fl/fl) was generated by breeding Plin2−/− mice with mouse strain B6.29S4-Gt(ROSA)26Sortm1(FLP1)Dym (Jackson Laboratory, Bar Harbor, ME) to create a floxed allele and restored Plin2 expression. Mice heterozygous for the floxed allele were bred together to obtain Plin2fl/fl mice that served as wild-type (WT) controls because neither the presence of the LoxP sites or the remaining FRT site altered the expression of Plin2 or the phenotype. Plin2fl/fl mice were then bred with mouse strain FVB.Cg-Tg(Alb1-cre)1Dlr/J (Jackson Laboratory), a liver-specific Cre recombinase-expressing mouse model to generate Plin2L+/− mice. Mice containing the liver-specific allele were then bred to homozygosity to produce Plin2L−/− mice, hereafter known as L-KO mice. All animal protocols were approved by the Institutional Animal Care and Use Committee at Michigan State University according to criteria outlined in Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
Dietary experiments.
One week before the start of the feeding experiments, age-matched WT and L-KO male mice (7–8 wk old) were placed on a control diet containing 10% fat (no. A02082003B; Research Diets, New Brunswick, NJ). After 1 wk, half of the mice in each group remained on the control diet, whereas the rest were switched to an isocaloric MCD diet, chemically defined to match the control diet (no. A02082002B; Research Diets). Mice were fed ad libitum for 15 days. Body weights and food intake of each mouse were monitored every 2 days. At the beginning and the end of the feeding study, the percentage of fat tissue mass and lean tissue mass of each mouse were determined in vivo using a TD-NMR-based Bruker Minispec LF50 body composition analyzer (Billerica, MA). At the end of the study, nonfasted mice were euthanized by CO2 asphyxiation and cervical dislocation. In keeping with other work (8, 35) and because of the severe weight loss the mice experienced on the MCD diet, we did not fast the mice at the end of the feeding study. Blood was collected, and livers were harvested and weighed. Portions of the liver were collected for histological examination. Remaining portions were snap frozen on dry ice and stored at −80°C.
Liver histology.
Liver samples (25–75-mm3 segments) were fixed in a 10% buffered formalin solution at room temperature overnight, then stored in alcohol until embedded in paraffin, sectioned (4–6-m thickness), and stained with hematoxylin and eosin (H and E) or Masson's Trichrome for histological evaluation. Sections were imaged with a Nikon LTX light microscope with a ×40 objective. Portions of the liver were frozen in optimal cutting temperature compound (Tissue-Tek; Sakura Finetek, Torrance, CA) for Oil red O staining. Morphometric analysis was performed using MetaMorph software (Sunnyvale, CA) to analyze the number and size of lipid droplets. Histological processing was done at the histopathology laboratory at Michigan State University.
Lipid analysis.
Lipids were extracted from mouse liver homogenates and resolved into individual lipid classes using thin-layer chromatography (TLC) as described previously (5, 6). Levels of total cholesterol, free fatty acids, triacylglycerol, cholesteryl esters, and total phospholipids were determined by TLC and densitometry following the method of Marzo (47). Protein concentration from the dried protein extract residue was digested overnight in 0.2 M KOH and analyzed using Bradford reagent (11). All lipid classes were identified by comparison to known TLC standards.
Mass spectrometry-based lipid analysis.
Before we performed high-resolution/accurate mass spectrometry (MS) and tandem MS, liver homogenates, spiked with synthetic phosphatidylcholine (PC) (14:0/14:0), phosphatidylethanolamine (PE) (14:0/14:0), and phosphatidylserine (PS) (14:0/14:0) as internal standards were subjected to monophasic lipid extraction with methanol:chloroform:water (2:1:0.74, vol:vol:vol) and amine group modification, as previously described (22, 44). Briefly, extracts (10 μl) were aspirated and directly infused at ∼250 nl/min by nanoelectrospray ionization (nESI) into a high-resolution/accurate mass Thermo Scientific model LTQ Orbitrap Velos mass spectrometer (San Jose, CA) using an Advion Triversa Nanomate nESI source (Advion, Ithaca, NY) with a spray voltage of 1.4 kV and a gas pressure of 0.3 psi. High-resolution mass spectra were acquired in positive-ion mode from derivatized lipid extracts and in negative-ion mode from underivatized lipid extracts, using the FT analyzer operating at 100,000 mass-resolving power. The mass spectrum signal was averaged for 2 min over the range of m/z 200-2,000. High-energy collision dissociation (HCD)-MS/MS product ion spectra were acquired to verify the identities of selected lipid ions of interest using the FT analyzer operating at 100,000 mass-resolving power and default activation times. HCD-MS/MS collision energies were individually optimized for each lipid class of interest using commercially available lipid standards whenever possible. Lipids were identified using the Lipid Mass Spectrum Analysis (LIMSA) v.1.0 software linear fit algorithm, in conjunction with a user-defined database of hypothetical lipid compounds for automated peak finding and correction of 13C isotope effects. Relative quantification of the abundances of lipid molecular species between samples was performed by normalization of target lipid ion peak areas to the internal standards, as previously described (21). Data were processed using mean-centering and Pareto-scaling before multivariate statistical analysis. The principal component analysis was performed using EZinfo software (Umetrics). Partial least-squares-discriminant analysis was carried out (EZ Info, Umetrics) to identify the differentially expressed phospholipids responsible for the separation of WT, L-KO, MCD diet-fed WT, and MCD diet-fed L-KO. The heat map was generated using Cluster 2.0 from the Eisen Laboratory modified by Michiel de Hoon (http://bonsai.hgc.jp/∼mdehoon/software/cluster/). Java Tree Viewer was used to view and color the heat map.
Serum lipids, lipoproteins, and lipolysis.
Total and free cholesterol, triacylglycerol, and nonesterified fatty acid levels in serum from nonfasted mice were determined using Wako Chemicals lipid assay systems (Wako Diagnostics, Richmond, VA). Levels of cholesteryl esters were determined by subtracting free cholesterol from total. Concentrations of cholesterol in high-density lipoprotein (HDL) and low-density (LDL)/very-low-density (VLDL) lipoproteins were quantified using a polyethylene glycol precipitation using the EnzyChrom HDL and LDL/VLDL kit from BioAssay Systems (Hayward, CA). To measure lipolysis in hepatic tissue, glycerol release was quantified using EnzyChrom Adipolysis Assay kit, from BioAssay Systems. Colorimetric analysis of lipids, lipoprotein, and lipolysis was measured at 570 nm on an Omega FLOUstar 96-well plate reader from BMG Labtech (Ortenberg, Germany).
Serum β-hydroxybutyrate measurement.
To measure acetyl CoA production from fatty acid oxidation, serum levels of the ketone body β-hydroxybutyrate were determined using the Wako Chemicals β-hydroxybutyrate kit (Wako Diagnostics). Levels were measured with high sensitivity and specificity according to the manufacturer's directions by measuring the rate of Thio-NADH (β-thionicotinamide adenine dinucleotide) production spectrophotometrically at 405 nm upon oxidation of β-hydroxybutyrate.
Western blot analysis.
Expression levels of Plin1, Plin2, Plin3, and Plin5, and phospholipase A2 (PLA2) were assessed by Western blot analysis, as previously described previously (6, 48). Rabbit polyclonal antibodies were purchased from the following sources: anti-Plin1, anti-Plin3, and anti-Plin5 from Thermo Scientific (Rockland, IL) and anti-PLA2 from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal antibodies against Plin2 were developed in house (48). Western blot analysis was performed on tissue [liver, white adipose tissue (WAT), heart, kidney, and brain] homogenates (20–40 μg) resolved on tricine gels (10%). Proteins were transferred to nitrocellulose membranes, and blots were stained with Ponceau S to confirm protein transfer and constant protein loading (60) before being blocked in 7% nonfat milk in TBST (10 mM Tris·HCl, pH 8, 100 mM NaCl, 0.05% Tween-20) overnight. Blots were incubated with specific polyclonal rabbit antibodies and were developed with IRDye 800CW anti-mouse (LI-COR) secondary antibodies. Blots were scanned using the LI-COR Odyssey imaging system (Lincoln, NE) to visualize the bands of interest. Protein bands were quantitated by densitometric analysis after image acquisition using NIH Scion Image to obtain relative protein levels expressed as integrated density values normalized to GAPDH expression.
Quantitative real-time PCR.
Total RNA was isolated from liver using Trizol (Qiagen, Valencia, CA) in accordance with the manufacturer's protocol. Total RNA (1 μg) from each liver sample was converted into first-strand cDNA using ABI high-capacity cDNA reverse transcription kit (Grand Island, NY). Expression levels of IL-1β, IL-6, TNF-α, cyclooxygenase 2 (COX2), C/EBP homologous protein (CHOP), eukaryotic translation initiation factor 2-α kinase 3 (PERK), phosphate cytidyltransferase 1 (CCT), sphingomyelin synthase (SMS), phosphatidylethanolamine N-methyltransferase (PEMT), glycine N-methyltransferase (GNMT), acyl-coenzyme A oxidase 1 (ACOX1), acyl-coenzyme A carboxylase (ACC), acyl-coenzyme A synthase (ACS), carnitine palmitoyltransferase 1-α (CPT1-α), fatty acid synthase (FASN), sterol-coenzyme A desaturase (SCD1), monoglycerol O-acyltransferase 1 (MGAT1), diacylglycerol O-acyltransferase 1 (DGAT1), DGAT2, microsomal triacylglycerol transfer protein (MTTP), HSL, and ATGL were analyzed by SYBR Green Technologies using commercially available primers from IDT (Coralville, IA) on a StepOne plus Real-Time PCR system (Life Technologies, Carlsbad, CA). Relative expression was calculated using the comparative 2−ΔΔCT method (42). Data were expressed as fold difference compared with WT mice on control diet.
Statistical analysis.
Values were expressed as the means ± SE. Statistical analyses were performed using two-way ANOVA with Newman Keuls post hoc test (Sigma Plot, San Jose, CA) to determine statistical significance. Values with P < 0.05 or less were considered significant.
RESULTS
Liver-specific deletion of Plin2.
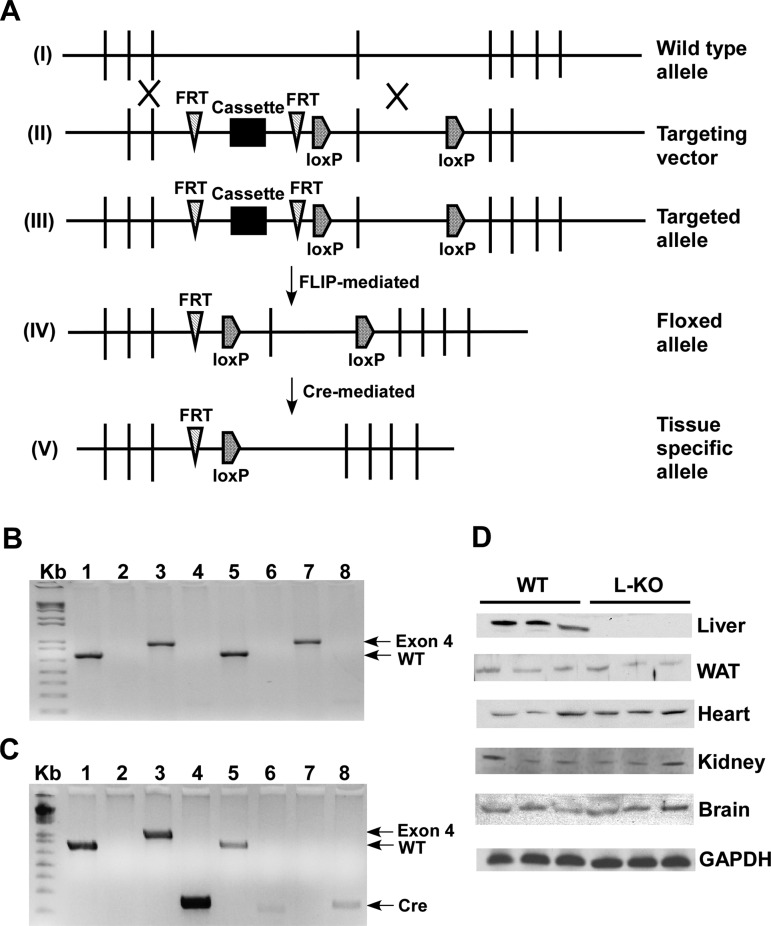
Conditional targeting of the mouse Plin2 gene was achieved as depicted in Fig. 1. Genotyping was confirmed by PCR to detect the presence or absence of the WT allele (850 bp), FRT site (204 bp), exon 4 (1 Kb), and the Cre gene (233 bp) in Plin2 floxed (Fig. 1B) and L-KO (Fig. 1C) mice. Western blot analysis showed ablation of Plin2 from liver homogenates but not from WAT, heart, brain, or kidney homogenates derived from WT and L-KO mice (Fig. 1D). For the experiments described, age-matched mice containing the floxed allele were designated as WT because pilot studies indicated neither the presence of the LoxP or FRT site altered Plin2 expression nor phenotype.
Fig. 1.
Conditional targeting of the Perilipin 2 (Plin2) allele allows for liver-specific deletion. A: targeting strategy of generating Plin2 liver-specific knockout (L-KO) mice. I: wild-type (WT) allele. II: targeting vector. III: targeted allele. IV: floxed allele. V: tissue-specific allele. B: PCR screening of WT mice. Primers were designed to detect the presence or absence of the WT allele (lane 1, 850 bp), FRT site (lane 2, 204 bp), exon 4 (lane 3, 1 Kb), and the Cre gene (lane 4, 233 bp) in tail (lanes 1–4) and liver (lanes 5–8) samples. Mice containing the floxed allele are positive for the WT gene (lane 1) and exon 4 (lane 3) but lack bands corresponding to the FRT site (lane 2) and Cre gene (lane 4). C: PCR screening of L-KO mice. Tail DNA (lanes 1–4) from L-KO mice shows bands corresponding to the WT gene (lane 1), exon 4 (lane 3), and the Cre gene (lane 4). L-KO liver DNA (lanes 5–8) is positive for the WT gene (lane 5) and the Cre gene (lane 8), but exon 4 is not detected (lane 7), indicating tissue-specific deletion. D: Western blot analysis of Plin2 protein expression in liver, white adipose tissue (WAT), heart, kidney, and brain using GAPDH as a loading control.
Hepatic Plin2 ablation alleviates MCD diet-induced lipid accumulation, inflammation, and fibrosis.
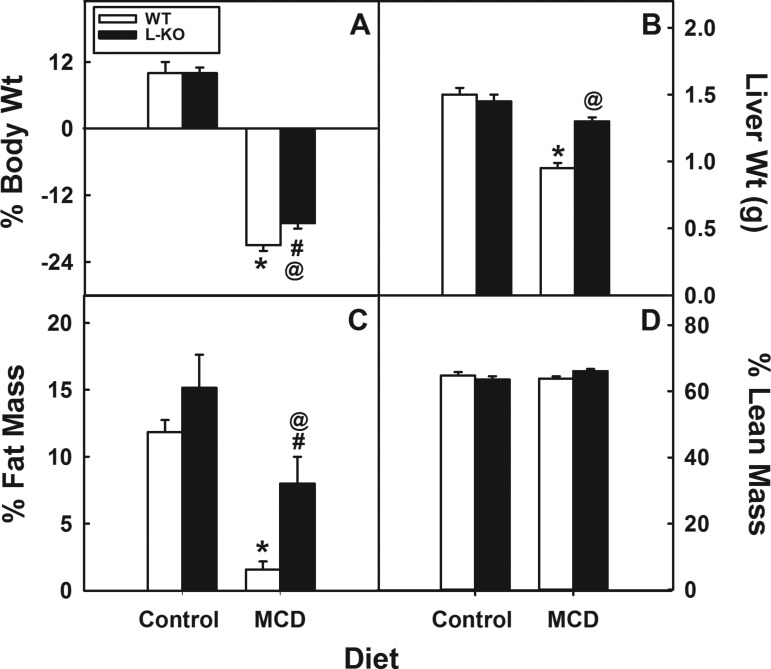
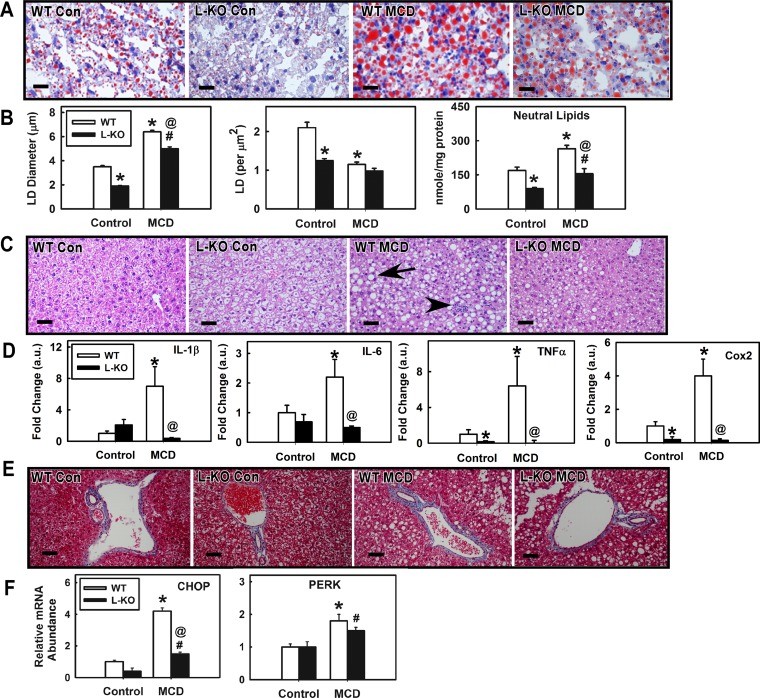
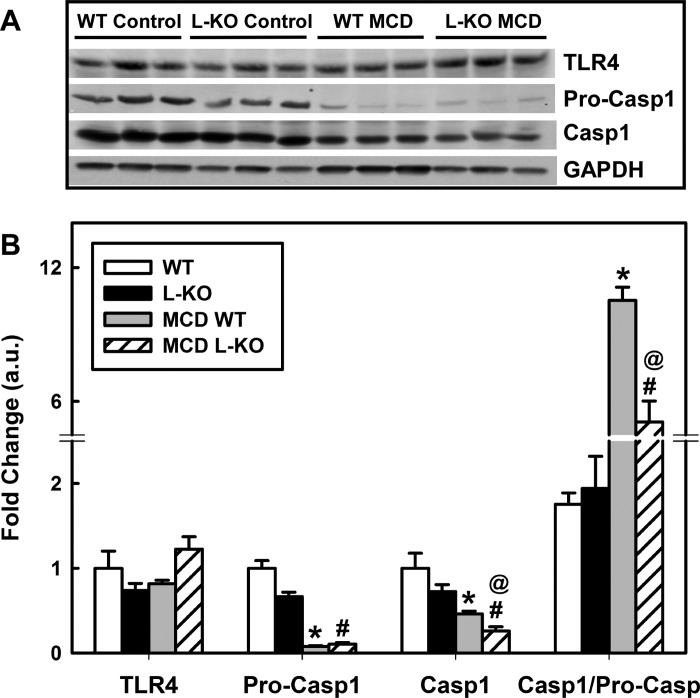
To study the role of Plin2 in hepatic function, we challenged Plin2 liver-specific KO mice with a MCD diet to induce a NASH phenotype of increased hepatic lipid accumulation, inflammation, and fibrosis (40, 59). Unlike high-fat or high-fat/high-fructose diets, the MCD diet produces the NASH phenotype of hepatic inflammation and fibrosis in mice rapidly. The MCD diet, however, does not generate the obese, insulin-resistant phenotype associated with the human NASH phenotype. In contrast, high-fat and high-fat/high-fructose diets will give rise to an obese, insulin-resistant state, yet these diets generally do not result in liver fibrosis and only mildly produce steatosis and inflammation (3, 27, 35, 58, 61). Moreover, lack of methionine-choline in the diet limits PC synthesis and VLDL secretion, resulting in weight loss. To keep the weight loss to <25% of body weight, the MCD diet was limited to 15 days. Both WT and age-matched L-KO mice lost body weight on the MCD diet, but L-KO mice were significantly less responsive, losing 19% less of their body weight (Fig. 2A, P < 0.03). The MCD diet significantly decreased liver weights of WT mice (37%, P < 0.002), but similarly fed L-KO mice showed little to no change (Fig. 2B). A substantial loss in percentage of fat tissue mass was observed in both L-KO mice and WT mice on the MCD diet (68% and 85%, respectively), but this effect was significantly reduced 20% in L-KO mice (Fig. 2C). The percentage of lean tissue mass was not affected by genotype in mice fed the control or MCD diets (Fig. 2D). Taken together, these findings suggest that the effects of the MCD diet on whole body phenotype are minimized in L-KO mice. We next investigated the effect of hepatic Plin2 deletion and the MCD diet on hepatic lipid accumulation. Liver sections stained with Oil red O showed decreased lipid content in L-KO livers compared with WT controls (Fig. 3A). Morphometric analysis revealed that lipid droplet size and number were respectively decreased 46% and 40% (Fig. 3B, left and middle). Consistent with this, lipid analysis revealed that neutral lipid content decreased 47% in L-KO livers (Fig. 3B, right). The MCD challenge resulted in enhanced Oil red O staining and neutral lipid accumulation in both L-KO and WT livers but significantly less with L-KO samples. H and E staining revealed marked histopathological lesions, including single-cell necrosis, increased vacuolization, and cell body inclusions in MCD WT livers, suggesting increased hepatic steatosis and inflammation (Fig. 3C). These lesions were not observed in livers from control-fed WT or L-KO mice and were significantly diminished in the livers from MCD diet-fed L-KO mice. Consistent with this, hepatic expression of genes associated with inflammation (IL-1β, IL-6, TNF-α, COX2) were increased in livers from WT mice on the MCD diet but decreased in similarly fed L-KO samples (Fig. 3D). Because steatosis has been identified as a risk factor for liver fibrosis, liver sections were also stained with Masson's Trichrome to visually determine increased collagen levels associated with diet-induced fibrosis (blue coloration, Fig. 3E). Although no genotype effect was observed in control-fed mice, the MCD diet significantly increased levels of fibrotic staining in WT, but not L-KO, livers near and around the arterial triad. Hepatic expression of CHOP and PERK, two enzymes involved in fibrosis and ER stress (39, 64), were increased 4.2- and 1.8-fold, respectively, in livers from MCD diet-fed WT mice, a response blunted in L-KO mice in which levels of PERK increased 1.5-fold and CHOP increased 1.6-fold (Fig. 3F). Plin2 ablation and the MCD diet had little effect on levels of Toll-like receptor 4 (TLR4) (Fig. 4A), the receptor responsible for activation of PERK under steatotic conditions (39). However, pro-caspase-1 was decreased in livers from both WT and L-KO mice but less so in L-KO mice. The ratio of caspase-1 to pro-caspase-1 was also determined to assess levels of active caspase-1 (Fig. 4B). The ratio in WT mice fed the MCD diet was increased sixfold compared with control-fed mice, but L-KO mice showed only a 2.6-fold increase. Taken together, these results suggest that Plin2 ablation may protect against diet-induced ER stress and fibrosis.
Fig. 2.
Effect of hepatic Plin2 ablation and methionine-choline-deficient (MCD) diet on body weight, liver weight, and fat mass. The percentage of change in body weight (A), liver weight (B), fat tissue mass (C), and lean tissue mass (D) was determined in WT and L-KO mice fed a control and MCD diet for 15 days. Values represent means ± SE, n = 4–5. *P < 0.05 vs. WT mice on the control diet. @P < 0.05 vs. WT mice on the MCD diet. #P < 0.05 vs. L-KO mice on the control diet.
Fig. 3.
Liver-specific ablation of Plin2 blunts MCD diet effects on hepatic lipid accumulation, inflammation, and fibrosis. A: oil red O-stained images of liver sections from WT and L-KO mice on control or MCD diets. B: morphometric analysis of hepatic lipid droplet size/number and neutral lipid content. C: representative hematoxylin and eosin (H and E)-stained images. D: gene expression of proinflammatory markers. E: representative Masson Trichrome-stained images. Cox, cyclooxygenase. F: gene expression of profibrotic markers. CHOP, C/EBP homologous protein; PERK, eukaryotic translation initiation factor 2-α kinase 3. Values represent means ± SE, n = 4–5. *P < 0.05 vs. WT mice on the control diet. @P < 0.05 vs. WT mice on the MCD diet. #P < 0.05 vs. L-KO mice on the control diet. Bars = 20 μm.
Fig. 4.
Ablation of hepatic Plin2 alleviates MCD diet-induced effects on Toll-like receptor 4 (TLR4) and caspase-1. A: Western blot analysis of TLR4, pro-caspase-1, and cleaved caspase-1 levels in hepatic tissue derived from L-KO and WT mice fed the control and MCD diet. B: relative protein expression levels were quantified by densitometric analysis. L-KO mice exhibited decreased levels of pro-caspase-1. The MCD diet increased the ratio of cleaved caspase-1/pro-caspase-1 in WT, an effect that was blunted in similarly fed L-KO mice. Values represent means ± SE, n = 4–5. *P < 0.05 vs. control diet-fed WT mice. @P < 0.05 vs. MCD diet-fed WT mice. #P < 0.05 vs. control diet-fed L-KO mice.
Hepatic Plin2 ablation blunts MCD diet effects on lipolysis, lipoprotein, and lipid levels in serum.
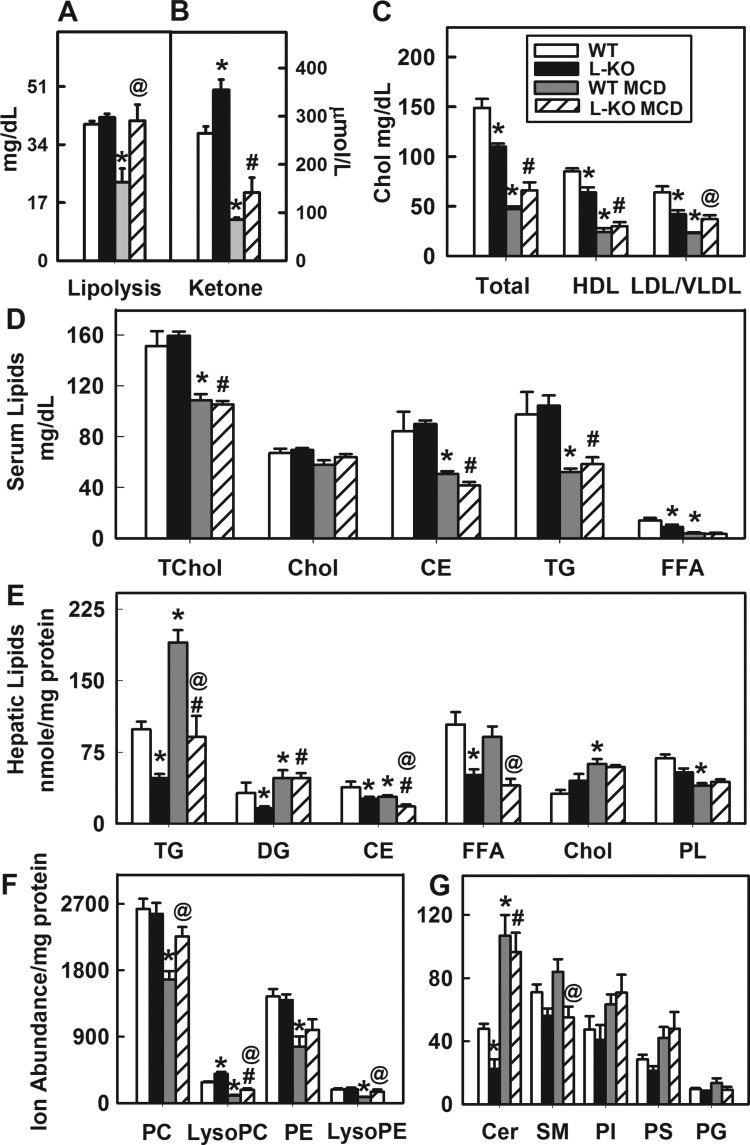
Because the MCD diet increases neutral lipid production and inhibits VLDL secretion (24, 40, 45, 46), we investigated the effects of the diet and hepatic Plin2 deletion on lipolysis and levels of serum lipoproteins and lipids. Lipolysis was not affected by Plin2 hepatic ablation in control-diet-fed mice. However, the MCD diet significantly decreased lipolytic activity in WT mice, a response not observed with similarly fed L-KO mice (Fig. 5A). Serum levels of β-hydroxybutyrate, a product of acetyl CoA produced by β-oxidation, were measured to assess fatty acid oxidation in WT and L-KO mice (7). Liver-specific ablation of Plin2 significantly increased β-hydroxybutyrate levels 1.4-fold in control-fed mice (Fig. 5B), indicating increased fatty acid oxidation. In contrast, the MCD diet challenge decreased β-hydroxybutyrate levels 3.1- and 2.7-fold, respectively, in WT and L-KO mice, suggesting that the effects of the diet were to limit oxidation. Levels of total serum lipoproteins, HDL, and LDL/VLDL were significantly decreased 26%, 25%, and 34%, respectively, when Plin2 was ablated (Fig. 5C, P < 0.05). The MCD diet challenge resulted in significantly reduced levels of total serum lipoproteins and HDL in WT and L-KO mice, but levels of LDL/VLDL were restored in L-KO mice. Similar decreases in serum levels of total cholesterol, cholesteryl esters, triglycerides, and free fatty acids in both WT and L-KO mice (Fig. 5D) were observed. Overall, these results suggested that lipolysis and LDL/VLDL production were protected against MCD insult in L-KO mice.
Fig. 5.
Hepatic Plin2 ablation and MCD diet alter lipid and lipoprotein profiles. A and B: lipolytic activity and ketone body levels in liver samples from WT and L-KO mice on control or MCD diets. C: serum lipoproteins levels. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein. D: serum lipid levels. TChol, total cholesterol; CE, cholesteryl esters; TG, triglyceride; FFA, free fatty acid. E: hepatic lipid levels. DG, diglyceride; PL, phospholipid. F and G: ion abundance of individual phospholipid classes with high and low abundance in liver. PC, phosphatidylcholine; PE, phosphatidylethanolamine; Cer, ceramide; SM, sphingomyelin; PI, phosphatidylinositol; PS, phosphatidylserine; PG, phosphatidylglycerol. Values represent means ± SE, n = 4–5. *P < 0.05 vs. WT mice on the control diet. @P < 0.05 vs. WT mice on the MCD diet. #P < 0.05 vs. L-KO mice on the control diet.
Plin2 liver-specific ablation reduces MCD diet effects on hepatic triglyceride and phospholipid levels.
Consistent with results from Oil red O staining (Fig. 3A), hepatic levels of triglycerides, diglycerides, cholesteryl esters, and free fatty acids were significantly reduced in L-KO mice compared with WT mice (Fig. 5E). The MCD diet increased hepatic levels of triglycerides in both WT and L-KO mice, but the response was significantly reduced in L-KO mice. In contrast, hepatic levels of total phospholipids were significantly decreased in WT mice on the MCD diet, but this effect was not observed with similarly fed L-KO mice. To investigate this further, a phospholipid lipidomic profile was generated. Liver-specific ablation of Plin2 increased levels of lyso-PC 1.5-fold and decreased ceramide levels 2.1-fold (Fig. 5, F and G). The MCD diet significantly decreased hepatic levels of PC and PE a respective 1.6- and 1.7-fold in WT, but not L-KO, mice (Fig. 5F). Similar decreases were observed with lyso-PC and lyso-PE levels. In contrast, ceramide levels were significantly increased in both WT and L-KO mice on the MCD diet (Fig. 5F, P < 0.05). In summary, the MCD diet challenge increased hepatic neutral lipid accumulation and decreased PC and PE levels in WT mice. The response of L-KO mice to the diet was less pronounced and, as shown with PC and PE levels, negligible.
NASH signature based on lipidomic data.
Despite the lack of methionine-choline in the MCD diet, L-KO mice did not exhibit decreased levels of hepatic PC or other phospholipid classes including PE, Lyso-PE, sphingomyelin (SM), PS, and phosphatidylinositol (PI). To investigate further, a lipidomic profile of individual phospholipid species was generated and subjected to principal component analysis (Fig. 6A). Lipidomic data were projected onto a plane depicting the two components representing greatest variance in the data set. Changes in the lipidomic profile were also visually observed in a heat map that presented the data in terms of fold change relative to control-diet-fed WT mice (Fig. 6B). Analysis of individual phospholipid species revealed changes across the classes in C16- and C18 fatty acid-containing phospholipid species, in addition to C20 and C22 species (Fig. 6C). Specifically, the effect of Plin2 liver-specific ablation on mice fed the control diet was to decrease levels of Cer 22:2 and SM 22:0 while increasing levels of PC 38:3 (18.1/20:2 Lyso-PC 20:4, Lyso-PC 22:6, and Cer 22:0) (Fig. 6C). On the MCD diet, WT mice exhibited significantly decreased levels of PC 34:3, PC 34:2 (16:1/18:1), Lyso-PC 16:0, Lyso-PC 22:6, PE 36:4 (16:0/20:4), PE 38:4 (18:0/20:4), Lyso-PE 18:2, Lyso-PE 18:1, Lyso-PE 20:4, phosphatidylglycerol 30:3, PI 40:7, PI 36:4, and PS 22:2, reflecting decreased levels of PC, Lyso-PC, PE, and Lyso-PE (Fig. 5F). Results were mixed with L-KO mice but yielded no net effect.
Fig. 6.
High-resolution/accurate-tandem mass spectrometry of individual phospholipid classes. A: principal component analysis of targeted lipidomics. B: heat map representing fold change of each lipid class relative to the mean in control-fed WT mice. LPC, Lyso-PC; LPE, Lyso-PE. C: ion abundance of individual phospholipid species and fatty acid composition. Lipid signals were normalized to synthetic internal standards PC (14:0/14:0), PE (14:0/14:0), and PS (14:0/14:0). UNSAT, unsaturated fatty acid; SAT, saturated fatty acid; PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid. Values represent means ± SE, n = 4–5. *P < 0.05 vs. WT mice on the control diet. @P < 0.05 vs. WT mice on the MCD diet. #P < 0.05 vs. L-KO mice on the control diet.
We next examined the impact of hepatic Plin2 ablation and the MCD diet on levels of phospholipid fatty acid classes including unsaturated fatty acid (UNSAT), saturated fatty acid (SAT), polyunsaturated fatty acid (PUFA), and monounsaturated fatty acid (MUFA). The MCD diet decreased levels of UNSAT, PUFA and MUFA in WT mice 1.7-fold, 1.5-fold, and 3.1-fold, respectively (Fig. 6C, bottom, right), consistent with previous reports of mice on MCD diets (33, 40). In L-KO mice, levels of MUFA, but not UNSAT or PUFA, decreased 2.1-fold on the MCD diet. Moreover, SAT levels in both WT and L-KO mice did not change, but the ratio of UNSAT to SAT decreased, reflecting higher SAT levels when mice were fed the MCD diet. These findings are consistent with NASH in humans in whom increased SAT levels in hepatic phospholipid pools is often observed (33).
Phospholipid remodeling directs hepatic PC biosynthesis in MCD fed L-KO mice.
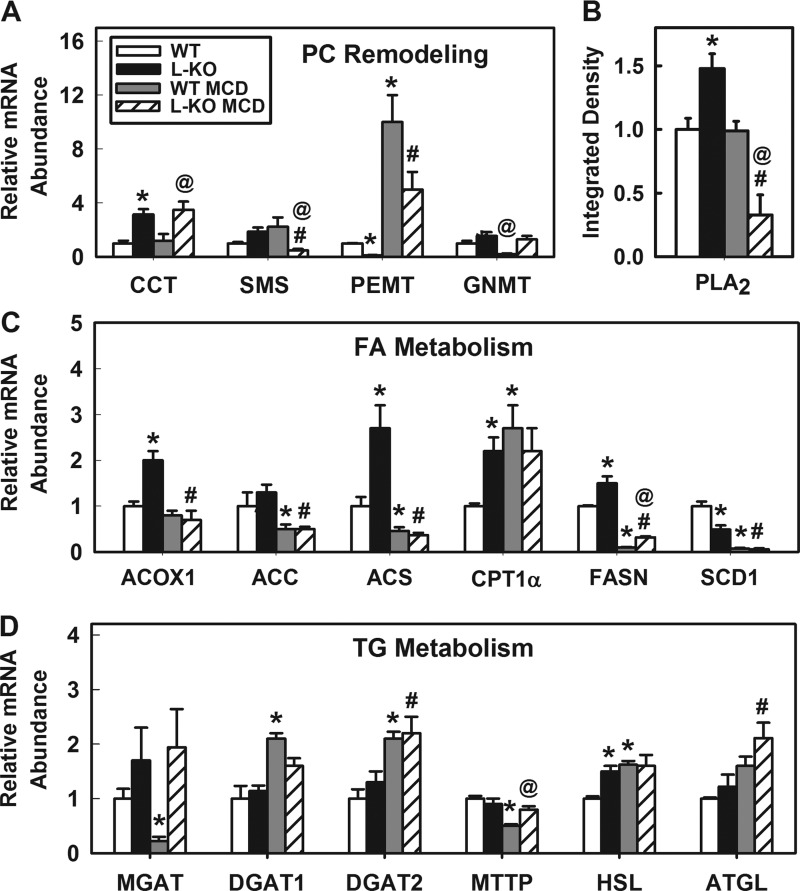
The MCD diet inhibited hepatic accumulation of PC and PE in WT, but not L-KO, mice. To investigate this further, expression levels of several phospholipid-remodeling enzymes including CCT, SMS, PEMT, and GNMT were determined. Levels of CCT, rate-limiting enzyme converting dietary choline and diglycerides to PC (37, 57), were increased 3.1-fold in L-KO mice, but no further effect was observed when mice were fed the MCD diet (Fig. 7A). In contrast, PEMT, a transferase that converts PE to PC, was downregulated 8.3-fold in L-KO mice compared with WT controls. The MCD diet increased PEMT expression 10- and 42-fold in WT and L-KO mice, respectively. In addition, expression levels of two enzymes that use a PC substrate, SM synthase, and PLA2, were decreased 3.8- and 11.3-fold, respectively, in MCD-fed L-KO mice (Fig. 7, A and B). Taken together, these results suggest that L-KO mice can increase production and limit catabolism of PC when choline and methionine are deficient through compensatory regulation of phospholipid-remodeling enzymes.
Fig. 7.
Plin2 liver-specific ablation reduces MCD diet effects on phospholipid, fatty acid, and triglyceride metabolism. A: gene expression of PC remodeling proteins. CCT, phosphate cytidyltransferase 1; SMS, sphingomyelin synthase; PEMT, phosphatidylethanolamine N-methyltransferase; GNMT, glycine N-methyltransferase. B: Western blot analysis of phospholipase A2 (PLA2). C: gene expression of proteins involved in FA metabolism. ACOX1, acyl-coenzyme A oxidase 1; ACC, acyl-coenzyme A carboxylase; ACS, acyl-coenzyme A synthase; CPT1-α, carnitine palmitoyltransferase 1-α; FASN, fatty acid synthase; SCD1, sterol-coenzyme A desaturase. D: gene expression of proteins involved in triglyceride metabolism. MGAT1, monoglycerol O-acyltransferase 1; DGAT1, diacylglycerol O-acyltransferase 1; MTTP, microsomal triacylglycerol transfer protein; HSL, hormone-sensitive lipase; ATGL, adipose triglyceride lipase. Values represent means ± SE, n = 4–5. *P < 0.05 vs. control diet-fed WT mice. @P < 0.05 vs. MCD diet-fed WT mice. #P < 0.05 vs. control diet-fed L-KO mice.
Hepatic Plin2 ablation blunts MCD diet effects on fatty acid and triglyceride metabolism.
To better understand the effect of hepatic Plin2 ablation on lipid metabolism, expression levels of genes involved in fatty acid and triglyceride metabolism were examined. ACOX1 and CPT1-α were significantly upregulated 2.0- and 2.2-fold, respectively, in control-fed L-KO mice (Fig. 7C). Likewise, FASN and ACS were significantly upregulated 1.5- and 2.7-fold, respectively. In contrast, SCD1, an enzyme that converts SAT to MUFA (59), was decreased twofold. However, no genotype effect was observed in mice fed the control diet (Fig. 7D) with regard to genes involved in triglyceride metabolism including MGAT, DGAT1, DGAT2, MTTP, or ATGL. These findings reflect observations of decreased hepatic lipid levels and decreased lipid droplet number/size in livers of L-KO mice. The MCD diet significantly decreased expression of hepatic genes involved in fatty acid oxidation including FASN (10-fold), SCD1 (14.3-fold), ACC (2-fold), and ACS (2.2-fold) in WT mice. In similarly fed L-KO mice, expression levels of these genes decreased but significantly less so with FASN (4.7-fold) and SCD (8.2-fold) expression. With genes related to triglyceride metabolism, the MCD challenge increased expression of DGAT1 (2.1-fold) and DGAT2 (2.2-fold) while decreasing levels of MGAT (4.5-fold) and MTTP (2.0-fold) in WT mice. Plin2 ablation blunted effects on MGAT, DGAT1, and MTTP in L-KO mice. Taken together, these findings indicate Plin2 ablation diminishes the influence of the MCD diet by alleviating the lipid oxidative and steatotic effects associated with the MCD diet.
Expression levels of Plin2 and other lipid droplet proteins including Plin1, Plin3, and Plin5 were also determined in liver, WAT, brain, and heart (Fig. 8). There was no genotype effect observed in hepatic expression of Plin1, Plin3, or Plin5 in control-fed mice. In contrast, levels of Plin5 in WAT were significantly decreased 2.5-fold, whereas, in brain, Plin2 was decreased 1.7-fold in L-KO mice compared with WT mice fed the control diet. The MCD diet increased and decreased, respectively, the hepatic expression of Plin1 (1.5-fold) and Plin3 (2-fold) in L-KO mice, whereas similarly fed WT mice exhibited a decrease in hepatic Plin5. In WAT, the MCD diet increased expression of Plin2 in WT animals 6.6-fold, whereas L-KO mice exhibited a respective 2.2- and 2.0-fold increase in Plin3 and Plin5. In brain and heart tissue, Plin2 expression was significantly decreased in WT mice, but no change was observed in L-KO mice. These findings indicate that WAT is most responsive to Plin2 ablation and challenge with the MCD diet.
Fig. 8.
Plin2 deletion and the MCD diet alter lipid droplet protein expression. Relative protein expression levels of Plin1, Plin2, Plin3, and Plin5 in liver (A), WAT (B), brain (C), and heart (D) tissue from WT mice and L-KO mice fed the control and MCD diets were determined by Western blotting and quantified by densitometric analysis. Values represent means ± SE, n = 4–5. *P < 0.05 vs. control diet-fed WT mice. @P < 0.05 vs. MCD diet-fed WT mice. #P < 0.05 vs. control diet-fed L-KO mice.
DISCUSSION
This study resolves a previously unknown role of Plin2 in the progression of NASH using a conditional Plin2 KO mouse model that selectively deletes Plin2 in hepatocytes.
We demonstrate for the first time the physiological importance of hepatic Plin2 on whole body lipid homeostasis and provide compelling evidence that hepatic ablation of Plin2 blunts the effects of diet-induced NASH related to hepatic lipid accumulation, inflammation, and fibrosis.
In terms of development, fertility, viability, and adiposity, hepatic Plin2 ablation had little effect on phenotype. Tissues were of normal size and weight. Analysis of hepatic lipid levels showed that neutral lipids were decreased by several times in L-KO mice, in line with previous studies with Plin2 global KO mice (15, 16, 46, 50) and mice treated with Plin2 antisense oligonucleotides (ASO) (14, 32, 63). These findings were explained in part by the L-KO mice exhibiting increased hepatic expression of genes involved in fatty acid oxidation, including ACOX1 and CPT1-α, suggesting that increased oxidation of lipids occurred, especially because little to no change was observed in expression levels of lipogenic enzymes MGAT, DGAT1, and DGAT2. In addition to increased hepatic expression of genes involved in fatty acid oxidation, levels of serum β-hydroxybutyrate were increased in L-KO mice, suggesting that fatty acid oxidation was increased in these mice. In support of these findings, recent studies have demonstrated that Hsc-70 targets Plin2 and Plin3 for chaperone-mediated autophagy and degradation, leading to increased lipolysis and fatty acid oxidation of lipids derived from lipid droplets (34). We also found that hepatic Plin2 ablation had little effect on expression of MTTP, a triglyceride transfer protein involved in the rate-limiting step of VLDL assembly. However, a decrease in total lipoprotein, HDL, and VLDL levels was observed in the serum. This was of interest because findings in literature are mixed with regard to VLDL levels and secretion in Plin2-null models. Global Plin2 KO mice exhibited similar VLDL secretion and lipid uptake/utilization as that of WT mice but showed increased expression of MTTP (15). In contrast, Plin2 knockdown in mice resulted in decreased hepatic VLDL secretion and production of MTTP (63). Interestingly, ablation of both Plin2 and GNMT, an enzyme involved in hepatic S-adenosylmethionine (SAMe) degradation, resulted in a mouse model with decreased lipogenesis and increased VLDL secretion (45, 46). Taken together, these findings demonstrated the differences and similarities between the different Plin2 deletion models and highlighted the importance of examining each mouse in the background of Plin2 deficiency-partial (ASO-treated), global, double (GNMT−/−/Plin2−/−), or liver-specific strains.
To investigate the effect of hepatic Plin2 ablation in the context of NASH, L-KO mice were placed on an MCD diet. MCD increases hepatic steatosis by a mechanism that inhibits VLDL secretion and PC synthesis derived from either the CDP-choline pathway involving the enzyme CCT (Kennedy pathway) (57) or by sequential methylation of PE by PEMT using methionine-derived substrates (SAMe) (30). We found that the effects of the MCD diet were blunted in L-KO mice. Levels of PC and VLDL were unchanged, and hepatic steatosis was reduced. These results were due, in part, to an increase in remodeling of PE to PC via the enzyme PEMT where a 10- and 42-fold increase in PEMT expression was observed in MCD-fed WT and L-KO mice, respectively. The MCD diet did not change levels of CCT in WT or L-KO mice, but expression of enzymes involved in PC catabolism was reduced when Plin2 was ablated, further bolstering PC levels in L-KO mice. These findings were consistent with the increased PE to PC flux observed in the GNMT/Plin2 KO mouse model, giving rise to increased PC- and triglyceride-rich VLDL secreted from the liver that helped alleviate hepatic steatosis (46). It should be noted that overexpression of Plin2 in mammalian cells increased PC levels via a CCT-mediated mechanism, which did not involve PEMT (48). In the present study, however, levels of CCT were unchanged in the presence of diet-induced NASH, despite that fact that CCT has been shown to target lipid droplets and to expand the phospholipid monolayer under conditions of lipid excess (37, 56). On the basis of our findings, we posit that hepatic Plin2 ablation blunts effects from the MCD diet through a mechanism that promotes PC synthesis, not at the lipid droplet surface as directed by the enzyme CCT (37), but by a PEMT-mediated mechanism. This hypothesis is consistent with our findings that hepatic Plin2 ablation alleviates diet-induced steatosis while maintaining PC and VLDL levels. To summarize the observed findings, a diagram illustrating the proposed mechanism is provided in Fig. 9.
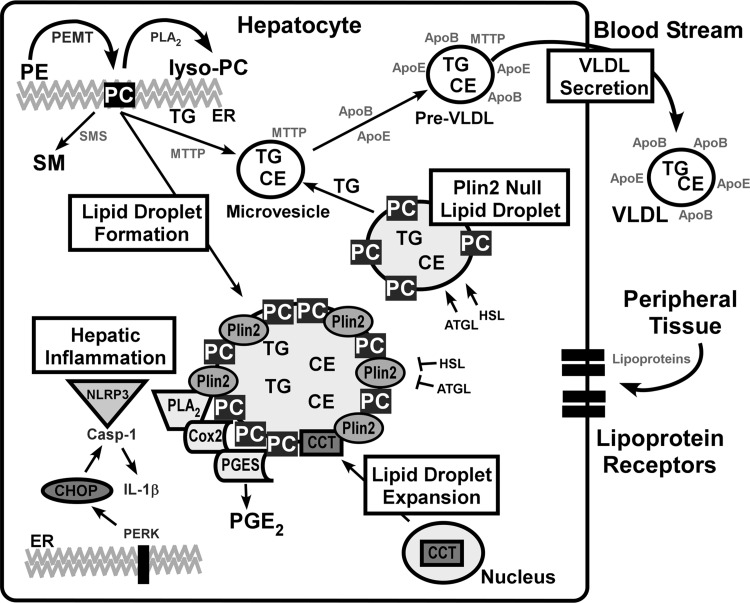
Fig. 9.
Schematic diagram illustrating lipid droplet formation, VLDL secretion, and pathways involving hepatic inflammation. For lipid droplet formation, phospholipids and triglycerides synthesized at the endoplasmic reticulum (ER) form naïve lipid droplets. Hepatic ablation of Plin2 decreases lipid droplet size and number. The MCD diet induces lipid accumulation, resulting in increased lipid droplet size, an effect blunted in L-KO mice. For Plin2-null lipid droplets, lack of Plin2 results in formation of smaller lipid droplets and production of MTTP-rich microsomes that are precursors of pre-VLDL and VLDL particles (15, 16). For VLDL secretion, partially lipidated ApoB100-containing microsomes constitute naive VLDL particles in the ER. MTTP transfers triglycerides and phospholipids to the nascent VLDL to form pre-VLDL that are stabilized by ApoE. The MCD diet inhibited PC synthesis in WT mice, leading to decreased levels of VLDL and increased hepatic steatosis. For lipid droplet expansion, CCT translocates to the lipid droplet surface and begins production of PC to expand the lipid monolayer (37). The lack of choline in the MCD diet favors PC synthesis via the PEMT pathway over CCT-mediated production. Plin2 may block lipase activity of HSL and ATGL (41). For hepatic inflammation, NLRP3 inflammasomes mediate caspase-1 activation of proinflammatory cytokine IL-1β. PERK, an ER stress marker, induces increased expression of CHOP, which then upregulates pro-caspase-1, which is cleaved by NLRP3 (18, 39) to generate the active form, caspase-1. Several proinflammatory markers target lipid droplets including COX2 and prostaglandin E synthase (PGES) (17, 18, 23). L-KO mice fed the MCD diet exhibit decreased expression of CHOP, resulting in decreased activation of caspase-1 and IL-1β.
Our investigations also demonstrated that hepatic Plin2 ablation reduced diet-induced inflammation and fibrosis. Signs of histopathological lesions and evidence of collagen were diminished in Plin2-null livers when mice were fed the MCD diet. In addition, expression levels of proinflammatory markers (IL-1β, TNF-α, IL-6, and COX2) were significantly decreased, and diet-induced effects on ER stress markers (CHOP and PERK) and proteins induced by CHOP (pro-caspase-1 and caspase-1) were blunted. These findings were consistent with other work showing decreased gene expression and secretion of TNF-α, IL-6, and MCP-1 when Plin2 was knocked down in THP-1 macrophages (17). Conversely, increased expression of IL-1β and caspase-1 was observed in LPS-stimulated C2C12 cells overexpressing Plin2 (18). These findings were significant because TLR4 activation of caspase-1 and NLRP3 links ER stress to inflammation and cell death, two cellular responses that ultimately lead to the onset of hepatic fibrosis and cirrhosis (64). In support of these findings, global ablation of Plin2 was found to protect against adipose inflammation in high-fat diet-fed mice (50). Plin2 deficiency reduced adipose inflammatory foci and macrophage invasion in visceral adipose; however, no hepatic inflammation or fibrosis was observed in WT or global KO mice, possibly due to the limitations of the high-fat diet. In other work, Plin2 ASO-treated mice fed a high-fat diet exhibited signs of fibrosis and increased expression of type 1α collage, but no change in expression of inflammation markers such as TNF-α or macrophage infiltration was observed (31). In all, we demonstrated that, although the MCD diet increased hepatic inflammation and fibrosis in WT mice yielding conditions consistent with a NASH phenotype, hepatic Plin2 ablation alleviated these effects and protected the liver from obvious signs of injury.
In conclusion, our findings present compelling evidence that lack of hepatic Plin2 alleviates lipid accumulation, inflammation, and fibrosis in the liver. Results support a PEMT-mediated mechanism that involves compensatory changes in proteins involved in PC remodeling, inflammation, and ER stress that work to alleviate diet-induced NASH. Overall, these findings support a role for Plin2 as a target for NASH therapy.
GRANTS
This work was supported in part by the USPHS, National Institutes of Health grant DK70965.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.P.N., S.S., T.A.L., A.D.J., and B.P.A. conception and design of research; C.P.N., S.S., M.B.A., K.A.F., S.D.O., J.R.B., and T.A.L. performed experiments; C.P.N., S.S., M.B.A., K.A.F., S.D.O., J.R.B., T.A.L., A.D.J., and B.P.A. analyzed data; C.P.N., T.A.L., A.D.J., and B.P.A. interpreted results of experiments; C.P.N. and B.P.A. prepared figures; C.P.N. and B.P.A. drafted manuscript; C.P.N., T.A.L., A.D.J., and B.P.A. edited and revised manuscript; C.P.N., S.S., M.B.A., K.A.F., S.D.O., J.R.B., T.A.L., A.D.J., and B.P.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The technical assistance of Brandon Kelly was much appreciated.
REFERENCES
- 1.Accioly MT, Pacheco P, Maya-Monterio CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res 68: 1732–1740, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Anderson N, Borlak J. Molecular mechanism and therapeutic targets in steatosis and steatohepatitis. Pharm Rev 60: 311–357, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Anstee Q, Goldin R. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol 87: 1–16, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Natl Rev Gastroenterol Hepatol 10: 330–344, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Atshaves BP, Gallegos A, McIntosh AL, Kier AB, Schroeder F. Sterol carrier protein-2 selectively alters lipid composition and cholesterol dynamics of caveolae/lipid raft vs non-raft domains in L-cell fibroblast plasma membranes. Biochemistry 42: 14583–14598, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Atshaves BP, McIntosh AL, Martin GG, Landrock D, Payne HR, Bhuvanendran S, Landrock KK, Lyuksyutova OI, Johnson JD, Marfarlane RD, Kier AB, Schroeder F. Overexpression of sterol carrier protein-2 differentially alters hepatic cholesterol accumulation in cholesterol-fed mice. J Lipid Res 50: 1429–1447, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atshaves BP, McIntosh AL, Storey SM, Landrock KK, Kier AB, Schroeder F. High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene-ablated mice. Lipids 45: 97–110, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartneck M, Fech V, Ehling J, Govaere O, Warzecha T, Hittatiya K, Vucur M, Gautheron J, Luedde T, Trautwein C, Lammers T, Roskams T, Jahnen-Dechent W, Tacke F. Histidine-rich glycoprotein promotes macrophage activation and inflammation in chronic liver disease. Hepatology 63: 1310–1324, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes 57: 2037–2045, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozza PT, Yu W, Penrose JF, Morgan ES, Dvorak AM, Weller PF. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med 186: 909–920, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 12.Brasaemle DL, Barber T, Wolins N, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38: 2249–2263, 1997. [PubMed] [Google Scholar]
- 13.Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3–L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid droplets. Biochim Biophys Acta 1483: 251–262, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Carr RM, Peralta G, Yin X, Ahima RS. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PLoS One 9: e97118, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol 26: 1063–1076, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang BH, Li L, Saha P, Chan L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J Lipid Res 51: 2132–2142, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen FL, Yang ZH, Wang XC, Liu Y, Yang YH, Li LX, Liang WC, Zhou WB, Hu RM. Adipophilin affects the expression of TNFα, MCP-1, and IL-6 in THP-1 macrophages. Mol Cell Biochem 337: 193–199, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Cho K, Kang PB. Plin2 inhibits insulin-induced glucose uptake in myoblasts through the activation of the NLRP3 inflammasome. Intern J Mol Med 36: 839–844, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Day CP, James OF. Steatohepatitis: a tale of two ‘hits’? Gastroenterology 114: 842–845, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Esterbauer H, Schaur R, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Fhaner CJ, Liu S, Ji H, Simpson RJ, Reid GE. Comprehensive lipidome profiling of primary and metastatic colon adenocarcinoma cell lines. Anal Chem 84: 8917–8926, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fhaner CJ, Liu S, Zhou X, Reid GE. Functional group selective derivatization and gas-phase fragmentation reactions of plasmalogen glycerophospholipids. Mass Spectrom (Tokyo) 2: S0015, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii H, Ikura Y, Arimoto J, Sugioka K, Lezzoni JC, Park SH, Naruko T, Itabe H, Kawada N, Caldwell SH, Ueda M. Expression of perilipin and adipohilin in nonalcoholic fatty liver disease: relevance to oxidative injury and hepatocyte ballooning. J Atheroscler Thromb 16: 1893–1901, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, Endo H, Takahashi H, Inamori M, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nakajima A. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology 50: 772–780, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Fukushima M, Enjoji M, Kohjima M, Sugimoto R, Ohta S, Kotoh K, Kuniyoshi M, Kobayashi K, Imamura M, Inoguchi T, Nakamuta M, Nawata H. Adipose differentiation related protein induces lipid accumulation and lipid droplet formation in hepatic stellate cells. In Vitro Cell Dev Biol Anim 41: 321–324, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Serrero G. Adipose differentiation related protein (ADRP) expressed in transfected COS-7 cells selectively stimulates long chain fatty acid uptake. J Biol Chem 274: 16825–16830, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Gauthier M, Favier R, Lavoie F. Time course of the development of non-alcoholic hepatic steatosis in response to high-fat diet-induced obesity in rats. Br J Nutr 95: 273–281, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest 121: 2102–2110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol 98: 2042–2047, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Horl G, Wagner A, Cole L, Malli R, Reicher H, Kotzbeck P, Kofeler H, Hofler G, Frank S, Bogner-Strauss J, Sattler W, Vance D, Steyrer E. Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. J Biol Chem 286: 17338–17350, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai Y, Boyle S, Varela G, Caron E, Yin X, Dhir R, Dhir R, Graham M, Ahima R. Effects of perilipin 2 antisense oligonucleotide treatment on hepatic lipid metabolism and gene expression. Physiol Genomics 44: 1125–1131, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai Y, Varela GM, Jackson MB, Graham MJ, Crook RM, Ahima RS. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology 132: 1947–1954, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Jump DB, Depner CM, Tripathy S, Lytle KA. Impact of dietary fat on the development of non-alcoholic fatty liver disease in Ldlr−/− mice. Proc Nutr Soc 75: 1–9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaushik S, Cuervo A. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol 17: 759–770, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirsch R, Clarkson V, Shepard E, Marais D, Jaffer M, Woodburne V, Kirsch R, Hall P. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol 18: 1272–1282, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Kos C. Cre/loxP system for generating tissue-specific knockout mouse models. Nutr Rev 62: 243–246, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, Newman H, Schmidt-Supprian M, Vance D, Mann M, Farese R, Walther T. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:Phosphocholine cytidylyltransferase. Cell Metab 14: 504–515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larigauderie G, Cuaz-Perolin C, Younes AB, Furman C, Lasselin C, Copin C, Jaye M, Fuchart JC, Rouis M. Adipophilin increases triglyceride storage in human macrophages by simulation of biosynthesis and inhibition of beta-oxidation. FEBS J 273: 3498–3510, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, Adam G, Lavallard VJ, Rovere C, Le Thuc O, Saint-Paul MC, Anty R, Schneck AS, Iannelli A, Gugenheim J, Tran A, Gual P, Bailly-Maitre B. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis 6: e1879, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee GS, Yan JS, Ng RK, Kakar S, Maher JJ. Polyunsaturated fat in the methionine-choline-deficient diet influences hepatic inflammation but not hepatocellular injury. J Lipid Res 48: 1885–1896, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res 48: 2751–2761, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Cell Dev Biol 10: 51–58, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Lydic TA, Busick JV, Reid GE. A monophasic extraction strategy for the simultaneous lipidome analysis of polar and nonpolar retina lipids. J Lipid Res 1797–1809, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez-Una M, Varela-Rey M, Cano A, Fernandez-Ares L, Beraza N, Aurrekoetxea I, Martinez-Arranz I, Garcia-Rodriguez JL, Buque X, Mestre D, Luka Z, Wagner C, Alonso C, Finnell RH, Lu SC, Martinez-Chantar ML, Aspichueta P, Mato JM. Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology 58: 1296–1305, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Una M, Varela-Rey M, Mestre D, Fernandez-Ares L, Fresnedo O, Fernandez-Ramos D, Gutierrez-de Juan V, Martin-Guerrero I, Garcia-Orad A, Luka Z, Wagner C, Lu S, Garcia-Monzon C, Finnell RH, Aurrekoetxea I, Buque X, Martinez-Chantar ML, Mato JM, Aspichueta P. S-adenosylmethionine increases circulating very-low-density lipoprotein clearance in non-alcoholic fatty liver disease. J Hepatol 62: 673–681, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzo A, Ghirardi P, Sardini D, Meroni G. Simplified measurement of monoglycerides, diglycerides, triglycerides, and free fatty acids in biological samples. Clin Chem 17: 145–147, 1971. [PubMed] [Google Scholar]
- 48.McIntosh AL, Senthivinayagam S, Moon KC, Gupta S, Lwande JS, Murphy CC, Storey S, Atshaves BP. Direct interaction of ADRP with lipids on the surface of lipid droplets: A live cell FRET analysis. Am J Physiol Cell Physiol 303: C728–C742, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McIntosh AL, Gallegos AM, Atshaves BP, Storey SM, Kannoju D, Schroeder F. Fluorescence and multiphoton imaging resolve unique structural forms of sterol in membrane of living cells. J Biol Chem 278: 6384–6403, 2003. [DOI] [PubMed] [Google Scholar]
- 50.McManaman JL, Bales ES, Orlicky DJ, Jackson MB, MacLean PS, Cain S, Crunk AE, Mansur A, Graham CE, Bowman TA, Greenberg AS. Perilipin-2 null mice are protected against diet-induced obesity, adipose inflammation and fatty liver disease. J Lipid Res 54: 1346–1359, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melo RCN, Weller PF. Unraveling the complexity of lipid body organelles in human eosinophils. J Leukoc Biol 96: 703–712, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris E, Rector R, Thyfault J, Ibdah J. Mitochondria and redox signaling in steatohepatitis. Antioxid Redox Signal 15: 485–504, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Najt CP, Lwande JS, McIntosh AL, Senthivinayagam S, Gupta S, Kuhn LA, Atshaves BP. Structural and functional assessment of Perilipin2 lipid binding domains. Biochemistry 53: 315–321, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ong JP, Elariny HC, Collantes R, Younoszai A, Chandhoke V, Reines HD, Goodman Z, Younossi ZM. Predictors of nonalcoholic steatohepatitis and advance fibrosis in morbidly obese patients. Obes Surg 15: 310–315, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Paul A, Chang B, Li L, Yechoor V, Chan L. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res 102: 1492–1501, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Payne F, Lim K, Girousse A, Brown R, Kory N, Robbins A, Xue Y, Sleigh A, Cochran E, Adams C, Dev Borman A, Russel-Jones D, Gorden P, Semple R, Saudek V, O'Rahilly S, Walther T, Barroso I, Savage D. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci USA 111: 8901–8906, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol 204: 635–646, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rinella M, Green R. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol 40: 47–51, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Rizki G, Arnaboldi L, Gabrielli B, Yan JS, Lee G, Ng RK, Turner SM, Badger TM, Pitas RE, Maher JJ. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism conincident with hepatic suppression of SCD-1. J Lipid Res 47: 2280–2290, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Romestaing C, Piquet M, Bedu E, Rouleau V, Dautresme M, Hourmand-Ollivier I, Filippi C, Duchamp C, Sibille B. Long term highly saturated fat diet does not induce NASH in Wistar rats. Nutr Metab (Lond) 4: 4, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subramanian V, Rothenberg A, Gomez C, Cohen A, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti M, Brasaemle D. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3–L1 adipocytes. J Biol Chem 279: 42062–42071, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Varela GM, Antwi DA, Dhir R, Yin X, Singhal NS, Graham MJ, Crook RM, Ahima RS. Inhibition of ADRP prevents diet-induced insulin resistance. Am J Physiol Gastrointest Liver Physiol 295: G621–G628, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19: 1880–1891, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wan HC, Melo RC, Dvorak AM, Weller PF. Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. FASEB J 21: 167–178, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weibel GL, Joshi MR, Wei C, Bates SR, Blair IA, Rothblat GH. Lipoxygenase-1 associates with neutral lipid droplets in macrophage foam cells: evidence of lipid droplet metabolism. J Lipid Res 50: 2371–2376, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolins N, Quaynor B, Skinner J, Tzekov A, Croce M, Gropler M, Varma V, Yao-Borengasser A, Rasouli N, Kern P, Finck B, Bickel P. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55: 3418–3428, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3-12, adipophilin and TIP47 package lipid in adipocytes. J Biol Chem 280: 19146–19155, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 278: 2461–2468, 2003. [DOI] [PubMed] [Google Scholar]