Abstract
The goals of this study were to evaluate the effects of ionizing radiation on apical junctions in colonic epithelium and mucosal barrier function in mice in vivo. Adult mice were subjected to total body irradiation (4 Gy) with or without N-acetyl-l-cysteine (NAC) feeding for 5 days before irradiation. At 2–24 h postirradiation, the integrity of colonic epithelial tight junctions (TJ), adherens junctions (AJ), and the actin cytoskeleton was assessed by immunofluorescence microscopy and immunoblot analysis of detergent-insoluble fractions for TJ and AJ proteins. The barrier function was evaluated by measuring vascular-to-luminal flux of fluorescein isothiocyanate (FITC)-inulin in vivo and luminal-to-mucosal flux in vitro. Oxidative stress was evaluated by measuring protein thiol oxidation. Confocal microscopy showed that radiation caused redistribution of occludin, zona occludens-1, claudin-3, E-cadherin, and β-catenin, as well as the actin cytoskeleton as early as 2 h postirradiation, and this effect was sustained for at least 24 h. Feeding NAC before irradiation blocked radiation-induced disruption of TJ, AJ, and the actin cytoskeleton. Radiation increased mucosal permeability to inulin in colon, which was blocked by NAC feeding. The level of reduced-protein thiols in colon was depleted by radiation with a concomitant increase in the level of oxidized-protein thiol. NAC feeding blocked the radiation-induced protein thiol oxidation. These data demonstrate that radiation rapidly disrupts TJ, AJ, and the actin cytoskeleton by an oxidative stress-dependent mechanism that can be prevented by NAC feeding.
Keywords: barrier function, oxidative stress, intestine, occludin, zona occludens-1, cytoskeleton, actin, protein thiol, adherens junction
radiotherapy or accidental exposure to ionizing radiation causes severe damage to healthy tissues. The gastrointestinal tract is one of the radiation-sensitive organs in the body, and the gastrointestinal complications of radiation are collectively referred to as gastrointestinal acute radiation syndrome (GI-ARS) (20). GI-ARS is characterized by nausea and diarrhea during the early stage of radiation injury and endotoxemia and bacteremia leading to septicemia in the later stage (9, 12, 16, 37). Total body irradiation (TBI) causes ablation of crypt cell proliferation, mitotic catastrophe, and apoptosis leading to gastrointestinal mucositis (33). The prevailing concept in this field is that the threshold for GI-ARS is 5–10 Gy (10); it is greater than 10 Gy according to the Fact Sheet for Physicians by the Center for Disease Control. According to the prevailing views in this field, the small intestine is the primary target of radiation, while the colon is relatively resistant to radiation injury (5, 14, 25). The facts that endotoxemia and bacteremia are important events in the pathogenesis of ARS and the colon is the primary source of endotoxins highlight the critical yet so far unrealized importance of colonic tissue injury in the pathogenesis of GI-ARS. Furthermore, colitis is one of the clinical problems associated with radiotherapy that originates from the gut microflora and induces colonic endotoxemia (16). Dysbiosis, infection, and endotoxemia involve disruption of the structural and functional integrity of gut mucosa, which affects selective permeability of important nutrients and endotoxins (22, 24).
Epithelial barrier function is the first line of defense in the gastrointestinal tract that prevents the diffusion of bacterial toxins into intestinal mucosa and eventually into the systemic circulation. Tight junctions (TJ), the highly specialized intercellular junctions, confer epithelial barrier function in the gastrointestinal tract (35). TJ are multiprotein complexes made up of transmembrane proteins such as occludin, claudins, and junctional adhesion molecules, which interact with the intracellular adapter proteins zona occludens (ZO)-1, ZO-2, and ZO-3 (1). The adapter proteins interact with other TJ-specific proteins such as cingulin, AF6, 7H6, and catenins. These protein-protein interactions are essential for the assembly of TJ and maintenance of their integrity. Adherens junctions (AJ), the junctional complexes that lie beneath the TJ, are also multiprotein complexes and composed of transmembrane and adapter proteins, such as E-cadherin and catenins (4). AJ are not diffusion barriers for macromolecules, but they indirectly regulate the integrity of TJ and therefore the barrier function. TJ and AJ protein complexes interact with the actin cytoskeleton, which is essential for the assembly and maintenance of TJ and AJ (8). Although there is an indication of TJ disruption by radiation in the small intestine, the radiation effect on colonic epithelial TJ and AJ is poorly understood.
Factors that prevent tissue damage by ionizing radiation are important in developing therapeutics for the prevention and treatment of GI-ARS. Oxidative stress is an important factor involved in the mechanism of radiation-induced tissue injury, and antioxidants are important considerations for development of therapeutics (32). N-acetyl-l-cysteine (NAC) is a derivative of cysteine amino acid and is one of the least toxic thiols known to reverse protein thiol oxidation in cells and tissues (2). Therefore, in this study, we tested the hypothesis that low-dose γ-irradiation (γ-IR) affects the colonic epithelial TJ and AJ in mice in vivo, and evaluated the effect of NAC feeding on radiation-induced TJ and AJ disruption and mucosal barrier dysfunction.
MATERIALS AND METHODS
Chemicals.
Maltose dextrin was purchased from Bioserv (Flemington, NJ). Regular Lieber DeCarli ethanol diet (Dyet no. 710260) was purchased from Dyets (Bethlehem, PA). Hoechst 33342 dye and BODIPY FL-N-(2-aminoethyl)maleimide were purchased from Life Technologies (Grand Island, NY). N-ethylmaleimide and tris(2-carboxyethyl)phosphine were from Sigma-Aldrich (St. Louis, MO). AlexaFlour-488-conjugated phalloidin and all other chemicals were purchased from either Sigma-Aldrich or Thermo Fisher Scientific (Tustin, CA).
Antibodies.
Anti-ZO-1, anti-occludin, and anti-claudin-3 (Cldn-3) antibodies were purchased from Invitrogen (Carlsbad, CA). Anti-E-cadherin and anti-β-catenin antibodies were purchased from BD Biosciences (Billerica, MA). Horseradish peroxidase-conjugated anti-mouse IgG and anti-rabbit IgG and anti-β-actin antibodies were obtained from Sigma-Aldrich. AlexaFlour-488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR).
Animals and diets.
Female C57BL/6 mice (12–14 wk; Harlan Laboratories, Houston, TX) were used for all experiments. All animal experiments were performed according to the protocols approved by the University of Tennessee Health Science Center Institutional Animal Care and Use Committee. Animals were housed in an institutional animal care facility with 12:2-h light-dark cycles. All mice had free access to regular laboratory chow and water until the start of experiments.
Study protocol.
In the first study, 12- to 14-wk-old adult female mice were subjected to TBI (4 Gy at a dose rate of ∼76 cGy/min) using a J. L. Shepherd & Associates Mark I, model 25, 137Cs source (San Fernando, CA). At 2–24 h post-IR, the integrity of colonic epithelial TJ, AJ, and actin cytoskeleton was examined. In a second study, mice were randomized to four groups [Sham (control), IR, NAC, and NAC + IR] and fed Lieber DeCarli liquid diet (catalog no. 710260; Dyets) with (NAC and NAC + IR) or without (Sham and IR) 20 mM NAC for 5 days before IR. IR and NAC + IR mice were subjected to TBI (4 Gy), whereas Sham and NAC mice were sham treated. At 2 h post-IR, colons were collected and examined for oxidative stress and epithelial junctional integrity. Radiation field mapping and calibration by ion chamber dosimetry was done by the manufacturer at installation. In addition, routine validation and quality control measurements of exposure rates and exposure rate mapping in the chamber at positions of interest were conducted by a Certified Health Physicist using a calibrated RadCal 0.6-ml therapy-grade ion chamber/electrometer system. High-dose thermo luminescent dosimeters were used in most IRs to validate the actual dose delivered to the mice (MD Anderson Cancer Center Radiation Dosimetry Services). The isodose field was validated using Gafchromic film for high-dose dosimetry (10–50 Gy; Ashland, Covington, KY). At the end of experiment, gut permeability was measured as described below. Colon and ileum segments were stored frozen for further analyses.
Gut permeability in vivo and in vitro.
Mucosal barrier dysfunction was evaluated by measuring gut permeability to FITC-inulin (6 kDa). On the last day of experiment, mice were intravenously injected with FITC-inulin (50 mg/ml solution; 2 μl/g body wt) via the tail vein. After injection (1 h), blood samples was collected by cardiac puncture under isoflurane anesthesia for plasma preparation. Luminal contents from colon and ileum were flushed with 0.9% saline (3 ml/segment). Fluorescence in plasma and luminal flushing was measured using a fluorescence plate reader. Fluorescence values in the luminal flushing were normalized to fluorescence values in corresponding plasma samples and calculated as percent of amount injected. For in vitro permeability at 3 h post-IR, colonic and ileal loops (4 cm) were prepared and filled with 0.15 ml of 0.9% saline containing FITC-inulin (0.05 mg/ml) and incubated in DMEM for 45 min in a cell culture incubator. Luminal contents flushed in 2 ml saline (0.9%) were analyzed for fluorescence to evaluate inulin absorption from the intestinal lumen. Previous studies have established that intestinal loops are viable for at least 60 min.
Histopathology.
Distal colon was fixed in 10% buffered formalin (Sigma Aldrich) for 24 h, and 6-μm-thick sections were collected by using a cryostat on glass slides and stained with hematoxylin and eosin (H&E) by a standard method. Briefly, after dehydration through graded ethanol washes (50, 70, 95, and 100%) and xylene washes, sections were mounted with permanent mounting medium (Vector Laboratories). The bright-field images were captured at ×10 magnification by using a Nikon Eclipse Ti microscope (Melville, NY).
Immunofluorescence microscopy.
Cryosections of colon (10 μm thickness) and ileum (12 μm thickness) were fixed in acetone-methanol mixture (1:1) at 20°C for 2 min and rehydrated in phosphate-buffered saline (PBS). Sections were permeabilized with 0.5% Triton X-100 in PBS for 15 min and blocked in 4% nonfat milk in 20 mM Tris, pH 7.2, and 150 mM NaCl. Sections were incubated for 1 h with primary antibodies (mouse monoclonal anti-occludin and rabbit polyclonal anti-ZO-1 antibodies or mouse monoclonal E-cadherin and rabbit polyclonal anti-β-catenin antibodies), followed by incubation for 1 h with secondary antibodies (AlexaFluor-488-conjugated anti-mouse IgG and Cy3-conjugated anti-rabbit IgG antibodies from Molecular Probes) containing Hoechst 33342. The fluorescence was examined by using a Zeiss 710 confocal microscope, and images from x–y sections (1 μm) were collected using Zen software. Images were stacked using the Image J software (National Institutes of Health, Bethesda, MD) and processed by Adobe Photoshop (Adobe Systems, San Jose, CA).
Preparation of the detergent-insoluble fraction.
Actin-rich detergent-insoluble fraction was prepared as described previously (30, 31). Mucosal scrapping from colon and ileum was incubated on ice for 15 min with lysis buffer-CS [Tris buffer containing 1% Triton X-100, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin A, 10 μl/ml of protease inhibitor cocktail, 1 mM sodium vanadate, and 1 mM phenylmethylsulfonyl fluoride (PMSF)]. Briefly, mucosal lysates were centrifuged at 15,600 g for 4 min at 4°C to sediment the high-density actin-rich detergent-insoluble fraction. The pellet was suspended in 100 μl of preheated (at 100°C) lysis buffer D (20 mM Tris buffer, pH 7.2, containing 10 μl/ml of protease inhibitor cocktail, 10 mM sodium fluoride, 1 mM sodium vanadate, and 1 mM PMSF) for 5 min, sonicated to homogenize the actin cytoskeleton, and heated at 100°C. Protein content was measured by the BCA method (Pierce Biotechnology, Rockford, IL). Triton-insoluble and -soluble fractions were mixed with an equal volume of 2× concentrated Laemmli's sample buffer and heated at 100°C for 5 min, and 25- to 40-μg protein samples were used for immunoblot analysis.
Immunoblot analysis.
Triton-soluble and -insoluble fractions were separated by SDS-polyacrylamide gel (7%) electrophoresis and transferred to polyvinylidene difluoride membranes as described before (31). Membranes were immunoblotted for different proteins using specific antibodies for different TJ and AJ proteins with β-actin as housekeeping protein in combination with horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG secondary antibodies. The blots were developed using the ECL chemiluminescence method (Pierce) and quantitated by densitometry using Image J software. The density for each band was normalized to the density of the corresponding actin band.
Protein thiol assay.
Protein thiols in intestinal sections were assessed as described before (17). Reduced protein thiols were evaluated by staining cryosections colon and ileum with BODIPY FL-N-(2-aminoethyl)maleimide (Flm) and confocal microscopy at excitation and emission wavelengths of 490 and 534 nm, respectively. For oxidized protein thiols, the reduced protein thiol was first alkylated with N-ethylmaleimide followed by reduction of oxidized protein thiols with tris(2-carboxyethyl)phosphine before staining with Flm. Control staining is done after N-ethylmaleimide treatment. Fluorescence images were collected, and fluorescence was quantitated by Image J software.
Statistical analyses.
All data are expressed as means ± SE. The differences among multiple groups were first analyzed by ANOVA. When a statistical significance was detected, Tukey's t-test was used to determine the statistical significance between multiple testing groups and the corresponding control. Statistical significance was established at 95%.
RESULTS
Ionizing radiation induces a rapid disruption of TJ and reorganization of actin cytoskeleton in the intestinal epithelium.
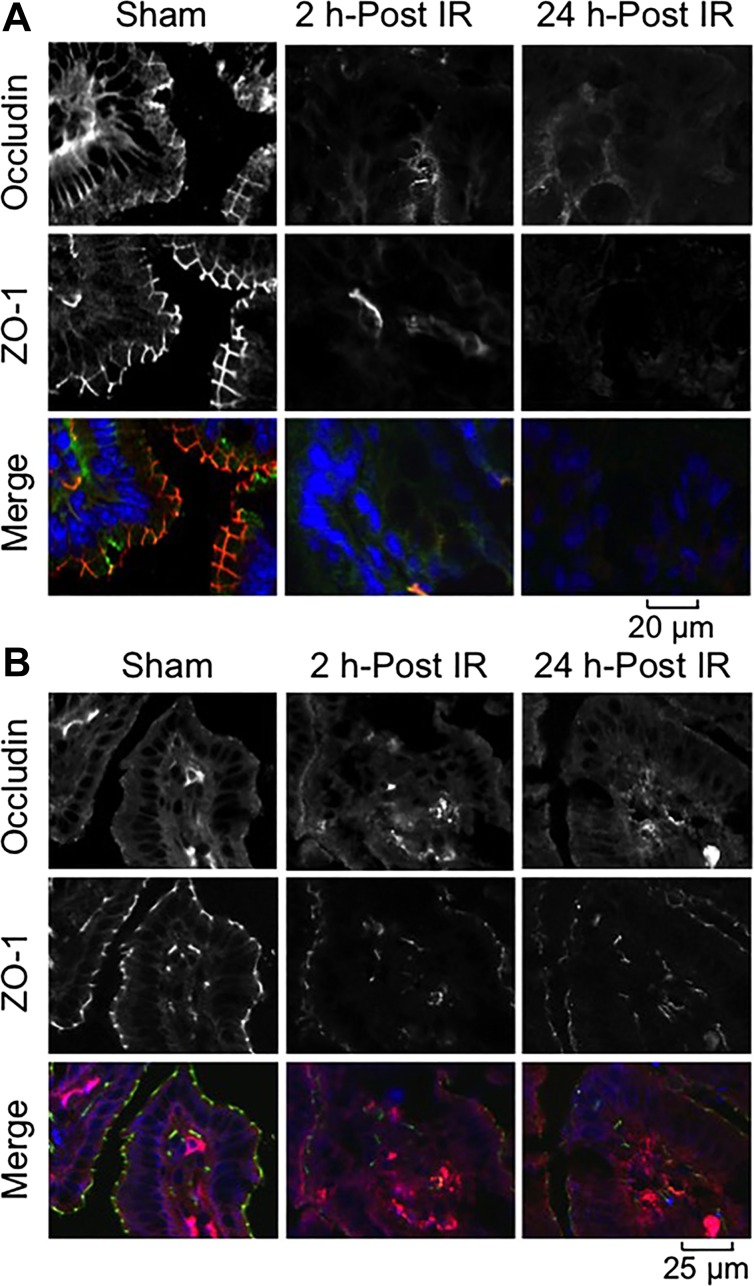
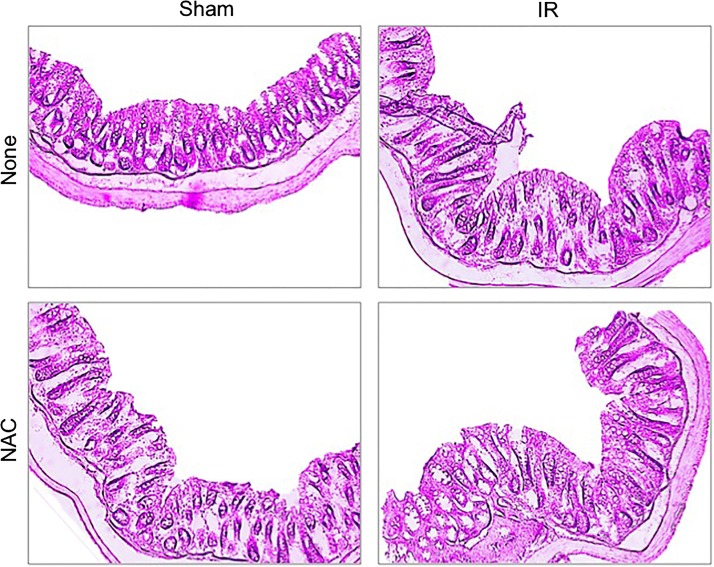
Although intestinal mucosal damage by ionizing radiation has been well established, the primary focus of investigations has been on the small intestine, whereas colon was found to be resistant to radiation-induced injury based on the histopathology, crypt damage, and cell proliferation markers (25). However, disruption of epithelial TJ without morphological or cellular damage, which is undetectable with a conventional light microscope, may lead to an increase in paracellular permeability and endotoxin flux in the mucosa. In our study, we chose low-dose γ-IR (4 Gy) on the intestinal epithelial TJ, AJ, and the actin cytoskeleton at varying time points on the 0- to 24-h scale. Bright-field images of H&E-stained colonic sections showed no gross morphological changes in colonic mucosa at 2 h post-IR (Fig. 1). However, at 24 h post-IR, there were signs of hematopoietic cell infiltration in the colonic mucosa. Confocal microscopy of colon (Fig. 2A) and ileum (Fig. 2B) from sham-treated mice showed a colocalization of occludin and ZO-1 at the intercellular junctions of epithelial cells. Radiation induced redistribution of both occludin and ZO-1 from the intercellular junctions in the intracellular compartment as early as 2 h post-IR. This redistribution caused by γ-IR sustained for at least 24 h in both the colon (Fig. 2A) and ileum (Fig. 2B). Redistribution of occludin and ZO-1 appeared to be more severe in the colon compared with that in ileum even at 2 h post-IR.
Fig. 1.
Temporal effect of γ-irradiation (γ-IR) on colonic mucosal morphology in mice. Mice were subjected to total body irradiation [TBI (IR)] or sham treatment (Sham). At 2 or 24 h postirradiation (post-IR), sections of formalin-fixed distal colon were stained with hematoxylin and eosin. Bright-field images were captured.
Fig. 2.
γ-IR induces rapid redistribution of tight junction (TJ) proteins in mouse intestine. Mice were subjected to TBI (IR) or sham treatment (Sham). At 2–24 h post-IR, cryosections of distal colon (A) and ileum (B) were stained for occludin (green in A and red in B) and zona occludens (ZO)-1 (red in A and green in B) by the immunofluorescence method, and nuclei were stained with Hoechst 33342 (blue). Fluorescence images were captured by confocal microscopy.
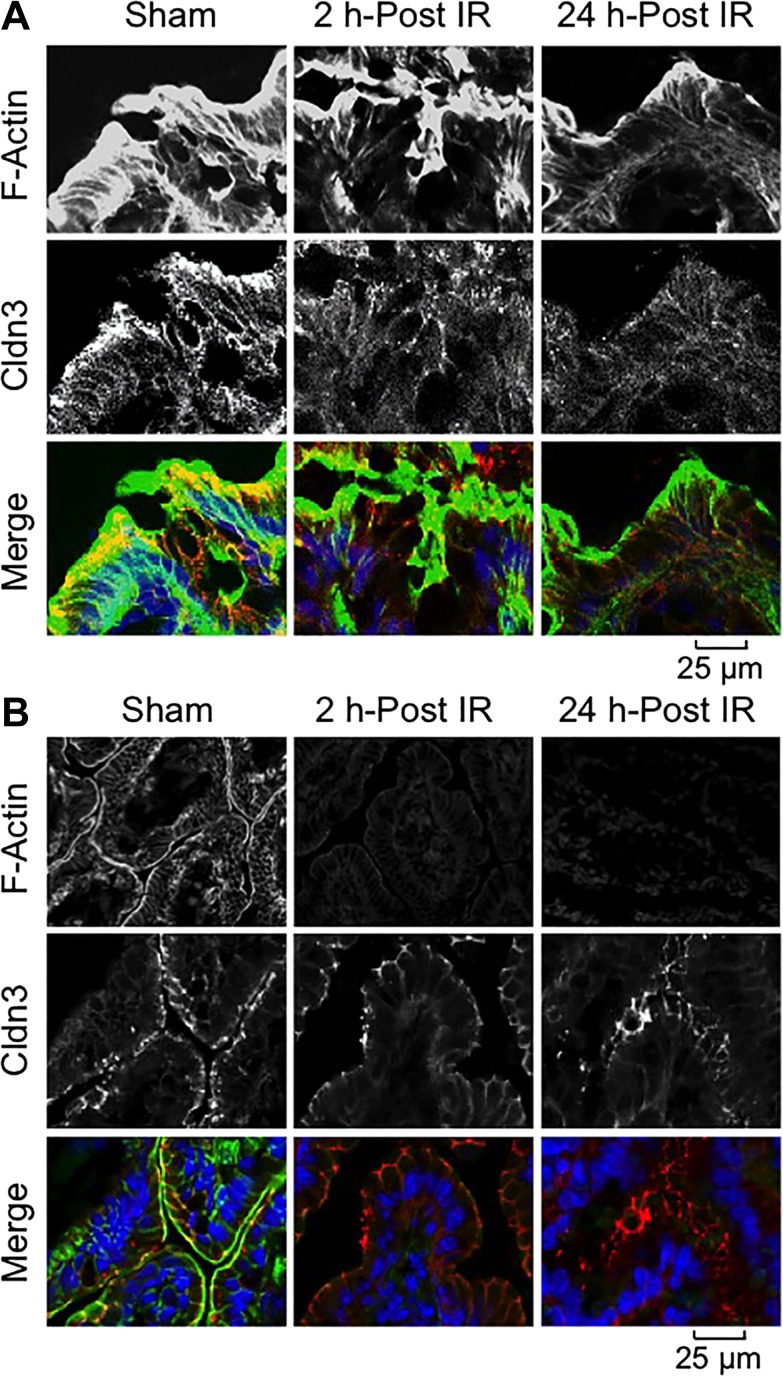
Claudins are a set of transmembrane proteins of TJ that play a crucial role in TJ assembly and maintenance of barrier function (36). Our data show that Cldn-3 is localized predominantly at the intercellular junctions of the colonic (Fig. 3A) and ileal (Fig. 3B) epithelia. Radiation induced a loss of junctional distribution of Cldn-3 in both the colon and ileum, but the damage appeared to be more severe in the colon. Staining for F-actin showed that the actin cytoskeleton is organized in the colonic (Fig. 3A) and ileal (Fig. 3B) epithelia with distinct distributions at the apical region and the lateral submembranous regions. Radiation induced a time-dependent loss of F-actin structure in the colonic epithelium (Fig. 3A), suggesting a depolymerization of the actin cytoskeleton. Similarly, F-actin levels were reduced by γ-IR in the ileal epithelium (Fig. 3B); the effect on F-actin appeared to be more severe in the ileum compared with that in colon.
Fig. 3.
γ-IR induces rapid redistribution of claudin (Cldn)-3 and reorganization of the actin cytoskeleton. Mice were subjected to TBI (IR) or sham treatment (Sham). At 2–24 h post-IR, cryosections of distal colon (A) and ileum (B) were stained for Cldn-3 (red) and F-actin (green) by the immunofluorescence method, and the nuclei were stained using Hoechst 33342 (blue).
Ionizing radiation induces a rapid disruption of AJ.
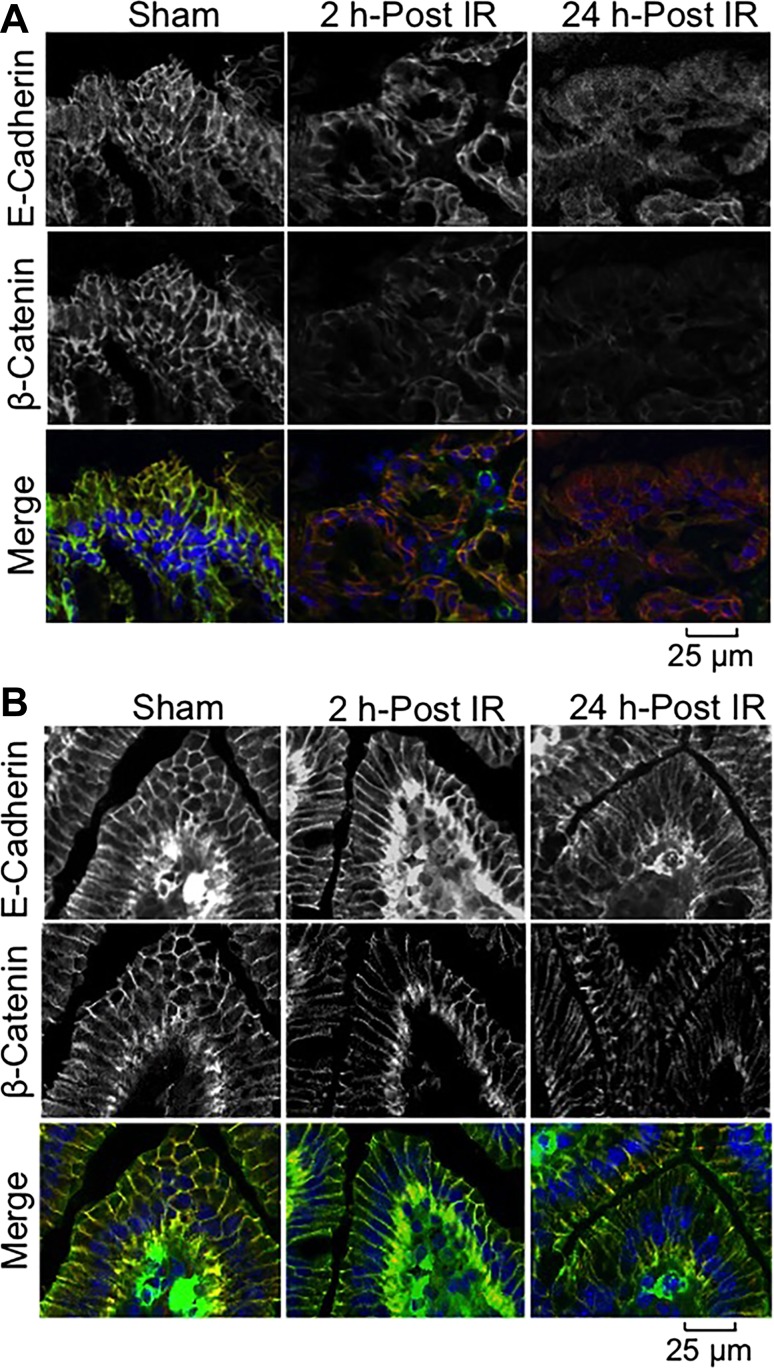
E-cadherin and β-catenin are the principal components of the epithelial AJ. Interaction between E-cadherin and β-catenin is crucial for the assembly and maintenance of AJ. Confocal microscopy showed that these two proteins are colocalized at the intercellular junctions of colonic (Fig. 4A) and ileal (Fig. 4B) epithelia. γ-IR induced a redistribution of E-cadherin and β-catenin from the colonic epithelial junctions as early as 2 h post-IR, and the damages were sustained at least for 24 h post-IR (Fig. 4A). There was a slight loss of E-cadherin and β-catenin in the epithelial junctions of ileum (Fig. 4B), but the effect was less severe compared with that in the colonic epithelium.
Fig. 4.
γ-IR induces rapid redistribution of adherens junction (AJ) proteins in mouse intestine. Mice were subjected to TBI (IR) or sham treatment (Sham). At 2–24 h post-IR, cryosections of distal colon (A) and ileum (B) were stained for E-cadherin (green) and β-catenin (red) by the immunofluorescence method, and the nuclei were stained with Hoechst 33342 (blue).
NAC feeding attenuates radiation-induced disruption of TJ, AJ, and the actin cytoskeleton.
Oxidative stress is one of the major mechanisms of ionizing radiation-induced tissue injury (32), and our previous studies demonstrated that oxidative stress disrupts intestinal epithelial TJ (26, 27, 29). NAC is an antioxidant that acts by restoring cellular protein thiols from oxidative depletion. Prophylactic treatment of IR and non-IR mice with 20 mM NAC in a liquid diet for 5 days before IR showed no obvious effect on colonic mucosal morphology (Fig. 5). NAC supplementation, however, resulted in almost a complete attenuation of radiation-induced redistribution of occludin and ZO-1 from the intercellular junctions in the colonic (Fig. 6A) and ileal (Fig. 6B) epithelia. NAC treatment also blocked radiation-induced redistribution of Cldn-3 in the colonic (Fig. 7A) and ileal (Fig. 7B) epithelia. Radiation induced loss of F-actin in the colonic (Fig. 7A) and ileal (Fig. 7B) epithelia that was blocked by NAC feeding. The protection by NAC was almost complete in the colon, but the actin cytoskeleton in the ileum was only partially protected. Radiation-induced redistribution of E-cadherin and β-catenin from the intercellular junctions of epithelium in the colon (Fig. 8A) and ileum (Fig. 8B) was also absent in NAC-fed mice.
Fig. 5.
N-acetyl-l-cysteine (NAC) treatment on colonic mucosal morphology in mice. Mice were fed a liquid diet with or without 20 mM NAC for 5 days before a 4 Gy dose of TBI (IR) or sham treatment (Sham). At 2 h post-IR, sections of formalin-fixed distal colon were stained with hematoxylin and eosin. Bright-field images were captured.
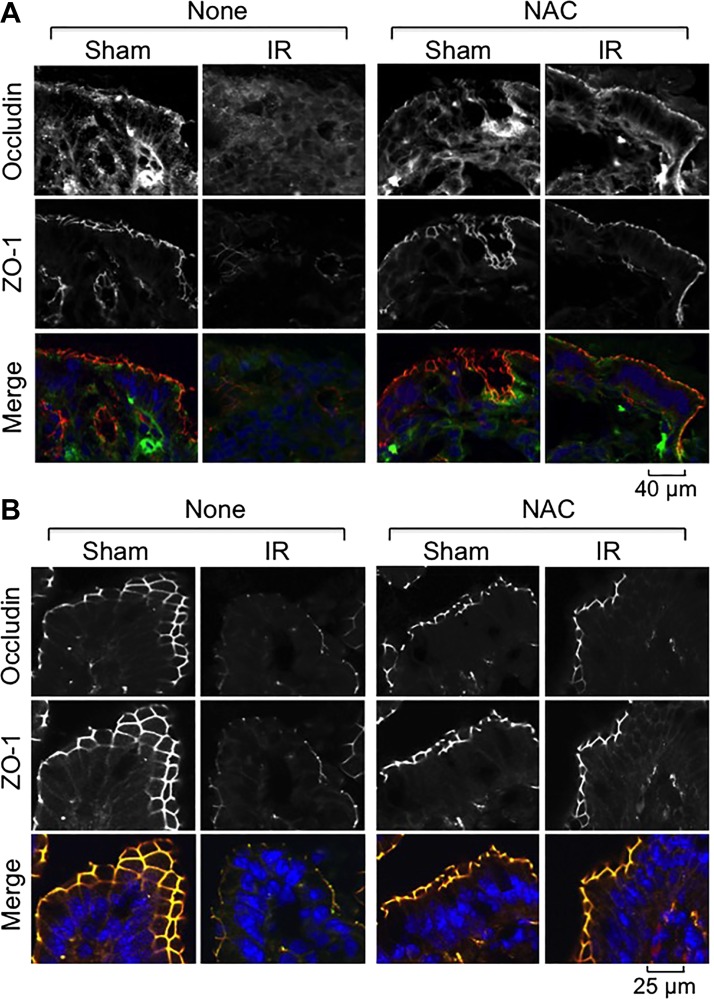
Fig. 6.
NAC feeding blocks γ-IR-induced redistribution of TJ proteins in mouse intestine. Mice were fed a liquid diet with or without 20 mM NAC for 5 days before a 4 Gy dose of TBI (IR) or sham treatment (Sham). At 2 h post-IR, cryosections of distal colon (A) and ileum (B) were stained for occludin (green) and ZO-1 (red) by the immunofluorescence method, and the nuclei were stained with Hoechst 33342 (blue).
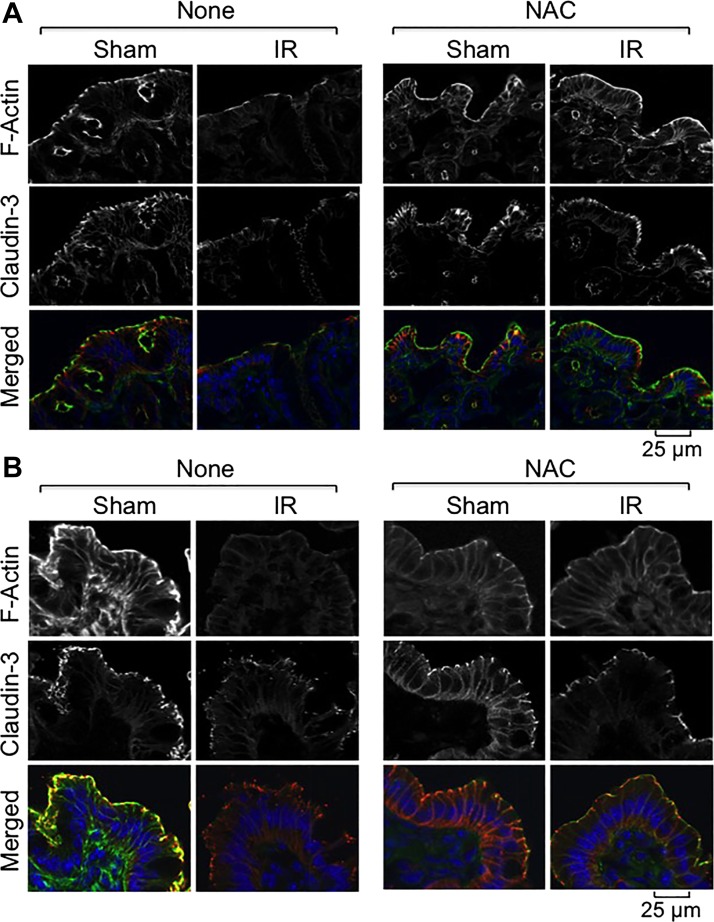
Fig. 7.
NAC feeding blocks γ-IR-induced redistribution of Cldn-3 and reorganization of actin cytoskeleton. Mice were fed a liquid diet with or without 20 mM NAC for 5 days before TBI (IR) or sham treatment (Sham). At 2 h post-IR, cryosections of distal colon (A) and ileum (B) were stained for F-actin (green) and Cldn-3 (red) by the immunofluorescence method, and the nuclei were stained with Hoechst 33342 (blue).
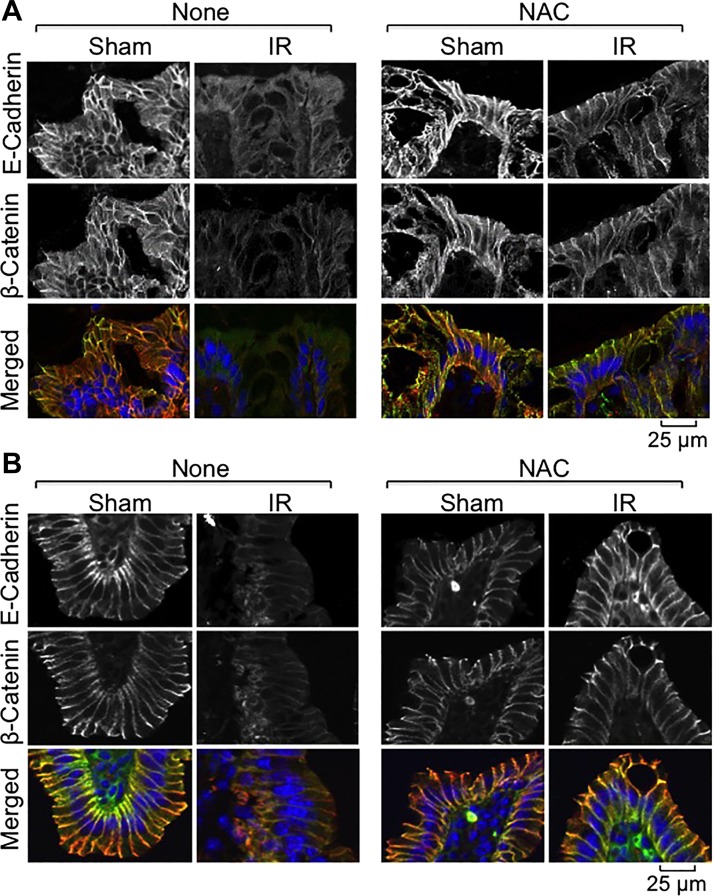
Fig. 8.
NAC feeding blocks γ-IR-induced redistribution of AJ proteins in mouse intestine. Mice were fed a liquid diet with or without 20 mM NAC for 5 days before TBI (IR) or sham treatment (Sham). At 2 h post-IR, cryosections of distal colon (A) and ileum (B) were stained for E-cadherin (green) and β-catenin (red) by the immunofluorescence method, and the nuclei were stained with Hoechst 33342 (blue).
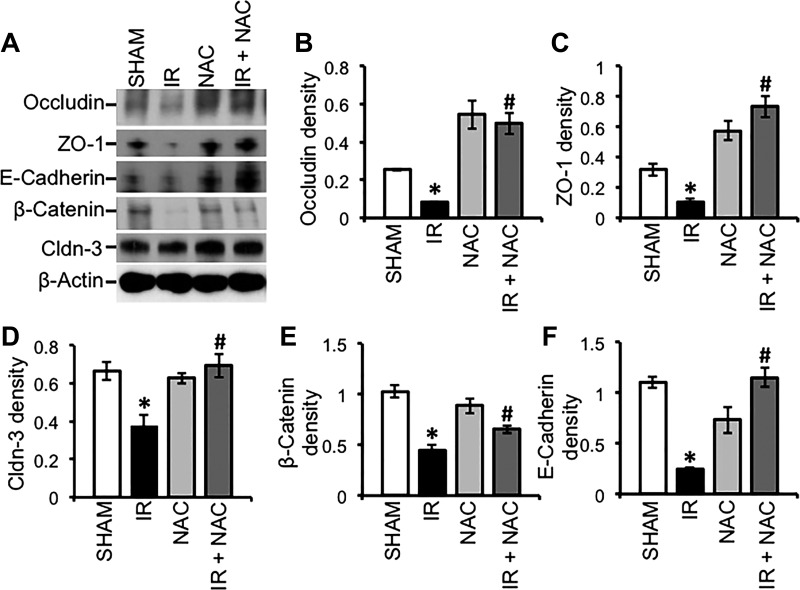
TJ and AJ protein complexes are attached to the actomyosin belt at the apical end of epithelial cells, and, therefore, TJ and AJ proteins are pulled down along with the actin-rich detergent-insoluble fractions (28). The level of TJ and AJ proteins in the detergent-insoluble fractions of epithelial cells is an excellent indicator of the integrity of TJ and AJ. Immunoblot analysis showed that radiation induced a loss of detergent-insoluble fractions of TJ and AJ proteins and that NAC treatment blocked this effect of radiation (Fig. 9A). Densitometric analysis of specific bands confirmed a significant reduction of the levels of detergent-insoluble fractions of occludin (Fig. 9B), ZO-1 (Fig. 9C), Cldn-3 (Fig. 9D), β-catenin (Fig. 9E), and E-cadherin (Fig. 9F). These results are consistent with the confocal microscopic data described above.
Fig. 9.
NAC feeding blocks γ-IR-induced depletion of AJ proteins in mouse intestine. Mice were fed a liquid diet with or without 20 mM NAC for 5 days before TBI (IR) or sham treatment (Sham). At 2 h post-IR, Triton-insoluble fractions were prepared from the distal colonic mucosa and immunoblotted for TJ and AJ proteins (A). Immunoblot bands for occludin (B), ZO-1 (C), Cldn-3 (D), E-cadherin (E), and β-catenin (F) were quantitated by densitometric analysis and normalized to band density of corresponding actin bands. Values are means ± SE (n = 3). *Significantly (P < 0.05) different from values for the corresponding control group. #Significantly (P < 0.05) different from values for the corresponding IR group.
γ-IR-induced increase in intestinal mucosal permeability is blocked by NAC feeding.
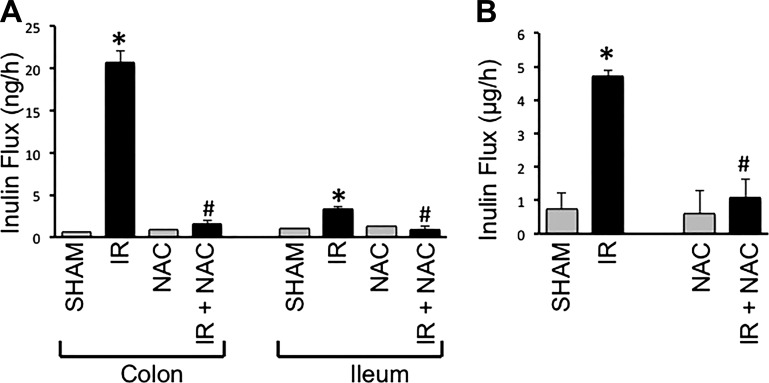
Measurement of vascular-to-luminal flux of FITC-inulin in vivo showed that γ-IR significantly increased mucosal permeability to FITC-inulin by nearly 30-fold in colon. NAC feeding effectively attenuated the radiation-induced increase in colonic mucosal permeability (Fig. 10A). γ-IR increased inulin permeability also in the ileum, although the effect was much less compared with that in the colon (Fig. 10A). The γ-IR-induced increase in inulin permeability in the ileum was also blocked by dietary supplementation of NAC. NAC, by itself, showed no significant influence on the mucosal permeability in colon or ileum.
Fig. 10.
NAC feeding blocks γ-IR-induced mucosal barrier dysfunction in mouse intestine. Adult mice were fed liquid diet with or without 20 mM NAC for 5 days before TBI (IR) or sham treatment (Sham). At 2 h post-IR, intestinal mucosal barrier function was evaluated by measuring vascular-to-luminal flux of fluorescein isothiocyanate (FITC)-inulin in vivo (A) and in vitro (B) as described in materials and methods. A: in vivo flux values are means ± SE (n = 6). *Significantly (P < 0.05) different from corresponding value for Sham-treated mice. #Significantly different from corresponding value for the IR group. B: at 3 h post-IR inulin absorption from the lumen of colonic loops was measured. Values for absorbed fluorescence are means ± SE (n = 5). *Significantly (P < 0.05) different from corresponding value for Sham-treated mice. #Significantly different from corresponding value for the IR group.
Analysis of luminal-to-mucosal permeability to FITC-inulin in vitro in the isolated loops of colon showed similar effects of radiation and NAC. The amount of inulin absorbed from the lumen was severalfold higher in the colonic loop prepared from γ-IR mice compared with that in loops prepared from Sham-treated mice (Fig. 10B). This effect of γ-radiation was absent in colonic loops prepared from NAC-fed IR mice.
NAC feeding prevents γ-IR-induced depletion of protein thiol oxidation.
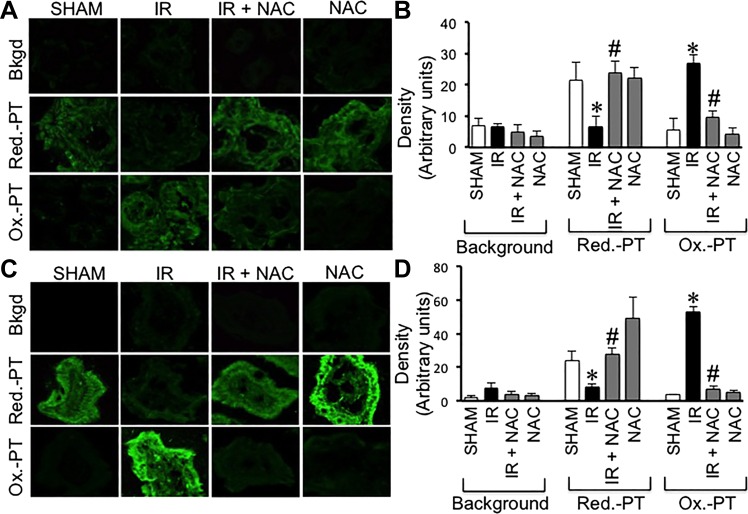
Our previous studies demonstrated that glutathione oxidation by hydrogen peroxide disrupts epithelial TJ leading to barrier dysfunction in the Caco-2 cell monolayers, the colonic carcinoma cell line (29). Therefore, it was hypothesized that one of the mechanisms involved in radiation-induced TJ disruption in mouse intestine involves protein thiol oxidation due to γ-radiation-induced oxidative stress. Results of our study show that IR induces a dramatic depletion of stain for reduced-protein thiols with a concomitant increase in the stain for oxidized protein thiols in both colon (Fig. 11A) and ileum (Fig. 11C). These effects of radiation were absent in the colon and ileum of NAC-fed mice. Densitometric analysis of fluorescence for reduced and oxidized protein thiols in samples from different mice confirmed that γ-IR significantly converts reduced protein thiols into oxidized protein thiols in the colon (Fig. 11B) and ileum (Fig. 11D) and that NAC feeding attenuates this effect of γ-IR.
Fig. 11.
NAC feeding blocks γ-IR-induced protein thiol oxidation in mouse intestine. Adult mice were fed liquid diet with or without 20 mM NAC for 5 days before TBI (IR) or sham treatment (Sham). At 2 h post-IR, the levels of reduced and oxidized protein thiols in distal colon (A and B) and ileum (C and D) were measured as described in materials and methods. Fluorescence images were collected by confocal microscopy (A and C). Fluorescence density was evaluated by using Image J software (B and D). Values are means ± SE (n = 5). *Significantly (P < 0.05) different from corresponding value for Sham-treated mice. #Significantly different from corresponding value for the IR group.
DISCUSSION
Gastrointestinal mucositis is a serious complication of radiotherapy and accidental radiation exposure (33). Ablation of crypt cell proliferation and apoptotic cell death in the small intestine are the well-known causes of GI-ARS (20). However, colon was found to be relatively resistant to radiation-induced mucosal damage (25). Therefore, most investigations have been directed to understand the mechanisms involved in the small intestinal tissue injury, and very little is known about the effect of radiation on colonic mucosal homeostasis. In this study, we show that colonic epithelial junctional complexes and barrier function are highly sensitive to γ-IR and that oxidative stress plays a key role in the mechanism of this acute colonic radiation subsyndrome.
Exposure of mice to low-dose γ-radiation (4 Gy, 76 cGy/min) impacts major effect on colonic epithelial TJ, AJ, and the actin cytoskeleton. Confocal immunofluorescence microscopy showed a rapid redistribution of occludin, ZO-1, and Cldn-3 from the intercellular junctions of colonic epithelium. These data indicate that the colonic epithelial TJ are disrupted by exposure to a lower dose of ionizing γ-radiation, that is less than the threshold of the hematopoietic acute radiation syndrome (generally 6 Gy). Disruption of TJ by 4 Gy dose suggests that colonic epithelial junctions are highly sensitive to radiation and are vulnerable at the radiation doses used for radiotherapy. Occludin and Cldn-3 are integral membrane proteins of TJ in different epithelia. The intracellular domains of these proteins interact with ZO-1, an adapter protein of TJ (1). A loss of interaction between these proteins and their redistribution from the junctions are critical indicators of TJ disruption. Disruption of TJ is not visible by gross microscopic imaging or analysis of crypt cell proliferation. It is likely that one of the initial events in radiation-induced injury to colon is disruption of epithelial TJ. Our data show that radiation effect on TJ occurred as early as 2 h post-IR and sustained for at least 24 h. This observation indicates that the effect of radiation on colonic epithelial TJ is rapid, and does not recover from it for at least 24 h. A breach in TJ integrity may lead to loss of barrier function and diffusion of endotoxins from the colonic lumen in the mucosal tissue. Disruption of TJ by 4 Gy dose of γ-radiation with a rapid time course that begins within 2 h of radiation exposure suggests that colonic epithelial junctions are highly sensitive to radiation and vulnerable at the radiation doses used for radiotherapy.
The low-dose sensitivity of TJ and the rapid time course of the disruption clearly distinguish this radiation syndrome from the hematopoietic-ARS or the GI-ARS that requires higher doses and several days to manifest. Previous studies by other laboratories have reported an increase in intestinal permeability by IR (15, 21). However, these studies measured small intestinal permeability at >3 days after IR at a dose of 10 Gy or higher. Under these conditions they also show significant inflammation in the mucosa, and, therefore, it is unclear whether the small intestinal permeability observed was an initial effect of IR or caused by the radiation-induced inflammation. Our study shows that colonic mucosal permeability is increased as early as 2 h after IR at a low dose of 2–4 Gy. No inflammation was evident in the colonic mucosa in this early post-IR stage. Our study also shows that the increase in small intestinal permeability was relatively low compared with radiation-induced colonic mucosal permeability, indicating that colonic mucosal barrier is highly sensitive to radiation and an initial event in the process of radiation damage. This is highly relevant, since colon is the primary source of toxins and pathogens in radiation-induced endotoxemia and bacteremia.
Our intent in this study was to demonstrate that low-dose IR is capable of causing barrier damage. We intentionally stayed away from the very high doses used in cancer therapy or radiation disasters. Instead, we used low doses (2–4 Gy) that can realistically occur as scatter from the focused pelvic IR used in the treatment of prostate, rectal, uterine, and other cancers. The novelty of our observation lies in that low doses of γ-IR elicit barrier damage in the colon that is considered relatively radioresistant compared with the small intestine. Based on these unique features, we propose to distinguish and recognize the acute colonic radiation syndrome as a subsyndrome of radiation damage to the gut.
γ-IR also disrupted AJ and the actin cytoskeleton in colon. E-cadherin and β-catenin are the major interacting proteins in the epithelial AJ. Redistribution of these proteins from the colonic epithelial junctions indicated that γ-IR at 4 Gy rapidly disrupts AJ. Although AJ is not a physical barrier for the diffusion of macromolecules, it has been shown to indirectly influence the integrity of TJ. Data also show that the organization of actin cytoskeleton was rapidly disrupted by γ-IR. Because the staining was targeted for filamentous actin, the loss of actin stain suggested a potential depolymerization of actin cytoskeleton. Organization of actin, particularly the formation of actomyosin belt near the apical end of cells, is crucial for the assembly and maintenance of TJ integrity, since TJ and AJ protein complexes are anchored to the actomyosin belt. Dissolution of actin architecture by cytochalasins has been shown to disrupt TJ and AJ (8). Therefore, disruption of actin cytoskeleton is likely an important mechanism involved in the radiation-induced TJ and AJ disruption.
TJ, AJ, and the actin cytoskeleton in the ileum were also rapidly (within 2 h) disrupted by γ-IR. The results of this study suggest that radiation-induced disruption of TJ and AJ is more severe in the colon, whereas the disruption of the actin cytoskeleton is more severe in the ileum. It is likely that γ-IR disrupts epithelial junctions in both colon and small intestine, but much more effective in the colonic epithelium. Disruption of colonic epithelium has higher pathophysiological significance in terms of endotoxin leak in mucosal tissue. Luminal bacteria are predominantly localized in the colon and, therefore, are the primary source of endotoxins. A breach in colonic barrier may lead to endotoxin flux in the mucosal tissue with a previously unrecognized rapid time course.
Radiotherapy is one of the treatment options for various malignancies. Despite much advancement in the treatment strategies, including intensity-modulated radiation therapy, it still causes damage to the normal tissues surrounding the tumors (11, 34). The gastrointestinal system is one of the major targets of radiotherapy causing diarrhea, malabsorption, and electrolyte imbalance (9, 37). Early disruption of TJ and barrier dysfunction may lead to endotoxin flux in the mucosa that may trigger diarrhea, malabsorption, and electrolyte imbalance. In the later stage, further damage to mucosa may lead to endotoxemia and eventually bacteremia in patients whose immunological defense is compromised by radiation and/or chemotherapy.
Oxidative stress is a leading mechanism in the pathogenesis of radiation injuries, including radiotherapy-mediated complications. Antioxidants, such as NAC, have been used to suppress oxidative stress and protection of various organs from acute radiation injury (23, 30). The results of our present study show that prophylactic feeding of 20 mM NAC in liquid diet blocks radiation-induced disruption of TJ and AJ, as indicated by the absence of radiation-induced redistribution of occludin, ZO-1, Cldn-3, E-cadherin, and β-catenin from the epithelial junctions in NAC-fed mice. This NAC dose is in the range of doses used previously by other investigators (6, 7, 19). Radiation caused a significant loss of F-actin stain and depletion of TJ and AJ proteins in the actin-rich detergent-insoluble fractions of colonic mucosa, indicating the loss of interaction of TJ and AJ proteins with the actin cytoskeleton. These data support the observation made by confocal microscopy and further confirm the disruption of TJ and AJ by radiation. NAC feeding blocked this effect of γ-IR, demonstrating that NAC feeding protects TJ and AJ from the radiation-induced damage. The detergent-insoluble fraction predominantly consists of actin cytoskeleton. In the epithelium with intact TJ and AJ, the actin cytoskeleton strongly binds to TJ and AJ protein complexes, and, hence, TJ and AJ proteins are recovered in the actin-rich detergent-insoluble fraction. Loss of detergent-insoluble fractions of TJ and AJ is an excellent indicator of TJ and AJ disruption, and to some extent this enables quantitative analysis of TJ and AJ disruption. Interestingly, NAC also blocks radiation-induced disruption of TJ, AJ, and the actin cytoskeleton in the ileum. However, in the ileum, prevention of actin reorganization by NAC was only partial, whereas a complete block was observed in the colon. All these data support the hypothesis that oxidative stress plays a crucial role in radiation-induced disruption of intestinal epithelial TJ, AJ, and the actin cytoskeleton and that the different gut tissues respond differentially to low-dose γ-IR.
In agreement with the disruption of TJ, the mucosal permeability to inulin was increased severalfold by γ-IR, confirming the colonic epithelial barrier dysfunction in IR mice; this effect was more severe in colon than in ileum. This observation is in contrast to a previous report that large intestine is resistant to acute ionizing radiation (25). It is likely that disruption of TJ and barrier dysfunction are the initial events of radiation injury to the gut, which then triggers further complications of GI-ARS. Sustained barrier dysfunction can lead to endotoxemia and bacterial translocation in the later stages of injury. This study also shows that NAC feeding almost completely blocks radiation-induced mucosal permeability to inulin, indicating the potential role of oxidative stress in radiation-induced barrier dysfunction. Protection by NAC indicates that radiation-induced TJ disruption and barrier dysfunction involve glutathione oxidation and protein thiol oxidation. Our previous studies have demonstrated that oxidative stress mediated by hydrogen peroxide disrupts intestinal epithelial TJ by glutathione oxidation-mediated inhibition of protein tyrosine phosphatases, leading to elevation of tyrosine-phosphorylated proteins (13, 18, 29). Tyrosine phosphorylation of occludin has been shown to result in loss of interaction with ZO-1, contributing to the loss of TJ integrity (13, 18). It is likely that a similar mechanism is involved in radiation-induced TJ disruption in mouse intestine.
NAC has been safely used in clinics to counteract acetaminophen and carbon monoxide poisoning for over four decades. Several clinically relevant effects have been attributed to NAC, such as reactive oxygen species scavenging activity, enhanced glutathione synthesis, and reduced inflammation. NAC has been reported to provide protection against the radiation-induced injury (30). It increases levels of radioprotective cytokines IL-1α, IL-1β, and IL-2 in IR human blood (3) and restores glutathione from radiation-induced depletion in liver, lung, and spleen (23). Our study shows that NAC feeding before IR prevents radiation-induced redistribution of TJ and AJ proteins from the epithelial junctions, indicating the preservation of TJ integrity from radiation. NAC feeding prevents radiation-induced reorganization of actin cytoskeleton and abrogates the γ-radiation-induced increase in mucosal permeability in both colon and ileum. These results indicate that oxidative stress mediates radiation-induced disruption of TJ and barrier dysfunction and that NAC prevents these radiation effects by restoring reduced glutathione and reduced protein thiol levels. Indeed, our data show that radiation depleted reduced protein thiols and elevated oxidized protein thiols in colon and ileum. NAC feeding blocked radiation-induced protein thiol oxidation. It is likely that radiation-induced oxidative stress induces protein thiol oxidation, leading to potential inhibition of protein tyrosine phosphatases and increase in tyrosine phosphorylation of TJ and AJ proteins.
In summary, this study demonstrates that γ-radiation, at a relatively low dose, rapidly disrupts TJ, AJ, and the actin cytoskeleton and causes barrier dysfunction in mouse colon in vivo. Radiation-induced disruption of epithelial junctions and barrier dysfunction is mediated by oxidative stress, which can be attenuated by NAC feeding before IR. This preclinical study implicates that NAC may serve as a potential prophylactic treatment to prevent radiotherapy-induced gastrointestinal mucositis and acute colonic radiation syndrome.
GRANTS
This study was supported by National Institutes of Health Grants DK-55532 (R. Rao) and AA-12307 (R. Rao), AI-080405 (G. Tigyi), and Grant I01-BX-007080 from the Biomedical Laboratory Research & Development Service of the Veterans Affairs Office of Research and Development (G. Tigyi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.K.S., R.G., B.M., A.S.M., N.Y., E.S., A.B., S.C.L., and G.J.T. performed experiments; P.K.S. analyzed data; P.K.S. and R.K.R. interpreted results of experiments; P.K.S. prepared figures; P.K.S. drafted manuscript; P.K.S., R.G., B.M., A.S.M., N.Y., E.S., A.B., S.C.L., G.J.T., and R.K.R. approved final version of manuscript; R.K.R. conception and design of research; R.K.R. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Terry Costello for technical help with tail vein injection and cardiac puncture.
REFERENCES
- 1.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med 6: 593–597, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Baier JE, Neumann HA, Moeller T, Kissler M, Borchardt D, Ricken D. Radiation protection through cytokine release by N-acetylcysteine. Strahlenther Onkol 172: 91–98, 1996. [PubMed] [Google Scholar]
- 4.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol 192: 907–917, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron S, Schwartz A, Sultan S, Schaefer IM, Hermann R, Rave-Frank M, Hess CF, Christiansen H, Ramadori G. Radiation-induced damage in different segments of the rat intestine after external beam irradiation of the liver. Exp Mol Pathol 92: 243–258, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Cao JJ, Picklo MJ. N-acetylcysteine supplementation decreases osteoclast differentiation and increases bone mass in mice fed a high-fat diet. J Nutr 144: 289–296, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Chan A, Shea TB. Effects of dietary supplementation with N-acetyl cysteine, acetyl-l-carnitine and S-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human ApoE4. Neuromolecular Med 9: 264–269, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Citalan-Madrid AF, Garcia-Ponce A, Vargas-Robles H, Betanzos A, Schnoor M. Small GTPases of the Ras superfamily regulate intestinal epithelial homeostasis and barrier function via common and unique mechanisms. Tissue Barriers 1: e26938, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damman CJ, Surawicz CM. The gut microbiota: a microbial arsenal protecting us from infectious and radiation-induced diarrhea. Gastroenterology 136: 722–724, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Driak D, Osterreicher J, Vavrova J, Rehakova Z, Vilasova Z. Morphological changes of rat jejunum after whole body gamma-irradiation and their impact in biodosimetry. Physiol Res 57: 475–479, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Drodge CS, Boychak O, Patel S, Usmani N, Amanie J, Parliament MB, Murtha A, Field C, Ghosh S, Pervez N. Acute toxicity of hypofractionated intensity-modulated radiotherapy for prostate cancer. Curr Oncol 22: e76–e84, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois A, Walker RI. Prospects for management of gastrointestinal injury associated with the acute radiation syndrome. Gastroenterology 95: 500–507, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem 284: 1559–1569, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman SL, Hossain M, MacNaughton WK. Radiation-induced acute intestinal inflammation differs following total-body versus abdominopelvic irradiation in the ferret. Int J Radiat Biol 77: 389–395, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Garg S, Wang W, Prabath BG, Boerma M, Wang J, Zhou D, Hauer-Jensen M. Bone marrow transplantation helps restore the intestinal mucosal barrier after total body irradiation in mice. Radiat Res 181: 229–239, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harb AH, Abou Fadel C, Sharara AI. Radiation enteritis. Curr Gastroenterol Rep 16: 383, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki T, Terrill J, Shavlakadze T, Grounds MD, Arthur PG. Visualizing and quantifying oxidized protein thiols in tissue sections: a comparison of dystrophic mdx and normal skeletal mouse muscles. Free Radic Biol Med 65: 1408–1416, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun 302: 324–329, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol 532: 759–769, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macia IGM, Lucas Calduch A, Lopez EC. Radiobiology of the acute radiation syndrome. Rep Pract Oncol Radiother 16: 123–130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monti P, Wysocki J, van der Meeren A, Griffiths NM. The contribution of radiation-induced injury to the gastrointestinal tract in the development of multi-organ dysfunction syndrome or failure. BJR Suppl 27: 89–94, 2005. [Google Scholar]
- 22.Naftalin R. Alterations in colonic barrier function caused by a low sodium diet or ionizing radiation. J Environ Pathol Toxicol Oncol 23: 79–97, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Neal R, Matthews RH, Lutz P, Ercal N. Antioxidant role of N-acetyl cysteine isomers following high dose irradiation. Free Radic Biol Med 34: 689–695, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Nejdfors P, Ekelund M, Westrom BR, Willen R, Jeppsson B. Intestinal permeability in humans is increased after radiation therapy. Dis Colon Rectum 43: 1582–1588, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Potten CS, Grant HK. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer 78: 993–1003, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci 13: 7210–7226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao RK, Baker RD, Baker SS, Gupta A, Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol Gastrointest Liver Physiol 273: G812–G823, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Rao RK, Basuroy S, Rao VU, Karnaky KJ Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368: 471–481, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao RK, Li L, Baker RD, Baker SS, Gupta A. Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 279: G332–G340, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Reliene R, Pollard JM, Sobol Z, Trouiller B, Gatti RA, Schiestl RH. N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals. Mutat Res 665: 37–43, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Samak G, Chaudhry KK, Gangwar R, Narayanan D, Jaggar JH, Rao R. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem J 465: 503–515, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirazi A, Mihandoost E, Mahdavi SR, Mohseni M. Radio-protective role of antioxidant agents. Oncol Rev 6: e16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somosy Z, Horvath G, Telbisz A, Rez G, Palfia Z. Morphological aspects of ionizing radiation response of small intestine. Micron 33: 167–178, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Thomas GM. Concurrent chemotherapy and radiation for locally advanced cervical cancer: the new standard of care. Semin Radiat Oncol 10: 44–50, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Van Itallie CM, Anderson JM. Claudin interactions in and out of the tight junction. Tissue Barriers 1: e25247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang A, Ling Z, Yang Z, Kiela PR, Wang T, Wang C, Cao L, Geng F, Shen M, Ran X, Su Y, Cheng T, Wang J. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study. PLoS One 10: e0126312, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]