Abstract
In liver injury, recruitment of bone marrow (BM) progenitors of liver sinusoidal endothelial cells (sprocs) is necessary for normal liver regeneration. Hepatic vascular endothelial growth factor (VEGF) is a central regulator of the recruitment process. We examine whether stromal cell-derived factor 1 [sdf1, or CXC ligand 12 (CXCL12)] acts downstream from VEGF to mediate recruitment of BM sprocs, what the sdf1 receptor type [CXC receptor (CXCR)-4 or CXCR7] is on sprocs, and whether sdf1 signaling is required for normal liver regeneration. Studies were performed in the rat partial hepatectomy model. Tracking studies of BM sprocs were performed in wild-type Lewis rats that had undergone BM transplantation from transgenic enhanced green fluorescent protein-positive Lewis rats. Knockdown studies were performed using antisense oligonucleotides (ASOs). Expression of sdf1 doubles in liver and liver sinusoidal endothelial cells (LSECs) after partial hepatectomy. Upregulation of sdf1 expression increases proliferation of sprocs in the BM, mobilization of CXCR7+ BM sprocs to the circulation, and engraftment of CXCR7+ BM sprocs in the liver and promotes liver regeneration. Knockdown of hepatic VEGF with ASOs decreases hepatic sdf1 expression and plasma sdf1 levels. When the effect of VEGF knockdown on sdf1 is offset by infusion of sdf1, VEGF knockdown-induced impairment of BM sproc recruitment after partial hepatectomy is completely attenuated and liver regeneration is normalized. These data demonstrate that the VEGF-sdf1 pathway regulates recruitment of CXCR7+ BM sprocs to the hepatic sinusoid after partial hepatectomy and is required for normal liver regeneration.
Keywords: CXC chemokine receptor 7, stromal cell-derived factor 1, endothelial cells, stem cells, liver regeneration
a long-standing hypothesis states that liver sinusoidal endothelial cells (LSECs) are an important driver of liver regeneration (7, 15, 24, 25, 34, 40). More recently, our laboratory refined the hypothesis, i.e., that it is not mature LSECs that drive liver regeneration but, rather, that the cells among the LSECs driving this process are the engrafted bone marrow (BM)-derived progenitors of the LSECs (BM sprocs) (5, 43, 44), derived from liver sinusoidal endothelial progenitor cells. It was recently reported that CXC receptor (CXCR) 7-positive LSECs drive liver regeneration (8); the nature of these LSECs was not examined (8), but the current study demonstrates that CXCR7 is expressed on sprocs, not on mature LSECs.
BM sprocs are recruited to the liver after partial hepatectomy and liver injury, and the recruited sprocs replace damaged LSECs (5, 18, 43, 44). These BM sprocs are the major source of increased LSEC hepatocyte growth factor (HGF) and promote hepatocyte proliferation during liver regeneration (43, 44). Recruitment of BM sprocs is necessary for normal liver regeneration: BM suppression causes prolonged suppression of hepatocyte proliferation and restoration of liver weight after partial hepatectomy, whereas infusion of BM sprocs in BM-suppressed rats completely normalizes liver regeneration (44).
Vascular endothelial growth factor (VEGF) increases after partial hepatectomy and liver injury (10, 20, 31, 35, 40, 43) and is the central regulator of BM sproc mobilization to the circulation, BM sproc recruitment to the liver, engraftment of BM sprocs in the liver, and differentiation of BM sprocs to LSECs (43). However, VEGF is a growth factor, not a chemokine. This suggests that VEGF must act through a downstream chemokine to recruit BM sprocs. Stromal cell-derived factor 1 [sdf1, or CXC ligand 12 (CXCL12)] is a chemokine that acts through its receptors CXCR4 and CXCR7. The VEGF-sdf1 pathway can recruit BM cells to the vasculature (16, 21). In the vasculature, sdf1 is found in endothelial cells (1, 4, 23, 27, 42) and perivascular mesenchymal cells, notably in pericytes (4, 37), arterial smooth muscle cells (1), and astrocytes (19, 46).
Data presented here demonstrate that BM sprocs are recruited to the liver after partial hepatectomy by the VEGF-sdf1 pathway and that recruitment of CXCR7+ BM sprocs by the VEGF-sdf1 pathway promotes liver regeneration.
METHODS
Materials.
All chemicals were obtained from Sigma-Aldrich Chemical (St. Louis, MO) unless stated otherwise. Antibodies were obtained as follows: rabbit anti-rat CXCR4 from Abcam (San Francisco, CA); TRITC-conjugated anti-proliferating cell nuclear antigen (PCNA), FITC-conjugated donkey anti-mouse IgG, goat anti-rabbit IgG-peridinin chlorophyll (PerCP), and TRITC-conjugated donkey anti-mouse IgG from Santa Cruz Biotechnology (Santa Cruz, CA); FITC-conjugated mouse anti-rat CD45, phycoerythrin (PE)-conjugated mouse anti-rat CD31, and PE-conjugated goat anti-mouse IgG from BD Biosciences (San Diego, CA); and allophycocyanin (APC)-conjugated mouse anti-CXCR7/RDC1 from R & D Systems (Minneapolis, MN). The CD133 cell isolation kit, the anti-FITC MultiSort Kit, and FcR blocking reagent were obtained from Miltenyi Biotec (Auburn, CA). Formaldehyde-treated serum albumin was a kind gift from Dr. Bård Smedsrød (University of Trømso, Norway). VEGF-A and sdf1 were knocked down in vivo using antisense oligonucleotides (ASOs). VEGF ASO, sdf1 ASO, and scrambled ASO control were a kind gift from Ionis Pharmaceuticals (Carlsbad, CA). Knockdown of sdf1 and VEGF was achieved by injection of ASO (20 mg/kg ip) twice weekly for 4 wk.
Animal studies.
Lewis rats were obtained from Harlan (Placentia, CA). Breeding pairs of Lew-Tg(CAG-EGFP)ys rats were obtained from the National Institutes of Health Rat Resource and Research Center at the University of Missouri. Male rats were used for the experiments. Rats were kept in conventional housing for rats, consisting of Allentown polycarbonate rat cages with filter tops and Sani-Chips bedding (wood product), in a 12:12-h light-dark cycle (lights on from 6 AM to 6 PM) at room temperature (21–23°C), with 5-μm-filtered water delivered to cages via an Edstrom automatic watering valve. Purina Lab Diet 5001 and water were provided ad libitum. Rats that showed distress postoperatively, based on activity level, behavior, appearance, reduced intake of food or water, or respiratory distress, were euthanized.
Partial (70%) hepatectomy was performed under general anesthesia with ketamine-xylazine (80–90 mg/kg ip). Buprenorphine SR (1 mg/kg) was used for analgesia.
Daily injections of sdf1 (100 ng/kg) via the tail were started 6 h before partial hepatectomy and continued through day 4 after partial hepatectomy. Plasma concentrations of sdf1 were measured on day 5, i.e., 24 h after the day 4 injection.
All protocols were reviewed and approved by the Animal Care and Use Committee at the University of Southern California to ensure ethical and humane treatment of the animals. This study followed the guidelines outlined in the Office of Laboratory Animal Welfare “Public Health Service Policy on Humane Care and Use of Laboratory Animals” (2015).
Liver cells.
LSECs were isolated by collagenase perfusion, iodixanol density gradient centrifugation, and centrifugal elutriation, as previously described (6, 38). Yields averaged 8.4 × 107 cells per normal rat liver with >95% viability. Purity of the cells was 99%, as determined by uptake of formaldehyde-treated serum albumin, a function specific to LSECs (2, 3, 11), and the presence of fenestrae organized in sieve plates. Uptake studies were performed as previously described (45).
The Nonparenchymal Liver Cell Core of the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases provided the rat hepatic stellate cells used for the sdf1 protein expression studies. The Cell Culture Core of the University of Southern California Research Center for Liver Diseases isolated rat hepatocytes as previously described (32).
Isolation of sprocs.
Sprocs in the BM and circulation were isolated by double-label immunomagnetic selection for CD133 and CD45 followed by fluorescein-activated cell sorting (FACS) for CD31 or by CD133 immunomagnetic selection followed by FACS for CD45 and CD31 (44). For double-label immunomagnetic selection, BM and circulating mononuclear cells were incubated with anti-CD45 FITC antibody (1:10 dilution) for 30 min at 4°C and then with anti-FITC microbeads (20 μl of beads for up to 107 cells) for 30 min at 4°C. After magnetic selection using an autoMACS Pro separator (Miltenyi Biotec), release reagent was used to clip off the magnetic bead. CD45+ cells were incubated with anti-CD133 microbeads (100 μl of beads for up to 108 cells) for 30 min at 4°C.
To investigate BM sproc proliferation, CD133+CD45+ BM cells were isolated by immunomagnetic selection, permeabilized, and incubated with TRITC-conjugated anti-PCNA antibody (1:100 dilution) and PE-conjugated anti-CD31 antibody (1:100 dilution) at 4°C for 30 min. The percentage of PCNA+ CD133+CD45+CD31+ cells was determined using a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed by CellQuest Pro software.
Resident sprocs are present in the same elutriation fraction as LSECs, i.e., at 27.6 ml/min at 2,500 rpm of the first elutriation step (44). Thus resident sprocs were obtained by isolation of LSECs as described above and selection for CD133+ cells by immunomagnetic separation with the autoMACS Pro separator.
BM transplantation.
Cells were obtained from the BM of one tibia and femur from the Lew-Tg(CAG-EGFP)ys donor rat. Recipients underwent 1,000-cGy total body irradiation and were injected via the tail vein with 5 × 107 BM cells. Rats received oxytetracycline (200 mg/ml) diluted 1:1,000 in the drinking water starting 2 days before irradiation and continuing until 1 wk after irradiation. BM was allowed to engraft for 2 mo before use.
Real-time PCR.
Total RNA of LSECs was prepared using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. cDNA was prepared using the RT2 First Strand Kit (Qiagen). Real-time PCR was performed using Real-Time RT2qPCR Primer Assays (Qiagen) and the StepOne Plus real-time PCR system (Applied Biosystems, Foster City, CA), with β-actin as a reference control. All primers were purchased from SABiosciences.
Immunoblot.
Frozen liver tissue (∼40 mg) was homogenized in triple lysis buffer [50 mM Tris-Cl, pH 8.0, 150 mM sodium chloride, 0.02% (wt/vol) sodium azide, 0.1% (wt/vol) SDS, 1% (vol/vol) NP-40, and 0.5% (wt/vol) sodium deoxycholate plus protease inhibitors]. Protein concentrations were quantified with DC reagent (Bio-Rad Laboratories, Richmond, CA). Total protein (100 μg) was separated by acrylamide gel and transferred to nitrocellulose. Immunoblotting was performed with a rabbit anti-sdf1 antibody (Cell Signaling, Boston, MA) and a secondary goat anti-rabbit antibody conjugated with IRDye 700CW (catalog no. 926-3221, LI-COR Biosciences, Lincoln, NE). Bands detected at 9 kDa were quantified using the Odyssey infrared imaging system (LI-COR Biosciences). Membranes were stripped and reblotted with β-actin antibody (Santa Cruz Biotechnology). Values were normalized to β-actin.
Flow cytometry.
To examine sdf1 receptors in circulating BM sprocs, cells were isolated from blood from Lewis rats by immunomagnetic selection for CD133 at 6 h after partial hepatectomy using the autoMACs Pro separator. Cell suspensions were preblocked with FcR blocking reagent and incubated for 60 min with antibodies recognizing rat CD45, CD31, CXCR4, and CXCR7: FITC-mouse anti-rat CD45, PE-mouse anti-rat CD31, rabbit anti-rat CXCR4, and APC-mouse anti-CXCR7/RDC1. After 60 min of incubation with a secondary antibody against CXCR4, goat anti-rabbit IgG-PerCP, cells were examined using the FACSCalibur flow cytometer. CXCR4 and CXCR7 expression of circulating BM sprocs was analyzed using CellQuest Pro software.
To examine sdf1 receptor expression in the liver in engrafted BM sprocs, BM-derived LSECs, resident sprocs, and resident LSECs after partial hepatectomy, LSECs were isolated from rats that had undergone BM transplantation with BM from Lew-Tg(CAG-EGFP)ys rats 2 mo beforehand and had undergone partial hepatectomy 24 h earlier. Cell suspensions were preblocked with FcR blocking reagent. CD133+ sprocs and CD133−LSECs were obtained by immunomagnetic selection for CD133 using the autoMACS Pro spearator and incubated for 60 min with rabbit anti-rat CXCR4 and APC-mouse anti-CXCR7/RDC1 (R & D Systems) and then for 60 min with a secondary antibody against CXCR4, goat anti-rabbit IgG-PerCP. CXCR4 and CXCR7 expression of green fluorescent protein (GFP)-positive and GFP− cells was analyzed by CellQuest Pro software.
Isotype control antibodies were used to determine appropriate gates, voltages, and compensation required for multivariate flow cytometry.
Statistics.
All studies were performed with the minimum number required to obtain valid results (per the 2015 Public Health Service Policy), or n ≥ 3. Values are reported as means ± SE. Data were compared by ANOVA with replication or by two-sided Student's t-test using Microsoft Excel with StatPlus. If ANOVA was significant, a posteriori comparison of individual points was performed by least significant difference. P < 0.05 was considered significant.
RESULTS
Expression of sdf1 after partial hepatectomy.
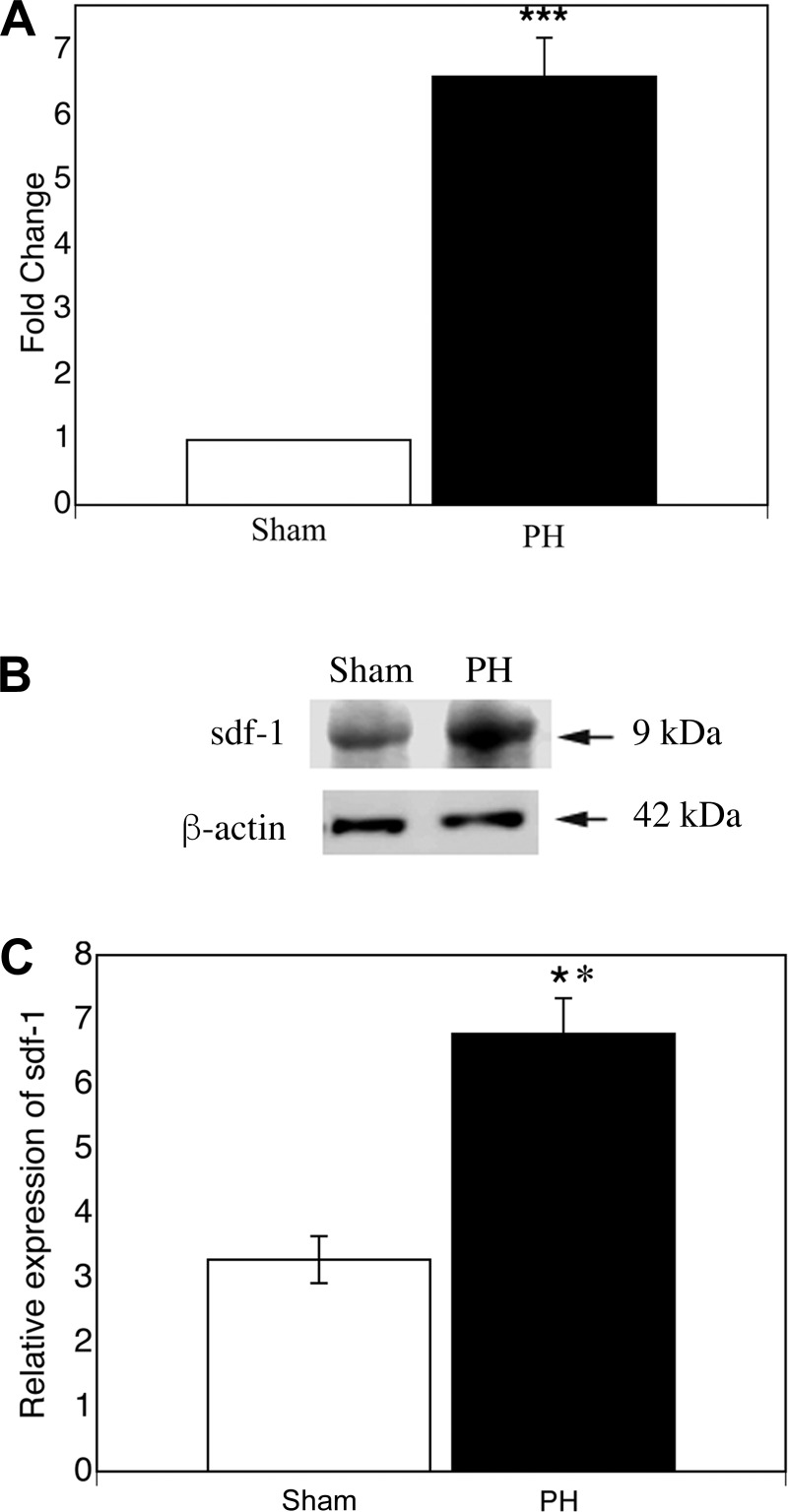
If sdf1 is necessary for BM sproc recruitment after partial hepatectomy, then hepatic sdf1 should increase after partial hepatectomy. Expression of sdf1 was examined at 72 h, the time of peak proliferation of the LSEC fraction and, thus, of proliferating BM sprocs (44). At 72 h after partial hepatectomy, hepatic sdf1 gene expression increased more than sixfold (P < 0.0005) and protein expression increased twofold (P < 0.01; Fig. 1). Expression of sdf1 was examined in freshly isolated LSECs, hepatocytes, and hepatic stellate cells after sham surgery and 72 h after partial hepatectomy to determine the cellular source of the increase along the sinusoid. Protein expression of sdf1 was detectable in LSECs from sham-operated rats and increased 2.3-fold after partial hepatectomy (P < 0.0005); sdf1 was undetectable in hepatic stellate cells and hepatocytes from sham-operated rats and rats subjected to partial hepatectomy (Fig. 2).
Fig. 1.
Partial hepatectomy (PH) increases hepatic stromal cell-derived factor 1 (sdf1) gene and protein expression. Hepatic sdf1 expression was examined 72 h after partial hepatectomy. A: RT-PCR of sdf1. B and C: immunoblot and quantification of immunoblot for sdf1. Values are means ± SE (n = 3). **P < 0.01, ***P < 0.0005.
Fig. 2.
Sinusoidal origin of increased sdf1 after partial hepatectomy. Sdf1 protein expression increases 2.3-fold in liver sinusoidal endothelial cells (LSECs) 72 h after partial hepatectomy (P < 0.0005) but remains undetectable in hepatocytes and hepatic stellate cells (n = 3). Lane a, hepatic stellate cells after sham surgery; lane b, hepatic stellate cells after partial hepatectomy; lane c, hepatocytes after sham surgery; lane d, hepatocytes after partial hepatectomy; lane e, LSECs after sham surgery; lane f, LSECs after partial hepatectomy.
BM sproc recruitment by sdf1.
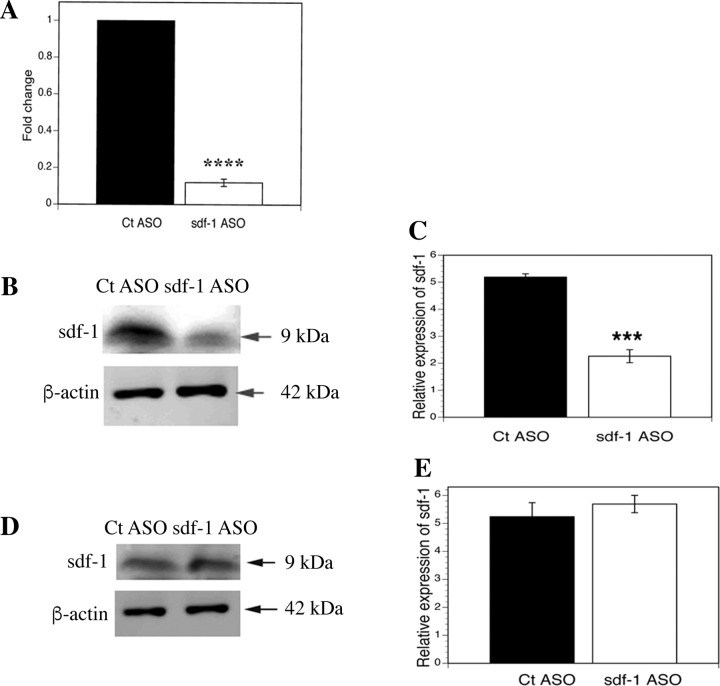
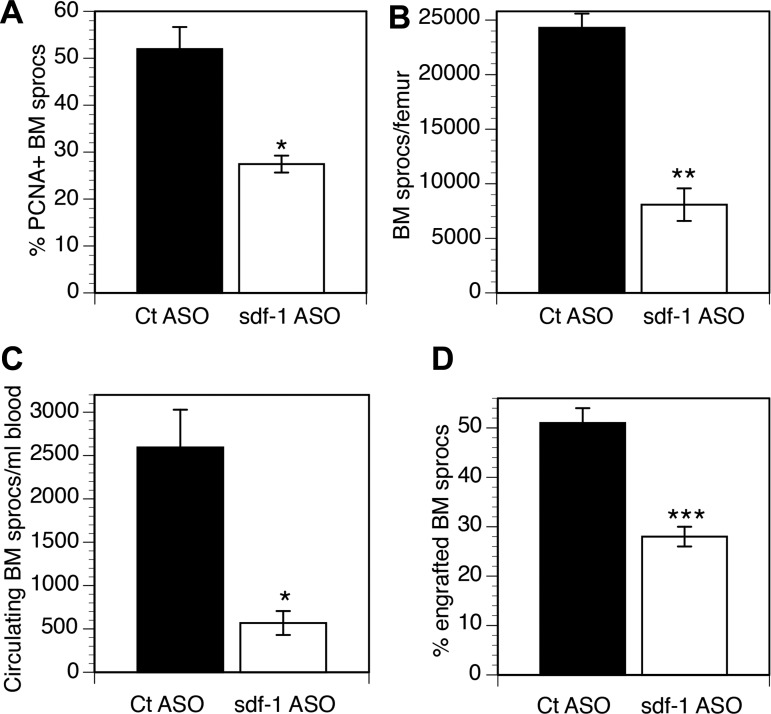
Sdf1 ASO decreased hepatic sdf1 gene expression by 88% (P < 0.0001) and protein expression by 50% (P < 0.0005) but did not decrease BM sdf1 expression (Fig. 3). [ASO biodistribution is greatest to the LSECs and Kupffer cells in the liver and to the kidney (12, 13, 30, 47).] The lack of effect on BM sdf1 is relevant, as sdf1 retains cells in the BM, and decreased sdf1 in the BM would promote mobilization to the circulation. In rats treated with sdf1 ASO for 4 wk followed by partial hepatectomy, the three phases of BM sproc recruitment were examined on day 3 after partial hepatectomy: 1) absolute number and proliferation of sprocs in the BM, 2) mobilization of BM sprocs to the circulation, and 3) engraftment of BM sprocs in the liver (Fig. 4, A–C). Compared with a scrambled ASO control, sdf1 knockdown decreased proliferation of sprocs in the BM after partial hepatectomy by 47% (P < 0.01) and decreased the number of sprocs in the BM by 67% (P = 0.001). Mobilization of BM sprocs to the circulation was decreased by 78% (P = 0.01). Similar to the effect of VEGF ASO in models of partial hepatectomy or toxin-induced injury, knockdown by sdf1 ASO brings the number of sprocs proliferating in the BM and circulating BM sprocs down to the baseline levels seen in uninjured control rats (43).
Fig. 3.
Selectivity of knockdown by sdf1 antisense oligonucleotide (ASO). A: sdf1 gene expression in liver after scrambled control (Ct) ASO and sdf1 ASO. B and C: sdf1 immunoblot and quantification of immunoblot in liver tissue after control and sdf1 ASO. D and E: sdf1 immunoblot and quantification of immunoblot in bone marrow (BM) after control and sdf1 ASO. Sdf1 ASO decreases hepatic sdf1 gene expression by 88% (****P < 0.0001) and sdf1 protein expression by 50% (***P < 0.0005) compared with scrambled ASO control but does not affect BM expression of sdf1 protein. Values are means ± SE (n = 3).
Fig. 4.
Knockdown of sdf1 attenuates increased hepatic recruitment and engraftment of BM sprocs after partial hepatectomy. Compared with scrambled ASO controls on day 3 after partial hepatectomy, sdf1 ASO decreases percentage of proliferating [proliferating cell nuclear antigen-positive (PCNA+)] sprocs in BM (A), number of BM sprocs per femur (B), number of sprocs mobilized to the circulation (C), and percentage of LSECs derived from engrafted BM sprocs (D). Values are means ± S (n = 3). *P = 0.01, **P < 0.001, ***P < 0.005.
To examine engraftment, wild-type Lewis rats were transplanted with BM from Lew-Tg(CAG-EGFP)ys donor rats. The enhanced GFP (EGFP) is expressed by a transgene in all transplanted cells, so that these cells can be tracked. At 2 mo after BM transplantation, rats were treated for 4 wk with sdf1 ASO and then subjected to partial hepatectomy. Knockdown of sdf1 decreased the percentage of LSECs derived from engrafted BM sprocs on day 3 after partial hepatectomy from 51% to 28% (45% decrease; P < 0.005; Fig. 4D). Earlier studies ruled out fusion of engrafted BM sprocs with LSECs (18) and found that only 1% of LSECs are GFP+ in control rats (44).
Functional significance of sdf1-mediated recruitment of BM sprocs.
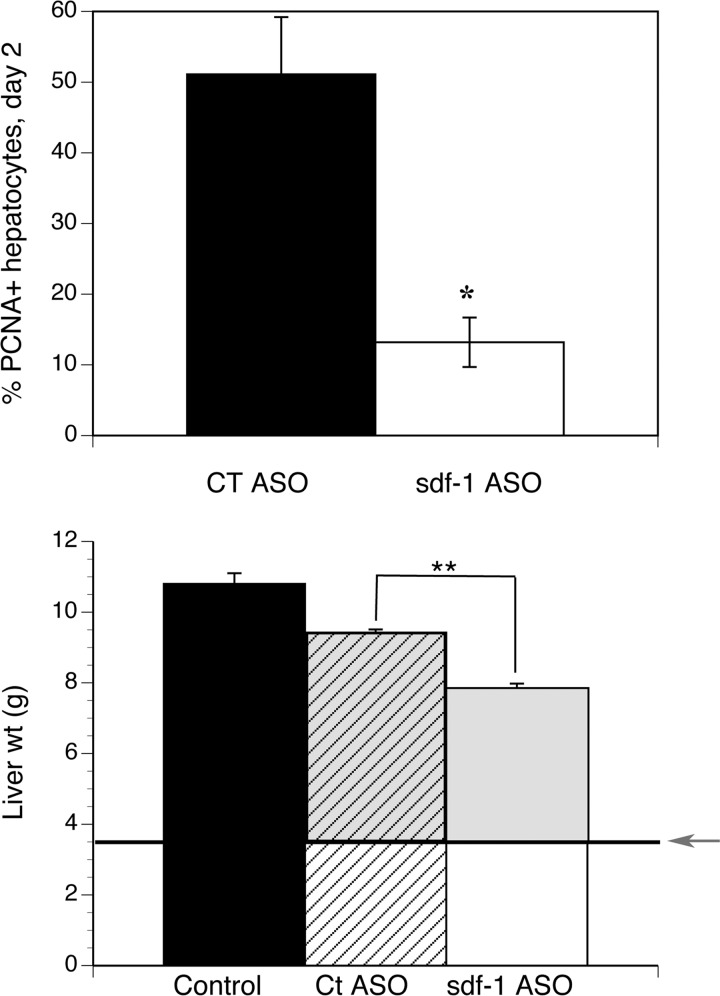
If sdf1-mediated recruitment of BM sprocs promotes liver regeneration, then liver regeneration should be impaired by sdf1 knockdown. In rats, the second round of hepatocyte proliferation occurs on day 2 (28). On day 2 after partial hepatectomy, hepatocyte proliferation, measured as the number of PCNA+ hepatocytes per high-power field, was 51% in control ASO-treated vs. 13% in sdf1 ASO-treated rats (P < 0.025; Fig. 5). Hematoxylin-eosin staining showed that sdf1 ASO did not cause any apparent injury compared with control ASO on day 2 after partial hepatectomy (data not shown). On day 5, when the partial hepatectomy control rats had regained 85% of liver weight, there was a significant decrease in liver weight in the sdf1 ASO-treated rats compared with controls (P < 0.001), and the amount of liver weight regained was 24% less than in controls (P < 0.001).
Fig. 5.
Sdf1 ASO decreases liver regeneration. Top: hepatocyte proliferation (%PCNA+ cells) on day 2 after partial hepatectomy. Bottom: liver weight on day 5. Arrow indicates approximate liver weight after partial hepatectomy, and gray area indicates liver weight regained after partial hepatectomy. Control, liver weights of littermates. Compared with scrambled ASO controls, sdf1 ASO decreases the percentage of PCNA+ hepatocytes (*P < 0.025), decreases liver weight (**P < 0.001), and reduces restoration of liver weight (gray shaded area) by 24% (P < 0.001). Values are means ± SE (n = 3).
Expression of sdf1 receptor.
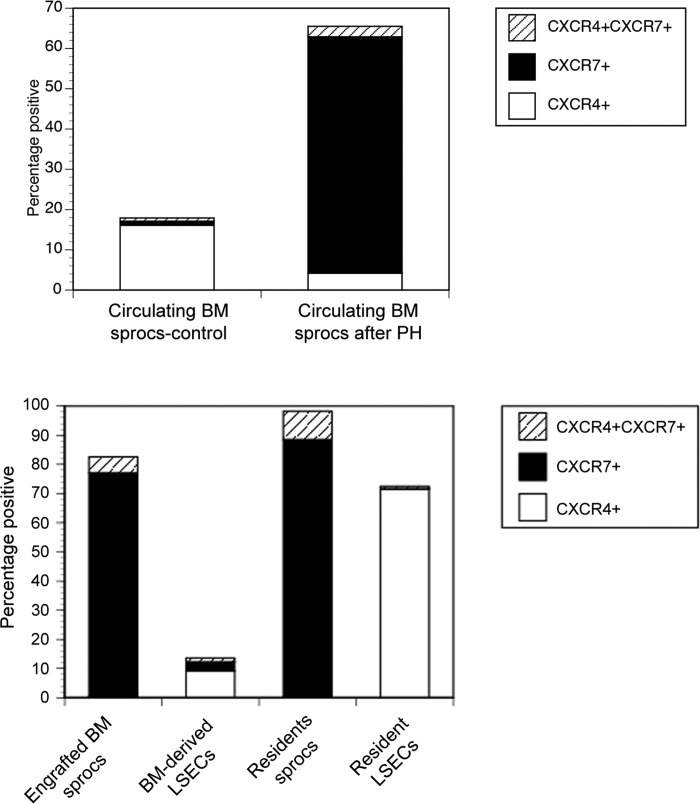
Sdf1 has two known receptors, CXCR4 and CXCR7. Rats transplanted with a transgenic EGFP+ BM underwent partial hepatectomy. Figure 6 (top) examines circulating BM sprocs mobilized to the circulation. Only 1% of circulating BM express CXCR7 in control rats, whereas 59% of circulating BM sprocs are CXCR7+ after partial hepatectomy, indicating that sdf1 mobilizes CXCR7+ BM sprocs after partial hepatectomy.
Fig. 6.
CXCR4, CXCR7, and dual CXCR4/CXCR7 expression determined by flow cytometry on circulating BM sprocs and on BM and resident sprocs and LSECs. Top: BM sprocs in the circulation after partial hepatectomy were isolated and examined for expression of sdf1 receptors (n = 3). y-Axis indicates percentage of cells positive for each of the receptors, i.e., CXCR4, CXCR7, or both. P < 0.0005 for expression of CXCR7 after partial hepatectomy compared with control rats (n = 3). Bottom: LSECs were isolated after partial hepatectomy and separated by immunomagnetic selection into sprocs (CD133+) and LSECs (CD133−) and separated by flow cytometry into CXCR4+ or CXCR7+ BM-derived (GFP+) or resident (GFP−) cells. P = 3.91373E-09 by ANOVA for CXCR7 expression. P < 0.001 by least significant difference for CXCR7 expression on either group of sprocs compared with resident LSECs or BM-derived LSECs. P = 1.4354E-09 by ANOVA for CXCR4 expression and P < 0.001 by least significant difference for CXCR4 expression on resident LSECs compared with the other 3 populations (n = 3).
Figure 6 (bottom) examines LSECs obtained from the liver after partial hepatectomy and separated into engrafted BM sprocs (GFP+CD133+), bone marrow-derived LSECs (GFP+CD133−), resident sprocs (GFP−CD133+), and resident (i.e., originating in the liver) LSECs (GFP−CD133−). The sdf1 receptor CXCR7 is highly expressed on engrafted BM sprocs and resident sprocs, but not on resident LSECs. Resident LSECs express CXCR4. Differentiation of engrafted BM sprocs to LSECs is accompanied by a significant decline in CXCR7 expression.
Sdf1 acts downstream from VEGF.
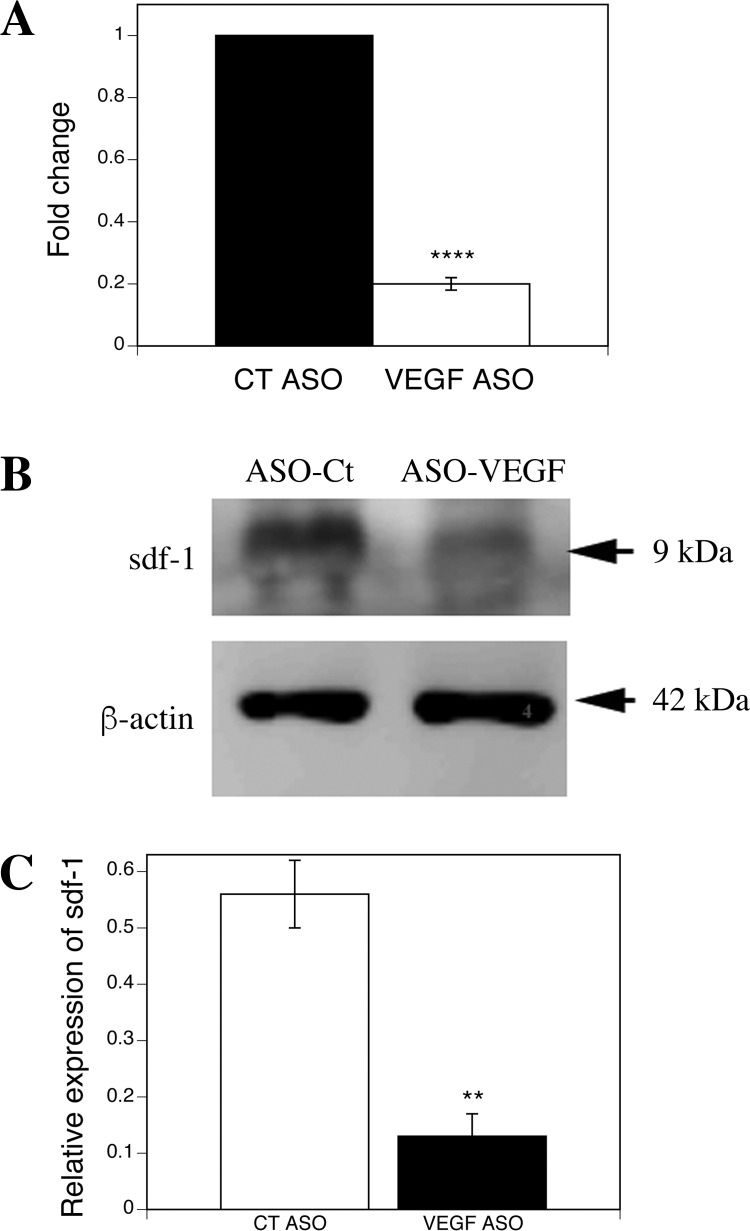
To examine whether sdf1 acts downstream from VEGF, we examined gene and protein expression of sdf1 in the liver after VEGF ASO knockdown. VEGF ASO decreased hepatic sdf1 gene expression by 79%, hepatic sdf1 protein expression by 77% (Fig. 7), and sdf1 plasma concentration after partial hepatectomy by 68% (Fig. 8A).
Fig. 7.
VEGF knockdown decreases hepatic sdf1 expression. A: RT-PCR. B: immunoblot. C: quantification of immunoblot. VEGF ASO decreases sdf1 gene expression by 80% (****P < 0.001) and protein expression by 77% (**P < 0.005) compared with scrambled ASO control (n = 3).
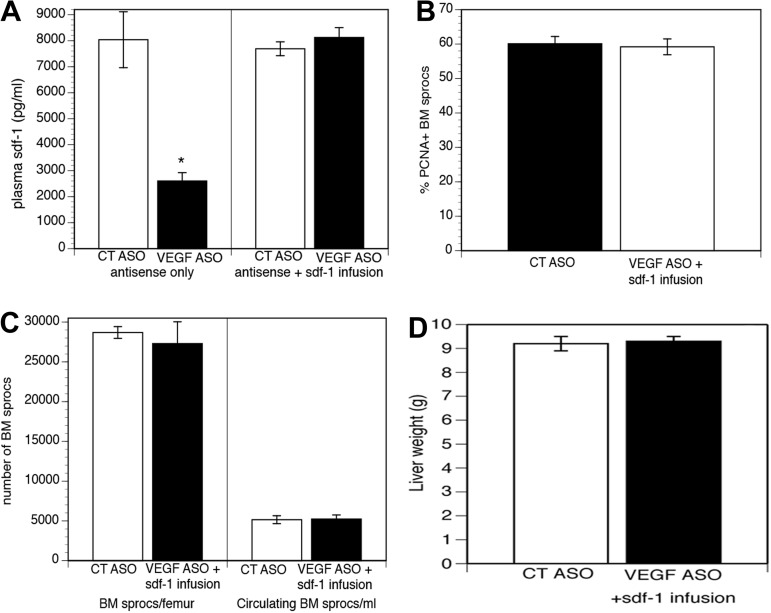
Fig. 8.
Maintenance of plasma sdf1 levels obviates the effect of VEGF knockdown. A: VEGF ASO reduces plasma sdf1 on day 5 after partial hepatectomy by 68% (left; *P < 0.01), and administration of sdf1 restores plasma sdf1 levels in rats treated with VEGF ASO to baseline (right). B and C: effect of VEGF knockdown on proliferation and number of sprocs in BM and mobilization of BM sprocs to the circulation are obviated by administration of sdf1. D: VEGF ASO does not impair liver regeneration when sdf1 is administered.
Hepatic VEGF increases after partial hepatectomy and after many forms of liver injury (10, 20, 31, 35, 40, 43). Knockdown of hepatic VEGF attenuates the recruitment and engraftment of BM sprocs that occur after partial hepatectomy (43), comparable to the effects shown above for sdf1 knockdown. If sdf1 acts downstream from VEGF to recruit BM sprocs, then restoration of plasma sdf1 concentration after VEGF knockdown should reverse the effect of VEGF knockdown. Infusion of sdf1 (100 ng/kg iv) restored plasma sdf1 concentrations, measured on day 5 after partial hepatectomy, in rats that had been treated for 4 wk with VEGF ASO (Fig. 8A). Administration of sdf1 to rats undergoing partial hepatectomy after VEGF knockdown completely blocked the effect of VEGF knockdown, i.e., maintained the increased percentage of proliferating sprocs in the BM, the number of sprocs in the BM, the mobilization of BM sprocs to the circulation, and the restoration of liver weight after partial hepatectomy (Fig. 8, B–D), indicating that sdf1 acts downstream from VEGF.
DISCUSSION
Liver regeneration permits the liver to maintain its mass during chronic liver injury and to restore its mass after acute injury and liver resection. Regulation of liver regeneration is a complex process, and the relative contribution of the factors involved is difficult to assess (29). Studies have demonstrated that LSECs promote liver regeneration (7, 8, 24, 25), but these studies did not distinguish between resident LSECs and BM sprocs that had engrafted after liver injury. Identification of the LSECs that drive liver regeneration subsequently demonstrated that BM sprocs engrafted in the liver are the major source of LSEC HGF (44). Recruitment of BM sprocs to the liver is essential for normal liver regeneration (5, 43, 44): suppression of 40% of the BM by irradiation inhibits restoration of liver regrowth by 40% after partial hepatectomy, and liver regeneration is fully restored by infusion of BM sprocs (44). Hepatic VEGF plays a central role in recruitment of BM sprocs to the liver after partial hepatectomy and liver injury (43). However, VEGF is a growth factor, not a chemokine. This suggests that there is a chemokine downstream from VEGF that recruits BM sprocs. In this study we examined sdf1, a chemokine that has been associated with VEGF (16).
After partial hepatectomy, VEGF promotes proliferation of sprocs in the BM, mobilizes sprocs from the BM to the circulation, and promotes BM sproc engraftment in the liver (43). Knockdown of VEGF (by 75%) (43) or sdf1 (by 50%) protein expression prior to partial hepatectomy reduces the increase in hepatic sdf1 levels, completely prevents the increased proliferation and mobilization of BM sprocs, and reduces hepatic engraftment of BM sprocs by 50%. Three pieces of evidence demonstrate that sdf1 acts downstream from VEGF. 1) The effects of VEGF and sdf1 knockdown are quantitatively extremely similar. 2) VEGF knockdown markedly decreases hepatic sdf1 gene and protein expression and plasma sdf1 levels. 3) The effect of VEGF knockdown on BM sproc recruitment is completely prevented by infusion of sdf1 to maintain plasma sdf1 concentration. The functional significance of sdf1-driven recruitment of BM sprocs is demonstrated by the observation that liver regeneration is impaired by sdf1 knockdown.
Sdf1 in BM mesenchymal cells and endothelial cells retains progenitor cells within the BM (9, 14, 22, 36). Thus a global endothelial knockout of sdf1 would decrease retention of progenitor cells in the BM, confounding the interpretation of the actions of hepatic sdf1. (LSEC-specific genes have not been identified, so knockout studies have been global endothelial cell knockout studies.) Fortunately for those of us doing hepatology research, ASOs are preferentially taken up in the liver (mainly LSECs and Kupffer cells) and the kidney and, to a lesser degree, in the BM (12, 13, 30, 47). Indeed, in the present studies the sdf1 ASO did not knock down sdf1 in the BM, but it allowed a selective knockdown of sdf1 in the liver.
Hepatic sdf1 increases twofold after partial hepatectomy. Expression of sdf1 increases in cholangiocytes and oval cells in liver injury (26, 39) and may contribute to the endocrine pool that leads to proliferation of sprocs in the BM and mobilization to the circulation. However, sdf1 in cholangiocytes recruits cells to the area surrounding bile ducts and adjacent portal veins, but not to the sinusoids (22). To promote vascular engraftment, an increased vascular or perivascular source of sdf1 is needed, so-called angiocrine regulation. After partial hepatectomy, sdf1 in the sinusoids is expressed by LSECs, but not by hepatic stellate cells or hepatocytes, and sdf1 protein expression increases more than twofold in LSECs after partial hepatectomy.
There are two known sdf1 receptors, CXCR4 and CXCR7. After partial hepatectomy, CXCR7+ BM sprocs appear in the circulation, and CXCR7 is expressed within the liver on engrafted BM sprocs and resident sprocs, whereas resident LSECs express CXCR4, but not CXCR7. Ding et al. (8) recently reported that CXCR7+ endothelial cells that appear in the sinusoid after injury promote liver regeneration. Our previous findings (43, 44) and the current study demonstrate that recruitment and engraftment of BM sprocs are necessary for normal liver regeneration. Together, these findings are consistent with the model that CXCR7+ BM sprocs, but not CXCR4+ resident LSECs, promote liver regeneration. Our previous studies demonstrated that BM sprocs are essential to normal liver regeneration, although resident sprocs also proliferate after injury and undoubtedly contribute to LSEC replacement.
Clinical implication.
The implication of our current study and our previous studies is that suppression of BM sprocs impairs liver regeneration and exacerbates liver injury (18, 44). Consistent with this idea, 10% of pediatric acute liver failure patients develop BM failure, which is a potentially life-threatening complication (17, 33, 41). A low number of circulating sprocs might be used to predict outcome in diseases such as acute liver failure or alcoholic hepatitis. Alternatively, since the current study demonstrates that very few (1%) circulating sprocs are CXCR7+ in controls and that over half of circulating sprocs are CXCR7+ after partial hepatectomy, another possibility would be to examine whether the lack of CXCR7+ sprocs in the circulation predicts poor outcome.
In summary, the VEGF-sdf1 pathway in the liver is upregulated after partial hepatectomy, and this increases BM production of sprocs, with increased mobilization of CXCR7+ BM sprocs to the circulation, hepatic engraftment of BM sprocs as LSECs, and promotion of liver regeneration. LSECs are responsible for the angiocrine regulation of homing to and engraftment in the sinusoid through sdf1 signaling.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-46357 (L. D. DeLeve), the Cell and Tissue Imaging Core and the Cell Separation and Culture Core of the University of Southern California Research Center for Liver Diseases (National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-048522), and the Nonparenchymal Liver Cell Core of the Southern California Research Center for Alcoholic Liver and Pancreatic Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.D.D. developed the concept and designed the research; L.D.D. and X.W. analyzed the data; L.D.D. interpreted the results of the experiments; L.D.D. prepared the figures; L.D.D. drafted the manuscript; L.D.D. edited and revised the manuscript; L.D.D. approved the final version of the manuscript; X.W. and L.W. performed the experiments.
REFERENCES
- 1.Berahovich RD, Zabel BA, Lewen S, Walters MJ, Ebsworth K, Wang Y, Jaen JC, Schall TJ. Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology 141: 111–122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomhoff R, Eskild W, Berg T. Endocytosis of formaldehyde-treated serum albumin via scavenger pathway in liver endothelial cells. Biochem J 218: 81–86, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomhoff R, Smedsrød B, Eskild W, Granum PE, Berg T. Preparation of isolated liver endothelial cells and Kupffer cells in high yield by means of an enterotoxin. Exp Cell Res 150: 194–204, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Cipriani P, Franca MA, Liakouli V, Pacini A, Manetti M, Marrelli A, Toscano A, Pingiotti E, Fulminis A, Guiducci S, Perricone R, Kahaleh B, Matucci-Cerinic M, Ibba-Manneschi L, Giacomelli R. Differential expression of stromal cell-derived factor 1 and its receptor CXCR4 in the skin and endothelial cells of systemic sclerosis patients: pathogenetic implications. Arthritis Rheum 54: 3022–3033, 2006. [DOI] [PubMed] [Google Scholar]
- 5.DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest 123: 1861–1866, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLeve LD, Wang X, McCuskey MK, McCuskey RS. Rat liver endothelial cells isolated by anti-CD31 immunomagnetic sorting lack fenestrae and sieve plates. Am J Physiol Gastrointest Liver Physiol 291: G1187–G1189, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468: 310–315, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505: 97–102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495: 231–235, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donahower B, McCullough SS, Kurten R, Lamps LW, Simpson P, Hinson JA, James LP. Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J Physiol Gastrointest Liver Physiol 291: G102–G109, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Eskild W, Kindberg GM, Smedsrod B, Blomhoff R, Norum KR, Berg T. Intracellular transport of formaldehyde-treated serum albumin in liver endothelial cells after uptake via scavenger receptors. Biochem J 258: 511–520, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 87: 46–51, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Geary RS, Yu RZ, Watanabe T, Henry SP, Hardee GE, Chappell A, Matson J, Sasmor H, Cummins L, Levin AA. Pharmacokinetics of a tumor necrosis factor-α phosphorothioate 2′-O-(2-methoxyethyl) modified antisense oligonucleotide: comparison across species. Drug Metab Dispos 31: 1419–1428, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495: 227–230, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene AK, Wiener S, Puder M, Yoshida A, Shi B, Perez-Atayde AR, Efstathiou JA, Holmgren L, Adamis AP, Rupnick M, Folkman J, O'Reilly MS. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann Surg 237: 530–535, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124: 175–189, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hadzic N, Height S, Ball S, Rela M, Heaton ND, Veys P, Mieli-Vergani G. Evolution in the management of acute liver failure-associated aplastic anaemia in children: a single centre experience. J Hepatol 48: 68–73, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Harb R, Xie G, Lutzko C, Guo Y, Wang X, Hill C, Kanel G, DeLeve LD. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology 137: 704–712, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol 63: 84–96, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa K, Mochida S, Mashiba S, Inao M, Matsui A, Ikeda H, Ohno A, Shibuya M, Fujiwara K. Expressions of vascular endothelial growth factor in nonparenchymal as well as parenchymal cells in rat liver after necrosis. Biochem Biophys Res Commun 254: 587–593, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Jin YL, Enzan H, Kuroda N, Hayashi Y, Toi M, Miyazaki E, Hamauzu T, Hiroi M, Guo LM, Shen ZS, Saibara T. Vascularization in tissue remodeling after rat hepatic necrosis induced by dimethyinitrosamine. Med Mol Morphol 39: 33–43, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest 112: 160–169, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai P, Li T, Yang J, Xie C, Zhu X, Xie H, Ding X, Lin S, Tang S. Upregulation of stromal cell-derived factor 1 (SDF-1) expression in microvasculature endothelial cells in retinal ischemia-reperfusion injury. Graefes Arch Clin Exp Ophthalmol 246: 1707–1713, 2008. [DOI] [PubMed] [Google Scholar]
- 24.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science 299: 890–893, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Maher JJ. Cell-specific expression of hepatocyte growth factor in liver: upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest 91: 2244–2252, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mavier P, Martin N, Couchie D, Preaux AM, Laperche Y, Zafrani ES. Expression of stromal cell-derived factor-1 and of its receptor CXCR4 in liver regeneration from oval cells in rat. Am J Pathol 165: 1969–1977, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendt M, Cardier JE. Stromal-derived factor-1 and its receptor, CXCR4, are constitutively expressed by mouse liver sinusoidal endothelial cells: implications for the regulation of hematopoietic cell migration to the liver during extramedullary hematopoiesis. Stem Cells Dev 21: 2142–2151, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalopoulos GK. Liver regeneration. J Cell Physiol 213: 286–300, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 176: 2–13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller CM, Donner AJ, Blank EE, Egger AW, Kellar BM, Østergaard ME, Seth PP, Harris EN. Stabilin-1 and Stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides (ASOs) in the liver. Nucleic Acids Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mochida S, Ishikawa K, Inao M, Shibuya M, Fujiwara K. Increased expressions of vascular endothelial growth factor and its receptors, flt-1 and KDR/flk-1, in regenerating rat liver. Biochem Biophys Res Commun 226: 176–179, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Moldeus P, Hogberg J, Orrenius S. Isolation and use of liver cells. Methods Enzymol 52: 60–71, 1978. [DOI] [PubMed] [Google Scholar]
- 33.Molina RA, Katzir L, Rhee C, Ingram-Drake L, Moore T, Krogstad P, Martin MG. Early evidence of bone marrow dysfunction in children with indeterminate fulminant hepatic failure who ultimately develop aplastic anemia. Am J Transplant 4: 1656–1661, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Nanji AA, Tahan SR, Wei Y, Sadrzadeh SM. Hepatic sinusoidal endothelial cell G1/S arrest correlates with severity of alcoholic liver injury in the rat. Gastroenterology 107: 818–823, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Papastefanou VP, Bozas E, Mykoniatis MG, Grypioti A, Garyfallidis S, Bartsocas CS, Nicolopoulou-Stamati P. VEGF isoforms and receptors expression throughout acute acetaminophen-induced liver injury and regeneration. Arch Toxicol 81: 729–741, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell 4: 62–72, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Seo J, Kim YO, Jo I. Differential expression of stromal cell-derived factor 1 in human brain microvascular endothelial cells and pericytes involves histone modifications. Biochem Biophys Res Commun 382: 519–524, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Steffan AM, Gendrault JL, McCuskey RS, McCuskey PA, Kirn A. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology 6: 830–836, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Swenson ES, Kuwahara R, Krause DS, Theise ND. Physiological variations of stem cell factor and stromal-derived factor-1 in murine models of liver injury and regeneration. Liver Int 28: 308–318, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem 49: 121–130, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Tung J, Hadzic N, Layton M, Baker AJ, Dhawan A, Rela M, Heaton ND, Mieli-Vergani G. Bone marrow failure in children with acute liver failure. J Pediatr Gastroenterol Nutr 31: 557–561, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Villalvilla A, Moro M, Arruza L, Redondo S, Fernandez-Cruz A, Fernandez-Durango R. Circulating endothelial progenitor cells are reduced in rat oxygen-induced retinopathy despite a retinal SDF-1/CXCR4 and VEGF proangiogenic response. Life Sci 91: 264–270, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Wang X, Wang L, Chui JD, van de Ven G, Gaarde WA, DeLeve LD. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology 143: 1555–1563, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest 122: 1567–1573, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie G, Wang L, Wang X, Wang L, DeLeve LD. Isolation of periportal, mid-lobular and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity. Am J Physiol Gastrointest Liver Physiol 299: G1204–G1210, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Q, Wang S, Jiang X, Zhao Y, Gao M, Zhang Y, Wang X, Tano K, Kanehara M, Zhang W, Ishida T. Hypoxia-induced astrocytes promote the migration of neural progenitor cells via vascular endothelial factor, stem cell factor, stromal-derived factor-1α and monocyte chemoattractant protein-1 upregulation in vitro. Clin Exp Pharmacol Physiol 34: 624–631, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Yu RZ, Kim TW, Hong A, Watanabe TA, Gaus HJ, Geary RS. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab Dispos 35: 460–468, 2007. [DOI] [PubMed] [Google Scholar]