Our study reveals that bone morphogenetic protein (BMP) signaling via ALK2 receptor promotes cardiac hypertrophy. Developing drugs that specifically inhibit ALK2 may offer promising therapeutic opportunities to treat cardiac hypertrophy. Furthermore, elucidating how BMP signaling regulates atrogin-1 expression and thereby alters calcineurin activity may provide novel insights into how BMP signaling triggers hypertrophy.
Keywords: cardiac hypertrophy, BMP signaling, calcineurin, BMP type I receptor, ALK2
Abstract
Bone morphogenetic protein (BMP) signaling contributes to the development of cardiac hypertrophy. However, the identity of the BMP type I receptor involved in cardiac hypertrophy and the underlying molecular mechanisms are poorly understood. By using quantitative PCR and immunoblotting, we demonstrated that BMP signaling increased during phenylephrine-induced hypertrophy in cultured neonatal rat cardiomyocytes (NRCs), as evidenced by increased phosphorylation of Smads 1 and 5 and induction of Id1 gene expression. Inhibition of BMP signaling with LDN193189 or noggin, and silencing of Smad 1 or 4 using small interfering RNA diminished the ability of phenylephrine to induce hypertrophy in NRCs. Conversely, activation of BMP signaling with BMP2 or BMP4 induced hypertrophy in NRCs. Luciferase reporter assay further showed that BMP2 or BMP4 treatment of NRCs repressed atrogin-1 gene expression concomitant with an increase in calcineurin protein levels and enhanced activity of nuclear factor of activated T cells, providing a mechanism by which BMP signaling contributes to cardiac hypertrophy. In a model of cardiac hypertrophy, C57BL/6 mice treated with angiotensin II (A2) had increased BMP signaling in the left ventricle. Treatment with LDN193189 attenuated A2-induced cardiac hypertrophy and collagen deposition in left ventricles. Cardiomyocyte-specific deletion of BMP type I receptor ALK2 (activin-like kinase 2), but not ALK1 or ALK3, inhibited BMP signaling and mitigated A2-induced cardiac hypertrophy and left ventricular fibrosis in mice. The results suggest that BMP signaling upregulates the calcineurin/nuclear factor of activated T cell pathway via BMP type I receptor ALK2, contributing to cardiac hypertrophy and fibrosis.
NEW & NOTEWORTHY
Our study reveals that bone morphogenetic protein (BMP) signaling via ALK2 receptor promotes cardiac hypertrophy. Developing drugs that specifically inhibit ALK2 may offer promising therapeutic opportunities to treat cardiac hypertrophy. Furthermore, elucidating how BMP signaling regulates atrogin-1 expression and thereby alters calcineurin activity may provide novel insights into how BMP signaling triggers hypertrophy.
cardiac hypertrophy is the leading cause of heart failure (13), affecting more than half a million individuals in the US (5). Elucidating the molecular mechanisms that regulate cardiac hypertrophy may facilitate the development of novel therapies for the treatment of heart failure. Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β family that play critical roles in cardiac development. BMP signaling, specifically BMP4, was recently reported to play an important role in the pathogenesis of cardiac hypertrophy (21). Blockade of BMP signaling with a dorsomorphine analog, DMH1, inhibited pressure overload-induced cardiac hypertrophy in mice (21). However, the underlying molecular mechanisms by which BMP signaling contributes to cardiac hypertrophy remain poorly understood.
BMP signal transduction is mediated by BMP type 1 [activin-like kinase 1 (ALK1), ALK2, ALK3, and ALK6] and type 2 [BMP receptor 2 (BMPR2), activin receptor (ACVR) 2A and ACVR2B] receptors. Upon ligand binding, the type 2 receptor phosphorylates the type 1 receptor, which in turn phosphorylates BMP-responsive Smads (small mothers against decapentaplegic) proteins 1, 5, and 8. Phosphorylated Smads 1/5/8 interact with Smad 4 and translocate into the nucleus to modulate the transcription of BMP-responsive genes, such as inhibitor of DNA binding 1 (Id1) (12, 19). The small molecule DMH1 binds and inhibits type I BMP receptor kinases, abrogating BMP signaling (26). However, the identity of the type I BMP receptor(s) in cardiomyocytes that is responsible for cardiac hypertrophy is not known.
Here, we report that BMP signaling was upregulated in, and required for, phenylephrine (PE)-induced hypertrophy in neonatal rat cardiomyocytes (NRCs). Blockade of BMP type I receptors, sequestration of BMP ligands, or silencing of BMP-responsive Smad 1 or 4 inhibited PE-induced hypertrophy. Activation of BMP signaling with BMP2 or BMP4 augmented the calcineurin-dependent nuclear factor of activated T cells (NFAT) pathway and induced hypertrophy in NRCs. In mice, inhibiting BMP signaling by treatment with LDN193189 (LDN) attenuated angiotensin II (A2)-induced cardiac hypertrophy and fibrosis. Moreover, deletion of ALK2, but not ALK1 or ALK3, in cardiomyocytes blocked BMP signaling and inhibited the development of A2-induced cardiac hypertrophy in mice.
MATERIAL AND METHODS
All mouse studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and reviewed and approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital Subcommittee on Research Animal Care. Mice were housed in micro-isolated sterile cages and were given normal chow and water ad libitum.
Generation of cardiac-specific ALK1-, ALK2-, or ALK3-deficient mice.
Mice with floxed ALK1 (ALK1fl/fl) or ALK2 (ALK2fl/fl) allele on a mixed C57BL/6; SV129 background or mice with floxed ALK3 allele (ALK3fl/fl) on a C57BL/6 background (2, 11, 16) were bred with mice bearing a transgene of α-MHC-MerCreMer (MCM) on a C57BL/6 background (Jackson Laboratories). Heterozygous animals were intercrossed to obtain mice homozygous for the ALK1fl/fl, ALK2fl/fl, or ALK3fl/fl alleles with the MCM transgene. At 8–10 wk of age, homozygous mice were administered tamoxifen (30 mg·k−1·day−1) or vehicle (corn oil with 10% ethanol) intraperitoneally for 5 days to delete ALK1, ALK2, or ALK3 selectively in cardiomyocytes. Tamoxifen administration in MCM mice has been reported to cause a transient decrease in cardiac function that resolved 10–14 days later (7). Therefore, we performed all of the experiments in tamoxifen-treated mice at least 3 wk after the treatment.
A2-induced cardiac hypertrophy.
Mice were anesthetized with ketamine (100 mg/kg) and fentanyl (75 μg/kg), and sufficient anesthesia was confirmed by reflex analysis and heart rate (HR) monitoring. A2-filled osmotic minipumps (Alzet 1002, Durect) were implanted subcutaneously into mice. A2 was infused at a dose of 1 μg·kg−1·min−1 for 2 wk to induce cardiac hypertrophy. Buprenorphine (0.05 mg/kg) was given subcutaneously 30 min before the beginning of the procedure as preemptive analgesia followed by another dose every 12 h for 3 days to alleviate pain. At the end of 2 wk, mice underwent echocardiographic analysis under sedation with ketamine (50 mg/kg). Mice then were euthanized with a high dose of pentobarbital (200 mg/kg ip), and the whole heart and left ventricle (LV) were weighed, and LV was divided in half and saved in liquid nitrogen or formalin.
Isolation of NRCs.
NRCs were isolated from the ventricles of 1- to 2-day-old pups of Sprague-Dawley rats after euthanasia with pentobarbital (100 mg/kg ip) using a cardiomyocyte isolation kit (Worthington, Lakewood, NJ). Cardiomyocytes were cultured for 48 h before any treatment in Dulbecco's modified Eagle's medium containing 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and thymidine (0.6 mg/ml). Cells were then serum starved, and hypertrophy was induced by treatment with PE (10 μM) or isoproterenol (ISO, 10 μM) for 24 h. To inhibit BMP signaling, NRCs were treated with BMP type I receptor inhibitors, LDN (100 nM), or noggin (100 ng/ml).
Quantitative real time-PCR.
Total RNA was extracted using Trizol. cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase and random primers (Promega). The mRNA levels were measured by quantitative PCR in a Mastercycler realplex 2 (Eppendorf) using hydrolysis probes (TaqMan Gene Expression Assays, Applied Biosystems) or Probe Fast Master Mix (Kapa Biosystems) using conventional Sybr or Taqman primers. Changes in relative gene expression were normalized to 18S mRNA levels and were determined using the relative Ct method. The sequences for Sybr probes used in this study are as follows: recombinant BMP2 (rBMP2)-forward CCAGGTTAGTGACTCAGAACAC, rBMP2-reverse TCATCTTGGTGCAAAGACCTGC; rBMP4-forward TGGACACTTCATCACACGACTA, rBMP4-reverse GCGACGGCAGTTCTTATTCTTC; rBMP7-forward AGACGCCAAAGAACCAAGAG, rBMP7-reverse GCTGTCGTCGAAGTAGAGGA; recombinant modulatory calcineurin-interacting protein (rMCIP)-forward AGCTCCCTGATTGCCTGTGT, rMCIP-reverse TTTGGCCCTGGTCTCACTTT; murine BMP2 (mBMP2)-forward TGGCCCATTTAGAGGAGAACC, mBMP2-reverse CTGTGTTCATCTTGGTGCAAAG; mBMP4-forward ATTCCTGGTAACCGAATGCTG, mBMP4-reverse CCGGTCTCAGGTATCAAACTAGC; mBMP7-forward ACCCTCGATACCACCATCGG, mBMP7-reverse GCTCCCGGATGTAGTCCTT.
Gene silencing.
To deplete Smad 1 and Smad 4, NRCs were transfected with small interfering RNA (siRNA) specific for Smad 1 and Smad 4, respectively (Silencer Select siRNA, Applied Biosystems, Life Technologies). As a negative control, cells were transfected with a nontargeting siRNA. All siRNA were transfected at a final concentration of 50 nM using Lipofectamine 2000 transfection reagent (Invitrogen), as described by the manufacturer. After 48 h, transfected cells were incubated overnight in Dulbecco's modified Eagle's medium without serum and were treated with PE for 24 h.
Immunoblotting.
Whole cell lysates were harvested from cultured NRCs or LVs with RIPA buffer containing proteinase and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO). Extracted proteins were separated by electrophoresis using 4–12% Bis-Tris gels (NuPAGE, Invitrogen, Grand Island, NY) and transferred to polyvinylidene fluoride membranes. Membranes were incubated with antibodies directed against total Smad 1 (Life Span Bio, catalog no. LS-C75853), phosphorylated-Smad 1 and 5 (Cell Signaling Technology, catalog no. 9516S), BMP4 (Santa Cruz Biotech, catalog no. 6896), BMP2 (Santa Cruz Biotech, catalog no. 6895), or calcineurin (BD Bioscience, catalog no. 610259). After washing, membranes were incubated with horseradish peroxidase-conjugated donkey anti-rabbit or anti-mouse IgG (Cell Signaling Technology, Beverly, MA). Membranes were incubated with ECLPlus (GE Healthcare, Wilmington, MA), and chemifluorescence was detected with a Versadoc 4000MP imager. For calcineurin immunoblots, membranes were incubated with IRDye 800CW donkey anti-mouse IgG (LI-COR, Lincoln, NE), and images were captured using LI-COR Odyssey detection system. Captured images were analyzed with ImageJ software (National Institutes of Health) for integrated density of protein bands.
Luciferase reporter assays.
NRCs were transfected with luciferase reporter gene constructs containing a BMP-response element or the human NPPB gene promoter using Lipofectamine 2000, according to the manufacturer's instructions. NRCs were also transfected with an NFAT luciferase reporter construct containing repetitive NFAT recognition sites or an atrogin-1 luciferase reporter construct containing 3.5 kb of atrogin-1 mouse promoter. As a control for transfection efficiency, cells were transfected with a plasmid directing constitutive expression of renilla luciferase. After 48 h, cells were treated with PE, BMP2, or BMP4 for 24 h. The cells were harvested, and firefly and renilla luciferase activities in cell extracts were measured using the Dual Luciferase Reporter Assay System (Promega, Madison, WI).
Immunofluorescent staining of NRCs.
NRCs were plated on gelatin-coated eight-well Lab-Tek II chamber slides and fixed with 2:5 acetone-methanol for 20 min at 4°C followed by permeabilization of cells for 10 min with 0.1% (vol/vol) Triton X-100. After blocking with 1% BSA solution in PBS, cells were incubated with either anti-NFATc3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:200 dilution) in PBS containing 2% BSA overnight at 4°C or with mouse anti-sarcomeric α-actinin (Abcam, Cambridge, MA, 1:1,000) for 1 h at room temperature. Bound primary antibodies were detected with Alexa Fluor 596-conjugated donkey anti-rabbit IgG or Alexa Fluor 488-conjugated donkey anti-mouse IgG (Life Technologies, 1:250 dilution).
Immunofluorescent histochemical staining of ventricular tissues.
To assess the size of myocardial cells, LV sections (5 μm) were incubated with FITC-conjugated wheat germ agglutinin (Sigma, St. Louis, MO) at 10 μg/ml for 2 h at room temperature. Myocardial interstitial fibrosis and collagen deposition were assessed on midventricular sections by staining with Masson trichrome and picro-sirius red, respectively. Images were captured using Nikon E800 inverted microscope (MVI, Avon, MA).
Statistical analysis.
All values were expressed as means ± SE. Data were analyzed using the Student's t-test or one-way ANOVA test followed by Bonferroni test for multiple comparisons, when applicable. Time course and dose response were analyzed by repeated-measure one-way ANOVA. P values < 0.05 were considered statistically significant.
RESULTS
Hypertrophic stimuli increase BMP signaling in NRCs.
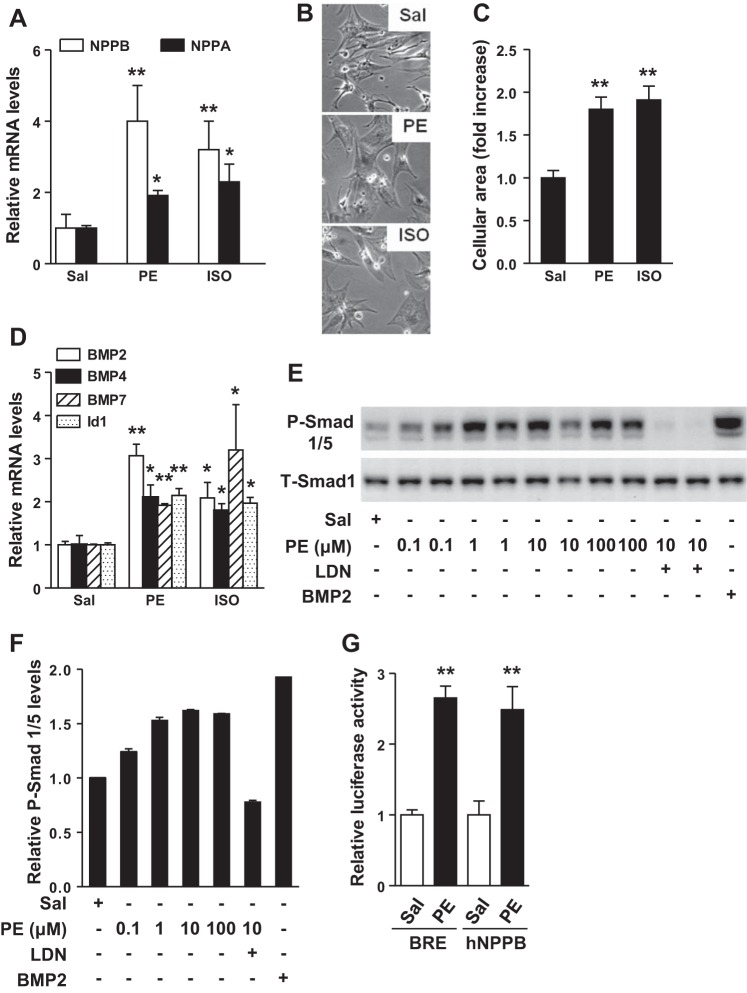
To investigate whether BMP signaling is altered during the development of cardiac hypertrophy, we treated NRCs with known hypertrophic stimuli, PE or ISO. Treatment with PE or ISO induced expression of the genes encoding atrial natriuretic peptide (NPPA) and B-type natriuretic peptide (NPPB; Fig. 1A) and increased the size of cardiomyocytes (Fig. 1, B and C). PE and ISO also increased the expression of genes encoding BMP2, BMP4, and BMP7, as well as BMP-responsive gene Id1 (Fig. 1D). To further investigate BMP signaling in cardiac hypertrophy, we focused on PE- (as opposed to ISO- or A2-) induced hypertrophy, because PE consistently produced a more robust response in NRCs. PE treatment of NRCs induced phosphorylation of Smads 1 and 5 (Smad 1/5), in a dose-dependent manner, without altering levels of total Smad 1 (Fig. 1, E and F). In cells transfected with a BMP response element luciferase reporter construct, PE increased the luciferase activity approximately threefold. PE also induced a more than twofold increase in luciferase activity in cells transfected with an human NPPB (hNPPB) promoter luciferase plasmid, demonstrating the ability of PE to increase BMP signaling under conditions that induce cardiac hypertrophy (Fig. 1G). Taken together, these data demonstrate that BMP signaling is activated in NRCs in response to agonist-induced hypertrophy.
Fig. 1.
Treatment with phenylephrine (PE) or isoproterenol (ISO) increases bone morphogenetic protein (BMP) signaling in neonatal rat cardiomyocytes (NRCs). Treatment of NRCs with PE (10 μM) or ISO (10 μM) for 24 h increased NPPB (B-type natriuretic peptide) and NPPA (atrial natriuretic peptide) mRNA levels (A) and increased the size of cardiomyocytes, as determined by phase-contrast microscopy (B). C: the cellular area of cardiomyocytes (no. of cells counted per treatment = 25–30) was quantified using Image J. D: treatment of NRCs with PE or ISO for 24 h induced BMP2, BMP4, BMP7, and Id1 gene expression. E: the levels of phosphorylated-small mothers against decapentaplegic 1/5 (p-Smad 1/5) in NRCs increased in a dose-dependent manner in NRCs treated with PE, as determined by immunoblot. Pretreatment of NRCs with LDN193189 (LDN; 100 nM) blocked the ability of PE to induce phosphorylation of Smad 1/5. As a positive control, NRCs were stimulated with BMP2. None of the treatments altered total Smad 1 (T-SMAD 1) levels. F: quantitative analysis of immunoblots shown in E. Immunoblots shown in E are representative of 2 independent experiments. Treatment of NRCs with PE (10 μM) for 24 h increased relative luciferase activity of reporter plasmids containing a BMP response element (BRE) or a human NPPB (hNPPB) promoter. G: cotransfection of a plasmid encoding renilla was used to normalize the results. Saline (Sal) treatment was used as a control. Values are means ± SE; n = 4, 4, and 6 samples per experimental group in A, D, and G, respectively. *P < 0.05 vs. saline. **P < 0.01 vs. saline treatment.
Blockade of BMP signaling inhibits cardiomyocyte hypertrophy.
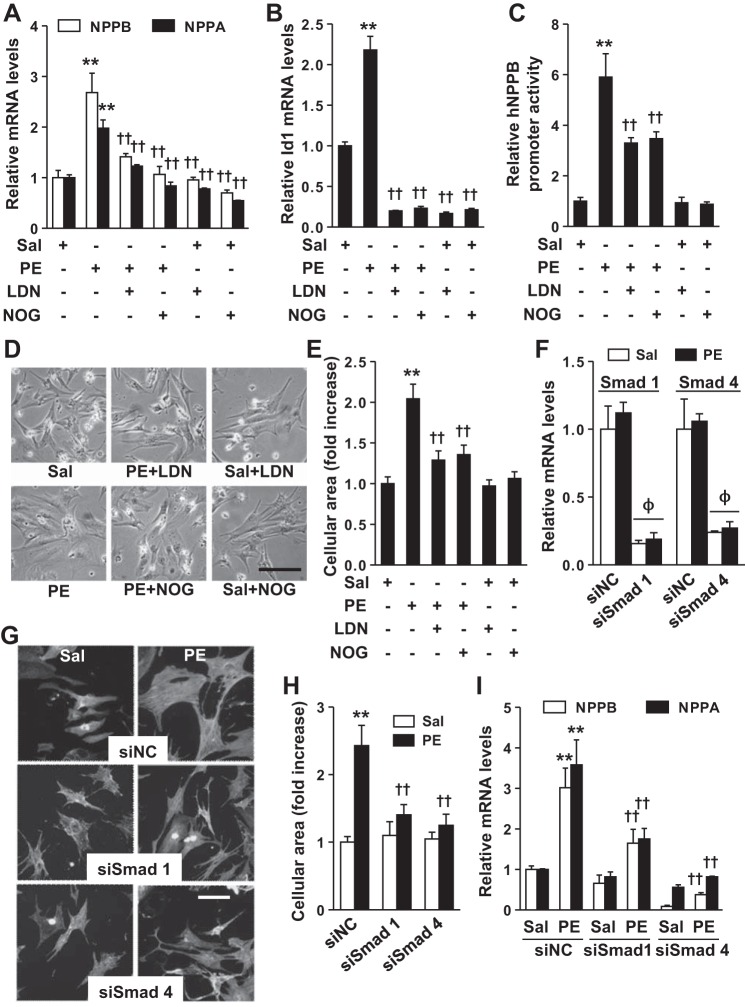
To further investigate whether increased BMP signaling contributes to PE-induced hypertrophy, we stimulated NRCs with PE in the absence or presence of inhibitors of BMP signaling, LDN (an inhibitor of type I BMP receptor kinases) or noggin (which sequesters BMP ligands and prevents their interaction with BMP receptors). Treatment of NRCs with LDN or noggin for 24 h markedly suppressed the ability of PE to induce NPPA and NPPB gene expression (Fig. 2A). LDN and noggin also inhibited PE-induced increases in Id1 mRNA levels (Fig. 2B). Moreover, LDN and noggin also partially inhibited PE-induced increases in hNPPB promoter activity (Fig. 2C) and cardiomyocyte size (Fig. 2, D and E). Although LDN and noggin both inhibited basal BMP signaling (Fig. 2C), neither treatment altered NPPA and NPPB gene expression or cell size in the absence of PE (Fig. 2, A and E), suggesting that basal BMP signaling does not affect NPPA and NPPB gene expression. siRNA-mediated silencing of BMP-responsive Smad 1 or Smad 4 in cardiomyocytes (Fig. 2F) reduced PE-induced increases in cell size (Fig. 2, G and H) and NPPA and NPPB gene expression (Fig. 2I). These data demonstrate that BMP signaling is required for PE-induced hypertrophy in NRCs.
Fig. 2.
In NRCs, inhibition of BMP signaling prevents the development of PE-induced hypertrophy. Treatment of NRCs with PE (10 μM) increased NPPB and NPPA (A), Id1 mRNA levels (B), and hNPPB promoter activity (C), an effect that was inhibited by pretreatment with LDN (100 nM) or noggin (NOG; 100 ng/ml). D: treatment with PE increased the cellular area of NRCs, an effect that was inhibited by treatment with LDN or NOG. E: quantification of the relative area of NRCs treated with PE, or PE and inhibitor. The no. of cells counted per treatment = 25–30. Depletion of Smad 1 or Smad 4 in NRCs using small interfering RNAs (siRNAs; F) inhibited the ability of PE to increase NRCs size (G and H) and induce NPPB and NPPA gene expression (I). In G, cells were stained with rabbit anti-actinin antibody and fluorescein-conjugated donkey anti-rabbit IgG antiserum, and 25–30 cells were counted per treatment. Values are means ± SE; n = 4 samples per experimental group in A, B, F, and I; n = 6 in C. Bar in D and G indicates 50 μm. **P < 0.01 vs. saline treatment. ††P < 0.01 vs. PE control. ΦP < 0.001 vs. corresponding siNC transfected cells. siNC, nontargeting siRNA; siSmad, siRNA against Smad.
Activation of BMP signaling is sufficient to induce hypertrophy in NRCs.
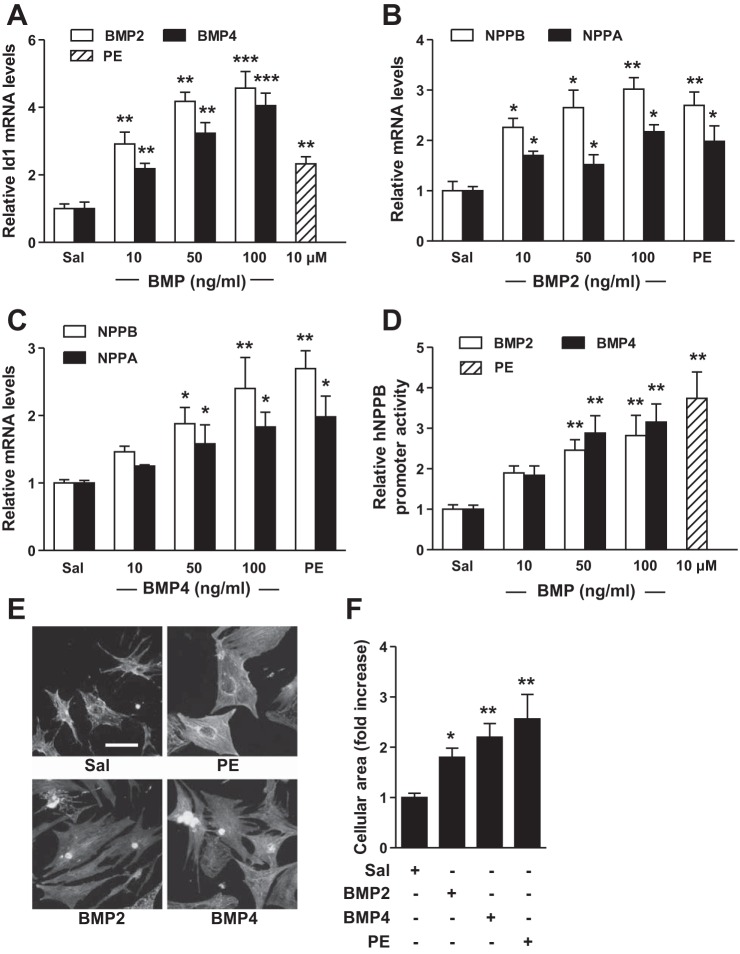
Expression of both BMP2 and BMP4 is increased during the development of cardiac hypertrophy in mice (10, 21). We hypothesized that if BMP signaling is the primary mediator of PE-induced hypertrophy, then activation of BMP signaling alone might induce hypertrophy. Treatment of NRCs with increasing doses of BMP2 or BMP4 activated BMP signaling, as reflected by increased Id1 gene expression (Fig. 3A), and increased NPPA and NPPB gene expression to levels comparable to those observed in response to PE (Fig. 3, B and C). Similarly, BMP2 and BMP4 induced hNPPB promoter activity (Fig. 3D) and increased cell size (Fig. 3, E and F). These results suggest that BMP signaling is sufficient to independently induce hypertrophy in NRCs.
Fig. 3.
BMP ligands induce hypertrophy in NRCs. Treatment of NRCs with BMP2 (10 ng/ml), BMP4 (10 ng/ml), or PE (10 μM) for 24 h induced Id1 (A) and NPPB and NPPA gene expression (B and C) and increased hNPPB promoter activity (D). E: BMP2, BMP4, or PE treatment for 24 h increased NRC size. F: quantification of the relative area of NRCs treated with BMP2, BMP4, or PE. No. of cells counted per treatment = 25–30. Values are means ± SE; n = 4 samples per treatment group in A–C, and n = 6 in D. *P < 0.05, **P < 0.01, and ***P < 0.01 vs. saline treatment. Bar in E = 50 μm.
BMP signaling stimulates the calcineurin/NFAT pathway in cardiomyocytes.
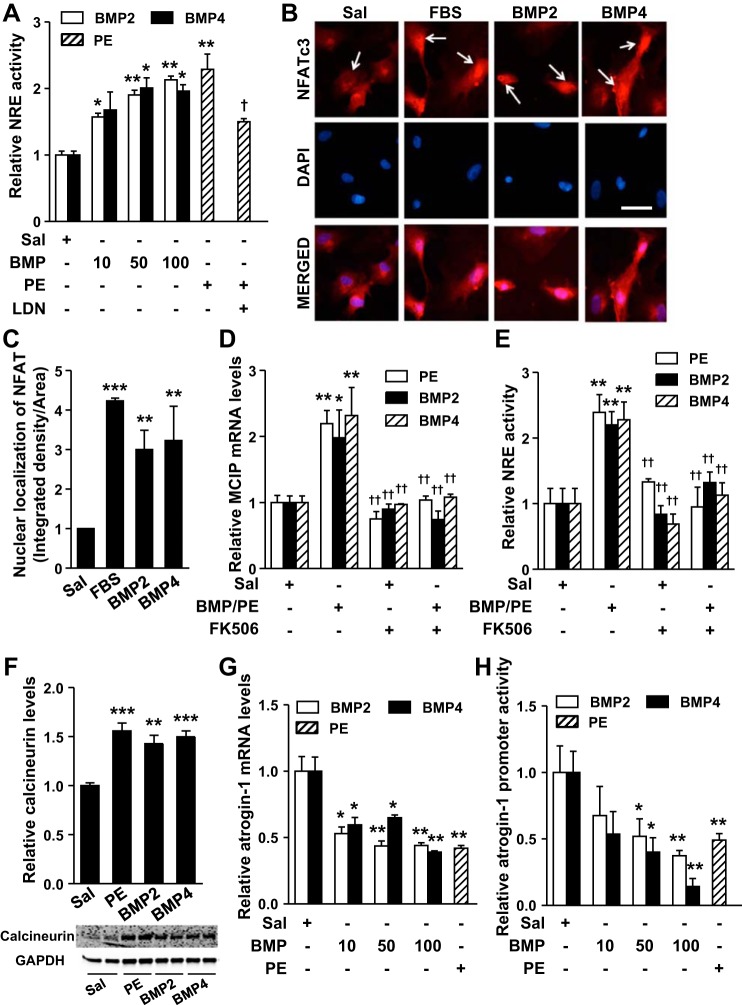
BMP signaling was previously reported to increase NFAT activity in mouse lung endothelial cells and keratinocytes (6, 8, 24). To investigate whether BMP signaling affects the NFAT pathway in cardiomyocytes, we determined the impact of BMP signaling on NFAT activity in NRCs. PE treatment for 24 h increased the activity of an NFAT response element (NRE) by 2 fold, as determined using a reporter plasmid and luciferase assay (Fig. 4A). Pretreatment with LDN inhibited the PE-induced increase in NRE activity by ∼60%. Similar to PE, both BMP2 and BMP4 treatment increased NRE activity (Fig. 4A). Immunofluorescence staining revealed that BMP2 or BMP4 treatment of NRCs increased the nuclear localization of NFATc3, a member of NFAT family known to play a critical role in pathogenesis of cardiac hypertrophy (Fig. 4, B and C) (14, 25). Treatment with 10% fetal bovine serum was used as a positive control for induction of nuclear localization of NFATc3.
Fig. 4.
BMP signaling upregulates the calcineurin/nuclear factor of activated T cell (NFAT) pathway. A: treatment of NRCs with PE (10 μM) for 24 h increased relative luciferase activity of a reporter plasmid containing an NFAT response element (NRE). Similarly, treatment of NRCs with increasing doses of BMP2 or BMP4 for 24 h increased relative luciferase activity of NRE. The concentration of BMP2 or 4 in A is in ng/ml. Pretreatment of NRCs with LDN (100 nM) inhibited the PE-induced increase in relative NRE activity. B: treatment of NRCs with BMP2 (10 ng/ml), BMP4 (10 ng/ml), or fetal bovine serum (FBS; 10%) increased nuclear localization of NFATc3. Cells were stained with rabbit anti-NFATc3 and Alex Fluor 596-conjugated donkey anti-rabbit IgG antiserum (red). 4′,6-Diamidino-2-phenylindole (DAPI; blue) was used to stain nuclei. C: quantification for nuclear localization of NFATc3 (red) of images given in B. Treatment of NRCs with BMP2 (10 ng/ml), BMP4 (10 ng/ml), or PE (10 μM) for 24 h induced MCIP (modulatory calcineurin-interacting protein) gene expression levels (D) and increased NRE activity (E), effects that were inhibited by FK506 (5 μM). F: treatment of NRCs with BMP2 (10 ng/ml), BMP4 (10 ng/ml), or PE (10 μM) for 24 h increased calcineurin protein levels, as determined by immunoblot and densitometric analysis. GAPDH was used as a loading control. Treatment of NRCs with BMP2, BMP4 (ng/ml), or PE (10 μM) for 24 h decreased atrogin-1 mRNA levels (G) and luciferase activity of a reporter plasmid containing a mouse atrogin-1 promoter (H). Immunoblots shown in inset (F) are representative of 2 independent experiments. Values are means ± SE; n = 4 samples per treatment group in A, D, E, G, and H. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. saline treatment. †P < 0.05 and ††P < 0.01 vs. PE or BMP treatment. Bar in B = 50 μm.
Consistent with the observed increase in NRE activity, BMP ligands and PE treatment induced the expression of the gene encoding MCIP, a direct transcriptional target of NFAT (17) (Fig. 4D). To elucidate whether PE- and BMP-induced activation of NFAT was dependent on calcineurin, we treated NRCs with FK506, a specific inhibitor of calcineurin (4). The presence of FK506 inhibited both PE- and BMP-induced increases in NRE activity (Fig. 4E) and MCIP mRNA levels (Fig. 4D). Moreover, treatment with PE, BMP2, or BMP4 induced a robust increase in calcineurin protein levels, as determined by immunoblotting (Fig. 4F and inset at the bottom). These data suggest that, similar to PE, BMP signaling activates NFAT by increasing calcineurin expression.
Calcineurin activity is negatively regulated by the ubiquitin ligase, atrogin-1, which promotes calcineurin degradation (9, 15). Because PE and BMP ligands increased calcineurin levels, we investigated whether exposure to PE, BMP2, or BMP4 inhibited atrogin-1 expression. PE treatment of NRCs reduced atrogin-1 mRNA levels by ∼60%. Similarly, treatment with BMP2 or BMP4 also diminished atrogin-1 mRNA levels (Fig. 4G). To investigate whether BMP signaling reduces atrogin-1 expression by altering its transcription, we measured atrogin-1 promoter activity using a luciferase reporter plasmid transfected into NRCs (18). Treatment of NRCs with PE reduced atrogin-1 promoter activity by >50%. Similarly, treatment of NRCs with BMP2 or BMP4 reduced atrogin-1 promoter activity in a dose-dependent manner (Fig. 4H). Collectively, these data suggest that the BMP-mediated increase in NFAT activity is linked to the ability of BMP to inhibit atrogin-1 and augment calcineurin expression levels.
Blockade of BMP signaling inhibits A2-induced cardiac hypertrophy.
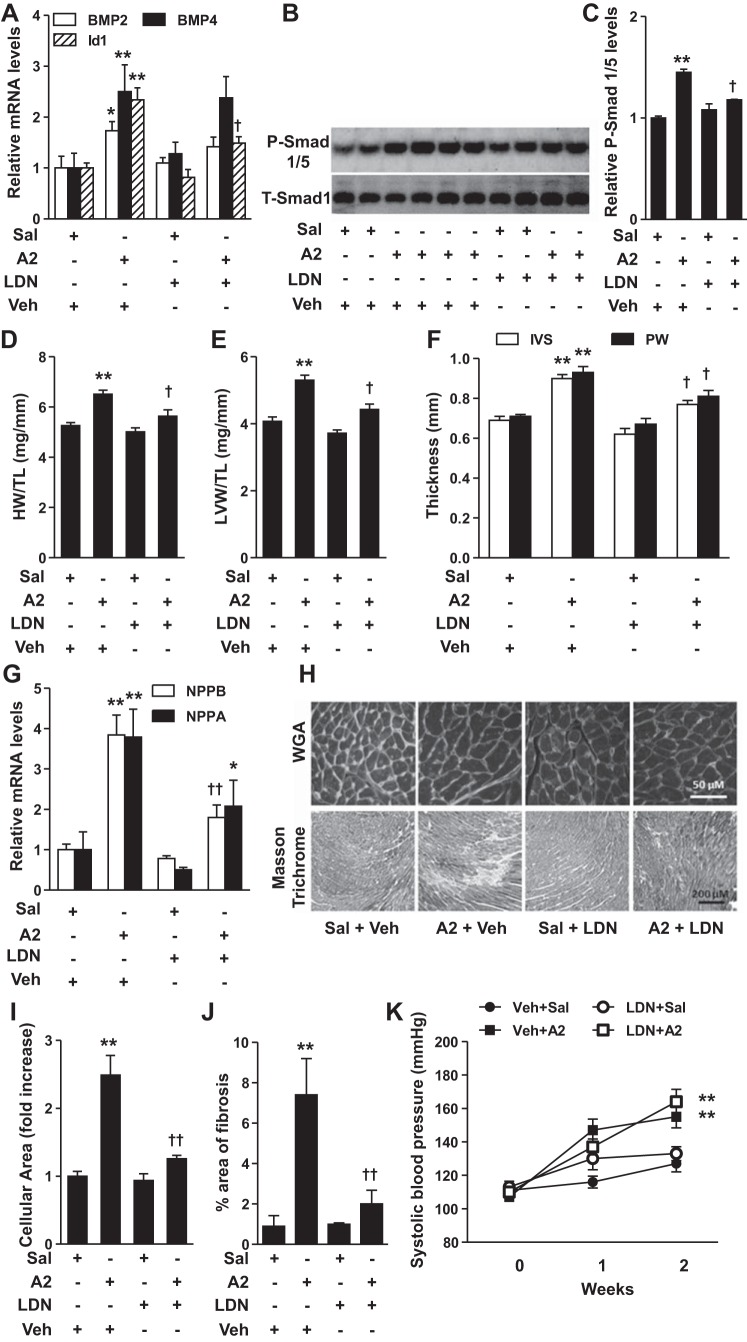
We used a mouse model of A2-induced cardiac hypertrophy to investigate the role of BMP signaling in the development of cardiac hypertrophy in vivo. Infusion of A2 in mice for 2 wk induced BMP2, BMP4, and Id1 gene expression (Fig. 5A) and increased phosphorylation of Smad 1/5 (Fig. 5, B and C) in the LV, indicating that A2 stimulated BMP signaling. A2 infusion increased the ratio of heart weight (HW) to tibial length (TL) (HW/TL) and LV weight (LVW) to TL (LVW/TL; Fig. 5, D and E). Compared with saline infusion, A2 also increased the thickness of the intraventricular septum (IVS) and posterior wall (PW) (Fig. 5F). Treatment with LDN inhibited A2-induced Id1 gene expression and phosphorylation of Smad 1/5 (Fig. 5, A–C). LDN treatment inhibited A2-induced increases in HW/TL, LVW/TL, and in the thickness of IVS and PW (Fig. 5, D–F). LDN also partially suppressed A2-induced NPPA and NPPB gene expression in LV (Fig. 5G). Moreover, LDN treatment markedly alleviated A2-induced increases in cardiomyocyte area (Fig. 5, H, top, and I) and collagen deposition (Fig. 5, H, bottom, and J) in LV sections. These results suggest that, consistent with our in vitro findings, inhibition of BMP signaling prevents the development of cardiac hypertrophy in mice. LDN treatment had no effect on systolic blood pressure in mice treated with or without A2 (Fig. 5K), suggesting that the LDN-induced protection against A2-cardiac hypertrophy was not due to attenuation of A2-induced hypertension. Moreover, HR, LV end-diastolic diameter, LV end-systolic diameter, and fractional shortening did not deteriorate with A2 treatment in any group (Table 1), suggesting that A2-induced hypertrophy in 2 wk was not sufficient to impair cardiac function, as has been reported previously (3).
Fig. 5.
Inhibition of BMP signaling attenuates angiotensin II (A2)-induced cardiac hypertrophy. Subcutaneous administration of A2 (1 μg·kg−1·min−1) for 2 wk in mice induced BMP2, BMP4, and Id1 expression (A) and increased phosphorylation of Smad 1/5 in left ventricles (LV; B). Treatment with LDN (3 mg·kg−1·day−1) inhibited A2-induced increases in Id1 gene expression (A) and P-Smad 1/5 protein levels (B). C: quantitative analysis of immunoblots shown in B. A2 administration increased the ratios of heart weight (HW) to tibial length (TL) (HW/TL; D), LV weight (LVW) to TL (LVW/TL; E), and the thickness of the intraventricular septum (IVS) and posterior wall (PW), as assessed by echocardiography (F), effects that were blocked by LDN. G: LDN also inhibited A2-induced increases in NPPB and NPPA gene expression. A2 administration for 2 wk in mice increased cardiomyocyte area (H, top, and I) and collagen deposition (H, bottom, and J) in LV, as assessed by wheat germ agglutinin (WGA) and Masson's trichrome staining, respectively. These effects were inhibited by LDN treatment. K: A2 administration (1 μg·kg−1·min−1) for 2 wk increased systolic blood pressure, an effect that was not affected by simultaneous treatment with LDN. Values are means ± SE; n = 6–7 mice/group. *P < 0.05 and **P < 0.01 vs. saline + vehicle (Veh) treatment. †P < 0.05 and ††P < 0.01 vs A2 + Veh treatment. Bar in WGA and Masson trichrome images represents 50 and 200 μm, respectively.
Table 1.
Echocardiographic analysis
| Sal + Veh | A2 + Veh | Sal+ LDN | A2 + LDN | |
|---|---|---|---|---|
| WT | ||||
| HR | 657 ± 14 | 657 ± 11 | 636 ± 14 | 635 ± 11 |
| LVEDD | 3.47 ± 0.1 | 3.24 ± 0.09 | 3.29 ± 0.1 | 3.28 ± 0.04 |
| LVESD | 1.62 ± 0.08 | 1.49 ± 0.1 | 1.56 ± 0.04 | 1.55 ± 0.08 |
| FS | 53 ± 1 | 54 ± 2 | 52.6 ± 2 | 53 ± 2 |
| Veh + Sal | Veh + A2 | Tam + Sal | Tam + A2 | |
|---|---|---|---|---|
| MCM-ALK2flox/flox | ||||
| HR | 659 ± 10 | 655 ± 9 | 676 ± 9 | 646 ± 10 |
| LVEDD | 3.43 ± 0.04 | 3.12 ± 0.04 | 3.4 ± 0.1 | 3.13 ± 0.04 |
| LVESD | 1.48 ± 0.04 | 1.2 ± 0.04 | 1.45 ± 0.06 | 1.23 ± 0.04 |
| FS | 56 ± 1 | 61 ± 1 | 57 ± 1 | 60 ± 1 |
Values are means ± SE; n = 6–7 mice in each group. Sal, saline; Veh, vehicle; A2, angiotensin II; LDN, LDN193189; Tam, tamoxifen; WT, wild type; HR, heart rate; LVEDD, left ventricular end-diastolic internal diameter; LVESD, left ventricular end-systolic internal diameter; FS, fractional shortening. No difference was found with or without LDN treatment or ALK2 deficiency in Sal- or A2-treated mice for 2 wk.
ALK2 is required for A2-induced cardiac hypertrophy.
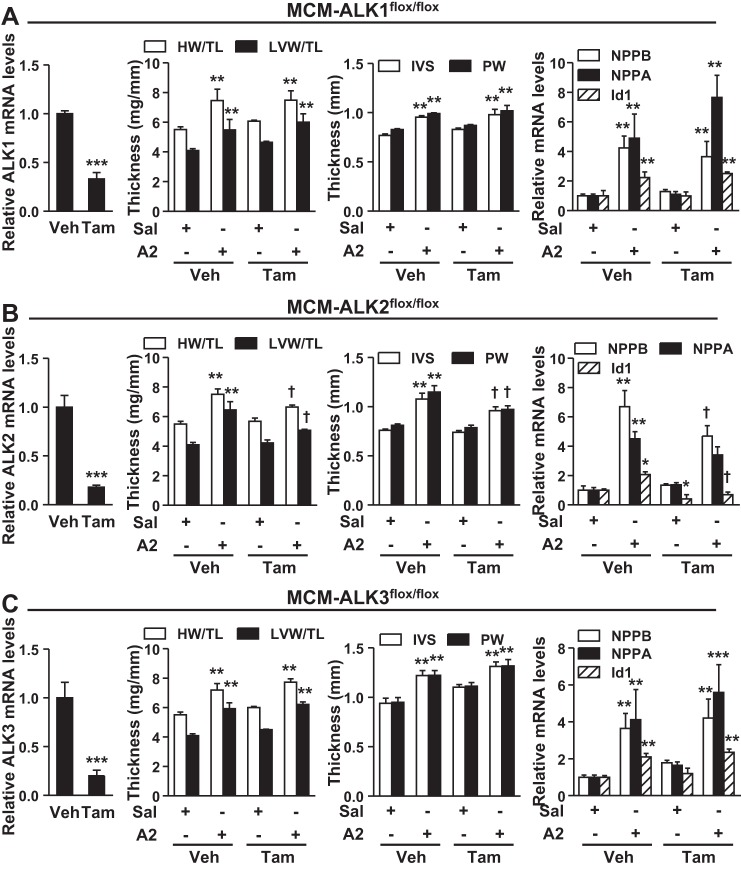
LDN prevents BMP signaling by inhibiting multiple BMP type I receptors (12, 19). Based on our quantitative PCR results, ALK2 and ALK3 were expressed at high levels, and ALK1 was expressed at modest levels in the heart. In contrast, ALK6 was detected at very low levels (data not shown). Therefore, to identify which type I receptor is required for A2-induced cardiac hypertrophy, we generated mice carrying the MCM transgene and floxed ALK1, ALK2, or ALK3 alleles. To selectively delete BMP type I receptor in cardiomyocytes, we treated mice with tamoxifen (1). In MCM-ALK1fl/fl, MCM-ALK2fl/fl, and MCM-ALK3fl/fl mice, administration of tamoxifen induced an ∼80% reduction in myocardial ALK1, ALK2, and ALK3 mRNA levels, respectively (left panels, Fig. 6, A–C, respectively). Compared with vehicle-treated mice, tamoxifen did not alter the baseline levels of markers of cardiac hypertrophy, including NPPA and NPPB gene expression, when measured 5 wk after tamoxifen administration (Fig. 6, A–C).
Fig. 6.
Activin-like kinase 2 (ALK2) is required for A2-induced cardiac hypertrophy. Tamoxifen (Tam) administration (30 mg·kg−1·day−1 ip) in α-MHC-MerCreMer (MCM)-ALK1flox/flox, MCM-ALK2flox/flox, and MCM-ALK3flox/flox mice for 5 days reduced mRNA levels of ALK1 (A), ALK2 (B), and ALK3 (C), respectively, in cardiomyocytes obtained from LV 5 wk after Tam injection (left). Before treatment with Tam and deletion of the floxed alleles, A2 administration in MCM-ALK1flox/flox (A), MCM-ALK2flox/flox (B), and MCM-ALK3flox/flox mice (C) increased the HW/TL and LVW/TL and increased IVS and PW thickness. A–C: A2 administration induced NPPB, NPPA, and Id1 expression in the LVs of these mice. After administration of Tam for 5 days to delete cardiac ALK1, ALK2, or ALK3, the effects of A2 on HW/TL and LVW/TL and IVS and PW thickness were inhibited in ALK2-deficient mice (B), but not in ALK1- (A) or ALK3-deficient animals (C). The effects of A2 on LV expression of NPPB, NPPA, and Id1 genes were inhibited in ALK2-deficient, but not ALK1- or ALK3-deficient mice. Values are means ± SE; n = 6–7 animals in each group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. corresponding saline or Veh treatment. †P < 0.05 vs. A2 + Veh treatment.
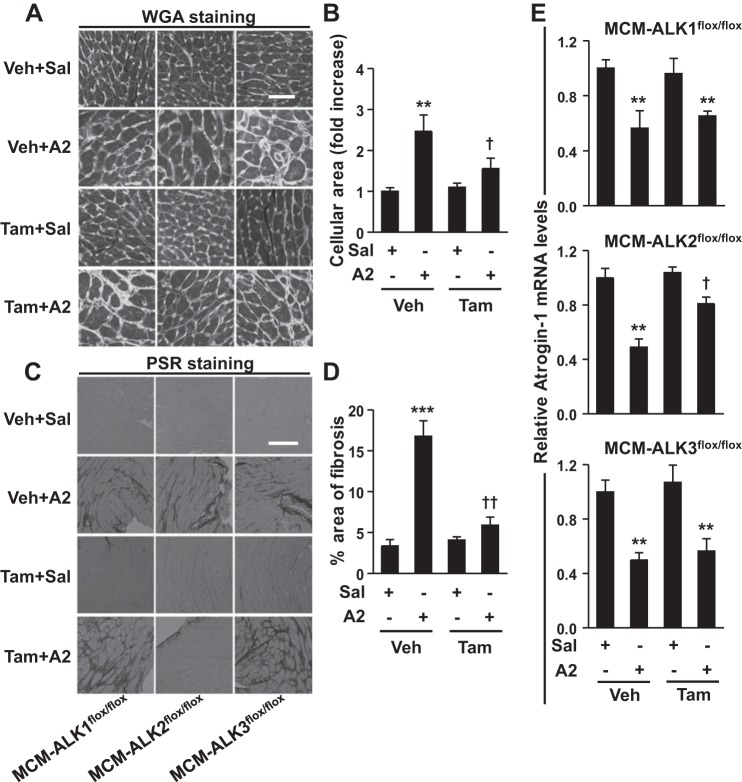
A2 infusion induced cardiac hypertrophy in control mice as evident by increased HW/TL and LVW/TL, increased IVS and PW thickness, and induction of NPPA and NPPB gene expression in LV (Fig. 6). Cardiomyocyte-specific deletion of ALK2 (Fig. 6B), but not ALK1 (Fig. 6A) or ALK3 (Fig. 6C), significantly attenuated the development of A2-induced cardiac hypertrophy, as reflected by marked reductions in these parameters. Similar to the observation in WT mice, HR, LV end-diastolic diameter, LV end-systolic diameter, and fractional shortening were not altered with A2 treatment in ALK2-deficient mice (Table 1). Deletion of ALK2, but not ALK1 or ALK3, inhibited both basal and A2-induced Id1 gene expression (Fig. 6, right), suggesting that ALK2 is required for basal and A2-induced cardiac BMP signaling. The A2-induced increases in cardiomyocyte size (Fig. 7, A and B) and collagen deposition (Fig. 7, C and D) in the LV were also decreased in mice with cardiomyocyte-specific ALK2 deficiency. A2 infusion repressed atrogin-1 gene expression by ∼50% in LV from control animals, and this effect was partially reversed in mice with ALK2 (Fig. 7E, middle), but not ALK1 or ALK3 (Fig. 7E, top and bottom), deficiency in cardiomyocytes. Collectively, these findings suggest that ALK2 is the primary BMP type I receptor that mediates A2-induced BMP signaling and cardiac hypertrophy.
Fig. 7.
Cardiac ALK2 deficiency inhibits A2-induced cardiomyocytes hypertrophy, fibrosis, and repression of atrogin-1 expression in the LV. A2 administration in MCM-ALK1flox/flox, MCM-ALK2flox/flox, and MCM-ALK3flox/flox mice increased the cardiomyocyte area (A) and collagen deposition (C) in LVs, as assess by WGA and picro-sirius red (PSR) staining, respectively. ALK2, but not ALK1 or ALK3, deficiency after Tam administration (30 mg·kg−1·day−1 for 5 days) inhibited A2-induced increases in cardiomyocytes area (B) and collagen deposition (D), as quantified in images shown in A and C. E: A2 administration repressed atrogin-1 gene expression in the LVs of MCM-ALK1flox/flox, MCM-ALK2flox/flox, and MCM-ALK3flox/flox mice. However, the A2-induced repression of atrogin-1 was partially restored in ALK2- but not ALK1- or ALK3-deficient mice (middle). Values are means ± SE; n = 6–7 animals in each group. **P < 0.01 and ***P < 0.001 vs. corresponding saline treatment. †P < 0.05 vs. A2 + Veh treatment. Bars in WGA and PSR images in A and C represent 50 and 200 μm, respectively.
DISCUSSION
This study demonstrates that BMP signaling is upregulated in both in vitro and in vivo models of cardiac hypertrophy, as evident by enhanced phosphorylation of Smad 1/5 protein levels and increased Id1 gene expression. Inhibition of BMP type I receptors, sequestration of BMP ligands, or silencing of BMP-responsive Smad 1 or Smad 4 prevents PE-induced hypertrophy in NRCs. Conversely, activation of BMP signaling in NRCs induces hypertrophy, likely via the atrogin-1/calcineurin/NFAT pathway. In a mouse model of cardiac hypertrophy, inhibition of BMP type I receptors with LDN attenuates A2-induced cardiac hypertrophy and collagen deposition. ALK2 in cardiomyocytes is the primary type I BMP receptor required for BMP signaling and A2-induced cardiac hypertrophy.
BMP signaling was previously shown to play a role in cardiac hypertrophy (21, 22). However, the mechanism by which BMP signaling contributes to cardiac hypertrophy remained unknown. We focused on the calcineurin/NFAT pathway because BMP signaling was recently shown to increase NFAT activity in pulmonary endothelial cells and keratinocytes (6, 8, 24), and because the calcineurin/NFAT pathway plays a central role in the pathogenesis of cardiac hypertrophy (14, 23). Calcineurin promotes translocation of NFAT into the nucleus, where it induces the transcription of fetal cardiac gene program, including NPPA and NPPB, to induce hypertrophy (15, 17). In this study, we demonstrated that BMP signaling increased calcineurin protein levels in cardiomyocytes and promoted the translocation of NFATc3 into the nucleus. NFATc3, a member of NFAT family, has an important role in the development of cardiac hypertrophy (25). Inhibition of calcineurin with FK506 (4) abrogated BMP signaling-induced increases in the activity of NRE and MCIP gene expression (a direct target of NFAT), suggesting that BMP signaling induced hypertrophy via calcineurin. Calcineurin activity is tightly regulated by atrogin-1, a ubiquitin ligase that targets calcineurin for degradation (9, 15, 20). Previous studies showed that overexpression of atrogin-1 in the hearts of transgenic mice suppressed calcineurin protein levels and blunted pressure overload-induced cardiac hypertrophy (9). Conversely, downregulation of atrogin-1 enhanced agonist-induced calcineurin activity and cardiomyocyte hypertrophy (9). In this study, we observed that activation of BMP signaling robustly suppressed atrogin-1 transcription, suggesting a mechanism by which BMP signaling increases calcineurin levels and induces hypertrophy.
Pharmacological inhibition of BMP signaling with LDN blunted A2-induced cardiac hypertrophy and collagen deposition, consistent with a previous report (21). Because LDN blocks multiple BMP type I receptors, we sought to identity which BMP type I receptor is required for the development of cardiac hypertrophy. Because ALK1, ALK2, and ALK3 are the major BMP type I receptors expressed in heart, we used a conditional knockout approach to delete ALK1, ALK2, or ALK3 selectively from cardiomyocytes. Decreased ALK2, but not ALK1 or ALK3, was sufficient to inhibit basal and A2-induced BMP signaling, suggesting that ALK2 is the primary BMP type I receptor required for BMP signaling in cardiomyocytes. Depletion of ALK2, but not ALK1 or ALK3, attenuated A2-induced cardiac hypertrophy and reduced collagen deposition in LV. A2-induced cardiac hypertrophy in mice was accompanied by a reduction in atrogin-1 gene expression in LV, as was reported previously (9, 15). Deficiency of ALK2 partially restored atrogin-1 expression, suggesting that BMP signaling via ALK2 receptor contributes to the downregulation of atrogin-1 expression during the development of cardiac hypertrophy. Because atrogin-1 has a critical role in the development of pressure overload cardiac hypertrophy and aging-related cardiomyopathy (9, 27), targeting the ALK2 receptor may provide an effective means to treat these disorders. A2 induces cardiac hypertrophy via both blood pressure-dependent and -independent pathways. Our study indicates that blockade of BMP signaling inhibits cardiac hypertrophy independent of blood pressure. However, it does not rule out a direct impact of pressure or volume overload on cardiac hypertrophy. Perhaps BMP signaling is activated secondarily as a consequence of pressure overload, regardless of stimuli, which then induces hypertrophy. The previous findings that both BMP signaling and atrogin-1 are also required for pressure overload-induced cardiac hypertrophy support this notion and suggest that BMP signaling-atrogin-1 pathway may represent an inherent characteristic of pathological cardiac hypertrophy.
In this study, we observed that BMP2 treatment for 24 h at a concentration of 10 ng/ml was able to induce hypertrophy in NRCs. In contrast, Lu and colleagues (10) recently reported that BMP2 treatment for 48 h at high concentration (50 ng/ml) did not induce hypertrophy in NRCs, but BMP2 instead antagonized BMP4-induced hypertrophy in an Akt-dependent manner. Whether the observed differences in the two studies are caused by the different BMP2 concentrations, different time points studied, or different culture conditions remains to be determined.
In conclusion, we report that BMP signaling is required for PE-induced hypertrophy in NRCs and A2-induced cardiac hypertrophy in mice. BMP signaling inhibits atrogin-1 expression and upregulates the calcineurin/NFAT pathway and contributes to the development of cardiac hypertrophy. ALK2 is the primary BMP type I receptor required for BMP signaling in cardiomyocytes and for A2-induced cardiac hypertrophy and fibrosis in mice. Pharmacological inhibition of ALK2 may prove to be a novel approach to the treatment of cardiac hypertrophy.
GRANTS
This work was supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases (R01DK082971 to K. D. Bloch, D. B. Bloch), the Leducq Foundation (C. Mayeur, P. A. Leyton, K. D. Bloch, D. B. Bloch), a LaDue Memorial Fellowship Award (D. K. Rhee), and from the French Federation of Cardiology (L. Ernande).
DISCLOSURES
The Massachusetts General Hospital has filed patents related to the use of small molecule inhibitors of BMP signaling to modulate iron metabolism, and the estate of K. D. Bloch may be eligible to receive royalties. The other authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: M.S., M.X.W., M.S.-C., E.S.B., W.M.Z., K.D.B., and D.B.B. conception and design of research; M.S., E.S., L.E., R.T., S.A.K., P.A.L., J.C., R.E.T.T., C.M., and D.K.R. performed experiments; M.S., E.S., L.E., R.T., S.A.K., P.A.L., J.C., R.E.T.T., C.M., and D.K.R. analyzed data; M.S., L.E., R.T., J.C., M.X.W., M.S.-C., E.S.B., W.M.Z., K.D.B., and D.B.B. interpreted results of experiments; M.S. prepared figures; M.S. drafted manuscript; M.S., M.X.W., M.S.-C., E.S.B., W.M.Z., K.D.B., and D.B.B. edited and revised manuscript; M.S., E.S., L.E., R.T., S.A.K., P.A.L., J.C., R.E.T.T., C.M., D.K.R., M.X.W., M.S.-C., E.S.B., W.M.Z., K.D.B., and D.B.B. approved final version of manuscript.
ACKNOWLEDGEMENTS
The authors thank Yushi Mishina and Vesa Kaartinen for providing Alk2+/flox and Alk3+/flox mice, respectively; and Hua Su for ALK1+/flox mice. Authors also thank Dr. J. Molkentin (University of Cincinnati) and Dr. C. Goodman (University of Wisconsin) for kind gifts of NFAT luciferase reporter and atrogin-1 promoter luciferase reporter constructs, respectively.
REFERENCES
- 1.Ali R, Huang Y, Maher SE, Kim RW, Giordano FJ, Tellides G, Geirsson A. miR-1 mediated suppression of Sorcin regulates myocardial contractility through modulation of Ca2+ signaling. J Mol Cell Cardiol 52: 1027–1037, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev 121: 173–182, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Frantz S, Klaiber M, Baba HA, Oberwinkler H, Volker K, Gabetaner B, Bayer B, Abebetaer M, Schuh K, Feil R, Hofmann F, Kuhn M. Stress-dependent dilated cardiomyopathy in mice with cardiomyocyte-restricted inactivation of cyclic GMP-dependent protein kinase I. Eur Heart J 34: 1233–1244, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao H, Wang F, Wang W, Makarewich CA, Zhang H, Kubo H, Berretta RM, Barr LA, Molkentin JD, Houser SR. Ca(2+) influx through L-type Ca(2+) channels and transient receptor potential channels activates pathological hypertrophy signaling. J Mol Cell Cardiol 53: 657–667, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129: e28–e292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132: 299–310, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koitabashi N, Bedja D, Zaiman AL, Pinto YM, Zhang M, Gabrielson KL, Takimoto E, Kass DA. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ Res 105: 12–15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156: 440–455, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest 114: 1058–1071, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Sun B, Huo R, Wang YC, Yang D, Xing Y, Xiao XL, Xie X, Dong DL. Bone morphogenetic protein-2 antagonizes bone morphogenetic protein-4 induced cardiomyocyte hypertrophy and apoptosis. J Cell Physiol 229: 1503–1510, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32: 69–72, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem 147: 35–51, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Molkentin JD, Dorn GW 2nd. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol 63: 391–426, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation 114: 1159–1168, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, Oh SP. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood 111: 633–642, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothermel BA, Vega RB, Williams RS. The role of modulatory calcineurin-interacting proteins in calcineurin signaling. Trends Cardiovasc Med 13: 15–21, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8: 970–982, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem 280: 20814–20823, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun B, Huo R, Sheng Y, Li Y, Xie X, Chen C, Liu HB, Li N, Li CB, Guo WT, Zhu JX, Yang BF, Dong DL. Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension 61: 352–360, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Sun B, Sheng Y, Huo R, Hu CW, Lu J, Li SL, Liu X, Wang YC, Dong DL. Bone morphogenetic protein-4 contributes to the down-regulation of Kv4.3 K+ channels in pathological cardiac hypertrophy. Biochem Biophys Res Commun 436: 591–594, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Vega RB, Bassel-Duby R, Olson EN. Control of cardiac growth and function by calcineurin signaling. J Biol Chem 278: 36981–36984, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Wen Z, Han L, Bamburg JR, Shim S, Ming GL, Zheng JQ. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J Cell Biol 178: 107–119, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol 22: 7603–7613, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4: 33–41, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaglia T, Milan G, Ruhs A, Franzoso M, Bertaggia E, Pianca N, Carpi A, Carullo P, Pesce P, Sacerdoti D, Sarais C, Catalucci D, Kruger M, Mongillo M, Sandri M. Atrogin-1 deficiency promotes cardiomyopathy and premature death via impaired autophagy. J Clin Invest 124: 2410–2424, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]