Vascular endothelial (VE)-cadherin is S-nitrosylated and phosphorylated by 1-O-alkyl-2-acetyl-sn-glycero-3-phospho-choline (PAF) at the endothelial cell junction. Importantly, we advance the concept that S-nitrosylation activity is necessary for VE-cadherin phosphorylation, which regulates its internalization. Our work supports the key function of S-nitrosylation in the complex dynamics of junctional proteins in the regulation of microvascular permeability.
Keywords: inflammation, endothelial permeability, VE-cadherin, S-nitrosylation, adherens junction
Abstract
The adherens junction complex, composed mainly of vascular endothelial (VE)-cadherin, β-catenin, p120, and γ-catenin, is the main element of the endothelial barrier in postcapillary venules. S-nitrosylation of β-catenin and p120 is an important step in proinflammatory agents-induced hyperpermeability. We investigated in vitro and in vivo whether or not VE-cadherin is S-nitrosylated using platelet-activating factor (PAF) as agonist. We report that PAF-stimulates S-nitrosylation of VE-cadherin, which disrupts its association with β-catenin. In addition, based on inhibition of nitric oxide production, our results strongly suggest that S-nitrosylation is required for VE-cadherin phosphorylation on tyrosine and for its internalization. Our results unveil an important mechanism to regulate phosphorylation of junctional proteins in association with S-nitrosylation.
NEW & NOTEWORTHY
Vascular endothelial (VE)-cadherin is S-nitrosylated and phosphorylated by 1-O-alkyl-2-acetyl-sn-glycero-3-phospho-choline (PAF) at the endothelial cell junction. Importantly, we advance the concept that S-nitrosylation activity is necessary for VE-cadherin phosphorylation, which regulates its internalization. Our work supports the key function of S-nitrosylation in the complex dynamics of junctional proteins in the regulation of microvascular permeability.
normal microvascular permeability depends on the maintenance of endothelial barrier by adherens junctions. In postcapillary venules, vascular endothelial (VE)-cadherin plays a significant role in barrier function (8). VE-cadherin is a single-pass transmembrane protein, with the amino terminal in the extracellular domain and the carboxy terminal in the intracellular domain. The amino terminal is the site of homotypic adhesion, whereas the carboxy terminal associates with several cytoplasmic proteins, including β-catenin, p120-catenin (p120), and γ-catenin. VE-cadherin is a fundamental component of adherens junctions to the extent that VE-cadherin knockout mice die during gestation because of inability to develop vascular structures (34). In addition, endothelial cells that are null for VE-cadherin have a very elevated basal permeability (6).
Microvascular permeability to macromolecules, which occurs mainly at postcapillary venules, is subject to regulation during inflammation and is increased by proinflammatory agents. We have used 1-O-alkyl-2-acetyl-sn-glycero-3-phospho-choline (PAF) to assess the mechanisms that regulate the onset of increased microvascular permeability. PAF increases endothelial permeability via endothelial nitric oxide (NO) synthase (eNOS) phosphorylation, eNOS translocation to cytosol, and NO production in vitro and in vivo (10, 11, 23, 28–31). We recently advanced the novel concept that PAF triggers hyperpermeability through S-nitrosylation of β-catenin and p120, and this modification correlates with disruption of the adherens junction complex and subcellular redistribution of these proteins from the plasma membrane to cytosol (23). S-nitrosylation is a post-translational modification that regulates several cellular functions (15, 17); however, only β-catenin and p120 have been described as targets of S-nitrosylation in regard to microvascular permeability (23, 33). In this report, we tested the hypotheses that 1) S-nitrosylation regulates VE-cadherin function in hyperpermeability as well as its associated internalization and that 2) S-nitrosylation is necessary for phosphorylation of VE-cadherin. Our results positively support both hypotheses.
MATERIALS AND METHODS
Reagents and antibodies.
PAF and N-acetyl-l-cysteine (NAC) were obtained from Calbiochem (San Diego, CA). NG-methyl-l-arginine (l-NMA) was from Sigma Chemicals (St. Louis, MO). NAC (2.5 mmol/l) was applied for 2.5 h before the addition of agonist. l-NMA (300 μmol/l) was applied for 1 h before PAF addition. Administration of both chemicals was continued throughout the experiment. Goat anti VE-cadherin and mouse anti-phosphotyrosine were from Santa Cruz Biotechnology (Dallas, TX). Rabbit anti-β-catenin and mouse anti-β-actin were from Sigma-Aldrich (St. Louis, MO). Mouse anti-p120 was from BD Biosciences (San Jose, CA).
Cell culture.
Immortalized human venous endothelial cells, EAhy926 (derived from human umbilical vein; kindly donated by Dr C. J. S. Edgell, University of North Carolina, Chapel Hill, NC) (12), were grown in basal media containing Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml fungizone and sodium hypoxanthine, aminopterin, and thymidine (HAT). Bovine coronary postcapillary venular endothelial cells, (CVECs; kindly donated by Dr. C. J. Meininger, Texas A&M University, Temple, TX) (32) were grown in the same medium with no HAT and additionally supplemented with 20 U/ml heparin.
Western blot analysis, immunoprecipitation, and cell surface biotinylation.
These methods are standard in our laboratory and were applied according to published protocols (29–31).
Biotin-switch assay.
Total protein (100 μg) obtained from cellular lysates from control and agonist-treated cells were denatured with sodium dodecyl sulfate (SDS) in the presence of methyl methanethiosulfonate (18). After acetone precipitation to remove excess methyl methanethiosulfonate, 1 mmol/l ascorbate and N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide (biotin-HPDP) were added to reduce the S-nitrosylated bond and label the reduced thiol with biotin, respectively. Biotinylated proteins were captured with streptavidin-agarose beads and then separated by SDS-PAGE and detected with specific antibodies by Western blot analysis.
Immunofluorescence microscopy.
We followed our established protocols (23, 28, 30). Cells were cultured on glass coverslips coated with fibronectin, treated with agonist, and then fixed in 4% paraformaldehyde for 20 min at room temperature and permeabilized with PBS-Triton X-100 (0.5%) for 10 min. The secondary Alexa Fluor-conjugated antibodies were added after incubation with corresponding primary antibodies for 1 h at room temperature. Nuclei were stained with 4,6-diamidino-2-phenylindole for 5 min at room temperature. Images were obtained using an epifluorescence microscope (Axioscop; Carl Zeiss) equipped with a 100× oil immersion objective lens and Axio Vision Rel. software (Zeiss, Germany). Eight-bit images were prepared for publication in Adobe Photoshop.
In vivo studies.
Protocols for experiments on animals were approved by the Institutional Bioethics and Biosecurity Committee of the Pontificia Universidad Católica de Chile and conducted according to National Institutes of Health guidelines for the use of animals in research. We measured microvascular permeability to macromolecules by intravital microscopy according to our published methods (4, 5, 14, 20, 21) in the cheek pouch of male Golden Syrian hamsters (110–130 g) and S-nitrosylation of VE-cadherin in the cremaster muscle of male mice (12–15 wk old, weighing 30–35 g) (23).
Statistical analysis.
Experiments were conducted in groups with minimum n = 3. Data are expressed as means ± SE. Apparent differences were assessed for statistical significance using paired Student's t-test. Significance was accepted at P ≤ 0.05.
RESULTS
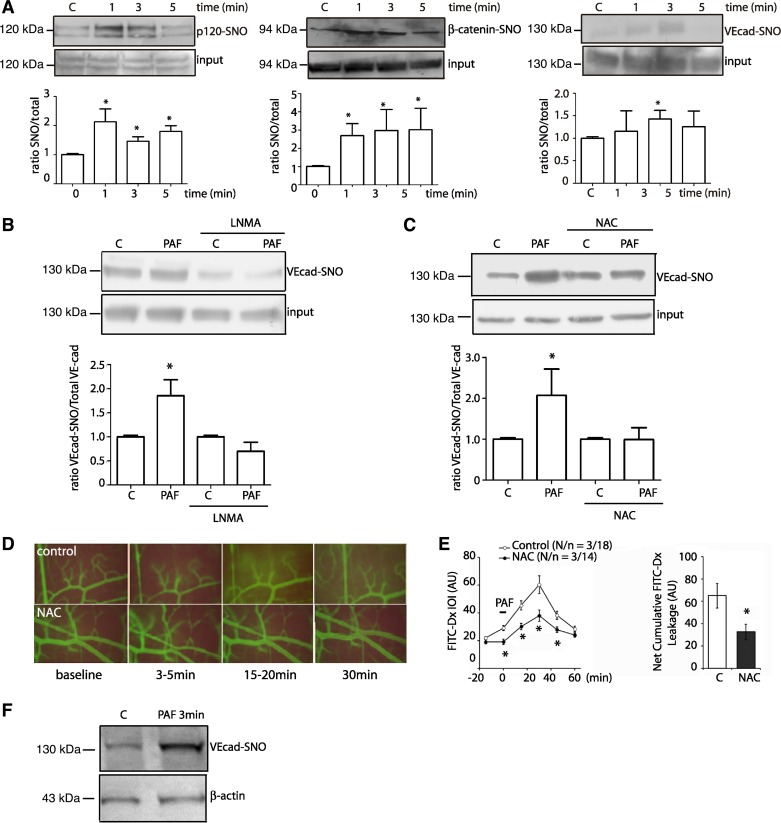
PAF increases permeability through S-nitrosylation of the adherens junction complex in vivo and in vitro. We tested the S-nitrosylation of VE-cadherin as well as p120 and β-catenin using EAhy926 endothelial cells, as we have shown that these cells faithfully recapitulate mechanisms involved in regulation of permeability (23). PAF induced S-nitrosylation of p120, β-catenin, and VE-cadherin (Fig. 1A) with significant changes observed at 1 min for p120 and β-catenin and at 3 min for VE-cadherin. We tested the dependence of S-nitrosylation on NO by administration of l-NMA to EAhy926 endothelial cells and that of NAC (which is a precursor for glutathione) in CVECs. Inhibition of NO synthase with l-NMA blocked PAF-induced S-nitrosylation of VE-cadherin (Fig. 1B), whereas NAC indirectly prevented PAF-induced S-nitrosylation of VE-cadherin (Fig. 1C). To determine in vivo the significance of S-nitrosylation of junctional proteins, we topically applied 100 nmol/l PAF to the hamster cheek pouch and assessed permeability to FITC-dextran 70 in control conditions and after NAC application. PAF significantly increased permeability, reaching peak magnitude at about 30 min and lasting for ∼60 min (Fig. 1, D and E). Topical application of NAC (2.5 mmol/l) did not influence basal permeability but significantly reduced the effect of PAF to a significantly smaller increase in permeability. Figure 1E shows the time course and the statistical analysis of changes in microvascular permeability in the hamster cheek pouch. To verify that S-nitrosylation of junctional proteins occurs in vivo across species and organs, we used the mouse cremaster model (23). PAF, applied for 3 min, induced S-nitrosylation of VE-cadherin in the mouse cremaster (Fig. 1F).
Fig. 1.
Platelet-activating factor (PAF) increases permeability through S-nitrosylation of the adherens junction complex in vitro and in vivo. A: S-nitrosylation of p120, β-catenin, and vascular endothelial-cadherin (VE-Cad) after PAF administration in EAhy926 cells. C, control; SNO, S-nitrosylated. B: NG-methyl-l-arginine (l-NMA) inhibits PAF-induced S-nitrosylation of VE-cadherin in EAhy926 cells. C: treatment with N-acetyl-l-cysteine (NAC) (2.5 mmol/l for 2.5 h) prevents PAF-induced S-nitrosylation in coronary postcapillary venular endothelial cells (PAF, 100 nmol/l for 3 min). D: microvascular permeability in the hamster cheek pouch. PAF (100 nmol/l) increased permeability to FITC-dextran 70 (FITC-Dx) in control (top). Pretreatment with NAC blocked PAF-induced hyperpermeability (bottom). E: time course of FITC-Dx leakage in the hamster cheek pouch, as determined by integrated optical intensity (IOI) in interstitium. PAF was applied topically at time −5 min. The statistical analysis bar graph shows the integral of the area under the curve (above baseline) for interstitial FITC-Dx fluorescence for control and NAC during the observation period. N/n, number of animals and number of microvascular areas analyzed, respectively; AU, arbitrary units. F: PAF topically applied in vivo for 3 min at 100 nmol/l induces S-nitrosylation of VE-cadherin in mouse cremaster muscle. For all the Western blots, the S-nitrosylated proteins and input were run in separate gels. Images were subsequently cropped to construct the final figure; n = 3. *P ≤ 0.05 compared with control. Data are expressed as means ± SE. Apparent differences were assessed for statistical significance using paired Student's t-test.
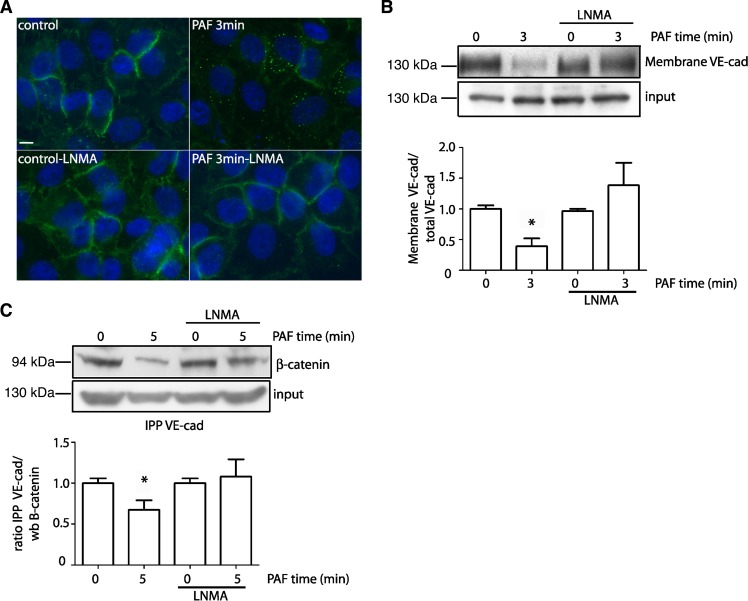
VE-cadherin internalization is dependent on S-nitrosylation. PAF-induced S-nitrosylation of p120 and β-catenin is associated with internalization of these proteins and disruption of the adherens junction complex (23). We tested whether PAF-induced S-nitrosylation modifies/regulates VE-cadherin localization at the adherens junction. Figure 2A shows that VE-cadherin is mainly distributed at the plasma membrane under control conditions. After PAF, the distribution of VE-cadherin at the plasma membrane decreases, as determined by fluorescence microscopy. Application of l-NMA, which blocks PAF-stimulated NO release, prevented PAF-induced subcellular redistribution of VE-cadherin (Fig. 2A). To provide an independent assessment, we examined the location of VE-cadherin by cell surface biotinylation. Figure 2B shows that PAF decreases the amount of VE-cadherin at the cell surface, indicating PAF-induced internalization of VE-cadherin. Inhibition of eNOS-derived NO production blocked PAF-induced internalization (Fig. 2B). Subsequently, we analyzed whether PAF-induced S-nitrosylation influenced the association between VE-cadherin and β-catenin. Figure 2C demonstrates that PAF reduces the association between VE-cadherin and β-catenin. Application of l-NMA to inhibit eNOS blocked the disruption of the adherens junction complex and preserved the association between VE-cadherin and β-catenin at the cell membrane. These data indicate that S-nitrosylation is an important mechanism for the internalization and interactions of VE-cadherin at the adherens junction complex.
Fig. 2.
VE-cadherin internalization depends on endothelial nitric oxide synthase (eNOS) activity and S-nitrosylation. A: PAF (100 nmol/l for 3 min) induces internalization of VE-cadherin in EAhy926 monolayers (top; VE-cadherin = green fluorescent label). Inhibition of eNOS with l-NMA pretreatment blocks PAF-induced VE-cadherin internalization and retains the fluorescent label at the cell membrane (bottom). B: VE-cadherin location examined by cell surface biotinylation in EAhy926 monolayers. PAF, applied for 3 min, reduces VE-cadherin at the cell surface. l-NMA inhibits PAF-induced redistribution of VE-cadherin. C: Association between VE-cadherin and β-catenin by coimmunoprecipitation. Cells extracts from EAhy926 cells were immunoprecipitated with VE-cadherin antibody and probed for β-catenin. PAF disrupts the interaction between VE-cadherin and β-catenin. Inhibition of eNOS with l-NMA pretreatment blocks PAF-induced dissociation of the interaction between VE-cadherin and β-catenin. For all the Western blots (wb), the modified proteins and input were run in separate gels. Images were cropped subsequently to construct the final figure. n = 3. *P ≤ 0.05 compared with control. Data are expressed as means ± SE. Apparent differences were assessed for statistical significance using paired Student's t-test.
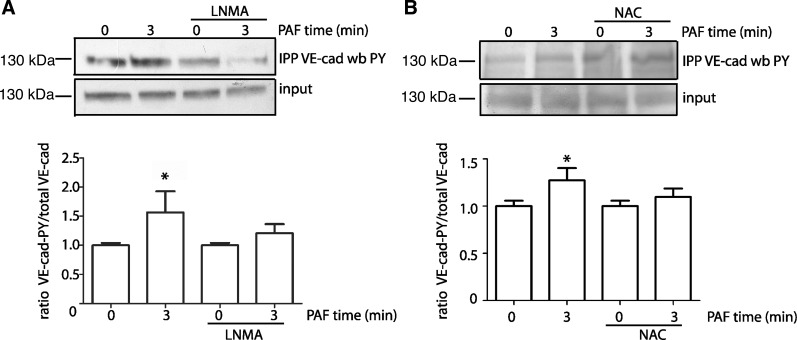
PAF induced VE-cadherin phosphorylation at tyrosine depends on S-nitrosylation. Phosphorylation is a universal classical regulatory mechanism and plays a role in VE-cadherin internalization (9, 13, 25). To determine if a mechanistic relationship exists between phosphorylation and S-nitrosylation, we examined VE-cadherin phosphorylation in response to PAF in the presence of l-NMA and NAC in EAhy926 cells. Figure 3A shows that PAF induces tyrosine phosphorylation of VE-cadherin at tyrosine residues and that eNOS blockade with l-NMA prevents this tyrosine phosphorylation. Similarly, indirect blockade of the S-nitrosylation with NAC inhibits PAF-induced phosphorylation of VE-cadherin at tyrosine residues (Fig. 3B).
Fig. 3.
PAF induces VE-cadherin phosphorylation dependent on S-nitrosylation activity. EAhy926 monolayers were treated with 100 nmol/l PAF for 3 min in the absence and presence of l-NMA (A) and NAC (B). VE-cadherin was immunoprecipitated (IPP) from the cell lysates and probed by Western blot analysis with anti-phospho-tyrosine (PY) antibodies. Inhibition of eNOS with l-NMA and NAC pretreatment block PAF-induced tyrosine phosphorylation of VE-cadherin. For all the Western blots, the phosphorylated proteins and input were run in separate gels. Images were cropped subsequently to construct the final figure. n = 3. *P ≤ 0.05 compared with control. Data are expressed as means ± SE. Apparent differences were assessed for statistical significance using paired Student's t-test.
To ensure that l-NMA did not cause changes in total VE-cadherin, we evaluated the amount of VE-cadherin in the input, as determined by Western blot analysis and arbitrary units of band density, in control and after l-NMA in all the experiments. The statistical analysis showed mean ± SE values of 51.4 ± 2.0 for control and 48.7 ± 2.0 for l-NMA (P = 0.35; n = 14). Thus l-NMA did not induce significant changes in VE-cadherin input.
DISCUSSION
Regulation of microvascular permeability is highly important for overall body homeostasis in health and disease. Our data provide important advances in our understanding of permeability regulatory mechanisms: 1) we demonstrate that VE-cadherin is another component of the endothelial adherens junction, besides p120 and β-catenin, that is modified by S-nitrosylation at the onset of hyperpermeability, and 2) blocking S-nitrosylation preserves the interaction of VE-cadherin with β-catenin and more importantly inhibits phosphorylation and internalization of VE-cadherin.
The onset of hyperpermeability depends on agonist-promoted translocation of eNOS to cytosol and S-nitrosylation of p120 and β-catenin (10, 11, 23, 27–31). These processes and onset of hyperpermeability are associated with disruption of the adherens junction complex and internalization of p120 and β-catenin (23). VE-cadherin, which is the most recognized junctional protein, also undergoes conformational changes and internalization (2, 3, 9, 13, 19, 22). However, it was unknown whether S-nitrosylation was involved in the dynamics of VE-cadherin. Our results demonstrate that VE-cadherin becomes S-nitrosylated after 3 min of exposing endothelial cells to 100 nmol/l PAF.
S-nitrosylation occurs in cysteines' free thiols. We do not know which cysteine residue (or residues) is relevant for VE-cadherin S-nitrosylation. Interestingly, VE-cadherin has one cysteine in the transmembrane domain very close to the intracellular domain and four additional cysteines in the extracellular domain (7). In a previous report, using an in vitro approach, we determined that p120 is S-nitrosylated preferentially at cysteine 579, which is located in the region where p120 binds to VE-cadherin (23). Cysteine 619 was identified as the cysteine S-nitrosylated by VEGF in β-catenin (33). As is the case for p120, Cys-619 is located in the putative binding region of β-catenin to VE-cadherin. Thus, it is possible that S-nitrosylation is the posttranslational modification that promotes disruption of the adherens junction complex and internalization of its constitutive proteins. This possibility is currently supported by the observations that blocking S-nitrosylation inhibits VE-cadherin internalization and disruption of the adherens junction complex.
It is yet unknown how junctional proteins become S-nitrosylated in the process triggering hyperpermeability. Translocation of eNOS to cytosol is required for NO production to increase permeability (29–31). Indeed, activation of eNOS anchored at the cell membrane leads to production of NO that neither causes S-nitrosylation of p120 and β-catenin nor hyperpermeability (23). One possibility to account for S-nitrosylation of VE-cadherin at the membrane is that eNOS-derived NO reaches p120 and β-catenin, which are on the cytosolic aspect of the cell membrane S-nitrosylating these proteins. It is plausible that S-nitrosylation of p120 and β-catenin causes conformational changes in VE-cadherin that allow access of NO to the VE-cadherin cysteine located in the transmembrane domain.
Phosphorylation is one of the most studied signaling mechanisms. PAF in fact induces tyrosine phosphorylation of VE-cadherin as a signal for internalization (16). We advance here the novel observation that S-nitrosylation activity is required for phosphorylation of VE-cadherin. The phosphorylation in tyrosine of VE-cadherin is dependent on src activity (1). Since VE-cadherin phosphorylation depends on src activity, it is possible that S-nitrosylation contributes to increase src kinase activity, as is the case for src in epithelial cells (26). However, other sites and signals may play a role as well in VE-cadherin internalization (9). Our observation that S-nitrosylation of VE-cadherin acts in tandem with its phosphorylation agrees with the report that tyrosine phosphorylation of VE-cadherin is needed but not enough to induce increase in permeability (1). Other mechanisms involved in VE-cadherin internalization include activation by Rac-p21-activated kinase (13) and inhibition by p120 binding to classical cadherins (24).
In summary, we provide evidence in support of the novel concept that S-nitrosylation of the adherens junction complex is required for onset of hyperpermeability and for activating phosphorylation signals leading to junctional protein internalization.
GRANTS
This study was supported by Fondecyt 1130769 (Chile) and National Heart, Lung, and Blood Institute Grant 5RO1-HL-070634.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.G., R.C., P.Z., L.R., P.B., J.S., M.P.B., A.K., and F.A.S. performed experiments; A.G., R.C., P.Z., L.R., P.B., J.S., M.P.B., and F.A.S. analyzed data; A.G., R.C., P.Z., L.R., P.B., J.S., M.P.B., W.N.D., and F.A.S. interpreted results of experiments; A.G., R.C., P.Z., L.R., P.B., M.P.B., and F.A.S. prepared figures; R.C., P.Z., J.S., M.P.B., W.N.D., and F.A.S. edited and revised manuscript; J.S., M.P.B., W.N.D., and F.A.S. conception and design of research; J.S., W.N.D., and F.A.S. approved final version of manuscript; W.N.D. and F.A.S. drafted manuscript.
REFERENCES
- 1.Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J Biol Chem 285: 7045–7055, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander JS, Alexander BC, Eppihimer LA, Goodyear N, Haque R, Davis CP, Kalogeris TJ, Carden DL, Zhu YN, Kevil CG. Inflammatory mediators induce sequestration of VE-cadherin in cultured human endothelial cells. Inflammation 24: 99–113, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Alexander JS, Jackson SA, Chaney E, Kevil CG, Haselton FR. The role of cadherin endocytosis in endothelial barrier regulation: involvement of protein kinase C and actin-cadherin interactions. Inflammation 22: 419–433, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Armenante PM, Kim D, Duran WN. Experimental determination of the linear correlation between in vivo TV fluorescence intensity and vascular and tissue FITC-DX concentrations. Microvasc Res 42: 198–208, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Bekker AY, Ritter AB, Duran WN. Analysis of microvascular permeability to macromolecules by video-image digital processing. Microvasc Res 38: 200–216, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98: 147–157, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J Cell Biol 154: 231–243, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejana E, Orsenigo F. Endothelial adherens junctions at a glance. J Cell Sci 126: 2545–2549, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo A, Lin MI, Murata T, Landskroner-Eiger S, Schleicher M, Kothiya M, Iwakiri Y, Yu J, Huang PL, Sessa WC. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J Cell Sci 126: 5541–5552, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duran WN, Beuve AV, Sanchez FA. Nitric oxide, S-nitrosation, and endothelial permeability. IUBMB Life 65: 819–826, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duran WN, Breslin JW, Sanchez FA. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc Res 87: 254–261, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 80: 3734–3737, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8: 1223–1234, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Hatakeyama T, Pappas PJ, Hobson RW 2nd, Boric MP, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol 574: 275–281, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron 46: 533–540, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hudry-Clergeon H, Stengel D, Ninio E, Vilgrain I. Platelet-activating factor increases VE-cadherin tyrosine phosphorylation in mouse endothelial cells and its association with the PtdIns3'-kinase. FASEB J 19: 512–520, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwakiri Y, Satoh A, Chatterjee S, Toomre DK, Chalouni CM, Fulton D, Groszmann RJ, Shah VH, Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci USA 103: 19777–19782, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001: pl1, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kevil CG, Ohno N, Gute DC, Okayama N, Robinson SA, Chaney E, Alexander JS. Role of cadherin internalization in hydrogen peroxide-mediated endothelial permeability. Free Radic Biol Med 24: 1015–1022, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Armenante PM, Duran WN. Mathematical modeling of mass transfer in microvascular wall and interstitial space. Microvasc Res 40: 358–378, 1990. [DOI] [PubMed] [Google Scholar]
- 21.Kim D, Armenante PM, Duran WN. Transient analysis of macromolecular transport across microvascular wall and into interstitium. Am J Physiol Heart Circ Physiol 265: H993–H999, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem 284: 5381–5394, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin N, Zamorano P, Carrasco R, Mujica P, Gonzalez FG, Quezada C, Meininger CJ, Boric MP, Duran WN, Sanchez FA. S-Nitrosation of beta-catenin and p120 catenin: a novel regulatory mechanism in endothelial hyperpermeability. Circ Res 111: 553–563, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol 199: 365–380, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Grazia Lampugnani M, Dejana E. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 3: 1208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman MA, Senga T, Ito S, Hyodo T, Hasegawa H, Hamaguchi M. S-nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J Biol Chem 285: 3806–3814, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez FA, Ehrenfeld IP, Duran WN. S-nitrosation of proteins: An emergent regulatory mechanism in microvascular permeability and vascular function. Tissue Barriers 1: e23896, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez FA, Kim DD, Duran RG, Meininger CJ, Duran WN. Internalization of eNOS via caveolae regulates PAF-induced inflammatory hyperpermeability to macromolecules. Am J Physiol Heart Circ Physiol 295: H1642–H1648, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez FA, Rana R, Gonzalez FG, Iwahashi T, Duran RG, Fulton DJ, Beuve AV, Kim DD, Duran WN. Functional significance of cytosolic endothelial nitric-oxide synthase (eNOS): regulation of hyperpermeability. J Biol Chem 286: 30409–30414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez FA, Rana R, Kim DD, Iwahashi T, Zheng R, Lal BK, Gordon DM, Meininger CJ, Duran WN. Internalization of eNOS and NO delivery to subcellular targets determine agonist-induced hyperpermeability. Proc Natl Acad Sci USA 106: 6849–6853, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez FA, Savalia NB, Duran RG, Lal BK, Boric MP, Duran WN. Functional significance of differential eNOS translocation. Am J Physiol Heart Circ Physiol 291: H1058–H1064, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schelling ME, Meininger CJ, Hawker JR Jr, Granger HJ. Venular endothelial cells from bovine heart. Am J Physiol Heart Circ Physiol 254: H1211–H1217, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Thibeault S, Rautureau Y, Oubaha M, Faubert D, Wilkes BC, Delisle C, Gratton JP. S-nitrosylation of beta-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol Cell 39: 468–476, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci USA 94: 6273–6278, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]