Age-related impairments in vascular function are associated with reduced nitric oxide bioavailability and augmented free radicals leading to oxidative stress. Ascorbic acid improved endothelium-dependent vasodilation during dynamic handgrip exercise without a concomitant attenuation of free radical outflow across the forearm.
Keywords: aging, endothelium, vascular function, l-NMMA
Abstract
The proposed mechanistic link between the age-related attenuation in vascular function and free radicals is an attractive hypothesis; however, direct evidence of free radical attenuation and a concomitant improvement in vascular function in the elderly is lacking. Therefore, this study sought to test the hypothesis that ascorbic acid (AA), administered intra-arterially during progressive handgrip exercise, improves brachial artery (BA) vasodilation in a nitric oxide (NO)-dependent manner, by mitigating free radical production. BA vasodilation (Doppler ultrasound) and free radical outflow [electron paramagnetic resonance (EPR) spectroscopy] were measured in seven healthy older adults (69 ± 2 yr) during handgrip exercise at 3, 6, 9, and 12 kg (∼13–52% of maximal voluntary contraction) during the control condition and nitric oxide synthase (NOS) inhibition via NG-monomethyl-l-arginine (l-NMMA), AA, and coinfusion of l-NMMA + AA. Baseline BA diameter was not altered by any of the treatments, while l-NMMA and l-NMMA + AA diminished baseline BA blood flow and shear rate. AA improved BA dilation compared with control at 9 kg (control: 6.5 ± 2.2%, AA: 10.9 ± 2.5%, P = 0.01) and 12 kg (control: 9.5 ± 2.7%, AA: 15.9 ± 3.7%, P < 0.01). NOS inhibition blunted BA vasodilation compared with control and when combined with AA eliminated the AA-induced improvement in BA vasodilation. Free radical outflow increased with exercise intensity but, interestingly, was not attenuated by AA. Collectively, these results indicate that AA improves BA vasodilation in the elderly during handgrip exercise through an NO-dependent mechanism; however, this improvement appears not to be the direct consequence of attenuated free radical outflow from the forearm.
NEW & NOTEWORTHY
Age-related impairments in vascular function are associated with reduced nitric oxide bioavailability and augmented free radicals leading to oxidative stress. Ascorbic acid improved endothelium-dependent vasodilation during dynamic handgrip exercise without a concomitant attenuation of free radical outflow across the forearm.
the causal link between an age-related increase in free radicals and endothelial dysfunction is an attractive hypothesis that is supported by improved vasodilation in the elderly in response to physiological [e.g., handgrip exercise and flow-mediated dilation (FMD)] and pharmacological (e.g., acetylcholine infusion) stimuli following ascorbic acid (AA) infusion or acute oral antioxidant administration (18, 47, 55). Importantly, the exogenous antioxidant-induced improvements in endothelial vasodilator function with advancing age are abolished after the inhibition of nitric oxide synthase (NOS), revealing a critical role of nitric oxide (NO) (14, 46, 47, 51). Thus the underlying mechanism of age-related endothelial dysfunction has been postulated to be a consequence of the degradation of NO by free radicals leading to a fall in NO bioavailability (9, 11, 24). However, direct evidence that improved endothelial vasodilatory function in the elderly treated with antioxidants is a result of attenuated free radicals is lacking.
Endothelial function has been commonly assessed by the measurement of brachial artery (BA) FMD following ischemic cuff occlusion (10, 20, 25, 33, 40, 56). However, the NO dependence of this type of FMD testing has been questioned (41, 43, 53, 58), potentially limiting the utility of this assessment. In contrast to FMD, progressive handgrip exercise evokes stepwise increases in shear rate and dilation of the BA and may therefore provide a more comprehensive approach to the study of BA vasodilatory capacity. Recently, our group utilized progressive handgrip exercise to examine the contribution of NO to BA dilation and documented that BA dilation is 70% NO dependent with this model (57). Furthermore, we have demonstrated that endothelial vasodilator function, assessed by progressive handgrip exercise, is attenuated in the elderly (18, 52), a segment of the population recognized to exhibit impaired endothelial function (16, 20, 22, 47, 48). These findings suggest that progressive handgrip exercise provides a robust assessment of NO-mediated endothelial function that can be used to further investigate the mechanisms contributing to endothelial dysfunction with age.
Therefore, using progressive handgrip exercise, this study sought to determine whether AA improves endothelial vasodilatory function in healthy older individuals by an NO-dependent mechanism that attenuates free radical outflow across the exercising forearm, thereby resulting in improved NO bioavailability. We hypothesized that 1) AA will improve endothelial function through an NO-mediated mechanism and 2) AA will attenuate the increase in free radical outflow from the forearm during progressive handgrip exercise. To test these hypotheses we measured BA vasodilation during progressive handgrip exercise at multiple workloads during the control condition, NOS inhibition with NG-monomethyl-l-arginine (l-NMMA), AA infusion, and combined NOS inhibition with AA infusion in healthy older subjects. Free radical outflow from the working forearm was assessed in each of the aforementioned conditions to determine whether AA infusion improved NO-dependent vascular function by directly quenching free radicals.
METHODS
Subjects.
Seven older healthy subjects (n = 3 men, n = 4 women; 69 ± 2 yr) were enrolled in this study. All subjects were nonsmokers, and none was performing any regular exercise. Subjects were not taking any prescription medications, including hormone replacement therapy in the women, and were free from overt cardiovascular disease. Protocol approval and written informed consent were obtained according to the University of Utah and the Salt Lake City Department of Veterans Affairs Medical Center (VAMC) Institutional Review Boards, in accordance with the principles outlined in the Declaration of Helsinki. All data collection took place at the Utah Vascular Research Laboratory (UVRL) located in the Salt Lake City VAMC. Peripheral and central hemodynamic responses to handgrip exercise in the control and l-NMMA conditions have been reported previously (52) and are included here to provide a reference for the AA conditions.
Protocols.
Subjects performed a minimum of two familiarization trials ∼1 wk prior to the experimental day. Maximal voluntary handgrip strength was determined, as measured by maximal voluntary contraction (MVC), and the progressive handgrip exercise protocol to be used during the experimental trials was performed during these familiarization trials.
On the experimental day subjects reported to the laboratory between 7:00 and 8:00 AM after an overnight fast. With sterile technique, an arterial catheter (Arrow, 18 gauge, 20 cm) was placed in the BA of the exercising arm after local anesthesia (2% lidocaine) ∼10 cm distal to the axilla and advanced 6–8 cm in the retrograde direction. The catheter was placed in the upper portion of the arm just below the axillary fossa to ensure that infusate entered the artery upstream to the ultrasound Doppler sample volume, allowing the direct local effect of the infusate on BA diameter and blood velocity to be assessed. A venous catheter (Arrow, 18 gauge, 20 cm) was placed in the antecubital vein of the exercising arm being studied and advanced in an antegrade direction ∼10 cm.
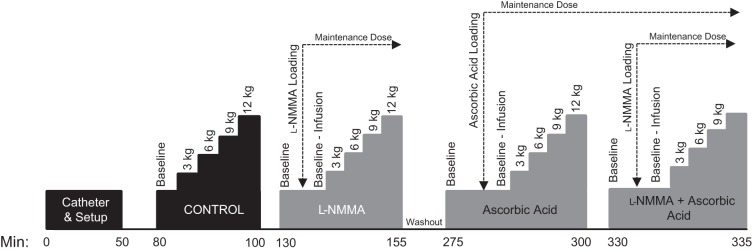
After a 30-min recovery from the catheter placement, baseline control measurements were made. Subjects then performed dynamic rhythmic handgrip exercise (1 Hz) with a commercially available handgrip dynamometer (TSD121C, Biopac Systems, Goleta, CA) interfaced with an analog-to-digital conversion system. Guidance was provided by a metronome, accompanied by real-time visual feedback of dynamometer force. Subjects were encouraged to perform rapid contractions with the goal of limiting contraction time to <25% of the duty cycle. Subjects exercised at 3, 6, 9, and 12 kg. Each exercise stage was performed for 2.5 min, and a 1-min rest was allotted between each work rate to limit fatigue. With the exception of the 2-h washout period following the l-NMMA trial, the rest period between trials was 30 min. The extended washout period following l-NMMA was employed because of previous data revealing that at least 1 h is required before forearm blood flow returns to baseline values after an infusion of l-NMMA (17). The experimental protocol is presented in Fig. 1.
Fig. 1.
Experimental timeline. After placement of the arterial and venous catheters and general setup, subjects performed progressive handgrip exercise at 3, 6, 9, and 12 kg under 4 experimental conditions: control, NG-monomethyl-l-arginine (l-NMMA), ascorbic acid (AA), and l-NMMA + AA. Venous and arterial blood samples were collected after resting baseline (both with and without the infusions) and during the final portion of 6- and 12-kg handgrip exercise [i.e., after Doppler assessment of brachial artery (BA) diameter and blood velocity]. After the control and AA trials subjects rested for 30 min. A 2-h washout period was performed after infusion of l-NMMA. AA and l-NMMA were infused at loading doses and then reduced to a maintenance dose 1 min before baseline measures. See text for further details.
l-NMMA and AA infusion.
Lower and upper arm volumes were determined anthropometrically (30) and then used for the calculation of infusion dosing. Total arm volume receiving infusate was calculated as follows: total arm volume (dl) = forearm volume + (upper arm × 0.5). A portion of the upper arm was included in this calculation because of the proximal location of the arterial catheter.
l-NMMA (Bachem) was diluted from 250 mg of lyophilized powder in normal saline to a concentration of 2.5 mg/ml. l-NMMA was infused at a priming dose of 0.48 mg/dl arm volume for 5 min prior to exercise. During baseline measurements and handgrip exercise l-NMMA was infused at a maintenance dose of 0.24 mg/dl arm volume. Previously, in young subjects, we demonstrated no further reduction in BA blood flow by increasing the infusion rate from 0.24 to 0.48 mg/dl arm volume (57). Handgrip exercise commenced 3 min after switching to the maintenance dose.
AA (Bioniche Pharma) was diluted from 50 mg/ml in normal saline. AA was infused at a priming dose of 8 mg/dl arm volume for 10 min. During baseline measurements and handgrip exercise AA was infused at a maintenance dose of 3.2 mg·dl arm volume−1·min−1. A similar AA infusion regimen has been used previously to improve exercise-induced vasodilation in older individuals during low-intensity handgrip exercise (14, 34).
Measurements.
Simultaneous measurements of BA blood velocity and vessel diameter were performed with a Logiq 7 ultrasound Doppler system (GE Medical Systems, Milwaukee, WI) operating in duplex mode. The Logiq 7 was equipped with a linear array transducer operating at an imaging frequency of 14 MHz. Blood velocity was obtained with the same transducer with a Doppler frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel on the basis of real-time ultrasound visualization. Mean velocity values (Vmean, angle-corrected and intensity-weighted area under the curve) were automatically calculated with commercially available software (Logiq 7). End-diastolic electrocardiogram (ECG) R wave-gated images were collected via video output from the Logiq 7 for off-line analysis of BA vasodilation using automated edge-detection software (Medical Imaging Applications, Coralville, IA). Using arterial diameter and Vmean, BA blood flow [Vmeanπ(vessel diameter/2)2 × 60] and shear rate (8Vmean/BA diameter) were calculated.
Heart rate (HR) was monitored from a standard three-lead ECG. Arterial blood pressure measurements were collected continually from within the BA, with the pressure transducer placed at the level of the catheter (Transpac IV, Abbott Laboratories). Mean arterial pressure (MAP) was calculated with the time integral of the directly measured arterial waveform. Stroke volume (SV) and cardiac output (CO) were determined with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). SV was calculated with the Modelflow method, a validated model (50) that uses an algorithm to compute the aortic flow waveform from an arterial blood pressure pulsation by simulating a nonlinear, self-adaptive (3-element windkessel) model of the aortic input impedance (Beatscope, version 1.1, Finapres Medical Systems). CO was then calculated as the product of HR and SV.
Blood assays.
In all trials arterial and venous blood samples were obtained at baseline, after infusion, and during 6 and 12 kg of handgrip exercise for the direct assessment of free radicals by electron paramagnetic resonance (EPR) spectroscopy (see EPR-specific methods below). Markers of oxidative stress and antioxidant status were also assayed in postinfusion venous blood samples to assess redox balance prior to handgrip exercise. Specifically, total antioxidant capacity was assessed by the ferric reducing ability of plasma (FRAP) assay (Cayman Chemical, Ann Arbor, MI) [coefficient of variation (CV) = 3.3%]. Plasma AA levels were determined with a commercially available assay kit (CosmoBio, Carlsbad, CA) (CV = 6.0%). Quantitative determination of thiobarbituric acid reactive substances (TBARS) was performed to assess lipid peroxidation (Bioassays Systems, Hayward, CA) (CV = 7.0%). Plasma nitrite levels were measured with a standard fluorometric assay kit (Cayman Chemical) (CV = 9.5%). Additionally, a lipid panel and complete blood chemistry assessment were performed by standard clinical techniques.
EPR spectroscopy and spin trapping.
Vascular free radical levels were directly assessed by spin trapping the free radicals in whole blood and then assessing the signal strength with EPR spectroscopy. Free radical outflow from the forearm was determined as the difference in venous minus arterial free radical EPR spectroscopy signal multiplied by blood flow. The EPR spectroscopy and spin trap methods have been described previously (4, 6). Briefly, 3.0 ml of venous or arterial blood was collected into a glass Vacutainer that contained 1 ml of the spin trap α-phenyl-tert-butylnitrone (PBN; 0.140 M). The PBN adduct was extracted from the whole blood with toluene, and the adduct (300 μl) was pipetted into a precision-bore quartz EPR spectroscopy sample tube (Wilmad-LabGlass, Vineland, NJ) that had been flushed with compressed N2. EPR spectroscopy was performed at 21°C with an EMX X-band spectrometer (Bruker, Billerica, MA) and analyzed with commercially available software (Bruker Xenon, version 1.1b.51) with the analyst blinded to experimental condition.
Data and statistical analysis.
Ultrasound images and Doppler velocity spectra were recorded continuously at rest and during each exercise stage. During the last 60 s of each ultrasound Doppler segment, Vmean was averaged across five 12-s intervals, which were matched with intima-to-intima BA diameter measurements evaluated during diastole. Based on previous work by our laboratory the CV for the assessment of endothelial function with this technique is ∼16% (57). The contribution of NO to BA vasodilation was determined for both control and AA conditions according to the following calculations: Control: ΔBAdiametercontrol − ΔBAdiameterl-NMMA and AA: ΔBAdiameterAA − ΔBAdiameterl-NMMA+AA. Statistics were performed with the use of commercially available software (SigmaPlot 11.0, Systat Software, Point Richmond, CA). A two-way repeated-measures analysis of variance (ANOVA) was used to identify significant changes in measured variables within and between drug conditions and groups and across exercise intensities, and post hoc analysis (Fisher least significant difference) was performed when appropriate. Main comparisons were made between control and all other conditions. To assess the impact of NO inhibition in AA conditions, a direct comparison between the AA and l-NMMA + AA conditions was also performed. The effect of the infusion at baseline within a specific condition was tested with a paired t-test. Potential sex-specific differences were not assessed because of the relatively small number of subjects in this study. All group data are expressed as means ± SE. Significance was established as P ≤ 0.05.
RESULTS
Subject characteristics.
Subject characteristics, including basic blood chemistry, are presented in Table 1. On average, all blood chemistry measures were within the normal range. All subjects were able to complete the handgrip exercise at a rate of 1 Hz with a resistance of 3, 6, 9, and 12 kg, which corresponded to 13 ± 1%, 26 ± 1%, 39 ± 2%, and 52 ± 2% of MVC.
Table 1.
Subject characteristics and blood chemistry
| Normal Range | ||
|---|---|---|
| Age, yr | 69 ± 2 | |
| Height, cm | 172 ± 4 | |
| Weight, kg | 76 ± 6 | |
| Body mass index, kg/m2 | 26 ± 1 | |
| Arm volume, dl | 13 ± 1 | |
| Glucose, mg/dl | 76 ± 3 | 64–128 |
| Sodium, mmol/l | 142 ± 1 | 136–144 |
| Potassium, mmol/l | 4.0 ± 0.1 | 3.7–5.2 |
| Chloride, mmol/l | 105 ± 1 | 101–111 |
| Creatinine, mg/dl | 0.9 ± 0.07 | 0.8–1.4 |
| Cholesterol, mg/dl | 185 ± 11 | 100–200 |
| Triglycerides, mg/dl | 72 ± 13 | <200 |
| HDL, mg/dl | 60 ± 4 | >50 |
| LDL, mg/dl | 115 ± 11 | <130 |
Values are means ± SE. Normal ranges for blood chemistry measures are presented for reference.
Peripheral hemodynamics and vascular responses at baseline and during arterial infusions.
BA diameter, shear rate, and blood flow were not different at baseline (i.e., prior to infusion) between conditions (Table 2). Baseline BA diameter was not altered by any of the infusions (Fig. 2A); however, compared with control, l-NMMA and l-NMMA + AA attenuated shear rate (Fig. 2B) (l-NMMA: −35 ± 3%, l-NMMA + AA: −30 ± 7%) and BA blood flow (see Fig. 4A) (l-NMMA: −36 ± 3%, l-NMMA + AA: −28 ± 7%). HR and MAP were not different at baseline between conditions (Table 3).
Table 2.
Peripheral hemodynamics at baseline
| Conditions |
||||
|---|---|---|---|---|
| Control | l-NMMA | Ascorbic acid | l-NMMA + ascorbic acid | |
| BA diameter, cm | 0.43 ± 0.03 | 0.43 ± 0.03 | 0.43 ± 0.03 | 0.43 ± 0.03 |
| Shear rate, s−1 | 79 ± 12 | 95 ± 12 | 72 ± 11 | 92 ± 13 |
| Blood flow, ml/min | 76 ± 22 | 95 ± 21 | 72 ± 22 | 90 ± 21 |
Values are means ± SE. l-NMMA, NG-monomethyl-l-arginine; BA, brachial artery.
Fig. 2.
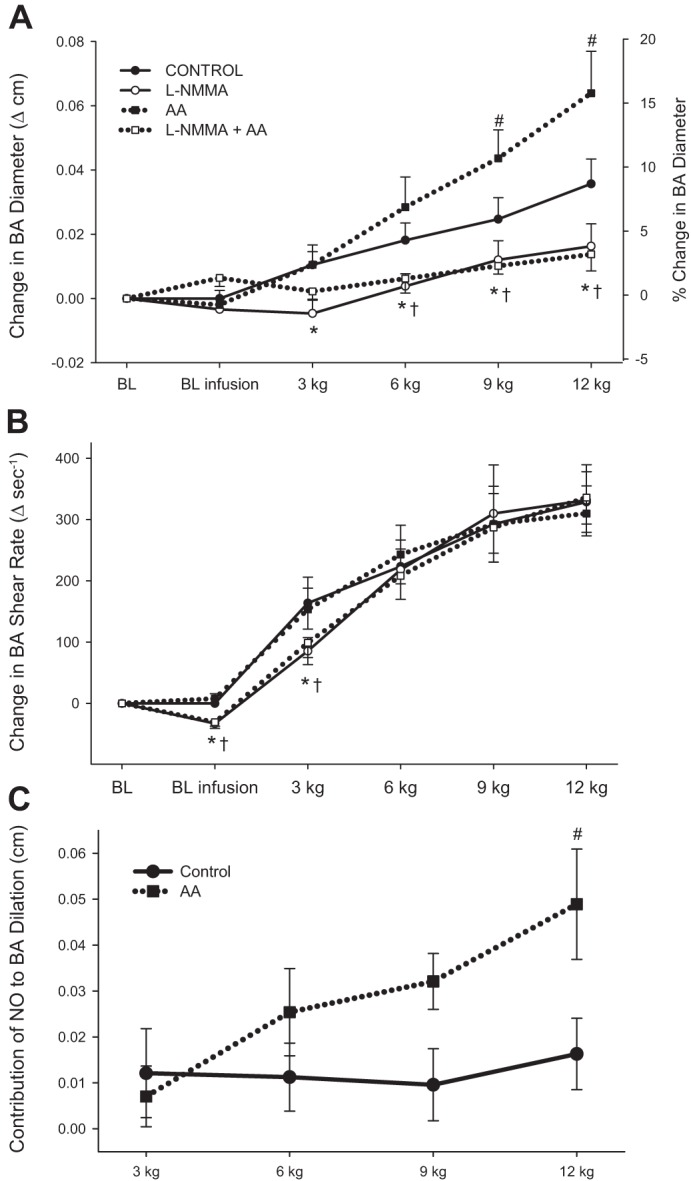
BA vasodilation (A), shear rate (B), and the contribution of nitric oxide (NO) to BA vasodilation (C) during progressive handgrip exercise. The contribution of NO to BA vasodilation was determined by the following equations: 1) NO contribution in control = ΔBAdiameterControl − ΔBAdiameterL-NMMA and 2) NO contribution in AA = ΔBAdiameterAA − ΔBAdiameterL-NMMA+AA. Absolute changes from baseline (BL) are presented for BA vasodilation and shear rate, and % change in BA diameter is expressed on right y-axis in A. Resting BL without (BL) and with infusion (BL infusion) is documented for each condition. All variables exhibited significant intensity-dependent increases during handgrip exercise (symbols denoting significant increases across workloads are not included for clarity). Values are means ± SE. *Significant difference between control and l-NMMA, #significant difference between control and AA, †significant difference between AA and l-NMMA + AA (P < 0.05).
Fig. 4.
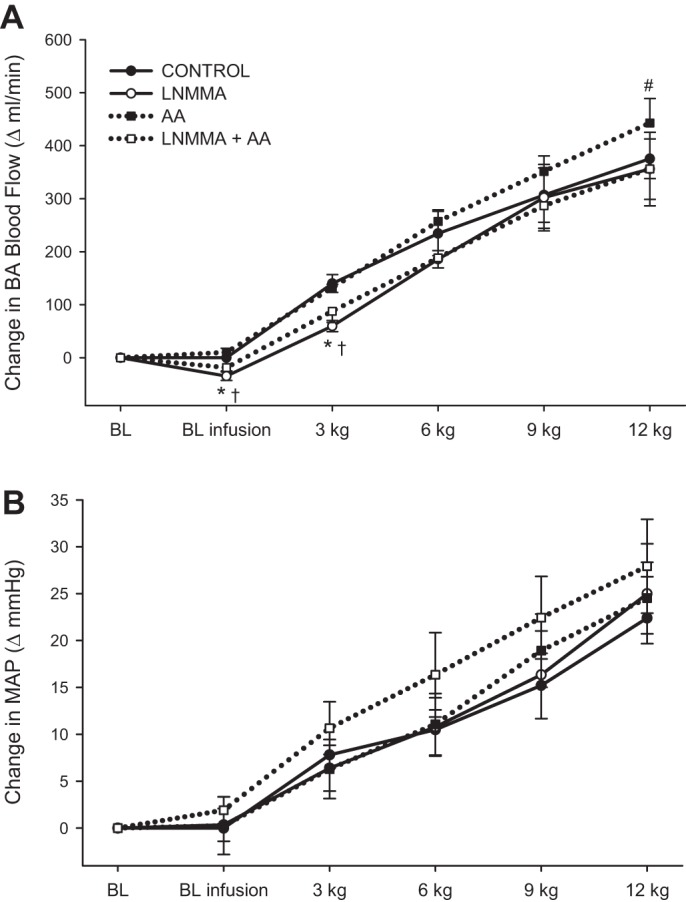
BA blood flow and blood pressure during progressive handgrip exercise. A: blood flow. B: mean arterial pressure (MAP). Resting baseline with and without the infusions is documented for each condition. All variables exhibited significant intensity-dependent increases during handgrip exercise (symbols denoting significant increases across workloads are not included for clarity). Values are means ± SE. *Significant difference between control and l-NMMA, #significant difference between control and AA, †significant difference between AA and l-NMMA + AA (P < 0.05).
Table 3.
Central hemodynamics at baseline and during progressive handgrip exercise
| Exercise Intensity |
|||||
|---|---|---|---|---|---|
| Baseline | 3 kg 13 ± 1% MVC | 6 kg 26 ± 1% MVC | 9 kg 39 ± 2% MVC | 12 kg 52 ± 3% MVC | |
| Control | |||||
| HR, beats/min | 57 ± 3 | 62 ± 4 | 64 ± 4* | 68 ± 5* | 73 ± 7* |
| SV, ml/beat | 111 ± 15 | 105 ± 19 | 106 ± 19 | 103 ± 19* | 103 ± 20* |
| CO, l/min | 6.3 ± 0.9 | 6.4 ± 1.2 | 6.6 ± 1.2 | 6.8 ± 1.2* | 7.2 ± 1.3* |
| MAP, mmHg | 102 ± 3 | 110 ± 4* | 112 ± 4* | 117 ± 5* | 124 ± 6* |
| l-NMMA | |||||
| HR, beats/min | 59 ± 4 | 63 ± 4 | 66 ± 4* | 68 ± 5* | 73 ± 7* |
| SV, ml/beat | 101 ± 17 | 96 ± 15 | 98 ± 15 | 96 ± 15 | 89 ± 14* |
| CO, l/min | 5.8 ± 1.0 | 5.9 ± 0.9 | 6.2 ± 0.9 | 6.4 ± 0.9* | 6.2 ± 0.9 |
| MAP, mmHg | 102 ± 3 | 108 ± 4* | 113 ± 5* | 118 ± 6* | 127 ± 6* |
| Ascorbic acid | |||||
| HR, beats/min | 57 ± 3 | 62 ± 3 | 67 ± 4* | 72 ± 6* | 75 ± 6* |
| SV, ml/beat | 100 ± 17 | 95 ± 17 | 97 ± 17 | 99 ± 21 | 90 ± 17* |
| CO, l/min | 5.8 ± 1.1 | 6.1 ± 1.1 | 6.5 ± 1.1* | 7.1 ± 1.5* | 6.6 ± 1.2* |
| MAP, mmHg | 99 ± 3 | 105 ± 5* | 110 ± 4* | 117 ± 5* | 123 ± 4* |
| l-NMMA + ascorbic acid | |||||
| HR, beats/min | 59 ± 5 | 63 ± 5 | 66 ± 5* | 69 ± 5* | 73 ± 6* |
| SV, ml/beat | 90 ± 17 | 89 ± 15 | 93 ± 17 | 84 ± 16 | 82 ± 15* |
| CO, l/min | 5.2 ± 1.0 | 5.4 ± 0.9 | 5.9 ± 1.0* | 5.6 ± 1.0 | 5.8 ± 1.0* |
| MAP, mmHg | 97 ± 4 | 108 ± 4* | 114 ± 6* | 120 ± 6* | 125 ± 7* |
Values are means ± SE. Absolute (kg) and relative [% maximal voluntary contraction (MVC)] exercise intensities are indicated.
HR, heart rate; CO, cardiac output; SV, stroke volume; MAP, mean arterial pressure.
Significantly different from baseline (P ≤ 0.05).
Peripheral hemodynamic and vascular responses to handgrip exercise.
During the control condition BA diameter increased linearly with increasing handgrip exercise intensity (Fig. 2A). AA improved BA dilation at 9 and 12 kg (P < 0.01). Individual BA vasodilatory responses to control and AA conditions at 12 kg are presented in Fig. 3. Compared with control, NOS inhibition by l-NMMA and l-NMMA + AA attenuated BA vasodilation across all workloads. Compared with AA, l-NMMA + AA also attenuated BA artery dilation at 6, 9, and 12 kg.
Fig. 3.
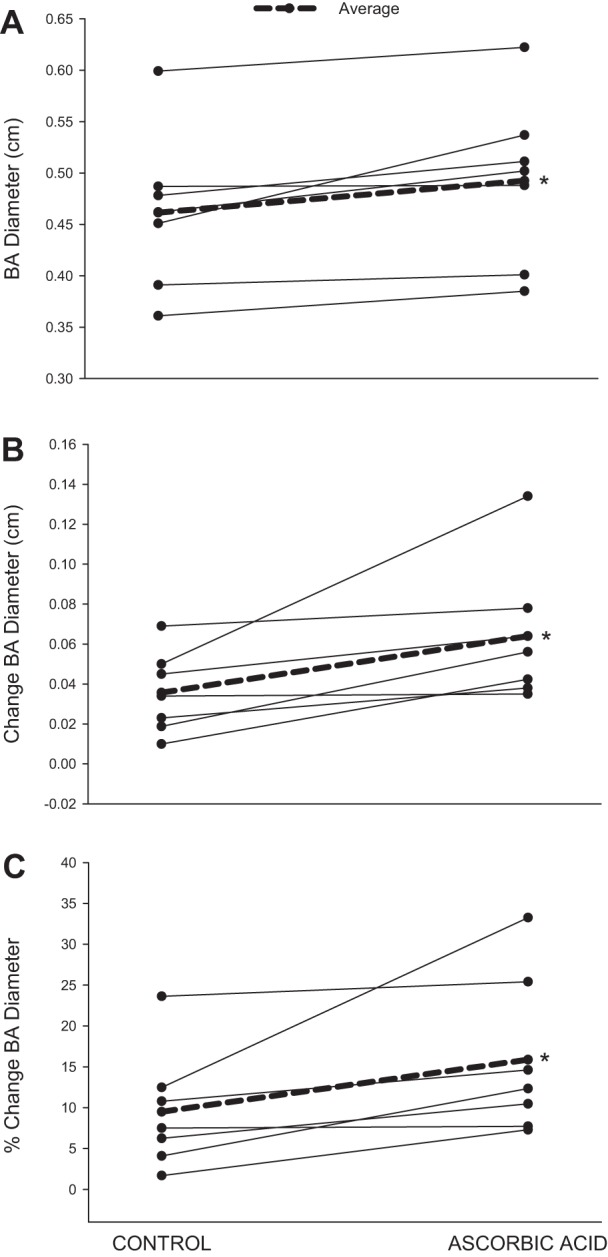
Individual BA vasodilatory responses to handgrip exercise at 12 kg in control and AA conditions: BA diameter (A), absolute change in BA diameter (B), and % change in BA diameter (C). On average, BA diameter was significantly increased in the AA condition. *Significant difference between conditions (P < 0.05); n = 7.
Shear rate was similar between control and AA conditions (Fig. 2B); however, l-NMMA and l-NMMA + AA attenuated shear rate at 3 kg by 26% and 22%, respectively. The change in shear rate was similar between conditions at 6, 9, and 12 kg. Compared with AA, l-NMMA + AA attenuated shear rate at 3 kg and tended to evoke a decrease at 6 kg (P = 0.07).
The contribution of NO to BA dilation remained constant during handgrip exercise in the control condition (Fig. 2C). Interestingly, during AA infusion, the contribution of NO to BA dilation increased with exercise intensity. At 12 kg the contribution of NO to BA dilation was greater during AA infusion than during the control condition.
BA blood flow increased with each progressive increase in intensity during handgrip exercise in the control condition (Fig. 4A). At peak intensity BA blood flow during control was 455 ± 55 ml/min. AA did not alter blood flow at the lower exercise intensities; however, blood flow at 12 kg was increased by 12% (AA: 512 ± 61 ml/min, P = 0.013). Individual BA blood flow responses to control and AA conditions at 12 kg are presented in Fig. 5. l-NMMA and l-NMMA + AA attenuated blood flow at 3 kg by 27% and 18%, respectively, and also tended to decrease blood flow at 6 kg (P = 0.054) compared with control. The impact of NOS inhibition on BA blood flow was completely abolished at the higher exercise intensities, as there were no differences between control and l-NMMA conditions at 9 and 12 kg. l-NMMA + AA, infused in combination, consistently attenuated blood flow across all workloads compared with AA by ∼12–16%.
Fig. 5.

Individual BA blood flow response to handgrip exercise at 12 kg in control and AA conditions. On average, BA blood flow was significantly increased in the AA condition. *Significant difference between conditions (P < 0.05); n = 7.
Central hemodynamic responses to handgrip exercise.
During handgrip exercise, independent of condition, HR increased linearly by ∼13–17 beats/min (Table 3). The increase in HR during handgrip exercise was not different between conditions. Similarly, SV decreased and CO increased with increasing exercise intensity; however, the changes in SV and CO from baseline were not different between conditions (Table 3). A significant exercise pressor response was observed during handgrip exercise, and the magnitude of the pressor response was similar between conditions (Fig. 4A).
Free radical outflow from the forearm and markers of oxidative stress.
Free radical outflow during preinfusion and infusion baselines was not different across conditions (Fig. 6). During progressive handgrip exercise free radical outflow from the forearm steadily increased (Fig. 6). AA alone had no impact on free radical outflow during handgrip exercise; however, during the combined l-NMMA + AA infusion free radical outflow was augmented at both 6 and 12 kg compared with control.
Fig. 6.
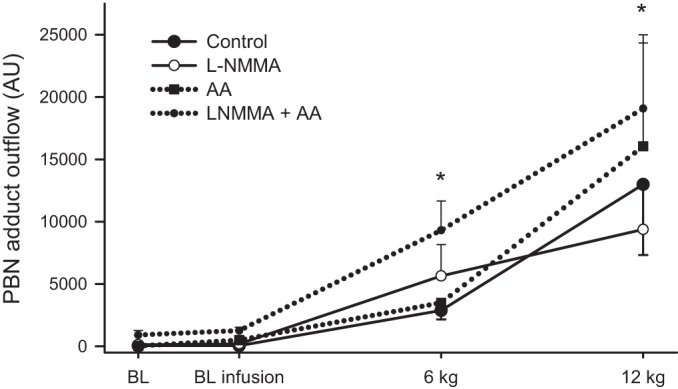
Forearm free radical outflow as measured by α-phenyl-tert-butylnitrone (PBN) adduct during progressive handgrip exercise. Resting baseline with and without the infusions is documented for each condition. Free radical outflow increased during progressive handgrip exercise in all conditions (symbols denoting significant increases across workloads are not included for clarity). AU, arbitrary units. Values are means ± SE. *Significant difference between control and l-NMMA + AA (P < 0.05).
AA alone or in combination with l-NMMA greatly increased postinfusion baseline levels of venous AA, FRAP, TBARS, and plasma nitrites (Table 4). Interestingly, coinfusion of l-NMMA + AA slightly reduced FRAP compared with AA alone; however, FRAP still remained well above the control and l-NMMA conditions.
Table 4.
Markers of oxidative stress and antioxidant capacity in venous blood
| Conditions |
||||
|---|---|---|---|---|
| Control | l-NMMA | Ascorbic acid | l-NMMA + ascorbic acid | |
| Ascorbic acid, μg/ml | 17 ± 2 | 15 ± 3 | 517 ± 34* | 519 ± 66* |
| FRAP, mM | 1.04 ± 0.06 | 1.06 ± 0.06 | 13.05 ± 1.51* | 8.51 ± 1.05*† |
| TBARS, μM | 1.4 ± 0.3 | 1.1 ± 0.2 | 8.2 ± 1.47* | 8.1 ± 3.0* |
| Nitrites, μM | 0.47 ± 0.04 | 0.48 ± 0.03 | 1.57 ± 0.14* | 1.91 ± 0.15* |
Values are means ± SE. Venous blood was collected immediately after intra-arterial infusion prior to the start of handgrip exercise. FRAP, ferric reducing ability of plasma; TBARS, thiobarbituric acid reactive substances.
Significantly different from control,
significantly different from ascorbic acid (P < 0.05).
DISCUSSION
This study sought to examine the mechanistic link between free radicals and the age-related attenuation in vascular function by determining whether AA, administered intra-arterially, improves BA vasodilation in an NO-dependent manner during progressive handgrip exercise by attenuating free radical production. The results of this investigation provide several novel findings that, in combination, question the hypothesis of a direct link between free radicals and vascular endothelial dysfunction in the elderly. First, AA infusion improved BA vasodilation during progressive handgrip exercise through an NO-dependent mechanism as evidenced by the complete elimination of the augmented vasodilatory response when AA was combined with NOS inhibition and AA-induced increase in plasma nitrites. Second, despite this improved NO-dependent vasodilation, AA did not attenuate free radical outflow from the vascular bed of the exercising forearm. Therefore, these findings reveal that AA-induced improvements in BA vasodilation occur through an NO-dependent mechanism that does not appear to be the consequence of attenuated free radicals.
Ascorbic acid improves BA vasodilation through an NO-dependent mechanism.
The causal link between age-related endothelial dysfunction and free radicals is supported by the finding that acute administration of AA augments endothelial function (20, 47). Moreover, improved vasodilation during AA infusion in healthy elderly subjects was negated by NOS inhibition, implying that the AA-induced improvement was directly related to an increase in NO bioavailability (47), which is recognized to be inversely related to free radical concentration. Additionally, AA infusion improved exercise hyperemia during small muscle mass exercise in elderly individuals (34) through an NO-dependent mechanism (14). Previously, our group reported improved BA vasodilation during progressive handgrip exercise following acute oral antioxidant supplementation with vitamins C and E and α-lipoic acid in healthy elderly men (18). This combination of antioxidants attenuated serum free radical levels at rest and after whole body maximal exercise, indicating a systemic curtailing of free radicals, as assessed by EPR spectroscopy and spin trapping with PBN. However, the NO dependence of the improved BA vasodilation and the impact of the antioxidant supplement on free radical outflow across the working muscle were not directly assessed.
In the present study the AA-induced improvement in BA vasodilation during handgrip exercise likely occurred through an NO-dependent mechanism as evidenced by the complete elimination of the improvement in BA vasodilation by the combined infusion of AA and l-NMMA (Fig. 2A). The dissociation between resting blood nitrites and the vasodilatory response, especially during the combined l-NMMA + AA trial, is not easily explained but may be due to the extremely short half-life of NO and the ubiquitous nature of this molecule compared with the more stable nitrite that is formed during NO metabolism. Indeed, several prior reports indicate that nitrite levels can actually remain relatively stable in the plasma after interventions that alter NO (23, 32); thus a temporal dissociation may limit the interpretation of nitrite as a definitive marker of NO availability in the l-NMMA + AA condition.
The NO dependence of the AA-induced improvement in vascular function is further demonstrated by the manner in which NOS inhibition differentially altered BA dilation under control and AA conditions (Fig. 2C). Specifically, during normal conditions the contribution of NO to BA vasodilation during handgrip exercise remained constant with increasing exercise intensity, despite concomitantly increasing shear stress. Interestingly, although not directly tested in the present study, this finding suggests that compensatory vasodilatory mechanisms may be operating to evoke the linear increase in BA diameter during handgrip exercise in the elderly. This observation supports the concept that with advancing age and disease vasodilatory mechanisms appear to shift from predominantly prostaglandins and NO to endothelium-derived hyperpolarizing factors (7, 8). In contrast, during the AA infusion the NO contribution to BA vasodilation was augmented in an exercise intensity-dependent manner (Fig. 2C), suggesting that, perhaps, the mechanotransduction of a given shear rate stimulus through NOS was amplified, leading to an improvement in NO-dependent vascular function. Furthermore, compared with reference data collected in young healthy subjects (57), AA improved the relationship between BA vasodilation and shear rate in the older subjects such that the age-related impairment in this relationship was abolished (Fig. 7).
Fig. 7.
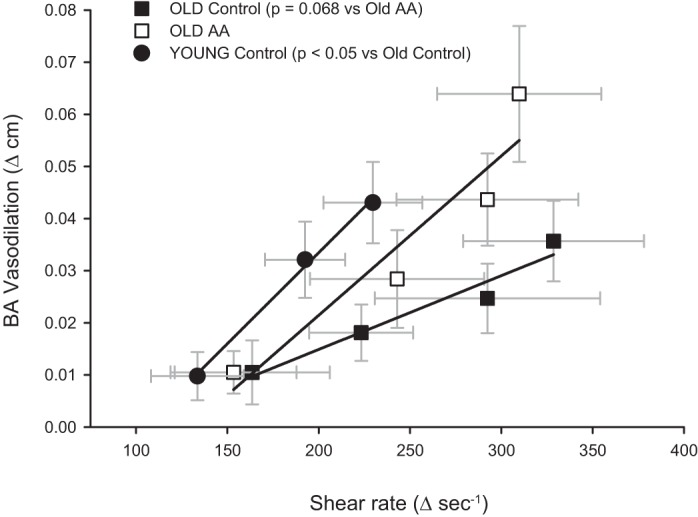
Relationship between shear rate and the change in BA diameter during progressive handgrip exercise. In addition to the present data from older subjects during both the control and AA infusion conditions, previously published control data from young subjects (57) are presented for reference. The slope of the relationship between shear rate and BA vasodilation was significantly attenuated in the old compared with the young (P < 0.05). AA increased the slope of the relationship between shear rate and BA vasodilation in the old such that the slope of this relationship was no longer different from that in the young (P = 0.98).
Improved BA vasodilation by AA may not be directly linked to attenuated free radicals.
AA is thought to improve vascular function by directly scavenging free radicals, thereby limiting the degradation of NO and improving NO bioavailability (28, 44). Contrary to our original hypothesis, AA did not attenuate forearm free radical outflow during exercise, revealing a disassociation between free radicals and endothelial function, as measured in this progressive handgrip exercise paradigm. This finding supports the notion that elevated circulating free radicals may not be a prerequisite for endothelial dysfunction (39). In light of these findings, it is apparent that the AA-induced functional outcome of improved BA vasodilation, while NO dependent, may not actually be directly linked to the level of free radicals flowing from the working muscle.
AA can act to chemically stabilize tetrahydrobiopterin (BH4), an essential cofactor for NOS (26, 27); therefore, whenever AA is used to improve in vivo endothelial function it is difficult to determine whether improvements are specific to the enzymatic function of NOS or due to altered NO bioavailability. Indeed, to further complicate matters, NOS function can shift between coupled (NO generation) and uncoupled (superoxide production) states depending on substrate and/or cofactor availability (13, 31, 54). Therefore, in the present study, it is possible that the augmented BA vasodilation during AA infusion is due, at least in part, to augmented enzymatic production of NO, as NOS inhibition completely abolished the AA-induced improvements (Fig. 2A). Interestingly, previous investigations reported that diminished BH4 availability contributes to the age-associated attenuation in NO-dependent vascular function by uncoupling NOS in both human and animal models (21, 45). In fact, in elderly humans acute BH4 supplementation improved BA FMD, signifying a direct association between BH4 and endothelial function (21). Additionally, AA may induce rapid changes in the phosphorylation state of NOS, independent of stabilizing BH4, leading to enhanced NOS activity (35). In light of these previous findings and the elevated nitrite levels following AA infusion (Table 4) coupled with a failure to attenuate muscle free radical outflow (Fig. 6), the present study suggests that AA improved BA vasodilation, at least in part, by enhancing and/or stabilizing NOS activity.
An alternative BH4-based explanation for the AA-induced improvement in vascular function in the present study may involve a lowering of oxidative stress resulting in increased BH4 levels. Elevated oxidative stress, specifically peroxynitrate, rapidly oxidizes BH4 to an inactive form, dihydrobiopterin, resulting in the uncoupling of NOS (12, 36, 37). However, in the present study such a role of AA is unlikely, as both free radical levels and TBARS, a measure of lipid peroxidation and an indirect marker of oxidative stress, were not attenuated by AA infusion (Table 4). In fact, AA elevated TBARS, supporting the prior suggestion that AA can have a prooxidant action when administered at high doses (42). Importantly, the inhibition of NOS in combination with AA increased free radical outflow during handgrip exercise (Fig. 6). Although the source of the elevated free radical production during combined l-NMMA and AA is not entirely clear, it is possible that the prooxidant properties of AA may be amplified in the presence of NOS inhibition by increasing the concentration of AA in the plasma. In the context of the present findings, the apparent prooxidant properties of AA were either overpowered by improvements in NO bioavailability or did not directly influence BA vasodilatory capacity.
Experimental considerations.
EPR spectroscopy provides the most direct, specific, and sensitive technique for the detection and identification of free radicals (3). Utilizing this technique, our group has previously reported age-related increases in free radicals at rest and during exercise in both the circulation and the active skeletal muscle (4–6). However, the chemical spin trap utilized in the ex vivo stabilization of reactants dictates which free radicals are identified. Therefore, the specificity of the spin trap has the potential to effect the conclusions of such a study. Indeed, albeit unlikely, it is possible that the specific free radicals formed during handgrip exercise, and subsequently quenched by AA, were “overlooked” by the PBN spin trap. However, the nature of the free radical species, as determined by the dominant signal of the hyperfine coupling constants, indicates that the O2-centered alkoxyl free radicals formed as a consequence of free radical-mediated damage to membrane phospholipids are the predominant species of radicals identified by PBN (4, 6). Furthermore, using PBN as a spin trap, Ashton et al. (2) reported attenuated plasma free radical levels after 1,000 mg of oral AA both at rest and during exercise. Additionally, in young healthy individuals free radical levels, as measured by PBN adducts, increased in proportion to exercise intensity during knee extension exercise (4, 6) and were, in another study, attenuated by an oral antioxidant at rest and after maximal whole body exercise (18). Accordingly, PBN appears to be an appropriate spin trap for the present investigation. Regardless, the possibility that non-O2-centered free radicals were produced by the handgrip exercise cannot be ruled out. Further investigation utilizing additional EPR spin traps, such as 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO), to specifically quantify superoxide, hydroxyl, and thiyl radicals is warranted to better understand the mechanisms by which AA improves BA vasodilation in the elderly. Finally, the lack of an assessment of endothelium-independent vasodilation could be considered a limitation of this study, but as the AA-induced improvement in BA vasodilation was fully abolished with NOS inhibition this is highly supportive of the conclusion that the improvement was, in fact, both NO and endothelium dependent.
Conclusions.
In summary, this investigation provides evidence that AA improves endothelial vascular function through an NO-dependent mechanism during progressive handgrip exercise in the elderly. This AA-mediated improvement in BA vasodilation occurred without a concomitant fall in PBN adducts, suggesting that mechanisms other than attenuated free radical outflow contribute to the AA-induced improvement in vasodilatory function. Of note, the role of free radicals not assessed as PBN adducts (non-O2-centered free radicals) in BA vasodilation is still unknown and will require further investigation using an array of EPR spin traps. However, this does not negate the novel finding that the attenuation in free radical outflow, as assessed by PBN adducts, is not obligatory for the AA-mediated improvement in vasodilatory function. Therefore, overall, the findings presented here question the attractive hypothesis that age-related vascular endothelial dysfunction is causally linked to free radicals, because an intra-arterial AA infusion during handgrip exercise improved BA vasodilation with no alteration in forearm free radical outflow.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute (PO1 HL-091830, R01 HL-118313), the Department of Veterans Affairs (RR&D E6910R, E1433-P), and the American Heart Association (0835209N, 1850039).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.T., D.W.W., Z.B.-O., and R.S.R. conception and design of research; J.D.T., D.W.W., M.A.W., G.L., Z.B.-O., S.J.I., J.D.C., V.R.R., J.Z., and R.S.R. performed experiments; J.D.T., M.A.W., Z.B.-O., S.J.I., V.R.R., and J.Z. analyzed data; J.D.T., D.W.W., M.A.W., G.L., S.J.I., V.R.R., and R.S.R. interpreted results of experiments; J.D.T. prepared figures; J.D.T. drafted manuscript; J.D.T., D.W.W., M.A.W., G.L., Z.B.-O., S.J.I., and R.S.R. edited and revised manuscript; J.D.T., D.W.W., M.A.W., G.L., Z.B.-O., S.J.I., J.D.C., V.R.R., J.Z., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Ashton T, Young IS, Peters JR, Jones E, Jackson SK, Davies B, Rowlands CC. Electron spin resonance spectroscopy, exercise, and oxidative stress: an ascorbic acid intervention study. J Appl Physiol 87: 2032–2036, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DM. Radical dioxygen: from gas to (unpaired!) electrons. Adv Exp Med Biol 543: 201–221, 2003. [PubMed] [Google Scholar]
- 4.Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS. EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. J Appl Physiol 94: 1714–1718, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, Pietri S, Young IS, Richardson RS. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol 109: 449–456, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey DM, Young IS, McEneny J, Lawrenson L, Kim J, Barden J, Richardson RS. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am J Physiol Heart Circ Physiol 287: H1689–H1699, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Beyer AM, Durand MJ, Hockenberry J, Gamblin TC, Phillips SA, Gutterman DD. An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. Am J Physiol Heart Circ Physiol 307: H1587–H1593, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyer AM, Gutterman DD. Regulation of the human coronary microcirculation. J Mol Cell Cardiol 52: 814–821, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bult H, Herman AG, Matthys KE. Antiatherosclerotic activity of drugs in relation to nitric oxide function. Eur J Pharmacol 375: 157–176, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med 48: 489–509, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Luscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol 21: 496–502, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res 43: 274–278, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dakak N, Husain S, Mulcahy D, Andrews NP, Panza JA, Waclawiw M, Schenke W, Quyyumi AA. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension 32: 9–15, 1998. [DOI] [PubMed] [Google Scholar]
- 16.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol 298: H671–H678, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 101: 629–635, 2001. [PubMed] [Google Scholar]
- 20.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27: 849–853, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Grube R, Kelm M, Motz W, Strauer B. The biology of nitric oxide. In: Enzymology, Biochemistry, and Immunology, edited by Moncada S, Feelisch M, Busse R, Higgs EA. London: Portland, 1994, p. 201–204. [Google Scholar]
- 24.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986. [DOI] [PubMed] [Google Scholar]
- 25.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. l-Ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276: 40–47, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Huang A, Vita JA, Venema RC, Keaney JF Jr. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem 275: 17399–17406, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Jackson TS, Xu A, Vita JA, Keaney JF Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 83: 916–922, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969. [PubMed] [Google Scholar]
- 31.Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci 30: 48–54, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta 1411: 273–289, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Kimura Y, Matsumoto M, Den YB, Iwai K, Munehira J, Hattori H, Hoshino T, Yamada K, Kawanishi K, Tsuchiya H. Impaired endothelial function in hypertensive elderly patients evaluated by high resolution ultrasonography. Can J Cardiol 15: 563–568, 1999. [PubMed] [Google Scholar]
- 34.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladurner A, Schmitt CA, Schachner D, Atanasov AG, Werner ER, Dirsch VM, Heiss EH. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med 52: 2082–2090, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in ApoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 263: 681–684, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Nightingale AK, James PP, Morris-Thurgood J, Harrold F, Tong R, Jackson SK, Cockcroft JR, Frenneaux MP. Evidence against oxidative stress as mechanism of endothelial dysfunction in methionine loading model. Am J Physiol Heart Circ Physiol 280: H1334–H1339, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Nishiyama SK, Walter Wray D, Berkstresser K, Ramaswamy M, Richardson RS. Limb-specific differences in flow-mediated dilation: the role of shear rate. J Appl Physiol 103: 843–851, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol 301: H1118–H1126, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature 392: 559, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol 298: H119–H126, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Sherman DL, Keaney JF Jr, Biegelsen ES, Duffy SJ, Coffman JD, Vita JA. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension 35: 936–941, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 82: 1535–1539, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Tam E, Azabji Kenfack M, Cautero M, Lador F, Antonutto G, di Prampero PE, Ferretti G, Capelli C. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clin Sci (Lond) 106: 371–376, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O'Keefe Z, Ives SJ, Conklin JD, Reese V, Richardson RS. Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. Am J Physiol Regul Integr Comp Physiol 305: R893–R899, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1237, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BSS, Karoui H, Tordo P, Pritchard KA Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Wray DW, Witman MA, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Progressive handgrip exercise: evidence of nitric oxide-dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300: H1101–H1107, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wray DW, Witman MA, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 345–351, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]