Abstract
Hyaluronan, a ubiquitous naturally occurring glycosaminoglycan, is a major component of the extracellular matrix, where it participates in biological processes that include water homeostasis, cell-matrix signaling, tissue healing, inflammation, angiogenesis, and cell proliferation and migration. There are emerging data that hyaluronan and its degradation products have an important role in the pathobiology of the respiratory tract. We review the role of hyaluronan in respiratory diseases and present evidence from published literature and from clinical practice supporting hyaluronan as a novel treatment for respiratory diseases. Preliminary data show that aerosolized exogenous hyaluronan has beneficial activity against airway inflammation, protects against bronchial hyperreactivity and remodeling, and disrupts the biofilm associated with chronic infection. This suggests a role in airway diseases with a predominant inflammatory component such as rhinosinusitis, asthma, chronic obstructive pulmonary disease, cystic fibrosis, and primary ciliary dyskinesia. The potential for hyaluronan to complement conventional therapy will become clearer when data are available from controlled trials in larger patient populations.
Keywords: asthma, bacterial biofilm, chronic obstructive pulmonary disease, cystic fibrosis, hyaluronan, inflammation, respiratory tract diseases
hyaluronan (also known as hyaluronic acid, hyaluronate, or HA) is a naturally occurring major component of the extracellular matrix (ECM) and is found in high concentrations in the mammalian connective tissue, including in the lung (3, 47).
There is emerging evidence that HA and its degradation products have an important role in lung pathobiology. Furthermore, HA supplementation may have beneficial effects in lung inflammation and airway hyperresponsiveness (AHR). The use of HA in the treatment of airway disease introduces a new class of therapeutic agents, which might be described as matrix modulators, and is therefore a very exciting development. The present article summarizes the available evidence and emerging data supporting a role of HA as a novel therapeutic in inflammatory airway disease (Fig. 1). The first half of this review provides a concise summary of available mechanistic data on the biological effects of HA in airway biology of the host (HA in inflammatory signaling and remodeling pathways) and the microbiome (HA in the formation of microbial biofilm). In the second half we detail the available evidence on the translational application of HA in the treatment of upper and lower respiratory tract diseases. Finally, we make suggestions about existing gaps in our knowledge of HA biology and therapeutic potential. In aggregate, we aim to provide the interested reader with a thoughtful bench-to-bedside review of HA biology, and hopefully stimulate further research into matrix biology of the lung.
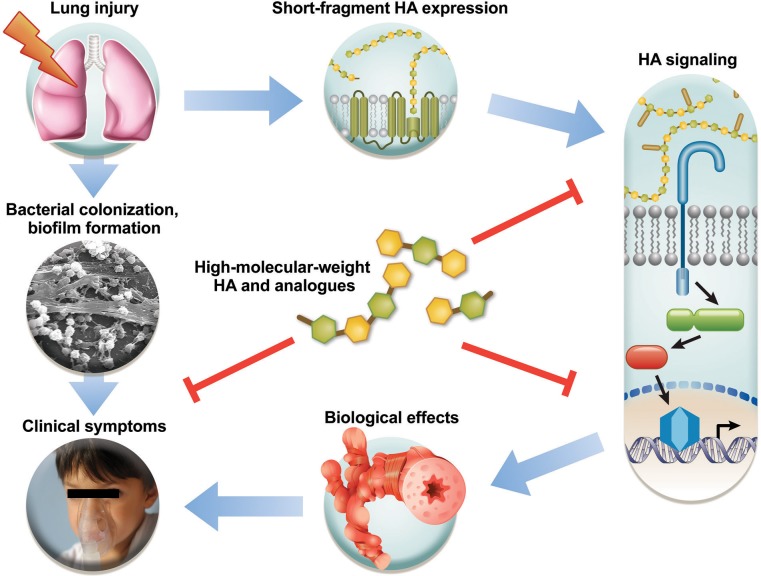
Fig. 1.
Concept of hyaluronan (HA) actions after environmental lung injury. Lung injury mediates the release of short-fragment HA, which activates innate immune receptors and leads to inflammation, remodeling, hyperresponsiveness, and clinical symptoms. Bacterial biofilms develop because of defective clearance in injury and compound the clinical presentation. The perpetuation of this vicious cycle can be broken by modulating short-fragment HA signaling, e.g., with high-molecular-weight HA.
HA Metabolism in the Respiratory Tract
HA is a linear polysaccharide polymer consisting only of simple repeating disaccharide units of d-glucuronic acid and N-acetylglucosamine (47, 94). Unlike other glycosaminoglycans such as chondroitin, heparin, heparan sulfate, and keratan sulfate, HA is synthesized by HA synthases at the inner surface of the plasma membrane, rather than in the Golgi apparatus within the cell. It further differs from other glycosaminoglycans in that it is an unmodified polysaccharide and is not attached to a protein core or further modified after secretion (47, 86, 88, 94). HA has exceptional hydrophilic characteristics and can bind approximately a thousand times its weight in water. After it is extruded onto the cell surface or into the intracellular matrix it produces a highly viscous gel that has an essential role in tissue homeostasis and biomechanical integrity (94). HA interacts with cell surfaces in two ways: by binding to specific cell-surface receptors and inducing intracellular signal transduction and by creating a matrix coat around the cell that may protect it from the environment and that allows other interactions with the cell.
HA is a unique molecule with properties dependent on its size. There is a clear distinction between high-molecular-weight HA (>1 million), which is the physiologically available form, and short-fragment HA (150,000–350,000 Da), produced during inflammation. High-molecular-weight HA has anti-inflammatory and antiangiogenic properties and promotes cell survival, in contrast to short-fragment HA, which has proinflammatory and proangiogenic properties and promotes cell migration (24, 81, 94). The products of digestion through hyaluronidases, HA oligosaccharides (3,000–7,000), have mixed effects, promoting inflammation in some situations and having a protective effect in others.
HA (which was short-fragment HA whenever the size was analyzed) has been detected in a number of lung diseases, both in the airways (bronchoalveolar lavage fluid) (10, 30, 59, 62, 66) and in the parenchyma (mainly in the perivascular space) (4, 5, 87, 97, 100). HA deposition and metabolism are thus a major component of inflammatory lung disease development, progression, and resolution (19, 20).
HA Signaling in the Airway
HA is localized in the areas of the airways that contribute to the development of hyperresponsiveness and inflammation. Distal airway imaging shows that HA is limited to the subepithelial layer in healthy individuals, in contrast to a much expanded accumulation of HA in the subepithelial spaces of severely asthmatic airways, which contributes to chronic inflammation and airway remodeling (62). Data from mouse models of acute and chronic lung inflammation and from human studies in asthmatic vs. healthy subjects show that short-fragment HA is being produced during the inflammatory process, localized in the alveolar walls and airway smooth muscle cells and around alveolar macrophages (10, 27, 30, 31, 62).
Extensive research in the last 20 years has shown that short-fragment HA signaling through receptors CD44 and RHAMM contributes to the accumulation of immune cells in inflammatory sites (69, 84, 102); furthermore, short-fragment HA activates immune cells and leads to the release of proinflammatory cytokines and metalloelastases and the inhibition of plasminogen activation (39–45, 70, 71, 73, 79). Importantly, HA also mediates experimentally induced AHR, with a clear size-dependent response. Short-fragment HA, but not high-molecular-weight HA or oligosaccharides of HA, replicates the inflammatory changes and hyperresponsiveness in the airway (30). Indeed, instillation of high-molecular-weight HA protects against AHR in experimental models (30, 58).
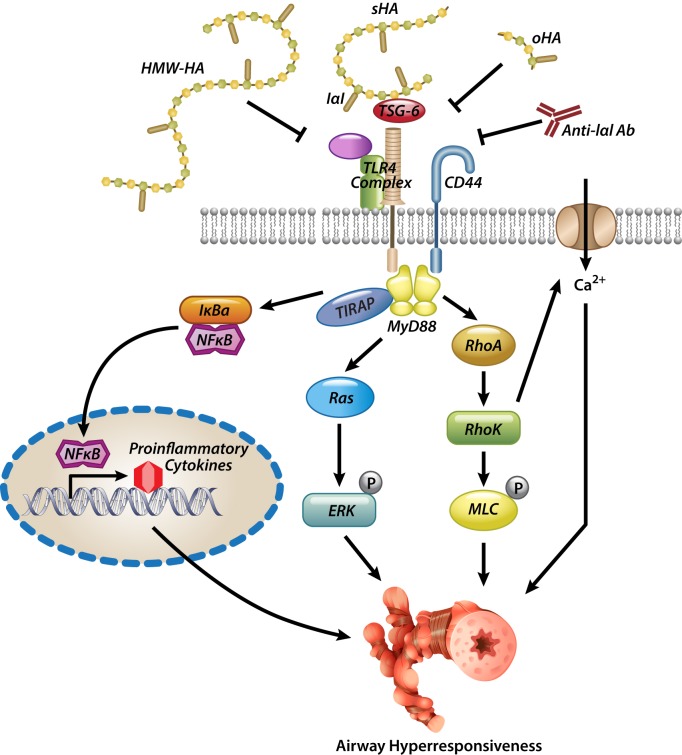
Short-fragment HA signaling in the lung.
The induction of airway inflammation, hyperresponsiveness, and remodeling by short-fragment HA appears to be mediated by interaction of short-fragment HA with a CD44 and Toll-like receptor 4 (TLR4) complex, leading to the activation of an intracellular signaling cascade involving, as far as is known, MyD88, TIRAP, and NF-κB and the release of proinflammatory cytokines and chemokines (31, 61). Extracellular proteins, such as inter-α-inhibitor (IαI) and TNF-α stimulated gene 6 (TSG-6), are involved in the receptor binding process. IαI is a complex protein found abundantly in serum but also expressed in many tissues including lung epithelia, fibroblasts, and airway smooth muscle and was originally described as a HA cross-linker that is necessary for the development of the cumulus oophorus in the ovaries (105). TSG-6 mediates the transfer of IαI heavy chains onto HA (72), which leads to better affinity of HA to its receptors (104). IαI is necessary for the development of AHR after ozone or chlorine exposure (30, 58), and the formation of a pathological HA matrix consisting of HA-IαI heavy chains is one of the hallmarks of asthmatic airway remodeling (57); on the other hand, genetic absence of IαI seems to exacerbate allergic asthma (103), suggesting complex interactions of IαI in the pathogenesis of allergic inflammation. TSG-6 itself is capable of cross-linking HA molecules (7); however, this is inhibited by IαI heavy chains (6), again underscoring the complex interactions and delicate balance of HA binding in the inflammatory matrix. Genetic absence of TSG-6 blocks the complexing of IαI heavy chains with HA and reduces inflammation and AHR in allergic asthma (90). HA [in association with IαI (104)] promotes the adhesion of activated lymphocytes onto the endothelial surface (51) and migration into the tissue, as well as persistence of immune cells such as eosinophils to apoptotic stimuli (74), which may be why HA deposition in asthmatic tissue correlates with influx of immune cells (19, 20). Short-fragment HA signaling through the involvement of IαI, TSG-6, CD44, and TLR4 leads to the activation of small GTPases (RhoA, Rho kinase) and calcium channels and, ultimately, AHR (Fig. 2) (58), while antibody neutralization of IαI or addition of high-molecular-weight HA inhibits these effects (30, 58). HA accumulation is also involved in the pathogenesis of acute and chronic airway rejection in transplantation (22, 89, 92, 93), wherein short-fragment HA activates innate and adaptive immunity (89, 92) while high-molecular-weight HA ameliorates immune activation in this condition (93). It is not clear at this point why different sizes of HA have disparate biological effects. It may be that short-fragment HA leads to its particular effects because it occupies the “Goldilocks” position for optimal receptor assembly, whereas high-molecular-weight and oligosaccharide HA competitively inhibit short-fragment HA effects by selectively occupying receptors but being unable to engage the full receptor complement. Other mechanisms may be at play as well. For example, HA oligosaccharides can “poach” IαI heavy chains away from higher-molecular-weight HA, thus inhibiting the development of pathological HA matrix and binding of HA to its receptors (56). Although the exact mechanisms for the airway effects of HA are not completely understood, it is becoming apparent that short-fragment HA is overexpressed in lung injury, whether from asthma, pollution, inflammation, infection, or remodeling processes (4, 5, 10, 12, 19, 20, 26, 30, 32, 59, 101), leading to activation of receptors and downstream signaling pathways that directly affect lung function. This cycle of hyperresponsiveness and remodeling can be ameliorated by high-molecular-weight HA or HA oligosaccharides.
Fig. 2.
Theoretical model of HA-induced airway hyperresponsiveness (AHR) at the airway smooth muscle level. Short-fragment HA (sHA) engages CD44 and Toll-like receptor 4 (TLR4) (and perhaps other receptors as well) and activates RhoA, Rho kinase, and ERK in the cell. RhoA activation also leads to calcium influx into the cell. All components are necessary for the development of smooth muscle contraction and hyperresponsiveness. High-molecular-weight HA (HMW-HA) and inter-α-inhibitor (IαI)-blocking antibodies (Anti-IαI Ab) inhibit this process. oHA, oligosaccharide HA.
High-molecular-weight HA signaling in the lung.
Studies in animal models of inflammatory lung conditions and in patients with asthma suggest a potential role for high-molecular-weight HA as a treatment for airway disease (16, 46, 77), including amelioration of inflammatory hyperresponsiveness, decreased expression of proinflammatory cytokines in a mouse model of allergic asthma (Fig. 3) (Garantziotis S, unpublished data), and protection against acute lung injury-induced inflammatory responses in mice (46). One possible mechanism is that high-molecular-weight HA promotes regulatory T-cell activity and suppression of adaptive immunity (8, 9), but high-molecular-weight HA also has stabilizing effects on airway myocytes and prevents repolarization and calcium flux-mediated contraction as well (58). In mouse models of chronic obstructive pulmonary disease (COPD), aerosolized HA protects endogenous HA in lung tissue against degradation by cigarette smoke-generated reactive oxygen species and produces significant reductions in air space enlargement and mitigation of elastic fiber injury, a marker for loss of alveoli (16). In humans, HA protects against exercise-induced AHR in patients with bronchial asthma (77).
Fig. 3.
High-molecular-weight HA ameliorates inflammatory AHR and remodeling. A and B: in a mouse model of allergic asthma, where mice were exposed to ovalbumin (OVA) + phosphate-buffered saline (PBS), OVA + HA 0.3% solution (Yabro), aluminium adjuvant (ALUM) + PBS, or ALUM + Yabro, HA reduced AHR (A) and reduced proinflammatory cytokines relevant to allergic asthma (B). C: in a model of chronic exposure, HA reduced the expression of specific genes relevant to airway remodeling. IL, interleukin; RL, total lung resistance; MCh, methacholine. *P < 0.05, **P < 0.01, ***P < 0.001.
Finally, high-molecular-weight HA applied to the airways may also protect the lung via interactions with the airway epithelium. Epithelium-expressed high-molecular-weight HA supports epithelial integrity in a lung injury model (46) through TLR2-TLR4 signaling and thus reduces lung injury and remodeling. HA promotes ciliary beating through the engagement of RHAMM (28, 68). HA further interacts with the cystic fibrosis transmembrane conductance regulator (85) and may act as an airway carrier for IαI, which blocks epithelial sodium channel activation, thus promoting mucus fluidity (59). The net effect of these actions, in addition to the shear hydrophilic potential of HA, may significantly improve airway clearance. HA also modifies epithelial immunity and the microbiome composition of the organism, which may also have relevance to the lung. For example, ingested HA isolated from human milk is a substrate for commensal bacteria (33) and enhances the innate intestinal epithelial antimicrobial defense, including protecting against Salmonella enteritis, by engaging with TLR4 and CD44 (38).
In summary, HA naturally occurs in the mammalian body, and its biology is strongly size dependent. Short-fragment HA is generated during tissue injury and mediates the inflammatory response, whereas high-molecular-weight HA ameliorates inflammation and lung injury in multiple models of respiratory disease. HA may therefore have potential as a novel treatment option in lung diseases where one or more of the following pathways are present: inflammation, epithelial survival, remodeling, or the microbiome. This suggests a possible role in asthma, COPD, cystic fibrosis (CF), primary ciliary dyskinesia (PCD), and other diseases with inflammation or epithelial dysfunction.
Role of HA in Bacterial Colonization and Biofilm Production
The biofilm is a complex microbial community protected by a self-produced polymeric matrix composed of polysaccharides, nucleic acid, and proteins (80). Biofilms firmly adhere to the surfaces of many materials used in a clinical setting. This is of particular relevance to airway infections, when biofilms occur on tracheostomy, endotracheal and tympanostomy tubes, sinus drainages, stents, valves, ossicular prostheses, and cochlear implants, but is also an issue with orthopedic prostheses, dental implants, catheters, and heart valves.
Role of the biofilm in infections.
The role of biofilms in chronic infections is well established; ∼65–80% of microbial infections in the body, including almost all chronic infections, involve bacterial biofilms (23). The principal biofilm-producing bacteria responsible for respiratory tract infections are Streptococcus pneumoniae, S. pyogenes, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa (37, 95, 98). In studies of children with chronic or recurrent otitis and sinusitis media, there was evidence of mucosal biofilm formation in 54–95% of cases (21, 48).
Biofilm resistance to antimicrobials is an important issue for clinicians. Bacteria embedded in biofilms are significantly more resistant to antimicrobials, as the biofilm protects bacteria embedded within the matrix against antibiotics (23, 80, 99). Thus strategies to reduce or prevent bacterial colonization and biofilm formation are essential.
Interfering with the biofilm: antiadhesive and antibiofilm activity of HA.
Evidence that HA has bacteriostatic activity against pathogens of the oral cavity (17, 78) prompted the evaluation of the in vitro antiadhesive and antibiofilm properties of HA against bacterial species responsible for respiratory tract infections. A proprietary preparation of HA 0.3% was tested at full strength and diluted to 50% for antibiofilm activity against S. aureus, M. catarrhalis, H. influenzae, and S. pneumoniae (25). The effect of HA on bacterial adhesion was assessed in Hep-2 cells, while antibiofilm activity was assessed by spectrophotometry following incubation of bacterial biofilms with HA. The study demonstrated that HA was able to interfere with bacterial adhesion to a cellular substrate, particularly at full strength. Biofilm produced by S. aureus was more sensitive to the antibiofilm action of HA than H. influenzae or M. catarrhalis (25) (Fig. 4). Thus HA has notable antiadhesive properties and moderate-to-high antibiofilm activity. “As bacterial adhesion to oral cells is the first step for colonization, these results further sustain the role of hyaluronic acid in the prevention of respiratory infections” (25).
Fig. 4.
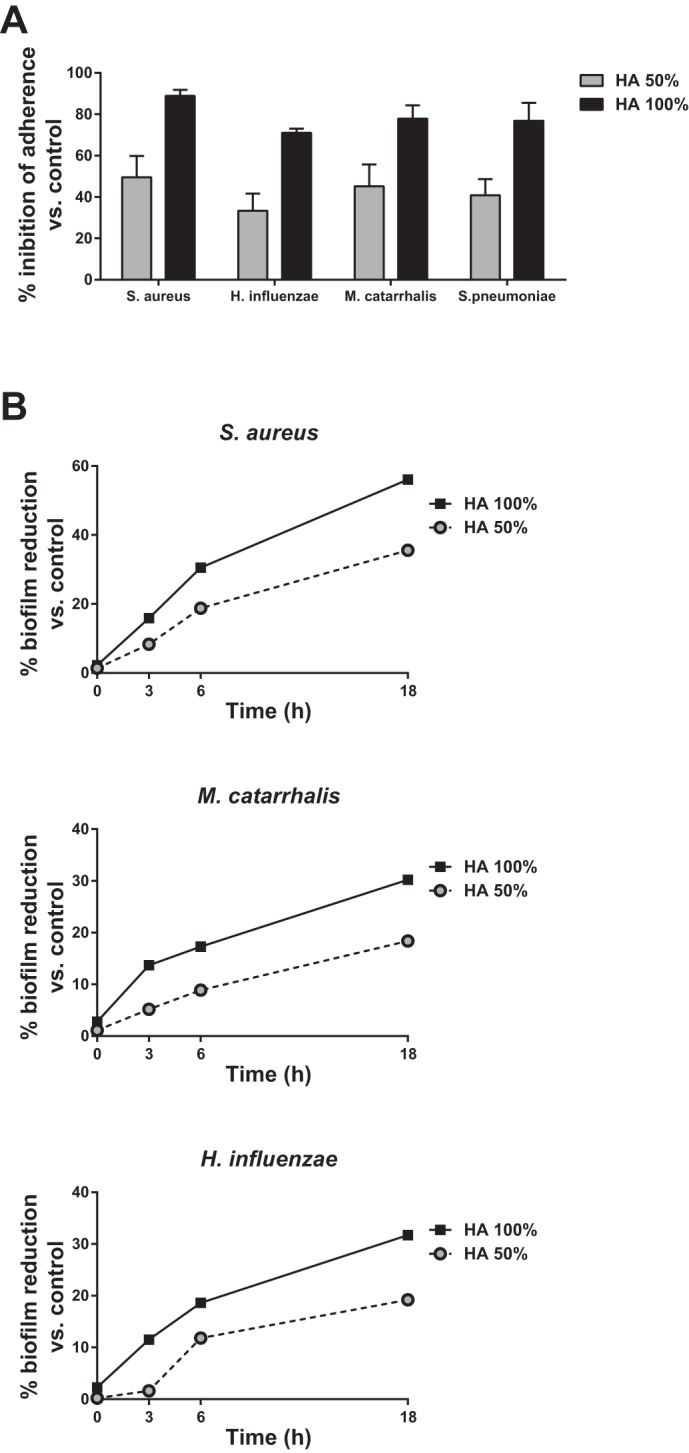
A: effect of HA on the adhesion of Staphylococcus aureus, Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae to Hep-2 cells. B: reduction of bacterial biofilm by HA for S. aureus, H. influenzae, and M. catarrhalis.
Clinical Use of HA
There is increasing evidence that administration of aerosolized exogenous HA may be useful for the prevention of recurrent chronic rhinosinusitis and may have an anti-inflammatory and protective effect in COPD, asthma, CF, and other respiratory conditions. We address current experience with the use of high-molecular-weight HA in the clinical setting below.
HA as Treatment in the Upper Airways
The mucociliary system is among the primary defense mechanisms of the airways, and impairment of mucociliary clearance predisposes to chronic airway infections. Chronic rhinosinusitis is characterized by a marked decrease in mucociliary clearance. In addition to its anti-inflammatory activity and ability to promote bacterial biofilm disruption, recent evidence that HA promotes mucociliary transport and helps to prevent the recurrence of chronic rhinosinusitis suggests a possible role for HA in diseases of the upper respiratory tract (18, 35, 65).
In a recent study, 75 children with recurrent upper respiratory tract infections were treated with aerosolized HA (0.3% solution in normal saline) or normal saline alone (64). HA improved the symptoms of rhinitis compared with saline alone (55.3% vs. 10.8%), postnasal drip (26.3% vs. 8.1%) and nasal dyspnea (31.6% vs. 10.8%), and reduced the presence of mycetes (26.3% vs. 8.1%) and biofilm (42.1% vs. 5.4%) (64). At 4 mo of treatment, ciliary motility was significantly improved in the HA group and adenoid hypertrophy, bacterial cell counts, and neutrophils were significantly reduced.
There is also evidence that aerosolized HA is beneficial in the postoperative management of patients with chronic rhinosinusitis by minimizing nasal crusting and edema and reducing the risk of infection, promoting restoration of the epithelium, mucociliary transport, and disruption of the microbial biofilm. In patients with nasal polyposis undergoing sinus surgery who were treated with HA (0.3% solution in saline) for 30 days from day 2 after surgery there were improvements in mucociliary clearance at 1 mo, and recipients of HA experienced a significantly lower incidence of rhinorrhea, lower nasal obstruction scores, and a lower presence of exudate on endoscopic examination (35). In another study of patients undergoing endoscopic sinus surgery for rhino-sinus remodeling, patients received HA (0.3% solution) nasal washes or saline alone for 15 days/mo over 3 mo. At the end of that period, HA was associated with significantly greater improvement in nasal dyspnea, mucociliary motility, presence of mycetes and appearance of nasal secretions and nasal mucosa, improvement in patency of the sinus ostia, reduction in mucosal edema, reduction in scarring, and improvement in the presence of biofilms (65). Finally, intranasal HA (0.3% solution) significantly improved short-term quality of life after sinus surgery for chronic rhinosinusitis with nasal polyposis, with improvements in both general and ENT-specific health status (13).
Although these studies suggest a useful role for aerosolized HA 0.3% solution in the management of chronic rhinosinusitis, further studies with larger patient numbers are needed to confirm these findings. A large, randomized study that plans to enroll at least 320 patients to investigate the use of HA in the postoperative period after functional endoscopic sinus surgery for chronic rhinosinusitis is currently underway in Germany, Italy, and Switzerland.
HA as Treatment in the Lower Airways
In addition to its role in the upper airways, HA has a functional role in various aspects of the bronchopulmonary pathology of the lower airways. Almost 20 years ago, subcutaneous administration of HA was shown to reduce the number of infectious acute exacerbations in patients with chronic bronchitis (96). Since then, various preclinical studies in animal models and lung tissue have found increased levels of HA degradation products in the disease and demonstrated a protective effect for exogenous high-molecular-weight HA, mostly when administered as inhaled HA (30, 60). Beneficial effects of HA have been cited in the literature in connection with asthma, COPD, emphysema, CF, PCD, extrinsic allergic alveolitis, interstitial lung disease, sarcoidosis, idiopathic pulmonary arterial hypertension, acute respiratory distress syndrome (ARDS), pneumonia, and tuberculosis (60, 88). The focus here is on inflammatory airway disease.
Asthma.
There is evidence that HA homeostasis is deranged in the asthmatic lung; the bronchial epithelium of asthmatic patients produces significantly higher concentrations of short-fragment HA than that of healthy subjects (62). Conversely, there is evidence for a beneficial effect of exogenous HA pretreatment in asthma, where some, but not all, studies have shown a significant protective effect (2, 54, 77). Any protective effect appears to be related to molecular size; in exercise-induced bronchoconstriction in asthmatic patients, inhaled HA with a mean molecular mass of 150,000 Da administered prior to exercise challenge did not protect against bronchoconstriction (54). However, aerosolized HA with a molecular mass varying between 400,000 and 4 million Da significantly reduced bronchial hyperreactivity to exercise and protected against exercise-induced bronchoconstriction compared with saline alone (P < 0.0001) (77).
In another randomized, crossover, double-blind study, pretreatment with aerosolized high-molecular-weight (∼1 million) HA 0.3% but not short-fragment (∼200,000) HA 0.3% significantly prevented methacholine-induced bronchoconstriction in asthmatic patients compared with placebo (2).
Pretreatment with inhaled exogenous HA appears to favorably modulate asthmatic bronchial hyperreactivity, suggesting that preseasonal and seasonal prophylactic administration of inhaled exogenous HA may increase the barrier function of bronchial mucosa and favorably modulate the course of seasonal allergic asthma. However, this is yet to be tested in a controlled clinical trial.
COPD.
HA is a significant constituent of lung ECM, and emphysema leads to loss of HA in human lungs (14, 53). In a mouse model of smoke-induced pulmonary emphysema, treatment with aerosolized HA led to reduction in the degree of alveolar injury and emphysema (15), an effect that persisted even when initiation of treatment was delayed until 1 mo after the onset of cigarette smoke exposure (16). Elastic fiber injury, a marker for loss of alveoli, was also mitigated in the HA group. The therapeutic possibility of inhaled HA as a chronic treatment in COPD is currently being tested in a safety trial (ClinicalTrials.gov identifier NCT00993707), while a study of inhaled HA in acute exacerbation of COPD is set to begin shortly in Italy.
Disorders of mucociliary clearance.
There is evidence that the use of inhaled HA has potential as anti-inflammatory therapy in CF, a condition in which biofilm has a significant role. In a mouse model of CF, nebulized HA was effective in controlling airway inflammation (34). This was also shown in vitro in human airway epithelial cells (34).
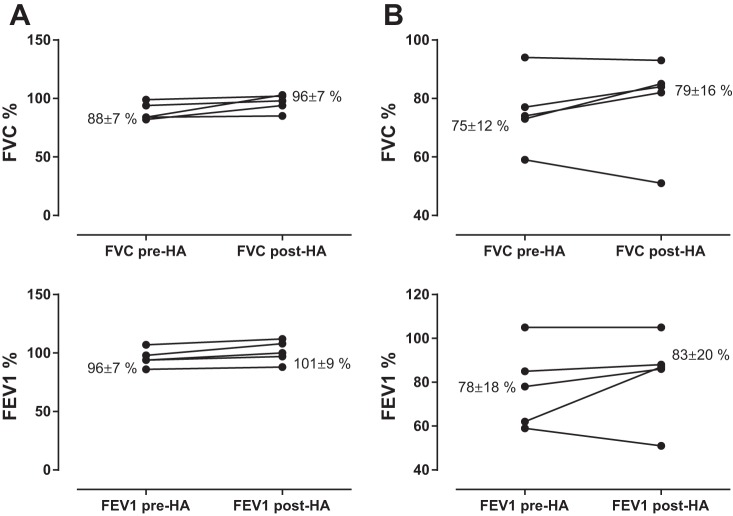
Inhaled HA has been used in CF patients for several years, mainly in association with hypertonic saline treatments. Addition of HA to the hypertonic solution improved patient perceptions of “pleasantness” and tolerability of nebulized hypertonic saline solution in patients with CF (11, 29, 67, 82). Notably, addition of HA also ameliorated the bronchoconstriction often caused by hypertonic saline and reduced the need for β-adrenergic bronchodilators (29). There have also been promising results in a real-life clinical setting in patients with PCD and CF treated with inhaled HA at the Clinic of Pediatric Pneumology, University Hospital, Bratislava, Slovakia. Stable patients underwent 40 days of alternate-day inhalatory administration of HA. If the patient was receiving a prescribed mucolytic, this was omitted on the day of inhalation; all other medications remained unchanged. Seven of ten patients showed clinically relevant improvements in lung function parameters, with increases in forced expiratory volume in 1 s (FEV1) of up to 10 percentage points in PCD patients and up to 25 percentage points in patients with CF (Fig. 5). Further study in a larger patient population would be very useful in elucidating the therapeutic potential of exogenous HA in CF.
Fig. 5.
Mean changes in lung function in patients with primary ciliary dyskinesia (A) and cystic fibrosis (B) before and after 40 days of alternate-day inhalatory administration of 3 ml of HA 0.3% solution. Improvements of ≥4% in lung function in patients with stable disease are considered clinically relevant. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
Gaps in Our Knowledge and New Research Directions
Although our understanding of HA biology has dramatically increased over the past decade, many gaps remain to be filled. Furthermore, the clinical research into applications of HA in human disease is only now starting. Therefore, there are many outstanding questions still to be elucidated.
As mentioned above, high-molecular-weight HA and short-fragment HA share the same molecular structure and appear to engage the same receptors. It is therefore important to understand what drives the different signaling behaviors. Are there differences in the affinity to receptors? Does size affect compartmentalization of HA or affinity to IαI or TSG-6 and thus dictate the differential effects? A related question, for clinical applications, is the ideal size of HA as a treatment agent. A common, and reasonable, concern about the application of high-molecular-weight HA in inflammatory disease is that it will be degraded to short-fragment HA, thus “adding fuel to the fire” in the intermediate or long term. Yet there is no evidence of this effect in either animal models or human studies that employed high-molecular-weight HA over several weeks. Additionally, although short-fragment HA does not protect from exercise-induced hyperresponsiveness in human asthma, and induces inflammation in naive mice, it seems to protect from the development of COPD in animal models and is being currently tested in clinical COPD trials. Thus it appears that we still do not fully understand the scope of HA signaling or effects in disease. It may be that pharmacological application of HA through the airway reaches a different compartment than short-fragment HA released in the interstitial space in inflammation, and this may account for the observed differences. Alternatively, it could be that short-fragment HA has adverse effects in naive tissues but acts as an antagonist to stronger inflammatory triggers, such as endotoxin and cigarette smoke. However, these hypotheses are yet to be experimentally tested.
Another related question with translational implications is whether other HA sizes, such as oligosaccharides, or HA analogs such as heparosan may play a role in the modulation of airway inflammation or remodeling. A potential benefit of these molecules is that they are not further degradable, thus bypassing questions of metabolite actions and also potentially prolonging the half-life time of the drug. HA oligosaccharides have been proposed as anticancer therapy agents (reviewed in Refs. 49, 63), and companies are developing both oligosaccharides and heparosan for human use. Thus new medical-grade compounds may soon be available for study.
With reference to compounding, it will also be interesting to investigate possible application routes. Notably, one of the first studies to investigate HA in human airway disease used a subcutaneous delivery to reduce exacerbations in patients with chronic bronchitis (96). Currently, HA is only available as a solution of nebulization in Europe. However, dry powder and systemic application may alter bioavailability, tissue delivery kinetics, and ease of use and are worth considering as HA-based drug formulations are developed.
Finally, it is worth noting that much of HA biology has focused on inflammatory cells or remodeling but there are significant HA effects on other cells, e.g., endothelia (52, 83, 87, 91) and platelets (1, 36, 75). Thus a role for HA in diseases of endothelial dysfunction or coagulation disorders, as they occur in sepsis and multiorgan dysfunction, is very probable. With it, therapeutic applications could be also envisioned. Even within the role of HA in remodeling, there are potential roles in vascular as much as airway remodeling. Indeed, HA metabolism and signaling have been found to play a role in pulmonary hypertension development (4, 5, 50, 55, 76). Thus it seems likely that therapeutic applications of HA may be expanded to the vascular compartment as well.
Summary and Conclusions
HA is a naturally occurring glycosaminoglycan macromolecule component of the ECM in the mammalian body, where it participates in a number of important biological processes, including water homeostasis, cell-matrix signaling, tissue healing, inflammatory processes, angiogenesis, and cell proliferation and migration. The physiological activity of HA is closely related to its molecular weight, and there is evidence that short-fragment HA, which is generated during tissue injury, augments inflammatory responses while high-molecular-weight HA may exert an anti-inflammatory and protective effect.
The anti-inflammatory, analgesic, and protective effects of exogenous HA are established in osteoarthritic conditions, where HA supplementation is widely accepted in clinical practice for the relief of pain and inflammation. Now there is increasing evidence that HA and its degradation products have an important role in the pathobiology of the respiratory tract and that the administration of aerosolized exogenous HA has beneficial activity against airway inflammation, protects against bronchial hyperreactivity and remodeling, and disrupts the biofilm associated with chronic infections. Exogenous HA may therefore be a novel treatment option to complement conventional medical or surgical therapy in diseases of the upper and lower respiratory tract that involve inflammation, epithelial survival, remodeling, and/or the microbiome, such as rhinosinusitis, asthma, COPD, CF and PCD, ARDS, or pulmonary hypertension. This role will become clearer when data are available from controlled trials in larger patient populations.
GRANTS
S. Garantziotis' research is funded by the Division of Intramural Research, National Institute of Environmental Health Sciences, National Institutes of Health (NIH).
DISCLOSURES
Some of the information in the present article was presented as scientific talks at the symposium “The Role of Hyaluronan in Respiratory Diseases” at the 2014 European Respiratory Society Meeting, in Munich, Germany, sponsored by IBSA Farmaceutici Italia Srl. M. Brezina, P. Castelnuovo, and L. Drago received an honorarium as reimbursement for their participation in the symposium. In accordance with NIH regulations, no speaker fee was provided to S. Garantziotis.
AUTHOR CONTRIBUTIONS
S.G., M.B., and L.D. prepared figures; S.G., M.B., P.C., and L.D. drafted manuscript; S.G., M.B., P.C., and L.D. edited and revised manuscript; S.G., M.B., P.C., and L.D. approved final version of manuscript.
REFERENCES
- 1.Albeiroti S, Ayasoufi K, Hill DR, Shen B, de la Motte CA. Platelet hyaluronidase-2: an enzyme that translocates to the surface upon activation to function in extracellular matrix degradation. Blood 125: 1460–1469, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allegra L, Abraham WM, Fasano V, Petrigni G. Methacholine challenge in asthmatics is protected by aerosolised hyaluronan at high (1,000 kDa) but not low (300 kDa) molecular weight. G Ital Mal Torace 62: 297–301, 2008. [Google Scholar]
- 3.Allegra L, Della Patrona S, Petrigni G. Hyaluronic acid: perspectives in lung diseases. Handb Exp Pharmacol 385–401, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Aytekin M, Comhair SA, de la Motte C, Bandyopadhyay SK, Farver CF, Hascall VC, Erzurum SC, Dweik RA. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 295: L789–L799, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aytekin M, Dweik RA. Low-molecular-mass of hyaluronan was detected in PASMCs from the patients with idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 300: L148, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Baranova NS, Foulcer SJ, Briggs DC, Tilakaratna V, Enghild JJ, Milner CM, Day AJ, Richter RP. Inter-alpha-inhibitor impairs TSG-6-induced hyaluronan cross-linking. J Biol Chem 288: 29642–29653, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baranova NS, Nileback E, Haller FM, Briggs DC, Svedhem S, Day AJ, Richter RP. The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J Biol Chem 286: 25675–25686, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol 86: 567–572, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol 179: 744–747, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Bracke KR, Dentener MA, Papakonstantinou E, Vernooy JH, Demoor T, Pauwels NS, Cleutjens J, van Suylen RJ, Joos GF, Brusselle GG, Wouters EF. Enhanced deposition of low-molecular-weight hyaluronan in lungs of cigarette smoke-exposed mice. Am J Respir Cell Mol Biol 42: 753–761, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Buonpensiero P, De Gregorio F, Sepe A, Di Pasqua A, Ferri P, Siano M, Terlizzi V, Raia V. Hyaluronic acid improves “pleasantness” and tolerability of nebulized hypertonic saline in a cohort of patients with cystic fibrosis. Adv Ther 27: 870–878, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Calve S, Isaac J, Gumucio JP, Mendias CL. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am J Physiol Cell Physiol 303: C577–C588, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantone E, Castagna G, Sicignano S, Ferranti I, Rega F, Di Rubbo V, Iengo M. Impact of intranasal sodium hyaluronate on the short-term quality of life of patients undergoing functional endoscopic sinus surgery for chronic rhinosinusitis. Int Forum Allergy Rhinol 4: 484–487, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Cantor J, Armand G, Turino G. Lung hyaluronan levels are decreased in alpha-1 antiprotease deficiency COPD. Respir Med 109: 656–659, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Cantor JO, Cerreta JM, Ochoa M, Ma S, Chow T, Grunig G, Turino GM. Aerosolized hyaluronan limits airspace enlargement in a mouse model of cigarette smoke-induced pulmonary emphysema. Exp Lung Res 31: 417–430, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Cantor JO, Cerreta JM, Ochoa M, Ma S, Liu M, Turino GM. Therapeutic effects of hyaluronan on smoke-induced elastic fiber injury: does delayed treatment affect efficacy? Lung 189: 51–56, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson GA, Dragoo JL, Samimi B, Bruckner DA, Bernard GW, Hedrick M, Benhaim P. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem Biophys Res Commun 321: 472–478, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Casale M, Sabatino L, Frari V, Mazzola F, Dell'Aquila R, Baptista P, Mladina R, Salvinelli F. The potential role of hyaluronan in minimizing symptoms and preventing exacerbations of chronic rhinosinusitis. Am J Rhinol Allergy 28: 345–348, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology 23: 43–58, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol 30: 126–134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coticchia J, Zuliani G, Coleman C, Carron M, Gurrola J 2nd, Haupert M, Berk R. Biofilm surface area in the pediatric nasopharynx: chronic rhinosinusitis vs. obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 133: 110–114, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, George G, Toprak D, Abdelnour E, D'Agostino E, Goldberg HJ, Perrella MA, Forteza RM, Rosas IO, Visner G, El-Chemaly S. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest 125: 4255–4268, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2: 114–122, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer 71: 251–256, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Drago L, Cappelletti L, De Vecchi E, Pignataro L, Torretta S, Mattina R. Antiadhesive and antibiofilm activity of hyaluronic acid against bacteria responsible for respiratory tract infections. APMIS 122: 1013–1019, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Eldridge L, Moldobaeva A, Wagner EM. Increased hyaluronan fragmentation during pulmonary ischemia. Am J Physiol Lung Cell Mol Physiol 301: L782–L788, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eurlings IM, Dentener MA, Mercken EM, de Cabo R, Bracke KR, Vernooy JH, Wouters EF, Reynaert NL. A comparative study of matrix remodeling in chronic models for COPD; mechanistic insights into the role of TNF-alpha. Am J Physiol Lung Cell Mol Physiol 307: L557–L565, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, Salathe M. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J 15: 2179–2186, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Furnari ML, Termini L, Traverso G, Barrale S, Bonaccorso MR, Damiani G, Piparo CL, Collura M. Nebulized hypertonic saline containing hyaluronic acid improves tolerability in patients with cystic fibrosis and lung disease compared with nebulized hypertonic saline alone: a prospective, randomized, double-blind, controlled study. Ther Adv Respir Dis 6: 315–322, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 31.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 181: 666–675, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 32.Garantziotis S, Zudaire E, Trempus CS, Hollingsworth JW, Jiang D, Lancaster LH, Richardson E, Zhuo L, Cuttitta F, Brown KK, Noble PW, Kimata K, Schwartz DA. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am J Respir Crit Care Med 178: 939–947, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrido D, Ruiz-Moyano S, Mills DA. Release and utilization of N-acetyl-d-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe 18: 430–435, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavina M, Luciani A, Villella VR, Esposito S, Ferrari E, Bressani I, Casale A, Bruscia EM, Maiuri L, Raia V. Nebulized hyaluronan ameliorates lung inflammation in cystic fibrosis mice. Pediatr Pulmonol 48: 761–771, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Gelardi M, Guglielmi AV, De Candia N, Maffezzoni E, Berardi P, Quaranta N. Effect of sodium hyaluronate on mucociliary clearance after functional endoscopic sinus surgery. Eur Ann Allergy Clin Immunol 45: 103–108, 2013. [PubMed] [Google Scholar]
- 36.Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L, Fiocchi A, Vacca M, Aiolfi R, Sammicheli S, Mainetti M, Cataudella T, Raimondi A, Gonzalez-Aseguinolaza G, Protzer U, Ruggeri ZM, Chisari FV, Isogawa M, Sitia G, Iannacone M. Immunosurveillance of the liver by intravascular effector CD8+ T cells. Cell 161: 486–500, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296: 202–211, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill DR, Rho HK, Kessler SP, Amin R, Homer CR, McDonald C, Cowman MK, de la Motte CA. Human milk hyaluronan enhances innate defense of the intestinal epithelium. J Biol Chem 288: 29090–29104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodge-Dufour J, Marino MW, Horton MR, Jungbluth A, Burdick MD, Strieter RM, Noble PW, Hunter CA, Pure E. Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci USA 95: 13806–13811, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, Pure E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol 159: 2492–2500, 1997. [PubMed] [Google Scholar]
- 41.Horton MR, Burdick MD, Strieter RM, Bao C, Noble PW. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-gamma in mouse macrophages. J Immunol 160: 3023–3030, 1998. [PubMed] [Google Scholar]
- 42.Horton MR, McKee CM, Bao C, Liao F, Farber JM, Hodge-DuFour J, Pure E, Oliver BL, Wright TM, Noble PW. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines mig and interferon-inducible protein-10 in mouse macrophages. J Biol Chem 273: 35088–35094, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Horton MR, Olman MA, Bao C, White KE, Choi AM, Chin BY, Noble PW, Lowenstein CJ. Regulation of plasminogen activator inhibitor-1 and urokinase by hyaluronan fragments in mouse macrophages. Am J Physiol Lung Cell Mol Physiol 279: L707–L715, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Horton MR, Olman MA, Noble PW. Hyaluronan fragments induce plasminogen activator inhibitor-1 and inhibit urokinase activity in mouse alveolar macrophages: a potential mechanism for impaired fibrinolytic activity in acute lung injury. Chest 116: 17S, 1999. [PubMed] [Google Scholar]
- 45.Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol 162: 4171–4176, 1999. [PubMed] [Google Scholar]
- 46.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Kakehi K, Kinoshita M, Yasueda S. Hyaluronic acid: separation and biological implications. J Chromatogr B Analyt Technol Biomed Life Sci 797: 347–355, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Kania RE, Lamers GE, Vonk MJ, Dorpmans E, Struik J, Tran Ba Huy P, Hiemstra P, Bloemberg GV, Grote JJ. Characterization of mucosal biofilms on human adenoid tissues. Laryngoscope 118: 128–134, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Karbownik MS, Nowak JZ. Hyaluronan: towards novel anti-cancer therapeutics. Pharmacol Rep 65: 1056–1074, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Karmouty-Quintana H, Weng T, Garcia-Morales LJ, Chen NY, Pedroza M, Zhong H, Molina JG, Bunge R, Bruckner BA, Xia Y, Johnston RA, Loebe M, Zeng D, Seethamraju H, Belardinelli L, Blackburn MR. Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 49: 1038–1047, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katoh S, Maeda S, Fukuoka H, Wada T, Moriya S, Mori A, Yamaguchi K, Senda S, Miyagi T. A crucial role of sialidase Neu1 in hyaluronan receptor function of CD44 in T helper type 2-mediated airway inflammation of murine acute asthmatic model. Clin Exp Immunol 161: 233–241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiene LS, Homann S, Suvorava T, Rabausch B, Muller J, Kojda G, Kretschmer I, Twarock S, Dai G, Deenen R, Hartwig S, Lehr S, Kohrer K, Savani RC, Grandoch M, Fischer JW. Deletion of hyaluronan synthase 3 inhibits neointimal hyperplasia. Arterioscler Thromb Vasc Biol 36: e9–e16, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konno K, Arai H, Motomiya M, Nagai H, Ito M, Sato H, Satoh K. A biochemical study on glycosaminoglycans (mucopolysaccharides) in emphysematous and in aged lungs. Am Rev Respir Dis 126: 797–801, 1982. [DOI] [PubMed] [Google Scholar]
- 54.Kunz LI, van Rensen EL, Sterk PJ. Inhaled hyaluronic acid against exercise-induced bronchoconstriction in asthma. Pulm Pharmacol Ther 19: 286–291, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Lauer ME, Aytekin M, Comhair SA, Loftis J, Tian L, Farver CF, Hascall VC, Dweik RA. Modification of hyaluronan by heavy chains of inter-alpha-inhibitor in idiopathic pulmonary arterial hypertension. J Biol Chem 289: 6791–6798, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lauer ME, Glant TT, Mikecz K, DeAngelis PL, Haller FM, Husni ME, Hascall VC, Calabro A. Irreversible heavy chain transfer to hyaluronan oligosaccharides by tumor necrosis factor-stimulated gene-6. J Biol Chem 288: 205–214, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lauer ME, Majors AK, Comhair S, Ruple LM, Matuska B, Subramanian A, Farver C, Dworski R, Grandon D, Laskowski D, Dweik RA, Erzurum SC, Hascall VC, Aronica MA. Hyaluronan and its heavy chain modification in asthma severity and experimental asthma exacerbation. J Biol Chem 290: 23124–23134, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazrak A, Creighton JR, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazrak A, Jurkuvenaite A, Ness EC, Zhang S, Woodworth BA, Muhlebach MS, Stober VP, Lim YP, Garantziotis S, Matalon S. Inter-alpha-inhibitor blocks epithelial sodium channel activation and decreases nasal potential differences in DeltaF508 mice. Am J Respir Cell Mol Biol 50: 953–962, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol 301: L137–L147, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z, Potts-Kant EN, Garantziotis S, Foster WM, Hollingsworth JW. Hyaluronan signaling during ozone-induced lung injury requires TLR4, MyD88, and TIRAP. PLoS One 6: e27137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 62.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol 128: 403–411, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lokeshwar VB, Mirza S, Jordan A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Cancer Res 123: 35–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macchi A, Castelnuovo P, Terranova P, Digilio E. Effects of sodium hyaluronate in children with recurrent upper respiratory tract infections: results of a randomised controlled study. Int J Immunopathol Pharmacol 26: 127–135, 2013. [DOI] [PubMed] [Google Scholar]
- 65.Macchi A, Terranova P, Digilio E, Castelnuovo P. Hyaluronan plus saline nasal washes in the treatment of rhino-sinusal symptoms in patients undergoing functional endoscopic sinus surgery for rhino-sinusal remodeling. Int J Immunopathol Pharmacol 26: 137–145, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Maile R, Jones S, Pan Y, Zhou H, Jaspers I, Peden DB, Cairns BA, Noah TL. Association between early airway damage-associated molecular patterns and subsequent bacterial infection in patients with inhalational and burn injury. Am J Physiol Lung Cell Mol Physiol 308: L855–L860, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maiz Carro L, Lamas Ferreiro A, Ruiz de Valbuena Maiz M, Wagner Struwing C, Gabilondo Alvarez G, Suarez Cortina L. [Tolerance of two inhaled hypertonic saline solutions in patients with cystic fibrosis.] Med Clin (Barc) 138: 57–59, 2012. [DOI] [PubMed] [Google Scholar]
- 68.Manzanares D, Monzon ME, Savani RC, Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol 37: 160–168, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonald B, McAvoy EF, Lam F, Gill V, de la Motte C, Savani RC, Kubes P. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med 205: 915–927, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, Choi AM, Noble PW. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor kappaB-dependent mechanism. J Biol Chem 272: 8013–8018, 1997. [DOI] [PubMed] [Google Scholar]
- 71.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest 98: 2403–2413, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milner CM, Tongsoongnoen W, Rugg MS, Day AJ. The molecular basis of inter-alpha-inhibitor heavy chain transfer on to hyaluronan. Biochem Soc Trans 35: 672–676, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappaB/I-kappaBalpha autoregulatory loop in murine macrophages. J Exp Med 183: 2373–2378, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ohkawara Y, Tamura G, Iwasaki T, Tanaka A, Kikuchi T, Shirato K. Activation and transforming growth factor-beta production in eosinophils by hyaluronan. Am J Respir Cell Mol Biol 23: 444–451, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Onclinx C, Dogne S, Jadin L, Andris F, Grandfils C, Jouret F, Mullier F, Flamion B. Deficiency in mouse hyaluronidase 2: a new mechanism of chronic thrombotic microangiopathy. Haematologica 100: 1023–1030, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ormiston ML, Slaughter GR, Deng Y, Stewart DJ, Courtman DW. The enzymatic degradation of hyaluronan is associated with disease progression in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 298: L148–L157, 2010. [DOI] [PubMed] [Google Scholar]
- 77.Petrigni G, Allegra L. Aerosolised hyaluronic acid prevents exercise-induced bronchoconstriction, suggesting novel hypotheses on the correction of matrix defects in asthma. Pulm Pharmacol Ther 19: 166–171, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Pirnazar P, Wolinsky L, Nachnani S, Haake S, Pilloni A, Bernard GW. Bacteriostatic effects of hyaluronic acid. J Periodontol 70: 370–374, 1999. [DOI] [PubMed] [Google Scholar]
- 79.Rockey DC, Chung JJ, McKee CM, Noble PW. Stimulation of inducible nitric oxide synthase in rat liver by hyaluronan fragments. Hepatology 27: 86–92, 1998. [DOI] [PubMed] [Google Scholar]
- 80.Romano CL, Toscano M, Romano D, Drago L. Antibiofilm agents and implant-related infections in orthopaedics: where are we? J Chemother 25: 67–80, 2013. [DOI] [PubMed] [Google Scholar]
- 81.Rooney P, Wang M, Kumar P, Kumar S. Angiogenic oligosaccharides of hyaluronan enhance the production of collagens by endothelial cells. J Cell Sci 105: 213–218, 1993. [DOI] [PubMed] [Google Scholar]
- 82.Ros M, Casciaro R, Lucca F, Troiani P, Salonini E, Favilli F, Quattrucci S, Sher D, Assael BM. Hyaluronic acid improves the tolerability of hypertonic saline in the chronic treatment of cystic fibrosis patients: a multicenter, randomized, controlled clinical trial. J Aerosol Med Pulm Drug Deliv 27: 133–137, 2014. [DOI] [PubMed] [Google Scholar]
- 83.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 276: 36770–36778, 2001. [DOI] [PubMed] [Google Scholar]
- 84.Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, Stern R, Greenberg AH, DeLisser HM, Khalil N. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol 23: 475–484, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Schulz T, Schumacher U, Prante C, Sextro W, Prehm P. Cystic fibrosis transmembrane conductance regulator can export hyaluronan. Pathobiology 77: 200–209, 2010. [DOI] [PubMed] [Google Scholar]
- 86.Singleton PA, Lennon FE. Acute lung injury regulation by hyaluronan. J Allergy Ther Suppl 4: S4-003, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singleton PA, Mirzapoiazova T, Guo Y, Sammani S, Mambetsariev N, Lennon FE, Moreno-Vinasco L, Garcia JG. High-molecular-weight hyaluronan is a novel inhibitor of pulmonary vascular leakiness. Am J Physiol Lung Cell Mol Physiol 299: L639–L651, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Souza-Fernandes AB, Pelosi P, Rocco PR. Bench-to-bedside review: the role of glycosaminoglycans in respiratory disease. Crit Care 10: 237, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stober VP, Szczesniak C, Childress Q, Heise RL, Bortner C, Hollingsworth JW, Neuringer IP, Palmer SM, Garantziotis S. Bronchial epithelial injury in the context of alloimmunity promotes lymphocytic bronchiolitis through hyaluronan expression. Am J Physiol Lung Cell Mol Physiol 306: L1045–L1055, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swaidani S, Cheng G, Lauer ME, Sharma M, Mikecz K, Hascall VC, Aronica MA. TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J Biol Chem 288: 412–422, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 279: 17079–17084, 2004. [DOI] [PubMed] [Google Scholar]
- 92.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant 6: 2622–2635, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, Pavlisko EN, Kelly FL, Huang H, Kreisel D, Palmer SM, Gelman AE. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med 189: 556–566, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4: 528–539, 2004. [DOI] [PubMed] [Google Scholar]
- 95.Torretta S, Drago L, Marchisio P, Gaffuri M, Clemente IA, Pignataro L. Topographic distribution of biofilm-producing bacteria in adenoid subsites of children with chronic or recurrent middle ear infections. Ann Otol Rhinol Laryngol 122: 109–113, 2013. [DOI] [PubMed] [Google Scholar]
- 96.Venge P, Pedersen B, Hakansson L, Hallgren R, Lindblad G, Dahl R. Subcutaneous administration of hyaluronan reduces the number of infectious exacerbations in patients with chronic bronchitis. Am J Respir Crit Care Med 153: 312–316, 1996. [DOI] [PubMed] [Google Scholar]
- 97.Venkatesan N, Ouzzine M, Kolb M, Netter P, Ludwig MS. Increased deposition of chondroitin/dermatan sulfate glycosaminoglycan and upregulation of beta1,3-glucuronosyltransferase I in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 300: L191–L203, 2011. [DOI] [PubMed] [Google Scholar]
- 98.Vergison A. Microbiology of otitis media: a moving target. Vaccine 26, Suppl 7: G5–G10, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol 182: 2675–2679, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science 228: 1324–1326, 1985. [DOI] [PubMed] [Google Scholar]
- 101.Wyatt HA, Dhawan A, Cheeseman P, Mieli-Vergani G, Price JF. Serum hyaluronic acid concentrations are increased in cystic fibrosis patients with liver disease. Arch Dis Child 86: 190–193, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, Savani RC. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol 33: 447–454, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu L, Zhuo L, Kimata K, Yamaguchi E, Watanabe H, Aronica MA, Hascall VC, Baba K. Deficiency in the serum-derived hyaluronan-associated protein-hyaluronan complex enhances airway hyperresponsiveness in a murine model of asthma. Int Arch Allergy Immunol 153: 223–233, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhuo L, Kanamori A, Kannagi R, Itano N, Wu J, Hamaguchi M, Ishiguro N, Kimata K. SHAP potentiates the CD44-mediated leukocyte adhesion to the hyaluronan substratum. J Biol Chem 281: 20303–20314, 2006. [DOI] [PubMed] [Google Scholar]
- 105.Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, Kawamura K, Suzuki T, Kimata K. Defect in SHAP-hyaluronan complex causes severe female infertility. A study by inactivation of the bikunin gene in mice. J Biol Chem 276: 7693–7696, 2001. [DOI] [PubMed] [Google Scholar]