Abstract
Fibrosis is a common pathological sequela of tissue injury or inflammation, and is a major cause of organ failure. Subsets of fibroblasts contribute to tissue fibrosis in multiple ways, including generating contractile force to activate integrin-bound, latent TGFβ and secreting excess amounts of collagens and other extracellular matrix proteins (ECM) that make up pathologic scar. However, the precise fibroblast subsets that drive fibrosis have been poorly understood. In the absence of well-characterized markers, α-smooth muscle actin (αSMA) is often used to identify pathologic fibroblasts, and some authors have equated αSMA+ cells with contractile myofibroblasts and proposed that these cells are the major source of ECM. Here, we investigated how well αSMA expression describes fibroblast subsets responsible for TGFβ activation and collagen production in three commonly used models of organ fibrosis that we previously reported could be inhibited by loss of αv integrins on all fibroblasts (using PDGFRβ-Cre). Interestingly, αSMA-directed deletion of αv integrins protected mice from CCl4-induced hepatic fibrosis, but not bleomycin-induced pulmonary or unilateral ureteral obstruction–induced renal fibrosis. Using Col-EGFP/αSMA-RFP dual reporter mice, we found that only a minority of collagen-producing cells coexpress αSMA in the fibrotic lung and kidney. Notably, Col-EGFP+αSMA-RFP− cells isolated from the fibrotic lung and kidney were equally capable of activating TGFβ as were Col-EGFP+αSMA-RFP+ cells from the same organ, and this TGFβ activation was blocked by a TGFβ-blocking antibody and an inhibitor of nonmuscle myosin, respectively. Taken together, our results suggest that αSMA is an inconsistent marker of contractile and collagen-producing fibroblasts in murine experimental models of organ fibrosis.
Keywords: αSMA, integrin, fibrosis
fibrosis of parenchymal organs is characterized by excessive accumulation of extracellular matrix proteins (ECM) including large amounts of fibrillar collagens (especially collagen I and III). Activated fibroblasts (fibroblasts that produce excess collagen) are key executors of this process, but the other molecular characteristics of these cells have not been well characterized. Transforming growth factor β (TGFβ), which recruits and activates fibroblasts, is well known as a potent profibrogenic cytokine that mediates fibrosis in multiple organs, including lung, liver, and kidney (6, 7, 11, 16, 31, 32, 35, 45). TGFβ is secreted as a latent complex, and extracellular activation of latent TGFβ is required for TGFβ activity and signaling. Two of the three mammalian TGFβ isoforms (TGFβ1 and TGFβ3) are principally activated by interaction with integrins, heterodimeric transmembrane receptors that bind to an arginine-glycine-aspartic acid (RGD) motif present in the latency associated peptide of TGFβ1 and TGFβ3 (15, 19, 22, 26, 31, 36, 41, 45).
Five integrins share the αv subunit (αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8), and three of these (αvβ1, αvβ6, and αvβ8) have been convincingly shown to activate latent TGFβ (3, 5–7, 17, 19, 33–36, 39, 48). In vivo loss of αvβ6 and αvβ8 reproduces the developmental abnormalities of loss of TGFβ1 and TGFβ3 (3), suggesting that αvβ6 and αvβ8 are crucial for the roles of these TGFβ isoforms in development. Emerging evidence indicates that αv integrins expressed on fibroblasts play a vital role in activating TGFβ and driving tissue fibrosis (6, 7, 18, 19, 33, 39). A previous study from our laboratory described an in vivo strategy to delete αv integrins from all fibroblasts using platelet-derived growth factor receptor β-Cre (PDGFRβ-Cre), and loss of αv integrins on fibroblasts protected mice from bleomycin-induced pulmonary fibrosis, CCl4-induced hepatic fibrosis, and unilateral ureteral obstruction (UUO) induced renal fibrosis (18). Recently, we found that blockade of the αvβ1 integrin by using a specific inhibitor results in the same degree of protection we observed with deletion of all αv integrins from fibroblasts, highlighting the critical role of the αvβ1 integrin in this process (39).
Integrins activate latent TGFβ by exerting mechanical force on the tethered latent complex, leading to conformational change that releases free active TGFβ to bind to its receptors on adjacent cells (21, 45, 51). Contractile myofibroblasts are widely believed to be the major contractile cells in fibrotic tissues and the key activated fibroblasts that produce excess collagens, and these cells are often identified in tissue based on expression of α-smooth muscle actin (αSMA) (10, 21, 27, 44, 50, 51). However, the effectiveness of αSMA as a marker of either contractile fibroblasts or collagen-producing fibroblasts has not been rigorously investigated in vivo.
In this study, we specifically targeted αSMA-expressing cells in vivo to examine whether they are the key subset responsible for the protective effects of αv deletion from fibroblasts in three widely used models of organ fibrosis: bleomycin-induced pulmonary, UUO-induced renal, and CCl4-induced hepatic fibrosis. We found that αSMA-directed deletion of αv integrins was only effective in preventing fibrosis in the liver, but not in the lung or kidney. Furthermore, using a reporter mouse that marks collagen I–expressing cells, we found that in the fibrotic lung and kidney, only a minority of collagen-producing cells coexpresses αSMA. Our results therefore suggest that αSMA is an inconsistent marker of both contractile fibroblasts and collagen-producing fibroblast in murine models of tissue fibrosis.
MATERIALS AND METHODS
Mouse lines.
Ai14 (Rosa-CAG-LSL-tdTomato-WPRE) (29) and tetO-Cre (37) mice were obtained from Jackson laboratories. PDGFRβ-Cre mice (12) were obtained from Dr. Ralf Adams at the University of Munster, Germany. αSMA-rtTA mice (9) were obtained from Dr. M. Shipley at the Washington University School of Medicine, St. Louis, MO. ItgαvFlox/Flox mice (25) were obtained from Dr. Adam Lacy-Hulbert at Harvard Medical School, Boston, MA. Col-EGFP (30, 52) and αSMA-RFP mice (30, 49) were obtained from Dr. David Brenner at the University of California, San Diego, CA. All mice were housed under specific pathogen-free conditions in the Animal Barrier Facility of the University of California, San Francisco, CA. Mice were maintained on C57BL/6 background, and genotyping of the mice was preformed by PCR. Mice used for experiments were 8–12 wk old and fed with grain-based doxycycline diet (2 gm/kg, BioServ) 2 wk before induction of fibrosis. All experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco, CA.
Fibrosis models.
To induce pulmonary fibrosis, mice were treated with a single dose of intratracheal bleomycin (3 U/kg) or water (control vehicle), delivered by a microsprayer (Penn-Century), and the lungs were harvested at 14 or 28 days as indicated. To induce hepatic fibrosis, mice were intraperitoneally injected with 1 μl/g body wt of CCl4 in a 1:3 ratio with olive oil or olive oil (control vehicle) twice a week as described previously (18), and the livers were harvested at 3 or 6 wk as indicated. To induce renal fibrosis, mice underwent UUO or sham operation on the left kidney as described previously (18), and the kidneys were harvested at 7 or 14 days as indicated.
Immunohistochemistry.
The tissue was harvested and fixed in 4% paraformaldehyde at 4°C for 3 h. Following immersion in 30% sucrose at 4°C overnight, the tissue was embedded with OCT compound (Tissue-Tek). Frozen sections were then stained by standard protocol described previously (18, 42). The following antibodies were used for immunohistochemistry: anti-αSMA (Sigma), anti-PDGFRβ (a gift from Dr. William Stallcup at Sanford-Burnham Medical Research Institute, La Jolla, CA), anti-RFP (Rockland), and anti-GFP (Abcam) primary antibodies, as well as Alexa Fluor 488-conjugated and 555-conjugated secondary antibodies (Invitrogen). Confocal imaging was performed on a Zeiss LSM 780 microscope.
Collagen content assay.
Determination of collagen content was performed by hydroxyproline assay of tissue lysates and picrosirius red staining of tissue sections. Hydroxyproline assay was conducted as described previously (18). In brief, the tissue was homogenized and precipitated with trichloroacetic acid. Following baking at 110°C overnight in HCl, samples were reconstituted in water, and hydroxyproline content was measured by a colorimetric chloramine T assay. For picrosirius red staining, the tissue was harvested and fixed in 10% formalin, followed by paraffin embedding. Paraffin-embedded sections were then dewaxed, hydrated, and stained with Weigert's haematoxylin for nuclei and picrosirius red (Sigma) for collagen.
Tissue dissociation and primary cell purification via FACS.
Tissue dissociation was conducted as described previously (18). In brief, mice were perfused with phosphate buffered saline (PBS) through the left ventricle to remove blood cells. The tissue was excised, minced with scissors, follow by digestion in Dulbecco's modified Eagle medium (DMEM, Invitrogen) containing liberase (0.13 IU/ml) (Roche) for lungs and livers, or by digestion in DMEM containing liberase (0.13 IU/ml) and collagenase 4 (0.5 mg/ml) (Sigma) for kidneys, at 37°C for 20 min. Single-cell suspensions were then prepared with a gentleMACS dissociator (Miltenyi Biotec) as described in manufacturer's instructions. The cell suspension was passed through a 70-μm cell strainer and centrifuged at 1,000 rpm for 5 min to form a pellet. To remove residual red blood cells (RBC), the cell pellet was resuspended in RBC lysis buffer (Sigma) and incubated at room temperature for 10 min. Cells were then passed through a 40-μm cell strainer and washed with DMEM twice to remove cell debris. After centrifuging at 1,000 rpm for 5 min, the cell pellet was resuspended in fluorescence-activated cell sorting (FACS) buffer (PBS supplemented with 3% fetal bovine serum). Following live/dead staining with DAPI (Sigma), live single cells with indicated reporters were sorted with a FACSAria (BD Biosciences).
TGFβ activation assay.
Primary cells of interest were purified via FACS, as described above, and then cocultured with transformed mink lung cells (TMLC), the mink lung epithelial cells expressing firefly luciferase downstream of a TGFβ sensitive portion of the plasminogen activator inhibitor 1 promoter (1, 4, 47), for 18 h. Cells were then washed and lysed in cell lysis buffer (Promega). The lysates were then processed with a luciferase assay kit (Roche) as described in the manufacturer's instructions, and luminescence was measured with a TECAN microplate reader. The pan-TGF-β blocking antibody 1D11 (40 μg/ml) and blebbistatin (20 μm) (Sigma) were used in this assay as indicated.
Statistics.
All error bars denote SE. Results were analyzed with SigmaStat software by using one-way ANOVA, followed by a Student t-test to determine P values. P values less than 0.05 were considered significant.
RESULTS
αSMA-positive cells are only a subset of PDGFRβ-positive cells within the fibrotic lung interstitium.
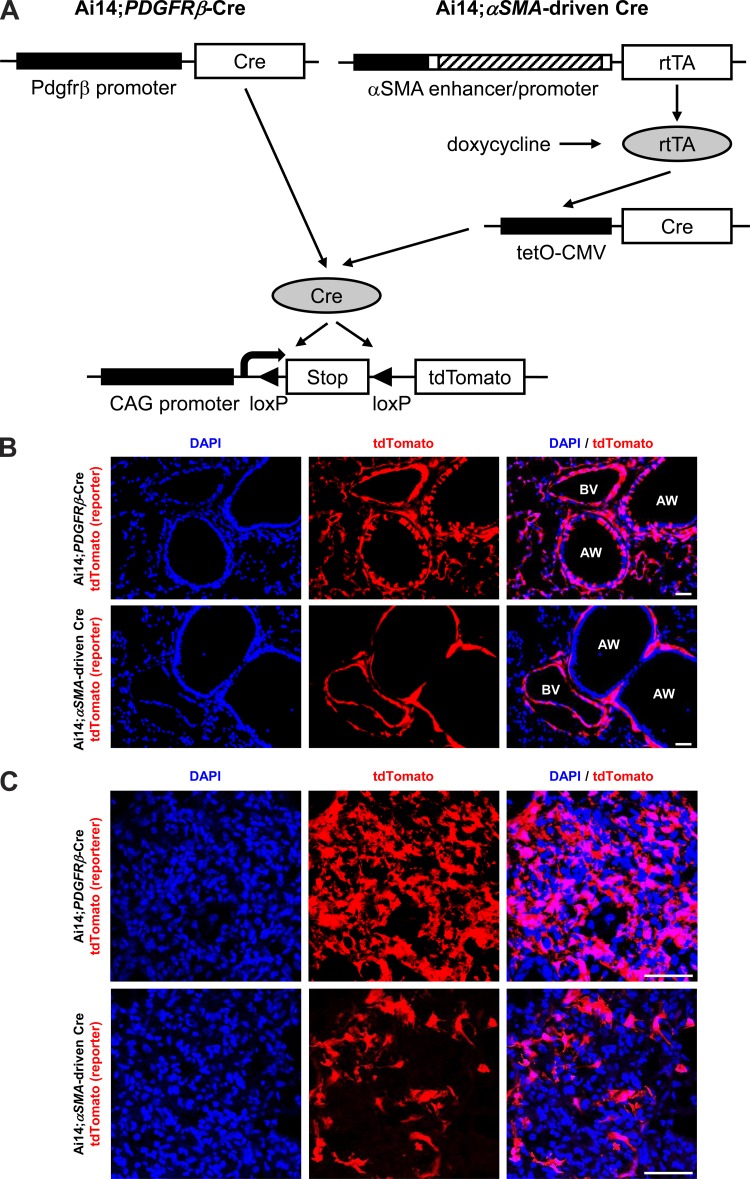
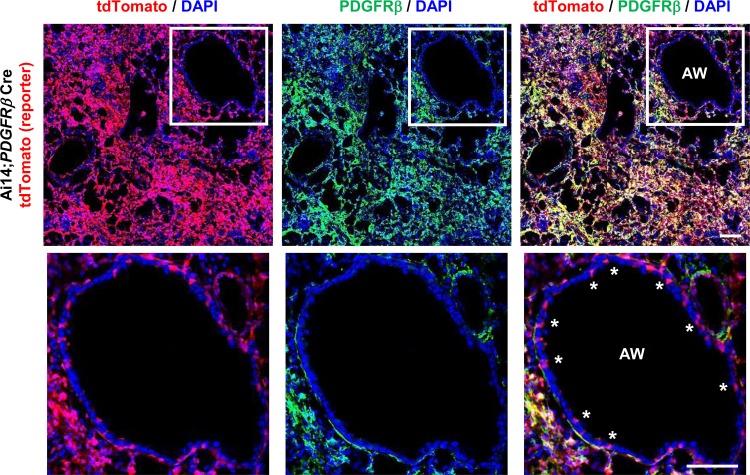
As noted above, our previous study used a strategy to broadly target fibroblasts by PDGFRβ-Cre (18). To directly examine the in vivo significance of αSMA-expressing fibroblasts in the setting of pulmonary fibrosis, we began by more thoroughly evaluating the recombination specificity of PDGFRβ-Cre in cells from uninjured and fibrotic lungs. For this purpose, we crossed PDGFRβ-Cre with Ai14 reporter mice (29) that express tandem dimer tomato (tdTomato) downstream of a lox-stop-lox cassette in the Rosa 26 locus after Cre-mediated recombination (Fig. 1A). A variety of types of cells, including cells in the alveolar interstitium (presumably pericytes), vascular smooth muscle cells, and some airway epithelial cells, demonstrated PDGFRβ-Cre-mediated recombination in uninjured lungs (Fig. 1B, panels at top). Fourteen days after treatment with bleomycin, there was also obvious recombination in aggregates of interstitial cells that we presume to be activated fibroblasts (Fig. 1C, panels at top). To further evaluate the accuracy of Ai14 reporting, we costained the bleomycin-treated lung sections with PDGFRβ antibody, and most of the cell types expressing tdTomato were also PDGFRβ-positive; however, airway epithelial cells were never found to express PDGFRβ by immunostaining (Fig. 2). These results suggest that either a subset of airway epithelial cells are derived from cells that expressed PDGFRβ during development, or that there was inappropriate expression of the construct we used in these cells. Otherwise, recombination appeared to be highly efficient and specific for cells that express PDGFRβ.
Fig. 1.
Comparison of tdTomato expression pattern between Ai14;PDGFRβ-Cre and Ai14;αSMA-rtTA;tetO-Cre mice in normal and fibrotic lungs. A: schematic of reporter mice generated by crossing Ai14 mice (29) with either PDGFRβ-Cre (12) or αSMA-driven Cre mice (9, 37). The αSMA enhancer/promoter construct contained that consisted of ∼1 kb of the 5′-flanking region (black box), the transcription start site, the 48-bp exon 1 (white box), the ∼2.5-kb intron 1 (hatched box), and the 15-bp exon 2 (white box) from the mouse αSMA gene (49). B: immunofluorescence micrographs of 14-day water-treated lung sections from the indicated reporter mice. AW, airway; BV, blood vessel. C: immunofluorescence micrographs of 14-day bleomycin-treated lung sections from the indicated reporter mice. Panels at left show DAPI (blue), panels in middle show endogenous tdTomato (red), and panels at right show merged images. Scale bar, 100 μm.
Fig. 2.
Inappropriate PDGFRβ-Cre-directed recombination in lung airway epithelial cells. Immunofluorescence micrographs of lung sections from 14-day bleomycin-treated Ai14;PDGFRβ-Cre mice, which was costained with PDGFRβ antibody. Panel at left shows DAPI (blue) and endogenous tdTomato (i.e., PDGFRβ) (red), panel in middle shows DAPI (blue) and PDGFRβ staining (green), and panel at right shows the merged image. Scale bar, 100 μm. AW, airway. Airway epithelial cells that were targeted by PDGFRβ-Cre indicated by *.
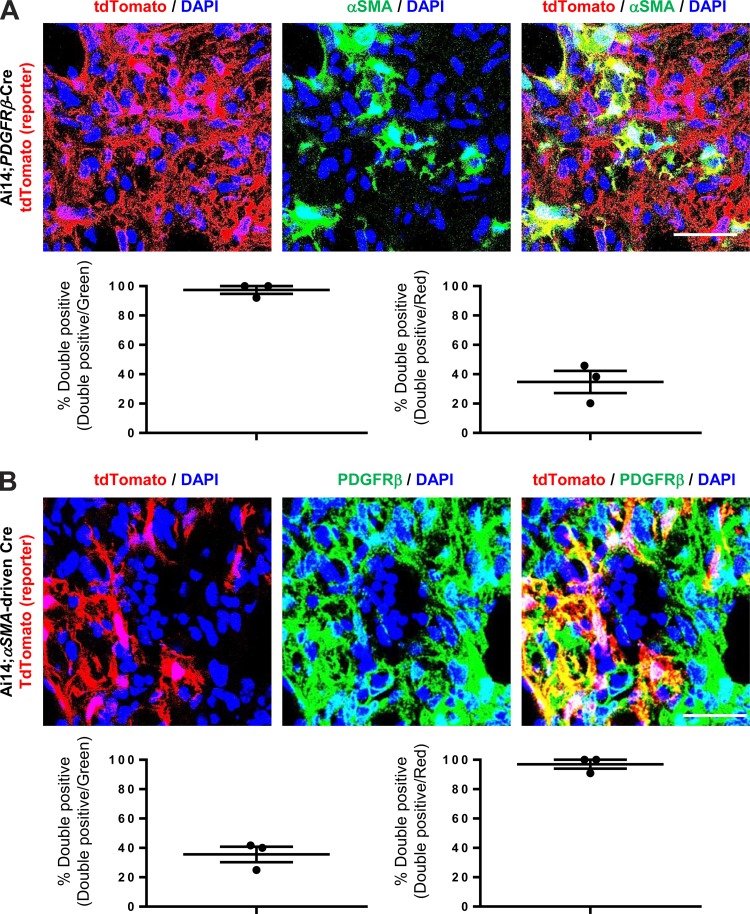
Notably, by coimmunostaining with αSMA in the fibrotic lung of bleomycin-treated Ai14;PDGFRβ-Cre mice, over 97% of the αSMA-positive cells observed were also tdTomato-positive (Fig. 3A), indicating a high degree of PDGFRβ-Cre recombination in αSMA+ myofibroblasts. However, upon quantification of images within the lung fibrotic interstitium, 35% of the tdTomato-positive cells were also αSMA-positive, indicating that only a minority of the expanded interstitial population of tdTomato-positive cells had detectible α-SMA by immunostaining (Fig. 3A).
Fig. 3.
αSMA-expressing cells are only a subpopulation of PDGFRβ-expressing cells within the fibrotic lung interstitium. A: immunofluorescence micrographs and image quantification of lung sections from 14-day bleomycin-treated Ai14;PDGFRβ-Cre mice, which were costained with αSMA antibody. Panel at left shows DAPI (blue) and endogenous tdTomato (i.e., PDGFRβ) (red), panel in middle shows DAPI (blue) and αSMA staining (green), and panel at right shows the merged image. B: immunofluorescence micrographs and image quantification of lung sections from 14-day bleomycin-treated Ai14;αSMA-rtTA;tetO-Cre mice, which were costained with PDGFRβ antibody. Panel at left shows DAPI (blue) and endogenous tdTomato (i.e., αSMA) (red), panel in middle shows DAPI (blue) and PDGFRβ staining (green), and panel at right shows the merged image. Scale bar, 50 μm.
To more specifically target and genetically manipulate αSMA-expressing fibroblasts in vivo, we used a doxycycline (dox)-inducible αSMA-driven Cre system (αSMA-rtTA × tetO-Cre) (9). This inducible αSMA-driven Cre allowed us to manipulate genes in adult mice by postnatal administration of dox in chow, which eliminates the possibility of compensation caused by lifelong genetic alteration. These mice were then crossed with Ai14 reporter mice for microscopic imaging (Fig. 1A). In uninjured Ai14;αSMA-rtTA;tetO-Cre mice, only smooth muscle cells of airways and vessels expressed tdTomato, and no tdTomato-positive cells were found within the lung interstitium (Fig. 1B, panels at bottom). After bleomycin treatment, tdTomato-positive cells began to appear within the lung interstitium of Ai14;αSMA-rtTA;tetO-Cre mice (Fig. 1C, panels at bottom). Upon image quantification, while over 96% of cells stained as PDGFRβ-positive expressed tdTomato, only 36% of PDGFRβ-positive cells within the fibrotic lung interstitium of Ai14;αSMA-rtTA;tetO-Cre mice were tdTomato-positive (Fig. 3B), similar to the results obtained by immunostaining for αSMA in bleomycin-treated Ai14;PDGFRβ-Cre mice (Fig. 3A). Taken together, our results suggest that αSMA-expressing fibroblasts are only a subset of PDGFRβ-positive interstitial cells.
αSMA-directed deletion of αv integrins does not confer protection against bleomycin-induced pulmonary fibrosis.
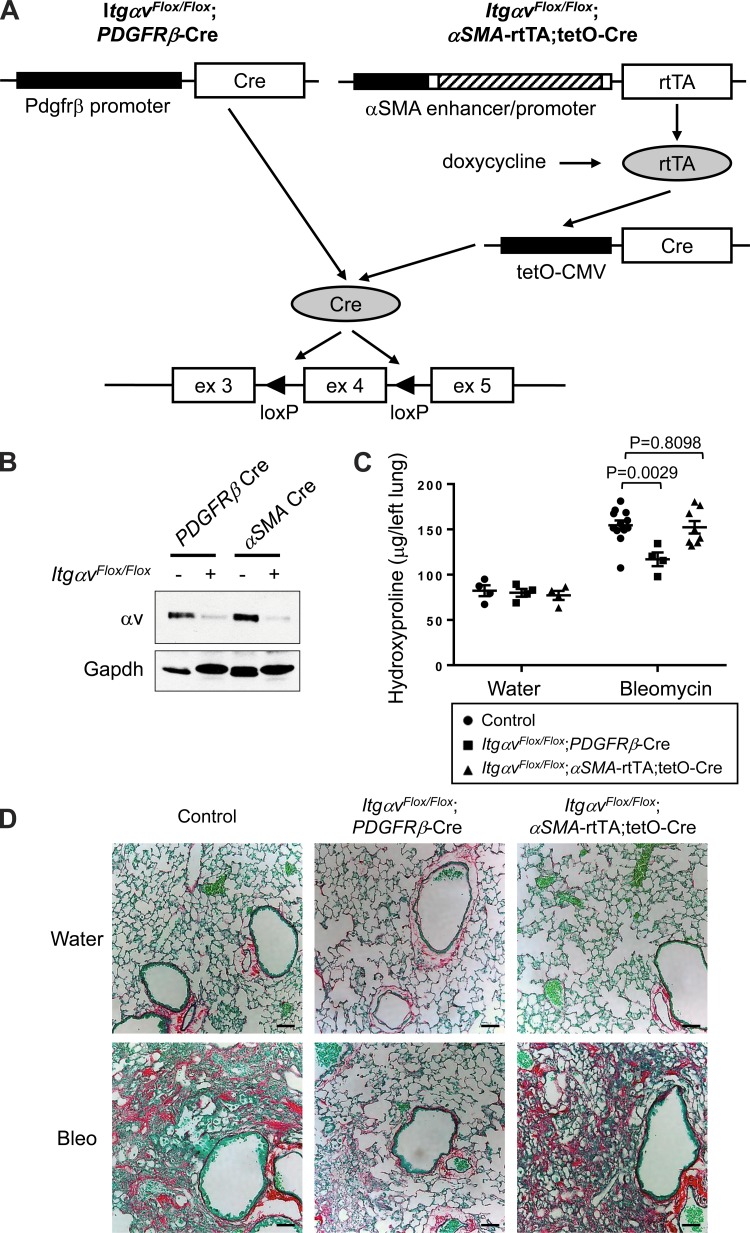
αv Integrins have been well characterized to activate latent TGFβ through force-dependent conformational change and to drive tissue fibrosis in multiple models (5–7, 17–19, 31, 33–36, 39, 48). Our previous study demonstrated that PDGFRβ-Cre-directed deletion of αv integrins protects mice from fibrosis in multiple organs, including the lung (18). To determine whether αSMA-expressing fibroblasts, a subset of PDGFRβ-positive interstitial cells in the fibrotic lung (Fig. 3), are the key subtype of cells responsible for this effect, we generated ItgαvFlox/Flox;αSMA-rtTA;tetO-Cre mice and compared results with those in the ItgαvFlox/Flox;PDGFRβ-Cre mice that we previously described (18) (Fig. 4A).
Fig. 4.
αSMA-directed deletion of αv integrins does not confer protective effects against bleomycin-induced pulmonary fibrosis. A: schematic of either PDGFRβ-Cre- (12) or αSMA-driven Cre- (9, 37) mediated recombination of the floxed αv integrin, which leads to removal of exon 4, shift in the translational reading frame in subsequent exons, and permanent inactivation of αv integrin expression (25). B: immunoblotting of expression of αv integrins in cells targeted by PDGFRβ-Cre or αSMA-driven Cre. Mice were treated with bleomycin for 14 days, and tdTomato-positive cells were purified by fluorescence-activated cell sorting (FACS), followed by immunoblotting with anti-αv integrin antibody. C: hydroxyproline assay of lung lysates. D: picrosirius red staining of lung sections from mice treated with water (control vehicle) or bleomycin (3 U/kg) for 28 days. Data are means ± SE. P value, Student's t-test. Scale bar, 100 μm.
To assess the efficiency of αv deletion by using either PDGFRβ-Cre or αSMA-driven Cre, it would be ideal to immunostain the tissues with an anti–αv integrin antibody that can distinguish wild-type and truncated αv integrins; however, no such antibody is available for immunohistochemistry. Alternatively, we crossed both lines with Ai14 reporter mice, which allows us to isolate tdTomato-positive cells by FACS. tdTomato-positive cells were purified from the lungs of bleomycin-treated Ai14;ItgαvFlox/Flox;PDGFRβ-Cre and Ai14;ItgαvFlox/Flox;αSMA-rtTA;tetO-Cre mice, as well as Ai14;PDGFRβ-Cre and Ai14;αSMA-rtTA;tetO-Cre mice that express wild-type αv integrins, followed by immunoblotting with an anti–αv integrin antibody. αv Integrins were deleted with similar efficiency from targeted cells by using either PDGFRβ-Cre or αSMA-driven Cre (Fig. 4B).
To determine whether loss of αv integrins on αSMA-expressing fibroblasts explained our previous results (18), we compared the effects of bleomycin-induced pulmonary fibrosis in ItgαvFlox/Flox;αSMA-rtTA;tetO-Cre and ItgαvFlox/Flox;PDGFRβ-Cre mice. Compared with their littermates expressing wild-type αv integrins, ItgαvFlox/Flox;PDGFRβ-Cre mice were protected from bleomycin-induced pulmonary fibrosis, whereas ItgαvFlox/Flox;αSMA-rtTA;tetO-Cre mice were not protected (Fig. 4, C and D). Our results suggest that αSMA-expressing fibroblasts are not the major subset of PDGFRβ-expressing cells responsible for αv integrin–mediated development of pulmonary fibrosis.
Heterogeneity: αSMA-directed deletion of αv integrins results in different outcomes in different models of organ fibrosis.
In addition to the lung, αSMA+ myofibroblasts in the liver and kidney are often considered the major mediators of collagen deposition and organ scarring after injury (10, 21, 27, 32, 44, 50). Our previous study has also shown that loss of αv integrins under the direction of PDGFRβ-Cre protects mice from CCl4-induced hepatic fibrosis and UUO-induced renal fibrosis (18). We therefore used the same tools to determine the relative contributions of αv integrins on αSMA-expressing fibroblasts to each of these models.
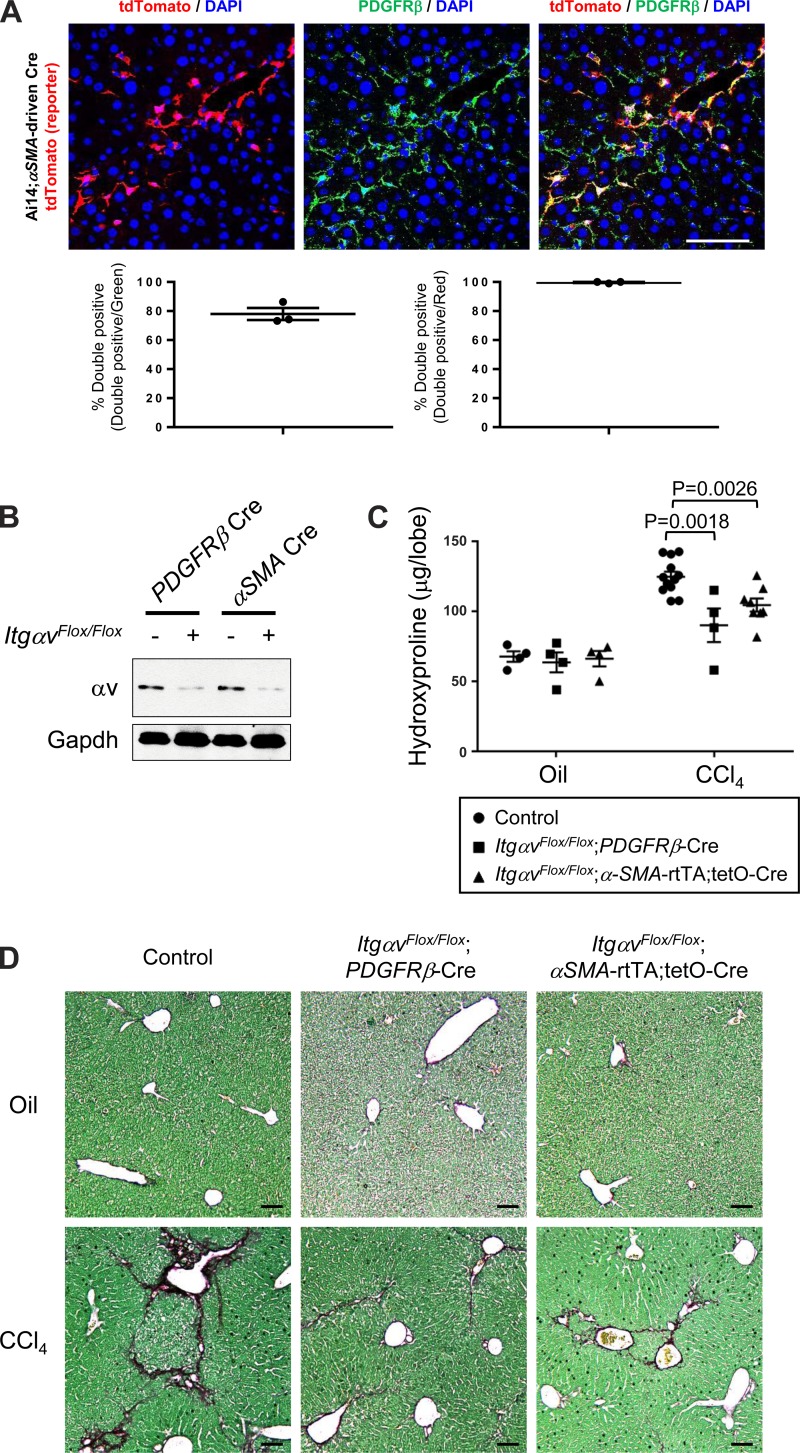
In the liver, hepatic stellate cells are well characterized as the source of myofibroblasts during hepatic fibrogenesis and the major source of extracellular matrix proteins (8, 13, 18, 21, 27, 40, 44, 50). We previously demonstrated that PDGFRβ-Cre effectively recombines hepatic stellate cells (HSCs) in both uninjured and injured livers (18). Indeed, in the fibrotic liver of CCl4-treated Ai14;PDGFRβ-Cre mice, virtually all of the αSMA-positive cells observed in liver fibrous septa and the surrounding liver parenchyma were also tdTomato-positive, indicating a high degree of PDGFRβ-Cre recombination in myofibroblasts (18). In the present study, after induction of hepatic fibrosis with CCl4 in Ai14;αSMA-rtTA;tetO-Cre mice, tdTomato-positive cells began to appear in liver fibrous septa and the surrounding liver parenchyma (Fig. 5A). Upon image quantification, 74% of cells immunostained as PDGFRβ-positive expressed tdTomato and 99% of these tdTomato-positive cells were also PDGFRβ-positive by immunostaining (Fig. 5A). Although a few PDGFRβ-positive and tdTomato-negative cells were also observed in CCl4-treated Ai14;αSMA-rtTA;tetO-Cre reporter mice, αSMA-expressing cells largely overlapped with PDGFRβ-positive cells in the fibrotic liver (Fig. 5A).
Fig. 5.
αSMA-directed deletion of αv integrins protects against CCl4-induced hepatic fibrosis. A: immunofluorescence micrographs and image quantification of liver sections from 3-wk CCl4-treated Ai14;αSMA-rtTA;tetO-Cre mice, costained with PDGFRβ antibody. Panel at left shows DAPI (blue) and endogenous tdTomato (i.e., αSMA) (red), panel in middle shows DAPI (blue) and PDGFRβ staining (green), and panel at right shows the merged image. Scale bar, 100 μm. B: immunoblotting of expression of αv integrins in cells targeted by PDGFRβ-Cre or αSMA-driven Cre. Mice were treated with CCl4 for 3 wk, and tdTomato-positive cells were purified by FACS, followed by immunoblotted with anti–αv integrin antibody. C: hydroxyproline assay of liver lysates. D: picrosirius red staining of liver from mice treated with olive oil (control vehicle) or CCl4 twice a week for 6 wk. Data are means ± SE. P value, Student's t-test. Scale bar, 200 μm.
To examine whether fibroblasts that were targeted by αSMA-inducible Cre are the key subset of cells responsible for protection from αv integrin–mediated hepatic fibrosis, we compared the effects of CCl4-induced hepatic fibrosis in ItgαvFlox/Flox;αSMA-rtTA;tetO-Cre and ItgαvFlox/Flox;PDGFRβ-Cre mice. αv Integrins were deleted with similar efficiency from targeted cells in the liver by either PDGFRβ-Cre or αSMA-driven Cre (Fig. 5B). In contrast to our results for bleomycin-induced pulmonary fibrosis, both ItgαvFlox/Flox;PDGFRβ-Cre and ItgαvFlox/Flox;αSMA-rtTA;tetO-Cre mice were similarly protected from CCl4-induced hepatic fibrosis, compared with their littermates expressing wild-type αv integrins (Fig. 5, C and D). It thus appears that αSMA-expressing fibroblasts are the major fibroblast subtype accounting for αv integrin–mediated development of CCl4-induced hepatic fibrosis.
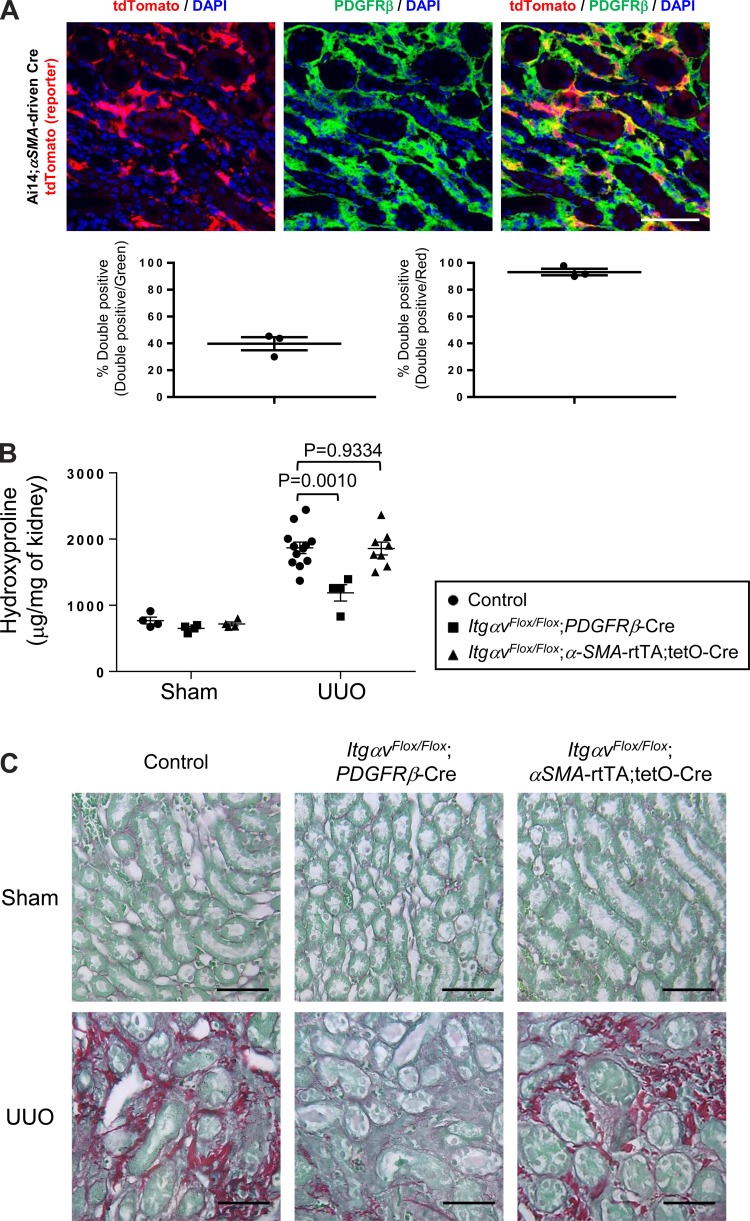
In the sham-operated kidney of the Ai14;PDGFRβ-Cre mice, PDGFRβ-Cre targets perivascular, intraglomerular cells and cells within renal interstitium, and induction of renal fibrosis via UUO results in massive expansion of tdTomato cells throughout the renal interstitium (18). Here, in the UUO-treated kidney of Ai14;αSMA-rtTA;tetO-Cre mice, PDGFRβ-positive cells were found throughout the renal interstitium (Fig. 6A). Upon image quantification, while over 98% of tdTomato-positive cells were stained as PDGFRβ-positive, only 43% of PDGFRβ-positive cells expressed tdTomato (Fig. 6A). Compared with their littermates expressing wild-type αv integrins, ItgαvFlox/Flox;PDGFRβ-Cre mice were protected from UUO-induced renal fibrosis, whereas ItgαvFlox/Flox;αSMA-rtTA;tetO-Cre mice were not protected (Fig. 6, B and C). These results suggest that in this model, as in bleomycin-induced pulmonary fibrosis, αSMA-expressing fibroblasts are not the major subset of PDGFRβ-expressing cell responsible for αv integrin–mediated fibrogenesis.
Fig. 6.
αSMA-directed deletion of αv integrins does not confer protective effects against unilateral ureteral obstruction (UUO) induced renal fibrosis. A: immunofluorescence micrographs and image quantification of kidney sections from 7-day UUO-treated Ai14;αSMA-rtTA;tetO-Cre mice, costained with PDGFRβ antibody. Panel at left shows DAPI (blue) and endogenous tdTomato (i.e., αSMA) (red), panel in middle shows DAPI (blue) and PDGFRβ staining (green), and panel at right shows the merged image. Scale bar, 50 μm. B: hydroxyproline assay of kidney lysates. C: picrosirius red staining of kidney sections from mice that underwent UUO or sham operation (control) on their left kidneys for 14 days. Data are means ± SE. P value, Student's t-test. Scale bar, 50 μm.
Only a minority of collagen-producing cells are αSMA-expressing fibroblasts in the fibrotic lung and kidney.
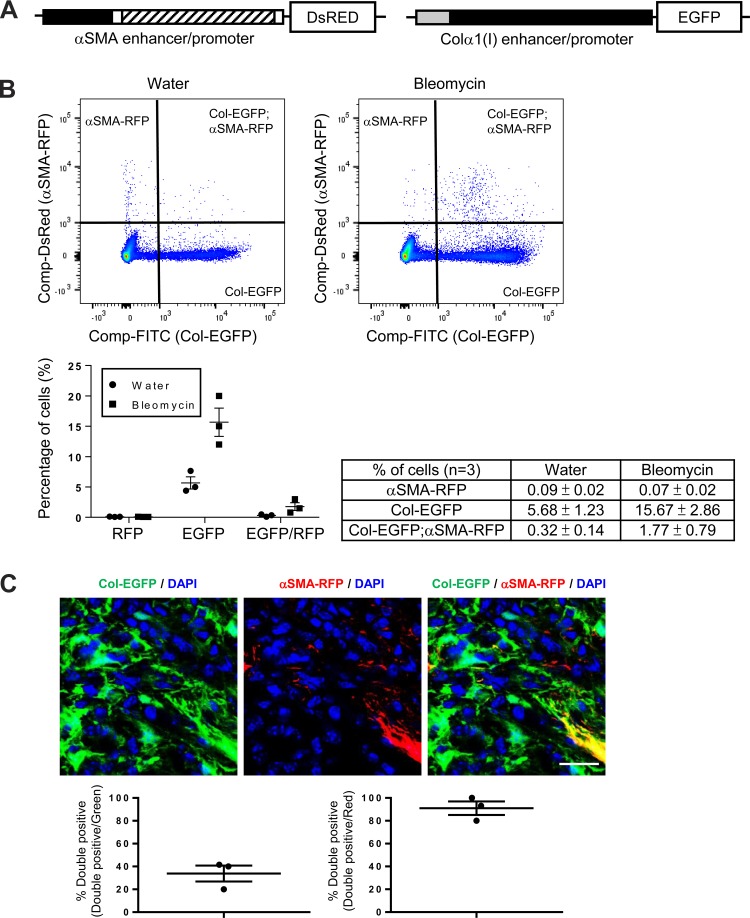
Our findings that αSMA-expressing fibroblasts were only a subset of PDGFRβ-expressing cells within the fibrotic interstitium in bleomycin-induced pulmonary fibrosis and UUO-induced kidney fibrosis, and that these cells were not critical for αv-mediated fibrogenesis in either organ, made us wonder how useful αSMA is as a marker of collagen-producing fibroblasts in the same models. To more clearly establish the in vivo relationship between αSMA expression and collagen production, a dual reporter mouse that expresses enhanced green fluorescence protein (EGFP) under direction of the collagen α1(I) enhancer/promoter (Col-EGFP) (30, 52) and red fluorescent protein (RFP) under direction of the αSMA enhancer/promoter (αSMA-RFP) (30, 49) was used (Fig. 7A). To more accurately assess the expression of the Col-EGFP and αSMA-RFP reporter genes, uninjured and injured tissues were dissociated, and expression of reporter genes was quantified by flow cytometry.
Fig. 7.
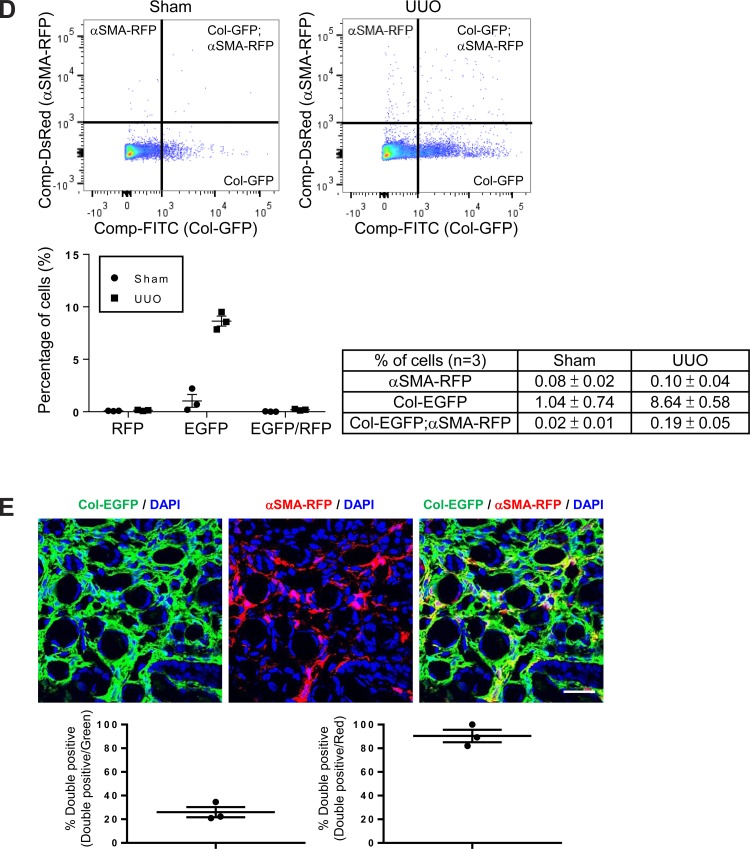
Only a minority of collagen-producing cells coexpress αSMA in the fibrotic lung and kidney. A: schematic of αSMA-RFP (30, 49) and Col-EGFP (30, 52) reporter mice. The αSMA enhancer/promoter construct consists ∼1-kb 5′-flanking region (black box), the transcription start site, the 48-bp exon 1 (white box), the ∼2.5-kb intron 1 (hatched box), and the 15-bp exon 2 (white box) (30, 49) from the mouse αSMA gene. The Colα1(I) enhancer/promoter contains an enhancer fragment, 5′-deoxyribonuclease (DNase) I-hypersensitive sites (HS) 4.5, followed by the ∼3-kb mouse Colα1(I) gene promoter (30, 52). B: flow cytometry–based quantification of cells isolated from the lungs of 14-day water- or bleomycin-treated Col-EGFP/αSMA-RFP mice. Data were collected from three mice. Representative dot plots are shown. Data are means ± SE. C: immunofluorescence micrographs and image quantification of lung sections from 14-day bleomycin-treated Col-EGFP/αSMA-RFP mice, costained with GFP and RFP antibodies to enhance the endogenous Col-EGFP and αSMA-RFP signals for microscopic imaging. Panel at left shows DAPI (blue) and Col-GFP (green), panel in middle shows DAPI (blue) and αSMA-RFP (red), and panel at right shows the merged image. Scale bar, 50 μm. D: flow cytometry-based quantification of cells isolated from the kidneys of 7-day sham- or UUO-treated Col-EGFP/αSMA-RFP mice. Data were collected from three mice. Representative dot plots are shown. Data are means ± SE. E: immunofluorescence micrographs and image quantification of kidney sections from 7-day UUO-treated Col-EGFP/αSMA-RFP mice, costained with GFP and RFP antibodies as above. Panel at left shows DAPI (blue) and Col-EGFP (green), panel in middle shows DAPI (blue) and αSMA-RFP (red), and panel at right shows the merged image. Scale bar, 50 μm.
Bleomycin treatment caused a dramatic expansion of lung cells expressing either Col-EGFP alone or both Col-EGFP and αSMA-RFP reporters. However, after injury, only a small fraction of the collagen-producing cells coexpressed αSMA (Fig. 7B). Evaluation of tissue sections from these dual reporter mice confirmed that only 42% of the Col-EGFP-positive cells within the interstitium of bleomycin-treated lung were also αSMA-RFP-positive, whereas over 93% of αSMA-RFP-positive cells expressed Col-EGFP (Fig. 7C). In the kidney, UUO resulted in a similar pattern in dual reporter mice. Seven days after UUO, an increase in the number of cells expressing either Col-EGFP alone or both Col-EGFP and αSMA-RFP reporters was found in the fibrotic kidney (Fig. 7D). Notably, while over 90% of the αSMA-RFP cells coexpressed Col-EGFP, only 26% of the Col-EGFP-positive cells coexpressed αSMA-RFP (Fig. 7E), and many interstitial Col-EGFP-positive cells were not αSMA-RFP-positive in tissue sections (Fig. 7E).
Taken together, in both the intratracheal bleomycin model and the UUO model, we detected abundant cells expressing Col-EGFP, but only a minority of Col-EGFP-positive cells were also αSMA-RFP-positive. Our data suggest that other subpopulations of fibroblasts that do not express αSMA are important contributors to both integrin-mediated TGFβ activation and collagen production during fibrogenesis in these models.
Col-EGFP+αSMA-RFP− cells from bleomycin-treated lungs and UUO-treated kidneys induce TGFβ activation at similar levels to Col-EGFP+αSMA-RFP+ cells.
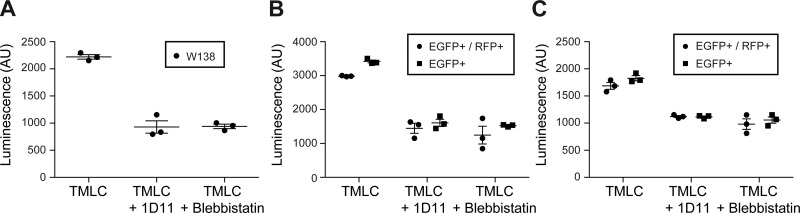
As loss of αv integrins on αSMA fibroblasts did not protect mice from fibrosis in response to bleomycin or UUO, we hypothesized that in addition to cells expressing both collagen I and αSMA, cells expressing only collagen I but not αSMA can also generate sufficient contractile force to activate TGFβ that drives fibrosis. To test this possibility, we examined TGFβ activation by sorted cells using coculture with TMLC expressing TGFβ-inducible luciferase. We used WI38 cells, a widely used human primary lung fibroblast cell line, as a positive control for this assay. WI38 cells generated force-dependent activation of TGFβ as demonstrated by a reduction in luciferase activity by 1D11 (a TGFβ blocking antibody) and blebbistatin (an inhibitor of nonmuscle myosin II), respectively (Fig. 8A).
Fig. 8.
Col-EGFP+αSMA-RFP− cells isolated from lung or kidney are equally capable of inducing TGFβ activation as Col-EGFP+αSMA-RFP+ cells. A: TGFβ activation assay of WI-38 cells. TGFβ activation assay of indicated cells sorted from fibrotic lungs (B) or fibrotic kidneys (C) of Col-EGFP/αSMA-RFP mice in the presence of indicated reagents. Col-EGFP/αSMA-RFP mice were treated with bleomycin for 14 days or UUO for 7 days. Following tissue disassociation, Col-EGFP+αSMA-RFP− cells (EGFP+) and Col-EGFP+αSMA-RFP+ cells (EGFP+/RFP+) were sorted by FACS and used in coculture TGFβ activation assays with mink lung epithelial cells stably transfected with a TGFβ-sensitive portion of the PAI-1 promoter driving firefly luciferase expression. TGFβ activation is expressed as luminescence. Data are means ± SE.
To avoid the fact that several days of in vitro culture of primary fibroblasts leads to fibroblast differentiation and αSMA expression, we isolated primary Col-EGFP+αSMA-RFP− and Col-EGFP+αSMA-RFP+ cells from fibrotic lung and kidney of Col-EGFP/αSMA reporter mice and immediately performed TGFβ activation assays. Col-EGFP+αSMA-RFP− and Col-EGFP+αSMA-RFP+ cells both activated TGFβ at similar levels (Fig. 8, B and C), and TGFβ activation by both types of fibroblasts was blocked by 1D11 and blebbistatin, respectively (Fig. 8, B and C). Our results suggest that like Col-EGFP+αSMA-RFP+, Col-EGFP+αSMA-RFP− cells activate TGFβ through force generated by actin/myosin contraction.
DISCUSSION
Interstitial lung fibrosis has diverse etiologies, and the pathogenesis of this disease remains incompletely understood. However, the excessive deposition of extracellular matrix with effacement of normal lung tissue architecture is a common feature of this process. Several previous reports have amply documented increased numbers of smooth muscle-like cells that express the smooth muscle contractile protein, αSMA, in the lung interstitium of patients with pulmonary fibrosis (2, 20, 24, 28, 38, 43, 46). These smooth muscle-like cells are defined as differentiated fibroblast which have contractile properties similar to smooth muscle cells and are commonly called myofibroblasts (38, 46). In the fibrotic lesion of patients, myofibroblasts are present at the sites undergoing active extracellular matrix deposition (24, 38, 46). Another important property of the myofibroblast is its contractility, which is believed to associate with wound contraction and perhaps to contribute to the altered mechanical characteristics of the fibrotic lung (2, 38, 46). The de novo appearance of αSMA-expressing myofibroblasts at active sites of fibrosis strongly suggests that they play an important role in fibrogenesis. Based on these findings, αSMA+ myofibroblasts are often presumed to be the major source of pathologic extracellular matrix in tissue fibrosis, and αSMA is widely used as a marker of activated fibroblasts. Our results suggest that αSMA expression is quite variable in collagen-producing cells across different models of tissue fibrosis.
We and others have shown that αv integrins on fibroblasts play an important role in activating TGFβ to drive the process of tissue fibrosis in multiple organs, and that this activation depends on cellular contraction and transmission of physical force from the fibroblasts to the tethered latent TGFβ (5–7, 17–19, 31, 33–36, 39, 48). We previously used PDGFRβ-Cre to induce highly efficient (but imprecise) αv deletion on all fibroblasts, and showed protection in the three models of organ fibrosis used in this current study (18). As αSMA is widely used as a marker of fibroblast subsets that drive fibrosis, we sought to test the idea that the key contractile cells involved in this process were indeed αSMA-expressing fibroblasts (as we expected) by more precisely inducing loss of αv integrins with a dox-inducible αSMA-driven Cre system. We confirmed that αSMA-directed deletion of αv integrins is sufficiently efficient to protect mice from CCl4-induced hepatic fibrosis, suggesting that in this model αSMA is a good marker of the fibroblast subset responsible for αv integrin–mediated hepatic fibrosis. However, much to our surprise, αSMA-directed deletion of αv integrins did not protect mice from either bleomycin-induced pulmonary or UUO-induced renal fibrosis, despite similar protection in ItgαvFlox/Flox;PDGFRβ-Cre mice to what we have previously reported (18). These findings suggest that there is another important population of contractile cells targeted by PDGFRβ-Cre that could substitute for αSMA-expressing fibroblasts to drive tissue fibrosis in these models.
One important limitation of the current study is the imprecise nature of recombination driven by PDGFRβ-Cre. We originally used this line because PDGFRβ-Cre is highly efficient in inducing recombination in the interstitial cells that accumulate in fibrotic organs (18). However, since this line is not inducible, it induces recombination in any cell that is derived from cells that at one time expressed PDGFRβ. This may be the reason we see some recombination in lung airway epithelial cells, although it is also possible that there is occasional inappropriate ectopic expression of Cre with this line. Aside from the inappropriate expression in airway epithelial cells, PDGFRβ-Cre-mediated recombination yields a similar pattern of expression to that seen by staining with anti-PDGFRβ antibody. Because PDGFRβ is expressed by some cells that are not pathologic fibroblasts, for example, pericytes (12), we cannot exclude the possibility that PDGFRβ-Cre-directed αv deletion from some nonfibroblasts contributes to the protective effects against bleomycin-induced pulmonary and UUO-induced renal fibrosis. However, our findings that αSMA-expressing fibroblasts largely overlap with PDGFRβ-expressing cells in CCl4-induced fibrotic liver but are only a minority of PDGFRβ-expressing cells within the fibrotic interstitium of bleomycin-treated lung and UUO-treated kidney, and that αSMA-directed deletion of αv integrins protects mice from fibrosis in response to CCl4, but not bleomycin or UUO, suggest that our results are likely explained by the dramatic differences in the relative contribution of myofibroblasts to the pathologic fibroblast pool in each model.
In this study, we used αSMA-RFP reporter mice (30, 49) to recapitulate the endogenous αSMA expression pattern in the lungs and kidneys we examined in this present study. However, it needs to be noted that the DNA construct of the αSMA promoter derived from ∼3-kb mouse smooth muscle cell α-actin enhancer/promoter fragment SMP8 (30, 49) might not fully recapitulate endogenous αSMA under all conditions. We also used Col-EGFP reporter mice (30, 52) to recapitulate the endogenous collagen I expression pattern in the lungs and kidneys we examined in this present study. Notably, this Col-EGFP construct was generated by linking EGFP with a ∼3-kb mouse collagen gene promoter together with an enhancer fragment, 5′-deoxyribonuclease (DNase) I-hypersensitive sites (HS) 4.5 (30, 52). DNase I-HS4.5 has been observed in the liver and been shown to be enhance the expression of α1(I) collagen gene promoter in HSCs in vitro and in vivo upon stimulation (52). Although DNase I-HS4.5 may not be present in the lung or kidney, our data showing that the number of Col-EGFP+ cells significantly increases in the lung or kidney upon injury have demonstrated to study the mechanisms regulating increased collagen expression during fibrogenesis.
A previous study using Col-EGFP mice showed that most of the collagen-producing cells in CCl4-induced hepatic fibrosis were αSMA-expressing fibroblasts (23). Here, by evaluating colocalization of Col-EGFP and αSMA-RFP in dual reporter mice, we found that in the bleomycin-induced fibrotic lung and UUO-induced fibrotic kidney, the fraction of Col-EGFP+αSMA-RFP+ is only a minority of the collagen-producing cells. Our observations in the lung were similar to those reported by Rock, et al. (42) who also analyzed the fibrotic lung by confocal microscopy and indicated that only a subset of collagen-producing cells coexpress αSMA. As such, these findings suggest that αSMA is an inconsistent marker for the pathologic fibroblast subset driving tissue fibrosis, at least in the murine models we used. They also underscore the importance of developing a better understanding of the heterogeneity that characterizes interstitial cells that have been loosely classified as fibroblasts, and the need to identify better markers to purify and characterize the diverse molecular phenotypes of these cells. For example, the results described here do not allow us to conclude that the same cells are principally responsible for integrin-mediated TGFβ activation and collagen production, and they simply show that many of the cells that perform each function in the models we employed to induce lung and kidney fibrosis do not express αSMA.
One interesting aspect of our findings using Col-EGFP/αSMA-RFP mice is the observation that Col-EGFP+αSMA-RFP− cells isolated from injured lung or kidney are equally capable of activating TGFβ as Col-EGFP+αSMA-RFP+ cells that are generally considered to be much more highly contractile. The importance of actin-myosin contraction in activating TGFβ by both types of cells was confirmed by inhibition of TGFβ activity by treatment with an inhibitor of nonmuscle myosin (blebbistatin). In some ways these results are not terribly surprising, because we have previously shown that epithelial cells, which also do not express αSMA, are perfectly capable of force-dependent activation of TGFβ through another integrin, αvβ6 (14).
In summary, we have extensively investigated whether αSMA serve as the marker of pathologic fibroblasts in three commonly used models of tissue fibrosis affecting the lung, liver, and kidney. Our results suggest that the significance of αSMA-expressing fibroblasts as central executors in driving tissue fibrosis differs substantially among these models. In CCl4-induced hepatic fibrosis, αSMA-expressing fibroblasts appear to be largely responsible for αv integrin–mediated fibrogenesis, in keeping with the widely held view of these cells. However, in the models of pulmonary and renal fibrosis examined here, αSMA-expressing fibroblasts represent only a minority of collagen-producing cells and do not appear to be the major cell type responsible for αv integrin–mediated TGFβ activation. These findings should serve as a stimulus to develop better tools to purify and characterize the heterogenous populations of interstitial cells that contribute to tissue fibrosis in various organs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 1F32HL-123267 (to K-H. Sun), HL-108794 (to D. Sheppard), and HL-053949 (to D. Sheppard).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.-H.S. and D.S. conception and design of research; K.-H.S., Y.C., and N.I.R. performed experiments; K.-H.S. and Y.C. analyzed data; K.-H.S., Y.C., and D.S. interpreted results of experiments; K.-H.S. and Y.C. prepared figures; K.-H.S. drafted manuscript; K.-H.S., Y.C., N.I.R., and D.S. edited and revised manuscript; K.-H.S., Y.C., N.I.R., and D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Yanli Wang, Chun Chen, Xiaozhu Huang, Xin Ren, Kieu-My Huynh, Kazuyuki Tsujino, Amha Atakilit, Thomas Arnold, and Nanyan Wu at UCSF for technical assistance and advice. We thank Sarah Elmes and Jane Gordon at the Nikon Imaging Center for assistance with FACS.
REFERENCES
- 1.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Adler KB, Low RB, Leslie KO, Mitchell J, Evans JN. Contractile cells in normal and fibrotic lung. Lab Invest 60: 473–485, 1989. [PubMed] [Google Scholar]
- 3.Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122: 227–232, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol 165: 723–734, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett 511: 65–68, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol 175: 7708–7718, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Asano Y, Ihn H, Yamane K, Jinnin M, Tamaki K. Increased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblasts. Am J Pathol 168: 499–510, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis 21: 437–451, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Kudo M, Rutaganira F, Takano H, Lee C, Atakilit A, Robinett KS, Uede T, Wolters PJ, Shokat KM, Huang X, Sheppard D. Integrin alpha9beta1 in airway smooth muscle suppresses exaggerated airway narrowing. J Clin Invest 122: 2916–2927, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc 9: 111–116, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A 82: 8681–8685, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giacomini MM, Travis MA, Kudo M, Sheppard D. Epithelial cells utilize cortical actin/myosin to activate latent TGF-beta through integrin alpha(v)beta(6)-dependent physical force. Exp Cell Res 318: 716–722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I, Rifkin DB. TGF-beta latency: biological significance and mechanisms of activation. Stem Cells 15: 190–197, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med 10: 76–99, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF 3rd Sheppard D, Gardner HA, Violette SM. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170: 110–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19: 1617–1624, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson NC, Sheppard D. Integrin-mediated regulation of TGFbeta in fibrosis. Biochim Biophys Acta 1832: 891–896, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heppleston AG. The pathology of honeycomb lung. Thorax 11: 77–93, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180: 1340–1355, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikushima H, Miyazono K. Biology of transforming growth factor-beta signaling. Curr Pharm Biotechnol 12: 2099–2107, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, Meng F, Asagiri M, Murray LA, Hofmann AF, Iida T, Glass CK, Brenner DA, Kisseleva T. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A 111: E3297–3305, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. AM J Pathol 138: 1257–1265, 1991. [PMC free article] [PubMed] [Google Scholar]
- 25.Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A 104: 15823–15828, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence DA. Latent-TGF-beta: an overview. Mol Cell Biochem 219: 163–170, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lepreux S, Desmouliere A. Human liver myofibroblasts during development and diseases with a focus on portal (myo)fibroblasts. Front Physiol 6: 173, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebow AA, Loring WE, Felton WL 3rd. The musculature of the lungs in chronic pulmonary disease. Am J Pathol 29: 885–911, 1953. [PMC free article] [PubMed] [Google Scholar]
- 29.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology 40: 1151–1159, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 11: 97–105, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng XM, Tang PM, Li J, Lan HY. TGF-beta/Smad signaling in renal fibrosis. Front Physiol 6: 82, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol 157: 493–507, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1. Mol Biol Cell 9: 2627–2638, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol 3: a005017, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A 99: 10482–10487, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phan SH. The myofibroblast in pulmonary fibrosis. Chest 122: 286S–289S, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, Sheppard D. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Scie Transl Med 7: 288ra279, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves HL, Friedman SL. Activation of hepatic stellate cells–a key issue in liver fibrosis. Front Biosci 7: d808–826, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem 280: 7409–7412, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A 108: E1475–1483, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scadding JG, Hinson KF. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax 22: 291–304, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci 22: 512–518, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature 474: 343–349, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair 5: 11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Waarde MA, van Assen AJ, Kampinga HH, Konings AW, Vujaskovic Z. Quantification of transforming growth factor-beta in biological material using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 247: 45–51, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology 46: 1404–1412, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest 100: 1425–1439, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells RG, Schwabe R. Origin and function of myofibroblasts in the liver. Semin Liver Dis 35: 97–106, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. DNase I-hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology 37: 267–276, 2003. [DOI] [PubMed] [Google Scholar]