Lymphatic valves are biased open, allowing multi-lymphangion operation with valves open (penalizing asynchronous contractions), even against adverse pressure. High-flow-rate intrinsic pumping arises from either synchronous contraction with some valves open, or sequential contraction with all valves operating (with adequate refractory period). The most efficient pumping results from antegrade sequential contraction.
Abstract
The observed properties of valves in collecting lymphatic vessels include transmural pressure-dependent bias to the open state and hysteresis. The bias may reduce resistance to flow when the vessel is functioning as a conduit. However, lymphatic pumping implies a streamwise increase in mean pressure across each valve, suggesting that the bias is then potentially unhelpful. Lymph pumping by a model of several collecting lymphatic vessel segments (lymphangions) in series, which incorporated these properties, was investigated under conditions of adverse pressure difference while varying the refractory period between active muscular contractions and the inter-lymphangion contraction delay. It was found that many combinations of the timing parameters and the adverse pressure difference led to one or more intermediate valves remaining open instead of switching between open and closed states during repetitive contraction cycles. Cyclic valve switching was reliably indicated if the mean pressure in a lymphangion over a cycle was higher than that in the lymphangion upstream, but either lack of or very brief valve closure could cause mean pressure to be lower downstream. Widely separated combinations of refractory period and delay time were found to produce the greatest flow-rate for a given pressure difference. The efficiency of pumping was always maximized by a long refractory period and lymphangion contraction starting when the contraction of the lymphangion immediately upstream was peaking. By means of an ex vivo experiment, it was verified that intermediate valves in a chain of pumping lymphangions can remain open, while the lymphangions on either side of the open valve continue to execute contractions.
NEW & NOTEWORTHY
Lymphatic valves are biased open, allowing multi-lymphangion operation with valves open (penalizing asynchronous contractions), even against adverse pressure. High-flow-rate intrinsic pumping arises from either synchronous contraction with some valves open, or sequential contraction with all valves operating (with adequate refractory period). The most efficient pumping results from antegrade sequential contraction.
the lymphatic vascular system is divided functionally into two main vessel types: initial lymphatics, small passive vessels with walls devoid of muscle cells, and collecting lymphatics, larger vessels with muscular walls, able to mount active contractions (38). Both types form converging manifolds, so that the single outlet of a manifold of initial lymphatics is one of many inlets to a manifold of collectors. Initial lymphatics begin at porous stumps formed of a single layer of specialized endothelial cells. By virtue of cell overlap, anchoring filaments to surrounding fibrous matrix and a lack of tight intercellular junctions, these cells constitute a primary valve encouraging one-way flow of interstitial fluid into the initial lymphatic (30).
In the collecting lymphatics, secondary valves are frequent, dividing each vessel into segments (lymphangions) some 3–10 diameters long. Pumping is then generated either by intrinsic mechanisms, whereby each lymphangion expels fluid volume into the segment downstream by contraction of its own lymphatic muscle cells, or as the result of relative movement of adjacent tissues [extrinsic pumping (26)]. The principle of intrinsic pumping is the same as that involved in ejection of blood from the cardiac ventricles, and cardiac terminology can be applied; the ejection fraction of lymphangions can reach an impressive 67% or more (4). Contractions can occur several times per minute, utilizing a muscle type that is intermediate between myocardium and arteriolar smooth muscle (25).
The properties of secondary lymphatic valves were investigated by Davis et al. (13). The most striking feature of the properties they observed is the offset: in rat mesentery at least, lymphatic valves are biased to the open state; they close only in response to a significant adverse (impelling fluid back through the valve) pressure difference across the valve ΔpV, implying that significant regurgitation before valve closure is unavoidable. At first sight, this property would appear disadvantageous, although it clearly aids the efficient operation of the vessel as a nonpumping conduit when there is no significant adverse pressure against which to pump. However, the normal situation of a collecting lymphatic vessel is to experience significant adverse pressure difference between its ends, i.e., outlet pressure greater than inlet, when it is actively pumping. This statement can be regarded as self-evident for a pump, but the need for pumping to raise the pressure is mainly because all collecting lymphatics except the largest trunks are transporting lymph toward lymph nodes, which are sites of high hydraulic resistance (10). In consequence, the pressure in nodal afferent vessels is high, and that in efferents low, and the mean pressure steps up along a lymphangion chain in the direction of flow (2). This is opposite to what happens in a blood vessel, where there is no pumping, and (all other things being equal) pressure declines in the streamwise direction as a result of blood viscosity. (Adverse pressure differences may also arise in both lymphatic and blood vessels from hydrostatic considerations, i.e., the effect of gravity.)
In this article, we investigate the consequences of the peculiar properties of lymphatic valves, particularly the bias, finding unexpectedly that this has major consequences even when each lymphangion along a chain is experiencing a step increase in mean internal pressure. Valve opening and closure are powerfully influenced by the relative timing of the contractions of the lymphangions on either side, and also by the degree of filling (distension) of those lymphangions, which, in turn, is the outcome of the time available for filling since the last contraction. Thus, we choose to investigate the valve function in the context of a study that varies systematically both the relative timing of lymphatic contractions and the time available for filling, while maintaining an adverse pressure difference across the lymphangion chain.
The coordination of the contractions of neighbouring lymphangions has been studied both experimentally and by numerical model. Physiologically, there are three possible local regulatory mechanisms, neural control having been excluded (22): conduction of a signal from one lymphangion to the next within excitable tissue in the wall (29, 36), contraction stimulated by stretch exceeding a threshold when fluid is ejected from the lymphangion upstream (15, 28), and convection of nitric oxide evolved from endothelium (35) of the lymphangion upstream. Nitric oxide dilates, so this last is postulated (19) to operate in conjunction with stretch. The last two both suggest propagation in a downstream direction, and with significant phase difference, whereas the first can potentially coordinate contractions in adjacent lymphangions to be almost simultaneous, and can operate in either direction.
McHale and Roddie (21) found contraction of all lymphangions in their isolated bovine mesenteric lymphatic segments to be near simultaneous. However, high-speed cine photography reveals that there is, indeed, a small phase difference (1, 3). Zawieja et al. (36) observed the rat small-intestinal mesentery in situ, finding that ∼80% of contractions propagated between lymphangions, with a conduction velocity of some 4–8 mm/s. The lymphangions averaged 1.04 mm in length and ∼100 μm in diameter, and contracted on average 5–10 times/min, with contractions lasting some 2 s and contraction onset taking some 0.5 s; thus, the inter-lymphangion delay or phase offset was typically less than the duration of contraction onset. Central conduction was only slightly more likely than peripheral, with individual vessels displaying wide variations over time. Working with isolated bovine mesenteric lymphatic segments of diameter 1–2 mm and length 70–80 mm (thus, including several lymphangions), McHale and Meharg (20) controlled contraction frequency independently at each end by varying bath temperature. Their results showed propagation in either direction, with no significant effect on volume pumped. The study suggested that local pacemaking can be entrained by a faster rate arriving from either adjacent lymphangion.
In a numerical model of a vessel consisting of three lymphangions, Venugopal et al. (31) found that mean flow-rate was higher when all lymphangions contracted at the same frequency, but largely insensitive to positive or negative time delay between contractions in adjacent lymphangions. Their model included a refractory period between contractions; conversely, the four-lymphangion model of Bertram et al. (6), in which sinusoidal contractions succeeded each other immediately, showed a strong effect of inter-lymphangion phase, with the least pumping occurring when lymphangions contracted in phase with each other. A later eight-lymphangion version of the model, with a different function describing the passive relation between transmural pressure Δptm and segment diameter D (16), included a refractory period tr. As before (6), a contraction was a smoothly varying sinusoid lasting 2s; each lymphangion's contraction started 0.5 s after that of the lymphangion immediately upstream. At all nonzero values of adverse pressure difference ΔP faced by the model, mean flow-rate Q̄ fell as the duration of refractory period between contractions increased out to 3 s. However, the decreases were proportionally modest when ΔP was small and Q̄ was large, such that contractions with nonzero tr could use the work done by active contraction more efficiently.
Our model has since been adapted (5, 8) to incorporate realistic properties of rat mesenteric lymphatic valves, as measured by Davis et al. (13); the changes also include incorporation of a length/tension relation to describe the varying force available from active contractions, and the adoption of parameter values based as closely as possible on measured values for rat mesentery. Previously, the offset from zero of the transvalvular pressure drop ΔpV for the open/close transition was a single fixed value Δpo; now, it depends on Δptm, such that the offset increases with increasing excess of internal pressure. The relation between offset Δpo and Δptm differs, according to whether the valve is currently open or closed, i.e., the valves exhibit hysteresis; a larger adverse ΔpV is needed to close the valve than to reopen it. These changes permit the model outputs to be compared closely with observations of real isolated pumping lymphatic segments.
We have described the consequences of the above valve properties for intrinsic pumping of a single lymphangion previously (5). Here, we explore the consequences for intrinsic pumping of multi-lymphangion segments, in the context of a systematic exploration of the two-dimensional parameter space defined by the refractory period between contractions tr and the interlymphangion contraction delay td, while also varying the adverse pressure difference across the segment.
METHODS
Computer model.
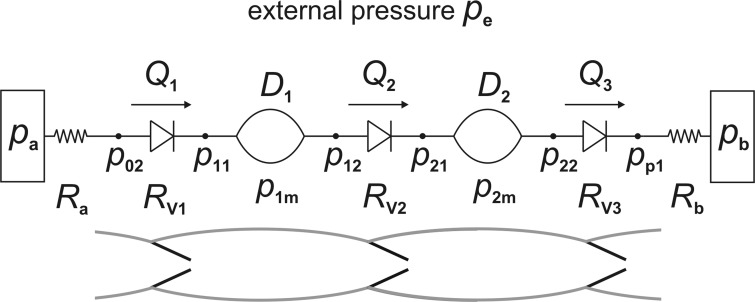
Pump function was investigated in a five-lymphangion series chain (see Fig. 1), using the equations (see appendix) that we have previously published (5), and as far as possible, the latest estimates for physiological parameter values everywhere (see Table 1). We chose to use five lymphangions, because this was the minimum number that showed the progressive development of pressure, etc., in those lymphangions internal to the chain (the two end-lymphangions are in an atypical situation). The equations expressed conservation of mass and of momentum, and a constitutive relation, for each lymphangion, plus the nonlinear dependence of valve resistance on valve state and transvalvular and transmural pressure. Muscular contraction created an extra contribution to the balance between transmural pressure and wall properties in the constitutive relation. One change from previous practice was that the model included fixed resistors at both ends of the chain (modeling the unavoidable hydraulic resistance of the cannulae in an isolated-vessel experiment). The pressures at the inlet side of the first valve (p02), and the outlet side of the last valve (pp1), thus became time variables, and two extra equations were required: pa − p02(t) = RaQ1(t) and pp1(t) − pb = RbQ6(t), where pa and pb are the (constant) inlet and outlet reservoir pressures, Ra and Rb are the corresponding cannula resistances, and Q1 and Q6 are the flow-rates through the first and last valves. The model was realized in MATLAB as vectorized code, which solved the differential equations of the system by a fourth-order-accurate Runge-Kutta scheme and the algebraic constraint equations by fixed-point iteration (7).
Fig. 1.
A schematic of a two-lymphangion version of the five-lymphangion model, showing the pressure, diameter, flow-rate, and valve-resistance time variables that the simulation tracked, as well as the constant-pressure reservoirs at each end and their associated fixed resistances. The corresponding lymphangions are shown below.
Table 1.
Values of adjustable parameters for the simulations reported in this study
| Parameter Description and Units | Symbol | Value | SI |
|---|---|---|---|
| Number of lymphangions | nla | 5 | 5 |
| Lymphangion length, cm | L | 0.3 | 3 × 10−3 |
| Pressure scale for passive Δptm/D-relation, dyn/cm2 | Pd | 732 | 73.2 |
| Diameter scale for passive Δptm/D-relation, cm | Dd | 0.0084534 | 8.45 × 10−5 |
| Normalizing diameter, cm | c9 | 0.02598 | 2.60 × 10−4 |
| Peak value of Md(D), dyn/cm | M0 | 150 | 0.15 |
| Contraction waveform frequency, Hz | f | 0.5 | 0.5 |
| Delay before first contraction, s | t0 | 0.25 | 0.25 |
| Refractory period, s | tr | 0 to 9 | 0 to 9 |
| Inter-lymphangion contraction delay, s | td | −1 to 2 | −1 to 2 |
| Inlet resistance, dyn·cm−5·s | Ra | 5 × 106 | 5 × 1011 |
| Outlet resistance, dyn·cm−5·s | Rb | 5 × 106 | 5 × 1011 |
| Minimum valve resistance, dyn·cm−5·s | RVn | 1.2 × 106 | 1.2 × 1011 |
| Valve resistance increase when closed, dyn·cm−5·s | RVx | 1010 − RVn | 1015 − RVn |
| Slope parameter for valve closure transition, cm2/dyn | so | 0.2 | 2 |
| Slope parameter for valve failure transition, cm2/dyn | sf | 0.2 | 2 |
| Valve pressure difference at failure, dyn/cm2 | Δpf | −5 × 104 | −5 × 103 |
| Attenuation factor at valve closure (see Ref. 5) | cfact | 0.22103 | 0.221 |
| Lymph viscosity (Poise) | μ | 0.01 | 10−3 |
| External pressure, cmH2O | pe | 2 | 196.2 |
| Inlet pressure, cmH2O | pa | 6 | 588.6 |
| Outlet pressure, cmH2O | pb | 0 to 14 | 0 to 1373 |
| No. of time points per unit simulated time, s−1 | nps | 1000 to 2000 | 1−2 × 103 |
| Duration of time simulated, s | T | 20 to 46 | 20 to 46 |
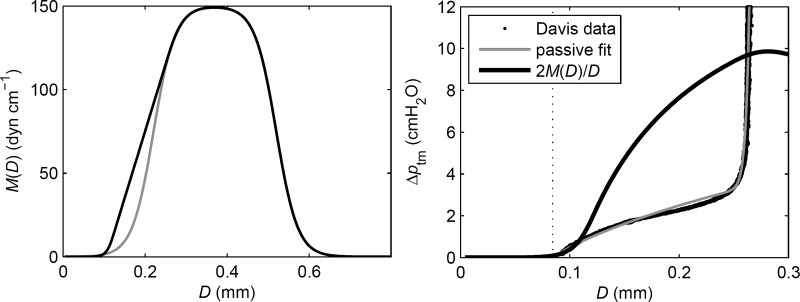
We have recently (Bertram CD, Macaskill C, Moore JE, Jr., unpublished data) described the effects on pumping of varying the shape of the relation Md(D) between muscle length and active tension, which has not been established for small lymphatic vessels. Here, we adopted the form shown in Fig. 2. The underlying form of the Md(D) relation is a symmetrical logistic curve defined by five constants (left panel, gray) that rises from zero to a maximum and decays again to zero, reflecting a generally well-accepted representation of the properties of muscle. However, because of the constraint of the passive elastic properties (right panel, gray curve), only the rising portion is exercised in lymphatic vessels. The rising portion is then modified with the use of further constants to yield a curve that is still differentiable everywhere but provides more tension at lower lengths (left panel, black curve). When divided by the lymphangion radius D/2, this curve becomes the active contribution to Δptm (right panel, black curve). With M0 = 150 dyn/cm, the active contribution peaks at 9.86 cmH2O.
Fig. 2.
Left: active tension vs. diameter, before (gray) and after (black) modification of the logistic relation for more tension at low diameter. Right: result converted to pressure (black curve) and compared with the curve (gray) fitted to the measured (13) passive pressure/diameter data (black points).
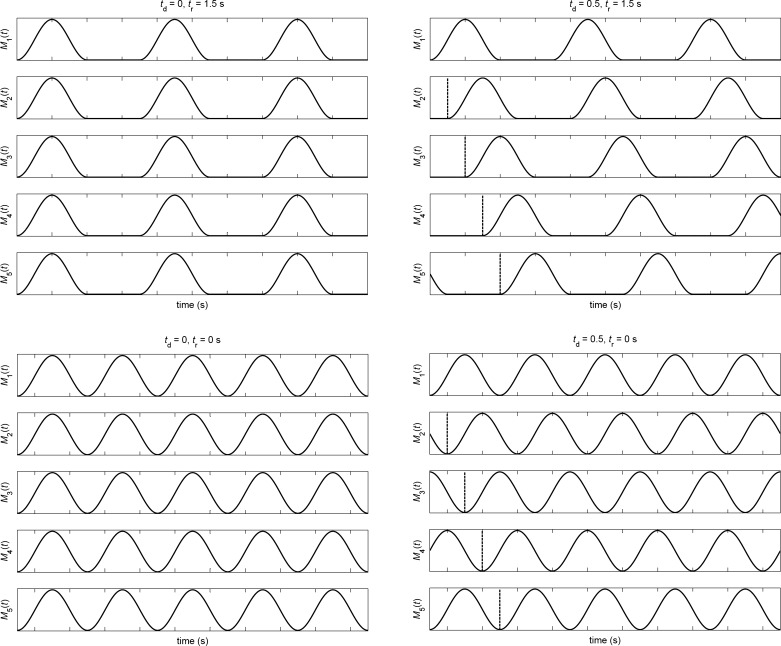
Lymphangion contractions occurred in a rhythm controlled by the refractory period tr and the inter-lymphangion delay td (Fig. 3). Each contraction was a waveform (1 − cos 2πf t)/2 of duration 2 s (f = 0.5 Hz). After an initial quiescent period of 0.5 s, contractions started in lymphangion 1 (that is closest to the inlet of the chain), with the next contraction starting after a further quiescent period of duration tr. The same pattern occurred in every lymphangion, but with successive delays td as shown. The solution was continued until the average flow-rate over a cycle 1/f + tr had become constant and was the same in every lymphangion. Such convergence took between 15 s and 35 s of simulated time to occur; the results come from the next cycle after that. Positive values of td out to 2 s and negative values out to −1 s were explored, but the overall cyclic pattern of contractions means that a sufficiently large positive value of td was equivalent to a small negative value once cyclic conditions had been reached; for example, at tr = 1 s, td = 2 s is equivalent to td = −1 s. Values of tr up to 9 s were explored, but at low values of td, a smaller tr range was sufficient to reveal all behaviors of interest.
Fig. 3.
The relative timing of active contractions in the five lymphangions, as controlled by tr and td; four examples are shown, arranged in the order of a plot with (td, tr)-axes. Refractory period tr = 1.5 s (top) and 0 (bottom). Inter-lymphangion delay td = 0 (left) and 0.5 s (right). In all cases, the contraction duration is 2 s.
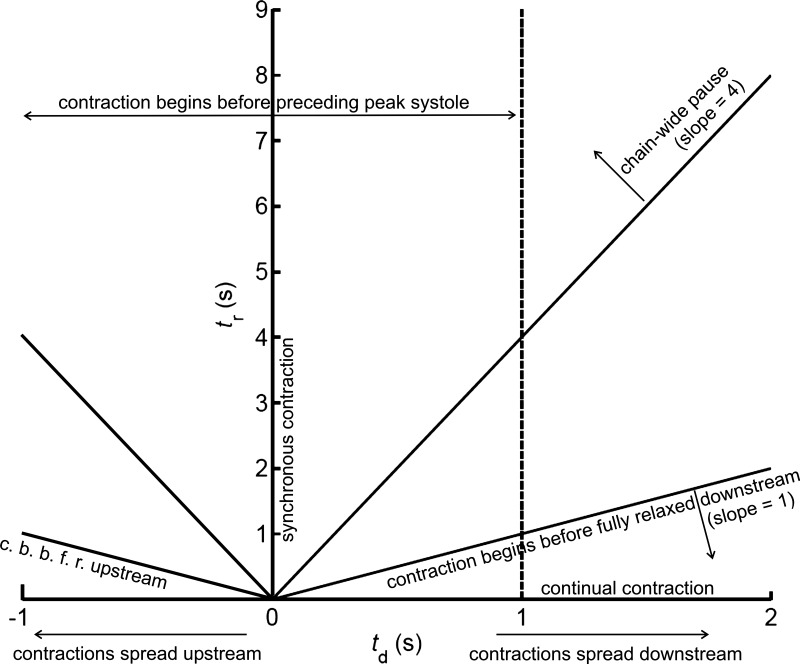
Certain qualitative subdivisions of the (td, tr) space are shown in Fig. 4. Somewhat misleadingly, the diagram suggests symmetry about td = 0. In fact, the main organizing principle of the space is that it wraps around horizontally with period 1/f + tr; thus, at tr = 0, −1 ≤ td ≤ 0 is equivalent to 1 ≤ td ≤ 2 s, but as tr increases, the wrapping quickly passes outside the td range investigated. The space investigated is not the entire unique space up to tr = 9 s; even the limited range −1 ≤ td ≤ 2 s is believed to extend beyond what is physiologically possible.
Fig. 4.
Qualitative subdivisions of (td, tr) space.
Biological experiment.
A segment of rat mesenteric collecting lymphatic vessel was isolated and mounted between micropipettes, following methods that are standard in our laboratory and that have been extensively documented in our previous publications (see, e.g., Ref. 13). The segment comprised four complete lymphangions bounded by five valves; in addition, sufficient part-lymphangion was left intact beyond the first and last valves that normal operation of these valves was not compromised by vessel ligature onto the micropipettes. After spontaneous contraction of all lymphangions had been reestablished, with the segment inlet-reservoir pressure and the external pressure both at 1 cmH2O and the outlet-reservoir pressure at 3 cmH2O, the segment was exposed to a parallel-ramp procedure, whereby both inlet and outlet pressures were progressively raised at an approximately constant rate of 3.6 cmH2O/min while maintaining the same small adverse pressure difference of 2 cmH2O between them. To obtain sufficient video image resolution to allow subsequent measurement of lymphangion diameter changes and valve motions, it was necessary to use a level of optical magnification, such that only part of the whole segment was recorded on video at a time. The ramp procedure was repeated identically three times, with the preparation repositioned each time relative to the video microscope to allow monitoring of a different part of the segment. Similar rates of spontaneous contraction were reestablished after each ramp, before starting the next. After the experiment, using the video footage, diameter change (ΔD) was measured upstream of the corresponding valve; thus, ΔD1 pertains to the part-lymphangion at the inlet. By combining diameter change and valve motion measurements across these three repetitions, an approximate picture was built up of the quasi-simultaneous behavior of all five valves and all four lymphangions (plus the part-lymphangion before the inlet valve) during one superposition of all three ramps. In reality, V1 and V2 (and ΔD1 and ΔD2) were measured during one ramp, V3 and ΔD3 during a second, and V4 and V5 (and ΔD4 and ΔD5) during a third.
RESULTS
Shortcomings of the pump function curve.
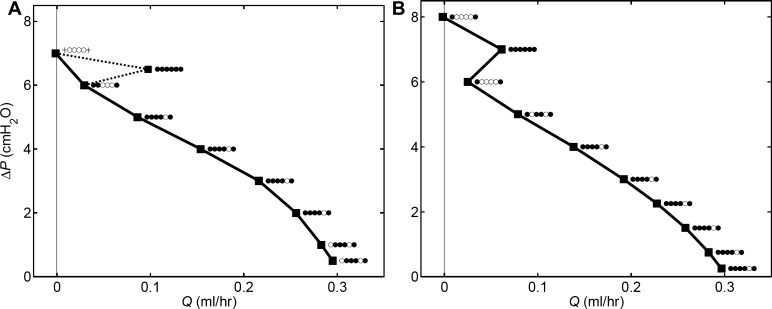
is usually described by a curve of negative slope on axes of time-averaged flow-rate Q̄ and adverse pressure difference ΔP overcome, and we have used this method previously (6, 16). Such a curve expresses the universal pump property that a larger adverse head leads to less flow, and vice versa. However, the properties of lymphatic valves, as incorporated here, cause exceptions to occur. We encountered one exception previously when varying the form of the length/active-tension relation (Bertram CD, Macaskill C, Moore JE, Jr., unpublished data) at ΔP = 0, when the maximum Q̄ might be expected, the lack of adverse pressure to close the valves, together with their bias to the open state, caused less Q̄ than at small positive ΔP. We have now found that valve bias makes pump function curves fundamentally unreliable. In determining a pump function curve, one necessarily visits a finite number of points, implicitly depending on the assumption that the underlying form is smooth. In the presence of valves with Δptm-dependent bias and hysteresis, this assumption is unsafe, as shown by Fig. 5.
Fig. 5.
Pump function in terms of Q̄ achieved at a given ΔP, with td = 0.5 s. At each operating point, a row of six symbols indicates valve states (left to right = inlet to outlet): ● = valve operates normally, ○ = valve stays open, + = valve stays shut. A: tr = 1 s. B: tr = 4 s.
Considering Fig. 5A, had only integer values of ΔP been investigated, an apparently smooth curve, as shown by the continuous line, would have resulted. The result for ΔP = 6.5 cmH2O is apparently anomalous, until one takes into account the fact that differing numbers of valves are operating (opening and closing during the cycle) at the nine (Q̄, ΔP) operating points shown. In Fig. 5B, even investigation of only integer values of ΔP shows up the jagged nature of the pump function “curve”.
These curves cannot be rendered smooth by investigation of further operating points. Our model specifies valve resistance RV changing smoothly and continuously with the valve pressure drop ΔpV. However, thanks to the observed hysteresis, now incorporated in our model, a valve cannot open or close partially beyond a certain limit (8). If ΔpV surpasses the instantaneously prevailing state-change threshold, the valve then obeys its other curve of state-change threshold, and the result is discontinuous change of valve resistance, with consequent adjustment of all other time variables. We have previously illustrated this process in detail (8). What this means in practice is that a given valve either opens and closes fully during repetitive pumping cycles or it does not; no halfway states occur that would act to make a continuous transition in some sense from having a valve operating to having it not operating. This behavior follows inevitably from the hysteresis displayed by real lymphatic valves (13).
Only (Q̄, ΔP) operating points involving the same set of valves operating normally belong entirely logically on the same pump function curve; the rest could be said to define parts of different curves. It is probable that further such discontinuities would be revealed if more values of ΔP were investigated, since there are many possible combinations of state over the six valves. Under these conditions, the generality of pump function curves becomes suspect. In particular, as a tool for investigating the effects of varying a parameter such as td or tr, they lose utility, since the valve states form another entity that is varying along with the parameter of interest and the value of ΔP, thus confounding the outcome.
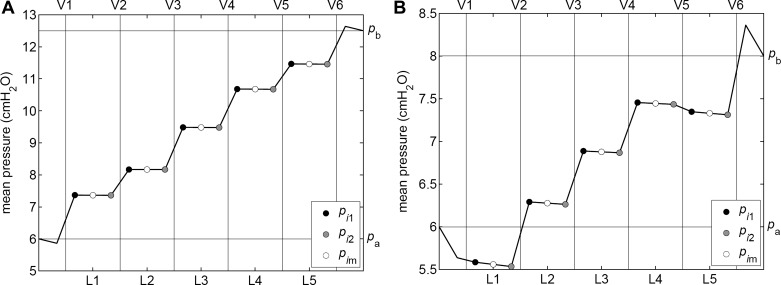
Average lymphangion pressure.
In general, under conditions causing valve opening and closing during repetitive pumping cycles, the result is an increase of cycle-average pressure across that valve; the mean pressure in the receiving lymphangion is higher than that in the sending one. However, exceptions occur, as illustrated by Fig. 6. Figure 6A shows the usual case, in which all valves are operating, and mean pressure steps up in each successive lymphangion. The (Q̄, ΔP) operating point shown here is the supposedly “anomalous” one in Fig. 5A, which, in fact, is the only point in that figure at which all valves were cyclically opening and closing. Figure 6B shows another (Q̄, ΔP) operating point from Fig. 5A, that for ΔP = 2 cmH2O. The only valve that stayed open all of the time during repetitive cycles of pumping was V5, yet Fig. 6B shows that cycle-average pressure also failed to increase across V1. Inspection of traces plotting valve state vs. time (not shown) reveals that V1 reopened shortly after closure, staying open until shortly before its normal reopening. Thus, it was open during most of the part of the cycle when lymphangion 1 was contracting, and it needed to be shut to make that contraction fully effective. Generalizing, we see that mean pressure may not increase across an operating valve if the cycle fraction during which the valve is closed is sufficiently small. Therefore, while an increase in mean pressure necessarily implies valve opening and closure, the reverse is not true.
Fig. 6.
Cycle-average pressure in each of the five lymphangions, for two of the operating points in Fig. 5A. ΔP = 6.5 cmH2O (A), ΔP = 2 cmH2O (B).
Contraction timing and mean flow-rate.
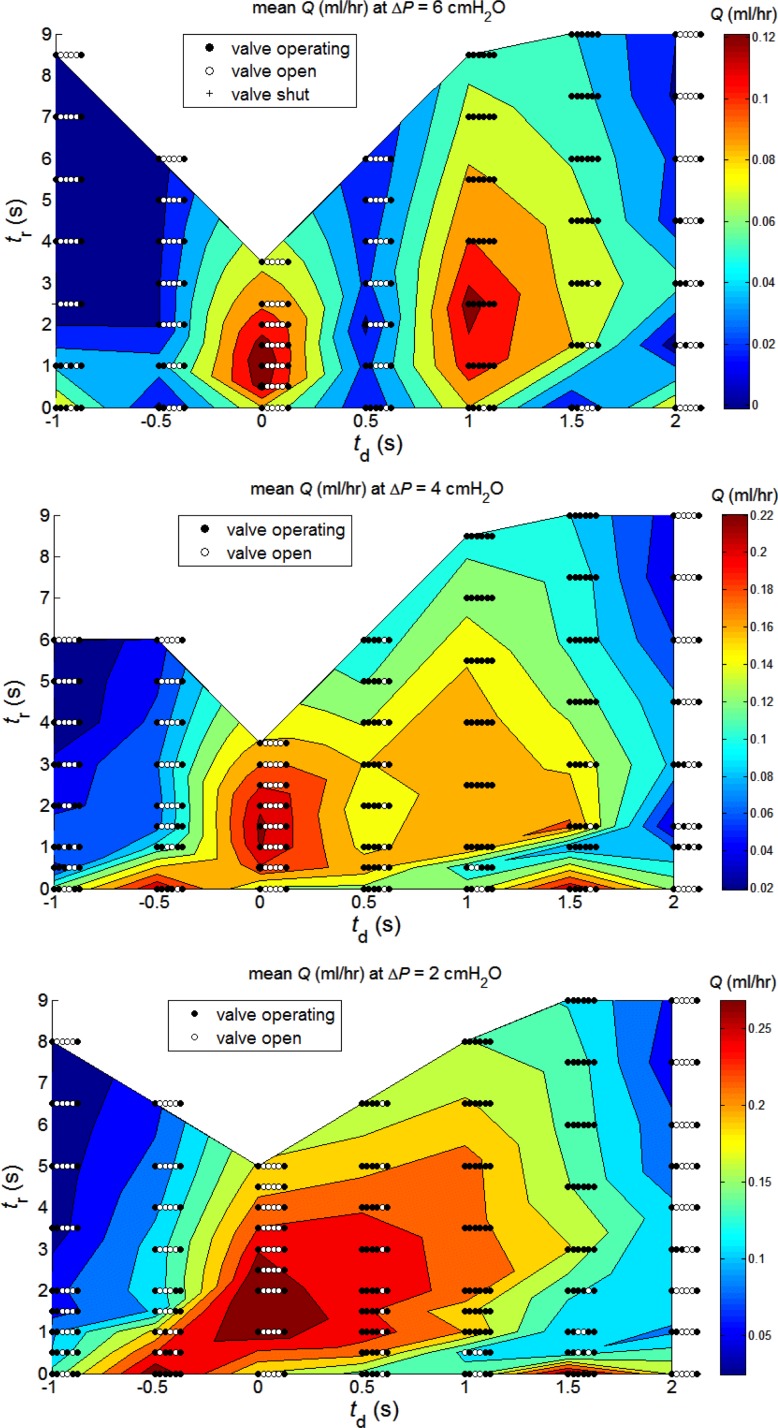
To overcome the identified deficiencies in pump function curves as a means of characterizing pumping in the presence of multiple valves, which may or may not operate, we decided to treat each value of ΔP separately. Accordingly, Fig. 7 presents color contours of Q̄ as a function of td and tr, each value of ΔP being a separate panel. The three panels span a ΔP range covering most of a conventional pump function curve as in Fig. 5. Valves still drop out of operation, but (reading either horizontally or vertically), only one parameter is being varied at a time.
Fig. 7.
Cycle-average flow-rate Q̄ (ml/h) as a function of inter-lymphangion delay td and refractory period tr, at three values of ΔP: from top to bottom, 6, 4, and 2 cmH2O. Valve function symbols are the same as in Fig. 5, but here, the leftmost of each row of six symbols is placed at the relevant (td, tr) coordinate.
At ΔP = 6 cmH2O (Fig. 7, top), maxima of Q̄ are achieved under two widely separated conditions. One (Q̄ = 0.128 ml/h), at td = 1 s, involves all lymphangions operating as separate pump stages in a series chain, as expected. The other (Q̄ = 0.138 ml/h), at td = 0, involves only valves 1 and 6; all of the intermediate valves stay perpetually open once repetitive cyclic pumping is established. The five-lymphangion chain here operates as a coordinated single-stage pump. Between these two peaks, at td = 0.5 s, there is a deep valley. Given the valve bias to remaining open, this value of delay works against the pump; the progressively later contraction of downstream lymphangion segments fails to close the pertinent inflow valve and, instead, merely displaces fluid to adjacent lymphangions in both directions. The contraction of the last lymphangion is almost entirely wasted in regurgitation to the relaxed lymphangions upstream. At td = 1.5 s, all valves are in use when tr > 4 s, but at lower tr values, valves near the outlet end of the chain progressively drop out, and the maximum Q̄ achieved is much lower than at td = 1 s. The zero-delay peak Q̄ was achieved at tr = 1 s, giving a cycle time of 3 s; one would, thus, recover the same peak again at td = 3 s. In summary, at ΔP = 6 cmH2O, a nonzero refractory period is beneficial to Q̄ whatever the value of td. Whatever the value of td, Q̄ decays monotonically once tr increases past the value giving peak Q̄; the contractions become entirely independent of each other, with only the length of the intervening pause increasing. Negative values of td are disadvantageous; Q̄ achieved is everywhere less than the value for the same tr and corresponding positive value of td.
At ΔP = 4 cmH2O (Fig. 7, middle), the Q̄ range is extended, relative to ΔP = 6 cmH2O; the pump can produce more flow-rate against a smaller adverse pressure difference. The advantage has now further shifted to the five-lymphangion segment working as a single coordinated pumping chamber; thus, the maximum Q̄ of 0.225 ml/h is achieved at td = 0 (and tr = 1.5 s); the maximum achieved at td = 1 s when all valves are working (0.177 ml/h at tr = 1s) is higher than at ΔP = 6 cmH2O, but now only 79% of what is produced by the single chamber. At td = 0.5 s, valve V5 drops out of use. The resulting pump has only four chambers, in one of which, there is some efficiency loss because the two halves do not contract simultaneously. The result is achievement of less Q̄ than at td = 0 and generally slightly less than at td = 1 s; only at tr = 0.5 s, where V2 and V3 stay open at td = 1 s, is there an exception. A single pumping chamber, with only V1 and V6 operating, occurs again at td = 2s for tr = 0 and tr ≥ 6 s. Because each lymphangion starts to contract only when the adjacent one has relaxed, this pattern is completely ineffective unless contractions are continuous. A second, highly localized, maximum of Q̄ (0.218 ml/h) was found at ΔP = 4 cmH2O when td = 1.5 s and tr = 0. This operating point recurs at td = −0.5 s. With the exception of this latter point, negative values of td always lead to lower Q̄ than their positive counterparts.
The trends seen in passing from ΔP = 6 cmH2O to ΔP = 4 cmH2O largely continue at ΔP = 2 cmH2O (Fig. 7, bottom). The valley at td = 0.5 s, which was deep at ΔP = 6 cmH2O and shallow at ΔP = 4 cmH2O, is now completely abolished. Maximum Q̄ increases again, to 0.296 ml/h and is achieved at td = 0, tr = 2 s (synchronous contraction everywhere, only V1 and V6 working). For comparison, the maximum Q̄ achieved with td = +0.5 s was only 0.261 ml/h (at tr = 3 s), but here, the chain was handicapped by V5 staying open perpetually. As at ΔP = 4 cmH2O, a secondary Q̄ maximum (0.290 ml/h) occurred at td = −0.5 s (recurring at +1.5 s) for tr = 0. Although the contour plot suggests a continuum of high Q̄ with that at td = 0; in fact, the mode of operation is completely different, since all valves are in use at td = −0.5 s and tr = 0. For all tr ≥ 1 s, Q̄ was very much less at td = −0.5 s than at td = +0.5 s. Pumping at td = −1 s was still worse, both in absolute terms and relative to td = +1 s. Thus, with the prominent exception of td = −0.5 s at tr = 0, simulated retrograde transmission of excitation always led to less mean flow than antegrade transmission.
One additional complexity of the operation of the multi-lymphangion pump under these varied conditions of contraction timing is not shown by Fig. 7. In the final cyclic condition, after start-up transients had died away, the outlet valve (V6) often opened and closed multiple times during a cycle period 1/f + tr. For instance, at ΔP = 2 cmH2O with td = −1s, as tr increased from 0 to 8 s, V6 opened twice per cycle at almost all points investigated, with the exceptions being 4 and 3 times/cycle at tr = 0.5 and 2 s, respectively. This behavior was not confined to operating points at which intermediate valves stayed open; thus, at ΔP = 2 cmH2O with td = 1.5 s, V6 opened 3, 4, 5, 5 and 5 times/cycle, as tr increased from 3 to 9 s, with all valves participating in pump operation. More rarely, the inlet valve V1 also opened and closed more than once during a cycle. Thus at td = 0.5, tr = 1 s (still at ΔP = 2 cmH2O), V1 opened twice; this occurred also at td = 1 s when tr = 0, 1 and 3.5 s. Sometimes, both V1 and V6 operated multiple times in a cycle; thus, at ΔP = 2 cmH2O and td = 2 s, as tr increased from 5 to 9 s, V1 operated 2, 3, 4, and 5 times, while V6 operated 4, 3, 5, and 5 times.
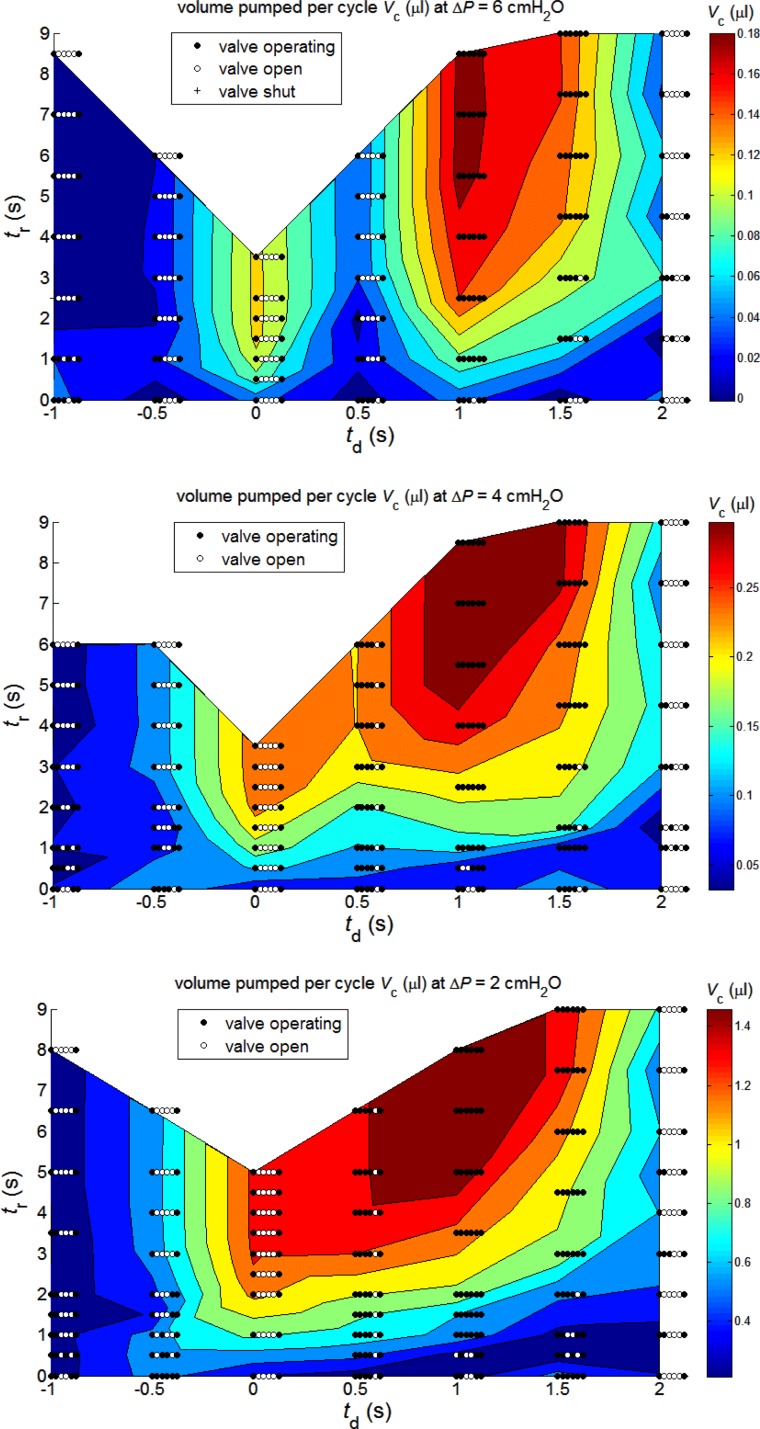
Volume pumped per cycle.
While continuous contraction with retrograde excitation spread may, at low ΔP, produce Q̄ to rival that achieved with a pause between synchronous contractions, it is clearly an extravagant mode, albeit on a minute scale, in terms of the local cellular metabolism. Such a mode may perhaps be advantageous when maximum lymph transport is needed, but otherwise one would expect economy of cellular metabolism through the use of less frequent contractions. An alternative measure of output is provided by the volume of fluid pumped per contraction cycle (1/f + tr). Whereas Q̄ must eventually decay as tr increases (Fig. 7), the volume pumped per cycle (Vc) asymptotes to a constant maximum, as shown in Fig. 8, as tr becomes large enough to allow diastolic lymphangion filling to proceed to completion before the start of the next round of contractile activity. Further increase in tr would only lengthen the period between these rounds when all time-variables are constant.
Fig. 8.
Volume pumped per cycle (μl) as a function of inter-lymphangion delay td and refractory period tr, for the same three values of ΔP as in Fig. 7.
Maximum Vc occurs when tr is long enough that diastolic lymphangion inflow from upstream has had time to decay to zero. Thus the (td, tr)-conditions for maximum Vc do not coincide with those for maximum Q̄; it is a measure of pumping efficiency instead of absolute performance. For all three values of ΔP, it reaches its global maximum at td = 1 s, with all lymphangions behaving as separate pump stages. The maximum Vc is 0.187 μl at ΔP = 6 cmH2O, 0.330 μl at 4 cmH2O, and 1.615 μl at 2 cmH2O. Continuous contractions (tr = 0) are, as expected, seen to be highly inefficient, whatever the relative phasing of contractions among lymphangions. Under these circumstances lymphangion filling is very far from complete before contraction restarts. The production of high Q̄ by the synchronous contraction (td = 0) of all lymphangions, at modest tr, with all intermediate valves open (Fig. 7), is likewise seen via Fig. 8 to be a relatively inefficient way to expend metabolic energy. When tr is sufficient to achieve the asymptotic value of Vc, the relative disadvantage of this contraction pattern is least at ΔP = 2 cmH2O and greatest at ΔP = 6 cmH2O.
Parallel-ramp experiment.
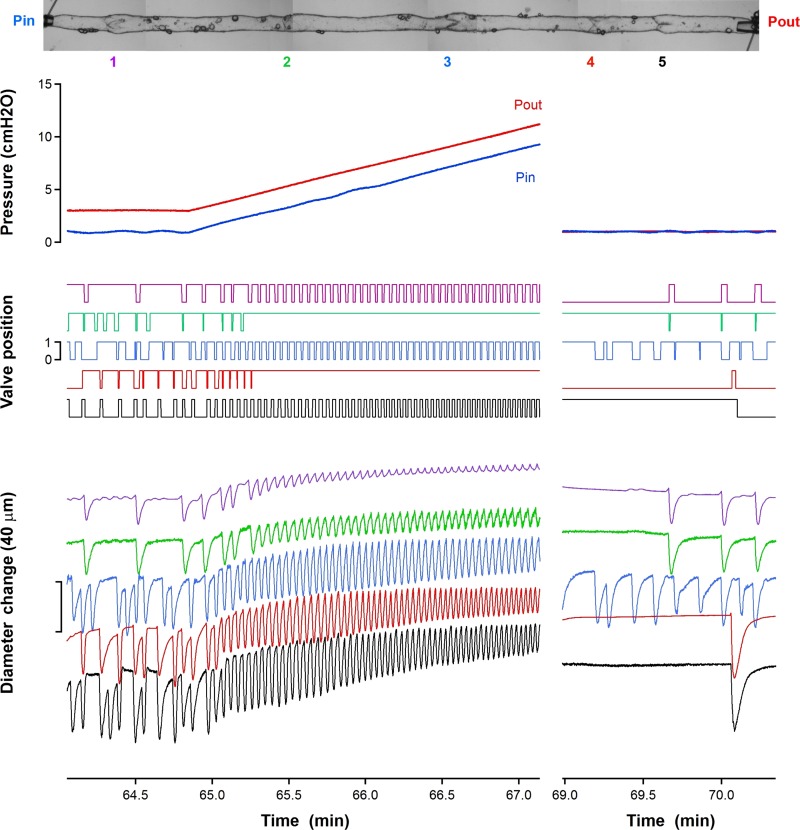
The 5-valve segment showed a strong response to the progressive increase in transmural pressure in all lymphangions that the ramping caused; see Fig. 9. Prior to the ramping, contractions were intermittent, with pauses as long as 0.4 min recorded from the first lymphangion where the transmural pressure would have been least. Once the ramp was under way, all lymphangions went over to rapidly repeated contractions, with the now-short refractory period between contractions continuing to decrease as the ramp proceeded. Although all four complete lymphangions, and the part-lymphangion beyond the last valve, continued to execute such contractions, first the valve between the first and second lymphangions (V2), and then shortly thereafter that between the third and fourth lymphangions (V4), ceased to shut, subsequently remaining open until the end of the ramp procedure and the restoration of minimal pressure everywhere. With these two valves out of operation, the segment, which nominally comprised four complete lymphangions, was in fact functioning as a 3-valve segment, with the remaining operating valves defining two pump chambers, each consisting of two lymphangions.
Fig. 9.
Reservoir pressure, valve state, and diameter-change traces during the composite ramp procedure built up by superposition of three ramp repetitions. The image at the top shows a photo-montage of the entire segment, with the five valves indicated by numbers.
DISCUSSION
Here, we have conducted a systematic exploration—in a numerical model of an isolated lymphatic vessel segment consisting of multiple lymphangions—of the effects of varying the timing of contractions. With contractions assumed to be of fixed length and to follow a regular pattern steadfastly, just two parameters control the timing: the duration of the pause between contractions, herein called the refractory period, and the time offset of contractions in adjacent lymphangions, herein called the inter-lymphangion delay. Such a study is impossible to do experimentally; the timing of lymphangion contractions is not under the experimenter's control. McHale and Meharg (20) achieved limited control of the average refractory period by lowering the temperature of fluid in their perfusing bath, but individual refractory periods varied autonomously. Unphysiologically low temperature would also be expected to affect other aspects of the contractile machinery, leading to possibly weaker and slower contractions. In biological experiments, many other physiological variables, such as variation of vessel and valve properties from one lymphangion to the next, are also impossible to exclude.
We find that, owing to the transmural pressure-dependent valve bias to the open state and the valve hysteresis (13), the pumping performance is very much bound up with the question of which valves are opening and closing at a given operating condition, as set by the adverse pressure difference faced. Unexpectedly, the greatest mean flow-rate was produced at all adverse pressure differences tested, when all lymphangions contracted simultaneously. Under these conditions, the four intermediate valves in the chain all stayed open continuously, which minimized their impediment to flow. In the supporting experiment, the second and fourth of five valves stayed open once the transmural pressure reached a suitable value, and subsequently remained open while the adjacent lymphangions continued to execute contractions. This constitutes proof of principle of the model prediction of intermediate valves staying open, even though the experiment was necessarily conducted under a different protocol. Lymphangion diameter in our model was of the order of 0.25 mm in diastole, and length was fixed at 3 mm, approximating what is found in the muscular lymphatic vessels of the rat mesentery. It is unlikely that multi-lymphangion segments of this length (some 60 diameters) could perform such perfectly synchronous contractions in vivo as implied by td = 0 in the model. In recent experiments on multi-lymphangion isolated segments of rat mesenteric lymphatic vessel (17), we have observed quasi-synchronous contractions, i.e., contractions that look synchronous to the eye, while aware that deployment of more sophisticated measurement means (1) would have revealed slight phase difference. On the other hand, such perfectly synchronous contraction can be regarded in modeling terms as not dissimilar to extrinsic pumping, where the external tissues in relative motion that give rise to the lymphatic squeezing are commonly of comparatively large dimension. Other intrinsic pumping possibilities, such as simultaneous contraction of two adjacent lymphangions while the next one contracts in antiphase, have been postulated (24). The model of Kunert et al. (19) predicts that activation will propagate streamwise when calcium influx dynamics dominate the pattern of contraction, and counter-streamwise when control is dominated by release of nitric oxide from lymphatic endothelium. Contraction patterns also depend on anatomical location, perhaps because of complex, time-varying external pressures (23, 26). Although our model could incorporate spatial and temporal variation of external pressure as a crude model of squeezing by adjacent tissues, this study has focused on exploring relative timings of active contraction.
The methods deployed in our supporting experiment did not permit analysis of relative timing of lymphangion contractions. The observations of Zawieja et al. (36) of rat mesentery in situ suggest that a lymphangion starts its contraction while the adjacent and preceding one is still in course of reducing its diameter. In the context of our model, this situation corresponds to a delay of ± 0.5 s, so it is interesting to find that a phase delay of +0.5 s leads in our results to particularly ineffective pumping when the chain is facing a large adverse pressure difference, and to less mean flow-rate than perfect synchrony when the chain is facing a moderate or small adverse pressure difference. The reason for the unsuccessful pumping in this circumstance was that the intermediate valves in the chain stayed open. The successive contraction of parts of the long chamber so formed simply moved fluid to and fro within the chamber for the most part; only a small part of the pumped volume was moved from the inlet reservoir to the outlet reservoir. It is not known whether any valves stayed open in the rat mesenteric lymphatics observed in situ (36). The circumstances of our simulations and the observations differ in that we included resistors at the ends of the chain to simulate cannulae leading to controlled-pressure reservoirs for an isolated vessel, whereas Zawieja et al. (36) observed intact vessels subject to the unknown impedances of connection to the rest of the lymphatic network.
Zawieja et al. (36) observed retrograde sequential contraction to be almost as prevalent as antegrade in vivo. This would suggest that retrograde propagation still allows good pumping. Alternatively, it may be that retrograde propagation does reduce pumping and is simply an unavoidable consequence of the arrival of activation impulses at the lymphatic vessel junction downstream from the other incoming branch. On the other hand, in isolated segments consisting of several lymphangions and exposed to equal pressure at inlet and outlet, McHale and Meharg (20) found that retrograde propagation of contraction pumped as much fluid as antegrade. In contrast, our study shows that for multi-lymphangion segments equipped with valves having the properties measured by Davis et al. (13), retrograde propagation, as modelled by td = −0.5 s, generally produced much less mean flow than antegrade. The sole exception to this, when the adverse pressure faced by the segment was moderate or low, occurred when contractions followed each other immediately. In this case, all (ΔP = 2 cmH2O) or all but one (ΔP = 4 cmH2O) valves operated, whereas when the refractory period was nonzero, two or more valves stayed open. Conversely, at td = +0.5 s, all but the fifth valve operated at all values of tr, and these values of ΔP; lack of refractory period conferred no advantage. At td = −0.5 s, the needed closure of each lymphangion's inlet valve at the start of contraction is made more difficult by the fact that the lymphangion upstream is itself building up to the peak of its contraction throughout the second half of the development of active tension for the lymphangion being considered. With nonzero refractory period, the situation is worse, because each contracting lymphangion expels fluid into a relaxing lymphangion downstream so easily. These two factors combine to limit the retrograde flow, which is needed to close the valve at the lymphangion inlet. This conclusion from our simulations can be reconciled to some extent with the experimental data of McHale and Meharg (20) if it be also noted that isolated segments tend to contract all lymphangions almost simultaneously (21). Thus, the data of McHale and Meharg may all relate to a very narrow band around td = 0 in our parameters. Again, it is unfortunate that no details were recorded experimentally of the behavior of the intermediate valves in the chain, since our simulations provide a strong testable prediction that the intermediate valves would have stayed open. However, the comparison is weakened also by species difference, since our model relates to valve properties measured in rat mesenteric lymphatics, while McHale et al. (20, 21) worked with bovine vessels, which are much larger and known to have qualitatively different properties.
Clearly, yet more fine detail would be found if the (td, tr) grid were to be investigated on a finer scale, but we are confident that we have mapped out the principal behaviors. We find that two different measures, the time-averaged flow-rate and the volume pumped per cycle, are needed to characterize different aspects of optimal pump performance. Experimentally, many have analyzed their results in terms of ejection fraction, a term borrowed from cardiac physiology. Ejection fraction is calculated from the maximum and minimum measured lymphangion diameter during a contraction, assuming (as in our model) that the segment is always a circular cylinder. The data can also be used to give an approximate flow-rate. An implicit assumption is that the calculated volume difference is, indeed, ejected, that is, that no retrograde flow occurs; Dixon et al. (14) found that true flow-rate in rat mesenteric lymphatics measured by particle tracking was only some 72% of that implied by the volume changes from such ejection calculations. Both of the measures we calculate here, mean flow-rate and volume per cycle, are true ones, in that they allow for regurgitation taking place. Ejection fraction cannot be directly compared with either measure, because it characterizes a single lymphangion; the concept cannot be generalized to produce a measure that applies to a whole multi-lymphangion segment, because the ejection fraction of each lymphangion is different (both in our model and in reality).
A limitation of our study is that it was necessarily confined to only one value of most model parameters (Table 1). Although many of these parameters describe aspects of the physiology that are indeed fixed, even if the physiological value is not known with any accuracy, there are others that can vary freely in vivo. An example is Δpae = pa − pe, which sets the level of transmural pressure [the actual transmural pressure experienced by a given lymphangion, Δptm,i(t) = pim(t) − pe, varies with time; see Ref. 5 for details]. We have previously described how the level of transmural pressure affects pump function (Ref. 6 and Bertram CD, Macaskill C, Moore JE, Jr., unpublished data). However, the description of valve function (8) is an area where our model is tied particularly closely to physiological experiment (13) on valves from rat mesentery. Therefore, we believe that the behavior manifested in these simulations, i.e., the tendency for one or more valves (which may or may not be adjacent) to remain open, which, in turn controlled the efficacy of pumping, is typical of that which would be seen if a study varying td and tr could be mounted biologically, and our experiment confirms this, in principle.
Our study also does not address the question of whether lymphangion activity might be chaotic rather than coordinated. Venugopal et al. (31) found decreased mean flow in their three-lymphangion model when contractions were uncoordinated. All local influences, which include those involving fluid-mechanical [pressure (12, 32) and wall shear (18)] and pharmacological [calcium and nitric oxide (19, 34)] stimuli, and those relating to electrotonic conduction of activation within the wall [via gap junctions between cells (11, 36)], will tend to produce sequential coordinated contraction of the lymphangions of a vessel. On the other hand, the arrival of such stimuli at lymphatic vessel junctions from different branches will tend to produce chaotic activity on the scale of a whole network, and so-called spontaneous depolarizations (33) may desynchronize contractions within a vessel.
The great strength of a computer model is that specific parameters can be varied systematically, providing a comprehensive answer to questions such as those posed here, under fully controlled conditions. Parameter space can be explored without regard to how much or little is feasible in reality. The range of inter-lymphangion delays used here exceeds the likely physiological space, probably by a large margin in the case of certain mechanisms of lymphangion coordination. Thus, for instance, if the frequency of contractions is set by the extent of lymphangion distension, as Fig. 9 exemplifies, this would suggest that the furthest downstream lymphangion, which under pumping conditions experiences the highest transmural pressure, would be likely to set the highest rate, and, in turn, would be expected to entrain the lymphangions further upstream. This means that contractions would always propagate retrogradely, while this mechanism dominated [This is contrary to the prediction by Kunert et al. (19).]. Antegrade propagation of an excitation impulse arriving (as in the previous paragraph) via an upstream junction would be possible only if the transmural pressure was low enough that the lymphangions of the excited vessel were not already responding to retrogradely propagating stimuli.
As indicated already, the properties of the model are as far as possible guided by physiological data for collecting lymphatics of rat mesentery. In other organs, lymphatic vessels can have quite profoundly different characteristics. For instance, Moriondo et al. (23) have described how diaphragmatic mesothelial lymphatic vessels may have the dimensions of collecting lymphatics, but at least some of the properties of initial lymphatics, with scanty smooth muscle cells and a wall mechanically dependent on the stress-strain features of surrounding tissues. Our model is not applicable to such vessels.
In conclusion, the question of the extent of lymphatic vascular pumping dependence on the synchronicity or otherwise of contractions turns out to be unexpectedly complex to answer, since pressure-dependent valve bias to the open position throws in a factor that cannot be fully predicted without doing the relevant computation. There is strong dependence on the extent of inter-lymphangion delay, as was indicated by our early study involving continuous contractions (6), but the dependence is nonmonotonic and also depends on the time between contractions and the adverse pressure difference faced by the vessel. At high and moderate adverse pressure difference, high mean flow-rate is generated by widely differentiated combinations of refractory period and inter-lymphangion delay, but it is not clear that these optima can be utilized in vivo, where the inter-lymphangion delay is set by the rate of inter-lymphangion excitation conduction. The situation that produces the highest flow-rate at low adverse pressure involves all lymphangions contracting simultaneously after a period of relaxation, which may not be feasible in vivo, but which may be interpreted as an approximate model of extrinsic pumping. This situation also involved all intermediate valves staying continuously open, where they create minimum impediment to flow, and may suggest a first partial answer to the question of why valves are biased to the open position at all. Experimentally, we found evidence that intermediate valves may, indeed, stay continuously open during pumping against an adverse pressure difference. Antegrade spread of contraction excitation, in which each lymphangion primes one that is about to contract, is generally better for fluid transport than retrograde conduction. The most efficient pumping occurs when the refractory period is long and the inter-lymphangion delay is such that each lymphangion begins to contract when the adjacent one upstream has just reached peak contraction.
Finally, in the face of the demonstrated inadequacy of conventional pump function curves, we have proposed new methods of plotting pump function and how it varies with alteration in parameters. The inadequacy arises specifically for multi-lymphangion segments in which the valves are given their proper hysteresis and transmural-pressure-dependent bias. Under other circumstances (single lymphangions, or simplified valve properties), conventional pump function curves continue to be an appropriate tool for quantifying pump function and, indeed, the only tool for showing explicitly how pump output varies with the adverse pressure difference faced.
GRANTS
J. E. Moore, Jr. gratefully acknowledges support from The Royal Society, The Royal Academy of Engineering, The Sir Leon Bagrit Trust, and U.S. National Institutes of Health (NIH) Grant R01-HL-094269. M. J. Davis's laboratory was supported by NIH Grant R01-HL-120867. All authors acknowledge support from NIH Grant U01-HL-123420.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.D.B. conception and design of research; C.D.B., C.M., and M.J.D. performed experiments; C.D.B., C.M., and M.J.D. analyzed data; C.D.B., C.M., and M.J.D. interpreted results of experiments; C.D.B. and M.J.D. prepared figures; C.D.B. drafted manuscript; C.D.B., C.M., M.J.D., and J.E.M. edited and revised manuscript; C.D.B., C.M., M.J.D., and J.E.M. approved final version of manuscript.
Appendix
The equations of the model are
where i is lymphangion number, t is time, D(t) is diameter, Q(t) is valve flow-rate, L is lymphangion length, p(t) is pressure, suffix 1, m, and 2 are lymphangion upstream end, midpoint, and downstream end, respectively, RVn = minimum valve resistance, RVx + RVn is maximum valve resistance, so is valve-opening slope constant, Δpo is valve-opening pressure difference, and pe is external pressure. Δpoi is a function of valve transmural pressure (alternately pi−1,2 − pe and pi1 − pe), as described in Ref. 8. The functions fp(Di) and Md(Di) are shown in Fig. 2, and Mt(t) is a waveform, as shown in Fig. 3.
REFERENCES
- 1.Akl TJ, Nepiyushchikh ZV, Gashev AA, Zawieja DC, Coté GL. Measuring contraction propagation and localizing pacemaker cells using high speed video microscopy. J Biomed Optics 16: 026016, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 73: 1–78, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Baez S. Flow properties of lymph—a microcirculatory study. In: Flow Properties of Blood and Other Biological Systems, edited by Copley AL and Stainsby G. Oxford, UK: Pergamon, 1960, p. 398–411. [Google Scholar]
- 4.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Bertram CD, Macaskill C, Davis MJ, Moore JE Jr. Development of a model of a multi-lymphangion lymphatic vessel incorporating realistic and measured parameter values. Biomech Model Mechanobiol 13: 401–416, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram CD, Macaskill C, Moore JE Jr. Simulation of a chain of collapsible contracting lymphangions with progressive valve closure. J Biomech Eng 133: 011008-1–011008-10, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertram CD, Macaskill C, Moore JE Jr. Towards a realistic model of a lymphatic network: improved methods of solution of the equations for many lymphangions in series. In: ASME 2013 Summer Bioengineering Conference (SBC2013), Sunriver, OR, abstract no. 14434 (2 pages), New York: ASME, 2013. [Google Scholar]
- 8.Bertram CD, Macaskill C, Moore JE Jr. Incorporating measured valve properties into a numerical model of a lymphatic vessel. Comp Methods Biomech Biomed Eng 17: 1519–1534, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browse NL, Doig RL, Sizeland D. The resistance of a lymph node to lymph flow. Br J Surg 71: 192–196, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Crowe MJ, von der Weid P-Y, Brock JA, Van Helden DF. Co-ordination of contractile activity in guinea-pig mesenteric lymphatics. J Physiol 500.1: 235–244, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol 587: 165–182, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE Jr. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 301: H48–H60, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE Jr., Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol Heart Circ Physiol 233: H57–H65, 1977. [DOI] [PubMed] [Google Scholar]
- 16.Jamalian S, Bertram CD, Richardson WJ, Moore JE Jr. Parameter sensitivity analysis of a lumped-parameter model of a chain of lymphangions in series. Am J Physiol Heart Circ Physiol 305: H1709–H1717, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamalian S, Jafarnajad M, Bertram CD, Zawieja DC, Davis MJ, Moore JE Jr. Suction effect produced by active contraction of collecting lymphatic vessels facilitates lymphatic filling. In: Abstracts of the Summer Biomechanics, Bioengineering and Biotransport Conference, (SB3C2015) 2015. [Google Scholar]
- 18.Kornuta JA, Nepiyushchikh ZV, Gasheva OY, Mukherjee A, Zawieja DC, Dixon JB. Effects of dynamic shear and transmural pressure on wall shear stress sensitivity in collecting lymphatic vessels. Am J Physiol Regul Integr Comp Physiol 309: R1122–R1134, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunert C, Baish JW, Liao S, Padera TP, Munn LL. Mechanobiological oscillators control lymph flow. Proc Natl Acad Sci USA 112: 10,938–10,943, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHale NG, Meharg MK. Co-ordination of pumping in isolated bovine lymphatic vessels. J Physiol 450: 503–512, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261: 255–269, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McHale NG, Roddie IC, Thornbury KD. Nervous modulation of spontaneous contractions in bovine mesenteric lymphatics. J Physiol 309: 461–472, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriondo A, Boschetti F, Bianchin F, Lattanzio S, Marcozzi C, Negrini D. Tissue contribution to the mechanical features of diaphragmatic initial lymphatics. J Physiol 588: 3957–3969, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munn LL. Mechanobiology of lymphatic contractions. Seminars Cell Dev Biol 38: 67–74, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17: 920–922, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Negrini D, Del Fabbro M. Subatmospheric pressure in the rabbit pleural lymphatic network. J Physiol 520: 761–769, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 239: H88–H95, 1980. [DOI] [PubMed] [Google Scholar]
- 28.Reddy NP, Krouskop TA, Newell PH Jr. Biomechanics of a lymphatic vessel. Blood Vessels 12: 261–278 [correction publ 14: 128], 1975. [DOI] [PubMed] [Google Scholar]
- 29.Scallan JP, Wolpers JH, Davis MJ. Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J Physiol 591: 443–459, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trzewik J, Mallipattu SK, Artmann GM, Delano FA, Schmid-Schönbein GW. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J 15: 1711–1717, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Venugopal AM, Stewart RH, Laine GA, Dongaonkar RM, Quick CM. Lymphangion coordination minimally affects mean flow in lymphatic vessels. Am J Physiol Heart Circ Physiol 293: H1183–H1189, 2007. [DOI] [PubMed] [Google Scholar]
- 32.von der Weid P-Y, Lee S, Imtiaz MS, Zawieja DC, Davis MJ. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphatic Res Biol 12: 66–75, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von der Weid P-Y, Rahman M, Imtiaz MS, van Helden DF. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol 295: H1989–H2000, 2008. [DOI] [PubMed] [Google Scholar]
- 34.von der Weid P-Y, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol 280: H2707–H2716, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Wilson JT, Wang W, Hellerstedt AH, Zawieja DC, Moore JE Jr. Confocal image-based computational modeling of nitric oxide transport in a rat mesenteric lymphatic vessel. J Biomech Eng 135: 51005, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol Heart Circ Physiol 264: H1283–H1291, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Zhang RZ, Gashev AA, Zawieja DC, Davis MJ. Length-tension relationships of small arteries, veins, and lymphatics from the rat mesenteric microcirculation. Am J Physiol Heart Circ Physiol 292: H1943–H1952, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Zweifach BW, Prather JW. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am J Physiol 228: 1326–1335, 1975. [DOI] [PubMed] [Google Scholar]