We have revealed EFNB3 as a novel component in blood pressure regulation. Its deletion caused increased blood pressure in a sex-dependent fashion. Vascular smooth muscle is the target tissue mediating such an effect of EFNB3. This finding may lead to sex hormone level-specific treatment of hypertension in individuals with EFNB3 mutations.
Keywords: ephrinb3, estrogen, blood pressure
Abstract
EPH kinases and their ligands, ephrins (EFNs), have vital and diverse biological functions, although their function in blood pressure (BP) control has not been studied in detail. In the present study, we report that Efnb3 gene knockout (KO) led to increased BP in female but not male mice. Vascular smooth muscle cells (VSMCs) were target cells for EFNB3 function in BP regulation. The deletion of EFNB3 augmented contractility of VSMCs from female but not male KO mice, compared with their wild-type (WT) counterparts. Estrogen augmented VSMC contractility while testosterone reduced it in the absence of EFNB3, although these sex hormones had no effect on the contractility of VSMCs from WT mice. The effect of estrogen on KO VSMC contractility was via a nongenomic pathway involving GPER, while that of testosterone was likely via a genomic pathway, according to VSMC contractility assays and GPER knockdown assays. The sex hormone-dependent contraction phenotypes in KO VSMCs were reflected in BP in vivo. Ovariectomy rendered female KO mice normotensive. At the molecular level, EFNB3 KO in VSMCs resulted in reduced myosin light chain kinase phosphorylation, an event enhancing sensitivity to Ca2+ flux in VSMCs. Our investigation has revealed previously unknown EFNB3 functions in BP regulation and show that EFNB3 might be a hypertension risk gene in certain individuals.
NEW & NOTEWORTHY
We have revealed EFNB3 as a novel component in blood pressure regulation. Its deletion caused increased blood pressure in a sex-dependent fashion. Vascular smooth muscle is the target tissue mediating such an effect of EFNB3. This finding may lead to sex hormone level-specific treatment of hypertension in individuals with EFNB3 mutations.
eph erythropoietin-producing human hepatocellular carcinoma receptor kinases are the largest family of receptor tyrosine kinases. They are divided into A and B subfamilies according to sequence homology (1). Ephrins (EFNs), which are also cell surface molecules, are ligands of EPHs. EFNs are also classified into A and B subfamilies; members of the A subfamily attach to the cell surface through glycosylphosphatidylinositol anchoring, whereas members of the B subfamily attach through transmembrane tails (12, 38, 48). Interactions among EPHs and EFNs are promiscuous, but in general, EPH A members interface preferentially with EFN A family members, and EPH B members with EFN B family members (12, 38, 48). EFNs can stimulate EPH receptors, and this is called forward signaling. Interestingly, EPHs are also capable of stimulating EFNs, which then transmit signaling reversely into cells, a phenomenon known as reverse signaling.
EPHs and EFNs are expressed in many tissues and organs. They play important roles in the central nervous system (12, 38, 48), immune system (27–29, 31, 32, 49, 51–54), digestive system (2), bone metabolism (6, 55), angiogenesis (45), and other processes (8, 17, 22).
Until recently, limited studies assessed the role of EPHs and EFNs in vascular smooth muscle cell (VSMC) function. VSMCs with an EFNB2 deletion show compromised migration (13). In cultured rat and human VSMCs, EFNA1 triggers EPHA4 signaling and actin stress fiber assembly (37). Whether such signaling elicits changes in VSMC contractility has not been investigated.
We recently reported that EPHB6, in concert with sex hormones, is crucial in VSMC contraction and blood pressure (BP) regulation (30). Male but not female Ephb6 gene knockout (KO) mice are hypertensive. VSMCs are targeted through which EPHB6 exerts its effect on BP control. Since EPHB6 and all its major ligands of the EFN B family, i.e., EFNB1, EFNB2, and EFNB3, are expressed in VSMCs (30), there exists a molecular framework for their function in these cells. We showed that while solid-phase recombinant EPHB6 reduces VSMC contraction in response to phenylephrine (PE) stimulation, solid-phase anti-EPHB6 antibody (Ab) does not (30). Since anti-EPHB6 Ab serves as an EPHB6 agonist surrogate, this indicates that reverse signaling from EPHB6 to EFNBs, but not forward signaling from EFNBs to EPHB6, is responsible for dampening VSMC contractility. Deletion of Efnb1 in smooth muscle cells also elicits hypertension in mice (50), while deletion of Ephb4 in these cells results in hypotension (46). Therefore, multiple members of the EPHB/EFNBs family compose a previously unknown BP regulation system.
So far, there is no report on the regulation of EPH/EFN function by sex hormones, except a study by Nikolova showing that estrogen could modulate EPHB4 and EFNB2 expression in mammary glands (36). The classic estrogen receptor (ER) is an intracellular protein. ER has α- and β-isoforms. They form heterodimers (αβ) or homodimers (αα or ββ) upon binding of estrogen, which enter the cells passively. The estrogen/ER complex then enters the nucleus serving as transcription factors, regulating target gene expression (25). In addition to the classical intracellular ERs, there is also GPER, a G protein-coupled cell membrane estrogen receptor, which mediates fast nongenomic responses to estrogens (39). Androgen receptors are also intracellular proteins, and when they bind to androgens, they will translocate into the nuclei, and serve as DNA-binding transcription factors, regulating genes with androgen-responsive elements (20). There are also cell membrane androgen receptors, which are less well-defined and might also be G protein-associated receptors (3), mediating fast nongenomic actions.
In the present study, we discovered that female but not male Efnb3 KO mice had higher BP compared with their WT counterparts. VSMCs were the major cell type responsible for these phenotypes. The nongenomic effect of estrogen via GPER promoted KO VSMC contractility while the genomic effect of testosterone reduced it.
MATERIALS AND METHODS
Efnb3 KO mice.
Efnb3 KO mice (referred to KO mice) were generously provided to us by Regeneron Pharmaceuticals (23). They had been backcrossed to the C57BL/6J background for more than 10 generations. Age (16–26 wk)- and sex-matched WT mice were served as controls and are referred to as WT mice. The animals were housed in specific pathogen-free rooms with 12:12-h light-dark cycles. Females were not monitored for their estrous cycles. For radiotransmitter implantation and castration/ovariectomy, mice were anesthetized with isoflurane (2% isoflurane with 0.75 l/min O2 flow). In some experiments, the mice with radiotransmitter implantation were castrated or ovariectomized, and telemetry was conducted at least 3 wk after the gonadectomy. The mice were euthanized with pentobarbital (400 mg/kg body wt ip) at the end of in vivo studies or for tissue retrieval.
VSMC isolation.
Mouse VSMCs were isolated, as described by Golovina and Blaustein (15) with modifications (30, 50). The aorta and mesenteric arteries, including their secondary branches, were pooled and digested with collagenase type II (347 U/ml; Worthington Biochemical, Lakewood, NJ). They were further digested with both collagenase type II (347 U/ml) and elastase type IV (6 U/ml; Sigma-Aldrich, St. Louis, MO). The dissociated cells were cultured at 37°C in Dulbecco's modified Eagle's medium (Wisent, St. Bruno, Quebec, Canada) supplemented with 15% fetal bovine serum for 4 to 5 days before experimentation.
Reverse transcription-quantitative polymerase chain reaction.
Efnb3 and GRER mRNA levels in VSMCs were measured by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) (30). For Efnb3, the 5′ primer was 5′-AGTTCCGATCCCACCACGATTACT-3′, and the 3′ primer was 5′-AGAAGCACCTTCATGCCTCTGGTT-3′. For GPR30, the 5′ primer was 5′- CATCATTGGCCTCTGCTACTC-3′, and the 3′ primer was 5′- GAAGATCATCCTCAGGGCTTTC −3′. β-actin mRNA served as an internal control, and primers for β-actin were described previously(50). The following qPCR condition was employed: 2 min at 50°C and then 2 min at 95°C followed by 45 cycles of 10 s at 94°C, 20 s at 58°C, and 20 s at 72°C. Signals between 20 and 30 cycles were analyzed. Samples were tested in triplicate, and the data are expressed as signal ratios of test gene mRNA/β-actin mRNA.
BP measurement.
Mice were implanted surgically with TA11PA-C10 radiotransmitters (Data Sciences International, St. Paul, MN) in the left carotid artery as described previously (24). At least 7 days were allowed for recovery before the measurement of systolic, diastolic and mean arterial pressure (MAP), systolic pressure (SP), diastolic pressure (DP), and heart rate (HR).
BP and HR in conscious, free-moving mice were then recorded continuously for 3 days with the Dataquest acquisition 3.1 system (Data Sciences International). Individual 10-s waveforms of SP, DP, MAP, and HR were sampled every 2 min throughout the monitoring period. The raw data were processed with the Dataquest A.R.T-Analysis program (24), and means + SE of hourly BP and HR are calculated and presented. ANOVA was used to compare the 72-h values of each group (area under the curve) for statistical significance.
VSMC contractility.
VSMC contractility was measured as described previously (30, 50). Briefly, primary VSMCs were cultured for 3–4 days and then stimulated with PE (20 μM). They were photographed continuously for 15 min at a rate of one picture per min. In experiments testing the effect of sex hormones, the VSMC were cultured charcoal-stripped sera in the presence of human 5α-dihydrotestosterone (6.49 ng/ml), cell membrane impermeable testosterone-3-(O-carboxymethyl)oxime-BSA (Aviva Systems Biology, San Diego, CA), or 17-β-estradiol (100 ng/ml) for periods indicated.
Fifteen or more cells were randomly selected, and their length was measured at each time point with Zeiss Axiovision software. Percent contraction was calculated as follows:
Immunofluorescence microscopy.
VSMC surface EFNB3, α1-adrenergic receptor (α1-AR), and GPR30 expression were assessed by immunofluorescence microscopy (30). Cells were blocked with 10% goat IgG in PBS for 20 min and then incubated with rabbit anti-mouse EFNB3 Ab (OAAF01784; Aviva Systems Biology), rabbit anti-mouse α1-AR Ab, (ab3462; Abcam, Cambridge, MA), or rabbit anti-mouse GPR30 Ab (ab39742; Abcam). Cells were then washed and reacted with FITC-conjugated sheep anti-rabbit IgG Ab (0.2 μg/ml; Chemicon International, Temecula, CA) at room temperature for 2 h and imbedded with ProLong Gold anti-fade reagent (Invitrogen). The stained cells were examined under a Zeiss microscope. The total fluorescence intensity of a cell and cell size were measured using AxioVision software from Zeiss; results from measurements of more than 15 cells are then presented as fluorescence intensity per arbitrary unit of cell area (1 pixel).
Immunoblotting.
VSMCs from WT and KO mice were isolated and cultured for 3–4 days. VSMCs were stimulated with PE (20 μM) for 3 s and then lyzed. Total and phosphorylated myosin light chain kinase (MLCK) and MLC phosphatase (MYPT) of the VSMCs were measured similarly, using rabbit anti-mouse MLCK mAb (clone EP1458Y; Abcam), rabbit anti-mouse phospho-MLCK Ab (Invitrogen, Camarillo, CA), rabbit anti-mouse MYPT Ab (no. 2634; Cell Signaling, Danvers, MA), and rabbit anti-mouse phospho-MYPT Ab (no. 5163; Cell Signaling).
Measurement of Ca2+ flux.
PE-stimulated Ca2+ flux in VSMCs was measured by immunofluorescence microscopy (15, 30) Briefly, VSMCs were cultured in medium containing Ca2+ for 4 days. They were then loaded with Fura-2-AM, stimulated with PE (20 μM) at 37°C in medium without Ca2+, as most of the Ca2+ flux in VSMCs was from sarcoendoplasmic reticulum. The cells were imaged for 60 s at a rate of one picture per 3 s. Excitation wavelengths were switched between 340 and 380 nm, and the emission wavelength was 510 nm. Signals from more than 15 randomly selected cells were recorded, and the results are expressed as ratios of fluorescence intensity at 510 nm excited by 340 nm vs. 380 nm.
Small interfering RNA transfection.
Gper small interfering RNAs (siRNAs) and negative control siRNAs were synthesized by Integrated DNA Technologies (Coralville, IA). Their sequences are presented in Table 1. VSMCs were cultured for 40 h in regular medium. They were then transfected with a mix of three pairs of Gper siRNAs (for each pair, the final concentration was 10 nM), or control siRNA, and cultured in medium free of antibiotics, as described before (50). Forty-eight hours after the siRNA transfection, VSMC contractility upon PE stimulation was assessed.
Table 1.
Gper siRNA sequences
|
Gper | |
|---|---|
| Sense sequence | Antisense sequence |
| 5′-GCUCUGUUAAUCUAACGAUCAGACUUA-3′ | 5′-CGAGACAAUUAGAUUGCUAGUCUGA-3′ |
| 5′-UUGAGAGUAUGACAUAGCUUAGCCCAC-3′ | 5′-AACUCUCAUACUGUAUCGAAUCGGG-3′ |
| 5′-AGAUCCAUUUACAGCUCGCUGUUCCCA-3′ | 5′-TCUAGGUAAAUGUCGAGCGACAAGG-3′ |
Urinary catecholamine measurement.
Twenty-four-hour urine under a fasting condition was collected by placing 15- to 17-wk-old KO and WT mice individually in metabolic cages. Urinary catecholamines were quantified by competitive enzyme immunoassays (3-CAT Research ELISA kits) according to the manufacturer's instructions (Labor Diagnostika Nord & Nordhorn). The reaction was monitored at 450 nm. Urine samples were measured in duplicate.
Ethics statement.
All animal studies were performed according to guidelines of Canadian Council for Animal Protection and were conducted according to the protocols approved by CRCHUM Animal Protection Committee.
Statistics.
Statistical significances were determined using a two-way ANOVA or paired Student's t-tests.
RESULTS
Efnb3 KO deletion in VSMCs.
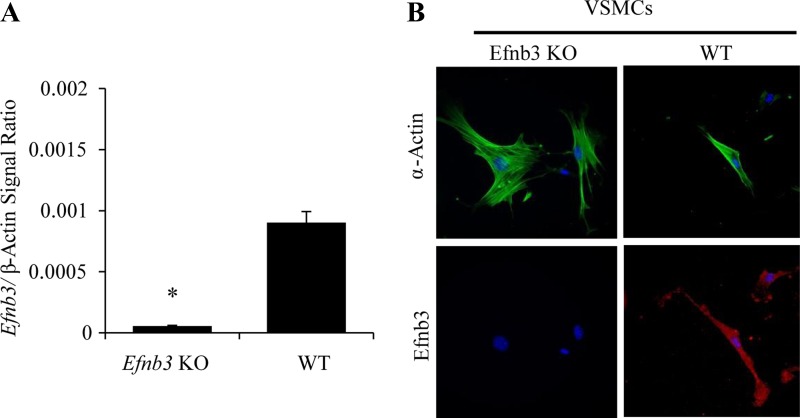
RT-qPCR demonstrated that Efnb3 mRNA was virtually absent in endothelium-stripped mesenteric arteries from Efnb3 KO mice (Fig. 1A). Efnb3 KO was also confirmed at the protein level in isolated EFNB3 KO VSMCs by immunofluorescence microscopy (Fig. 1B).
Fig. 1.
Efnb3 deletion in vascular cells from Efnb3 knockout (KO) mice. The experiments were conducted twice, and data from representative experiments are reported. A: Efnb3 mRNA deletion in mesenteric arteries of Efnb3 KO mice. Efnb3 mRNA expression in mesenteric arteries from WT and Efnb3 KO mice was measured by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) with β-actin mRNA as internal control. Samples in RT-qPCR were in triplicate, and means ± SE of Efnb3 signal/β-actin signal ratios are reported. B: EFNB3 deletion in vascular smooth muscle cells (VSMCs). WT and EFNB3 KO VSMCs cultured for 4–5 days were stained with mouse anti-human α-actin mAb (in pseudo-green color) followed by rhodamine-conjugated goat anti-mouse IgG Ab, and goat anti-mouse-EFNB3 Ab followed by rhodamine-conjugated bovine anti-goat IgG Ab (in pseudo-red color). Nuclei were identified by DAPI staining. *P < 0.01, Student's t-test.
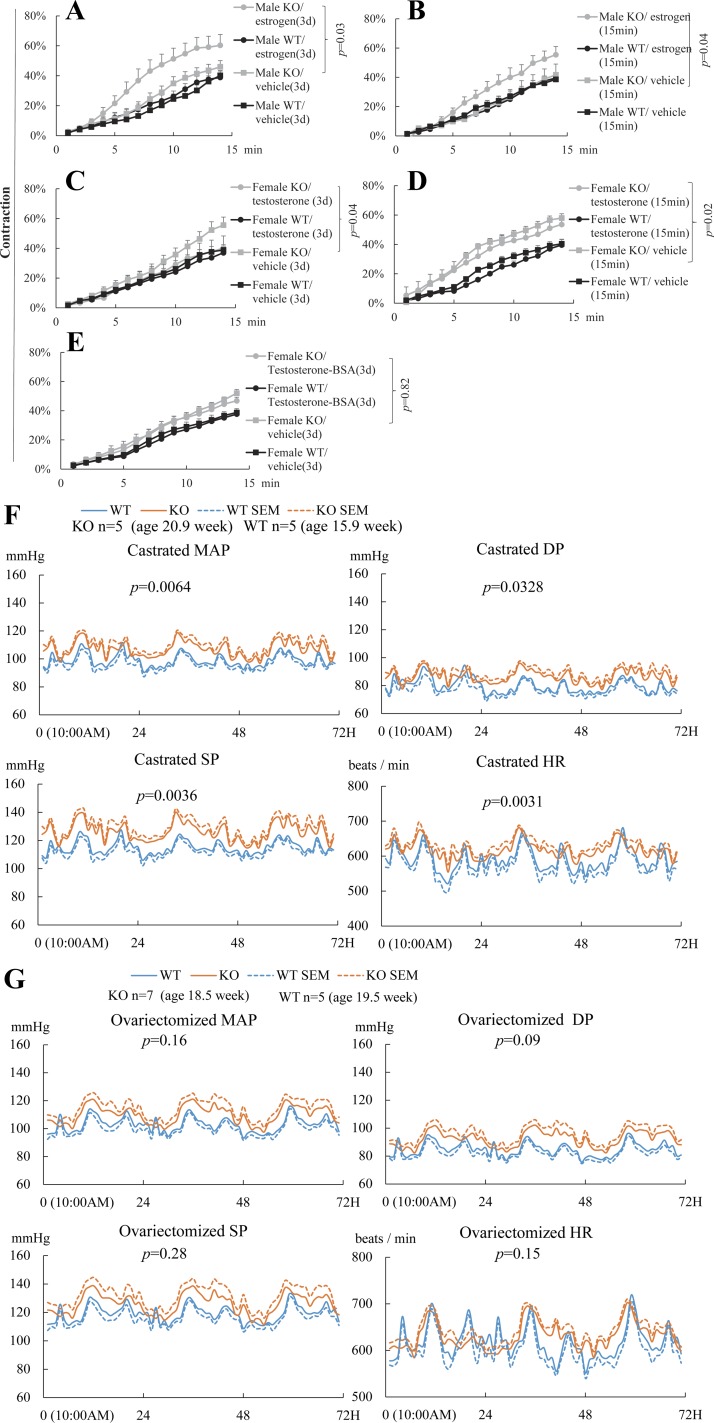
EFNB3 deletion caused increased BP in females but not males.
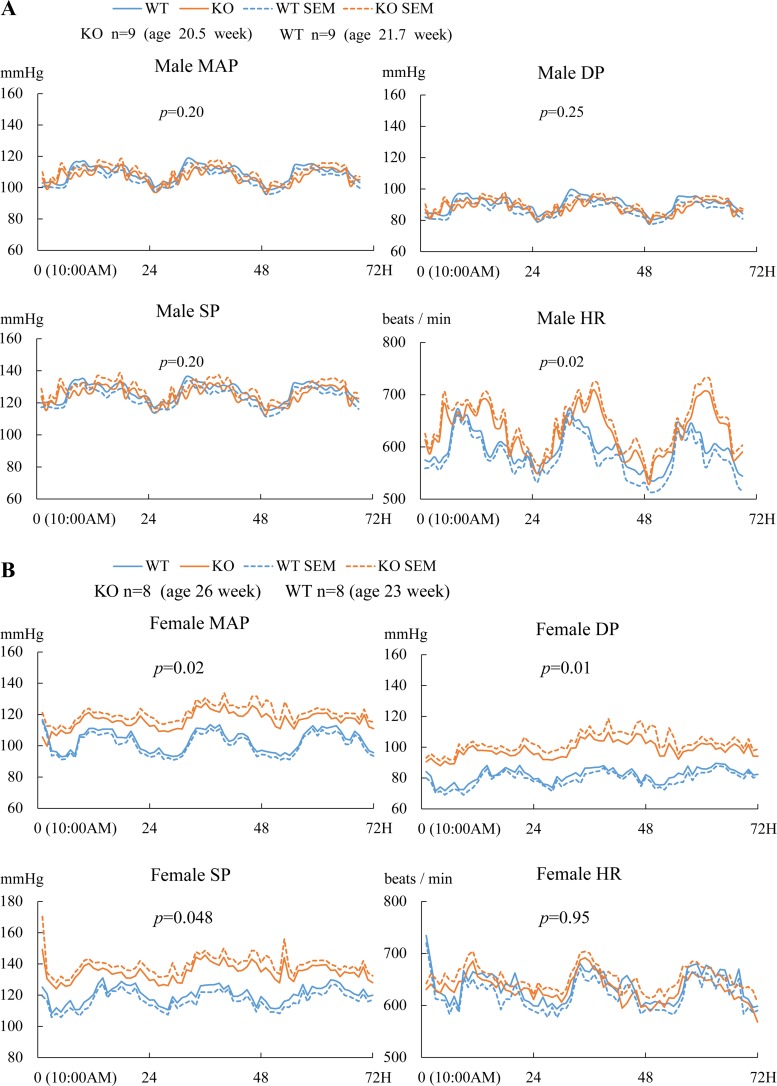
BP of the aged-matched Efnb3 KO and WT mice was measured by radiotelemetry. The MAP, SP, and DP of male KO mice showed no significant differences from their WT controls, although the HR of the KO mice was higher (Fig. 2A). Females KO had increased MAP, SP, and DP compared with their WT counterparts, while their HR were normal (Fig. 2B). These results clearly show that EFNB3 deletion caused higher BP in a sex-dependent way.
Fig. 2.
Increased blood pressure (BP) in female Efnb3 KO mice. BP and heart rate (HR) of male (A) and female (B) KO and WT mice were measured by radiotelemetry starting at least 7 days after transmitter implantation. Number per group and the mean ages of the mice at the time of BP measurement are indicated. The BP and HR of all mice were measured for 72 h. Means ± SE of hourly BP and HR are plotted. MAP, mean arterial pressure; SP, systolic pressure; DP, diastolic pressure; HR, heart rate. The 72-h values were used for statistical analysis (ANOVA). P values are indicated.
Increased contractility of VSMCs from female KO mice.
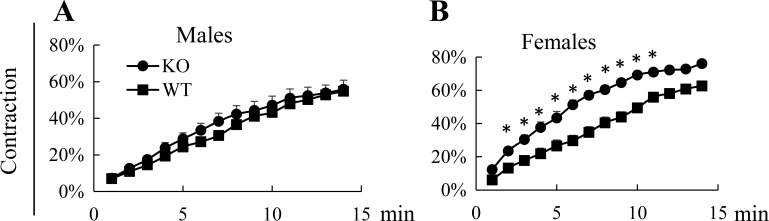
When isolated VSMCs were stimulated with PE, those derived from female Efnb3 KO mice but not male KO mice showed increased contractility compared with their WT counterparts (Fig. 3, A and B), indicating that VSMCs are a functional target tissue of the BP-regulating effect of EFNB3 deletion.
Fig. 3.
Female but not male VSMC contractility is augmented by EFNB3 deletion. All experiments were conducted 3 times independently. Data from representative experiments are presented. VSMCs were isolated from the mesenteric arteries and aorta of male (A) or female (B) WT and KO mice and cultured for 4 days. They were then stimulated with 20 μM phenylephrine (PE) at 37°C and imaged every min for 15 min. Means ± SE of percentage contraction of more than 15 cells per group are reported. The experiment was conducted 3 times, using 3 pairs of WT and KO mice in 3 different days, and data from a representative one are shown. Statistically significant differences were assessed by Student's t-test. *P < 0.05.
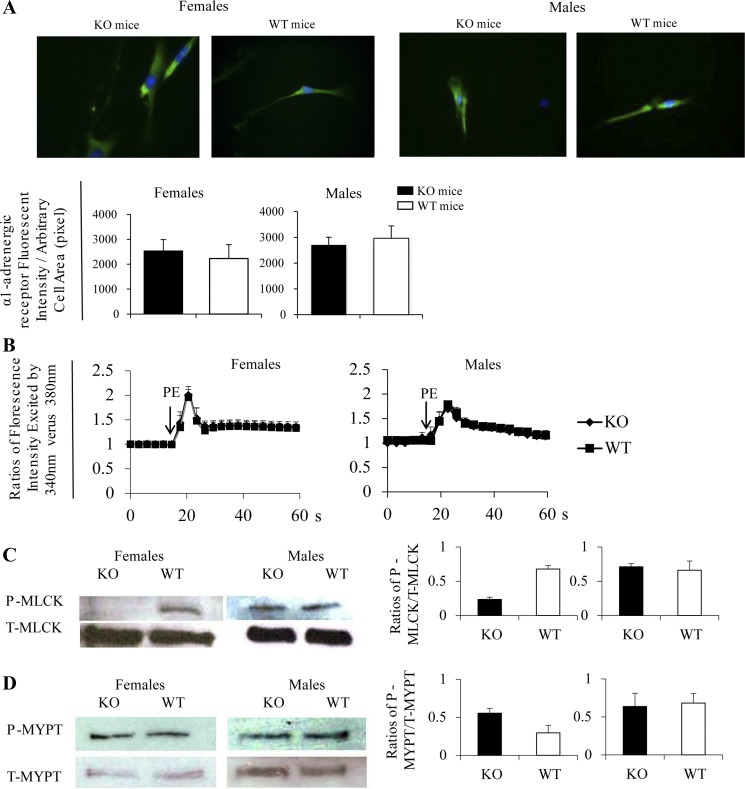
The increased contractility of female KO VSMCs upon PE stimulation might be due to heightened adrenoreceptor signaling strength. However, we found no difference in α1-AR expression in KO and WT VSMCs, regardless of the sex, according to either immunofluorescence (Figs. 4A).
Fig. 4.
Signaling events related to contractility of KO and WT VSMCs. A: normal α1-adrenergic receptor (α1-AR) expression in Efnb3 KO VSMCs according to immunofluorescence microscopy. VSMCs from female (left) and male (right) Efnb3 KO or WT mice were cultured for 4 days and then stained with Ab against α1-AR and α-actin. For each experiment, more than 15 α-actin-positive cells were randomly selected, and their total α1-AR immunofluorescence intensity and cell size were measured. Top: micrographs of α1-AR immunofluorescent staining. Bottom: means ± SE of α1-AR fluorescence intensity per unit of arbitrary cell area (pixel) of all cells examined in a group of a representative experiment. The data were analyzed by paired Student's t-test, but no significant difference was found. The experiment was performed 3 times independently and data of a representative 1 are shown. B: normal Ca2+ flux in VSMCs from Efnb3 KO mice. VSMCs from male (right) and female (left) Efnb3 KO or WT mice were cultured for 4 days and loaded with Fura2. They were then placed in HBSS at 37°C and stimulated with PE. Arrows indicate the time points at which PE (20 μM) was added. The ratio of emissions at 510 nm triggered by 340-nm vs. 380-nm excitation in each cell was registered every 3 s for 1 min. The experiments were conducted 3 times independently. Means ± SE of the ratio of more than 15 randomly selected VSMC of a representative experiment are illustrated. No statistical significant difference between the KO and WT groups are found according to ANOVA. C and D: phosphorylation of myosin light chain (MLC) kinase (MLCK) and MLC phosphatase (MYPT) in VSMCs from KO and WT mice. VSMC from female and male KO and WT mice were cultured for 4 days, then stimulated with 20 μM PE for 3 s and immediately lysed. Total (T-) and phosphorylated (P-) MLCK (C) and MYPT (D) were analyzed by immunoblotting, which was conducted twice. Immunoblotting from representative experiments are illustrated. Data from densitometry of all the immunoblots are presented as bar graphs with means ± SD shown.
Ca2+ flux (Fig. 4B) in KO VSMCs was comparable to that of the WT counterparts, whether they were from males or females. Thus the increased contractility of VSMCs from female KO mice was likely not due to increased Ca2+ flux but augmented Ca2+ responsiveness, which is regulated by MLCK. When MLCK is phosphorylated at Ser1760 by calmodulin-dependent protein kinase II or protein kinase A, its MLCK enzymatic activity is reduced (14, 33, 40). Conversely, reduced phosphorylation at Ser1760 will lead to increased VSMC contractility. We assessed MLCK phosphorylation at Ser1760 and observed that VSMCs from female but not male KO mice had significantly lower values upon PE stimulation than WT controls (Fig. 4C). This result implies higher MLCK activity and hence higher Ca2+ responsiveness in VSMCs from female KO mice, compatible with the finding that female VSMCs presented augmented contractility. VSMC contractility is also modulated by MYPT, which dephosphorylates MLC and reduces VSMC contractility (19). MYPT phosphorylation at T695 prevents its binding to MLC and thus diminishes its phosphatase activity (34). We investigated the outcome of EFNB3 deletion on MYPT phosphorylation in VSMCs. After PE stimulation (3 s), MYPT phosphorylation at T695 in VSMCs from both female and male KO mice was similar to that of their WT counterparts (Fig. 4D). This observation suggests that MYPT is not influenced by EFNB3 deletion and does not participate in the contractility upregulation of female KO arteries.
Nongenomic effect of estrogen augments while genomic effect of testosterone reduces KO VSMC contractility.
The default function of EFNB3 signaling seemed to reduce VSMC contractility, and hence, EFNB3 KO deletion led to the increased VSMC contractility (Fig. 3B). However, such an increase only occurred in VSMCs from female but not male KO mice, suggesting that either estrogen is permissive for this phenotype, or androgen antagonizes it, or both.
To assess the role of estrogen in this regard, we cultured VSMCs from male KO mice in the presence of a physiological concentration of estrogen (17β-estradiol). VSMCs from male but not female mice were used to minimize intracellular estrogen carryover from in vivo exposure. The fetal calf sera used in these sex hormone studies were absorbed by charcoal to remove residual sex hormones in the sera.
We found that after a 3- to 4-day culture in the presence of 17β-estradiol, the contractility of male KO VSMCs, which had no difference from WT counterparts in the absence of estrogen, was increased compared with vehicle controls, while estrogen did not affect the contractility of male WT VSMCs (Fig. 5A).
Fig. 5.
Estrogen enhances but testosterone suppresses and VSMC contractility and hence BP in the absence of EFNB3. VSMCs were cultured in medium containing charcoal-stripped FCS for 3–4 days. Means ± SE of percentage contraction of more than 15 cells per group are registered. Statistically significant differences were assessed by ANOVA followed by post hoc examination. P values between groups with significant differences are indicated. All experiments were conducted 3 times independently. Data from representative experiments are presented. A: long-term estrogen treatment augments contractility of VSMCs from KO but not WT mice. VSMCs from WT and KO males were cultured for 3–4 days in the presence of 17β-estradiol (100 pg/ml) or vehicle and then stimulated with PE (20 μM). Cell contractility was registered. B: short-term estrogen treatment augments contractility of VSMCs from KO but not WT male mice. VSMCs from male KO or WT mice were cultured for 3–4 days and then stimulated with 17β-estradiol (100 pg/ml) or vehicle for 15 min. In the same last 15 min of culture, PE (20 μM) was also added and VSMC contractility was recorded. C: long-term testosterone treatment suppresses contractility of VSMCs from female KO but not WT mice. VSMC from WT and KO females were cultured for 3–4 days in the presence of 5α-dihydrotestosterone (6.49 ng/ml) or vehicle and then stimulated with PE (20 μM). VSMC contractility was recorded. D: short-term testosterone treatment has no effect on contractility of VSMCs from female KO or WT mice. VSMCs from female KO or WT mice were cultured for 3–4 days and then stimulated with 5α-dihydrotestosterone (6.49 ng/ml) or vehicle for 15 min. In the last 15 min of culture, PE (20 μM) was also added and VSMC contractility was recorded. E: cell membrane-impermeable testosterone-3-(O-carboxymethyl)-oxime-BSA has not effect on contractility of VSMCs from female KO or WT male mice. VSMCs from female KO or WT mice were cultured for 3–4 days in the presence of cell membrane-impermeable testosterone-3-(O-carboxymethyl)-oxime-BSA (1.1 μg/ml) and then stimulated with PE (20 μM). VSMC contractility was recorded. F and G: castrated male KO mice are hypertensive and ovariectomized female KO mice are normotensive. BP and HR of castrated male (F) and ovariectomized female (G) KO and WT mice were measured by radiotelemetry as described in Fig. 2. Telemetry transmitters were implanted in the mice, and after at least 1 mo, they were castrated or ovariectomized. Telemetry was conducted 3 wk after gonadectomy. Number per group and the mean ages of the mice at the time of BP measurement are indicated. The BP and HR of all mice were measured for 72 h. The 72-h values were used for statistical analysis (ANOVA). P values are indicated.
In the above-described experiments, estrogen was present in the culture for 3–4 days. Therefore, its effect could be either genomic or nongenomic or both. A short-term estrogen treatment was employed to discern these two possibilities. When VSMCs from male KO mice were incubated with 17β-estradiol, their contractility were increased within as short as 15 min, while the contractility of WT VSMCs was not influenced by such a treatment (Fig. 5B). The speed of the estrogen effect suggests that the nongenomic pathway is involved in the effect of estrogen on male KO VSMCs.
To assess whether androgen has a protective effect on the augmented VSMC contractility after EFNB3 deletion, we cultured VSMCs from KO females in the presence of a physiological concentration of 5α-dihydrotestosterone for 3–4 days. The female VSMCs but not male VSMCs were used in this experiment to minimize the intracellular androgen carryover from the in vivo environment. 5α-Dihydrotestosterone effectively reduced the augmented female KO VSMC contractility to the WT level (Fig. 5C), while it had no effect on female WT VSMCs. However, short-term (15 min) 5α-dihydrotestosterone (6.49 ng/ml) treatment (Fig. 5D) or a long-time (3 days) treatment of a cell membrane-impermeable BSA-conjugated testosterone [testosterone-3-(O-carboxymethyl)-oxime-BSA; testosterone-BSA; Aviva Systems Biology] used at an equal molar concentration (1.1 μg/ml) as 5α-dihydrotestosterone (6.49 ng/ml) could not reduce the female KO VSMC contractility (Fig. 5E). These results suggest that the genomic but not nongenomic effect of testosterone is responsible for countering the VSMC contractility-augmenting effect of EFNB3 deletion and explain the normal contractility of VSMCs from male KO mice, and the normal BP of those mice.
The above in vitro study showed that in the absence of EFNB3, estrogen promoted VSMC contractility, while testosterone reduced it. Is this conclusion valid in vivo where VSMC contractility is reflected in BP? Indeed, when male KO mice were castrated, they had higher BP compared with the WT counterparts (Fig. 5F), and when females KO mice were ovariectomized, their higher BP returned to the normal range. (Fig. 5G). The BP of WT and KO mice before and after gonadectomy was also compared and results are shown in Table 2. The BP of WT females basically had no change after ovariectomy, except SP, which was increased after ovariectomy. The BP of KO female was reduced after ovariectomy, as expected. For the males, castration leads to reduced BP in WT mice. On the other hand, castration did not change BP in KO mice. The interpretations of these findings in conjunction with that of VSMC data are presented in the discussion.
Table 2.
BP comparison before and after gonadectomy
| Female WT |
Female KO |
Male WT |
Male KO |
|||||
|---|---|---|---|---|---|---|---|---|
| Naïve | Ovariectomized | Naive | Ovariectomized | Naive | Castrated | Naive | Castrated | |
| DP, mmHg | 81.9* | 84.7 | 98.4 | 92.4 | 90.5 | 79.9 | 87.8 | 86.5 |
| P value | 0.0002‡§ | 6.5E-15§ | 7.3E-24§ | 0.058 | ||||
| SP, mmHg | 120.4* | 119.0 | 134.8 | 126.2 | 126.8 | 115.0 | 124.4 | 126.0 |
| P value | 0.061 | 5.9E-17§ | 7.6E-28§ | 0.058§ | ||||
| MAP, mmHg | 103.4* | 102.9 | 116.3 | 110.1 | 109.4 | 98.8 | 106.7 | 107.0 |
| P value | 0.408 | 3.4E-14§ | 7.4E-26§ | 0.093 | ||||
| HR, beats/min | 636.6† | 618.3 | 632.4 | 630.3 | 596.9 | 590.1 | 626.3 | 617.6 |
| P value | 0.0001§ | 0.432 | 0.165 | 0.153 | ||||
WT, wild type; KO, knockout; DP, diastolic pressure; SP, systolic pressure; MAP, mean arterial pressure; HR, heart rate.
Mean of 72-h BP.
Mean of 72-h HR.
Data were analyzed by two-way ANOVA.
Statistically significant differences.
GPER mediates the nongenomic effect of estrogen on KO VSMC contractility.
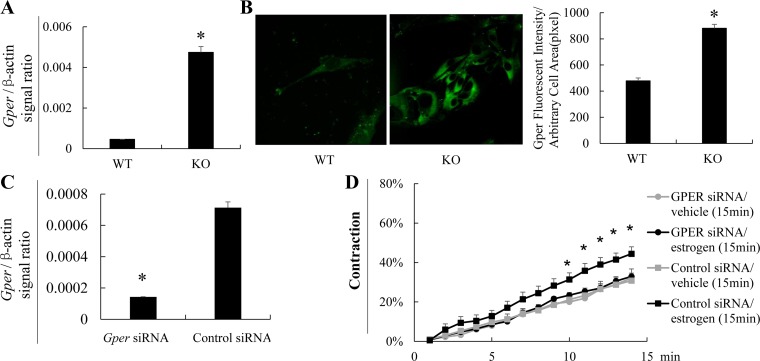
GPR30 is a G protein-associated cell surface molecule mediating the nongenomic effects of estrogen (10). We found that its mRNA (Fig. 6A) and protein expression (Fig. 6B), as detected by RT-qPCR and immunofluorescence, respectively, were significantly augmented KO VSMCs, compared with the WT counterparts, consistent with the obvious nongenomic effect in KO VSMC.
Fig. 6.
EFNB3 deletion enhances GPER expression and results in increased VSMC contractility in the presence of estrogen. A: EFNB3 KO VSMCs present enhanced Gper mRNA expression according to RT-qPCR. VSMCs from male KO or WT mice were cultured in medium containing charcoal-stripped FCS for 4 days, and their Gper mRNA levels were measured by RT-qPCR. The samples were in triplicate. Data from 3 independent experiments are pooled and means ± SD of signal ratios of Gper vs. β-actin are shown. The data were analyzed by Student's t-test. *P < 0.05. B: EFNB3 KO VSMCs present enhanced GPER protein expression according to immunofluorescence. VSMCs from male KO or WT mice were prepared as described in A, and their GPER protein expression was detected by immunofluorescence. Micrographs are shown at left. For each experiment, more than 15 α-actin-positive cells per type of VSMCs were randomly selected, and their total immunofluorescence intensity and cell size were recorded AxioVision software. Means ± SE of fluorescence intensity per unit of arbitrary cell area (pixel) of all cells examined in a given type of VSMCs of 3 independent experiments are pooled and summarized in a bar graph at right. The data were analyzed by Student's t-test. *P < 0.05. C and D: GPER knockdown by siRNA abolishes estrogen-promoted KO VSMC contractility. VSMCs from male KO and WT mice were cultured for 40 h in regular medium containing charcoal-stripped FCS. They were then transfected with a mix of 3 pairs of Gper siRNAs (for each pair, the final concentration was 10 nM), or control siRNA, and cultured in medium free of antibiotics, as described before (50). Forty-eight hours after the siRNA transfection, VSMCs were treated with 17β-estradiol (100 pg/ml) or vehicle for 15 min. In the same last 15 min of culture, PE (20 μM) was also added and VSMC contractility was assessed (D). The cells were then harvested, their Gper mRNA levels were determined by RT-qPCR (C). The experiment was conducted 3 times. Data (means ± SE) of a representative 1 are shown. *P < 0.05 (Student's t-test) between cells treated with Gper siRNA plus estrogen vs. control siRNA plus estrogen.
To further prove GPER's role in mediating estrogen's effect on VSMC contractility, we transfected VSMCs with Gper siRNA. The knockdown Gper expression at the mRNA level was confirmed by RT-qPCR (Fig. 6C). Such knockdown resulted in failed augmentation of male KO VSMC contractility by estrogen (Fig. 6D).
Investigation of the effect of EFNB3 KO on the sex hormone levels and levels of BP-related endocrine molecules.
The serum testosterone and estrogen levels of males and females, respectively, in KO and WT mice were measured, but no apparent difference between the KO and WT mice was observed (data not shown).
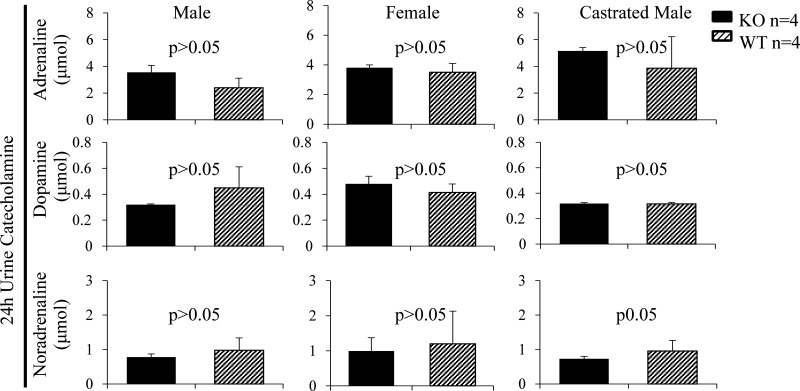
We showed that in male or female mice, EFNB3 KO did not alter 24-h urine catecholamine contents (Fig. 7). Serum ANG II levels in KO and WT mice, whether males or females, were comparable (data not shown).
Fig. 7.
Normal 24-h urinary catecholamine levels in Efnb3 KO mice. Male and female Efnb3 KO and WT mice were placed in metabolic cages. Urine was collected during a 24-h fasting period. Urinary catecholamines were measured by competitive enzyme immunoassay. Means ± SE of hormones excreted during the 24-h period and mouse number per group (n) are presented. No statistically significant differences were found between KO and their WT counterparts (unpaired Student's t-test).
DISCUSSION
Our data indicate that VSMCs are a target tissue for EFNB3 function in BP regulation. In the absence of EFNB3, the VSMCs contracted stronger, hence increased BP. However, such phenotype was only revealed under permissive conditions, i.e., the presence of estrogen.
In our in vivo BP measurement, in some test and control groups, there was 3- to 5-wk age difference. The BP difference between the KO and WT mice is not caused by such age difference, as we have confirmed that the BP in mice with 10- to 20-wk age difference are similar (data not shown).
The HR of male but not female KO mice was significantly higher than that of the WT counterparts, especially during the night when the mice were active (Fig. 2A). The EFNB3 KO mice used in this study have a general deletion of the gene. One of the possible reasons of the increased HR is that EFNB3 has a functional role in heart rhythm pacing cells and such a role is sex dependent. Another possibility is that as EFNB3 has a known function in the central nervous system (23), it might influence the circadian neural outflow to the pacing cells. These possibilities are under investigation. However, such a HR increase did not influence BP in males, likely due to multiple BP compensation mechanisms. Such HR phenotype did not appear in KO females and, therefore, does not contribute to the increased BP in them. In castrated KO males, the HR also increased and such an increase might contribute to the higher BP. However, increased VSMC contractility remains a significant contributor to the elevated BP, as evidence by our in vitro VSMC data.
Since sex hormones are steroids with long half-lives, in our VSMC experiments, we used female VSMCs to test the function of testosterone, and male VSMCs to test the function of estrogen. This cross-over approach minimizes the carryover of sex hormones to be tested from in vivo to in vitro and hence reduces the background noise. Like any reductionist strategy in vitro experiments, we are aware of the limitation of this cross-over approach in that under physiological conditions female VSMCs do not encounter high concentrations of testosterone, and male VSMCs, estrogen.
We demonstrated EFNB3 can modulate VSMC contractility by reverse signaling. EFNB3 is a transmembrane protein without enzymatic activity in its intracellular tail. How does it regulate VSMC contractility? EFNB intracellular tails are known to associate with GRIP1(7), Dishevelled (44), PDZ-RGS3 (26), TIAM1 (43), and GRB4 (42). We have demonstrated that the association of EFNB3 with GRIP1 is critical for its function in regulating VSMC contractility (unpublished observations, Wang Y, Wu Z, Luo H, Peng J, Raelson J, Ehret GB, Munroe PB, Stoyanova E, Qin Z, Cloutier G, Bradley WE, Wu J). In VSMCs, EFNB3 might also associate with other so-far unidentified binding proteins. During reverse singling, some of these proteins might interact with and modulate the functions of other pathways that control VSMC contractility, the MLCK pathway being one of them. As shown in Fig. 4C, in female KO VSMCs, MLCK phosphorylation was reduced compared with that of the WT counterparts. Reduced MLCK phosphorylation augments the MLCK activity, and then MLC phosphorylation, which promotes Ca2+ sensitivity of VSMCs, hence increased contraction. MLCK can be phosphorylated by Cam-kinase II, kinase A, or kinase C, resulting in its inactivation. In our previous publication related to the role of EphB4 in VSMC contractility, we reported that EphB4 deletion modulates Cam-kinase II (46). As EphB4 interacts with EFNB2, a molecule belonging to the same subfamily as EFNB3 does, it is possible that EFNB3 deletion also modulates Cam-kinase II. The intermediate signaling molecules between EFNB3/GRIP1 and Cam-kinase II/MLCK/MLC remain to be identified. In addition, EFNB3 could also modulate MLC function through other pathways. For example, Zip kinase in the Rho kinase pathway could directly activate MLC (4). The putative connection between EFNB3 and the Rho pathway is quite relevant, as among EFNB-associating proteins, Tiam1 is a guanine exchange factor for Rho GTPase (43).
The default function of EFNB3 with regard to VSMC contractility should be an inhibitory one, but such a default function was only revealed in the presence of estrogen or absence of testosterone. What are the possible mechanisms by which sex hormones in concert with EFNB3 modulate VSMC contractility? A possible one is that, in the absence of EFNB3, sex hormones regulate the expression of certain cell surface receptors or their signaling involved in VSMC contractility. These receptors include vasoconstrictor receptors, such as type 1a α-AR AT1 receptor (the major ANG II receptor), and ETα/ETβ2 (endothelin-1 receptors for constriction). Another possible mechanism is that that some EFNB3-associating proteins start to respond to sex hormones only when EFNB3 is deleted, with regard to their downstream connection/modulation of VSMC contracting machinery. In other words, although they are capable of associating with EFNB3, they still regulate VSMC contractility in the absence of EFNB3, but such regulation is now being modulated by sex hormones. The above-described two mechanisms are not mutually exclusive.
We discovered that contractility of KO VSMCs could be enhanced by the nongenomic effect of estrogen. This is consistent with the finding that KO VSMCs presented augmented GPER expression (18, 39, 41). With that said, our results could not rule out the involvement of the genomic effect of estrogen on KO VSMC contractility.
The effect of estrogen on enhancing KO VSMC contractility might have relevance to our current health problems: estrogen levels in males tend to increase in industrialized countries. This is caused by two factors. Firstly, adipose tissue is a major periphery source of aromatase activity, which converts testosterone into estrogen (35), hence increased estrogen levels locally (particularly in the perivascular adipose tissue) or systemically in overweight/obese individuals. Secondly, our exposure to environmental xenoestrogens (chemical compounds mimicking endogenous estrogen) in the industrialized world is on the rise (5). Therefore, for male patients with loss-of-function EFNB3 mutation, if their GPER expression is increased as is the case in mice, they will have increased hypertension risk if they are overweight or obese, as would be expected in metabolic syndrome. Similarly, due to exposure to xenoestrogens, living in industrialized countries also poses increased risks of hypertension for males carrying EFNB3 mutations.
There is existing literature related to the inhibitory effect of estrogen on vessel contraction and BP. However, most of such data come from rat or rabbit experiments (16, 21), and such an effect is often attributed to the regulation of nitric oxide production by endothelium (11). Controversies remain in this regard. For example, α2-AR antagonist-induced mouse tail artery constriction is not affected by estrogen (9); estrogen has no effect on the PE-induced constriction in aortic rings from inducible nitric oxide synthase KO mice (56). Furthermore, in women taking estrogen-containing contraceptives, ∼5% will develop de novo hypertension. Possible explanations for such controversy include species differences and the difference in how completely the endothelial cells are removed from the vessels during experimentation. Therefore, we regard the effect of estrogen on BP regulation and VSMC contractility as unresolved controversy. In our VSMC experiments, the cells are totally devoid of endothelial cells, and probably for this reason, the contractility of WT VSMCs was not affected by estrogen; on the other hand, we revealed that KO VSMCs showed increased contractility in the presence of estrogen, suggesting that the EFNB3 deletion will cause increased vessel tone, but this only occurs under a permissive condition, i.e., the presence of estrogen. The in vivo BP findings (Figs. 2 and 5 and Table 2) in female WT and KO mice before and after ovariectomy are basically consistent with the VSMC data: KO females had higher BP than WT females, and KO VSMCs presented higher contractility than WT VSMCs. BP of WT females after ovariectomy was basically unchanged (except an increase in SP, and this is not inconsistent with many previous reports showing some BP-reducing effects of estrogen), and estrogen had no effect on WT VSMC contractility. For KO mice after ovariectomy, their higher BP was lowered to the normal range, consistent with the increased contractility in KO VSMCs treated with estrogen.
For the males, castration led to reduced BP in WT mice. This is consistent with other reports in animal models that testosterone is prohypertension. On the other hand, castration did not change BP in KO mice. The end result was that KO mice after castration had higher BP than their WT counterparts, but such BP difference was caused by reduced BP in castrated WT males, rather than an increase of BP in KO males after castration. These data are not consistent with our VSMC results, which showed that testosterone caused reduced KO VSMC contractility. It is likely that there exists an EFNB3-independent BP regulation by testosterone, due to its effect on various other organs, tissues (including endothelium), or the endocrine system. This EFNB3-independent effect is prohypertensive, as reported by many other studies using WT animals, hence reduced BP in WT males after castration. This BP-reducing effect by castration should also occur in KO males, as it is EFNB3 independent. Therefore, in theory, castration in KO mice should lead to reduced BP. However, this putative BP decrease is likely neutralized by the BP increase due to augmented VSMC contractility caused by EFNB3 deletion and reduced testosterone levels in castrated KO males. The net result is that BP of castrated KO males remained unchanged.
Our additional human genetic study revealed that five single nucleotide polymorphisms in the EFNB3 gene present higher frequency in hypertensive patients than in normotensive ones, and are thus significantly associated with hypertension risks (data not shown). Such data corroborate our findings in the EFNB3 KO mice and demonstrate the relevance of the data from our animal studies to human hypertension. Based on this, we could propose the following therapeutic options.
Elevated estrogen levels, either due to overweight/obesity or environmental xenoestrogens in males, might represent a hypertension risk if they carry the EFNB3 mutations, and estrogen antagonists might be an effective therapy. For females with loss-of-function EFNB3 mutations, avoiding oral contraceptives during their reproduction-active age and estrogen replacement therapy after menopause might reduce their risk of hypertension. Of course, a large amount of clinical study will be needed to validate our proposed therapeutic strategies. For the hypogonadal males with EFNB3 loss-of-function mutations, the net effect of testosterone replacement therapy on BP will be minimal, as although it will reduce the VSMC contractility. Hence, with a reduction in the vessel tone and BP, the EFNB3-independent BP-increasing effect of testosterone will cancel such a beneficial outcome.
Our previous work has shown that several members of the Eph/EFN family (e.g., EphB6 and EFNB1) have default negative impacts on VSMC contractility, as their deletion leads to higher BP (30, 50). This study has added a new member to this category. On the other hand, certain other members of the family, such as EphB4, has defaulted positive impacts on VSMC contractility and its deletion results in reduced VSMC contractility and lower BP (46). Collectively, our research has revealed a previously unknown Eph/EFN-based mechanism for BP regulation. An interesting question is why there needs to be opposing forces from members of the same families to regulate BP. In the biological systems, such opposing forces are widely present. Examples are protein phosphorylation vs. dephosphorylation; calcium influx vs. outflux in a cell; sympathetic vs. parasympathetic neurological activities, etc. Such opposing forces, which can also be called Yin and Yang, provide a mechanism for achieving the rejuvenation or homeostasis of biological processes and systems to cope with ever-changing external environments. Coexisting positive and negative regulatory effects on VSMC contractility by different members of the EPH/EFN family likely provide an additional layer of stabilizing force to maintain BP within an acceptable range. EFN and Eph members are constitutively expressed on VSMCs and engaged with each other constantly. Therefore, they are probably not for rapid regulation of BP or for rejuvenation of each other's signaling pathways, but rather for some chronic fine-tuning of vascular tone.
GRANTS
This work was supported by Canadian Institutes of Health Research Grants MOP57697, MOP69089, and MOP123389 (to J. Wu), MOP97829 (to H. Luo), MOP14496 (to E. Thorin), and ISO106797 (to J. Tremblay). It was also financed by grants from Heart and Stroke Foundation of Quebec, Natural Sciences and Engineering Research Council of Canada (203906-2012), Juvenile Diabetes Research Foundation (17–2013-440), Fonds de Recherche du Quebec-Santé (Ag-06), and the J.-Louis Levesque Foundation (to J. Wu). This study was also supported by National Sciences Foundation of China Grant 81361120264 (to J. Shen, S. Hu, and T. Wu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.W., Z.W., E.T., J.T., J.L.L., H.L., J.P., S.Q., T.W., F.C., and S.H. performed experiments; Y.W., Z.W., E.T., J.T., J.L.L., H.L., and J.S. analyzed data; Y.W. and Z.W. prepared figures; Y.W., Z.W., E.T., J.T., J.L.L., H.L., J.P., S.Q., T.W., F.C., and J.W. approved final version of manuscript; E.T., J.T., J.L.L., and J.P. interpreted results of experiments; J.W. conception and design of research; Y.W. and J.W. drafted manuscript.
ACKNOWLEDGMENTS
We thank Regeneron Pharmaceuticals for generously providing Efnb3 KO mice.
REFERENCES
- 1.Anonymous. Unified nomenclature for Eph family receptors, and their ligands, the ephrins. Eph Nomenclature Committee. Cell 90: 403–404, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251–263, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. FASEB J 13: 123–133, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Brozovich FV. Myosin light chain phosphatase–it gets around. Circ Res 90: 500–502, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Danzo BJ. The effects of environmental hormones on reproduction. Cell Mol Life Sci 54: 1249–1264, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol 4: e315, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong HL, OBrien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: A synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature 386: 279–284, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Dravis C, Wu T, Chumley MJ, Yokoyama N, Wei S, Wu DK, Marcus DC, Henkemeyer M. EphB2 and ephrin-B2 regulate the ionic homeostasis of vestibular endolymph. Hear Res 223: 93–104, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Eid AH, Maiti K, Mitra S, Chotani MA, Flavahan S, Bailey SR, Thompson-Torgerson CS, Flavahan NA. Estrogen increases smooth muscle expression of α2C-adrenoceptors and cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 293: H1955–H1961, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Evans PD, Bayliss A, Reale V. GPCR-mediated rapid, nongenomic actions of steroids: comparisons between DmDopEcR and GPER1 (GPR30). Gen Comp Endocrinol 195: 157–163, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J 10: 615–624, 1996. [PubMed] [Google Scholar]
- 12.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci 21: 309–345, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Giembycz MA, Newton R. Beyond the dogma: novel beta2-adrenoceptor signalling in the airways. Eur Respir J 27: 1286–1306, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Golovina VA, Blaustein MP. Preparation of primary cultured mesenteric artery smooth muscle cells for fluorescent imaging and physiological studies. Nat Protoc 1: 2681–2687, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Gui Y, Zheng XL, Zheng J, Walsh MP. Inhibition of rat aortic smooth muscle contraction by 2-methoxyestradiol. Am J Physiol Heart Circ Physiol 295: H1935–H1942, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto T, Karasawa T, Saito A, Miyauchi N, Han GD, Hayasaka K, Shimizu F, Kawachi H. Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int 72: 954–964, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci 117: 4619–4628, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol 147: 1023–1038, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerkhofs S, Denayer S, Haelens A, Claessens F. Androgen receptor knockout and knock-in mouse models. J Mol Endocrinol 42: 11–17, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Koga M, Hirano K, Nishimura J, Nakano H, Kanaide H. Endothelium-dependent and independent enhancement of vascular contractility in the ovariectomized rabbit. J Soc Gynecol Investig 11: 272–279, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S, Lammert E. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 129: 359–370, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev 15: 877–888, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol 286: F965–F971, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19: 1951–1959, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Q, Lu Z, Liu Q, Guo L, Ren H, Fu J, Jiang Q, Clarke PR, Zhang C. Chromatin-bound NLS proteins recruit membrane vesicles and nucleoporins for nuclear envelope assembly via importin-alpha/beta. Cell Res 22: 1562–1575, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo H, Charpentier T, Wang X, Qi S, Han B, Wu T, Terra R, Lamarre A, Wu J. Efnb1 and Efnb2 proteins regulate thymocyte development, peripheral T cell differentiation, and antiviral immune responses and are essential for interleukin-6 (IL-6) signaling. J Biol Chem 286: 41135–41152, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo H, Wan X, Wu Y, Wu J. Cross-linking of EphB6 resulting in signal transduction and apoptosis in Jurkat cells. J Immunol 167: 1362–1370, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Luo H, Wu Z, Qi S, Jin W, Han B, Wu J. Ephrinb1 and Ephrinb2 are associated with interleukin-7 receptor alpha and retard its internalization from the cell surface. J Biol Chem 286: 44976–44987, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo H, Wu Z, Tremblay J, Thorin E, Peng J, Lavoie JL, Hu B, Stoyanova E, Cloutier G, Qi S, Wu T, Cameron M, Wu J. Receptor tyrosine kinase Ephb6 regulates vascular smooth muscle contractility and modulates blood pressure in concert with sex hormones. J Biol Chem 287: 6819–6829, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo H, Yu G, Tremblay J, Wu J. EphB6-null mutation results in compromised T cell function. J Clin Invest 114: 1762–1773, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo H, Yu G, Wu Y, Wu J. EphB6 crosslinking results in costimulation of T cells. J Clin Invest 110: 1141–1150, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JR, Silver PJ, Stull JT. The role of myosin light chain kinase phosphorylation in beta-adrenergic relaxation of tracheal smooth muscle. Mol Pharmacol 24: 235–242, 1983. [PubMed] [Google Scholar]
- 34.Muranyi A, Derkach D, Erdodi F, Kiss A, Ito M, Hartshorne DJ. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: inhibitory effects and occurrence in A7r5 cells. FEBS Lett 579: 6611–6615, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol 45: S116–124, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Nikolova Z, Djonov V, Zuercher G, Andres AC, Ziemiecki A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J Cell Sci 111: 2741–2751, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Ogita H, Kunimoto S, Kamioka Y, Sawa H, Masuda M, Mochizuki N. EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ Res 93: 23–31, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell 133: 38–52, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol 389: 71–83, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raina H, Zacharia J, Li M, Wier WG. Activation by Ca2+/calmodulin of an exogenous myosin light chain kinase in mouse arteries. J Physiol 587: 2599–2612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roman-Blas JA, Castaneda S, Largo R, Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther 11: 241, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci 10: 301–310, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Kamata R, Yanagihara K, Sakai R. Suppression of gastric cancer dissemination by ephrin-B1-derived peptide. Cancer Sci 101: 87–93, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka M, Kamo T, Ota S, Sugimura H. Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. EMBO J 22: 847–858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93: 741–753, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Thorin E, Luo H, Tremblay J, Lavoie JL, Wu Z, Peng J, Qi S, Wu J. EPHB4 protein expression in vascular smooth muscle cells regulates their contractility, and EPHB4 deletion leads to hypotension in mice. J Biol Chem 290: 14235–14244, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson DG. Eph receptors and ephrins: regulators of guidance and assembly. Int Rev Cytol 196: 177–244, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Luo H. Recent advances on T-cell regulation by receptor tyrosine kinases. Curr Opin Hematol 12: 292–297, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Wu Z, Luo H, Thorin E, Tremblay J, Peng J, Lavoie JL, Wang Y, Qi S, Wu T, Wu J. Possible role of Efnb1 protein, a ligand of Eph receptor tyrosine kinases, in modulating blood pressure. J Biol Chem 287: 15557–15569, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu G, Luo H, Wu Y, Wu J. Ephrin B2 induces T cell costimulation. J Immunol 171: 106–114, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Yu G, Luo H, Wu Y, Wu J. EphrinB1 is essential in T-cell-T-cell co-operation during T-cell activation. J Biol Chem 279: 55531–55539, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Yu G, Luo H, Wu Y, Wu J. Mouse ephrinB3 augments T-cell signaling and responses to T-cell receptor ligation. J Biol Chem 278: 47209–47216, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Yu G, Mao J, Wu Y, Luo H, Wu J. Ephrin-B1 is critical in T-cell development. J Biol Chem 281: 10222–10229, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4: 111–121, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295: 505–508, 2002. [DOI] [PubMed] [Google Scholar]