We have investigated the effects of aging on the heart using a genetically uniform large animal model, maintained under highly regulated conditions. Our results indicate that the myocyte compartment undergoes physiological alterations with age that negatively interfere with ventricular function.
Keywords: aging, myocardium, contractile reserve
Abstract
Studies of myocardial aging are complex and the mechanisms involved in the deterioration of ventricular performance and decreased functional reserve of the old heart remain to be properly defined. We have studied a colony of beagle dogs from 3 to 14 yr of age kept under a highly regulated environment to define the effects of aging on the myocardium. Ventricular, myocardial, and myocyte function, together with anatomical and structural properties of the organ and cardiomyocytes, were evaluated. Ventricular hypertrophy was not observed with aging and the structural composition of the myocardium was modestly affected. Alterations in the myocyte compartment were identified in aged dogs, and these factors negatively interfere with the contractile reserve typical of the young heart. The duration of the action potential is prolonged in old cardiomyocytes contributing to the slower electrical recovery of the myocardium. Also, the remodeled repolarization of cardiomyocytes with aging provides inotropic support to the senescent muscle but compromises its contractile reserve, rendering the old heart ineffective under conditions of high hemodynamic demand. The defects in the electrical and mechanical properties of cardiomyocytes with aging suggest that this cell population is an important determinant of the cardiac senescent phenotype. Collectively, the delayed electrical repolarization of aging cardiomyocytes may be viewed as a critical variable of the aging myopathy and its propensity to evolve into ventricular decompensation under stressful conditions.
NEW & NOTEWORTHY
We have investigated the effects of aging on the heart using a genetically uniform large animal model, maintained under highly regulated conditions. Our results indicate that the myocyte compartment undergoes physiological alterations with age that negatively interfere with ventricular function.
studies of myocardial aging in humans are complex, as it is difficult to separate the effects of time from genetic, ethnic, lifestyle, and environmental factors, which may modify physiological cardiac aging. Additionally, the incidence of cardiovascular diseases increases with age (24) and intervening pathologies may change the natural temporal evolution of the organ. Therefore, the mechanisms involved in the age-related deterioration of ventricular performance and decreased functional reserve of the old heart (9, 12, 17, 18, 39) remain to be properly defined.
The general belief has been that abnormalities in ventricular compliance with age occur as a result of collagen deposition and diffuse interstitial fibrosis, which, together with cardiomyocyte loss, lead to depressed systolic and diastolic function (17, 18, 26, 39). Additionally, myocardial hypertrophy has been proposed as a critical variable of the senescent myopathy (5), in spite of the lack of organ hypertrophy in older humans (25). Current understanding of the pathophysiology of the aging myopathy and the mechanisms involved in the increased incidence of heart failure and sudden death in older adults is limited and a characterization of the process is needed.
In the current study, a major effort was made to acquire information on a genetically uniform, large animal model maintained under controlled conditions during the organism lifespan. This research aimed at defining physiological defects of the senescent heart and recognizing novel targets for the management of the aging myopathy. A large colony of beagle dogs, raised and kept in a highly regulated environment, has been investigated at multiple levels. A comprehensive approach has been introduced to integrate information on global ventricular performance with data on the electromechanical properties of the myocardium and individual cardiomyocytes. This analysis has been combined with measurements of the size and shape of the organ, structural composition of the myocardium, myocyte death, and cell volume and number to recognize determinants of the aging myopathy and their potential implications in the development of ventricular dysfunction.
METHODS
In vivo studies.
Beagle dogs of either sex ranging from 3 to 14.3 yr of age (n = 41) were maintained and studied in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals; animal experiments were approved by the local animal care committee (Institutional Animal Care and Use Committee at New York Medical College, Albert Einstein College of Medicine, and Lovelace Respiratory Research Institute). Fifteen beagle dogs were chronically instrumented for evaluation of cardiac function, as previously described (30, 33, 56). Cardiomyocytes obtained from male hound type dogs 7–9 mo old (n = 4) were employed to test the rate dependency of the late Na+ current (INaL) by patch-clamp technique in voltage-clamp mode. Data obtained from these animals were not included in comparisons with results from beagle dogs.
Beagle dogs were raised and kept at Lovelace Respiratory Research Institute (LRRI) for a period of time averaging 85.6 ± 1.9% of their life. Animals were on a 2025C Certified (Harlan) diet before death, with the exception of one dog on k/d prescription diet (Hill's) due to suspected renal disease. This animal was not included in physiological tests. For the determination of cardiac troponin I (cTnI) and blood chemistry screen, blood samples were collected by jugular vein puncture into a serum separator tube and allowed to clot for ∼30 min. Blood samples were centrifuged at 1,200 g followed by collection of serum and freezing at −80°C. Samples were shipped on dry ice to Minneapolis Medical Research Foundation (Minneapolis, MN) for analysis of cTnI using TnI-Ultra assay (Siemens Advia Centaur) or to Antech Diagnostic (Irvin, TX) for blood chemistry screening.
Surgical instrumentation.
Dogs were sedated with acepromazine maleate (1 mg/kg body wt im), anesthetized with propofol (4 mg/kg body wt iv), and ventilated with room air. Anesthesia was maintained by 2% isoflurane. Body temperature was monitored every 15 min with a rectal thermometer, together with electrocardiograms, oxygen saturation, blood pressure, and fluid intake. The chest of each dog was scrubbed with a sterilizing soap and sterilized with iodine solution. A thoracotomy was performed in the fifth intercostal space. A fluid-filled Tygon catheter (Cardiovascular Instruments) was inserted into the descending thoracic aorta for measurement of mean arterial blood pressure and blood sampling; another fluid-filled Tygon catheter was inserted into the left atrial appendage for delivery of drugs; a silicon catheter was placed in the coronary sinus for blood sampling. Blood gases and lactate were measured with a blood gas analyzer (IL-682 CO-Oximeter) (46). A solid-state pressure gauge (P4; Konigsberg Instruments) was implanted into the left ventricle (LV) through the apex for measurement of LV pressure. Two myocardial pacing leads were attached to the LV free wall. Two pairs of piezoelectric crystals were implanted in the mid-myocardium of the LV free wall, orthogonal to the ventricular long axis, 10–15 mm apart, to assess regional circumferential shortening. The chest was closed in layers, and catheters and wires were tunneled subcutaneously and externalized through the skin in the interscapular region. Antibiotics were given after surgery, and dogs were allowed to fully recover for 10–14 days. Animals were then trained to lay quietly on the laboratory table. Dogs laid undisturbed, either awake or asleep, during the experiment.
Hemodynamics, LV regional shortening, echocardiographic, and electrocardiographic recordings.
To ensure controlled conditions of animals, body temperature was evaluated in dogs on the day of data collection and heart rate, electrocardiograms, and mean arterial pressure were monitored during data acquisition. Hemodynamic parameters were recorded on paper and also stored in computer memory at a sampling rate of 250 Hz. The piezoelectric crystals were connected to a sonomicrometer to measure cyclic changes in the segmental length. This parameter served as an index of regional contractile function. Two-dimensional and M-mode echocardiography was performed (Sequoia C256; Acuson) to measure ejection fraction, LV dimensions, and wall thickness. Images were obtained from a right parasternal approach at the midpapillary muscle level, according to the criteria of the American Society of Echocardiography. Increasing doses of dobutamine were administered intravenously, followed by isoproterenol bolus. For treadmill test, animals were subjected to progressive faster running speed, ranging from 1–4 miles per hour. Twelve-lead electrocardiograms were obtained in aged dogs using a HP Hewlett Packard 4760A ECG EKG Cardiograph (16).
At completion of the protocol, dogs were euthanized with an overdose of sodium pentobarbital. The heart was explanted and cardioplegia was flushed through the aorta with a 20-ml syringe. Subsequently, the heart was stored in cold cardioplegic solution and shipped from New York Medical College (Valhalla, NY) to the Brigham and Women's Hospital (Boston, MA). Similarly, hearts from hound type dogs were shipped from Montefiore Medical Center, Albert Einstein College of Medicine (New York, NY) to the Brigham and Women's Hospital.
Immunohistological analysis.
LV myocardial tissue was fixed in phosphate-buffered formalin (10%, Sigma) and embedded in paraffin. Sections, 4-μm thick, were trichrome-stained for detection of connective tissue (Gomori's One Step Trichrome Method; Poly Scientific R&D) following manufacturer's instructions. Images were acquired using an upright microscope (Olympus BX60) with ×20 objective equipped with a color camera (Olympus DP73). Interstitial fibrosis was quantified with respect to total tissue area using ImageJ software. Apoptosis in myocyte nuclei was evaluated by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay in tissue sections stained with α-sarcomeric actin (mouse monoclonal antibody, Sigma), DAPI (Sigma), and a TUNEL-based assay kit (ApoAlert DNA Fragmentation Assay Kit). On average, ∼38,000 myocyte nuclei were analyzed per animal. Images were obtained with a confocal microscope (Olympus Fluoview FV1000).
Ex vivo electrical properties of LV myocardium.
LV samples were perfused through a large branch of the left coronary artery in a Langendorff apparatus at a constant pressure of 80 mmHg with Krebs-Henseleit buffer (KHB) containing the following (in mM): 118 NaCl, 4.7 KCl, 11 glucose, 1.2 MgSO4, 1.2 KH2PO4, 1.8 CaCl2, and 25 NaHCO3, gassed with 95% O2-5% CO2 (pH 7.4) (41, 42). The temperature was maintained at 37°C by immersing the myocardial tissue in a water-heated glassware reservoir (Radnoti), containing preheated KHB. LV myocardium was stimulated with a 2-ms square pulse at two times its threshold level (4 channels stimulator; BMS 414; Crescent Electronics), using a minicoaxial electrode placed on the endocardium (Harvard Apparatus). Myocardial electrical activity was monitored employing a two-lead mini ECG system (Harvard Apparatus), in which electrodes were placed on the endocardial and epicardial aspects of the canine LV sample. Monophasic action potentials (MAPs) were recorded using a micro MAP-Tip electrode (Harvard Apparatus) (41, 42). ECG and MAP signals were amplified (Animal Bio Amp; ADInstruments), digitized using a 4 kHz A/D converter (Power Lab 8/30; ADInstruments), and recorded using LabChart 7 Pro software (ADInstruments) with low- and high-pass filtering at 1 kHz and 0.3 Hz, respectively. Amplitude of extracellular electrical signals was influenced by quality of contact of electrodes to myocardial specimens. Overall, QRS amplitude was comparable in adult (<10 yr of age, 2.1 ± 0.6 mV) and old (>10 yr of age, 2.0 ± 0.6 mV) LV tissue. Also, MAP amplitude was similar in tissue from adult (<10 yr of age, 8.1 ± 2.1 mV) and old (>10 yr of age, 7.8 ± 1.5 mV) animals. The effects of pharmacological agents on electrical properties of the perfused myocardium were tested at fixed cycle lengths.
Myocyte isolation.
LV samples were perfused through the left anterior descending coronary artery using a Langendorff apparatus (42). Initially, the tissue was perfused with a solution containing the following (mM): 126 NaCl, 4.4 KCl, 5 MgCl2, 5 HEPES, 22 glucose, 20 taurine, 5 creatine, 5 Na pyruvate, 5 NaH2PO4, and 10 2,3-butanedione monoxime (pH 7.4, adjusted with NaOH). A constant temperature of 37°C was maintained, and the buffer was gassed with 85% O2-15% N2. After ∼10 min, 0.015 mM CaCl2, 274 units/ml collagenase (type 2; Worthington Biochemical), and 0.57 units/ml protease (type XIV; Sigma) were added to the perfusate for enzymatic dissociation of the tissue. At completion of digestion, LV myocardium was cut in small pieces and these fragments were shaken in resuspension solution and filtered using a 200-μm nylon mesh (Spectrum Labs). Aliquots of cell suspensions were centrifuged for 5 min at 100 g (Eppendorf 5702 R) at 4°C and frozen for biochemical assays. For electrophysiological and mechanical studies, only rod-shaped myocytes exhibiting cross striations and showing no spontaneous contractions or contractures were selected.
Myocyte number, volume, and nucleation.
Following enzymatic digestion, LV myocytes were fixed in paraformaldehyde (4%; Electron Microscopy Sciences) and nuclei were stained with Hoechst (10 μM; Sigma). Images were collected with a fluorescent inverted microscope (Olympus IX71) equipped with a CCD camera (Hamamatsu ORCA-R2). Cell length (long axis), area, and number of nuclei were evaluated using ImageJ software. Average cell width was computed by dividing cell area by the myocyte length (long axis). Cell thickness (Z-axis) was evaluated in a subset of myocytes using images acquired by two-photon microscopy (BX51WI Olympus microscope coupled with a Bio-Rad Radiance 2100MP system). Three-dimensional reconstructions were obtained using second-harmonic generation signal of sarcomeric structures (32, 41). The ratio between cell width and cell thickness was calculated and found to be comparable in myocytes from adult and old dogs. A value of 1.81 as ratio between cell width and thickness was employed in the morphological computation. Cell volume was calculated assuming the shape of myocytes as a flattened cylinder with elliptical cross section. In the crosssection, the major axis corresponds to the average cell width and the minor axis (cell thickness, Z-axis) corresponds to average cell width divided by 1.81. Myocyte volume was calculated in cells obtained from dogs at 4, 7–8, and 12–14 yr of age. The relation between dog age and average myocyte volume was established by fitting the calculated average volumes with linear regression.
The number of myocytes of individual hearts was determined by converting weight to mass employing myocardium specific gravity of 1.04 mg/ml; then, myocyte volume fraction of the myocardium was calculated based on microscopic examination and point-counting technique of myocytes and nonmyocytes in 10 random fields of 4-μm-thick sections stained by hematoxylin and eosin, using an upright microscope (Olympus BX60) with ×100 objective. The number of myocytes was calculated by dividing myocyte mass of the heart by the computed average myocyte volume.
Patch-clamp studies.
Isolated LV myocytes were placed in the bath on the stage of inverted microscopes (IX51 and IX71; Olympus) for patch-clamp measurements (36, 42). Experiments were conducted at 37°C and, for a subset of data, at room temperature. Data were acquired by means of the whole cell patch-clamp technique in voltage- and current-clamp modes using Multiclamp 700A and 700B amplifiers (Molecular Devices). Electrical signals were digitized using 250-kHz 16-bit resolution A/D converters (Digidata 1322 and 1440A; Molecular Devices) and recorded using pCLAMP 9.0 and 10 software (Molecular Devices) with low-pass filtering at 2 kHz. Pipettes were pulled by means of a vertical (PB-7; Narishige) or horizontal (P-1000; Sutter Instruments) glass microelectrode pullers; when filled with intracellular solution, pipettes had a resistance of 1–2 MΩ. Membrane capacitance (Cm) was measured in voltage-clamp mode using a 5-mV voltage step and pCLAMP software algorithm (41, 42). Cm was comparable in myocytes obtained from adult (<10 yr of age, 129.6 ± 7.4 pA/pF, n = 38) and old (>10 yr of age, 112.7 ± 5.2 pA/pF, n = 39) hearts.
For action potential measurements, current-clamp mode was employed (36, 42). Cells were stimulated with current pulses 1.5 times threshold. Myocytes were bathed with Tyrode solution containing the following (in mM): 140 NaCl, 5.4 KCl, 1 MgCl2, 5 HEPES, 5.5 glucose 5.5, and 1 CaCl2 (pH 7.4, adjusted with NaOH). The composition of the pipette solution was the following (in mM): 10 NaCl, 113 KCl, 0.5 MgCl2, 5 K2-ATP, 5.5 glucose, 10 HEPES, 10 EGTA, and 1 CaCl2 (pH 7.2 with KOH). INaL was studied in voltage-clamp mode (36, 43, 53). Myocytes were bathed with a modified Tyrode solution in which KCl was replaced with CsCl and 4 μM nicardipine were added to block L-type Ca2+ current. Composition of the pipette solution was the following (in mM): 10 NaCl, 113 CsCl, 0.5 MgCl2, 5 Tris-ATP, 5.5 glucose, 10 HEPES, 5 EGTA, and 20 tetraethylammonium chloride (pH 7.2 with CsOH). Myocytes were held at Vh of −120 mV and INaL was elicited using a 500-ms depolarizing pulses to −30 mV. The pulse was preceded by a 5-ms prepulse to +50 mV to optimize voltage control (43). Measured currents were normalized by Cm.
For simultaneous measurement of electrical and Ca2+ cycling properties in myocytes, cells were loaded with 10 μM Fluo-3 AM (Invitrogen). Excitation length was 480 nm with emission collected at 535 nm, and the fluorescence signal intensity of Fluo was collected using a photomultiplier, and a photon to voltage converter (IonOptix) connected to the patch-clamp A/D converter (42). EGTA and Ca2+ were omitted from the pipette solution.
INaL was blocked with low dose of tetrodotoxin (TTX; 1 μM; Sigma), mexiletine (10 μM; Sigma), or ranolazine (10 μM; Sigma) (10, 40, 50, 53). When possible, tests were repeated with different inhibitors to minimize off-target effects of these compounds (23, 27). The effects of pharmacological agents on electrical and Ca2+ transient properties of myocytes were tested at fixed cycle lengths.
Cell shortening.
Isolated LV myocytes were placed in a bath on the stage of a microscope (Axiovert, Zeiss; BH-2, Olympus) for the evaluation of cell contractility (42). Experiments were conducted at 37°C. Cells were bathed continuously with a Tyrode solution containing the following (in mM): 140 NaCl, 5.4 KCl, 1 MgCl2, 5 HEPES, 5.5 glucose, and 1.8 CaCl2 (pH 7.4, adjusted with NaOH). Measurements were collected in field-stimulated (S88 and SD9 Grass stimulators) cells paced at 0.25–0.5 Hz by IonOptix contractility systems (IonOptix) or by video edge detection (VED-205; Crescent Electronics; PowerLab 8/35; AdInstruments) (41). Contractions were elicited by rectangular depolarizing pulses, 2 ms in duration, and 1.5 times threshold in intensity, with platinum electrodes. The effects of mexiletine (10 μM) on myocytes contractility were tested at fixed cycle lengths.
Isometric force in ventricular trabeculae carneae.
Thin ventricular trabeculae obtained from the right ventricle were mounted in a horizontal tissue bath (Steiert; Hugo Sachs Elektronik-Harvard Apparatus) connected to a force transducer (F10; Harvard Apparatus) (42). Muscles were superfused with KHB solution with composition identical to the solution employed to perfuse LV tissue. Experiments were conducted at 37°C. The myocardium was stimulated by two platinum electrodes employing field stimulation (isolated stimulator output: pulse duration, 2 ms; intensity, 1.5-fold threshold; UISO; Hugo Sachs Elektronik-Harvard Apparatus). Each muscle was stretched to the length at which force of contraction was maximal. Muscle preparations were allowed to equilibrate for at least 1 h. Developed tension was measured isometrically with the force transducer attached to a Bridge Amp (ADInstruments) and a 4-kHz A/D converter (Power Lab 4/30; ADInstruments). Tension signal was recorded using LabChart 7 Pro software (ADInstruments). Based on the premise that each muscle had a cylindrical shape, force measurements were normalized by the cross-sectional area of the muscle (mN/mm2). Digital images of sections were acquired using a micro-zoom system stereo microscope (SZX16, Olympus) and a camera (DP73) and analyzed by ImageJ software (42). The length at which the muscle developed maximal twitch tension (Lmax) was identified by progressively stretching muscles stimulated at a cycle length of 400 ms, beginning from near slack length (L0). The late Na+ current was blocked with mexiletine (10 μM; Sigma) or a low dose of tetrodotoxin (1 μM; Sigma; Enzo Life Sciences) or ranolazine (10 μM; Sigma) or enhanced with anemonia toxin II (ATX-II; 10 nM; Sigma) (7, 40, 50). β-Adrenergic stimulation was induced with isoproterenol (100 nM) (42). The effects of pharmacological agents on isometric twitching muscles were tested at fixed cycle lengths.
Quantitative RT-PCR.
Total RNA was extracted from snap-frozen LV myocardial tissue utilizing QIAzol Lysis Reagent (Qiagen) and digested with DNase I and cleaned up with RNeasy Mini Kit (Qiagen) to eliminate genomic DNA. cDNA was obtained from 1 μg total RNA using MultiScribe reverse transcriptase kit (Applied Biosystems). Real-time RT-PCR was performed with primers indicated in Table 1. The StepOnePlus Real-Time PCR system (Applied Biosystems) was employed for quantitative RT-PCR. In each case, cDNA was combined with Power SYBR Green Master Mix (Applied Biosystems) in a 20-μl reaction. Cycling conditions were as follows: 95°C for 10 min followed by 40 cycles of amplification (95°C denaturation for 15 s, 60°C annealing and extension for 1 min). The melting curve was then obtained. Ct values were normalized with respect to hypoxanthine guanine phosphoribosyl transferase (HPRT). To avoid the influence of genomic contamination, forward and reverse primers for each gene were located in different exons.
Table 1.
Primers for PCR analysis
| Gene/Primers |
|---|
| Dog COL1A1 |
| F: 5′-AGAGGAGGGCCAAGAAGAAG-3′ |
| R: 5′-CTCGGGTTTCCATACGTCTC-3′ |
| Dog COL1A2 |
| F: 5′-GCCCAGTATGATGGAAAAGG-3′ |
| R: 5′-CAGGTCCTTGGAAACCTTGA-3′ |
| Dog COL3A1 |
| F: 5′-TCCTGGTATTCCTGGGAGAA-3′ |
| R: 5′-TAGGACTCGAACTGGGGAGA-3′ |
| Dog TGFB1 |
| F: 5′-GAGCAGCATGTGGAGCTGTA-3′ |
| R: 5′-CACGACTCCAGTGACATCAAA-3′ |
| Dog MMP2 |
| F: 5′-CACGGCCAACTATGATGATG-3′ |
| R: 5′-CAGAATGCTCCAGTCCCATT-3′ |
| Dog POSTN |
| F: 5′-GCCATCTGTGGAAGGAAAAC-3′ |
| R: 5′-TCCCACAATGCCCAGAGTA-3′ |
| Dog FN1 |
| F: 5′-GAGGGGAGTGGAAGTGTGAA-3′ |
| R: 5′-AGTAAACCACGCCACTGTCC-3′ |
| Dog CTGF |
| F: 5′-TGTGAAGCTGACCTGGAAGA-3′ |
| R: 5′-CCACAGAACTTAGCCCGGTA-3′ |
| Dog HPRT |
| F: 5′-CAGACTTTGCTTTCCTTGGTCA-3′ |
| R: 5′-CAGGTTTATAGCCAACACTTCGA-3′ |
F, forward; R, reverse; COL1A1, collagen type I α1; COL1A2, collagen type I α2; COL3A1, collagen type III α1; TGFB1, transforming growth factor-β1; MMP2, matrix metalloproteinase-II; POSTN, periostin; FN1, fibronectin; CTGF, connective tissue growth factor; HPRT, hypoxanthine guanine phosphoribosyl transferase.
Western blotting.
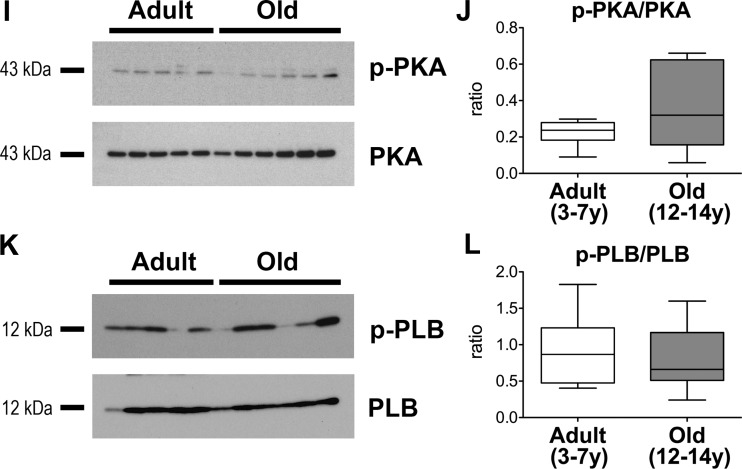
Whole protein extracts from LV myocardium were prepared using RIPA buffer (Sigma), supplemented with a cocktail of protease inhibitors (Roche) and phosphatase inhibitors (Sigma). The equivalent of 1–10 μg of proteins was separated on SDS-PAGE, transferred onto PVDF membrane, blocked with 5% BSA, and exposed to PKA (dilution 1:1,000; rabbit monoclonal antibody no. 5842; Cell Signaling Technologies), phospho-PKA [Phospho-PKA C (Thr197) (D45D3); dilution 1:1,000; rabbit monoclonal antibody no. 5661; Cell Signaling Technologies], phospholamban (dilution 1:2,000; rabbit phospholamban antibody no. 8495; Cell Signaling Technologies), and phospho-phospholamban [dilution 1:2,000; Phospho-Phospholamban (Ser16/Thr17) rabbit antibody no. 8496; Cell Signaling Technologies] antibodies. Horseradish peroxidase-conjugated secondary antibodies (1:5,000 dilution; Cell Signaling Technology) and Pierce ECL 2 Western Blotting chemiluminescent substrate (Thermo Scientific) were utilized for signal detection (41, 42). Western blotting protocols with various antibodies were optimized before quantitative analysis. For molecular weight identification, Precision Plus Protein Kaleidoscope Prestained Protein Standards (Bio-Rad) was employed. Optical density of bands was measured using ImageJ.
Data analysis.
Data are presented as means ± SE or median and interquartile ranges. Linear regressions were calculated with Prism 6.0c software. For comparison of data pertaining to adult (4 to 7 yr of age) and old (>10 yr of age) dogs, median and interquartile ranges for animal age in each group are reported in Fig. 1. Statistical analysis was performed using SigmaPlot 11.0 software. Data were initially tested for normality (Shapiro-Wilk) and equal variance for assignment to parametric or nonparametric analysis. Parametric test included t-test or ANOVA followed by Bonferroni test for nonpaired comparison between two or among multiple groups, respectively. For paired statistical analysis, paired t-test or one-way repeated-measures ANOVA followed by Bonferroni test were employed for two groups or multiple comparisons, respectively. When normality or equal variance were not met, nonparametric analysis was performed using Mann-Whitney rank sum test or Kruskal-Wallis one-way ANOVA on ranks followed by Dunn's method, for nonpaired comparison between two or among multiple groups, respectively. Wilcoxon signed rank test or Friedman repeated-measures ANOVA on ranks was employed for paired comparison between two or among multiple groups, respectively (41). P < 0.05 was considered significant.
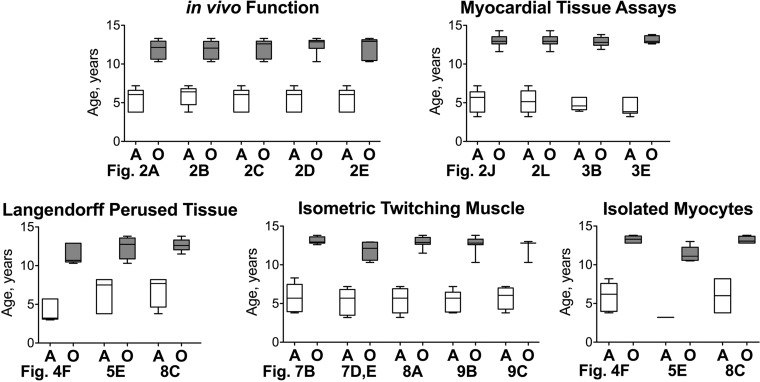
Fig. 1.
Age of adult (A) and old (O) dogs employed in various tests is shown as median and interquartile ranges. References to figure panels are reported.
RESULTS
Physiological aging alters ventricular performance.
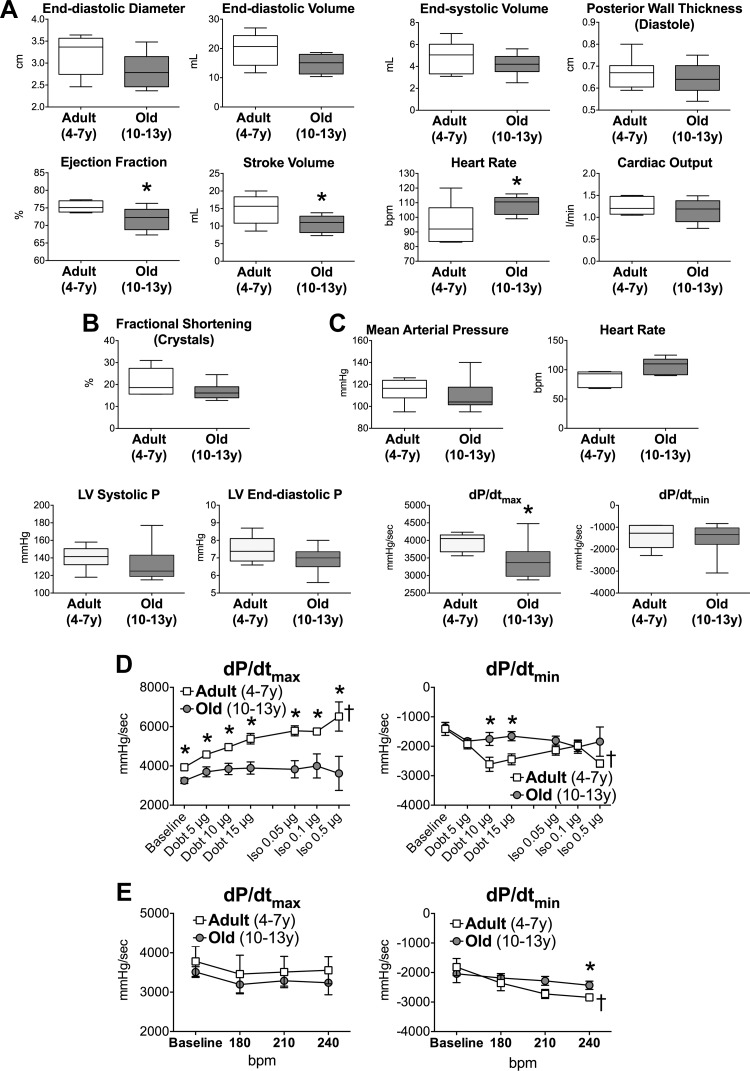
To define the effects of aging on LV function, beagle dogs were divided in adults, from 4 to 7 yr of age, and old, from 10 to 13 yr of age (see Fig. 1). Based on a previous report (29), these ranges correspond to 37–52 and 67–83 in human year equivalents, respectively. Animals were instrumented to assess cardiovascular parameters in the conscious state (30). LV wall thickness, internal diameter, and chamber volume were similar in adult and old dogs. Although circumferential fractional shortening and LV end-diastolic and systolic pressures were preserved with aging, stroke volume, maximal rate of LV isovolumic contraction (dP/dtmax), and ejection fraction (EF) were decreased in old animals (Fig. 2, A–C). Importantly, the significant increase in heart rate in old dogs appears to contribute to the slightly lower LV filling volume, decreased stroke volume, and preservation of cardiac output. The in vivo infusion of the β-adrenergic agonist dobutamine, followed by isoproterenol bolus, revealed that the inotropic and lusitropic response of the senescent heart to catecholaminergic-like stimulation was attenuated with age; these defects were observed in part with the overdrive maneuver (Fig. 2, D and E). Moreover, three of the seven old dogs, but only one of eight adult animals, failed to complete the treadmill protocol.
Fig. 2.
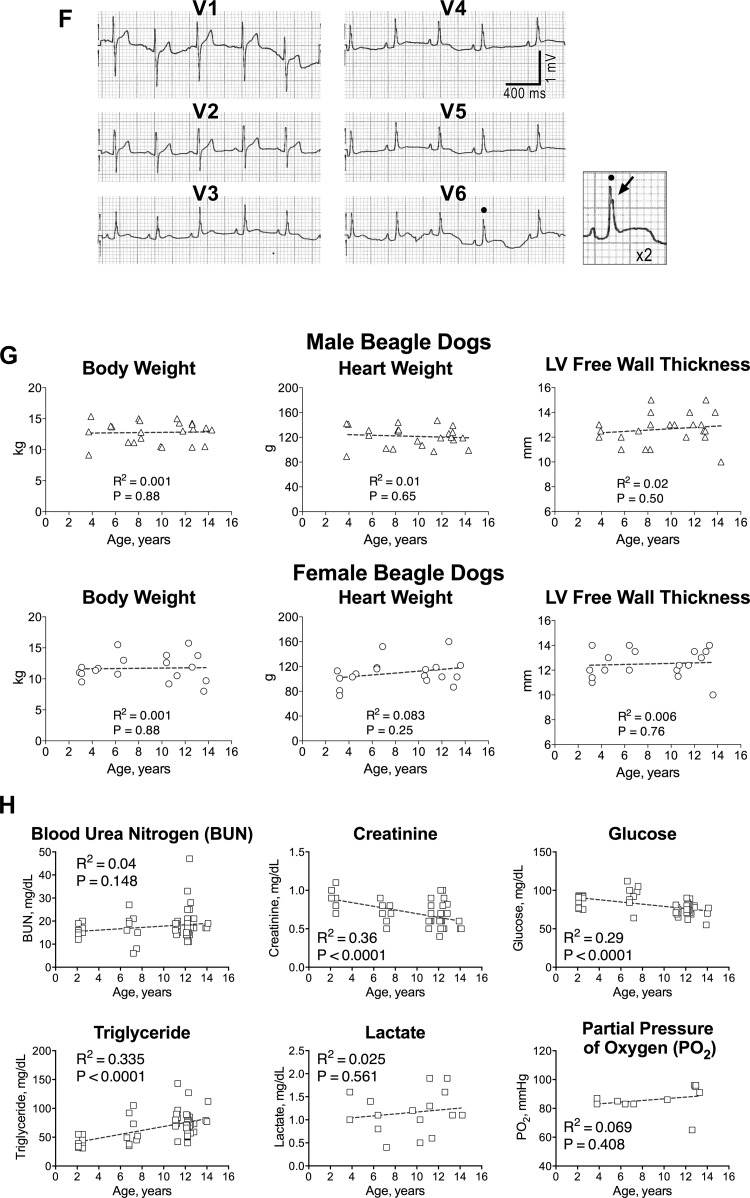
Aging negatively interferes with cardiac function. A: echocardiographic parameters in aging dogs; data are shown as median and interquartile ranges (adult: n = 6, 5.5 ± 0.6 yr; old: n = 8, 11.9 ± 0.4 yr). *P < 0.05 vs. adult; y, yr of age. B: radial left ventricular (LV) contractility in aging dogs; data are shown as median and interquartile ranges (adult: n = 5, 5.9 ± 0.6 yr; old: n = 8, 11.8 ± 0.4 yr). *P < 0.05 vs. adult. C: hemodynamic parameters in aging dogs; data are shown as median and interquartile ranges (adult: n = 6, 5.5 ± 0.6 yr; old: n = 9, 12.0 ± 0.4 yr). *P < 0.05 vs. adult. D: maximal rate of LV contraction and relaxation in aging dogs following infusion of dobutamine (Dobt, dosage referred to kg of body wt per minute of infusion) and bolus of isoproterenol (Iso, dosage referred to kg of body wt) are shown as mean ± SE (adult: n = 6, 5.5 ± 0.6 yr; old: n = 6, 12.5 ± 0.4 yr). *P < 0.05 vs. adult; †P < 0.001 within the same group E: effects of overdrive pacing are shown as mean ± SE (adult: n = 6, 5.5 ± 0.6 yr; old: n = 5 12.0 ± 0.6 yr). *P < 0.05 vs. adult; †P < 0.01 within the same group. F: ECGs from precordial leads obtained in a dog at 11 yr of age. Notched R waves are present in V6. Arrow points to the notched R wave in the magnified trace. G: gross anatomical parameters in aging dogs. Body weight before death and heart weight and LV free wall thickness measured in the explanted organ (male: n = 21; female: n = 18). Data are fitted with linear regression. R2 and P value for each fitting are reported. H: biochemical parameters of blood samples obtained from the jugular vein, with the exception of lactate and partial pressure of oxygen, measured in blood collected from the coronary sinus. Data are fitted with linear regression. R2 and P values for each fitting are reported. I–L: Western blots analysis for total and phosphorylated PKA and phospholamban (PLB) in the LV myocardium of aging dogs. Quantitative data for p-PKA/PKA ratio (J) in the LV myocardium of adult (n = 11, 5.2 ± 0.4 yr) and old (n = 12, 12.9 ± 0.2 yr) beagle dogs are shown as median and interquartile ranges. Quantitative data for p-PLB/PLB ratio (L) in the LV myocardium of adult (n = 10, 5.2 ± 0.5 yr) and old (n = 12, 12.9 ± 0.2 yr) beagle dogs are shown as median and interquartile ranges.
The presence of left bundle branch block increases with age and is likely to be associated with cardiovascular disease (39). Thus 12-lead ECGs were obtained in a cohort of six dogs at 10–14 yr of age. In two out of the six old animals a notched R wave was observed in lead V6, together with a relatively deep S wave in V1 and V2 (Fig. 2F). QRS duration, measured in lead II, averaged 70.6 ± 3.3 ms and reached 85 ms in one of the two dogs with notched R wave. These findings suggest that conduction delays occur in aged animals possibly contributing to alteration in mechanical function (1, 2).
Body weight, heart weight, and thickness of the LV free wall were preserved with age (Fig. 2G). By biochemical analysis of blood samples, blood urea nitrogen was maintained, whereas creatinine and glucose decreased and level of triglyceride increased in old dogs (Fig. 2H). Also lactate and partial pressure of O2 in blood samples from the coronary sinus were not affected by age. These data suggest that renal function (4, 28) is preserved in aging beagles and metabolic alterations are in place. Additionally, LV myocardium was examined for general molecular markers related to the cyclic AMP (cAMP)/cAMP-dependent protein kinase (PKA) pathway, critical in modulating myocardial function. Phosphorylation status of the catalytic subunit of PKA and Ca2+ handling protein phospholamban, a target of PKA, were comparable in tissue from adult and old dogs (Fig. 2, I–L) minimizing the possibility that alterations of the cAMP/PKA axis contribute to the observed differences in cardiac function at baseline.
Thus cardiac aging in beagle dogs manifests with abnormalities in ventricular performance and reduced functional reserve in the absence of organ hypertrophy.
Physiological aging has modest effects on the structure of the myocardium.
The contribution of myocyte death to myocardial aging in beagle dogs was then established. Myocyte apoptosis was identified in adult and aged dogs by TUNEL assay and found comparable in the two groups (Fig. 3, A and B). Myocyte necrosis, evaluated by the high sensitivity troponin I assay (19), was detected in adult animals and increased with age (Fig. 3C). These data are consistent with results in humans showing that circulating levels of troponin are commonly found in apparently healthy individuals (19), indicating that myocyte necrosis is an ongoing process and is enhanced with aging and cardiac pathology.
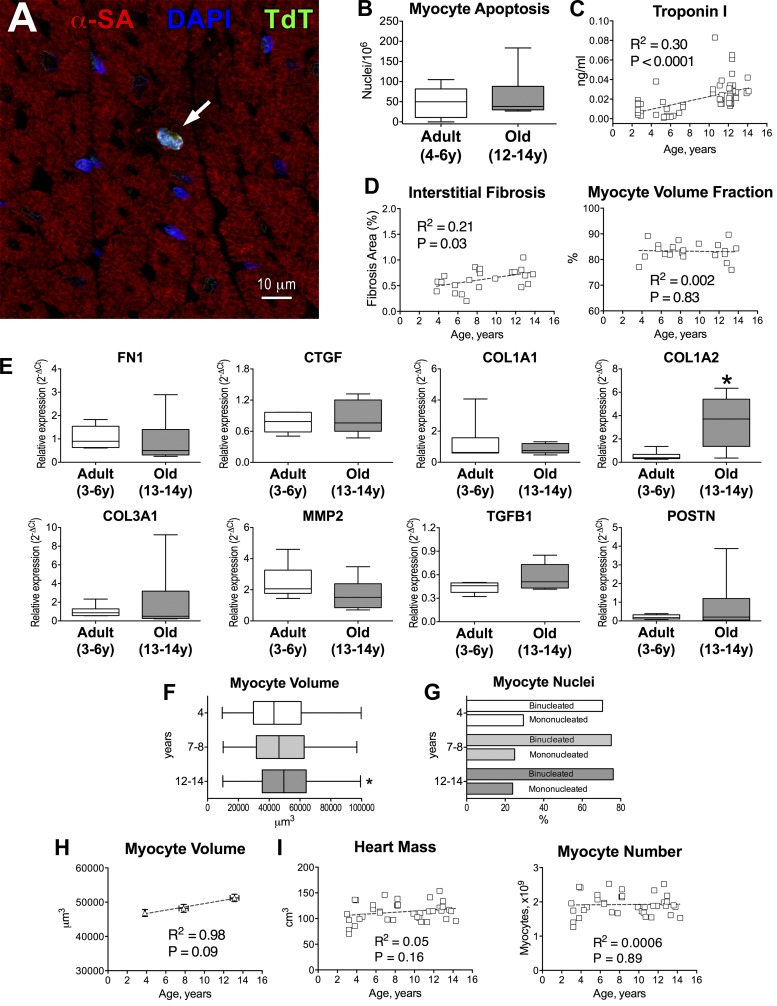
Fig. 3.
Structural properties of the myocardium are modestly affected by aging in beagle dogs. A: apoptotic myocyte (arrow) in the adult canine myocardium by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL; TdT; green) assay. Myocytes are identified by α-sarcomeric actin (α-SA; red). Nuclei are stained by DAPI (blue). B: quantitative data for myocyte apoptosis in the LV myocardium of adult (n = 5, 4.8 ± 0.4 yr) and old (n = 6, 12.9 ± 0.3 yr) beagle dogs are shown as median and interquartile ranges. C: circulating levels of cardiac troponin I in beagle dogs (n = 63). Data are fitted with linear regression. R2 and P values are reported. D: quantitative measurements of interstitial fibrosis (n = 23) and volume fraction (n = 22) of cardiomyocytes. Data are fitted with linear regression. R2 and P values for each fitting are reported. E: quantitative data for level of transcript for fibronectin (FN1), connective tissue growth factor (CTGF), collagen type I α1 (COL1A1), collagen type I α2 (COL1A2), collagen type III α1 (COL3A1), matrix metalloproteinase-II (MMP2), transforming growth factor-β1 (TGFB1), and periostin (POSTN) in the LV of adult and old beagle dogs. Data are shown as median and interquartile ranges (adult: n = 6 specimens, 6 dogs, 4.4 ± 0.4 yr; old: n = 6 specimens, 6 dogs, 13.1 ± 0.2 yr). *P < 0.05 vs. adult. F: volume of cardiomyocytes obtained from beagle dogs at 4, 7–8 and 12–14 yr of age (n = 404 myocytes, 3 dogs, 3.8 ± 0.03 yr; n = 309 myocytes, 4 dogs, 7.9 ± 0.2 yr; n = 360 myocytes, 4 dogs, 13.1 ± 0.2 yr, respectively) is shown as median and interquartile ranges. *P < 0.01 vs. 4 yr. G: fraction of mononucleated and binucleated myocytes for cells shown in F. H: quantitative data for myocyte volume shown in F are reported as mean ± SE and fitted with linear regression. R2 and P values are reported. I: myocardial mass and computed myocyte number in aging beagle dogs (n = 38). Data are fitted with linear regression. R2 and P values for each fitting are reported.
To evaluate the consequences of myocyte death in the aging heart, interstitial fibrosis and the volume fraction of cardiomyocytes were measured quantitatively in animals 4–14 yr of age. Interstitial fibrosis slightly increased with age, although the relative proportion of cardiomyocytes did not change (Fig. 3D). Moreover, gene expression profile of various fibrotic markers (6) was not altered in the aged myocardium. However, collagen type I α2 was significantly increased and transcripts for collagen type III α1 and periostin displayed large variability in old dogs (Fig. 3E).
Myocyte volume, evaluated at 4, 7–8, and 12–14 yr of age, increased minimally (8% at 12–14 yr vs. 4), whereas the fraction of mono- and binucleated myocytes remained constant (Fig. 3, F and G). The relationship between organ age and myocyte volume was then established, and this parameter was employed to compute the number of cardiomyocytes in each heart. As expected by the preservation of the volume fraction of the myocyte compartment and the modest changes in myocyte volume, the aggregate myocyte number was not affected by age (Fig. 3, H and I). Thus cardiac aging in this model is characterized by a largely intact myocardial structure and a modest increase in interstitial fibrosis.
Physiological aging prolongs the electrical recovery of the myocardium.
The anatomical and structural properties of the myocardium have explained only in part the defects in cardiac performance and contractile reserve of the aged heart. Therefore, a detailed electrophysiological analysis of the myocardium was performed. LV tissue was perfused in a Langendorff system to measure transmural pseudo-ECG and monophasic action potentials (MAP). This protocol allowed us to evaluate the repolarization properties of the myocardium at predetermined pacing rates in the absence of circulating factors that may influence the electrical behavior of the cardiac muscle (42, 53).
A progressive prolongation of the QT interval and time to 90% repolarization of local MAP (APD-90) was observed in the aging myocardium (Fig. 4, A–D). In the adult and old muscle, increases in stimulation frequency, from 0.5 to 2.5 Hz, resulted in a progressive shortening of the duration of the QT interval (Fig. 4, E and F). The protracted electrical recovery of the old myocardium was more pronounced at low pacing frequencies, while it decreased progressively at faster rates of stimulation. The reverse rate dependency of the protracted repolarization phase of the old myocardium suggests that cellular alterations are involved in the process, rather than structural abnormalities of the senescent heart.
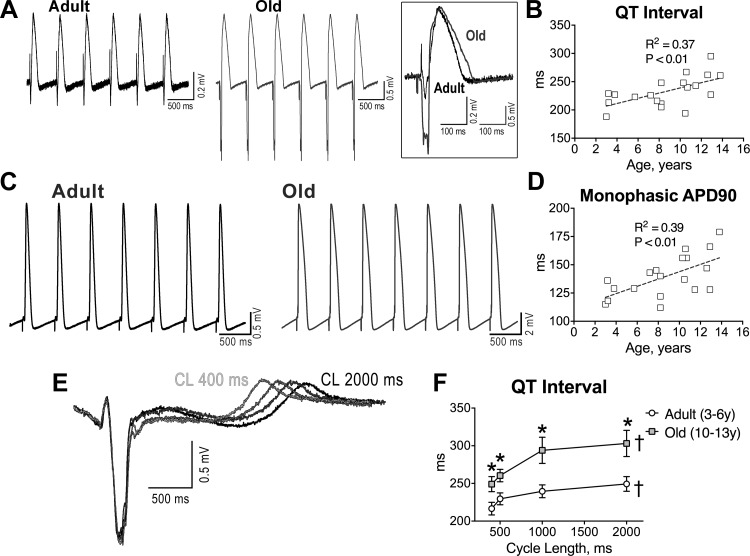
Fig. 4.
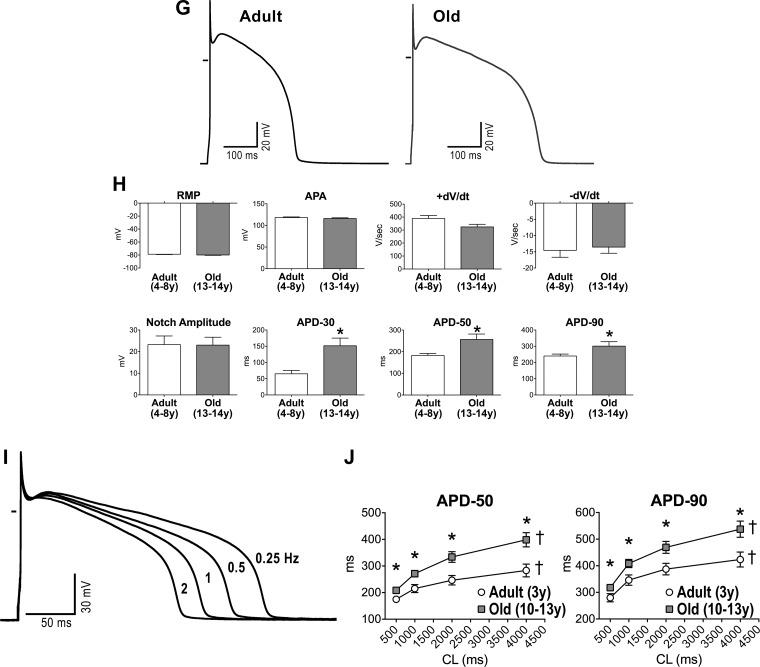
Aging results in prolongation of the electrical recovery of myocardium and myocytes. A: pseudo-ECGs in the perfused adult and old LV myocardium stimulated at 2 Hz. Superimposed traces are shown in the inset. B: quantitative measurements of QT interval duration in the myocardium of beagle dogs (n = 19 muscles, 19 dogs) are fitted with linear regression. R2 and P values are reported. C: monophasic action potentials (MAPs) measured in adult and old LV tissue. D: quantitative measurements of duration of the action potential (AP) at 90% repolarization (APD90; n as in B) are fitted with linear regression. R2 and P values are reported. E: superimposed pseudo-ECGs obtained at different pacing cycle lengths (CL). F: data are shown as mean ± SE (adult: n = 11 muscles, 6 dogs, 3.8 ± 0.4 yr; old: n = 8 muscles, 7 dogs, 11.5 ± 0.4 yr). *P < 0.05 vs. adult; †P < 0.001 for various frequencies within the same group. G: APs of LV myocytes from adult and old dogs. H: data are shown as mean ± SE (adult: n = 22 cells, 8 dogs, 5.6 ± 0.4 yr; old: n = 10 cells, 5 dogs, 13.1 ± 0.1 yr). *P < 0.05 vs. adult. RMP, resting membrane potential; APA, AP amplitude. I: superimposed APs at various pacing frequencies in a LV myocyte from an adult dog. J: data are shown as mean ± SE (adult: n = 16 cells, 2 dogs, 3.2 ± 0 yr; old: n = 26 cells, 6 dogs, 10.9 ± 0.1 yr). *P < 0.05 vs. adult; †P < 0.001 for various frequencies within the same group.
To establish the role of the myocyte action potential (AP) in the repolarization defects of the old myocardium, LV cardiomyocytes were analyzed by patch clamp. The plateau and late repolarization phases of the AP were significantly longer in old myocytes. However, resting membrane potential, amplitude of the AP and phase 1 notch, and maximal and minimal rate of depolarization and repolarization were similar in adult and senescent cardiomyocytes (Fig. 4, G and H). Again, at high stimulation frequencies, repolarization was faster and the differences in AP duration between adult and old cardiomyocytes were attenuated (Fig. 4, I and J). Thus, cardiac aging in beagle dogs is characterized by a slower repolarization of the AP in cardiomyocytes, which contributes to the protracted electrical recovery of the old myocardium.
Function of the late Na+ current in the remodeled electrical properties of the senescent myocardium.
The reduced repolarization reserve of old cardiomyocytes may have enhanced the function of the late Na+ current (INaL), which is operative in the plateau and repolarization phases of the AP. The prolonged repolarization may have sustained Na+ influx via INaL, a factor that may contribute to the slower electrical recovery in old cells, with important implication on intracellular ionic homeostasis (3, 22, 54, 55).
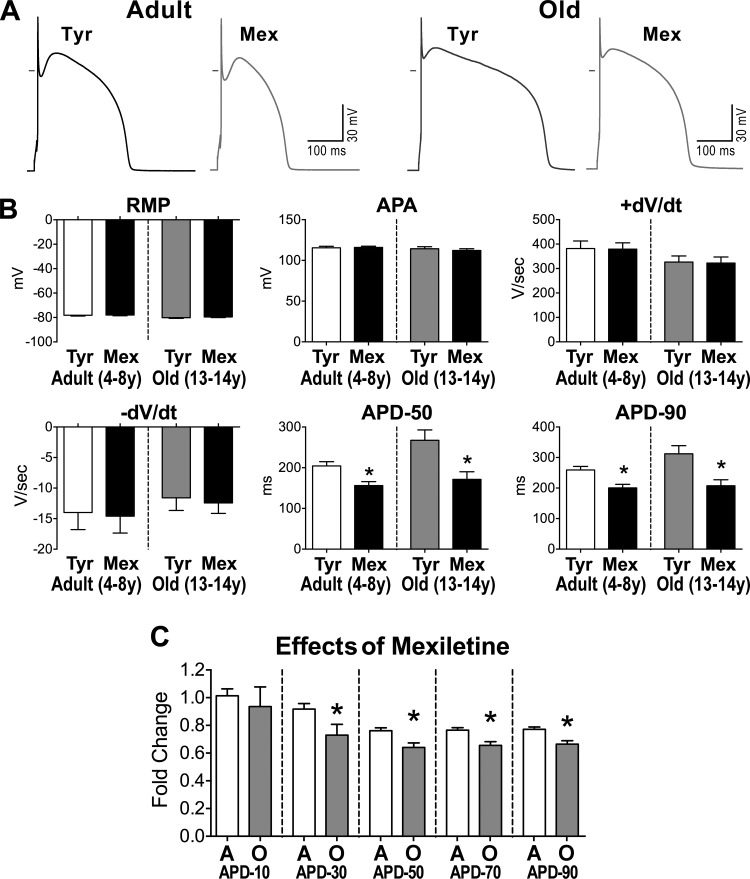
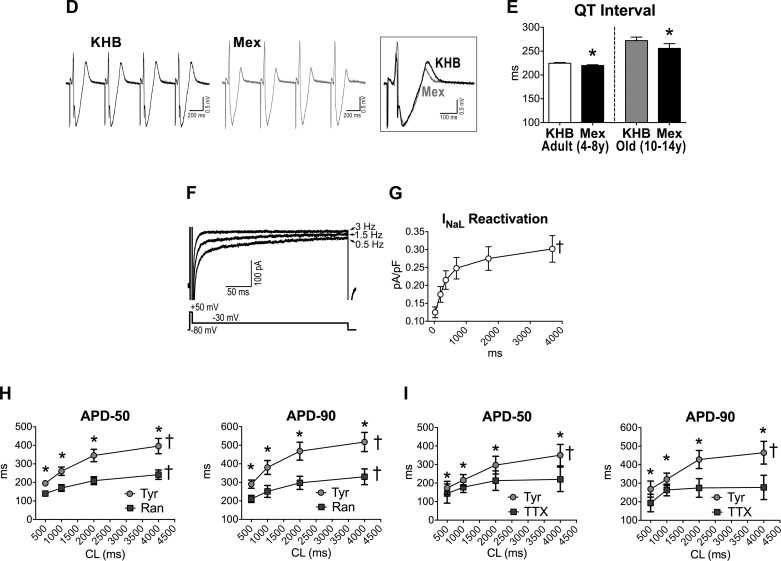
To test the function of INaL in the AP profile of aging myocytes, this ionic current was inhibited pharmacologically (mexiletine, 10 μM) (10, 40) in isolated cells. This intervention shortened the intermediate and late repolarization phases of the AP, a phenomenon that was more apparent in old cell (Fig. 5, A–C). However, resting membrane potential, AP amplitude, and maximal and minimal dV/dt were not modified by blockade of INaL. Importantly, the effects of inhibition of INaL on the electrical recovery of cardiomyocytes were equally observed at the level of the intact LV myocardium; the duration of the QT interval in the old muscle was markedly reduced, mimicking the response observed in senescent cardiomyocytes (Fig. 5, D and E).
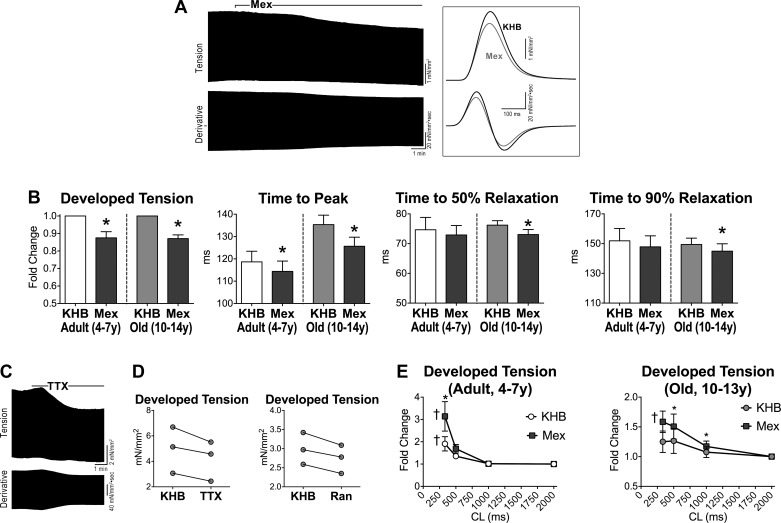
Fig. 5.
A: APs recorded in 1 adult and 1 old myocyte in Tyrode (Tyr) and after exposure to mexiletine (Mex; 10 μM). B: data are shown as mean ± SE (adult: n = 19 cells, 8 dogs, 5.2 ± 0.4 yr; old: n = 10 cells, 4 dogs, 13.1 ± 0.1 yr). *P < 0.001 vs. Tyr. C: comparison of the effects of late Na+ current (INaL) inhibition on AP duration between adult (A) and old (O) myocytes reported in B. data are shown as mean ± SE. *P < 0.01 vs. A. D: pseudo-ECG in the perfused LV myocardium of an adult dog. Traces in Krebs-Henseleit buffer (KHB) solution and after exposure to Mex (10 μM) are reported and superimposed in inset. E: data are shown as mean ± SE (adult: n = 6 muscles, 6 dogs, 6.5 ± 0.9 yr; old: n = 6 muscles, 4 dogs, 12.7 ± 0.5 yr). *P < 0.05 vs. KHB. F: superimposed traces of INaL (top traces) measured in voltage-clamp using a voltage-command protocol (bottom trace) at 0.5, 1.5, and 3 Hz, in a juvenile hound type dog myocyte. INaL was progressively reduced at higher frequencies. G: quantitative data for INaL reactivation in LV myocytes form juvenile (0.6–0.8 year old) hound type dogs are reported as mean ± SE (n = 29 cells, 4 dogs). †P < 0.001 for various frequencies. Amplitude of INaL is reported as positive value. H and I: data relative to effects of INaL inhibitors ranolazine (Ran; 10 μM) (H) and low doses of tetrodotoxin (TTX; 1 μM) (I) on AP profile of cardiomyocytes from old beagle dogs (11–13 yr of age) are shown as mean ± SE (ranolazine: n = 10 cells, 2 dogs 11 yr old; TTX: n = 5 cells, 2 dogs, 11–13 yr). *P < 0.05 vs. Tyr; †P < 0.001 for various frequencies within the same group.
As documented previously (20, 53), INaL presents a significant reverse rate dependency (Fig. 5, F and G). This behavior, identified in myocytes from juvenile hound-type dogs, may have critical consequences on the rate adaptation of the AP profile in myocytes from old beagles. Therefore, electrical signals were evaluated in old cells at different pacing frequencies in the absence and presence of INaL inhibitors ranolazine (10 μM) or TTX at low dose (1 μM) (36, 41, 50, 53). These compounds preferentially shortened the AP at basal stimulation frequency, attenuating the reverse rate dependency of the repolarization phase of the AP (Fig. 5, H and I). Thus cardiac aging in beagle dogs leads to a prolongation of the action potential favoring INaL, which contributes to the steep reverse rate dependency of the repolarization of the aging myocardium.
Prolongation of the AP duration enhances the contractile function of aging myocytes.
The prolongation of the AP has important implications in the excitation contraction-coupling of old cardiomyocytes. The lengthening of the plateau phase of the AP may 1) increase Ca2+ entry via L-type Ca2+ channels and reverse mode Na+/Ca2+ exchanger (NCX); and 2) favor Na+ entry via INaL and subsequent raise of [Na+]i, which in turn promotes the extrusion of Na+ and the entry of Ca2+ via the NCX. These conditions enhance the Ca2+ load and potentiate the Ca2+-induced Ca2+ release process in cardiomyocytes (8, 13, 22, 31, 38, 41–44, 48).
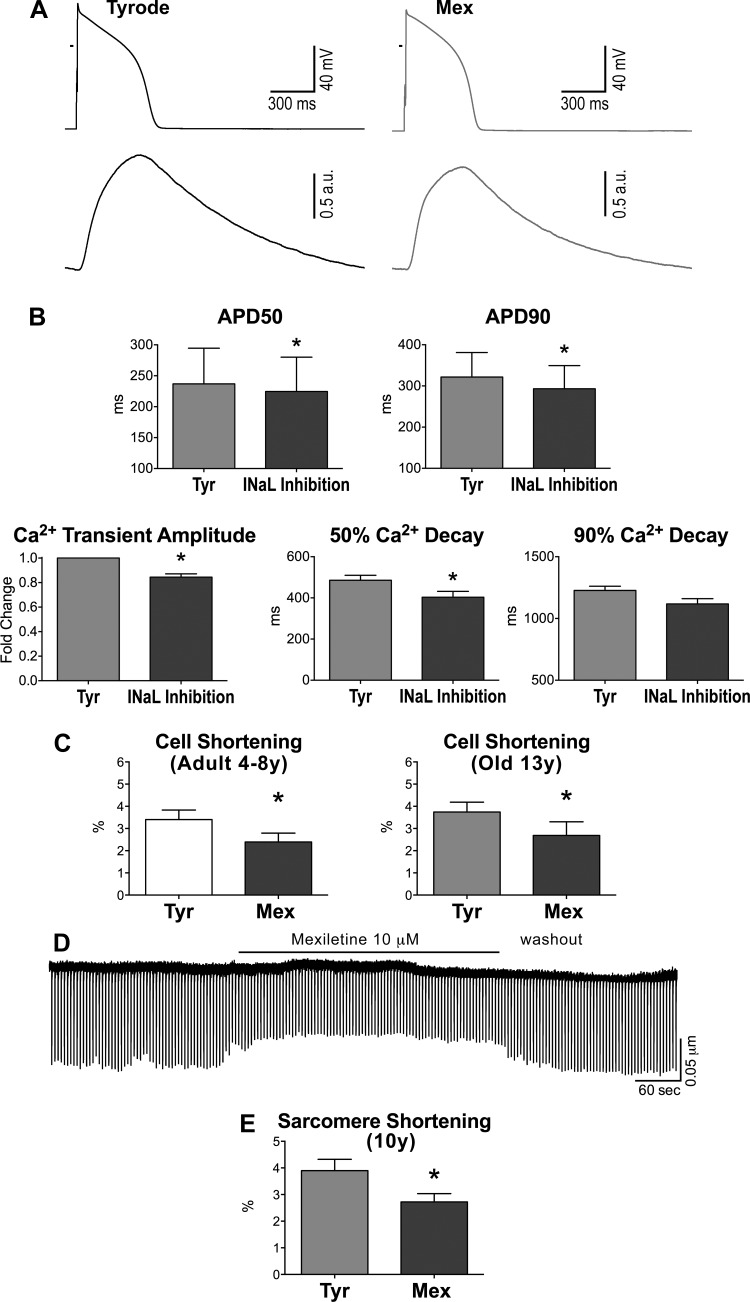
To validate whether these mechanisms of inotropic support, previously reported in canine cardiomyocytes (48), were operative in cells of the aged heart, AP and Ca2+ transients were simultaneously measured in isolated cells. Inhibition of INaL with low dose of TTX or mexiletine led to a faster repolarization of the AP and to a 15% decrease in Ca2+ transient amplitude (Fig. 6, A and B). Similarly, attenuation of INaL in cardiomyocytes reduced significantly cell contractility (Fig. 6, C–E). Thus myocardial aging in beagle dogs is characterized by a prolongation of the AP enhancing the contractile behavior of senescent cardiomyocytes.
Fig. 6.
INaL has inotropic effects on cardiomyocytes. A: AP (top traces) and Ca2+ transients (bottom traces) recorded simultaneously in a myocyte from an old dog in Tyr and after exposure to the INaL inhibitor Mex (10 μM). B: quantitative data for AP and Ca2+ transient properties in old myocytes, in Tyr, and in the presence of INaL inhibition (10 μM Mex, n = 1, or 1 μM TTX, n = 3); data are shown as mean ± SE (n = 4 cells, 2 dogs, 11–14 yr old). *P < 0.05 vs. Tyr. C: data on contractile (cell shortening) properties in adult (left: n = 14 cells, 6 dogs, 6.3 ± 0.9 yr) and old (right: n = 6 cells, 2 dogs, 13.1 ± 0.3 yr) myocytes stimulated at 0.25–0.5 Hz in Tyr and following exposure to Mex (10 μM) are shown as mean ± SE. *P < 0.05. D: trace of sarcomere shortening for an old myocyte during the exposure to Mex and during the washout phase. E: data of contractility (sarcomere shortening) for old myocytes are shown as mean ± SE (n = 7 cells, 1 dog, 10 yr old). *P < 0.001 vs. Tyr.
Inotropic reserve is impaired in the aging myocardium.
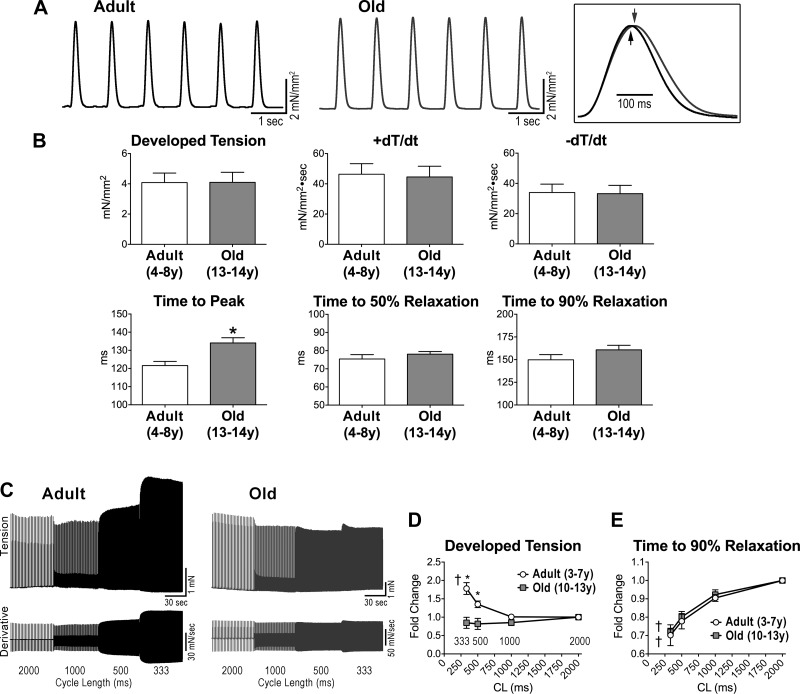
Based on the results discussed above, chronological age leads to adaptations in the electrical properties of the myocardium, which tends to sustain the mechanical performance of the old organ. To establish the efficiency of the process, ventricular trabeculae were analyzed in an isometric system. At 1-Hz stimulation frequency, developed tension, and positive and negative dT/dt were similar in adult and old muscle samples. Time to peak tension was delayed in the old myocardium, but the time of relaxation was comparable in the two sets of muscles (Fig. 7, A and B).
Fig. 7.
The old myocardium presents blunted contractile force frequency relationship. A: isometric contraction of ventricular trabeculae obtained from adult and old dogs stimulated at 1-Hz pacing frequency. Twitches are superimposed in the inset. B: data are shown as mean ± SE (adult: n = 9 muscles, 6 dogs, 5.8 ± 0.6 yr; old: n = 10 muscles, 7 dogs, 13.2 ± 0.1 yr). *P < 0.05. C: isometric contraction (top traces) and first derivative of developed tension (bottom traces) recorded at progressively higher stimulation frequencies in adult and old trabeculae. D and E: quantitative data for developed tension (D) and time to 90% relaxation (E) normalized with respect to CL 2,000 ms are shown as mean ± SE (adult: n = 7 muscles, 5 dogs, 5.1 ± 0.6 yr; old: n = 6 muscles, 4 dogs, 12.2 ± 0.4 yr). *P < 0.05 vs. adult; †P < 0.001 for various frequencies within the same group.
Increasing stimulation rates were employed to assess the contractile reserve of the myocardium. Reductions in pacing cycle length revealed a positive contractile force-frequency relationship (FFR) in adult muscles; in fact, a 1.8-fold increase in developed tension was detected. Conversely, developed tension remained essentially constant in old muscles (Fig. 7, C and D). The accelerated relaxation at high frequency, however, was comparable in adult and old muscles (Fig. 7E). This phenomenon has been attributed to an increase in sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump activity and enhanced SR Ca2+ reuptake at high pacing rates (14, 15).
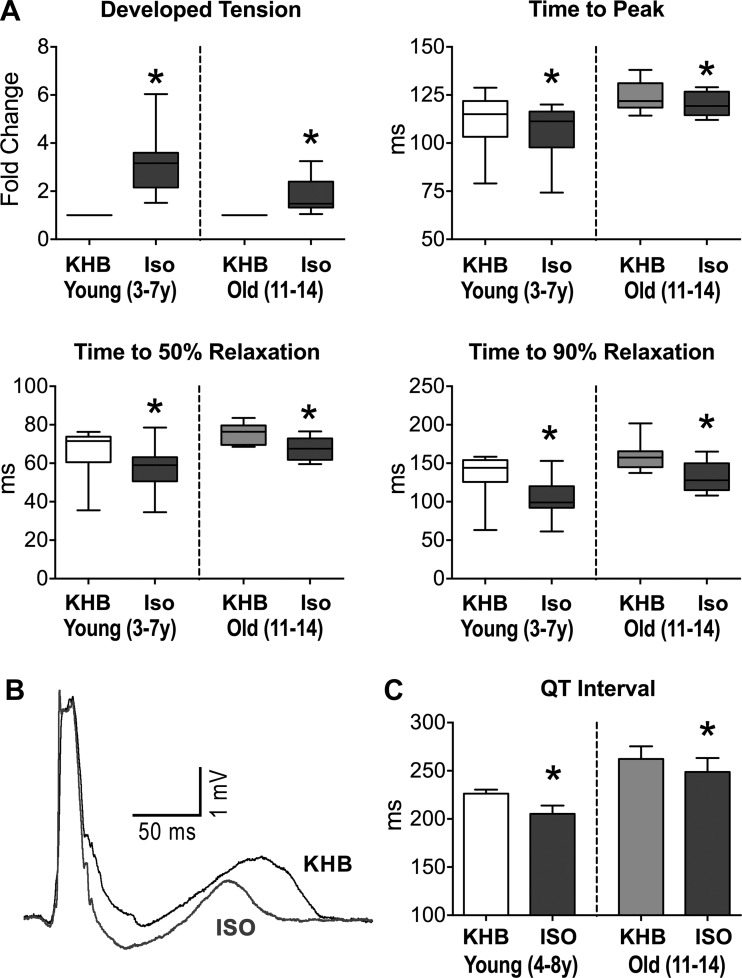
The lack of a positive staircase in the old myocardium was coupled with an attenuated β-adrenergic response. At fixed cycle length, exposure to isoproterenol increased developed tension more in adult than in old trabeculae; P < 0.05 with respect to old (Fig. 8A). Also, isoproterenol decreased time to peak tension and relaxation time in both muscles, and shortened the QT interval in the perfused myocardium paced at 2 Hz (Fig. 8, A–C). Thus myocardial aging in beagle dogs shows preserved contractile function at rest, but defective inotropic reserve following fast pacing and β-adrenergic stimulation.
Fig. 8.
The old myocardium presents blunted contractile response to β- adrenergic receptor (β-AR) stimulation. A: data for twitch properties in aging muscles in KHB and after exposure to isoproterenol (Iso; 100 nM) are shown as median and interquartile ranges (adult: n = 9 muscles, 7 dogs, 5.5 ± 0.5 yr; old: n = 10 muscles, 8 dogs, 13.0 ± 0.2 yr). *P < 0.01 vs. KHB. B: superimposed pseudo-ECGs obtained in the perfused adult LV myocardium in KHB and after exposure to the β-AR agonist Iso (100 nM). C: quantitative data for QT interval in adult and old LV myocardium in KHB and after exposure to Iso are shown as mean ± SE (adult: n = 4 muscles, 4 dogs, 6.9 ± 1 yr; old: n = 7 muscles, 5 dogs, 12.8 ± 0.3 yr). *P < 0.05 vs. KHB.
The prolonged electrical recovery provides inotropic support to the aging myocardium.
Collectively, these findings suggest that the blunted positive contractile FFR of the senescent myocardium was dictated by the reverse rate dependency of electrical recovery. At low stimulation frequency, the prolonged duration of the AP and activity of INaL were capable of providing inotropic support to the aging muscle. This mechanism, however, was no longer operative at high pacing rates when the AP was significantly shortened, partly by INaL inactivation. To investigate this relationship, ventricular trabeculae paced at 1 Hz were studied at baseline and following pharmacological inhibition of INaL with mexiletine. This strategy led to a reduction in developed tension, time to peak tension, and relaxation kinetics of old muscles; these changes were less apparent in trabeculae from adult hearts (Fig. 9, A and B). Inhibition of INaL with a low dose of TTX or ranolazine had comparable effects to those observed with mexiletine (Fig. 9, C and D). Importantly, attenuation of INaL restored the positive contractile FFR of the senescent myocardium and potentiated this phenomenon in the adult tissue (Fig. 9E).
Fig. 9.
INaL alters mechanical properties of the ventricular myocardium. A: isometric contraction (top trace) and first derivative of developed tension (bottom trace) recorded in a trabecula from an old dog before and after exposure to Mex (10 μM). Twitches and first derivative of developed tension are superimposed in inset. B: data are shown as mean ± SE (adult: n = 6 muscles, 5 dogs, 5.0 ± 0.6 yr; old: n = 10 muscles, 7 dogs, 12.6 ± 0.4 yr). *P < 0.01 vs. KHB. C: isometric contraction (top trace) and first derivative of developed tension (bottom trace) recorded in a trabecula from an old dog before and after exposure to low dose of TTX (1 μM). D: data for developed tension in 3 trabeculae before and after exposure to TTX (1 μM) or Ran (10 μM). Trabeculae were obtained from 2 old dogs (11.3 and 14.3 yr old). E: data for developed tension, normalized with respect to CL 2,000 ms, are shown as mean ± SE (adult: n = 5 muscles, 4 dogs, 5.3 ± 0.6 yr; old: n = 4 muscles, 3 dogs, 12.3 ± 0.7 yr). *P < 0.05 vs. KHB; †P < 0.01 for various frequencies within the same group.
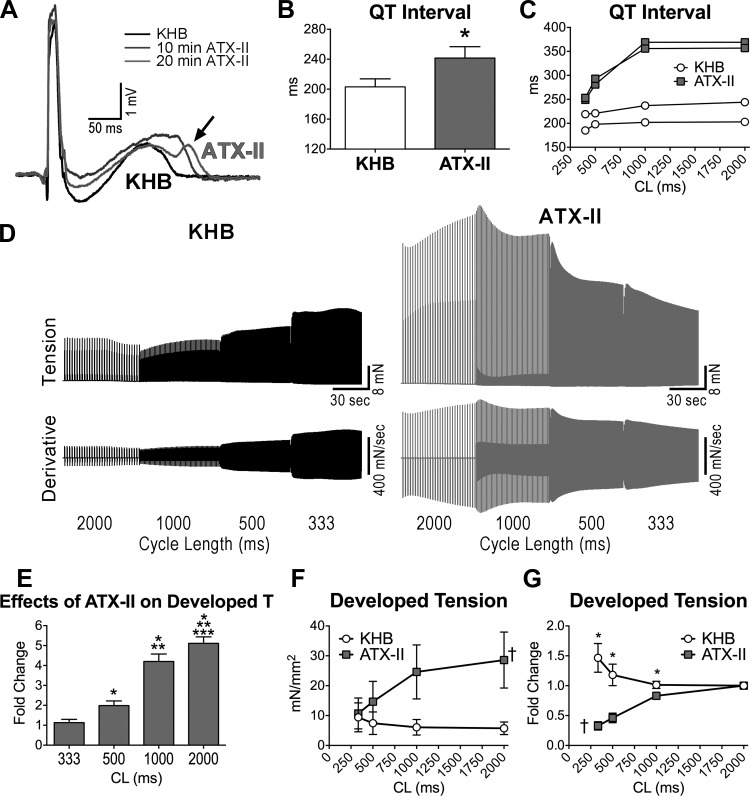
To define the rate dependency of the inotropic support of INaL, this current was increased pharmacologically with anemonia toxin-II (ATX-II) (40, 44) in the adult myocardium. In the perfused muscle stimulated at 2 Hz, the increase in INaL led to a 20% prolongation of the QT interval and the appearance of notched T waves in 2 of the 4 myocardial preparations (Fig. 10, A and B). Importantly, protracted repolarization was more pronounced at slower pacing rates (Fig. 10C). Similarly, the inotropic action of the enhancement of INaL in twitching trabeculae was apparent at low pacing rates and was attenuated at shorter stimulating intervals (Fig. 10, D and E). The preferential function of INaL at basal cycle lengths resulted in a steep negative FFR (Fig. 9, D–G).
Fig. 10.
INaL influences the contractile force frequency relationship. A: superimposed pseudo-ECGs obtained in the perfused adult LV myocardium before (KHB) and after exposure to the INaL enhancer ATX-II (5 nM). Arrow points to notched T wave occurring after prolonged exposure to ATX-II. B: data obtained in adult dogs are shown as mean ± SE (n = 4 muscles, 4 dogs, 4–8 yr old). *P < 0.05. Notched T waves were present in 50% of muscles exposed to ATX-II. C: examples of QT interval duration at various stimulation frequencies for LV myocardium obtained from two adult dogs (6 and 7 yr old) in KHB and after exposure to ATX-II. D: isometric contraction (top traces) and first derivative of developed tension (bottom traces) recorded at progressively higher stimulation frequencies in 1 trabecula from an adult dog before (KHB) and after exposure to ATX-II (10 nM). E: data for the effects of ATX-II on developed tension at the various frequencies of stimulation, with respect to baseline, are reported as mean ± SE (n = 3 muscles, 2 dogs, 6 and 7 yr old). *P < 0.05 vs. 333 ms; **P < 0.05 vs. 500 ms; ***P < 0.05 vs. 1,000 ms. F and G: quantitative data for developed tension (F) and developed tension normalized with respect to CL 2,000 ms (G) in adult trabeculae presented in E are shown as mean ± SE. *P < 0.05 vs. KHB; †P < 0.01 for various frequencies within the same group.
Thus myocardial aging leads to a prolonged electrical recovery favoring INaL activity, factors that enhance the inotropic state of the senescent myocardium. However, the reverse rate dependency of INaL and duration of the AP restrict the action of this beneficial mechanism to low pacing rates.
DISCUSSION
The results of the current study indicate that myocardial aging in a large animal model is coupled to alterations in the myocyte compartment, resulting in a loss of the contractile reserve typically present in young hearts. The defects in the electrical and mechanical characteristics of cardiomyocytes with aging suggest that this cell population is a critical determinant of the cardiac senescent phenotype. Ventricular hypertrophy has not been observed with myocardial aging in beagle dogs, a phenomenon consistent with previous observations in humans (25). The significant increase in circulating troponin I and elevated myocardial transcripts for collagen type I α2 in old animals suggest that necrotic cell death and replacement fibrosis are active processes in the aged heart. However, the structural composition of the myocardium is modestly altered with aging in this model, weakening the possibility that increased fibrotic depositions act as the mechanism responsible for the depressed cardiac performance of the old heart. The decreases in stroke volume, dP/dtmax, and EF measured at baseline in senescent animals in the conscious state may reflect alterations in cardiac autonomic control (52) and increased heart rate, together with abnormalities in the physiological properties of senescent myocytes and myocardium. Although not investigated here, impaired myocardial substrate metabolism may represent an important factor contributing to the defective contractile function observed in aged dogs (45).
Changes in the duration of the AP in cardiomyocytes have been recognized as important modulators of Ca2+ transient amplitude with maturation, aging, and myocardial infarction and following stimulation of Gq-protein coupled receptors (13, 38, 42). The prolongation of the AP duration in old myocytes is consistent with protracted QT interval and electrical recovery of the old dog myocardium, a phenomenon frequently observed in elderly patients (34, 39). The increase in Ca2+ transient with prolongation of the AP is mediated by the protracted kinetics of Ca2+ entry and Ca2+-induced Ca2+ release mechanism (13, 38). However, lengthening of the AP also causes increased activity of INaL and Na+ entry resulting in abnormal cytosolic Na+ load (54). The extrusion of Ca2+ via forward mode NCX is reduced and cytosolic Ca2+ accumulates; these factors improve myocardial contraction but impact negatively on muscle relaxation (8, 41, 44, 48, 49, 54). The results of the current study in the old dog heart are consistent with this possibility. The delayed temporal dynamics of myocyte and myocardial contractility may reflect the prolonged repolarization and the increased cytosolic Na+ load induced by INaL. In fact, inhibition of INaL reduces cell shortening and developed tension in cardiomyocytes and the isometric twitch in isolated trabeculae. Moreover, these changes are characterized by decreases in the timing parameters of developed tension and relaxation. We have previously documented in rodents that alterations in the AP profile using AP-clamp mode or by inhibiting repolarizing K+ currents affect the amplitude, time to peak, and decay of Ca2+ transients and contractility (35, 41, 42). However, modulation of INaL has peculiar effects on intracellular Ca2+ cycling and contractility, which reflect the ability of this current to alter the profile of the AP and promote Na+ influx (41).
The inability of the old myocardium to adjust the developed force to increases in stimulation frequency may be due to the steep reverse rate dependency of the duration of the AP in cardiomyocytes together with the biophysical properties of INaL reactivation. At high pacing rates, the AP is shortened and INaL is reduced; these factors weaken the inotropic support derived from protracted repolarization, Na+ entry, and load. Additionally, in old muscles, the basal cytoplasmic Na+ level may prevent further increases in Na+ load, a key regulator of the positive FFR and other mechanisms of contractile reserve (49). This possibility is supported by the diminished inotropic responsiveness of the intact senescent dog heart to the cardiac glycoside acetylstrophantidin (11), whose positive contractile action is due to enhanced Ca2+ load secondary to inhibition of the Na+/K+ pump, and intracellular accumulation of Na+ (49). In a comparable manner, the pharmacological potentiation of the INaL with ATX-II results in an increase in developed force of the adult myocardium at basal cycle length, but it suppresses the positive contractile FFR. Although not tested in the current study, prolonged electrical recovery and Na+ entry via INaL in old cardiomyocytes may be in part implicated in the attenuated contractile response of the senescent heart to β-adrenergic agonists. Activation of this signaling pathway shortens myocardial electrical recovery, reducing the temporal availability of Na+ influx and the inotropic support of Na+ load.
Although a formal review of the cause of death has not been performed in our beagle dog colony, there is no indication that heart failure or arrhythmias are major factors influencing the death of aging dogs (C. Royer and E. G. Barrett, personal communication). This observation raises the possibility that the enhanced occurrence of sudden cardiac death in the elderly population (47) may be secondary to intervening cardiac pathologies enhancing electrical instability of the heart. The protracted electrical recovery of the myocardium and prolonged AP duration of myocytes from old dogs are features shared with the failing heart. Specifically, chronic heart failure in dogs induced by multiple sequential coronary microembolizations or by bundle-branch radiofrequency ablation and tachypacing manifests with reduced EF and ventricular arrhythmias (1, 2, 21, 37, 48). These defects are coupled with an increase in INaL and reduced inward rectifier (IK1), delayed rectifier (IK), and transient outward (Ito) K+ currents in cardiomyocytes (1, 2, 21). Similar changes have been identified in myocytes of the old rodent heart (41, 51) raising the possibility that enhanced INaL and reduced repolarizing K+ currents are initial alterations during the early phase toward cardiac failure. Further studies are warranted to properly define alterations of ionic currents occurring in aging myocytes, leading to changes in the AP profile in the various anatomical regions of the large mammalian heart.
In conclusion, the results of current study in the old beagle dog heart raise the possibility that the prolongation of the electrical recovery and promotion of INaL activity may be viewed as important determinants of the aging myopathy and its propensity to evolve into ventricular decompensation under stressful conditions.
GRANTS
This work was supported by National Institute of Health Grants R01-HL-091021, R01-HL-114346, P01-AG-043353, P01-HL-092868, R01-AG-037495, R01-HL-111183, R01-AG-037490, R01-HL-105532, R01-AG-044455, and R37-HL-081737.
DISCLOSURES
P. Anversa is a member of Autologous LLP; P. Anversa and A. Leri are members of AAL Scientifics Corp.
AUTHOR CONTRIBUTIONS
Author contributions: A.S., S.S., K.Q., G.B., M.M., A.C., Y.Z., D.A.D., O.W., R.E.M., C.M.R., P.G., A.L., E.G.B., P.A., T.H.H., and M.R. conception and design of research; A.S., S.S., K.Q., G.B., M.M., A.C., Y.Z., E.W., M.L., R.K., E.Z., A.M., A.W., M.C., E.K., C.M.R., E.G.B., T.H.H., and M.R. performed experiments; A.S., S.S., K.Q., G.B., M.M., A.C., Y.Z., E.W., M.L., R.K., E.Z., A.W., M.C., E.K., T.H.H., and M.R. analyzed data; A.S., S.S., K.Q., G.B., M.M., A.C., Y.Z., T.H.H., and M.R. interpreted results of experiments; A.S., S.S., E.W., R.K., and M.R. prepared figures; A.S., S.S., and M.R. drafted manuscript; A.S., S.S., K.Q., G.B., M.M., A.C., Y.Z., E.W., E.Z., A.W., D.A.D., P.G., A.L., P.A., T.H.H., and M.R. edited and revised manuscript; A.S., S.S., K.Q., G.B., M.M., A.C., Y.Z., E.W., M.L., R.K., E.Z., A.M., A.W., M.C., E.K., D.A.D., O.W., R.E.M., C.M.R., P.G., A.L., E.G.B., P.A., T.H.H., and M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Nicola Alesi and Cuiqi Li at the Brigham and Women's Hospital and Dr. Xiaobin Xu and Manuel Ochoa at New York Medical College for technical assistance.
REFERENCES
- 1.Aiba T, Barth AS, Hesketh GG, Hashambhoy YL, Chakir K, Tunin RS, Greenstein JL, Winslow RL, Kass DA, Tomaselli GF. Cardiac resynchronization therapy improves altered Na channel gating in canine model of dyssynchronous heart failure. Circ Arrhythm Electrophysiol 6: 546–554, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, Dimaano VL, Abraham TP, O'Rourke B, Akar FG, Kass DA, Tomaselli GF. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation 119: 1220–1230, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belardinelli L, Giles WR, Rajamani S, Karagueuzian HS, Shryock JC. Cardiac late Na(+) current: proarrhythmic effects, roles in long QT syndromes, and pathological relationship to CaMKII and oxidative stress. Heart Rhythm 12: 440–448, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein RD, Zhang X, Zhao G, Forfia P, Tuzman J, Ochoa F, Ochoa M, Vogel T, Hintze TH. Mechanisms of nitrate accumulation in plasma during pacing-induced heart failure in conscious dogs. Nitric Oxide 1: 386–396, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Boyle AJ, Shih H, Hwang J, Ye J, Lee B, Zhang Y, Kwon D, Jun K, Zheng D, Sievers R, Angeli F, Yeghiazarians Y, Lee R. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol 46: 549–559, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardin S, Libby E, Pelletier P, Le Bouter S, Shiroshita-Takeshita A, Le Meur N, Leger J, Demolombe S, Ponton A, Glass L, Nattel S. Contrasting gene expression profiles in two canine models of atrial fibrillation. Circ Res 100: 425–433, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cesselli D, Beltrami AP, D'Aurizio F, Marcon P, Bergamin N, Toffoletto B, Pandolfi M, Puppato E, Marino L, Signore S, Livi U, Verardo R, Piazza S, Marchionni L, Fiorini C, Schneider C, Hosoda T, Rota M, Kajstura J, Anversa P, Beltrami CA, Leri A. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol 179: 349–366, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppini R, Ferrantini C, Yao L, Fan P, Del Lungo M, Stillitano F, Sartiani L, Tosi B, Suffredini S, Tesi C, Yacoub M, Olivotto I, Belardinelli L, Poggesi C, Cerbai E, Mugelli A. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 127: 575–584, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Correia LC, Lakatta EG, O'Connor FC, Becker LC, Clulow J, Townsend S, Gerstenblith G, Fleg JL. Attenuated cardiovascular reserve during prolonged submaximal cycle exercise in healthy older subjects. J Am Coll Cardiol 40: 1290–1297, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Xue X, Hu D, Liu W, Yuan Y, Sun H, Li L, Timothy KW, Zhang L, Li C, Yan GX. Inhibition of late sodium current by mexiletine: a novel pharmotherapeutical approach in timothy syndrome. Circ Arrhythm Electrophysiol 6: 614–622, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Guarnieri T, Spurgeon H, Forehlich JP, Weisfeldt ML, Lakatta EG. Diminished inotropic responses but unaltered toxicity to acetylstrophanthidin in the senescent beagle. Circulation 60: 1548–1554, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Hees PS, Fleg JL, Mirza ZA, Ahmed S, Siu CO, Shapiro EP. Effects of normal aging on left ventricular lusitropic, inotropic, and chronotropic responses to dobutamine. J Am Coll Cardiol 47: 1440–1447, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Janczewski AM, Spurgeon HA, Lakatta EG. Action potential prolongation in cardiac myocytes of old rats is an adaptation to sustain youthful intracellular Ca2+ regulation. J Mol Cell Cardiol 34: 641–648, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Janssen PM, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol 43: 523–531, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res 110: 1646–1660, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus MS, Moise NS, Rishniw M, Dykes N, Erb HN. Morphology of ventricular arrhythmias in the boxer as measured by 12-lead electrocardiography with pace-mapping comparison. J Vet Intern Med 16: 153–158, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Mahajan VS, Jarolim P. How to interpret elevated cardiac troponin levels. Circulation 124: 2350–2354, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation 98: 2545–2552, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail 9: 219–227, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maltsev VA, Undrovinas A. Late sodium current in failing heart: friend or foe? Progr Biophys Mol Biol 96: 421–451, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno JD, Clancy CE. Pathophysiology of the cardiac late Na current and its potential as a drug target. J Mol Cell Cardiol 52: 608–619, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation 131: e29–e322, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol 26: 1068–1079, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res 68: 1560–1568, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Ono K, Kiyosue T, Arita M. Comparison of the inhibitory effects of mexiletine and lidocaine on the calcium current of single ventricular cells. Life Sci 39: 1465–1470, 1986. [DOI] [PubMed] [Google Scholar]
- 28.Osorio JC, Xu X, Vogel T, Ochoa M, Laycock S, Hintze TH. Plasma nitrate accumulation during the development of pacing-induced dilated cardiac myopathy in conscious dogs is due to renal impairment. Nitric Oxide 5: 7–17, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci 52: B171–178, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Pepe M, Mamdani M, Zentilin L, Csiszar A, Qanud K, Zacchigna S, Ungvari Z, Puligadda U, Moimas S, Xu X, Edwards JG, Hintze TH, Giacca M, Recchia FA. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res 106: 1893–1903, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pieske B, Maier LS, Piacentino V, Weisser J 3rd, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation 106: 447–453, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Plotnikov SV, Millard AC, Campagnola PJ, Mohler WA. Characterization of the myosin-based source for second-harmonic generation from muscle sarcomeres. Biophys J 90: 693–703, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qanud K, Mamdani M, Pepe M, Khairallah RJ, Gravel J, Lei B, Gupte SA, Sharov VG, Sabbah HN, Stanley WC, Recchia FA. Reverse changes in cardiac substrate oxidation in dogs recovering from heart failure. Am J Physiol Heart Circ Physiol 295: H2098–H2105, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reardon M, Malik M. QT interval change with age in an overtly healthy older population. Clin Cardiol 19: 949–952, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Rota M, Hosoda T, De Angelis A, Arcarese ML, Esposito G, Rizzi R, Tillmanns J, Tugal D, Musso E, Rimoldi O, Bearzi C, Urbanek K, Anversa P, Leri A, Kajstura J. The young mouse heart is composed of myocytes heterogeneous in age and function. Circ Res 101: 387–399, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Rota M, Vassalle M. Patch-clamp analysis in canine cardiac Purkinje cells of a novel sodium component in the pacemaker range. J Physiol 548: 147–165, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabbah HN, Goldberg AD, Schoels W, Kono T, Webb C, Brachmann J, Goldstein S. Spontaneous and inducible ventricular arrhythmias in a canine model of chronic heart failure: relation to haemodynamics and sympathoadrenergic activation. Eur Heart J 13: 1562–1572, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Sah R, Ramirez RJ, Kaprielian R, Backx PH. Alterations in action potential profile enhance excitation-contraction coupling in rat cardiac myocytes. J Physiol 533: 201–214, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz J, Zipes D. Cardiovascular disease in the elderly. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (9th ed), edited by Bonow R, Mann D, Zipes D, Libby P. Philadelphia, PA: Saunders, 2011, p. 1727–1753. [Google Scholar]
- 40.Sicouri S, Antzelevitch D, Heilmann C, Antzelevitch C. Effects of sodium channel block with mexiletine to reverse action potential prolongation in in vitro models of the long term QT syndrome. J Cardiovasc Electrophysiol 8: 1280–1290, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Signore S, Sorrentino A, Borghetti G, Cannata A, Meo M, Zhou Y, Kannappan R, Pasqualini F, O'Malley H, Sundman M, Tsigkas N, Zhang E, Arranto C, Mangiaracina C, Isobe K, Sena BF, Kim J, Goichberg P, Nahrendorf M, Isom LL, Leri A, Anversa P, Rota M. Late Na(+) current and protracted electrical recovery are critical determinants of the aging myopathy. Nat Commun 6: 8803, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Signore S, Sorrentino A, Ferreira-Martins J, Kannappan R, Shafaie M, Del Ben F, Isobe K, Arranto C, Wybieralska E, Webster A, Sanada F, Ogorek B, Zheng H, Liu X, Del Monte F, D'Alessandro DA, Wunimenghe O, Michler RE, Hosoda T, Goichberg P, Leri A, Kajstura J, Anversa P, Rota M. Inositol 1, 4, 5-trisphosphate receptors and human left ventricular myocytes. Circulation 128: 1286–1297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sossalla S, Maurer U, Schotola H, Hartmann N, Didie M, Zimmermann WH, Jacobshagen C, Wagner S, Maier LS. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIdelta(C) can be reversed by inhibition of late Na(+) current. Basic Res Cardiol 106: 263–272, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts–role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 45: 32–43, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Suematsu N, Ojaimi C, Kinugawa S, Wang Z, Xu X, Koller A, Recchia FA, Hintze TH. Hyperhomocysteinemia alters cardiac substrate metabolism by impairing nitric oxide bioavailability through oxidative stress. Circulation 115: 255–262, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Tung P, Albert CM. Causes and prevention of sudden cardiac death in the elderly. Nat Rev Cardiol 10: 135–142, 2013. [DOI] [PubMed] [Google Scholar]
- 48.Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci 60: 245–257, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vassalle M, Lin CI. Calcium overload and cardiac function. J Biomed Sci 11: 542–565, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res 108: 555–565, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker KE, Lakatta EG, Houser SR. Age associated changes in membrane currents in rat ventricular myocytes. Cardiovasc Res 27: 1968–1977, 1993. [DOI] [PubMed] [Google Scholar]
- 52.White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, Port JD, Anderson F, Campbell D, Feldman AM, Bristow MR. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation 90: 1225–1238, 1994. [DOI] [PubMed] [Google Scholar]
- 53.Wu L, Ma J, Li H, Wang C, Grandi E, Zhang P, Luo A, Bers DM, Shryock JC, Belardinelli L. Late sodium current contributes to the reverse rate-dependent effect of IKr inhibition on ventricular repolarization. Circulation 123: 1713–1720, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac “late sodium current”. Pharmacol Ther 119: 326–339, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Zaza A, Rocchetti M. The late Na+ current–origin and pathophysiological relevance. Cardiovasc Drugs Ther 27: 61–68, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao G, Walsh E, Shryock JC, Messina E, Wu Y, Zeng D, Xu X, Ochoa M, Baker SP, Hintze TH, Belardinelli L. Antiadrenergic and hemodynamic effects of ranolazine in conscious dogs. J Cardiovasc Pharmacol 57: 639–647, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]