Abstract
Background
Complications and postoperative pain are major care problems that can affect the quality of health care plan.
Objectives
According to the use of multimodal therapy the current study aimed to compare the efficacy of gabapentin and celecoxib in pain management and complications after laminectomy at Ilam University of Medical Sciences, Ilam, Iran, in 2015.
Patients and Methods
In this randomized double-blind clinical trial, 114 patients scheduled for elective laminectomy with simple random sampling design received gabapentin (n = 38, 900 mg/day), celecoxib (n = 38, 600 mg/day) and placebo (n = 38, capsule contain starch). Visual analog scale (VAS) was used to determine the intensity of pain. Complications after surgery, anxiety scores before surgery and patient’s satisfaction 24 hours after the surgery were recorded.
Results
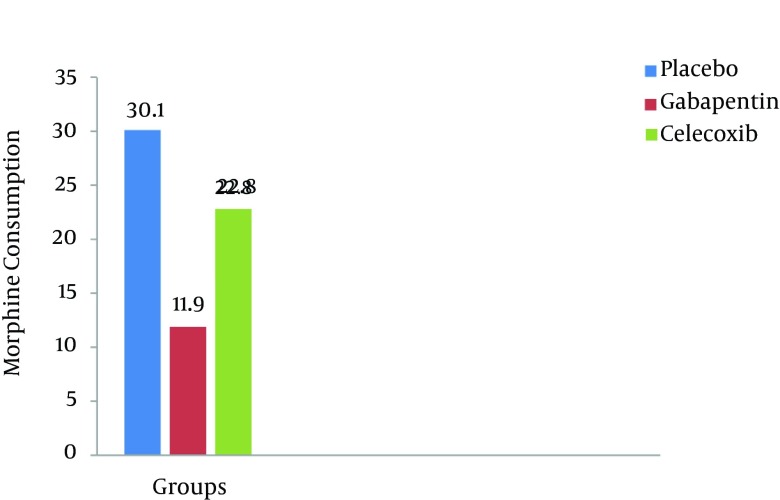
The mean pain intensity in the gabapentin group was lower compared to those of the placebo and celecoxib groups respectively at different time durations (P < 0.001). The means of morphine consumption were 11.9 mg, 22.8 mg and 30.1 mg in the gabapentin, celecoxib and placebo groups, respectively (P < 0.001). The prevalence of shivering, nausea, vomiting and pruritus were 10.5%, 12.8%, 10.3% and 18.4% in the gabapentin group vs 31.5%, 29.8%, 32.4% and 28.9% in the celecoxib group and 42.1%, 44.7%, 39.5% and 44.7% in the placebo group (P < 0.001). The mean anxiety score in the gabapentin group was 2.4 vs those of the celecoxib group 3 and placebo group 3.6 (P < 0.001). The frequencies of drowsiness were 42.1%, 13.2% and 5.3% in the gabapentin, celecoxib and placebo groups, respectively (P < 0.001). In the gabapentin group, patient satisfaction was significantly higher compared to those of the placebo and celecoxib groups (P < 0.05).
Conclusions
According to the effect of gabapentin on pain management, complications after laminectomy and increased patients satisfaction, it can be regarded as an alter native in multimodal analgesia.
Keywords: Multimodal Therapy, Analgesia, Laminectomy, Gabapentin, Celecoxib
1. Background
Postoperative pain causing several complications such as delay in wound healing, infections, prolong hospital stay, readmission after discharge, increase in morbidity and cost of hospital stay is one of the most common challenges in postoperative care process for physicians (1, 2). Therefore, management of post-surgical pain plays a critical role in the welfare of patients (3-5). Patients experiencing postoperative pain are exposed to decreased lung function and the risk of developing thromboembolism due to immobility, nausea and vomiting. Also cardiac workload, systemic vascular resistance, and myocardial oxygen consumption increase due to catecholamine release (6). The literature shows that 80% of the patients undergoing surgery, experience postoperative pain (1, 7); most (approximately 80%) of these patients reported moderate to severe pain intensity (8). Laminectomy following lumbar herniation is one of the most common surgeries with an incidence of 10% to 40% in neurosurgery. Annually, 300,000 to 400,000 lumbar surgeries are conducted (9); about 13,000 cases in the UK and over 250,000 cases in the US. In the United States alone $2.5 billion is spent on lumbar surgery (10) and back pain annually causes loss of about 150 million days of work (11). Postoperative pain in these patients can continue for up to three days and 13% - 43% may cause chronic pain (12). Opioids are the first choice to treat pain after the surgery. The use of opioids due to a number of side effects such as respiratory depression, nausea, vomiting, excessive sedation, dizziness, drowsiness, pruritus, and urinary retention is limited (1, 12-15). Recently, there is emphasis on the use of non-opioid analgesic drugs (16, 17), the use of two or more analgesics, and multimodal therapy (1, 12). One of these multimodal analgesia methods is the use of anti-epileptic drugs such as gabapentin (1) and other non-steroidal anti-inflammatory drugs (NSAID) such as celecoxib. Gabapentin is an anticonvulsant drug that has analgesic effect in post-herpetic neuralgia, diabetic neuropathy, and neuropathic pain. Celecoxib is one of the NSAIDs, that its analgesic effect is reported in various studies by cyclooxygenase-2 (COX-2) inhibitor (1, 18, 19).
2. Objectives
The current study aimed to investigate the comparative effect of gabapentin versus celecoxib on pain and complications after laminectomy based on the hypothesis that the effect of gabapentin and celecoxib on pain and complication after laminectomy are different.
3. Patients and Methods
It was a randomized double-blind clinical trial carried out at the Imam-Khomeini hospital (center of surgery and trauma) affiliated to Ilam University of Medical Sciences, Ilam, Iran, in 2015. This hospital is a teaching and academic hospital with a level 1 trauma center with 170 general beds and 12 intensive care unit (ICU) beds. The statistical population included all the patients referred to the department due to laminectomy.
3.1. Sample Collection
The sample size was calculated according to the data from a pilot study with 10 patients and the following formula:
Equation 1.
Z1 = 95% = 1.96; Z2 = 80% = 0.84 (test power); S, an estimate of the standard deviation of visual analog scale (VAS) in the groups; 1.67 in a pi-lot study; d, the minimum of the mean differences of VAS between the groups which showed a significant difference 1.1.
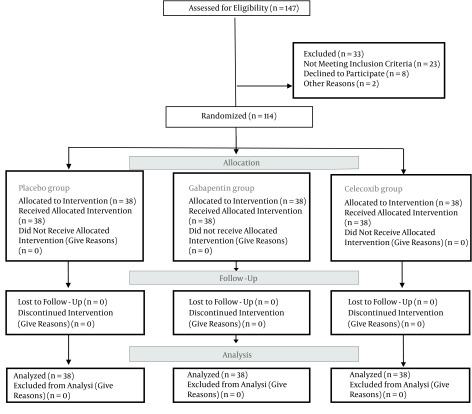
In the current randomized, double-blind clinical trial, there were 114 patients of grade I or II according to the American society of anesthesiologists (ASA), aged 20 - 60 years, scheduled for elective laminectomy under general anesthesia. Patients were randomized to three groups. In each group, there were 38 patients: group A (gabapentin), group B (celecoxib) and group C (placebo). A simple random sampling design was used (Figure 1). Sampling was performed with a sealed envelopes technique and coding. Coded as: code 1 = gabapentin, code 2 = celecoxib, code 3 = placebo. The patients, anesthesiologists and surgeons were blinded to the drug administered. Coding and sealed envelopes technique for the double-blind was prepared by a nurse who was not participating in the study.
Figure 1. CONSORT Diagram of Participant in the Clinical Trial.
3.2. Protocols
The patients in the group A (gabapentin) received 600 mg gabapentin two hours before surgery and 300 mg six hours after surgery, group B (celecoxib) received 400 mg celecoxib two hours before surgery and 200 mg six hours after surgery and group C (placebo) received a placebo capsule orally two hours before surgery and six hours after surgery. The patients with drug abuse, history of allergic reaction to any of the understudy drugs, under use of non-steroidal anti-inflammatory analgesic, pregnancy, cardiovascular, metabolic, respiratory, peptic ulcer and renal failure, or coagulation abnormalities were excluded from the study. The neurosurgeon and anesthesiologist were the same for all patients. Anesthesia was carried out with thiopentone (5 mg/kg IV), atracurium (0.5 mg/kg IV), and maintained with Isoflurane (1% - 1.5%) and nitrous oxide (50%) in oxygen. Fentanyl was given in the operation room according to the patient’s need and clinical discretion. Patients were reversed with 0.05 mg/kg neostigmine combined with 0.02 mg/kg Atropine. Standard monitoring included electrocardiogram, noninvasive blood pressure, and pulse oximetry was done. In order to achieve the same VAS scores after surgery in the groups the extra morphine was used in the patients with pain in the placebo group.
3.3. Measurements
The VAS was used to determine severity of pain. The pain severity was assessed in the 2, 4, 6, 8, 12 and 24 hours after surgery. The patients’ mean blood pressure (BP), heart rate (HR), respiratory rate (RR), saturation (SPO2), urine retention, vomiting, shivering, headache, dizziness, nausea, drowsiness, pruritus and morphine consumption were recorded. Anxiety scores before surgery and patients’ satisfaction 24 hours after surgery were recorded. Preoperative anxiety was assessed according to a seven-point scale (1 = relaxed, 2 = apprehension, 3 = mild anxiety, 4 = moderate anxiety, 5 = manifest anxiety, 6 = severe anxiety, 7 = very severe anxiety). The patients` satisfaction with pain management was assessed on a 5-point scale; 0 = poor, 1 = fair average, 2 = moderate, 3 = good and 4 = excellent) and recorded in postoperative periods. Shivering was assessed on a scale with 0 = no shivering observed; 1 = shivering observed (1).
3.4. Ethical Issues
The current study was approved by the vice chancellor for research at the Ilam University of Medical Sciences, Ilam, Iran (EC: 94/H/269); the informed consent was obtained from all subjects. This study was registered at the Iranian registry of clinical trials (IRCT2015071222870N3).
3.5. Validity and Reliability
VAS rating is a valid scale to evaluate pain severity with ratings from 0 to 10 (0 means no pain and 10 means the maximum pain in this scale). In order to determine the validity and reliability of the scale, content validity (content validity index (CVI) and content validity ratio (CVR) and Cronbach’s alpha test were used. The CVI and CVR indexes of the scale were 0.79 and 0.87 respectively. The reliability of the questionnaire was 0.83.
3.6. Statistical Analysis
Collected data were analyzed using the statistical software SPSS, ver.16. (SPSS Inc., Chicago, IL, USA). Descriptive statistics, the Chi-square test, one-way ANOVA, the Fisher least significant difference (LSD), Tukey test and repeated measurement were carried out to analyze the results. P < 0.05 was considered significant.
4. Results
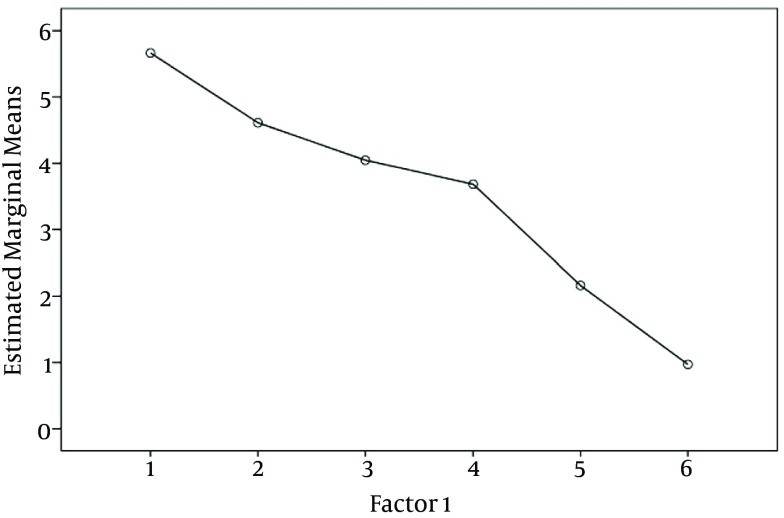
Baseline characteristics of the patients are shown in Table 1. None of the 114 enrolled patients was withdrawn for any reasons. In the quantitative data according to Kolmogorov-Smirnov test, data distribution was normal and the parametric method was used (P > 0.05) Subjects characteristics were not different among the groups (P > 0.5) (Table 1). One-way ANOVA showed that the mean pain severity score in the gabapentin group was less than those of the placebo and celecoxib groups respectively at different intervals (P < 0.001) (Table 2). According to LSD and Tukey test, the mean pain severity in the gabapentin group was significantly lower than those of the placebo and celecoxib groups respectively at various intervals (P < 0.001, P < 0.05). In the eighth hour after the intervention, although the mean pain severity in the celecoxib group was lower than that of the placebo group, no statistically significant difference between the two groups was observed (P > 0.05). Repeated measurement analysis showed that the mean pain score in the gabapentin (P < 0.001), placebo (P < 0.05) and celecoxib groups (P < 0.05) were significantly different in various intervals (Figure 2). The means of morphine consumption, anxiety score, shivering, nausea, vomiting and pruritus in the gabapentin group were significantly lower than those of the celecoxib and placebo groups respectively (P < 0.001, P < 0.05) (Tables 2 and 3 Figure 3). The frequencies of drowsiness (42.1%) in the gabapentin group were significantly higher than those of the celecoxib and placebo groups (P < 0.001). No statically significant differences were observed between the groups in relation to headache and dizziness (P > 0.05) (Table 3), Patient satisfaction was significantly higher in the gabapentin group than those of the placebo and celecoxib groups (P < 0.05).
Table 1. Baseline Characteristics of the Patientsa,b.
| Characteristic | Placebo | Gabapentin | Celecoxib | P Valuec |
|---|---|---|---|---|
| Age, y | 50.2 ± 7.2 | 49.5 ± 5.9 | 50.2 ± 4.2 | > 0.881 |
| Gender | > 0.467 | |||
| Male | 28(73.7) | 31(81.6) | 29(76.3) | |
| Female | 10(26.3) | 7(18.4) | 9(23.7) | |
| Married | 34 (89.5) | 35 (92.1) | 36 (94.7) | > 0.871 |
| Duration of surgery, h | 2.11 ± 0.23 | 2.27 ± 0.28 | 2.17 ± 0.35 | > 0.654 |
| Duration of anesthesia, h | 2.35 ± 0.14 | 2.57 ± 0.18 | 2.49 ± 0.25 | > 0.537 |
| BP, mm/Hg | 132 ± 3.2 | 122 ± 3.6 | 126 ± 3.1 | > 0.863 |
| PR, per/min | 72.8 ± 3.4 | 73.6 ± 5.6 | 74.4 ± 5.4 | > 0.736 |
| Spo 2 | 95 ± 3.4 | 95 ± 2.2 | 96 ± 2.7 | > 0.741 |
Abbreviation: BP, blood pressure.
aValues are expressed as mean ± SD unless otherwise indicated as No. (%).
bN = 38.
cP > 0.05.
Table 2. Severity of Pain, Morphine Consumption and Anxiety Score in the Groupsa,b.
| Characteristic | Placebo | Gabapentin | Celecoxib | P Value |
|---|---|---|---|---|
| Pain score by vas at various intervals, h | ||||
| 2 h after intervention | 7.6 ± 1 | 4.9 ± 0.7 | 6 ± 1 | 0c |
| 4 h after intervention | 6.6 ± 0.9 | 3.8 ± 0.8 | 4.8 ± 0.5 | 0c |
| 6 h after intervention | 5.4 ± 0.5 | 3.4 ± 0.5 | 4.4 ± 0.7 | 0c |
| 8 h after intervention | 4.5 ± 0.6 | 3 ± 0.8 | 4.4 ± 1 | 0c |
| 12 h after intervention | 2.9 ± 0.7 | 1.6 ± 0.5 | 2.5 ± 0.4 | 0c |
| 24 h after intervention | 1.4 ± 0.4 | 0.7 ± 0.3 | 1 ± 0.4 | 0c |
| Morphine consumption, mg | 30.1 ± 0.6 | 11.9 ± 4.4 | 22.8 ± 6.8 | 0c |
| Anxiety score | 3.6 ± 0.7 | 2.4 ± 0.5 | 3 ± 0.9 | 0c |
| Patient satisfaction | ||||
| Good | 4 (10.5) | 19 (50) | 8 (21) | 0.03d |
| Excellent | 0 (0) | 9 (23.6) | 3 (7.8) | 0.04d |
Abbreviation: VAS, visual analog scale.
aValues are expressed as mean ± SD unless otherwise indicated as No. (%).
bN = 38.
cP < 0.001.
dP < 0.05.
Figure 2. Repeated Measurement Analysis of Pain Between Groups.
Table 3. Frequencies of Adverse Effects Between the Groupsa.
| Outcome Parameters | Placebo | Gabapentin | Celecoxib | P Value |
|---|---|---|---|---|
| Vomiting | 15 (39.5) | 4 (10.3) | 12 (32.4) | 0.008b |
| Shivering | 16 (42.1) | 4 (10.5) | 12 (31.5) | 0.004b |
| Headache | 4 (10.5) | 5 (13.2) | 5 (13.2) | 0.930c |
| Dizziness | 8 (21.1) | 13 (34.2) | 10 (26.3) | 0.519c |
| Nausea | 17 (44.7) | 5 (12.8) | 11 (29.8) | 0.02b |
| Drowsiness | 2 (5.3) | 16 (42.1) | 5 (13.2) | 0d |
| Pruritus | 17 (44.7) | 7 (18.4) | 11 (28.9) | 0.04b |
| Urine retention | 9 (23.7) | 5 (13.1) | 7(18.4) | 0.637c |
aValues are expressed as No. (%).
bP < 0.05.
cP > 0.05.
dP < 0.001.
Figure 3. Morphine Consumption in the Groups.
5. Discussion
In the past decade, despite the knowledge of pain physiology, about 80% of the patients after surgery experience postoperative pain (18). Postoperative pain, shivering, nausea, vomiting and urine retention are common complications impairing the quality of postoperative recovery such as readmissions after discharge, chronic pain after surgery and increasing morbidity and costs (8). Although opioids are the first choice to manage pain after surgery, they are associated with some complications (20). Recently, Interest in multimodal analgesia, using two or more analgesics and modalities with multi-mechanisms to treat analgesia and decrease the prevalence of complication is rising. One of these analgesia methods is the use of anticonvulsant drugs such as gabapentin and pregabalin (1). Therefore, the multimodal analgesic approach is recommended as an alternative treatment to manage postoperative pain (18). Management of pain after surgery and complications such as shivering and emesis are common challenges in care process and the basic principles in the early mobilization and the quality of postoperative care in the patients undergoing surgeries (5). The current study results suggested that gabapentin versus celecoxib significantly reduced pain, overall morphine consumption, preoperative anxiety, pruritus, postoperative shivering, nausea and vomiting in patients following laminectomy under general anesthesia. Patient satisfaction was significantly higher in the gabapentin group compared to the placebo and celecoxib ones. This finding was consistent with the previous studies on the effects of gabapentin on postoperative pain and complications (21-24). A study by Ozgencil et al. (1) on 90 patients undergoing laminectomy found that gabapentin and pregabalin significantly reduced preoperative anxiety, pruritus, overall opioid consumption and postoperative shivering; patient’s satisfaction was significantly higher compared to the placebo group. Kumar et al. (6) showed that after laminectomy, pregabalin significantly decreased pain compared to the placebo, but its impact was less than that of tramadol. Demand for morphine was less in tramadol group. In pregabalin group, the postoperative complications such as nausea, vomiting and drowsiness were less in comparison to those of the tramadol. In another study, Reuben et al. (18) concluded that following laminectomy, the patients in the pregabalin/celecoxib composition group had more analgesia and lower postoperative complications. In the study by Gianesello et al. (12) on the spinal surgery, pregabalin significantly decreased VAS scores, prevalence of constipation and emesis compared to the placebo group. Liporaci Juniot in the spinal surgery found that gabapentin significantly reduced the pain intensity after surgery (25). Rahimi et al. (26) concluded that daily consumption of 800 mg gabapentin can decrease the pain severity after laminectomy in the patients compared to the patients in the placebo group and the group that received 400 mg gabapentin daily. Studies emphasized on the use of non-opioid analgesic drugs and multimodal therapy to manage pain after surgery. Pain management, decreases in preoperative stress, reduced length of hospital stay and costs, increased patient’s satisfaction, and a reduction in postoperative complications are the benefits of multimodal therapy (12). Pandey et al. (27) concluded that prophylactic gabapentin in patients undergoing laparoscopic cholecystectomy significantly decreased nausea and vomiting and fentanyl consumption. Syal et al. (28) in a study on patients undergoing open cholecystectomy found that gabapentin was effective on pain and morphine consumption reduction, but increased the prevalence of nausea and vomiting and sedation. Bafna et al. (29) in a study on patients undergoing elective gynecological surgeries under spinal anesthesia found that preemptive use of gabapentin and pregabalin significantly reduced the postoperative rescue analgesic requirement and increased the duration of postoperative analgesia. The sedation levels in the gabapentin group were significantly higher compares to those of the placebo and celecoxib groups. This finding suggests that gabapentin can raise the patients’ sedation levels (1). Gabapentin, a structural feature of gamma amino butyric acid, is an antiepileptic drug with known analgesic effects on diabetic neuropathy, post-herpetic neuralgia and neuropathic pain (19, 27). The mechanism of gabapentin action is to reduce the release of several excitatory neurotransmitters (e.g. glutamate, substance P, calcitonin, noradrenaline, gene-related peptide) by binding to the α2δ subunit of voltage dependent calcium channels (1, 30). Gabapentin has antiallodynic and antihyperalgesic properties that decrease the hyperexcitability of dorsal horn neurons due to tissue injury. Decreasing the central sensitization by gabapentin can decrease acute pain after surgery. Gabapentin can be decrease morphine consumption (31). The anxiolytic effects of gabapentin are reported in previous studies (1). On the other hand, the effect of gabapentin to treat nausea and vomiting in patients under cytotoxic therapy and patients under laparoscopic cholecystectomy is shown. The mechanism of gabapentin in the prevention of emesis due to decreases of tachykinin neurotransmitter activity is reported (27). Decrease of the severity of shivering is due to anticonvulsive, anxiolytic and analgesic effects of gabapentin (1). The limitations of the current research include relatively small sample size and the subjective perception of pain by patients. All patients enrolled in this study underwent surgery by a single surgeon; and the data were collected from a single center that was the strengths of this study. To conclude, the current study finding revealed that to manage the postoperative pain after laminectomy, gabapentin 900 mg/daily was the alternative in multimodal analgesia. It was observed that gabapentin had less adverse effects, decreased the amount of morphine consumption for postoperative pain management and increased patient satisfaction.
Acknowledgments
Authors thank Ilam University of Medical Sciences, patients and all people who participated in this study.
Footnotes
Authors’ Contribution:Aminolah Vasigh and Javaher Khajavikhan participated in patient’s anesthesia. Fatemeh Najafi, Molouk Jaafarpour and Ali Khani participated in the study design, control of patients and data analysis.
Funding/Support:This study was supported by Ilam University of Medical Sciences (EC: 94/H/269).
References
- 1.Ozgencil E, Yalcin S, Tuna H, Yorukoglu D, Kecik Y. Perioperative administration of gabapentin 1,200 mg day-1 and pregabalin 300 mg day-1 for pain following lumbar laminectomy and discectomy: a randomised, double-blinded, placebo-controlled study. Singapore Med J. 2011;52(12):883–9. [PubMed] [Google Scholar]
- 2.Yavuz O. The Effect of Gabapentin on Postoperative Pain and Opioid-Related Side Effects in Patients Undergoing Combined Spinal-Epidural Anesthesia (a Preliminary Study): Cukurova University; 2013. [Google Scholar]
- 3.Warner DO. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology. 2000;92(5):1467–72. doi: 10.1097/00000542-200005000-00037. [DOI] [PubMed] [Google Scholar]
- 4.Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Current Opinion in Clinical Nutrition & Metabolic Care. 2005;8(1):23–32. doi: 10.1097/00075197-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mathiesen O, Moiniche S, Dahl JB. Gabapentin and postoperative pain: a qualitative and quantitative systematic review, with focus on procedure. BMC Anesthesiol. 2007;7:6. doi: 10.1186/1471-2253-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar KP, Kulkarni DK, Gurajala I, Gopinath R. Pregabalin versus tramadol for postoperative pain management in patients undergoing lumbar laminectomy: a randomized, double-blinded, placebo-controlled study. J Pain Res. 2013;6:471–8. doi: 10.2147/JPR.S43613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesthesia & Analgesia. 2003;97(2):534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 8.Melemeni A, Staikou C, Fassoulaki A. Gabapentin for acute and chronic post-surgical pain. Signa Vitae. 2007;2(Suppl. 1):42–51. [Google Scholar]
- 9.Rahimzadeh P, Sharma V, Imani F, Faiz HR, Ghodraty MR, Nikzad-Jamnani AR, et al. Adjuvant hyaluronidase to epidural steroid improves the quality of analgesia in failed back surgery syndrome: a prospective randomized clinical trial. Pain Physician. 2014;17(1):E75–82. [PubMed] [Google Scholar]
- 10.Kim SB, Lee KW, Lee JH, Kim MA, An BW. The effect of hyaluronidase in interlaminar lumbar epidural injection for failed back surgery syndrome. Ann Rehabil Med. 2012;36(4):466–73. doi: 10.5535/arm.2012.36.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porchet F, Vader J-P, Larequi-Lauber T, Costanza M, Burnand B, Dubois R. The assessment of appropriate indications for laminectomy. Journal of Bone and Joint Surgery. J Bone Joint Surg Br. 1999;2(81):234–9. doi: 10.1302/0301-620x.81b2.8871. [DOI] [PubMed] [Google Scholar]
- 12.Gianesello L, Pavoni V, Barboni E, Galeotti I, Nella A. Perioperative pregabalin for postoperative pain control and quality of life after major spinal surgery. J Neurosurg Anesthesiol. 2012;24(2):121–6. doi: 10.1097/ANA.0b013e31823a885b. [DOI] [PubMed] [Google Scholar]
- 13.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 14.White PF. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94(3):577–85. doi: 10.1097/00000539-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritus, and urinary retention. Evidence from published data. Br J Anaesth. 2005;95(5):584–91. doi: 10.1093/bja/aei227. [DOI] [PubMed] [Google Scholar]
- 16.White PF. Multimodal analgesia: its role in preventing postoperative pain. Curr Opin Investig Drugs. 2008;9(1):76–82. [PubMed] [Google Scholar]
- 17.White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005;101(5 Suppl):S5–22. doi: 10.1213/01.ANE.0000177099.28914.A7. [DOI] [PubMed] [Google Scholar]
- 18.Reuben SS, Buvanendran A, Kroin JS, Raghunathan K. The analgesic efficacy of celecoxib, pregabalin, and their combination for spinal fusion surgery. Anesth Analg. 2006;103(5):1271–7. doi: 10.1213/01.ane.0000237279.08847.2d. [DOI] [PubMed] [Google Scholar]
- 19.Serpell MG. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99(3):557–66. doi: 10.1016/S0304-3959(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 20.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. The American journal of surgery. 2002;183(6):630–41. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 21.Dahl JB, Mathiesen O, Moiniche S. 'Protective premedication': an option with gabapentin and related drugs? A review of gabapentin and pregabalin in in the treatment of post-operative pain. Acta Anaesthesiol Scand. 2004;48(9):1130–6. doi: 10.1111/j.1399-6576.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 22.Seib RK, Paul JE. Preoperative gabapentin for postoperative analgesia: a meta-analysis. Can J Anaesth. 2006;53(5):461–9. doi: 10.1007/BF03022618. [DOI] [PubMed] [Google Scholar]
- 23.Hurley RW, Cohen SP, Williams KA, Rowlingson AJ, Wu CL. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med. 2006;31(3):237–47. doi: 10.1016/j.rapm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain: A systematic review of randomized controlled trials. Pain. 2006;126(1-3):91–101. doi: 10.1016/j.pain.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Liporaci Junior JL. Assessment of preemptive analgesia efficacy in surgical extraction of third molars. Rev Bras Anestesiol. 2012;62(4):502–10. doi: 10.1016/S0034-7094(12)70148-4. [DOI] [PubMed] [Google Scholar]
- 26.Rahimi M, Naser Zareh M, Siavashi B, Amiri SR. The Effectiveness of Different Doses of Gabapentin in Controlling Postoperative Pain. Iranian J of Surgery. 2014;22(1):44–49. [Google Scholar]
- 27.Pandey CK, Priye S, Ambesh SP, Singh S, Singh U, Singh PK. Prophylactic gabapentin for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled study. J Postgrad Med. 2006;52(2):97–100. [PubMed] [Google Scholar]
- 28.Syal K, Goma M, Dogra RK, Ohri A, Gupta AK, Goel A. "Protective premedication": a comparative study of acetaminophen, gabapentin and combination of acetaminophen with gabapentin for post-operative analgesia. J Anaesthesiol Clin Pharmacol. 2010;26(4):531–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Bafna U, Rajarajeshwaran K, Khandelwal M, Verma AP. A comparison of effect of preemptive use of oral gabapentin and pregabalin for acute post-operative pain after surgery under spinal anesthesia. J Anaesthesiol Clin Pharmacol. 2014;30(3):373–7. doi: 10.4103/0970-9185.137270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003;60(4):742–50. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104(6):1545–56. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]