Abstract
To investigate age-associated impairments in fluid homeostasis, 4-mo (young) and 32-mo (old) Fischer 344/BN male rats were studied before and after a dietary sodium load. Transferring young rats from a low-sodium (LS) to a high-sodium (HS) diet increased water intake and urine volume by 1.9- and 3.0-fold, respectively, while urine osmolality and plasma aldosterone decreased by 33 and 98%. Concomitantly, adrenocortical angiotensin type 1 receptor (AT1R) density decreased by 35%, and AT1bR mRNA decreased by 39%; no changes were observed in AT1aR mRNA. In contrast, the increase in water intake (1.4-fold) was lower in the old rats, and there was no effect of the HS diet on urine volume or urine osmolality. AT1R densities were 29% less in the old rats before transferring to the HS diet, and AT1R densities were not reduced as rapidly in response to a HS diet compared with the young animals. After 6 days on the HS diet, plasma potassium was lowered by 26% in the old rats, whereas no change was detected in the young rats. Furthermore, while plasma aldosterone was substantially decreased after 2 days on the HS diet in both young and old rats, plasma aldosterone was significantly lower in the old compared with the young animals after 2 wk on the LS diet. These findings suggest that aging attenuates the responsiveness of the adrenocortical AT1R to a sodium load through impaired regulation of AT1bR mRNA, and that this dysregulation contributes to the defects in water and electrolyte homeostasis observed in aging.
Keywords: angiotensin type 1 receptor and angiotensin type 2 receptor messenger ribonucleic acid, urine volume, urine osmolality, aldosterone, adrenal cortex
individuals 65 yr and older are one of the most rapidly growing segments of the United States population. Change in the control of sodium and water balance is a major characteristic of the normal human aging process and includes a decrease in thirst, urinary-concentrating ability, and capacity to excrete water and electrolytes (32). These age-related changes in humans are also observed in animals. Aging impairs the ability of rats to excrete a sodium load (11) and to maximally concentrate urine (12). These changes in fluid and electrolyte regulation can put the elderly at increased risk for disorders of hyponatremia (due to water retention) or hypernatremia (as a result of sodium retention), which can cause central nervous system dysfunction and also negatively impact medication effectiveness, resulting in adverse clinical events and surgical outcomes as well as other physiological functions (34, 38). Indeed, it has been shown that excess salt intake in rats increases the ability of centrally administered ANG II to increase sympathetic nerve activity (1).
The adrenal steroid hormone aldosterone plays a key role in the homeostatic mechanisms controlling fluid and electrolyte balance (28, 40). In humans (10, 21–23) and experimental studies of animal models (9), aging is associated with decreased plasma aldosterone levels. Aging-related changes in aldosterone are magnified under conditions that stimulate aldosterone secretion, indicating that not only is plasma aldosterone reduced in the old, the aldosterone responsiveness to appropriate stimuli is diminished. Sitting upright increases plasma aldosterone in both young adult and old individuals, but the magnitude of this increase is smaller in the elderly (35, 55, 63). Likewise, when sodium intake is restricted or plasma volume is reduced, plasma aldosterone levels rise to a greater degree in young adult compared with old individuals (14, 66).
Aldosterone is synthesized in adrenal glomerulosa cells within the adrenal cortex, and secretion of this hormone is regulated by sodium, potassium, adrenocorticotropic hormone, and angiotensin II (ANG II). One likely contributor to the aging-associated decrease in plasma aldosterone is an attenuation in adrenal responsiveness to ANG II since ANG II is the major controller of aldosterone production when dietary sodium is altered (30). ANG II infusion in young adult 8–10 mo of age and old (28–32 mo) Long-Evans rats increased plasma aldosterone; however, the response to ANG II was significantly smaller in the old rats compared with the young adult rats (51). These findings are not restricted to rodents, since ANG II-induced aldosterone production was lower in adrenal glomerulosa cell suspensions from old cows compared with those from young cows (50).
Aldosterone release from the adrenal cortex is primarily mediated by activation of the angiotensin type 1 receptor (AT1R). Many studies in young adult animals have shown that the adrenal AT1R plays a key role in maintaining electrolyte balance in response to changes in dietary sodium. A high-sodium (HS) diet downregulates adrenal AT1R expression and aldosterone release, whereas a low-sodium (LS) diet has the reverse effects (2, 3). What is not well known is how aging alters the adrenal AT1R response to dietary sodium manipulation. This study investigated the regulation of adrenocortical AT1R protein and mRNA during the adaptation response to a sodium load as a function of age to increase our understanding of the mechanisms influencing age-associated impairments in fluid homeostasis. To maximize the sodium load, we maintained the rats on a LS diet for 2 wk before transferring them to a HS diet. We chose the Fischer 344/BN rat to avoid the confounds of age- and sodium-induced hypertension since these animals remain normotensive throughout their lifespan (7) and their blood pressure increases only marginally on a HS diet (16).
MATERIALS AND METHODS
Animals.
Male Fischer 344BN rats at 4 mo (young) and 32 mo (old) of age were purchased from the National Institutes of Aging and individually housed in a temperature-controlled animal facility. All rats were maintained on a LS (0.13% NaCl) diet for 2 wk. Subsequently, all rats were then transferred to a HS (4% NaCl) diet for up to 6 days (Teklad, Madison, WI). The animals were given tap water ad libitum under controlled conditions (12:12-h light-dark schedule at 24°C). Body weight was measured daily. Animals were placed in metabolic cages for determination of daily water and food intake and collection of urine. Under isoflurane anesthesia, the adrenal was removed, trunk blood was collected by cardiac puncture, and the animals were killed by exsanguination. The Georgetown University Animal Care and Use Committee approved all procedures.
Urine and plasma analysis.
Urine osmolality was measured by freezing-point depression (model 3900 osmometer; Advanced Instruments, Norwood, MA). Plasma was collected from heparinized trunk blood, and plasma sodium and potassium were determined by an Easylyte Na/K Analyzer (MEDICA, Bedford, MA). Plasma aldosterone was measured by RIA (Coat-a-count, Siemens, Los Angeles, CA). Plasma vasopressin (AVP) content was measured by radioimmunoassay after extraction, as previously described (56, 65).
AT1R radioligand binding.
Membranes were prepared from the adrenal cortex as described previously (25, 67). Membranes (5–10 μg protein/tube) were incubated for 1–2 h at room temperature with increasing concentrations of the ANG II antagonist 125I-labeled [Sar1,Ile8]ANG II in the presence of 1 μM PD-123319, an AT2R antagonist, to ensure only AT1R expression was measured (67). 125I-labeled [Sar1,Ile8]ANG II was prepared as previously described (61). Binding reactions were terminated by rapid filtration through a Brandel cell harvester. Specific AT1R binding was determined by the total amount of radioligand bound minus nonspecific binding (defined as the amount bound in the presence of 200 nM ANG II, i.e., 100 × Kd for ANG II). Data points were obtained in triplicate. Kd and Bmax values were determined using the one-site saturation binding nonlinear regression analysis program, PRISM (GraphPad Software).
Real-time PCR.
Total RNA from adrenal cortex was extracted using TRIzol reagent (Life Technologies). First-strand cDNA was made from total RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) with Moloney murine leukemia virus RNase H+ reverse transcriptase, oligo(dT), and random hexamers. Quantitation of specific mRNAs and 18S rRNA (for control) was performed by real-time PCR using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) (67). The PCR reaction mixture consisted of RNase-free water, TaqMan Universal PCR Master Mix (Perkin Elmer Applied Biosystems), and 300 nM specific primers and 10 μM probe [for AT1aR primers and probe: forward (F) primers 3′-untranslated region (UTR)-47F, 5′-GCA GCC TCT GAC TAA ATG GCT T-3′, reverse (R) primer 3′-UTR-191R, 5′-CAA GAC GGC TTT GCT TGG TTA-3′, and probe 3′-UTR-70T, 6 FAM-CGA CCA AAG GAC CAT TCA CCC TGC-TAMRA; for AT1bR primers and probe: forward primers 3′-UTR-38F, 5′-AGC AGA AGC CAG AGG ACC ATT-3′, reverse primer 3′-UTR-142R, 5′-CAC TGA GTG CTT TCT CTG CTT CA-3′, and probe 3′-UTR-89T, 6 FAM-AGT GTT CAA CCT CCA GCA ATC CTT TCA GG-TAMRA] and cDNA samples. PCR conditions were optimized for the two probes (232T and 89T) and sets of primers (47F and 191R and 38F and 142R) using control cDNAs. The specificity of these primers was confirmed using the AT1R and AT1bR in pCR3 (Invitrogen, Grand Island, NY); we did not detect any amplified products using AT1aR specific primers in the AT1bR-expressing cells and vice versa. The expression of 18S rRNA, AT1aR, and AT1bR mRNA in each sample was quantitated using the specific primers specified above. PCR reactions without reverse transcription were included to control for contamination by genomic DNA. The standard curves for 18S rRNA, AT1aR, and AT1bR mRNA were made from a series of 10 times dilutions (53, 54, 55, 56, 57, and 58) for each cDNA. The tissue levels of these cDNAs were calculated based on the standard curves.
Statistics.
Data are expressed as means ± SE and in some cases as the ratio of the parameters measured under HS and LS dietary conditions at day 0. Statistical significance of the differences between groups was assessed by Student's t-test and two-way ANOVA. Differences were considered significant at P < 0.05.
RESULTS
Body weight.
Young male rats were approximately one-half the body weight of old rats (Fig. 1A). Switching from a LS to a HS diet reduced body weight in the old but not the young rats (P < 0.0001, young vs. old by 2-way ANOVA) (Fig. 1, A and B). When the data were normalized to body weight on day 0 of the HS diet, the body weight of the old rats dropped by 12% on day 2 and remained reduced on day 6 (Fig. 1B).
Fig. 1.
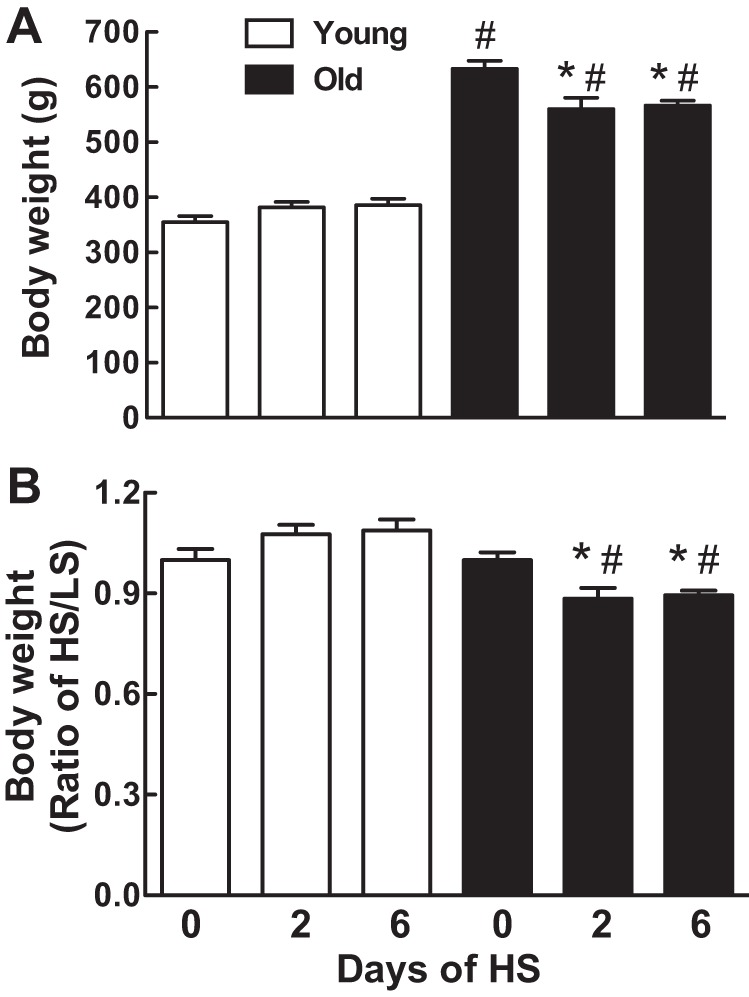
Effect of age on body weight changes in response to a high-sodium (HS) diet. Body weight (g) is expressed as mean ± SE (A) or HS-to-low sodium (LS) ratio (B) on days 0, 2, and 6 after the onset of a HS diet; *P < 0.05 vs. day 0 within the same age groups; #P < 0.05 vs. young rats on the same day; n = 6–8 rats/group.
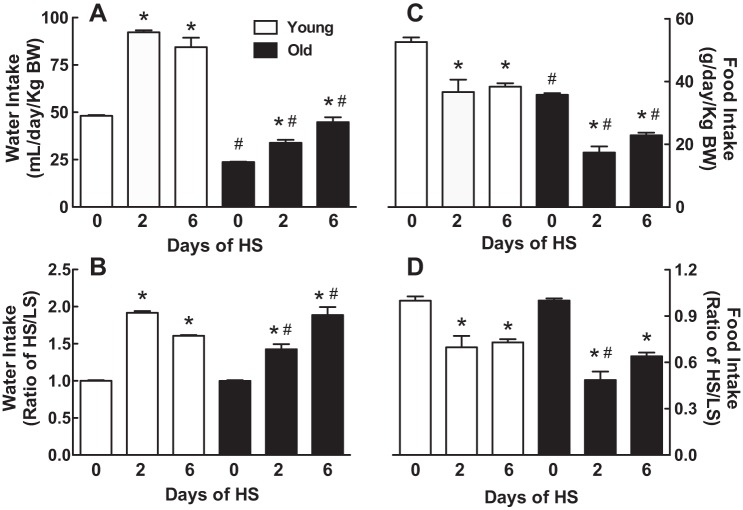
Water and food intake.
Young rats rapidly increased their water intake by 1.9-fold on day 2 after switching to a HS diet and remained increased on day 6 (Fig. 2, A and B). Water intake on the LS diet was 51% less in the old rats and remained lower after switching to a HS diet compared with the young rats (P < 0.0001, young vs. old by 2-way ANOVA) (Fig. 2A). In response to the HS diet, the old rats also increased their water intake although in a slower manner compared with the young rats; water intake increased by 1.4-fold on day 2 and by 1.9-fold on day 6 (Fig. 2B).
Fig. 2.
Effect of age on water and food intake in response to a HS diet. Water (A and B) or food intake (C and D) was normalized to body weight, and the results are expressed as means ± SE (A and C) or HS-to-LS ratio (B and D) on days 0, 2, and 6 after the onset of a HS diet; *P < 0.05 vs. day 0 within the same age groups; #P < 0.05 vs. young rats on the same day; n = 6/group. BW, body weight.
Young rats responded to the HS diet by decreasing their food intake by ~30% on days 2 and 6 (Fig. 2, C and D). Old rats decreased their food intake to a larger extent than young rats: 52% less food on day 2 of HS and 36% less food on day 6 (P < 0.01, young vs. old by 2-way ANOVA). This accounts for the loss of body weight in old rats in contrast to the minimal changes in body weight observed in the young rats.
There was no difference in the NaCl intake per kilogram body weight per day between the young and old rats on the LS diet (Fig. 3). The consumption of NaCl per kilogram of body weight increased dramatically in both age groups with the HS diet on days 1-2 and 3-6, but the young rats increased their consumption of NaCl more than the old rats (P < 0.01 by 2-way ANOVA). The young rats continued to increase their NaCl consumption on days 3-6 relative to days 1-2 on the HS diet (P < 0.01), but the old rats did not significantly increase their NaCl consumption from days 1-2 to days 3-6.
Fig. 3.
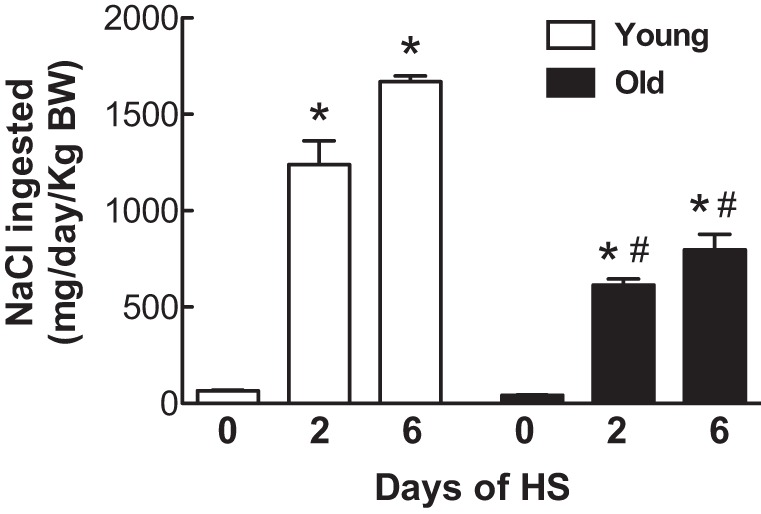
Effect of age on dietary sodium intake in response to a HS diet. Dietary sodium intake was normalized to body weight, and the results are expressed as means ± SE on days 0, 2, and 6 after the onset of a HS diet; *P < 0.05 vs. day 0 within the same age groups; #P < 0.05 vs. young rats on the same day; n = 6/group.
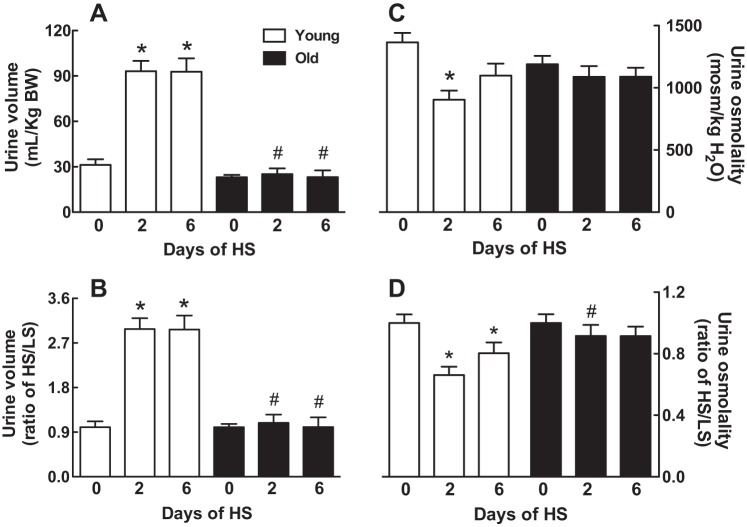
Urine volume and osmolality.
In young rats, urine volume increased by threefold 2 days after being transferred from a LS to a HS diet and remained elevated on day 6 (Fig. 4, A and B). Urine volume in the old rats was similar to the young rats on day 0 of the HS diet; however, urine volume did not increase after switching to the HS diet (P < 0.0001, young vs. old by 2-way ANOVA) (Fig. 4A).
Fig. 4.
Effect of age on urine volume and osmolality in response to a HS diet. Urine volume (A and B) or urine osmolality (C and D) was normalized to body weight, and the results are expressed as means ± SE (A and C) or HS-to-LS ratio (B and D) on days 0, 2, and 6 after the onset of a HS diet; *P < 0.05 vs. day 0 within the same age group; #P < 0.05 vs. young rats on the same day; n = 7–8/group.
Urine osmolality in 4-mo-old rats decreased by 33% 2 days after being transferred from a LS to a HS diet (Fig. 4, C and D). Four days later, the urine osmolality was indistinguishable from day 0 in the young rats (Fig. 4, C and D). No significant differences in urine osmolality were detected between young and old rats maintained on a LS diet for 2 wk (Fig. 4, C and D). In contrast to the young animals, urine osmolality in the old rats was not attenuated in response to switching to a HS diet (Fig. 4D).
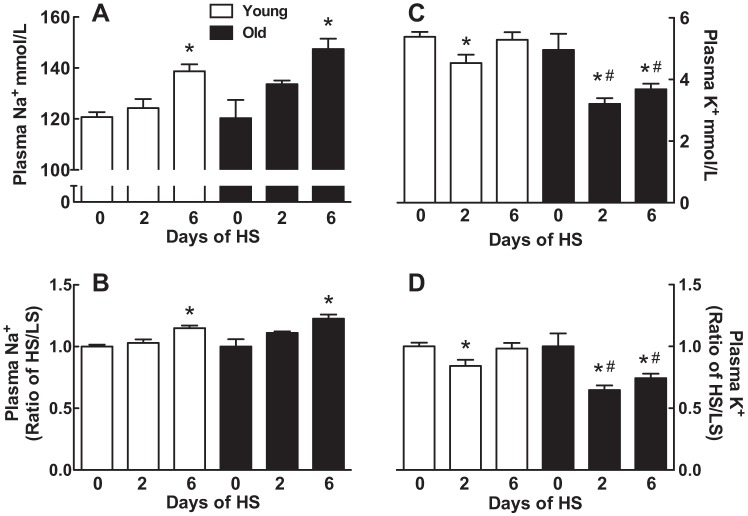
Plasma sodium and potassium.
There were no differences in plasma sodium between the young and old rats (Fig. 5A) or in plasma potassium (Fig. 5C) between young and old rats maintained on the LS diet. There were also no detectable differences in the magnitude of the plasma sodium increase in response to the HS diet in young (1.2-fold) and old (1.2-fold) rats on day 6 (Fig. 5B).
Fig. 5.
Effect of age on plasma sodium and potassium in response to a HS diet. Plasma sodium (A and B) or plasma potassium (C and D) is expressed as means ± SE (A and C) or HS-to-LS ratio (B and D) on days 0, 2, and 6 after the onset of a HS diet; *P < 0.05 vs. day 0 within the same age groups; #P < 0.05 vs. young rats on the same day; n = 7–8/group.
Transferring to a HS diet lowered plasma potassium in young and old rats at 2 days (Fig. 5C). On day 6, plasma potassium returned to levels observed before the sodium load in the young rats. In contrast, plasma potassium remained lower than before the sodium load in the old rats (P < 0.002, young vs. old by 2-way ANOVA) (Fig. 5D).
Plasma aldosterone.
In the young rats, plasma aldosterone decreased by 98% 2 days after being transferred from a LS to a HS diet (Fig. 6, A and B). Six days later, plasma aldosterone remained reduced to the same extent. Although the amount of the reduction in plasma aldosterone after switching to HS was similar in the old rats (87% by day 2 and 84% by day 6), plasma aldosterone was 45% less in the old rats compared with the young rats before switching to the HS diet.
Fig. 6.
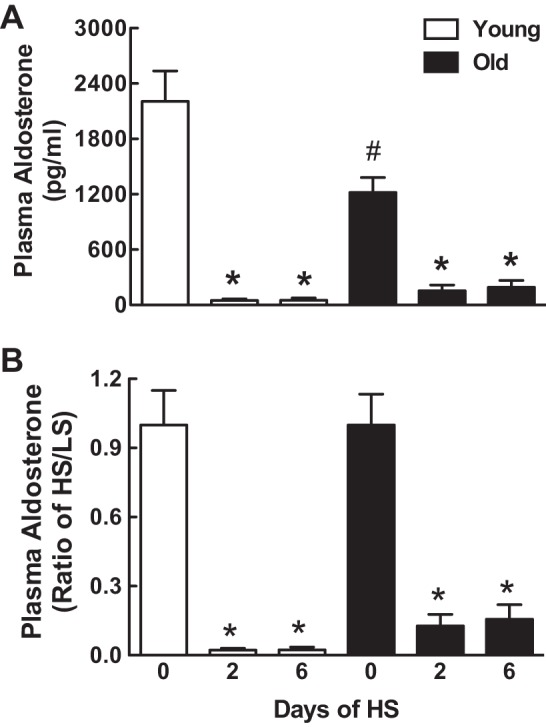
Effect of age on plasma aldosterone in response to a HS diet. Plasma aldosterone is expressed as means ± SE (A) or HS-to-LS ratio (B) on days 0, 2, and 6 after the onset of a HS diet; *P < 0.05 vs. day 0 within the same age groups; #P < 0.05 vs. young rats on the same day; n = 7–8/group.
Plasma AVP.
In young rats, plasma AVP levels increased after 6 days on HS, but the difference was not statistically significant (LS: 3.0 ± 0.8 pg/ml, n = 4; HS: 3.9 ± 0.4, n = 4). Similarly, there was no significant difference in plasma AVP levels in old rats on LS (7.2 ± 1.4, n = 4) vs. HS (6.5 ± 1.1, n = 3). Plasma AVP levels were significantly higher in old vs. young animals on either the LS or HS diets (P < 0.05).
Adrenocortical AT1R density.
Radioligand binding assays on adrenocortical membranes using 125I labeled [Sar1,Ile8]ANG II revealed that the density of AT1R in the adrenal cortex of young rats was decreased by 35 and 43%, respectively, on days 2 and 6 after switching to the HS diet (Fig. 7, A and B). AT1R densities were 29% less in the old rats before switching to the HS diet, and AT1R densities were not reduced as rapidly in response to a HS diet compared with the young animals (P < 0.001, young vs. old by 2-way ANOVA). In fact, a significant drop in adrenocortical AT1R density was not observed until day 6 in the old rats (Fig. 7). Two-way ANOVA showed no significant differences in binding affinity as a function of age or dietary sodium, and there was no interaction (the average Kd was 0.14 ± 0.02 nM).
Fig. 7.
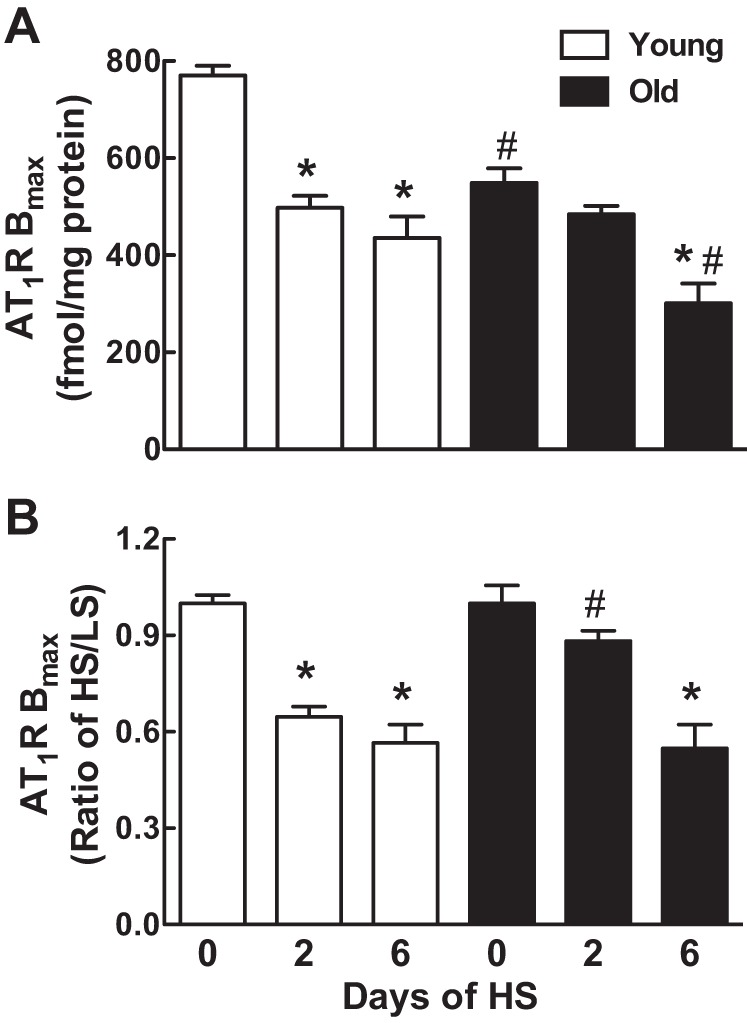
Effect of age on adrenocortical angiotensin type 1 receptor (AT1R) density in response to a HS diet. AT1R densities are expressed as means ± SE (A) or HS-to-LS ratio (B) on days 0, 2, and 6 after the onset of a HS diet; *P < 0.05 or **P < 0.01 vs. day 0 within the same age groups; #P < 0.05 vs. young rats on the same day; n = 7–8/group.
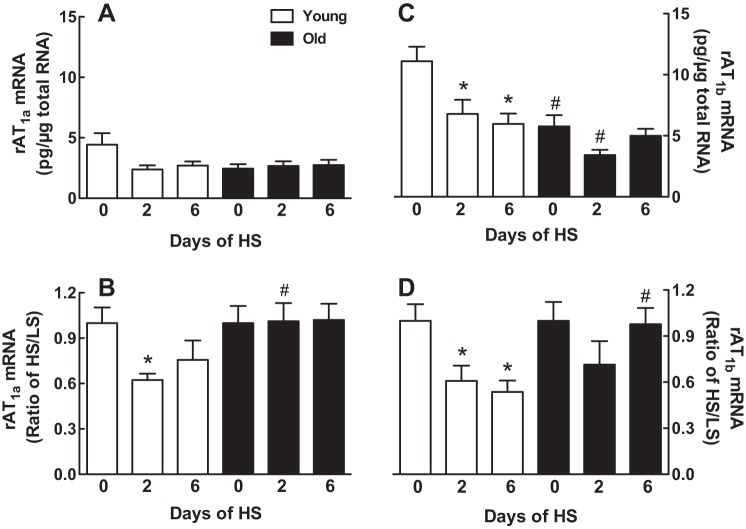
Adrenocortical AT1aR and AT1bR mRNA.
No differences in the AT1aR mRNA expression levels were detected as a function of age or days on the HS diet (Fig. 8A); however, after normalizing AT1aR mRNA expression to day 0 of the HS diet, we found the ratio of HS/LS was reduced by 38% on day 2 in the young rats although no significant inhibition was observed on day 6 (Fig. 8B). There was no effect of the HS diet on AT1aR mRNA levels or on the ratio of HS/LS in the old rats (Fig. 8, A and B).
Fig. 8.
Effect of age on AT1aR and AT1bR mRNA expression in response to a HS diet. AT1aR (A and B) or AT1bR mRNA (C and D) is expressed as means ± SE (A and C) or HS-to-LS ratio (B and D) on days 0, 2, and 6 after the onset of a HS diet; *P < 0.05 vs. day 0 within the same age groups; #P < 0.05 vs. young rats on the same day; n = 6–8/group. rAT1b, rat ANG II type 1b receptor.
AT1bR mRNA expression was 48% less in the old rats compared with the young rats before switching to the HS diet (P < 0.05, by 2-way ANOVA) (Fig. 8C). Expression of the AT1bR mRNA was decreased in the young rats by 39 and 46%, respectively, on days 2 and 6 after transferring to the HS diet (Fig. 8C). While transferring to the HS diet showed a trend to decrease AT1bR mRNA in the old rats on day 2, this effect did not reach statistical significance even after normalizing the data to LS levels (Fig. 8, C and D).
DISCUSSION
The main findings of this study are that aging impaired the adrenal AT1R response to a dietary sodium load in male Fischer rats. Adrenal AT1R densities and AT1bR mRNA were 29 and 48% less, respectively, in old rats before the sodium load, and AT1R densities were not reduced as rapidly in response to a HS diet compared with the young animals. These age-associated effects on adrenal AT1R densities and AT1bR mRNA levels correlated with reduced water intake and plasma aldosterone with little change in urine volume, urine osmolality, or plasma AVP.
In response to an increase in dietary sodium, urine volume increases in an effort to rid the body of excess sodium; however, this ability to increase urine volume is impaired in the elderly (35). These findings in humans are also observed in experimental models of aging. Previous studies have shown that an intracarotid injection of hypertonic sodium chloride resulted in a blunted antidiuretic response in the old rat (20 mo) compared with the young (<6 mo) male (19). Our study in male Fischer 344/BN rats extends these findings by demonstrating that, in response to a dietary sodium load, compared with the young rats, the old animals exhibited a diminished ability to rapidly increase water intake (Fig. 2, A and B) and urine volume (Fig. 4, A and B), which resulted in a decreased ability to rapidly lower urine osmolality (Fig. 4, C and D) and maintain plasma potassium homeostasis (Fig. 4, C and D). This diminished ability to handle a dietary sodium load may have contributed to the greater reduction in NaCl intake (mg/kg body wt) in the old rats relative to the young rats (Fig. 3).
Our findings support previous studies in male Fischer 344 rats subjected to dehydration. Maximum urine electrolyte concentration after 40 h of dehydration was significantly lower in old, 23-mo rats compared with 4-mo rats (12). Furthermore, the fraction of infused sodium excreted during and after expansion with isotonic saline was attenuated in old (22–24 mo) compared with the young (4–6 mo) rats (11). Similar findings of an impaired ability to excrete sodium with volume expansion are observed in humans. Elderly men placed on a sodium-restricted diet had lower urine osmolality than young men (36).
Previous studies have shown that aging alters aldosterone metabolism. While aging had little effect on plasma aldosterone on an unrestricted sodium diet, men and women greater than 50 yr of age had markedly lower plasma aldosterone levels on a LS diet compared with those who were 20–30 yr old (23). Furthermore, urinary aldosterone excretion in 70- to 90-yr-old men was significantly less than found in 18- to 28-yr-old men (21). Urinary excretion of other adrenal mineralocorticoids was shown to be inversely correlated with increasing age from 20 to 70 (64). Consistent with this clinical research, we found that plasma aldosterone in the old rats maintained for 2 wk on a LS diet was nearly one-half the level found in the young rats (Fig. 6).
Not only did we observe lower levels of plasma aldosterone in the old rats on a sodium-restricted diet, we also found the density of adrenocortical AT1R was markedly lower in the old rats compared with the young animals (Fig. 7). We previously demonstrated that a positive correlation exists between the modulation of adrenal AT1R density and plasma aldosterone; a 30% reduction in adrenocortical AT1R density induced by 17β-estradiol in ovariectomized female rats was associated with significant reductions in plasma aldosterone (53). Taken together, our findings suggest the age-associated decline in plasma aldosterone is due to reduced adrenocortical AT1R densities. This observation supports previous studies showing that the adrenal zona glomerulosa in humans (42, 45), rats (9, 20), and cows (50) undergoes an age-dependent impairment in its aldosterone secretory response to ANG II. While our study was conducted solely in male rats, we expect adrenocortical AT1R densities would also be decreased in old female Fischer 344/BN rats since aging (22–24 mo) in female Long-Evans rats was associated with diminished aldosterone secretion in response to ANG II (51).
In contrast to our findings in the adrenal cortex, a previous study in Fisher 344 rats reported higher immunoreactive AT1R protein expression in the adrenal medulla in 24-mo-old rats compared with young adult animals (17). However, this previous study measured immunoreactive AT1R protein expression by Western blot rather than receptor density by radioligand binding, and several studies have challenged the specificity of available AT1R antibodies (13, 24). Thus, it is possible that immunoreactive AT1R protein expression does not correlate with receptor density due to antibody nonspecificity, posttranslational regulation, and/or because of discordant regulation of the AT1R in the adrenal cortex and adrenal medulla. Moreover, it is important to note that the AT1R that mediates aldosterone secretion is located in the zona glomerulosa cells and not the adrenal medulla. The adrenal cortex is not the only tissue reported to exhibit an age-related decline in AT1R density. AT1R densities determined by autoradiography were found to be diminished by >50% in the paraventricular nucleus and by ∼35% in the organum vasculosum laminae terminalis in 20-mo Fischer 344 rats compared with 5- to 15-mo rats (54).
ANG II (via its actions on AT1Rs in the adrenal cortex) is the major regulator of plasma aldosterone during altered sodium intake. Early studies showed that adrenal AT1Rs are upregulated by ANG II and sodium restriction (4–6). Thus, the age-related reduction in adrenocortical AT1Rs is likely a response to decreased plasma ANG II as a function of aging. Although we had insufficient sample material to measure plasma ANG II in this study, reports in male rats have shown that there is an age-related decrease in the rate-limiting step in ANG II formation, i.e., plasma renin activity (37) as well as serum angiotensin-converting enzyme activity (41). Furthermore, plasma ANG II and plasma renin activity are both lower in the elderly compared with young individuals (44). Studies have also shown that the dipsogenic response to ANG II administered subcutaneously was more robust in rats at 3 mo of age compared with 12, 20, or 24 mo of age (60).
Aging is associated with reduced plasma potassium levels (47) and lower fractional excretion of potassium in men and women (43). The elderly are at greater risk for impaired potassium homeostasis than young adults (48, 49). The AT1R plays a critical role in maintaining plasma potassium levels. In this study, we found that the HS diet substantially reduced adrenal AT1R densities after switching from the LS to the HS diet in both young and old rats; however, young rats were able to reduce the density of adrenocortical AT1Rs within 48 h while old rats took at least 6 days to achieve the same magnitude effect (Fig. 7). These data suggest that aging impairs the ability to rapidly downregulate the AT1R in response to an increase in dietary sodium. Therefore, impairment in adrenocortical AT1R regulation could contribute to the reduced ability of the elderly to respond appropriately to a sodium load. Furthermore, the attenuation of rapid AT1R regulation was coincident with the impairment in urine-concentrating ability and the finding that plasma potassium fell in response to increased dietary sodium in old but not young rats (Fig. 5, C and D). Taken together, these findings suggest that the slower ability of adrenal AT1Rs to downregulate in response to sudden increases in dietary sodium results in a more sluggish water intake and urine-concentrating response and subsequent impaired potassium homeostasis.
There are two subtypes of the AT1R (AT1aR and AT1bR) in rats and mice. These receptor subtypes share 95% amino acid homology (57), and there are no currently available pharmacological agents that can effectively differentiate between their protein expression; however, the mRNA levels of these receptor subtypes can be distinguished (33). Studies have shown that the AT1aR mRNA is widely expressed throughout rodent tissues and, in general, is far more abundant than the AT1bR mRNA except in a few tissues, including the adrenal cortex where PCR amplification (27, 59) and in situ hybridization (26) showed the majority (80%) of adrenal AT1R is of the AT1bR subtype. Therefore, we expect the adrenal AT1bR plays a greater role in the response of the adrenal AT1R to a sodium load than the AT1aR subtype; however, we cannot rule out that the minority population of adrenal AT1aRs also contributed to the age-associated dysregulation of adrenal AT1Rs.
Old rats express 48% less AT1bR mRNA in the adrenal cortex than the young rats under conditions of sodium restriction. Therefore, the lower density of adrenocortical AT1Rs is likely the result of an age-related reduction in the transcription of the AT1bR mRNA, since lower mRNA levels could lead to less mRNA translation into AT1bR protein. As a consequence, fewer AT1bRs would be available in the old rat adrenal cortex to stimulate aldosterone secretion in response to ANG II. This age effect on plasma aldosterone would be magnified under LS conditions, since ANG II levels would be elevated and ANG II-induced aldosterone secretion would be increased compared with high dietary sodium conditions in which ANG II levels are suppressed and aldosterone secretion is maximally inhibited (4). In contrast, the AT1aR subtype is not likely to contribute to age effects on adrenal AT1R function, including aldosterone secretion, since no differences were observed in the mRNA expression of the AT1aR transcript in the adrenal cortex between young and old rats on a LS diet. This differential regulation of the two subtypes is not surprising given that these two receptor subtypes have been shown to be discoordinately regulated by dietary sodium in other tissues, including the brains of adult rats (58) and mice (15), suggesting that tissue-specific receptor subtype regulation occurs.
Our finding that the HS more rapidly downregulates the AT1bR transcript in young compared with old rats and strongly correlates with changes in AT1R densities in the young and old animals suggests that impairment in the transcriptional regulation of the AT1bR contributes to the age-associated defect in AT1R regulation in response to increased dietary sodium. Decreased mRNA expression levels could also reflect increased receptor mRNA turnover since receptor expression is known to be regulated at the level of mRNA stability. For example, ANG II downregulates AT1R densities in vascular smooth muscle cells by decreasing mRNA stability (31).
Aging blunts thirst and alters AVP responsiveness to physiologically relevant stimuli (8). Although originally controversial, it has now been demonstrated in numerous studies that, in rodents and humans, plasma AVP increases with aging, and indeed aging has been characterized as a state of relative AVP resistance (8, 39). Our results (Fig. 2, A and B) support prior findings and demonstrate not only an age-associated decrease in water intake, but also a blunted drinking response to HS diet. Additionally, our results indicate that AVP levels, while elevated in old animals compared with young animals, do not change in response to a dietary sodium load. Although much more work is needed, in particular a detailed examination of the time course of potential changes in plasma AVP as animals adjust to the increased salt intake, our preliminary data suggest that, in the case of the young rats, renal mechanisms accommodate the increased salt load so that there is no stimulus for additional AVP secretion. In old animals, on the other hand, it is possible that AVP secretion is already maximally elevated, and the switch to HS fails to alter the already activated state. It would be interesting as well to determine at the cellular level whether production of AVP and the other neurohypophysial hormone, oxytocin, changes with HS in young vs. old rats. Oxytocin is known to exert anorexigenic actions in rodents (46), and central release of oxytocin has been demonstrated in rats following osmotic stimuli (29, 62). If oxytocin released centrally in response to HS in our animals is the reason for the maintained suppression of food intake in the old rats as in the young animals, then, unlike AVP, it would not appear that aging is an oxytocin-resistant state. There is evidence that, unlike AVP, plasma levels of oxytocin do not significantly differ between 3- and 32-mo-old rats (18).
We did not measure blood pressure in this study; however, the male Fischer 344/BN rat is not a model of dietary sodium-induced hypertension. Chugh et al. (16) showed in young (2 mo) and old (20 mo) male Fischer 344/BN rats that a HS (8% NaCl) diet for 4 wk increased systolic blood pressure by <5 mmHg. Therefore, it is unlikely that the age-associated effects were due to hypertension.
Perspectives and Significance
In conclusion, the age-related decline in AT1bRs in the adrenal cortex contributes to reduced water intake and plasma aldosterone levels during conditions of sodium restriction and dysregulation of AT1R-mediated responses to sodium loading, including water intake, urine-concentrating ability, and potassium homeostasis. These findings suggest that dysregulation of adrenocortical AT1bRs in response to dietary sodium manipulation contributes to the defects in water and electrolyte homeostasis observed in the old Fischer male rat. These findings may have clinical implications for dietary sodium consumption by the elderly and suggest this population could be more susceptible to the adverse consequences of a HS diet.
GRANTS
This research was supported by a National Kidney Foundation Grant-in-Aid (H. Ji) and National Institutes of Health Grants R21-AG-037832 (H. Ji), R01-HL-57502 (K. Sandberg), R01-AG-19291 (K. Sandberg), and R01-HL-121456 (K. Sandberg and W. K. Samson), and the Peptide Radioiodination Service Center of the University of Mississippi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.J., W.K.S., and K.S. conception and design of research; H.J., W.Z., X.W., L.M.S., and G.L.C.Y. performed experiments; H.J. analyzed data; H.J., R.C.S., J.G.V., W.K.S., and K.S. interpreted results of experiments; H.J. prepared figures; H.J. and K.S. drafted manuscript; H.J., R.C.S., J.G.V., W.K.S., and K.S. edited and revised manuscript; H.J. and K.S. approved final version of manuscript.
REFERENCES
- 1.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension 52: 932–937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilera G. Role of angiotensin II receptor subtypes on the regulation of aldosterone secretion in the adrenal glomerulosa zone in the rat. Mol Cell Endocrinol 90: 53–60, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Aguilera G, Catt KJ. Regulation of aldosterone secretion by the renin-angiotensin system during sodium restriction in rats. Proc Natl Acad Sci USA 75: 4057–4061, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilera G, Hauger RL, Catt KJ. Control of aldosterone secretion during sodium restriction: adrenal receptor regulation and increased adrenal sensitivity to angiotensin II. Proc Natl Acad Sci USA 75: 975–979, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguilera G, Menard RH, Catt KJ. Regulatory actions of angiotensin II on receptors and steroidogenic enzymes in adrenal glomerulosa cells. Endocrinology 107: 55–60, 1980. [DOI] [PubMed] [Google Scholar]
- 6.Aguilera G, Schirar A, Baukal A, Catt KJ. Angiotensin II receptors. Properties and regulation in adrenal glomerulosa cells. Circ Res 46: I118–I127, 1980. [PubMed] [Google Scholar]
- 7.Baskin S, Robert J, Kendrick Z. Effect of age on body weight, heart rate and blood pressure in pair-caged, male, Fischer 344 rats. Age 2: 47–50, 1979. [Google Scholar]
- 8.Baylis PH. Osmoregulation and control of vasopressin secretion in healthy humans. Am J Physiol Regul Integr Comp Physiol 253: R671–R678, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Belloni AS, Rebuffat P, Malendowicz LK, Mazzocchi G, Rocco S, Nussdorfer GG. Age-related changes in the morphology and function of the zona glomerulosa of the rat adrenal cortex. Tissue Cell 24: 835–842, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Belmin J, Levy BI, Michel JB. Changes in the renin-angiotensin-aldosterone axis in later life. Drugs Aging 5: 391–400, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Bengele HH, Mathias RS, Alexander EA. Impaired natriuresis after volume expansion in the aged rat. Ren Physiol 4: 22–29, 1981. [DOI] [PubMed] [Google Scholar]
- 12.Bengele HH, Mathias RS, Perkins JH, Alexander EA. Urinary concentrating defect in the aged rat. Am J Physiol Renal Fluid Electrolyte Physiol 240: F147–F150, 1981. [DOI] [PubMed] [Google Scholar]
- 13.Benicky J, Hafko R, Sanchez-Lemus E, Aguilera G, Saavedra JM. Six commercially available angiotensin II AT1 receptor antibodies are non-specific. Cell Mol Neurobiol 32: 1353–1365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brudieux R, Rakotondrazafy J. Effect of aging and sodium deprivation on plasma concentration of aldosterone and on plasma renin activity in the rat. Gerontology 42: 229–234, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Liu-Stratton Y, Hassanain H, Cool DR, Morris M. Dietary sodium regulates angiotensin AT1a and AT1b mRNA expression in mouse brain. Exp Neurol 188: 238–245, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Chugh G, Asghar M, Patki G, Bohat R, Jafri F, Allam F, Dao AT, Mowrey C, Alkadhi K, Salim S. A high-salt diet further impairs age-associated declines in cognitive, behavioral, and cardiovascular functions in male Fischer brown Norway rats. J Nutr 143: 1406–1413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdos B, Broxson CS, Landa T, Scarpace PJ, Leeuwenburgh C, Zhang Y, Tumer N. Effects of life-long caloric restriction and voluntary exercise on age-related changes in levels of catecholamine biosynthetic enzymes and angiotensin II receptors in the rat adrenal medulla and hypothalamus. Exp Gerontol 42: 745–752, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fliers E, Swaab DF. Activation of vasopressinergic and oxytocinergic neurons during aging in the Wistar rat. Peptides 4: 165–170, 1983. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SM, Hinke JA, Friedman CL. Neurohypophyseal responsiveness in the normal and senescent rat. J Gerontol 11: 286–291, 1956. [DOI] [PubMed] [Google Scholar]
- 20.Frolkis VV, Verkhratsky NS, Magdich LV. Regulation of aldosterone secretion in old rats. Gerontology 31: 84–94, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Gordon R, Doran F, Thomas M, Thomas F, Cheras P. Solitary kidney and ageing as causes of low renin and aldosterone concentrations: relevance to “low-renin” essential hypertension. Clin Sci Mol Med Suppl 3: 177s–180s, 1976. [DOI] [PubMed] [Google Scholar]
- 22.Hallengren B, Elmstahl S, Galvard H, Jerntorp P, Manhem P, Pessah-Rasmussen H, Stavenow L. 80-year-old men have elevated plasma concentrations of catecholamines but decreased plasma renin activity and aldosterone as compared to young men. Aging (Milano) 4: 341–345, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Hegstad R, Brown RD, Jiang NS, Kao P, Weinshilboum RM, Strong C, Wisgerhof M. Aging and aldosterone. Am J Med 74: 442–448, 1983. [DOI] [PubMed] [Google Scholar]
- 24.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 61: 253–258, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji H, Leung M, Zhang Y, Catt KJ, Sandberg K. Differential structural requirements for specific binding of nonpeptide and peptide antagonists to the AT1 angiotensin receptor. Identification of amino acid residues that determine binding of the antihypertensive drug losartan. J Biol Chem 269: 16533–16536, 1994. [PubMed] [Google Scholar]
- 26.Johren O, Golsch C, Dendorfer A, Qadri F, Hauser W, Dominiak P. Differential expression of AT1 receptors in the pituitary and adrenal gland of SHR and WKY. Hypertension 41: 984–990, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Kakar SS, Riel KK, Neill JD. Differential expression of angiotensin II receptor subtype mRNAs (AT-1A and AT-1B) in the brain. Biochem Biophys Res Commun 185: 688–692, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Kuhnle U, Lewicka S, Fuller PJ. Endocrine disorders of sodium regulation. Role of adrenal steroids in genetic defects causing sodium loss or sodium retention. Horm Res 61: 68–83, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Landgraf R, Neumann I, Schwarzberg H. Central and peripheral release of vasopressin and oxytocin in the conscious rat after osmotic stimulation. Brain Res 457: 219–225, 1988. [DOI] [PubMed] [Google Scholar]
- 30.Laragh JH, Sealey J, Brunner HR. The control of aldosterone secretion in normal and hypertensive man: abnormal renin-aldosterone patterns in low renin hypertension. Am J Med 53: 649–663, 1972. [DOI] [PubMed] [Google Scholar]
- 31.Lasségue B, Alexander RW, Nickenig G, Clark M, Murphy TJ, Griendling KK. Angiotensin II down-regulates the vascular smooth muscle AT1 receptor by transcriptional and post-transcriptional mechanisms: evidence for homologous and heterologous regulation. Mol Pharmacol 48: 601–609, 1995. [PubMed] [Google Scholar]
- 32.Leosco D, Ferrara N, Landino P, Romano G, Sederino S, Cacciatore F, Longobardi G, Dal Canton A, Rengo F. Effects of age on the role of atrial natriuretic factor in renal adaptation to physiologic variations of dietary salt intake. J Am Soc Nephrol 7: 1045–1051, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension 24: 538–548, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Luckey AE, Parsa CJ. Fluid and electrolytes in the aged. Arch Surg 138: 1055–1060, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Luft FC, Fineberg NS, Weinberger MH. The influence of age on renal function and renin and aldosterone responses to sodium-volume expansion and contraction in normotensive and mildly hypertensive humans. Am J Hypertens 5: 520–528, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Luft FC, Weinberger MH, Fineberg NS, Miller JZ, Grim CE. Effects of age on renal sodium homeostasis and its relevance to sodium sensitivity. Am J Med 82: 9–15, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Magro AM, Rudofsky UH. Plasma renin activity decrease precedes spontaneous focal glomerular sclerosis in aging rats. Nephron 31: 245–253, 1982. [DOI] [PubMed] [Google Scholar]
- 38.Miller M. Fluid and electrolyte homeostasis in the elderly: physiological changes of ageing and clinical consequences. Baillieres Clin Endocrinol Metab 11: 367–387, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Miller M. Increased vasopressin secretion: an early manifestation of aging in the rat. J Gerontol 42: 3–7, 1987. [DOI] [PubMed] [Google Scholar]
- 40.Miller M. Nocturnal polyuria in older people: pathophysiology and clinical implications. J Am Geriatr Soc 48: 1321–1329, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Mooradian AD, Lieberman J. Age-related decrease in serum angiotensin converting enzyme activity: the role of thyroidal status and food intake. J Gerontol 45: B24–27, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Morimoto S, Uchida K, Miyamoto M, Kigoshi T, Morise T, Takimoto H, Takeda R. Plasma aldosterone response to angiotensin II in sodium-restricted elderly subjects with essential hypertension. J Am Geriatr Soc 29: 302–307, 1981. [DOI] [PubMed] [Google Scholar]
- 43.Musso CG, Miguel R, Algranati L, Farias Edos R. Renal potassium excretion: comparison between chronic renal disease patients and old people. Int Urol Nephrol 37: 167–170, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Noth RH, Lassman MN, Tan SY, Fernandez-Cruz A Jr, Mulrow PJ. Age and the renin-aldosterone system. Arch Intern Med 137: 1414–1417, 1977. [PubMed] [Google Scholar]
- 45.Ogihara T, Hata T, Maruyama A, Mikami H, Nakamaru M, Mandai T, Kumahara Y. Studies on the renin-angiotensin-aldosterone system in elderly hypertensive patients with an angiotensin II antagonist. Clin Sci 57: 461–463, 1979. [DOI] [PubMed] [Google Scholar]
- 46.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129: 785–791, 1991. [DOI] [PubMed] [Google Scholar]
- 47.Osterlind PO, Alafuzoff I, Lofgren AC, Marklund S, Nystrom L, Sandman PO, Steen B, Winblad B. Blood components in an elderly population. Gerontology 30: 247–252, 1984. [DOI] [PubMed] [Google Scholar]
- 48.Perazella MA. Hyperkalemia in the elderly: a group at high risk. Conn Med 60: 195–198, 1996. [PubMed] [Google Scholar]
- 49.Perazella MA, Mahnensmith RL. Hyperkalemia in the elderly: drugs exacerbate impaired potassium homeostasis. J Gen Intern Med 12: 646–656, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potter CL, Goodfriend TL. Aldosterone production and hormone responsiveness in adrenal glomerulosa cells from cows of different ages. Gerontology 33: 77–86, 1987. [DOI] [PubMed] [Google Scholar]
- 51.Rakotondrazafy J, Brudieux R. Age-related change in plasma aldosterone response to exogenous angiotensin II in the rat. Horm Res 39: 156–160, 1993. [DOI] [PubMed] [Google Scholar]
- 52.Rodeck H, Lederis K, Heller H. The hypothalamoneurohypophysial system in old rats. J Endocrinol 21: 225–228, 1960. [DOI] [PubMed] [Google Scholar]
- 53.Roesch DM, Tian Y, Zheng W, Shi M, Verbalis JG, Sandberg K. Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats. Endocrinology 141: 4629–4636, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Rowland NE, Morien A, Garcea M, Fregly MJ. Aging and fluid homeostasis in rats. Am J Physiol Regul Integr Comp Physiol 273: R1441–R1450, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Salvetti A, Pedrinelli R, Poli L, Giannessi D, Luche AD, Duranti P. The relationship of plasma and urinary aldosterone with sodium balance, plasma renin activity and age in normal subjects. J Nucl Med Allied Sci 21: 7–17, 1977. [PubMed] [Google Scholar]
- 56.Samson WK. Atrial natriuretic factor inhibits dehydration and hemorrhage-induced vasopressin release. Neuroendocrinology 40: 277–279, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Sandberg K. Structural analysis and regulation of angiotensin II receptors. Trends Endo Metab 5: 28–35, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Sandberg K, Ji H, Catt KJ. Regulation of angiotensin II receptors in the rat brain during dietary sodium changes. Hypertension 23: I137–141, 1994. [DOI] [PubMed] [Google Scholar]
- 59.Sandberg K, Ji H, Clark AJ, Shapira H, Catt KJ. Cloning and expression of a novel rat angiotensin II receptor subtype. J Biol Chem 267: 9455–9458, 1992. [PubMed] [Google Scholar]
- 60.Silver AJ, Morley JE, Ishimaru-Tseng TV, Morley PM. Angiotensin II and fluid ingestion in old rats. Neurobiol Aging 14: 519–522, 1993. [DOI] [PubMed] [Google Scholar]
- 61.Speth RC, Harding JW. Radiolabeling of Angiotensin peptides. Methods Mol Med 51: 275–295, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Stricker EM, Verbalis JG. Interaction of osmotic and volume stimuli in regulation of neurohypophyseal secretion in rats. Am J Physiol Regul Integr Comp Physiol 250: R267–R275, 1986. [DOI] [PubMed] [Google Scholar]
- 63.Takeda R, Morimoto S, Uchida K, Miyamori I, Hashiba T. Effect of age on plasma aldosterone response to exogenous angiotensin II in normotensive subjects. Acta Endocrinol (Copenh) 94: 552–558, 1980. [DOI] [PubMed] [Google Scholar]
- 64.Takeda Y, Miyamori I, Yoneda T, Iki K, Takeda R. Effect of aging on urinary excretion of 19-noraldosterone and 18,19-dihydroxycorticosterone. J Steroid Biochem Mol Biol 52: 383–386, 1995. [DOI] [PubMed] [Google Scholar]
- 65.Taylor MM, Baker JR, Samson WK. Brain-derived adrenomedullin controls blood volume through the regulation of arginine vasopressin production and release. Am J Physiol Regul Integr Comp Physiol 288: R1203–R1210, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Weidmann P, De Myttenaere-Bursztein S, Maxwell MH, de Lima J. Effect on aging on plasma renin and aldosterone in normal man. Kidney Int 8: 325–333, 1975. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Ji H, Elena Fabucci M, Falconetti C, Zheng W, Sandberg K. Translational control of the rat angiotensin type 1a receptor by alternative splicing. Gene 341: 93–100, 2004. [DOI] [PubMed] [Google Scholar]