Abstract
This study examines how chronically hyperglycemic rainbow trout modulate glucose kinetics in response to graded exercise up to critical swimming speed (Ucrit), with or without exogenous glucose supply. Our goals were 1) to quantify the rates of hepatic glucose production (Ra glucose) and disposal (Rd glucose) during graded swimming, 2) to determine how exogenous glucose affects the changes in glucose fluxes caused by exercise, and 3) to establish whether exogenous glucose modifies Ucrit or the cost of transport. Results show that graded swimming causes no change in Ra and Rd glucose at speeds below 2.5 body lengths per second (BL/s), but that glucose fluxes may be stimulated at the highest speeds. Excellent glucoregulation is also achieved at all exercise intensities. When exogenous glucose is supplied during exercise, trout suppress hepatic production from 16.4 ± 1.6 to 4.1 ± 1.7 μmol·kg−1·min−1 and boost glucose disposal to 40.1 ± 13 μmol·kg−1·min−1. These responses limit the effects of exogenous glucose to a 2.5-fold increase in glycemia, whereas fish showing no modulation of fluxes would reach dangerous levels of 114 mM of blood glucose. Exogenous glucose reduces metabolic rate by 16% and, therefore, causes total cost of transport to decrease accordingly. High glucose availability does not improve Ucrit because the fish are unable to take advantage of this extra fuel during maximal exercise and rely on tissue glycogen instead. In conclusion, trout have a remarkable ability to adjust glucose fluxes that allows them to cope with the cumulative stresses of a glucose overload and graded exercise.
Keywords: fish exercise physiology, carbohydrate metabolism, hepatic glucose production, continuous tracer infusion, Oncorhynchus mykiss
in mammals, glucose plays an essential role as a fuel for the brain and contributes significantly to energy metabolism in working muscles (40). During aerobic exercise, glucose oxidation accounts for 10 to 40% of metabolic rate (43, 45), and glucose fluxes can be stimulated five-fold over resting values (37, 45). By contrast, the role of glucose has not been clearly characterized in fish, and the evidence available for a model species like rainbow trout is still ambiguous (34). The high sensitivity of trout glycemia to various hormones, water osmolarity, and diet suggests that glucose is an important substrate (34). However, the opposite could also be argued because trout are generally renowned for their poor glucoregulation (26) and only normalize glycemia very slowly in glucose tolerance tests (22). In addition, they decrease glucose flux during prolonged, low-intensity swimming, whereas mammals doing equivalent exercise show a two- to four-fold increase (39). Glycogen in red and white muscle has also been shown to decrease significantly during low- and high-intensity swimming (32, 39). This suggests that glucose plays a minor role as a fuel for locomotor muscles, but the glucose kinetics of fish have never been measured at high exercise intensities when carbohydrates become preferred.
Trout were previously considered to have a limited capacity for modulating glucose fluxes because the only changes demonstrated were a twofold increase with epinephrine (46), and more minor effects of temperature (18), and low-intensity swimming (39). However, we have recently put the plasticity of trout glucose kinetics to a more stringent test by infusing exogenous glucose at twice the normal rate of hepatic production in resting, already hyperglycemic animals (8). Chronically hyperglycemic fish were selected for these experiments because they already have elevated glucose fluxes (8). This chronic state of hyperglycemia is obtained by raising the animals on a high-fat diet, which reduces glucose phosphorylation and hepatic lipogenesis and increases hepatic glucose release (13). Therefore, they face a particularly important challenge when they must modulate their fluxes to cope with an additional glucose overload. Surprisingly, they completely suppressed endogenous production (Ra glucose) and stimulated disposal by 2.6-fold (Rd glucose). Such mammal-like regulation is particularly surprising because it occurred in hyperglycemic fish with chronically elevated baseline glucose kinetics, and possibly with some degree of insulin resistance. Under such conditions, it is unclear whether Rd glucose was pushed to its upper limit because mammals receiving exogenous glucose can boost disposal to higher levels during exercise (1, 19, 24, 25) than at rest (12, 20, 28). However, the effect of this combination of exercise and exogenous glucose has never been measured in rainbow trout.
Most human studies testing the effects of exogenous glucose on athletic performance show an improvement, although some report no change or even a decrease (7). It is not known whether enhancing glucose availability could increase critical swimming speed (Ucrit) in trout, but a previous study testing exogenous lactate showed no effect (30). The supply of exogenous glucose could also affect fuel selection by causing a switch from lipids to carbohydrates. Therefore, it could potentially decrease metabolic rate (MO2) because 15 to 30% less oxygen is needed to produce the same amount of ATP when oxidizing carbohydrates rather than lipids (38, 47). This could result in a lower metabolic cost of transport (COT) measured as the amount of oxygen needed to move one unit body mass by one unit distance (42). Therefore, the goals of this study are 1) to quantify the effects of graded swimming on the glucose kinetics of rainbow trout, 2) to determine how the supply of exogenous glucose modulates the changes in glucose fluxes caused by exercise alone, and 3) to see whether exogenous glucose increases swimming performance (Ucrit) or decreases COT. We anticipate that glucose fluxes will show a greater decrease at high swimming speeds than previously observed during sustained, low-intensity exercise (39), that exogenous glucose will suppress Ra and stimulate Rd to higher values than reported for rest (8), and that it will fail to improve Ucrit but decrease COT.
METHODS
Animals.
Rainbow trout of both sexes (Oncorhynchus mykiss Walbaum) were purchased from Linwood Acres Trout Farm (Campbellcroft, ON, Canada). Because no sex differences were observed, all of the results comprise pooled data from both sexes. They were fed commercial food pellets (44% protein, 26% fat, 15% carbohydrate) (5.5 Optimum mix from Corey Nutrition, Fredericton, NB, Canada). This feed contains 26% lipids, and such a high-fat diet causes chronic hyperglycemia in rainbow trout (13), which was verified in our animals (8). The fish were held in a 1,200-liter flow-through tank in dechlorinated Ottawa tap water maintained at 13°C and were exposed to a 12:12 h light-dark photoperiod. They were acclimated to these conditions for a minimum of 2 wk before experiments. The animals were randomly divided into two groups for 1) glucose kinetics measurements (316 ± 12 g; n = 22) and 2) for tissue glycogen and glucagon measurements (318 ± 19 g; n = 20). The fish used for glucose kinetics were divided into “control” and “exogenous glucose,” while the tissue glycogen and glucagon group was divided into “nonexercised controls” and “exercised.” Table 1 shows the average mass, length, Fulton's condition factor (3), and water velocity at rest and at Ucrit. All of the procedures were approved by the Animal Care Committee of the University of Ottawa and adhered to the guidelines established by the Canadian Council on Animal Care.
Table 1.
Average mass, length, condition factor, resting water velocity and Ucrit water velocity for fish receiving no exogenous glucose (control), with exogenous glucose, and fish used for tissue glycogen and glucagon analysis
| Control Group | Exogenous Glucose Group | Glycogen and Glucagon Group | |
|---|---|---|---|
| Mass, g | 314 ± 14.0 (10) | 318 ± 20.0 (12) | 318 ± 19.2 (20) |
| Length, cm | 31.7 ± 0.4 (10) | 32.1 ± 0.3 (12) | 31.0 ± 0.5 (20) |
| Fulton's condition factor, K | 0.98 ± 0.03 (10) | 0.95 ± 0.03 (12) | 1.0 ± 0.03 (20) |
| Average resting water velocity, cm/s | 15.8 ± 0.2 (10) | 16.0 ± 0.2 (12) | 15.3 ± 0.5 (10) |
| Average Ucrit water velocity, cm/s | 73.3 ± 4.9 (10) | 75.1 ± 5.5 (12) | 87.4 ± 4.1 (10) |
Values are expressed as means ± SE; sample size (n) appears in parentheses. There were no differences between any of the treatment groups (P > 0.05, using one-way ANOVA).
Catheterizations.
Fish were fasted for 24 h prior to surgery. They were anesthetized with ethyl 3-aminobenzoate methanesulfonate (MS-222; 60 mg/l) (Syndel Laboratories, Nanaimo, BC, Canada), and the fish used for glucose kinetics measurements were doubly cannulated with BTPE-50 catheters (Instech Laboratories, Plymouth Meeting, PA) in the dorsal aorta, as described previously (17). Fish used for tissue glycogen and glucagon measurements were implanted with a single dorsal aorta catheter. All catheters were kept patent by flushing with Cortland saline containing 50 U/ml heparin (Sigma-Aldrich, St. Louis, MO). Only animals with a hematocrit >20% after recovery from surgery were used in experiments.
Swim tunnel respirometry and Ucrit protocol.
After surgery, each animal was allowed to recover overnight in a 90-liter swim tunnel respirometer (Loligo Systems, Tjele, Denmark) with a water velocity of 0.5 body length per second (BL/s). This low water flow rate minimizes stress, and it requires no swimming, allowing the fish to rest quietly at the bottom of the respirometer. The swim tunnel was filled with the same quality of water as the holding tank and was kept at 13°C. It was well oxygenated (>95% saturation) by bubbling air through a column containing glass beads. MO2 was measured by intermittent flow respirometry using galvanic oxygen probes (Loligo Systems) connected to a DAQ-PAC-G1 instrument (Loligo Systems) controlled with AutoResp software (version 2; Loligo Systems). The probes were calibrated before measurements using air-saturated water (20.9% O2). Both groups (with or without exogenous glucose) performed a stepwise critical swimming speed (Ucrit) protocol, as detailed by Teulier et al. (42).
Glucose kinetics.
The catheters were made accessible through the swim tunnel lid by channeling them through a water-tight port. The rates of glucose appearance (Ra) and glucose disposal (Rd) were measured by continuous infusion of 6-[3H]glucose (PerkinElmer, Boston, MA; 1.691 TBq/mmol). Infusates were freshly prepared immediately before each experiment by drying an aliquot of the solution obtained from the supplier under N2 and resuspending in Cortland saline. A priming dose equivalent to 6 h of infusion was injected as a bolus at the start of each infusion (time −60 min) to reach isotopic steady state in <45 min (8). With this priming dose, glucose-specific activity reached steady state as follows: 399 ± 29.3 Bq/μmol (50 min after the start of tracer infusion), 400 ± 28.6 Bq/μmol (55 min), 401 ± 28.4 Bq/μmol (60 min) (n = 22). For both experimental groups, glucose kinetics were quantified by infusing labeled glucose at 1 ml/h using a calibrated syringe pump (Harvard Apparatus, South Natick, MA). Infusion rates for labeled glucose averaged 5,952 ± 209 Bq·kg−1·min−1 (n = 22), and these trace amounts only accounted for 0.00005% of the baseline rate of hepatic glucose production in normoglycemic fish (39). In addition, the group receiving exogenous glucose was supplied with unlabeled glucose at a rate of 20 μmol·kg−1·min−1. The exact infusion rate (∼1 ml/h) was determined individually for each fish to adjust for differences in body mass. Blood samples (100 μl each) were taken after 50, 55, and 60 min of tracer infusion to quantify baseline glucose kinetics (and to confirm isotopic steady state), as well as every 20 min thereafter corresponding to the stepwise increase in swimming speed. Because there were no differences between the three baseline values, only one value was plotted in each graph. The amount of blood sampled from each fish accounted for <10% of total blood volume. Samples were immediately deproteinized in 200 μl of perchloric acid (6% wt/wt) and centrifuged for 5 min at 13,523 g (Eppendorf 5425, Brinkman, Rexdale, Canada). Supernatants were kept frozen at −20°C until analyses.
Tissue glycogen and plasma glucagon.
Tissue glycogen and plasma glucagon were measured in a separate batch of fish. After surgery, nonexercised fish were placed in individual opaque Plexiglas boxes that were filled with well-oxygenated water, while the swimming individuals recovered in a 90-liter swim tunnel respirometer (Loligo Systems) with a low water velocity of 0.5 BL/s requiring no swimming. The Ucrit protocol used for glucose kinetics measurements was also used here for the exercised group. Plasma glucagon measurements were only made on the exercised group, using their resting condition as a control. Blood samples (200 μl) were taken at rest (baseline) and at 2.0 BL/s, and every 0.2 BL/s increment thereafter until Ucrit was reached. Samples were centrifuged for 5 min at 13,523 g (Eppendorf 5425; Brinkman, Rexdale, ON, Canada) to separate plasma that was kept frozen at −80°C until analyses. For tissue glycogen measurements, fish from the nonexercised and exercised groups were killed with an overdose of pentobarbital sodium through the catheter (Euthanyl; Abrax Pharmaceutical Products, Schaumburg, IL). One gram of red muscle and white muscle as well as the whole heart, liver, kidney, and brain were removed and freeze-clamped with aluminum tongs precooled in liquid nitrogen. White muscle was always sampled below the dorsal fin. All tissue samples were frozen within 5 min of death. They were stored at −80°C until subsamples were homogenized (Polytron, Kinematica, Littau, Switzerland) and analyzed.
Sample analyses.
All metabolites and glucagon were measured spectrophotometrically using a Spectra Max Plus384 Absorbance Microplate Reader (Molecular Devices, Sunnyvale, CA). To measure glucose activity, samples were dried under N2 to eliminate tritiated water and resuspended in distilled water. Radioactivity was measured by scintillation counting (Beckman Coulter LS 6500, Fullerton, CA) in Bio-Safe II scintillation fluid (RPI, Mount Prospect, IL). Plasma glucagon was measured using a commercially available human glucagon ELISA kit (Crystal Chem, Downers Grove, IL). This specific kit uses a particular COOH-terminal anti-glucagon fragment, and it has been previously validated for fish glucagon (29). Tissue glycogen was measured using the amyloglucosidase hydrolysis method with a commercially available glucose oxidase assay kit (Sigma-Aldrich) (14).
Calculations and statistics.
Critical swimming speed (Ucrit), total cost of transport (TCOT), and net cost of transport (NCOT) were calculated as previously described by Teulier et al. (42). TCOT is the total amount of oxygen required to move one unit body mass by one unit distance, which includes the cost of sustaining life in resting tissues. NCOT is the oxygen cost to power locomotion alone and excludes all resting costs. Glucose fluxes were calculated using the equations of Steele (41). The steady-state equation was used to calculate baseline flux under resting conditions, while the nonsteady-state equation was used during exercise to calculate Ra and Rd glucose separately. The rate of endogenous glucose production (endogenous Ra) was calculated by subtracting the rate of exogenous glucose infusion from measured values for total Ra glucose. Statistical comparisons were performed using one-way ANOVA and one- or two-way repeated-measures analysis of variance (RM-ANOVA) with the Dunnett's post hoc test to determine which values were significantly different from control. One- or two-tailed t-tests and paired t-tests were also used to compare means. Two-tailed t-test was used to compare Ucrit between groups, and paired t-test was used to compare hematocrit before and after Ucrit (SigmaPlot v.12, Systat Software, San Jose, CA). When the assumptions of normality or equality of variances were not met, Friedman's nonparametric RM-ANOVA on ranks was used, or the data were normalized by log10 transformation before parametric analysis. Values are presented as means ± SE, and a level of significance of P < 0.05 was used in all tests.
RESULTS
Metabolic rate, critical swimming speed, and cost of transport.
MO2 for control fish (no exogenous glucose) and fish receiving exogenous glucose are shown in Fig. 1A as a function of speed in BL/s. MO2 was lower in fish receiving exogenous glucose than in controls (P = 0.040; Fig. 1A), but increased with swimming speed in both groups (P < 0.001). MO2 increased from a resting value of 67.4 ± 5.0 μmol·kg−1·min−1 to a maximum of 149 ± 31 μmol·kg−1·min−1 in the controls, and from 54.3 ± 2.6 to 169 ± 5.9 μmol·kg−1·min−1 in fish receiving glucose. Figure 1B shows changes in MO2 as a function of exercise intensity expressed as %Ucrit. The data were fitted with a second-order polynomial regression (r2 = 0.289; P < 0.001): MO2 = 61.563 − 0.0544 (%Ucrit) + 0.00614 (%Ucrit)2. Exogenous glucose had no effect on Ucrit, which was 2.3 ± 0.1 BL/s in controls and 2.3 ± 0.2 BL/s in the treatment group (P = 0.873). TCOT and NCOT for control fish (no exogenous glucose) and fish receiving exogenous glucose are shown in Fig. 2. TCOT was lower in fish receiving glucose than in controls (P = 0.036; Fig. 2A). It decreased with speed from 5.0 ± 0.5 to 2.0 μmol O2·kg−1·m−1 in the control fish (P < 0.001), and from 3.8 ± 0.2 to a minimal value of 2.2 ± 0.1 μmol O2·kg−1·m−1 in fish receiving glucose (P < 0.001). NCOT was not different between the treatment groups (P = 0.595; Fig. 2B). It increased from 0.4 ± 0.2 to 1.3 ± 0.3 μmol O2·kg−1·m−1 in the control fish (P < 0.001) and from 0.2 ± 0.1 to a maximal value of 1.7 ± 0.1 μmol O2·kg−1·m−1 in fish receiving glucose (P < 0.001).
Fig. 1.

Effects of graded swimming on the metabolic rate (MO2) in fish receiving no exogenous glucose (control group; ●) and fish receiving exogenous glucose (○). A: MO2 as a function of swimming speed [in body lengths (BL)/s]. Values are expressed as means ± SE; n = 10 for controls and 12 for exogenous glucose. Within each treatment group, MO2 increased with speed [P < 0.001; two-way repeated-measures (RM) ANOVA on ranks]. MO2 was lower for exogenous glucose than for controls (P < 0.05; two-way RM-ANOVA on ranks). B: MO2 as a function of exercise intensity (expressed as %Ucrit). The thick line was fitted by a second-order polynomial regression (r2 = 0.289; P < 0.001): MO2 = 61.563-0.0544 (%Ucrit) + 0.00614 (%Ucrit)2. Dashed lines indicate confidence intervals (± 95%).
Fig. 2.
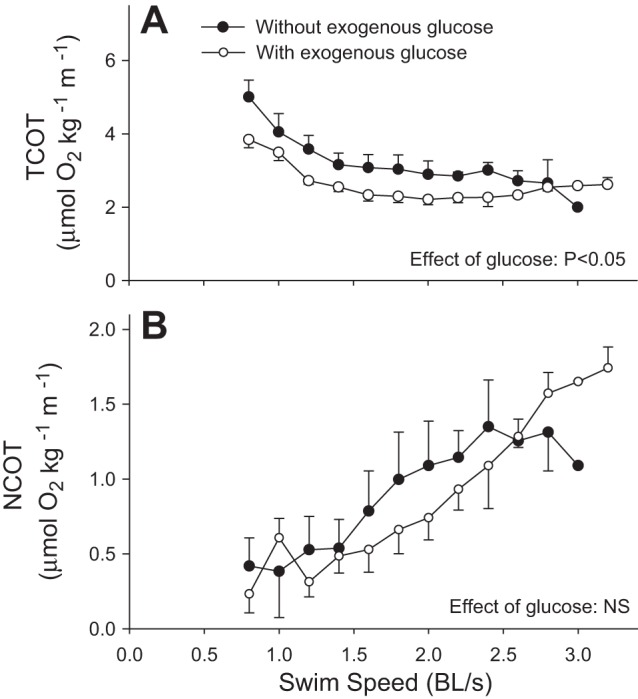
Total cost of transport (TCOT) (A), and net cost of transport (NCOT) (B) in fish receiving no exogenous glucose (control group; ●) and fish receiving exogenous glucose (○) during graded swimming. Values are expressed as means ± SE (n = 10 for controls and 12 for exogenous glucose). Within each treatment group, NCOT increased with speed, while TCOT decreased (P < 0.001; two-way RM-ANOVA). TCOT was lower for exogenous glucose than for controls (P < 0.05; two-way RM-ANOVA), but no significant difference in NCOT was detected between treatments.
Carbohydrate metabolism in control fish.
In control fish receiving no exogenous fuel, glucose concentration was independent of swimming speed and remained constant at 15.6 ± 0.4 mM throughout the experiment (P = 0.305; Fig. 3A). Lactate concentration increased from a resting value of 1.0 ± 0.2 to a maximum of 3.1 mM at the highest speed (P = 0.002; Fig. 3A). Glucose-specific activity decreased with speed from 415 ± 35 to 235 Bq/μmol (P = 0.039; Fig. 3B). Figure 4 shows the effects of graded swimming on glucose fluxes. Because glucose concentration remained constant, Ra and Rd of glucose were never different from each other (P > 0.05), and they showed the same changes with speed. Ra glucose remained constant until 2.4 BL/s, where there was an increase from 15.6 ± 1.4 to 22.1 μmol·kg−1·min−1 (P = 0.012). Rd glucose followed the same pattern with an increase from 15.6 ± 1.4 to 21.8 μmol·kg−1·min−1 at speeds greater than 2.4 BL/s (P = 0.047).
Fig. 3.

Blood glucose and lactate concentrations (A) and blood glucose specific activity (B) in control fish receiving no exogenous glucose during graded swimming. Values are expressed as means ± SE. Numbers indicated under mean glucose values are sample sizes (n) for each speed (n decreases with speed because individual fish reached different maximal exercise intensities). Lactate concentration increased with speed, while glucose specific activity decreased with speed (P < 0.05; one-way RM-ANOVA). Dunnett's post hoc test could not identify specific means that were statistically different from baseline.
Fig. 4.
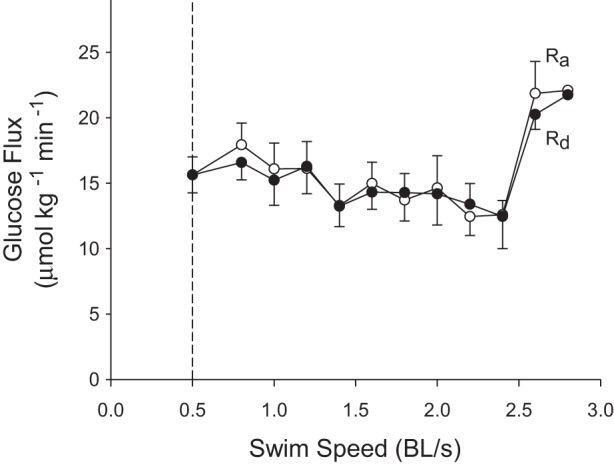
Effects of graded swimming on the glucose fluxes of control fish receiving no exogenous glucose. The rate of endogenous (hepatic) glucose production (endogenous Ra) is shown by open circles, and the rate of glucose disposal (Rd) by solid circles. Values are expressed as means ± SE; n = 10 below 1.8 BL/s, but <10 at higher speeds because individual fish reached different maximal exercise intensities. Both endogenous Ra and Rd increased with speed (P < 0.05; one-way RM-ANOVA). Dunnett's post hoc test could not identify specific means that were statistically different from baseline.
Carbohydrate metabolism in fish receiving exogenous glucose.
In fish receiving exogenous fuel, glucose concentration increased from a resting value of 17.7 ± 1.6 to a maximum of 43.8 ± 11.3 mM at the highest speed (P < 0.001; Fig. 5A). Lactate concentration increased from a resting value of 1.1 ± 0.4 to a maximum of 3.1 ± 1.3 mM at the highest swimming speed (P < 0.001; Fig. 5A). Glucose-specific activity decreased with speed from 390 ± 44 to 145 ± 56 Bq/μmol (P < 0.001; Fig. 5B). Figure 6 shows the effects of graded swimming and exogenous glucose on glucose fluxes. Measured total Ra (= rate of endogenous hepatic glucose production + rate of exogenous glucose administration) increased progressively from 16.4 ± 1.6 to 42.7 ± 13.4 μmol·kg−1·min−1 as exogenous glucose was supplied and speed increased (P < 0.001). Rd glucose increased from 16.4 ± 1.6 to 40.1 ± 13 μmol·kg−1·min−1 as speed increased (P < 0.001). Endogenous Ra glucose decreased from 16.4 ± 1.6 to 4.1 ± 1.7 μmol·kg−1·min−1 before increasing to 22.7 ± 13.4 μmol·kg−1·min−1 at higher speeds than 2.6 BL/s (P < 0.001).
Fig. 5.
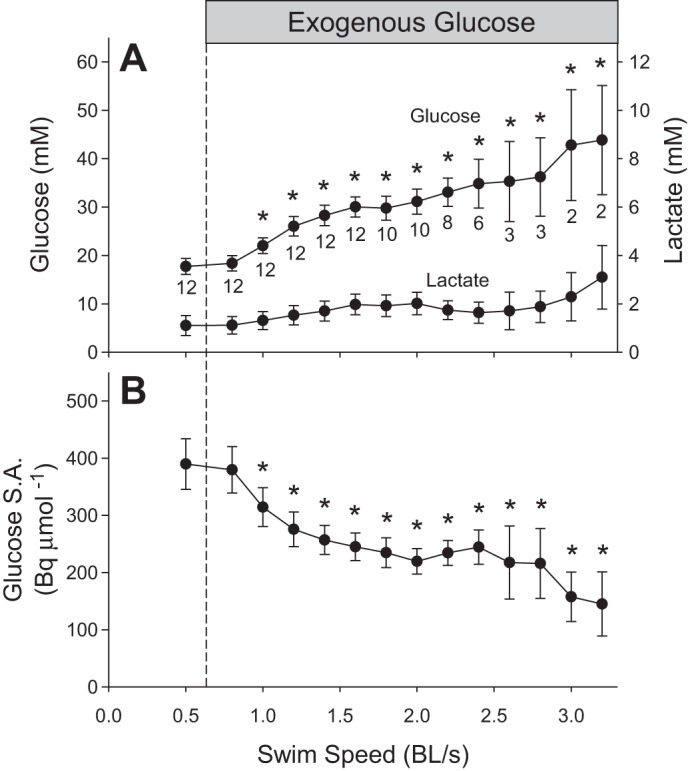
Blood glucose and lactate concentrations (A) and blood glucose specific activity (B) in fish receiving exogenous glucose during graded swimming. Values are expressed as means ± SE. Numbers indicated under mean glucose values are sample sizes for each speed. These three parameters changed with speed (P < 0.001; one-way RM-ANOVA on log10 transformation). *Significant differences from baseline. For lactate concentration, Dunnett's post hoc test could not identify specific means that were different from baseline.
Fig. 6.
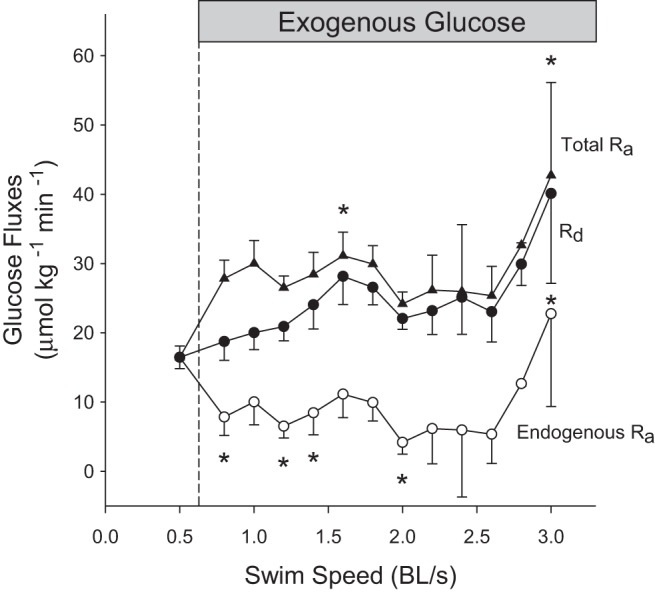
Effects of graded swimming on the glucose fluxes of fish receiving exogenous glucose. The measured total rate of appearance of glucose (Total Ra; ▲) is the sum of endogenous glucose production (endogenous Ra; ○) and exogenous glucose supply. The rate of glucose disposal (Rd) is indicated with solid circles. Values are expressed as means ± SE; n = 12 below 1.6 BL/s, but <10 at higher speeds because individual fish reached different maximal exercise intensities. Total Ra, endogenous Ra, and Rd glucose changed with speed (P < 0.001; one-way RM-ANOVA). *Significant difference from baseline for each parameter.
Relative changes in Rd glucose with exercise.
Control fish receiving no exogenous fuel decreased Rd glucose to 81.1 ± 6.9% of baseline values before increasing to 195% at higher speeds than 2.4 BL/s (P = 0.017; Fig. 7). Fish receiving exogenous fuel increased Rd glucose progressively from 100 to 275 ± 143% of resting values before exogenous glucose was supplied (P < 0.001).
Fig. 7.

Relative changes in Rd glucose for control fish receiving no exogenous glucose (solid circles; n = 10) and fish supplied with exogenous glucose (○; n = 12). Values are expressed as means ± SE. Speed had an effect on relative Rd glucose in both treatment groups (P < 0.05; one-way RM-ANOVA). Dunnett's post hoc test could not identify specific means that were different from baseline.
Potential contribution of glucose oxidation to MO2.
The relative importance of glucose as an oxidative fuel was calculated after assuming either that 100% or only 50% of Rd glucose is oxidized (100% Rd provides the highest possible contribution of glucose to metabolic rate; 50% Rd is the average value observed in resting mammals). For the control group, the relative contribution of glucose to MO2 varied with swimming speed (P = 0.002; Fig. 8A). If 100% of Rd is oxidized, the maximal possible contribution of glucose decreases progressively from 136 ± 16% to 51 ± 11%, before increasing to 111% at speeds higher than 2.4 BL/s. If 50% of Rd is oxidized, the maximal contribution of glucose decreases from 67.9 ± 7.8% to a minimum of 25.5 ± 5.4%, before rising back to 55.5% at speeds higher than 2.4 BL/s. In the fish receiving exogenous glucose, the relative contribution of this fuel to MO2 did not vary with swimming speed (P = 0.12; Fig. 8B). If 100% of Rd is oxidized, glucose alone could account for total MO2 because all calculated values are above 100%. If 50% of Rd is oxidized, the relative contribution of glucose to MO2 would average 88.3 ± 4.4%.
Fig. 8.
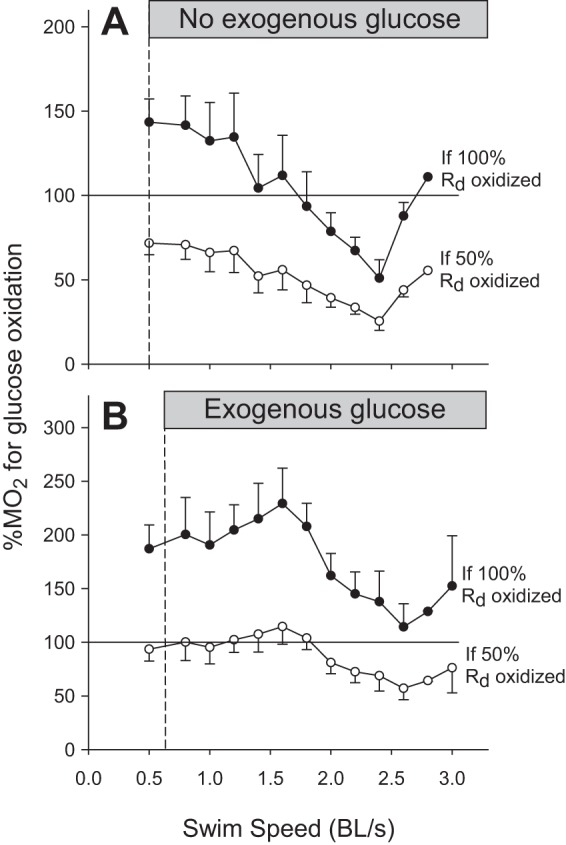
Calculated percentages of MO2 that could be accounted for by glucose oxidation in control fish receiving no exogenous glucose (A) and fish receiving exogenous glucose (B) during graded swimming. Values were calculated assuming that either 100% (●) or only 50% (○) of Rd glucose was oxidized. Values are expressed as means ± SE; n = 10 for controls and 12 for exogenous glucose. Percentage of MO2 changed in control fish with speed (P < 0.05; one-way RM-ANOVA) and Dunnett's post hoc test could not identify specific means that were different from baseline.
Impact of flux regulation on blood glucose concentration.
To evaluate the effects of the changes in glucose kinetics reported here, Fig. 9 provides a comparison of observed concentrations with theoretical concentrations if glucose fluxes had not responded to the administration of exogenous glucose. Three different scenarios were used to calculate these hypothetical changes in glycemia: 1) if Ra glucose had not been suppressed, 2) if Rd glucose had not been stimulated, and 3) if both, Ra and Rd, had remained constant throughout the infusion of exogenous glucose. Observed blood glucose concentrations reached 43.8 mM after ∼4 h of exogenous glucose infusion. However, hypothetical fish would have reached 74 (if Ra was not suppressed), 77 (if Rd was not stimulated), and 114 mM (if glucose fluxes had not responded at all to exogenous supply).
Fig. 9.
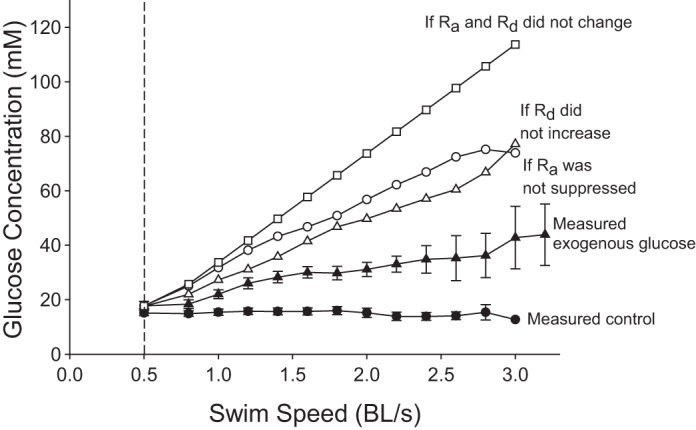
Comparison of measured blood glucose concentrations with theoretical values calculated for hypothetical fish that would fail to regulate their glucose fluxes when exogenous glucose is provided during graded swimming. Measured blood glucose concentrations are presented for control fish (●) and for those receiving exogenous glucose (▲). Theoretical concentrations are given for three different scenarios: 1) if Ra glucose had not been suppressed (○); 2) if Rd glucose had not been stimulated (△), and 3) if both, Ra and Rd, had remained at baseline throughout the infusion of exogenous glucose (□).
Changes to plasma glucagon and tissue glycogen during graded swimming.
In a separate batch of fish, we have measured plasma glucagon during graded swimming and the effects of a Ucrit test on tissue glycogen. Rainbow trout showed no change in plasma glucagon concentrations that averaged 143.7 ± 11 pg/ml (P = 0.8; Fig. 10). Table 2 shows body mass and glycogen concentration for red and white muscle, heart, liver, kidney, and brain in nonexercised and exercised trout. White muscle, heart, liver, kidney, and brain glycogen concentrations were all lower in the exercised group (P < 0.05). We also found that hematocrit decreased from 25.1 ± 1.8% to 21.4 ± 1.9% (P = 0.001) after reaching Ucrit.
Fig. 10.
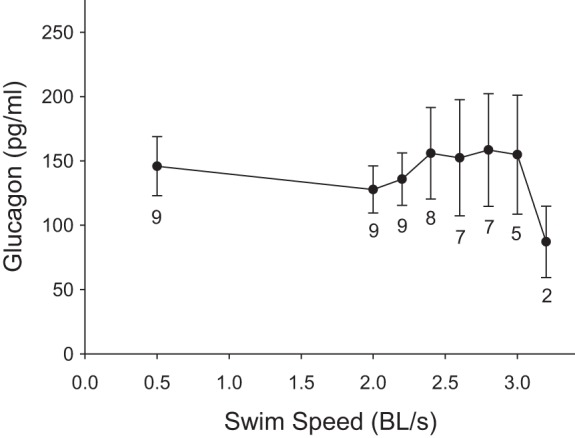
Effects of graded swimming on plasma glucagon concentrations. Values are expressed as means ± SE. Numbers indicated under mean glucose values are sample sizes (n) for each speed (n decreases with speed because individual fish reached different maximal exercise intensities). There was no effect of swimming speed on glucagon concentration (P = 0.074; one-way RM-ANOVA).
Table 2.
Mean body mass and glycogen concentration in nonexercised and exercised fish to Ucrit
| Nonexercised | Exercised | |
|---|---|---|
| Mass, g | ||
| Whole body | 329 ± 26.7 | 306 ± 28.4 |
| Glycogen Concentration, μmol glucosyl units/g | ||
| Red muscle | 46.2 ± 1.8 | 58.8 ± 9.5 |
| White muscle | 106.4 ± 26.6 | 51.7 ± 7.9* |
| Heart | 96.8 ± 16.1 | 52.3 ± 8.8* |
| Liver | 76.6 ± 9.4 | 42.8 ± 13.4* |
| Kidney | 94.2 ± 19.3 | 36.2 ± 3.7* |
| Brain | 70.7 ± 14.4 | 36.4 ± 8.7* |
Red muscle and white muscle mass were assumed to be 5% and 60% of body mass, respectively (27). The sample size for each tissue was n = 10.
Significant differences between exercised and nonexercised groups (P < 0.05, using one-tailed t-test).
DISCUSSION
This study shows that the glucose fluxes of rainbow trout are unaffected at exercise intensities below 2.5 BL/s but may be stimulated at the highest swimming speeds. By comparison, mammals already start increasing fluxes at low work intensities to reach up to five times baseline values during intense exercise. When trout receive exogenous glucose during swimming, they are able to stimulate disposal more strongly than with exogenous supply alone or exercise alone, reaching maximal values of 40 μmol·kg−1·min−1. In addition, they suppress hepatic glucose production, but not completely as they do at rest (8). Supplying extra carbohydrates in the form of glucose does not improve swimming performance because Ucrit remains constant, as previously observed for exogenous lactate (30). However, the metabolic rate of fish receiving exogenous glucose is consistently lower than in controls, and the total cost of transport is reduced accordingly. A switch from lipids to more carbohydrates allows them to use oxygen more efficiently for ATP production because carbohydrate oxidation is accomplished with a higher P/O ratio than lipid oxidation (38, 47).
Glucose kinetics during graded swimming.
This paper is the first to characterize the effects of swimming intensity up to Ucrit on the glucose fluxes of rainbow trout. The only information previously available was that, unlike mammals, trout progressively decrease Ra and Rd glucose during sustained, mild exercise of constant intensity (−33% over 4 h) (39). Therefore, we had anticipated that intense swimming would cause an even stronger inhibition of glucose fluxes. This hypothesis is now rejected because trout show no decrease in fluxes at speeds below 2.5 BL/s and even appear to stimulate fluxes at maximal exercise intensities (Fig. 4). In mammals, both sustained and incremental exercise gradually increase Ra glucose (37, 45). Plasma epinephrine may play an important role in regulating glucose fluxes in trout because the levels of this hormone decrease below baseline during prolonged, low-intensity swimming, potentially explaining the inhibition of Ra observed previously (39). Low epinephrine levels have been shown to impair gluconeogenesis and glycogenolysis in isolated trout hepatocytes (48). At high swimming speeds, there appears to be a large increase in Ra and Rd glucose. Such a response could be mediated by circulating glucagon and catecholamines that are known to stimulate gluconeogenesis and glycogenolysis (15, 34). However, no change in glucagon concentration was observed, even at maximal swimming speeds (Fig. 10). Therefore, catecholamines could be the major regulator of hepatic glucose production in trout. Given the nature of the experiments, high swimming speeds have lower sample sizes because very few fish can reach the highest Ucrit. Closer statistical scrutiny reveals that different sample sizes across speeds prevent the data from meeting the assumption of sphericity (Mauchly's test; P < 0.05). Therefore, the apparent stimulation of glucose fluxes observed here during intense swimming should be taken with caution. It is still unclear whether circulating glucose plays a significant role in allowing trout to reach maximal swimming speeds. After reaching Ucrit, however, we found that glycogen concentration in white muscle, heart, liver, kidney, and brain was significantly lower than in control, nonexercised fish (Table 2). The observed 52% decrease in white muscle glycogen stores shows that this intramuscular fuel is essential to reach Ucrit.
Pushing the limits of glucose disposal.
This study demonstrates that the response of Rd glucose to high-speed swimming (Fig. 4) is weaker than in resting trout infused with exogenous glucose that increase Rd to 28 μmol·kg−1·min−1 (8). The different magnitude of the responses can be explained by considering what signals are involved in each situation. In swimming trout, glucose disposal could be stimulated because exercise itself could cause an insulin-independent migration of GLUT4 from intracellular stores to the membranes of myocytes and adipocytes (23). In resting trout receiving glucose, GLUT4 migration could be mediated by elevated insulin levels (23) that may also activate liver GLUT2 (35), glycolysis (11, 34, 35), and glycogen synthesis (33, 35). In addition, exogenous glucose causes further hyperglycemia that could activate brain GLUT2 (34) and maintains higher blood-to-tissue glucose gradients that drive disposal. Some glucose will also be lost in urine because the kidney is not able to deal with extreme hyperglycemia. The rate of appearance of glucose in urine was previously measured as 2.1 μmol·kg−1·min−1 in adult rainbow trout supplied with exogenous glucose that increased glycemia to similar levels as in our experiments (6). This rate of urinary loss would have accounted for 5.2% of Rd (=2.1/40.1 μmol·kg−1·min−1). When intense exercise and exogenous glucose are combined, trout can push Rd to 40 μmol·kg−1·min−1 (Figs. 6 and 7), and this result is consistent with the response reported for mammals (19, 24, 25). In trout, the simultaneous effects of exercise, insulin, and hyperglycemia could provide the necessary signals to reach this high rate of glucose disposal.
Exogenous glucose inhibits hepatic glucose production.
Trout receiving exogenous glucose are able to lower hepatic glucose production by 75% for several hours during graded exercise (Fig. 6). This response is not as dramatic as in the resting state when Ra glucose is completely suppressed (8). Current information shows that elevated insulin levels caused by strong hyperglycemia play a major role in inhibiting Ra glucose (2). In mammals, as well as in trout, insulin reduces hepatic glucose production by inhibiting glucose 6-phosphatase (33, 36) and glycogen phosphorylase (11, 35, 40). In mammals, insulin also inhibits phosphoenolpyruvate carboxykinase (36), but this only occurs in trout when they are fed a high-protein diet (40–60%) (9–11). This mechanism could operate here because the trout were fed a diet containing 46% protein. During exercise, exogenous glucose does not cause the complete inhibition of Ra as it does at rest. This is probably because swimming triggers additional hormonal signals that are absent at rest, like increases in circulating catecholamines (15). These stress hormones can stimulate Ra by activating glycogenolysis and gluconeogenesis (34). This occurs in mammals and explains why they only decrease Ra to baseline values during intense exercise (1, 19, 24, 25), but completely suppress it at rest (12, 20, 28). It is unknown whether chronically hyperglycemic fish show any degree of insulin resistance. If they do, the fact that they are still able to suppress Ra so strongly would mean that normoglycemic animals could have an even better ability for glucoregulation than observed in this study.
Swimming performance and energetics.
The metabolic rate of trout receiving exogenous glucose is significantly lower compared with controls receiving no glucose (−16%) (Fig. 1A). This suggests that exogenous supply causes a shift in fuel selection toward a higher relative use of glucose because it takes between 15 and 30% less oxygen to produce the same amount of ATP when oxidizing carbohydrates compared with lipids (38, 47). Consequently, fish receiving glucose have a lower total cost of transport than controls (TCOT = basal metabolic cost + cost of locomotion) (see Fig. 2A). A lower net cost of transport (NCOT = cost of locomotion only) could not be demonstrated, but the animals supplied with exogenous glucose show a nonsignificant trend toward a decrease at submaximal speeds (Fig. 2B). At maximal intensities, trout are not able to use the additional circulatory glucose and are most likely relying on intramuscular glycogen. This is supported by the fact that exogenous glucose does not improve exercise performance because it does not increase Ucrit.
Capacity for glucoregulation.
In a previous study, rainbow trout showed a good ability to regulate glucose fluxes and to minimize the effects of a glucose load in the resting state (8). Here, graded exercise provides a greater regulatory challenge given the potentially changing demand for glucose by locomotor muscles. Even among mammals, some species are unable to maintain glycemia and will triple blood glucose concentrations during intense exercise (45). Against all expectations, trout showed perfect glucoregulation by maintaining constant blood glucose levels at all swimming speeds including Ucrit (Fig. 3A). This glucoregulation is particularly impressive for chronically hyperglycemic fish and suggests that normoglycemic animals would show the same capacity.
In the second part of this study, we evaluated how the fish cope with the simultaneous stresses of graded exercise and exogenous glucose. After 4 h of exogenous glucose infusion, they were able to limit glucose accumulation to a 2.5-fold increase in circulating concentration (Fig. 5A). This was achieved through changes in glucose kinetics: the inhibition of endogenous Ra for the first ∼3 h (−75%), and a 145% increase in Rd (Fig. 6). To illustrate the consequences of these large changes in flux, Fig. 9 shows calculated values for glycemia if glucose kinetics had not been modulated. If only partial changes had occurred (i.e., if only Ra or only Rd had responded), glycemia would have increased by 4.3-fold, whereas no modulation of fluxes at all would have resulted in a 6.4-fold increase to dangerous levels of 114 mM (Fig. 9). This study demonstrates that rainbow trout subjected to graded swimming are able to modulate glucose fluxes rapidly to minimize the metabolic stress caused by a glucose overload.
Glucose as an oxidative fuel.
Rd glucose is the sum of glucose oxidation and nonoxidative disposal. Therefore, it is a measure of the highest possible rate of glucose oxidation when nonoxidative disposal is nil. In the resting state, mammals only oxidize about 50% of Rd as reported for rats (43%) (5), dogs (30–50%) (31, 44), and humans (40–60%) (16, 21). However, during exercise, this can increase to close to 100% as in humans (4). The fraction of Rd glucose actually oxidized in swimming trout has never been measured, but it is likely to be in the range of 50 to 100% depending on the work intensity. To start characterizing the potential importance of glucose as an oxidative fuel in trout, we have tried to estimate the contribution made by this fuel to total metabolic rate, assuming that either 100 or only 50% of Rd is oxidized (Fig. 8). In fish receiving no exogenous glucose, this fuel alone could not account for total metabolic rate at speeds above 1.8 BL/s (Fig. 8A). At such high exercise intensities, most of the ATP is produced from carbohydrates, and, therefore, a significant contribution from intramuscular glycogen will have to make up the shortfall. In fish receiving exogenous glucose, a maximum of 50% Rd can be oxidized between 0.8 and 1.8 BL/s (Fig. 8B) because it is sufficient to explain 100% of MO2. At these submaximal speeds, therefore, at least 50% of Rd must go to nonoxidative disposal (glycogen and lipid synthesis). If other fuels besides glucose are also oxidized, the relative importance of glucose oxidation will decrease below 50% of Rd and nonoxidative disposal will increase accordingly. Experiments involving the direct measurement of glucose oxidation will be necessary to quantify the exact contribution of this fuel to the metabolic rate of swimming fish.
Perspectives and Significance
Graded swimming does not inhibit glucose fluxes like steady, low-intensity exercise (39), but may actually stimulate them at the highest intensities. Rainbow trout are able to show perfect glucoregulation at all exercise intensities because the rates of glucose production and disposal change in synchrony. Ra and Rd appear to be stimulated at high swimming speeds, possibly through catecholamines, and insulin-independent movement of GLUT4. When fish are given exogenous glucose during swimming, they have the capacity to boost disposal while they deal with the high total Ra caused by exogenous supply and residual hepatic production. Under these conditions, Rd glucose is probably stimulated by a combination of signals, including exercise itself, insulin, and hyperglycemia that could activate GLUT4, GLUT2, and key enzymes of carbohydrate metabolism. Fish receiving exogenous glucose show a lower MO2 because they may use more carbohydrates that allow them to produce ATP at a higher P/O ratio. However, the high glucose availability does not improve Ucrit, because trout are unable to take advantage of this extra fuel during maximal exercise. This study shows that rainbow trout have a remarkable capacity to adjust glucose fluxes that allows them to cope with the cumulative stresses of a glucose overload and graded exercise.
GRANTS
This work was supported by grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada to J. M. Weber (NSERC Discovery Grant no. 105639-2012 and NSERC Research Tools and Instruments Grant no. 315429-05).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.C. and J.-M.W. conception and design of research; K.C. performed experiments; K.C. analyzed data; K.C. and J.-M.W. interpreted results of experiments; K.C. prepared figures; K.C. drafted manuscript; K.C. and J.-M.W. edited and revised manuscript; K.C. and J.-M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bill Fletcher and Christine Archer for taking care of the animals.
REFERENCES
- 1.Angus DJ, Febbraio MA, Hargreaves M. Plasma glucose kinetics during prolonged exercise in trained humans when fed carbohydrate. Am J Physiol Endocrinol Metab 283: E573–E577, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Banos N, Baro J, Castejon C, Navarro I, Gutierrez J. Influence of high-carbohydrate enriched diets on plasma insulin levels and insulin and IGF-I receptors in trout. Regul Pept 77: 55–62, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell BG, Brown ML, Willis DW. Relative weight (Wr) status and current use in fisheries assessment and mangement. Rev Fish Sci 8: 1–44, 2000. [Google Scholar]
- 4.Brooks GA. Mammalian fuel utilization during sustained exercise. Comp Biochem Physiol B 120: 80–107, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Brooks GA, Donovan CM. Effect of endurance training on glucose kinetics during exercise. Am J Physiol Endocrinol Metab 244: E505–E512, 1983. [DOI] [PubMed] [Google Scholar]
- 6.Bucking C, Wood CM. Renal regulation of plasma glucose in the freshwater rainbow trout. J Exp Biol 208: 2731–2739, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cermak NM, van Loon LJ. The use of carbohydrates during exercise as an ergogenic aid. Sports Med 43: 1139–1155, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Choi K, Weber JM. Pushing the limits of glucose kinetics: how rainbow trout cope with a carbohydrate overload. J Exp Biol 218: 2873–2880, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Cowey CB, Higuera Mdl, Adron JW. The effect of dietary composition and of insulin on gluconeogenesis in rainbow trout (Salmo gairdneri). Br J Nutr 38: 385–395, 1977. [DOI] [PubMed] [Google Scholar]
- 10.Cowey CB, Knox D, Walton MJ, Adron JW. The regulation of gluconeogenesis by diet and insulin in rainbow trout (Salmo gairdneri). Br J Nutr 38: 463–470, 1977. [DOI] [PubMed] [Google Scholar]
- 11.Enes P, Panserat S, Kaushik S, Oliva-Teles A. Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem 35: 519–539, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Ferrannini E, Bjorkman O Jr., GAR, Pilo A, Olsson M, Wahren J, DeFronzo RA. The disposal of an oral glucose load in healthy subjects. Diabetes 34: 580–588, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Figueiredo-Silva AC, Panserat S, Kaushik S, Geurden I, Polakof S. High levels of dietary fat impair glucose homeostasis in rainbow trout. J Exp Biol 215: 169–178, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Fournier RA, Weber JM. Locomotory energetics and metabolic fuel reserves of the Virginia opossum. J Exp Biol 197: 1–16, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Galbo H, Holst JJ, Christensen NJ. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol 38: 70–76, 1975. [DOI] [PubMed] [Google Scholar]
- 16.Glamour TS, McCullough AJ, Sauer PJJ, Kalhan SC. Quantification of carbohydrate oxidation by respiratory gas exchange and isotopic tracers. Am J Physiol Endocrinol Metab 268: E789–E796, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Haman F, Weber JM. Continuous tracer infusion to measure in vivo metabolite turnover rates in trout. J Exp Biol 199: 1157–1162, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Haman F, Zwingelstein G, Weber JM. Effects of hypoxia and low temperature on substrate fluxes in fish: plasma metabolite concentrations are misleading. Am J Physiol Regul Integr Comp Physiol 273: R2046–R2054, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Howlett K, Angus D, Proietto J, Hargreaves M. Effect of increased blood glucose availability on glucose kinetics during exercise. J Appl Physiol 84: 1413–1417, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Jackson RA, Roshania RD, Hawa MI, Sim BM, DiSilvio L. Impact of glucose ingestion on hepatic and peripheral glucose metabolism in man: An analysis based on simultaneous use of the forearm and double isotope techniques. J Clin Endocrinol Metab 63: 541–549, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Katz H, Homan M, Butler P, Rizza R. Use of [3-3H]glucose and [6-14C]glucose to measure glucose turnover and glucose metabolism in humans. Am J Physiol Endocrinol Metab 263: E17–E22, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Legate NJ, Bonen A, Moon TW. Glucose tolerance and peripheral glucose utilization in rainbow trout (Oncorhynchus mykiss), American eel (Anguilla rostrata), and black bullhead catfish (Ameiurus melas). Gen Comp Endocrinol 122: 48–59, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Marín-Juez R, Capilla E, Carvalho-Simoes F, Camps M, Planas JV. Structural and functional evolution of glucose transporter 4 (GLUT4): a look at GLUT4 in fish. In: Glucose Homeostasis, edited by Szablewski L. Vienna, Austria: InTech, 2014, p. 37–67. [Google Scholar]
- 24.Marmy-Conus N, Fabris S, Proietto J, Hargreaves M. Pre-exercise glucose ingestion and glucose kinetics during exercise. J Appl Physiol 81: 853–857, 1996. [DOI] [PubMed] [Google Scholar]
- 25.McConell G, Fabris S, Proietto J, Hargreaves M. Effect of carbohydrate ingestion on glucose kinetics during exercise. J Appl Physiol 77: 1537–1541, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Moon TW. Glucose intolerance in teleost fish: fact or fiction? Comp Biochem Physiol 129: 243–249, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Moyes CD, West TG. Exercise metabolism of fish. Biochem Mol Biol Fishes 4: 367–392, 1995. [Google Scholar]
- 28.Muller MJ, Moring J, Seitz HJ. Regulation of hepatic glucose output by glucose in vivo. Metabolism 37: 55–60, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Navarro I, Gutierrez J, Planas J. Estimates of fish glucagon by heterologous radioimmunoassay: antibody selection and cross-reactivities. Comp Biochem Physiol 110: 313–319, 1995. [Google Scholar]
- 30.Omlin T, Langevin K, Weber JM. Exogenous lactate supply affects lactate kinetics of rainbow trout, not swimming performance. Am J Physiol Regul Integr Comp Physiol 307: R1018–R1024, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul P, Bella Issekutz J. Role of extramuscular energy sources in the metabolism of the exercising dog. J Appl Physiol 22: 615–622, 1967. [DOI] [PubMed] [Google Scholar]
- 32.Pearson MP, Spriet LL, Stevens ED. Effect of sprint training on swim performance and white muscle metabolism during exercise and recovery in rainbow trout (Salmo gairdneri). J Exp Biol 149: 45–60, 1990. [Google Scholar]
- 33.Polakof S, Moon TW, Aguirre P, Skiba-Cassy S, Panserat S. Effects of insulin infusion on glucose homeostasis and glucose metabolism in rainbow trout fed a high-carbohydrate diet. J Exp Biol 213: 4151–4157, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Polakof S, Panserat S, Soengas JL, Moon TW. Glucose metabolism in fish: a review. J Comp Physiol B Biochem Syst Environ Physiol 182: 1015–1045, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Polakof S, Skiba-Cassy S, Choubert G, Panserat S. Insulin-induced hypoglycaemia is co-ordinately regulated by liver and muscle during acute and chronic insulin stimulation in rainbow trout (Oncorhynchus mykiss). J Exp Biol 213: 1443–1452, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Rojas JM, Schwartz MW. Control of hepatic glucose metabolism by islet and brain. Diabetes Obes Metab 16: 33–40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romijn JA, Coyle EF, Sidossis LS, Rosenblatt J, Wolfe RR. Subtrate metabolism during different exercise intensities in endurance-trained women. J Appl Physiol 88: 1707–1714, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Schippers MP, Ramirez O, Arana M, Pinedo-Bernal P, McClelland Grant B. Increase in carbohydrate utilization in high-altitude Andean mice. Curr Biol 22: 2350–2354, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Shanghavi DS, Weber JM. Effects of sustained swimming on hepatic glucose production of rainbow trout. J Exp Biol 202: 2161–2166, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Shrayyef MZ, Gerich JE. Normal glucose homeostasis. In: Principles of Diabetes Mellitus, 2nd ed., edited by Poretsky L. New York: Springer, 2010, p. 19–35. [Google Scholar]
- 41.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959. [DOI] [PubMed] [Google Scholar]
- 42.Teulier L, Omlin T, Weber JM. Lactate kinetics of rainbow trout during graded exercise: do catheters affect the cost of transport? J Exp Biol 216: 4549–4556, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest 50: 2715–2725, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasserman DH, Lacy DB, Bracy D, Williams PE. Metabolic regulation in peripheral tissues and transition to increased gluconeogenic mode during prolonged exercise. Am J Physiol Endocrinol Metab 263: E345–E354, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Weber JM, Roberts TJ, Vock R, Weibel ER, Taylor CR. Design of the oxygen and substrate pathways III. Partitioning energy provision from carbohydrates. J Exp Biol 199: 1659–1666, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Weber JM, Shanghavi DS. Regulation of glucose production: role of epinephrine in vivo and in isolated hepatocytes. Am J Physiol Regul Integr Comp Physiol 278: R956–R963, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Welch KC Jr, Altshuler DL, Suarez RK. Oxygen consumption rates in hovering hummingbirds reflect substrate-dependent differences in P/O ratios: carbohydrate as a “premium fuel”. J Exp Biol 210: 2146–2153, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Wright PA, Perry SF, Moon TW. Regulation of hepatic gluconeogenesis and glycogenesis by catecholamines in rainbow trout during environmental hypoxia. J Exp Biol 147: 169–188, 1989. [DOI] [PubMed] [Google Scholar]


