Abstract
In human cancers, the autocrine and paracrine loop mediated by the aberrantly activation of endothelin-1 (ET-1) receptor (ET-1R) elicits pleiotropic effects, preferentially mediated by the scaffold protein β-arrestin 1 (β-arr1), on tumor cells and on the host microenvironment, providing a strong rationale for targeting ET-1 receptors. This review describes the most up-to-date preclinical and clinical results obtained by using ET-1 therapeutics. The previous negative clinical results of ET-1 therapeutics should not prevent us from setting the standard of this class of drugs for future well-designed clinical trials. The preclinical data obtained with the dual ETAR and ETBR antagonist macitentan indicate that this molecule, which targets cancer cells and tumor-associated microenvironmental elements, could be a cancer therapeutic option. The field of ET-1 therapeutics will be improved in the next decade, facilitated by the new knowledge on the genomic landscape of the human stroma and tumor, and by the low invasive approaches based on liquid biopsies for the discovery of predictive biomarkers. The information obtained from preclinical studies in patient-derived models and from the Cancer Genome Atlas will set the scene of precision medicine for cancer. Results from these studies are expected to open the possibility that ET-1R antagonists might be more efficacious as molecular cancer therapeutics, able to hamper the functional β-arr1-dependent signaling complexes, either alone or coupled with new targeted approaches.
Keywords: β-arrestin, cancer, endothelin-1, endothelin-1 receptor, target therapy
recent investigations have consistently suggested that endothelin-1 (ET-1), a small bioactive peptide of 21 residues derived from longer prepro-ET-1 precursor, is an important signal messenger in different pathological conditions, including human cancers (24, 35). ET-1 belongs to a family of closely related peptides that include ET-2 and ET-3, which are all similar in structure to ET-1, but are separate gene products, with tissue-specific expression. ETs act mainly via G protein-coupled receptors (GPCR) from endothelin receptor type A (ETAR) and B (ETBR) (24). The two receptors can be differentiated by agonists and antagonists binding affinity and in their cellular distributions. The ETAR binds ET-1 with the greatest affinity, whereas ET-1, ET-2, and ET-3 all have equal affinity for the ETBR.
It is now well known that ET-1 receptors can activate several signaling pathways in a G protein-independent manner via complexes with β-arrestin (β-arr)-1 or -2 (18, 24). The scaffold protein β-arr1 serves as a copilot in ET-1 signaling to organize complex networks driving tumor progression (7, 23, 25, 26, 28, 30, 33). Thus ET-1 axis confers pleiotropic effects on both tumor cells and microenvironment, modulating different processes by activating β-arr-mediated pathways (Fig. 1A). A lesson learned from various tumor models is that there are different expression profiles of the ET-1 receptors compared with normal tissues. There are cancers expressing predominantly ETAR such as ovarian, nasopharyngeal, thyroid, prostate, colon, pancreatic, gastric, renal, and breast cancers, and others predominately expressing ETBR such as melanoma, glioblastoma multiform (GBM), and astrocytoma, and those expressing both ETAR and ETBR such as oral, lung, bladder, vulvar cancers, and Kaposi's sarcoma (24).
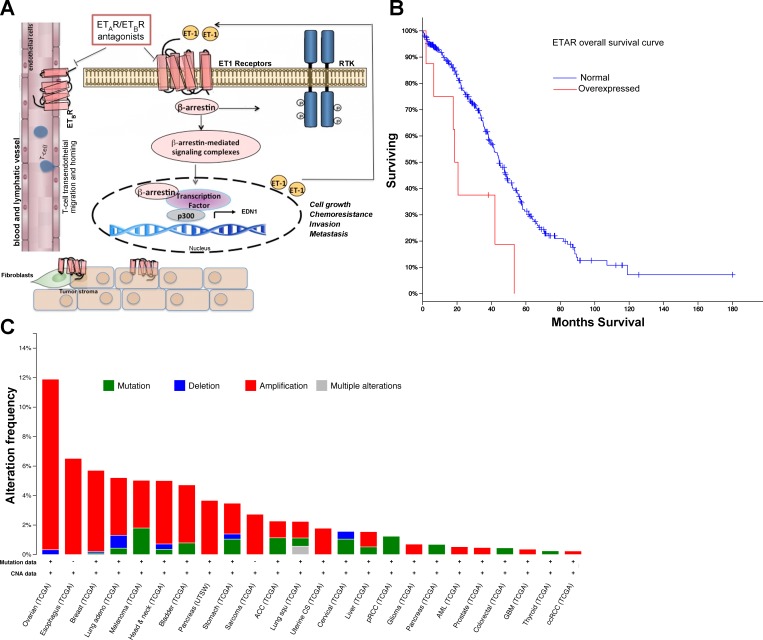
Fig. 1.
A: schematic model describing the potential mechanism by which β-arrestin-1 (β-arr1) regulates endothelin-1 (ET-1)-induced signaling pathways. In tumor cells ET-1 binding on receptors leads to recruitment of β-arr1. Then β-arr1 shuttles into the nucleus, where it interacts with a different transcriptional factor to form a complex with p300 required for histone acetylation and for the transcription of target genes, such as ET-1. This mechanism leads to the amplification of the ET-1 autocrine loop. As a signal transducer, β-arr1 initiates the transactivation of different receptor tyrosine kinase (RTK). In parallel, the autocrine/paracrine production of ET-1 activates ETBR expressed on endothelial cells to promote angiogenesis and lymphangiogenesis and on microenvironmental cells. The dual ETAR and ETBR antagonist targets not only cancer cells (which express ETAR and ETBR) but also targets tumor-associated stromal elements, which express ETBR, impairing growth, invasion, neovascularization, chemoresistance, and metastatic spread, and enhancing antitumor immune mechanisms. B: ETAR overexpression is associated with improved survival of patients with ovarian cancer. Overall survival of 315 patients with high-grade serous ovarian cancer and ETAR overexpression compared with normal expression (P < 0.01). Data were retrieved from TCGA data portal. C: β-arr1 is amplified in various human cancers. The cBio Cancer Genomics Portal (http://www.cbioportal.org/public-portal/) was queried for ARBB1 across various tumor datasets. The alteration frequency, type of alterations of β-arr1 gene (ARRB1), availability of mutation analysis, and Copy Number Alteration (CNA) in the various tumor cohorts are shown.
ET-1-controlled steps in cancer include cell survival and proliferation, angiogenesis, epithelial-to-mesenchymal transition (EMT), osteogenesis, immune modulation, invasion, and metastasis (24).
Relevant advances have been made toward our understanding of the ET-1 pathway. Selective or nonselective ET-1 receptor antagonists have already been used in cancer treatment. This approach is based on results that were obtained through basic and translation research more than two decade ago, but the clinical potential of this pathway in cancer treatment is just now becoming evident. In this review, we will describe the history of ET-1 therapeutics from the benchwork to the latest clinical findings.
New and Old Roles of ET-1 Axis in Cancer
Role of ET-1 in cell proliferation and survival.
Previous reports describe the proliferative and antiapoptotic effects of ET-1 in tumor cells, and ET-1 receptor antagonists have been evaluated as molecular target agents in cancer. Indeed, autocrine ET-1 signaling alone or in cooperation with well-known growth factors, including epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) conveying mitogenic signals to the nucleus, promotes cell proliferation in different tumor cells (24). In the majority of these cells, ET-1 acts by modulating cell survival pathways, and ET-1 receptor blockade with different receptor antagonists results in antitumor activity, by concomitant growth inhibition and apoptosis induction. At a therapeutic level, apoptotic responses of different tumor cells are potentiated when chemotherapeutic agents are combined with ETAR-selective antagonists (BQ123, atrasentan, and zibotentan) or dual ETAR and ETBR antagonists (macitentan), suggesting that combination of ET-1 therapeutics with chemotherapeutic drugs represents a viable option to stop compensatory pathways activated in response to therapeutics (17, 24, 26).
Role of ET-1 in the interactions of tumor cells with the microenvironment.
The interactions of tumor cells with their adjacent microenvironment are determined by structural and biochemical properties of the extracellular matrix (ECM) as well as by communication with surrounding endothelial cells (EC), cancer-associated fibroblasts (CAF), mesenchymal stem cells (MSC), and a variety of different immune cells, including lymphocytes and tumor-associated macrophages (TAM). The deep knowledge of these interactions is mandatory to understand the mechanisms involved in tumor growth and progression and chemoresistance. In this context, many of these critical interactions are emerging to be modulated by ET-1 axis.
ET-1, FIBROBLASTS, AND MESENCHYMAL CELLS.
ET-1 modulates tumor stroma remodelling by acting on both ET-1 receptors expressed on cancer-associated fibroblasts, as shown in colon, ovarian, and breast cancers (24). Moreover, ET-1 promotes growth, migration, contraction, and the production of ECM-modifying proteins in fibroblasts adjacent to colon cancer, suggesting a role of ET-1 in the formation of a supportive stroma (16). More recently, it has been shown that when colorectal cancer cells were mixed with nontumorigenic MSC, they secrete enhanced levels of ET-1, thereby increasing the capacities of EC for recruitment and angiogenesis, demonstrating a further integration of tumor-associated stroma with ET-1 signaling (12).
ET-1 AND IMMUNE SYSTEM.
The complexity of the tumor immunoenvironment is underscored by the discovery of different subsets of immune effectors and regulatory cells. The regulation of immunoenvironment seems to be mediated also by ET-1 axis at different levels. Dendritic cells (DC) play a key role in inducing and maintaining the antitumor immunity for their antigen-presenting function. ET-1 axis seems to be involved in DC behavior and function. Indeed, these cells produce ET-1 and express functional ETAR and ETBR, which are both highly expressed during DC maturation (11). However, whereas ETAR blockade significantly reduces the expression of CD83 (a marker of mature dendritic cell marker), decreases the production of interleukin-12, and promotes DC apoptosis, ETBR blockade results in increased expression of CD83 and improved DC survival (11). Within the complex microenvironment, the orchestrated actions of ET-1 can accelerate the mobilization of macrophages and the conversion of these cells to TAM and their recruitment to tumors. Indeed, macrophages expressing ET-1 receptors migrate toward inflamed tissues under the influence of autocrine or paracrine ET-1 release through ETBR (24). Experimental studies in bladder cancer have also revealed an ET-1/ETAR-dependent production of pro-inflammatory mediators and infiltration of macrophages into tumors, which promotes breaching of vascular integrity and dissemination of cancer cells to the lungs (24). Macrophages also stimulate the expression of ET-1 and its receptors in breast cancer cells and EC, thus stimulating transendothelial migration of cancer cells (6).
Characterization of the microenvironment in human cancer patients reveals a T cell-infiltrated phenotype that has been activated spontaneously in response to the growing tumor. Transcriptional profiling of microdissected EC from ovarian cancers (OC) revealed that overexpression of the ETBR was associated with the absence of tumor-infiltrating lymphocytes (TIL). Moreover, ETBR blockade increased T cell adhesion to human endothelium in vitro, and, in mice, increased T cell homing to tumors; this homing required intercellular adhesion molecule-1 and enabled response to otherwise ineffective immunotherapy in vivo. These findings highlight a molecular switch operating within the blood vessels to keep T lymphocytes out, a mechanism with the potential to be utilized to enhance the efficacy of immunotherapy (4, 8). Therefore, the dual ETAR and ETBR antagonists might offer the advantage of simultaneously targeting the tumor cells (through ETAR) and enhancing antitumor immune mechanisms (through inhibiting vascular ETBR) by augmenting T cell infiltration and homing to tumors. Recently, a study demonstrated that gliomas overexpressing ETBR exhibited fewer TIL, suggesting that ETBR expression may interfere with the homing of TIL around the tumor and be involved in the immune escape mechanism of gliomas (19).
These findings suggest that ET-1-dependent coordination of the events required to trigger different subpopulations of immune cells to the tumor bed is clearly more complex than expected and include the management of multiple mechanisms. A deep knowledge of these mechanisms will guide our efforts to develop more efficacious combination of ET-1 therapeutics and immunotherapy approaches.
ET-1 AND BLOOD AND LYMPHATIC ENDOTHELIAL CELLS.
In the tumor microenvironment, blood and lymphatic EC increase angiogenesis and lymphangiogenesis in response to ET-1/ETBR activation. ET-1 induces various stages of neovascularization, also promoting induction of VEGF and displaying a potent additive effect with VEGF family members, in the angiogenic and lymphangiogenic processes (24, 32, 34).
In different tumors, increased expression of ET-1 and its cognate receptors is significantly associated with the expression of VEGF and its receptors as well as with microvessel density (24).
Several observations linked ET-1 action in cancer cells and EC in response to hypoxic microenvironment, particularly involving a reciprocal relationship between ET-1 and hypoxia-inducible factor-1α (HIF-1α), which is a crucial hypoxia-responsive transcriptional factor (24, 31). ET-1 activates the hypoxia-responsive pathway by inducing stabilization of HIF-1α under normoxic conditions, inducing the expression of different members of VEGF family (31). Interestingly, HIF1α also activates the transcription of ET-1 in different cell types. HIF-1α and VEGF production can thus be amplified by ET-1 under normoxic or hypoxic conditions (24). In cancer cells, ET-1 can itself be upregulated under hypoxia. This may lead to a positive feedback system, whereby ET-1 expression stimulates hypoxic pathways, which promote further ET-1 expression, maintaining constant angiogenic and protumoral responses.
Role of ET-1 in epithelial-to-mesenchymal transition, migration, and invasion.
A plethora of evidences indicate that ET-1 displays a key role in tumor migration, invasion, EMT, and metastatic growth. Several signaling pathways have been shown to be important for cell motility induced by ET-1, including those controlling β-catenin signaling, EMT-related factors, integrin signaling, as well as gap junction intercellular communication disruption (22, 24). In particular, activation of the ET-1 pathway induces EMT through multiple distinct mechanisms, including the stabilization of the β-catenin protein, to engage transcription of genes related to EMT, through the ability of β-arr1 to serve as signal transducers in OC cells (23, 25, 26). Moreover, coexpression of ETAR and β-arr1 in human OC tissues is indicative of the malignant phenotype, and ETAR overexpression is associated with the switch of cadherin expression, indicating the relevant role of ETAR/β-arr1 in the regulation of EMT and aggressive behavior (24, 26).
It is now widely accepted that the formation of actin-rich proteolytic structures, called invadopodia protrusions, is a key step during cancer cell invasion. These dynamic organelle-like structures adhere to and digest ECM through secretion of matrix metalloproteinases (MMP). In several tumors, including ovarian, epatocellular, colon carcinoma, melanoma, and osteosarcoma, ET-1-dependent invasion occurs through increase transcription, secretion, and activation of MMP (24). Additional studies demonstrated further that ET-1-dependent nuclear function of β-arr1 induces epigenetic modification regulating MMP-2 transcription in OC cells (25). According with the ability of ET-1 to control MMP activity, ECM degradation, and cell invasion, a recent work demonstrated that ET-1/ETAR activates a member of the Rho GTPase family, RhoC, through the direct interaction of β-arr1 with one of the Rho activators belonging to the guanine nucleotide exchange factors (GEFs) PDZ (postsynaptic density protein 95/disc-large/zonula occludens)-RhoGEF. This interaction is driven by ETAR and is functional to the formation and activation of invadopodia, as demonstrated by treatment with macitentan, which significantly impairs invadopodia function, MMP activity, and invasion (28).
Role of ET-1 in drug resistance and stem cell-like behavior.
Important evidences recently defined a key role of ET-1 axis in drug resistance. In a model of brain metastasis from breast cancer, it has been demonstrated that bidirectional signaling between astrocytes and tumor cells involves upregulation and activation of ET-1 axis, which protects tumor cells from cytotoxicity induced by chemotherapeutic drugs such as taxol (15, 24). Moreover, macitentan abolishes astrocyte- and endothelial cell-mediated chemoprotection (15). More recently, by using orthotopic models of human GBM, it has been shown that astrocytes and brain EC protect glioma cells from the alkylating agent temozolomide (TMZ) through an ET-1-dependent signaling mechanism, and that macitentan enhances their sensitivity to TMZ, producing durable responses (14). A similar mechanism has been observed in chronic lymphocytic leukemia (CLL). CLL cells express higher levels of ET-1 and ETAR compared with normal B cells. These cells, when in contact with endothelial layers, acquire the survival advantage, drug-resistance, and growth signals that can be blocked by ETAR inhibition (17).
A critical role of β-arr1 in chemoresistance has been reported in OC cells, identifying a novel bypass mechanism through which ETAR/β-arr1 links nuclear β-catenin signaling to acquire chemoresistant and EMT phenotype, resulting in histone acetylation, chromatin reorganization, and enhanced transcription of genes, such as ET-1, enhancing the network that sustains chemoresistance (24–26). Of note, treatment with macitentan prevented this signaling and restored drug sensitivity. Moreover, the combination of macitentan and cisplatinum resulted in the potentiation of the cytotoxic effect, suggesting new combinatorial treatment with chemotherapy plus ET-1 therapeutics to improve sensitivity to chemotherapy (26). The strengths of the preclinical data driven by analysis of OC tissues from sensitive and resistant patients demonstrating that coexpression of ETAR and β-arr1, and the co-occupancy of β-arr1 and β-catenin on ET-1 gene promoter, appear to be indicative of the chemoresistant phenotype, further supporting the pathobiologic relevance of ETAR/β-arr1 in the regulation of chemoresistance (26).
Recent studies suggest the involvement of ET-1 in cancer stem cells (CSC) within the tumor, such as OC, colon, and GBM (24). Moreover, ETAR is preferentially expressed in CD133+ OC cell lines and primary human OC cells, hence ETAR may play a part in the chemoresistance of CSC (8).
Role of ET-1 in metastasis.
Supportive evidence for the involvement of ET-1 in tumor progression and metastasis comes from different studies. The first ones have uncovered a prominent role for ET-1 in the formation of osteoblastic lesions in patients with metastatic prostate and breast cancer, by stimulating mitogenesis in osteoblasts and by decreasing osteoclast activity and motility (24). Bone metastasis in experimental models is inhibited by the ETAR antagonists, as well as by the dual ETAR and ETBR antagonist (24). Interestingly, ET-1 contributes to the pain associated with metastatic disease. This occurs through ET-1 causing the excitation of nociceptors through ETAR (24). In this regard, it has been previously demonstrated that ETAR antagonist potentiates opioid analgesia and eliminates analgesic tolerance (2), suggesting that ETAR blockade might be therapeutically useful not only to control bone metastasis but also to reduce metastasis-associated pain.
Overexpression of ETBR is associated with increased incidence of brain metastases in a preclinical model of melanoma, which was inhibited by ETBR antagonist (9).
In the bladder cancer microenvironment, ET-1 primes cancer cells for pulmonary metastasis (24). Of note, ETAR antagonist reduced the development of lung metastases, and this was associated with a significant decrease in macrophage infiltration and cytokine production in the lung before the development of metastases (24), suggesting that ETAR inhibitors might be more effective as adjuvant therapeutic agents before metastases become clinically apparent.
In orthotopic OC models, it has been demonstrated direct involvements of ET-1 axis in promoting metastatic phenotype (13, 23–26, 28). Moreover, deeper studies showed that the metastatic phenotype is linked to the ETAR-β-arr1-mediated pathways. Most importantly, although metastatic progression can be controlled by specific ETAR antagonists, more recently, studies showed a superior inhibition by using ETAR/ETBR antagonist (13, 26, 28).
Endothelin Axis Members as Tumor Biomarker
All clinical development programs with ET-1 therapeutics will face the fact that there are no validated biomarkers yet predicting responses to treatment with ET-1 receptor antagonists to stratify patients into subgroups that are most likely to benefit from treatment.
Levels of ET-1 are higher in patients with muscle-invasive bladder cancers, which are prone to metastasize and which correlate with reduced patient survival, thus indicating ET-1 could be a potential biomarker for lung metastasis (27).
With the use of data from the Cancer Genome Atlas (TCGA) portal (1, 5, 10), it has been demonstrated that ETAR overexpression is associated with worse survival in high-grade serous OC (Fig. 1B). These data are strongly supported by analysis of human OC tissues demonstrating that ETAR overexpression is associated with poor survival and is more frequent in advanced stages of OC (22, 23). Recently, the univariate and multivariate analyses of clinicopathological parameters from sensitive and platinum-resistant OC patients showed that advanced stage, serous histotype, presence of ascites, short progression-free interval, and high ETAR expression were significantly associated with poor clinical outcome, demonstrating that the association between high ETAR expression and worse survival is to be ascribed to the unfavorable prognostic role played by high ETAR expression, in particular, in the subset of platinum-resistant OC cases (26).
Intriguingly, a recent work in translational research seeking to identify epigenetic regulators whose aberrant activity contributes to ETAR-driven oncogenesis has underscored for the first time a critical microRNA (miRNA), miR-30a, as a tumor suppressor in chemoresistant OC by targeting ETAR signaling axis (29). Univariate and multivariate analyses, employing the TCGA data set, revealed the prognostic role of low miR-30a expression that was independent from other clinical variables (tumor stage, grade, and treatment response). Interestingly, it has been shown that ETAR gene expression inversely correlated with miR-30a expression when comparing sensitive and resistant patients (29). These findings encourage furthering evaluation of ET-1/ETAR and associated miR expression as biomarkers in larger samples to validate their predictive value.
β-Arrestin as Critical Tranducer of ET-1 Signaling
ET-1 receptors, among GPCRs, are prominent drug targets in cancer, and conventionally the primary focus has been on designing antagonists to turn the receptors “off” (24). To improve the use of these antagonists in cancer treatment, it is important to focus on the the ability of some ligands, referred to as biased ligands, to selectively trigger G protein- and β-arr1-mediated signaling pathways, raising the possibility of selective induction of a subset of signaling pathways (18). This characteristic opens the opportunity of manipulating ET-1 therapeutics to achieve selective inhibition of oncogenic pathways. There are many emerging instances where β-arr-1 appears to play a central role in cancer onset (3, 20, 23–26, 28, 30, 33). Prompted by these findings, we analyzed the expression of β-arr-1 by querying TGCA database (5, 10). This analysis revealed that β-arr1 is amplified in various tumors (Fig. 1C). Among the different tumors, β-arr1 is most frequently amplified in OC. In the ET-1 receptor field, considering the rapidly expanding repertoire of β-arr1 functions in tumor-promoting pathways as cytosolic and nuclear scaffold and signaling proteins (Table 1), we are likely to witness many more examples of functional specialization that are impaired by ET-1 therapeutics. A key focus area going forward is likely to be the use of this class of antagonists to block ET-1/β-arr1-dependent molecular complexes, and the recent findings in OC represent a start in this direction (23, 25, 26, 28). Furthermore, complementary studies in other tumors to decipher functional specialization will also be essential to fully appreciate the depth of ET-1-biased signaling and should also be considered while designing clinical approaches by using ET-1 therapeutics.
Table 1.
Roles of endothlin-1-receptor-driven β-arrestin functions in human cancers
| Localization | Tumor Type | Interactors | Ref |
|---|---|---|---|
| Cytosolic | |||
| β-arr1/β-arr2 | Ovarian cancer | C-Src/EGFR | 23 |
| β-arr1 | Ovarian cancer | PDZ-RhoGEF/RHOA, C GTPase | 28 |
| β-arr1 | Melanoma | c-Src/VEGFR-3 | 30 |
| Nuclear | |||
| β-arr1 | Ovarian cancer | β-Catenin | 25, 26 |
| β-arr1 | Ovarian cancer | Nuclear factor-κB | 7 |
| β-arr1 | Ovarian cancer | Angiogenic/metastic genes (Calcrl, Ece1, Foxc1, Icam2, Igfbp1, Igfbp6, Itga5 and Mfng) | 33 |
Therapeutic Approaches
Both basic and preclinical evidences indicate that ET-1 receptors play an important role in the overall pathways and in drug resistance in a wide range of human cancers (24). Many approaches targeting ET-1 have been developed, including the selective inhibition of ET-1 synthesis by endothelin-converting enzyme (ECE) inhibitors or by natural products, such as green tea and red wine, biologically active polyphenols, and the modulation of ET-1 degradation by upregulating neutral endopeptidase (NEP) activity (24). However, major efforts were done in the field of ET-1 receptor antagonists. Driven by positive preclinical results, different clinical trials were initiated, reaching phases II to III with selective or specific ETAR antagonists or with the dual ETAR and ETBR antagonist or by the selective ETBR agonist IRL-1620, which may be used for drug delivery, by increasing tumor perfusion to potentiate the therapeutic efficacy of anticancer agents and of radiation therapy (21, 24).
Following compelling preclinical studies in prostate cancer models and the promising phase II trials in patients with advanced metastatic prostate cancer, atrasentan and zibotentan have been evaluated as single agents in phase III trials carried out in patients with nonmetastatic prostate cancer or advanced metastatic prostate cancer and in combination with standard chemotherapy in patients with metastatic prostate cancer. A phase II trial assessing zibotentan plus carboplatin and paclitaxel in patients with advanced OC reported no improvements in the primary end point or in secondary end points (24). Unfortunately, all trials have proved to be unsuccessful in patients with established disease (24). Contributory factors to account for the lack of overall clinical benefit and the discrepancies with the encouraging preclinical data are difficult to identify. It could also be argued that specific ETAR blockade could tilt the balance toward increased ETBR signaling, hampering antitumor immunity. This could partly explain the failure of specific ETAR antagonists to produce significant clinical results in tumors in which the inhibition of an antitumor immune response might affect overall survival. Negative trial results also underpin the observation that ETAR blockade does not affect established high-volume disease because these advanced tumors are no longer dependent on macrophages or their inflammatory response for maintenance and growth (27). These observations suggest that ETAR antagonists should be investigated in the adjuvant setting in nonmetastatic patients to impair the metastatic colonization driven by critical factors released by the tumor microenvironment.
Given the negative results of specific ETAR antagonism in cancer, different groups investigated concomitant ETAR and ETBR blockade by using macitentan, a dual antagonist approved for the treatment of pulmonary hypertension (24). Preclinical studies indicate that combined ETAR and ETBR antagonism, compared with a selective ETAR antagonist, would be preferable in cancer. Indeed, this class of drug offers a unique advantage by inducing concomitant suppression of invasive properties of tumor cells and simultaneously by controlling stromal elements, offering also the opportunity to enhance antitumor immune mechanisms. More studies are also needed to investigate and to optimize new combination strategies by combining macitentan with chemotherapy or other molecular target therapy, including those using small molecule tyrosine kinase inhibitors, because of the interconnected signaling network of ET-1 with tyrosine kinase receptor, like EGFR or VEGFR (Fig. 1A). From the promising activity of macitentan in different preclinical models and the well-tolerated toxicity profile, this small molecule might be considered a good candidate for future cancer therapy. The outcome of the ongoing phase I trials conducted in primary and recurrent GBM in monotherapy or in combination with TMZ and radiotherapy followed by maintenance with macitentan and TMZ, are likely to open a novel area for ET-1 therapeutics.
Conclusive Remarks
Predictive preclinical models that most closely recapitulate tumor heterogeneity will be useful to cover the gap between clinical observation and preclinical data. New opportunity in ET-1 therapeutic development might derive from the use of deeply developing patient-derived models, including patient-derived xenograft and organoids, that represent the histological, biological, molecular feature of human cancer, more reliably proxy of prospective findings in patients. Patient-derived models have become one of the preferred approaches for preclinical drug development and biomarker discovery, representing an important tool for translational cancer research and personalized medicine application. In these patient-derived models, understanding whether ET-1 receptor antagonist could also hamper the β-arr1-dependent propagation of pleiotropic signals to generate functionally selective response is mandatory to exploit tumor cell vulnerabilities to ET-1 cancer therapeutics.
Perspectives and Significance
The field of ET-1 cancer therapeutics will be transformed in the next decade, facilitated by the knowledge of the genomic landscape of the human microenvironment and tumor and by the noninvasively detection of biomarkers for the early cancer detection and prediction of drug response. This information, together with that obtained with efforts of preclinical studies in patient-derived models and from TGCA, will set the scene of future precision medicine for cancer, providing the rationale that ET-1 receptor antagonists might be more efficacious as cancer therapeutics, either alone or in combination with molecular targeted and/or immunotherapy approaches.
GRANTS
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC) to A. Bagnato (AIRC 14199) and L. Rosanò (AIRC 16965).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.R. and A.B. conception and design of research; L.R. and A.B. analyzed data; L.R. and A.B. interpreted results of experiments; L.R. prepared figures; L.R. and A.B. drafted manuscript; L.R. and A.B. edited and revised manuscript; A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the members of the laboratory for support and helpful comments.
REFERENCES
- 1.The. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474: 609–615, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhalla S, Pais G, Tapia M, Gulati A. Endothelin ETA receptor antagonist reverses naloxone-precipitated opioid withdrawal in mice. Can J Physiol Pharmacol 93: 935–944, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of beta-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA 103: 1492–1497, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O'Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nature Med 14: 28–36, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2: 401–404, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CC, Chen LL, Hsu YT, Liu KJ, Fan CS, Huang TS. The endothelin-integrin axis is involved in macrophage-induced breast cancer cell chemotactic interactions with endothelial cells. J Biol Chem 289: 10029–10044, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianfrocca R, Tocci P, Semprucci E, Spinella F, Di Castro V, Bagnato A, Rosanò L. β-Arrestin 1 is required for endothelin-1-induced NF-κB activation in ovarian cancer cells. Life Sci 118: 179–184, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Coffman L, Mooney C, Lim J, Bai S, Silva I, Gong Y, Yang K, Buckanovich RJ. Endothelin receptor-A is required for the recruitment of antitumor T cells and modulates chemotherapy induction of cancer stem cells. Cancer Biol Ther 14: 184–92, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Muñoz W, Jaramillo ML, Man S, Xu P, Banville M, Collins C, Nantel A, Francia G, Morgan SS, Cranmer LD, O'Connor-McCourt MD, Kerbel RS. Roles for endothelin receptor B and BCL2A1 in spontaneous CNS metastasis of melanoma. Cancer Res 72: 4909–4919, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6: p11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guruli G, Pflug BR, Pecher S, Makarenkova V, Shurin MR, Nelson JB. Function and survival of dendritic cells depend on endothelin-1 and endothelin receptor autocrine loops. Blood 104: 2107–2115, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 32: 4343–4354, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Kim JS, Kim SW, Yun SJ, He J, Brantley E, Fan D, Strickner P, Lehembre F, Regenass U, Fidler IJ. Antivascular therapy for multidrug-resistant ovarian tumors by macitentan, a dual endothelin receptor antagonist. Transl Oncol 5: 39–47, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SJ, Lee HJ, Kim MS, Choi HJ, He J, Wu Q, Aldape K, Weinberg JS, Yung WK, Conrad CA, Langley RR, Lehembre F, Regenass U, Fidler IJ. Macitentan, a dual endothelin receptor antagonist, in combination with temozolomide leads to glioblastoma regression and long-term survival in mice. Clin Cancer Res 21: 4630–4641, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SW, Choi HJ, Lee HJ, He J, Wu Q, Langley RR, Fidler IJ, Kim SJ. Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro Oncol 12: 1585–1598, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles JP, Shi-Wen X, Haque SU, Bhalla A, Dashwood MR, Yang S, Taylor I, Winslet MC, Abraham DJ, Loizidou M. Endothelin-1 stimulates colon cancer adjacent fibroblasts. Int J Cancer 130: 1264–1272, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Maffei R, Bulgarelli J, Fiorcari S, Martinelli S, Castelli I, Valenti V, Rossi D, Bonacorsi G, Zucchini P, Potenza L, Vallisa D, Gattei V, Del Poeta G, Forconi F, Gaidano G, Narni F, Luppi M, Marasca R. Endothelin-1 promotes survival and chemoresistance in chronic lymphocytic leukemia B cells through ETA receptor. PLos One 9: e98818, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maguire JJ, Kuc RE, Pell VR, Green A, Brown M, Kumar S, Wehrman T, Quinn E, Davenport AP. Comparison of human ETA and ETB receptor signalling via G-protein and β-arrestin pathways. Life Sci 91: 544–549, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima S, Sugita Y, Miyoshi H, Arakawa F, Muta H, Ishibashi Y, Niino D, Ohshima K, Terasaki M, Nakamura Y, Morioka M. Endothelin B receptor expression in malignant gliomas: the perivascular immune escape mechanism of gliomas. J Neurooncol 2015 Dec 8. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Pillai S, Trevino J, Rawal B, Singh S, Kovacs M, Li X, Schell M, Haura E, Bepler G, Chellappan S. β-arrestin-1 mediates nicotine-induced metastasis through E2F1 target genes that modulate epithelial-mesenchymal transition. Cancer Res 75: 1009–1020, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajeshkumar NV, Matwyshyn G, Gulati A. IRL-1620, a tumor selective vasodilator, augments the uptake and efficacy of chemotherapeutic agents in prostate tumor rats. Prostate 67: 701–713, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Rosanò L, Spinella F, Di Castro V, Nicotra MR, Dedhar S, de Herreros AG, Natali PG, Bagnato A. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res 65: 11649–11657, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Rosanò L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA 106: 2806–2811, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosanò L, Spinella F, Bagnato A. Endothelin 1 in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 13: 637–651, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Rosanò L, Cianfrocca R, Tocci P, Spinella F, Di Castro V, Caprara V, Semprucci E, Ferrandina G, Natali PG, Bagnato Aβ-arrestin-1. is a nuclear transcriptional regulator of endothelin-1-induced β-catenin signaling. Oncogene 32: 5066–5077, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Rosanò L, Cianfrocca R, Tocci P, Spinella F, Di Castro V, Caprara V, Semprucci E, Ferrandina G, Natali PG, Bagnato A. Endothelin A receptor/β-arrestin signaling to the Wnt pathway renders ovarian cancer cells resistant to chemotherapy. Cancer Res 74: 7453–7464, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Said N, Smith S, Sanchez-Carbayo M, Theodorescu D. Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J Clin Invest 121: 132–147, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semprucci E, Tocci P, Cianfrocca R, Sestito R, Caprara V, Veglione M, Castro VD, Spadaro F, Ferrandina G, Bagnato A, Rosanò L. Endothelin A receptor drives invadopodia function and cell motility through the β-arrestin/PDZ-RhoGEF pathway in ovarian carcinoma. Oncogene 2015. doi: 10.1038/onc.2015.403. [DOI] [PubMed] [Google Scholar]
- 29.Sestito R, Cianfrocca R, Rosanò L, Tocci P, Semprucci E, Di Castro V, Caprara V, Ferrandina G, Sacconi A, Blandino G, Bagnato A. miR-30a inhibits endothelin A receptor and chemoresistance in ovarian carcinoma. Oncotarget 2015. doi: 10.18632/oncotarget.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinella F, Caprara V, Di Castro V, Rosanò L, Cianfrocca R, Natali PG, Bagnato A. Endothelin-1 induces the transactivation of vascular endothelial growth factor receptor-3 and modulates cell migration and vasculogenic mimicry in melanoma cells. J Mol Med 91: 395–405, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Spinella F, Caprara V, Cianfrocca R, Rosanò L, Di Castro V, Garrafa E, Natali PG, Bagnato A. The interplay between hypoxia, endothelial and melanoma cells regulates vascularization and cell motility through endothelin-1 and vascular endothelial growth factor. Carcinogenesis 35: 840–848, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka T, Sho M, Takayama T, Wakatsuki K, Matsumoto S, Migita K, Ito M, Hamada K, Nakajima Y. Endothelin B receptor expression correlates with tumour angiogenesis and prognosis in oesophageal squamous cell carcinoma. Br J Cancer 110: 1027–1033, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teoh JP, Park KM, Wang Y, Hu Q, Kim S, Wu G, Huang S, Maihle N, Kim IM. Endothelin-1/endothelin A receptor-mediated biased signaling is a new player in modulating human ovarian cancer cell tumorigenesis. Cell Signal 26: 2885–2895, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu MH, Huang CY, Lin JA, Wang SW, Peng CY, Cheng HC, Tang CH. Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene 33: 1725–1735, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988. [DOI] [PubMed] [Google Scholar]