Abstract
Prolonged food deprivation in mammals typically reduces glucose, insulin, and thyroid hormone (TH) concentrations, as well as tissue deiodinase (DI) content and activity, which, collectively, suppress metabolism. However, in elephant seal pups, prolonged fasting does not suppress TH levels; it is associated with upregulation of adipose TH-mediated cellular mechanisms and adipose-specific insulin resistance. The functional relevance of this apparent paradox and the effects of glucose and insulin on TH-mediated signaling in an insulin-resistant tissue are not well defined. To address our hypothesis that insulin increases adipose TH signaling in pups during extended fasting, we assessed the changes in TH-associated genes in response to an insulin infusion in early- and late-fasted pups. In late fasting, insulin increased DI1, DI2, and THrβ-1 mRNA expression by 566%, 44%, and 267% at 60 min postinfusion, respectively, with levels decreasing by 120 min. Additionally, we performed a glucose challenge in late-fasted pups to differentiate between insulin- and glucose-mediated effects on TH signaling. In contrast to the insulin-induced effects, glucose infusion did not increase the expressions of DI1, DI2, and THrβ-1 until 120 min, suggesting that glucose delays the onset of the insulin-induced effects. The data also suggest that fasting duration increases the sensitivity of adipose TH-mediated mechanisms to insulin, some of which may be mediated by increased glucose. These responses appear to be unique among mammals and to have evolved in elephant seals to facilitate their adaptation to tolerate an extreme physiological condition.
Keywords: hypothalamic-pituitary-thyroid axis, thyroid stimulating hormone, metabolism
prolonged food deprivation is associated with a decrease in cellular thyroid hormone (TH)-mediated events, in an effort to abate substrate depletion and starvation. It is characterized by a suppression of circulating levels of TH coupled with a decrease in deiodinase type 1 (DI1), deiodinase type 2 (DI2), thyroid hormone receptor beta 1(THrβ-1), and the transcription of certain TH-targeted genes, including mitochondrial uncoupling protein 2 (UCP2) and peroxisome proliferator-activated receptor coactivator 1-alpha (PGC1α) in most fasting mammals, including humans, rats, and bulls (3, 23, 24, 43). It is also coupled with an increase in deiodinase type 3 (DI3) expression and, concomitantly, plasma reverse triiodothyronine (rT3) (5, 13). In humans serum triiodothyronine (T3) levels decrease as a consequence of a reduced peripheral deiodination from thyroxine (T4) to T3 (24, 46). During fasting in bull calves, TH secretion is nearly halted (44). Similar effects have been observed in fasted chickens (45), rats (19, 23), and black bears (4, 21), suggesting that the cellular, TH-mediated responses to prolonged food deprivation in higher vertebrates is conserved. Conversely, in hibernating ground squirrels, plasma T3 concentration increase as a result of marked reductions in nuclear receptors (dampening TH function despite the increase in circulating levels), rendering them cryptically hyperthyroid (25, 26).
Elephant seals naturally fast from food and water for up to three months while on land (15, 31, 32, 36). Contrary to the typical responses to food deprivation in other mammals, prolonged fasting in elephant seals is associated with 1) a lack of a decrease in circulating concentrations of T4 and T3, 2) an increase in the mRNA expressions of DI1, DI2, THrβ-1, and UCP2, and 3) an increase in the translation of these genes to higher protein levels (28). Furthermore, fasting is not associated with increased levels of rT3 (28, 33, 34), suggesting that the cellular TH-mediated activity is potentially functional and that DI3 activity is not increased, if not reduced altogether. Therefore, suppression of DI3, in a mammal undergoing prolonged fasting, contributes to a unique endocrine adaptation, given that most, if not all other mammals that fast and/or hibernate, undergo a systematic dampening of TH signaling.
While blood glucose has been shown to increase in response to hyperthyroidism (8, 9), the effects of elevated glucose on circulating TH and TH-mediated cellular events remain elusive. Although some studies show that fasting blood glucose levels in hyperthyroidism are normal (55), another study demonstrated elevated blood glucose resulting from an increase in hepatic glucose production in patients with hyperthyroidism (12), suggesting that glucose may stimulate TH secretion. If so, then elevated glucose and the concomitant increase in insulin do not likely induce a negative-feedback effect on TH levels and TH-mediated cellular effects. Regardless, the regulatory effects of insulin and glucose on cellular TH-associated mechanisms are not well-defined in mammals. Therefore, to address the complex interrelationships among glucose, insulin, and TH, and our hypothesis that insulin increases adipose TH signaling in pups during extended fasting, we separately infused fasting elephant seal pups with either insulin or glucose and thoroughly examined the cellular TH-associated components.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committees of both the University of California Merced and Sonoma State University. All work was conducted under the National Marine Fisheries Service marine mammal permit no. 87-1743. The current study complements and extends our previous study elucidating the cellular mechanisms of lipid metabolism in fasting northern elephant seal pups within the same cohort of animals (49).
Animals.
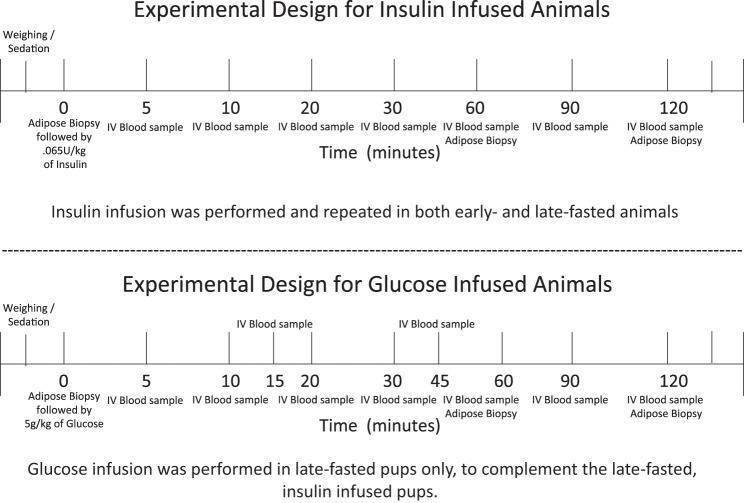
Northern elephant seal (Mirounga angustirostris) pups were studied at the Año Nuevo State Reserve (30 km north of Santa Cruz, CA) while they are on land during their natural, postweaning fast. In preparation of the infusion studies (insulin or glucose), pups were weighed, and initially sedated with an intramuscular injection of Telazol (tiletamine/zolazepam HCl, Fort Dodge Labs, Ft. Dodge, IA) (49–51). Once immobilized, an 18-gauge, 3.5-inch spinal needle was inserted into the extradural spinal vein to facilitate the infusion of insulin or glucose (35). Initially, body mass was estimated on the basis of length and girth measurements, which has been validated in elephant seals (11, 18) (Fig. 1).
Fig. 1.
Protocol diagram representing the sampling period for both insulin- and glucose-treated animals. The pups were sampled during either the early (1–2 wk postweaning; insulin infusion only) and the late fasting period (6–8 wk postweaning; glucose and insulin infusions).
Insulin infusion.
For the insulin study, pups were infused during the early (1–2 wk postweaning; n = 5; 127 ± 1 kg) and the late (6–8 wk postweaning; n = 6 late; 93 ± 4 kg) fasting periods. Prior to infusion, a predose adipose biopsy and blood sample were collected, immediately followed by the bolus infusion, and subsequent blood sampling at 5, 10, 20, 30, 60, 90, and 120 min (Fig. 1). Subsequent subcutaneous adipose biopsies were collected at 60 and 120 min (Fig. 1). Procedures were terminated at 120 min to avoid potential concerns associated with insulin-induced hypoglycemia. Immediately following the collection of the 120-min samples, glucose was infused (iv) slowly to assist in the restoration of preinfusion levels, and the animals were monitored closely.
Intravenous glucose infusion.
Because the analysis of the effects of glucose on TH-mediated cellular events was conducted to complement our previous study (48, 49, 51), sufficient samples (plasma and biopsies) to perform complete measurements were only available for the late-fasting portion of the study. Thus, only data from this group of animals are provided. However, this data set is still valuable to the interpretation of the results for the following reasons: 1) a direct comparison of the effects of insulin vs. glucose can be made because complete data are available for both insulin and glucose in late-fasted animals, 2) no other data exist on the effects of glucose on cellular, TH-mediated events in any naturally adapted mammal, and 3) federal restrictions on the number of animals that can be used is limited, making replication of such studies difficult. Furthermore, because the most profound changes in response to insulin were observed in the late-fasted pups, the impact of the lack of glucose-infusion data during the early fasting period is minimized. Therefore, for the glucose infusion study, only data from late-fasted (6–8 wk post-weaning; n = 8 late; 83 ± 7 kg) pups are presented. The animals studied in the glucose infusion protocol were different from those used in the insulin infusion study. The inclusion of this data allowed us to better evaluate the cellular responses to the two infusion protocols and provided an opportunity to distinguish between insulin- and glucose-mediated effects on cellular TH-associated genes.
As with the insulin infusion study, once the animals were sedated a preinfusion blood sample and adipose biopsy was collected from each animal. Following the preinfusion sample collection, animals were infused with a mass-specific dose of glucose (0.5g/kg) over a 2-min period (48, 49). Immobilization of the animal was maintained with 100 mg iv bolus injections of ketamine as needed. Subsequent blood samples were collected at 5, 10, 15, 20, 30, 45, 60, 90, and 120 min postinfusion, and subsequent adipose biopsies were collected at 60 and 120 min postinfusion (48–51) (Fig. 1). Immediately after collection, blood glucose was measured using a commercially available blood glucose monitor (49).
Sample collection and preparation.
Blood samples obtained from the extradural spinal vein were collected in chilled, EDTA-treated vacutainer sample tubes containing a protease inhibitor cocktail (PIC; Sigma-Aldrich) and kept on ice until they could be centrifuged (49). Blood samples were centrifuged for 15 min at 3,000 g, and the plasma was transferred to cryogenic vials, immediately frozen by immersion in liquid nitrogen, and stored at −80°C upon return to the laboratory (48–51).
Adipose biopsies were collected by first cleaning a small region in the flank of the animal near the hind flipper with alternating wipes of isopropyl alcohol and betadine, followed by a subcutaneous injection of 2–3 ml lidocaine (Henry Schein, Melville, NY). A small (<1.5 cm) incision was made using a sterile scalpel, and a biopsy (∼100–200 mg) collected with a sterile biopsy punch needle (Henry Schein) (48, 49). The biopsy samples were rinsed with cold, sterile saline, placed in cryogenic vials, immediately immersed in liquid nitrogen, and stored at −80°C until later analyses. Frozen tissue samples were homogenized in 500 μl hypotonic buffer containing a PIC and phosphatase inhibitor cocktail (Halt PIC; Thermo, Waltham, MA) (48, 49). The homogenate was then centrifuged at 16,100 g for 15 min, and the aqueous layer was aliquoted into a separate tube. The pellet was reconstituted with TBS (500 μl) containing 1% vol/vol Triton X-100, 1% wt/vol SDS, and 1% vol/vol PIC and sonicated. The resulting suspension was then centrifuged at 16,100 g for 15 min, and the aqueous layer was again transferred to a separate tube. Total protein content in nuclear, cytosolic, and membrane-bound fractions was measured by Bradford assay (Bio-Rad Laboratories), and quantities were used to normalize loading of samples into gel wells.
Quantification of mRNA expressions.
Total RNA was isolated from adipose samples using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. RNA integrity was confirmed by measuring the absorbance at 260 nm and 280 nm and by evaluating the bands run on 1% agarose gel electrophoresis (38). Contamination of genomic DNA in total RNA was eliminated by digestion with DNase I (Roche, Indianapolis, IN), as specified by the manufacturer. Different cDNAs from each tissue were synthesized from total DNA-free RNA (1 μg) using oligo-dT and the QuantiTect Reverse Transcription kit (Qiagen, Valencia, CA). Specific primers for DI1, DI2, DI3, THrβ-1, UCP2, and PGC1α were designed on the basis of homologous mammalian nucleotide sequences and partial sequences confirmed (Table 1). The expression of GAPDH was used as an internal standard to normalize the expression of each target gene. Gene expression was measured by quantitative qPCR using DI1Fw2 + DI1Rv2, DI2Fw1 + DI2Rv2, DI3Fw1 + DI3Rv1, UCP2Fw1 + UCP2Rv2, THrβ1Fw1 + THrβ1Rv1, PGC1αFw1 + PCG1αRv1, and GAPDHFw1 + GAPDHRv1 primers, respectively. The PCR reactions of each tissue sample were run on a 7500 real-time PCR system (Applied Biosystems, Foster City, CA) in a final volume of 20 μl containing 10 μl of SYBR Green PCR Master Mix (Applied Biosystems), 6 μl of H2O, 0.5 μl of each primer (20 μmol/l), and 3 μl of cDNA (equivalent to 150 ng of total RNA). After an initial denaturing step at 94°C for 5 min, amplifications were performed for 40 cycles at 94°C for 30 s, 58–63°C for 30 s, and a final step of 30 s at 72°C, with a single fluorescence measurement and a final melting curve program decreasing 0.3°C every 20 s from 95 to 60°C. Positive (with cDNA) and negative (no cDNA) controls were included in each assay. Standard curves for each gene of interest were run to determine the efficiency of amplification using dilutions from 5E−3 to 5E−8 ng/μl of PCR fragments. For each measurement, expression levels (ng/μl) were normalized to the expression of GAPDH. To confirm that GAPDH expression did not change with fasting duration or in response to the exogenous infusions, additional quantitative PCR analyses were performed, and the data confirmed its utility for normalizing as a reference gene.
Table 1.
Concentrations of plasma thyroid stimulating hormone, total and free T3, T4, insulin, and glucose from northern elephant seal pups before (0 min) and 10, 30, 60, 90, and 120 min postinsulin infusion during the early and late fasting period, and postglucose infusion during the late fasting period
| 0 min | 10 min | 30 min | 60 min | 90 min | 120 min | |
|---|---|---|---|---|---|---|
| Early Insulin Infusion | ||||||
| TSH, ng/ml | 0.09 ± 0.07 | 0.08 ± 0.04 | 0.09 ± 0.05 | |||
| tT3, ng/ml | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.1 | 1.4 ± 0.5 | 1.4 ± 0.4 |
| tT4, ng/ml | 37 ± 6 | 34 ± 4 | 33 ± 5 | 38 ± 6 | 33 ± 5 | 39 ± 7 |
| Insulin,a μU/ml | 3.2 ± 0.02 | 171 ± 9* | 25 ± 1* | 6 ± 1* | 2.4 ± 0.4 | 2.1 ± 0.5 |
| Glucosea mg/dl | 174 ± 8 | 159 ± 7 | 133 ± 11* | 107 ± 16* | 101 ± 11* | 109 ± 9* |
| Late Insulin Infusion | ||||||
| TSH, ng/ml | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | |||
| tT3, ng/ml | 1.4 ± 0.1 | 2.3 ± 0.7 | 1.7 ± 0.9 | 1.5 ± 0.1 | 1.6 ± 0.2 | 1.3 ± 0.2 |
| tT4, ng/ml | 36 ± 3 | 38 ± 7 | 32 ± 5 | 41 ± 4 | 31 ± 5 | 44 ± 9 |
| Insulina, μU/ml | 1.9 ± 1 | 138 ± 30* | 21 ± 4* | 4 ± 1* | 2.1 ± 0.4 | 1.4 ± 0.2 |
| Glucosea, mg/dl | 135 ± 9 | 128 ± 8 | 105 ± 5* | 77 ± 5* | 64 ± 5* | 62 ± 5* |
| Late Glucose Infusion | ||||||
| TSH, ng/ml | 0.10 ± 0.08 | 0.10 ± 0.04 | 0.09 ± 0.03 | |||
| fT3, pg/ml | 1.2 ± 0.10 | 1.1 ± 0.08 | 1.0 ± 0.10 | 1.0 ± 0.03 | 1.0 ± 0.04 | |
| tT3, ng/ml | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.04 | 1.2 ± 0.1 | 1.2 ± 0.1 | |
| fT4, pg/ml | 10.8 ± 1.0 | 10.5 ± 0.6 | 9.8 ± 0.3 | 9.9 ± 0.8 | 10.6 ± 0.4 | |
| tT4, ng/ml | 43 ± 2 | 40 ± 2 | 42 ± 1 | 40 ± 2 | 43 ± 1 | |
| Insulinb, μU/ml | 1.3 ± 0.1 | 1.8 ± 0.3 | 2.1 ± 0.3* | — | 2.0 ± 0.2* | |
| Glucoseb, mg/dl | 128 ± 9 | 287 ± 13‡ | 222 ± 12‡ | 187 ± 8‡ | 175 ± 9‡ | |
Values are expressed as means ± SE. At 30 min, concentrations for glucose and hormones are not shown because they were not available. TSH, plasma thyroid stimulating hormone; t, total; f, free; T3, triiodothyronine; T4, thyroxine;
Insulin and glucose values were adapted from Viscarra et al. (49).
Glucose values were adapted from Viscarra et al. (48).
P < 0.05;
P < 0.01.
Quantification of protein expression by Western blot analysis.
The quantity of the biopsy samples limited our ability to quantify the expressions of all the proteins of interest. Because our hypothesis focused on the changes in the deiodinases and THrβ-1, these proteins were given priority. Protein expression was quantified by standard Western blot analysis, as previously described (48, 51). The primary antibodies for DI1, DI2, THrβ1, β-actin, and TATA binding protein (TBP) (Santa Cruz Biotechnology, Santa Cruz, CA) were diluted 1:500 to 1:5,000. The HRP-conjugated secondary antibody (Pierce, Rockford, IL) was diluted 1:10,000, and blots were developed using the Immun-Star Western C kit (Bio-Rad). Blots were visualized and semi-quantified using a Kodak 440 digital science imager. In addition to consistently loading the same amount of total protein (20 μg) per well, densitometry values were further normalized by the densitometry values of β-actin or TBP.
Plasma analyses.
The plasma concentrations of thyroid-stimulating hormone (TSH), total thyroxine, free thyroxine, total triiodothyronine (tT3), and free triiodothyronine (fT3) were measured by radioimmunoassay and previously validated for elephant seals, with internal control provided by the manufacturer (33, 36). Plasma sample volumes limited our analyses of TH to six time points in the present study and to only three time points (0, 10, or 120 min) for TSH. The validation of TSH assay for elephant seal plasma was confirmed by diluting two separate pools of plasma (Fig. 2). All samples were analyzed in duplicate and run in a single assay with intra-assay percent coefficients of variability of <10% for all assays.
Fig. 2.
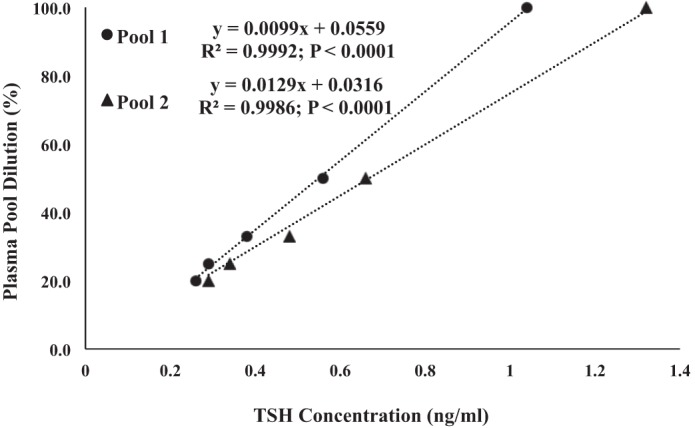
Thyroid-stimulating hormone (TSH) dilutions hold well in two different high pools, indicating the specificity of the TSH antibody used for elephant seal TSH.
Statistics.
The baseline (or time 0) measurements (plasma, mRNA, or tissue protein content) of the early- and late-fasted groups were used to assess changes as a function of fasting duration (insulin infusion study only). Means ± SE were compared by ANOVA using a Fisher's protected least significant difference (PLSD) post hoc test. Repeated-measures ANOVA was used to determine changes in parameters following the infusions of glucose and insulin. Mean percent changes were calculated for each time point and compared by repeated-measures ANOVA to identify the changes in response to the infusions using a Fisher's PLSD post hoc test. Changes were considered significantly different at P < 0.05. Statistical analyses were performed with StatView software (SAS Institute, Cary, NC).
RESULTS
Insulin infusion increased adipose DI1 and THrβ-1 mRNA expression.
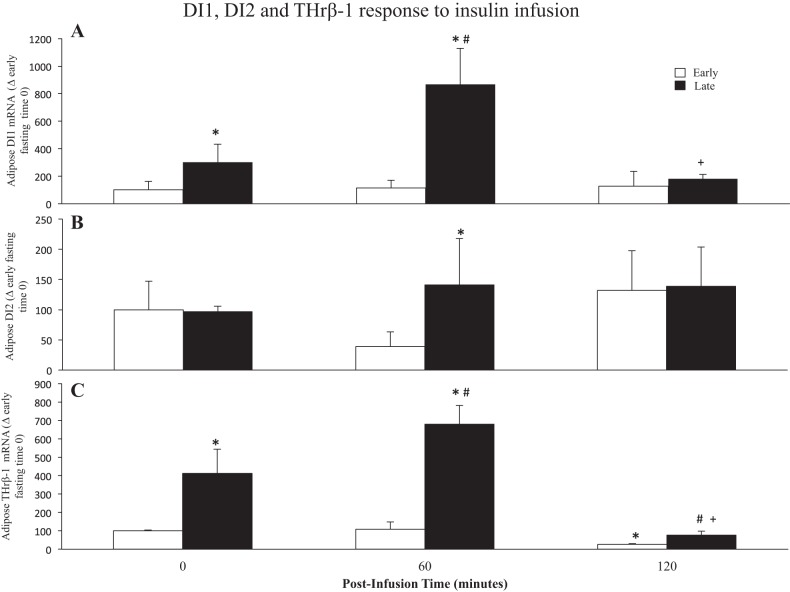
The expression of adipose DI1, DI2, and THrβ-1 was influenced both by fasting and by infusion of exogenous insulin during fasting. Fasting duration (early vs. late, T0) was associated with three- and four-fold increases (P < 0.05) in mRNA expressions of DI1 (Early group: 100 ± 62 vs. 301 ± 132) and THrβ-1, respectively (Early group: 100 ± 5 vs. 413 ± 131) (Fig. 3). Infusion of insulin early in fasting had little to no effect on any of these three variables, suggesting that the cellular TH-associated signaling is desensitized to insulin during this period. However, infusion of insulin late in fasting increased the expressions of all three at 60 min postinfusion (Fig. 3). Insulin infusion in late fasting increased (P < 0.05) the mRNA expression of DI1 2.8-fold at 60 min (time 60: 867 ± 264 vs. 301 ± 132) with levels returning to baseline (T0) by 120 min (Fig. 3A). Insulin infusion in late fasting increased (P < 0.05) the mRNA expression of DI2 1.4-fold at 60 min with levels remaining elevated at 120 min (time 60: 141 ± 77 vs. 97 ± 9) (Fig. 3B). Insulin infusion in late fasting initially (T60) increased (P < 0.05) the mRNA expression of THrβ-1 1.6-fold (time 60: 680 ± 101 vs. 413 ± 131) with levels decreasing to 19% of baseline (T0) at 120 min (Fig. 3C). Nonetheless, these reduced THrβ-1 expression levels at T120 were 2.8-fold greater (P < 0.05) than the lower levels in early fasting (Early group: 27 ± 4 vs. 77 ± 22). These results suggest that prolonged fasting increases the adipose expressions of DI1 and THβ-1 and increases the tissues sensitivity to insulin with regard to its ability to enhance the expressions of D1, D2, and THβ-1. Levels of DI3 mRNA expression were not detectably expressed in adipose before or during insulin infusion in both early and late fasting.
Fig. 3.
Values are expressed as means ± SE. mRNA expressions of adipose deiodinase type I (DI1) (A), type II (DI2) (B), and thyroid hormone receptor β-1 (THrβ-1) (C) from early (2 wk postweaning) and late (7–8 wk postweaning) fasting elephant seal pups before (0) and (60 and 120 min) following an insulin infusion. *Significant difference (P < 0.05) from early. #Significant difference (P < 0.05) from T0 within a group. +Significant difference (P < 0.05) from T60.
Insulin infusion altered adipose UCP2 mRNA expression but not PGC1α.
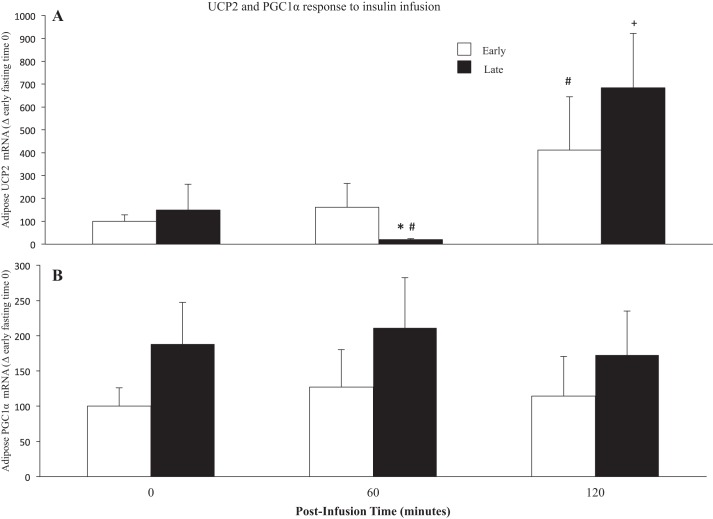
While insulin infusion had no significant effect on adipose mRNA expression of UCP2 in early fasting, insulin infusion in late fasting decreased (P < 0.05) UCP2 expression 7.5-fold at 60 min (time 60: 20 ± 3 vs. 148 ± 113) (Fig. 4A). At 120 min, the expression of UCP2 increased (P < 0.05) six-fold in early fasting and nearly five-fold in late fasting (time 120: 683 ± 238 vs. 148 ± 113) (Fig. 4A). While an increasing trend with fasting duration (T0) is evident, insulin had no significant effect on PGC1α expression, whether it was infused early or late in fasting. (Fig. 4B).
Fig. 4.
Values are expressed as means ± SE. mRNA expressions of adipose uncoupling protein 2 (UCP2) (A) and peroxisome proliferator activated receptor gamma co-activator 1 alpha (PGC1α) (B) from early (2 wk postweaning) and late (7–8 wk postweaning) fasting elephant seal pups before (0) and following (60 and 120 min) an insulin infusion. *Significant difference (P < 0.05) from Early. #Significant difference (P < 0.05) from T0 within a group. +Significant difference (P < 0.05) from T60.
Glucose delays the insulin-induced increase in mRNA expression of DI1, DI2 and THrβ-1.
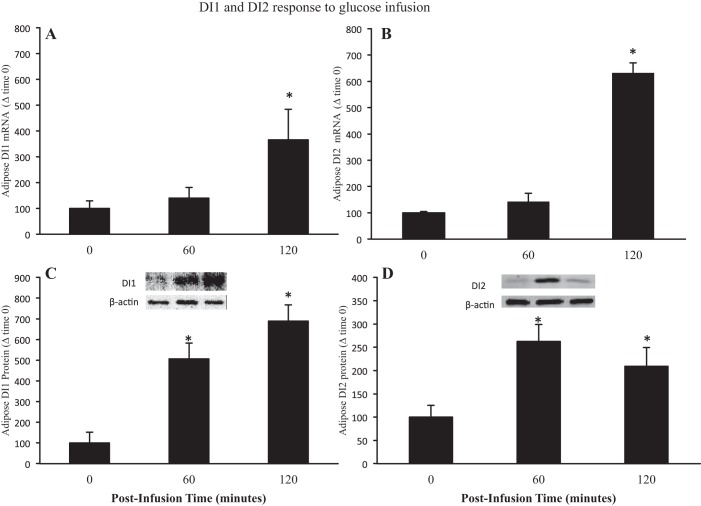
Effects of glucose infusion in late fasting (Figs. 5–7) were similar to those of late-fasting insulin infusion (Figs. 3 and 4). Expressions of adipose DI1 and DI2 mRNA increased (P < 0.05) 3.6- (time 120: 366 ± 118 vs. 100 ± 29) and 6.3-fold (time 120: 632 ± 38 vs. 100 ± 4), respectively, at 120 min (Fig. 5, A and B). The protein expression of DI1 increased (P < 0.05) five-fold at 60 min (time 60: 506 ± 76 vs. 100 ± 51), and seven-fold at 120 min (time 120: 688 ± 77 vs. 100 ± 4) (Fig. 5C). Similarly, the protein expression of DI2 increased (P < 0.05) 2.6-fold at 60 min (time 60: 262 ± 36 vs. 100 ± 25) and remained elevated (P < 0.05) at 120 min (time 120: 209 40 vs. 100 ± 25) (Fig. 5D). Similar to DI1 and DI2 expressions, the mRNA expression of THrβ-1 was not increased initially (T60), but mean levels increased (P < 0.05) four-fold at 120 min (time 120: 436 ± 134 vs. 100 ± 28) (Fig. 6A). The protein content of THrβ-1 increased (P < 0.05) 40% at 60 min and (time 60: 140 ± 4 vs. 100 ± 11), an additional 40% at 120 min (time 120: 180 ± 23 vs. 100 ± 11) (Fig. 6B). In general, the effects of glucose infusion were delayed, prolonged, or both compared with those of insulin infusion.
Fig. 5.
Values are expressed as means ± SE. mRNA expression of adipose DI1 (A) and DI2 (B) and of protein content levels of adipose DI1 (C) and DI2 (D) from late (7–8 wk postweaning) fasting elephant seal pups before (0) and (60 and 120 min) following a glucose infusion. Insets: representative Western blots for each protein. *Significant difference (P < 0.05) from T0.
Fig. 7.
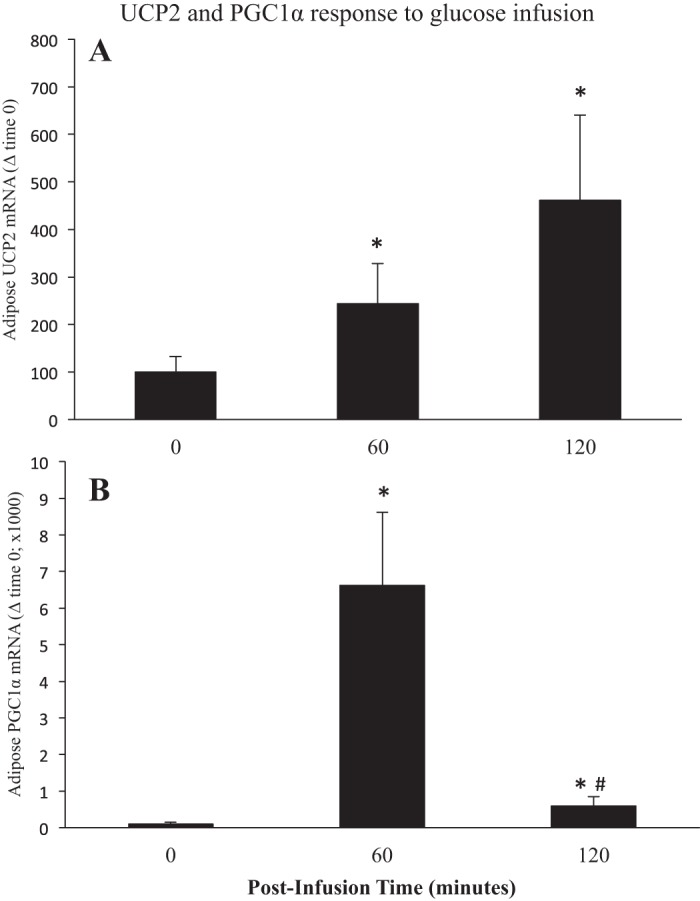
Values are expressed as means ± SE. mRNA expressions of adipose uncoupling protein 2 (UCP2) (A) and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) (B) from late (7–8 wk postweaning) fasting elephant seal pups before (0) and following (60 and 120 min) a glucose infusion. *Significant difference (P < 0.05) from T0. #Significant difference (P < 0.05) from T60.
Fig. 6.
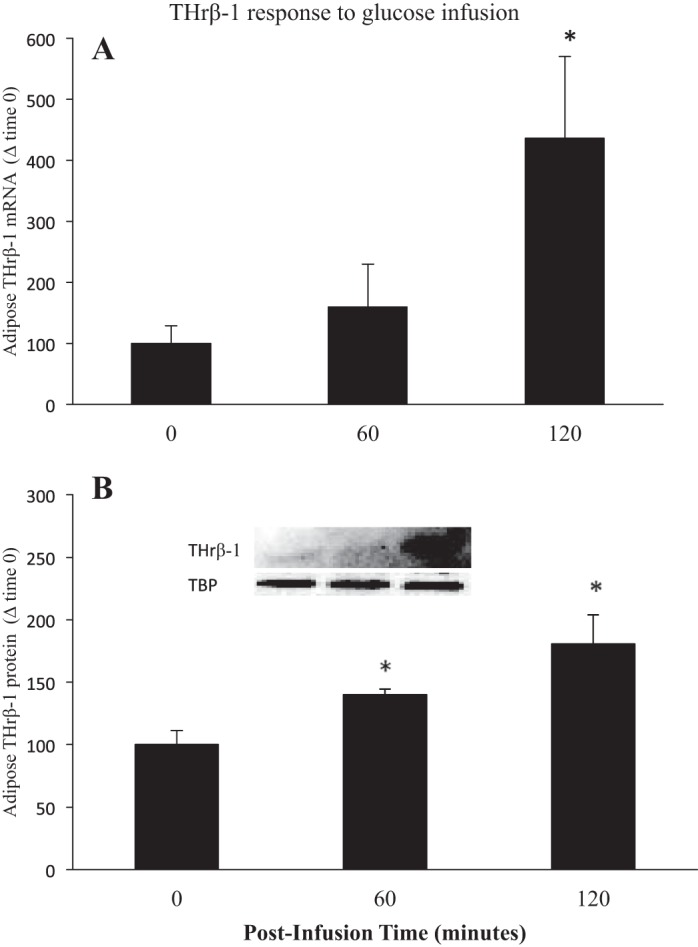
Values are expressed as means ± SE. Adipose thyroid hormone receptor β-1 (THrβ-1) mRNA expression (A) and protein content levels (B) from late (7–8 wk postweaning) fasting elephant seal pups before (0) and (60 and 120 min) following a glucose infusion. Inset: representative Western blot for THrβ-1. *Significant (P < 0.05) difference from T0.
Adipose UCP2 mRNA expression responds differentially to glucose.
While insulin induced an initial decrease in UCP2 mRNA expression, glucose increased (P < 0.05) mean mRNA expression of adipose UCP2 2.4-fold at 60 min (time 60: 243 ± 84 vs. 100 ± 32) and 4.6-fold at 120 min (time 120: 461 ± 179 vs. 100 ± 32) (Fig. 7A). Unlike the effects of insulin, glucose increased (P < 0.05) the mean mRNA expression of adipose PGC1α 68-fold at 60 min (time 60: 6,621 ± 1,996 vs. 100 ± 46), and despite the 91% decrease between 60 and 120 min, the mean expression levels remained (P < 0.05) six-fold higher (time 120: 591 ± 252 vs. 100 ± 46) at time 120. (Fig. 7B).
Neither insulin nor glucose infusion altered circulating TH levels.
Neither insulin nor glucose infusion had remarkable effects on the circulating levels of any of the TH measured (Table 1).
DISCUSSION
Although thyroid hormones have profound and direct effects on glucoregulatory pathways (22, 40–42, 52), the relationships between insulin and glucose on cellular TH-mediated signaling is more complex and not well established. To address the complexity in these relationships, the more significant contributions of the present study are that 1) fasting duration is associated with increased responsiveness of cellular TH-mediated signaling to insulin in adipose, 2) elevated glucose delays the insulin-induced increase in TH-associated genes in late-fasted seals, 3) cellular TH pathways via UCP2 respond differentially to insulin depending on the presence or absence of elevated glucose, and 4) potentially redundant, but independent, activation of TH-mediated signaling by both glucose and insulin.
Insulin upregulates cellular thyroid hormone-associated genes.
The contributions of TH on substrate-level metabolism (glucose) have been described (20, 37, 41, 52, 40), but are incongruent. Both hypo- and hyperthyroidism may affect insulin actions, directly altering insulin secretion, and ultimately, glucose levels. In hypothyroid subjects, glucose-stimulated insulin secretion (GSIS) was decreased (39), while in hyperthyroid subjects, the β-cell response to glucose was increased (29), demonstrating the spectrum of glucose effects on insulin secretion in the presence or absence of elevated TH levels. However, the reciprocal changes in insulin levels on cellular TH-mediated responses are less defined. In the present study, infused insulin in the absence of high glucose and unaltered TH levels increased the mRNA expressions of DI1 and THrβ-1 (and to a lesser extent DI2) at 60 min, but the insulin-induced increases were not sustained, returning to baseline levels by 120 min. The quantification of deiodinases can provide an accurate assessment of cellular thyroidal status where elevated DI2 corresponds to an active thyroid status, elevated DI3 to a preferential downregulation of the system, and elevated DI1 representing either condition (16). Here, we show that elevated insulin increases TH-mediated signaling, with the timing of the cellular events associated with reduced extracellular glucose.
The peripheral thyroid hormone receptor, THrβ-1, has been shown to affect insulin signaling; however, the inverse is not well defined. Because UCP2 is a target gene of THrβ-1 (6), the insulin-induced increase in adipose UCP2 expression suggests that the increase in THrβ-1 was functional and biologically relevant. While the contribution of UCP2 to regulating cellular metabolism is not yet fully understood, decreased hepatic UCP2 is associated with impaired lipid metabolism and antioxidant capacity in insulin-resistant rats (30). Furthermore, in β-cells, UCP2 may regulate GSIS and protect against oxidative stress by decreasing mitochondrial reactive oxygen species (ROS) in insulinoma cells (1, 2). Therefore, the increase in cellular TH-mediated signaling with fasting duration may contribute to UCP2-associated lipid metabolism and to the alleviation of mitochondrial ROS generation (30, 47). This is corroborated by our previous studies in fasted elephant seal pups demonstrating that oxidative damage is alleviated, while antioxidant enzyme activities and protein content are increased (47).
Changes in the mRNA expression of PGC1α can provide further indication of the activation of THrβ-1. While the mRNA expression of PGC1α did not increase in response to insulin, glucose infusion increased its expression, suggesting that some aspect (direct or indirect) of a hyperglycemic environment contributes to the regulation of adipose PGC1α expression during late fasting. Although T3 directly regulates PGC1α in vivo and in vitro (54), the glucose-induced increase in adipose PGC1α protein was independent of changes in T3 (or any other TH), suggesting that glucose directly regulates adipose PGC1α in late-fasted seals. PGC1α may also promote the induction of UCPs in both adipose and muscle (53); however, the simultaneous increase in UCP2 and PGC1α was only observed in response to the glucose infusion, suggesting that the effects of PGC1α on UCP2 in adipose during prolonged fasting is a function of elevated glucose (and not insulin) in seals.
Glucose delays the insulin-induced increase in TH-associated genes.
Because profound effects of insulin on cellular TH-mediated signaling were observed only during late fasting, when animals develop adipose-specific insulin resistance (48–51), a supplemental, glucose-infusion study was performed to complement the insulin infusion data. This additional protocol provides a more robust assessment of the contributions of glucose to cellular TH-mediated signaling. While DI2 may contribute to glucose metabolism, its role in insulin resistance is not well defined. Where DI2 increases glucose metabolism in human cardiac tissue (20) and has protective effects against diet-induced obesity (27), DI2 knockout mice have reduced glucose uptake, consistent with insulin resistance, independent of diet-induced obesity (27). In the present study, glucose infusion increased the mRNA expressions of both DI1 and DI2, but not until the 120-min measurement period. In contrast, insulin infusion increased these expressions at 60 min with levels returning to baseline by 120 min. The distinct timing of the increases in DI1 and DI2 expressions between the two infusion protocols, which differ in circulating levels of glucose, suggests that glucose delays the insulin-induced increased expressions of DI1 and DI2 in late-fasted animals. This is further corroborated by the observation that the elevated glucose and insulin induced with glucose infusion remain so at 120 min (48). Moreover, these dynamic changes in deiodinase and receptor expression suggest that increases in cellular TH-mediated signaling are functional and possibly contribute to reciprocal regulation of substrate metabolism.
Hyperglycemia, independent of insulin, stimulates similar TH pathways as insulin: redundant activation in adipose?
Alternatively, given that the increases in the expression of these TH-associated genes during insulin infusion are associated with an increase in plasma insulin, peaking at 138 μU/ml, but with a decline in glucose below baseline, glucose and insulin may redundantly activate some of the same genes, with the principal distinction being that glucose delayed and prolonged the effects compared with those of insulin. Moreover, given that peak plasma insulin levels are substantially higher in the insulin-infused group than they are in the glucose-infused group, the hyperglycemia, independent of insulin, may activate the observed increases in TH-associated genes. This alternative hypothesis is supported by the difference in plasma insulin levels measured between the two infusion groups. Also supporting this alternative explanation are the differing transcriptional responses of cellular TH-target genes, UCP2 and PGC1α. Specifically, although the mRNA expression of UCP2 decreased and then increased with the insulin infusion and no significant effect on PGC1α, both UCP2 and PGC1α expression increased with the glucose infusion.
Responsiveness of TH signaling genes to insulin is increased with fasting.
Although we showed that the thyroid hormone-associated genes increased in response to insulin during the late fast, we also showed that the mRNA expression of adipose DI1 and THrβ-1 increased with fasting duration, which coincides with the development of adipose-specific insulin resistance in northern elephant seal pups (49). A significant and major contribution of the present study is the change in the responsiveness of the TH-associated genes to insulin. During the early fasting period, the responses of the measured genes to insulin were wholly unremarkable with only a couple of detectable changes. However, the responsiveness of the genes of interest to insulin was starkly contrasted during late fasting, and much more dynamic in nature. Interestingly, this stark contrast in responsiveness to insulin in adipose, which we have previously described as insulin resistant (49) suggests that adipose may be resistant to insulin's glucoregulatory effects, but is not resistant to its secondary effects (i.e., promotion of TH signaling). The dynamic variability of insulin's effects in adipose in this fashion sheds new light on the complexity of insulin-mediated signaling during the manifestation of fasting-induced insulin resistance.
Neither insulin nor glucose alter circulating TH levels: change in clearance?
Previous studies have noted a correlation between TH levels and insulin signaling. For example, in humans the prevalence of impaired fasting glucose increases linearly with levels of fT3, and impaired glucose tolerance decreases with levels of fT3 (22). The present study demonstrated that neither insulin nor glucose infusions remarkably altered any of the plasma thyroid hormones over the course of the measurement period. However, this lack of treatment-induced changes in TH may be indicative of increased clearance of hormones from circulation, which can be facilitated by increased deiodinase and receptor availability (7). The increases in adipose DI1 (and lesser extent DI2) and THrβ-1 expressions could potentially contribute to increased hormone clearance/metabolism, explaining the lack of changes in circulating levels. Regardless, measuring plasma TH alone does not provide an accurate assessment of functional potential, especially in regard to TH-mediated mechanisms. This concept is further supported by data from hibernating ground squirrels. Plasma T3 concentrations increase as a result of reduced TH metabolism, which is accompanied by a reduction in nuclear receptors, indicating that, despite the increase in circulating levels (i.e., cryptically hyperthyroid), TH function is suppressed (25, 26). Conversely, in the present study, the upregulation of cellular TH-associated genes and proteins despite the lack of an increase in circulating hormone levels suggests that TH activity is upregulated, regardless of the lack of changes in hormone levels.
Limitations
While the significant new revelations elucidate novel signaling pathways and interactions, we are cognizant of the study's limitations. Glucose infusion studies in early-fasted seals to parallel the data in the late-fasted animals would have been ideal. However, a comparison of the effects of insulin vs. glucose infusions in late-fasted animals alone, when the most dynamic and interesting changes were detected, revealed highly significant contributions to our understanding of these interactions, especially as they relate to insulin resistance. Another limitation associated with working with federally protected animals, is the inability to sample other tissues besides peripheral adipose, muscle, and blood. Given that adipose is stored in different sites and functionally distinct (14), distinguishing the potential contributions of these different fat stores (e.g., subcutaneous vs. visceral vs. brown) is not possible here. However, elephant seals have little visceral fat compared with other mammals, including other phocid seals (10, 17), with most of their adipose stored in subcutaneous blubber, from which we sampled.
Perspectives and Significance
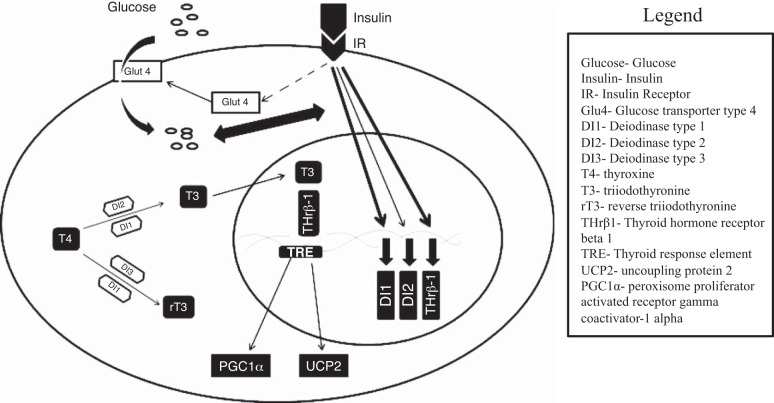
Unlike many fasting-adapted mammals, the fast of northern elephant seal pups is paradoxically associated with increases in cellular TH-mediated events. Furthermore, these TH-mediated events are associated with the onset of adipose-specific insulin resistance. In this study, we show that there is a defined shift in the responsiveness of adipose to insulin with fasting duration that may represent a “conditioning” state as the animals prepare to depart the rookery soon after this measurement period to feed. This is especially significant because fasting duration is associated with reduced plasma insulin. Therefore, elucidation of the mechanisms that contribute to maintaining the integrity of insulin signaling may have profound implications in the study of understanding insulin resistance. Additionally, because feeding stimulates insulin secretion, maintaining the responsiveness of peripheral tissues to insulin is paramount to sustaining cellular metabolism and function. Conversely, desensitization of the insulin-mediated effects during early fasting would be adaptive and help to abate the metabolic burden induced by insulin signaling, resulting in the conservation of circulating glucose. This study highlights the capacity of glucose to delay the insulin-induced increases in TH-associated genes despite the development of fasting-induced insulin resistance. This is significant because it elucidates a novel signaling pathway that contributes to the complexity of the interrelationships among glucose, insulin, and thyroid hormones (Fig. 8).
Fig. 8.
Schematic representation for proposed insulin-induced regulation of cellular thyroid hormone-associated pathways.
GRANTS
J. G. Soñanez-Organis was supported by a postdoctoral fellowship from the University of California Institute for Mexico as well as the United States and Mexico's National Council for Science and Technology (UC MEXUS-CONACYT). J. A. Viscarra was supported by a Supplement to Support Diversity National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) R01HL-09176-S. R. M. Ortiz was partially supported by NHLBI (K02HL-103787). Research was funded by the NIH NHLBI Grant R01HL-09176. Bridget Martinez was supported by the Dennis R. Washington Graduate Achievement Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.M., J.A.V., D.E.C., and R.M.O. conception and design of research; B.M., J.G.S.-O., J.A.V., J.T.J., and D.E.C. performed experiments; B.M., J.G.S.-O., and J.A.V. analyzed data; B.M. and R.M.O. interpreted results of experiments; B.M. and J.T.J. prepared figures; B.M. drafted manuscript; B.M., J.G.S.-O., J.T.J., D.S.M., D.E.C., and R.M.O. edited and revised manuscript; B.M., J.G.S.-O., J.A.V., J.T.J., D.S.M., D.E.C., and R.M.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. Tift, S. Tavoni, C. Champagne, and J. Cutler for their help sedating the seals. We also thank M. Thorwald for assisting with sample collection.
REFERENCES
- 1.Affourtit C, Brand MD. Uncoupling protein-2 contributes significantly to high mitochondrial proton leak in INS-1E insulinoma cells and attenuates glucose-stimulated insulin secretion. Biochem J 409: 199–204, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Affourtit C, Jastroch M, Brand MD. Uncoupling protein-2 attenuates glucose-stimulated insulin secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radic Biol Med 50: 609–616, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azizi F. Effect of dietary composition on fasting-induced changes in serum thyroid hormones and thyrotropin. Metabolism 27: 935–942, 1978. [DOI] [PubMed] [Google Scholar]
- 4.Azizi F, Mannix JE, Howard D, Nelson RA. Effect of winter sleep on pituitary-thyroid axis in American black bear. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E227–E230, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Baqui M, Botero D, Gereben B, Curcio C, Harney JW, Salvatore D, Sorimachi K, Larsen PR, Bianco AC. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem 278: 1206–1211, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Barbe P, Larrouy D, Boulanger C, Chevillotte E, Viguerie N, Thalamas C, Trastoy MO, Roques M, Vidal H, Langin D. Triiodothyronine-mediated upregulation of UCP2 and UCP3 mRNA expression in human skeletal muscle without coordinated induction of mitochondrial respiratory chain genes. FASEB J 15: 13–15 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116: 2571–2579, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boerner A, Voth E, Theissen P, Wienhard K, Wagner R, Schicha H. Glucose metabolism of the thyroid in autonomous goiter measured by F-18-FDG-PE. Exp Clin Endocrinol Diabetes 108: 191–196, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Boerner A, Voth E, Theissen P, Wienhard K, Wagner R, Schicha H. Glucose metabolism of the thyroid in Graves' disease measured by F-18-fluoro-deoxyglucose positron emission tomography. Thyroid 8: 765–772, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Bonner W. The Natural History of Seals. New York, NY: Facts on File, 1990. [Google Scholar]
- 11.Bryden MM. Body size and composition of elephant seals (Mirounga leonine): absolute measurements and estimates from bone dimensions. J Zool 167: 265–276, 1972. [Google Scholar]
- 12.Cavallo-Perin P, Bruno A, Boine L, Cassader M, Lenti G, Pagano G. Insulin resistance in Graves' disease: a quantitative in-vivo evaluation. Eur J Clin Invest 18: 607–613, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Ciavardelli D, Bellomo M, Crescimanno C, Vella V. Type 3 deiodinase: role in cancer growth, stemness, and netabolism. Front Endocrinol 5: 215, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen CA, Shea AA, Heffron CL, Schmelz EM, Roberts PC. Intra-abdominal fat depots represent distinct immunomodulatory microenvironments: a murine model. PLoS One 8: e66477, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crocker DA, Webb PM, Costa DP, and Le Boeuf BJ. Protein catabolism and renal function in lactating northern elephant seals. Physiol Zool 71: 485–491, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Darras VM, Van Herck SLJ. Iodothyronine deiodinase structure and function: from ascidians to humans. J Endocrinol 215: 189–206, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Feldhamer GA, Drickamer LC, Vessey SH, Merritt JF, Krajewski C. Mammalogy: Adaptation, Diversity, and Ecology, Baltimore, MD: Johns Hopkins University, 2015. [Google Scholar]
- 18.Haley MP, Deutsch CJ, Boeuf BJL. A method for estimating mass of large pinnipeds. Mar Mammal Sci 7: 157–164, 1991. [Google Scholar]
- 19.Herlihy JT, Stacy C, Bertrand HA. Long-term food restriction depresses serum thyroid hormone concentrations in the rat. Mech Age Dev 53: 9–16, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Hong EG, Kim BW, Young Jung D, Hun Kim J, Yu T, Seixas Da Silva W, Friedline RH, Bianco SD, Seslar SP, Wakimoto H, Berul CI, Russell KS, Won Lee K, Larsen PR, Bianco AC, Kim JK. Cardiac expression of human type 2 iodothyronine deiodinase increases glucose metabolism and protects against doxorubicin-induced cardiac dysfunction in male mice. Endocrinology 154: 3937–3946, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janan J, Rudas P, Bartha T, Bozó S, Gábor G. Effect of severe energy restriction and refeeding on thyroid hormones in bulls. Acta Veterinaria Hung 43: 173–177, 1995. [PubMed] [Google Scholar]
- 22.Jing S, Xiaoying D, Ying X, Rui L, Mingyu G, Yuting C, Yanhua Y, Yufan W, Haiyan S, Yongde P. Different levels of thyroid hormones between impaired fasting glucose and impaired glucose tolerance: free T3 affects the prevalence of impaired fasting glucose and impaired glucose tolerance in opposite ways. Clin Endocrinol 6: 890–898, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Kmiec Z, Kotlarz G, Smiechowska B, Mysliwski A. Thyroid hormones homeostasis in rats refed after short-term and prolonged fasting. J Endocrinol Invest 19: 304–311, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Lopresti JS, Gray D, Nicoloff JT. Influence of fasting and refeeding on 3,3′,5′-triiodothyronine metabolism in man. J Clin Endocrinol Metab 72: 130–136, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Magnus TH. Thyroid hormone resistance in hibernating ground squirrels, Spermophilus richardsoni. I. Increased binding of triiodo-l-thyronine and l-thyroxine by serum proteins. Gen Comp Endocrinol 69: 352–360, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Magnus TH, Henderson NE. Thyroid hormone resistance in hibernating ground squirrels, Spermophilus richardsoni: II. Reduction of hepatic nuclear receptors. Gen Comp Endocrinol 69: 361–371, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Marsili A, Aguayo-Mazzucato C, Chen T, Kumar A, Chung M, Lunsford EP, Harney JW, Van-Tran T, Gianetti E, Ramadan W, Chou C, Bonner-Weir S, Larsen PR, Silva JE, Zavacki AM. Mice with a targeted deletion of the Type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PLoS One 6: e20832, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez B, Soñanez-Organis JG, Vázquez-Medina JP, Viscarra JA, MacKenzie DS, Crocker DE, Ortiz RM. Prolonged food deprivation increases mRNA expression of deiodinase 1 and 2, and thyroid hormone receptor β-1 in a fasting-adapted mammal. J Exp Biol 216: 4647–4654, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitrou P, Raptis SA, Dimitriadis G. Insulin action in hyperthyroidism: a focus on muscle and adipose tissue. Endocr Rev 31: 663–679, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Montez P, Vázquez-Medina JP, Rodríguez R, Thorwald MA, Viscarra JA, Lam L, Peti-Peterdi J, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade recovers hepatic UCP2 expression and aconitase and SDH activities and ameliorates hepatic oxidative damage in insulin-resistant rats. Endocrinology 153: 5746–5759, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortiz CL, Costa D, Le Boeuf BJ. Water and energy flux in elephant seal pups fasting under natural conditions. Physiol Zool 51: 166–178, 1978. [Google Scholar]
- 32.Ortiz RM, Crocker DE, Houser DS, Webb PM. Angiotensin II and aldosterone increase with fasting in breeding adult male northern elephant seals (Mirounga angustirostris). Physiol Biochem Zool 79: 1106–1112, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz RM, Houser DS, Wade CE, Ortiz CL. Hormonal changes associated with the transition between nursing and natural fasting in northern elephant seals (Mirounga angustirostris). Gen Comp Endocrinol 130: 78–83, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz RM, MacKenzie DS, Worthy GA. Thyroid hormone concentrations in captive and free-ranging West Indian manatees (Trichechus manatus). J Exp Biol 203: 3631–3637, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz RM, Noren DP, Ortiz CL, Talamantes F. GH and ghrelin increase with fasting in a naturally adapted species, the northern elephant seal (Mirounga angustirostris). J Endocrinol 178: 533–539, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz RM, Wade CE, Ortiz CL. Effects of prolonged fasting on plasma cortisol and TH in postweaned northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol 280: R790–R795, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Casanova B, Pulido N, Suarez A, Rodriguez E, Rovira A. Stimulation of glucose transport by thyroid hormone in 3T3–L1 adipocytes: increased abundance of GLUT1 and GLUT4 glucose transporter proteins. J Endocrinol 164: 187–195, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Russell DW. SDS-polyacrylamide gel electrophoresis of proteins. CSH Prot pii: pdb.prot4540, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Stanická S, Vondra K, Pelikánová T, Vlček P, Hill M, Zamrazil V. Insulin sensitivity and counter-regulatory hormones in hypothyroidism and during thyroid hormone replacement therapy. Clin Chem Lab Med 43: 715- 720, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Teixeira SS, Tamrakar AK, Goulart-Silva F, Serrano-Nascimento C, Klip A, Nunes MT. Triiodothyronine acutely stimulates glucose transport into L6 muscle cells without increasing surface GLUT4, GLUT1, or GLUT3. Thyroid 22: 747–754, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torrance CJ, deVente JE, Jones JP, Dohm GL. Effects of thyroid hormone on GLUT4 glucose transporter gene expression and NIDDM in rats. Endocrinology 138: 1204–1214, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Tuzcu A, Bahceci M, Gokalp D, Tuzun Y, Gunes K. Subclinical hypothyroidism may be associated with elevated high-sensitive C-reactive protein (low-grade inflammation) and fasting hyperinsulinemia. Endocr J 52: 89–94, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Tveit B, Almlid T. T4 degradation rate and plasma levels of TSH and thyroid hormones in ten young bulls during feeding conditions and 48 h of starvation. Acta Endocrinol 93: 435–439, 1980. [DOI] [PubMed] [Google Scholar]
- 44.Tveit B, Larsen F. Suppression and stimulation of TSH and thyroid hormones in bulls during starvation and refeeding. Acta Endocrinol 103: 223–226, 1983. [DOI] [PubMed] [Google Scholar]
- 45.Van der Geyten S, Van Rompaey E, Sanders JP, Visser TJ, Kühn ER, Darras VM. Regulation of thyroid hormone metabolism during fasting and refeeding in chicken. Gen Comp Endocrinol 116: 272–280, 1999. [DOI] [PubMed] [Google Scholar]
- 46.van Haasteren GAC, Linkels E, van Toor H, Klootwijk W, Kaptein E, de Jong FH, Reymond MJ, Visser TJ, de Greef WJ. Effects of long-term food reduction on the hypothalamus-pituitary-thyroid axis in male and female rats. J Endocrinol 150: 169–178, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Vázquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J Exp Biol 213: 2524–2530, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viscarra JA, Champagne CD, Crocker DE, Ortiz RM. 5′AMP-activated protein kinase activity is increased in adipose tissue of northern elephant seal pups during prolonged fasting-induced insulin resistance. J Endocrinol 209: 317–325, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viscarra JA, Rodriguez R, Vazquez-Medina JP, Lee A, Tift MS, Tavoni SK, Crocker DE, Ortiz RM. Insulin and GLP-1 infusions demonstrate the onset of adipose-specific insulin resistance in a large fasting mammal: potential glucogenic role for GLP-1. Physiol Rep 1: e00023, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Viscarra JA, Vázquez-Medina JP, Crocker DE, Ortiz RM. Glut4 is upregulated despite decreased insulin signaling during prolonged fasting in northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol 300: R150–R154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viscarra JA, Vázquez-Medina JP, Rodriguez R, Champagne CD, Adams SH, Crocker DE, Ortiz RM. Decreased expression of adipose CD36 and FATP1 are associated with increased plasma non-esterified fatty acids during prolonged fasting in northern elephant seal pups (Mirounga angustirostris). J Exp Biol 215: 2455–2464, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinstein SP, O'Boyle E, Fisher M, Haber RS. Regulation of GLUT2 glucose transporter expression in liver by thyroid hormone: evidence for hormonal regulation of the hepatic glucose transport system. Endocrinology 135: 649–654, 1994. [DOI] [PubMed] [Google Scholar]
- 53.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Wulf A, Harneit A, Kröger M, Kebenko M, Wetzel MG, Weitzel JM. T3-mediated expression of PGC-1α via a far upstream located thyroid hormone response element. Mol Cell Endocrinol 287: 90–95, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Yang L, Shen X, Yan S, Yuan X, Lu J, Wei W. HbA1c in the diagnosis of diabetes and abnormal glucose tolerance in patients with Graves' hyperthyroidism. Diabetes Res Clin Pract 101: 28–34, 2013. [DOI] [PubMed] [Google Scholar]