Abstract
Recent findings suggest that the intestinal sodium-glucose transporter 1 (SGLT1) glucose transporter and sensor mediates, in part, the appetite-stimulation actions of intragastric (IG) glucose and nonmetabolizable α-methyl-d-glucopyranoside (MDG) infusions in mice. Here, we investigated the role of SGLT1 in sugar conditioning using SGLT1 knockout (KO) and C57BL/6J wild-type (WT) mice. An initial experiment revealed that both KO and WT mice maintained on a very low-carbohydrate diet display normal preferences for saccharin, which was used in the flavored conditioned stimulus (CS) solutions. In experiment 2, mice were trained to drink one flavored solution (CS+) paired with an IG MDG infusion and a different flavored solution (CS−) paired with IG water infusion. In contrast to WT mice, KO mice decreased rather than increased the intake of the CS+ during training and failed to prefer the CS+ over the CS− in a choice test. In experiment 3, the KO mice also decreased their intake of a CS+ paired with IG glucose and avoided the CS+ in a choice test, unlike WT mice, which preferred the CS+ to CS−. In experiment 4, KO mice, like WT mice preferred a glucose + saccharin solution to a saccharin solution. These findings support the involvement of SGLT1 in post-oral glucose and MDG conditioning. The results also indicate that sugar malabsorption in KO mice has inhibitory effects on sugar intake but does not block their natural preference for sweet taste.
Keywords: post-oral sugar conditioning, glucose, α-methyl-d-glucopyranoside, SGLT1 knockout
the appetite for sugar begins with the stimulation of sweet taste receptors in many species, including the fly, mouse, and human (12). It is also documented in these same species that sugar appetite is enhanced by the post-oral actions of the nutrient (22). This is most extensively demonstrated in laboratory rodents by the ability of gastric or intestinal sugar infusions to increase the intake of and preference for flavored nonnutritive solutions. We refer to this process as appetition, to distinguish it from the satiation process that suppresses feeding (21). The discovery of the subunits of the lingual sweet taste receptors (T1r2, T1r3) in the gut suggested the possibility that these gut “taste” receptors mediate post-oral sugar appetition (2). This conjecture is not supported, however, by the findings that mice missing the T1r3 receptor or other critical sweet taste-signaling elements (Trpm5, gustducin) demonstrate normal flavor-conditioning responses to intragastric (IG) sugar infusions (22, 24). Instead, a recent study suggests that the intestinal sodium-glucose transporters (SGLTs), which also function as glucose sensors (“transceptors”), may be critically involved in post-oral sugar appetition (35). In this study, C57BL/6J (B6) mice were trained to drink a flavored saccharin solution [the conditioned stimulus + (CS+)] that was paired with matched IG self-infusions of sugars or nonmetabolizable sugar analogs that varied in their affinity for SGLTs. A different flavored saccharin solution (the CS−) was paired with IG water self-infusions. Infusions of the SGLT1 ligands glucose, galactose, α-methyl-d-glucopyranoside (MDG), and 3-O-methyl-d-glucopyranoside stimulated CS+ intakes above CS− baseline levels. In addition, glucose, galactose, and MDG infusions conditioned preferences for the CS+ over the CS−; glucose and MDG, which are ligands for both SGLT1 and SGLT3, were the most effective. In contrast, infusions of fructose, which is not an SGLT ligand, failed to stimulate CS+ intake or preference in B6 mice.
The present study further investigated the involvement of SGLT1 in post-oral sugar appetition. This was accomplished using the SGLT1 knockout (KO) mouse, which is missing SGLT1 in intestinal and other cells (kidney, taste) (9). Our prior study indicated that SGLTs are critical for MDG-induced flavor conditioning, because MDG conditioning was blocked by coinfusion of the SGLT inhibitor phloridzin (35). Glucose conditioning, however, was not attenuated by this inhibitor alone but was blocked by a combination of phloridzin and phloretin, an antagonist of the GLUT2 glucose transporter. If SGLT1 is a critical transceptor to post-oral sugar appetition, then the ability of IG MDG and glucose infusions to stimulate CS+ intake or preference in SGLT1 KO mice should be attenuated or completely prevented. A preliminary experiment was first conducted to determine whether SGLT1 KO mice display a normal preference for saccharin-sweetened solutions, which are used in the IG flavor-conditioning procedure.
Experiment 1: Sweetener Preferences in SGLT1 KO and WT Mice
Recent reports indicate that SGLT1 is colocalized in taste cells that express the T1r3 sweet receptor (14, 32). The functional significance of this colocalization is not known, but it is conceivable that SGLT1 KO mice differ from B6 wild-type (WT) mice in their sweet taste preferences. Another concern is that SGLT1 KO mice, because they do not absorb glucose, must be maintained on a very low-carbohydrate diet, which may influence their sweet taste preferences. To control for potential diet effects, the WT mice were maintained on the same low-carbohydrate diet, which might also affect their sweet taste preferences. Therefore, the present experiment compared saccharin preferences in SGLT1 KO and WT mice using standard two-day, two-bottle tests, which we have previously conducted with B6 mice fed a standard chow diet (20). In addition, the preference for MDG, which has a palatable taste to chow-fed B6 mice, as measured in brief lick tests (1), was evaluated.
MATERIALS AND METHODS
Animals.
SGLT1 KO mice (3 male, 2 female) on a C57BL/6 background (9) and C57BL/6J wild-type mice (B6 WT, 3 male, 3 female; Jackson Laboratories, Bar Harbor, ME) were singly housed in plastic tub cages kept in a room maintained at 22°C with a 12:12-h light-dark cycle. All mice were fed a low-carbohydrate diet (% kcal: 65% protein, 33.2% fat, 1.9% carbohydrate as maltodextrin; 3.2 kcal/g; TD.08212, Harlan Laboratories, Madison, WI) to limit glucose/galactose malabsorption in the SGLT1 KO mice. The pellet diet was presented in a food hopper inside the cage that collected spilled pellets in a pan. Fluid was available through sipper spouts attached to 50-ml plastic tubes that were placed on top of the cage. The sipper spouts were inserted through holes positioned 3.7 cm apart in a stainless-steel plate, and the drinking tubes were fixed in place with clips. Food and fluid intakes were measured to the nearest 0.1 g by weighing the food hoppers and drinking bottles on an electronic balance interfaced to a laptop computer. Daily fluid spillage was estimated by recording the change in weight of two bottles that were placed on an empty cage, and intake measures were corrected by this amount.
Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Test solutions.
Saccharin solutions were prepared at 0.025, 0.05, 0.1, and 0.2% wt/wt concentrations using sodium saccharin (Sigma Chemical, St. Louis, MO) and deionized water. The MDG (Sigma Chemical) solutions were prepared at 4% and 8% wt/wt concentrations.
Procedure.
The mice were adapted to the tub cages with diet and water for 8 days. Then they were given 2-day, two-bottle tests with saccharin vs. water, with saccharin concentrations presented in an ascending order for 2 days each. Water only was available for 3 days followed by two-bottle tests with 4% MDG and 8% MDG vs. water for 2 days each. The left-right positions of the bottles were alternated daily to control for side preferences.
Data analysis.
Intakes during the 2 days at each saccharin concentration were averaged and evaluated with an ANOVA with a group (KO vs. WT), fluid (saccharin vs. water), and concentration factors (0.025–0.2%). Saccharin preference was also evaluated as percent intakes (saccharin/total intake × 100) and evaluated with an ANOVA (group and concentration factors). Absolute and percent MDG intakes were evaluated in a similar manner. In addition, MDG solute intakes were analyzed in a separate ANOVA (group and concentration factors).
RESULTS
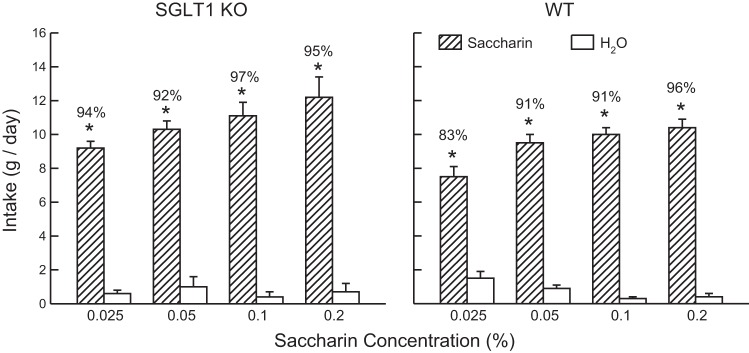
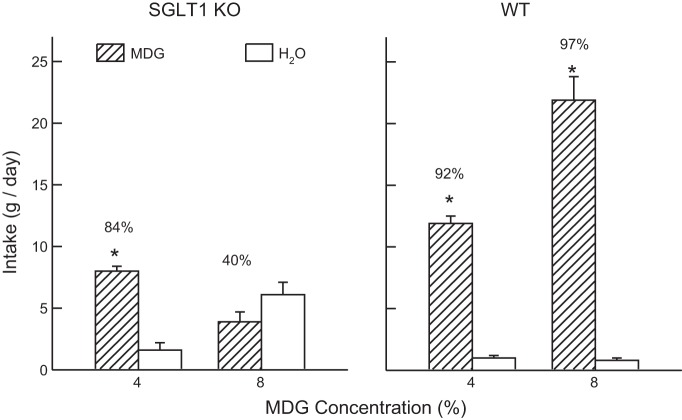
Overall, the SGLT1 KO and WT mice did not differ in their saccharin intakes (Fig. 1); they consumed more saccharin than water [F(1,9) = 571.5, P < 0.001] and increased their saccharin intake with concentration [F(3,27) = 21.5, P < 0.001]. They differed in their percent saccharin preference in that the preference for 0.025% saccharin was lower in the WT mice than KO mice [83% vs. 94%, group × concentration interaction, F(3,27) = 3.0, P < 0.050]. The groups differed more substantially, however, in their MDG intakes, particularly as a function of concentration [group × fluid × concentration interaction, F(1,9) = 112.8, P < 0.001] (Fig. 2). At the 4% concentration, both groups drank more (P < 0.01) MDG than water but the SGLT1 KO mice consumed less (P < 0.01) MDG than the WT mice; they did not significantly differ in their percent MDG intakes (84% vs. 92%). At the 8% concentration, however, the WT mice drank substantially more (P < 0.001) MDG than water, whereas the KO mice drank somewhat less MDG than water. In addition, the percent MDG intake was much higher in the WT than KO mice (97% vs. 40%, P < 0.001). Analysis of MDG solute intakes revealed that the KO mice consumed less MDG solute than WT mice at both concentrations [F(1,9) = 67.4, P < 0.001]. Furthermore, whereas the WT mice consumed substantially more solute at the 8% than 4% concentration (1.76 vs. 0.48 g/day), the KO mice consumed similar amounts at the two concentrations (0.31 vs. 0.32 g/day) [group × concentration interaction, F(1,9) = 70.4, P < 0.001].
Fig. 1.
Experiment 1. Mean (+SE) intakes of saccharin solution and water in two-bottle tests at sweetener concentrations of 0.025 to 0.2%. Left: Sodium-glucose transporters (SGLT1) knockout (KO) data. Right: B6 wild-type (WT) data. Numbers atop bars represent mean percent preference for saccharin. Significant intake differences (*P < 0.05) are indicated within each test.
Fig. 2.
Experiment 1. Mean (+SE) intakes of α-methyl-d-glucopyranoside (MDG) solution and water in two-bottle tests at MDG concentrations of 4% and 8%. Left: SGLT1 KO data. Right: B6 WT data. Numbers atop bars represent mean percent preference for MDG. Significant intake differences (*P < 0.05) within each test are indicated.
Overall, the SGLT1 KO and WT mice did not differ in their body weights during the saccharin and MDG tests (23.0 vs. 21.2 g), and they consumed similar amounts of diet (3.8 vs. 3.5 g/day).
DISCUSSION
The SGLT1 KO mice displayed a robust preference for saccharin that was, at the lowest concentration, stronger than that of the WT mice. Thus, saccharin intake and preference are not impaired by the lack of SGLT1 in lingual taste receptors or in the gut. The findings also indicate that maintaining normal B6 mice on a low-carbohydrate diet does not reduce saccharin preference. We previously reported (19) that B6 mice fed a standard carbohydrate-rich chow diet displayed preferences for 0.025, 0.05, 0.1, and 0.2% saccharin solutions of 73%, 83%, 94%, and 92%, respectively, which are similar to the preferences of the B6 WT mice in this experiment of 83%, 91%, 91%, and 96%, respectively.
The SGLT1 KO mice, however, differed from WT mice in their ingestive response to MDG. While they preferred 4% MDG to water, their preference was weaker and they consumed less than did the WT mice. The response of the KO mice to 8% MDG was more substantially impaired; they drank slightly less 8% MDG than water, while the WT mice consumed substantially more 8% MDG than water.
The reduced intakes and preferences of the KO mice for MDG are presumably related to their inability to absorb this glucose analog into intestinal cells. Consistent with this interpretation, the KO mice consumed similar amounts of MDG solute at the 4% and 8% concentrations, which may represent the amount of the sugar analog that they could consume each day without experiencing intestinal discomfort.
It is also possible that the lack of SGLT1 in lingual sweet taste cells, while not reducing saccharin preference, may attenuate the palatable taste of MDG. We tested this possibility by conducting 3-min, two-bottle MDG vs. water preference tests that minimize post-oral effects (1). Naive KO and WT mice (7 male and 7 female of each genotype) were food-restricted to motivate them to drink in the brief lick tests. The KO and WT groups did not differ in their robust preferences for 4% MDG (84% and 90%) and 8% MDG (95% and 94%) over water, and both groups increased (P < 0.01) their preference with concentration. Thus, genetic deletion of SGLT1 does not suppress the taste palatability of MDG, and reduced 24-h intakes and preferences can be attributed to post-oral factors related to the sugar's malabsorption.
Experiment 2: Flavor Preferences Conditioned by IG MDG Infusions
We previously reported in B6 mice that IG self-infusions of 8% MDG, like 8% glucose infusions, stimulated the intake of and preference for a CS+ flavor relative to that of a CS− flavor paired with IG water infusions (35). MDG is absorbed into intestinal cells via SGLT1 but is not metabolized, is not actively transported into the blood, and does not elevate blood glucose or insulin levels (16, 31, 35). These findings suggest that signals generated by MDG binding to SGLT1 and/or SGLT3 are responsible for post-oral appetition actions of MDG. Consistent with this view, coinfusions of the SGLT1/3 inhibitor phloridzin blocked MDG conditioning in B6 mice. The present experiment further investigated the role of SGLT1 in MDG appetition by comparing the flavor conditioning response of SGLT1 KO and WT mice to IG 8% MDG infusions. Note that the orally consumed CS+ dilutes the IG 8% MDG to a 4% solution, which is the MDG concentration preferred by the SGLT1 KO mice in the 24-h two-bottle tests in experiment 1.
MATERIALS AND METHODS
Animals.
New SGLT1 KO mice (7 male, 5 female) and WT mice (7 male, 8 female) were individually housed in plastic tub cages and fed the low-carbohydrate diet as in experiment 1, except that the pellets were available at the top of the cage. The mice were surgically fitted with chronic gastric catheters, as previously described (24).
Apparatus.
IG infusion tests were conducted in plastic test cages (24). The sipper spouts were interfaced via electronic lickometers to a computer that operated a syringe pump, which infused fluid into the gastric catheters as the animals licked; the oral-to-infusion intake ratio was maintained at ∼1:1. The pump rate was nominally 0.5 ml/min, but the overall infusion rate and volume were controlled by the animal's licking behavior. Daily oral fluid intakes were measured to the nearest 0.1 g, and IG infusions were recorded to the nearest 0.5 ml.
Test solutions.
The mice were initially trained in the infusion cages to drink unflavored 0.025%-0.05% saccharin solutions. They were then trained with flavored solutions (CS) containing 0.025% or 0.05% saccharin flavored with 0.02% ethyl acetate or propyl acetate (Sigma). During training, the CS− solution was paired with IG infusion of water while the CS+ solution was paired with IG infusion of 8% MDG. For about half the animals the CS− solution contained ethyl acetate and the CS+ solution contained propyl acetate; the flavors were reversed for the remaining animals.
Procedure.
Two weeks after surgery, the mice were water restricted (1 h/day) and trained in the test cages to drink unflavored 0.025% saccharin paired with matched IG infusions of water for two 1 h/day sessions. They were then given ad libitum water and switched to a food restriction schedule that maintained them at ∼90% body weight by giving them limited rations of their low-carbohydrate diet each day. During the first two sessions under food restriction, the KO mice licked more than the WT mice for the 0.025% saccharin (738.3 vs. 513.8 licks/h, P < 0.05). To stimulate the licking response of the WT mice and equate baseline intake, their saccharin concentration was doubled to 0.05%, while the KO mice continued with the 0.025% solution for two sessions. The KO and WT mice did not significantly differ in their licks in these sessions (844.5 vs. 748.1 licks/h). The KO and WT groups were, therefore, tested with flavored CS solutions containing 0.025% and 0.05% saccharin, respectively, for the remainder of the experiment.
The mice were given a series of one-bottle training sessions (1 h/day) with the CS− paired with IG water (sessions 1–3) and the CS+ paired with IG MDG (sessions 4–6). This was followed by four additional 1-h sessions with the CS− (sessions 7 and 9) and CS+ (sessions 8 and 10). In the final CS− and CS+ sessions, the mice were given a second sipper tube containing water not paired with IG infusions to familiarize them with the presence of two sipper tubes in the subsequent two-bottle test; water licks were minimal. A two-bottle choice test was conducted in sessions 11 to 14 (1 h/day) with the CS+ vs. CS− solutions without IG infusions. The left-right positions of the CS+ and CS− solutions were counterbalanced throughout training and testing. This training series was based on our prior studies (35, 36).
Data analysis.
Licks during the last two CS− training sessions (referred to as CS− test 0) were averaged and compared with licks during the subsequent three CS+ sessions (referred to as CS+ tests 1 to 3). These data were evaluated with an ANOVA with a group (KO vs. WT) and test (tests 0–4) factors. In addition, CS− and CS+ licks were averaged during the last two sessions with each CS and evaluated with a separate ANOVA (group × CS). Mean CS+ and CS− licks during the two-bottle choice test were also evaluated with an ANOVA. Preliminary analyses revealed no main or interactive effects of sex and, therefore, the data for male and female mice were combined.
RESULTS
In test 0, the KO and WT licked similar amounts for the CS− solution, but the WT mice licked more (P < 0.05) than the KO mice for the CS+ in tests 1–3 [Fig. 3; group × test interaction, F(3,75) = 29.0, P < 0.001]. Within-group analyses indicated that WT mice increased their licks from tests 0 to 3, and they licked more (P < 0.05) in test 3 than in tests 0–2 [F(3,42) = 13.5, P < 0.01]. In contrast, the KO mice decreased their licks from test 0 to 3, and they licked less (P < 0.05) in tests 2 and 3 than in tests 0 and 1 [F(3,33) = 18.2, P < 0.01]. Parallel changes were observed in the total amount of solutions consumed (oral + IG). The WT mice increased their intakes from 2.0 to 2.6 g/h from tests 0 to 3 [F(3,42) = 8.6, P < 0.001], while the KO mice decreased their intakes from 1.9 to 1.0 g/h [F(3,33) = 21.1, P < 0.001].
Fig. 3.
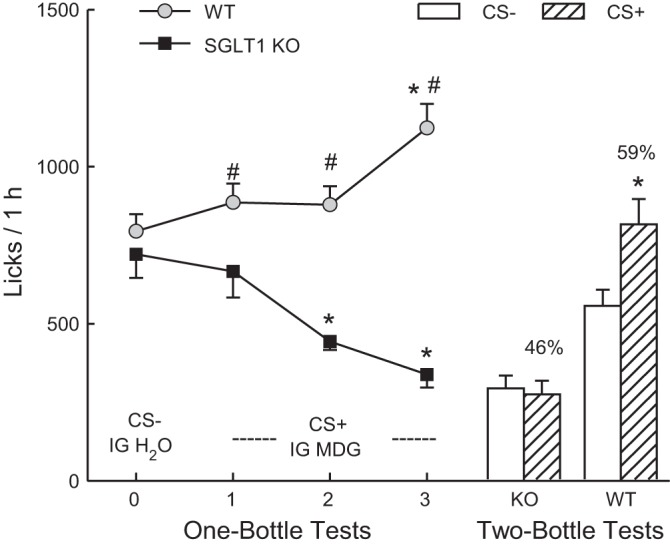
Experiment 2. Mean licks (+SE) during one-bottle tests 0–3 (left curves) and two-bottle tests (right bars) conducted with SGLT1 KO and WT mice. In test 0, the mice drank (1 h/day) a conditioned stimulus (CS)-flavored solution paired with intragastric (IG) self-infusions of water, and in tests 1–3, they drank a CS+-flavored solution paired with IG self-infusions of 8% MDG. In the two-bottle preference test, the mice drank the CS+ and CS− solutions not paired with IG infusions. Significant within-group differences (*P < 0.05) between CS+ and CS− licks are indicated. Significant between-group differences (#P < 0.05) in CS+ licks are indicated.
In CS tests 5–8, the WT mice licked more overall than the KO mice, and licked more for the CS+ than CS− (1,007.1 vs. 861.0 licks/h, P < 0.01), whereas the KO mice licked somewhat less for the CS+ than CS− (366.8 vs. 419.3 licks/h) [group × CS interaction, F(1,25) = 15.2, P < 0.01].
In the two-bottle choice test (Fig. 3), the WT mice licked significantly more (P < 0.01) for the CS+ than CS−, whereas the KO mice did not differ in their CS+ and CS− licks [group × CS interaction, F(1,25) = 11.2, P < 0.01]. In addition, the WT mice licked more (P < 0.01) for the CS+ and the CS− than the KO mice. The percent CS+ intake of the WT mice also exceeded that of the KO mice (59% vs. 47%, P < 0.01).
DISCUSSION
Consistent with prior findings with B6 mice (35), the IG infusions of 8% MDG stimulated CS+ intake and conditioned a CS+ preference in the WT mice. The new finding here is that SGLT1 KO mice failed to develop a preference for the CS+ flavor, and the IG MDG infusions suppressed rather than increased their CS+ intakes during training. The KO mice did not significantly reduce their CS+ training intakes until tests 2 and 3, but even in test 1, they licked less for the CS+ than did the WT mice. The KO mice may have reduced their CS+ training intakes, relative to their CS− baseline and relative to the WT mice, because of the inhibitory actions of the unabsorbed MDG in the lower intestinal tract. In CS+ tests 1 and 3, the KO mice self-infused 0.065 g and 0.031 g, respectively, of MDG solute, compared with their solute intake of 0.32 g/day or, on average, 0.013 g/h in the first experiment. The finding that the KO mice consumed similar amounts of CS+ and CS− in the two-bottle choice test indicates that they did not develop an aversion to the CS+ flavor, which was presumably due, in part, to their self-limiting the MDG infusions.
Experiment 3: Flavor Preferences Conditioned by IG Glucose
Our previous finding that the SGLT inhibitor phloridzin did not block glucose conditioning in B6 mice indicated that SGLTs alone are not essential for glucose appetition or alternatively that phloridzin did not completely inhibit SGLT activity (35). In this experiment, therefore, we determined whether IG glucose infusions would condition a CS+ preference in SGLT1 KO mice.
MATERIALS AND METHODS
At the end of experiment 2, the mice were given two 1-h sessions with unflavored saccharin paired with IG water infusions. One WT mouse with a defective catheter was excluded from this experiment. In the first session, in which the KO and WT mice were offered 0.025% and 0.05% saccharin solutions, respectively, as in the first experiment, the KO mice consumed less saccharin than did the WT mice. In the second session, both groups were given 0.05% saccharin solutions, which equated their intakes; this concentration was, therefore, used for the remainder of the experiment.
The mice were trained and tested with new flavored CS− and CS+ solutions using the training protocol of experiment 2. The CS flavors were 0.05% grape and cherry Kool Aid (unsweetened mix, Kraft Foods, Rye Brook, NY) with half of the animals having the grape as the CS+ paired with IG infusions of 8% glucose (food grade, Honeyville Food Products, Rancho Cucamonga, CA) and cherry as the CS− paired with IG water infusions; the flavor-infusion pairs were reversed for the remaining animals.
Statistical analyses were performed as in experiment 2.
RESULTS
In test 0 CS− licks were similar for the KO and WT mice but the WT mice licked more (P < 0.05) than the KO mice for the CS+ in tests 1–3 [Fig. 4; group × test interaction, F(3,72) = 14.0, P < 0.001]. Within-group analyses indicated that WT mice increased their licks from tests 0 to 3, and they licked more (P < 0.05) in tests 2 and 3 than in test 0 [F(3,39) = 11.4, P < 0.01]. In contrast, the KO mice decreased their licks from test 0 to 3, and they licked less (P < 0.05) in test 3 than in test 0 [F(3,33) = 4.4, P < 0.05]. Parallel changes were observed in the total (oral + IG) intake. The WT mice increased their intakes from 2.1 to 2.8 g/h from tests 0 to 3 [F(3,39) = 20.1, P < 0.001], while the KO mice decreased their intakes from 2.2 to 1.5 g/h [F(3,33) = 6.6, P < 0.01].
Fig. 4.
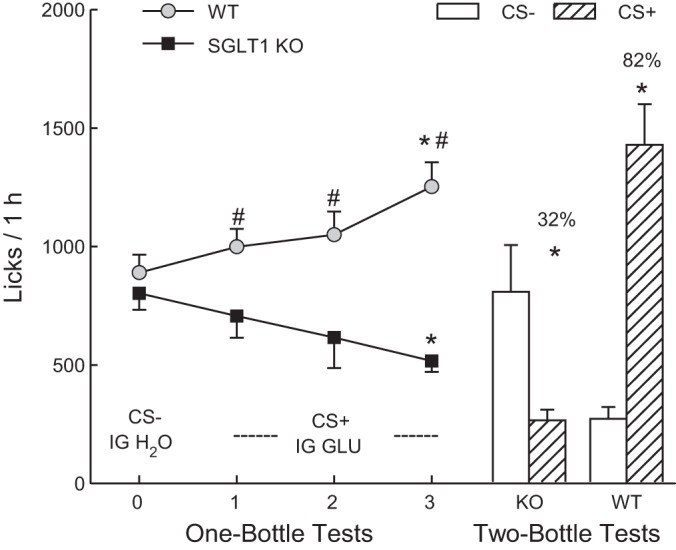
Experiment 3. Mean licks (+SE) during one-bottle tests 0–3 (left curves) and two-bottle tests (right bars) conducted with SGLT1 KO and WT mice. In test 0, the mice drank (1 h/day) a CS−-flavored solution paired with IG self-infusions of water, and in tests 1–3, they drank a CS+-flavored solution paired with IG self-infusions of 8% glucose. In the two-bottle preference test, the mice drank the CS+ and CS− solutions not paired with IG infusions. Significant within-group differences (*P < 0.05) between CS+ and CS- licks are indicated. Significant between-group differences (#P < 0.05) in CS+ licks are indicated.
In CS tests 5–8, the WT mice licked more overall than the KO mice, and licked more for the CS+ than CS− (1,158.6 vs. 1,045.4 licks/h, P < 0.05), whereas the KO mice licked less (P < 0.01) for the CS+ than CS− (374.8 vs. 583.5 licks/h, P < 0.01) [group × CS interaction, F(1,24) = 20.3, P < 0.001].
In the two-bottle choice test (Fig. 4), the WT mice licked more (P < 0.01) for the CS+ than CS−, whereas the KO mice licked less (P < 0.05) for the CS+ than for the CS− [group × CS interaction, F(1,24) = 33.4, P < 0.01]. In addition, the WT mice licked more (P < 0.01) for the CS+ and less (P < 0.01) for the CS− compared with the KO mice. The percent CS+ intake of the WT mice also exceeded that of the KO mice (82% vs. 32%, P < 0.01).
DISCUSSION
As in our prior studies with B6 mice, the IG infusions of 8% glucose stimulated CS+ intakes and conditioned a strong preference for the CS+ in the WT mice (34–36). In contrast, the IG glucose suppressed CS+ intakes in the SGLT1 KO mice and conditioned a significant avoidance of the CS+ relative to the CS−. Their reduction in CS+/glucose training intakes relative to the CS− (test 3 vs. test 0) was less pronounced than that observed in experiment 2 with the CS+/MDG (30% vs. 49%). Consequently, the KO mice self-infused more glucose solute in this experiment than MDG in the prior experiment (0.058 vs. 0.046 g/h, mean of CS+ tests 1 to 5). This may be related, in part, to testing with CS flavors that contained 0.05% rather than 0.025% saccharin.
Experiment 4. Glucose Preferences in SGLT1 KO and WT Mice
In experiments 2 and 3, the SGLT1 KO mice failed to acquire preferences for the CS+ flavors paired with IG infusions of 8% MDG or glucose. This may have occurred because in the absence of functional SGLTs, MDG and glucose do not generate positive signals to reinforce the CS+ preference. An alternative explanation is that sugars continue to generate reinforcing signals, but the inhibitory and/or aversive effects of the unabsorbed MDG and glucose in the gut counteract these positive signals, resulting in no CS+ preference or, in the case of IG glucose, in a CS+ avoidance. Because the identity of the post-oral positive signals generated by sugars remains unknown, it is not possible to distinguish between these alternative explanations by measuring the sugar-generated signals in KO and WT mice. Instead, the present experiment used an indirect approach and determined whether the post-oral inhibitory actions of glucose are sufficient to block the natural preference that mice have for the sugar's sweet taste. If so, this would support the idea that the inhibitory actions also blocked the post-oral glucose-conditioned preference. In experiment 1, the KO mice significantly preferred 4% MDG to water, although they consumed less MDG than did WT mice. This may not be the case for 4% glucose, however, or with KO mice that are food restricted and given only 1 h/day access to the sugar as in experiment 3.
MATERIALS AND METHODS
Naive SGLT1 KO mice (5 males, 5 females) and WT mice (5 males, 5 females) were individually housed as in experiment 2 and fed the low-carbohydrate diet. The mice were initially water deprived and trained 1 h/day for 2 days in the test cages to drink unflavored 0.2% saccharin and water from two sipper tubes. The mice were given 1-h access to water in their home cages following the first session and unlimited water following the second session but restricted food rations designed to maintain them at 90% of their ad libitum weight. The mice were then given four daily sessions with saccharin vs. water. This was followed by five daily sessions with 4% glucose + 0.2% saccharin (G+S) vs. water; the rationale for adding saccharin to the glucose solution is explained in the discussion. These sessions correspond to the 5 CS+ training sessions of experiment 3, in which the animals self-infused 8% glucose that was diluted to 4% glucose by the ingested CS+-flavored saccharin solution. The mice were then given a two-bottle test with the G+S vs. 0.2% saccharin for two 1-h/day sessions. This test determined whether the KO mice acquired an aversion or avoidance to the G+S relative to the plain saccharin solution. The left-right position of the G+S and water or saccharin bottles was alternated daily.
Licks and intakes during the last two saccharin training sessions were averaged (test 0) and compared with the licks/intakes during the subsequent G+S sessions (tests 1–5) in separate ANOVAs (group × tests). Licks during the two G+S vs. saccharin sessions (test 6) were also averaged. Sweetener preferences were calculated as sweetener licks divided by total licks × 100. The absolute and percent lick data were analyzed in separate ANOVAs.
RESULTS
In test 0, the KO mice licked somewhat more for 0.2% saccharin than did the WT mice (Fig. 5). Both groups then increased their licks when offered G+S in test 1, which was followed by a slight decline in G+S licks in the WT mice and a more substantial decline in the KO mice in tests 2–5. The ANOVA confirmed that both groups increased (P < 0.01) their licks from test 0 to 1 and did not differ in these tests, but that the KO mice licked less (P < 0.01) than the WT mice in tests 2–5 [group × test interaction, F(5,90) = 11.6, P < 0.001]. Within-group analyses indicated that the WT mice licked more (P < 0.01) for G+S in tests 1–5 than for saccharin in test 0. In contrast, the KO mice licked more (P < 0.01) for G+S than saccharin in test 1 but not more G+S in tests 2 to 5. Analysis of sweetener intakes revealed similar patterns: the WT mice increased their intake from 1.2 to 2.8 g/h in tests 0 to 1 and then consumed, on average 2.4 g/h G+S in tests 2–5 [F(5,45) = 33.1, P < 0.001]. In contrast, the KO mice increased their sweetener intakes from 1.5 to 2.1 g/h from test 0 to 1, and then consumed 1.2 g/h of G+S in tests 2–5 [F(5,45) = 25.1, P < 0.001]. The mice in both groups licked very little water in tests 0–5 (<80 licks/h), and overall, their percent G+S licks, relative to water, exceeded 94% in these tests.
Fig. 5.
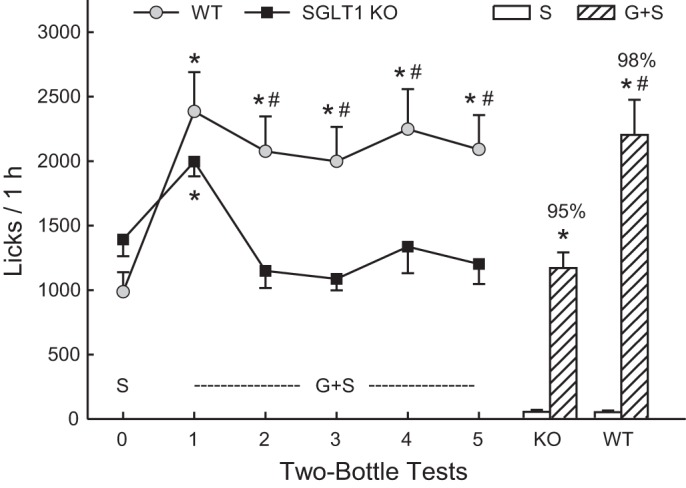
Experiment 4. Mean licks (+SE) during two-bottle tests with 0.2% saccharin (S; test 0) and 4% glucose + 0.2% saccharin (G+S; tests 1–5) vs. water conducted with SGLT1 KO and WT mice. Water licks in these tests were minimal (<40/h) and are not presented. The bars on the right present G+S and S licks during the two-bottle test 6 with these two sweeteners. Significant within-group differences (*P < 0.05) between G+S and S licks are indicated. Significant between-group differences (#P < 0.05) in G+S licks are indicated by a number sign.
Examination of G+S cumulative lick curves from test 1 revealed that KO and WT mice licked at nearly identical levels during the first 35 min of the session, and then the KO mice decreased their rate of licking below that of the WT mice (data not presented). In tests 2–5, the KO mice licked at slower rates for G+S than did the WT mice from the beginning of each session; the G+S cumulative lick curves in these tests were similar to that for saccharin in test 0.
In test 6, both groups licked substantially more for G+S than saccharin [F(1,18) = 121.4, P < 0.001], and the WT mice licked more for G+S than did the KO mice; the groups did not differ in their saccharin licks [group × solution interaction, F(1,18) = 12.2, P < 0.01]. The G+S preferences of the WT and KO mice were 98% and 95%, respectively.
DISCUSSION
Like the WT mice, the KO mice were very attracted to the G+S solution and significantly increased licking and intakes when switched from saccharin to G+S. The KO mice licked less for G+S than WT mice in the last half of test 1, presumably due to the inhibitory actions of the unabsorbed glucose. In subsequent sessions (tests 2–5), the KO mice licked at a lower rate than WT mice from the beginning of each session, which may represent a learned response to slow the accumulation of unabsorbed glucose in their gut. Nevertheless, the G+S licks and intakes of the KO mice remained stable during the last four sessions at a level comparable to that for saccharin in test 0. Furthermore, like WT mice, the KO mice strongly preferred G+S to saccharin in the final two-bottle test.
In a preliminary study we obtained similar findings with KO mice switched from 0.025% saccharin solution in test 0 to a 4% glucose solution (without saccharin) in tests 1–5. That is, the mice increased their licks when first switched from saccharin to glucose and then reduced their glucose licks in tests 2–5 to the level of their saccharin licks in test 0. Their glucose solute intake over the five sessions was less than that self-infused by the KO mice in experiment 3 (0.030 vs. 0.058 g/h, P < 0.01). To induce higher glucose intakes, the KO mice in the present experiment were tested with the G+S solution, which is highly attractive to rodents (8). This manipulation was effective, and the KO mice self-administered 0.057 g/h glucose solute in tests 1–5, which closely matched the amount self-infused by the KO mice in experiment 3. Although the KO mice consumed less G+S than the WT mice, their stable intakes in tests 2–5 and their strong preference for G+S over saccharin indicates that the post-oral inhibitory actions of the glucose did not condition an aversion to the G+S solution.
GENERAL DISCUSSION
Recent findings implicated the glucose transporters/sensors SGLT1 and SGLT3 in the appetite-stimulating actions of glucose-based carbohydrates in mice (35). We observed that IG infusions of glucose and nonmetabolizable MDG, which are ligands for SGLT1 and SGLT3, stimulated the intake of and preference for a CS+-flavored saccharin solution in B6 mice, whereas IG fructose, which does not bind to the SGLTs, was ineffective. Furthermore, coinfusing the SGLT antagonist phloridzin blocked MDG-induced appetition, although both phloridzin and phloretin antagonists were required to block glucose appetition. The present results obtained with SGLT1 KO mice indicate that SGLT1 is essential for post-oral glucose and MDG appetition.
Consistent with our prior findings (35), IG infusions of 8% MDG stimulated CS+ intake and conditioned a preference for the CS+ over the CS− in B6 WT mice. In contrast, MDG infusions decreased CS+ intakes in SGLT1 KO mice and did not condition a CS+ preference. The indifference displayed by the KO mice to the CS+ in the choice test is consistent with the finding that MDG infusions containing the SGLT1 inhibitor phloridzin blocked CS+ preference conditioning. The phloridzin also blocked MDG-induced stimulation of CS+ intake during training, whereas the KO mice reduced their CS+ training intakes in experiment 2. This indicates that genetic deletion of SGLT1 has a more profound effect on MDG absorption than does pharmacological blockade with phloridzin.
The MDG-conditioned preference displayed by the WT mice in experiment 2, while significant, was weaker than that observed in our prior study with chow-fed B6 mice (59% vs. 70%) (35). Also, the MDG infusions did not stimulate CS+ intakes until CS+ test 3, whereas they stimulated CS+ intakes in the first test in chow-fed B6 mice. This may have resulted because, compared with B6 mice fed carbohydrate-rich chow, the WT mice fed the very low-carbohydrate diet had low expression levels of intestinal SGLT1 (13), which would have reduced the appetition signals generated by the MDG infusions. Adding a sweetener, such as sucralose, to the low-carbohydrate diet, which is reported to stimulate SGLT1 expression (13), would be expected to enhance MDG conditioning in WT mice fed a low-carbohydrate diet.
The SGLT1 KO mice, unlike B6 WT, also failed to acquire a preference for the CS+ flavor paired with IG glucose infusions. Instead, they avoided the CS+ and drank more CS− in the two-bottle choice test. They also decreased, rather than increased their CS+ intake during the one-bottle training sessions. The finding that KO mice avoided the glucose-paired CS+ but were indifferent to the MDG-paired CS+ suggests that IG glucose infusions were more discomforting than the MDG infusions. This may have resulted, in part, because the mice self-infused more glucose solute than MDG solute in the two experiments. In addition, unabsorbed glucose in the lower intestinal tract is subject to bacterial fermentation to short-chain fatty acids, which are potent stimuli for intestinal GLP-1 and PYY secretion. Powell et al. (18) reported that a gastric glucose load produced much higher and prolonged glucose levels in the small intestine, cecum, and colon in SGLT1 KO mice than WT mice and increased plasma levels of GLP-1 and PYY for 1 to 6 h after a glucose infusion. High levels of GLP-1 and PYY are reported to condition taste aversions in rodents (4, 10, 29).
The finding that glucose preference conditioning is blocked by genetic deletion of SGLT1 contrasts with the failure of the SGLT inhibitor phloridzin to attenuate glucose conditioning (35). Glucose conditioning was blocked, however, by a mixture of phloridzin and the GLUT2 inhibitor phloretin (35). This finding suggested that, in the presence of SGLT1 inhibition by phloridzin, intestinal luminal glucose absorption and/or signaling by GLUT2 is sufficient to generate appetition signals that mediate flavor preference conditioning. The expression of luminal GLUT2 is blocked in SGLT1 KO mice, which would explain why glucose conditioning is blocked in SGLT1 KO mice but not in B6 mice treated with phloridzin alone (9).
We previously reported that glucose and MDG, which are ligands for SGLT1 and SGLT3, conditioned stronger preferences than did galactose and 3-O-methyl-d-glucopyranoside, which are ligands for SGLT1 only (35). This suggested that stimulation of SGLT3 contributes to appetition effects of IG glucose and MDG infusions. The present findings, however, indicate that in the absence of SGLT1, glucose and MDG stimulation of SGLT3 is not sufficient to condition flavor preferences in mice. It may be that SGLT3 expression is reduced in SGLT1 KO mice. Alternatively, appetition effects of SGLT3 stimulation by glucose or MDG may be counteracted by feeding-inhibitory signals generated by the accumulation of the unabsorbed sugars in the intestinal tract of KO mice. A limitation of the SGLT1 KO mouse model, therefore, is that in addition to eliminating the generation of appetite stimulatory signals by ingested glucose, it also promotes the generation of appetite inhibitory signals by blocking sugar absorption.
It is possible, however, the genetic deletion of SGLT1 did not block glucose-induced appetition signals but that the inhibitory actions of the unabsorbed sugar counteracted these positive signals and blocked the acquisition of the CS+ preference or induced an avoidance with IG MDG and glucose infusions, respectively. This possibility was evaluated in experiment 4 by determining the willingness of KO mice to consume a G+S solution that has an attractive taste. The mice initially overconsumed the solution to almost the same degree as the WT mice but then limited their intakes to that of plain saccharin presumably in response to inhibitory post-oral feedback. Most importantly, they significantly preferred G+S to saccharin in test 6, indicating that the post-oral effects of glucose did not induce a glucose aversion. In contrast, thirsty animals trained to drink 5% glucose paired with intraperitoneal lithium chloride injections reduce their intake to 0 ml over several training sessions and prefer water to glucose (28). The G+S findings (and preliminary 4% glucose results) indicate that the inhibitory actions of unabsorbed glucose did not override the natural preference of KO mice for the sweet taste of glucose. This suggests that the failure of KO mice to display preferences for the CS+ paired with IG MDG or glucose was not due to malabsorption effects overriding post-oral sugar appetition but rather resulted because SGLT1 deletion prevented the generation of sugar appetition signals. We hypothesize that the KO mice in experiment 3 avoided the glucose-paired CS+ in the choice test because the CS+ and CS− were equally sweet (both contained 0.05% saccharin) and the CS+ was associated only with the post-oral signals generated by the unabsorbed sugar and not with its positive oral or postoral signals.
Perspectives and Significance
The present findings along with recent data indicate that SGLT1 functions as an intestinal sugar sensor that promotes the appetite for glucose-rich foods. As discussed elsewhere, the identity of the appetition signal generated by SGLT1 remains to be identified (22, 26, 35). Hormonal rather than neural signals are implicated by the failure of visceral nerve deafferentation to block glucose appetition (13, 23, 25, 34, 34). However, most gut hormones released by glucose suppress rather than stimulate feeding, and ghrelin, the only known orexigenic hormone, is not implicated in glucose appetition (22, 26). Conceivably, there may be an as yet unidentified orexigenic hormone released by intestinal glucose that stimulates appetite. Signals generated by glucose sensors in the portal vein region in rodents and by brain sensors in flies may also contribute to learned food preferences (7, 17, 30). The present focus on glucose reflects the ineffectiveness of fructose, the other common dietary monosaccharide, to promote post-oral appetition in B6 mice. However, fructose has post-oral conditioning effects in other inbred mouse strains (FVB, SWR), as well as in flies (6, 11, 15, 27). Human food preferences are influenced by the post-oral actions of glucose, but evidence for fructose appetition is lacking (3, 5, 33).
GRANTS
This research was supported by grant DK-031135 from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S. and K.A. conception and design of research; A.S. and K.A. analyzed data; A.S., H.K., and K.A. interpreted results of experiments; A.S. prepared figures; A.S. and K.A. drafted manuscript; A.S., H.K., and K.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Martin Zartarian and Kwame McCartney for their expert technical assistance. We also thank Dr. Volker Vallon, University of California San Diego, who provided the breeding stock of SGLT1 KO mice.
REFERENCES
- 1.Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning in rats by glucose but not a non-metabolizable glucose analog. Physiol Behav 133: 92–98, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20: 64–72, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch LL, McPhee L, Steinberg L, Sullivan S. Conditioned flavor preferences in young children. Physiol Behav 47: 501–505, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3–36) on conditioned taste aversion in rats. Peptides 27: 3193–3201, 2006. [DOI] [PubMed] [Google Scholar]
- 5.de Araujo IE, Lin T, Veldhuizen MG, Small DM. Metabolic regulation of brain response to food cues. Curr Biol 23: 878–883, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dus M, Ai M, Suh GSB. Taste-independent nutrient selection is mediated by a brain-specific Na+/solute co-transporter in Drosophila. Nat Neurosci 16: 526–528, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dus M, Lai JS, Gunapala KM, Min S, Tayler TD, Hergarden AC, Geraud E, Joseph CM, Suh GS. Nutrient sensor in the brain directs the action of the brain-gut axis in Drosophila. Neuron 87: 139–151, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glendinning JI, Beltran F, Benton L, Cheng S, Gieseke J, Gillman J, Spain HN. Taste does not determine daily intake of dilute sugar solutions in mice. Am J Physiol Regul Integr Comp Physiol 299: R1333–R1341, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61: 187–196, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10,470–10,476, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraft TT, Huang D, Lolier M, Warshaw D, Lamagna S, Natanova E, Sclafani A, Bodnar RJ. BALB/c and SWR inbred mice differ in post-oral appetition as revealed by sugar versus non-nutritive sweetener tests. Physiol Behav 153: 64–69, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liman E, Zhang Y, Montell C. Peripheral coding of taste. Neuron 81: 984–1000, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas F, Sclafani A. Capsaicin attenuates feeding suppression but not reinforcement by intestinal nutrients. Am J Physiol Regul Integr Comp Physiol 270: R1059–R1064, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Merigo F, Benati D, Cristofoletti M, Amarú F, Osculati F, Sbarbati A. Glucose transporter/T1R3-expressing cells in rat tracheal epithelium. J Anat 221: 138–150, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto T, Chen Y, Slone J, Amrein H. Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLos One 8: e56304, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 297: E1358–E1365, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira-Maia AJ, Roberts CD, Walker QD, Luo B, Kuhn C, Simon SA, Nicolelis MA. Intravascular food reward. PLos One 6: e24992, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell DR, Smith M, Greer J, Harris A, Zhao S, DaCosta C, Mseeh F, Shadoan MK, Sands A, Zambrowicz B, Ding ZM. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther 345: 250–259, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Sclafani A. Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav 87: 745–756, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav 87: 734–744, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite 71: 454–458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol 302: R1119–R1133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav 78: 285–294, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol 299: R1643–R1650, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sclafani A, Lucas F. Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol Behav 60: 447–453, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Sclafani A, Touzani K, Ackroff K. Ghrelin signaling is not essential for sugar or fat conditioned flavor preferences in mice. Physiol Behav 149: 14–22, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sclafani A, Zukerman S, Ackroff K. Fructose and glucose conditioned preferences in FVB mice: Strain differences in post-oral sugar appetition. Am J Physiol Regul Integr Comp Physiol 307: R1448–R1457, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith ME, Norgren R, Grigson PS. A mixed design reveals that glucose moieties facilitate extinction of a conditioned taste aversion in rats. Learn Behav 32: 454–462, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Thiele TE, Vandijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol Regul Integr Comp Physiol 272: R726–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Tordoff MG, Friedman MI. Hepatic-portal glucose infusions decrease food intake and increase food preference. Am J Physiol Regul Integr Comp Physiol 251: R192–R196, 1986. [DOI] [PubMed] [Google Scholar]
- 31.Wright EM, Sala-Rabanal M, Loo DDF, Hirayama BA. Sugar absorption. In: Physiology of the Gastrointestinal Tract, edited by Johnson L. Boston, MA: Academic, 2012, p. 1583–1593. [Google Scholar]
- 32.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA 108: 5431–5436, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol Behav 93: 798–806, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol 301: R1635–R1647, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol 305: R840–R853, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zukerman S, Ackroff K, Sclafani A. Post-oral glucose stimulation of intake and conditioned flavor preference in C57BL/6J mice: A concentration-response study. Physiol Behav 109: 33–41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]