Abstract
The present study examined the effect of intensive training in combination with marked reduction in training volume on phospholemman (FXYD1) expression and phosphorylation at rest and during exercise. Eight well-trained cyclists replaced their regular training with speed-endurance training (10–12 × ∼30-s sprints) two or three times per week and aerobic high-intensity training (4–5 × 3–4 min at 90–95% of peak aerobic power output) 1–2 times per week for 7 wk and reduced the training volume by 70%. Muscle biopsies were obtained before and during a repeated high-intensity exercise protocol, and protein expression and phosphorylation were determined by Western blot analysis. Expression of FXYD1 (30%), actin (40%), mammalian target of rapamycin (mTOR) (12%), phospholamban (PLN) (16%), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) γ/δ (25%) was higher (P < 0.05) than before the training intervention. In addition, after the intervention, nonspecific FXYD1 phosphorylation was higher (P < 0.05) at rest and during exercise, mainly achieved by an increased FXYD1 Ser-68 phosphorylation, compared with before the intervention. CaMKII, Thr-287, and eukaryotic elongation factor 2 Thr-56 phosphorylation at rest and during exercise, overall PKCα/β, Thr-638/641, and mTOR Ser-2448 phosphorylation during repeated intense exercise as well as resting PLN Thr-17 phosphorylation were also higher (P < 0.05) compared with before the intervention period. Thus, a period of high-intensity training with reduced training volume increases expression and phosphorylation levels of FXYD1, which may affect Na+/K+ pump activity and muscle K+ homeostasis during intense exercise. Furthermore, higher expression of CaMKII and PLN, as well as increased phosphorylation of CaMKII Thr-287 may have improved intracellular Ca2+ handling.
Keywords: phospholemman, intense exercise training, protein signaling
changes in muscle ion homeostasis during intense contraction reduce membrane excitability, which may lead to development of fatigue (30). Exercise training improves performance during intense exercise and reduces the accumulation of potassium in both blood (25) and muscle interstitium (32), which has been associated with elevated levels of Na+/K+ (NaK) pump subunit expression (25, 31–33). However, training studies have shown improved work capacity without adaptations in the NaK pump content and isoform abundance but with a higher maximal NaK pump activity (3). Thus, factors other than NaK pump subunits expression may affect the capacity of the NaK pump.
Phospholemman (FYXD1) is a regulatory protein associated with the NaK pump, and changes in its expression and phosphorylation affect pump activity (7, 13, 27, 35). It is well known that muscle NaK pump activity increases markedly with exercise (9), which may be regulated partly by an increased FXYD1 phosphorylation observed during both moderate-intensity (5) and high-intensity acute exercise in humans (52). The effect of endurance training on muscle FXYD1 expression and phosphorylation during and after exercise has been examined (5). Ten days of moderate-intensity cycle training including 6 × 5 min at 90–100% of an intensity corresponding to V̇o2 max did not affect FXYD1 expression or FXYD1 phosphorylation during long-term, low-intensity exercise in untrained healthy individuals (5). In contrast, a 2-wk period of high-intensity exercise training elevated resting levels of FXYD1 phosphorylation (54), indicating that intensity during training may be important for the adaptations of FXYD1. However, the effect of intense training on muscle FXYD1 expression and exercise-induced phosphorylation has not been examined. We hypothesize that intensified training does lead to higher expression of FXYD1 and increased FXYD1 phosphorylation during intense exercise, which can explain the finding of a lower femoral venous potassium concentration after intense exercise (23).
Exercise training leads to multiple adaptations in human skeletal muscles as a result of molecular events, including exercise-induced activation of signaling pathways, which regulate changes of muscle structure and function. AMPK is known as a key protein for exercise-mediated muscle adaptations and particular regulation of mitochondrial and GLUT4 biogenesis (44). AMPK content, activity, and phosphorylation are markedly regulated during a few weeks of endurance training (17, 29). On the other hand, AMPK Thr-172 phosphorylation is elevated after high-, but not low-, intensity exercise (15). Furthermore, AMPK and acetyl-CoA carboxylase (ACC) phosphorylation are increased after four 30-s bouts of intense exercise (21), indicating that high-intensity exercise training, including training intensities, exceeding V̇o2 max, may lead to adaptations in the AMPK signaling pathway, but this issue has not been investigated.
Regulation of muscle Ca2+ fluxes during exercise does affect the development of fatigue (1). In human skeletal muscles, the multifunctional Ca2+/calmodulin-dependent protein kinase (CaMK) II is the major CaMK and was shown to be activated during low-intensity exercise (48). Furthermore, endurance training alters CaMKII cell signaling in human skeletal muscles (47). In contrast, CaMKII Thr-287 phosphorylation is only elevated after high-, and not low-, intensity exercise (15). Therefore, high-intensity exercise training may induce adaptations in the CaMKII pathway via changes in CaMKII Thr-287 phosphorylation, which will affect phospholamban (PLN) Thr-17 phosphorylation and thereby Ca2+ fluxes via the SERCA pumps (48).
Mammalian target of rapamycin (mTOR) is part of the multiprotein complex, mTORC1, and plays via e.g., eukaryotic initiation factor 4E-binding protein (4E-BP1) and ribosomal protein S6 p70 kinase 1 (p70S6K1) an essential role in the regulation of muscle mass and protein synthesis (22). Phosphorylation of mTOR Ser-2448 and activation of mTORC1 have been associated with both atrophy and hypertrophy of skeletal muscles (22, 42). Endurance exercise induces an increased mTOR signaling via phosphorylation of mTOR Ser-2448 (4), and heavy resistance exercise induces increases in mTOR signaling and protein synthesis (22). On the other hand, four 30-s sprints did not activate mTOR signaling (21), while other studies implementing high-intensity exercise do report activation of mTOR signaling (22). Because of the ambiguous findings, it is of value to examine whether intense exercise induces mTOR signaling and how intensified training affects mTOR signaling.
Thus, the aim of the present study was to examine the effects of intense training with reduced volume on FXYD1 expression and phosphorylation during repeated high-intensity exercise in trained individuals. In addition, to examine the effect of intensified training with a reduced volume on activation of signaling pathways involving mTOR, AMPK, and CaMKII in human skeletal muscles.
MATERIALS AND METHODS
Ethical approval and subjects.
The study was approved by the local ethical committee of the capital region of Copenhagen (Region Hovedstaden) and was performed in accordance to the principles of the Declaration of Helsinki. The subjects and training intervention were the same as in a study, focusing on adaptations of ion transport proteins and ion kinetics (23) and a study focusing on adaptations in oxygen kinetics (8) during repeated high-intensity exercise. Eight well-trained male cyclists, who had been training and competing on a regular basis for at least 3 years, with an average (mean ± SD) age, weight, and maximum oxygen uptake of 33 ± 8 yr, 81 ± 8 kg, and 59 ± 4 ml·min−1·kg−1, respectively, participated in the study. The subjects were informed of any risks and discomforts associated with the experiments before giving their written, informed consent to participate.
Training intervention.
A 7-wk intensive training intervention, including a volume reduction was performed, as a one-group longitudinal design immediately after the regular cycling season, as described in detail previously (8, 23). All training sessions were supervised and performed on public roads and on the subjects' own bikes. Briefly, the subjects replaced all their regular training with two or three sessions of speed-endurance training a week performed as 10–12 × ∼30-s maximal uphill (∼6% gradient) cycle sprinting interspersed by 4.5 min of low-intensity exercise and 1–2 sessions a week of aerobic high-intensity training consisting of 4–5 × ∼4 min of cycling (2-km flat course) at 90–95% of maximal heart rate interspersed by 2 min of rest with a work-to-rest ratio of ∼2:1. During the training intervention, subjects reduced the training volume by ∼70% (62 vs. 211 km/wk).
Experimental design.
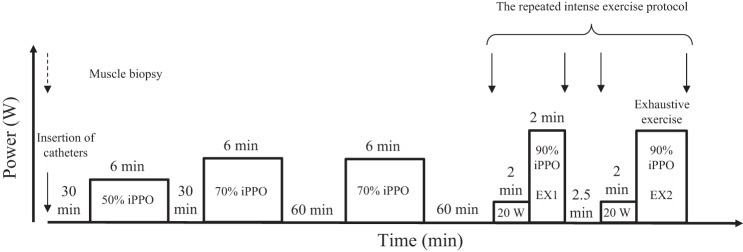
Subjects carried out two experimental days, as well as two performance testing days, before and after the 7-wk training intervention, as described in detail previously (8, 23). Briefly, on the first experimental day, subjects arrived at the laboratory in the morning at least 60 min after consumption of a standardized breakfast. After 30 min of supine rest, catheters were inserted into the femoral artery and vein under local anesthesia, using the Seldinger technique. The catheters were used to measure blood flow and for blood sampling. After 30 min of rest, subjects cycled for 6 min at 50% of peak power output on an ergometer bike (Monark, Ergomedic 839E, Vansbro, Sweden), then after 30 min of rest, for 6 min at 70% of peak power output, and 60 min later for 6 min at 70% of peak power output. Then, after another 60 min of rest, subjects performed a repeated intense exercise protocol, consisting of 2 min at low-intensity (20 W), then intense exercise for 2 min (EX1), followed by 2.5 min of recovery and 2 min of low-intensity exercise (20 W), and then another intense exercise bout performed to exhaustion (EX2). The intensity during the intense exercise was 90% of peak aerobic power output (356 ± 6 W). This article focuses on training adaptations and changes in relation to the repeated intense exercise protocol performed at the end of one of the two experimental days (Fig. 1).
Fig. 1.
A: schematic illustration of the protocol performed on the experimental day. Muscle biopsies were obtained at the time points indicated by solid arrows. A fifth biopsy was also obtained at rest in the morning, indicated by the dashed arrow, but data from this biopsy are not included in the article. The present article only includes data related to the repeated intense exercise protocol performed at the end of the experimental day. iPPO, incremental peak power output.
Before the repeated intense exercise protocol, a muscle biopsy (n = 7 as one subject did not have biopsies taken) was obtained from the musculus vastus lateralis (6) under local anesthesia (1 ml of lidocaine, 20 mg/ml without epinephrine), and incisions were made as a preparation for the following three biopsies. A biopsy was collected immediately after EX1, just prior to the low-intensity exercise before EX2 and at exhaustion in EX2 within 10 s of exercise cessation with the subjects still placed on the bike (Fig. 1). All muscle samples were immediately frozen in liquid N2 and stored at −80°C until analyses were initiated.
Protein expression in muscle homogenate lysates.
Protein expression was determined as described previously (54). In short, samples of ∼2.5 mg of freeze-dried human muscle tissue were dissected free from blood, fat, and connective tissue. Samples were homogenized for 1 min at 28.5 Hz (Qiagen Tissuelyser II; Retsch) in a fresh batch of ice-cold buffer containing (in mM): 10% glycerol, 20 Na-pyrophosphate, 150 NaCl, 50 HEPES (pH 7.5), 1% NP-40, 20 β-glycerophosphate, 2 Na3VO4, 10 NaF, 2 PMSF, 1 EDTA (pH 8), 1 EGTA (pH 8), 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 3 benzamidine, afterward rotating for 1 h at 4°C and centrifuged at 18,320 g for 20 min at 4°C to exclude nondissolved structures. The supernatant (lysate) was collected and used for further analysis. Total protein concentration in each sample was determined by a BSA standard kit (Thermo Scientific), and samples were mixed with 6 × Laemmli buffer (7 ml 0.5 M Tris-base, 3 ml glycerol, 0.93 g DTT, 1 g SDS, and 1.2 mg bromophenol blue) and ddH2O to reach equal protein concentration before protein expression were determined by Western blot analysis.
Western blot analysis.
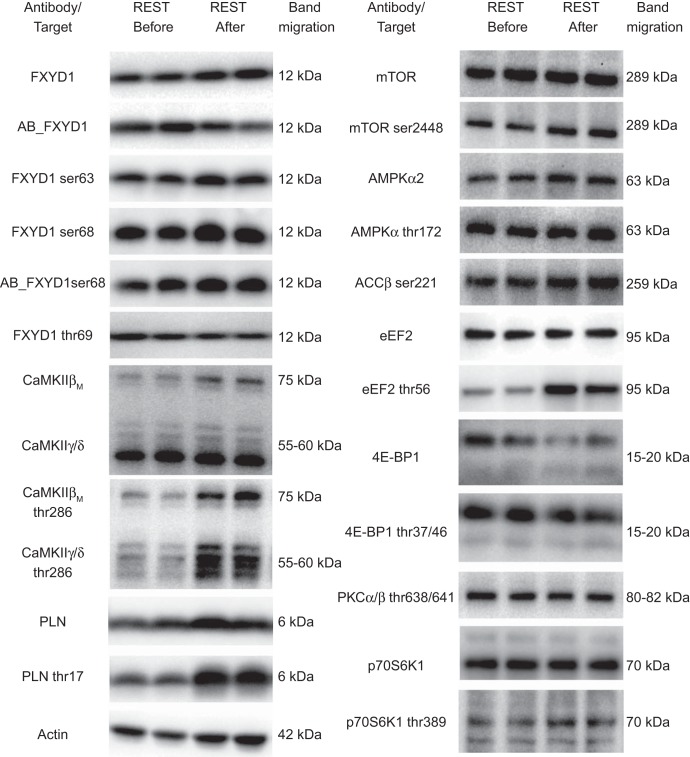
Equal amounts of total protein were loaded in each well of precast gels (Bio-Rad Laboratories). All samples from each subject were loaded on the same gel. Proteins were separated according to their molecular weight by SDS-PAGE and semi-dry transferred to a PVDF membrane (Bio-Rad). The membranes were blocked in either 2% skim milk or 3% BSA in TBS, including 0.1% Tween-20 (TBST) before an overnight incubation in primary antibody at 4°C and a subsequent 1-h incubation in horseradish peroxidase-conjugated secondary antibody at room temperature. The bands were visualized with ECL (Millipore) and recorded with a digital camera (ChemiDoc MP Imaging System, Bio-Rad Laboratories). Densitometry quantification of the Western blot band intensity was done using Image Lab version 4.0 (Bio-Rad Laboratories) and determined as the total band intensity adjusted for background intensity. Representative blots are shown in Fig. 2.
Fig. 2.
Representative Western blots, including the molecular weight of band migration. 4E-BP1, eukaryotic initiation factor 4E-binding protein 1; ACCβ Ser-221 phos, acetyl-CoA carboxylase β serine-221 phosphorylation; AMPKα2, AMP-activated protein kinase α2; CaMKII, Ca2+/calmodulin-dependent protein kinase II; eEF2, eukaryotic elongation factor 2; FXYD1, phospholemman; mTOR, mammalian target of rapamycin; PKCα/β Thr-638/641 phos, protein kinase Cα/β threonine 638/641 phosphorylation; p70S6K1, ribosomal protein S6 p70 kinase 1; PLN, phospholamban.
Antibodies.
The primary antibodies used in the present experiment were optimized by use of mixed human muscle standard lysates to ensure that the protein amount loaded would result in band signal intensities localized on the steep and linear part of a standard curve. To determine total and phospho-specific protein expression, the antibodies that are included in Table 1 were used with the localization of the quantified signal noted. The phospho-specific ACCα Ser-79 antibody (no. 07–303; Millipore) was previously shown to recognize the equivalent Ser-221 in human ACCβ (45, 57), and therefore, it was used to determine ACCβ Ser-221 phosphorylation. The secondary antibodies used were horseradish peroxidase-conjugated rabbit anti-sheep (P-0163), rabbit anti-goat (P-0449), goat anti-mouse (P-0447, DAKO) and goat anti-rabbit IgM/IgG (4010-05; Southern Biotech).
Table 1.
Antibody overview
| Protein Target | Ab cat. Number or Name | Company or Donor | Ab Source | Migration, MW |
|---|---|---|---|---|
| 4E-BP1 | 9452 | Cell Signaling Technology | rabbit | 15–20 kDa |
| 4E-BP1 Thr-37/46 phos | 2855 | Cell Signaling Technology | rabbit | 15–20 kDa |
| ACCβ Ser-221 phos | 07–303 | Millipore | rabbit | 259 kDa |
| Actin | A2066 | Sigma Aldrich | rabbit | 42 kDa |
| AMPKα2 | AMPK α2 | Dr. J. Birk, University of Copenhagen | sheep | 63 kDa |
| AMPKα Thr-172 phos | 2531 | Cell Signaling Technology | rabbit | 63 kDa |
| CaMKII | 611293 | BD Transduction Laboratories | mouse | 55–75 kDa |
| CaMKII Thr-286 phos | 3361 | Cell Signaling Technology | rabbit | 55–75 kDa |
| eEF2 | ab130187 | Abcam | mouse | 95 kDa |
| eEF2 Thr-56 phos | 2331 | Cell Signaling Technology | rabbit | 95 kDa |
| FXYD1 | 13721-1-AP | Proteintech | rabbit | 12 kDa |
| FXYD1 unphosphorylated | AB_FXYD1–C2 | Dr. J. Randall Moorman, University of Virginia | rabbit | 12 kDa |
| FXYD1 Ser-68 phos | AB_FXYD1 ser68–CP68 | Dr. D. Bers, Loyola University | rabbit | 12 kDa |
| FXYD1 Ser-63 phos | FXYD1 Ser-63 phos | Professor M. Shattock, King's College London | rabbit | 12 kDa |
| FXYD1 Ser-68 phos | FXYD1 Ser-68 phos | Professor M. Shattock, King's College London | rabbit | 12 kDa |
| FXYD1 Thr-69 phos | FXYD1Thr-69 phos | Professor M. Shattock, King's College London | sheep | 12 kDa |
| mTOR | 2972 | Cell Signaling Technology | rabbit | 289 kDa |
| mTOR Ser-2448 phos | 2971 | Cell Signaling Technology | rabbit | 289 kDa |
| p70S6K1 | 2708 | Cell Signaling Technology | rabbit | 70 kDa |
| p70S6K1 Thr-389 phos | 9234 | Cell Signaling Technology | rabbit | 70 kDa |
| PKCα/β Thr-638/641 phos | 9375 | Cell Signaling Technology | rabbit | 80–82 kDa |
| PLN | PA5-19351 | Pierce–ThermoScientific | goat | 6 kDa |
| PLN Thr-17 phos | Sc-17024 | Santa Cruz Biotechnology | rabbit | 6 kDa |
4E-BP1, eukaryotic initiation factor 4E-binding protein 1; ACCβ Ser-221 phos, Acetyl-CoA carboxylase β serine-221 phosphorylation; AMPKα2, AMP-activated protein kinase α2; CaMKII, Ca2+/calmodulin-dependent protein kinase II; eEF2, eukaryotic elongation factor 2; FXYD1, phospholemman; mTOR, mammalian target of rapamycin; PKCα/β Thr-638/641 phos, protein kinase Cα/β threonine 638/641 phosphorylation; p70S6K1, ribosomal protein S6 p70 kinase 1; PLN, phospholamban.
FXYD1 antibody phospho-specificity.
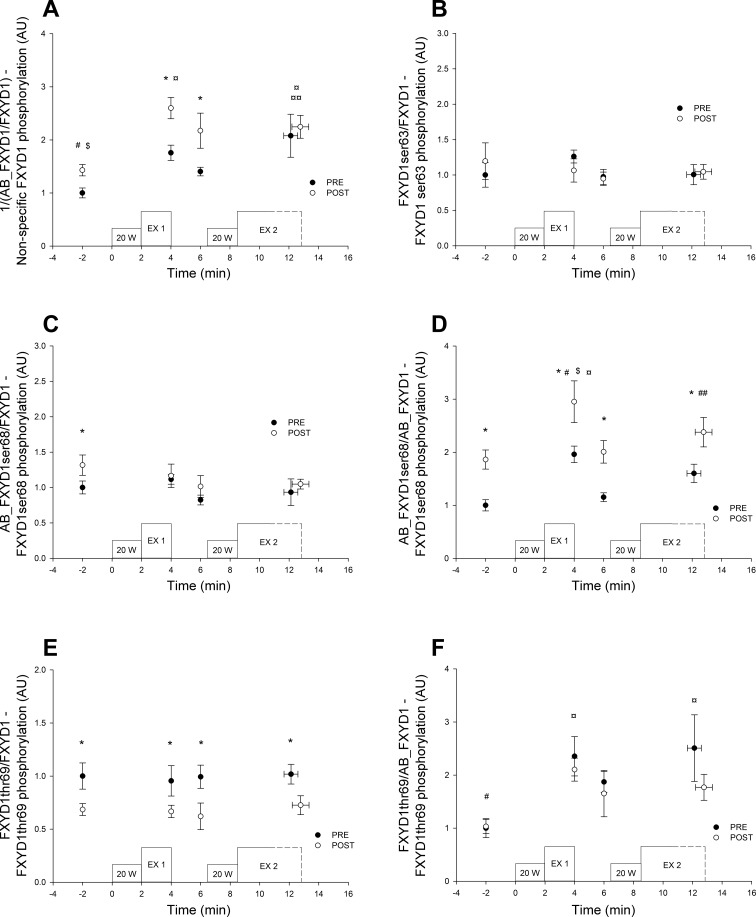
All of the FXYD1 antibodies used in the present study were previously shown to detect FXYD1 in human skeletal muscle (5, 52), as well as FXYD1 in other tissues (18, 41, 50). To interpret the data meaningfully, it should be noted that AB_FXYD1 recognizes mainly unphosphorylated FXYD1; however, phosphorylation at Ser-63, Ser-68, and Thr-69 reduces the AB_FXYD1 signal intensity, as the antibody epitope is located in the COOH-terminal region of FXYD1 protein, where the phosphorylation sites are also located (5, 41, 50, 53). This was confirmed in the present study by dephosphorylation of the membrane proteins (43) after the original Western blot analysis with AB_FXYD1. The original PVDF membrane was first reactivated in ethanol and afterward incubated in TBST. Then the membrane was incubated in a stripping buffer (0.5 M Tris·HCL; pH 6.7, 2% SDS, and 100 mM 2-mercaptoethanol) at 50°C for 2 h. After 3 × 10 min washing in TBST in another container, the membrane was blocked with TBST, including 2% skimmed milk in 15 min and incubated in secondary antibody for 1 h. Membranes were then washed again for 3 × 15 min, and the stripping procedure was confirmed by exposure of the membrane. When the entire primary antibody was removed by the stripping protocol, the dephosphorylation protocol was conducted by incubating membranes for 2 h at 37°C in the dephosphorylation buffer (50 mM Tris·HCl, 0.1 mM Na2EDTA, 5 mM DTT, 0.01% Brij 35 and 2 mM MnCl2; pH 7.5), including 500 U/ml lambda protein phosphatase (P07535; New England BioLabs). Then the membrane was blocked with TBST, including 2% skimmed milk, incubated overnight in AB_FXYD1, washed 2 × 5 min in TBST, incubated for 1 h in secondary antibody, and exposed by ECL. Following this procedure, total FXYD1 expression (using AB_FXYD1 on dephosphorylated proteins) was shown to be significantly increased (0.91 ± 0.05 vs. 1.04 ± 0.06) after the training intervention versus before. A similar result (30% increase) was obtained with the total FXYD1 antibody (Table 2), raised against the NH2 terminal region of the FXYD1, confirming the AB_FXYD1 phospho-specificity. For clarity purposes, data obtained with AB_FXYD1 are inverted and shown as 1/AB_FXYD1; thus, an increase in the Fig. 3A represents an increase in nonspecific FXYD1 phosphorylation.
Table 2.
Muscle protein expression before and after 7 wk of high-intensity training in combination with a reduced training volume in trained cyclist
| Main Statistical P Values for a Two-Way Repeated-Measures ANOVA |
|||||
|---|---|---|---|---|---|
| Protein/Antibody | Before | After | Training | Acute Exercise | Interaction |
| 4E-BP1 | 0.99 ± 0.06 | 0.75 ± 0.05* | 0.013 | 0.896 | 0.850 |
| Actin | 0.86 ± 0.05 | 1.26 ± 0.09* | 0.018 | 0.399 | 0.828 |
| AMPKα2 | 1.00 ± 0.03 | 1.04 ± 0.04 | 0.325 | 0.285 | 0.965 |
| CaMKII βM | 0.96 ± 0.07 | 1.19 ± 0.13# | 0.072 | 0.563 | 0.771 |
| CaMKII γ/δ | 0.92 ± 0.07 | 1.17 ± 0.11** | 0.006 | 0.382 | 0.179 |
| eEF2 | 0.74 ± 0.05 | 0.80 ± 0.06 | 0.357 | 0.143 | 0.051 |
| FXYD1 | 0.98 ± 0.05 | 1.28 ± 0.08** | 0.005 | 0.215 | 0.081 |
| mTOR | 0.95 ± 0.05 | 1.07 ± 0.06* | 0.015 | 0.630 | 0.211 |
| p70S6K1 | 0.87 ± 0.03 | 0.88 ± 0.04 | 0.570 | 0.106 | 0.030 |
| PLN | 1.06 ± 0.05 | 1.22 ± 0.06** | 0.007 | 0.470 | 0.328 |
Values are expressed as means ± SE in arbitrary units; n = 7. The main statistical P values obtained from a two-way repteated-measures ANOVA statistical analysis are expressed. Bolded values denote overall significant P values. Protein expression is different after compared to before the training intervention
P < 0.05, and
P < 0.01. Protein expression tended to be different after compared to before the training intervention
P < 0.10.
Fig. 3.
Muscle FXYD1 phosphorylation at rest and during repeated intense exercise (EX1 and EX2) before (PRE) and after (POST) 7 wk of high-intensity training in combination with a reduced training volume (IT) in trained cyclist (n = 7). Data are normalized to the mean at rest before the intervention period (PRE) and expressed as means ± SE. A: nonspecific FXYD1 phosphorylation. For overall statistical effects: Acute exercise: P > 0.001; Training: P = 0.012, and Interaction: P = 0.232. *Post higher than Pre. #Rest lower than all other time points. $Rest lower than all other time points after IT (Post). ¤End of EX1 and EX2 higher than rest before IT (PRE) and ¤¤ End of EX2 higher than before EX2 before IT (PRE). B: FXYD1 Ser-63 phosphorylation For overall statistical effects: Acute exercise: P = 0.359; Training: P = 0.938; and Interaction: P = 0.165. C: FXYD1 Ser-68 phosphorylation. For overall statistical effects: Acute exercise: P = 0.053, Training: P = 0.046, and Interaction: P = 0.520. *Post higher than Pre. D: FXYD1 Ser-68 phosphorylation, considering antibody phospho-sensitivity, For overall statistical effects: Acute exercise: P < 0.001, Training: P = 0.004, and Interaction: P = 0.920. *Post higher than Pre. #End of EX1 higher than all other time points. ##End of EX2 higher than Rest and before EX2. $End of EX1 higher than all other time points after IT (Post). ¤End of EX1 higher than Rest and before EX2 before IT (PRE). E: FXYD1 Thr-69 phosphorylation For overall statistical effects: Acute exercise: P = 0.824, Training: P = 0.001, and Interaction: P = 0.937. *POST lower than PRE. F: FXYD1 Thr-69 phosphorylation, considering antibody phospho-sensitivity For overall statistical effects: Acute exercise: P = 0.006; Training: P = 0.071; and Interaction: P = 0.723. #Rest lower than all other time points. ¤End of EX1 and End of EX2 higher than Rest before IT (Pre).
AB_FXYD1 Ser-68 (originally named CP68) is phospho-specific for Ser-68 residue in humans (52), although it should be noted that the affinity for Ser-68 residue is affected by the phosphorylation status of the adjacent Thr-69. Thus, the amount of Ser-68 phosphorylation, as determined by AB_FXYD1 Ser-68 (Fig. 3C), can be underestimated if Thr-69 is phosphorylated (18). Similarly, FXYD1 Thr-69 phosphorylation (Fig. 3E) can be affected by the phosphorylation status of the Ser-68 residue.
Furthermore, a new batch of FXYD1 phospho-specific antibodies—FXYD1 Ser-63, FXYD1 Ser-68, and FXYD1 Thr-69 (developed by Will Fuller and Michael Shattock)—have also been used. These antibodies were used in mouse and rat ventricular myocytes, where FXYD1 is poorly phosphorylated at Thr-69 (16); however, in vitro phosphorylation data indicate (18) that the FXYD1 Ser-68 and FXYD1 Thr-69 antibodies are affected to a similar extent as the older generation of antibodies, AB_FXYD1 Ser-68, and AB_FXYD1. Indeed, in our study, FXYD1 Ser-68 phosphorylation data obtained by the FXYD1 Ser-68 and AB_FXYD1 Ser-68 antibodies were similar and, thus, for simplicity, only data obtained using AB_FXYD1 Ser-68 are included in the results.
To take into account the phospho-specificity and -sensitivity of the antibodies used, the AB_FXYD1 Ser-68/AB_FXYD1 ratio (Fig. 3D) was used as an alternative to determine Ser-68 phosphorylation (Fig. 3C), as done in the past (54), whereas, FXYD1Thr-69/AB_FXYD1 (Fig. 3F) was used as an alternative to determine Thr-69 phosphorylation (Fig. 3E). These ratios may overcome the fact that the determination of FXYD1 Ser-68 and Thr-69 phosphorylation probably are affected by simultaneous phosphorylation at the two sites located next to each other. Data obtained from the ratio FXYD1 Ser-68/AB_FXYD1 were similar to AB_FXYD1 Ser-68/AB_FXYD1 and not included.
Data treatment.
For each muscle sample, protein expression and phosphorylation were determined in duplicate (except for three muscle samples, which only had one measurement due to limited muscle tissue), and the average intensities were calculated. Values for all of the individual time points were compared with the average resting value before the training intervention.
Training-induced changes in total protein expression and phosphorylation are shown in relation to the total expression of the same protein, in which both are determined, e.g., mTOR phosphorylation and mTOR expression. Determination of the specific phosphorylation level and total protein expression was performed on separate membranes in separate analyses.
Statistics.
Changes in protein phosphorylation and expression were evaluated by a two-way repeated-measures ANOVA. If overall significant main effects were observed, a Student-Newman-Keuls post hoc analysis was conducted to identify differences in protein phosphorylation within specific time points (SigmaPlot 11.0). P < 0.05 was chosen as the level of significance.
RESULTS
Effect of the training intervention on protein expression.
Total expression of muscle FXYD1, CaMKII γ/δ, PLN, mTOR, and actin was 30% (P < 0.01), 25% (P < 0.01), 16% (P < 0.01), 12% (P < 0.05), and 40% (P < 0.05) higher after than before the training intervention. The expression of 4E-BP1 was 24% lower (P < 0.05) after than before the training intervention. CaMKII βM expression tended (P = 0.072) to be higher after compared with before the training intervention, whereas the expression of AMPKα2, eukaryotic elongation factor 2 (EF2), and p70S6K1 was not changed with the training intervention (Table 2).
Effect of the training intervention on protein phosphorylation during intense exercise.
Nonspecific FXYD1 phosphorylation was higher (P < 0.05) at all time points during the repeated intense exercise, compared with rest. After the training intervention period, nonspecific FXYD1 phosphorylation was higher (P < 0.05) after EX1 and before EX2, than before the training intervention (Fig. 3A). FXYD1 Ser-63 phosphorylation was not altered during the repeated intense exercise, nor was it changed with the training intervention (Fig. 3B). FXYD1 Ser-68 phosphorylation was higher (P < 0.001) at the end of EX1, compared with rest, decreased (P < 0.001) after EX1, and then increased (P < 0.05) after compared with before EX2 (Fig. 3D). Furthermore, FXYD1 Ser-68 phosphorylation was higher (P < 0.05) at rest and throughout the repeated intense exercise protocol after compared with before the training intervention (Fig. 3, C and D). FXYD1 Thr-69 phosphorylation was higher (P < 0.05) after EX1, before and after EX2 compared with rest, while the training intervention did not affect FXYD1 Thr-69 phosphorylation (Fig. 3F).
PKCα/β Thr-638/641 phosphorylation.
PKCα/β Thr-638/641 phosphorylation did not change during the repeated intense exercise, but after the training intervention, it was higher (P < 0.01) before EX2 compared with rest (Table 3). After the training intervention, PKCα/β Thr-638/641 phosphorylation was higher at the end of EX1 (P < 0.05) and before EX2 (P < 0.01) compared with before the training intervention.
Table 3.
Changes in protein phosphorylation at rest and during the repeated intense exercise protocol before and after 7 wk of high-intensity training in combination with a reduced training volume in trained cyclist
| Target | Main effects ANOVA P Values | Time | Rest | End of EX1 | Before EX2 | End of EX2 |
|---|---|---|---|---|---|---|
| PKCα/β Thr-638, Thr-641 | E: P = 0.240 | PRE | 1.00 ± 0.12 | 0.75 ± 0.09 | 0.88 ± 0.09 | 0.93 ± 0.07 |
| T: P = 0.036 | POST | 0.81 ± 0.09 | 1.03 ± 0.20** | 1.27 ± 0.16**$ | 1.05 ± 0.16 | |
| I: P = 0.029 | ||||||
| CaMKIIβ Thr-287 | E: P = 0.156 | PRE | 1.00 ± 0.24 | 4.37 ± 2.12 | 1.08 ± 0.15 | 2.45 ± 0.68 |
| T: P = 0.014 | POST | 6.03 ± 1.04 | 8.95 ± 2.34 | 8.61 ± 2.23** | 11.32 ± 3.77** | |
| I: P = 0.337 | ||||||
| CaMKIIγ/δ Thr-287 | E: P = 0.279 | PRE | 1.00 ± 0.27 | 3.81 ± 1.38 | 1.06 ± 0.13 | 1.96 ± 0.35 |
| T: P = 0.004 | POST | 5.48 ± 1.21** | 6.10 ± 1.37 | 6.22 ± 1.52** | 7.30 ± 2.09** | |
| I: P = 0.263 | ||||||
| PLN Thr-17 | E: P = 0.117 | PRE | 1.00 ± 0.16 | 1.37 ± 0.05 | 1.52 ± 0.12$ | 1.14 ± 0.10 |
| T: P = 0.235 | POST | 1.42 ± 0.07** | 1.31 ± 0.07 | 1.44 ± 0.14 | 1.21 ± 0.09 | |
| I: P = 0.039 | ||||||
| eEF2 Thr-56 | E: P = 0.007 | PRE | 1.00 ± 0.19 | 2.90 ± 0.37 | 2.75 ± 0.32 | 3.52 ± 0.24##$ |
| T: P = 0.002 | POST | 3.16 ± 0.64** | 2.87 ± 0.47 | 2.60 ± 0.51 | 5.29 ± 0.99**##$$ | |
| I: P = 0.086 | ||||||
| mTOR Ser-2448 | E: P = 0.064 | PRE | 1.00 ± 0.13 | 1.63 ± 0.25 | 0.99 ± 0.13 | 1.28 ± 0.15 |
| T: P = 0.018 | POST | 1.32 ± 0.22 | 1.59 ± 0.19 | 1.62 ± 0.30** | 1.72 ± 0.14** | |
| I: P = 0.139 | ||||||
| p70S6K1 Thr-389 | E: P = 0.021 | PRE | 1.00 ± 0.12 | 2.36 ± 0.39#$ | 1.96 ± 0.34$ | 2.27 ± 0.26$ |
| T: P = 0.524 | POST | 1.47 ± 0.29 | 2.60 ± 0.36#$ | 2.09 ± 0.46 | 1.95 ± 0.23 | |
| I: P = 0.178 | ||||||
| 4E-BP1 Thr-37/46 | E: P = 0.271 | PRE | 1.00 ± 0.16 | 0.67 ± 0.11 | 0.82 ± 0.09 | 0.59 ± 0.10 |
| T: P = 0.197 | POST | 1.01 ± 0.25 | 0.73 ± 0.11 | 0.93 ± 0.17 | 0.88 ± 0.18 | |
| I: P = 0.296 | ||||||
| AMPKα Thr-172 | E: P = 0.003 | PRE | 1.00 ± 0.09 | 0.74 ± 0.08 | 0.74 ± 0.09 | 1.46 ± 0.19*$$## |
| T: P = 0.210 | POST | 0.90 ± 0.10 | 0.65 ± 0.14 | 0.79 ± 0.11 | 1.03 ± 0.10## | |
| I: P = 0.047 | ||||||
| ACCβ Ser-221 | E: P < 0.001 | PRE | 1.00 ± 0.18 | 3.25 ± 0.76#$ | 2.98 ± 0.84#$ | 3.99 ± 0.86#$¤ |
| T: P = 0.182 | POST | 1.00 ± 0.15 | 2.51 ± 0.37#$ | 1.84 ± 0.37# | 3.00 ± 0.60#$¤ | |
| I: P = 0.558 |
Data are expressed as means ± SE.
ACCβ, acetyl-CoA carboxylase β; AMPKα2, AMP-activated protein kinase α2; PKCα/β, protein kinase C α/β; E, acute exercise; T, training; I, interaction; Bolded values denote overall significant P values. End of EX1, after the first intense exercise bout lasting 2 min; Before EX2, before the second exercise bout; End of EX2, after the second high-intensity exercise bout performed to exhaustion.
PRE higher than POST.
POST higher than PRE.
Higher than Rest within PRE or POST.
Higher than all other time points within PRE or POST.
Higher than Rest;
Higher than all other time points. ¤Higher than before EX2.
CaMKII Thr-287, PLN Thr-17, and eEF2 Thr-56 phosphorylation.
Neither CaMKII βM nor γ/δ subunit Thr-287 phosphorylation was altered during the repeated high-intensity exercise. After the training intervention, CaMKII γ/δ Thr-287 phosphorylation was higher (P < 0.01) at rest, and both CaMKII βM and γ/δ Thr-287 phosphorylation were higher (P < 0.01) before and after EX2, compared with before the training intervention (Table 3).
After the training intervention Phospholamban (PLN) Thr-17 phosphorylation, was higher at rest (P < 0.01) compared with before the intervention. Furthermore, before the training intervention, PLN Thr-17 phosphorylation was higher before EX2 compared with rest, while there were no changes in PLN Thr-17 phosphorylation with exercise after the training intervention (Table 3).
Another CaMKII downstream target, eEF2 Thr-56 phosphorylation, was increased at rest (P < 0.01) and after EX2 (P < 0.05) after the training intervention compared with before. Before the training intervention eEF2 Thr-56 phosphorylation after EX2 was higher (P < 0.05) than at rest, while after the intervention, the eEF2 Thr-56 phosphorylation after EX2 was higher (P < 0.05) than at all other time points (Table 3).
mTOR Ser-2448, p70S6K1 Thr-389, and 4E-BP1 Thr-37/46 phosphorylation.
Phosphorylation of mTOR Ser-2448 tended (P = 0.064) overall to change during the exercise bouts. After the training intervention, mTOR Ser-2448 phosphorylation was higher before EX2 (P < 0.01) and after EX2 (P < 0.05), compared with before the training intervention (Table 3).
Before the training intervention mTORC1 activity determined by p70S6K1 Thr-389 phosphorylation at all time points was higher (P < 0.05) compared with rest. After the training intervention, p70S6K Thr-389 phosphorylation was higher (P < 0.05) after EX1 compared with rest (Table 3). The mTOR substrate eukaryotic initiation factor 4E-BP1 Thr-37/46 phosphorylation was not changed with either exercise or training (Table 3).
AMPKα Thr-172 and ACCβ Ser-221 phosphorylation.
Before the training intervention, AMPKα Thr-172 phosphorylation was higher (P < 0.01) after EX2 compared with the other time points. After the training intervention, AMPKα Thr-172 phosphorylation after EX2 was lower (P < 0.01) than before the training intervention (Table 3). As a downstream target of AMPK, the ACCβ Ser-221 phosphorylation was higher after EX1 (P < 0.001) and before EX2 (P < 0.01) compared with rest and was further increased (P < 0.05) at exhaustion, but was not affected by the training intervention (Table 3).
DISCUSSION
The main findings of the present experiment were that 7 wk of intensive training, with a reduced training volume, increased the total expression of FXYD1 and elevated the resting nonspecific FXYD1 phosphorylation level in an endurance-trained cyclist. In addition, repeated intense exercise after the training intervention induced a higher level of nonspecific FXYD1 phosphorylation than before the intervention. This was dominated by higher phosphorylation at FXYD1 Ser-68 residues. Other important findings were that the training intervention elevated the expression of actin, mTOR, PLN, and CaMKII γ/δ, while it lowered the 4E-BP1 expression. Furthermore, the resting PLN Thr-17 phosphorylation, the overall PKCα/β Thr-638/641, and mTOR Ser-2448 phosphorylation during repeated intense exercise as well as CaMKII, Thr-287, and eEF2 Thr-56 phosphorylation at rest and during exercise, was higher after compared with before the training intervention.
Total FXYD1 expression was higher after compared with before the intensified training period, with no change in NaK pump α- and β-isoform expression [NaKα1: −11%, NaKα2: −8%, NaKβ1: −3%; (23)]. In contrast, no change in total FXYD1 expression, but elevated NaK pump α1-, α2- and β1-isoform protein expressions were shown after 10 days of moderate intensity (75–100% of V̇o2 peak) cycle training in recreationally active subjects (5). Thus, it appears that the intensity of training and/or the training status of the subjects are important for adaptation of muscle FXYD1. In support of the first notion, sprint training in rats induced higher muscle FXYD1 levels, while endurance training did not have any effect on FXYD1 expression (38). Treadmill running with a 10% grade, 5 days/wk for 45 min in ∼14 wk elevated FXYD1 expression in rat skeletal muscles (40). The different effect of the various training forms may have been caused by the degree of the fast twitch (FT) muscle fiber stimulation, as FT muscle fibers are expected to be more activated during the intense training. In agreement, it has been demonstrated in humans, that the exercise (5-min cycling at 95% of V̇o2 max)-induced change in FXYD1 phosphorylation is more pronounced in type II fibers than in type I fibers (51).
In the resting state, nonspecific FXYD1 phosphorylation and Ser-68 phosphorylation was higher after compared with before the training intervention. In agreement, a higher level of FXYD1 Ser-68 phosphorylation at rest was observed after 2 wk of intensified training in soccer players (54). In contrast, 10 days of moderate intensity exercise training did not induce changes in the resting FXYD1 phosphorylation level (5), indicating that exercise intensity is also important for the training adaptations of FXYD1 phosphorylation at rest.
During repeated intense exercise, the nonspecific FXYD1 phosphorylation increased because of greater Ser-68 and Thr-69 phosphorylation, which is also observed during exercise with moderate intensity (52). On the other hand, FXYD1 Ser-63 phosphorylation did not change during the short and intense repeated exercise protocol as shown after 20–30 min of moderate-intensity exercise (5, 52). This may be explained by the lack of increase in PKCα/β Thr-638/641 phosphorylation level, as Ser-63 phosphorylation is PKC-mediated (7, 36). The duration of the repeated intense exercise protocol may have been too short or the intensity too high to induce Ser-63 phosphorylation. FXYD1 Thr-69 phosphorylation increased after EX1 and stayed elevated during the repeated intense exercise protocol, while Ser-68 phosphorylation increased during both exercise bouts and decreased in recovery from EX1. These marked increases in FXYD1 phosphorylation levels during exercise suggest that FXYD1 phosphorylation may play a crucial role in regulation of the NaK pump, and hence, K+ regulation during and after intense exercise, where K+ fluxes are pronounced (24, 28). Thus, in the same study, it was observed that the average venous K+ concentration during the first 2 min of recovery from the intense exercise bouts was lower (P < 0.05) after compared with before the training intervention (4.2 ± 0.2 vs. 4.9 ± 0.2 and 4.3 ± 0.2 vs. 5.1 ± 0.1 mM), suggesting an enhanced muscle K+ reuptake, without changes in the expression of NaK pumps subunits (20). Furthermore, performance during repeated intense exercise was improved with the training intervention (256 vs. 217 s) (23).
After the training intervention, nonspecific FXYD1 phosphorylation was higher at the end of EX1 and before EX2, due to higher FXYD1 Ser-68 phosphorylation, compared with before the intervention. The training intervention did not affect FXYD1 Thr-69 phosphorylation, which is in agreement with findings after a period of moderate-intensity training (5). The training-induced increase in PKCα/β Thr-638/641 phosphorylation may have contributed to the elevated FXYD1 phosphorylation, since PKCα activity has been shown to be required for contraction-induced FXYD1 phosphorylation in mouse skeletal muscles (52) and other tissues (7, 18, 35).
The higher expression of FXYD1 and FXYD1 phosphorylation after compared with before the training intervention may have affected the NaK pump activity and, hence, muscle potassium reuptake at rest and during contractions (10). In rat skeletal muscles, around 30% of the α-subunits were coexpressed with FXYD1 (39), and the finding of a larger amount of FXYD1 may suggest a higher degree of NaK pumps found as α/β/FXYD1 or a higher pool of free FXYD1 proteins. It has been shown in Xenopus oocytes that the affinity for potassium (K+) and especially sodium (Na+) is lower for α/β/FXYD1 pumps compared with α/β pumps (both α1/β1 and α2/β1) without differences in the maximal pump activity (13). Thus, at rest, a potential higher amount of α/β/FXYD1 pumps after compared with before the training intervention may per se lower the NaK pump activity, but it may also have been counterbalanced by an increased Na+ affinity expected from a higher resting FXYD1 phosphorylation (7, 35).
Incubation of rat muscle tissue homogenates with an anti-FXYD1 antibody lowered the NaK enzymatic activity by more than 50% compared with samples with no treatment (40), indicating that more FXYD1 increases the activity of NaK pumps in muscles through a higher amount of NaK pumps found as α/β/FXYD1. In addition, a higher pool of free FXYD1 after compared with before the training intervention, may have elevated the NaK pump activity during contractions. Indeed, FXYD1 has been suggested to translocate from an intracellular pool to the sarcolemma membrane during contractions, concomitant with an increased association between FXYD1 and the α1-subunit and a higher pump activity in the sarcolemma membrane fraction (39). Furthermore, the higher FXYD1 phosphorylation after the training intervention may have improved the pump activity through both a higher Na+ affinity (27) and a higher Vmax (34, 35). Thus, during exercise, both the higher FXYD1 expression and phosphorylation may have contributed to an increased NaK pump activity after the training intervention compared with before. Unfortunately, the maximal NaK pump activity could not be determined due to lack of muscle tissue. Nevertheless, a higher activity of the NaK pump during and after exercise may explain the observation of lowered femoral venous plasma K+ concentration in the first 2 min of recovery after EX1 and EX2 as a result of the training intervention (23).
An increased exercise-induced extracellular K+ concentration has been linked to depolarization of the muscle membranes, decreased excitability, and muscle fatigue. Therefore, higher muscle K+ reuptake is expected to improve performance. Improved K+ handling and exercise performance have been related to higher NaK pump content after a period of training (25, 31–33). On the other hand, high-intensity training has augmented maximal pump activity despite unchanged total pump content and protein isoform expression (3). FXYD1 expression and phosphorylation were not determined in either of these studies, and adaptations in the FXYD1 proteins may be the missing link explaining increased NaK pump activity without changes in pump content or isoform expression (3). Concomitant adaptations in the NaK pump α2-subunit and FXYD1 phosphorylation have previously been demonstrated after intensified training (54). Thus, the adaptations in FXYD1 expression and FXYD1 phosphorylation shown here may have improved K+ handling during exercise, despite no changes in NaK pump subunit expression. It is interesting to hypothesize that these adaptations in the FXYD1 protein may be the cause of the improved performance during repeated high-intensity exercise of already trained athletes after the intensified training intervention with reduced training volume, as observed in the present study (23).
An improved performance as a result of the training intervention (23) may also have been related to an improved intracellular Ca2+ handling (20). The high-intensity training intervention with reduced volume induced increases in the CaMKII γ/δ isoforms, while the CaMKII βM tended to be higher. The elevated CaMKII expression was associated with a higher expression of PLN and a higher resting phosphorylation of the substrate phospholamban Thr-17, which relieves the phospholamban inhibition on SERCA, allowing a higher Ca2+ affinity and, thus, a higher rate of Ca2+ uptake (48). A higher content of PLN with the same degree of Thr-17 phosphorylation would most likely lead to better Ca2+ homeostasis in the trained muscle (47), as observed previously in rats (26). It should be noted, however, that the changes in CaMKII expression in the present study were less pronounced than with 10 days of endurance training (4) and 3 wk of one-legged endurance exercise training, which doubled the CaMKII activity, CaMKII kinase isoform expression, and CaMKII autophosphorylation in resting muscles (47). On the other hand, the changes in PLN expression and Thr-17 phosphorylation at rest, as well as in CaMKII Thr-287 phosphorylation (up to eight-fold increases) at rest and throughout the repeated intense exercise protocol shown after the intense training intervention, were either not seen after 10 days of endurance training (4) or were less pronounced after 3 wk of endurance training (47), even though the subjects in the present study were trained before the intervention period. Thus, adaptations in PLN expression and CaMKII Thr-287 phosphorylation seem to be intensity dependent. CaMKII phosphorylation accelerates ATP provision via glycogenolysis and glycolysis during contractions (48) and may explain why higher muscle lactate levels were observed during exercise after the training intervention (23).
AMPK Thr-172 phosphorylation at exhaustion was lower after the intervention period. In accordance, 10 days of endurance exercise training abolished a nine-fold increase in AMPK α2 activity, observed during prolonged exercise before the training period (29). On the other hand, in the present experiment, the downstream target of AMPK, ACC Ser-221 phosphorylation was not affected by the training intervention, which was observed after a period of endurance training (4, 29). These findings indicate that high-intensity training has an impact on AMPK signaling, but the effect is less pronounced than seen after endurance training. When the energy sensing and signaling protein AMPK is activated, it increases ATP production by stimulation of glucose uptake and fatty acid oxidation. Furthermore, activation of AMPK inhibits ATP-consuming processes, such as protein synthesis (56). The observed decrease in the exercise-induced AMPK Thr-172 phosphorylation after the training intervention may indicate an abolished AMPK activity during high-intensity exercise, even though other factors are involved. A decrease in AMPK activity will improve the ability for ATP-consuming processes in the muscle cell, such as an increased NaK pump activity, which may contribute to improved K+ handling and improved performance. In support for a link between AMPK and NaK pump activity, repeated treatment of mice with the AMPK activator AICAR increased FXYD1 phosphorylation and affected the NaK pump activity by increasing the Na+ affinity (27).
AMPK may be involved in the regulation of mTOR, as elevated AMPK signaling lowers mTOR signaling in mouse skeletal muscles (14), while it is presently unclear whether it also occurs in humans (19). Thus, the abolished AMPK phosphorylation after the training intervention may have caused the increased expression of mTOR, as well as mTOR Ser-2448 phosphorylation. These increases in mTOR and Ser-2448 phosphorylation were similar to the adaptations seen after moderate-intensity training (4) and appear not to be intensity dependent. The mTOR signaling pathway is involved in many processes in the muscle cell, including pathways controlling protein synthesis and muscle hypertrophy (12, 22). The increased actin expression may indicate muscle hypertrophy. It is supported by a training-induced decrease in 4E-BP1 expression, which may have reduced eIF4E/4E-BP1 binding and elevated translation initiation (22). The mTORC1 readout p70S6K1 Thr-389 phosphorylation was in the present study higher during 2 × 2–4 min of high-intensity exercise, which is in contrast to shorter high-intensity exercise bouts (11, 21). During the training intervention, both 30-s and 4-min bouts were performed; thus, mTORC1 may have been activated during the training and may have induced hypertrophy. On the other hand, both exercise and training induced an increase in the downstream target of CaMKII, eEF2 Thr-56 phosphorylation (19, 37), which is expected to lower protein synthesis by lowering the eEF2 interaction with the ribosome and, thereby, impairing the elongation rate (37). Likewise, acute endurance exercise and endurance exercise training intervention, where hypertrophy is not expected, do lead to higher eEF2 Thr-56 phosphorylation levels (55). The higher eEF2 Thr-56 phosphorylation observed at rest after the training intervention is expected to blunt the overall muscle protein synthesis (49) and does not indicate hypertrophy. In support, mean or peak power output during the initial sprint was not changed with the training intervention (23). Thus, it is unclear whether the intervention did lead to mTORC1-induced muscle hypertrophy, and further studies are warranted to examine whether high-intensity exercise training can lead to hypertrophy in already endurance-trained individuals.
In summary, 7 wk of high-intensity training with reduced training volume in endurance-trained cyclists increased FXYD1 expression and FXYD1 phosphorylation levels and may have caused the improved K+ reuptake during the intense repeated exercise, thus, possibly contributing to the improved performance. Furthermore, the intense training intervention induced adaptations in CaMKII and PLN expression as well as CaMKII phosphorylation that may improve intracellular Ca2+ handling during exercise, which may potentially contribute to the improved performance.
Perspectives and Significance
The present study showed that high-intensity exercise training in combination with a reduced training volume can induce significant adaptations in already endurance-trained cyclists. It also demonstrated that it is important to examine changes in muscle protein phosphorylation and signaling during acute exercise before and after a training intervention. Higher FXYD1 expression and phosphorylation, as well as CaMKII signaling, may have elevated K+ reuptake (23), via increased NaK pump activity (13, 27, 34, 35), and improved Ca2+ handling (26, 47, 48), respectively, but these effects need to be examined, and possible links to improved excitation-contraction coupling should be investigated. Further studies are also warranted to clarify the effects of high-intensity exercise training with reduced training volume on muscle hypertrophy and the signaling mechanisms regulating protein synthesis.
GRANTS
The study was supported by grants from the Danish Ministry of Culture, Team Danmark and the British Heart Foundation (to M. J. Shattock: RG/12/4/29426).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.T., T.P.G., P.M.C., D.P., M.J.S., and J.B. conception and design of research; M.T., T.P.G., P.M.C., D.P., M.J.S., and J.B. performed experiments; M.T., T.P.G., and P.M.C. analyzed data; M.T., T.P.G., P.M.C., D.P., M.J.S., and J.B. interpreted results of experiments; M.T. and J.B. prepared figures; M.T. and J.B. drafted manuscript; M.T., T.P.G., P.M.C., D.P., M.J.S., and J.B. edited and revised manuscript; M.T., T.P.G., P.M.C., D.P., M.J.S., and J.B. approved final version of manuscript.
REFERENCES
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Apro W, Wang L, Ponten M, Blomstrand E, Sahlin K. Resistance exercise induced mTORC1 signaling is not impaired by subsequent endurance exercise in human skeletal muscle. Am J Physiol Endocrinol Metab 305: E22–E32, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Aughey RJ, Murphy KT, Clark SA, Garnham AP, Snow RJ, Cameron-Smith D, Hawley JA, McKenna MJ. Muscle Na+,K+ATPase activity and isoform adaptations to intense interval exercise and training in well-trained athletes. J Appl Physiol 103: 39–47, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Benziane B, Burton TJ, Scanlan B, Galuska D, Canny BJ, Chibalin AV, Zierath JR, Stepto NK. Divergent cell signaling after short-term intensified endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 295: E1427–E1438, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Benziane B, Widegren U, Pirkmajer S, Henriksson J, Stepto NK, Chibalin AV. Effect of exercise and training on phospholemman phosphorylation in human skeletal muscle. Am J Physiol Endocrinol Metab 301: E456–E466, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bergstrom J. Muscle electrolytes in man. Scan J Clin Lab Invest 68: 1–110, 1962. [Google Scholar]
- 7.Bibert S, Roy S, Schaer D, Horisberger JD, Geering K. Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K-ATPase isozymes. J Biol Chem 283: 476–486, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Christensen PM, Gunnarsson TP, Thomassen M, Wilkerson DP, Nielsen JJ, Bangsbo J. Unchanged content of oxidative enzymes in fast-twitch muscle fibers and V̇o2 kinetics after intensified training in trained cyclists. Physiol Rep 3: pii: e12428, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83: 1269–1324, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Clausen T. Quantification of Na+,K+ pumps and their transport rate in skeletal muscle: functional significance. J Gen Physiol 142: 327–345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, Phillips SM, Hawley JA. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol 111: 1473–1483, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 20: 190–192, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Crambert G, Fuzesi M, Garty H, Karlish S, Geering K. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc Natl Acad Sci USA 99: 11,476–11,481, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshmukh AS, Treebak JT, Long YC, Viollet B, Wojtaszewski JF, Zierath JR. Role of adenosine 5'-monophosphate-activated protein kinase subunits in skeletal muscle mammalian target of rapamycin signaling. Mol Endocrinol 22: 1105–1112, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O'Gorman DJ. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol 588: 1779–1790, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Armouche A, Wittkopper K, Fuller W, Howie J, Shattock MJ, Pavlovic D. Phospholemman-dependent regulation of the cardiac Na/K-ATPase activity is modulated by inhibitor-1 sensitive type-1 phosphatase. FASEB J 25: 4467–4475, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5'-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 286: E411–E417, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Fuller W, Howie J, McLatchie LM, Weber RJ, Hastie CJ, Burness K, Pavlovic D, Shattock MJ. FXYD1 phosphorylation in vitro and in adult rat cardiac myocytes: threonine 69 is a novel substrate for protein kinase C. Am J Physiol Cell Physiol 296: C1346–C1355, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fyfe JJ, Bishop DJ, Stepto NK. Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med 44: 743–762, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Gejl KD, Hvid LG, Frandsen U, Jensen K, Sahlin K, Ortenblad N. Muscle glycogen content modifies SR Ca2+ release rate in elite endurance athletes. Med Sci Sports Exerc 46: 496–505, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol (1985) 106: 929–934, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Goodman CA. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev Physiol Biochem Pharmacol 166: 43–95, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Gunnarsson TP, Christensen PM, Thomassen M, Nielsen LR, Bangsbo J. Effect of intensified training on muscle ion kinetics, fatigue development, and repeated short-term performance in endurance-trained cyclists. Am J Physiol Regul Integr Comp Physiol 305: R811–R821, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Hallen J, Gullestad L, Sejersted OM. K+ shifts of skeletal muscle during stepwise bicycle exercise with and without β-adrenoceptor blockade. J Physiol 477: 149–159, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iaia FM, Thomassen M, Kolding H, Gunnarsson T, Wendell J, Rostgaard T, Nordsborg N, Krustrup P, Nybo L, Hellsten Y, Bangsbo J. Reduced volume but increased training intensity elevates muscle Na+-K+ pump α1-subunit and NHE1 expression as well as short-term work capacity in humans. Am J Physiol Regul Integr Comp Physiol 294: R966–R974, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Inashima S, Matsunaga S, Yasuda T, Wada M. Effect of endurance training and acute exercise on sarcoplasmic reticulum function in rat fast- and slow-twitch skeletal muscles. Eur J Appl Physiol 89: 142–149, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Ingwersen MS, Kristensen M, Pilegaard H, Wojtaszewski JF, Richter EA, Juel C. Na,K-ATPase activity in mouse muscle is regulated by AMPK and PGC-1α. J Membr Biol 242: 1–10, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol 278: R400–R406, 2000. [DOI] [PubMed] [Google Scholar]
- 29.McConell GK, Lee-Young RS, Chen ZP, Stepto NK, Huynh NN, Stephens TJ, Canny BJ, Kemp BE. Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol 568: 665–676, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Physiol 104: 288–295, 2008. [DOI] [PubMed] [Google Scholar]
- 31.McKenna MJ, Schmidt TA, Hargreaves M, Cameron L, Skinner SL, Kjeldsen K. Sprint training increases human skeletal muscle Na+-K+-ATPase concentration and improves K+ regulation. J Appl Physiol 75: 173–180, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen JJ, Mohr M, Klarskov C, Kristensen M, Krustrup P, Juel C, Bangsbo J. Effects of high-intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J Physiol 554: 857–870, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordsborg N, Ovesen J, Thomassen M, Zangenberg M, Jons C, Iaia FM, Nielsen JJ, Bangsbo J. Effect of dexamethasone on skeletal muscle Na+,K+ pump subunit specific expression and K+ homeostasis during exercise in humans. J Physiol 586: 1447–1459, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlovic D, Fuller W, Shattock MJ. The intracellular region of FXYD1 is sufficient to regulate cardiac Na/K ATPase. FASEB J 21: 1539–1546, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Pavlovic D, Fuller W, Shattock MJ. Novel regulation of cardiac Na pump via phospholemman. J Mol Cell Cardiol 61: 83–93, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Pavlovic D, Hall AR, Kennington EJ, Aughton K, Boguslavskyi A, Fuller W, Despa S, Bers DM, Shattock MJ. Nitric oxide regulates cardiac intracellular Na+ and Ca2+ by modulating Na/K ATPase via PKCepsilon and phospholemman-dependent mechanism. J Mol Cell Cardiol 61: 164–171, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J 403: 217–234, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen MK, Juel C, Nordsborg NB. Exercise-induced regulation of muscular Na+-K+ pump, FXYD1, and NHE1 mRNA and protein expression: importance of training status, intensity, and muscle type. Am J Physiol Regul Integr Comp Physiol 300: R1209–R1220, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen MK, Kristensen M, Juel C. Exercise-induced regulation of phospholemman (FXYD1) in rat skeletal muscle: implications for Na+/K+-ATPase activity. Acta Physiol (Oxf) 194: 67–79, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Reis J, Zhang L, Cala S, Jew KN, Mace LC, Chung L, Moore RL, Ng YC. Expression of phospholemman and its association with Na+-K+-ATPase in skeletal muscle: effects of aging and exercise training. J Appl Physiol 99: 1508–1515, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Rembold CM, Ripley ML, Meeks MK, Geddis LM, Kutchai HC, Marassi FM, Cheung JY, Moorman JR. Serine 68 phospholemman phosphorylation during forskolin-induced swine carotid artery relaxation. J Vasc Res 42: 483–491, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds TH, Bodine SC, Lawrence JC Jr. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277: 17,657–17,662, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Richter EA, Vistisen B, Maarbjerg SJ, Sajan M, Farese RV, Kiens B. Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle. J Physiol 560: 909–918, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life 60: 145–153, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JFP, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab 288: E133–E142, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Ronnestad BR, Hansen EA, Raastad T. Effect of heavy strength training on thigh muscle cross-sectional area, performance determinants, and performance in well-trained cyclists. Eur J Appl Physiol 108: 965–975, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Rose AJ, Frosig C, Kiens B, Wojtaszewski JF, Richter EA. Effect of endurance exercise training on Ca2+ calmodulin-dependent protein kinase II expression and signalling in skeletal muscle of humans. J Physiol 583: 785–795, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol 574: 889–903, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose AJ, Alsted TJ, Jensen TE, Kobbero JB, Maarbjerg SJ, Jensen J, Richter EA. A Ca2+-calmodulin-eEF2K-eEF2 signalling cascade, but not AMPK, contributes to the suppression of skeletal muscle protein synthesis during contractions. J Physiol 587: 1547–1563, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silverman BZ, Fuller W, Eaton P, Deng J, Moorman JR, Cheung JY, James AF, Shattock MJ. Serine 68 phosphorylation of phospholemman: acute isoform-specific activation of cardiac Na/K ATPase. Cardiovasc Res 65: 93–103, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Thomassen M, Murphy RM, Bangsbo J. Fibre type-specific change in FXYD1 phosphorylation during acute intense exercise in humans. J Physiol 591: 1523–1533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomassen M, Rose AJ, Jensen TE, Maarbjerg SJ, Bune L, Leitges M, Richter EA, Bangsbo J, Nordsborg NB. Protein kinase Cα activity is important for contraction-induced FXYD1 phosphorylation in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 301: R1808–R1814, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Thomassen M. Regulation of Na+/K+ Pump Activity and Importance of Skeletal Muscle Ion Transporting Proteins for Performance in Humans (PhD thesis). Department of Exercise and Sport Sciences, Faculty of Science, University of Copenhagen, 2010, p. 1–160.
- 54.Thomassen M, Christensen PM, Gunnarsson TP, Nybo L, Bangsbo J. Effect of 2-wk intensified training and inactivity on muscle Na+-K+ pump expression, phospholemman (FXYD1) phosphorylation, and performance in soccer players. J Appl Physiol 108: 898–905, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Van PK, De BK, Hespel P. Training in the fasted state facilitates re-activation of eEF2 activity during recovery from endurance exercise. Eur J Appl Physiol 111: 1297–1305, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys 47: 332–347, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab 284: E813–E822, 2003. [DOI] [PubMed] [Google Scholar]