Abstract
Nitric oxide synthase 1 (NOS1)-derived nitric oxide (NO) production in collecting ducts is critical for maintaining fluid-electrolyte balance. Rat collecting ducts express both the full-length NOS1α and its truncated variant NOS1β, while NOS1β predominates in mouse collecting ducts. We reported that dynamin-2 (DNM2), a protein involved in excising vesicles from the plasma membrane, and NOS1α form a protein-protein interaction that promotes NO production in rat collecting ducts. NOS1β was found to be highly expressed in human renal cortical/medullary samples; hence, we tested the hypothesis that DNM2 is a positive regulator of NOS1β-derived NO production. COS7 and mouse inner medullary collecting duct-3 (mIMCD3) cells were transfected with NOS1β and/or DNM2. Coimmunoprecipitation experiments show that NOS1β and DNM2 formed a protein-protein interaction. DNM2 overexpression decreased nitrite production (index of NO) in both COS7 and mIMCD-3 cells by 50–75%. mIMCD-3 cells treated with a panel of dynamin inhibitors or DNM2 siRNA displayed increased nitrite production. To elucidate the physiological significance of IMCD DNM2/NOS1β regulation in vivo, flox control and CDNOS1 knockout mice were placed on a high-salt diet, and freshly isolated IMCDs were treated acutely with a dynamin inhibitor. Dynamin inhibition increased nitrite production by IMCDs from flox mice. This response was blunted (but not abolished) in collecting duct-specific NOS1 knockout mice, suggesting that DNM2 also negatively regulates NOS3 in the mouse IMCD. We conclude that DNM2 is a novel negative regulator of NO production in mouse collecting ducts. We propose that DNM2 acts as a “break” to prevent excess or potentially toxic NO levels under high-salt conditions.
Keywords: collecting duct, nitric oxide synthase 1 splice variant, nitric oxide, dynamin-2, human
in the collecting duct, a number of paracrine, autocrine, and endocrine factors are known to balance sodium and water intake with excretion to maintain homeostasis. The nitric oxide (NO) pathway functions as a natriuretic and diuretic factor. NO is produced by the NO synthase (NOS) family of enzymes and expressed throughout the kidney with the inner medullary collecting ducts (IMCD) having the greatest NOS activity (33). NOS1 (neuronal NOS, or nNOS) is highly expressed in both mouse and rat collecting ducts (12, 28, 29, 33). NOS3 (endothelial NOS, or eNOS) is also expressed in the IMCD, but at much lower levels than NOS1 (12, 28, 29, 33). NOS1 is also expressed as alternative splice variants; NOS1α is the 155-kDa full-length variant with a PDZ domain, while NOS1β and NOS1γ are NH2-terminal truncated variants without a PDZ domain (4, 7). We recently reported that in the rat IMCD, both NOS1α and NOS1β are expressed, yet in the mouse, NOS1β is predominantly expressed (12, 16). In humans, NOS1α expression has been described for the renal cortex (18), although an evaluation of all the NOS1 splice variants in the human kidney has not been completed.
NOS activity and NO production are regulated by a variety of pathways. For example, NOS undergoes a multitude of posttranslational modifications and/or protein-protein interactions that can either inhibit or stimulate NOS activity and NO production (9). Recently, it has been reported that the protein enabling excision of vesicles from the plasma membrane or “pinchase”, dynamin-2 (DNM2), interacts with the reductase domain of NOS1 (sequence is identical in all NOS1 variants) and leads to a significant increase in rat collecting duct NO production (15). However, it is unknown whether DNM2 also interacts with NOS1β and what effect this may have on NO production.
In the present study, we first elucidated that NOS1β is predominantly expressed in the human kidney, indicating that the human kidney is more similar to the mouse kidney than the rat kidney with respect to NOS1 expression. We next designed studies to test the hypothesis that DNM2 interacts with NOS1β, leading to an increase in NO production. However, as opposed to our initial hypothesis, the findings indicate that DNM2 acts as a negative regulator of NOS1β. Thus, we further designed studies to test the hypothesis that DNM2 is a negative regulator of collecting duct NOS1β in vivo under high-salt conditions using the novel mouse model with collecting duct-specific NOS1 knockout (CDNOS1KO).
METHODS
Human kidney samples.
Human kidney lysate samples were purchased from Origene (Rockville, MD). Origene collected these samples from U.S. institutions under strict Institutional Review Board and ethical consent practices. The samples contained 40–80% medulla and were from nontumor, normal renal structures, as determined by a board-certified pathologist. The samples were homogenized by Origene in modified RIPA buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP40, and 0.25% deoxycholate) plus protease inhibitor cocktail (P2714; Sigma, St. Louis, MO), 0.4 mM PefaBlock SC Plus (Roche 11873601001; Roche, Minneapolis, MN), and PhosStop phosphatase inhibitor (Roche 04906845001). They were then centrifuged to remove nonsoluble material and stored at −80°C until purchased. The samples included 3 females and 2 males, of whom there was one African-American, two Caucasians, and 2 patients with unrecorded race. Their ages ranged from 44 to 79 yr. Lysate (10 μg) was separated on an 8% SDS-PAGE gel, as previously described with polyclonal COOH-terminal NOS1-specific antibody (R20, sc-648; Santa Cruz Biotechnology, Santa Cruz, CA) that detects all NOS1 variants (12, 15, 16).
Cell culture.
COS7 and mIMCD-3 cells were purchased from American Type Culture Collection (Manassas, VA) and were cultured as previously described (15). Only passages 4–6 were used. All experiments were completed in triplicate and replicated on at least three different days (n = 3 unless noted differently).
Transfection studies.
Rat NOS1β in pcDNA 3.1 was purchased from Origene, and the DNM2-GFP or NOS1α constructs used were previously described (15). For coimmunoprecipitation studies, 5 μg of DNM2-GFP construct or empty vector was transfected in 100-mm dishes of confluent cells at a ratio of 1 μg:8 μl of linear polyethylenimine transfection agent (22). The medium was changed after 24 h, and experiments commenced at 48 h posttransfection.
To determine nitrite production, cells were serum-starved for 3 h, washed twice with Hank's balanced salt solution (HBSS; Mediatech, Manassas, VA), and then incubated in HBSS + 20 U/ml superoxide dismutase + 250 μM l-arginine for 1 h at 37°C at 5% CO2. A subset of cultures was stimulated with 3 μM ionomycin (Sigma) during the hour incubation. HBSS was then snap frozen until analysis for nitrite concentrations by HPLC, as previously described (12, 14, 15). Cells were digested with 20 min of incubation of 0.1 N NaOH, and protein concentrations were determined by Bradford assay (Quickstart, Bio-Rad, Hercules, CA).
Dynamin-2 inhibition and siRNA knockdown.
mIMCD-3 cells were grown in 12-well plates and allowed to reach 100% confluency. Cells were then serum starved for 3 h, at which point they were treated for 30 min with various dynamin inhibitors (ab120468; Abcam, Cambridge, MA) [final concentration of 80 μM (15) dissolved in 0.8% DMSO] in HBSS + 20 U/ml superoxide dismutase + 250 μM l-arginine. This panel also includes negative controls. After 30 min, the HBSS was replaced with fresh HBSS + inhibitors or negative controls for an additional 1 h at 37°C at 5% CO2. Afterward, the HBSS was snap-frozen until analysis of nitrite concentrations by HPLC, the cells were digested with 0.1 N NaOH, and protein concentrations were determined by the Bradford assay.
Mouse DNM2 siRNA (no. SR414809) and scramble control siRNA (no. SR30004) were purchased from Origene. All three DNM2 siRNA were combined for maximal inhibition of DNM2. mIMCD-3 cells were siRNA transfected using Lipofectamine RNAiMax (Life Technologies, Carlsbad, CA) using the manufacturer's reverse transfection protocol. Forty-eight hours after transfection, cell lysates were processed for Western blot analysis to determine knockdown efficiency or nitrite concentration in the cell supernatants. All cells were serum-starved for 3 h before experimentation.
Immunoprecipitation, Western blot, and antibodies.
Immunoprecipitations were performed to detect protein-protein interactions between NOS1β and DNM2, and Western blots were performed as previously described (15). IgG controls were performed with mouse or rabbit IgG (Santa Cruz Biotechnology, Dallas, TX) and DNM2/NOS1β lysates passed over the IgG-conjugated beads. Immunogens for human DNM2 antibody generation were CSPTPQRRPVSSIHPPGRPPA (residues 760–779) and were generated in rabbits by ProSci (Poway, CA). Immunoreactive sera were affinity-purified using antigen cross-linked protein A/G beads. This antigen is 95% homologous to mouse DNM2. Commercially available antibodies included monoclonal and polyclonal anti-GFP (Santa Cruz Biotechnology, Dallas, TX; sc-9996, sc-8334), polyclonal anti-NOS1 (R20, Santa Cruz, sc-648), monoclonal β-actin (A1978; Sigma).
Identification of dynamin-2 domains.
The various domains of mouse dynamin-2 protein (NP_001240822.1) were predicted with the program InterPro (21), and the sequence annotated with the freeware program, GeneDoc (Nicholas KB, Nicholas JR, and Deerfield II, DW unpublished data, available at http://genedoc.software.informer.com).
Mouse studies.
All animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of the University of Alabama at Birmingham. In house, 3–6-mo-old flox control and CDNOS1KO male and female mice were used in the study (12). No statistically significant effects of sex were seen in our study; thus, male and female mice were randomly assigned to the treatment groups. Mice were randomly assigned to either normal-salt (TD.96208, 0.49% NaCl diet, Teklad, Frederick, MD) (Flox mice, n = 24; CDNOS1KO mice, n = 18) or high-salt (TD.92034, 4.0% NaCl diet, Teklad) diet for 7 days (Flox mice, n = 38; CDNOS1KO mice, n = 24). All mice were euthanized with 50 mg/kg ip of FatalPlus, followed by thoracotomy, and the kidneys were immediately excised.
Fresh IMCD were isolated from two mice (four inner medullas) per preparation, as previously described (12), each preparation representing a single sample (n = 1). IMCD pellets were resuspended in HBSS with final concentrations of 250 μM l-arginine and 20 U/ml superoxide dismutase and +/− vehicle (0.8% DMSO) or 80 μM dynasore for 30 min at 37°C while shaking. After 30 min, the IMCDs were pelleted at 300 g for 5 min, and the HBSS was removed and replaced with fresh HBSS + l-arginine and SOD. After 1 h, the cells were pelleted, and the HBSS and IMCDs were separated, snap frozen, and kept at −80°C until analysis for nitrite and protein concentration, respectively. To determine protein concentration, the IMCD pellet was homogenized in 100 μl of lysis buffer (20 mM Tris, 150 mM NaCl, 0.5% Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 μM leupeptin, 2 μM pepstatin A, and 1 mM PMSF, at pH 7.5) with a hand-held disposable pestle system (Fisher Scientific) on ice. The samples were then sonicated with 10 × 1 s bursts on ice. Total protein was determined by Bradford assay. Final sample sizes of independently replicated experiments for each group were as follows: Flox NS: n = 10; Flox HS: n = 10; Flox HS+dynasore: n = 6; CDNOS1KO NS: n = 5; HS: n = 4; and HS+dynasore: n = 6.
A coimmunoprecipitation experiment was performed with the control flox IMCD lysates to determine the putative NOS1β-DNM2 interaction in the IMCD. IMCD protein (200 μg) was passed over anti-DNM2-conjugated protein A/G beads or rabbit IgG protein A/G beads, as previously described (15). Western blot analyses were then performed with anti-NOS1 and anti-DNM2 antibodies, as described above.
In a separate group of mice (n = 4 mice per genotype and diet), the inner medulla from both kidneys was dissected and pooled from flox control and CDNOS1KO mice on NS and HS diets, and homogenized in lysis buffer, centrifuged 5,000 g at 4°C to pellet debris, and used in Western blots to determine DNM2 expression.
Statistical analyses.
For analyses comparing the effect control versus DNM2 overexpression, siRNA knockdown, or inhibitor, an unpaired, two-tailed Student's t-test was performed. To determine whether there was a significant effect of the dynamin inhibitors mitTMAB or octTMAB compared with the negative control on nitrite production or with increasing concentrations of DNM2 siRNA, a one-way ANOVA was performed with Dunnett's post hoc test. To test for the effect of transfection and ionomycin stimulation or in the physiological IMCD nitrite experiments for the effect of genotype or diet, two-way ANOVAs were performed with Sidak-Bonferroni post hoc test (α = 0.05).
RESULTS
Human kidney expresses NOS1β.
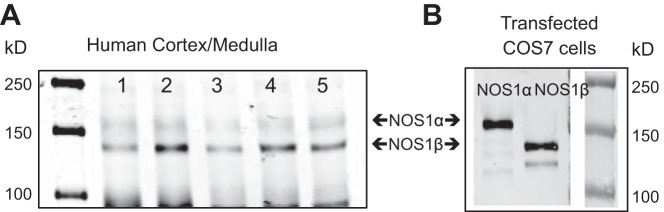
In all 5 human samples of cortex and medulla, we found expression of both NOS1α (155 kDa) and NOS1β (130 kDa) (Fig. 1A). NOS1β was more prominent than the NOS1α, suggesting that NOS1β is the predominant splice variant expressed in these human renal homogenates. As a positive control for specificity, COS7 cells were transfected with either NOS1α or NOS1β (Fig. 1B).
Fig. 1.
Protein expression of NOS1 splice variants in human cortical and medullary homogenates by Western blot analysis. A: five different human samples (see methods for demographic information) express both NOS1α (155 kDa) and NOS1β (130 kDa). B: COS7 cells transfected with either NOS1α or NOS1β as a positive control for the COOH-terminal, NOS1-specific antibody.
NOS1β and DNM2 interact.
COS7 cells were transfected with NOS1β and DNM2-GFP (Fig. 2, A and B) and as depicted in Fig. 2A, results of the coimmunoprecipitation studies indicated that NOS1β and DNM2 formed a protein-protein interaction. Also, in both empty vector transfected mIMCD-3 cells and mIMCD-3 overexpressing DNM2-GFP (Fig. 2, C and D), NOS1β and DNM2 formed a protein-protein interaction (Fig. 2C). An interaction between both the endogenous DNM2 (100 kDa) and transfected DNM2-GFP (130 kDa) with NOS1β was observed (Fig. 2C).
Fig. 2.
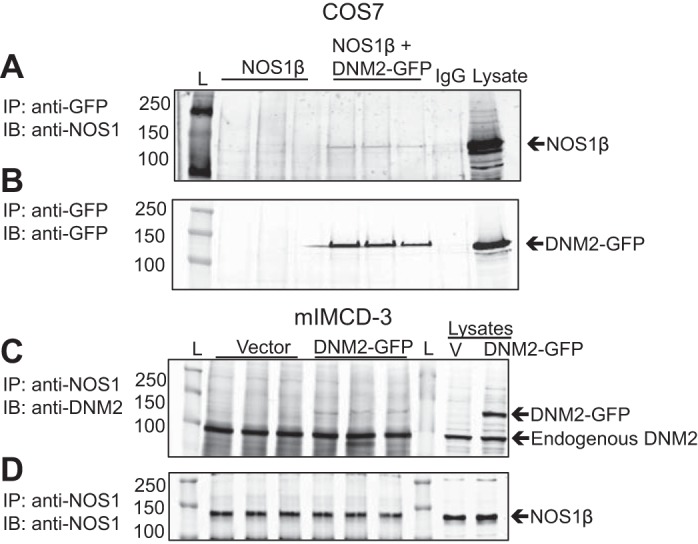
Dynamin-2 (DNM2) and NOS1β interactions in COS7 and mouse inner medullary collecting duct-3 (mIMCD-3) cells. A: COS7 cells were transfected with either NOS1β ± DNM2-GFP. Coimmunoprecipitation experiments were performed by immunoprecipitation (IP) with anti-GFP or mouse IgGs and immunoblot (IB) with anti-NOS1. An interaction is observed in the NOS1β+DNM2 cells only. B: confirmation of the anti-GFP IP with IB with anti-GFP. GFP only expressed in the DNM2-GFP transfected cells. C: mIMCD-3 cells transfected with empty vector or DNM2-GFP. Coimmunoprecipitation experiments were performed by IP with anti-NOS1 and IB with anti-DNM2. NOS1β interacts with both the endogenous DNM2 (100 kDa) and transfected DNM2-GFP (130 kDa), as shown in the bottom panel. D: confirmation of the anti-NOS1 IP with IB with anti-NOS1 (n = 3). L, molecular ladder; V, empty vector transfected cells.
DNM2 inhibits NOS1β-mediated nitrite production.
In aqueous solutions, NO is rapidly oxidized to nitrite (19); hence, nitrite levels were measured as an index of NO production. COS7 cells overexpressing NOS1β produced nitrite (Fig. 3A). When DNM2 was also overexpressed with NOS1β in COS7 cells, there was a significant 60% reduction in the nitrite production (Fig. 3A; P = 0.007). mIMCD-3 cells were also transfected with and without DNM2. Vector-expressing control cells produced 421 ± 38 pmol·mg−1 protein·h−1 of nitrite and overexpression of DNM2 significantly reduced the mIMCD-3 nitrite production to 224 ± 54 pmol·mg protein−1·h−1 (Fig. 3B; P = 0.04). Finally, to determine whether DNM2 also inhibits stimulated nitrite production, COS7 cells expressing either NOS1β or NOS1β+DNM2 were acutely stimulated with ionomycin. Ionomycin led to a significant twofold increase in nitrite production by the NOS1β-transfected cells; however, coexpression with DNM2 prevented the ionomycin-dependent increase in nitrite (Fig. 3C, two-way ANOVA: Ptransfection < 0.001, Pionomycin < 0.001, and Pinteraction < 0.01).
Fig. 3.
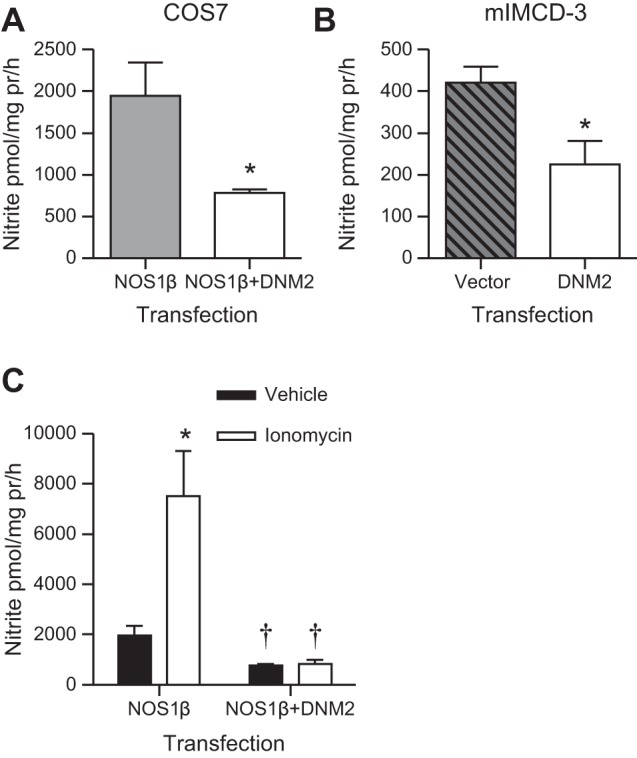
Nitrite production in COS7 and mIMCD-3 cells. A: COS7 cells were transfected with NOS1β ± DNM2 and DNM2 significantly reduced nitrite production (n = 3, *P = 0.007). B: mIMCD-3 cells overexpressing DNM2 produced significantly less nitrite compared with vector control cells (n = 3; *P = 0.04). C: acute stimulation of transfected COS7 cells resulted in a significant increase in nitrite in the NOS1β transfected cells, but no significant effect in the NOS1β+DNM2 cells. *P < 0.05 compared with vehicle. †P < 0.05 compared with corresponding NOS1β vehicle or ionomycin treated cells.
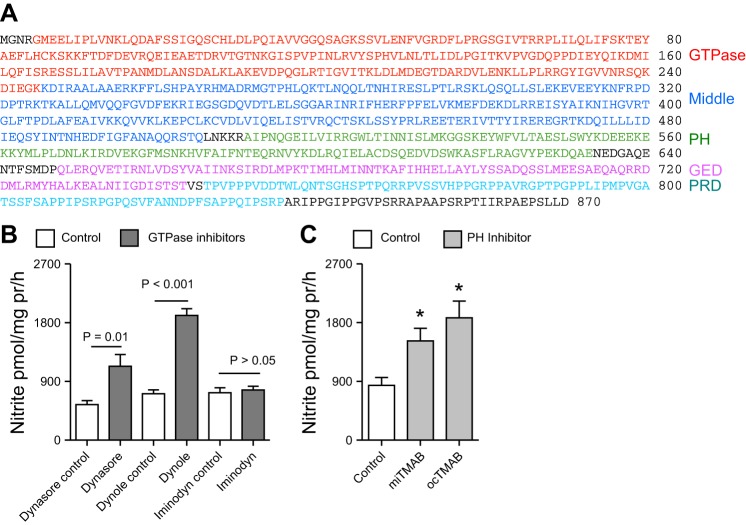
DNM2 inhibition increases nitrite production.
Two strategies were employed to inhibit DNM2. First, we used a pharmacological approach with a panel of DNM2 inhibitors. These inhibitors targeted different domains of DNM2 (Fig. 4A) and have different chemical scaffolds. Dynamin-2 contains a GTPase, middle, plekstrin homology (PH), GTPase effector domain, and proline-rich (PRD) domains. The panel of inhibitors targets the GTPase or PH domains. Compared with their respective negative controls, dyansore and dynole (GTPase domain inhibitors) resulted in a significant increase in mIMCD-3 nitrite production (Fig. 4B, P < 0.01). Among the GTPase domain inhibitors used, only iminodyn failed to significantly alter mIMCD3 nitrite production (Fig. 4B, P > 0.05). Inhibition of the PH domain (miTAMB or ocTAMB treatment) also resulted in a significant increase in mIMCD-3 nitrite production (Fig. 4C, P = 0.01).
Fig. 4.
The domains of DNM2 and pharmacological inhibition of DNM2. A: illustration of the different domains in mouse DNM2. PH, plekstrin homology; GED, GTPase effector domain; PRD, proline-rich domain; B: acute pharmacological blockade of the GTPase domain of DNM2 with a panel of inhibitors, resulted in a significant increase in mIMCD-3 nitrite production, compared with their respective controls. C: acute pharmacological inhibition of the PH domain of DNM2 resulted in a significant increase in mIMCD-3 nitrite production, compared with control (P = 0.01). Two-factor ANOVA with Sidak's multiple (6) comparisons were used. *Significant difference compared with control.
Given that the IMCD also expresses DNM3 (15), siRNA experiments were performed to further confirm the specificity of the inhibition of DNM2. mIMCD-3 cells were transfected with DNM2 or scramble control siRNA, achieving 92 ± 2% knockdown of DNM2 (Fig. 5A). The mIMCD-3 cells with DNM2 knockdown produced significantly more nitrite than scramble control mIMCD-3 cells (Fig. 5B, P < 0.001).
Fig. 5.
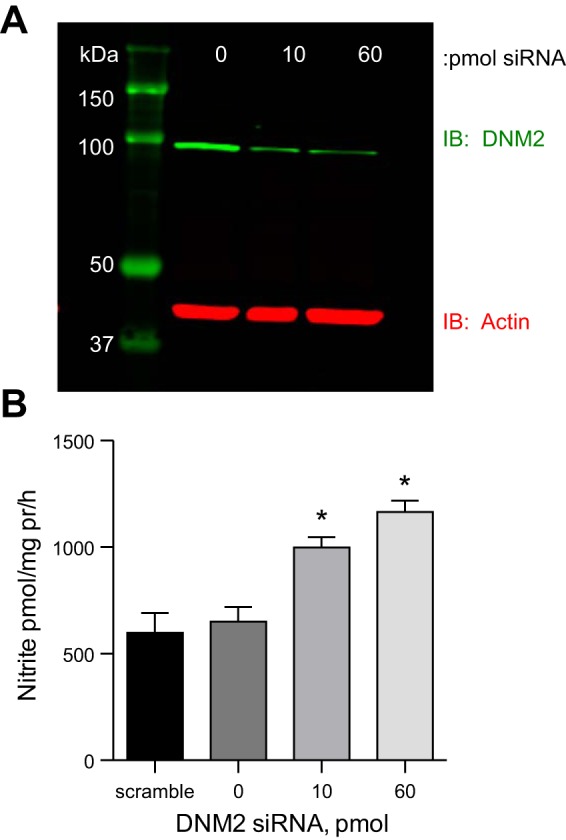
Knockdown of DNM2 by siRNA in mIMCD-3 cells. A: representative image of DNM2 knockdown with 0, 10, or 60 pmol of DNM2 siRNA. B: DNM2 siRNA knockdown results in a significant increase in mIMCD-3 nitrite production compared with scramble siRNA. *P < 0.05 compared with scramble siRNA.
DNM2 and NOS1β in vivo significance.
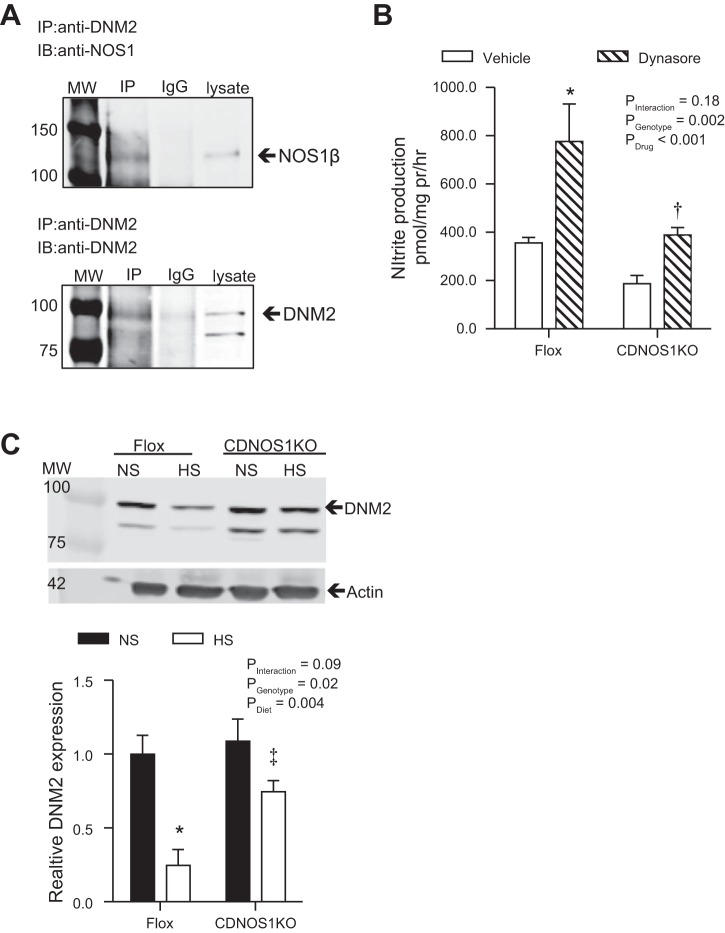
Coimmunoprecipitation studies indicated protein-protein interaction between NOS1β and DNM2 in lysates of freshly isolated mouse IMCD (Fig. 6A). Next, flox control mice and mice that lack NOS1 in the CD (CDNOS1KO) (12) provided normal-salt (NS) or high-salt diets (HS) for 7 days, after which IMCDs were isolated. In accord with our previous finding (16), HS diet led to a significant increase in nitrite production in IMCD from flox control mice (NS = 128.3 ± 3.5 pmol·mg protein−1·h−1; HS = 355.7 ± 22.9 pmol·mg protein−1·h−1; P < 0.001). Furthermore, nitrite production by IMCDs from CDNOS1KO mice on the HS diet exceeded that of the NS diet (NS = 91.1 ± 9.4 pmol·mg protein−1·h−1, HS = 187.5 ± 34.0 pmol·mg protein−1·h−1, P < 0.05); however, it was significantly blunted compared with flox control mice fed the HS diet (Pgenotype < 0.001, Pdiet < 0.001, Pinteraction < 0.01). To further elucidate whether DNM2 regulates NO production in vivo, similar experiments utilized acute inhibition of DNM2 with the GTPase inhibitor dynasore. Acute incubation of IMCD with dynasore significantly increased nitrite production by IMCDs from flox control mice fed a HS diet (Pgenotype < 0.001, Ptreatment < 0.001, Pinteraction = 0.02; Fig. 6B). In contrast, although dynasore tended to increase nitrite production by IMCD from CDNOS1KO mice fed a HS diet, this effect did not achieve statistical significance (P > 0.05) and was blunted compared with the response to dynasore in IMCD from flox (Fig. 6B).
Fig. 6.
NOS1β and DNM2 form a protein-protein interaction in vivo. Freshly isolated IMCD from flox and collecting duct-specific NOS1 knockout (CDNOS1KO) mice on different salt diets for 7 days. A: coimmunoprecipitation (IP) experiments were performed with anti-DNM2 or rabbit IgGs followed by immunoblot (IB) with anti-NOS1 or anti-DNM2 with IMCD from flox control mice on a HS diet. An interaction is observed between NOS1β and DNM2. B: nitrite production from freshly isolated IMCDs from flox and CDNOS1KO mice on a 7-day high-salt diet (n = 4–10; see methods for detailed explanation of sample). Acute 30-min inhibition of DNM2 resulted in a significant increase in nitrite production by flox and tended to increase nitrite production in CDNOS1KO mice (CDNOS1KO vehicle compared with dynasore P > 0.05); however, it was blunted in the CDNOS1KO IMCD. Two-factor ANOVA with Sidak's multiple (6) comparisons were used. *P < 0.05 compared with flox vehicle. †P < 0.05 compared with flox dynasore. C: flox control mice fed a HS diet for 7 days had significantly less inner medullary DNM2 than flox mice on NS diet or CDNOS1KO mice on HS diet (n = 4 mice per group). Two-factor ANOVA with Sidak's multiple (6) comparisons were used. *P < 0.05 compared with flox NS. ‡P < 005 compared with flox HS.
Although we previously reported that HS feeding does not increase IMCD NOS expression (16), further studies examined the impact of HS feeding on DNM2 expression. As shown in Fig. 6C, HS feeding resulted in a significant reduction in inner medullary DNM2 expression (Pgenotype = 0.02, Pdiet < 0.001, Pinteraction = 0.089). Flox control mice on a HS diet presented with a significant 75% reduction in inner medullary DNM2 expression compared with flox control mice fed a NS diet (Fig. 6C). CDNOS1KO mice were used to determine whether CD NOS1 influences the HS-induced decrease in inner medullary DNM2 expression. These studies revealed that CDNOS1KO and flox control mice fed a NS diet displayed similar levels of DNM2 expression. Compared with NS diet, the HS diet did not significantly reduce inner medullary DNM2 expression in CDNOS1KO mice (Fig. 6C); however, DNM2 expression in CDNOS1KO mice on HS diet was significantly greater than that of flox control mice fed the same diet (Fig. 6C).
DISCUSSION
In this study, we aimed to test the hypothesis that DNM2 is a positive regulator of NOS1β, as we previously determined that DNM2 increased NOS1α-derived NO production. However, we found that DNM2 was a negative regulator of NOS1β, such that inhibition or knockdown of DNM2 increased NO production. Moreover, overexpression of DNM2 resulted in a decrease in NO production. Thus, we have identified DNM2 as a novel negative regulator of NOS1β activity.
NOS1 is highly expressed in the IMCD (12, 28, 29, 33), with both NOS1α and NOS1β expressed in the rat, yet mice express predominantly NOS1β in the IMCD (12, 16). In humans, NOS1α has been found in the renal cortex (18); however, this was established using an NH2-terminal antibody that only detects NOS1α. Here, we present evidence, using a COOH-terminal NOS1 antibody that detects all NOS1 variants, that humans express NOS1β. From our five human samples, it appears NOS1β has a greater expression than NOS1α, which suggests that the human kidney is more similar to the mouse kidney than the rat kidney with regard to NOS1 variant expression. Recently, we reported that collecting duct NOS1 is critical for maintaining fluid-electrolyte balance when consuming a HS diet (12). Furthermore, much of the urinary nitrite/nitrate (NOx) is derived from collecting duct NOS1 (12, 31). In both normotensive humans (17) and mice (24, 31), urinary excretion of NOx is increased when fed a high-salt diet. However, urinary NOx is inappropriately low in salt-sensitive hypertensive patients (2, 3, 6, 8, 11), similar to the collecting duct-specific NOS1 knockout mouse that produces inappropriately low urinary NOx and presents with a salt-sensitive blood pressure phenotype (12). Thus, taken together these studies support the utility of the mouse as an appropriate model for humans. Moreover, mouse models are beneficial for testing mechanistic questions about NOS1 activation and regulation of fluid-electrolyte balance and blood pressure control.
Protein-protein interactions lead to regulation of NOS1 activity (34). NOS1α contains a PDZ domain, from which a number of proteins have been determined to interact with NOS1 (34). Recently, our laboratory determined that DNM2 interacts with the reductase domain of NOS1α (15). This novel protein-protein interaction led to a significant increase in NO production. On the contrary, the NOS1β variant lacks the NH2-terminal PDZ domain of NOS1α, but it is otherwise 100% identical in primary sequence with NOS1α. Given that the mouse and human kidney express NOS1β and the reductase domain is conserved in all NOS1 variants, we sought to determine whether DNM2 is also a positive regulator of NOS1β. We confirmed that DNM2 does form a protein-protein interaction with NOS1β; however, using three different approaches, in two different cell lines, we determined that DNM2 inhibits NOS1β-derived NO production. Moreover, increases in intracellular calcium with ionomycin lead to significantly increased NO levels; however, elevated DNM2 levels blocked the calcium-mediated activation of NOS1β. Thus, we have identified a novel NOS1β regulatory pathway.
During high-salt feeding, there are a number of natriuretic and diuretic factors that work to maintain fluid-electrolyte balance. Previously, we demonstrated that collecting duct NOS1 is a modulator in the pressure-natriuresis mechanism and is critical for maintaining fluid-electrolyte balance during HS feeding (12, 13). With a week of HS feeding, the IMCD significantly increases NO production (16), but this increase is significantly blunted in CDNOS1KO mice (Fig. 6). This is also reflected in urinary NO metabolite excretion (NOx), which we previously reported as being significantly blunted in CDNOS1KO mice on a HS diet compared with flox control (12). Thus, IMCD NOS1 and NOS3 contribute to the HS-dependent NO production, although NOS3 is unable to fully compensate for the loss of CD NOS1. NOS2 is not normally expressed in the mouse inner medulla (12). To determine whether DNM2 influences NOS1 and/or NOS3 activity in vivo, we placed flox control and CDNOS1KO mice on a HS diet for 7 days. To further determine whether DNM2 activity as it relates to NOS1-dependent NO production is altered with HS feeding, we acutely inhibited DNM2 activity in IMCDs from flox and CDNOS1KO mice on HS diets with dynasore (20). Acute DNM2 inhibition in isolated IMCDs from mice on a HS diet resulted in a statistically significant increase in NO production in flox mice but not CDNOS1KO mice. However, there was a tendency for DNM2 inhibition to increase IMCD NO production in the CDNOS1KO mouse, suggesting a small contribution of NOS3. This finding is in contrast to the published report that used bovine aortic endothelial cells and purified DNM2 to demonstrate that DNM2 activates NOS3 (5). Our current findings suggest that, in the IMCD, DNM2 acts as an inhibitor of NO production and a negative regulator of NOS1β and, most likely, NOS3. We determined that an HS diet leads to a decrease in inner medullary DNM2 expression in flox control animals, but not in the inner medulla from CDNOS1KO mice. This observation suggests that DNM2 expression is regulated by NOS1 activity during HS feeding, although the mechanism remains to be determined. Taken together, these data suggest that inner medullary DNM2 expression is reduced with HS feeding, and the residual DNM2 activity acts as a break to regulate NO production. We speculate that this process is critical to fine-tune and balance NO production, to match the natriuretic and redox states of the nephron, and to ensure that the excess salt is excreted, while preventing aberrant NO production. Excess NO leads to nitrosative stress, including the formation of peroxynitrite, which can lead to DNA damage, protein nitration, fatty acid nitration, and ultimately cytotoxicity (26, 32). In the endothelium, alterations in DNM2 activity result in increased permeability under hypoxic conditions, which is mediated by a peroxynitrite-dependent mechanism (25).
Dynamin-2 plays a critical role in a variety of cellular processes. The classic function is to regulate endocytosis of plasma membrane proteins, via its scission of budding vesicles (for review, see Ref. 10), although studies suggest DNM2 also regulates Golgi network vesicles, trafficking, and fusion processes (10). Moreover, a novel role of DNM2 regulation of the release of apical vesicular carriers in the apical recycling endosome pathway was recently elucidated (30). Thus, it is evident that DNM2 functions in more than just scission of vesicles from the plasma membrane. Dynamin isoforms contain a PRD that interacts with a variety of proteins. For example, proteins that contain an SH3 domain interact with the DNM2 PRD and result in an increased DNM2 GTPase activity (23). Interestingly, DNM2 also increases enzymatic activity of its binding partner. Previous studies determined that the reductase domain of NOS3 (endothelial NOS) interacts with the PRD domain of DNM2, resulting in an increase in NOS3 activity (5). Furthermore, we reported that the NOS1 reductase domain interacts with DNM2, stimulating NOS1α activity (15). Inhibition of the DNM2 GTPase domain with dynasore resulted in reduced NO production by NOS1α (15). In the current study, we demonstrated that DNM2 interacts with NOS1β and likely NOS3 to inhibit IMCD NO production. Inhibition of either the GTPase or PH domains by a variety of inhibitors led to a stimulation of NOS1 activity. Knockdown of endogenous DNM2 also led to a stimulation of mouse IMCD NO production. From these findings, we conclude that the GTPase and PH domains of DNM2 are involved in NO production by the IMCD.
The mechanism(s) of DNM2 activation of NOS1α and inhibition of NOS1β remain speculative. Cao et al. (5) determined that the PRD domain of DNM2 interacts with the reductase domain of NOS3, resulting in NOS3 activation due to an increase in electron transfer between the FAD and FMN domains of NOS3. We previously determined that the reductase domain of NOS1 (which is 100% conserved between NOS1α and NOS1β) interacts with DNM2; however, the DNM2 domain involved in this interaction remains to be determined. From the current study with NOS1β, it is the GTPase and PH domains of DNM2 that inhibit NO production; thus, we would propose that a reduction in electron transfer occurs. Other possibilities include changes in substrate affinity, including a reduction in calcium/calmodulin binding, BH4, or perhaps even l-arginine. Subcellular localization, posttranslational modifications, and protein-protein interactions are all critical for NOS activity (1). It is well established that NOS3 undergoes multiple posttranslational modifications that can either increase or decrease activity. On the contrary, NOS1 regulation is regulated through protein-protein interactions. Although, purified NOS1β has ∼80% of the activity of NOS1α (4), we know very little about the regulatory pathways of NOS1β. For example, Ser/Thr phosphorylation sites are distinct between NOS3 and NOS1 (1) and may be further distinct between NOS1α and NOS1β. Furthermore, there may be unique protein-protein interactions among DNM2, the NOS isoforms, and splice variants, and other yet to be determined proteins that confer distinct regulation of NOS activity. These findings clearly demonstrate a need for further investigation into the molecular mechanisms involved in the distinct patterns of DNM2 regulation of the NOS isoforms and splice variants.
Perspectives and Significance
In summary, the renal medulla expresses NOS1 splice variants, with mice and human medullas expressing greater levels of the NOS1β variant, as well as rats expressing both NOS1α and NOS1β (16, 27). NOS1α and NOS1β form protein-protein interactions with DNM2 resulting in activation of NOS1α but inhibition of NOS1β. Thus, DNM2 is a novel NOS1-interacting protein that can either activate or inhibit NOS1 depending on the splice variant that is involved in the association. This complexity may be important for regulation of NO production in response to different stimuli. We previously reported that mice and rats regulate medullary NO production in distinct manners; in response to a HS diet, rats increase expression of NOS1β over time, while mice maintain a constant level of NOS1β expression (16). HS feeding leads to a significant increase in mouse IMCD NO production (16), and here, we present evidence that although DNM2 expression is reduced with HS feeding, there is residual DNM2 activity that limits the increase in NO production. We speculate that this may regulate the redox state of the IMCD and prevent the production of toxic levels of NO. In renal diseases, increases in nitrosylation and oxidative stress result in significant renal damage (26, 32). Alternatively, salt-sensitive hypertension is associated with renal NO deficiency (3, 11). Thus, we have identified DNM2 as a new target that warrants further investigation to determine whether deranged DNM2 regulation is causative in renal diseases.
GRANTS
K. A. Hyndman is the recipient of an American Heart Association Postdoctoral Fellowship. A. M. Arguello is the recipient of the University of Evansville Undergraduate Summer Fellowship and UAB PARAdiGM. S. K. H. Morsing is the recipient of the American Physiological Society Undergraduate Summer Research Fellowship. This study was also supported by an National Institutes of Health Program Project Grant on Endothelin Control of Renal Excretory and Hemodynamic Function (P01 HL95499) (to J. S. Pollock).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.A.H. and J.S.P. conception and design of research; K.A.H., A.M.A., and S.K.M. performed experiments; K.A.H., A.M.A., S.K.M., and J.S.P. analyzed data; K.A.H., A.M.A., S.K.M., and J.S.P. interpreted results of experiments; K.A.H. prepared figures; K.A.H. drafted manuscript; K.A.H. and J.S.P. edited and revised manuscript; K.A.H., A.M.A., S.K.M., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Dr. Pamela Carmines for her thorough and expert editorial advice on the manuscript.
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function, and inhibition. Biochem J 357: 593–615, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armas-Padilla MC, Armas-Hernandez MJ, Sosa-Canache B, Cammarata R, Pacheco B, Guerrero J, Carvajal AR, Hernandez-Hernandez R, Israili ZH, Valasco M. Nitric oxide and malondialdehyde in human hypertension. Am J Ther 14: 172–176, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Baumann M, Schmaderer C, Kuznetsova T, Bartholome R, Smits JF, Richart T, Struijker-Boudier H, Staessen JA. Urinary nitric oxide metabolites and individual blood pressure progression to overt hypertension. Eur J Cardiovasc Prev Rehabil 18: 656–663, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Brenman JE, Xia H, Chao DS, Black SM, Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci 19: 224–231, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Cao S, Yao J, Shah V. The proline-rich domain of dynamin-2 is responsible for dynamin-dependent in vitro potentiation of endothelial nitric-oxide synthase activity via selective effects on reductase domain function. J Biol Chem 278: 5894–5901, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Cubeddu LX, Alfieri AB, Hoffmann IS, Jimenez E, Roa CM, Cubeddu R, Palermo C, Baldonedo RM. Nitric oxide and salt sensitivity. Am J Hypertens 13: 973–979, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci USA 94: 3396–3401, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH. Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension 33: 1008–1012, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 837a–837d, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Jamett AM, Momboisse F, Haro-Acuna V, Bevilacqua JA, Caviedes P, Cardenas AM. Dynamin-2 function and dysfunction along the secretory pathway. Front Endocrinol (Lausanne) 4: 126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann IS, Alfieri AB, Cubeddu LX. Effects of lifestyle changes and metformin on salt sensitivity and nitric oxide metabolism in obese salt-sensitive Hispanics. J Hum Hypertens 21: 571–578, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension 62: 91–98, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyndman KA, Bugaj V, Mironova E, Stockand JD, Pollock JS. NOS1-dependent negative feedback regulation of the epithelial sodium channel in the collecting duct. Am J Physiol Renal Physiol 308: F244–F251, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyndman KA, MacDonell AH, Pollock JS. Extracellular signal-regulated kinases 1/2 signaling pathways are not involved in endothelin regulation of mouse inner medullary collecting duct nitric oxide production. Life Sci 91: 578–582, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyndman KA, Musall JB, Xue J, Pollock JS. Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1. Am J Physiol Renal Physiol 301: F118–F124, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyndman KA, Xue J, MacDonell A, Speed JS, Jin C, Pollock JS. Distinct regulation of inner medullary collecting duct nitric oxide production from mice and rats. Clin Exp Pharmacol Physiol 40: 233–239, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imanishi M, Okada N, Konishi Y, Morikawa T, Maeda I, Kitabayashi C, Masada M, Shirahashi N, Wilcox CS, Nishiyama A. Angiotensin II receptor blockade reduces salt sensitivity of blood pressure through restoration of renal nitric oxide synthesis in patients with diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 14: 67–73, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Jarry A, Renaudin K, Denis MG, Robard M, Buffin-Meyer B, Karam G, Buzelin F, Paris H, Laboisse CL, Vallette G. Expression of NOS1 and soluble guanylyl cyclase by human kidney epithelial cells: morphological evidence for an autocrine/paracrine action of nitric oxide. Kidney Int 64: 170–180, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta 1411: 273–289, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhausen T, Macia E, Pelish HE. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol 438: 77–93, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell A, Chang HY, Daugherty L, Fraser M, Hunter S, Lopez R, McAnulla C, McMenamin C, Nuka G, Pesseat S, Sangrador-Vegas A, Scheremetjew M, Rato C, Yong SY, Bateman A, Punta M, Attwood TK, Sigrist CJ, Redaschi N, Rivoire C, Xenarios I, Kahn D, Guyot D, Bork P, Letunic I, Gough J, Oates M, Haft D, Huang H, Natale DA, Wu CH, Orengo C, Sillitoe I, Mi H, Thomas PD, Finn RD. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res 43: D213–D221, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed SE, Staley EM, Mayginnes JP, Pintel DJ, Tullis GE. Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J Virol Methods 138: 85–98, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Scaife RM, Margolis RL. The role of the PH domain and SH3 binding domains in dynamin function. Cell Signal 9: 395–401, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 51: 1605–1610, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seerapu H, Subramaniam GP, Majumder S, Sinha S, Bisana S, Mahajan S, Kolluru GK, Muley A, Siamwala JH, Illavazagan G, Chatterjee S. Inhibition of dynamin-2 confers endothelial barrier dysfunctions by attenuating nitric oxide production. Cell Biol Int 34: 755–761, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan D, Rainville LC, Tyther R, McDonagh B. Redox proteomics in study of kidney-associated hypertension: new insights to old diseases. Antioxid Redox Signal 17: 1560–1570, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Smith C, Merchant M, Fekete A, Nyugen HL, Oh P, Tain YL, Klein JB, Baylis C. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant 24: 1422–1428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan JC, Pardieck JL, Hyndman KA, Pollock JS. Renal NOS activity, expression, and localization in male and female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 298: R61–R69, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan JC, Smart EJ, Pollock DM, Pollock JS. Influence of salt on subcellular localization of nitric oxide synthase activity and expression in the renal inner medulla. Clin Exp Pharmacol Physiol 35: 120–125, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Thuenauer R, Hsu YC, Carvajal-Gonzalez JM, Deborde S, Chuang JZ, Romer W, Sonnleitner A, Rodriguez-Boulan E, Sung CH. Four-dimensional live imaging of apical biosynthetic trafficking reveals a post-Golgi sorting role of apical endosomal intermediates. Proc Natl Acad Sci USA 111: 4127–4132, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, Ecelbarger CM. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci USA 105: 6469–6474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyther R, McDonagh B, Sheehan D. Proteomics in investigation of protein nitration in kidney disease: technical challenges and perspectives from the spontaneously hypertensive rat. Mass Spectrom Rev 30: 121–141, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Wu F, Park F, Cowley AW Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol Renal Physiol 276: F874–F881, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20: 223–230, 2009. [DOI] [PubMed] [Google Scholar]