Abstract
The loss of skeletal muscle mass is observed in many pathophysiological conditions, including aging and obesity. The loss of muscle mass and function with aging is defined as sarcopenia and is characterized by a mismatch between skeletal muscle protein synthesis and breakdown. Characteristic metabolic features of both aging and obesity are increases in intramyocellular lipid (IMCL) content in muscle. IMCL accumulation may play a mechanistic role in the development of anabolic resistance and the progression of muscle atrophy in aging and obesity. In the present study, aged and high-fat fed mice were used to determine mechanisms leading to muscle loss. We hypothesized the accumulation of bioactive lipids in skeletal muscle, such as ceramide or diacylglycerols, leads to insulin resistance with aging and obesity and the inability to activate protein synthesis, contributing to skeletal muscle loss. We report a positive association between bioactive lipid accumulation and the loss of lean mass and muscle strength. Obese and aged animals had significantly higher storage of ceramide and diacylglycerol compared with young. Furthermore, there was an attenuated insulin response in components of the mTOR anabolic signaling pathway. We also observed differential increases in the expression of inflammatory cytokines and the phosphorylation of IκBα with aging and obesity. These data challenge the accepted role of increased inflammation in obesity-induced insulin resistance in skeletal muscle. Furthermore, we have now established IκBα with a novel function in aging-associated muscle loss that may be independent of its previously understood role as an NF-κB inhibitor.
Keywords: aging, insulin, inflammation, sarcopenia, obesity, intramuscular lipids, ceramide, diacylglycerol
the loss of skeletal muscle mass is observed in many pathophysiological conditions, including aging and obesity (2, 23). The loss of muscle mass and function with aging has been defined as sarcopenia and is characterized by a mismatch between skeletal muscle protein synthesis (MPS) and breakdown (21, 23, 35). The concurrence of obesity and sarcopenia, termed sarcopenic obesity, increases the risk of metabolic impairments and physical disability more than just either sarcopenia or obesity alone (4, 35). Sarcopenia and obesity magnify one another as the loss of muscle reduces the mass of available insulin-responsive tissue, promoting insulin resistance, which, in turn, promotes the metabolic syndrome and obesity (4). A characteristic metabolic feature of both aging and obese skeletal muscle is an increase in intramyocellular lipid (IMCL) content (40, 41). Increases in IMCL content may play an important mechanistic role in the development of the muscle's resistance to anabolic stimuli and the progression of sarcopenia with aging and muscle atrophy in obesity (20, 22, 41, 46). In support of this concept, studies performed in cultured skeletal muscle cells have revealed that the accumulation of bioactive lipid metabolites (i.e. ceramide) can inhibit MPS response to anabolic stimuli (26). Furthermore, previous investigations have shown that reducing IMCL content improves the sensitivity of the muscle in obese animals to anabolic stimuli, such as insulin (25). However, the underlying mechanisms by which age- and obesity-related IMCL accumulation diminish insulin-stimulated anabolic signaling have not been identified. Furthermore, while the physiology seen in both aged and obese individuals is similar, the mechanisms leading to muscle atrophy and fat accumulation may be different. Thus, the overall objective of this study will be to examine the role of IMCL accumulation on age and diet-induced changes in skeletal muscle anabolic response to insulin stimulation.
Insulin is a powerful anabolic factor that stimulates MPS through signaling pathways that induce hypertrophy (38). In fact, recent work has shown that both amino acids and insulin are required for anabolic events such as stimulating protein synthesis, inhibiting protein degradation, and activation of translation machinery (6, 36, 37). The mechanistic target of rapamycin (mTOR) serves as a sensor and activator for protein synthesis in response to anabolic stimuli through downstream kinases such as p70S6 kinase (S6K1) or binding proteins like eIF4E-binding protein 1 (4E-BP1), which initiate protein translation (38). Insulin resistance refers to the inability of insulin to induce an appropriate cellular response. Insulin resistance is highly coupled with aging and obesity and results in decreases in glucose uptake, MPS, and the inability to inhibit lipid uptake (38, 46). This occurs because of a blunted activation of important insulin-activated proteins, such as Akt and mTOR. Akt is activated in an insulin-dependent manner and can affect multiple anabolic processes (18). It is hypothesized that increases in intramuscular fat seen in aged and obese states can lead to insulin resistance through ceramide synthesis and lipotoxicity (10, 12, 16, 30).
The chronic proinflammatory state observed with aging and obesity may play a critical role in the decline of muscle mass and function observed in these conditions (23). The master transcription factor NF-κB accompanied by inflammatory cytokines that are its transcriptional targets are reported as being regulated by bioactive lipids, such as ceramide and diacylglycerols (DAG) (23, 45, 53). In its inactive state, NF-κB is sequestered in the cytoplasm by its inhibitor IκBα. Upon degradation of IκBα, NF-κB can enter the nucleus and, once phosphorylated, increase transcription of its target genes (27). Increases in inflammatory pathways can lead to dysregulation of anabolic regulators, such as Akt and mTOR (28, 31). Lipid accumulation in the muscle may also be involved in inflammation through targets like TNF-α and Toll-like receptor 2 (TLR2), and IL-1β (15, 31, 33). Understanding the mechanisms initiating the chronic activation of these proinflammatory pathways could be essential for understanding muscle loss with aging and obesity.
In the present study, we used aged and high-fat-fed mice to determine the mechanisms that lead to muscle loss associated with aging and obesity. Muscle loss can occur due to either decreased MPS or increased MPB, and it is still unclear which plays a greater role in aging and obesity. We hypothesized that the accumulation of intramuscular fat leads to insulin resistance in both the aged and high-fat-fed animals, and this inability to activate protein synthesis in response to insulin may be a contributing factor in skeletal muscle loss.
METHODS
Study Design
Male C57BL/6 mice aged 3 mo (YNG, n = 32) and 21 mo (OLD, n = 14) were purchased from the National Institute on Aging. Mice were housed in a temperature-controlled animal room (21°C) maintained on a 12:12-h light-dark cycle with free access to food and water. All animal experimentation procedures were approved by the Institutional Animal Use and Care Committee of the Jean Mayer USDA Human Nutrition Research Center at Tufts University.
Old mice on the control-fed diet (OLD CFD) were assigned a 10% fat/kcal isocaloric diet (D12450B; Research Diets New Brunswick, NJ), and young mice were randomly assigned to either a 10% fat/kcal isocaloric diet (YNG CFD; D12450B, Research Diets) or a high-fat 60% fat/kcal isocaloric diet (YNG HFD; D12492, Research Diets). After 12 wk of ad libitum feeding, mice were fasted for 5 h and then received either a intraperitoneal injection of 0.85 U/g body wt insulin (INS; Humulin R, Eli Lilly, Indianapolis, IN) or an equivalent volume of saline (VEH). It has been previously reported that there are 10 times higher circulating insulin levels at equivalent doses of insulin (10 mU/g body wt) to the current study, compared with physiological events, such as refeeding (1). Thirty minutes after the administration of INS or VEH, tissue was collected, snap frozen in liquid nitrogen, and stored at −80°C for later analysis.
Fasting Glucose and Lean Mass
Blood glucose was determined after a 5-h fast using the Contour blood glucose monitoring system (Bayer Diabetes Care, Whippany, NJ). Determination of the body composition of each animal was performed using quantitative magnetic resonance EchoMRI-900 whole body composition analyzer (Echo Medical Systems, Houston, TX).
Grip Strength
A Chatillon E-DFE digital force gauge (Ametek, Largo, FL) was used to measure grip strength. Briefly, the forelimb of the mouse was allowed to grasp onto the pull bar assembly. The animal was then gently pulled backward by the tail leading away from the sensor until the grip was released, and maximum force attained was stored on the display. Three trials were performed for each mouse with at least a 10-min resting period between trials.
Lipidomics
Lipidomics was analyzed using a high-performance liquid chromatography/tandem mass spectrometry (LC/MS2), as previously described (41), with modifications. Briefly, lipids were extracted in isopropanol:water:ethylacetate (30:10:60), evaporated under N2 gas stream, and then resuspended in chloroform:methanol (2:1). Separation by HPLC was carried out using a C8 X-Bridge (150 mm) column (Waters, Milford, MA). Levels of individual lipids were quantified using spiked internal standards, including D-erythro-sphingosine-d7 (860657), D-erythro-sphingosine-1-phosphate (860492), N-palmitoyl-D-erythro-sphingosine (860516), N-stearoyl-D-erythro-sphingosine (860518) for sphingolipid analysis and d5-DG internal standard mixture I (LM-6001), and d5-DG internal standard mixture II (LM-6004) for diacylglycerol analysis (Avanti Polar Lipids, Alabaster, AL). Mass spectrometry was carried out in an AB QTRAP 5500 mass spectrometer (Agilent Technologies, Lexington, MA) following the expected fragmentation pattern of each of the lipids under specific conditions.
Cellular Signaling and NF-κB Activity Measures
The phosphorylation and concentration of signaling proteins were quantified via Western blot analysis, as performed previously (42). The gastrocnemius muscle was cut and weighed and then homogenized at 1:10 wt/vol with ice-cold homogenization buffer [50 mM Tris·HCl (pH 7.5), 5 mM Na-pyrophosphate, 50 mM NaF, 1 mM EDTA, 1 mM EGTA, 10% glycerol (vol/vol), 1% Triton X, 1 mM DTT, cOmplete ULTRA (Roche Applied Science, Indianapolis, IN) and PhosSTOP inhibitors (from Roche Applied Science) (1 tablet/10 ml)]. Following centrifugation (10,000 g at 4°C) for 10 min, the supernatant was collected and assayed for protein content. The supernatant was solubilized in Laemmli buffer (10 mM DTT), separated by SDS-PAGE, and transferred to PVDF membranes. The membranes were then blocked (5% nonfat dry milk) and incubated overnight at 4°C with primary antibodies specific for phospho-Akt (9271), Akt (9272), phospho-mTOR (2971), mTOR (2972), phospho-S6K (9205), S6K (9202), phospho-4E-BP1 (2855), phospho-IκBα (9246), IκBα (9242), phospho-NF-κB (3033 and 3039), and NF-κB (8242). Membranes were probed with GAPDH 2118 or histone 3 (4499) to monitor protein loading; all antibodies were from Cell Signaling Technology (Danvers, MA). The immunoreactive proteins were detected with Supersignal chemiluminescent substrate (Thermo Scientific, Rockford, IL), and intensities were quantified by densitometry (Chemi-doc XRS+ system; Bio-Rad, San Leandro, CA) and analyzed, as previously described (39). Nuclear fractionations were carried out using NE-PER nuclear and cytoplasmic extraction kit, as per the manufacturer's instructions (Thermo Scientific), and probed as described previously. NF-κB DNA binding assay was obtained from Abcam (ab133128; Cambridge, MA) and was performed according to the manufacturer's instructions.
mRNA Expression Analysis
RNA was extracted from gastrocnemius muscle using Aurum Total RNA fatty and fibrous tissue pack per manufacturer's instructions (732–6830; Bio-Rad, Hercules, CA). mRNA concentration and purity were determined by spectrophotometry (Nanodrop 1000; Thermo Scientific, Wilmington, DE). cDNA was generated via iScript Reverse Transcription Supermix for RT-qPCR (170–8840; Bio-Rad). cDNA levels were measured using validated primer pairs from PrimerBank for the following genes: Tlr2 (31981333a1), Il1b (6680415a1), Trim63 (21523717a1), Fbxo32 (13385848a1), Capn3 (3661585a1), IL6 (13624311a1), Tnfα (31560799a1), Fbox32 (13385848a1) Rela (6677709a1), and Nfkbia (6754840a1) (51). All reactions were run using a commercially available reaction mixture (iTaq Universal SYBR Green Supermix; Bio-Rad) on a CFX-96 Touch (Bio-Rad). Fold changes in gene expression were normalized to the reference gene β-transducin repeat containing E3 ubiquitin protein ligase (Btrc) (6753210a1).
Statistical Analysis
Lipidomics, gene expression, IκBα ubiquitination, and NF-κB activity.
Differences between groups were identified using a one-way ANOVA with Tukey post hoc test with GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, http://www.graphpad.com). Results are expressed as means ± SE, and statistical significance was accepted at P < 0.05.
Cellular signaling.
Differences between groups were identified using a two-way ANOVA with Bonferroni post hoc test with GraphPad Prism version 5.00 for Windows (GraphPad Software). Results are expressed as means ± SE, and statistical significance was accepted at P < 0.05.
RESULTS
Obese and aged mice have significantly reduced muscle function and mass.
The metabolic, functional, and compositional consequence of obesity and aging were first determined in the groups of mice. Twelve weeks of high-fat feeding increased body weight and fasting blood glucose levels compared to control feeding and aging (Table 1). Obesity and aging are associated with significantly lower percent lean mass compared with lean mice (Table 1). Grip strength in humans has previously been shown to be a potential predictor of future mortality and morbidity and is related to total muscle strength (9, 43, 52). We now show that grip strength is significantly lowered with aging and obesity (Table 1). These data establish that with aging and obesity, there is a significant reduction in muscle mass and function as measured by grip strength.
Table 1.
Whole body characteristics of age and diet groups
| YNG CFD | YNG HFD | OLD CFD | |
|---|---|---|---|
| Body weight, g | 36.2 ± 0.6 | 49.1 ± 0.5* | 34.5 ± 0.6 |
| Fasting blood glucose, mmol/dl | 173.4 ± 8.9 | 353.0 ± 25.3* | 134.6 ± 4.7 |
| % Lean mass, g/BW | 77.5 ± 3.0 | 51.8 ± 1.6* | 74.8 ± 1.1* |
| Grip strength, N/g LM | 8.4 ± 0.9 | 6.3 ± 0.5* | 5.3 ± 0.8* |
BW, body weight; LM, lean mass. CFD, control-fed diet; HFD, high-fat diet; YNG, young, BW, body weight; LM, lean mass.
P < 0.05 vs. YNG CFD; n = 14/group.
Obesity and aging increase the intramuscular storage of sphingolipid and diacylglycerol.
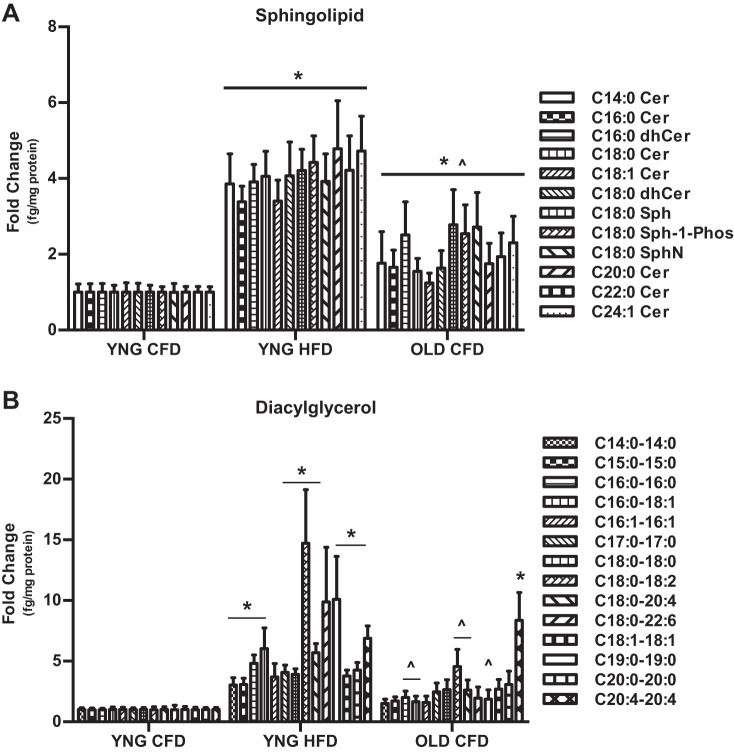
In addition to declines in metabolic rate, obesity and aging are associated with changes to skeletal muscle composition. Using lipidomic analysis, we determined the baseline intramuscular storage of multiple moieties of the bioactive lipids, ceramide (Cer), and diacylglycerol (DAG). Aging significantly increased the intramuscular storage of all 12 Cer species approximately twofold compared with lean animals (Fig. 1A). In obesity, ceramide storage was significantly increased approximately fourfold vs. YNG CFD and approximately twofold vs. OLD CFD (Fig. 1A). The storage of DAG species C14:0–14:0, C15:0–15:0, C16:0–16:0, C16:0–18:1, C17:0–17:0, C18:0–18:0, C18:0–18:2, C18:0–20:4, C18:1–18:1, C19:0–19:0, and C20:0–20:0 was significantly different in obese animals compared with the young lean animals (Fig. 1B), while the DAG species C16:0–16:0, C16:0–18:1, C18:0–18:2, C18:0–20:4, and C18:1–18:1 were significantly different between YNG HFD and OLD CFD animals (Fig. 1B). The only DAG species that was significantly increased in OLD CFD vs. YNG CFD was C20:4–20:4 (Fig. 1B). These data show a significant change in the storage of bioactive lipids in aged and obese skeletal muscle.
Fig. 1.
Lipidomic analysis of skeletal muscle from lean (YNG CFD), obese (YNG HFD), and aged (OLD CFD) C57BL/6 mice. Intramuscular ceramide (Cer), dihydroceramide (dhCer), sphingosine (Sph), sphingosine-1-phosphate (Sph-1-Phos), sphingonine (SphN) (A), and diacylglycerol (B) content were determined in the gastrocnemius via liquid chromatography/tandem mass spectrometry. (*P < 0.05 vs. YNG CFD, ∧P < 0.05 vs. YNG HFD; n = 14/group).
The response of anabolic signaling to insulin is attenuated with high-fat feeding.
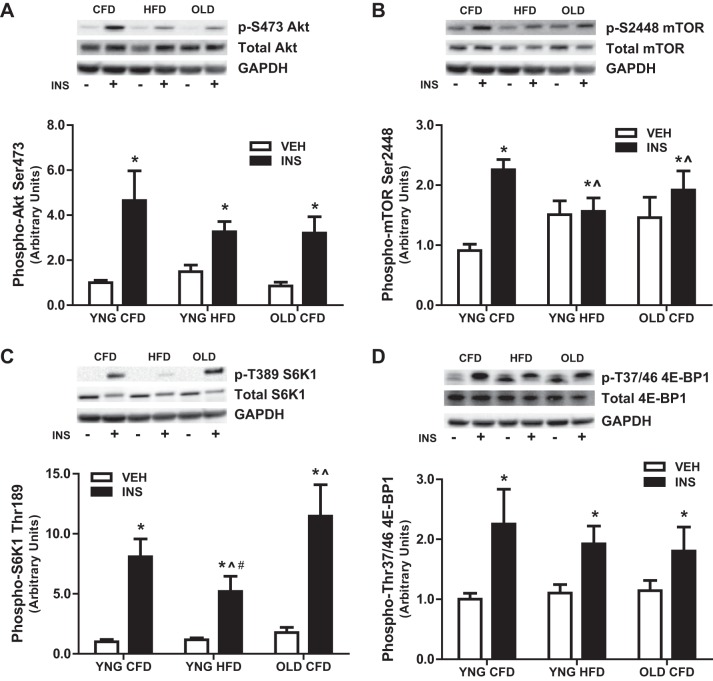
Anabolic resistance is a consequence of both aging and obesity and can precipitate the loss of skeletal muscle mass. Therefore, we determined the response of anabolic signaling in skeletal muscle to a maximal dose of insulin. After insulin stimulation, the phosphorylation of the anabolic regulator Akt was increased similarly in all groups compared with vehicle (Fig. 2A). However, insulin-stimulated activation of the nutrient sensor mTOR was significantly impaired by age and high-fat feeding with a 120% increase mTOR phosphorylation in YNG CFD and only a 23% and 27% increase in YNG HFD and OLD CFD, respectively (Fig. 2B). Downstream of mTOR, the insulin-stimulated phosphorylation of S6K1 was attenuated in obese animals, as seen with an eight-fold increase and eleven-fold increase in lean and aged mice, respectively compared with only a five-fold increase observed with obesity (Fig. 2C). The phosphorylation of 4E-BP1 was similarly increased in all groups after insulin stimulation (Fig. 2D). These data demonstrate anabolic resistance with aging, and these effects are greater with obesity.
Fig. 2.
Anabolic signaling before insulin (VEH) and after insulin (INS) stimulation. Relative protein levels of phosphorylated Akt (A), mTOR (B), S6K1 (C), and 4E-BP1 (D) were quantified using Western blot analysis in skeletal muscle. (*P < 0.05 vs. VEH, ∧P < 0.05 vs. YNG CFD + INS, #P < 0.05 vs. OLD CFD + INS; n = 7–12/group).
Increased IκBα phosphorylation and ubiquitination in aged mice.
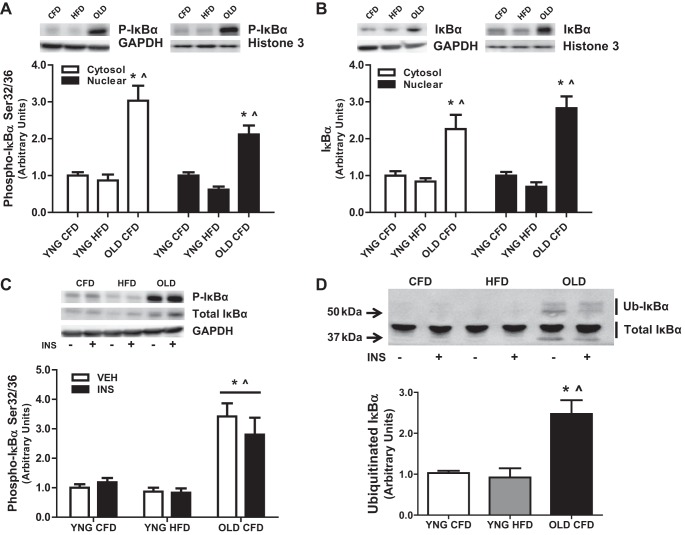
Chronic inflammation accompanies both aging and obesity, an event that may precede the metabolic dysfunction and muscle loss observed during these conditions. We determined the baseline activation of the important transcription factor for inflammatory cytokines NF-κB and its inhibitor IκBα in the skeletal muscles of animals that were fasted 5 h. We observed a significantly higher total protein concentration and phosphorylation of IκBα in both the cytosolic and nuclear fraction of OLD CFD compared with YNG CFD and YNG HFD (Fig. 3, A and B). These results were mirrored in the total fractions showing higher IκBα total protein concentration and phosphorylation in OLD CFD compared with YNG HFD and YNG CFD (Fig. 3C). When determining the ubiquitination of IκBα via mobility shift, we observed significantly higher ubiquitinated IκBα in OLD CFD compared with YNG HFD and YNG CFD (Fig. 3D). These data show a significant activation of IκBα with aging but not obesity compared with control.
Fig. 3.
Phosphorylation, total protein content, and ubiquitination of IκBα in total, cytosolic, and nuclear cell fractions with INS and VEH insulin stimulation in skeletal muscle. Relative protein levels of phospho-IκBα (A), total IκBα in the cytosolic and nuclear fraction (B) and phospho-IκBα (C) and ubiquitinated IκBα (D) in the total fraction were quantified using Western blot analysis. The cytosolic protein GAPDH and the nuclear protein histone 3 were used to determine proper separation. (*P < 0.05 vs. YNG CFD, ∧P < 0.05 vs. YNG HFD; n = 8–14/group).
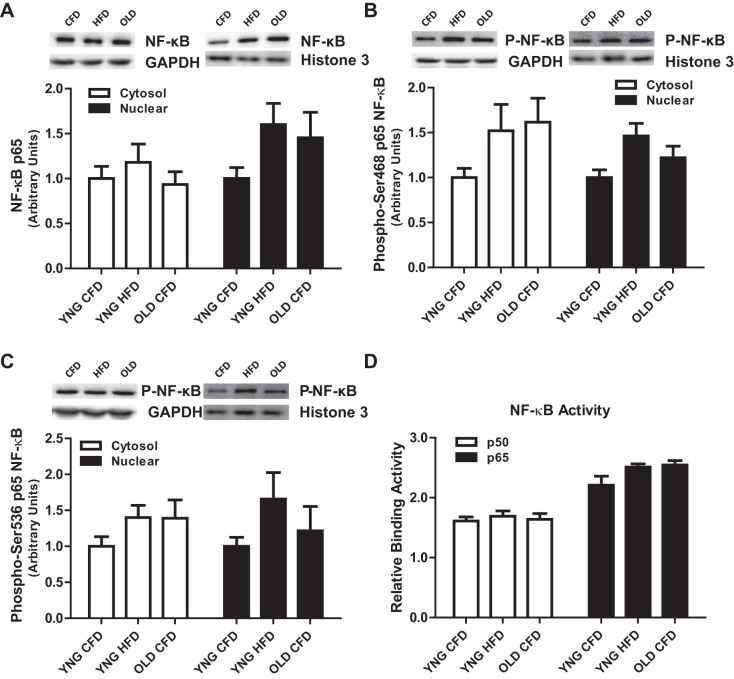
The phosphorylation and subsequent inhibition of IκBα precede the activation of the critical transcription factor NF-κB, conferring the transcription of its multiple target genes. As a result of the significantly higher phosphorylation of IκBα found with aging, we determined the cytosolic and nuclear activation phosphorylation and activation of NF-κB. There were no differences in cytosolic and nuclear, total protein content (Fig. 4A), phosphorylation of NF-κB on its Ser-468 (Fig. 4B) or Ser-536 (Fig. 4C) sites, and the DNA binding activity of NF-κB (Fig. 4D).
Fig. 4.
Phosphorylation, total protein content, and DNA binding activity of NF-κB in skeletal muscle. Relative protein levels of cytosolic and nuclear cell fractions of total NF-κB (A), phospho-Ser-468 NF-κB (B), phospho-Ser-536 NF-κB (C), and NF-κB DNA binding activity (D) in the total fraction were quantified using Western blot analysis. The cytosolic protein GAPDH and the nuclear protein histone 3 were used to determine proper separation. (n = 6–14/group).
Alteration in the expression of NF-κB target genes.
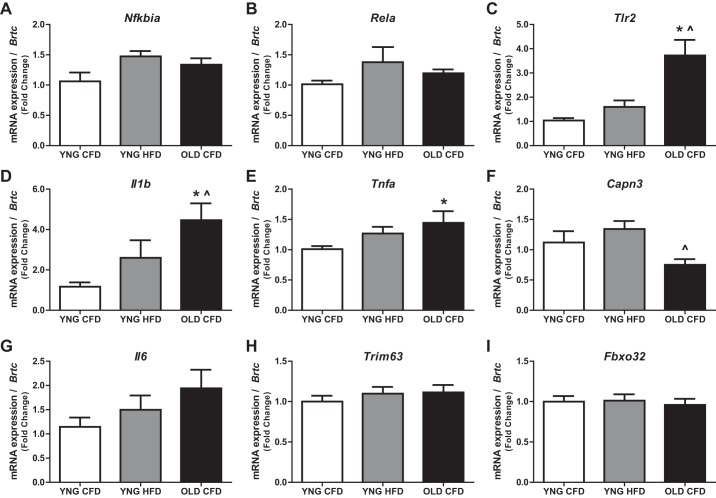
NF-κB is a master transcriptional regulator of genes responsible for immune response, inflammation, cellular growth, apoptosis, and other developmental processes. Therefore, we determined the gene expression of both IκBα (Nfkbia) and NF-κB (Rela), observing no difference between any of the groups (Fig. 5, A and B). We next determined the mRNA expression of NF-κB transcriptional targets and observed a fourfold increased expression of both immune receptor Tlr2 and the cytokine Il1b in OLD CFD compared with both young groups (Fig. 5, C and D), 1.5-fold increased expression of the cytokine Tnfa in OLD CFD vs. YNG CFD (Fig. 5E) and a twofold decreased expression of the protease Capn3 with aging compared with obesity (Fig. 5F), although we observed no difference in the expression of the inflammatory cytokine Il6 (Fig. 5G).
Fig. 5.
mRNA expression of the NF-κB pathway components Nfkiba (A) and Rela (B); its transcriptional targets Tlr2 (C), Il1b (D), Tnfa (E), Il6 (G); and Capn3 (F), and the E3 ligases Trim63 (H) and Fbxo32 (I) were measured via quantitative PCR (*P < 0.05 vs. YNG CFD, P̂ < 0.05 vs. YNG HFD; n = 10/group).
Potential downstream targets of TNF-α previously found to be elevated with chronic inflammation are the E3 ligases MuRF1 (Trim63) and atrogin-1 (Fbxo32) that have a recognized role in muscle atrophy (5). We now report no differences in either of the E3 ligases in any group (Fig. 5, H and I).
DISCUSSION
In the current study, we report that increases in the bioactive lipids ceramide and diacylglycerols are associated with decreases in lean mass and muscle strength in aged and obese animals. Furthermore, there was an attenuated insulin response to components of the mTOR anabolic signaling pathway. Interestingly, we also observed differential increases in the expression of some inflammatory cytokines and the phosphorylation of IκBα between the aged and obese animals with no change in markers of protein degradation, MuRF1, and atrogin-1 in either group. These data provide evidence that the decreases in lean mass and strength observed in these groups may be associated with the increased accumulation of bioactive lipid metabolites and independent of increased inflammation.
In agreement with the current study, we have recently reported an attenuated growth signaling response after anabolic stimulation in older compared with younger men (41). These differences were associated with increases in both ceramide accumulation and NF-κB signaling with aging. In the current study, we determined the accumulation of two major bioactive lipids, ceramide and diacylglycerol, which both have a known inhibitory role in skeletal muscle growth and metabolism (3, 14, 29, 41). We had the ability to detect 12 different sphingolipids, including ceramide, sphingosine, and their respective metabolites dihydroceramide, sphingonine, and sphingosine-1-phosphate. Aging significantly increased the accumulation of sphingolipids in skeletal muscle twofold to fourfold compared with lean mice, while sphingolipid accumulation was higher in obesity compared with both lean and aged mice. Furthermore, we detected 14 different DAG moieties in skeletal muscle, and these were all increased with aging compared with lean mice and were higher with obesity compared with both aged and lean mice. These results are intriguing since ceramide accumulation is required for proinflammatory induction of insulin resistance in skeletal muscle (17, 19, 24, 47), and increased DAG accumulation is predictive of insulin resistance in humans (8).
Chronic inflammation associated with aging has been referred to as “inflammaging” and can have several consequences, such as anabolic resistance and increased catabolic effects (13, 44, 48). Furthermore, increased lipid accumulation with obesity is associated with an increased inflammatory state through interaction with members of Toll-like receptor (TLR) family and the secretion of cytokines, including TNF-α, IL-1β, and IL-6 (32). In the current study, only aged mice showed an increase in the expression of the inflammatory markers TLR2, TNF-α, and IL-1β. When determining the pathway activation of NF-κB, the central inflammatory transcription factor, we similarly observed a higher phosphorylation and ubiquitination of its key inhibitor IκBα with aging, but not in obesity. In spite of these data, the phosphorylation and activity of NF-κB were not different between the groups. However, it has been previously reported that the expression of IκBα is a good indicator of the transactivation potential of NF-κB (11), and, therefore, a limitation of our experiments may be the insensitivity of our NF-κB analysis. Increases in the inflammatory components, such as IκBα, TLR2, IL-1β, and TNF-α were only observed with aging and not obesity. These unobserved changes of inflammation with obesity may be a result of the length of the dietary intervention, since it has been previously reported that 16 wk of high-fat feeding is required to observe increased inflammation in mice, despite the increase in lipid accumulation and the development of insulin resistance (49). Importantly, we now show in this study an increase in the accumulation of ceramide and DAG lipid moieties in obese and aged mice, leading to a lower activation of mTOR signaling in response to insulin. Therefore, it is possible that fat feeding leads to insulin resistance and muscle loss via a different mechanism than in aged mice.
We are, to the best of our knowledge, the first to show an increase in phosphorylation, total protein concentration, and ubiquitination of IκBα in skeletal muscle of aged mice. The reasons and consequences of this increase are unclear and warrant further research, as IκBα may play a key role in the development of sarcopenia. Recently, it has been shown that IκBα may have roles beyond acting as an inhibitor of NF-κB and act as a cotranscription factor affecting the transcription of various histone deacetylases (HDACs) (50). HDACs are well known for their regulation of muscle atrophy through the regulation of histone acetylation, making our result a novel target for future studies (7, 34). There are some limitations and weaknesses associated with this study. A huge superphysiological dose of insulin was used, and this may mask some of the subtle differences in insulin sensitivity, especially between the aged and lean mice. However, because the dose was normalized to body weight, this means that the lack of a response observed in obese mice is a true decrease, because they received a large dose appropriate for their increase in mass.
Perspectives and Significance
We show that both aging and obesity increase the accumulation of critical bioactive lipids in the skeletal muscle, leading to decreases in lean mass and muscular strength and an attenuated anabolic response to insulin in these conditions. Further, we show these effects were associated with inflammation in the skeletal muscle of aged but not obese mice. These data challenge the accepted role of increased inflammation in obesity-induced insulin resistance in skeletal muscle. Furthermore, we have now established IκBα with a novel function in aging-associated skeletal muscle loss that may be independent of its previously understood role as an NF-κB inhibitor.
GRANTS
This material is based on the work supported by the U.S. Department of Agriculture, under agreement 58-1950-0014. The study was also supported by the Boston Claude D. Pepper Center Older American Independence Centers (Grant 1P30AG031679).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.A.R., G.G.D., and R.A.F. conception and design of research; D.A.R., D.J.M., N.P.R., P.H.H., and G.G.D. performed experiments; D.A.R., D.J.M., N.P.R., and P.H.H. analyzed data; D.A.R., D.J.M., and N.P.R. interpreted results of experiments; D.A.R. prepared figures; D.A.R., D.J.M., N.P.R., and R.A.F. drafted manuscript; D.A.R., D.J.M., N.P.R., G.G.D., and R.A.F. edited and revised manuscript; D.A.R., D.J.M., N.P.R., P.H.H., G.G.D., and R.A.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture (USDA). Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
REFERENCES
- 1.Agouni A, Owen C, Czopek A, Mody N, Delibegovic M. In vivo differential effects of fasting, re-feeding, insulin and insulin stimulation time course on insulin signaling pathway components in peripheral tissues. Biochem Biophys Res Commun 401: 104–111, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Akhmedov D, Berdeaux R. The effects of obesity on skeletal muscle regeneration. Front Physiol 4: 371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amati F, Dube JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60: 2588–2597, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton SD, Karabetian C, Naugle K, Buford TW. Obesity and diabetes as accelerators of functional decline: can lifestyle interventions maintain functional status in high risk older adults? Exp Gerontol 48: 888–897, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem 41: 173–186, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Balage M, Sinaud S, Prod'homme M, Dardevet D, Vary TC, Kimball SR, Jefferson LS, Grizard J. Amino acids and insulin are both required to regulate assembly of the eIF4E. eIF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab 281: E565–E574, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Beharry AW, Sandesara PB, Roberts BM, Ferreira LF, Senf SM, Judge AR. HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy. J Cell Sci 127: 1441–1453, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 55: 1140–1150, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Physical Ther 31: 3–10, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Bollheimer LC, Buettner R, Pongratz G, Brunner-Ploss R, Hechtl C, Banas M, Singler K, Hamer OW, Stroszczynski C, Sieber CC, Fellner C. Sarcopenia in the aging high-fat fed rat: a pilot study for modeling sarcopenic obesity in rodents. Biogerontology 13: 609–620, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Bottero V, Imbert V, Frelin C, Formento JL, Peyron JF. Monitoring NF-κB transactivation potential via real-time PCR quantification of I κB-α gene expression. Mol Diagn 7: 187–194, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Campbell TL, Mitchell AS, McMillan EM, Bloemberg D, Pavlov D, Messa I, Mielke JG, Quadrilatero J. High-fat feeding does not induce an autophagic or apoptotic phenotype in female rat skeletal muscle. Exp Biol Med (Maywood) 240: 657–668, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chazaud B, Mouchiroud G. Inflamm-aging: STAT3 signaling pushes muscle stem cells off balance. Cell Stem Cell 15: 401–402, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Coen PM, Hames KC, Leachman EM, DeLany JP, Ritov VB, Menshikova EV, Dube JJ, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severe obesity. Obesity (Silver Spring) 21: 2362–2371, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 300: E145–E154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Larichaudy J, Zufferli A, Serra F, Isidori AM, Naro F, Dessalle K, Desgeorges M, Piraud M, Cheillan D, Vidal H, Lefai E, Nemoz G. TNF-α- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skel Muscle 2: 2, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demarchi F, Bertoli C, Greer PA, Schneider C. Ceramide triggers an NF-κB-dependent survival pathway through calpain. Cell Death Differ 12: 512–522, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol 103: 378–387, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Gamard CJ, Dbaibo GS, Liu B, Obeid LM, Hannun YA. Selective involvement of ceramide in cytokine-induced apoptosis. Ceramide inhibits phorbol ester activation of nuclear factor κB. J Biol Chem 272: 16,474–16,481, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol 90: 2157–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Gordon BS, Kelleher AR, Kimball SR. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol 45: 2147–2157, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gueugneau M, Coudy-Gandilhon C, Theron L, Meunier B, Barboiron C, Combaret L, Taillandier D, Polge C, Attaix D, Picard B, Verney J, Roche F, Feasson L, Barthelemy JC, Bechet D. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J Gerontol Ser A Biol Sci Med Sci 70: 566–576, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Haran PH, Rivas DA, Fielding RA. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia Sarcopenia Muscle 3: 157–162, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121: 1858–1870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167–179, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19: 461–463, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Jackman RW, Cornwell EW, Wu CL, Kandarian SC. Nuclear factor-κB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol 98: 19–24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legrand D, Adriaensen W, Vaes B, Mathei C, Wallemacq P, Degryse J. The relationship between grip strength and muscle mass (MM), inflammatory biomarkers and physical performance in community-dwelling very old persons. Arch Gerontol Geriatr 57: 345–351, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Lessard SJ, Lo Giudice SL, Lau W, Reid JJ, Turner N, Febbraio MA, Hawley JA, Watt MJ. Rosiglitazone enhances glucose tolerance by mechanisms other than reduction of fatty acid accumulation within skeletal muscle. Endocrinology 145: 5665–5670, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB 3rd, Hawley JA. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes 56: 1856–1864, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Liang H, Tantiwong P, Sriwijitkamol A, Shanmugasundaram K, Mohan S, Espinoza S, Defronzo RA, Dube JJ, Musi N. Effect of a sustained reduction in plasma free fatty acid concentration on insulin signalling and inflammation in skeletal muscle from human subjects. J Physiol 591: 2897–2909, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins AR, Nachbar RT, Gorjao R, Vinolo MA, Festuccia WT, Lambertucci RH, Cury-Boaventura MF, Silveira LR, Curi R, Hirabara SM. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis 11: 30, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michot C, Mamoune A, Vamecq J, Viou MT, Hsieh LS, Testet E, Laine J, Hubert L, Dessein AF, Fontaine M, Ottolenghi C, Fouillen L, Nadra K, Blanc E, Bastin J, Candon S, Pende M, Munnich A, Smahi A, Djouadi F, Carman GM, Romero N, de Keyzer Y, de Lonlay P. Combination of lipid metabolism alterations and their sensitivity to inflammatory cytokines in human lipin-1-deficient myoblasts. Biochim Biophys Acta 1832: 2103–2114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minetti GC, Feige JN, Bombard F, Heier A, Morvan F, Nurnberg B, Leiss V, Birnbaumer L, Glass DJ, Fornaro M. Galphai2 signaling is required for skeletal muscle growth, regeneration, and satellite cell proliferation and differentiation. Mol Cell Biol 34: 619–630, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson MI, Dobson JP, Greene NP, Wiggs MP, Shimkus KL, Wudeck EV, Davis AR, Laureano ML, Fluckey JD. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J 27: 3905–3916, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans 34: 213–216, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Proud CG, Denton RM. Molecular mechanisms for the control of translation by insulin. Biochem J 328: 329–341, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivas DA, Lessard SJ, Coffey VG. mTOR function in skeletal muscle: a focal point for overnutrition and exercise. Appl Physiol Nutr Metab 34: 807–816, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, Parnell LD, Fielding RA. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J 28: 4133–4147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivas DA, Morris EP, Fielding RA. Lipogenic regulators are elevated with age and chronic overload in rat skeletal muscle. Acta Physiol (Oxf) 202: 691–701, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivas DA, Morris EP, Haran PH, Pasha EP, Morais Mda S, Dolnikowski GG, Phillips EM, Fielding RA. Increased ceramide content and NF-κB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J Appl Physiol 113: 1727–1736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivas DA, Yaspelkis BB 3rd Hawley JA, Lessard SJ. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside. J Endocrinol 202: 441–451, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanderson WC, Scherbov S. Measuring the speed of aging across population subgroups. PloS One 9: e96289, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schakman O, Dehoux M, Bouchuari S, Delaere S, Lause P, Decroly N, Shoelson SE, Thissen JP. Role of IGF-I and the TNFα/NF-κB pathway in the induction of muscle atrogenes by acute inflammation. Am J Physiol Endocrinol Metab 303: E729–E739, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silveira LR, Fiamoncini J, Hirabara SM, Procopio J, Cambiaghi TD, Pinheiro CH, Lopes LR, Curi R. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol 217: 1–12, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Stephens FB, Chee C, Wall BT, Murton AJ, Shannon CE, van Loon LJ, Tsintzas K. Lipid induced insulin resistance is associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men. Diabetes 64: 1615–1620, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology 145: 4592–4602, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L, Sacco A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med 20: 1182–1186, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Viatour P, Legrand-Poels S, van Lint C, Warnier M, Merville MP, Gielen J, Piette J, Bours V, Chariot A. Cytoplasmic IκBα increases NF-κB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J Biol Chem 278: 46,541–46,548, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res 31: e154, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wind AE, Takken T, Helders PJ, Engelbert RH. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr 169: 281–287, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Wu CL, Cornwell EW, Jackman RW, Kandarian SC. NF-κB but not FoxO sites in the MuRF1 promoter are required for transcriptional activation in disuse muscle atrophy. Am J Physiol Cell Physiol 306: C762–C767, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]