Abstract
Immaturity of respiratory controllers in preterm infants dispose to recurrent apnea and oxygen deprivation. Accompanying reductions in brain oxygen tensions evoke respiratory depression, potentially exacerbating hypoxemia. Central respiratory depression during moderate hypoxia is revealed in the ventilatory decline following initial augmentation. This study determined whether the thalamic parafascicular nuclear (Pf) complex involved in adult nociception and sensorimotor regulation (Bentivoglio M, Balerecia G, Kruger L. Prog Brain Res 87: 53–80, 1991) also becomes a postnatal controller of hypoxic ventilatory decline. Respiratory responses to moderate isocapnic hypoxia were studied in conscious lambs. Hypoxic ventilatory decline was compared with peak augmentation. Pf and/or adjacent thalamic structures were destroyed by the neuron-specific toxin ibotenic acid (IB). IB lesions involving the thalamic Pf abolished hypoxic ventilatory decline. Lesions of adjacent thalamic nuclei that spared Pf and control injections of vehicle failed to blunt hypoxic respiratory depression. Our findings reveal that the thalamic Pf region is a critical controller of hypoxic ventilatory depression and thus a key target for exploring molecular concomitants of forebrain pathways regulating hypoxic ventilatory depression in early development.
Keywords: brain, biphasic ventilation, hypoxia, respiratory inhibition, thalamus, sheep
the critical transition from fetus to newborn depends upon timely transfer of respiratory gas exchange from the placenta to lung and an essential change in respiratory control. In contrast to postnatal respiration, fetal breathing involves fluid-filled lungs, minimal pulmonary expansion, prolonged apneas, modulation by glycemia, independence of normal fluctuations in respiratory gases, expression limited to specific behavioral states, and inhibition by moderate hypoxia (47). Thus, parturitional changes critical for survival involve the onset of continuous breathing and hypoxic hyperpnea. Pioneering studies at Cambridge (5) and Oxford (20, 21, 39) revealed the uniqueness of the control of respiratory muscles in fetal sheep. The ovine model had considerable advantages in resistance to surgery-induced labor, and the large fetus facilitated instrumentation and measurement of blood gases and pH. This work and subsequent discoveries in sheep (e.g., Refs. 10, 12, 13, 40, 41, 42, 43, 44, 45, 46, 71, 92) were instrumental in establishing the foundation for perinatal medicine.
Immaturity of central respiratory controllers dispose newborns to recurrent apnea and thus O2 deprivation. Respiratory responses to hypoxic stress include a rapid-onset stimulation (augmentation) followed by a gradual decline (depression). Putative O2 sensors in the supramedullary brain stem (9, 52, 58, 81, 82, 90) mediate the depressant effects of hypoxia, an adaptation involved in reducing O2 consumption but also potentially increasing infant vulnerability to acute O2 deprivation. Brain stem sectors implicated in hypoxic depression have been derived from studies largely involving anoxic reduction/brain slice preparations in vitro and small, anesthetized, or decerebrate mammals in vivo (see Refs. 9 and 83). Such studies would reflect neither the magnitude of brain O2 deprivation nor necessarily the executive neuronal network orchestrating hypoxic ventilatory decline in normal conscious mammals.
Among its roles, the thalamic parafascicular nuclear complex (Pf) is a critical regulator of adult nociception and sensorimotor function (8). These features are consistent with a forebrain controller of energy metabolism in oxygen deficiency, although its functional role in development is largely unexplored. Our studies in sheep have identified Pf as being critically involved in hypoxic inhibition of fetal breathing movements (41, 42, 43). Although breathing is regulated quite differently before parturition, the thalamic Pf of newborn lambs has a moderate density of adenosine (ADO) A2A receptors that mediate hypoxic ventilatory decline (44, 95). These features are consistent with involvement of Pf in transduction mechanisms linking modest reductions in brain Po2 to hypoxic ventilatory depression and thus would account for Pf as a critical controller of O2 homeostasis in the newborn. Experiments in this study were principally designed to examine the effects of neuronal destruction within the thalamic Pf sector on quantitative changes of hypoxic hyperpnea in unanesthetized awake lambs.
The findings indicate that thalamic Pf constitutes a forebrain controller of hypoxic ventilatory depression. The critical involvement of ADO A2A receptors in hypoxic respiratory inhibition (40, 44, 95) and recent identification of metabolic components reflecting changes in activity of Pf neurons (4, 33, 51, 63, 75) suggest that the metabolic profile of Pf neurons determines the extent of hypoxic ventilatory decline and enable future studies with powerful new tools to explore the molecular intermediates and pathways involved in activating Pf neurons (40).
MATERIALS AND METHODS
All surgical and experimental procedures were carried out in accordance with the guidelines of the American Physiological Society and approved by the University of California Los Angeles (UCLA) Chancellor's Animal Research Committee and the Institutional Animal Care and Use Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Surgery
Thirteen lambs up to 8 days of age underwent aseptic surgery under general anesthesia (10 mg/kg im ketamine, 0.06 mg/kg im atropine, and 1–3% isoflurane in O2). After the hair was clipped, the skin was scrubbed with betadine followed by an alcohol rinse. A penicillin G, procaine penicillin, and benzathine penicillin mixture (Combi-Pen 48, 10,000 U/kg) was administered intramuscularly, and the lamb was placed on a heating pad in the surgical suite and connected to instruments for monitoring the electrocardiogram, cutaneous oxygen saturation, body temperature, and heart rate. Two stainless steel guide cannulas (0.8 mm OD, shaft length 21 mm) were inserted through two symmetrically placed burr holes in parietal bone 3 mm lateral to the sagittal suture and 6–7 mm caudal to the coronal suture. The cannulas were advanced stereotaxically at a 70° angle to the horizontal plane toward Pf or surrounding thalamus. The polycarbonate holders of the guide cannulas were secured to stainless steel screws in parietal bone with dental acrylic and cyanoacrylate cement. A protective plastic dome was placed over the polycarbonate holders. Polyvinyl catheters were inserted in the right external jugular vein and carotid artery. Catheters were passed subcutaneously and exteriorized in a nylon pouch over the right dorsolateral thorax. Postoperatively, the lamb was warmed by a heating lamp and was continuously observed for 1 h until able to remain standing without assistance and transferred back to the ewe to nurse. Buprenorphine (0.005–0.01 mg/kg) was intramuscularly injected after surgery for analgesic control. Injections of penicillin antibiotics (Combi-Pen, 10,000 U/kg im) were administered for the first three postoperative days, and catheter patency was maintained by daily injections of heparinized saline (4 U/ml). The lambs were weighed daily and resumed gaining weight by the second postoperative day.
Experimental Design
Physiological measurements.
Conditioning to the respiratory apparatus began at least 2 days after surgery. Biphasic ventilatory responses to isocapnic hypoxia were achieved 7–10 days after surgery. Studies were performed in unanesthetized awake lambs restrained in a sling at ambient temperature (∼22°C). Arterial Po2 (PaO2), Pco2 (PaCO2), and pH were measured (ABL 700; Radiometer, Copenhagen, Denmark), with values corrected to 40°C. Rectal temperature was measured in five lambs by a thermometer probe (Thermalert model TH-8; Physitemp, Clifton, NJ).
Gases (O2, CO2, N2) from mass flowmeters (302 series; Hastings Instruments, Hampton, VA) were mixed by a model 400 Hastings Instruments controller. The humidified gas mixture flowed (18 l/min) through respiratory tubing and a Y-connector to a rubber face mask (Jorgensen Laboratories, Loveland, CO) that was secured with hydrophilic vinyl polysiloxane to the lamb's snout, and end-tidal O2 was monitored by a Vacu-Med (Ventura, CA) O2 analyzer.
Ventilation was measured via a turbine transducer flowmeter (Universal Ventilation Meter; Vacu-Med) attached to the face mask. The analog output signal was processed to calculate inspiratory increases in lung volume. Breath interval was calculated from the onset of each breath. The flowmeter had a resolution of 0.5 ml and a linear accuracy of ±1.5%. The respiratory signal was sampled at 200 Hz (PowerLab/16 SP chart data recorder; ADInstruments, Colorado Springs, CO) and stored on the hard drive of a microcomputer.
Hypoxia protocol.
The lambs breathed air [fraction of inspired O2 (FiO2) = 0.21] during the control period before the onset of O2 deprivation. Arterial blood gases and pH were measured in normoxia (control), at 1, 5, 10, and 15 min after onset of hypoxia (FiO2 ∼0.07). Carbon dioxide flow (0.2–0.6 l/min) was adjusted to minimize changes in PaCO2 during the 15 min of O2 deficiency.
Ibotenic acid lesions.
After the biphasic ventilatory response to isocapnic hypoxia was established in 11 lambs, localized destruction of thalamic neurons was achieved by overstimulation of N-methyl-d-aspartate and metabotropic receptors with ibotenic acid (IB) enabling specific destruction of neuron somata (24, 38, 61, 73), sparing surrounding vasculature and fibers of passage.
A microinjection pump (CMA/200; CMA/Microdialysis) was used for bilateral microinjections of 0.19 M IB in synthetic cerebrospinal fluid (Harvard Apparatus, Holliston, MA) to sling-restrained conscious lambs. Stainless steel needles (0.6 mm OD, 38 mm long) were inserted through symmetrically implanted cannulas and advanced 3 mm beyond the shaft. IB was injected (2 μl at 0.35 μl/min) in thalamic Pf or surrounding structures of 11 lambs. The needle remained in place for 10 min after injection to facilitate IB dispersion into neuropil. The needle was advanced 1 mm for a second 2-μl injection in seven lambs (nos. 40, 41, 325, 338, 436, 481, and 468). The hypoxia experiments were conducted two or more days after IB administration because the neurotoxin releases GABA and activates glutamate receptors (73). Upon completion of the studies, lambs were killed by lethal intravenous injection of pentobarbital sodium (180 mg/kg).
Analysis
Data analysis.
Mean values were obtained at 20-s intervals for inspiratory duration, respiratory period (total breath duration), tidal volume, and temperature (PowerLab software; ADInstruments). Measurements for control, maximum augmentation (1–2 min of hypoxia), and ventilatory decline (≥10 of hypoxia) were compared, as previously reported (44). Ventilatory changes from control (Δventilation) were calculated for each experiment. Hypoxic ventilatory decline was expressed as ventilatory decrement from peak augmentation divided by peak augmentation.
Brain analysis.
Lamb brains were perfused in situ with a buffered 4% formaldehyde solution. The cryopreserved brains were cut in 35-μm coronal sections, and alternate sections were Nissl stained with cresyl violet. Our neuroanatomist, blinded to the study results, identified and reconstructed the needle tracks and outlines of structures in the zone of thalamic neuronal destruction. The neuroanatomical reconstruction was based on the description of the sheep thalamus by Rose (69), with nomenclature conforming to contemporary usage (41, 42, 43).
Statistical analysis.
Physiological measurements before the start of hypoxia were subtracted from values during hypoxia to correct for baseline differences. Temporal profiles for ventilatory phases (augmentation, decline at 10 min, and decline at 15 min) were compared within and between groups using a two-way repeated-measures ANOVA (mixed) model and the Tukey-Fisher's least-significant difference post hoc comparison criterion (JMP version 10; SAS Institute, Cary, NC). Ventilatory responses to hypoxia were determined for two groups based on whether the lesions involved thalamic Pf. The two fixed factors in the repeated-measures analyses were type of brain lesion (cf. extent of Pf involvement) and time pre- and postlesion. Site of brain lesion (Pf, non-Pf) was a between-group factor. Time and presence or absence of brain lesions were “within-animal” factors. Comparisons of age between Pf vs. non-Pf groups were performed by Student's t-test. Gender was compared between groups using Fisher's exact test. A log transformation of some of the continuous data was carried out before analysis to normalize skewed distributions. Differences were considered significant at the P < 0.05 level. All continuous data summaries are expressed as means ± SE.
RESULTS
Thalamic Lesions Involving Pf
Five lambs (2 males, 3 females) exhibited lesions extensively involving Pf. Hypoxia studies were carried out at 10 ± 0.5 days of age in these lambs before IB administration and repeated after IB injection at 13 ± 0.5 days of age.
Arterial blood gases and pH.
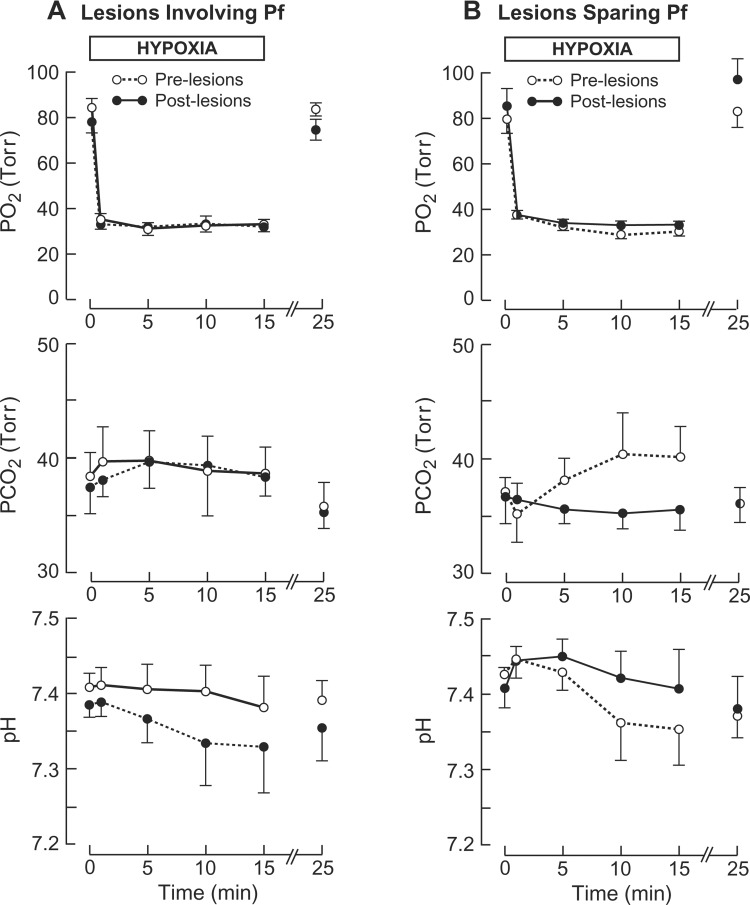
In pre- and postlesion experiments, mean arterial blood gases and pH were within the normal range for lambs in normoxia (Fig. 1A). In both experiments (pre- and postlesion), PaO2 fell to ∼31 Torr during hypoxia without significantly altering PaCO2 or arterial pH from their respective control values of ∼38 Torr and ∼7.39.
Fig. 1.
Effects of hypoxia on arterial blood gases and pH before and after ibotenic acid (IB)-induced brain lesions. A: lambs (n = 5) with thalamic lesions involving parafascicular nuclear complex (Pf). B: lambs (n = 6) with thalamic lesions sparing Pf.
Temperature.
Core temperature was measured in one lamb in which Pf lesions were reconstructed histologically. In pre- and postlesion experiments, rectal temperature was reduced by ∼0.1°C over 15 min of hypoxia compared with the respective controls of 39.9 and 40.6°C.
Ventilation.
In control normoxia, the respiratory period was 1.37 ± 0.15 s before lesions and thus significantly less (P < 0.02) than the postlesion value of 1.64 ± 0.15 s. Respiration was similar before and after lesions with respect to inspiratory duration (prelesion: 0.52 ± 0.06 s; postlesion: 0.59 ± 0.06 s), tidal volume (prelesion: 12.5 ± 3.7 ml/kg; postlesion 13.2 ± 3.7 ml/kg), and minute ventilation (prelesion: 0.57 ± 0.28 l·min−1·kg−1; postlesion: 0.49 ± 0.28 l·min−1·kg−1).
ΔVENTILATION.
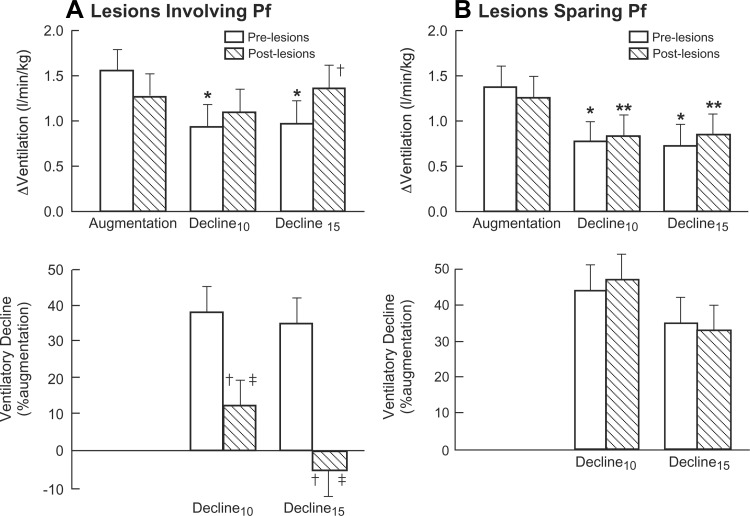
Ventilatory changes during hypoxia relative to control (Δventilation) are revealed in Fig. 2A, top. In prelesion studies, ventilation rapidly rose to a maximum augmentation of ∼1.55 l·min−1·kg−1. After 10 min hypoxia, ventilation subsequently declined by ∼38% relative to maximum increment.
Fig. 2.
Hypoxic ventilatory responses of lambs for pre- and postlesion experiments. ΔVentilation represents change in ventilation from normoxic control. Hypoxic ventilatory decline is represented by %decrement in Δventilation from maximum increase referenced to peak augmentation. A: lambs with thalamic lesions involving Pf (n = 5). B: lambs with thalamic lesions external to Pf (n = 6). *P < 0.005 and **P < 0.03 compared with augmentation. †P < 0.005 compared with prelesion at the same time. ‡P < 0.05 compared with thalamic lesions sparing Pf at the same time.
After lesions, hypoxia-evoked maximum ventilation was not significantly altered compared with prelesion experiments. In prelesion studies, ventilation after 10 min hypoxia was significantly less than peak values. Postlesion ventilatory increments after ≥10 min hypoxia did not differ significantly from peak augmentation (Figs. 2A, top). Ventilation at 15 min was significantly greater in postlesion compared with prelesion studies.
VENTILATORY DECLINE.
The decline in Δventilation relative to peak augmentation is illustrated (Fig. 2A, bottom). In prelesion studies, Δventilation after ≥10 min hypoxia declined ∼40% from peak values. Following Pf lesions, Δventilation following ≥10 min hypoxia approximated augmentation and was clearly significantly greater than before Pf lesions.
Thalamic Lesions External to Pf
In six lambs (5 males, 1 female) with thalamic lesions external to Pf, pre- and postlesion experiments were performed at 11 ± 1.5 and 15 ± 1.7 days of age, respectively.
Arterial blood gases and pH.
Normoxic means for PaO2, PaCO2, and pH were similar for pre- and postlesion studies (Fig. 1B). Hypoxic averages for PaO2 were also similar for pre- and postlesion experiments. The average PaCO2 in hypoxia was not significantly greater than control in prelesion experiments.
Temperature.
Averaging 40.0 ± 0.5°C in normoxia, mean core temperature declined nonsignificantly by 0.2 ± 0.1°C during hypoxia (n = 4). Similar minimal changes in mean rectal temperature occurred in postlesion experiments (n = 5) from the normoxic mean of 39.7 ± 0.1°C.
Ventilation.
In normoxia, a prelesion respiratory period of 1.61 ± 0.13 s was significantly less (P < 0.001) than the postlesion mean of 1.97 ± 0.13 s. Lesions did not significantly alter inspiratory duration (prelesion: 0.56 ± 0.06 s; postlesion: 0.64 ± 0.06 s), tidal volume (prelesion: 15.5 ± 3.4 ml/kg; postlesion: 14.1 ± 3.4 ml/kg), or minute ventilation (prelesion: 0.63 ± 0.25 l·min−1·kg−1; postlesion: 0.44 ± 0.25 l·min−1·kg−1).
ΔVENTILATION.
In pre- and postlesion experiments, hypoxia induced a similar increase in maximum ventilation (Fig. 2B, top). In both circumstances, average Δventilation after ≥10 min hypoxia was significantly less than augmentation.
VENTILATORY DECLINE.
Relative to maximum augmentation, Δventilation fell ∼40% after 10 min of hypoxia for both pre- and postlesion experiments (Fig. 2B, bottom). In two additional lambs, the ventilatory decline was similarly reduced before and after vehicle injections.
Pf vs. non-Pf Lesions
In normoxia, both lesion groups revealed similar breathing patterns (inspiratory duration, breath interval, amplitude, and minute ventilation) for the respective pre- and postlesion experiments.
ΔVentilation.
In pre- and postlesion experiments, the maximum ventilation increment did not differ significantly between lambs with or without Pf lesions (P = 0.6 and 0.17, respectively), as shown in Fig. 2, A and B, top. ΔVentilation at 10 and 15 min of hypoxia for pre- or post-Pf lesions did not differ significantly from pre- or post-Pf-sparing lesions (Fig. 2, A and B).
Ventilatory decline.
Normalizing the decline in Δventilation to peak augmentation revealed that the ventilatory reduction at 10 and 15 min hypoxia was significantly less in lambs with Pf lesions (P < 0.05 and P = 0.004, respectively), as depicted in Fig. 2, A and B, bottom.
Other comparisons.
The respective lamb age for pre- and postlesion experiments was similar for both groups. No male/female differences were observed. Postoperative weight gain of 218 ± 35 g/day was not significantly affected by lesion site (Pf, non-Pf).
Anatomical Analysis
Thalamic lesions.
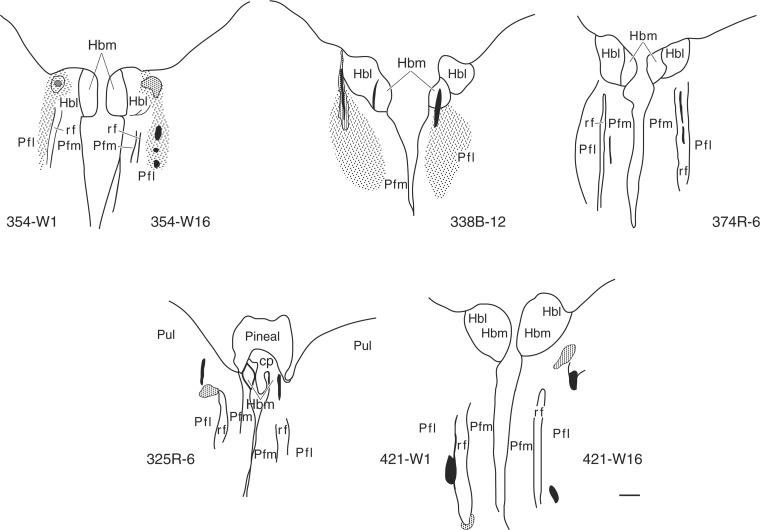
Figure 3 shows typical examples of outline drawings of needle tracks and sites of IB-induced neuronal degeneration that involved Pf. The anatomical depiction of disruptions that abolished the hypoxic ventilatory decline is more clearly shown in lambs 354, 338, and 374 where the critical lesions involve a dorsolateral sector of Pf. Findings in lambs 325 and 421 unilaterally illustrate lateral Pf lesions (Fig. 3).
Fig. 3.
Outline drawings of representative Nissl-stained histological sections depicting lesions that abolished the hypoxic ventilatory decline in 5 lambs. Needle tracks are black. Stippled areas represent neuronal destruction. Hbl, lateral habenula nuclei; Hbm, medial habenula nuclei; Pfl, lateral parafascicular nucleus; Plm, medial parafascicular nucleus; Pul, pulvinar; rf, fasciculus retroflexus.
In six lambs, the brain lesions did not include Pf. These disruptions involved more rostral thalamic structures, including mediodorsal, ventromedial, anteroventral (nos. 481 and 440), and anteromedial and medioventral nuclei (no. 468). Other nearby unrelated sectors included the habenula nuclei (no. 440), rostral thalamus (nos. 40 and 41), and cingulate cortex (no. 436).
Vehicle control injections.
Needle tracks were identified between medial and lateral Pf in no. 268. In no. 253B, the tracks ended immediately lateral to the left lateral habenula and the right medial pretectal nuclei; the parafascicular sectors remained intact bilaterally.
DISCUSSION
Respiratory responses of human infants to hypoxia (FiO2 = 0.15) were first reported in the early 1950s (15, 16). Unlike adults, hyperventilation declined after 1–2 min of O2 deficiency, a pattern confirmed in many other mammalian newborns (see Refs. 9 and 83).
Stimulation
Ventilation rapidly peaks within the first 1–2 min of acute O2 deprivation. This respiratory augmentation is developmentally regulated, since it is attenuated in preterm human infants and is not expressed in very premature infants (3). Hypoxic hyperpnea is almost exclusively a chemoreflex evoked by carotid body O2 sensors (see Ref. 83). In term human infants, the magnitude of the augmentation in quiet sleep progressively increases over the first 2–3 mo (67). The developmental progression in the strength of ventilatory stimulation involves complex changes (see Ref. 15), including maturation of O2 sensing by the carotid bodies (10, 12), integration of carotid sinus nerve activity into respiratory output (1, 10, 12), and rise of facilitatory input from the posterior hypothalamus.
Decline
Following peak ventilation, respiration gradually falls toward or even below normoxic values because of central dampening of excitatory input of peripheral O2 sensors (1). The magnitude of the ventilatory decline depends on the severity of hypoxia, peak augmentation, species, level of consciousness, anesthesia, sex, ambient temperature, maturity of respiratory control mechanisms, and plasticity related to prolonged stable or intermittent changes in PaO2 (see Refs. 9, 32, 40, 60, and 83). In human adults, the time to the onset of the decline (5–10 min) in moderate isocapnic hypoxia (SaO2 80%) is longer, with ventilation ultimately decreasing 20–25% of peak values (22).
A number of mechanisms have been proposed for hypoxic ventilatory decline (9, 83). The direct effects of lowered brain Po2 play a critical role (11, 52, 57, 58, 74, 85a, 90), although progressive attenuation of carotid sinus nerve activity has not been excluded in all cases (14). Chemical mediators in the medulla implicated in hypoxic respiratory depression include ADO, γ-aminobutyric acid, glycine, taurine, serotonin, opioids, and platelet-derived growth factor (30, 78).
In small mammals with high metabolic rates, hypoxia resets the thermoregulatory set point to a lower body temperature (60). The resulting decline in metabolism diminishes CO2 production, contributing to a fall in ventilation. Hypoxic ventilatory decline occurs independently of changes in PaCO2 because it is observed in both poikilocapnic and hypercapnic hypoxia (27, 66, 80).
Hypoxic ventilatory depression appears to be driven by a central locus acting as an O2 sensor (1, 40, 83). Large brain lesions implicated supramedullary brain stem involvement in depressant effects of hypoxia on ventilation in newborn rats, rabbits, and piglets (23, 58, 82). More discrete lesions identified putative O2 sensory neurons in the mesencephalic red nucleus of decerebrate young rabbits (1, 91) and caudal ventromedial pons of decerebrate piglets (81). Focal cooling revealed involvement of a locus ceruleus sector in anesthetized neonatal lambs (59). These nonselective techniques disrupted function of cell bodies, fibers of passage, and vasculature and thus lacked specificity for identifying neuronal somata mediating hypoxic ventilatory decline.
A ventral zone of the hypothalamus has also been implicated in hypoxia-evoked respiratory depression in diencephalon-brain stem-spinal cord preparations from newborn rats (89). However, observations from anoxic reduction preparations have questionable relevance to hypoxic ventilatory decline of moderately hypoxic conscious intact neonates.
In summary, O2-sensing neurons involved in hypoxic ventilatory decline have been surmised to reside in supramedullary brain stem primarily based on indiscriminate disruption of fiber tracts and somata. In the current study, selective destruction of thalamic Pf neurons by IB abolished hypoxic ventilatory decline in conscious lambs, which constitutes a true advance in identifying forebrain neurons involved in hypoxic ventilatory depression.
Thalamic Pf and Hypoxic Inhibition
In 1936, Kabat (35) reported slower shallower breathing in anesthetized cats could be elicited by electrical stimulation of the habenula or its projections in the fasciculus retroflexus. Although a remarkable achievement at the time given the use of larger electrodes and the smaller cat brain, the inhibitory effects could be attributable to spread of current to Pf neurons surrounding the fasciculus.
The current study in chronically catheterized awake lambs reveals that neurons in the Pf sector are involved in hypoxic ventilatory decline, establishing this locus as a critical forebrain controller of hypoxic ventilatory decline. A functional role for Pf in hypoxic ventilatory depression is further bolstered by: 1) nociception and sensorimotor function (8), 2) efferent connections to second-order respiratory motoneurons (25, 26), 3) projections to regions of the supramedullary brain stem implicated in respiratory inhibition (1, 34, 53, 58, 62, 65, 91, 92), and 4) increased neural activity of this sector in human subjects during mild-moderate O2 deprivation (55).
The identity and position of Pf are clearly defined by its location surrounding the fasciculus retroflexus (the habenulointerpeduncular tract). Pf forms the posterior cap of the mediodorsal nucleus, and ventrally it rests upon the rostral periaqueductal gray (Fig. 4). A lateral expansion, called the centre median or centrum medianum in the early literature, is prominent in the thalamus of primates, and especially in the human brain, and to a lesser extent in the thalamus of cetaceans and other large brain mammals (48), although there is no evident link to respiratory function.
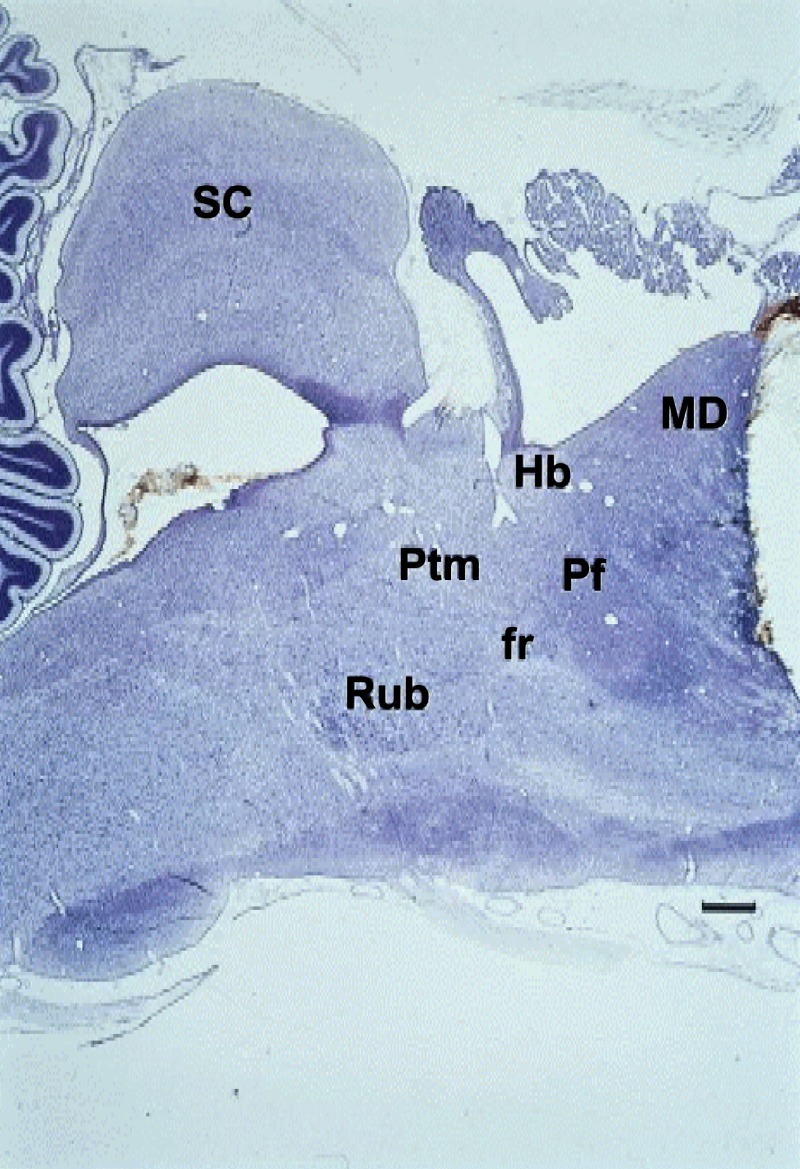
Fig. 4.
A Nissl-stained parasagittal section of a near-term fetal sheep brain that depicts the Pf in relation to other brain sectors. The rostral cut represents a transection that left intact hypoxic inhibition of fetal breathing movements. MD, mediodorsal thalamic nucleus; Hb, habenula; SC, superior colliculus; Rub, red nucleus; Ptm, medial pretectal area; fr, fasciculus retroflexus. Horizontal bar = 1 mm.
Electrophysiological mapping and single unit recording studies revealed a projection from slowly conducting C-fiber nociceptor afferents to this region of the cat thalamus (2, 49) and possibly related to the effects of pain on respiratory function, but there is no persuasive evidence of relevant forebrain projections of Pf neurons, or any other thalamic nucleus that can be readily linked to a compensatory role in breathing behavior.
During the last two decades the thalamic Pf has been recognized as a functional entity distinct from the loosely classified thalamic “intralaminar” nuclear system, partly based on its connectivity and molecular idiosyncrasies (8). This derived primarily from early recognition of degeneration of substantia nigra neurons (79), its role in Parkinson's disease (4, 31, 33, 75), and evidence of its segregated striatal projections (51, 84, 85, 88, 94), as well as widespread interest in recognition of its distinctive role in metabolic regulation (4, 33, 51, 63, 75, 94). The classification of Pf together with the centre median nucleus as part of the intralaminar group (8) or the “postmedial group” proposed by Rose (69) shall presumably rely upon recent findings now emerging from identification of molecular components and their regulators in conserving the energetics that sustain respiration and tissue oxygenation (75, 76).
Baseline ADO concentrations vary widely among whole brain sectors and strains in mice (64), reflecting heterogeneity of enzyme-mediated ADO formation and/or degradation. Hypoxia activates cellular enzymes that support ATP levels while increasing ADO formation (see Ref. 40). Brain stem ADO A1 receptors have long been implicated in hypoxic respiratory depression in newborns (19, 29, 50). Our studies with highly selective and potent antagonists for A1 and A2A receptors indicated for the first time that ADO mediates hypoxic ventilatory depression via activation of A2A receptors likely expressed within the Pf sector (40, 42, 44, 95).
Physiological Implications
Compared with other neurons, the putatively enhanced indirect O2 sensitivity of the thalamic Pf likely involves differences in mitochondrial energy state, enzymes linking energy state to membrane depolarization, and/or ATP dependency on mitochondrial oxidative phosphorylation (40). Such functional differences are consistent with forebrain control of energy homeostasis in which a fall in O2 availability triggers a transduction cascade involving activation of 5′-nucleotidase, formation of ADO, stimulation of A2A receptors, and excitation of Pf neurons that depress respiration (40, 42, 44). Activity of Pf neurons is reflected in changes in expression of molecular intermediates in mitochondrial O2 consumption and other elements and pathways of cellular energetics (4, 31, 51, 63, 75). These advances now enable the study of molecular components of Pf related to neuronal activation and control of energy metabolism.
The functional characteristics of Pf are consistent with those of other discrete neuronal populations that act as O2 sensors, such as the nucleus tractus solitarius, pre-Bötzinger complex, rostral ventrolateral medulla, and posterior hypothalamus (see Refs. 40 and 83). Although translational mechanisms vary, they appear to involve depolarization of sensory neurons (83). Besides peripheral O2 sensors, these central loci are involved in cardiorespiratory control of oxygen delivery, illustrating the regulatory complexity of tissue oxygenation.
Hypoxic arrest of breathing activity has a significant survival value in utero because fetal breathing movements account for up to 30% of total O2 consumption (71). Postnatally, the O2 cost of respiration is minimal under resting conditions, but the O2 demands of breathing expand exponentially with increments in respiratory work (6, 68, 70). Thus, hypoxic ventilatory decline itself contributes at least marginally to the fall in total O2 consumption.
Postnatally, the thalamic Pf has important relevance to hypoxic respiratory depression of birth asphyxia and to apnea and pulmonary disease in immature newborns. The thalamic Pf also has pertinence to pathophysiological states involving hypoxic injury to the brain and other tissues. Recurrent apnea due to neurologic immaturity, brain pathophysiology, or upper airway obstruction dispose to respiratory instability, sympathetic overactivity, hypertension, arrhythmias, depression, diabetes mellitus, brain lesions, and sudden death (18, 36, 40, 56, 77, 83, 87). By blunting respiratory defenses to acute O2 deprivation, hypoxic ventilatory depression may increase vulnerability to such sequelae, particularly during critical stages of development.
Measurement of Hypoxic Ventilatory Decline
The magnitude of hypoxic ventilatory decline relates directly to peak augmentation (see Ref. 83). Thus, hypoxic ventilatory decline was calculated as the decrement in Δventilation (augmentation − decline) referenced to augmentation. The dependency of hypoxic ventilatory depression on augmentation probably accounts for the absence of a ventilatory decline in lambs that lack hypoxic hyperpnea because of carotid body denervation (13), although stimulatory input from the posterior hypothalamus may be involved (see Ref. 83).
Limitations
IB-induced demyelination cannot be excluded, although the fasciculus retroflexus was visibly intact even when adjacent to degenerated neuropil. That the respiratory effects may have resulted from injury to fiber tracts and/or neurons by insertion of the injection needle itself seems unlikely 1) because of the variability of probe placement within the Pf sector and 2) because vehicle injections into Pf did not alter the roll off.
In prelesion experiments, mean PaCO2 during hypoxia was up to 3 Torr greater than the normoxic control. Although not significant, this difference may have slightly increased phase 1 ventilation. Nevertheless, hypoxic ventilatory depression was still observed under these conditions.
The relatively large lamb brain allows for greater specificity and spatial resolution than can be achieved in species with much smaller brains, greatly facilitating the identification of neuronal substrate involved in respiratory inhibition. The role of thalamic Pf in hypoxic ventilatory decline has been clearly demonstrated in the lamb, but it is yet to be determined whether this can be generalized to other mammalian species.
Perspectives and Significance
Over the past 80 years, brain transections have been performed in the study of 1) apnea and respiratory responses to asphyxia in fetal sheep exteriorized in saline baths (5), 2) apnea and hypoxic inhibition of breathing in chronically catheterized fetal sheep with measurement of arterial blood gases and pH (21, 39), and 3) hypoxic ventilatory depression in neonatal rabbits (58). More localized nonspecific disruptions of neural elements have subsequently been used in chronically catheterized fetal sheep (28) and in anesthetized or decerebrate young mammals (1, 59, 81, 82, 91). Together, these studies have established the involvement of the supramedullary brain stem in the depressant effects of hypoxia on breathing. We now have used IB to destroy neurons without significantly disrupting fiber tracts or vascularity. Along with our previous studies (42, 44, 46, 95), the results indicate that thalamic Pf acts as an O2 sensor for hypoxic ventilatory decline (40). Metabolic markers are now available to track changes in metabolic activity of Pf neurons (1, 31, 33, 51, 63, 75, 76). Future studies can now be directed toward the molecular energetics of Pf neurons and functional alterations during hypoxia.
GRANTS
This study was supported in part by National Institute of Child Health and Human Development Grant HD-18478 and by the Department of Obstetrics and Gynecology, David Geffen School of Medicine at UCLA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.J.K. conception and design of research; B.J.K., A.R., B.O.I., and C.G. performed experiments; B.J.K., A.R., B.O.I., and L.K. analyzed data; B.J.K., A.R., and B.O.I. interpreted results of experiments; B.J.K. prepared figures; B.J.K. drafted manuscript; B.J.K., B.O.I., and L.K. edited and revised manuscript; B.J.K., A.R., B.O.I., C.G., and L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marianne Cilluffo for preparation of the brain sections, Shaemion McBride for participation in experiments, and Jeffrey Gornbein for assistance with statistical analysis.
REFERENCES
- 1.Ackland GL, Noble R, Hanson MA. Red nucleus inhibits breathing during hypoxia in neonates. Respir Physiol 110: 251–260, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Albe-Fessard D, Kruger L. Duality of unit discharges from cat centrum medianum in response to natural and electrical stimulation. J Neurophysiol 25: 3–20, 1962. [DOI] [PubMed] [Google Scholar]
- 3.Alvaro R, Alvarez J, Kwiatkowsi K, Cates D, Rigatto H. Small preterm infants (≤1500 g) have only a sustained decrease in ventilation in response to hypoxia. Pediat Res 32: 403–406, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Aymerich MS, Barroso-Chinea P, Perez-Manso M, Munoz-Patino AM, Moreno-Igoa M, Gonzalez-Hernandez T, Lanciego JL. Consequences of unilateral nigrostriatal denervation on the thalamostriatal pathway in rats. Eur J Neurosci 23: 2099–2108, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Barcroft J, Barron DH. Movements in mid-foetal life in the sheep embryo. J Physiol 91: 329–351, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett RG Jr, Specht H. Energy cost of breathing determined with a simplified technique. J Appl Physiol 11: 84–86, 1957. [DOI] [PubMed] [Google Scholar]
- 8.Bentivoglio M, Balerecia G, Kruger L. The specificity of the nonspecific thalamus: the midline nuclei. Prog Brain Res 87: 53–80, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Bissonnette JM. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol 278: R1391–R1400, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Blanco CE, Dawes GS, Hanson MA, McCooke MB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol 351: 25–37, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco CE, Hanson MA, Johnson P, Rigatto H. Breathing pattern of kittens during hypoxia. J Appl Physiol 56: 12–17, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Blanco CE, Hanson MA, McCooke MB. Effects on carotid chemoreceptor resetting of pulmonary ventilation in the fetal lamb in utero. J Dev Physiol 10: 167–174, 1988. [PubMed] [Google Scholar]
- 13.Bureau MA, Lamarche J, Foulon P, Dalle D. The ventilatory response to hypoxia in the newborn lamb after carotid body denervation. Respir Physiol 60: 109–119, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Carroll JL, Donnelly DF. Postnatal development of carotid chemoreceptor function. In: Sleep and Breathing in Children (2nd. ed.), edited by Marcus CL, Carroll JL, Donnelly DF, Loughlin GM. London, UK: Informa, 2008. [Google Scholar]
- 15.Carroll JL, Kim I. Carotid chemoreceptor “resetting” revisited. Respir Physiol Neurobiol 185: 30–43, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross KW, Oppé TE. The effect of inhalation of high and low concentrations of oxygen on the respiration of the premature infant. J Physiol 117: 38–55, 1952. [PMC free article] [PubMed] [Google Scholar]
- 17.Cross KW, Warner P. The effect of inhalation of high and low oxygen concentrations on the respiration of the newborn. J Physiol 114: 283–295, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross RL, Kumar R, Macey, Doering LV, Alger JR, Yan-Go FL, Harper RM. Neural alterations and depressive sleep apnea. Sleep 31: 1103–1109, 2008. [PMC free article] [PubMed] [Google Scholar]
- 19.Darnall DA., Jr Aminophylline reduces hypoxic ventilator depression: possible role of adenosine. Pediatr Res 19: 706–710, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Dawes GS. The central control of fetal breathing and skeletal muscle movements. J Physiol 346: 1–18, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawes GS, Gardner WM, Johnston BM, Walker DW. Breathing in fetal lambs: the effects of brain stem section. J Physiol (Lond) 335: 535–553, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Easton PA, Slykerman LJ, Anthoninsen NR. Ventilatory response to sustained hypoxia in normal adults. J Appl Physiol 61: 906–911, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Fung ML, Wang W, Darnall RA, St. John WM. Characterization of ventilatory responses to hypoxia in neonatal rats. Respir Physiol 103: 57–66, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Garey LJ, Hornung JP. The use of ibotenic acid lesions for light and electron microscopic study of antegrade degeneration in the visual pathway of the cat. Neurosci Lett 19: 117–123, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Gaytán SP, Pásaro R. Connections of the rostral ventral respiratory neuronal cell group: an anterograde and retrograde tracing study in the rat. Brain Res Bull 47: 625–642, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Gaytán SP, Pásaro R, Coulon P, Bevengut M, Hilaire G. Identification of central nervous system neurons innervating the respiratory muscles of the mouse: a transneuronal study. Brain Res Bull 57: 335–339, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Georgopoulos D, Berezanski D, Anthonisen NR. Effects of CO2 breathing on ventilatory response to sustained hypoxia in normal adults. J Appl Physiol 66: 1071–1078, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Gluckman PD, Johnston BM. Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanesthetized fetal lambs in utero. J Physiol 382: 373–383, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedner T, Hedner J, Wessberg P, Jonason J. Regulation of breathing in the rat: indication for a role of central adenosine mechanisms. Neurosci Lett 382: 373–383, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Hehre DA, Devia CJ, Bancalari E, Suguihara C. Brainstem amino acid neurotransmitters and ventilatory response to hypoxia in piglets. Pediatr Res 63: 46–50, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Henderson JM, Carpenter K, Cartwright H, Halliday GM. Degeneration of the centre median-parafascicular complex in Parkinson's disease. Ann Neurol 47: 345–352, 2000. [PubMed] [Google Scholar]
- 32.Hill CB, Grandgeorge SH, Bavis RW. Developmental hyperoxia alters CNS mechanisms underlying hypoxic ventilatory depression in neonatal rats. Respir Physiol Neurobiol 189: 498–505, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch EC, Perier C, Orieux G, Francois C, Feger J, Yelnik J, Vila M, Levy R, Tolosa ES, Marin C, Trinidad Herrero M, Obeso JA, Agid Y. Metabolic effects of nigrostriatal denervation in basal ganglia. Trends Neurosci 23: S78–S85, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Hugelin A. Forebrain and midbrain influences on respiration. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Am Physiol Soc, 1986, sect. 3, vol II, pt. 1, chapt. 2, p. 33–68. [Google Scholar]
- 35.Kabat H. Electrical stimulation of points in the forebrain and mid-brain: the resultant alterations in respiration. J Comp Neurol 64: 187–208, 1936. [Google Scholar]
- 36.Katz ES, Mitchell RB, D'Ambrosio CM. Obstructive sleep apnea in infants. Am J Respir Crit Care Med 185: 805–816, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawahara K, Suzuki M. Descending inhibitory pathway responsible for simultaneous suppression of postural tone and respiration in decerebrate cats. Brain Res 538: 303–309, 1991. [DOI] [PubMed] [Google Scholar]
- 38.Köhler C, Schwarcz R. Comparison of ibotenate and kainite neurotoxicity in rat brain: a histological study. Neuroscience 8: 819–835, 1983. [DOI] [PubMed] [Google Scholar]
- 39.Koos BJ. Central stimulation of breathing movement in fetal sheep of prostaglandin synthetase inhibitors. J Physiol 362: 455–466, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koos BJ. Adenosine A2a receptors and O2 sensing in development. Am J Physiol Regul Integr Comp Physiol 301: R601–R622, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koos BJ, Chau A, Matsuura M, Punla O, Kruger L. A thalamic locus mediates hypoxic inhibition of breathing in fetal sheep. J Neurophysiol 79: 2383–2393, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Koos BJ, Chau A, Matsuura M, Punla O, Kruger L. Thalamic lesions dissociate breathing inhibition by hypoxia and adenosine in fetal sheep. Am J Physiol Regul Integr Comp Physiol 278: R831–R837, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Koos BJ, Kawasaki Y, Hari A, Bohorquez F, Jan C, Roostaeian J, Wilson CL, Kruger L. Electrical stimulation of the posteromedial thalamus modulates breathing in unanesthetized fetal sheep. J Appl Physiol 96: 115–123, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Koos BJ, Kawasaki Y, Kim YH, Bohoroquez F. Adenosine A2A receptor blockade abolishes the roll-off respiratory response to hypoxia in awake lambs. Am J Physiol Regul Integr Comp Physiol 288: R1185–R1194, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Koos BJ, Maeda T. Adenosine A2A receptors mediate cardiovascular responses to hypoxia in fetal sheep. Am J Physiol Heart Circ Physiol 280: H83–H89, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Koos BJ, Maeda T, Lopez G. Adenosine A2A receptors mediate hypoxic inhibition of fetal breathing in sheep. Am J Obstet Gynecol 186: 663–668, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Koos BJ, Rajaee A. Fetal breathing movements and changes at birth. Adv Exper Med Biol 814: 89–101, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Kruger L. The thalamus of the dolphin (Tursiops truncatus) and comparison with other mammals. J Comp Neurol 111: 133–194, 1959. [Google Scholar]
- 49.Kruger L, Albe-Fessard D. The distribution of responses to somatic afferent stimuli in the diencephalon of the cat under chloralose anesthesia. Exp Neurol 2: 442–467, 1960. [DOI] [PubMed] [Google Scholar]
- 50.Lagercrantz H, Yamamoto Y, Fredholm BB, Prabhakar NR, von Euler C. Adenosine analogues depress ventilation in rabbit neonates. Theophylline stimulation of respiration via adenosine receptors? Pediatr Res 18: 387–390, 1984. [DOI] [PubMed] [Google Scholar]
- 51.Lanciego JL, Gonzalo N, Castle M, Sanchez-Escobar C, Aymerich MS, Obeso JA. Thalamic innervation of striatal and subthalamic neurons projecting to the rat entopeduncular nucleus. Eur J Neurosci 19: 1267–1277, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Lawson EE, Long WA. Central origin of biphasic breathing pattern during hypoxia in newborns. J Appl Physiol 55: 483–488, 1983. [DOI] [PubMed] [Google Scholar]
- 53.Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol 106: 138–152, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep 31: 967–977, 2008. [PMC free article] [PubMed] [Google Scholar]
- 55.Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, Harper RM. Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic defects in congenital central hypoventilation syndrome. J Appl Physiol 98: 958–969, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Martin RJ, DiFiore JM, Jana V, Davis RL, Miller MJ, Coles SK, Dick TE. Persistence of biphasic ventilatory response to hypoxia in preterm infants. J Pediatr 132: 960–964, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Martin-Body RL. Brain transections demonstrate the central origin of hypoxic ventilatory depression in carotid-denervated rats. J Physiol 407: 41–52, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin-Body RL, Johnston BM. Central origin of the hypoxic depression of breathing in the newborn. Respir Physiol 71: 25–32, 1988. [DOI] [PubMed] [Google Scholar]
- 59.Moore PJ, Parkes MJ, Hanson MA. Unilateral cooling in the region of the locus coeruleus blocks the fall in respiratory output during hypoxia in anesthestized neonatal sheep. Exp Physiol 81: 983–994, 1996 1996. [DOI] [PubMed] [Google Scholar]
- 60.Mortola JP, Maskrey M. Metabolism M, temperature, ventilation. Comp Physiol 1: 1679–1709, 2011. [DOI] [PubMed] [Google Scholar]
- 61.Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Methods 121: 199–209, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Noble R. Brainstem mechanisms mediating the neonatal ventilatory response to hypoxia. In: The Fetal and Neonatal Brainstem: Developmental and Clinical Issues, edited by Hanson MA. Cambridge, MA: Cambridge Univ Press, 1991. [Google Scholar]
- 63.Orieux G, Francois C, Feger J, Yelnik J, Vila M, Ruberg M, Agid Y, Hirsch EC. Metabolic activity of excitatory parafascicular and pedunculopontine inputs to the subthalamic nucleus in a rat model of Parkinson's disease. Neuroscience 97: 79–88, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Pani A, Jiao Y, Sample KJ, Smeyne RJ. Neurochemical measurement of adenosine in discrete brain regions of five strains of inbred mice. PloS one 9: e92422, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrovický P. Thalamic descendents from anterior and parafascicular nuclei to the brainstem. An experimental study using the HRP technique in the rat. J Hirnforsch 23: 329–339, 1983. [PubMed] [Google Scholar]
- 66.Rehan V, Haider AZ, Alvaro RE, Nowaczyk B, Cates DB, Kwiatkowski K, Rigatto H. The biphasic ventilator response to hypoxia in preterm infants is not due to a decrease in metabolism. Pediatr Pulmonol 22: 287–294, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Richardson HL, Parslow PM, Walker AM, Harding R, Horne RS. Maturation of the initial ventilatory response to hypoxia in sleeping infants. J Sleep Res 16: 117–127, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Robertson CH Jr, Foster GH, Johnson RL Jr. The relationship of respiratory failure to the oxygen consumption of, lactate production by, and the distribution of blood flow among respiratory muscles during increasing inspiratory resistance. J Clin Invest 59: 31–42, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rose JE. The thalamus of the sheep: cellular and fibrous structure and comparison with pig, rabbit and cat. J Comp Neurol 401: 163–186, 1942. [Google Scholar]
- 70.Rozé JC, Chambille B, Fleury MA, Debillon T, Gaultier C. Oxygen cost of breathing in newborn infants with long-term ventilatory support. J Pediatr 127: 984–987, 1995. [DOI] [PubMed] [Google Scholar]
- 71.Rurak DW, Gruber NC. Increased oxygen consumption associated with breathing activity in fetal lambs. J Appl Physiol Respir Environ Exerc Physiol 54: 701–707, 1983. [DOI] [PubMed] [Google Scholar]
- 72.Schmid K, Böhmer G, Fallert M. Influence of rubrospinal tract and the adjacent mesencephalic reticular formation on the activity of medullary respiratory neurons and phrenic nerve discharge in the rabbit. Pfügers Arch 413: 23–31, 1988. [DOI] [PubMed] [Google Scholar]
- 73.Schwarcz R, Hökfelt T, Fuxe G, Jonsson G, Goldstein M, Terrenius L. Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Exp Brain Res 37: 199–216, 1979. [DOI] [PubMed] [Google Scholar]
- 74.Schwieler GH. Respiratory regulation during postnatal development in cats and rabbits and some of its morphological substrate. Acta Physiol Scand Suppl 304: 1–123, 1968. [PubMed] [Google Scholar]
- 75.Sedaghat K, Finkelstein DI, Gundlach AL. Effect of unilateral lesion of the nigrostriatal dopamine pathway on survival, and neurochemistry of parafascicular nucleus neurons in the rat-evaluation of time-course and L.GR8 expression. Brain Res 1271: 83–94, 2009. [DOI] [PubMed] [Google Scholar]
- 76.Sedaghat K, Shen P-J, Finkelstein DI, Henderson JM, Gundlach AL. Leucine-rich repeat-containing G.-protein-coupled receptor 8 (LGR8) in the rat brain:enrichment in thalamic neurons and their efferent projections. Neuroscience 156: 319–333, 2008. [DOI] [PubMed] [Google Scholar]
- 77.Shpirer I, Rapoport MJ, Stav D, Elizer A. Normal and elevated HbA1c levels correlate with severity of hypoxemia in patients with obstructive sleep apnea and decrease following CPAP treatment. Sleep Breath 16: 461–466, 201. [DOI] [PubMed] [Google Scholar]
- 78.Simakajornboon N, Kuptanon T. Maturational changes in neuromodulation of central pathways underlying hypoxic ventilatory response. Resp Physiol Neurobiol 149: 273–286, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Stanic D, Parish CL, Zhu WM, Krstew EV, Lawrence AJ, Drago J, Finkelstein DI, Horne MK. Changes in function and ultrastructure of striatal dopaminergic terminals that regenerate following partial lesions of the SNpc. J Neurochem 86: 329–343, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Steinback CD, Poulin MJ. Ventilatory responses to isocapnic and poikilocapnic hypoxia in humans. Respir Physiol Neurobiol 155: 104–113, 2007. [DOI] [PubMed] [Google Scholar]
- 81.St.-Jacques R, Filiano JJ, Darnall RA. Characterization of hypoxia induced ventilatory depression in newborn piglet. Exp Physiol 88: 509–515, 2003. [DOI] [PubMed] [Google Scholar]
- 82.St.-John WM, St.-Jacques R, Li A, Darnall RA. Modulation of hypoxic depressions of ventilatory activity in the newborn piglet by mesencephalon mechanisms. Brain Res 819: 147–149, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: mechanisms, measurement, and analysis. Physiol Rev 90: 675–754, 2010. [DOI] [PubMed] [Google Scholar]
- 84.Tsumori T, Yokota S, Lai H, Yasui Y. Monosynaptic and disynaptic projections from the substantia nigra pars reticulate to the parafascicular thalamic nucleus in the rat. Brain Res 858: 429–435, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Tsumori T, Yokota S, Ono K, Yasui Y. Synaptic organization of GABAergic projections from the substantia nigra pars reticulata and the reticular thalamic nucleus to the parafascicular thalamic nucleus in the rat. Brain Res 957: 231–241, 2002. [DOI] [PubMed] [Google Scholar]
- 85a.van Beek JHGM, Berkenbosch A, De Goede J, Olievier CN. Effects of brainstem hypoxemia on the regulation of breathing. Respir Physiol 57: 171–188, 1984. [DOI] [PubMed] [Google Scholar]
- 86.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res 39: 107–140, 2002. [DOI] [PubMed] [Google Scholar]
- 87.Verbeck MM, Richardson HL, Parslow PM, Walker AM, Harding R, Horne RS. Arousal and ventilatory responses to mild hypoxia in sleeping preterm infants. J Sleep Res 17: 344–353, 2008. [DOI] [PubMed] [Google Scholar]
- 88.Vercelli A, Marini G, Tredici G. Anatomical organization of the telencephalic connections of the parafascicular nucleus in adult and developing rats. Eur J Neurosci 18: 275–289, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Voituron N, Frugierem Gros F, Macron JM, Bonineau L. Diencephalic and mesencephalic influences on ponto-medullary respiratory control in normoxic and hypoxic conditions: an in vitro study on central nervous system preparations from newborn rat. Neuroscience 132: 843–854, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Vizek M, Pickett CK, Weil JV. Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. J Appl Physiol 63: 1658–1664, 1987. [DOI] [PubMed] [Google Scholar]
- 91.Waites BA, Ackland GL, Noble R, Hanson MA. Red nucleus lesions abolish the biphasic respiratory response to isocapnic hypoxia in decerebrate young rabbits. J Physiol 495: 217–225, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker DW, Lee B, Nitsos I. Effect of hypoxia on respiratory activity in the foetus. Clin Exp Pharmacol Physiol 27: 110–113, 2000. [DOI] [PubMed] [Google Scholar]
- 93.Wisden W, Morris BJ. In situ hypbridization with oligonucleotide probes. Int Rev Neurobiol 47: 3–59, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Yan W, Zhang QJ, Liu J, Wang T, Wang S, Liu X, Chen L, Gui ZH. The neuronal activity of thalamic parafascicular nucleus is conversely regulated by nigrostriatal pathway and pedunculopontine nucleus in the rat. Brain Res 1240: 204–212, 2008. [DOI] [PubMed] [Google Scholar]
- 95.Yan X, Koos BJ, Kruger L, Linden J, Murray TF. Characterization of [125I]ZM 241385 binding to adenosine A2A receptors in the pineal of sheep brain. Brain Res 1096: 30–39, 2006. [DOI] [PubMed] [Google Scholar]