This study is the first demonstration of perinexal width and conduction velocity modulation in hearts by varying extracellular calcium without measurable changes in gap junctional (GJ) coupling. Also, provided here is the first experimental evidence for ephaptic self-attenuation, as predicted by mathematical models that incorporate the effects of ephaptic coupling.
Keywords: ion concentration, sodium, calcium, conduction, ephaptic coupling
Abstract
Our laboratory previously demonstrated that perfusate sodium and potassium concentrations can modulate cardiac conduction velocity (CV) consistent with theoretical predictions of ephaptic coupling (EpC). EpC depends on the ionic currents and intercellular separation in sodium channel rich intercalated disk microdomains like the perinexus. We suggested that perinexal width (WP) correlates with changes in extracellular calcium ([Ca2+]o). Here, we test the hypothesis that increasing [Ca2+]o reduces WP and increases CV. Mathematical models of EpC also predict that reducing WP can reduce sodium driving force and CV by self-attenuation. Therefore, we further hypothesized that reducing WP and extracellular sodium ([Na+]o) will reduce CV consistent with ephaptic self-attenuation. Transmission electron microscopy revealed that increasing [Ca2+]o (1 to 3.4 mM) significantly decreased WP. Optically mapping wild-type (WT) (100% Cx43) mouse hearts demonstrated that increasing [Ca2+]o increases transverse CV during normonatremia (147.3 mM), but slows transverse CV during hyponatremia (120 mM). Additionally, CV in heterozygous (∼50% Cx43) hearts was more sensitive to changes in [Ca2+]o relative to WT during normonatremia. During hyponatremia, CV slowed in both WT and heterozygous hearts to the same extent. Importantly, neither [Ca2+]o nor [Na+]o altered Cx43 expression or phosphorylation determined by Western blotting, or gap junctional resistance determined by electrical impedance spectroscopy. Narrowing WP, by increasing [Ca2+]o, increases CV consistent with enhanced EpC between myocytes. Interestingly, during hyponatremia, reducing WP slowed CV, consistent with theoretical predictions of ephaptic self-attenuation. This study suggests that serum ion concentrations may be an important determinant of cardiac disease expression.
NEW & NOTEWORTHY
This study is the first demonstration of perinexal width and conduction velocity modulation in hearts by varying extracellular calcium without measurable changes in gap junctional (GJ) coupling. Also, provided here is the first experimental evidence for ephaptic self-attenuation, as predicted by mathematical models that incorporate the effects of ephaptic coupling.
the synchronized spread of electrical activity is important for coordinating efficient cardiac contraction, and slowed conduction is a well-established substrate for lethal cardiac arrhythmias. GJ coupling, tissue excitability, intercalated disk micro-architecture, and extracellular ion concentrations have been identified as important determinants of cardiac conduction (1, 11, 19, 29, 46). Many studies have now demonstrated that simultaneously altering more than one determinant of cardiac conduction can synergistically modulate conduction velocity (CV) significantly more than even pathological alterations of a single parameter (12, 42, 47, 48).
More interestingly, our laboratory recently determined that altering perfusate composition within concentration ranges consistent with murine plasma ionic concentrations produces complex effects on CV that are interdependent on sodium, potassium, and functional expression of the principal ventricular gap junction protein connexin 43 (Cx43) (11, 12). In previous papers, our laboratory suggested that the effects are consistent with the theory of ephaptic coupling (EpC), as well as GJ coupling. EpC, which can facilitate electrical coupling between myocytes through electric fields that develop in restricted extracellular spaces like the perinexus (12, 47), is dependent on the volume of intercalated disk microdomains, as well as rate of charge depletion from these regions (12, 26). Specifically, our laboratory previously demonstrated that wider perinexi, increased intercellular separation, with low extracellular sodium concentration ([Na+]o) and high extracellular potassium concentration, both of which can reduce rate of charge depletion from the perinexus, was associated with slower impulse propagation through myocardium (12).
The perinexus has been suggested to be a candidate structure of a cardiac ephapse and an important modulator of CV (12, 47). Specifically, previous studies demonstrated that changing tissue hydration in guinea pig with mannitol or albumin can alter bulk interstitial edema (48), and, in the case of mannitol, also increase perinexal width (WP) (47) to modulate cardiac conduction in a manner most consistent with the theoretical predictions of EpC. More recently, our laboratory found that perfusate ion composition can also modulate WP and CV in mouse (12), and we speculated that increasing extracellular calcium concentration ([Ca2+]o) might decrease WP, since intercellular adhesion is calcium sensitive. Therefore, in this study, we hypothesize that [Ca2+]o modulates WP.
Mathematical models of EpC also predict a phenomenon termed self-attenuation, which is similarly dependent on intercellular separation within the intercalated disk and extracellular ion composition (22, 26, 28). More specifically, the mechanism of self-attenuation has been described as the process by which sodium current (INa) attenuates itself by reducing driving force when the cleft between myocytes is sufficiently narrow (22). This delays myocyte activation and manifests as macroscopic conduction slowing. The driving force for INa is the difference between the transmembrane potential (Vm) and the Nernst potential for sodium (ENa). Either increasing Vm or decreasing ENa can reduce the driving force and thereby INa. Therefore, two important components that are required to support self-attenuation are 1) reduced sodium driving force, and 2) reduced width of intercalated disk spaces like the perinexus. Based on these predictions, we also hypothesize that decreasing WP and reducing sodium driving force slows cardiac conduction by a mechanism consistent with ephaptic self-attenuation.
The results of this study demonstrate that increasing [Ca2+]o decreases WP. Furthermore, decreasing WP during normonatremia increased CV, consistent with our laboratory's previous studies (12, 47), and decreasing WP during hyponatremia decreased CV, consistent with computational predictions of ephaptic self-attenuation.
METHODS
All protocols were approved by the Institutional Animal Care and Use Committee at Virginia Polytechnic Institute and State University and conform to the guidelines of the National Institutes of Health Guide for the Care and Usage of Laboratory Animals.
Langendorff Preparations
Mice were anesthetized by inhalation of isoflurane vapors from an isoflurane-soaked cotton gauze in a custom-designed closed chamber. Cervical dislocation was performed upon cessation of respiration and was immediately followed by thoracotomy and excision of the heart. Wild-type (WT; 100% Cx43) and heterozygous (HZ; ∼ 50% Cx43) mice, 10–30 wk old, on the C57BL/6 background were cannulated and Langendorff perfused, as previously described (12), with solutions containing (in mM) 118.3 NaCl, 29 NaHCO3, 4.7 KCl, 1.4 KH2PO4, 1 MgSO4, 10 glucose at pH 7.4. CaCl2 concentration was varied from 1 to 3.4 mM. The perfusate and the bath solution were the same at any given time. The perfusion pressure was maintained at ∼70 mmHg, and the heart was suspended in a bath maintained at ∼37°C along with the perfusates. The process was repeated during hyponatremia (NaCl 91 mM). The osmolarities of the various perfusates were measured using a Wescor VAPRO5520 Vapor Pressure Osmometer and are reported in Table 1.
Table 1.
Perfusate osmolarity
| [Na+]o, mM | [Ca2+]o, mM | Osmolarity, mosM |
|---|---|---|
| 147.3 | 1.0 | 304.3 |
| 147.3 | 1.8 | 303.0 |
| 147.3 | 2.6 | 301.3 |
| 147.3 | 3.4 | 301.3 |
| 120 | 1.0 | 250.3 |
| 120 | 1.8 | 253.0 |
| 120 | 2.6 | 250.3 |
| 120 | 3.4 | 246.3 |
The osmolarity of the perfusates with varying [Na+]o and [Ca2+]o are listed.
Optical Mapping
Hearts (8 solutions × 2 mouse types × 5/7 replicates, N = 96) were perfused with the voltage-sensitive dye, di-4-ANEPPS at a concentration of 4 μM, and excess dye was washed out. Each heart was serially perfused with four solutions of either increasing or decreasing calcium concentration. Hearts were perfused with each solution for ∼8 min before being optically mapped. Motion was reduced by 2,3-butanedionemonoxime (10 mM), and the heart was stabilized against the front glass of the bath by applying slight pressure to the posterior surface. Hearts were paced using a unipolar silver wire placed on the anterior surface and a reference wire at the back of the bath. Stimuli of 1-V amplitude and 1-ms duration at a basic cycle length of 150 ms were applied. CV longitudinal (CVL) and transverse (CVT), anisotropic ratio (AR), and action potential duration (APD) were quantified as previously described (12). Briefly, the heart was excited by 510-nm light, and the excited light passed through a 610-nm filter and then was recorded using the MiCam Ultima CMOS L-camera at a sampling rate of 1,000 frames/s. The maximum rate of rise of the optical action potentials was assigned as activation times and CV vectors were determined by fitting the activation times at every pixel to a parabolic surface. Vectors up to 5 pixels away from a user-defined line indicating the direction of propagation, which fell within ∼3 or 5 mm (CVT and CVL, respectively) from the pacing site, not including the first 2 rows, and whose direction was not more than 7.5° from the direction of propagation, were included in the analysis. This region is roughly illustrated by the orange and green boxes in the isochrones maps (see Figs. 2 and 4). APD was calculated as the time interval between activation and 90% repolarization.
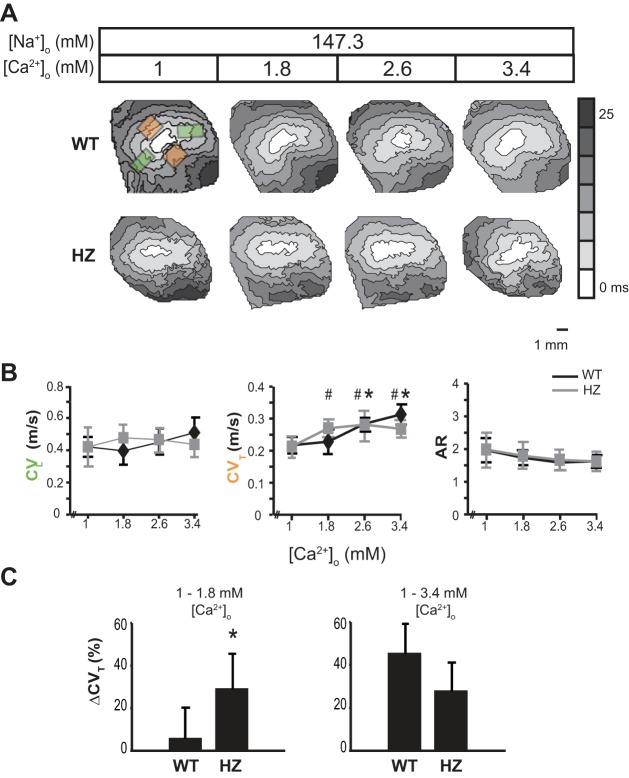
Fig. 2.
Extracellular calcium modulates conduction velocity during normonatremia. A: representative activation maps obtained by optically mapping a single heart during the serial perfusion of solutions with increasing [Ca2+]o. Orange boxes to the top and bottom of the pacing site indicate the regions from which CVT was averaged, and green boxes to the left and right indicate regions from which CVL was averaged. B: average CVT, CVL, and AR (anisotropic ratio) from WT (black line) and HZ (gray line) hearts. C: percent change in CVT between [Ca2+]o = 1–1.8 mM and 1–3.4 mM in WT and HZ hearts demonstrates increased sensitivity of HZ hearts to WP in the former and not the latter range. Values are means ± SD; n = 5. P < 0.05 relative to [Ca2+]o = 1 mM in *WT and #HZ by paired Student's t-test.
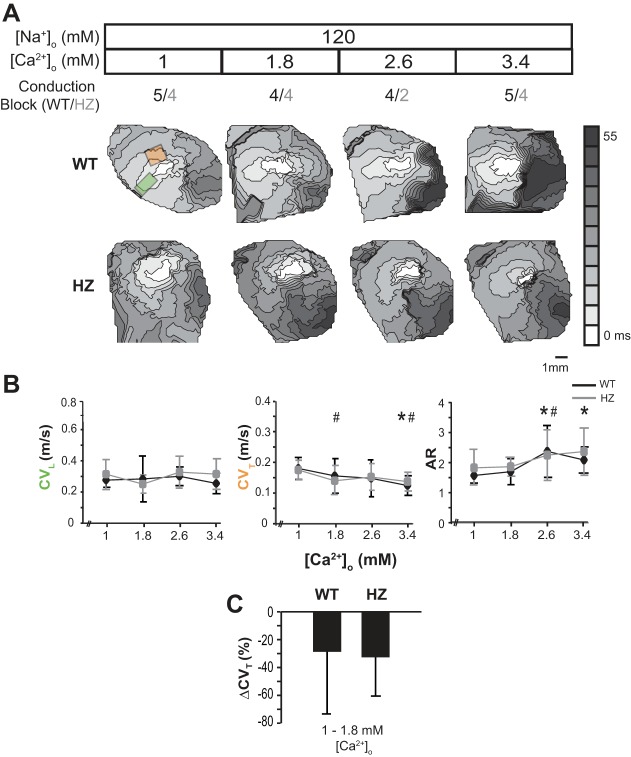
Fig. 4.
Extracellular calcium modulates conduction velocity during hyponatremia. A: representative activation maps obtained by optically mapping a single heart during the serial perfusion of solutions with increasing [Ca2+]o during hyponatremia. The orange box to the top of the pacing site indicates the region from which CVT was averaged, and the green box to the left indicates the region from which CVL was averaged in hearts with CV block over the left ventricle. B: average CVT, CVL, and AR (anisotropic ratio) from WT (black line) and HZ hearts (gray line). C: percent change in CVT between [Ca2+]o = 1 and 1.8 mM in WT and HZ hearts. Values are means ± SD; n = 5. P < 0.05 relative to [Ca2+]o = 1 mM in *WT and #HZ by paired Student's t-test.
Transmission Electron Microscopy
Hearts (7 solutions × 2 mouse types × 3 replicates × 15 images, N = 630) were perfused with the respective solution for 30 min, and 1-mm3 cubes were then fixed overnight in 2.5% glutaraldehyde at 4°C. Samples were washed in PBS and processed for transmission electron micrographs, as previously described (12). The samples were then sectioned onto copper grids, and the sections were imaged at ×150,000 magnification using a JEOL JEM 1400 transmission electron microscope. Perinexal images were then analyzed in a blinded manner by ImageJ to determine WP. It has to be noted here that the WP measurements in this study refer to the intermembrane separation adjacent to the GJ plaque as our laboratory previously reported in mice (12) and guinea pigs (47) and not the spatial extent (distance from the gap junction edge) of the perinexus, as reported by Rhett et al. (35). Additionally, based on our previous studies, WP changes in the plateau portion of this nanodomain (30–105 nm away from the edge of the gap junction) best correlated with CV, and, therefore, values reported here are averages of six measurements at 15-nm intervals from within this region.
Western Immunoblotting
Hearts (4 solutions × 2 mouse types × triplicates run twice, N = 48), perfused with the respective solutions for 30 min, were snap frozen. Tissue was homogenized in a lysis buffer containing (in mM) 50 HEPES, 150 KCl, 1 EDTA, 1 EGTA, 1 DTT, 1 NaF, 0.1 Na3VO4, and 0.5% Triton X-100. Protein concentration was normalized following quantification with the Bio-Rad DC protein assay. SDS-PAGE electrophoresis was performed as previously described (40) using 4–20% NuPage Bis-Tris gels, which were then transferred to a PVDF membrane and blocked with 5% BSA in TNT buffer (0.1% Tween 20, 150 mM NaCl, 50 mM Tris, pH 8.0) at room temperature for 1 h. Membranes were then incubated with rabbit anti-phospho-Cx43Ser368 (1:1,000 in 5% BSA TNT, no. 3511 Cell Signaling Technology) primary antibody overnight at 4°C. Membranes were washed and incubated with goat anti-rabbit horseradish peroxidase secondary antibody (1:5,000, Abcam) for 1 h at room temperature, washed, and bound antibody detected using Clarity Western ECL Substrate (Bio-Rad) and imaged using the Bio-Rad Chemidoc MP system. To detect total Cx43, membranes were first stripped with Re-Blot Plus Strong (Millipore), according to the manufacturer's instructions. Following blocking for 1 h at room temperature in 5% milk in TNT buffer, membranes were incubated with primary antibodies against Cx43 (1:4,000, C6219 rabbit; Sigma Aldrich), and mouse anti-GAPDH (1:4,000 RDI-TRK5G4-6C5, mouse; Research Diagnostics) overnight at 4°C diluted in 5% milk TNT. Membranes were then washed and incubated with the secondary antibodies goat anti-mouse Alexa Fluor 555 and goat anti-rabbit Alexa Fluor 647 (both 1:1,000 in milk TNT, Life Technologies) for 1 h at RT. Following several washes in TNT, membranes were imaged using the Bio-Rad Chemidoc MP System, and protein expression was quantified by densitometry using ImageLab software (Bio-Rad). For quantification, all samples were run together on 26-well gels. Cx43 expression levels were normalized to GAPDH and pCx43 to total Cx43 to compare between samples.
Impedance Spectroscopy
GJ resistance (RGJ) was estimated using the four-point electrode technique as previously described in cardiac tissue (5, 16, 31, 38, 39, 43). The electrical impedance spectra in the mice hearts (4 solutions × 2 mouse types × 5/3 replicates for WT/HZ, N = 64) were measured during perfusion with different solutions. The four-point measurement technique was implemented to reduce the effect of electrode polarization and facilitate measurement at low frequencies. Due to the small size of the heart, a custom-made electrode array with interelectrode distance of ∼200 μm was used. For each measurement, the electrodes penetrated to a depth of 500 μm from the heart surface in an orientation approximately parallel to the epicardial fiber orientation. The rest of the electrode length was insulated to minimize the parasitic conductivity path induced by the perfusion fluid on the heart exterior. The Gamry Instruments Interface 1000 was used in the galvanostatic mode to introduce a 500-μA alternating current between the two outer electrodes, and the two inner electrodes measured the change in voltage, over a range of 1 Hz to 1 MHz.
The resistance-reactance curves between 1 and 100 kHz, obtained from these recordings, were fitted with a circle to determine an index of RGJ, as previously described (2, 4–6). The x-intercepts of the circle were determined, as they correspond to the intracellular resistance (right intercept) and the cytoplasmic resistance (left intercept). The difference between the two is reported as RGJ below. All resistance values are reported as percent change from control (solution containing, 147.3 mM [Na+]o and 1 mM [Ca2+]o), except for the comparison between WT and HZ RGJ, where absolute values were used.
Due to potential inconsistencies associated with anisotropic nature of the tissue and different fiber orientations between animals, data are compared in a paired fashion within hearts to determine whether impedance spectroscopy can detect changes in RGJ due to perfusate composition. Unpaired comparisons between WT and HZ heart were used to determine whether the measurement could detect a 50% reduction in Cx43 protein. Finally, positive control impedance estimates were made in hearts treated with 50 μM carbenoxolone (CBX) or subjected to 30 min of no-flow ischemia (ISC).
Statistical Analysis
All data are reported as means ± SD, unless stated otherwise. Single-factor ANOVA was used to detect differences between WT and HZ WP. Two-tailed, equal sample size and variance, paired/unpaired Student's t-test was performed to determine significant difference in WP, CV, protein expression, as well as RGJ data. Bonferroni correction was applied for multiple comparisons.
RESULTS
Normonatremia-Perinexal Width
To investigate whether [Ca2+]o alters intercellular separations within the intercalated disk, perfusate [Ca2+]o was increased from 1 to 3.4 mM, and WP was quantified from transmission electron micrographs (Fig. 1A) in hearts perfused with normonatremic solutions. WP was not significantly different between WT and Cx43 HZ hearts at any [Ca2+]o, as revealed by a single-factor ANOVA (P = 0.45), and all reported data and comparisons include both WT and HZ WP values.
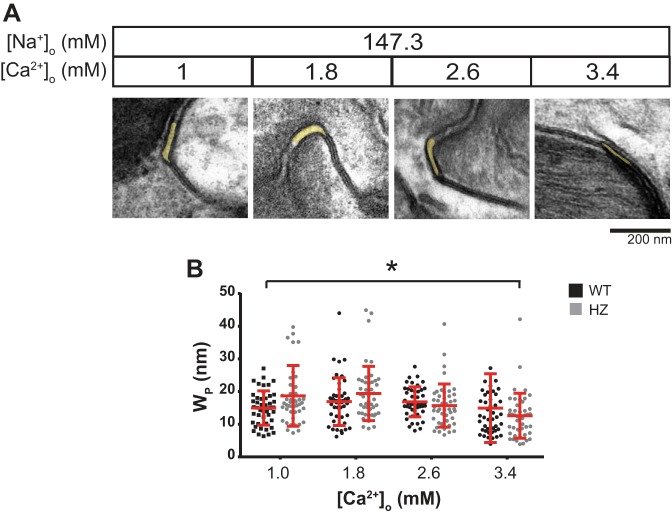
Fig. 1.
Extracellular calcium-perinexal width relationship during normonatremia. A: representative electron micrographs of hearts perfused with varying [Ca2+]o. Perinexi are highlighted in yellow. B: summary data of WP as a function of [Ca2+]o from WT (black) and HZ hearts (gray). Means and SD are indicated by red lines. n = 3 × 15 images. *P < 0.05 between [Ca2+]o = 1 and 3.4 mM by unpaired Student's t-test.
Summary data demonstrate that WP was inversely correlated to [Ca2+]o in the range of 1 to 3.4 mM (Fig. 1B). A single-factor ANOVA reveals a significant relationship between [Ca2+]o and WP (P < 0.001). Post hoc comparison between 1 and 3.4 mM [Ca2+]o reveals that WP is significantly narrower at the higher calcium concentration (17.8 ± 0.8 and 13.8 ± 0.9 nm, means ± SE, respectively). Additionally, increasing [Ca2+]o from 1 to 1.8 mM visually appears to increase in WP; however, this was not significant (P = 0.252). Importantly, the WP values reported here are consistent with those observed in our laboratory's previous study in mouse hearts exposed to a similar range of [Ca2+]o (12).
Normonatremia-Conduction Velocity
Our laboratory previously demonstrated under conditions of physiological [Na+]o in mice as well as guinea pig hearts that WP and CV are inversely correlated (12, 47). Specifically, decreasing WP was associated with faster CV. Representative conduction isochrones in Fig. 2A demonstrate the response of CV to increasing [Ca2+]o, decreasing WP. CVL and CVT vectors were averaged from two regions each that are indicated by green and orange boxes, respectively.
The relationship between CV, [Ca2+]o, and Cx43 expression is complex, as illustrated in Fig. 2B. Increasing [Ca2+]o over the range of 1–3.4 mM did not significantly alter CVL in either WT or HZ hearts. On the other hand, increasing [Ca2+]o over the same range appears to sigmoidally modify CVT in WT hearts. Specifically, increasing [Ca2+]o from 1 to 1.8 mM did not significantly alter CVT. However, increasing [Ca2+]o to 2.6 or 3.4 mM significantly increased CVT (0.22 ± 0.03 to 0.28 ± 0.04 or 0.31 ± 0.03 m/s, respectively) relative to 1 mM [Ca2+]o.
In HZ hearts, increasing [Ca2+]o over the range of 1–3.4 mM only increased CVT for very low [Ca2+]o. Specifically, CVT significantly increased for [Ca2+]o raised from 1 to 1.8 mM (0.21 ± 0.03 and 0.27 ± 0.03, respectively), but CVT was not different between [Ca2+]o of 1.8 and 3.4 mM (0.27 ± 0.03, 0.28 ± 0.02, and 0.27 ± 0.03 m/s). While summary data of AR visually suggest that AR may decrease in both WT and HZ hearts with increasing [Ca2+]o, this relationship was not significant.
Our laboratory's previous studies have suggested that loss of functional gap junctions increases CV sensitivity to cardiac hydration. To explore the unique responses of CVT to [Ca2+]o, the relative change in CVT for WT and HZ hearts is compared for [Ca2+]o between 1 and 1.8, and 1 and 3.4 mM (Fig. 2C). The data reveal that CVT increases more in HZ hearts for [Ca2+]o between 1 and 1.8 mM. Interestingly, the difference is attenuated when comparing CVT for [Ca2+]o between 1 and 3.4 mM, suggesting that CV is more sensitive to WP changes during reduced Cx43 GJ coupling (12, 47). The sensitivity of Cx43 HZ hearts to [Ca2+]o appears to be limited by a mechanism preventing further CV increases even as WP decreases.
Hyponatremia-Perinexal Width
Interestingly, computational models of EpC predict that the relationship between CV and intercalated disk separation may be biphasic, particularly when GJ coupling is reduced (22, 28). In short, for very narrow intercellular separations, conduction can also slow by a process termed “self-attenuation.” To probe whether ephaptic self-attenuation can occur, WP was reduced in the setting of reduced sodium reversal potential and driving force (hyponatremia).
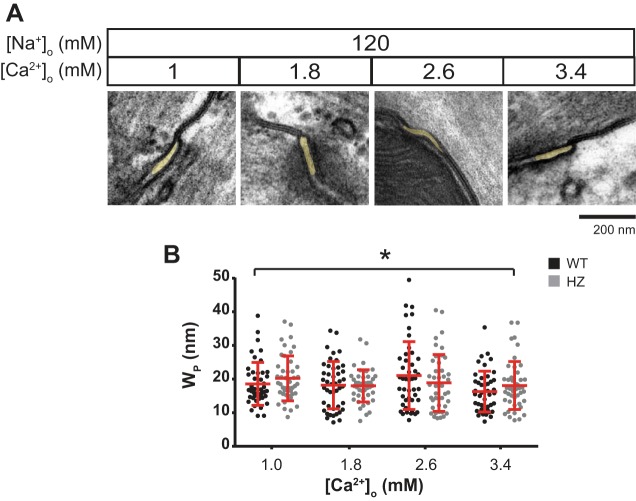
First, the structural relationship between WP-[Ca2+]o was quantified in hearts perfused with a hyponatremic solution ([Na+]o = 120 mM), and representative micrographs are presented in Fig. 3A. Once again, a single-factor ANOVA reported significant differences in WP between solutions (P < 0.05) and [Ca2+]o (1–3.4 mM, Fig. 3B). Further post hoc analysis revealed significantly wider WP at the lowest relative to highest [Ca2+]o (19.4 ± 0.6 vs. 17.2 ± 0.6 nm, means ± SE). Additionally, WP was not different between WT and HZ hearts (P = 0.83) during hyponatremia as well.
Fig. 3.
Extracellular calcium-perinexal width relationship during hyponatremia. A: representative electron micrographs of hearts perfused with varying [Ca2+]o during hyponatremia. Perinexi are highlighted in yellow. B: summary data of WP as a function of [Ca2+]o from WT (black) and HZ hearts (gray). Means and SD are indicated by red lines. n = 3 × 15 images. *P < 0.05 between [Ca2+]o = 1 and 3.4 mM by unpaired Student's t-test.
Hyponatremia-Conduction Velocity
First, it is important to note that hyponatremia was associated with significantly slower CVT relative to normonatremia in both WT and HZ hearts at all [Ca2+]o. Furthermore, representative isochrones maps in Fig. 4A suggest that increasing [Ca2+]o during hyponatremia now decreases CV, and this relationship is different from the normonatremic cases presented in Fig. 2. Lastly, the isochrones suggest increased likelihood of conduction block into the left ventricle in both WT and HZ hearts, particularly at the highest [Ca2+]o. Under these conditions, CV was measured from the right ventricle. The number of hearts in which conduction block was observed is indicated above the isochrones in Fig. 4A, and, in these hearts, CVL and CVT were averaged only in one direction each, as indicated by the green and orange box in Fig. 4A.
In WT hearts, increasing [Ca2+]o from 1 to 1.8 or 2.6 mM did not significantly alter CVL, CVT, or AR. However, increasing [Ca2+]o from 1 to 3.4 mM slowed CVT (0.17 ± 0.03 to 0.12 ± 0.03 m/s) and increased AR (1.6 ± 0.2 vs 2.0 ± 0.5) without measurably changing CVL. The finding that increasing [Ca2+]o during hyponatremia has the opposite effect on CV relative to normonatremia is consistent with theoretical predictions of ephaptic self-attenuation. Specifically, CV increased in hearts with narrow perinexi and high ENa, but decreased in hearts with narrow perinexi and low ENa.
CV slowing was also observed in HZ hearts in response to increasing [Ca2+]o during hyponatremia. Increasing [Ca2+]o slowed CVT (0.17 ± 0.02 to 0.11 ± 0.03, 0.12 ± 0.03 or 0.12 ± 0.03 m/s, respectively) without measurably altering CVL or AR. Interestingly, hyponatremia did not produce a significantly greater CVT response in HZ relative to WT hearts at the lowest [Ca2+]o of 1 and 1.8 mM (Fig. 4C). This finding is consistent with the predictions of mathematical models that, in the self-attenuation range, the effect of GJ coupling on CV is reduced (22, 26, 28).
Extracellular Calcium and GJ Coupling
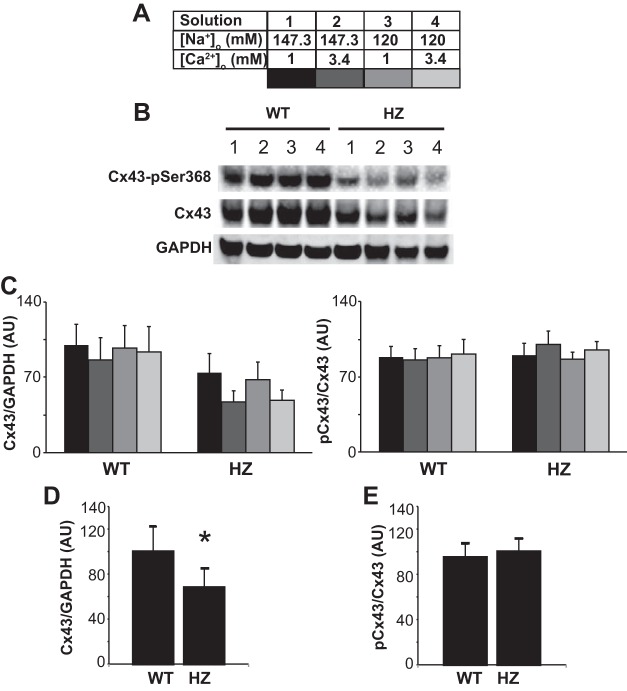
Cx43 expression and phosphorylation.
Calcium ions are an important component of several cell signaling pathways and can modulate the functional states of proteins like Cx43 (17, 27, 36). The effect of varying [Ca2+]o and [Na+]o on total Cx43 and phosphorylated Cx43 at Ser368 (pCx43) were quantified in this study to determine whether [Ca2+]o might alter gap junction expression levels after 30 min of perfusion. Representative and summary data are illustrated in Fig. 5.
Fig. 5.
Cx43 expression during variation in [Na+]o and [Ca2+]o. A: upper and lower limits of the range over which [Na+]o and [Ca2+]o were varied in this study and the color code for the panels below. B: representative Western immunoblots of total and pSer368 Cx43. C: total Cx43 normalized to GAPDH (left) and pCx43 normalized to total Cx43 (right) illustrate that varying [Na+]o and [Ca2+]o do not significantly alter Cx43 expression. D: total Cx43 is significantly reduced in HZ hearts relative to WT. E: the ratio of pCx43 to total Cx43 is not different between WT and HZ hearts. Values are means ± SD; n = 3. *P < 0.05 by unpaired Student's t-test. AU, arbitrary units.
Perfusion with the extremes of the [Ca2+]o and [Na+]o used in this study (Fig. 5A) did not alter the expression of total Cx43 or pCx43 (Fig. 5, B and C). However, it must be noted that, in the HZ hearts, increasing [Ca2+]o to 3.4 mM trended to decrease total Cx43 expression at both high and low [Na+]o (P = 0.080 and 0.094, respectively), but these changes did not reach statistical significance after correction for multiple comparisons. As expected, HZ hearts expressed significantly less total Cx43 expression (Fig. 5D, data normalized to GAPDH), but the ratio of pCx43 to total Cx43 was similar to that of WT hearts (Fig. 5E, data normalized to total Cx43).
GJ resistance.
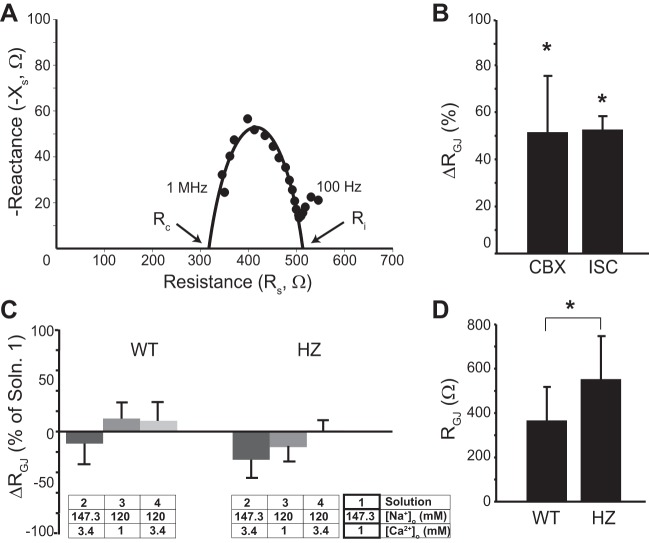
Since protein levels do not necessarily correlate to its function, GJ coupling was estimated by tissue impedance spectroscopy. Figure 6A shows a representative resistance-reactance curve with the best circle fit. In Fig. 6B, the percent change in RGJ relative to control, during perfusion of 50 μM CBX and after 30 min of no-flow ISC, are reported as positive controls. As expected, both CBX and ISC significantly increased RGJ relative to control. Furthermore, the increase in RGJ during inhibition of Cx43 by CBX is consistent with a previous study with guinea pig that reported a similar increase in RGJ in the presence of CBX (5).
Fig. 6.
Tissue impedance during variation in [Na+]o and [Ca2+]o. A: representative resistance (Rs)-reactance (Xs) curve with circle fit. B: percent change (Δ) in gap junctional resistance (RGJ) increases after treatment with 50 μM carbenoxolone (CBX) and 30 min of ischemia (ISC). C: average RGJ values determined during perfusion of solutions with different [Na+]o and [Ca2+]o. D: RGJ resistance is significantly increased in HZ hearts relative to WT. Values are means ± SD. *P < 0.05 by paired Student's t-test in B and C, and unpaired Student's t-test in D.
The percent change in RGJ relative to control, during perfusion of different solutions in WT and HZ hearts, are presented in Fig. 6C. Varying [Na+]o and [Ca2+]o in the range used in this study did not significantly change RGJ. As an additional positive control for demonstrating that impedance spectroscopy can detect loss of functional Cx43, as previously demonstrated (6), RGJ was compared between WT and HZ hearts. Summary data in Fig. 6D demonstrate that RGJ in WT hearts was significantly lower relative to HZ, as expected.
The impedance data coupled with the immunoblotting suggest that the perfusates used in this study either did not alter Cx43 functional expression, or altered it below the resolution for detection. Taken together, the data suggest that varying [Ca2+]o and [Na+]o in the specified range may not change GJ coupling, while these interventions did produce measureable changes in cardiac conduction.
Action Potential Duration
Altering calcium concentration has been demonstrated to modify the functioning of several ion channels like Nav1.5 (21, 44) and the small-conductance calcium-activated potassium channels (52). Modulation of these ion channels can then manifest as changes in APD and morphology, and also alter CV. However, alterations in these proteins have been reported for much larger [Ca2+]o, and the effect of small variations, as used in this study, has not been explored. Therefore, we quantified APD in WT and HZ hearts perfused with 1 and 3.4 mM [Ca2+]o solutions.
Representative action potentials from the same heart perfused with 1 and 3.4 mM [Ca2+]o and 120 mM [Na+]o are presented in Fig. 7A and the summary of APD in Fig. 7B. Importantly, APD did not significantly change in response to [Ca2+]o or [Na+]o, as determined from a single-factor ANOVA (P = 0.9 and 0.2 for WT and HZ, respectively). Additionally, Fig. 7B also demonstrates that Cx43 expression levels do not significantly alter APD for any concentration of [Ca2+]o or [Na+]o.
Fig. 7.
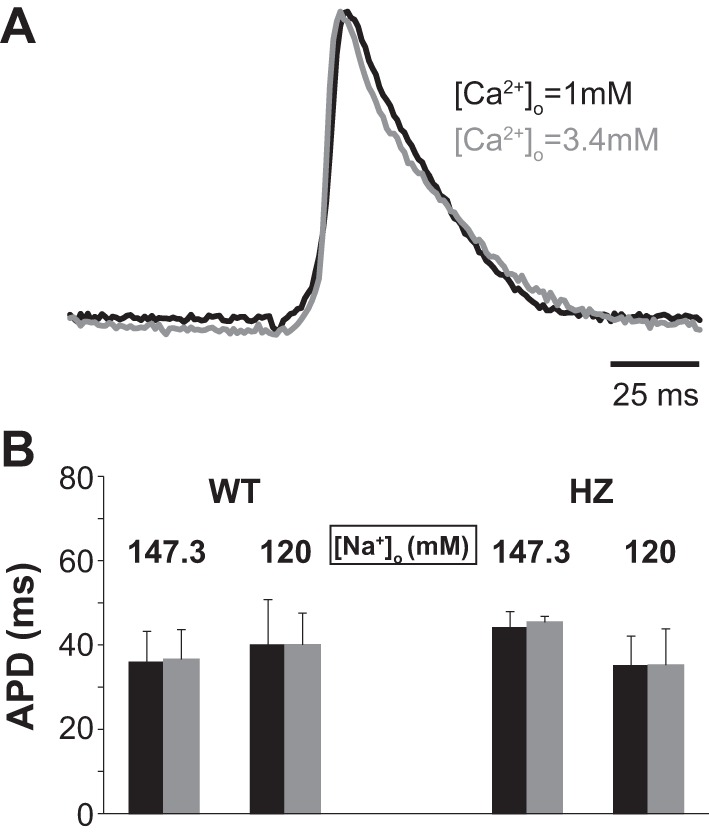
Action potential duration (APD) not affected by [Na+]o, [Ca2+]o, or Cx43 expression. A: representative action potentials from the same heart perfused with [Ca2+]o = 1 mM (black) and 3.4 mM (gray). B: neither [Na+]o, [Ca2+]o, nor Cx43 expression alter mean epicardial APD.
DISCUSSION
The results of this study demonstrate that [Ca2+]o can modulate WP and CV as hypothesized. More specifically, increasing [Ca2+]o from 1 to 3.4 mM reduces WP during both normonatremia as well as hyponatremia. However, the relationship between [Ca2+]o and CV is more complex and dependent on [Na+]o. Specifically, increasing [Ca2+]o can increase CV during normonatremia and decrease CV during hyponatremia. Taken together, these findings suggest that [Ca2+]o modulates WP, which modulates CV differently during normonatremia and hyponatremia by a mechanism that is not gap junction dependent.
Extracellular Calcium and Perinexal Width
At several points along the intercalated disk, transmembrane proteins that form gap junctions, desmosomes, or adherens junctions bind to their counterparts on the apposing cell membrane, structurally holding the two membranes together (24). Several proteins that compose these junctions like N-cadherins, desmocollin, and desmoglein, among others, have calcium-dependent adhesion properties (3, 49, 51). In the absence of calcium or during hypocalcemia, the binding affinity of these proteins could be reduced, making these junctions looser points of contact, which in turn results in greater separation between the membranes at the intercalated disk (10, 14).
Varying [Ca2+]o in this study resulted in WP modulation. Specifically, increasing calcium ion concentration was associated with narrower perinexi or, in other words, WP and [Ca2+]o were inversely correlated. However, this correlation was weaker during hyponatremia.
Ephaptic Coupling
Cellular excitation of cardiac myocytes occurs when transmembrane potential (Vm), which is the difference between the intracellular and extracellular potentials (Vm = Φi − Φo), depolarizes the cell sufficiently to activate voltage-gated sodium channels. The postjunctional cell Vm can rise by either decreasing Φo or increasing Φi. The most commonly accepted method for raising postjunctional Vm is by electrotonically increasing Φi via gap junctions. On the other hand, Φo can be altered by the depletion or accumulation of charge in the extracellular space caused by, for example, withdrawal of sodium or increased potassium in intercellular clefts during the activation of the prejunctional cell. Such a non-GJ, nonsynaptic form of electrical coupling between myocytes is what is termed as EpC, and several more mechanisms for EpC have been mathematically proposed (41).
One important requirement for EpC is the presence of microdomains with closely abutting cell membranes. Several such intercalated disk structures like the perinexus (47) and connexome (23) have been proposed as possible cardiac ephapses with volumes that can be modulated. The study by Veeraraghavan et al. (47) also identified the consequences that WP modulation has on CV by pharmacological as well as mathematical methods. Specifically, narrower perinexi promote faster transmission of electrical impulses from the pre- to the postjunctional cell due to closer spacing of the two membranes. Thus increasing [Ca2+]o, as we did in this study, could increase CV in normonatremic hearts by narrowing perinexi and improving EpC between cells consistent with our laboratory's previous works (12, 47).
In this study, transverse CV was more sensitive to experimental interventions that altered WP and sodium driving force. To understand this finding, it is important to consider that gap junctional uncoupling preferentially alters transverse CV, because the propagating wavefront in the transverse direction encounters more GJs per unit length relative to the longitudinal direction. Parallel to this concept, perinexi, which are theoretical EpC junctions, are also localized to the intercalated disk. Therefore, a wavefront propagating transverse to fibers will encounter more gap and ephaptic junctions relative to the longitudinal direction of propagation. In short, inhibiting EpC is expected to likewise preferentially affect transverse CV.
Self-attenuation
Self-attenuation was previously suggested in mathematical models of cardiac conduction incorporating extracellular field effects as well as a polarized voltage-gated sodium ion channel distribution to the intercalated disk, both of which are essential to support EpC (22, 28). Self-attenuation was described as the process by which peak INa attenuates itself due to reduced cleft width and INa driving force. The INa driving force, as described above, can be reduced by either increasing Vm or decreasing ENa. While two models (22, 26) predict self-attenuation solely based on changes in Vm, the Peskin (28) model additionally tracks ion concentrations and Nernst potentials. Therefore, the models that incorporate both Vm overshoot and changes in ENa within the intercalated disk may be more sensitive to ephaptic self-attenuation (28).
In the present study, cleft width was reduced by increasing [Ca2+]o, and sodium driving force was reduced by reducing [Na+]o. In the case of the high [Na+]o solutions, where sodium driving force was relatively larger, altering WP alone increased CV, consistent with our previous work in guinea pigs and mice, possibly due to modulation of EpC outside of the self-attenuation range reported by mathematical models. Importantly, this study for the first time identifies that, in the setting of reduced sodium driving force (low [Na+]o solutions), the relationship between [Ca2+]o, WP, and CV is consistent with self-attenuation. Specifically, our data are consistent with the hypothesis that reducing WP during reduced [Na+]o further reduces available [Na+]o in the perinexus during sodium-mediated depolarization. Under these conditions, depolarization of the prejunctional cell withdraws a large number of available sodium ions from the intercalated disk microdomain. This charge withdrawal greatly reduces the INa driving force for the postjunctional cell. The reduced driving force then delays propagation of the electrical impulse from the pre- to the postjunctional myocyte, resulting in CV slowing.
It is difficult to compare the results of this work with previous studies investigating the relationship between CV and [Ca2+]o due to significant experimental differences. For example, elevating [Ca2+]o from 1 to 2 mM did not alter CV, while raising [Ca2+]o from 2 to 8 mM significantly slowed CV in false tendons isolated from dog ventricles (33). Another study reported nonsignificant changes in CVL and CVT in dog papillary muscle when [Ca2+]o was varied similar to concentrations used in our study (45). Therefore, while previous studies in different animal models, tissue preparations, and with different perfusate compositions demonstrated that raising [Ca2+]o within some narrow window can decrease or not change CV, to our knowledge this is the first study to demonstrate that CV and [Ca2+]o can positively or negatively correlate, depending on [Na+]o, in a manner consistent with the mathematical predictions of ephaptic self-attenuation (22, 26, 28).
GJ Coupling
Calcium ions are involved in the regulation of several physiological functions and play a key role in various signaling pathways (55). Intracellular calcium homeostasis is tightly regulated by a highly developed system that involves several intracellular calcium stores (9, 20). However, during certain pathophysiological states, intracellular calcium increases, which then results in modulation of several factors that can then alter CV (7). For example, intracellular calcium concentration has been shown to modulate GJ coupling (17, 27, 36). Elevated [Ca2+]i alters Cx43 gating by a Ca2+/calmodulin-dependent pathway and reduces intercellular coupling (54). Cx43 uncoupling has been associated with slow CV when the extracellular ionic composition is altered to weaken EpC (8, 12, 15, 19). However, this study demonstrates that modulating [Ca2+]o (1–3.4 mM) does not change either total or phosphorylated Cx43 expression during normonatremia as well as hyponatremia. Additionally, varying [Ca2+]o did not alter RGJ, and increasing [Ca2+]o was also associated with faster CV during normonatremia. Furthermore, the changes in electrophysiology were measured at a much earlier time point (8 min) compared with Cx43 expression measurements (30 min). These findings suggest that GJ coupling may not be the mechanism by which [Ca2+]o modulates CV in this study.
Limitations
Modulating [Ca2+]o could have several physiological implications in the heart, which includes altered contraction, calcium handling, cell signaling pathways, and apoptosis (55). [Ca2+]o was varied from 1 to 3.4 mM in this study, which is a range wider than the normal physiological [Ca2+]o, which typically falls between 1.7 and 2.5 mM in mice (34). Previous studies investigating the effects of hypercalcemia have reported significant changes in GJ coupling at much higher [Ca2+]o (>5 mM) (27, 53). Such [Ca2+]o increases could also alter ionic currents, which could in turn modulate CV (21, 44, 52), even though changes in APD were not significantly different between interventions.
The perinexus has been identified as the site of dense localization of several ion channels, and it is possible that downregulation of Cx43 in HZ mice could affect the structure of the perinexus. First, this study demonstrates that intercellular separation within the perinexus (WP) is not different between WT and HZ hearts for any intervention. Furthermore, it has been previously demonstrated in the same mouse model that the structure of GJ plaques in HZ mice was not different compared with WT, although the number of GJ plaques was reduced (37). This suggests that at least the GJ plaque and intercellular separation are conserved, even when GJs are genetically reduced. However, this does not exclude the possibility that the spatial extent of the perinexus, distance from the GJ edge, is altered in HZ animals, and this requires further investigation, specifically of the three-dimensional structure of the perinexus.
Finally, application of the four-electrode tissue impedance spectroscopy technique in whole heart preparations to estimate GJ coupling has many limitations based on anisotropic cell size, space constant, and extracellular conductance. These factors can significantly confound estimates of RGJ (18). Therefore, RGJ in this paper should be interpreted as an index of GJ coupling, and not an absolute estimate of gap junction conductance. Importantly, recent studies by Drs. Pollard and Barr demonstrated that impedance spectroscopy can well estimate RGJ in cardiac tissue when electrode spacing and diameters fall below the space constant for cardiac tissue (32, 50). Specifically, we used electrodes made with 180-μm-diameter Kingli acupuncture needles with 200 μM spacing between electrodes and an exposed needle depth of 500 μM. Furthermore, estimates of RGJ that are compared in this study are made between recordings obtained from the same electrode position in the same tissue (except WT to HZ) to minimize the aforementioned confounding variables.
Conclusions
Extracellular ion concentration is a crucial component in modulating cardiac conduction. This study identifies that extracellular sodium and calcium composition could differently modulate CV during health and conditions like hyponatremia commonly associated with several diseases that include congestive heart failure (30), kidney (25), and liver (13) disorders. Specifically, while reducing WP by increasing [Ca2+]o was beneficial and increased CV during normonatremia, it slowed CV during hyponatremia. Furthermore, the changes in CV reported in this study were independent of Cx43 protein expression, phosphorylation, or RGJ, suggesting the role of a non-GJ-mediated mechanism for CV modulation like EpC.
These data reinforce the importance of regulating serum ion concentration during health and, more importantly, during disease. Furthermore, there is now mounting evidence that modulating parameters predicted to alter EpC can be used as a tool in the treatment of conduction-based disorders in the heart.
GRANTS
This work was supported by an R01-HL-102298 awarded to S. Poelzing, and a Virginia Tech Carilion Research Institute Medical Research Scholar Award, an American Heart Association Predoctoral fellowship, and the David W. Francis and Lillian Francis Scholarship Fund awarded to S. A. George. R. Davalos acknowledges the support from the Institute for Critical Technologies and Applied Sciences of Virginia Tech.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.A.G., M.B., M.Z., R.D., J.W.S., and S.P. conception and design of research; S.A.G., M.B., M.Z., and J.W.S. performed experiments; S.A.G., M.B., M.Z., R.D., and J.W.S. analyzed data; S.A.G., M.B., M.Z., R.D., J.W.S., and S.P. interpreted results of experiments; S.A.G., M.B., M.Z., J.W.S., and S.P. prepared figures; S.A.G., M.B., M.Z., J.W.S., and S.P. drafted manuscript; S.A.G., M.B., M.Z., R.D., J.W.S., and S.P. edited and revised manuscript; S.A.G., M.B., M.Z., R.D., J.W.S., and S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Robert G. Gourdie for assistance in determining tissue morphology from electron microscopy images.
REFERENCES
- 1.Ballantyne F 3rd, Davis LD, Reynolds EW Jr. Cellular basis for reversal of hyperkalemic electrocardiographic changes by sodium. Am J Physiol 229: 935–940, 1975. [DOI] [PubMed] [Google Scholar]
- 2.Chapman RA, Fry CH. An analysis of the cable properties of frog ventricular myocardium. J Physiol 283: 263–282, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chitaev NA, Troyanovsky SM. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein, and desmocollin, contributes to cell-cell adhesion. J Cell Biol 138: 193–201, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooklin M, Wallis WR, Sheridan DJ, Fry CH. Changes in cell-to-cell electrical coupling associated with left ventricular hypertrophy. Circ Res 80: 765–771, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon PS, Chowdhury RA, Patel PM, Jabr R, Momin AU, Vecht J, Gray R, Shipolini A, Fry CH, Peters NS. Relationship between connexin expression, and gap-junction resistivity in human atrial myocardium. Circ Arrhythm Electrophysiol 7: 321–329, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Dhillon PS, Gray R, Kojodjojo P, Jabr R, Chowdhury R, Fry CH, Peters NS. Relationship between gap-junctional conductance, and conduction velocity in mammalian myocardium. Circ Arrhythm Electrophysiol 6: 1208–1214, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Di Diego JM, Antzelevitch C. High [Ca2+]o-induced electrical heterogeneity, and extrasystolic activity in isolated canine ventricular epicardium. Phase 2 reentry. Circulation 89: 1839–1850, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res 51: 681–690, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Fearnley CJ, Roderick HL, Bootman MD. Calcium signaling in cardiac myocytes. Cold Spring Harb Perspect Biol 3: a004242, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganote CE, Grinwald PM, Nayler WG. 2,4-Dinitrophenol (DNP)-induced injury in calcium-free hearts. J Mol Cell Cardiol 16: 547–557, 1984. [DOI] [PubMed] [Google Scholar]
- 11.George SA, Poelzing S. Cardiac conduction in isolated hearts of genetically modified mice–connexin43 and salts. Prog Biophys Mol Biol 120: 189–198, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George SA, Sciuto KJ, Lin J, Salama ME, Keener JP, Gourdie RG, Poelzing S. Extracellular sodium, and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene. Pflugers Arch 467: 2287–2297, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gines P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology 48: 1002–1010, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Greve G, Rotevatn S, Saetersdal T, Oksendal AN, Jynge P. Ultrastructural studies of intercalated disc separations in the rat heart during the calcium paradox. Res Exp Med (Berl) 185: 195–206, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest 99: 1991–1998, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain SK, Schuessler RB, Saffitz JE. Mechanisms of delayed electrical uncoupling induced by ischemic preconditioning. Circ Res 92: 1138–1144, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Kagiyama Y, Hill JL, Gettes LS. Interaction of acidosis, and increased extracellular potassium on action potential characteristics and conduction in guinea pig ventricular muscle. Circ Res 51: 614–623, 1982. [DOI] [PubMed] [Google Scholar]
- 18.Kleber AG, Riegger CB. Electrical constants of arterially perfused rabbit papillary muscle. J Physiol 385: 307–324, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation, and associated arrhythmias. Physiol Rev 84: 431–488, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kohlhaas M, Maack C. Calcium release microdomains and mitochondria. Cardiovasc Res 98: 259–268, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, Leymaster ND, Dun W, Wright PJ, Cardona N, Qian L, Mitchell CC, Boyden PA, Binkley PF, Li C, Anderson ME, Mohler PJ, Hund TJ. Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation 126: 2084–2094, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res 91: 1176–1182, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leo-Macias A, Liang FX, Delmar M. Ultrastructure of the intercellular space in adult murine ventricle revealed by quantitative tomographic electron microscopy. Cardiovasc Res 107: 442–452, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Radice GL. A new perspective on intercalated disc organization: implications for heart disease. Dermatol Res Pract 2010: 207835, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CS, Cheng CJ, Shih KC, Lin SH. Recurrent hyponatremia in a patient with chronic kidney disease. J Nephrol 19: 394–398, 2006. [PubMed] [Google Scholar]
- 26.Lin J, Keener JP. Microdomain effects on transverse cardiac propagation. Biophys J 106: 925–931, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer P, Weingart R. Cell pairs isolated from adult guinea pig and rat hearts: effects of [Ca2+]i on nexal membrane resistance. Pflügers Arch 409: 394–402, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc Natl Acad Sci U S A 105: 6463–6468, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nygren A, Giles WR. Mathematical simulation of slowing of cardiac conduction velocity by elevated extracellular. Ann Biomed Eng 28: 951–957, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Oren RM. Hyponatremia in congestive heart failure. Am J Cardiol 95: 2B–7B, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Padilla F, Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M, Inserte J, Soler-Soler J. Protection afforded by ischemic preconditioning is not mediated by effects on cell-to-cell electrical coupling during myocardial ischemia-reperfusion. Am J Physiol Heart Circ Physiol 285: H1909–H1916, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Pollard AE, Barr RC. A structural framework for interpretation of four-electrode microimpedance spectra in cardiac tissue. Conf Proc IEEE Eng Med Biol Soc 2014: 6467–6470, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pressler ML, Elharrar V, Bailey JC. Effects of extracellular calcium ions, verapamil, and lanthanum on active and passive properties of canine cardiac purkinje fibers. Circ Res 51: 637–651, 1982. [DOI] [PubMed] [Google Scholar]
- 34.Research Animal Resources University of Minnesota. Reference Values for Laboratory Animals. Normal Hematology Values (Online). http://www.ahc.umn.edu/rar/refvalues.html [2013].
- 35.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol 245: 411–422, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudisuli A, Weingart R. Electrical properties of gap junction channels in guinea-pig ventricular cell pairs revealed by exposure to heptanol. Pflügers Arch 415: 12–21, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Saffitz JE, Green KG, Kraft WJ, Schechtman KB, Yamada KA. Effects of diminished expression of connexin43 on gap junction number, and size in ventricular myocardium. Am J Physiol Heart Circ Physiol 278: H1662–H1670, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez JA, Rodriguez-Sinovas A, Fernandez-Sanz C, Ruiz-Meana M, Garcia-Dorado D. Effects of a reduction in the number of gap junction channels or in their conductance on ischemia-reperfusion arrhythmias in isolated mouse hearts. Am J Physiol Heart Circ Physiol 301: H2442–H2453, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Smith WT, Fleet WF, Johnson TA, Engle CL, Cascio WE. The Ib phase of ventricular arrhythmias in ischemic in situ porcine heart is related to changes in cell-to-cell electrical coupling. Experimental Cardiology Group, University of North Carolina. Circulation 92: 3051–3060, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human, and mouse myocardium. J Clin Invest 120: 266–279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sperelakis N. An electric field mechanism for transmission of excitation between myocardial cells. Circ Res 91: 985–987, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Stein M, van Veen TA, Remme CA, Boulaksil M, Noorman M, van Stuijvenberg L, van der Nagel R, Bezzina CR, Hauer RN, de Bakker JM, van Rijen HV. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction. Cardiovasc Res 83: 52–60, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Stiles DK, Oakley BA. Four-point electrode measurement of impedance in the vicinity of bovine aorta for quasi-static frequencies. Bioelectromagnetics 26: 54–58, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AA, Anderson ME, Balser JR. A calcium sensor in the sodium channel modulates cardiac excitability. Nature 415: 442–447, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Veenstra RD, Joyner RW, Rawling DA. Purkinje and ventricular activation sequences of canine papillary muscle. Effects of quinidine and calcium on the Purkinje-ventricular conduction delay. Circ Res 54: 500–515, 1984. [DOI] [PubMed] [Google Scholar]
- 46.Veeraraghavan R, Gourdie RG, Poelzing S. Mechanisms of cardiac conduction: a history of revisions. Am J Physiol Heart Circ Physiol 306: H619–H627, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veeraraghavan R, Lin J, Hoeker G, Keener J, Gourdie R, Poelzing S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch 467: 2093–2105, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veeraraghavan R, Salama ME, Poelzing S. Interstitial volume modulates the conduction velocity-gap junction relationship. Am J Physiol Heart Circ Physiol 302: H278–H286, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vleminckx K, Kemler R. Cadherins, and tissue formation: integrating adhesion and signaling. Bioessays 21: 211–220, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Waits CM, Barr RC, Pollard AE. Sensor spacing affects the tissue impedance spectra of rabbit ventricular epicardium. Am J Physiol Heart Circ Physiol 306: H1660–H1668, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol Biol Cell 11: 1077–1092, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Li T, Zhang L, Yang Y, Zeng XR. [Effects of intracellular calcium alteration on SK currents in atrial cardiomyocytes from patients with atrial fibrillation]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 30: 296–300, 305, 2014. [PubMed] [Google Scholar]
- 53.Weingart R. The actions of ouabain on intercellular coupling, and conduction velocity in mammalian ventricular muscle. J Physiol 264: 341–365, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Q, Kopp RF, Chen Y, Yang JJ, Roe MW, Veenstra RD. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am J Physiol Cell Physiol 302: C1548–C1556, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarain-Herzberg A, Fragoso-Medina J, Estrada-Aviles R. Calcium-regulated transcriptional pathways in the normal and pathologic heart. IUBMB Life 63: 847–855, 2011. [DOI] [PubMed] [Google Scholar]