We used mice with specific deletion of the endothelial small-conductance Ca2+-activated potassium 3 (SK3) channel in vascular endothelium to study the SK3 subcellular localization, interaction with transient receptor potential vanilloid 4 (TRPV4) channels, and contribution to vasodilation. We found that Ca2+ signals with large-amplitude and slow-decay kinetics associated with SK3-TRPV4 microdomains modulate vascular tone and animal blood pressure.
Keywords: small conductance Ca2+-activated potassium channel, intermediate-conductance Ca2+-activated potassium channel, transient receptor potential vanilloid 4, calcium microdomain, endothelium
Abstract
Activation of vascular endothelial small- (KCa2.3, SK3) or intermediate- (KCa3.1, IK1) conductance Ca2+-activated potassium channels induces vasorelaxation via an endothelium-derived hyperpolarization (EDH) pathway. Although the activation of SK3 and IK1 channels converges on EDH, their subcellular effects on signal transduction are different and not completely clear. In this study, a novel endothelium-specific SK3 knockout (SK3−/−) mouse model was utilized to specifically examine the contribution of SK3 channels to mesenteric artery vasorelaxation, endothelial Ca2+ dynamics, and blood pressure. The absence of SK3 expression was confirmed using real-time quantitative PCR and Western blot analysis. Functional studies showed impaired EDH-mediated vasorelaxation in SK3−/− small mesenteric arteries. Immunostaining results from SK3−/− vessels confirmed the absence of SK3 and further showed altered distribution of transient receptor potential channels, type 4 (TRPV4). Electrophysiological recordings showed a lack of SK3 channel activity, while TRPV4-IK1 channel coupling remained intact in SK3−/− endothelial cells. Moreover, Ca2+ imaging studies in SK3−/− endothelium showed increased Ca2+ transients with reduced amplitude and duration under basal conditions. Importantly, SK3−/− endothelium lacked a distinct type of Ca2+ dynamic that is sensitive to TRPV4 activation. Blood pressure measurements showed that the SK3−/− mice were hypertensive, and the blood pressure increase was further enhanced during the 12-h dark cycle when animals are most active. Taken together, our results reveal a previously unappreciated SK3 signaling microdomain that modulates endothelial Ca2+ dynamics, vascular tone, and blood pressure.
NEW & NOTEWORTHY
We used mice with specific deletion of the endothelial small-conductance Ca2+-activated potassium 3 (SK3) channel in vascular endothelium to study the SK3 subcellular localization, interaction with transient receptor potential vanilloid 4 (TRPV4) channels, and contribution to vasodilation. We found that Ca2+ signals with large-amplitude and slow-decay kinetics associated with SK3-TRPV4 microdomains modulate vascular tone and animal blood pressure.
endothelium-derived hyperpolarization (EDH) (16) is dependent on SK3 (small-) and IK1 (intermediate-conductance Ca2+-activated potassium) channel activation. However, the contribution of SK3 and IK1 channels to EDH varies in different vascular beds and states (13, 33). Recent evidence suggests one mechanism by which SK3 and IK1 channels induce vasodilation in systemic vessels and reduces blood pressure (7, 26, 48) is via Ca2+ coupling to transient receptor potential vanilloid type 4 (TRPV4) (2, 31, 42, 45). Ca2+ influx or release from internal stores activates SK3/IK1 and induces membrane hyperpolarization that creates a positive driving force for more Ca2+ influx via TRPV4. Thus, TRPV4 and SK3/IK1 form a positive feedback loop. Endothelial membrane hyperpolarization spreads into the smooth muscle causing vasorelaxation; this is the EDH pathway.
Both SK3 and IK1 channels are activated by intracellular Ca2+ binding to calmodulin, which is constitutively bound to the COOH-terminal domain of each subunit. They are highly sensitive to calcium activation with submicromolar KD values (∼0.5 μM) (20, 50). However, there are also important distinctions between SK3 and IK1 channels. For example, the conductance of IK1 channels is ∼4 times larger than that of SK3 channels. More importantly, recent studies in the endothelium have shown that IK1 and SK3 channels differ in their subcellular distribution (40, 49), trafficking (28), gene regulation (51), and Ca2+ sources for activation. For example, IP3-dependent internal Ca2+ release preferentially activates IK1 channels (27). Dependent upon their subcellular localization, TRPV4-induced SK3 or IK1-mediated EDH would differ. Both SK3 and TRPV4 channels associate with caveolin-1 (1, 39), suggesting the formation of caveolar SK3-TRPV4 Ca2+ microdomains. Moreover, TRPV4-dependent activation of IK1 is also evident and may dominate in certain conditions (46, 51).
Our previous studies using global IK1 knockout models, with or without SK3 knockdown, have shown that their activity modulates vascular tone. Notably, reducing global expression of these channels leads to hypertension (7, 48). An assumption was that hypertension was a result of reduced EDH signaling due to the lack of SK3/IK1 channels. Because SK3 channels are also expressed in other cell types and organs, such as liver, kidney, and brain (3, 5, 6, 21, 38), in this study, we tested the hypothesis that selectively removing SK3 channels from vascular endothelium alone results in elevated blood pressure. We generated a novel endothelium-specific SK3 knockout (SK3−/−) mouse by crossing a floxed SK3 mouse with a mouse that expresses endothelial Cre recombinase driven by the Tie2 promoter (23, 37). Studies using mesenteric arteries obtained from these SK3−/− mice showed 1) a lack of SK3 channels in endothelial cells, 2) an absence of SK3-mediated vasorelaxation, 3) redistribution of TRPV4 channels, 4) modulation of Ca2+ signals, and 5) elevated blood pressure. Thus, the present study uncovers a distinct involvement of endothelial SK3 channels in Ca2+ signaling, vascular tone, and blood pressure regulation.
METHODS
Animals and tissue.
All animal experimental procedures were approved by the University of South Alabama Institutional Animal Care and Use Committee and were conducted according to the “Guide for the Care and Use of Laboratory Animals.” Two groups of adult mice (8–16 wk of age) with C57BL/6J background were used in the present study: wild-type (WT; Charles River Laboratories, Willimantic, CT) and endothelium-specific knockout of SK3 channel (SK3−/−) mice. Using a strategy similar to the generation of global SK3 knockout mice (12), we generated these endothelium-specific SK3−/− mice using the floxed SK3 mice crossed to an endothelium-specific Cre-expressing mouse (Jackson Laboratories, Bar Harbor, ME) (23). Transgenic pups were genotyped for Cre and floxed SK3 (SK3f/f) expressions.
Mice were euthanized with isoflurane overdose and collected tissues were placed in ice-cold HEPES buffer solution containing (in mM): 134 NaCl, 6 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 glucose, and 10 HEPES (pH 7.4). Mesenteric arteries were carefully dissected from surrounding tissues in ice-cold HEPES solution with low MgCl2 and CaCl2 (0.2 and 0.1 mM, respectively). Depending on the protocol, vessels were homogenized, digested enzymatically to obtain dispersed endothelial cells, pinned en face on a piece of silicone gel (Sylgard 184, Dow Corning, Midland, MI), or mounted in a wire myograph.
Western blot analysis.
Mouse mesenteric arteries (first- to fourth- order branches) were isolated and homogenized in ice-cold lysis buffer (in mM: 50 Tris-base, 150 NaCl, 1 EDTA, 1% NP-40, 0.5% sodium deoxycholate; and 1× protease inhibitor). Samples from organ lysates were prepared similarly. Cerebral vessels were pooled from six mice to obtain sufficient protein. Lysates were sonicated for 30 s on ice and then centrifuged at 13,000 rpm for 10 min. Supernatant was collected and analyzed for the protein concentration using Coomassie Plus protein assay reagent (Thermo Scientific, Pittsburgh, PA). Sample buffer containing 5% β-mercaptoethanol was boiled for 5 min to denature proteins before loading. For each sample, 40 μg of protein were separated with 10% SDS-polyacrylamide gel. The resolved proteins were electrophoretically transferred to a nitrocellulose membrane using Bio-Rad Mini Trans-Blot cell at 110 V for 60 min at 4°C. Membranes were blocked with 5% nonfat milk dissolved in PBS containing 0.5% vol/vol Tween-20 for 60 min at room temperature. Membranes were immunoblotted with primary antibodies against SK3 (APC-025, lot no. AN0802; Alomone, Jerusalem, Israel), TRPV4 (ACC-034, lot no. ACC034AN1550, Alomone), Cre-recombinase (MAB3120, lot no. 2387475; Millipore, Temecula, CA), IK1 (ALM-051, lot no.AN03100), and caveolin-1 (BD610407, lot no. 41652; BD Biosciences, San Jose, CA). A horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or fluorescence labeled (Dylight) secondary antibodies from Thermo Scientific (Pittsburgh, PA) were used. Blots were developed using a Kodak automated film processing system and imaged using a digital imager (GE Healthcare Bio-Sciences, Pittsburgh, PA), or visualized using an Odyssey infrared imaging system (LI-COR Biosciences), according to manufacturer's instructions.
Real-time quantitative PCR.
Total RNA was extracted from WT and SK3−/− mouse mesenteric arteries (first- to fourth-order branches) using TRIzol as per the manufacturer's instructions. The quality of the purified RNA was controlled by measurement of the A260/A280 nm ratio. Only RNA samples exhibiting an A260/A280 ratio >1.70 were used in further experiments. The concentration was quantified using NanoDrop Spectrophotometer (Thermo Scientific). cDNA was synthesized from 100 ng of total RNA using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time qPCR was performed in an iCycler thermal cycler system (Bio-Rad) using SYBR Green qPCR Master Mix kit (Thermo Scientific). Amplification temperatures and calibration curves (Cx vs. logarithm of the starting concentration) were predetermined for each primer sets from a dilution series of lysates. Primer sequences, programmed annealing temperature, and size of PCR products are listed in Table 1. Three independent reactions were performed for each set, prepared from four mice of each genotype. The thermal cycling conditions included an initial heat denaturing step at 95°C for 9 min, followed by denaturation at 95°C for 15 s, annealing at optimal programmed temperature of primers (as shown in Table 1) for 30 s and polymerization at 72°C for 30 s for 40 cycles. Following amplification, the melting curves of PCR products were determined from 55 to 95°C to determine the specificity of amplification. The expression levels were normalized to expression of GAPDH as an endogenous control by using 2ΔΔCT comparative method:
Table 1.
qPCR summary
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product, bp | Annealing, °C | SK3−/−/WT |
|---|---|---|---|---|---|
| Cre | GCGGTCTGGCAGTAAAAACTATC | GTGAAACAGCATTGCTGTCACTT | 100 | 56 | 18.45 ± 1.39 |
| SK3 | GCTCTGATTTTTGGGATGTTTG | CGATGATCAAACCAAGCAGG | 148 | 55 | 0.10 ± 0.03 |
| IK1 | TGCACGCTGAGATGTTGTGG | GTGTCTGTGAGGTGCCCCGT | 584 | 63 | 1.61 ± 0.34 |
| TRPV4 | CGTCCAAACCTGCGAATGAAGTTC | CCTCCATCTCTTGTTGTCACTGG | 186 | 60 | 1.25 ± 0.02 |
| Cav1 | GGGCAACATCTAGAAGCCCAACAA | CTGATGCACTGAATTCCAATCAGGAA | 371 | 53 | 1.39 ± 0.07 |
| GADPH | GGTGCCAACCCCAAACGTAT | CTTTCACAGCCTCCTTGATAGCA | 88 | 51 |
qPCR conditions and results are presented with list of primer sequences, size of PCR products, programmed annealing temperatures for the primers used in this study, as well as the normalized results comparing mRNA expression in SK3−/− and wild-type (WT) mesenteric arteries using ΔΔCT comparative method. n = 4 mice from each genotype.
where X is the gene of interest, SK denotes SK−/− mice, WT, denotes wild-type mice, CT is the cycle count, and Ref denotes GAPDH.
Confocal Ca2+ imaging and analysis.
First- and second-order mesenteric arteries were cut into 2-mm sections and opened longitudinally; en face fillets were pinned on a silicone block with the endothelium facing up. Blocks were submerged in HEPES solution containing membrane-permeable Ca2+-sensitive fluorescent dye Fluo-4 AM (10 μM) for 30 min at room temperature. Tissue blocks were kept in the dark during incubation. After a 5-min wash, blocks (intima facing down) were placed on two parallel pins in a custom glass-bottom chamber containing HEPES solution. Recordings of transient changes in fluorescence intensity were captured at 20× using an inverted microscope fitted with a PerkinElmer spinning disk RS-3 confocal unit. Fluo-4 was excited at 488 nm, and fluorescent images were collected at 510 nm. Using a lower-magnification objective enabled us to perform population study of Ca2+ signals in a layer of 100–150 endothelial cells (18). The basal activity of intracellular Ca2+ was captured for 200 s, and histograms were generated from these recordings (n = 14 WT, n = 9 SK3−/−). Additional 300-s recordings were used to compare Ca2+ activity in the absence and presence of TRPV4 agonist. GSK was added at 120 s, and the difference in Ca2+ signal prior and after GSK was quantified. Ca2+ events were pooled to generate histogram plots to study GSK response (n = 6 each WT and SK3−/− vessels). Data were acquired at eight frames per second at 25°C using PerkinElmer Ultraview software. Analysis of changes in Ca2+ fluorescence intensities was performed offline using custom automated region of interest (ROI) algorithm ImageJ plugin software (LC_Pro, http://rsb.info.nih.gov/ij/plugins/lc-pro/index.html) to detect and track Ca2+ events (17, 18). Individual Ca2+ traces were further analyzed using IgorPro. Fluorescence signal (F) from each ROI (6.3-μm diameter) is subtracted from its background signal (F0) and normalized to obtain ΔF/F0. Seven WT mice and five SK3−/− mice were used in these Ca2+ studies.
Immunostaining.
Mesenteric arteries (first- and second-order branches) were prepared and pinned on silicone blocks, as described above. Vessels were fixed in 4% paraformaldehyde for 5 min at room temperature. Following fixation, vessels were washed with PBS for 40 min (4 × 10 min washes). Sections were blocked in PBS containing 5% goat serum, 5% BSA and 0.1% Triton X-100 for 1 h at room temperature. Sections were then incubated overnight at 4°C with antibodies directed against caveolin-1, SK3, and TRPV4 (1:300 in PBS containing 1% BSA and 0.02% Triton X-100). The same primary antibodies were used as in Western blot studies. Finally, sections were washed with PBS and incubated with secondary antibody (Dylight 550-conjugated goat anti-rabbit IgG and Dylight 633-conjugated goat anti-mouse IgG; Thermo Scientific). Fluorescent images were acquired on a Nikon A-1 Spectral confocal microscope using a water immersion objective magnification of ×60 (NA: 1.2). Immunolabeled TRPV4 signals were expressed as 85-μm line-scan intensity plots, drawn perpendicular to the length of vessel (using ImageJ). The signal distance intervals and counts were compared between WT and SK3−/− tissues. For quantification of immunolabeling and internal elastic lamina (IEL) holes, images of combined z stacks were adjusted by contrast to clearly define the edges immunolabeling and holes via the ImageJ threshold and particle analyzer commands and converted into two-dimensional binary maps, as described previously (22). Binary maps were compared, and the amount of signal colocalization was calculated as a percentage of immunolabeled signals overlapping with the IEL holes. Five WT and four SK3−/− mice, with two mesenteric artery segment from each mouse, were used for this study. One to two samples were recorded from each segment, depending on the immunostaining quality.
Blood pressure measurement.
A group of age-matched mice (12–16 wk) underwent surgical implantation of a PA-C10 radiotelemetry transmitter (Data Sciences International, St. Paul, MN). The implantation surgery was performed under general anesthesia using a mixture of ketamine and xylazine, followed a standard procedure and guideline provided by the manufacturer. Briefly, a 1-cm pressure-sensitive catheter was inserted from the left carotid artery into the aortic arch and then secured with sutures. The wireless transmitter was placed subcutaneously in the abdominal region. Immediately postoperatively, animals were placed in a clean cage on top of a warming pad to recover and monitored until ambulatory. Mice received daily subcutaneous injections of Buprenex for 2 days after surgery and recovered for 4 days before measurements were initiated. Animals were free to roam in their individual cages, and blood pressures were recorded using Ponemah Physiology Platform (Data Sciences International) every 30 s, at a sampling frequency of 500 Hz, 24 h/day in a room with a 12:12-h light-dark cycle for 7 days.
Myography.
Small second- and third-order mesenteric arteries (∼100–200 μm outer diameter) were used for myography studies. Previous studies have shown that the contribution of SK3 channel activity to vasodilation is enhanced in smaller arteries and arterioles (44). On average, four vessel segments were used from each mouse. Arteries mounted in a wire myograph (Danish Myo Technology, DMT, Aarhus, Denmark) were bathed in 37°C bicarbonate-based physiological salt solution (PSS; in mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 0.026 EDTA, 10.5 glucose, and 23 NaHCO3, constantly bubbled with 95% O2-5% CO2. Vessels were equilibrated for 20 min and stretched to their optimal resting tension of ∼2 mN, as determined in previous studies (48, 51), followed by equilibration for another 10 min before the start of experiments. For cumulative concentration-response studies, arteries were bathed in different concentrations of phenylephrine (PE), followed by bath incubation in 60 mM KCl PSS (replacement of 60 mM NaCl with 60 mM KCl) containing (in mM): 59 NaCl, 64.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 0.026 EDTA, 10.5 glucose, and 23 NaHCO3 at 37°C to obtain maximum tension. PE concentration-response curves were normalized to KCl-induced maximum force for each vessel. After bath washout for at least three times, vessels were precontracted with PE to ∼80% (EC80) of maximum tension and cumulative concentrations of ACh were bath applied to determine its concentration responses.
To study the contribution of SK3 channels to ACh-induced vasorelaxation, following the same equilibration periods as described, arteries were precontracted with 3 μM PE (EC80), relaxed with subsequent addition of 1 μM ACh, followed by brief incubation in 60 mM KCl PSS. After at least three washes, vessels were preincubated in the presence of 1 μM TRAM-34 [1-[(2-cholorophenyl)diphenylmethyl]−1H-pyrazole) to block IK1 channels], 100 μM l-NAME (NG-nitro-l-arginine methyl ester) to block nitric oxide production, and 10 μM indomethacin to block prostacyclin production for 20 min. A small amount of PE was added empirically to the bath to induce ∼50% precontraction, and then 1 μM ACh was added. ACh-induced vasorelaxation were normalized to PE-induced constriction and compared in the absence and presence of inhibitors. Arteries that did not show ACh-induced endothelium-dependent vasorelaxation, hence, indicating damage to the endothelium, were discarded. Myography data were both acquired and analyzed using LabChart 7 (DMT).
Cell isolation and electrophysiology.
To obtain dispersed endothelial cells (ECs), cleaned mesenteric arteries (first- and second-order branches) were placed in 37°C HEPES solution containing (in mM): 55 NaCl, 80 Na-glutamate, 5.9 KCl, 2 MgCl2, 0.1 CaCl2, 10 glucose, and 10 HEPES (pH 7.3), with 0.5 mg/ml protease, 0.5 mg/ml elastase for 50 min, followed by an additional 5 min in the same solution containing 0.5 mg/ml collagenase (51). The tissue was then washed several times with ice-cold Ca2+-free HEPES solution and triturated with a fire-polished pasture pipette. Isolated ECs were kept in the ice-cold solution; recordings were completed within 6 h.
Whole cell voltage-clamp recordings were performed on isolated ECs using an Axopatch 200B amplifier, Digidata 1322A, and data were acquired using pClamp 8 software (all from Molecular Devices, Sunnyvale, CA). Cells were clamped at their resting membrane potential, and whole cell currents were evoked every 30 s with a voltage protocol consisting of three segments: a 20-ms hyperpolarizing step for membrane capacitance measurement and a 200-ms voltage ramp from −80 to +60 mV (30). Currents were sampled at 2 kHz and filtered at 1 kHz. We obtained current density by normalizing currents to the membrane capacitance, which was calculated from a 5-mV hyperpolarizing step. External bath solution contained (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES (pH 7.3, 280 mosmol). For whole cell recordings, patch pipettes (∼4 MΩ) were filled with (in mM): 130 K+ gluconate, 4 KCl, 4 NaCl, 4 EDTA, 10 HEPES, 5.13 MgCl2, 1.01 CaCl2 1.01, 4 MgATP, 0.3 GTP, and phosphocreatine 10 (pH 7.2, 308 mosmol). The concentrations of free Mg2+ (0.9 mM) and Ca2+ (3 μM) were calculated using Patcher's Power Tools (Department of Membrane Biophysics at MPI Biophysical Chemistry in Göttingen, Germany). For perforated patch recordings, patch pipettes solution contained amphotericin B (150 μg/ml). Perforated patch recordings were performed to study direct TRPV4 activation of SK3 and/or IK channels using a similar voltage ramp protocol. Recordings were performed after obtaining a stable access resistance (∼14 min after gigaseal), monitored with a hyperpolarizing pulse (28, 29, 47). GSK was used to activate TRPV4, followed by TRAM-34 and apamin to block IK and SK3 channels, respectively. Osmolarity for all solutions was verified with Osmette III osmometer (Precision systems, Natick MA). Series resistance and membrane capacitance were monitored to ensure recording quality and were not compensated. Patch-clamp results were obtained from endothelial cells isolated in this manner from four animals of each genotype.
Data and analysis.
Excel (Microsoft, Redmond, WA) was used for general data computation. Myography data were analyzed using LabChart (DMT). IgorPro (WaveMetrics, Lake Oswego, OR) were used to analyze electrophysiological data and to plot figures shown in this study, unless noted otherwise. A selective TRPV4 antagonist, HC067047 (50 nM), was used as negative control for GSK. Averaged and normalized data are expressed as means ± SE. Paired two-sample t-tests were used to determine significance of data from the same vessel or cell; t-tests, ANOVA, or Wilcoxon Rank tests were used to determine significance among different groups of data. P < 0.05 was considered significant.
RESULTS
Deletion of vascular endothelial SK3 channels.
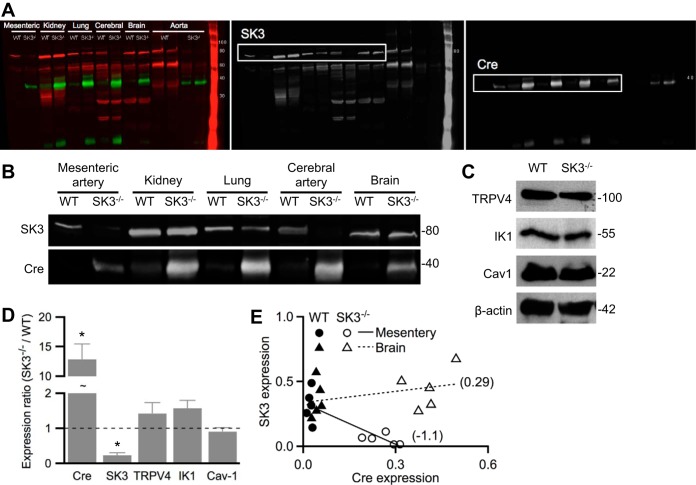
To selectively delete SK3 channels from vascular endothelium, we crossed floxed homozygous SK3 (SK3f/f) mice with those expressing an endothelium-specific Tie2-driven Cre recombinase (12, 23). Endothelial SK3 gene expression is deleted (SK3−/−) in progeny expressing both the floxed allele and endothelial Cre recombinase. Successful ablation of endothelial SK3 expression was confirmed by real-time quantitative PCR performed using mesenteric arteries isolated from SK3−/− and WT mice. As expected, results showed negatively correlated Cre and SK3 mRNA levels (SK3−/−/WT ΔΔCT for Cre = 18.5 ± 1.4, and for SK3 = 0.10 ± 0.03; n = 4 mice per genotype; Table 1).
Western blot analysis further confirmed that in SK3−/− mice, isolated arteries lacked SK3 proteins (Fig. 1, A and B). In contrast, SK3 expression was not different in whole organ lysates obtained from brains, lungs, and kidneys of SK3−/− and WT mice, supporting endothelium-specific deletion of SK3 channels. These results are consistent with previous studies that showed SK3 channels are only expressed in the ECs but not smooth muscle cells (40, 48). We next determined whether the expressions of caveolin-1, TRPV4, and IK1 change in SK3−/− mesenteric artery. Results showed unchanged mRNA and protein levels of TRPV4, IK1, and caveolin-1 (Table 1, Fig. 1, C and D; P > 0.05); however, SK3 protein expression was largely absent in SK3−/− (SK3−/−/WT: 0.23 ± 0.07; n = 5 mice each; P < 0.05; Fig. 1D). Linear regression plots of normalized protein levels showed negative correlation between SK3 and Cre in mesenteric artery but not in brain lysate, indicating SK3 expression was abolished in a tissue-specific manner in SK3−/− (Fig. 1E).
Fig. 1.
Lack of endothelial small-conductance Ca2+-activated potassium 3 (SK3) channels in tissue-specific SK3−/− mice. Western blot analysis performed on tissues obtained from homozygous floxed SK3 expressing endothelium-specific Cre-recombinase (SK3−/−) or wild-type (WT) mice. A: representative blots prepared from whole brain, lung, or kidney lysate, or lysates of aortas or cerebral or mesenteric arteries, were probed with anti-SK3 or anti-Cre recombinase antibodies. Color channels were separated to show SK3 (red) and Cre (green) expression in black and white. B: black and white representation of the indicated sections in A with enhancement of brightness/contrast. C: representative blots prepared from WT and SK3−/− mesenteric arteries were probed for transient receptor potential vanilloid 4 (TRPV4), IK1, and caveolin-1 (cav-1), and the expression densitometry analyses were normalized to β-actin signal. D: protein expression ratios in SK3−/− vs. WT. Asterisks (*) denote statistical significance using Wilcoxon rank test (P < 0.05) comparing SK3−/− and WT protein densitometry normalized to β-actin. Data are expressed as means ± SE; n = 5 mice from each genotype. E: expression of SK3 and Cre normalized to β-actin in WT (solid) and SK3−/− (empty) mesenteric artery (circle) or brain lysate (triangle). Linear regression fits (lines) and slopes (numbers in brackets) show negative correlation of SK3 and Cre in mesenteric arteries. Each point represents an animal; n = 5 animals from each genotype.
Loss of SK3 contribution to mesenteric vasorelaxation in SK3−/−.
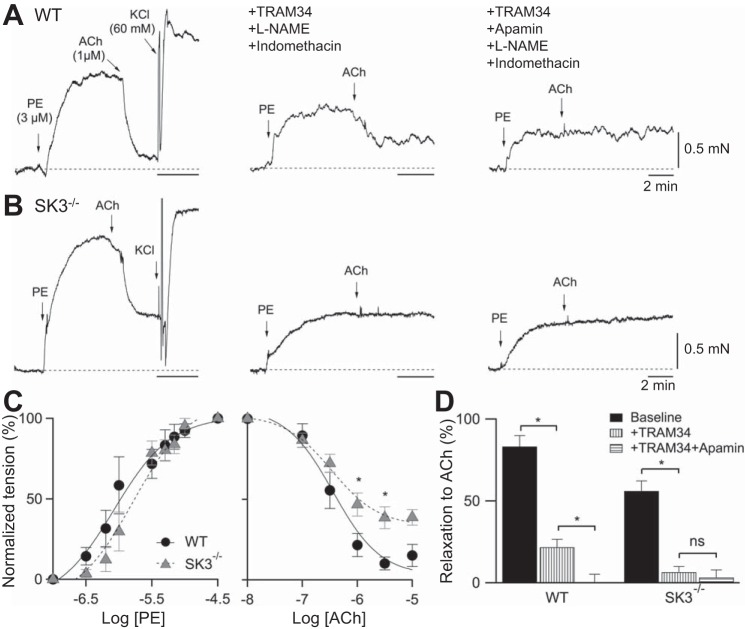
Functional studies using global transgenic SK3 knockdown or overexpressing mice, or using pharmacological blockade of SK3 channels with the specific blocker apamin, have shown that SK3 channel activity contributes to ACh-induced vasorelaxation (4, 11, 45, 51). To verify the functional absence of SK3-dependent EDH in SK3−/−, we studied mesenteric artery ACh vasorelaxation. Application of 3 μM of PE induced similar vasocontraction, while notably, 1 μM of ACh induced vasorelaxation was reduced in SK3−/− vessels (Fig. 2, A and B, left; WT: 87% vs. SK3−/−: 58%). Application of 60 mM of KCl (replacement of 60 mM NaCl with 60 mM KCl) also caused a comparable, maximal increase in vascular tension (WT: 2.1 ± 0.4 mN, n = 7; SK3−/−: 2.5 ± 0.4 mN, n = 8; P > 0.05), indicating similar maximal depolarization-induced contractile responses in these vessels. In a separate experiment, we also determined the concentration response for PE and ACh. Although the amount of ACh relaxation was reduced in SK3−/−, half-maximal responses for PE and ACh were not significantly different between WT and SK3−/− vessels (PE: EC50: WT = 1.1 ± 0.4 μM, SK3−/− = 1.7 ± 0.6 μM; n = 6. ACh: IC50: WT = 0.36 ± 0.06 μM, n = 6; SK3−/− = 0.34 ± 0.04 μM; n = 11; Fig. 2C).
Fig. 2.
Lack of SK3-dependent vasorelaxation in SK3−/− vessels. A and B: representative force myograph traces showing isometric tension plotted against time for WT and SK3−/− mesenteric arteries. Tension responses to ACh (1 μM) induced vasorelaxation in the absence (left), and presence of inhibitors and TRAM-34 (middle), and apamin (right). Bath application of PE (phenylephrine, 3 μM) induced vasoconstriction and ACh-induced vasorelaxation, were followed by high KCl (60 mM NaCl:KCl replacement) bath solution replacement that induced vasoconstriction (left). After bath washout, the vessels were preincubated in l-NAME, and then indomethacin (inhibitors) and TRAM-34 for 20 min, followed by PE to induce 50% maximum contraction. 1 μM ACh induced vasorelaxation (middle). Force measurements were further recorded in the presence of inhibitors TRAM-34 and apamin (right). Horizontal bars, 2 min; vertical bars, 0.5 mN. C: normalized concentration-responses of mesenteric arteries to PE-induced vasorelaxation and ACh-induced vasoconstriction. n = 6 vessels from each genotype mice. *P < 0.05; t-test. D: summary of the normalized basal ACh-induced relaxation in baseline conditions, in the presence of inhibitors and TRAM-34 with or without apamin, for WT and SK3−/− vessels. Statistical significance comparing within each genotype (*P < 0.05, paired t-test; ns, not significant). Data are expressed as means ± SE. Baseline: n = 13; TRAM-34: n = 5 vessels from each genotype.
To further examine whether the reduced ACh-dependent vasorelaxation in SK3−/− is attributable to the loss of SK3 channels, we used selective inhibitors to block NO, prostacyclin (PGI2), and EDH. In the presence of l-NAME (100 μM), indomethacin (10 μM), and TRAM-34 (1 μM; selective IK1 antagonist), ACh-induced vasorelaxation was reduced in WT (53%), and absent in SK3−/− vessels (Fig. 2, A and B, middle). Notably, ACh-mediated vasorelaxation was abolished in both vessels in the presence of additional 300 nM apamin (Fig. 2, A and B, right). Summary results demonstrated that SK3/IK channels contribute to EDH responses and that the SK3 component is lost in SK3−/− mice (Fig. 2D).
Loss of SK3 currents in SK3−/− ECs.
We next examined SK3 channel activity in ECs isolated from mesenteric arteries using patch-clamp techniques. Conventional whole cell recordings were performed on acutely dispersed ECs (51) in the presence of 3 μM free internal Ca2+ to activate both SK3 and IK1 channels. Currents were elicited with a 200-ms voltage ramp and were normalized to the membrane capacitance as current density (Fig. 3). Bath application of TRAM-34 (1 μM) decreased current density by 50.8%, and subsequent addition of apamin (300 nM) further decreased it by 31.4%. Isolated IK1 and SK3 current densities obtained from digital subtraction were 53.9 and 16.4 pA/pF at +60 mV, respectively (Fig. 3A). Whole cell recordings on SK3−/− ECs showed that TRAM-34 reduced the current density by 58.5%, and digitally isolated IK1 current density was 62.1 pA/pF at +60 mV. Importantly, additional apamin did not further reduce the current, indicating the absence of SK3 channels in SK3−/− cells (Fig. 3B).
Fig. 3.
Absence of TRPV4-induced SK3 activation in SK3−/− ECs. A and B: representative WT and SK3−/− ECs current densities elicited by a voltage ramp (200 ms, −80 to +60 mV) using whole cell recordings. After a stable baseline recording (control), sequential additions of TRAM-34 (T34, 1 μM) and apamin (Apa, 300 nM) selectively blocked IK1 and SK3 channels, respectively. Bottom: digitally isolated IK1 and SK3 current density. C and D: representative WT and SK3−/− current densities recorded using a perforated patch. Baseline whole cell currents were elicited by a similar 200-ms voltage ramp in the absence (control) and presence of GSK (10 nM). GSK, TRAM-34, and apamin-sensitive current density were digitally isolated as TRPV4-activated IK1 and SK3 currents shown at the bottom panels, respectively. E and F: summary of SK3 and IK1 current densities using whole-cell (E) or perforated-patch (F) recordings from WT and SK3−/− ECs. Asterisk (*) denotes statistical significance using t-tests (P < 0.05) between the two animal groups. Data are expressed as means ± SE. Whole-cell: n = 6; WT cells: n = 7; SK3−/− cells, perforated: n = 7; WT cells, n = 8: SK3−/− cells.
We performed perforated-patch whole cell recordings to study whether TRPV4 activation provides a Ca2+ source for SK3 and IK1 activation, and whether TRPV4-SK3 coupling is lost in SK3−/− ECs. Without dialyzing Ca2+ to disrupt the intracellular milieu, recordings using the perforated-patch configuration did not elicit large outward currents in basal conditions. Bath-applied 10 nM GSK (GSK1016790A, a selective agonist for TRPV4 channels), elicited whole cell currents that were sensitive to IK and SK3 antagonists (Fig. 3, C and D). TRAM-34 reduced the current density by 35.9% and 57.2%, and subsequent apamin further reduced it by 32.1% and 5.0% in WT and SK3−/− ECs, respectively. Current density summaries are shown in Fig. 3, E and F for conventional and perforated-patch recordings. Notably, the current density of SK3 was absent in SK3−/− ECs (whole cell: 2.3 ± 2.7 pA/pF, n = 7; perforated: 1.2 ± 2.4 pA/pF, n = 8 cells). Together, these results indicate TRPV4 Ca2+ influx activates SK3/IK channels and directly confirmed the lack of SK3 currents in SK3−/− ECs.
Changes in endothelial TRPV4 distribution in SK3−/− mesenteric arteries.
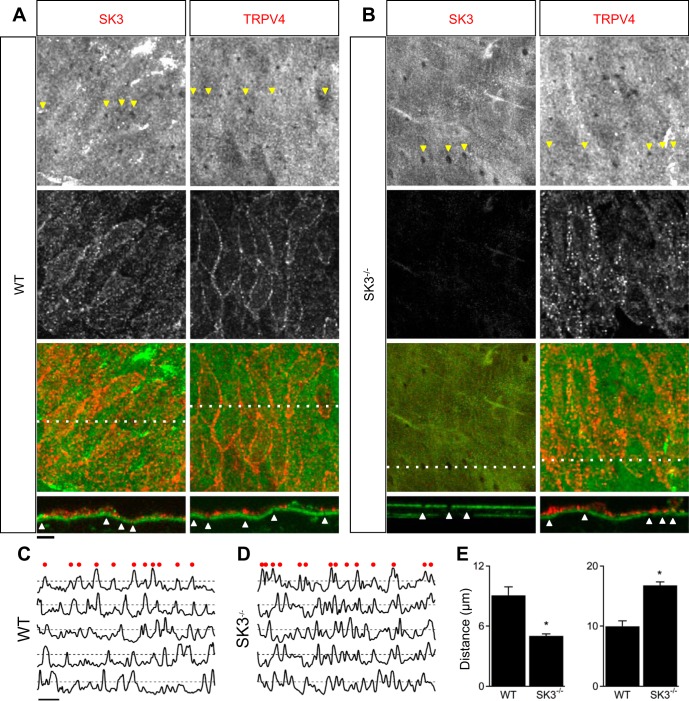
Results from previous studies showing coimmunoprecipitation of endothelial caveolin-1 and SK3 (1), as well as caveolin-1 and TRPV4 (39), suggest that SK3 and TRPV4 channels may associate with the scaffolding protein caveolin-1 and colocalize in caveolae. We performed immunostaining to visualize endothelial SK3 and TRPV4 distribution in WT and SK3−/− mesenteric arteries. Figure 4 shows z stack labeling of SK3 (left) and TRPV4 (right) in WT (Fig. 4A) and SK3−/− vessels (Fig. 4B). Green autofluorescence of IEL that separates endothelial and smooth muscle layers showed holes (arrowheads) in the IEL that may correspond to myoendothelial projection sites (Fig. 4, A and B, top). SK3 and TRPV4 channels were independently detected using their respective rabbit primary antibodies, followed by red fluorescence-tagged secondary antibody (Fig. 4, A and B, middle). Combined green/red fluorescence is shown in Fig. 4, A and B, bottom. A cross section through the tissue is indicated by the dotted line; the orthogonal view is shown below, with arrowheads highlighting IEL holes. Unfortunately, some indicated holes are not apparent in two dimensions due to convolution of the holes, fenestration, and/or thickness of sections. Our results showed that SK3 and TRPV4 expression was limited to ECs (above the IEL), being absent from smooth muscle cells (below the IEL). Labeling of SK3 showed homogeneous distribution with the channels expressed on both basolateral and apical membranes. TRPV4 expression was more apparent at EC-EC borders and mostly on the basolateral membrane in WT (Fig. 4A). As expected, SK3 labeling was absent in SK3−/− mesenteric arteries (Fig. 4B, left). Importantly, TRPV4 labeling in SK3−/− showed a distinct pattern different from that observed in WT. Instead of outlining EC-EC borders, TRPV4 channels showed punctate, as well as diffuse, labeling in SK3−/− ECs (Fig. 4B, right). TRPV4 expression patterns were further quantified using line-scan of fluorescent signals. Normalized signals with fluorescent intensity above an arbitrary 50% threshold were counted as positive signals (Fig. 4, C and D). Both distance between TRPV4 signals (Fig. 4E; WT: 9.1 ± 0.8 μm; SK3−/−: 5.0 ± 0.2 μm), and positive counts (WT: 10.0 ± 1; SK3−/−: 16.8 ± 0.6) were significantly different (n = 5 WT vessels; n = 4 SK3−/− vessels; P < 0.05, t-test). These results suggest that the distribution of TRPV4 channels is influenced by SK3 channel expression. We further examined whether TRPV4 distribution corresponds to IEL holes, but fluorescence binary threshold and proximity analysis failed to show positive correlation (see methods).
Fig. 4.
Ablation of endothelial SK3 disrupts TRPV4 subcellular localization. Immunostaining of SK3 and TRPV4 using WT (A) and SK3−/− mesenteric arteries (B). Top: autofluorescence from the internal elastic lamina (IEL) shows IEL holes of putative myoendothelial projections shown in grayscale. Middle: immunolabeling of SK3 or TRPV4 shown in grayscale. Bottom: overlay of green IEL autofluorescence and red SK3 or TRPV4 immunolabeling. Dotted lines indicate cross sections with the orthogonal view shown as bottom insets. Arrowheads indicate IEL holes. C and D: normalized TRPV4 fluorescence intensity measurements across a line drawn perpendicular to the longitudinal direction of vessel. Top traces show TRPV4 intensity in WT (A) and SK3−/− (B) vessels measured across the lines. Dashed lines indicate 50% threshold, and red dots above indicate positive signals. Bottom traces show measurements taken from other preparations. E: summary of distance between positive signals (left) and total positive signals per 85-μm line scan. Horizontal scale bars = 10 μm. *P < 0.05, t-test. WT vessels: n = 5; SK3−/− vessels: n = 4.
SK3 channels contribute to endothelial Ca2+ signal.
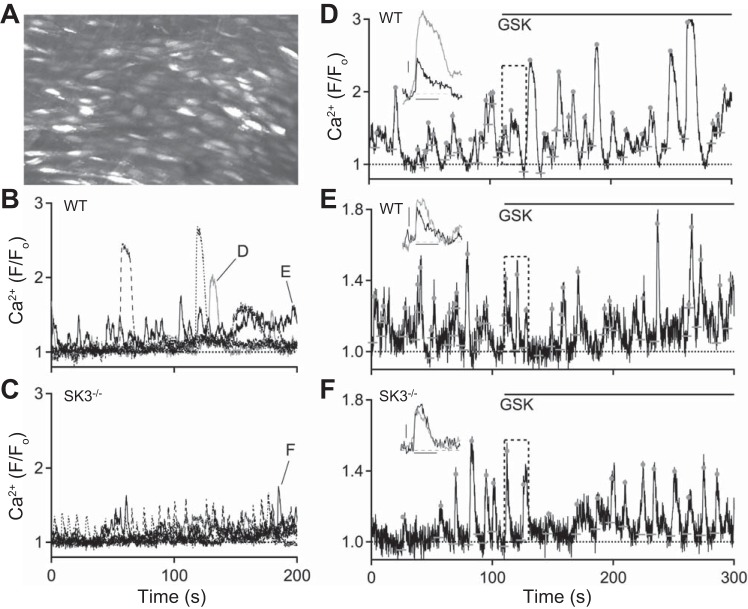
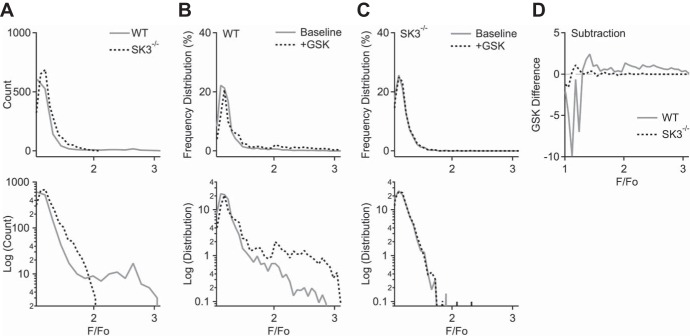
In WT endothelium Ca2+ sparklets, via activation of a cluster of TRPV4 channels, are sufficient to activate IK1 and SK3 channels (45, 46). Our results suggest that the subcellular distribution of TRPV4, and thus the Ca2+ signaling microdomains orchestrated by them, may be dependent upon SK3 expression. Therefore, we next examined endothelial Ca2+ signals in basal conditions and in the presence of GSK (30 nM). Figure 5A shows a representative maximum Ca2+ intensity projection over 200 s obtained from a WT vessel (35). Bright spots show EC Ca2+ signals. Automated LC_Pro analysis was used to detect, quantify, and identify Ca2+ event ROIs. Figures 5, B and C show five Ca2+ signals plotted against time for WT and SK3−/− vessels, respectively. WT Ca2+ signals consisted of both large-and small-amplitude Ca2+ events in basal conditions. Notably, the large-amplitude Ca2+ signals were absent in SK3−/− endothelium. Table 2 shows the quantification of Ca2+ events in basal conditions. SK3−/− ECs showed a twofold increase in both number of Ca2+ sites and events from a field containing similar number of ECs (WT: n = 14, SK3−/−: n = 9 vessels; P < 0.05). Of note, while large-amplitude Ca2+ events were present in WT vessels, these events were infrequent compared with small-amplitude events. As a result, although the averaged F/F0 amplitude of all Ca2+ events did not differ in WT and SK3−/− ECs (WT: 1.26 ± 0.03; SK3−/−: 1.36 ± 0.03, P = 0.051; Table 2), the maximum event amplitude was consistently larger in WT vs. SK3−/−. This point is further expanded in Fig. 6.
Fig. 5.
Absence of large-amplitude Ca2+ events in SK3−/− endothelium. A: representative z stack of maximum fluorescent Ca2+ signal in an en face preparation of WT mesenteric artery focusing on the endothelial layer. B and C: representative Ca2+ dynamics obtained from WT and SK3−/−. Notice the absence of large Ca2+ events in arteries obtained form SK3−/− mice. D and E: Ca2+ analysis from same regions of interest (ROI; indicated in B) that show large (D) or small (E) Ca2+ amplitude. 30 nM GSK, added at 110 s, induces changes in Ca2+ dynamics. Insets show averaged Ca2+ events (aligned to horizontal baseline bars; dots denote peaks) before (black) and after (gray) GSK. Activation of TRPV4 increases the amplitude and duration of large Ca2+ events in WT endothelium (D, inset). F: Ca2+ analysis from same ROI shown in C (indicated by black trace; SK3−/− vessel). Inset: averaged Ca2+ events before (black) and after (gray) 30 nM GSK. In SK3−/− vessel, GSK increases Ca2+ event frequency, but not amplitude. Dashed rectangular marquees indicate GSK bath exchange and traces within the marquees were not included in analysis. Inset scale bars: vertical 0.1, horizontal 5 s. WT vessels: n = 14; SK3−/− vessels: n = 9.
Table 2.
Summary of Ca2+ events
| Site | Event | Amplitude | Duration, s | Spread, μm2 | AUC | |
|---|---|---|---|---|---|---|
| Summary of Basal Ca2+ Activity | ||||||
| WT | 51 ± 6 | 103 ± 19 | 1.26 ± 0.03 | 7.2 ± 1.2 | 13.5 ± 3.0 | |
| SK3−/− | 101 ± 15* | 181 ± 24* | 1.36 ± 0.03 | 7.1 ± 0.9 | 16.9 ± 2.1 | |
| Normalized Activity Change in Response to GSK, % | ||||||
| WT | 138 ± 14 | 109 ± 14 | 100 ± 0.3 | 174 ± 14 | 121 ± 8 | 203 ± 32 |
| SK3−/− | 169 ± 12 | 126 ± 18 | 101 ± 0.8 | 124 ± 14 | 106 ± 14 | 219 ± 78 |
Summary of Ca2+ dynamic analysis using LC_Pro obtained from WT and SK3−/− endothelium. Ca2+ dynamics were analyzed using the automated Ca2+ detection program LC_Pro, including number of Ca2+ sites, total number of events, amplitudes (F/FO), duration, and spread of Ca2+ signal. WT: n = 14; SK3−/−: n = 9.
P < 0.05 comparing WT and SK3−/− using t-tests. A summary of each of these Ca2+ parameters in the presence of GSK is normalized to that prior to the application of GSK. n = 6 each. AUC, area under curve.
Fig. 6.
SK3-TRPV4 mediate large Ca2+ signal. A: histogram plots of Ca2+ event amplitude distribution in WT and SK3−/− endothelium. Ca2+ event amplitudes (F/F0), pooled from WT (n = 14) and SK3−/− (n = 9) 200-s recordings, were plotted on linear (top) and log-scale y-axes (bottom). X-axes were binned with 0.07 width units. B and C: relative frequency distribution of Ca2+ amplitudes before (baseline, solid line) and after the application of 30 nM GSK (dotted line) in WT (B) and SK3−/− endothelium (C), on linear (top) and log scale (bottom). Analyses were pooled from 300-s recordings (n = 6 recordings from each genotype). X-axes were binned with 0.06 width units. D: relative frequency distribution differences induced by GSK in WT (solid) and SK3−/− endothelium (dashed). GSK-dependent increase in Ca2+ event amplitude was larger in WT.
Next, we studied whether TRPV4 activation affects endothelial Ca2+ dynamics. 30 nM GSK increased the number of Ca2+ sites by 138 ± 14% in WT and 169 ± 12% in SK3−/− endothelium (Table 2). The WT Ca2+ signals from ROIs that generated large or small amplitude were plotted in Fig. 5, D and E, respectively. Bath-applied GSK further increased the amplitude and duration of the large Ca2+ events (Fig. 5D; before GSK: F/F0 amplitude = 1.33 ± 0.05, half-time = 2.7 ± 0.4 s, n = 15 events; after GSK: amplitude = 1.67 ± 0.11; half-time = 4.8 ± 0.5 s, n = 18 events; P < 0.05 for both amplitude and half-time; t-test). In contrast, GSK did not affect small Ca2+ amplitude (from 1.19 ± 0.03, n = 17 events to 1.26 ± 0.04, n = 15 events) or half-time (from 2.1 ± 0.3 to 2.5 ± 0.4 s; P > 0.05). Similarly, in SK3−/− ECs, GSK did not change the shape of the small Ca2+ events (Fig. 5F; amplitude from 1.33 ± 0.06, n = 6 events to 1.27 ± 0.03, n = 11 events; half-time from 2.6 ± 0.7 to 2.4 ± 0.5 s; P > 0.05). Thus, the large-amplitude Ca2+ events, present only in WT ECs, are sensitive to TRPV4 activation.
A linear histogram plot summarizing Ca2+ amplitude distribution in basal conditions (Fig. 6A, top), and the same plot in log-scale reveal an additional larger peak amplitude in WT, but not in SK3−/− ECs (Fig. 6A, bottom). These events were quantified from pooled 200-s recordings of basal Ca2+ activities (WT: n = 14; SK3−/−: n = 9 recordings). Together, these plots show that Ca2+ events with small amplitudes occurred much more frequently, by several orders of magnitude. GSK-induced changes in Ca2+ amplitude were summarized with relative frequency distribution plots for WT and SK3−/− vessels (Fig. 6, B and C, respectively; n = 6 recordings each). Distribution plots on linear scale (Fig. 6, B and C, top) hinted subtle difference in GSK; the same plots on log-scale showed that in basal conditions large-amplitude Ca2+ events existed only in WT but not in SK3−/− (solid lines, Fig. 6, B and C, bottom, respectively). GSK activation up-shifted the frequency distribution in WT but not in SK3−/− (dotted lines in Fig. 6, B and C). Digital subtractions of the frequency distribution traces before and after GSK also revealed a shift in the peak Ca2+ amplitude (Fig. 6D; peak WT: 1.42 and SK3−/−: 1.18). Thus, TRPV4 activation induces SK3 feedback and generates larger-amplitude Ca2+ events in WT.
Endothelial SK3 channels contribute to systemic blood pressure.
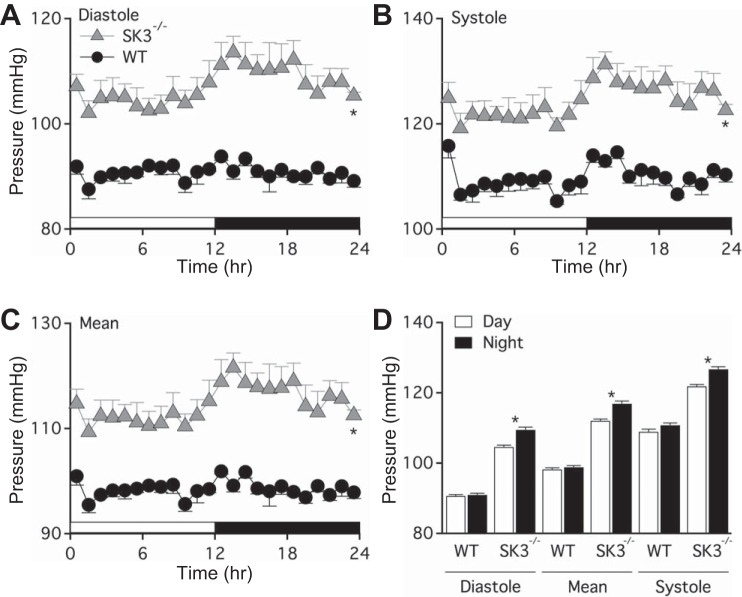
To determine whether the absence of endothelial SK3 channels is sufficient to increase blood pressure, we performed telemetric blood pressure recordings. Figure 7 shows diastolic (WT = 91 ± 2 mmHg; SK3−/− = 107 ± 4 mmHg; P < 0.05), systolic (WT = 110 ± 3 mmHg; SK3−/− = 124 ± 4 mmHg; P < 0.05), and mean (WT = 99 ± 2 mmHg; SK3−/− = 114 ± 4 mmHg; P < 0.05) arterial blood pressure for both WT and SK3−/− mice, indicating SK3−/− mice were hypertensive (n = 4 male mice each). Changes in blood pressure of WT mice during light and dark cycles were insignificant. Interestingly, the blood pressure of SK3−/− mice significantly increased during dark cycles when nocturnal mice are most active (Fig. 7D).
Fig. 7.
Vascular endothelial SK3 channels contribute to regulation of systemic blood pressure. A–C: in vivo telemetric blood pressure recordings comparing WT and endothelium-specific SK3−/− mice. Male mice (12–16 wk of age) were implanted with radiotelemetry transmitters in the left common carotid artery. After 4 days of recovery, basal systemic blood pressure data were collected for seven consecutive days under a 12:12-h light-dark cycle. Data are presented as the time-averaged day (empty horizontal bar, 12 h) and night (black horizontal bar, 12 h) diastolic pressure (A), systolic pressure (B), and mean arterial pressure (C). *P < 0.05 comparing SK3−/− to WT using t-tests. D: averaged mouse diastolic, mean, and systolic blood pressure measured in daytime (empty bars) and in night time (black bars). *P < 0.05 comparing day to night blood pressure measurement of same animal using paired t-tests. Data are expressed as means ± SE; n = 4 mice from each genotype.
DISCUSSION
In this study, we used a novel endothelium-specific SK3 knockout mouse model to test the hypothesis that endothelial SK3 channel activity contributes to blood pressure regulation. We confirmed the absence of vascular endothelial SK3: 1) mRNA and protein, 2) channel activity, and 3) functional contribution to EDH. Further examination showed 4) disrupted TRPV4 distribution, 5) lack of large-amplitude, low-frequency Ca2+ events, and 6) systemic hypertension in the SK3−/− mice. Taking these results together, we conclude that endothelial SK3 channels, likely through a feedback interaction with TRPV4 Ca2+ influx, constantly modulate endothelial Ca2+ signaling, vascular tone, and blood pressure.
Endothelium-derived hyperpolarization is an important mechanism in vasorelaxation of small arteries and arterioles, playing a critical role in regulating blood flow (19). This is especially pertinent during physical activity as activation of endothelial SK3 in skeletal muscles contributes to exercise hyperemia (34). However, the proportional contribution of SK3 vs. IK1 channels to EDH appears to vary, depending on animal species, particular vascular bed, and vessel size (9, 33, 44). In mouse mesenteric arteries, blockade of SK3 or IK1 channels alone does not abolish EDH, but concomitant inhibition of these channels abolishes EDH (15), suggesting both are necessary, and neither one is sufficient (Fig. 2). This could be attributable to differential subcellular channel distribution and localized Ca2+ sources, such as TRPV4-mediated Ca2+. In addition, mesenteric ECs express several other TRP channels, including TRPC1 (canonical), C3, C6, V1, and several M (melastatin) channels (14, 25). TRPA1 (36), C1 (41), C3 (24, 43), and V4 (45) channels have been shown to interact with SK3/IK1 in various vascular beds. In mesenteric artery, previous studies have further shown that IK1 channels may be concentrated at the IEL holes, where endothelial projections protrude and contact smooth muscle cells (40). At that site, IK channels are preferentially activated by Ca2+ emerging from clusters of IP3-sensitive Ca2+ stores in the endoplasmic reticulum situated at the base of the projections (49). In addition, Ca2+ influx through TRPV4 channels in or around the base of endothelial projections also provide Ca2+ ions for IK1 channel activation (46). In contrast, SK3 channels associate with caveolin-1 (1) and distribute more evenly across the plasma membrane (40). Evidence for caveolar trafficking and colocalization of SK3 strongly suggests a molecular interaction between SK3 and caveolin-1 (1, 28). Previous studies have also shown association of TRPV4 and caveolin-1 (8, 32, 39), and activation of SK3 by TRPV4-mediated Ca2+ influx (30, 45), suggesting caveolar SK3-TRPV4 microdomains. The present results showed that both SK3 and TRPV4 are expressed at EC-EC borders. Notably, ablation of SK3 channel expression disrupted TRPV4 distribution (Fig. 4); however, whether SK3 and TRPV4 colocalize in caveolae is currently not clear.
We measured Ca2+ dynamics distributed across 170 μm2 of the endothelial layers. In studying endothelial Ca2+ events, we found that the numbers of active Ca2+ sites and total Ca2+ events were approximately doubled in SK3−/− compared with WT vessels. Ca2+ spread was also significantly increased in SK3−/− vessels. Importantly, our analysis of Ca2+ events revealed the absence of large-amplitude-low frequency Ca2+ events in SK3−/−, suggesting that SK3 channels are required for their development (Figs. 5 and 6). These results are consistent with previous studies using IK1 knockout mice, which showed that IK1 channels promote more Ca2+ sites and events, while SK3 channels tend to increase event size (35). Similarly, in our hands, TRPV4 activation increased the Ca2+ amplitude and duration in WT mice, but only increased low-amplitude Ca2+ event frequency in SK3−/−. Thus, the elevated Ca2+ activity in response to TRPV4 activation is most likely modulated by Ca2+ activated K+ channels. The underlying mechanism may involve a molecular interaction directly or indirectly between SK3 and TRPV4 proteins or via caveolin1, and is beyond the scope of this study. Furthermore, results obtained from whole cell recordings, in the presence of 3 μM intracellular Ca2+ to fully activate SK3 and IK channels, provide direct evidence that SK3 channels are absent in SK3−/− ECs. Without dialyzing Ca2+ to activate SK3 and IK channels, perforated-patch clamp studies not only support the notion that Ca2+ influx via TRPV4 activates SK3 and IK channels, but also confirm the absence of SK3 in SK3−/− ECs (Fig. 3, C and D). Taken together Ca2+ and electrophysiological studies, our results demonstrate distinct EC Ca2+ signals in the presence and absence of SK3 channels, suggesting that SK3 channels play a role in Ca2+ microdomain and/or signaling.
There are several potential limitations of our findings. First, the SK3-dependent large-amplitude Ca2+ events are infrequent and may be undersampled because of the short time course studied, as shown on a log scale (Fig. 6A). Ca2+ event frequency distribution further highlights a shift in Ca2+ event amplitude in WT, but not SK3−/− ECs (Fig. 6, C and D). Whether the difference between WT and SK3−/− Ca2+ signal is due to TRPV4 distribution and/or interaction with other TRP channels are not yet clear and outside the scope of this study. Definitive SK3-TRPV4-caveolin-1 localization and proximity analysis is a high priority in the future. Moreover, this study used only mesenteric arteries of various sizes because of technical limitations, e.g., we had to combine large vessels to get enough tissue mass for Western blot analysis. It will be interesting to compare these results with those obtained from other beds. Although it is unclear whether a total or specific loss of endothelial SK3 channels occurs in humans, endothelial SK3 upregulation by estrogen could have significant impact on vascular health in postmenopausal women (10, 51).
The results obtained from blood pressure measurements show that endothelial SK3 channels contribute to blood pressure in vivo, consistent with previous studies that show reduced SK3 or IK1 expression results in hypertension. Furthermore, our results showed that during the 12-h dark cycle when mice are most active, the blood pressure increase was significant in SK3−/− mice, but not in WT mice. This may reflect the loss of SK3-associated Ca2+ signals and/or EDH vasorelaxation. Interestingly, the increased small and rapid Ca2+ events and sites in SK3−/− EC did not compensate for the loss of infrequent large and slow Ca2+ or EDH, supporting the idea that Ca2+ dynamics, patterns, and microdomains play distinct roles in endothelial function. The current study suggests differential SK3- and IK1-associated Ca2+ signaling contributes to EDH pathways. Together, these results highlight the importance of these specific channels in shaping subcellular EC Ca2+ dynamics and endothelial function.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: F.C.Y., D.S.W., M.I.T., B.S.C., and M.T.L. performed experiments; F.C.Y., B.S.C., J.M., and M.T.L. analyzed data; F.C.Y., D.S.W., M.S.T., M.I.T., B.S.C., J.M., J.P.A., and M.T.L. approved final version of manuscript; D.S.W., M.I.T., J.M., J.P.A., and M.T.L. edited and revised manuscript; M.S.T., J.P.A., and M.T.L. conception and design of research; M.I.T., B.S.C., J.M., and M.T.L. interpreted results of experiments; J.M. and M.T.L. prepared figures; M.T.L. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Michael Francis for his help on ImageJ and LC_Pro analyses. This work was supported by grants from the National Institutes of Health R00HL102056 to M. T. Lin and S10RR027535 to support the College of Medicine BioImaging Facility.
REFERENCES
- 1.Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SKCa channels and caveolin-rich domains. Br J Pharmacol 151: 332–340, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18,174–18,179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barfod ET, Moore AL, Lidofsky SD. Cloning and functional expression of a liver isoform of the small conductance Ca2+-activated K+ channel SK3. Am J Physiol Cell Physiol 280: C836–C842, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Behringer EJ, Segal SS. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca2+-activated K+ channels. Circ Res 110: 1311–1321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrout J, Mamenko M, Zaika OL, Chen L, Zang W, Pochynyuk O, O'Neil RG. Emerging role of the calcium-activated, small conductance, SK3 K+ channel in distal tubule function: regulation by TRPV4. PLos One 9: e95149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosch MA, Kelly MJ, Ronnekleiv OK. Distribution, neuronal colocalization, and 17β-E2 modulation of small conductance calcium-activated K+ channel (SK3) mRNA in the guinea pig brain. Endocrinology 143: 1097–1107, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Kohler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119: 2323–2332, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem 278: 27,208–27,215, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Chan MV, Bubb KJ, Noyce A, Villar IC, Duchene J, Hobbs AJ, Scotland RS, Ahluwalia A. Distinct endothelial pathways underlie sexual dimorphism in vascular auto-regulation. Br J Pharmacol 167: 805–817, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de Wit C, Jensen BL, Simonsen U, Bie P, Wulff H, Kohler R. Pharmacological activation of KCa3.1/KCa23 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol 165: 223–234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deignan J, Lujan R, Bond C, Riegel A, Watanabe M, Williams JT, Maylie J, Adelman JP. SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons. Neuroscience 217: 67–76, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res 102: 1247–1255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earley S. Vanilloid and melastatin transient receptor potential channels in vascular smooth muscle. Microcirculation 17: 237–249, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Francis M, Qian X, Charbel C, Ledoux J, Parker JC, Taylor MS. Automated region of interest analysis of dynamic Ca2+ signals in image sequences. Am J Physiol Cell Physiol 303: C236–C243, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis M, Waldrup J, Qian X, Taylor MS. Automated analysis of dynamic Ca2+ signals in image sequences. J Vis Exp, doi: 10.3791/51560, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschberg B, Maylie J, Adelman JP, Marrion NV. Gating of recombinant small-conductance Ca2+-activated K+ channels by calcium. J Gen Physiol 111: 565–581, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keating DJ, Rychkov GY, Giacomin P, Roberts ML. Oxygen-sensing pathway for SK channels in the ovine adrenal medulla. Clin Exp Pharmacol Physiol 32: 882–887, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kirby BS, Bruhl A, Sullivan MN, Francis M, Dinenno FA, Earley S. Robust internal elastic lamina fenestration in skeletal muscle arteries. PLos One 8: e54849, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 230: 230–242, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Kochukov MY, Balasubramanian A, Abramowitz J, Birnbaumer L, Marrelli SP. Activation of endothelial transient receptor potential C3 channel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J Am Heart Assoc 3, pii: e000913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler R, Brakemeier S, Kuhn M, Degenhardt C, Buhr H, Pries A, Hoyer J. Expression of ryanodine receptor type 3 and TRP channels in endothelial cells: comparison of in situ and cultured human endothelial cells. Cardiovasc Res 51: 160–168, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Kohler R, Degenhardt C, Kuhn M, Runkel N, Paul M, Hoyer J. Expression and function of endothelial Ca2+-activated K+ channels in human mesenteric artery: A single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res 87: 496–503, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin MT, Adelman JP, Maylie J. Modulation of endothelial SK3 channel activity by Ca2+-dependent caveolar trafficking. Am J Physiol Cell Physiol 303: C318–C327, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MT, Hessinger DA, Pearce WJ, Longo LD. Developmental differences in Ca2+-activated K+ channel activity in ovine basilar artery. Am J Physiol Heart Circ Physiol 285: H701–H709, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Lin MT, Jian MY, Taylor MS, Cioffi DL, Yap FC, Liedtke W, Townsley MI. Functional coupling of TRPV4, IK, and SK channels contributes to Ca2+-dependent endothelial injury in rodent lung. Pulm Circ 5: 279–290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma X, Du J, Zhang P, Deng J, Liu J, Lam FF, Li RA, Huang Y, Jin J, Yao X. Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension 62: 134–139, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol 30: 851–858, 2010. [DOI] [PubMed] [Google Scholar]
- 33.McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke 37: 1277–1282, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Milkau M, Kohler R, de Wit C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J 24: 3572–3579, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Qian X, Francis M, Kohler R, Solodushko V, Lin M, Taylor MS. Positive feedback regulation of agonist-stimulated endothelial Ca2+ dynamics by KCa3.1 channels in mouse mesenteric arteries. Arterioscler Thromb Vasc Biol 34: 127–135, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation 20: 138–148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao L, Nishimura T, Shi L, Sessions D, Thrasher A, Trudell JR, Berry GJ, Pearl RG, Kao PN. Endothelial fate mapping in mice with pulmonary hypertension. Circulation 129: 692–703, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci 22: 9698–9707, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: possible relationship to vasodilator function? J Anat 209: 689–698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt K, Dubrovska G, Nielsen G, Fesus G, Uhrenholt TR, Hansen PB, Gudermann T, Dietrich A, Gollasch M, de Wit C, Kohler R. Amplification of EDHF-type vasodilatations in TRPC1-deficient mice. Br J Pharmacol 161: 1722–1733, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senadheera S, Bertrand PP, Grayson TH, Leader L, Murphy TV, Sandow SL. Pregnancy-induced remodelling and enhanced endothelium-derived hyperpolarization-type vasodilator activity in rat uterine radial artery: transient receptor potential vanilloid type 4 channels, caveolae and myoendothelial gap junctions. J Anat 223: 677–686, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senadheera S, Kim Y, Grayson TH, Toemoe S, Kochukov MY, Abramowitz J, Housley GD, Bertrand RL, Chadha PS, Bertrand PP, Murphy TV, Tare M, Birnbaumer L, Marrelli SP, Sandow SL. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc Res 95: 439–447, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal 7: ra66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao X, Lin MT, Thorington GU, Wilson SM, Longo LD, Hessinger DA. Long-term hypoxia increases calcium affinity of BK channels in ovine fetal and adult cerebral artery smooth muscle. Am J Physiol Heart Circ Physiol 308: H707–H722, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res 93: 124–131, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol 302: C1226–C1242, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Yap FC, Taylor MS, Lin MT. Ovariectomy-induced reductions in endothelial SK3 channel activity and endothelium-dependent vasorelaxation in murine mesenteric arteries. PLos One 9: e104686, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]