The present study provides compelling evidence that early life stress in mice programs increased superoxide generation in the vasculature, leading to endothelial dysfunction in adulthood. This translational study provides mechanistic insight into vascular dysfunction in adult humans who are exposed to adverse childhood experiences.
Keywords: maternal separation with early weaning, early life stress, endothelial dysfunction, superoxide, NADPH oxidase
Abstract
Early life stress (ELS) is a risk for cardiovascular disease in adulthood although very little mechanistic insight is available. Because oxidative stress and endothelial dysfunction are major contributors to cardiovascular risk, we hypothesized that ELS induces endothelial dysfunction in adult male mice via increased superoxide production. Studies employed a mouse model of ELS, maternal separation with early weaning (MSEW), in which litters were separated from the dam for 4 h/day [postnatal days (PD) 2–5] and 8 h/day (PD6-16), and weaned at PD17. Control litters remained undisturbed until weaning at PD21. When compared with control mice, thoracic aortic rings from adult male MSEW mice displayed significant endothelial dysfunction that was reversed by the superoxide scavenger, polyethylene glycol-superoxide dismutase (PEG-SOD). PEG-SOD-inhibitable superoxide production by aortae from MSEW mice was significantly greater than observed in control aortae, although unaffected by nitric oxide synthase inhibition, suggesting that uncoupled nitric oxide synthase was not responsible for the accelerated superoxide production. Aortic SOD expression, plasma SOD activity, and total antioxidant activity were similar in MSEW and control mice, indicating unaltered antioxidant capacity in MSEW mice. Increased expression of the NADPH oxidase subunits, NOX2 and NOX4, was evident in the aortae of MSEW mice. Moreover, endothelial dysfunction and superoxide production in MSEW mice was reversed with the NADPH oxidase inhibitor, apocynin, indicating increased NADPH oxidase-dependent superoxide production and endothelial dysfunction. The finding that MSEW induces superoxide production and endothelial dysfunction in adult mice may provide a mechanistic link between ELS and adult cardiovascular disease risk.
NEW & NOTEWORTHY
The present study provides compelling evidence that early life stress in mice programs increased superoxide generation in the vasculature, leading to endothelial dysfunction in adulthood. This translational study provides mechanistic insight into vascular dysfunction in adult humans who are exposed to adverse childhood experiences.
adverse childhood experiences (ACEs), also known as early life stress (ELS), are associated with increased risk of cardiovascular disease (22). Retrospective studies strongly link chronic separation from parents during childhood with elevated systolic and diastolic blood pressures (2), as well as increased prevalence of ischemic heart disease and type 2 diabetes in late adulthood (3, 8). Moreover, our group recently reported that ACEs were associated with increases in pulse wave velocity, total peripheral resistance, and diastolic blood pressure in relatively healthy, young adults (35), indicating that vascular dysfunction may be a mediator in the ELS-induced increase in cardiovascular disease risk. Currently, there is a need for mechanistic studies to elucidate the relationship between ELS and vascular dysfunction.
In humans, ELS is associated with increased oxidative stress (33). Oxidative stress due to increased production of free radicals, reactive oxygen species (ROS), and/or loss of antioxidants has been shown to increase cardiovascular disease risk through induction of cellular damage in a wide range of organ systems, especially the vasculature (38). Components of the vasculature, such as endothelial cells, vascular smooth muscle cells, and resident monocytes and macrophages produce ROS and have the potential to contribute to overall oxidative stress in a biological system (38). Superoxide anion accumulation due to increased NADPH oxidase activity, nitric oxide (NO) synthase (NOS) uncoupling, and/or loss of antioxidant capacity, as well as the resulting decrease in NO bioavailability and endothelial dysfunction, are well-described mechanisms of vascular pathology (7). Indeed, endothelial dysfunction is associated with virtually every condition predisposing to cardiovascular disease (6) and is considered to represent an important mechanism of cardiovascular risk (11). Given that both ELS and oxidative stress induce vascular dysfunction and that ELS is associated with increased oxidative stress markers in humans (30), it is tempting to speculate that oxidative stress and/or endothelial dysfunction may contribute to the ELS-induced cardiovascular disease risk in adulthood.
In this study, experiments were performed to test the hypothesis that ELS induces endothelial dysfunction and/or increased oxidative stress. Maternal separation with early weaning in C57Bl/6J mice is a model of ELS involving extended maternal separation time and early weaning that generates robust effects on anxiety, hyperactivity, and behavioral despair in adulthood, of which all are contributors to the development and progression of cardiovascular disease (13, 41). Using this model, we elucidated whether ELS induces oxidative stress, as well as changes in cardiovascular physiological parameters such as endothelial function, blood pressure, heart rate, and blood glucose in adult mice.
METHODS
Mouse model of early life stress.
Studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Animals and approved by the Institutional Animal Care and Use Committee at Augusta University and the University of Alabama at Birmingham. C57BL/6J mice (Jackson, Bar Harbor, ME) were acclimated for 2 wk before breeding. Mice were maintained under a 12-h/12-h light-dark cycle. The breeding scheme consisted of a male-female pair with males removed after 17 days, and females were observed to determine exact day of birth [postnatal day (PD) 0]. Litters were assigned to maternal separation with early weaning (MSEW) or control groups. From PD2 to PD5, MSEW litters were separated from dams for 4 h each day (0900 h to 1300 h) by transferring them to an incubator (37.5 ± 1°C, 60% humidity). From PD6 to PD16, MSEW litters were separated from dams for 8 h a day (0900 h to 1700 h). On PD17, MSEW litters were weaned to clean cages. Control litters remained undisturbed with dams until PD21 at which time they were weaned to clean cages. Litters consisting of 6–10 pups were included. Each experiment included mice from at least 4 different litters to control for litter effects. Body weights were determined at postnatal days 2, 5, 10, 15, and 28 and again at 12 wk of age. Baseline parameters and vascular reactivity experiments were performed in male and female mice, whereas all other experiments were performed in male mice only.
Tissue collection, plasma analyses, and organ weights.
At 12 wk old, mice were anesthetized with methohexital sodium (Brevital; 50 mg/kg ip), after which blood was drawn by tail snip, and nonfasting blood glucose levels were determined using a glucometer (Accu-Chek Advantage Meter, Indianapolis, IN). Blood was drawn by cardiac puncture and placed in heparinized tubes for centrifugation and plasma collection. Subsequently, the left kidney and heart were excised and weighed, and the thoracic aorta was harvested for functional and/or biochemical studies. Plasma nitrate + nitrite (NOx) levels were measured by high performance liquid chromatography (ENO20, Eicom, Japan). Plasma superoxide dismutase (SOD) activity and total antioxidant capacity were assessed using commercially available kits (Antioxidant Assay Kit, Superoxide Dismutase Assay Kit; Cayman Chemical, Ann Arbor, MI). Kit instructions were followed as specified by the manufacturer.
Telemetry.
Blood pressure (mean arterial, systolic, and diastolic), heart rate, and activity were recorded by telemetry transmitters (model PA-C20, Data Sciences International, St. Paul, MN) implanted in 11- to 12-wk old male mice. Surgical procedures were performed as previously described (18). Animals were allowed to recover for 4 days before measurements were taken. Parameters were recorded every 2 min during the 48-h period, which started at 0600 h (lights on). Averages (12 h) for each animal were calculated by averaging all values generated during the active (1800 h to 0600 h) or inactive (0600 h to 1800 h) 12-h period.
Vascular reactivity protocol.
Thoracic aortae were excised and perivascular fat was removed. Aortae were cut into concentric rings (2 to 3 mm) and mounted on pins for wire myography (Danish Myo Technology; Aarhus, Denmark) as previously described (17). For assessment of endothelium-dependent vasorelaxation, the mounted aortic segments were constricted with 1 μmol/l phenylephrine (PE) followed by evaluation of vasorelaxation based on cumulative concentration-response curves to acetylcholine (ACh; 1 × 10−9 mol/l to 10−5 mol/l), and then to sodium nitroprusside (SNP; 1 × 10−10 mol/l to 10−5 mol/l) in the same aortic segment. Rings were incubated in the presence or absence of either SOD conjugated to polyethylene glycol (PEG-SOD, 100 U/ml; Sigma-Aldrich, St. Louis, MO) or apocynin (300 μM; Sigma-Aldrich) for 20 min before construction of cumulative concentration-response curves. Vasorelaxation data are presented as percent relaxation (%PE constriction) as analyzed by the equation [(maximum PE response − ACh response)/(maximum PE response − baseline before PE constriction)] × 100. GraphPad Prism data analysis software was used to calculate the sensitivity (−logEC50) and maximum response (Emax) to the vasoactive agonists.
Western blot analysis.
Thoracic aortae were cleaned of adherent fat and homogenized in 50 mM Tris (pH 7.4), 250 mM sucrose, 0.1 mM EDTA, 0.1 mM EGTA, 10% glycerol, 0.1% SDS, 0.5% Triton X-100, 0.5% sodium deoxycholate, 0.1% β-mercaptoethanol, and 0.01 mg/ml each of leupeptin, pepstatin, and aprotinin using a handheld motorized pestle. The samples then were freeze thawed, sonicated for 10 × 1-s bursts on ice, and incubated on a rocker at 4°C for 30 min. After centrifugation at 17,000 g at 4°C for 20 min, supernatant was collected and stored at −80°C. Samples were run on 8 or 15% SDS gels, transferred to polyvinylidene flouride membranes (Immobilon-FL), and blotted using antibodies against NOS3 (No. 610297; BD Biosciences, San Jose, CA), pS1177 (No. 9571S; Abcam, Cambridge, MA), SOD1 (No. SOD-101; StressGen, Farmingdale, NY), SOD2 (No. SOD-111; StressGen), SOD3 (No. SOD-105; StressGen), and actin (Sigma-Aldrich). Secondary antibodies were used at a concentration of 1 μg/ml. Membranes were imaged using the Odyssey CLx Infared Imaging System, and band intensities were quantified using Image Studio Software (LI-COR Imaging Systems, Lincoln, NE).
Quantitative real-time RT-PCR.
Thoracic aortae were cleaned of adherent fat, flash frozen, and homogenized in TRIzol (Invitrogen, Grand Island, NY) using a glass mortar and Teflon pestle. RNA isolation was performed according to the TRIzol method established by Invitrogen, and reverse transcription of RNA to cDNA was performed using a commercial kit (QuantiTect Reverse Transcription Kit; Qiagen, Valencia, CA). Quantitative real-time RT-PCR was performed for detection of Cybb (encodes NOX2), Nox4, Ncf1 (encodes p47phox), Ncf2 (encodes p67phox), and Nos3 (encodes NO synthase 3) expression using commercial kits (QuantiFast SYBR Green RT-PCR Kit, QuantiTect primers; Qiagen).
Aortic superoxide production.
PEG-SOD-inhibitable and apocynin-inhibitable superoxide production in thoracic aorta were assessed using the dihydroethidium (DHE) method previously described by Fink and colleagues (10). Briefly, aortae were cleaned of perivascular fat and cut into 2-mm rings. The rings were preincubated with either vehicle (Krebs-Henseleit buffer, KHB), PEG-SOD (100 U/ml), or apocynin (300 μM) for 20 min, and then DHE was added for an additional 40 min. Aortic rings were then rinsed in KHB, thoroughly homogenized, filtered, and processed for HPLC analysis (Elite LaChrome, Hitachi High-Technologies, Tokyo, Japan). To calculate PEG SOD-inhibitable superoxide production, the value obtained for PEG-SOD-treated rings was subtracted from the value obtained for vehicle-treated rings. The same approach was used to calculate apocynin-inhibitable superoxide production. To assess the contribution of NOS activity to aortic superoxide production, aortic rings were incubated with NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) (100 μM in KHB; Sigma-Aldrich) for 20 min before DHE incubation.
Statistical analyses.
Data are expressed as means ± SE. Baseline parameters, superoxide production, antioxidant measurements, mRNA expression, and protein expression data were analyzed using unpaired t-tests (GraphPad Prism, La Jolla, CA). Differences in −logEC50 and Emax of cumulative concentration curves for vascular reactivity were analyzed using two-way ANOVA with subsequent Bonferroni post hoc analysis (GraphPad Prism). For real-time quantitative RT-PCR, the 2−ΔΔCT method of analysis was used. Statistical significance was defined as P < 0.05.
RESULTS
Body weight, blood pressure, and basal parameters.
No significant difference in body weight between control and MSEW mice was observed up to PD5 (values in grams reported as control vs. MSEW, PD2: 1.6 ± 0.1 vs. 1.5 ± 0.1; PD5: 2.8 ± 0.1 vs. 2.6 ± 0.1, n = 14/group). At PD10, pups were easily distinguishable by sexually dimorphic features, and thus body weights were calculated accordingly. Body weights continued to show no significant differences between groups in both males (values in grams reported as control vs. MSEW, PD10: 4.7 ± 0.2 vs. 4.3 ± 0.2; PD15: 6.3 ± 0.3 vs. 6.1 ± 0.3; PD28: 11.4 ± 0.4 vs. 11.4 ± 1.2; n = 6–7/group) and females (values in grams reported as control vs. MSEW, PD10: 5.1 ± 0.2 vs. 4.5 ± 0.2; PD15: 6.8 ± 0.4 vs. 6.2 ± 0.3; PD28: 11.1 ± 0.3 vs. 11.9 ± 0.5, n = 7–8/group). Adult body weight, kidney and heart weight, blood pressure, heart rate, activity, and nonfasting blood glucose were comparable between control and MSEW male mice (Table 1). Also, female control and MSEW mice showed no significant difference in adult body weight (control: 19.9 ± 0.47 g vs. MSEW: 19.1 ± 0.11 g, n = 4–7/group), kidney weight (control: 0.11 ± 0.003 g vs. MSEW: 0.11 ± 0.004 g, n = 3/group), heart weight (control: 0.09 ± 0.008 g vs. MSEW: 0.09 ± 0.006, n = 3/group), and nonfasting blood glucose (control: 206 ± 14 mg/dl vs. MSEW: 202 ± 10 mg/dl, n = 5–7/group).
Table 1.
Baseline parameters of 12-wk-old male mice
| Parameter | Control | MSEW |
|---|---|---|
| Body weight, g | 25.7 ± 2.16 | 26.0 ± 1.58 |
| Heart weight, g | 0.13 ± 0.01 | 0.14 ± 0.02 |
| Kidney weight, g | 0.15 ± 0.02 | 0.15 ± 0.02 |
| Blood glucose, mg/dl | 208 ± 23 | 210 ± 34 |
| 12-hour mean arterial pressure, mmHg | ||
| Active period | 120.7 ± 1.2 | 123.0 ± 1.0 |
| Inactive period | 108.5 ± 0.9 | 108.7 ± 1.2 |
| 12-hour systolic pressure, mmHg | ||
| Active period | 135.2 ± 1.6 | 139.3 ± 1.2 |
| Inactive period | 123.2 ± 1.9 | 122.6 ± 1.9 |
| 12-hour diastolic pressure, mmHg | ||
| Active period | 106.3 ± 0.7 | 106.7 ± 2.8 |
| Inactive period | 93.8 ± 0.8 | 93.3 ± 2.6 |
| 12-hour heart rate, beats/min | ||
| Active period | 605 ± 11 | 601 ± 4 |
| Inactive period | 567 ± 14 | 557 ± 6 |
| 12-hour activity, counts/min | ||
| Active period | 15.6 ± 1.4 | 14.5 ± 3.5 |
| Inactive period | 4.1 ± 1.1 | 3.5 ± 1.1 |
Values are means ± SE. An unpaired, two-tailed t-test was performed for each parameter: n = 5–8 mice/group for body weight, heart weight, kidney weight, and blood glucose, and n = 4 mice/group for blood pressure, heart rate, and activity.
MSEW, maternal separation with early weaning.
Aortic superoxide production and endothelial dysfunction.
PEG-SOD-inhibitable superoxide production was significantly greater (∼3-fold) in MSEW than control aortae (Fig. 1A). To determine the contribution of increased superoxide levels in MSEW aortae to endothelial dysfunction (defined as impaired NO-dependent relaxation), responses to cumulative increasing concentrations of ACh were assessed in the absence or presence of PEG-SOD. The maximum relaxation response to ACh was significantly blunted in the aortae of MSEW mice compared with control mice with no difference in −logEC50 (Fig. 1B; Table 2). Preincubation of aortae with PEG-SOD reversed the blunted response to ACh in aortae from MSEW mice without significantly affecting SNP-induced vasorelaxation (Fig. 1, B and C; Table 2). SNP-induced vasorelaxation was similar in the MSEW and control groups (Fig. 1C; Table 2). ACh- or SNP-induced vasorelaxation in control aortae was not significantly affected by pretreatment with PEG-SOD (Fig. 1, B and C; Table 2). Pretreatment of aortic rings with PEG-SOD significantly decreased the level of preconstriction in response to 1 μmol/l phenylephrine; however, there was no difference between control and MSEW aortae in the degree of decrease in preconstriction [FTreatment (1, 16) = 27.93, P < 0.0001, FGroup (1, 16) = 0.90, P = 0.36, FInteraction (1, 16) = 0.41, P = 0.53]. These results indicate that enhanced superoxide production significantly contributed to MSEW-induced endothelial dysfunction.
Fig. 1.
Maternal separation with early weaning (MSEW) induced aortic superoxide production and endothelial dysfunction in adult male mice without affecting plasma total antioxidant capacity, plasma SOD activity, or plasma nitrite + nitrate levels (NOx). A: PEG-SOD-inhibitable superoxide production by aortae from control (n = 4) and MSEW (n = 4) mice. *P = 0.002. B: aortic relaxation in response to acetylcholine (ACh) in presence of vehicle (VEH) or PEG-SOD (100 U/ml). Refer to Table 2 for statistical analyses (n = 5–7 per group). *P < 0.05 vs. control VEH, †P < 0.05 vs. MSEW PEG-SOD. C: aortic relaxation in response to sodium nitroprusside (SNP) in the presence of vehicle (VEH) or PEG-SOD (100 U/ml). Refer to Table 2 for statistical analyses (n = 5–7 per group). D: total antioxidant capacity of plasma from control (n = 19) and MSEW (n = 17) mice. E: plasma SOD activity in plasma from control (n = 6) and MSEW (n = 6) mice. F: NOx levels in plasma from control (n = 12) and MSEW (n = 12) mice.
Table 2.
Effect of APO and PEG-SOD on −logEC50 and Emax of mouse thoracic aorta relaxation in response to ACh and SNP
| Control |
MSEW |
|||
|---|---|---|---|---|
| Agonist/Treatment | −logEC50 | Emax | −logEC50 | Emax |
| ACh | ||||
| VEH | 6.99 ± 0.12 | 73.2 ± 4.3 | 6.86 ± 0.07 | 53.5 ± 6.9* |
| APO | 7.27 ± 0.11 | 87.0 ± 5.8 | 7.31 ± 0.09† | 95.0 ± 2.1††† |
| PEG-SOD | 7.20 ± 0.10 | 81.5 ± 4.3 | 7.14 ± 0.12 | 77.3 ± 4.7† |
| SNP | ||||
| VEH | 8.54 ± 0.13 | 101.8 ± 0.7 | 8.23 ± 0.11 | 101.5 ± 0.7 |
| APO | 8.48 ± 0.18 | 102.9 ± 1.1 | 8.17 ± 0.11 | 100.4 ± 0.7 |
| PEG-SOD | 8.41 ± 0.14 | 102.3 ± 0.7 | 8.30 ± 0.09 | 100.4 ± 0.5 |
Values are means ± SE. Sensitivity (−logEC50) and maximal effect (Emax) values determined from %relaxation from phenylephrine preconstriction in response to agonist. Two-way ANOVA with Bonferroni post hoc analyses were performed for each agonist group [acetylcholine (ACh) or sodium nitroprusside (SNP)] with factors of group (Control, MSEW) and treatment [vehicle (VEH), apocynin (APO) or VEH, superoxide dismutase (PEG-SOD)]; n = 5–7 mice/group.
P < 0.05 compared with VEH Control;
P < 0.05, ††P < 0.01, and
P < 0.001 compared with VEH MSEW.
Aortae of control and MSEW female mice displayed no significant difference in maximum relaxation or −logEC50 in response to ACh [values reported as control (n = 7) vs. MSEW (n = 4); Emax: 57.2 ± 6.87 vs. 51.9 ± 12.22, P = 0.69, −logEC50: 6.78 ± 0.187 vs. 7.19 ± 0.147; P = 0.17] or SNP (Emax: 99.3 ± 1.04 vs. 97.6 ± 2.55, P = 0.47, −logEC50: 8.42 ± 0.097 vs. 8.62 ± 0.108, P = 0.25), thus precluding them from further study.
Systemic and aortic antioxidant biomarkers.
Plasma total antioxidant activity and SOD activity did not differ between groups (Fig. 1, D and E). In addition, aortic protein expression of SOD isoforms (SOD1, SOD2, and SOD3) were not different between groups [values (fold change relative to control) reported as control vs. MSEW, SOD1: 1.0 ± 0.1 vs. 0.9 ± 0.1; SOD2: 1.0 ± 0.01 vs. 1.0 ± 0.1; SOD3: 1.0 ± 0.2 vs. 0.9 ± 0.2; n = 4–6/group, P > 0.58]. Plasma NOx also did not differ between groups (Fig. 1F). Furthermore, no difference was found in expression of NO synthase 3 (NOS3) mRNA [values (fold change relative to control) reported as control vs. MSEW, control 1.0 ± 0.2 vs. MSEW 1.5 ± 0.4, n = 5–8/group, P = 0.49] or NOS3 protein (control 1.0 ± 0.04 vs. MSEW 1.0 ± 0.1, n = 11–12/group, P = 0.91) or phosphorylated Ser1177NOS3 (control 1.0 ± 0.1 vs. MSEW 1.1 ± 0.2, n = 6/group, P = 0.55) in aortae from control and MSEW mice.
Aortic NADPH oxidase activity and subunit expression.
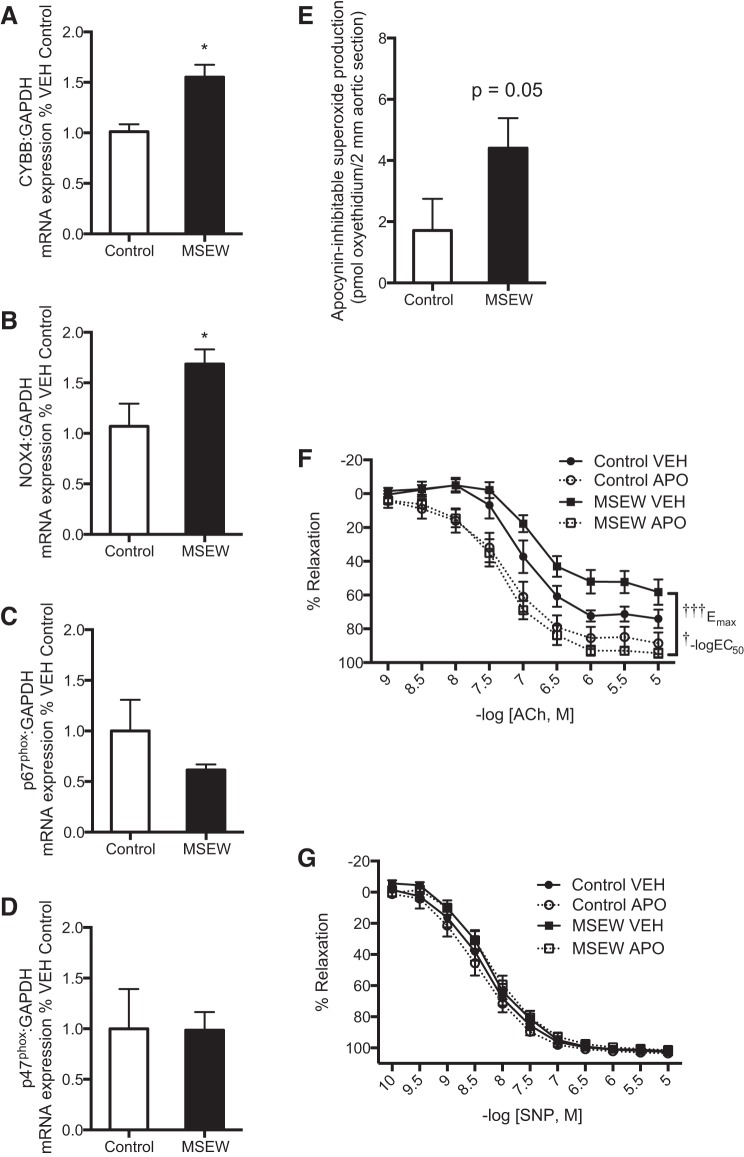
At the mRNA level, expression of the membrane-bound NADPH oxidase enzyme subunits, Cybb and Nox4, was upregulated in aorta of MSEW mice (Fig. 2, A and B). In contrast, MSEW did not significantly alter aortic expression of Ncf1 and Ncf2, which encode cytosolic NADPH oxidase enzyme subunits, p47phox and p67phox, respectively (Fig. 2, C and D). Preincubation of aortae with the NADPH oxidase inhibitor, apocynin, abolished the endothelial dysfunction in aortae from MSEW mice without affecting SNP-induced vasorelaxation (Fig. 2, F and G; Table 2). Neither ACh- nor SNP-induced vasorelaxation was significantly affected by apocynin treatment of control aortae (Fig. 2, F and G; Table 2). Pretreatment of aortic rings with apocynin significantly decreased the level of preconstriction in response to 1 μmol/l phenylephrine; however, there was no difference between control and MSEW aortae in the degree of decrease in preconstriction [FTreatment (1, 16) = 33.39, P < 0.0001, FGroup (1, 16) = 0.07, P = 0.79, FInteraction (1, 16) = 0.002, P = 0.96]. Moreover, apocynin-inhibitable superoxide production was ∼2.5 fold greater in aortae of MSEW mice compared with control (Fig. 2E). These results suggest that enhanced NADPH oxidase activity likely contributed to aortic endothelial dysfunction in MSEW mice. Additionally, l-NAME preincubation did not significantly influence superoxide production in aortae of MSEW mice (vehicle: 12.8 ± 3.10 vs. l-NAME: 18.6 ± 6.48 pmol/2-mm section, P = 0.49, n = 6–7/group), indicating that uncoupled NOS3 most likely is not the source of increased aortic superoxide production.
Fig. 2.
MSEW induced NADPH oxidase subunit expression and activity in aorta of adult male mice. Quantitative RT-PCR analysis of Cybb (NOX2; A), Nox4 (NOX4; B), Ncf2 (p67phox; C), and Ncf1 (p47phox; D) in aorta of control (n = 3–5) and MSEW (n = 4–9) mice. *P < 0.05. E: apocynin-inhibitable superoxide production by aortae from control (n = 4) and MSEW (n = 4) mice. F: aortic relaxation in response to acetylcholine (ACh) in presence of vehicle (VEH) or apocynin (APO, 300 μM). Refer to Table 2 for statistical analyses (n = 5–7 per group). †P < 0.05, †††P < 0.001 vs. MSEW APO. G: aortic relaxation in response to sodium nitroprusside (SNP) in presence of vehicle (VEH) or apocynin (APO, 300 μM). Refer to Table 2 for statistical analyses (n = 5–7 per group).
DISCUSSION
The major finding of this study is that MSEW, a mouse model of early life stress, induced aortic endothelial dysfunction in male adult mice through increased superoxide production. Endothelial dysfunction, a hallmark of increased cardiovascular disease risk, may be due to a number of mechanisms that results in reduction of nitric oxide (NO) bioavailability (23, 34). The results of the present study indicate that endothelial dysfunction of the thoracic aorta in MSEW mice is likely due to increased NADPH oxidase-generated superoxide production and subsequent loss of NO bioavailability, rather than loss of antioxidant capacity (i.e., superoxide dismutase activity or NOS3 production), or increased superoxide generation resulting from NOS3 uncoupling.
NADPH oxidase is a predominant contributor to endothelial oxidative stress via production of ROS and has been shown to directly mediate vascular dysfunction (24, 25, 31). The NADPH oxidase complex consists of several components including membrane-bound subunits (NOX isoforms and p22phox) and cytosolic components (p67phox, p47phox, and rac), all of which are expressed in aortic tissue (5). The present study revealed that key components of the complex, NOX2 and NOX4, were upregulated in aorta at adulthood by MSEW. Studies indicate that overexpression of vascular endothelial NOX2 increases aortic superoxide and macrophage recruitment without altering blood pressure in mice (9), whereas overexpression of vascular NOX4 can either increase superoxide or hydrogen peroxide and induce protection or damage, depending on the vessel type and the nature of the stress condition under question [reviewed in Touyz and Montezano (39)]. More recently, NOX4 has been shown to produce predominately hydrogen peroxide in vitro (27). Interestingly, in this study, inhibition of NADPH oxidase activity (apocynin treatment) or scavenging of superoxide (PEG-SOD treatment) restored endothelial function and reduced superoxide production to a greater extent in MSEW aortae, suggesting a prominent role of NOX-driven superoxide production in mediating MSEW-induced endothelial dysfunction. It must be noted that the use of apocynin as a selective NADPH oxidase inhibitor in vascular cells is controversial, and interpretation of its specificity must be approached with caution (15, 29). However, the observation of significant upregulation of two NADPH oxidase subunits in conjunction with the apocynin-reversible endothelial dysfunction as well as increased superoxide production in MSEW aortas lends credence to our conclusion that endothelial dysfunction in adult MSEW mice is largely due to an increase in NADPH oxidase-driven superoxide generation.
NADPH oxidase may drive superoxide generation from the transfer of electrons from NAPDH to oxygen or from downstream effector molecules. Previous studies from our laboratory and others have shown that NOS3 uncoupling is facilitated by activated NADPH oxidase and the resulting increase in superoxide levels (37); however, NOS inhibition did not alter superoxide production by aortae from MSEW mice, thus indicating that NADPH oxidase-derived superoxide generation did not potentiate superoxide production via NOS3 uncoupling in these mice. Additionally, MSEW mice had normally functioning systemic and aortic antioxidant capacity and plasma SOD activity, and they showed no changes in expression of SOD1, SOD2, or SOD3 in aortic tissue, suggesting a fully functional SOD system. To an extent, NO has the capacity to reduce oxidative stress and may play an important role in counterbalancing superoxide production. Neither NOS3 expression nor its phosphorylation status was affected by MSEW, indicating that the endothelial dysfunction is not likely due to reduced NOS3 expression or activity. These data further support the contention that the predominant mechanism underlying MSEW-induced endothelial dysfunction is the direct generation of superoxide by NADPH oxidase. Given that NADPH oxidase is expressed in endothelial cells, vascular smooth muscle cells, and vascular macrophages (38), additional studies are necessary to fully understand the cellular source of MSEW-driven superoxide production. In this study, we observed no change in endothelium-independent relaxation after treatment with an intracellular superoxide scavenger (PEG-SOD); thus, we propose that the endothelium is the likely source of MSEW-driven superoxide production and that the increase in intracellular superoxide reduces intracellular NO, rather than directly affecting vascular smooth muscle superoxide production.
The goal of this study was to investigate whether endothelial function was disturbed by ELS using a mouse model of MSEW. Flow-mediated dilation of the brachial artery, a noninvasive measure of endothelial function of a conduit vessel, is a popular choice of assessing endothelial function in humans (12). Results from the current study revealed that the MSEW mouse model may serve as an excellent model by which to investigate the mechanisms by which ELS may induce endothelial dysfunction and vascular disease of conduit vessels in humans. Studies have shown that correlation between endothelial function of conduit and resistance vessels is dependent on the condition or stressor under investigation (16, 40, 42); thus, we recognize that ELS may have varying effects on different vessel beds in the MSEW model and that this topic warrants further study.
Notably, MSEW triggered endothelial dysfunction in adult male mice without alterations in blood pressure, heart rate, activity, blood glucose, body weight, or organ weight. The absence of overt cardiovascular pathology in MSEW mice at 12 wk of age suggests that endothelial dysfunction is a mechanism by which early life stress increases the risk for cardiovascular disease in young adulthood. The current study is in agreement with our human studies showing that adolescents and young adults (13–29 yr old) exposed to ACEs exhibited relatively mild but significant vascular dysfunction without overt signs of cardiovascular disease (35). Additionally, in a longitudinal study, we found that blood pressure of people exposed to ACEs significantly deviated from people with no exposure to ACEs after 30 yr of age, indicating that underlying vascular dysfunction caused by ELS did not immediately manifest in blood pressure dysregulation but may still pose as a significant risk factor for disease (36).
In humans, it is well known that there exists prominent sex differences in the response to psychosocial stressors during childhood and adulthood [reviewed in Bale and Epperson (4)]. Also, rodent studies have shown that maternal separation has disparate sex-dependent effects on angiotensin II-mediated hypertension and renal function (21), behavioral reactivity to subsequent stressors (26), as well as neurogenesis (19). The present study is the first to show that MSEW caused adult endothelial dysfunction in male, but not female, mice. In our previous human cohort studies, we found that sex difference did not significantly affect ELS-mediated vascular dysfunction or blood pressure trajectory; however, endothelial function was not directly measured in these studies (35, 36). Results of the current study highlight the need to investigate the potential effect of sex difference on ELS-regulated endothelial dysfunction in humans.
ELS can significantly modulate components of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal-medullary (SAM) system in rodents (20). Specifically, rodents that have been subjected to maternal separation before weaning show altered circulating plasma corticosterone levels, increased HPA axis responsiveness to stressors, altered hippocampal glucocorticoid receptor density, increased corticotropin-releasing factor expression in hypothalamus, and decreased serotonin transporter and tryptophan hydrolyase expression in brain (1, 28, 32). Many of the components altered by ELS can play an important role in regulating vascular tone, senescence, and inflammation (14) and thus are possible mechanisms by which MSEW-induced superoxide in mouse aorta is orchestrated. Whether MSEW directly or indirectly mediates vascular NADPH oxidase-mediated superoxide production remains to be investigated. We propose future studies that test whether in vivo inhibition of NADPH oxidase activity or treatment with antioxidants during exposure to MSEW would abolish endothelial dysfunction in adulthood.
In conclusion, the results of the present study provide compelling evidence for ELS-induced endothelial dysfunction through NADPH oxidase-mediated superoxide production in adult male mice. The current findings indicate that psychosocial stress in early life acts to program the vasculature, and in particular the endothelium, toward higher susceptibility to the development of vascular disease through oxidative stress pathways. We propose that this mechanism may precede overt disease and may lead to a predisposition toward cardiovascular disease risk later in life when secondary stressors are present.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants F32-HL-116145 (to D. H. Ho) and P01-HL-69999 (to J. S. Pollock); American Heart Association Grant 11POST7240008 (to K. A. Hyndman); Augusta University Experimental Medicine summer research internship (to B. Musall); and Augusta University STAR summer undergraduate research program and University of Alabama at Birmingham Cardio-Renal Physiology & Medicine Gap Year research internship (to M. L. Burch).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.H.H. and J.S.P. conception and design of research; D.H.H., M.L.B., B.M., and J.B.M. performed experiments; D.H.H., M.L.B., B.M., K.A.H., and J.S.P. analyzed data; D.H.H., K.A.H., and J.S.P. interpreted results of experiments; D.H.H. and J.S.P. prepared figures; D.H.H. and J.S.P. drafted manuscript; D.H.H., M.L.B., K.A.H., and J.S.P. edited and revised manuscript; D.H.H., M.L.B., B.M., J.B.M., K.A.H., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Michael W. Brands for assistance with the radiotelemetry surgery and Dr. Pamela K. Carmines for critical and thorough evaluation of the manuscript.
Present address of D. H. Ho: Tripler Army Medical Ctr., Dept. of Clinical Investigation, 1 Jarrett White Rd., MCHK-CI, Bldg. 40, Rm. 216, Honolulu, HI 96859.
REFERENCES
- 1.Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 32: 256–266, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Heinonen K, Kajantie E, Eriksson JG. Early life stress and blood pressure levels in late adulthood. J Hum Hypertens 27: 90–94, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Kajantie E, Heinonen K, Forsen TJ, Eriksson JG. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med 41: 66–72, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci 18: 1413–1420, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Lüscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ; Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens 23: 233–246, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 110: 1761–1766, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Douglas G, Bendall JK, Crabtree MJ, Tatham AL, Carter EE, Hale AB, Channon KM. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE(-)/(-) mice. Cardiovasc Res 94: 20–29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation 126: 753–767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci 11: 123, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girod JP, Brotman DJ. Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiovasc Res 64: 217–226, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Houghton JL, Davison CA, Kuhner PA, Torossov MT, Strogatz DS, Carr AA. Heterogeneous vasomotor responses of coronary conduit and resistance vessels in hypertension. J Am Coll Cardiol 31: 374–382, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase–dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 49: 893–901, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Lee DL, Leite R, Fleming C, Pollock JS, Webb RC, Brands MW. Hypertensive response to acute stress is attenuated in interleukin-6 knockout mice. Hypertension 44: 259–263, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Loi M, Koricka S, Lucassen PJ, Joels M. Age- and sex-dependent effects of early life stress on hippocampal neurogenesis. Front Endocrinol (Lausanne) 5: 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loria AS, Ho DH, Pollock JS. A mechanistic look at the effects of adversity early in life on cardiovascular disease risk during adulthood. Acta Physiol (Oxf) 210: 277–287, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol 304: R121–R129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull 137: 959–997, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montezano AC, Touyz RM. Molecular mechanisms of hypertension–reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol 28: 288–295, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Montezano AC, Touyz RM. Reactive oxygen species and endothelial function–role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol 110: 87–94, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Munzel T, Hink U, Heitzer T, Meinertz T. Role for NADPH/NADH oxidase in the modulation of vascular tone. Ann NY Acad Sci 874: 386–400, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Nishinaka T, Kinoshita M, Nakamoto K, Tokuyama S. Sex differences in depression-like behavior after nerve injury are associated with differential changes in brain-derived neurotrophic factor levels in mice subjected to early life stress. Neurosci Lett 592: 32–36, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry 53: 5111–5120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Own LS, Iqbal R, Patel PD. Maternal separation alters serotonergic and HPA axis gene expression independent of separation duration in mice. Brain Res 1515: 29–38, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Petronio MS, Zeraik ML, Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules 18: 2821–2839, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry 73: 15–23, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roque S, Mesquita AR, Palha JA, Sousa N, Correia-Neves M. The behavioral and immunological impact of maternal separation: a matter of timing. Front Behav Neurosci 8: 192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiavone S, Colaianna M, Curtis L. Impact of early life stress on the pathogenesis of mental disorders: relation to brain oxidative stress. Curr Pharm Des 21: 1404–1412, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res 34: 665–673, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Su S, Wang X, Kapuku GK, Treiber FA, Pollock DM, Harshfield GA, McCall WV, Pollock JS. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension 64: 201–207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su S, Wang X, Pollock JS, Treiber FA, Xu X, Snieder H, McCall WV, Stefanek M, Harshfield GA. Adverse childhood experiences and blood pressure trajectories from childhood to young adulthood: the Georgia stress and Heart study. Circulation 131: 1674–1681, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan JC, Pollock JS. Coupled and uncoupled NOS: separate but equal? Uncoupled NOS in endothelial cells is a critical pathway for intracellular signaling. Circ Res 98: 717–719, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42: 1075–1081, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Touyz RM, Montezano AC. Vascular Nox4: a multifarious NADPH oxidase. Circ Res 110: 1159–1161, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Triggle CR, Ding H. The endothelium in compliance and resistance vessels. Front Biosci (Schol Ed) 3: 730–744, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Vetulani J. Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacol Rep 65: 1451–1461, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Walsh JH, Yong G, Cheetham C, Watts GF, O'Driscoll GJ, Taylor RR, Green DJ. Effects of exercise training on conduit and resistance vessel function in treated and untreated hypercholesterolaemic subjects. Eur Heart J 24: 1681–1689, 2003. [DOI] [PubMed] [Google Scholar]