The current study provides evidence that pharmacological depolarization of mitochondria activates neuronal nitric oxide (NO) synthase (nNOS) in neurons and vascular wall. Generation of NO from nNOS activation affords protection against lethal stress in neurons and promotes cerebral vasodilation, thus indicating functional significance of mitochondria-nNOS signaling pathway.
Keywords: mitochondrial ATP-sensitive potassium channels, diazoxide, BMS-191095, membrane potential, superoxide anion
Abstract
The diverse signaling events following mitochondrial depolarization in neurons are not clear. We examined for the first time the effects of mitochondrial depolarization on mitochondrial function, intracellular calcium, neuronal nitric oxide synthase (nNOS) activation, and nitric oxide (NO) production in cultured neurons and perivascular nerves. Cultured rat primary cortical neurons were studied on 7–10 days in vitro, and endothelium-denuded cerebral arteries of adult Sprague-Dawley rats were studied ex vivo. Diazoxide and BMS-191095 (BMS), activators of mitochondrial KATP channels, depolarized mitochondria in cultured neurons and increased cytosolic calcium levels. However, the mitochondrial oxygen consumption rate was unaffected by mitochondrial depolarization. In addition, diazoxide and BMS not only increased the nNOS phosphorylation at positive regulatory serine 1417 but also decreased nNOS phosphorylation at negative regulatory serine 847. Furthermore, diazoxide and BMS increased NO production in cultured neurons measured with both fluorescence microscopy and electron spin resonance spectroscopy, which was sensitive to inhibition by the selective nNOS inhibitor 7-nitroindazole (7-NI). Diazoxide also protected cultured neurons against oxygen-glucose deprivation, which was blocked by NOS inhibition and rescued by NO donors. Finally, BMS induced vasodilation of endothelium denuded, freshly isolated cerebral arteries that was diminished by 7-NI and tetrodotoxin. Thus pharmacological depolarization of mitochondria promotes activation of nNOS leading to generation of NO in cultured neurons and endothelium-denuded arteries. Mitochondrial-induced NO production leads to increased cellular resistance to lethal stress by cultured neurons and to vasodilation of denuded cerebral arteries.
NEW & NOTEWORTHY
The current study provides evidence that pharmacological depolarization of mitochondria activates neuronal nitric oxide (NO) synthase (nNOS) in neurons and vascular wall. Generation of NO from nNOS activation affords protection against lethal stress in neurons and promotes cerebral vasodilation, thus indicating functional significance of mitochondria-nNOS signaling pathway.
mitochondrial membrane potential is a critical regulator of cellular activity and survival and is controlled by a variety of factors including the ATP-sensitive potassium (mitoKATP) channels located on the inner mitochondrial membrane (2). Pharmacological activators of mitoKATP channels, such as BMS-191095 (BMS) and diazoxide, decrease the mitochondrial membrane potential by facilitating the influx of potassium from cytosol into the matrix (2). Signaling events following mitochondrial depolarization appear to be diverse in the various cell types comprising the neurovascular unit (cerebral vascular endothelium and smooth muscle, perivascular neurons, parenchymal neurons, and glia). Both BMS and diazoxide applications result in dilation of isolated cerebral arteries; however, individual effects on vascular smooth muscle and endothelium, which contribute to the overall vascular effect, are completely different (20, 22, 35, 42). Thus BMS and diazoxide relax cerebral vascular smooth muscle cells via generation of localized calcium sparks by sarcoplasmic reticulum linked to mitochondrial depolarization, resulting in decreased global intracellular Ca2+ ([Ca2+]i) (20, 42). Conversely, mitochondrial depolarization in cerebral endothelial cells leads to activation of endothelial nitric oxide synthase (eNOS) and production of nitric oxide (NO) by increasing [Ca2+]i as well as by increasing phosphorylation of eNOS via the phosphatidylinositol 3-kinase (PI3K)/Akt (protein kinase B) pathway (22). Endothelial NO then diffuses to adjacent vascular smooth muscle and enhances the intrinsic relaxant effects through a cGMP-dependent mechanism (33). Little is known about the relationships between mitochondrial membrane potential and NOS activation in other cell types of the neurovascular unit such as cortical neurons and perivascular nerves. Substances such as NO could be produced by cortical neurons or perivascular nerves and promote relaxation of cerebral vascular smooth muscle (41). Cerebral arteries are heavily invested by NOS-containing perivascular nerves and are closely associated to parenchymal neurons (1, 39, 40). Diazoxide and BMS depolarize mitochondria in neurons resulting in enhanced cellular protection in the form of pre- and postconditioning (9, 16, 23, 24). However, the signaling events following mitochondrial depolarization are not fully elucidated and no previous studies have examined the effects of mitochondrial depolarization on neuronal NOS (nNOS) activity.
In this study, we examined the effects of mitochondrial depolarization on nNOS activation and NO production in primary cultures of rat neurons as well as subsequent signaling events. Specifically, we determined the effects of diazoxide and BMS on mitochondrial membrane potential, mitochondrial reactive oxygen species (ROS) production, mitochondrial oxygen consumption rate (OCR), [Ca2+]i, and NO production. Furthermore, we examined the functional role of nNOS resulting from diazoxide application in promoting cellular protection of cultured neurons. We also examined the effects of BMS on relaxation of endothelium-denuded cerebral arteries, which contain intact perivascular innervation, to demonstrate that our studies in cultured neurons are supported by results from naturally occurring neurons. nNOS is located in perivascular nerves supplying the cerebral vasculature (39), and NO from both the perivascular (7, 8, 39) and parenchymal neurons (31) has access via diffusion to the cerebral vasculature. Although several investigators have indicated that nNOS or other NOS isoforms are present in mitochondria as well as in the cytosol, the prevailing evidence does not support a mitochondrial localization of NOS under basal conditions in neurons or cardiac cells (25, 44).
MATERIALS AND METHODS
The animal protocols were approved by the Institutional Animal Care and Use Committee of Tulane University School of Medicine and comply with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996, Bethesda, MD). Timed-pregnant dams (n = 14; for culturing neurons) and 10-wk-old male (n = 16; for vascular studies) Sprague-Dawley (SD) rats were obtained from Harlan (Indianapolis, IN). Rats were housed in the animal care facility and received standard rat chow and water ad libitum. Vehicle for drugs were either H2O, DMSO, or NaOH.
Rat primary cortical neuronal cell culture.
Timed-pregnant rat dams with embryonic day 18 fetuses were anesthetized with 5% isoflurane (VetOne, Boise, ID) and decapitated. Rat primary cortical neurons were isolated and cultured as previously described (9, 24). Briefly, cortical neurons were isolated and plated onto poly-d-lysine-coated dishes, multiwell plates, or coverslips and maintained in a humidified 5% CO2 incubator. After cell attachment, plating medium was replaced with Neurobasal Medium (Waltham, MA) supplemented with B27 (2%), l-glutamine (0.5 mM), 2-mercaptoethanol (55 μM), and KCl (25 mM). ARA-C (10 μM) was used to inhibit astrocyte growth. Positive immunostaining for microtubule-associated protein-2 and negative immunostaining for glial fibrillary acidic protein verified that the cultures consisted of more than 99% of neurons. Neurons were studied on 7–11 days in vitro (DIV).
Mitochondrial respiration of neurons.
Mitochondrial OCR in cultured cortical neurons was determined using a Seahorse Bioscience XFe24 Analyzer as described previously (9, 35). The Seahorse Bioscience XFe24 Analyzer determines mitochondrial OCR by measuring the rate of change in oxygen and proton concentrations in the medium surrounding the neurons that were cultured in poly-d-lysine coated 24-well plates. For the experiments, Neurobasal Medium was replaced with Seahorse XF Assay Medium (no. 102365-100; Seahorse Bioscience), containing 5.0 mmol/l glucose and 2.0 mmol/l pyruvate at pH 7.4. Experiments were conducted at 37°C. The neurons were exposed to medium alone, medium containing vehicle, or medium containing 500 μmol/l diazoxide. Individual components of mitochondrial respiration were evaluated using serial injections of the drugs oligomycin, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), and antimycin A plus rotenone. Mathematical calculations were made using the raw OCR values. Nonmitochondrial respiration equals the minimum value of the five OCR measurements after antimycin A and rotenone injection. Basal respiration equals the values for OCR measurements before the first injections minus nonmitochondrial respiration. Proton leak equals OCR measurements after oligomycin injection before FCCP injection minus nonmitochondrial respiration. ATP production equals basal respiration minus proton leak. Maximal respiration equals OCR values after FCCP injection before antimycin A/rotenone injection minus nonmitochondrial respiration. Spare respiratory capacity equals maximal respiration minus basal respiration. For mitochondrial function, we expressed OCR data in picomoles per minute.
Fluorescence confocal microscopy.
We determined the effects of vehicle, diazoxide (100 μmol/l), and BMS (50 μmol/l) on mitochondrial membrane potential, NO levels, mitochondrial ROS production, or levels of [Ca]i on neurons cultured on glass coverslips using protocols described previously (18, 20–22). In some experiments, we also coapplied 7-nitroindazole (7-NI, a selective nNOS inhibitor; 100 μmol/l). The concentrations of diazoxide, BMS, and other drugs were chosen based on our prior studies (9, 16, 20, 22, 23, 35, 36).
Mitochondrial membrane potential was determined using rhodamine 123 (20, 22). MitoSOX was used to measure mitochondrial ROS, specifically superoxide anion (34). Fluo-4 AM was used to determine [Ca2+]i (20). Diaminorhodamine-4M (DAR-4M) was used to determine NO (18, 22). All fluoroprobes were obtained from Molecular Probes (Eugene, OR). Confocal microscopy and imaging were performed using a laser scanning confocal system (7 Live; Zeiss, Jena, Germany) or a Leica SP2 AOB laser confocal microscope attached to an inverted microscope with optics and filters specific to the fluoroprobe. Imaging conditions such as gain levels and laser power were held constant for each protocol. Offline analysis of images was performed using ImageJ software (NIH) to determine the average pixel intensity of neurons in each defined field (10–20 fields per coverslip), and the results are expressed in relative fluorescence units. Fluorescence measurements in relative units are expressed as percent change from the baseline images before administration of vehicle, BMS, or diazoxide. The n value represents the number of cover slips containing neurons for each treatment.
Electron spin resonance spectroscopy for NO measurements.
The NO measurements were performed using electron spin resonance (ESR) spectroscopy, as previously described (4, 11, 22). Cultured neurons were incubated in 1 ml K-H buffer in the presence and absence of vehicle, diazoxide (100 μmol/l), or N-(2-aminoethyl)-N-(2-hydroxy-2-nitrosohydrazino)-1,2-ethylenediamine (spermine NONOate, a NO donor; 10 μmol/l). After incubation with vehicle or drugs, neurons were washed and collected for analysis. The ESR spectra were obtained using a benchtop X-band EMX series ESR spectrometer (Bruker Biospin, Karlsruhe, Germany) using a high-sensitivity SHQ microwave cavity in a finger Dewar filled with liquid nitrogen. The ESR spectrometer settings included the following: microwave power: 40 mW; modulation amplitude: 8-G center-field 2.03 g; sweep width: 80 G; conversion time: 80 ms; time constant: 20 ms; and sweep time: 10.24 s, with 60 scans. For calculation of baseline, signals were quantified by measuring the total amplitude after correction. The amount of NO was determined by measuring the total ESR signal amplitude for each treatment and comparing it with the ESR signal amplitude following NO-donor spermine NONOate. Diazoxide-induced NO production in neurons was expressed as percent change from basal levels (vehicle treated). The n value represents the sample size for normalized ESR signal amplitude of neurons grown to confluence in a 35-mm culture dish for each treatment.
Western blotting for nNOS.
Cultured neurons were treated with vehicle, 25 μmol/l BMS, or with 100 μmol/l diazoxide at 37°C for 15–20 min. Cells were washed and homogenates were prepared from the cells in lysis buffer containing protease and phosphatase inhibitors. Subsequently, cellular signaling was determined by Western blotting, as previously described (19). We used primary antibodies directed against phosphorylated nNOS (serine 1417, 160 kDa, Abcam; and serine 847, 160 kDa, Abcam) and total nNOS (160 kDa, BD Transduction Laboratories, San Jose, CA). Each immunoband intensity of phosphorylated nNOS was normalized to the corresponding immunoband intensity of total nNOS. The n value represents the sample size for normalized intensity of immunobands from the lysate of neurons grown to confluence in a 35-mm culture dish for each treatment.
Preconditioning experiments.
Primary cortical neurons were cultured in 96-well plates for 7 DIV. On the seventh day, cells were treated with 1) vehicle, 2) 500 μmol/l diazoxide, 3) diazoxide plus 100 μmol/l nitro-l-arginine methyl ester (l-NAME), and 4) diazoxide plus l-NAME and the NO donors, sodium nitroprusside (SNP; 1 μmol/l), or diethylamine nitric oxide (DEANO; 5 μmol/l), for 3 consecutive days. On 10 DIV, neurons were subjected to either oxygen glucose deprivation (OGD) or maintained at normoxia as previously described (23, 24). For OGD, 96-well cell culture plates and dishes were rinsed with PBS and the culture medium was replaced with glucose-free DMEM and placed in a Shel Lab Bactron Anaerobic Chamber (Sheldon Manufacturing, Cornelius, OR) containing anaerobic mixed gas (AMG; 5% CO2 -5% H2-90% N2) at 37°C for 3 h. The 5% H2 in the chamber removed remaining traces of oxygen-forming water on a platinum catalyst. The oxygen level was continuously monitored with an infrared gas analyzer (Illinois Instruments, Ingleside, IL) and maintained at <0.1% during the experiments. Control cell cultures were treated identically and incubated in glucose-containing DMEM (4.5 mg/ml) in a 5% CO2 cell culture incubator. Oxygen-glucose deprivation was terminated by removing the cell culture plates and dishes from the anoxic chamber and replacing the glucose-free medium with regular culture medium containing glucose. The cells were then maintained in a 5% CO2 incubator until determination of cell viability. Twenty-four hours after OGD, cell viability was assessed using the tetrazolium-based CellTiter 96 AQueous One Solution Assay (Promega, Madison, WI). Cell viability was expressed as the percentage of the corresponding control culture (untreated and not exposed to OGD). The n value represents the sample size for normalized cell viability of neurons grown to confluence in an each 96-well plate.
Vascular reactivity.
Vasoreactivity of isolated cerebral arteries following endothelial denudation was measured as described previously (20, 22). Briefly, rats were killed under deep anesthesia and posterior cerebral arteries were isolated from rat brains. Endothelium was removed by injecting a 1-ml bolus of air through the arteries. Arteries were transferred to a vessel bath filled with oxygenated, warm physiological salt solution, cannulated with glass pipettes, and secured with 10-0 ophthalmic suture. Intraluminal diameter was measured by video dimension analyzer (Living Systems Instrumentation, Burlington, VT). After an equilibration period of ∼30 min, arteries were slowly pressurized to 70 mmHg with PSS until they developed a stable myogenic tone (40–45% of passive diameter). Subsequently, drugs were administered abluminally via the bath solution and cumulative concentration responses to drugs were determined. Endothelial denudation was verified by lack of dilator response to bradykinin (10 μmol/l) and viability was tested by intact vasodilator response to SNP (10 μmol/l) and by contraction to KCl. Vascular responses to 50 μmol/l BMS were determined in endothelium denuded arteries pretreated with and without 7-NI (a selective nNOS inhibitor; 100 μmol/l) or tetrodotoxin (5 μmol/l). The n value represents the sample size for each segment of isolated cerebral artery for each treatment.
Electron microscopy.
For identification of perivascular nerves in cerebral arteries, rats were euthanized by deep anesthesia with 5% isoflurane and perfused with a PBS solution containing 2% of glutaraldehyde and 3% of formaldehyde. Arteries were removed and kept in the perfusion solution for 1 h and were fixed in a PBS solution containing 2% of glutaraldehyde and 3% of formaldehyde. Later, arteries were postfixed in 1% osmium tetroxide and embedded in Spurr's resin. Ultrathin sections (80–90 nm) were mounted on formvar-coated copper grids (200 mesh), air dried, and stained with uranyl acetate and lead citrate (at 7 and 7 min, respectively). The sections were put on grids and viewed at a magnification of ×11,000 using a FEI Tecnai BioTwin 120 keV TEM with a digital imaging setup (Wake Forest University Health Sciences, Winston-Salem, NC).
Data analysis and statistics.
Results are expressed as mean ± SE; n indicates the number of independent experiments. Means were compared by one-way ANOVA. Post hoc analysis was done by Tukey's test. A P < 0.05 was considered as statistically significant.
RESULTS
Mitochondrial membrane potential and ROS measurements.
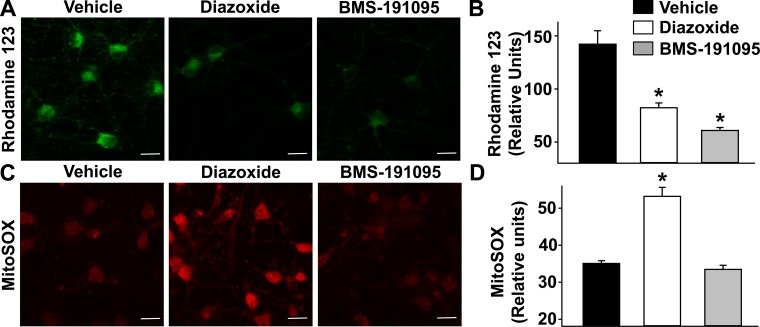
Diazoxide and BMS depolarized mitochondria in cultured neurons as indicated by the decreased rhodamine 123 fluorescence intensity (relative units) from 142 ± 13 following vehicle treatment to 82 ± 4.5 and 61 ± 2.7 following diazoxide and BMS, respectively (n = 6 each; P < 0.05, compared with vehicle; Fig. 1, A and B). On the other hand, BMS (33 ± 1.1, n = 5; P = NS) did not increase mitochondrial ROS levels whereas diazoxide increased ROS levels (53 ± 2.4, n = 8; P < 0.05, compared with vehicle), indicated by the increase in MitoSOX fluorescence intensity, compared with vehicle (35 ± 0.7, n = 6) in neurons (Fig. 1, C and D).
Fig. 1.
Mitochondrial depolarization and generation of mitochondrial reactive oxygen species (ROS). Fluorescence images of cultured neurons loaded with fluorescent probes and treated with vehicle or 50 μmol/l BMS-191095 (BMS) or 100 μmol/l diazoxide. A: fluorescence images of cultured neurons loaded with rhodamine 123, a mitochondrial membrane potential marker. Mitochondrial depolarization is demonstrated by the decrease in fluorescence. C: fluorescence images of cultured neurons loaded with MitoSOX, a mitochondrial ROS-sensitive dye. Mitochondrial ROS generation, indicated by increase in red fluorescence, is increased by diazoxide but not by BMS. B and D: cumulative data of rhodamine 123 and MitoSOX fluorescence intensities plotted as a bar graph for the neurons examined. Scale bar = 20 μm. *P < 0.05, compared with vehicle.
Mitochondrial OCR.
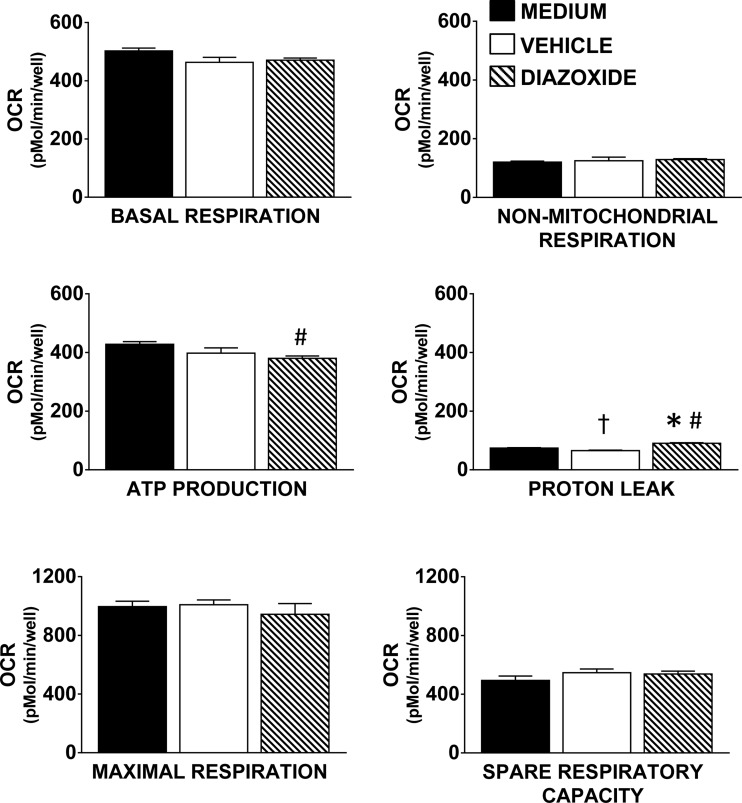
Diazoxide had no significant effects on raw values for OCR as well as for the calculated values for basal respiration, maximal respiration, spare respiratory capacity, or nonmitochondrial respiration (Fig. 2). However, diazoxide had statistically significant but modest effects on ATP production and proton leak (Fig. 2, n = 5 for each group; P < 0.05, DZ compared with vehicle, P < 0.05, DZ compared with medium, P < 0.05, vehicle compared with medium).
Fig. 2.
Components of oxygen consumption rate (OCR) in neurons. Mitochondrial bioenergetic parameters including basal respiration, nonmitochondrial respiration, ATP production, maximal respiration, and spare respiratory capacity were similar among medium, vehicle, and diazoxide groups. Diazoxide administration significantly decreased ATP production and significantly increased proton leak, but the effects were relatively modest. Data are expressed as mean ± SE; n = 5 plates for each group. *P < 0.05, diazoxide vs. vehicle; #P < 0.05, diazoxide vs. medium, †P < 0.05, vehicle vs. medium.
Cytosolic Ca2+.
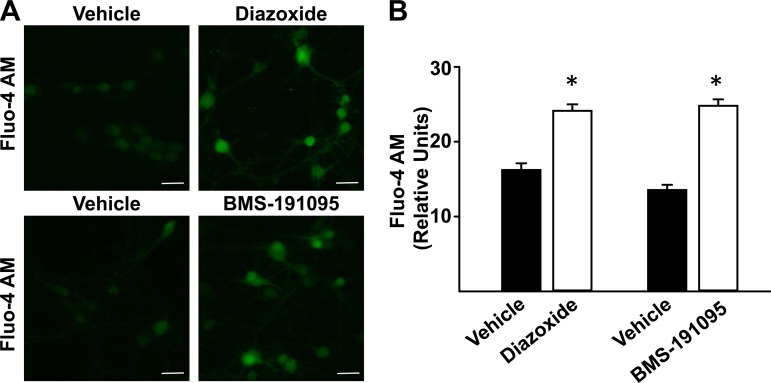
Diazoxide and BMS increased [Ca2+]i indicated by the increased fluo-4AM fluorescence intensity (relative units) to 24.1 ± 0.9 (n = 8; P < 0.05) and 24.8 ± 0.9 (n = 8; P < 0.05), respectively, compared with vehicle treated cultured neurons (16.2 ± 0.9 and 13.6 ± 0.7 for respective controls, n = 12 for each; Fig. 3, A and B).
Fig. 3.
Diazoxide and BMS increase intracellular Ca2+ ([Ca2+]i) in cultured neurons. A: representative fluorescence images of cultured neurons loaded with fluo-4 AM and treated with vehicle, 100 μmol/l diazoxide, or 50 μmol/l BMS are shown. Increase in fluo-4 AM fluorescence indicates increase in [Ca2+]i. B: cumulative data for all of the neurons studied. Scale bar = 20 μm. *P < 0.05, diazoxide or BMS treatment vs. vehicle.
nNOS phosphorylation.
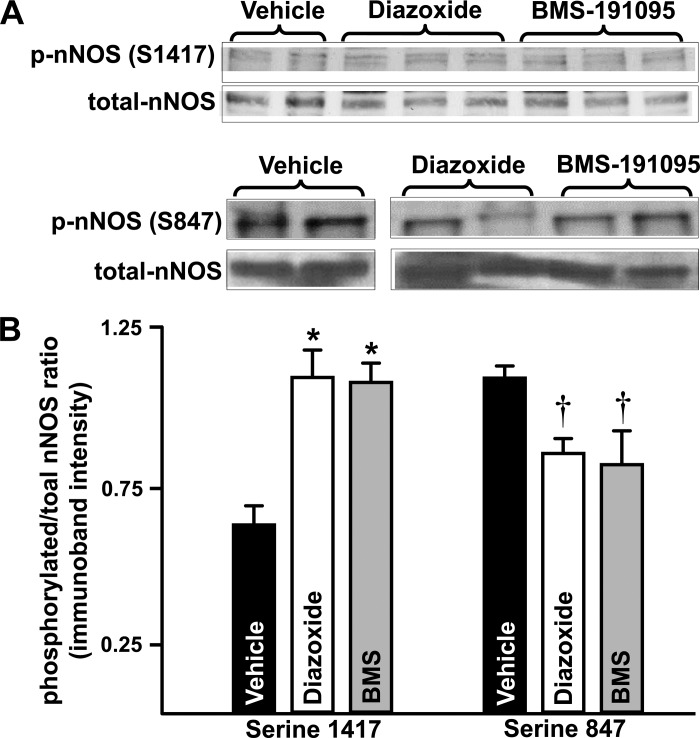
Treatment of neurons with diazoxide (1.1 ± 0.08, n = 6; P < 0.05) and BMS (1.09 ± 0.06, n = 5; P < 0.05) led to an increased phosphorylated serine 1417-nNOS/total-nNOS ratio of immunoband intensity compared with vehicle treated neurons (0.66 ± 0.06, n = 6; Fig. 4, A and B). In contrast, treatment of neurons with diazoxide (0.87 ± 0.04, n = 6; P < 0.05) and BMS (0.84 ± 0.1, n = 5; P < 0.05) induced a decreased phosphorylated-nNOS/total-nNOS ratio of immunoband intensity compared with vehicle-treated neurons (1.1 ± 0.04, n = 6; Fig. 4, A and B).
Fig. 4.
Diazoxide and BMS promote differential neuronal nitric oxide synthase (nNOS) phosphorylations favoring activation of the enzyme. Western blots of total and phosphorylated forms of nNOS in homogenates of neurons treated with vehicle (control) or diazoxide or BMS are shown (A). The cumulative data (mean ± SE) from the immunoband intensities were expressed as ratio of phosphorylated nNOS to total nNOS (B). *P < 0.05 and †P < 0.05 indicate significant difference compared with corresponding vehicle-treated neurons for serine 1417 and serine 847 immunobands, respectively.
NO production.
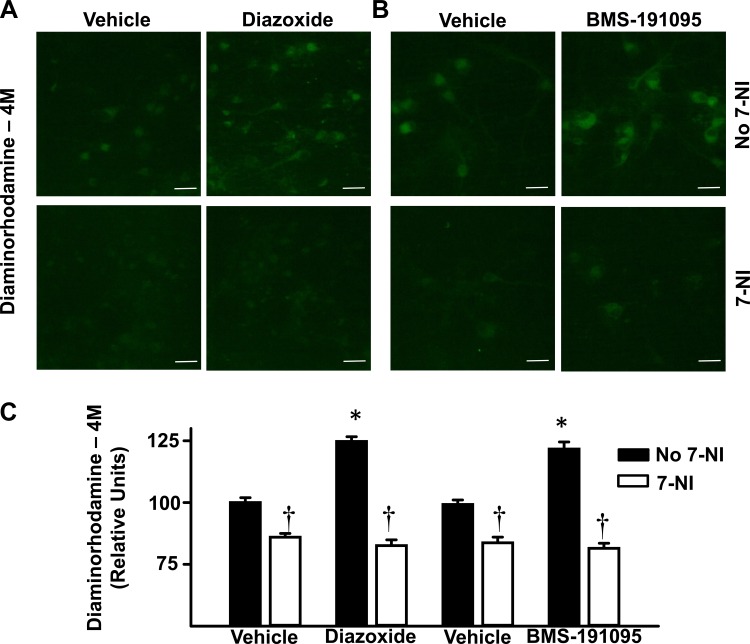
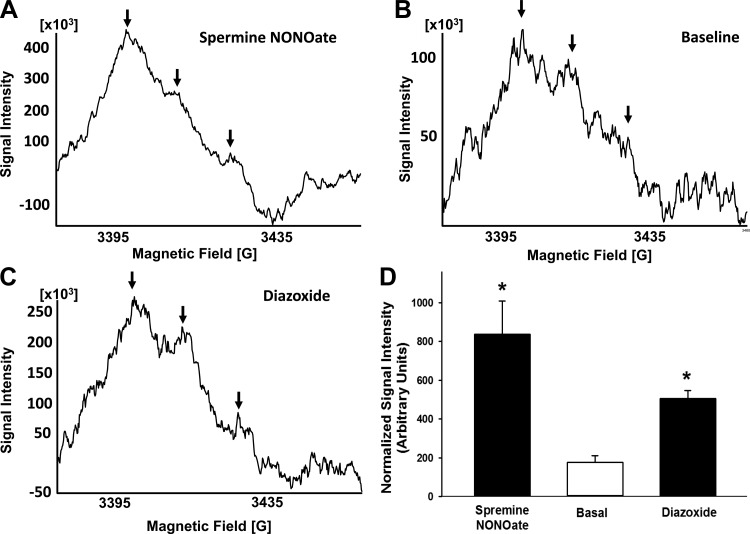
Diazoxide (125 ± 2, n = 7) and BMS (122 ± 3, n = 8) increased NO levels in cultured neurons indicated by the increased DAR-4M fluorescence intensity (relative units) compared with vehicle (100 ± 2 and 99 ± 2 for respective controls, n = 12 for each; Fig. 5, A–C; P < 0.05, compared with vehicle). Inhibition of nNOS with 7-NI pretreatment blocked NO increases to diazoxide (83 ± 2, n = 6; P = NS) and BMS (82 ± 2, n = 6; P = NS) compared with vehicle (83 ± 2 and 84 ± 2 for respective controls, n = 5 for each; Fig. 5, A–C). NO levels (relative units) also increased in the presence of diazoxide (507 ± 39, n = 5) or spermine NONOate (834 ± 172, n = 4) compared with basal levels in the presence of vehicle (177 ± 33, n = 5) using the ESR method (Fig. 6, A–D; P < 0.05, compared with basal).
Fig. 5.
Diazoxide and BMS increase NO production in cultured neurons as measured by diaminorhodamine-4M (DAR-4M). A and B: representative fluorescence images of cultured neurons loaded with DAR-4M indicates increased generation of NO. Neurons were pretreated with or without 7-nitroindazole (7-NI), before administration of vehicle, 100 μmol/l diazoxide, or 50 μmol/l BMS. C: cumulative data of fluorescence intensity for all of the neurons studied. Diazoxide and BMS increased NO production in cultured neurons and this effect was blocked by 7-NI. Scale bar = 20 μm. *P < 0.05, compared with vehicle. †P < 0.05, compared with absence of 7-NI.
Fig. 6.
Diazoxide and BMS increase NO production in cultured neurons as measured by electron spin resonance (ESR). A–C: representative ESR spectra of cultured neurons incubated for 90 min at 37°C with 0.4 mmol/l colloid Fe(DETC)2. The vertical axis represents signal intensity in arbitrary units and the horizontal axis represents the magnetic field (G). A characteristic NO-Fe(DETC)2 signal with 3 peaks (indicated by arrows) was detected in cultured neurons incubated with Fe(DETC)2 and various drugs. The representative ESR spectra of the basal (vehicle-treated), 100 μmol/l diazoxide-treated, and 10 μmol/l spermine NONOate-treated neurons are shown along with the cumulative data in arbitrary units are represented as a bar graph. D: cumulative data for all experiments. *P < 0.05, compared with basal level of normalized NO-Fe(DETC)2.
Preconditioning.
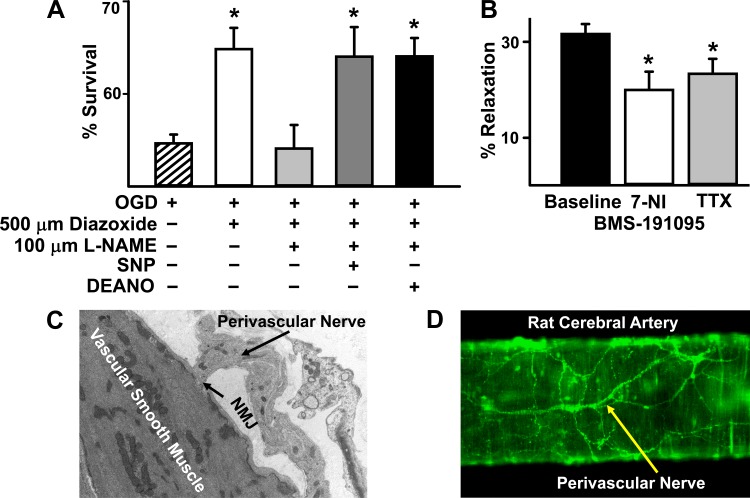
None of the treatments in DMEM containing glucose during normoxia significantly affected neuronal survival compared with untreated neurons (data not shown). After OGD, untreated neurons showed a significant decrease in viability (Fig. 7A). Diazoxide preconditioning improved neuronal survival significantly after OGD, compared with untreated neurons (Fig. 7A, P < 0.05, compared with OGD). Coadministration of l-NAME during preconditioning abolished the protective effects of diazoxide (Fig. 7A). Exogenously supplying NO using donors such as SNP and DEANONOate along with the combined diazoxide and l-NAME treatment rescued the protective effects of diazoxide (Fig. 7A, P < 0.05, compared with OGD).
Fig. 7.
Physiological consequences of mitochondrial depolarization induced NO production. A: preconditioning: oxygen glucose deprivation resulted in ∼50% cell death, which was attenuated by diazoxide treatment. Cotreatment of diazoxide with nitro-l-arginine methyl ester (l-NAME) prevented protection of neurons. Addition of the NO donors sodium nitroprusside (SNP) or diethylamine nitric oxide (DEANO) in the presence of diazoxide and l-NAME improved cell viability following oxygen glucose deprivation (OGD) comparable to diazoxide alone. *P < 0.05, compared with OGD alone. B: vasoreactivity: the basal intraluminal diameters of the cerebral arteries were similar for each group of experiments. Administration of 50 μmol/l BMS elicited vasodilation in endothelium-denuded cerebral arteries and coadministration of 7-NI or tetrodotoxin (TTX) diminished vasodilation in response to BMS. *P < 0.05, compared with baseline. C: electron microscopy. Electron microscopy image of rat cerebral artery showing the vascular smooth muscle cell with numerous mitochondria and a perivascular nerve showing a neuromuscular junction (NMJ). Rats were euthanized with anesthesia and perfused with a PBS solution containing 2% glutaraldehyde and 3% formaldehyde. Arteries were removed and kept in the perfusion solution for 1 h and postfixed in 1% osmium tetroxide and embedded in Spurr's resin. Ultrathin sections (80–90 nm) were mounted on formvar-coated copper grids (200 mesh), air dried, and stained with uranyl acetate and lead citrate (at 7 and 7 min, respectively). The sections were put on grids and viewed using a FEI Tecnai Bio Twin 120 keV TEM with a digital imaging setup (Wake Forest University Health Sciences). D: fluorescence microscopy. Fluorescence confocal microscopy image of rat middle cerebral artery loaded with fluo-4 AM, the calcium binding fluoroprobe, showing a perivascular nerves. Vascular smooth muscle cells also appear underneath the perivascular nerves.
A previous study by our laboratory demonstrated the involvement of Akt/mammalian target of rapamycin (mTOR)/S6K in diazoxide induced preconditioning, and we extended these studies by examining effects of l-NAME treatment. Administration of diazoxide enhanced the levels of phosphorylated/total of Akt, mTOR, and S6K and coadministration of l-NAME reversed this effect. We found that phosphorylated Akt protein/total protein expression was elevated from 16.8 ± 2.1 arbitrary units in the vehicle group to 59.4 ± 70.9 arbitrary units with diazoxide preconditioning and that l-NAME coapplication with diazoxide attenuated this increase (40.2 ± 4.9 arbitrary units; data not shown; P < 0.05, for diazoxide preconditioned vs. vehicle or diazoxide plus l-NAME). Similarly, we found that phosphorylated mTOR protein/total protein expression was elevated from 41.9 ± 4.2 arbitrary units in the vehicle group to 77.6 ± 6.8 arbitrary units with diazoxide preconditioning and that l-NAME coapplication with diazoxide attenuated this increase (42.1 ± 7.1 arbitrary units; data not shown; P < 0.05, for diazoxide preconditioned vs. vehicle or diazoxide plus l-NAME). Lastly, we found that S6K protein expression was elevated from 53.4 ± 11.4 arbitrary units in the vehicle group to 362.7 ± 70.9 arbitrary units with diazoxide preconditioning and that l-NAME coapplication with diazoxide attenuated this increase (70.3 ± 3.9 arbitrary units; data not shown; P < 0.05, for diazoxide preconditioned vs. vehicle or diazoxide plus l-NAME). Data are derived from 4–18 independent neuronal cultures per treatment.
Intraluminal diameter measurements.
The basal diameters of the cerebral arteries were similar for each group of experiments (data not shown). Administration of 50 μmol/l BMS elicited a robust vasodilation in endothelium-denuded cerebral arteries with 31.6 ± 2% relaxation (n = 14; Fig. 7B). Coadministration of 7-NI diminished vasodilation to 20.8 ± 2% in response to 50 μmol/l BMS (n = 11; P < 0.05, compared with baseline) and tetrodotoxin reduced vasodilation to 23.3 ± 3.1% (n = 6; P < 0.05, compared with vehicle; Fig. 7B).
Demonstration of perivascular nerves.
Electron microscopy identified perivascular nerve terminals and neuromuscular junctions on the surface of the cerebral arteries; a representative image is shown in Fig. 7C. Furthermore, fluorescence images of cerebral arteries loaded with a calcium binding fluoroprobe, fluo-4AM, showed perivascular nerve terminals morphologically consistent with previous reports by Segal et al. (38) and Yokomizo et al. (43) (Fig. 7D).
DISCUSSION
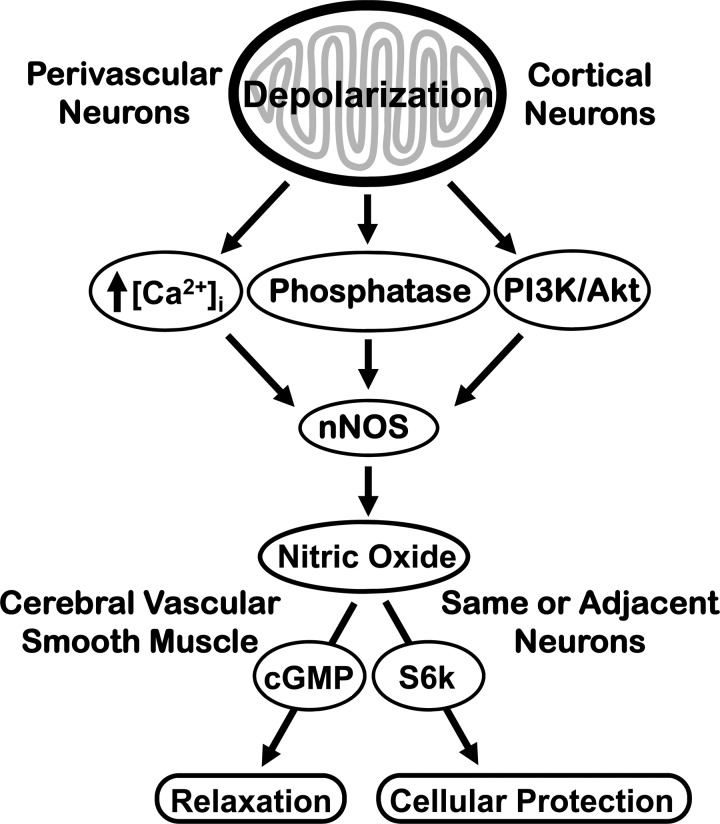
There are four major, new findings of the study. First, mitochondrial depolarization is a potent activator of nNOS in neurons and mitochondrial depolarization-induced NO generation in neurons is associated with increased [Ca2+]i and activating nNOS phosphorylation. Second, mitochondrial depolarization has minimal effects on mitochondrial respiration indicating that mitochondria can maintain energy production despite pertubations in mitochondrial status. Third, NO is an important mediator of diazoxide-induced preconditioning in cultured neurons demonstrating that NO is an important signaling agent for inducing cellular protection against potentially lethal stresses. Fourth, endothelium-independent vasodilation induced by mitochondrial depolarization is substantially mediated by NO, probably derived from the nNOS in perivascular nerves, establishing that perivascular and cultured parenchymal neurons respond similarly to mitochondrial activation. Our observations provide strong support for the view that the mitochondria-nNOS-NO signaling pathway represents an important, previously unknown, determinant of diverse responses in the neurovascular unit (Fig. 8).
Fig. 8.
Schematic illustration showing consequences of mitochondrial depolarization in neurons: The consequences of mitochondrial depolarization include activation of nNOS by increased [Ca]i, protein phosphatases, and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt pathway leading to augmented NO production and neuroprotection or arterial vasodilation. Previous studies have indicated that arterial dilation to NO is mediated by increased levels of cGMP in vascular smooth muscle (13), and results indicate that S6K phosphorylation mediate NO-induced neuronal preconditioning (10).
The current findings on neurons complement and extend our previous studies on other cell types and demonstrate the heterogeneity of mitochondrial potential-linked signal pathways in the neurovascular unit (14, 16, 23, 24). Thus nNOS activation in neurons following mitochondrial depolarization is similar in some respects to eNOS activation in cerebral vascular endothelium but is completely different from the NO-independent signaling pathways involving mitochondria that we have observed in cerebral vascular smooth muscle. Many structural, regulatory, and functional similarities exist between eNOS and nNOS isoforms (13). For example, both isoforms exhibit similar regulation of their activities by caveolins (37). Similarly, both isoforms are catalytically activated by increased [Ca2+]i. In addition, both isoforms have similar phosphorylation sites containing serine residues. We have shown that the PI3K-Akt pathway is activated by BMS and diazoxide to eNOS phosphorylation (22). Diazoxide also activates the PI3K-Akt signaling pathway in neurons (28). We observed that mitochondrial depolarization in neurons induces elevation of [Ca2+]i. In addition, we found that mitochondrial depolarization not only promotes nNOS phosphorylation at serine 1417 (positive regulatory site) but also diminishes phosphorylation at serine 847 (the negative regulatory site). The phosphorylation of nNOS at serine 1417 is likely to be due to activation of PI3K/Akt pathway. Notably, we previously have shown that mitochondrial depolarization activates PI3K/Akt pathway in neurons and endothelial cells (16, 22). In contrast, diminished phosphorylation at serine 847 has been shown to be due to promotion of protein phosphatase activity (45). It appears that mitochondrial depolarization promotes phosphatase activity in neurons that activates nNOS by reducing negative regulatory phosphorylation at serine 847. Further studies are required to confirm the identity and the role of mitochondria-activated phosphatases. Thus, for the first time, we found that mitochondrial depolarization in neurons leads to activation of nNOS and generation of NO. Although several studies have suggested the idea that NOS isoforms are present normally in mitochondria, we and others have been unable to find evidence for this concept in quiescent neurons and cardiomyocytes (25, 44). Nonetheless, mitochondrial as well as nuclear localization of NOS isoforms may occur following chronic stress such as inflammation (6). In contrast to endothelium and neurons, the predominant early event following mitochondrial depolarization in vascular smooth muscle cells is the localized generation of calcium sparks from sarcoplasmic reticulum resulting in a decrease in [Ca2+]i (20, 42).
BMS has selective effects on mitoKATP channels; however, it appears that diazoxide has dual effects on mitochondria, which involve activation of mitoKATP channels as well as generation of ROS from inhibition of complex II (2). We have documented that mitochondria are the source of superoxide anion in response to diazoxide application using the selective fluoroprobe MitoSOX. Based on our earlier studies, the formation of ROS after diazoxide application is probably via the inhibition of succinate dehydrogenase. Thus, although diazoxide produces two different effects, these effects are still limited to mitochondria. These findings confirm and extend our original observations with BMS and diazoxide in cerebral endothelial and vascular smooth muscle cells as well as in cultured neurons and astroglia and isolated mitochondria (20, 22). The consistent results from our studies as well as several investigations by others clearly demonstrate our original finding that mitochondrial depolarization with agents such as BMS can occur without increasing intracellular or mitochondrial ROS (2, 3, 16, 20, 22, 23). It is interesting that although diazoxide and BMS differ in their effect on mitochondrial ROS generation in various cell types we studied, they activate identical signaling events downstream of mitochondrial depolarization (3, 15, 16, 20, 22). Further studies are needed to examine the ROS-dependent and -independent effects of mitochondrial depolarization. Furthermore, it is noted that the only difference that we observed between diazoxide and BMS actions was the generation of acute production of superoxide only by diazoxide. Diazoxide and BMS elicit identical responses in activation of every signaling event that we measured. With regards to ONOO−, we have previously demonstrated in isolated mitochondrial from brain and heart that ONOO− formed in the mitochondria is an endogenous opener of mitoKATP channels (26, 27). Thus it is very likely that simultaneous formation of NO and superoxide in the mitochondria could lead to peroxynitrite formation and subsequent effects on neurons. However, diazoxide-induced superoxide is formed in the mitochondria and is therefore mostly segregated in the mitochondrial compartment whereas diazoxide-induced nitric oxide is formed in the cytosol. Therefore, diazoxide-induced formation of peroxynitrite in the mitochondria is a possible but unlikely event. However, future studies will measure the peroxynitrite formation following diazoxide treatment in mitochondria and the cytosol.
We previously showed that BMS-induced mitochondrial depolarization had minimal effects on ATP production in cultured neurons (23). We have extended these results by a more comprehensive examination of the effects of diazoxide on mitochondrial respiration in cultured neurons. Diazoxide did not affect basal respiration, maximal respiration, spare respiratory capacity, or nonmitochondrial respiration. Diazoxide had only minimal, but statistically significant, effects on ATP production and proton leak. Although mitochondrial effects underlie the neuroprotection afforded by diazoxide, acute treatment of diazoxide has minimal effects on the mitochondrial respiration in neurons. It appears that diazoxide does not affect the normal mitochondrial functioning in neurons. In contrast, we have previously demonstrated that diazoxide treatment increases basal, ATP synthesis-linked, and maximal respiration in neurons subjected to oxygen-glucose deprivation (9). Thus diazoxide-afforded neuroprotection does not involve alteration of mitochondrial function under normal conditions but utilize other mitochondrial-initiated signaling pathways as previously described (9). We can conclude that mitochondrial respiratory dynamics are robust and are only minimally affected by depolarization with BMS or by depolarization and succinate dehydrogenase inhibition by diazoxide.
We also previously demonstrated that exposure of isolated cerebral arteries to diazoxide or BMS leads to relaxation that involves distinct and overlapping mechanisms originating from vascular smooth muscle and endothelium (20, 22, 36). Our current study extends these observations with the demonstration that enhanced NO production by perivascular nerves in the adventitia and its diffusion to vascular smooth muscle also contributes to the integrated arterial response to diazoxide and BMS. Large cerebral arteries are heavily invested with many types of nerves (1). Furthermore, it is likely that NO from parenchymal neurons can promote dilation of cerebral resistance vessels, as previously shown, with application of glutamate receptor agonists to the intact cortical surface (32). Notably, nNOS has been identified in the vascular smooth muscle (12) and thus may also contribute to the mitochondrial depolarization induced vasodilation in endothelium-denuded arteries. However, the exact mechanisms underlying the activation of vascular smooth muscle nNOS are unclear and need further study. Mitochondria in various cell types within the neurovascular unit could be depolarized simultaneously by global events such as ischemia, but individual cells types may be activated by more restricted events. For example, increased shear stress has been shown to selectively activate mitochondria in endothelium and secondarily promote relaxation of smooth muscle of human coronary arteries (29, 30). In addition, cortical spreading depression depolarizes mitochondria in neurons but not in astroglia and we have shown that NO is an important mediator of vasodilation (7, 8, 16). Thus we propose that cell specific mitochondrial depolarization in the neurovascular unit during changes in physiological status, such as occurs during neuronal activation, will lead to appropriate blood flow changes due to production of NO.
Our results showing a critical role of endogenous NO in diazoxide-induced protection of cultured neurons is consistent with our earlier findings showing that NO is critical for in vivo preconditioning following cortical spreading depression (16, 17). An important role for NO in the development of neuronal protection has also been shown by other investigators but has not been linked to mitochondrial membrane potential (5). Together with our earlier findings (9), it appears that diazoxide induces preconditioning via a NO-S6K signaling pathway.
In summary, our study has uncovered a novel mitochondria-mediated mechanism of NO generation in neurons and cerebral arteries that involves the activation of nNOS in response to mitochondrial depolarization. Activation of both ROS-dependent and ROS-independent signaling pathways following mitochondrial depolarization leads to complex cellular events, which appear to match neuronal activity and cerebral blood flow with metabolism.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-077731, HL-030260, HL093554, and HL-065380 (to D. W. Busija), Louisiana Board of Regents Support Fund-Research Competitiveness Subprogram [LEQSF(2014-17)-RD-A-11; to P. V. Katakam], American Heart Association National Center Scientist Development Grant 14SDG20490359 (to P. V. Katakam), and American Heart Association Post-Doctoral Fellowship Grant 15POST23040005 (to I. Rutkai). This research was supported in whole or in part by the Louisiana Board of Regents Endowed Chairs for Eminent Scholars Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.V.G.K., S.D., and D.W.B. conception and design of research; P.V.G.K., S.D., V.N.S., S.M.G., A.O.G., N.R.P., and I.R. performed experiments; P.V.G.K., S.D., V.N.S., S.M.G., A.O.G., and N.R.P. analyzed data; P.V.G.K., S.D., V.N.S., N.R.P., and D.W.B. interpreted results of experiments; P.V.G.K., S.D., I.R., and D.W.B. prepared figures; P.V.G.K. and D.W.B. drafted manuscript; P.V.G.K., S.D., V.N.S., A.O.G., N.R.P., I.R., and D.W.B. edited and revised manuscript; P.V.G.K., S.D., V.N.S., S.M.G., A.O.G., N.R.P., I.R., and D.W.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nancy Busija for editing the manuscript.
REFERENCES
- 1.Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol 101: 161–211, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Busija DW, Katakam P, Rajapakse NC, Kis B, Grover G, Domoki F, Bari F. Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Res Bull 66: 85–90, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Busija DW, Katakam PV. Mitochondrial mechanisms in cerebral vascular control: shared signaling pathways with preconditioning. J Vasc Res 51: 175–189, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H, Li Z, Dikalov S, Holland SM, Hwang J, Jo H, Dudley SC Jr, Harrison DG. NAD(P)H oxidase-derived hydrogen peroxide mediates endothelial nitric oxide production in response to angiotensin II. J Biol Chem 277: 48311–48317, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8: 766–775, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Chan CK, Jiang Y, Davidge ST. Neuronal nitric oxide synthase regulates endothelial inflammation. J Leukoc Biol 91: 947–956, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Colonna DM, Meng W, Deal DD, Busija DW. Nitric oxide promotes arteriolar dilation during cortical spreading depression in rabbits. Stroke 25: 2463–2470, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Colonna DM, Meng W, Deal DD, Gowda M, Busija DW. Neuronal NO promotes cerebral cortical hyperemia during cortical spreading depression in rabbits. Am J Physiol Heart Circ Physiol 272: H1315–H1322, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Dutta S, Rutkai I, Katakam PV, Busija DW. The mechanistic target of rapamycin (mTOR) pathway and S6 kinase mediate diazoxide preconditioning in primary rat cortical neurons. J Neurochem 134: 845–856, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta S, Wappler E, Katz PS, Carvalho CI, Katakam PV, Busija DW. mTOR pathway mediates diazoxide preconditioning in cultured neurons. FASEB J 27: 691.691, 2013. [Google Scholar]
- 11.Fink B, Dikalov S, Fink N. ESR techniques for the detection of nitric oxide in vivo as an index of endothelial function. Pharmacol Rep 58, Suppl: 8–15, 2006. [PubMed] [Google Scholar]
- 12.Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23: 1121–1131, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 837a-837d, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaspar T, Domoki F, Lenti L, Institoris A, Snipes JA, Bari F, Busija DW. Neuroprotective effect of adenoviral catalase gene transfer in cortical neuronal cultures. Brain Res 1270: 1–9, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaspar T, Katakam P, Snipes JA, Kis B, Domoki F, Bari F, Busija DW. Delayed neuronal preconditioning by NS1619 is independent of calcium activated potassium channels. J Neurochem 105: 1115–1128, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaspar T, Snipes JA, Busija AR, Kis B, Domoki F, Bari F, Busija DW. ROS-independent preconditioning in neurons via activation of mitoK(ATP) channels by BMS-191095. J Cereb Blood Flow Metab 28: 1090–1103, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Horiguchi T, Snipes JA, Kis B, Shimizu K, Busija DW. The role of nitric oxide in the development of cortical spreading depression-induced tolerance to transient focal cerebral ischemia in rats. Brain Res 1039: 84–89, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Katakam PV, Domoki F, Lenti L, Gaspar T, Institoris A, Snipes JA, Busija DW. Cerebrovascular responses to insulin in rats. J Cereb Blood Flow Metab 29: 1955–1967, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katakam PV, Domoki F, Snipes JA, Busija AR, Jarajapu YP, Busija DW. Impaired mitochondria-dependent vasodilation in cerebral arteries of Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol 296: R289–R298, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katakam PV, Gordon AO, Sure VN, Rutkai I, Busija DW. Diversity of mitochondria-dependent dilator mechanisms in vascular smooth muscle of cerebral arteries from normal and insulin-resistant rats. Am J Physiol Heart Circ Physiol 307: H493–H503, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katakam PV, Snipes JA, Steed MM, Busija DW. Insulin-induced generation of reactive oxygen species and uncoupling of nitric oxide synthase underlie the cerebrovascular insulin resistance in obese rats. J Cereb Blood Flow Metab 32: 792–804, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katakam PV, Wappler EA, Katz PS, Rutkai I, Institoris A, Domoki F, Gaspar T, Grovenburg SM, Snipes JA, Busija DW. Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler Thromb Vasc Biol 33: 752–759, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kis B, Nagy K, Snipes JA, Rajapakse NC, Horiguchi T, Grover GJ, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 induces neuronal preconditioning. Neuroreport 15: 345–349, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J Neurochem 87: 969–980, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Lacza Z, Pankotai E, Busija DW. Mitochondrial nitric oxide synthase: current concepts and controversies. Front Biosci 14: 4436–4443, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacza Z, Snipes JA, Kis B, Szabo C, Grover G, Busija DW. Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Res 994: 27–36, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lacza Z, Snipes JA, Miller AW, Szabo C, Grover G, Busija DW. Heart mitochondria contain functional ATP-dependent K+ channels. J Mol Cell Cardiol 35: 1339–1347, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Liu RG, Song N, Li JM, Cui X, Chen YQ. [Akt involved in diazoxide preconditioning against rat hippocampal neuronal apoptosis induced by anoxia-reoxygenation injury]. Zhonghua Yi Xue Za Zhi 90: 492–495, 2010. [PubMed] [Google Scholar]
- 29.Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Med Biol Eng Comput 46: 469–478, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Meng W, Colonna DM, Tobin JR, Busija DW. Nitric oxide and prostaglandins interact to mediate arteriolar dilation during cortical spreading depression. Am J Physiol Heart Circ Physiol 269: H176–H181, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Meng W, Tobin JR, Busija DW. Glutamate-induced cerebral vasodilation is mediated by nitric oxide through N-methyl-d-aspartate receptors. Stroke 26: 857–862; discussion 863, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Qian J, Fulton D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front Physiol 4: 347, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc 3: 941–947, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Rutkai I, Dutta S, Katakam PV, Busija DW. Dynamics of enhanced mitochondrial respiration in female compared with male rat cerebral arteries. Am J Physiol Heart Circ Physiol 309: H1490–H1500, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutkai I, Katakam PV, Dutta S, Busija DW. Sustained mitochondrial functioning in cerebral arteries after transient ischemic stress in the rat: a potential target for therapies. Am J Physiol Heart Circ Physiol 307: H958–H966, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato Y, Sagami I, Shimizu T. Identification of caveolin-1-interacting sites in neuronal nitric-oxide synthase. Molecular mechanism for inhibition of NO formation. J Biol Chem 279: 8827–8836, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Segal SS. Integration and modulation of intercellular signaling underlying blood flow control. J Vasc Res 52: 136–157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toda N, Okamura T. Cerebral blood flow regulation by nitric oxide in Alzheimer's disease. J Alzheimers Dis 32: 569–578, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Toda N, Okamura T. Modulation of renal blood flow and vascular tone by neuronal nitric oxide synthase-derived nitric oxide. J Vasc Res 48: 1–10, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Westcott EB, Segal SS. Perivascular innervation: a multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation 20: 217–238, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res 97: 354–362, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokomizo A, Takatori S, Hashikawa-Hobara N, Goda M, Kawasaki H. Characterization of perivascular nerve distribution in rat mesenteric small arteries. Biol Pharm Bull 38: 1757–1764, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Zaobornyj T, Ghafourifar P. Strategic localization of heart mitochondrial NOS: a review of the evidence. Am J Physiol Heart Circ Physiol 303: H1283–H1293, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Zhou C, Li C, Yu HM, Zhang F, Han D, Zhang GY. Neuroprotection of gamma-aminobutyric acid receptor agonists via enhancing neuronal nitric oxide synthase (Ser847) phosphorylation through increased neuronal nitric oxide synthase and PSD95 interaction and inhibited protein phosphatase activity in cerebral ischemia. J Neurosci Res 86: 2973–2983, 2008. [DOI] [PubMed] [Google Scholar]