Abstract
OBJECTIVES
Video-assisted thoracoscopic (VATS) lobectomy is increasingly accepted for the management of early-stage non-small cell lung cancer (NSCLC), but its role for locally advanced cancers has not been as well characterized. We compared outcomes of patients who received induction therapy followed by lobectomy, via VATS or thoracotomy.
METHODS
Perioperative complications and long-term survival of all patients with NSCLC who received induction chemotherapy (ICT) (with or without induction radiation therapy) followed by lobectomy from 1996–2012 were assessed using Kaplan–Meier and Cox proportional hazard analysis. Propensity score-matched comparisons were used to assess the potential impact of selection bias.
RESULTS
From 1996 to 2012, 272 patients met inclusion criteria and underwent lobectomy after ICT: 69 (25%) by VATS and 203 (75%) by thoracotomy. An ‘intent-to-treat’ analysis was performed. Compared with thoracotomy patients, VATS patients had a higher clinical stage, were older, had greater body mass index, and were more likely to have coronary disease and chronic obstructive pulmonary disease. Induction radiation was used more commonly in thoracotomy patients [VATS 28% (n = 19) vs open 72% (n = 146), P < 0.001]. Thirty-day mortality was similar between the VATS [3% (n = 2)] and open [4% (n = 8)] groups (P = 0.69). Seven (10%) of the VATS cases were converted to thoracotomy due to difficulty in dissection from fibrotic tissue and adhesions (n = 5) or bleeding (n = 2); none of these conversions led to perioperative deaths. In univariate analysis, VATS patients had improved 3-year survival compared with thoracotomy (61% vs 43%, P = 0.010). In multivariable analysis, the VATS approach showed a trend towards improved survival, but this did not reach statistical significance (hazard ratio, 0.56; 95% confidence interval, 0.32–1.01; P = 0.053). Moreover, a propensity score-matched analysis balancing patient characteristics demonstrated that the VATS approach had similar survival to an open approach (P = 0.56).
CONCLUSIONS
VATS lobectomy in patients treated with induction therapy for locally advanced NSCLC is feasible and effective and does not appear to compromise oncologic outcomes.
Keywords: VATS, Thoracoscopic lobectomy, Lung cancer
INTRODUCTION
Video-assisted thoracoscopic (VATS) lobectomy is a safe and accepted treatment for early-stage non-small cell lung cancer (NSCLC) [1]. When compared with open lobectomy, VATS is associated with decreased morbidity, shorter hospital stay, decreased chest tube duration, reduced inflammation, reduced postoperative pain, improved preservation of pulmonary function and shorter recovery time [2–11]. However, there have only been a few small retrospective studies evaluating the role of VATS lobectomy for patients with locally advanced NSCLC who have been treated with preoperative chemotherapy. These preliminary studies have shown that VATS is feasible and safe and is associated with reduced hospital stay and reduced chest tube duration when compared with open lobectomy, but evidence regarding long-term outcomes is limited [2, 12–14]. The purpose of this study was to assess short- and long-term outcomes among patients who are treated with preoperative chemotherapy followed by open versus VATS lobectomy for patients with NSCLC. Our objective was to test the hypothesis that the VATS approach in appropriately selected patients is not associated with significant differences in oncologic outcomes when compared with open lobectomy.
MATERIALS AND METHODS
A retrospective analysis was performed of patients with NSCLC who received preoperative chemotherapy with or without radiation therapy and had a lobectomy within 1 year of preoperative chemotherapy at the Duke University Medical Center from 1996 to 2012. The study received Institutional Review Board approval with individual patient consent being waived. The preoperative choice of therapy between chemotherapy alone or chemotherapy with concurrent radiation was based on physician preference and availability of induction therapy protocols. The surgical technique for open and VATS lobectomy was performed in a manner as previously described [2].
Survival and recurrence data were available for all patients. Baseline variables (collected at the time of induction therapy) and outcome variables included demographics, comorbidities, pulmonary function, preoperative clinical stage, histology, pathological stage, chest tube duration, length of hospitalization, postoperative bleeding requiring reoperation, postoperative bleeding requiring blood transfusion, pneumonia, atrial fibrillation, prolonged air leak (present for more than 5 days), respiratory failure, other major complications (myocardial infarction, pulmonary embolism, chylothorax, empyema, bronchopleural fistula, sepsis) and overall and recurrence-free survival.
As this study was focused on outcomes after resection, overall survival was determined from the time of lobectomy to death from any cause with patients censored at the time of last known follow-up. Recurrence-free survival was determined from the time of lobectomy to death or recurrence as evaluated by biopsy or imaging seen during close follow-up with patients censored at the time of last follow-up.
Post-discharge follow-up data were collected through clinic notes, direct contact with patients and physicians, a national death registry and postal questionnaire as collected by the Duke Cancer Institute. Preoperative staging was based on information prior to resection, including computed tomographic (CT) scan, positron emission tomographic (PET) scan, brain imaging with CT or magnetic resonance imaging, bronchoscopy, mediastinoscopy, thoracoscopic staging and needle biopsy. The postoperative pathological stage was determined from the final pathology report. In patients with N2 disease, restaging after induction therapy and prior to resection was routinely performed.
Statistical analysis
Baseline characteristics and outcomes were compared between the VATS and open groups using the Pearson's χ2-test or Fisher's exact test when applicable for categorical variables and Student's unpaired t-test or the Wilcoxon rank-sum test when applicable for continuous variables. Patients who underwent conversions from VATS to open lobectomy were assessed using an intent-to-treat analysis.
Overall and recurrence-free survival was evaluated using the Kaplan–Meier method and the log-rank test. A Cox proportional hazards model was then used to compare overall survival between open and VATS groups, adjusting for variables chosen based on clinical relevance, which include age, sex, forced expiratory volume in 1 s (FEV1), diffusing capacity of the lung for carbon monoxide (DLCO), cerebrovascular disease, diabetes, Zubrod score, renal insufficiency, congestive heart failure, coronary artery disease, prior thoracic surgery, radiation, chronic obstructive pulmonary disease (COPD), pretreatment histology, preinduction therapy clinical stage, history of smoking and operative year. Complete case analysis was used for this adjusted model and the proportional hazards assumption was tested for all Cox models using smooth scaled Schoenfeld residual plots (there were no violations of assumptions) with linearity confirmed for all continuous predictors included in Cox regression analysis using Martingale residuals.
A propensity-matched analysis of open versus VATS lobectomy was also performed to further evaluate if there were differences in perioperative outcomes and survival between groups. Propensity scores were developed, defined as the probability of treatment with the VATS approach versus open approach conditional on measured covariates. Variables included in the propensity score model were age, sex, coronary artery disease, COPD, pathological T and N status, distant metastases, tumour location (central versus peripheral, as previously defined [15]), pretreatment histology, radiation and operative date. Patients were then matched on propensity score using a 1 : 1 nearest neighbour matching algorithm with a calliper distance of 0.01 and no replacement. Patient demographics and outcomes were assessed following propensity matching using Pearson's χ2-test or Fisher's exact test when applicable for categorical variables and Student's unpaired t-test or the Wilcoxon rank-sum test when applicable for continuous variables. Further, overall and recurrence-free survival was evaluated using the Kaplan–Meier method and the log-rank test. Statistical significance was set as a P-value of less than 0.05. A Cox proportional hazards model was then used to compare overall survival between the propensity-matched open and VATS groups, adjusting for variables that did not meet the 20% threshold used to confer adequate balance [16]. All statistical analyses were performed using Stata version 13.0 (StataCorp LP, College Station, TX, USA).
RESULTS
Patient and treatment characteristics
Of the 272 patients who met study inclusion criteria, 203 (75%) underwent lobectomy by thoracotomy. The remaining 69 (25%) patients underwent thoracoscopic lobectomy. Table 1 gives the baseline characteristics of the patients in each group. When compared with the open group, the VATS group was older, more likely to be white, had higher body mass index, had a higher clinical stage, and were more likely to have hypertension, COPD and coronary artery disease (Table 1). Preoperative radiation was used in more patients in the open group [72% (n = 146)] when compared with the VATS group [28% (n = 19); P < 0.001]. Operative year also differed significantly between the groups (P < 0.001). Figures displaying the type of surgery by operative year and the type of preoperative treatment (ICT or induction chemoradiation) by operative year can be found in Supplementary Figs S1 and S2, respectively. There were no other significant differences in baseline characteristics between the two groups.
Table 1:
Patient characteristics
| Characteristic | Open approach (n = 203) | VATS approach (n = 69) | P-value |
|---|---|---|---|
| Female sex (n, %) | 84 (41) | 37 (54) | 0.077 |
| Age (mean ± SD) (years) | 58.3 ± 0.7 | 63.7 ± 1.2 | <0.001 |
| Ethnicity (n, %) | 0.024 | ||
| White | 176 (76) | 56 (81) | |
| Black | 26 (24) | 10 (14) | |
| Native American | 0 (0) | 3 (4) | |
| FEV1 (mean ± SD) (% predicted) | 71.4 ± 18.0 | 74.9 ± 18.9 | 0.17 |
| DLCO (mean ± SD) (% predicted) | 69.8 ± 18.9 | 73.6 ± 17.4 | 0.16 |
| BMI at operation (mean ± SD) (kg/m2) | 25.8 ± 4.7 | 27.7 ± 5.5 | 0.027 |
| History of diabetes (n, %) | 25 (12) | 13(19) | 0.18 |
| Renal insufficiency (n, %) | 2 (1) | 2 (3) | 0.27 |
| Hypertension (n, %) | 61 (30) | 38 (55) | <0.001 |
| COPD (n, %) | 45 (22) | 24 (35) | 0.037 |
| Peripheral vascular disease (n, %) | 10 (5) | 4 (6) | 0.76 |
| Prior thoracic surgery (n, %) | 43 (21) | 21 (30) | 0.12 |
| Clinical T statusa | 0.001 | ||
| 1a | 13 (7) | 13 (22) | |
| 1b | 20 (11) | 7 (12) | |
| 2a | 40 (21) | 18 (31) | |
| 2b | 29 (15) | 10 (17) | |
| 3 | 73 (38) | 7 (12) | |
| 4 | 15 (8) | 4 (0.7) | |
| Clinical N statusb | 0.10 | ||
| 0 | 74 (39) | 15 (27) | |
| 1 | 11 (6) | 4 (7) | |
| 2 | 102 (54) | 34 (61) | |
| 3 | 2 (1) | 3 (5) | |
| Clinical stage prior to induction therapy (n, %) | 0.004 | ||
| IA | 0 (0) | 0 (0) | |
| IB | 3 (1) | 1 (1) | |
| IIA | 14 (7) | 6 (9) | |
| IIB | 50 (25) | 3 (4) | |
| IIIA | 107 (53) | 44 (64) | |
| IIIB | 10 (5) | 3 (4) | |
| IV | 14 (7) | 5 (7) | |
| Unknown | 5 (2) | 7 (10) | |
| Congestive heart failure (n, %) | 5 (2) | 1 (1) | 0.62 |
| Cerebrovascular disease (n, %) | 10 (5) | 0 (0) | 0.062 |
| Coronary artery disease (n, %) | 21 (10) | 16 (23) | 0.007 |
| Zubrod score (n, %) | 0.32 | ||
| 0 | 62 (31) | 26 (38) | |
| 1 | 136 (68) | 40 (59) | |
| 2 | 2 (1) | 2 (3) | |
| 3 | 1 (0) | 0 (0) | |
| History of cancer (n, %) | 28 (14) | 12 (17) | 0.49 |
| History of smoking (n, %) | 192 (95) | 63 (91) | 0.33 |
| Radiation (n, %) | 146 (72) | 19 (28) | <0.001 |
| Transient ischemic attack/cerebrovascular accident (n, %) | 11 (5) | 3 (4) | 0.73 |
| Central versus peripheral tumour (n, %) | 0.78 | ||
| Central | 67 (33) | 15 (22) | |
| Peripheral | 136 (67) | 54 (78) | |
| Time from preoperative therapy to surgery (days) | 0.61 | ||
| Median (Interquartile range) | 92 (76–128) | 89 (73–115) | |
| Operative year | <0.001 | ||
| 1996–2001 | 58 (29) | 1 (1) | |
| 2002–2007 | 100 (49) | 12 (17) | |
| 2008–2012 | 45 (22) | 56 (81) |
FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; COPD: chronic obstructive pulmonary disease; VATS: video-assisted thoracoscopic surgery; SD: standard deviation; BMI: body mass index.
aData only available for 249 of 272 patients (open n = 190, VATS n = 59).
bData only available for 245 of 272 patients (open n = 189, VATS n = 56).
The majority of patients were clinical stage IIIA (Table 1), although a small percentage of clinical stage I patients had preoperative therapy. The reasons that Stage I patients received preoperative therapy included poor medical fitness (n = 2), initial refusal of surgery (n = 1) or a protocol for a clinical trial (n = 1). There was a difference between the VATS and open groups in terms of the indications for induction therapy (P = 0.001). In the open group, 39% (n = 79) had biopsy-proven N2 disease, 6% (n = 12) had clinical N2 disease, 8% (n = 16) underwent induction therapy for definitive treatment, 42% (n = 85) underwent induction therapy for local extent (e.g. T3–T4, N0–N1), 3% (n = 7) initially had chemotherapy or radiation because they were not deemed medically fit for surgery and 2% (n = 4) underwent induction for unknown reasons. In the VATS group 52% (n = 36) had biopsy-proven N2 disease, 9% (n = 6) had clinical N2 disease, 9% (n = 6) underwent induction therapy for definitive treatment, 14% (n = 10) underwent induction therapy for local extent, 13% (n = 9) underwent induction therapy because they were not deemed medically fit and 3% (n = 2) underwent induction therapy for unknown reasons.
Table 2 presents treatment and tumour characteristics for the two groups. The open group had higher pathological T status when compared with the VATS group. There were no significant differences in pathological N status, preinduction therapy tumour size, postinduction therapy tumour size, pathological stage and down-staging between the two groups (Table 2).
Table 2:
Treatment and tumour characteristics
| Characteristic | Open approach (n = 203) | VATS approach (n = 69) | P-value |
|---|---|---|---|
| Induction chemotherapy (n, %) | 0.47 | ||
| Platinum doublet therapy | 164 (81) | 57 (84) | |
| Platinum only | 1 (0.5) | 0 (0) | |
| Epidermal growth factor receptor inhibitor | 1 (0.5) | 1 (1) | |
| Taxane only | 5 (2) | 0 (0) | |
| Other | 6 (3) | 0 (0) | |
| Unknown | 26 (13) | 10 (15) | |
| Pathological T status (n, %) | 0.017 | ||
| T0 | 59 (29) | 15 (22) | |
| T1a | 40 (20) | 10 (14) | |
| T1b | 11 (5) | 12 (17) | |
| T2a | 34 (17) | 18 (26) | |
| T2b | 16 (8) | 6 (9) | |
| T3 | 39 (19) | 7 (10) | |
| T4 | 4 (2) | 1 (1) | |
| Pathological N status (n, %) | 0.23 | ||
| N0 | 146 (72) | 42 (61) | |
| N1 | 23 (11) | 11 (16) | |
| N2 | 34 (17) | 16 (23) | |
| Pathological M status (n, %) | 0.74 | ||
| M0 | 188 (93) | 65 (94) | |
| M1 | 14 (7) | 4 (6) | |
| Histology (n, %) | 0.012 | ||
| Adenocarcinoma | 75 (37) | 35 (51) | |
| Adenosquamous | 2 (1) | 2 (3) | |
| Squamous | 61 (30) | 24 (35) | |
| Large cell | 12 (6) | 3 (4) | |
| Non-small cell, not otherwise specified | 53 (26) | 5 (7) | |
| Complete pathological response (n, %) | 52 (26) | 15 (22) | 0.52 |
| Preinduction therapy tumour sizea (cm), mean ± SD | 4.8 ± 2.5 | 4.2 ± 2.4 | 0.22 |
| Postinduction therapy tumour size (cm), mean ± SD | 2.7 ± 2.8 | 2.8 ± 2.2 | 0.42 |
| Down-staged from PN2 to PN0b (n, %) | 54 (59) | 20 (48) | 0.21 |
| Down-staged from PN2 to PN1 or PN0b (n, %) | 64 (70) | 28 (67) | 0.67 |
| Overall down-stagec (n, %) | 121 (61) | 36 (58) | 0.67 |
FEV1: forced expiratory volume in 1 s; VATS: video-assisted thoracoscopic surgery; SD: standard deviation.
aData only available for 169 of 272 patients (open n = 124, VATS n = 45).
bDenominator used is the number of patients who had N2 disease in the open (n = 91) and VATS (n = 42) groups.
cData only available for 260 of 272 patients (open n = 198, VATS n = 62).
Anatomic distribution of lobectomies
The anatomic distribution of lobectomies performed significantly differed between the open and VATS groups (P < 0.001). When compared with thoracotomy, there were fewer right upper lobectomies and more lower lobectomies performed by VATS. In the open group, 59% (n = 120) underwent right upper lobectomy, 7% (n = 15) underwent right middle lobectomy, 2% (n = 5) underwent right lower lobectomy, 27% (n = 54) underwent left upper lobectomy and 4% (n = 9) underwent left lower lobectomy. In the VATS group, 35% (n = 24) underwent right upper lobectomy, 9% (n = 6) underwent right middle lobectomy, 20% (n = 14) underwent right lower lobectomy, 17% (n = 12) underwent left upper lobectomy and 19% (n = 13) underwent left lower lobectomy.
Perioperative outcomes
Table 3 reports short-term perioperative outcomes for the two groups. Patients who underwent thoracoscopic lobectomy had shorter length of hospital stay, shorter chest tube duration and fewer cases of pneumonia than the thoracotomy group. There were no significant differences in 30-day all-cause mortality, overall morbidity, postoperative bleeding requiring reoperation, postoperative bleeding requiring blood transfusion, atrial fibrillation, prolonged air leak, respiratory failure and other major complications.
Table 3:
Perioperative outcomes
| Characteristic | Open approach (n = 203) | VATS approach (n = 69) | P-value |
|---|---|---|---|
| 30-day mortality (n, %) | 8 (4) | 2 (3) | 0.69 |
| Overall morbiditya (n, %) | 97 (48) | 28 (41) | 0.30 |
| Postoperative bleeding requiring blood transfusion (n, %) | 21 (10) | 4 (6) | 0.34 |
| Postoperative bleeding requiring reoperation (n, %) | 3 (1) | 1 (1) | 1.00 |
| Pneumonia (n, %) | 23 (11) | 2 (3) | 0.036 |
| Atrial fibrillation (n, %) | 40 (20) | 13 (19) | 0.88 |
| Prolonged air leaks (n, %) | 29 (14) | 10 (14) | 0.97 |
| Respiratory failure (n, %) | 7 (3) | 0 (0) | 0.20 |
| Other major complications (n, %) | 20 (10) | 6 (9) | 1.00 |
| Length of hospitalization (days), median (IQR) | 5 (4–7) | 4 (4–5) | <0.001 |
| Chest tube duration (days), median (IQR) | 4 (3–5) | 3 (2–4) | <0.001 |
VATS: video-assisted thoracoscopic surgery; SD: standard deviation; IQR: interquartile range.
aOverall morbidity represents the number (%) of patients with at least one postoperative complication.
Overall, there were seven conversions from VATS to thoracotomy (10%). Six (9%) of the conversions occurred in patients who underwent ICT and were due to difficulty from fibrotic tissue and adhesions (n = 4) or bleeding (n = 2). One (1%) of the VATS patients who underwent induction chemoradiation therapy was converted to thoracotomy due to difficulty from fibrotic tissue and adhesions. None of the patients who required a conversion had a perioperative death.
Survival analysis
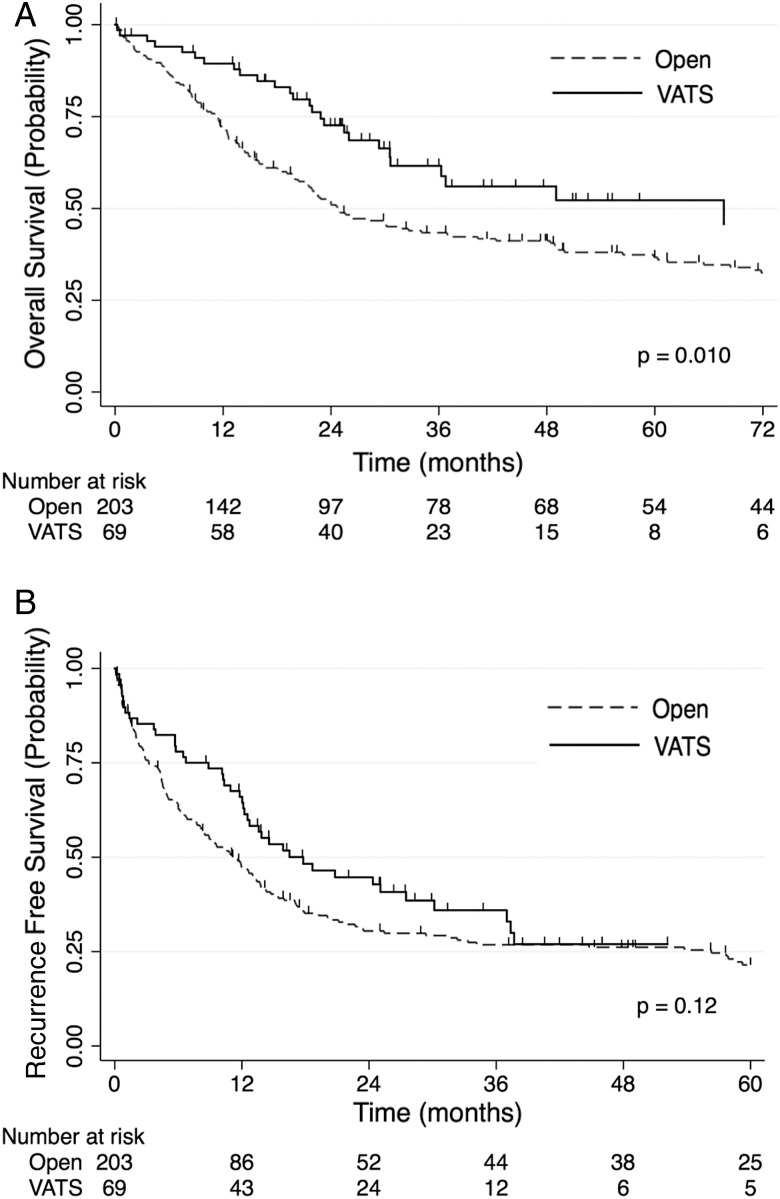
In univariable analysis and after a median follow-up of 24.0 (range: 0.1–184) months for the entire cohort, improvements in overall survival were associated with VATS compared with open surgery {27 of 69 events vs 143 of 203 events; 3-year survival 61% [95% confidence interval (CI), 47–73%] vs 43% (95% CI, 36–50%); log-rank, P = 0.010} (Fig. 1). The VATS group had a median survival of 67.7 months (95% CI, 30.6–83.5) and a median follow-up of 25.8 (range: 0.1–117) months, while the open group had a median survival of 24.8 months (95% CI, 21.3–36.9) and a median follow-up of 22.6 (range: 0.2–184) months. As shown in Table 4, there was a trend towards improved overall survival for the VATS approach when compared with the open approach after multivariable adjustment, but this did not reach statistical significance [hazard ratio (HR), 0.56; 95% CI, 0.32–1.01; P = 0.053].
Figure 1:
(A) Overall survival stratified by surgical approach: this figure depicts the Kaplan–Meier overall survival estimates of the open group (dashed line) versus the VATS group (solid line). (B) Recurrence-free survival stratified by surgical approach: this figure depicts the Kaplan–Meier recurrence-free survival estimates of the open group (dashed line) versus the VATS group (solid line). Tick marks represent censored subjects. VATS: video-assisted thoracoscopic surgery.
Table 4:
Multivariable Cox proportional hazards model
| Characteristic | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Approach | 0.56 | 0.32–1.01 | 0.053 |
| Age | 1.01 | 0.99–1.03 | 0.17 |
| Sex | 0.90 | 0.61–1.33 | 0.61 |
| FEV1 | 1.00 | 0.99–1.02 | 0.56 |
| DLCO | 0.99 | 0.98–1.00 | 0.11 |
| Cerebrovascular disease | 0.96 | 0.35–2.66 | 0.94 |
| Diabetes | 1.09 | 0.65–1.83 | 0.73 |
| Zubrod score | |||
| 0 | Ref | Ref | Ref |
| 1 | 0.68 | 0.46–1.01 | 0.055 |
| 2 and 3a | 2.49 | 0.73–8.52 | 0.15 |
| Renal insufficiency | 1.08 | 0.25–4.72 | 0.92 |
| Congestive heart failure | 1.45 | 0.49–4.27 | 0.50 |
| Coronary artery disease | 0.95 | 0.54–1.68 | 0.86 |
| Prior thoracic surgery | 1.49 | 0.96–2.30 | 0.077 |
| Radiation | 1.22 | 0.78–1.19 | 0.39 |
| COPD | 1.62 | 1.01–2.62 | 0.047 |
| Pretreatment histology | |||
| Adenocarcinoma | Ref | Ref | Ref |
| Adenosquamous | 0.99 | 0.13–7.45 | 0.99 |
| Squamous | 1.01 | 0.66–1.53 | 0.98 |
| Large cell | 0.89 | 0.41–1.92 | 0.76 |
| Non-small cell, not otherwise specified | 0.57 | 0.34–0.96 | 0.033 |
| Clinical stage prior to induction therapy | 1.00 | 0.99–1.01 | 0.68 |
| History of smoking | 2.09 | 0.62–7.03 | 0.23 |
| Operative year | 1.02 | 0.96–1.08 | 0.45 |
VATS: video-assisted thoracoscopic surgery; COPD: chronic obstructive pulmonary disease; SD: standard deviation; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide.
aZubrod scores 2 and 3 were combined into one category to simplify the output because there was only 1 patient in the entire cohort with a Zubrod score of 3.
There were no significant differences in recurrence-free survival between the VATS and open groups in 3-year survival [44 of 69 events vs 150 of 203 events; 36% (95% CI, 24–48%) vs 27% (95% CI, 21–33%); log-rank, P = 0.12]. There were also no differences in recurrence-free survival between the VATS and open groups regarding median survival [17.8 months (95% CI, 12.2–30.2) vs 11.5 months (95% CI, 8.2–13.7)]. After multivariable adjustment, there was no significant difference in recurrence-free survival between the VATS and open lobectomy groups (HR, 0.68; 95% CI, 0.42–1.09; P = 0.11).
Propensity analysis
The propensity analysis created groups of well-matched VATS and thoracotomy patients. Comparison of baseline patient characteristics after propensity matching between patients who had VATS versus open lobectomy following induction therapy shows no statistically significant differences in baseline characteristics between the two groups (Table 5). After propensity matching, patients who underwent thoracoscopic lobectomy continued to have shorter length of hospital (P = 0.007) stay and shorter chest tube duration (P = 0.023) than the thoracotomy group (Table 6). There continued to be no significant differences in 30-day mortality (P = 0.55), overall morbidity (P = 0.20), postoperative bleeding requiring reoperation (1.00), postoperative bleeding requiring blood transfusion (P = 0.64), pneumonia (P = 0.39), atrial fibrillation (P = 1.00), prolonged air leak (P = 0.28), respiratory failure (P = 0.31) and other major complications (P = 1.00) between the two groups (Table 6). A characterization of the unmatched VATS patients can be found in Supplementary Table S1.
Table 5:
Baseline characteristics after propensity score matching
| Characteristics | Open approach Matched patients (n = 30) |
VATS approach Matched patients (n = 30) |
P-value | Absolute standardized difference (%) |
|---|---|---|---|---|
| Female sex (n, %) | 13 (43) | 12 (40) | 0.79 | 6.7 |
| Age (mean ± SD) (years) | 60.7 ± 8.9 | 61.6 ± 11.4 | 0.72 | 9.3 |
| Ethnicity (n, %) | 0.15 | |||
| White | 26 (87) | 27 (90) | 9.1 | |
| Black | 4 (13) | 1 (3) | 29.0 | |
| Native American | 0 (0) | 2 (7) | 45.9 | |
| FEV1 (mean ± SD) (% predicted) | rm14 | 70.4 ± 20.5 | 0.47 | 20.5 |
| DLCO (mean ± SD) (% predicted) | 68.0 ± 21.5 | 71.3 ± 17.0 | 0.54 | 17.7 |
| History of diabetes (n, %) | 5 (17) | 4 (13) | 0.72 | 9.2 |
| Renal insufficiency (n, %) | 0 (0) | 0 (0) | 1.00 | 0.0 |
| COPD (n, %) | 10 (33) | 10 (33) | 1.00 | 0.0 |
| Prior thoracic surgery (n, %) | 5 (17) | 8 (27) | 0.35 | 22.9 |
| Clinical stage prior to induction therapya (n, %) | 0.51 | |||
| IA | 0 (0) | 0 (0) | 0.0 | |
| IB | 0 (0) | 1 (3) | 27.6 | |
| IIA | 2 (7) | 2 (7) | 0.0 | |
| IIB | 4 (13) | 1 (3) | 29.6 | |
| IIIA | 15 (50) | 18 (60) | 20.3 | |
| IIIB | 1 (3) | 1 (3) | 0.0 | |
| IV | 6 (20) | 4 (13) | 25.9 | |
| Unknown | 2 (7) | 3 (10) | 13.8 | |
| Congestive heart failure (n, %) | 0 (0) | 0 (0) | 1.00 | 0.0 |
| Cerebrovascular disease (n, %) | 1 (3) | 0 (0) | 0.31 | 21.7 |
| Coronary artery disease (n, %) | 3 (10) | 5 (17) | 0.45 | 18.0 |
| History of cancer (n, %) | 5 (17) | 5 (17) | 1.00 | 0.0 |
| History of smoking | 27 (90) | 28 (93) | 0.64 | |
| Radiation (n, %) | 5(17) | 5 (17) | 1.00 | 7.4 |
| Transient ischemic attack/cerebrovascular accident (n, %) | 0 (0) | 0 (0) | 1.00 | 0.0 |
| Central versus peripheral tumour (n, %) | 0.77 | |||
| Central | 9 (30) | 8 (27) | 7.5 | |
| Peripheral | 21 (70) | 22 (73) | 7.5 | |
| Operative year | 0.061 | |||
| 1996–2001 | 0 (0) | 1 (3) | 10.1 | |
| 2002–07 | 11 (37) | 10 (33) | 7.5 | |
| 2008–12 | 19 (63) | 19 (63) | 0.0 | |
| Pathological T status (n, %) | 0.49 | |||
| T0 | 8 (27) | 6 (20) | 15.3 | |
| T1 | 6 (20) | 10 (33) | 29.5 | |
| T2 | 11 (37) | 9 (30) | 14.6 | |
| T3 | 5 (17) | 4 (13) | 9.5 | |
| T4 | 0 (0) | 1 (3) | 25.6 | |
| Pathological N status (n, %) | 0.78 | |||
| N0 | 21 (70) | 23 (77) | 14.1 | |
| N1 | 3 (10) | 3 (10) | 0.0 | |
| N2 | 6 (20) | 4 (13) | 16.6 | |
| Pathological M status (n, %) | 0.69 | 13.6 | ||
| M0 | 26 (87) | 27 (90) | ||
| M1 | 4 (13) | 3 (10) | ||
| Pretreatment histology (n, %) | 0.36 | |||
| Adenocarcinoma | 15 (50) | 12 (40) | 20.2 | |
| Adenosquamous | 1 (3) | 1 (3) | 0.0 | |
| Squamous | 5 (17) | 11 (37) | 42.6 | |
| Large cell | 4 (13) | 1 (3) | 45.2 | |
| Non-small cell, not otherwise specified | 5 (17) | 5 (17) | 0.0 |
FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; COPD: chronic obstructive pulmonary disease; VATS: video-assisted thoracoscopic surgery; SD: standard deviation.
aData only available for 56 of 60 patients (open n = 28, VATS n = 28).
Table 6:
Perioperative outcomes and tumour characteristics after propensity matching
| Characteristics | Open approach (n = 30) | VATS approach (n = 30) | P-value |
|---|---|---|---|
| 30-day mortality (n, %) | 2 (7) | 1 (3) | 0.55 |
| Overall morbiditya (n, %) | 17 (57) | 12 (40) | 0.20 |
| Postoperative bleeding requiring blood transfusion (n, %) | 3 (10) | 2 (7) | 0.64 |
| Postoperative bleeding requiring reoperation (n, %) | 1 (3) | 1 (3) | 1.00 |
| Pneumonia (n, %) | 4 (13) | 2 (7) | 0.39 |
| Atrial fibrillation (n, %) | 7 (23) | 7 (23) | 1.00 |
| Prolonged air leaks (n, %) | 6 (20) | 3 (10) | 0.28 |
| Respiratory failure (n, %) | 1 (3) | 0 (0) | 0.31 |
| Other major complications (n, %) | 6 (9) | 20 (10) | 1.00 |
| Length of hospitalization (days), median (IQR) | 5 (4–8) | 4 (3–5) | 0.007 |
| Chest tube duration (days), median (IQR) | 4 (3–5) | 3 (2–3) | 0.023 |
| Complete pathological response (n, %) | 7 (23) | 6 (20) | 0.75 |
| Preinduction therapy tumour sizeb (cm), mean ± SD | 5.2 ± 3.0 | 4.4 ± 2.8 | 0.38 |
| Postinduction therapy tumour size (cm), mean ± SD | 3.2 ± 2.7 | 3.3 ± 2.5 | 0.89 |
| Down-staged from PN2 to PN0c (n, %) | 7 (50) | 10 (71) | 0.25 |
| Down-staged from PN2 to PN1 or PN0c (n, %) | 8 (57) | 11 (79) | 0.26 |
| Overall down-stagedd (n, %) | 15 (54) | 17 (63) | 0.48 |
VATS: video-assisted thoracoscopic surgery; SD: standard deviation; IQR: interquartile range.
aOverall morbidity represents the number (%) of patients with at least one postoperative complication.
bData only available for 37 of 60 patients (open n = 16, VATS n = 21).
cDenominator used is the number of patients who had N2 disease in the open (n = 14) and VATS (n = 14) groups.
dData only available for 55 of 60 patients (open n = 28, VATS n = 27).
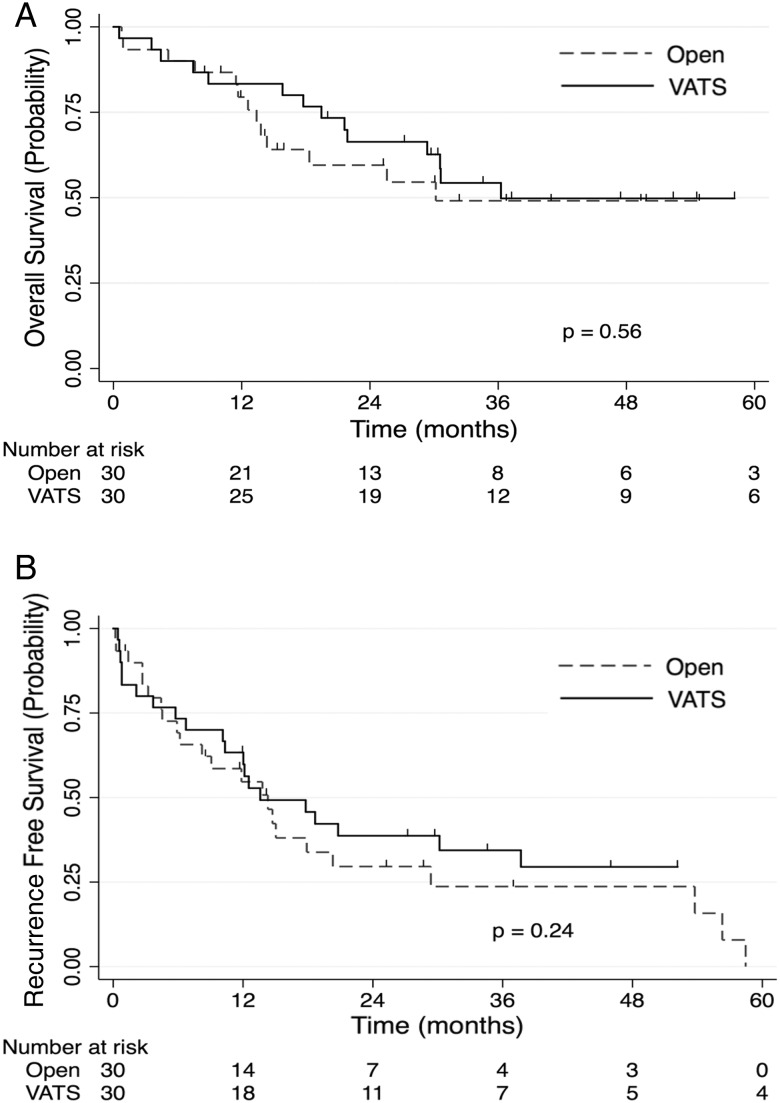
After propensity score matching, there were also no significant differences in overall survival between the two groups (Fig. 2). There were no significant differences in overall survival between the VATS and open groups in 3-year survival [54% (95% CI, 34–71%) vs 49% (95% CI, 28–67%)]. In multivariable analysis, adjusting for variables not sufficiently balanced by the propensity score matching (FEV1, prior thoracic surgery, pretreatment clinical stage, pathological T status, history of cerebrovascular disease and pretreatment histology), there remained no significant difference in overall survival between the open and VATS lobectomy groups (HR 0.88; 95% CI, 0.39–1.97; P = 0.76). After propensity score matching, there were no significant differences in recurrence-free survival between the VATS and open groups in 3-year survival [34% (95% CI, 18–52%) vs 24% (95% CI, 9–42%)]. In multivariable analysis, adjusting for variables not sufficiently balanced by the propensity score matching (FEV1, prior thoracic surgery, pretreatment clinical stage, pathological T status, history of cerebrovascular disease and pretreatment histology), there remained no significant difference in recurrence-free survival between the VATS and open lobectomy groups (HR 0.91; 95% CI, 0.46–1.83; P = 0.80).
Figure 2:
(A) Propensity-matched survival stratified by surgical approach: this figure depicts the Kaplan–Meier overall survival estimates of the open group (dashed line) versus the VATS group (solid line) using the propensity-matched data. (B) Propensity-matched recurrence-free survival stratified by surgical approach: this figure depicts the Kaplan–Meier recurrence-free survival estimates of the open group (dashed line) versus the VATS group (solid line) using the propensity-matched data. Tick marks represent censored subjects. VATS: video-assisted thoracoscopic surgery.
DISCUSSION
This study compared outcomes of patients who received induction therapy for NSCLC and then underwent lobectomy by a VATS or thoracotomy approach. In the present study, the VATS approach was associated with a shorter length of hospital stay and shorter chest tube duration when compared with open lobectomy. The groups did not differ significantly in perioperative outcomes including perioperative complications and 30-day mortality. In unadjusted analysis, the VATS approach was associated with improved overall survival but after multivariable adjustment, there were no significant differences in overall and recurrence-free survival between the VATS and open approaches. In addition, in propensity score-matched analysis, there were no significant differences in overall and recurrence-free survival between the VATS and open approaches. These results support the use of VATS in appropriately selected patients who have been treated with induction therapy.
To date, only few studies have detailed the outcomes of VATS lobectomy in patients with NSCLC who underwent induction therapy. Our study shows results consistent with these previous studies in a large cohort that allowed better comparison of both short-term and long-term outcomes. Huang et al. evaluated the outcomes of 43 patients with stage IIA–IIIB NSCLC who underwent induction therapy followed by thoracoscopic lung resection (including 28 lobectomies) and reported a conversion rate of 17%, complication rate of 9.5%, and 1- and 3-year survival rates of 94% and 65%, respectively [14]. Gonzalez-Rivas et al. evaluated the outcomes of 43 advanced NSCLC patients who underwent uniportal thoracoscopic resection (including 37 lobectomies) of whom approximately two thirds had induction therapy prior to resection. The authors reported a conversion rate of 6.5%, complication rate of 14% and 30-month survival rate of 74% for those patients [13]. Shaw et al. conducted a study of patients undergoing VATS treatment for early-stage NSCLC that included 10 patients who underwent neoadjuvant induction therapy [17]. The authors reported a median length of hospital stay and median chest tube duration of 3.5 and 3 days, respectively, a complication rate of 30% and a perioperative mortality rate of 0% [17]. In addition, we had previously performed a preliminary study comparing the outcomes of 12 VATS patients to 85 patients who underwent open lobectomy following induction therapy and found a median overall survival of 28 months [2]. The present study findings are largely consistent with those reported by other groups above. In addition, our conversion rate of 10% reported in this study compares favourably to the rates reported by studies of VATS lobectomy for early-stage NSCLC. Yan et al. performed a meta-analysis of 21 studies of VATS lobectomy for early-stage NSCLC, and found a median conversion rate of 8.1% and conversion rates in the range of 0–15.7% [18].
The present study findings are also comparable with studies of open lobectomy after induction therapy. Kim et al. conducted a study of 233 patients who underwent neoadjuvant chemoradiation for clinically advanced NSCLC followed by lung resection (pneumonectomy, sleeve lobectomy, bilobectomy and standard lobectomy). The authors reported a 5-year overall survival rate for lobectomy patients of 51%, a 90-day mortality rate of 8% and 5-year overall survival rates of 50, 41 and 32% for Stage I, II and III, respectively [19]. Paul et al. conducted a retrospective review of 136 patients with clinical stage IIIA NSCLC who underwent surgical resection after ICT or chemoradiation. The authors reported down-staging rates to N0 or N1 of 52% and a 5-year survival rate of 36% for patients undergoing lobectomy [20]. Stefani et al. conducted a retrospective review of 175 N2 NSCLC patients who underwent induction therapy followed by surgical resection [96 (55%) lobectomies and 79 (45%) pneumonectomies] and reported an overall median survival of 34.7 months and an overall 5-year survival rate of 30% [21].
One potential concern of preoperative radiation is that patients who have received preoperative radiation could have a fibrotic hilum, which could increase the difficulty of dissection and performing a lobectomy thoracoscopically. Our study found that the majority of conversions (6 of 7) occurred in patients who underwent ICT and that none of the patients who required a conversion had a perioperative death. These study findings suggest that the VATS approach can be safely used by experienced surgeons even for patients who have undergone induction radiation.
This study has several limitations. First, this is a single institution, retrospective study, and while efforts were made through multivariable adjustment and propensity matching to reduce the potential effects of selection bias, unobserved confounding could still be present. Second, the study included all patients who had undergone induction therapy followed by lobectomy, and although the majority of patients received induction therapy for Stage IIIA disease, patients in the study had undergone induction therapy for a wide range of reasons including for superior sulcus tumours and tumours invading the chest wall. Of note, there was a significantly higher proportion of patients in the open group who had cT3N0 tumours when compared with the VATS group, and there were more patients in the VATS group who underwent induction therapy for biopsy-proven Stage IIIA-N2 NSCLC compared with the open group. This would actually bias the results against VATS. We tried to account for these differences in staging by using multivariable models and propensity matching (where we matched by pathological T and N status and tumour location) but we could have been underpowered to detect differences in particular subgroups. Third, the power of the propensity analysis is limited because there are only 30 patients in each arm of the propensity-matched analysis and, therefore, the study may have been underpowered to detect if there was truly a difference between the two groups; larger sample sizes would strengthen findings. Further, some of the variables in the propensity-matched analysis were not sufficiently balanced. Fourth, these findings are from a single institution that performs a high volume of VATS lobectomies and may be limited in its generalizability. Fifth, there has been an evolution in induction therapy for NSCLC during the study period that may have affected our outcomes, although we did try to account for differences in treatment ‘eras’ by including the operative year in multivariable analysis. Sixth, there was obvious temporal bias to the selection of approach that may have affected the outcomes. Finally, the follow-up time for patients was relatively short, which may have limited the long-term findings of the study; longer follow-up time would help strengthen findings.
In conclusion, VATS lobectomy in patients treated with induction therapy for locally advanced NSCLC is feasible and effective. The VATS approach is associated with a shorter length of hospitalization and decreased chest tube duration and does not appear to compromise oncologic outcomes. On the basis of these results, surgeons comfortable with VATS should not consider induction therapy to be a contraindication to using this approach in NSCLC patients who require a lobectomy.
SUPPLEMENTARY MATERIAL
Funding
Matthew G. Hartwig is supported by the NIH funded Cardiothoracic Surgery Trials Network, 5U01HL088953-05. Chi-Fu Jeffrey Yang is supported by the American College of Surgeons Resident Research Scholarship. Robert Ryan Meyerhoff is supported by an MSTP T32 grant (Medical Scientist Training Program NSRA T32GM007171).
Conflict of interest: Thomas D'Amico is a consultant for Scanlan (<$10 000).
Supplementary Material
REFERENCES
- 1.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10:1236–71. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RP, Pham D, Toloza EM, Burfeind WR, Harpole DH Jr, Hanish SI et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg 2006;82:214–9. [DOI] [PubMed] [Google Scholar]
- 3.Loscertales J, Quero Valen zuela F, Congregado M, Jiménez Merchán R, Gallardo Varela G, Trivino Ramírez A et al. Video-assisted thoracic surgery lobectomy: results in lung cancer. J Thorac Dis 2010;2:29–35. [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels LJ, Balderson SS, Onaitis MW, D'Amico TA. Thoracoscopic lobectomy: a safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg 2002;74:860–4. [DOI] [PubMed] [Google Scholar]
- 5.Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194–200. [DOI] [PubMed] [Google Scholar]
- 6.McKenna RJ., Jr New approaches to the minimally invasive treatment of lung cancer. Cancer J 2005;11:73–6. [DOI] [PubMed] [Google Scholar]
- 7.Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362–5. [DOI] [PubMed] [Google Scholar]
- 8.Nomori H, Horio H, Naruke T, Suemasu K. What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg 2001;72:879–84. [DOI] [PubMed] [Google Scholar]
- 9.Walker WS, Codispoti M, Soon SY, Stamenkovic S, Carnochan F, Pugh G. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397–402. [DOI] [PubMed] [Google Scholar]
- 10.Yim APC, Wan S, Lee TW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243–7. [DOI] [PubMed] [Google Scholar]
- 11.Demmy TL, Plante AJ, Nwogu CE, Takita H, Anderson TM. Discharge independence with minimally invasive lobectomy. Am J Surg 2004;188:698–702. [DOI] [PubMed] [Google Scholar]
- 12.Onaitis MW, Petersen RP, Balderson SS, Toloza E, Burfeind WR, Harpole DH et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Rivas D, Fieira E, Delgado M, Mendez L, Fernandez R, de la Torre M. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Xu X, Chen H, Yin W, Shao W, Xiong X et al. Feasibility of complete video-assisted thoracoscopic surgery following neoadjuvant therapy for locally advanced non-small cell lung cancer. J Thorac Dis 2013;5:S267-S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks DR, Austin JH, Heelan RT, Ginsberg MS, Shin V, Olson SH et al. Influence of type of cigarette on peripheral versus central lung cancer. Cancer Epidemiol Biomarkers Prev 2005;14:576–81. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33–8. [Google Scholar]
- 17.Shaw JP, Dembitzer FR, Wisnivesky JP, Litle VR, Weiser TS, Yun J et al. Video-assisted thoracoscopic lobectomy: state of the art and future directions. Ann Thorac Surg 2008;85:S705-S09. [DOI] [PubMed] [Google Scholar]
- 18.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553–62. [DOI] [PubMed] [Google Scholar]
- 19.Kim AW, Liptay MJ, Bonomi P, Warren WH, Basu S, Farlow EC et al. Neoadjuvant chemoradiation for clinically advanced non-small cell lung cancer: an analysis of 233 patients. Ann Thorac Surg 2011;92:233–43. [DOI] [PubMed] [Google Scholar]
- 20.Paul S, Mirza F, Port JL, Lee PC, Stiles BM, Kansler AL et al. Survival of patients with clinical stage IIIA non–small cell lung cancer after induction therapy: age, mediastinal downstaging, and extent of pulmonary resection as independent predictors. J Thorac Cardiovasc Surg 2011;141:48–58. [DOI] [PubMed] [Google Scholar]
- 21.Stefani A, Alifano M, Bobbio A, Grigoroiu M, Jouni R, Magdeleinat P et al. Which patients should be operated on after induction chemotherapy for N2 non-small cell lung cancer? Analysis of a 7-year experience in 175 patients. J Thorac Cardiovasc Surg 2010;140:356–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.