Abstract
Mesothelin, C-ERC/mesothelin is a 40-kDa cell surface glycoprotein that is normally present on normal mesothelial cells lining the pleura, peritoneum, and pericardium. Moreover, mesothelin has been shown to be overexpressed in several human cancers, including virtually all mesothelioma and pancreatic cancer, approximately 70% of ovarian cancer and extra bile duct cancer, and 50% of lung adenocarcinomas and gastric cancer. The full-length human mesothelin gene encodes the primary product, a 71-kDa precursor protein. The 71-kDa mesothelin precursor is cleaved into two products, 40-kDa C-terminal fragment that remains membrane-bound via glycosylphosphatidylinositol anchor, and a 31-kDa N-terminal fragment, megakaryocyte potentiating factor, which is secreted into the blood. The biological functions of mesothelin remain largely unknown. However, results of recent studies have suggested that the mesothelin may play a role of cell proliferation and migration. In pancreatic cancer, mesothelin expression was immunohistochemically observed in all cases, but absent in normal pancreas and in chronic pancreatitis. Furthermore, the expression of mesothelin was correlated with an poorer patient outcome in several human cancers. The limited mesothelin expression in normal tissues and high expression in many cancers makes it an attractive candidate for cancer therapy. The present review discusses the expression and function of mesothelin in cancer cells and the utility of mesothelin as a target of cancer therapy.
Keywords: Mesothelin, Luminal membrane expression, Cytoplasmic expression, Tumor marker, Cancer therapy
Core tip: Mesothelin is a 40-kDa cell surface glycoprotein expressed on normal mesothelial cells lining the pleura, pericardium, and peritoneum. Moreover, mesothelin has been shown to be overexpressed in several cancer types. Recent studies have suggested that the overexpression of mesothelin increases cell proliferation and migration. Furthermore, the expression of mesothelin was related to an unfavourable patient outcome in several human cancers. The limited mesothelin expression in normal tissues and high expression in many cancers makes it an attractive candidate for cancer therapy.
INTRODUCTION
Mesothelin is a 40-kDa cell surface glycoprotein that is normally present on normal mesothelial cells lining the pleura, peritoneum, and pericardium[1,2]. Moreover, mesothelin has been shown to be overexpressed in several human cancers, including virtually all mesothelioma and pancreatic cancer, approximately 70% of ovarian cancer and extra bile duct cancer, and 50% of lung adenocarcinomas and gastric cancer[3-6] (Table 1). The full-length human mesothelin gene (Full-ERC/mesothelin) encodes a 71-kDa precursor protein. The 71-kDa mesothelin precursor is cleaved into two products, 40-kDa C-terminal fragment (C-ERC/mesothelin) that remains membrane-bound via glycosylphosphatidylinositol anchor[7], and a 31-kDa N-terminal fragment (N-ERC/mesothelin, megakaryocyte potentiating factor), which is secreted into the blood (Figure 1)[1]. The function of mesothelin in cancer is still unclear. However, results of recent studies have suggested that the mesothelin may play a role of tumor progression in vitro[8-11] and in vivo[11,12].
Table 1.
Mesothelin expression in human cancer detected by immunohistochemistry
| Tumour | Mesothelin expressions (%) | Comments | Ref. |
| Pancreatic cancer | 86-100 | Co-expression of mesothelin and CA125 group associated with a poorer patient prognosis | [6,16,17] |
| Gastric cancer | 29-59 | Luminal membrane expression is one of the poor prognostic factors | [6,18,27,35] |
| Extrahepatic bile duct cancer | 72-100 | Luminal membrane expression or cytoplasmic expression of mesothelin could be a reliable prognostic factor | [6,21] |
| Colorectal cancer | 28-58 | Luminal membrane expression was associated with lymphatic invasion | [6,20] |
| Intraductal papillary mucinous neoplasm | 57 | Luminal membrane expression was correlated with the histological classification of the tumor and the recurrence rate | [19] |
Figure 1.
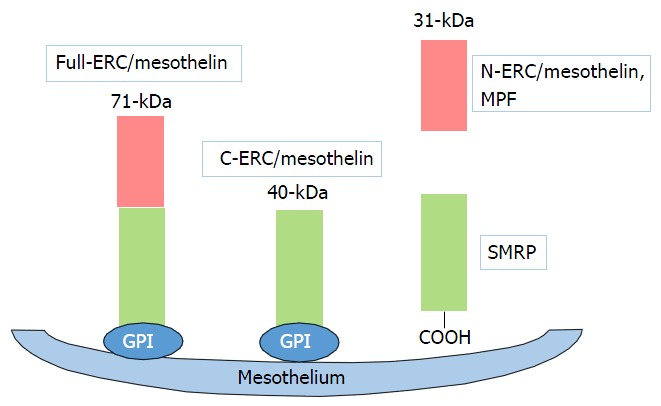
Schematics showing the maturation of mesothelin protein. The primary product of the full-ERC/mesothelin gene is a 71-kDa precursor protein. This protein is physiologically cleaved, releasing a 31-kDa fragment, N-ERC/mesothelin, into the blood. MPF: Megakaryocyte potentiating factor; SMRP: Soluble mesothelin-related peptide; GPI: Glycosylphosphatidylinositol.
MESOTHELIN EXPRESSION IN GASTROINTESTINAL CANCERS
Co-expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma
Mesothelin could play a role of the binding to CA125[13-15]. Mesothelin and CA125 binding may be important in the peritoneal spread[13,15]. In ovarian cancer, advanced clinical stage and/or high histological grade patients showed mesothelin expression and CA125 expressions[15]. Our group showed that the co-expression of mesothelin and CA125 group was a higher histological grade and a higher level of blood vessel permeation and correlated with recurrence rate and poor patient outcome in pancreatic ductal adenocarcinoma[16]. These findings suggest that the co-expression of mesothelin and CA125 may lead to tumor development, metastasis, and a poorer patient prognosis.
Luminal membrane expression of mesothelin is a prominent poor prognostic factor
The expression of mesothelin was related to an unfavorable patient outcome in pancreatic ductal adenocarcinoma[16,17]. Our group investigated mesothelin expression in gastric cancers by using immunohistochemistry, especially focusing on the localization of mesothelin, i.e., “luminal membrane-positive” and/or “cytoplasm-positive” (Figure 2)[18].
Figure 2.
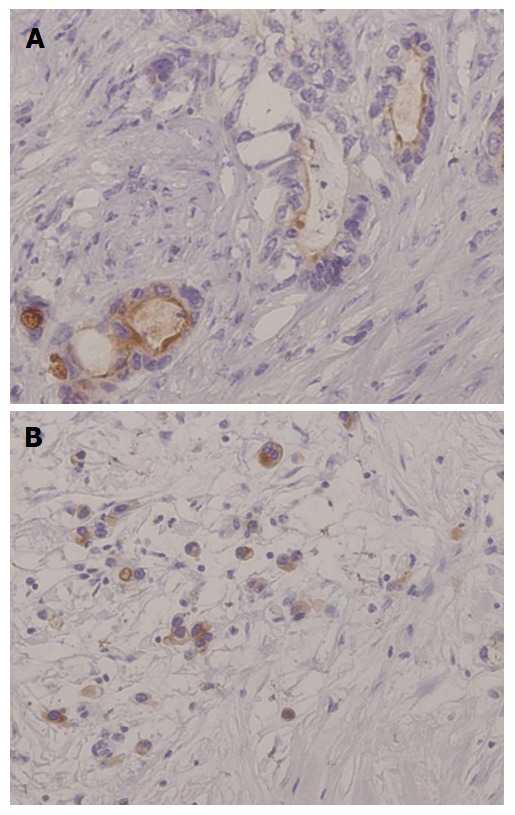
The expression of mesothelin in gastric cancer. A: Luminal membrane expression. The entire circumference of the cell membrane was stained; B: “Cytoplasmic expression” with granular cytoplasmic staining in cancer cells.
The overall survival revealed that the “luminal membrane-positive” group showed a significantly poorer outcome compared to the “luminal membrane-negative” group. On the other hand, the “mesothelin-positive” group and the “cytoplasmic-positive” group were not correlated with overall survival in the gastric cancer patients.
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas has a histological spectrum ranging from benign adenoma to invasive cancer. We performed an immunohistochemical analysis of mesotheelin expression in IPMN. Mesothelin was absent in all of the normal pancreatic tissues. But, mesothelin was expressed in both adenoma and carcinoma cells. Most of mesothelin expressed adenoma cells exhibited slight “cytoplasmic-positive”, and the “luminal membrane-positive” group has a tendency of poor prognosis and high recurrence rate[19].
Based on these results, the “luminal membrane-positive” of mesothelin is a useful prognostic factor, implying that membrane-localized mesothelin might have the significant function of the aggressive behavior in the cancer cells.
THE ROLE OF MESOTHELIN EXPRESSION IN TUMOR BIOLOGY
Our study generated the novel finding, the potential role of the “luminal membrane-positive” mesothelin in the malignant behavior of tumor cells[18-21]. The human mesothelin gene encodes a 71-kDa precursor protein (Full-ERC/mesothelin). This precursor protein is cleaved by furin-like proteases into a 31-kDa N-terminal secreted form (N-ERC/mesothelin) and a C-terminal fragment, 40-kDa mesothelin (C-ERC/mesothelin)[1,7,22]. The 5B2 anti-mesothelin antibody, which we used in our studies, can detect the 71-kDa precursor protein (Full-ERC/mesothelin) and the 40-kDa C-terminal fragment (C-ERC/mesothelin), but not the 30-kDa N-terminal fragment (N-ERC/mesothelin). Based on the specificity of this antibody, the “luminal membrane-positive” mesothelin observed in our study might have indicated the existence of 40-kDa mesothelin (C-ERC/mesothelin) membrane-bound form, while the “cytoplasmic-positive” mesothelin might have indicated the presence of the the 71-kDa precursor protein (Full-ERC/mesothelin). To demonstrate the mechanism of the membranous localization of mesothelin, we enforced the expression of Full-, C-, and N-ERC/mesothelin in human colorectal cancer (CRC) cell lines[20]. The 7E7 antibody, which recognizes the 30-kDa N-terminal fragment (N-ERC/mesothelin), revealed the diffuse cytoplasmic expression of Full- and N-ERC/mesothelin in Full-WiDr and N-WiDr. In contrast, the 22A31 antibody, which recognizes 40-kDa mesothelin (C-ERC/mesothelin), demonstrated a dot-like expression of Full- and C-ERC/mesothelin in Full-WiDr and C-WiDr. Moreover, some of the dot-like spots along with the cellular membrane were merged with actin, showing yellow signals. According to these results, we confirmed the membranous expressions of C-ERC/mesothelin in CRC cell lines.
To demonstrate the biological role of Full-, C-, and N-ERC/mesothelin in the lymphatic invasion of CRC, we performed an in vitro lymphatic invasion assay. C-ERC/mesothelin, the 40-kDa membrane-localized fragment, promoted the lymphatic invasion by increasing cell adhesion to lymphatic endothelial cells.
THE PATHWAYS OF MESOTHELIN INVOLVED IN CANCER
Recent studies reported that mesothelin is not only associated with increased cell proliferation and the migration of pancreatic cancer cells in vitro[11,23], but also contributes to tumor progression in vivo[11]. Mesothelin protects cancer cells from paclitaxel-induced apoptosis through both the concomitant activation of PI3K/Akt and MAPK/ERK pathways[24]. Overexpression of mesothelin in pancreatic cancer cells leads to constitutive activation of signal transducer and activator of transcription 3, which results in enhanced expression of cyclin E and cyclin E/cyclin-dependent kinase 2 complex formation as well as increased G1-S transition[23]. Mesothelin expression correlated closely with interleukin (IL)-6 in human pancreatic cancer specimens and cell lines. Cancer cell with forced mesothelin expression grow faster than control cells by producing higher quantities of IL-6[9,10].
BLOOD TEST FOR MESOTHELIN
Several ELISAs have been developed to measure the levels of soluble mesothelin-related peptide (SMRP) and megakaryocyte potentiating factor (MPF, N-ERC/mesothelin). The soluble form of mesothelin is likely due to an abnormal splicing event resulting in a frameshift mutation and premature termination at amino acid 600 deleting the amino acids at the COOH terminus that are responsible for its association with the cell membrane. The full-length human mesothelin gene encodes the primary product, a 71-kDa precursor protein. It can be physiologically cleaved by some furin-like proteases into a 40-kDa C-terminal fragment that remains membrane-bound, and a 31-kDa N-terminal fragment, which is secreted into the blood. The C-terminal 40-kDa fragment is referred to as mesothelin. In contrast, the N-terminal 31-kDa fragment is a secreted protein identified as MPF. SMRP has proven to be a promising cancer biomarker in the sera of patients with tumors of mesothelial origin[25,26]. MPF has been reported to be expressed in gastrointestinal cancers[27,28].
Wu et al[29] revealed that SMRP performs better than CA125 as a tumor marker for epithelial ovarian cancer, it increases only in malignant patients and not in benign patients or healthy volunteers. Furthermore, the sensitivity is enhanced when combined with CA125. Hassan et al[30] identified a positive correlation with the tumor burden and SMRP levels, as a marker for monitoring the response to treatment o malignant mesothelioma.
MESOTHELIN TARGET IMMUNOTHERAPY
Because of the high expression of mesothelin in many malignancies and its limited expression in normal tissues, mesothelin has been suggested as an attractive target for immunotherapy. Several therapeutic agents that target mesothelin have been developed and some are being evaluated in preclinical and clinical studies. SS1P is an immunotoxin being clinically tested as a systemic agent in solid tumor patients. Two phase I trials of single-agent SS1P have been performed[31,32]. The majority of patients developed antidrug antibodies by the end of their first cycle, resulting in non-therapeutic drug levels if any additional cycles were given. MORAb-009 (amatuximab) is a chimeric antibody. A phase I clinical trial of MORAb-009 for mesothelioma, pancreatic cancer, and ovarian cancer patients has been completed[33]. Eleven of 24 subjects had stable disease. Phase II studies of MORAb-009 in different mesothelin-expressing cancers are ongoing. The mesothelin tumor vaccine in clinical development is CRS-207. The safety of this vaccine was established in a phase I clinical trial of patients with mesothelin-expressing refractory cancers[34].
CONCLUSION
Mesothelin is an attractive antigen that is expressed in several gastrointestinal cancers. Recent studies have revealed oncogenic functions of mesothelin in cancer proliferation and invasion and drug resistance. Also, soluble mesothelin could be useful as a tumor marker. The limited mesothelin expression in normal tissues and high expression in many cancers makes it an attractive candidate for cancer therapy.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 2, 2015
First decision: November 4, 2015
Article in press: January 29, 2016
P- Reviewer: Munoz M, Zhu YL S- Editor: Qiu S L- Editor: A E- Editor: Li D
References
- 1.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50:373–381. doi: 10.1002/ijc.2910500308. [DOI] [PubMed] [Google Scholar]
- 3.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 4.Hassan R, Kreitman RJ, Pastan I, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- 5.Ordóñez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 6.Ordóñez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Bodempudi V, Liu Z, Borrego-Diaz E, Yamoutpoor F, Meyer A, Woo RA, Pan W, Dudek AZ, Olyaee MS, et al. Inhibition of mesothelin as a novel strategy for targeting cancer cells. PLoS One. 2012;7:e33214. doi: 10.1371/journal.pone.0033214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis. 2011;32:1013–1024. doi: 10.1093/carcin/bgr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin confers pancreatic cancer cell resistance to TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and IL-6/Mcl-1 overexpression. Mol Cancer. 2011;10:106. doi: 10.1186/1476-4598-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Bharadwaj U, Zhang R, Zhang S, Mu H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther. 2008;7:286–296. doi: 10.1158/1535-7163.MCT-07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Servais EL, Colovos C, Rodriguez L, Bograd AJ, Nitadori J, Sima C, Rusch VW, Sadelain M, Adusumilli PS. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res. 2012;18:2478–2489. doi: 10.1158/1078-0432.CCR-11-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, Bera TK, Connor J, Sathyanarayana BK, Lee B, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko O, Gong L, Zhang J, Hansen JK, Hassan R, Lee B, Ho M. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M, Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279:9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 16.Einama T, Kamachi H, Nishihara H, Homma S, Kanno H, Takahashi K, Sasaki A, Tahara M, Okada K, Muraoka S, et al. Co-expression of mesothelin and CA125 correlates with unfavorable patient outcome in pancreatic ductal adenocarcinoma. Pancreas. 2011;40:1276–1282. doi: 10.1097/MPA.0b013e318221bed8. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu A, Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Kitahata Y, Nakamura Y, Noda T, Yokoyama S, et al. Coexpression of MUC16 and mesothelin is related to the invasion process in pancreatic ductal adenocarcinoma. Cancer Sci. 2012;103:739–746. doi: 10.1111/j.1349-7006.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Einama T, Homma S, Kamachi H, Kawamata F, Takahashi K, Takahashi N, Taniguchi M, Kamiyama T, Furukawa H, Matsuno Y, et al. Luminal membrane expression of mesothelin is a prominent poor prognostic factor for gastric cancer. Br J Cancer. 2012;107:137–142. doi: 10.1038/bjc.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einama T, Kamachi H, Nishihara H, Homma S, Kanno H, Ishikawa M, Kawamata F, Konishi Y, Sato M, Tahara M, et al. Importance of luminal membrane mesothelin expression in intraductal papillary mucinous neoplasms. Oncol Lett. 2015;9:1583–1589. doi: 10.3892/ol.2015.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamata F, Homma S, Kamachi H, Einama T, Kato Y, Tsuda M, Tanaka S, Maeda M, Kajino K, Hino O, et al. C-ERC/mesothelin provokes lymphatic invasion of colorectal adenocarcinoma. J Gastroenterol. 2014;49:81–92. doi: 10.1007/s00535-013-0773-6. [DOI] [PubMed] [Google Scholar]
- 21.Kawamata F, Kamachi H, Einama T, Homma S, Tahara M, Miyazaki M, Tanaka S, Kamiyama T, Nishihara H, Taketomi A, et al. Intracellular localization of mesothelin predicts patient prognosis of extrahepatic bile duct cancer. Int J Oncol. 2012;41:2109–2118. doi: 10.3892/ijo.2012.1662. [DOI] [PubMed] [Google Scholar]
- 22.Cheng WF, Huang CY, Chang MC, Hu YH, Chiang YC, Chen YL, Hsieh CY, Chen CA. High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. Br J Cancer. 2009;100:1144–1153. doi: 10.1038/sj.bjc.6604964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharadwaj U, Li M, Chen C, Yao Q. Mesothelin-induced pancreatic cancer cell proliferation involves alteration of cyclin E via activation of signal transducer and activator of transcription protein 3. Mol Cancer Res. 2008;6:1755–1765. doi: 10.1158/1541-7786.MCR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang MC, Chen CA, Hsieh CY, Lee CN, Su YN, Hu YH, Cheng WF. Mesothelin inhibits paclitaxel-induced apoptosis through the PI3K pathway. Biochem J. 2009;424:449–458. doi: 10.1042/BJ20082196. [DOI] [PubMed] [Google Scholar]
- 25.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, Winzell P, Hellstrom KE, Hellstrom I. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 26.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellström KE, Hellström I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96:11531–11536. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito T, Kajino K, Abe M, Sato K, Maekawa H, Sakurada M, Orita H, Wada R, Kajiyama Y, Hino O. ERC/mesothelin is expressed in human gastric cancer tissues and cell lines. Oncol Rep. 2014;31:27–33. doi: 10.3892/or.2013.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inami K, Kajino K, Abe M, Hagiwara Y, Maeda M, Suyama M, Watanabe S, Hino O. Secretion of N-ERC/mesothelin and expression of C-ERC/mesothelin in human pancreatic ductal carcinoma. Oncol Rep. 2008;20:1375–1380. [PubMed] [Google Scholar]
- 29.Wu X, Li D, Liu L, Liu B, Liang H, Yang B. Serum soluble mesothelin-related peptide (SMRP): a potential diagnostic and monitoring marker for epithelial ovarian cancer. Arch Gynecol Obstet. 2014;289:1309–1314. doi: 10.1007/s00404-013-3128-x. [DOI] [PubMed] [Google Scholar]
- 30.Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, Alexander R, Willingham M, Pastan I, Onda M. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 31.Hassan R, Broaddus VC, Wilson S, Liewehr DJ, Zhang J. Anti-mesothelin immunotoxin SS1P in combination with gemcitabine results in increased activity against mesothelin-expressing tumor xenografts. Clin Cancer Res. 2007;13:7166–7171. doi: 10.1158/1078-0432.CCR-07-1592. [DOI] [PubMed] [Google Scholar]
- 32.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, Schweizer C, Weil S, Laheru D. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–6138. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baba K, Ishigami S, Arigami T, Uenosono Y, Okumura H, Matsumoto M, Kurahara H, Uchikado Y, Kita Y, Kijima Y, et al. Mesothelin expression correlates with prolonged patient survival in gastric cancer. J Surg Oncol. 2012;105:195–199. doi: 10.1002/jso.22024. [DOI] [PubMed] [Google Scholar]


