Abstract
Central circadian timing influences mental and physical health. Research in nocturnal rodents has demonstrated that when alcohol is consumed, it reaches the central hypothalamic circadian pacemaker (suprachiasmatic nuclei) and can directly alter circadian phase shifts to light. In two separate studies, we examined, for the first time, the effects of a single dose of alcohol on circadian phase advances and phase delays to light in humans. Two 23-day within-subjects placebo-controlled counterbalanced design studies were conducted. Both studies consisted of 6 days of fixed baseline sleep to stabilize circadian timing, a 2-day laboratory session, a 6-day break, and a repeat of 6 days of fixed sleep and a 2-day laboratory session. In the phase advance study (n = 10 light drinkers, 24–45 yr), the laboratory sessions consisted of a baseline dim light phase assessment, sleep episode, alcohol (0.6 g/kg) or placebo, 2-h morning bright light pulse, and final phase assessment. In the phase-delay study (n = 14 light drinkers, 22–44 yr), the laboratory sessions consisted of a baseline phase assessment, alcohol (0.8 g/kg) or placebo, 2-h late night bright light pulse, sleep episode, and final phase assessment. In both studies, alcohol either increased or decreased the observed phase shifts to light (interaction P ≥ 0.46), but the effect of alcohol vs. placebo on phase shifts to light was always on average smaller than 30 min. Thus, no meaningful effects of a single dose of alcohol vs. placebo on circadian phase shifts to light in humans were observed.
Keywords: alcohol, circadian, human, light, melatonin
the central mammalian circadian clock, which regulates many circadian rhythms, is located in the suprachiasmatic nuclei in the hypothalamus (SCN; Ref. 24). In humans, the central circadian clock has an average endogenous period slightly greater than 24 h (∼24.2 h) (9, 14) and, therefore, requires daily adjustment to remain synchronized to the external 24-h day. Much of this daily resetting is accomplished through light exposure captured by retinal photoreceptors and transmitted to the SCN (22). Whether light shifts the timing of the circadian clock earlier or later is critically dependent on when the light is received (39). Light in the evening or first part of the night causes the clock to shift rhythms later (phase delay), and light in the morning causes the clock to shift rhythms earlier (phase advance). Thus, morning light is essential for producing corrective daily phase advances, while evening light can produce phase delays, which often exacerbate the clock's endogenous tendency to drift later and promotes circadian misalignment. Correct alignment between the timing of the circadian clock and sleep/wake is essential for optimal mental and physical health (e.g., 15, 37).
Interestingly, a delay in circadian timing is associated with an increase in alcohol consumption in humans, even after controlling for factors such as age, sex, socioeconomic status, and educational level. For example, adults with an evening preference (proxy for delayed circadian timing) are more likely to be alcohol drinkers (45, 47), and to consume more alcohol daily [11.9 cc in evening types vs. 5.1 cc in neither types, (1)]. In one study, adults in the top quintile of eveningness drank 3.1 more standard drinks/day than those in the lowest quintile (17). Possible mechanisms that may explain this association include a delay in circadian timing leading to worse mood (19, 31), sleep onset difficulties (30), and/or reduced reward functioning (16), all of which may drive increased drinking (26, 32, 44). It also remains possible that alcohol directly affects the circadian system's response to light, further contributing to alcohol use disorders. For example, alcohol levels peak in the SCN about 20 min after hamsters consume alcohol (34). Additionally, alcohol can directly alter phase shifts to light in rodents, when alcohol is chronically consumed (34, 38) or when an acute dose of alcohol is injected into the SCN extracellular space (6, 35). This effect of alcohol on phase shifts to light is proposed to be due primarily to alcohol directly altering levels of GABA and glutamate in the SCN (6, 29, 35). However, the effect of alcohol on circadian phase shifts to light in humans remains to be investigated. Thus, we present the first proof-of-concept experiments to examine the effects of alcohol on phase shifts to light in humans, using a single dose, representative of social drinking. We measured circadian phase shifts with the dim light melatonin onset (DLMO), which is a reliable marker of human central circadian timing (20, 21). In line with the association between later circadian timing in humans and increased alcohol consumption, we hypothesized that a single dose of alcohol would increase phase delays to light but decrease phase advances to light.
MATERIALS AND METHODS
Subjects.
Thirteen healthy people were enrolled in the Phase Advance study, although two did not complete the protocol: one due to a death in the family and the other due to an illness that developed before the first laboratory session. Fifteen healthy people were enrolled in the Phase Delay study, although one did not complete the protocol as a result of accepting a full-time job offer. Thus, 11 subjects completed the Advance protocol (5 males, 6 females, age range: 24–45 yr) and 14 subjects completed the Delay study (7 males, 7 females, age range: 22–44 yr). All subjects were medication-free, with a body mass index between 17.9–31.0 kg/m2. On the basis of an in-person interview and their responses to screening questionnaires, all subjects had no medical (41), psychiatric (4, 11, 42), or sleep disorders (2, 3, 27), and reported good sleep quality (13). All subjects passed a urine drug screen for nicotine and other common drugs for abuse. Subjects consumed only moderate doses of caffeine (<300 mg/day). Nonsteroidal anti-inflammatory drugs were not permitted throughout the study, as they suppress melatonin (25). All subjects had not worked any night shifts or traveled across more than three time zones in the month preceding the study. All subjects were not color blind, as determined from the Ishihara test.
For both studies, subjects were required to habitually drink alcohol, but not be at-risk drinkers (28). Thus, males had to report consuming alcohol ≥2 occasions/month and between 2–4 drinks/occasion, but ≤14 drinks/wk. Females had to report consuming alcohol ≥2 occasions/month and 2–3 drinks/occasion, but ≤7 drinks/wk. Because of the difficulty in recruiting females, the criterion for females was adjusted to ≤10 drinks/wk. All subjects had no personal or family history of drug/alcohol abuse and were instructed not to consume alcohol during the study apart from what they were given as part of the protocol. All females were also required to pass a urine pregnancy test at the start of each laboratory session and were informed of their responsibility to use effective birth control during the study. None of the females reported breastfeeding. All subjects gave written informed consent prior to participation. The study was approved by the Rush University Medical Center Institutional Review Board.
Protocols.
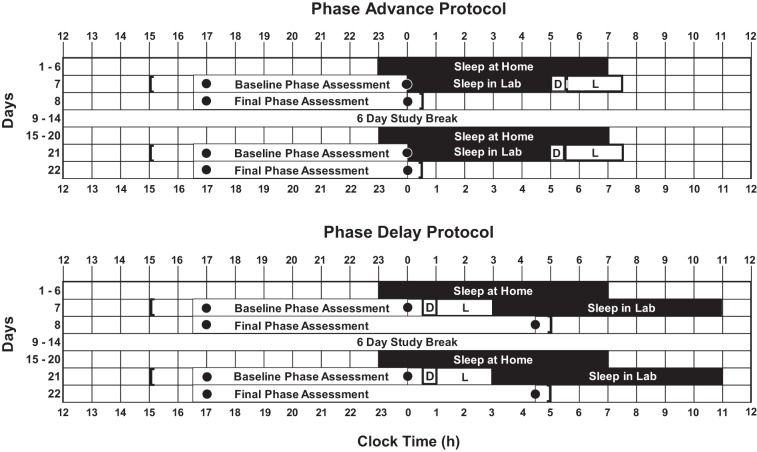
Both studies were 23 days long and consisted of a within-subjects, placebo-controlled counterbalanced design with two parts (Fig. 1). Each part consisted of 6 days of fixed sleep at home to stabilize circadian phase followed by a 2-day laboratory session. There was a 6-day study break between the two parts, during which subjects returned to their baseline sleep times ± 30 min. During the laboratory sessions, alcohol or placebo was administered prior to a bright light pulse (more details below), with the order of drink type (alcohol or placebo) counterbalanced across subjects. During each laboratory session in the Advance study, there was a baseline circadian phase assessment, a 5-h sleep episode, a 30-min drink window (alcohol, placebo), and a 2-h bright light pulse, followed by a final circadian phase assessment. Five subjects received alcohol in the first laboratory session, followed by placebo in the second laboratory session, and six subjects received placebo in the first laboratory session, followed by alcohol in the second laboratory session. During the laboratory session in the Delay study, there was a baseline circadian phase assessment, a 30-min drink window (alcohol, placebo), a 2-h bright light pulse, and an 8-h sleep episode, followed by a final circadian phase assessment. Seven subjects received alcohol in the first laboratory session, followed by placebo in the second laboratory session, and seven subjects received placebo in the first laboratory session, followed by alcohol in the second laboratory session. The timing of each protocol was tailored to each individual's habitual sleep times collected in the week before the study start with sleep diaries, which, on average, was 23:43 ± 0:50 (SD) − 7:32 ± 0:52 (SD) h.
Fig. 1.
A sample protocol for the Phase Advance study (top) and the Phase Delay study (bottom) for a subject who typically slept from 2300 to 0700. Both the Phase Advance and Phase Delay protocols consisted of 6 days of fixed sleep at home to stabilize circadian phase, a 2-day laboratory session, a 6-day study break, another 6 days of fixed sleep at home followed by another 2-day laboratory session. During the laboratory session in the Phase Advance study there was a baseline circadian phase assessment, a sleep episode, a 30-min drink window (alcohol, placebo), a 2-h bright light pulse, followed by a final circadian phase assessment. During the laboratory session in the Phase Delay study, there was a baseline circadian phase assessment, a 30-min drink window (alcohol, placebo), a 2-h bright light pulse, a sleep episode, followed by a final circadian phase assessment. The black rectangles represent scheduled sleep times. The dots represent the first and last saliva sample during the dim light circadian phase assessments. The D represents the 30-min drinking window. The L represents the 2-h bright light pulse. Square brackets indicate approximate arrival and departure times from the laboratory.
Sleep at home.
All subjects slept at home except during the 2-day laboratory sessions. During their scheduled 8-h sleep episodes at home, subjects were instructed to lie in bed and try to sleep. Subjects were not permitted to read, use any electronic devices, or talk at this time. Daytime naps were not permitted. To ensure compliance with the sleep schedule, all subjects were required to call the laboratory voice mail (time and date of call was recorded) before turning off their lights at night and at their wake time each morning. Subjects also completed daily sleep logs, noting bedtime, estimated sleep onset time, any awakenings during the night, and time of final awakening. Each subject also wore a waterproof actigraphy monitor with photosensor (Actiwatch Spectrum, Philips Respironics, Bend, OR; 30-s epochs) on their nondominant wrist throughout the protocol. Subjects came to the laboratory every fourth day of the fixed at-home sleep episodes to have their sleep logs and activity data inspected in their presence to ensure compliance to the sleep schedule.
Phase assessments.
Each subject participated in four dim-light phase assessments in the laboratory to determine their endogenous melatonin profiles (Fig. 1). Subjects were required to be caffeine-free for the 6 days prior to each laboratory session and were breathalyzed for alcohol at the start of each laboratory session. During the phase assessments, subjects remained awake and seated in dim light (<5 lux, at the level of the subjects' eyes, in the direction of gaze, measured every 2 h, Extech 403125 light meter, Nashua, NH) and were continuously monitored by staff. After 30 min in the dim light, subjects gave a saliva sample every 30 min using Salivettes (Sarstedt, Newton, NC). Toothpaste or mouthwash were not allowed during the phase assessments. Small snacks and fluids were permitted, except in the 10-min before each sample, and subjects were required to rinse and brush their teeth with water while remaining seated 10 min before each sample if they had consumed food or drink. The samples were centrifuged immediately upon collection and frozen. The samples were later shipped in dry ice to Solidphase (Portland, ME), which radioimmunoassayed the samples for melatonin using commercially available kits (ALPCO, Salem, NH). The assay sensitivity was 0.5 pg/ml. Intra-assay and inter-assay coefficients of variation for low levels of salivary melatonin are 20.1%, and 16.7%, respectively. A dim-light melatonin onset (DLMO) was calculated for each phase assessment, as the clock time (with linear interpolation) when the melatonin concentration exceeded the mean of three low, consecutive, daytime values plus twice the standard deviation of these points (5, 43). This low threshold more closely tracks the initial rise of melatonin (23). The phase shift during each 2-day laboratory stay was calculated as the difference between the baseline DLMO and the final DLMO.
Alcohol and placebo drinks.
The alcohol and placebo drinks were administered to seated subjects in a private, temperature-controlled, bedroom. In the Advance study, in which subjects were required to drink in the early morning, males received 0.60 g/kg of alcohol, and females received 0.51 g/kg, after a 5-h fast during the scheduled sleep episode. Given the slower metabolism of alcohol in the morning (46), females received lower doses of alcohol in the morning to reduce the risk of adverse events. In the Delay study, where subjects were required to drink late at night, both males and females received 0.80 g/kg, after a 2.5-h fast. The drinks were prepared about 1 h before administration, out of sight of the subjects. The alcohol drinks consisted of a 1:4 mix of vodka (80 proof) and tonic water, with a splash of lime juice to mask the taste (33, 36). The placebo drink consisted of the same volume of liquid, but was primarily tonic water with the same splash of lime juice and three drops of vodka dropped on the top of the drink with an eye dropper (33, 36). The total volume of the alcohol or placebo drink was divided into three cups, and each cup was consumed in a 10-min window, for a total drinking window of 30 min. Subjects were breathalyzed (Alco-sensor III, Intoximeters, Saint Louis, MO) every 30 min after the first drink to the end of the bright light exposure, but were kept blinded from the breath alcohol concentration (BrAC) readings. Thus, the study was single-blind, with subjects but not staff blind to condition, as staff had to record the BrAC readings. On average, the BrAC readings recorded after the third drink were 0.053 ± 0.034 g/210 l (SD) in the Advance study and 0.063 ± 0.047 g/210 l (SD) in the Delay study. In the alcohol condition, the BrAC readings always remained above zero for the duration of the light pulse in both the Advance and Delay studies. In the placebo condition, all BrAC readings were 0.00 g/210 l as expected. Subjects were breathalyzed at the start of each phase assessment to ensure a BrAC of zero prior to subsequent saliva collection.
Bright light.
During each laboratory stay, subjects were exposed to a continuous 2-h bright light pulse [mean intensity 4,763 ± 689 (SD) lux, measured periodically at angle of gaze with Minolta TL-1 light meter, Ramsey, NJ]. The bright light was produced by a single light box (61 × 61 × 10 cm, Enviro-Med, Vancouver, WA) placed on a desk about 45 cm in front of the subject's eyes. Each light box had a diffuser screen and contained four 54-cm long 40-W fluorescent horizontal tubes (Philips PL-L40W/41/RS/IS, 4100K). At this distance, subjects received 5.1 × 1015 photons·cm−2·s−1, and specifically 1.1 × 1015 photons·cm−2·s−1 in the blue range (400–490 nm), with an irradiance of 1,741 μW/cm2 (400–750 nm). Subjects were permitted to read materials flat on the desk in front of the light box. Subjects were monitored continuously by staff either in person or via video cameras to ensure they were not closing their eyes, looking away, or falling asleep during the bright light exposure.
Statistical analysis.
In each study, the baseline DLMOs measured in the baseline phase assessment in each part of the study were compared with a post hoc paired samples t-test to ensure subjects received the alcohol or placebo drink and bright light at approximately the same circadian phase. This verification was important, as circadian phase is an important predictor of subsequent phase shifts to light (39).
For each study, the DLMOs were initially analyzed with a three-way repeated-measures ANOVA with within-subjects factor “drink” (alcohol vs. placebo), within-subjects factor “time” (before vs. after light), and between-subjects factor “order” (alcohol condition first vs. second). However, in all cases, the between-subjects factor “order” was not significant (all P ≥ 0.15), so this factor was subsequently removed from the analysis. The drink × time interaction for each study was of most interest, as it would indicate if the magnitude of the phase shifts to bright light differed between conditions. Statistical significance for all analyses was determined with two-tailed tests at P < 0.05. Results are reported as means ± SD.
RESULTS
The distributions of the DLMOs in both studies were normal (Kolmogorov-Smirnov test P ≥ 0.20).
Advance study.
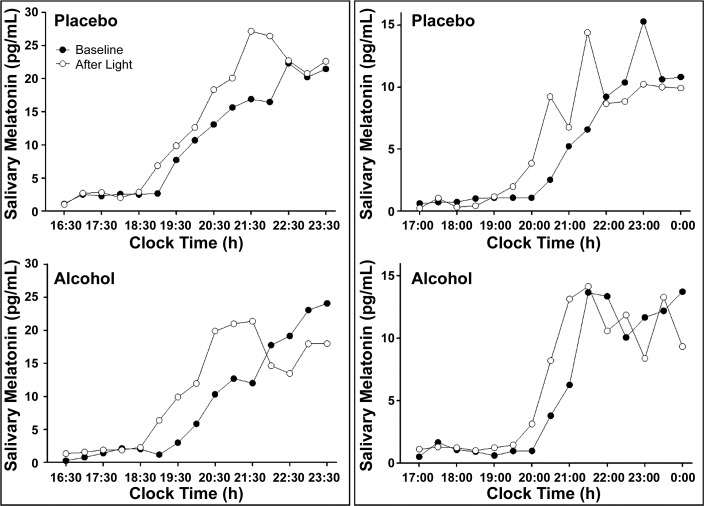
Of the 11 subjects who completed the Advance study, one subject displayed an initial rise in melatonin, a decrease, and then a second rise in melatonin, at baseline, in the placebo condition. As the DLMO could not be reliably calculated, this subject was removed from subsequent analyses. In the remaining 10 subjects, the average absolute difference between the baseline DLMOs was 0.27 ± 0.16 (SD) h, and the baseline DLMOs were not significantly different from each other (P = 0.28). In the Advance study, there were individual cases where the phase advances to bright light were larger with alcohol than placebo (Fig. 2). However, we also observed the opposite effect in other subjects (Fig. 2). Overall, in the Advance study, five subjects showed larger phase advances to light with alcohol (mean increase in phase advance 0.39 h), four subjects showed smaller phases advances to light with alcohol (mean decrease in phase advance 0.26 h), and one subject phase delayed with alcohol (by 0.42 h, Fig. 4). All these changes were smaller than 0.5 h, and less than the 30-min saliva sampling rate. In addition to these changes not being meaningful in size (d = 0.08), the drink × time interaction was not significant (P = 0.74), indicating no statistical effect of alcohol on phase advances to light.
Fig. 2.
The evening rise in melatonin levels observed in two subjects in the Phase Advance study. The baseline evening rise in melatonin levels before the bright light is shown by solid circles (●), and the evening rise in melatonin levels after the bright light on the next day is shown with open circles (○). One subject (left) showed greater phase advances with alcohol than with placebo. The other subject (right) showed smaller phase advances with alcohol than with placebo.
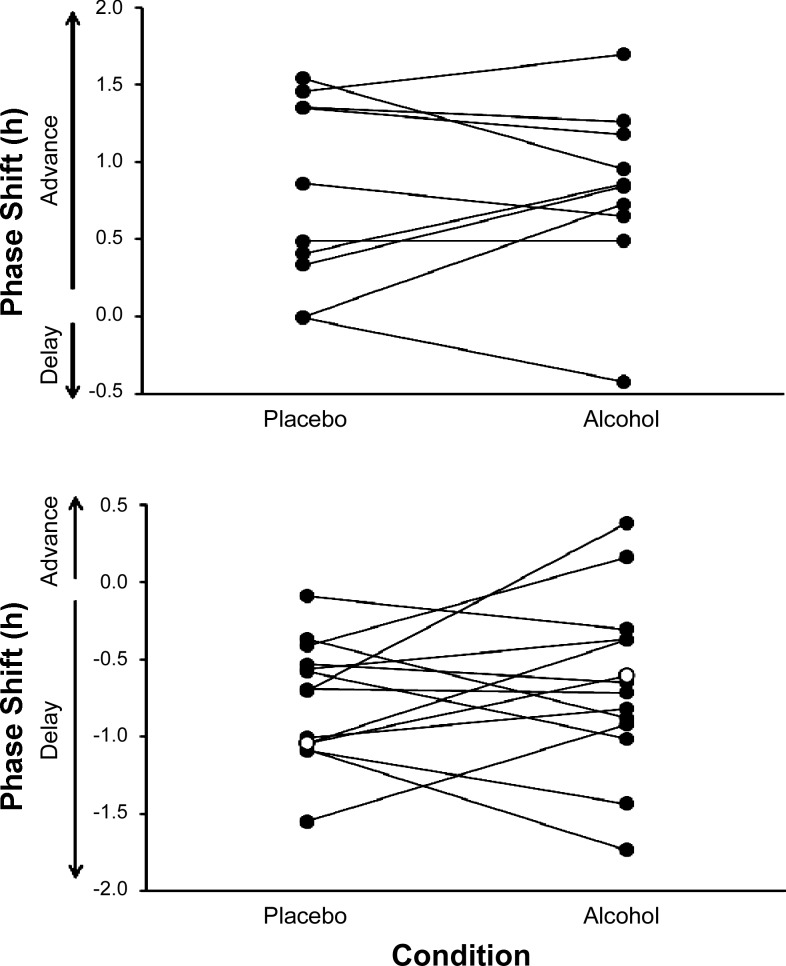
Fig. 4.
The phase shifts in the dim-light melatonin onset (DLMO) observed in each individual subject in the Phase Advance study (top) and phase delay study (bottom). The phase shifts for an individual subject are connected by a line. The open circles (○) represent the observed phase shift in the single subject whose baseline DLMOs varied by more than 1 h between conditions. No systematic effect of the single dose of alcohol on phase advances or phase delays to the bright light were observed (drink × time interactions in both studies P ≥ 0.46).
Delay study.
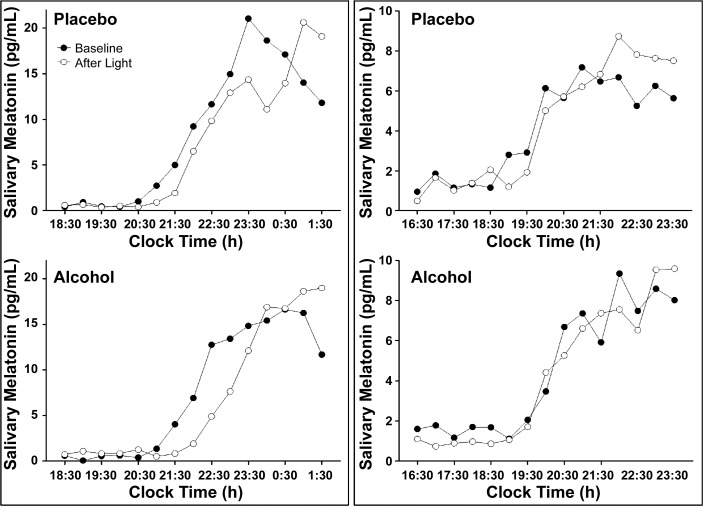
In the Delay study, the average absolute difference between the baseline DLMOs was 0.44 ± 0.41 (SD) h, and the baseline DLMOs were not significantly different from each other (P = 0.90). In the Delay study, there were individual cases in which the phase delays to bright light were larger with alcohol than placebo (Fig. 3). However, we also observed the opposite effect in other subjects (Fig. 3). Overall, in the Delay study seven subjects showed larger phase delays to light with alcohol (mean increase in phase delay 0.33 h), five subjects showed smaller phase delays to light with alcohol (mean decrease in phase delay 0.42 h), and two subjects showed phase advances to light with alcohol (by less than 0.38 h, Fig. 4). All of these changes were smaller than 0.5 h, and less than the 30-min saliva sampling rate. In addition to these changes not being meaningful in size (d = 0.20), the drink × time interaction was not significant (P = 0.46), indicating no statistical effect of alcohol on phase delays to light. One subject in the Delay study had baseline DLMOs that differed by 1.5 h, due to him sleeping 25 min past his scheduled wake time on the day of the first baseline phase assessment in the placebo condition. When the analysis was repeated after removing this subject, the drink × time interaction remained nonsignificant (P = 0.60). Overall, a power analysis revealed low statistical power due to the small sample size in each study (power ≤ 0.20). However, given the small effect sizes, a sample size of at least n = 120 in each study would have been required to detect any statistical significance associated with these small effects.
Fig. 3.
The evening rise in melatonin levels observed in two subjects in the Phase Delay study. The baseline evening rise in melatonin levels before the bright light is shown by solid circles (●), and the evening rise in melatonin levels after the bright light on the next day is shown with open circles (○). One subject (left) showed greater phase delays with alcohol than with placebo. The other subject (right) showed smaller phase delays with alcohol than with placebo.
DISCUSSION
The results of the two studies reported here are the first investigations of the effects of alcohol on phase shifts to light in humans. A single high dose of alcohol was administered late at night, and a single moderate dose of alcohol was administered in the morning to account for time-of-day differences in the metabolism of alcohol (46). In both studies, the alcohol or placebo was administered to light drinkers immediately before exposure to a 2-h bright light pulse. In both studies, the average changes in phase shifts to light observed with a single dose of alcohol vs. placebo were consistently of small magnitude, less than 30 min in size, and no consistent effects were observed.
The 6 days of fixed sleep prior to each laboratory session were included in the study design to increase the likelihood that the bright light would occur at approximately the same circadian phase in both conditions. This is important because the phase shifting effect of light is dependent on the circadian time at which light is received (39). This approach, which has been used in other within-subjects design circadian studies (7, 8), was successful in that the baseline DLMOs between conditions in both studies were not significantly different. Indeed, only one subject had baseline DLMOs that were more than 1 h apart, and this was likely due to him not following the fixed sleep schedule on the morning of the first baseline phase assessment. Nonetheless, the results remained statistically nonsignificant, even after excluding this subject from the analyses.
The finding that a single dose of alcohol did not systematically alter circadian phase shifts to light in humans is in contrast to results reported in studies that tested a single dose of alcohol in nocturnal rodents. For example, in Syrian hamsters, a single dose of alcohol reduced phase advances to light, but did not significantly alter phase delays to light (35). In mice, a single dose of alcohol reduced phase delays to light but did not significantly change phase advances to light (6). Notably, the alcohol in these studies was injected directly into the SCN extracellular fluid compartment, rather than consumed by the rodents, probably reducing the variability in the bioavailability of alcohol that normally occurs after oral consumption. Indeed, chronic intake of alcohol in rodents can alter phase shifts to light (34, 38), suggesting multiple doses of alcohol could meaningfully alter phase shifts to light in humans.
Similarly, we have previously reported that a single dose of alcohol (0.8 g/kg) does not increase intestinal permeability in healthy humans (lactulose/mannitol ratio; Ref. 18), whereas chronic exposure to alcohol (0.4 g/kg or 1–3 glasses of red wine, per night for 1 wk), does increase intestinal permeability in healthy humans by more than 50% (40). Therefore, despite our negative results associated with a single dose of alcohol, it remains quite possible that chronic exposure to alcohol may meaningfully alter circadian phase shifts to light in humans. Given the association of later circadian timing with drinking more alcohol (1, 17, 45, 47), one might predict larger phase delays to light with alcohol, especially as humans most typically consume alcohol in the evening. However, to date, alcohol has only ever reduced phase shifts to light in nocturnal rodents, or produced no change (29). Therefore, how chronic exposure to alcohol influences circadian phase shifts to light in diurnal humans remains an important gap in our knowledge that should be addressed by future research.
There were limitations to this study. Both studies consisted of a within-subjects design, with a study break (wash out) in between the conditions, which led to relatively long 23-day studies. We chose a within-subjects design to ensure that the alcohol and placebo drinks were given at approximately the same circadian phase, and to reduce the high between-subjects variability often observed in phase shifts to light. Indeed, even when light is administrated at the same circadian phase in humans, phase shifts in response to a 1-h pulse of bright light can vary by ∼1–1.5 h (39). Our within-subjects design led to smaller sample sizes of 10 subjects in the Phase Advance study and 14 subjects in the Phase Delay study, sample sizes that are commonly seen in human circadian research that report positive findings [e.g., n = 5 per group (10), n = 6–7 per group (12)]. Despite the small sample sizes, we noted that, on average, the change in phase shifts with alcohol were not more than 30 min different to those observed with placebo, and, therefore, were not meaningful. We also chose the initial step of testing a single dose of alcohol on phase shifts to light, as per the rodent literature (34). Other studies have observed a significant effect of a single dose of caffeine, for example, on human circadian timing in similarly small sample sizes [n = 5 per group (10)]. Thus, while our sample sizes were small, they were adequate to show that a single episode of social drinking does not meaningfully change human circadian phase shifts to light.
Perspectives and Significance
Circadian timing has a significant influence on mental and physical health in humans. Light is the strongest environmental influence on circadian timing. Previous research in rodents reveals that alcohol can directly alter the response of the central circadian clock to light. Here, we examined for the first time, the effect of a single moderate to high dose of alcohol on phase shifts to evening light and morning light in humans. No notable changes were observed, demonstrating that a single social drinking episode does not meaningfully change the circadian system's response to light in humans. The effect of chronic alcohol exposure or multiple doses of alcohol on the circadian response to light in humans remains to be tested.
GRANTS
The research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R21 AA021762 awarded to H. J. Burgess. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.J.B. and A.K. conception and design of research; H.J.B. and M.R. performed experiments; H.J.B., M.R., and L.F.F. analyzed data; H.J.B., M.R., L.F.F., and A.K. interpreted results of experiments; H.J.B. and M.R. prepared figures; H.J.B. and M.R. drafted manuscript; H.J.B., M.R., and A.K. edited and revised manuscript; H.J.B., M.R., L.F.F., and A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Holly Hunt, Toni Iurcotta, Catherine Keefner, Athanasios Kondilis, Devon Langston, Thomas Molina, Philip Sanchez, and Haein Sung for their assistance with data collection. Enviro-Med donated the light boxes. We thank Dr. Todd Arnedt for his assistance with the grant application that funded this research and with the instructions on how to prepare the alcohol and placebo drinks.
REFERENCES
- 1.Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction 89: 455–462, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, Zucconi M, Ferri R, Trenkwalder C, Lee HB. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—history, rationale, description, and significance. Sleep Med 15: 860–873, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2: 297–307, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 4: 53–63, 1961. [DOI] [PubMed] [Google Scholar]
- 5.Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med 4: 66–69, 2008. [PMC free article] [PubMed] [Google Scholar]
- 6.Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcohol Clin Exp Res 35: 1467–1474, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess HJ. Evening ambient light exposure can reduce circadian phase advances to morning light independent of sleep deprivation. J Sleep Res 22: 83–88, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess HJ. Partial sleep deprivation reduces phase advances to light in humans. J Biol Rhythms 25: 460–468, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol Rhythms 23: 374–376, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke TM, Markwald RR, McHill AW, Chinoy ED, Snider JA, Bessman SC, Jung CM, O'Neill JS, Wright KP. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med 7: 305ra146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher JN, Dahlstrom WG, Graham JR, Tellegen A, Kaemmer B. MMPI-2 (Minnesota Multiphasic Personality Inventory-2): Manual for Administration and Scoring. Minneapolis, MN: University of Minnesota Press, 1989. [Google Scholar]
- 12.Buxton OM, L'Hermite-Baleriaux M, Turek FW, Van Cauter E. Daytime naps in darkness phase shift the human circadian rhythms of melatonin and thyrotropin secretion. Am J Physiol Regul Integr Comp Physiol 278: R373–R382, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psych Res 28: 193–213, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Duffy JF, Cain SW, Chang AM, Phillips AJ, Munch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP Jr, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA 108 Suppl 3: 15,602–15,608, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psych Res 168: 259–261, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res 37: 558–565, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanerva N, Kronholm E, Partonen T, Ovaskainen ML, Kaartinen NE, Konttinen H, Broms U, Mannisto S. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int 29: 920–927, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol 89: 2205–2211, 1994. [PubMed] [Google Scholar]
- 19.Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, Kamei Y, Mishima K. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol Int 27: 1797–1812, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms 17: 181–193, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Lewy A, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms 14: 227–236, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Lucas RJ, Lall GS, Allen AE, Brown TM. How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock. Prog Brain Res 199: 1–18, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int 28: 714–718, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore RY. Organization and function of a central nervous system circadian oscillator: The suprachiasmatic hypothalamic nucleus. Fed Proc 42: 2783–2789, 1983. [PubMed] [Google Scholar]
- 25.Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav 59: 133–139, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature's clocks and human mood: the circadian system modulates reward motivation. Emotion 9: 705–716, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 131: 485–491, 1999. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor PG, Schottenfeld RS. Patients with alcohol problems. N Engl J Med 338: 592–602, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Prosser RA, Glass JD. Assessing ethanol's actions in the suprachiasmatic circadian clock using in vivo and in vitro approaches. Alcohol 49: 321–339, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid KJ, Burgess HJ. Circadian rhythm sleep disorders. Prim Care 32: 449–473, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Reid KJ, Jaksa AA, Eisengart JB, Baron KG, Lu B, Kane P, Kang J, Zee PC. Systematic evaluation of Axis-I DSM diagnoses in delayed sleep phase disorder and evening-type circadian preference. Sleep Med 13: 1171–1177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roehrs T, Papineau K, Rosenthal L, Roth T. Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology 20: 279–286, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Roehrs T, Zwyghuizen-Doorenbos A, Knox M, Moskowitz H, Roth T. Sedating effects of ethanol and time of drinking. Alcohol Clin Exp Res 16: 553–557, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. Am J Physiol Regul Integr Comp Physiol 297: R729–R737, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. Am J Physiol Regul Integr Comp Physiol 296: R411–R418, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rupp TL, Acebo C, Carskadon MA. Evening alcohol suppresses salivary melatonin in young adults. Chronobiol Int 24: 463–470, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106: 4453–4458, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacol Biochem Behav 87: 297–305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1h pulse of bright white light. J Physiol 590: 3035–3045, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson GR, Tieu V, Shaikh M, Forsyth C, Keshavarzian A. Is moderate red wine consumption safe in inactive inflammatory bowel disease? Digestion 84: 238–244, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tasto DL, Colligan MJ, Skjei EW, Polly SJ. Health Consequences of Shift Work. Cincinnati, OH: NIOSH, Publication # 78–154, 1978. [Google Scholar]
- 42.Terman M, Williams J. Personal inventory for depression and SAD (PIDS). J Prac Psych Behav Health 5: 301–303, 1998. [Google Scholar]
- 43.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms 12: 457–466, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry 19: 245–250, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Whittier A, Sanchez S, Castaneda B, Sanchez E, Gelaye B, Yanez D, Williams MA. Eveningness chronotype, daytime sleepiness, caffeine consumption, and use of other stimulants among Peruvian university students. J Caffeine Res 4: 21–27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson RH, Newman EJ, Newman HW. Diurnal variation in rate of alcohol metabolism. J Appl Physiol 8: 556–558, 1956. [DOI] [PubMed] [Google Scholar]
- 47.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int 23: 497–509, 2006. [DOI] [PubMed] [Google Scholar]