Abstract
We tested the hypothesis that an increase in the anti-inflammatory cholinergic pathway, when induced by pyridostigmine (PY), may modulate subtypes of lymphocytes (CD4+, CD8+, FOXP3+) and macrophages (M1/M2) soon after myocardial infarction (MI) in rats. Wistar rats, randomly allocated to receive PY (40 mg·kg−1·day−1) in drinking water or to stay without treatment, were followed for 4 days and then were subjected to ligation of the left coronary artery. The groups—denominated as the pyridostigmine-treated infarcted (IP) and infarcted control (I) groups—were submitted to euthanasia 3 days after MI; the heart was removed for immunohistochemistry, and the peripheral blood and spleen were collected for flow cytometry analysis. Noninfarcted and untreated rats were used as controls (C Group). Echocardiographic measurements were registered on the second day after MI, and heart rate variability was measured on the third day after MI. The infarcted groups had similar MI areas, degrees of systolic dysfunction, blood pressures, and heart rates. Compared with the I Group, the IP Group showed a significant higher parasympathetic modulation and a lower sympathetic modulation, which were associated with a small, but significant, increase in diastolic function. The IP Group showed a significant increase in M2 macrophages and FOXP3+ cells in the infarcted and peri-infarcted areas, a significantly higher frequency of circulating Treg cells (CD4+CD25+FOXP3+), and a less extreme decrease in conventional T cells (CD25+FOXP3−) compared with the I Group. Therefore, increasing cholinergic modulation with PY induces greater anti-inflammatory cell recruitment soon after MY in rats.
Keywords: myocardial infarction, T lymphocytes, macrophage activation, anticholinesterase drugs, cholinergic anti-inflammatory reflex
the marked advances in recent decades in the management of patients suffering from myocardial infarction (MI) have significantly reduced these patients' short-term mortality. However, the development of heart failure after MI is still high, which leads to a significant increase in mortality in this population (20).
Myocardial infarction triggers a sterile inflammatory reaction characterized by sequential recruitment and activation of innate and adaptive immune cells.(10, 41) A deregulated immune reaction can lead to changes in ventricular shape, size, and function and can, thus, induce the development of heart failure syndrome.(4, 15) Therefore, it would be desirable to develop new therapeutic strategies to modulate immune response and improve healing after MI (1).
Classically, macrophages and lymphocytes are the leading infiltrating cells in the infarcted myocardium, and these cells show a functional and phenotypic plasticity during the resolution phase of inflammation (15, 27). M1 macrophages that produce high amounts of proinflammatory cytokines mediate the initial inflammatory response, and M2 macrophages that produce mainly anti-inflammatory cytokines involved in repair functions dominate the healing phase (36). Subpopulations of T lymphocytes with proinflammatory and anti-inflammatory profiles have been found in the myocardium after MI (15, 27). Likewise, the adaptive immune system plays a central role in the pathogenesis of ventricular remodeling after myocardial ischemic injury (33, 36). Splenocytes from infarcted rats contain a subset of CD8+ T lymphocytes with cytotoxic activity against healthy cardiomyocytes ex vivo (37). Experimental studies have demonstrated that insufficient recruitment of a subgroup of CD4+ T lymphocytes with potent immunosuppressive properties and denominated regulatory T cells (Treg cells, CD4+CD25+FOXP3+) can result in worsening ventricular remodeling after MI (13, 32). Clinical studies showed that in patients with acute coronary syndrome and heart failure, the frequency of circulating Treg cells is reduced, and their suppressive function is compromised (14, 33). Additionally, infiltration of CD4+ and CD8+ T lymphocytes was found in both MI and non-MI areas in clinical studies (1). In vitro studies have been recently performed to manipulate macrophage polarization and Treg cells and control the duration and extent of the inflammatory phase following MI (11, 39).
Tracey's group proposed the concept that the parasympathetic nervous system modulates the inflammatory response by acting on effector cells related to innate and acquired immunity (28–31). The cholinergic anti-inflammatory pathway has been attracting the attention of investigators as a therapeutic strategy for protecting against myocardial ischemia reperfusion injury (7, 16) and MI damage (8, 9). Accumulated evidence indicates that stimulation of the efferent vagus nerve can release the important neurotransmitter ACh, which acts through the α-7 subunit of the nicotinic ACh receptor (α-7 nAChR) that is expressed in macrophages and regulatory T lymphocytes (6, 30, 37) to modulate the anti-inflammatory response.
Our group (8, 27) and others (9, 18) have demonstrated that baroreflex dysfunction, reduction in parasympathetic modulation, and increased sympathetic response identified after MI may be restored with physiological and pharmacological interventions that act on the cholinergic anti-inflammatory pathway.
Pyridostigmine (PY), a peripheral cholinesterase inhibitor agent used to treat myasthenia gravis, reduces the ACh hydrolysis released by cholinergic neurons and increases parasympathetic modulation in normal rats (30). Administration of PY improves the autonomic profile (8, 9, 18, 27) and decreases left ventricular (LV) dysfunction following MI in some experimental studies (8, 9, 18). Two to four weeks of PY administration may ameliorate cardiac remodeling induced by MI via multiple mechanisms, including by increasing mediators of angiogenesis (41), by decreasing proinflammatory cytokine production, and via cardiac fibrosis of the surviving myocardium (21). Moreover, in vitro, ACh reduces apoptosis in various cell types (19, 42).
Considering that the protective effects of PY may depend on the inflammatory milieu after myocardial ischemia, data on PY's effect on the mobilization of immune cells after MI are needed. Therefore, in the present study, we tested the hypothesis that PY-mediated increase in the anti-inflammatory cholinergic pathway may modulate the total CD4+ and CD8+ T lymphocytes and the Treg cells in the peripheral blood, spleen, and heart and that it may alter the M1/M2 macrophages in the ischemic myocardium soon after MI in rats.
MATERIALS AND METHODS
Animal model.
All procedures and animal care were approved by the Committee on the Ethics of Animal Experimentation at University of São Paulo Medical School and were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health.
Male Wistar-Kyoto rats (200–250 g) were housed in collective plastic cages (four animals per cage) with a controlled temperature (23°C), a 12:12-h light-dark cycle and with rat chow provided ad libitum. The animals were randomly assigned to one of three groups, with 20 animals in each group: control rats (C), untreated infarcted rats (I), and PY-treated infarcted rats (IP). The groups were followed for a total of 7 days.
Rats in the C and I Groups had unlimited access to tap water, and those in the IP Group had unlimited access to water containing PY bromide (40 mg/ml; Sigma-Aldrich, St Louis, MO), as described previously (8, 30). PY treatment started 4 days prior to MI and continued for 3 days after this procedure. The dose and period of PY administration (7 days) were chosen according to a prior study (30) that established that this treatment protocol is adequate to inhibit ∼40% of plasma acetylcholinesterase activity. Water consumption was monitored during the experimental period in all groups.
Myocardial infarction.
Rats in the I and IP Groups were anesthetized (80 mg/kg ketamine and 12 mg/kg xylazine ip) and subjected to induction of MI by surgical occlusion of the left coronary artery, as previously described (7, 24). A left thoracotomy was performed, the third intercostal space was dissected, and the heart was exposed. Then, the left coronary artery was occluded with a single nylon (6.0 mm) suture 1 mm distal to the left atrial appendage. The chest was then sutured. The rats were maintained under ventilation until recovery. The Control Group underwent the same procedure except that MI was not induced.
Echocardiographic evaluation and arterial catheterization.
Echocardiography was performed by an observer who was blinded to the group allocation on the second day after thoracotomy, according to the guidelines of the American Society of Echocardiography.
The rats were anesthetized (80 mg/kg ketamine and 12 mg/kg xylazine ip), and images were obtained with a 10–14-MHz linear transducer in a SEQUOIA 512 (ACUSON, Mountain View, CA) for measurements of LV ejection fraction (LVEF), LV fractional shortening, left atria diameter, and isovolumetric relaxation time (IVRT), as described previously (5, 8, 22). MI was defined by echocardiography as any abnormal segmental wall motion, including hypokinesis, akinesis, and dyskinesis. On each echocardiographic transverse plane (basal, middle, and apical), the arcs corresponding to segments with infarction (AI) and the total perimeter of the endocardial border (PE) were measured 3 times at the end-diastole, and the MI area was estimated as MI area = AI/PE·100.
After echocardiographic evaluation, while the rats were still under anesthesia, a catheter filled with 0.06 ml of saline solution was implanted into the femoral artery of the rats, as described in another article (8, 22).
Hemodynamic measurements.
On the third day after thoracotomy, the arterial cannula was connected to a strain gauge transducer (Blood Pressure XDCR; Kent Scientific, Torrington, CT), and arterial pressure (AP) signals and pulse interval [heart rate (HR)] were digitally recorded over a 30-min period in conscious animals using a data acquisition system (WinDaq, 2 kHz; DATAQ, Springfield, OH), as described elsewhere (7, 19). This basal acquisition was used to evaluate heart rate variability (HRV) and systolic arterial pressure variability (as described below).
HRV.
The overall variability of the pulse interval (HRV) was measured in the time and frequency domains using spectral estimation. For this, a 20-min time series of pulse intervals was cubic-spline interpolated (250 Hz) and decimated to be equally spaced in time. Following linear trend removal, the power spectral density was obtained with a fast Fourier transformation using Welch's method over 16,384 points with a Hanning window and 50% overlapping. The pulse-interval variability was evaluated in the time domain using standard deviation of the RR interval. The spectral power was calculated for very low-frequency (VLF: 0.00–0.20 Hz), low-frequency (LF: 0.20–0.75 Hz), and high-frequency (HF: 0.75–4.0 Hz) bands using power-spectrum density integration within each frequency bandwidth and a customized routine (MATLAB 6.0; MathWorks, Natick, MA). The autonomic balance was evaluated as the ratio of the absolute values of LF and HF (8, 22).
Immunohistochemistry for immune cells.
On the third day after thoracotomy, seven animals from each group were anesthetized (80 mg/kg ketamine and 12 mg/kg xylazine ip) and perfused with 0.9% NaCl plus 14 mmol/l KCl solution (iv; with a pressure equal to 13 cmH2O) to arrest the heart in diastole, followed by a perfusion of 4% buffered formalin for tissue fixation. Excised hearts were immersed in formalin for 24 h. Transverse slices were processed and embedded in paraffin. Serial sections of paraffin-embedded tissues (3 μm) were placed on glass slides coated with 2% 3-aminopropyltriethylsilane (Sigma-Aldrich) and deparaffinized in xylene, then immersed in alcohol and incubated with 3% hydrogen peroxide diluted in TBS (pH 7.4). The sections were blocked by incubation with 3% normal goat serum for 20 min and immersed in a citrate buffer (pH 6.0; Sigma-Aldrich) at 95°C for 20 min for antigen retrieval. Nonspecific signals were blocked using specific antibody diluents (Antibody Diluent, cat. no. S0809; Dako, Glostrup, Denmark). The slides were then incubated with the following antibodies: CD4 (W3/25, cat. no. 111815; Abcam, Cambridge, UK), CD8 (X-8RT; Millipore, Merk-Millipore, Darmstadt, Germany), FOXP3 (cat. no. 22510; Abcam), CD11b/c (cat. no. 75476; Abcam), and CD206 (cat. no. 64693; Abcam); see Table 1.
Table 1.
Antibodies and antigen retrieval
| Antibody | Antigen | Brand | Dilution | Antigen Retrieval |
|---|---|---|---|---|
| CD4+ | T helper lymphocytes | W3/25, Abcam | 1/40 | Citrate buffer pH 6.0 95–100°C |
| CD8+ | T cytotoxic lymphocytes | X-8RT, Millipore | 1/40 | Citrate buffer pH 6.0 95–100°C |
| Foxp3 | T regulatory | Abcam | 1/600 | Citrate buffer pH |
| lymphocytes | (cat. no. 54501) | 6.0 95–100°C | ||
| CD11b/c | M1 | Abcam | 1/300 | Citrate buffer pH |
| macrophages | (cat. no. 75476) | 6.0 95–100°C | ||
| CD206 | M2 | Abcam | 1/300 | Citrate buffer pH |
| macrophages | (cat. no. 64693) | 6.0 95–100°C |
The samples were kept overnight at 4°C in a humidified chamber. The sections then were washed with TBS and incubated with N-Histofine Simple Stain (Nichirei Biosciences, Tokyo, Japan) for 30 min. The sections were then incubated in 3,3′-diaminobenzidine in a chromogen solution (Dako) at room temperature for 2–5 min. The sections were then stained with Mayer's hematoxylin (Sigma-Aldrich) and covered. For the negative controls, the primary antibodies were replaced with 1% PBS/BSA and nonimmune mouse serum (X501-1, Dako).
Cell counts in the infarcted and peri-infarcted zones.
Ten consecutive microscope fields (magnification: ×400) of the infarcted and peri-infarcted zones were photographed (Leica Microsystems, Wetzlar, Germany). An experienced pathologist with no prior knowledge of the samples analyzed the images and manually counted the cells with the aid of the ImageJ 1.45 program (NIH, Bethesda, MD), using the “cell counter” plug-in.
Flow cytometry for T lymphocytes.
A set of 12 animals in each group was euthanized by decapitation on the third day after thoracotomy, to collect fresh spleen, heart, and blood samples for FACS measurements.
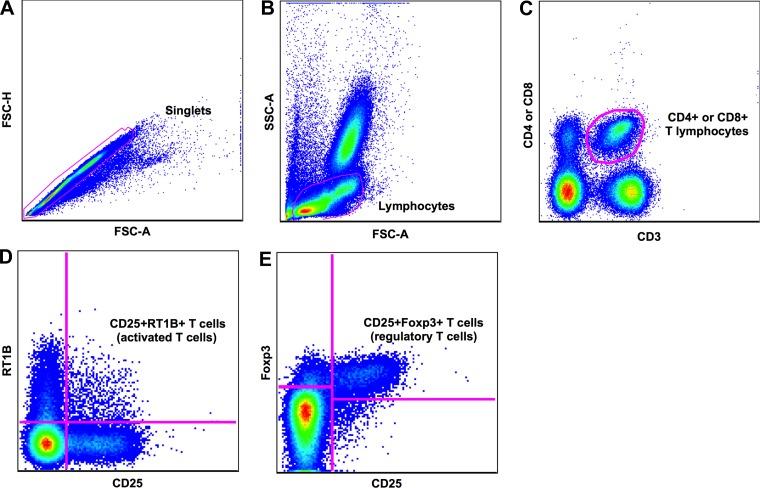
The cells were gently dissociated from the organ by gentle maceration through a 100-μm cell strainer (BD Biosciences, Franklin Lakes, NJ) in RPMI medium. The red blood cells were lysated under room temperature incubation with a commercial ACK lysis buffer. After washing, 3 million splenocytes from each animal were surface-stained using the following monoclonal antibodies: CD3 (APC), CD4 (PE-Cy5) or CD8 (PercP), CD25 (PE), and RT1B (FITC; all from BD Biosciences) for 30 min at 4°C. For peripheral blood cells, 100 μl of each sample was used, and a doubled concentration of each antibody was used. After surface staining, splenocytes and peripheral cells were fixed and permeabilized using buffers from FOXP3 set per the manufacturer's instructions (eBioscience, San Diego, CA). The cells were washed twice with a FACS buffer after the surface-staining incubation, and 100 μl of the first buffer was added. The cells were then incubated for 1 h at room temperature in the dark. After incubation, the cells were washed twice with buffer B2 and intracellularly stained with FOXP3 PE-Cy7 monoclonal antibody (eBioscience) for 1 h at room temperature in the dark. Following intracellular staining, the cells were washed twice with the second buffer and resuspended in 150 μl of FACS buffer for acquisition. Samples were acquired on a FACS Canto flow cytometer (BD Biosciences) and analyzed using the FlowJo Software (version 9.0.2; Tree Star, San Carlo, CA). Over 500,000 events were acquired on the lymphocyte gate. For analysis, cells were gated on forward/side scatter (FSC/SSC) to define the lymphocyte population, and CD3+ T cells were selected for the T lymphocyte definition. A subsequent gate on CD4+ or CD8+ populations was drawn (26). After the identification of CD4+ and CD8+ populations, a CD25 vs. RT1B gate (to discriminate activation) or CD25 vs. FOXP3+ gate (to discriminate regulatory T cells) was imposed (Fig. 1). For each flow cytometry experiment performed, unstained and single-color controls were used to allow for proper voltage adjustment and compensation. On the basis of the above gating strategy, the total frequency of CD4+ and CD8+ T lymphocytes, activated T cells (CD25+RT1B+), and regulatory T cells (FOXP3+) were defined for the peripheral blood and splenocytes.
Fig. 1.
Gating strategy. Peripheral blood mononuclear cells or splenocytes were obtained and stained for activation or FOXP3-marker evaluation. Three million cells were surface-stained with CD3/CD4 or CD8/CD25/RT1B and intracellularly stained with FOXP3. Cells were acquired in a LSRII-Fortessa, and 500,000 events were recorded in the lymphocyte gate. A: cells were gated on FSC-H and FSC-A to allow the selection of single cells. B: cells were gated on the basis of size (FSC-A) and granularity (SSC-A) to select the lymphocyte population. C: subsequently, cells were gated on CD3 and either CD4 or CD8, defining CD4 or CD8 T lymphocytes, respectively. D: further gating on CD25 × RT1B allowed us to discriminate the activated T cells. E: regulatory T cells were defined on the basis of the double expression of CD25+ FOXP3+.
Statistical analysis.
Data analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA). All data were represented as means ± the SE. For parametric data, two-way ANOVA was performed with Tukey's multiple-comparison test. For nonparametric data, the Mann-Whitney U-test was used. P values less than 0.05 were considered significant.
RESULTS
Water consumption was similar among the C, I, and IP Groups (48 ± 8, 46 ± 5, and 45 ± 5 ml/day, respectively). Treated animals had a PY intake of 8.0 ± 1.8 mg/day, corresponding to ∼29.5 mg/day.
Ventricular function.
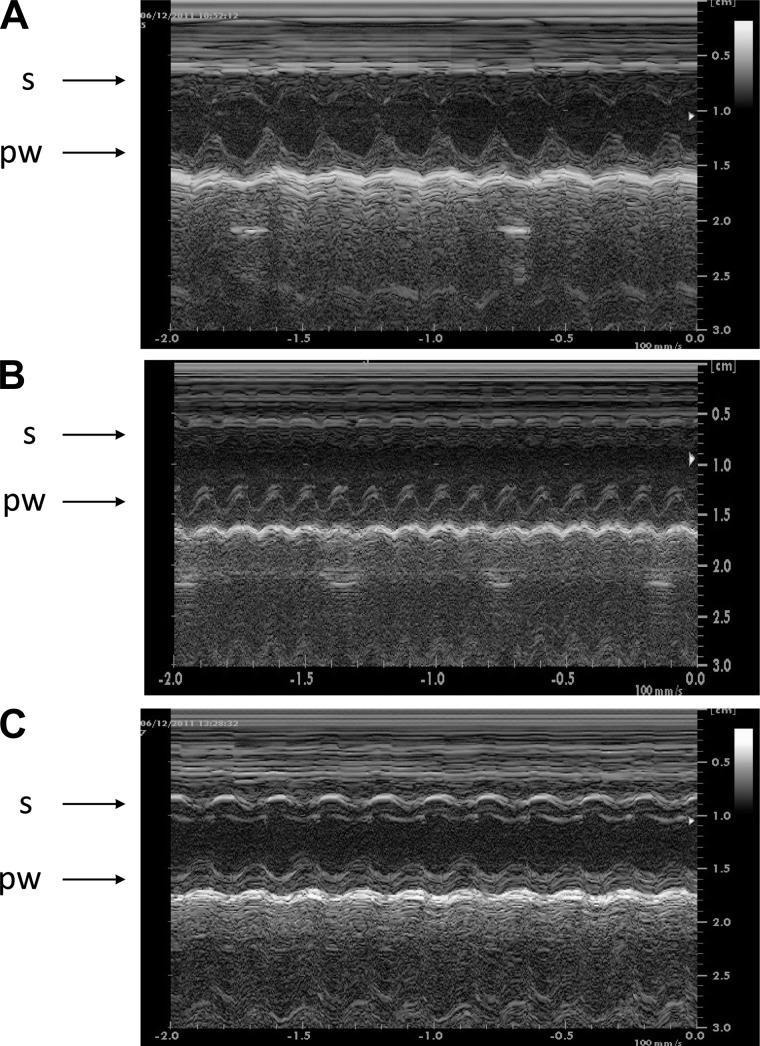
The echocardiographic morphological and function data were obtained on the second day after thoracotomy (Fig. 2). Compared with the C Group, the infarcted groups (I and IP) showed a similar area of hypokinesia/akinesia and an equivalent reduction of LV systolic function, assessed as LVEF (Table 2). Moreover, an abnormal diastolic dysfunction, assessed as LV filling patterns, was also detected in both the I and IP Groups, compared with C and I Groups. However, the IP Group showed a small, but significant, improvement of the isovolumetric relaxation time normalized by heart rate (IVRT/RR0.5) compared with the I Group, suggesting a positive impact of PY treatment on LV diastolic function at the second day after MI. No significant differences on atrial and ventricular diameters and volumes were detected among groups at this time of observation.
Fig. 2.
Echocardiography images of the left ventricle cavity delimited by the septum (s) and posterior wall (pw). A: normal contraction of the septum and the posterior wall in a control (C) rat. B: akinetic septum and normal contraction of the posterior wall in an untreated, infarcted (I) rat. C: hypokinesia of the septum and normal contraction of the posterior wall in a pyridostigmine (PY)-treated rat.
Table 2.
Echocardiographic parameters in anesthetized control and infarcted rats with and without pyridostigmine treatment
| C | I | IP | P | |
|---|---|---|---|---|
| MI area, % LV | 0 | 46 ± 3* | 48 ± 2* | P < 0.001 |
| LAD, cm | 0.36 ± 0.01 | 0.38 ± 0.05 | 0.36 ± 0.02 | P = 0.6 |
| LVDV, cm3 | 0.41 ± 0.05 | 0.37 ± 0.02 | 0.39 ± 0.01 | P = 0.4 |
| IVRT, ms | 22 ± 2 | 33 ± 5* | 32 ± 3* | P < 0.001 |
| IRVT/RR0,5 | 52.4 ± 0.5 | 84.0 ± 0.7* | 80.4 ± 1.0*† | P < 0.001 |
| LVEF, % | 77 ± 2 | 30 ± 4* | 29 ± 5* | P < 0.001 |
MI, myocardial infarction; LAD, left atrial diameter; LVDV, left ventricular diastolic volume; IVRT, isovolumetric relaxation time; IRVT/RR0.5, isovolumetric relaxation time normalized by heart rate; LVEF, left ventricular ejection fraction; IP, pyridostigmine-treated infarcted rats.
P < 0.05 compared with the control (C) group.
P < 0.05 compared with the infarcted (I) group (two-way ANOVA followed by a Tukey post hoc test).
Hemodynamic and autonomic measurements.
Awake hemodynamic parameters, measured on the second day after thoracotomy, are shown in Table 3. Baseline values of systolic blood pressure were lower (P = 0.002), and HR values were higher (P < 0.001) in the I and IP Groups relative to those in the C Group. Moreover, similar values of diastolic blood pressure were detected among all groups. Therefore, PY administration did not prevent the hemodynamic changes associated with this acute phase of MI.
Table 3.
Hemodynamic and heart rate variability parameters evaluated in awake control and infarcted rats with and without pyridostigmine treatment
| C | I | IP | P | |
|---|---|---|---|---|
| SBP, mmHg | 132 ± 3 | 124 ± 2* | 118 ± 3* | P = 0.002 |
| DBP, mmHg | 89 ± 2 | 90 ± 1 | 85 ± 2 | P = 0.2 |
| HR, bpm | 341 ± 6 | 389 ± 7* | 379 ± 9* | P < 0.001 |
| SDNN, ms | 6 ± 1 | 7 ± 1 | 7 ± 1 | P = 0.4 |
| LF, % | 14 ± 2 | 27 ± 2* | 17 ± 2† | P < 0.001 |
| HF, % | 86 ± 2 | 73 ± 2* | 83 ± 2† | P < 0.001 |
| LF/HF ratio | 0.17 ± 0.03 | 0.39 ± 0.04* | 0.22 ± 0.03† | P < 0.001 |
Data are expressed as means ± SE.
P < 0.05 compared with the control (C) group.
P < 0.05 compared with the infarcted (I) group (two-way ANOVA followed by Turkeyś post hoc test). IP, pyridostigmine-treated infarcted group; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SDNN, standard deviation of the RR interval; LF, normalized low-frequency band of the RR interval; HF, normalized high-frequency band of the RR interval; LF/HF ratio, ratio of absolute values of LF/HF.
The results of the spectral analysis of HRV, also shown in Table 3, demonstrated that the I Group had significantly higher (P < 0.001) normalized LF-band values (%), lower normalized HF-band values (%), and a higher LF/HF ratio than the C Group. PY significantly increased the HF-normalized components and decreased the LF component of HRV in the IP Group compared with that of the I Group. Furthermore, the sympathovagal balance, expressed as the LF/HF ratio, was significantly reduced relative to that of the I Group (P < 0.001).
Infiltration of macrophages and T lymphocytes in the infarcted and peri-infarcted zones.
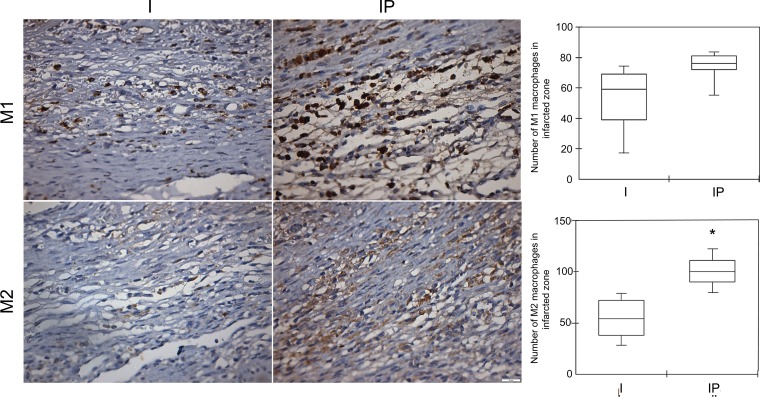
As expected, we observed massive inflammatory cell infiltration into the infarcted and peri-infarcted zones on the third day after MI (in the I and IP Groups); this infiltration was made up of polymorphonuclear and mononuclear cells. The C Group did not present a cluster of inflammatory cell infiltration in the heart, but it did present a few resident macrophages, mainly next to blood vessels. The absolute cell count of M1 macrophages was similar in the infarcted groups (P = 0.09). On the other hand, the M2 macrophages were significantly higher in the infarcted and peri-infarcted zones of the IP Group than in those of the I Group (P = 0.0054; see Fig. 3).
Fig. 3.
Photomicrographs of the infarcted control (I) and pyridostigmine-treated infarcted group (IP) showing the M1 macrophages within the peri-infarcted zone. The cytoplasmic stain showed no difference in the M1 cell count between the groups (P = 0.09). There were scarce M2 macrophages in the I Group and a dense diffuse infiltration of M2 cells in the infarcted zone in the IP Group (*P < 0.05). Ten microscopic fields of seven animals per group were analyzed and displayed using means ± SE. (Immunohistochemistry, diaminobenzidine: scale bar equals 50 μm).
The C Group did not show T lymphocytes in the heart, as expected (data not shown). There was no difference (P = 0.18) between the I and IP Groups in the number of CD4+ and CD8+ cells in the infarcted and peri-infarcted zones.
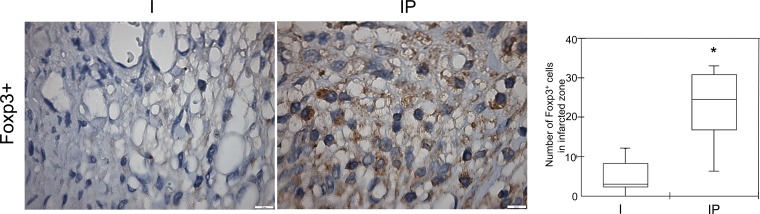
We also evaluated a subset of T lymphocytes, the FOXP3+ cells in the myocardium. A significant increase in these cells was observed in the IP Group (P = 0.0045) compared with the untreated infarcted group, as shown in Fig. 4.
Fig. 4.
Photomicrographs of the I and IP Groups showing few FOXP3 cells within the peri-infarcted zone of the I Group and a moderate number of positive cells in the IP Group. Right: number of the CD4+CD25+FOXP3 cells in both groups, which is significantly different (*P = 0.0045). Ten microscopic fields of seven animals per group were analyzed and displayed using means ± SE. (Immunohistochemistry, diaminobenzidine: scale bar equals 50 μm)
Frequency of T lymphocytes in the blood and spleen.
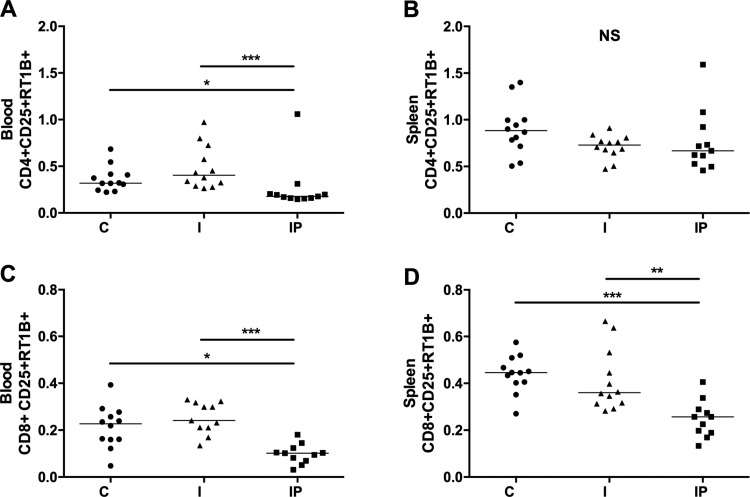
The analysis of the frequencies of total CD4+ and CD8+ T cells in the peripheral sites investigated [peripheral blood mononuclear cells and splenocytes] showed that treatment with PY did not alter these frequencies (data not shown). However, PY treatment had an impact on the frequency of activated T cells. On the basis of the double expression of CD25 and RT1B markers, we observed a significant reduction in the frequency of activated CD4+ and CD8+ T cells in both blood (Fig. 5, A and C) and spleen cells (on CD8+ T-cell compartment; Fig. 5, B and D).
Fig. 5.
PY treatment reduces CD4+ and CD8+ T-cell activation. Blood and spleen cells were collected from the C Group and from the I and IP Groups and then were stained to evaluate the activation. CD25 and RT1B double expression of the CD3+CD4+ and CD3+CD8+ cells were evaluated. A and B: CD4+ T-cell activation in the blood and spleen, respectively. C and D: CD8+ T-cell activation in the blood and spleen, respectively. *P < 0.05; **P < 0.01; ***P < 0.001; NS, nonsignificant.
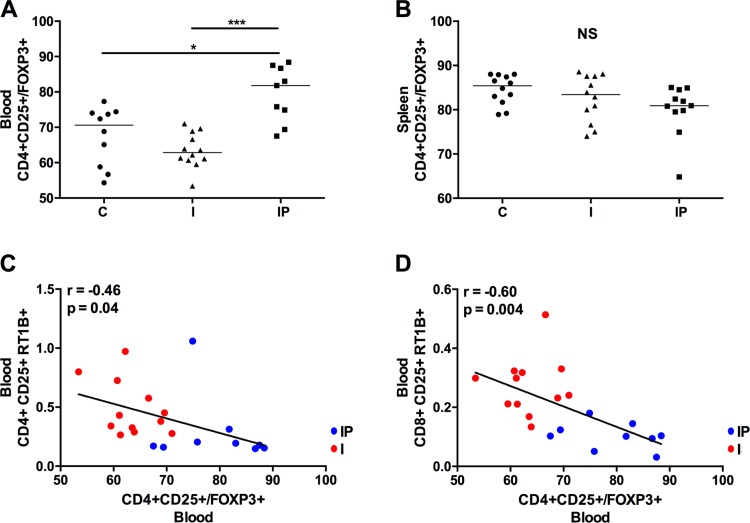
Considering the importance of regulatory T cells in controlling activation of the immune system, we evaluated the frequency of these cells in all tested groups. On the basis of the expression of FOXP3+ in CD4+CD25+ T cells, we observed that the treatment with PY propitiated higher numbers of FOXP3+ T cells in the blood relative to those in the noninfarcted control group and the infarcted, untreated group (Fig. 6A). The same effect was not observed in splenocytes (Fig. 6B). When correlating the activation levels of CD4+ and CD8+ T cells with the frequency of circulating regulatory T cells in blood (Fig. 5, C and D, respectively), we observed an inverse correlation, indicating that the presence of these cells can have an impact on the control of immune activation (r = −0.46 and P = 0.04 for CD4+CD25+RT1B+ vs. CD4+CD25+/FOXP3+; r = −0.60 and P = 0.004 for CD8+CD25+RT1B+ vs. CD4+CD25+/FOXP3+). We also observed a clear segmentation of the treated and untreated infarcted groups (blue and red dots, respectively) on the correlation plots (Fig. 6, C and D).
Fig. 6.
PY treatment increases Treg in blood cells. FOXP3 expression was evaluated in CD4+CD25+ T cells to define Treg cells in blood (A) and spleen (B). Correlations were measured between Treg cells from blood and CD4+ (C) and between Treg cells from blood and CD8+ after T-cell activation in blood cells (D) (r and P values are depicted). In the correlation plots, blue dots correspond to the IP Group, and red dots correspond to the I Group. *P < 0.05; ***P < 0.001; NS, nonsignificant.
DISCUSSION
This study reports the first evidence that increasing cholinergic modulation with PY is associated with greater anti-inflammatory cell recruitment 3 days after MI in rats. According to the literature, the predominance of immune cells with an anti-inflammatory profile in the myocardium was expected to occur a few days later, during the complex resolution of the inflammatory process triggered by MI (15, 40) compared with infarcted but untreated animals, the infarcted rats treated with PY presented more M2 macrophages and FOXP3+ T lymphocytes in the MI zone and a higher frequency of CD4+CD25+FOXP3+ T lymphocytes (Treg cells) in peripheral blood observed on the third day after MI. In addition, the presence of peripheral Treg cells was inversely correlated with CD4+ and CD8+ T-cell activation. The presence of a higher number of anti-inflammatory immune cells in infarcted and peri-infarcted zones may alter the milieu of chemokines/cytokines, thus altering the proinflammatory/anti-inflammatory balance and ultimately assuring tissue repair and the return of physiological function (4, 15, 21, 25, 35, 40, 41). This PY-triggered mechanism may contribute to ameliorating ventricular function and remodeling after MI, as previously described by our group and others (8, 9).
In the present study, water consumption was similar among all groups. Treated animals had a PY intake of ∼29.5 mg/day. Considering previous studies (8, 30), we estimated that 7 days with this treatment reduced plasma acetylcholinesterase activity inhibition by ∼40%. The PY treatment, which was started 4 days before MI, increased the high-frequency (vagal) component of HRV, indicating an increase in cholinergic stimulation. PY also decreased the low-frequency (sympathetic) component of HRV and, thus, normalized the sympathovagal balance in the heart. These results are in agreement with previous studies (27, 39) that had a longer follow-up period. Administration of PY is effective in blunting the hyperactivation of the sympathetic nervous system and the inhibition of the parasympathetic nervous system that both follow acute MI (8, 18, 27). Since a depression in vagal tone may predict a poor outcome after MI (17), parasympathetic stimulation with an anticholinesterase drug (e.g., PY) is considered a meaningful therapeutic approach to reduce postinfarct mortality (19, 33). In this scenario, recent evidence suggests that the use of acetylcholinesterase inhibitors is associated with a reduced risk of myocardial infarction in subjects diagnosed with Alzheimer's disease (23).
Another important outcome of increased vagal modulation due to treatment with anticholinesterase drugs is the improvement in myocardial remodeling in experiments using rats with chronic heart failure and healed MI (8, 9). We analyzed ventricular function by performing echocardiographic measurements on the second day after thoracotomy. Considering the short follow-up, the diameters and volumes of the cardiac chambers were not different among the infarcted and control groups. As expected, the systolic and diastolic left ventricular functions were significantly compromised in the infarcted (I and IP) groups; compared with the C Group, reduced LVEF characterized the systolic dysfunction, and abnormal LV filling patterns—both absolute and normalized IRVT—characterized the diastolic dysfunction. However, infarcted animals treated with PY showed a small, but significant, decrease in IVRT/RR0.5 compared with the I Group, suggesting a positive impact of PY on LV diastolic function in the very early acute phase of MI. Noninvasive parameters of diastolic function measured by echocardiography present a good correlation with invasive measurements of LV cardiac function measured by catheterization in rat models of myocardial hypertrophy and MI (22); these noninvasive parameters may predict further ventricular dysfunction (5). We can speculate that keeping rats under PY treatment for a few more days would intensify the differences between the infarcted groups, leading to further improvement in echocardiographic parameters related to systolic and diastolic function in the PY Group.
The cardiac protector mechanisms related to administration of PY and other anticholinesterase drugs deserve investigation. In this scenario, interventions on the cholinergic anti-inflammatory pathway have been attracting the attention of investigators as therapeutic strategies for treating acute and chronic inflammatory conditions, including cardiovascular diseases (19).
It is under investigation whether the anti-inflammatory effects produced by acetylcholinesterase inhibitors are related mainly to central cholinergic pathways. Hofer et al. (12) demonstrated that the peripheral acetylcholinesterase inhibitor neostigmine was equally effective as physostigmine (an acetylcholinesterase inhibitor that crosses the blood-brain barrier) in attenuating the inflammatory response in murine sepsis; however, other studies suggest otherwise (12). Akinci et al. (2) reported a deleterious effect of neostigmine in a model of endotoxemia. Moreover, Kox et al. (16) reported that neostigmine had no anti-inflammatory effects on the inflammation and lung injury induced by the mechanical ventilation (MV) injury model in mice. Nevertheless, the selective pharmacological stimulation of the peripheral branch of the cholinergic anti-inflammatory pathway with the selective partial α-7 nAChR agonist GTS-2 attenuates MV-induced TNF-α production at the transcriptional level and improves lung function. Kox et al. (16) hypothesized that the anti-inflammatory effects of GTS may be attributable to its effect on immune cells, particularly on alveolar macrophages. These data suggest the anti-inflammatory effects associated with anticholinesterase drugs depends on the drugs' pharmacological characteristics: the dose and timing of drug delivery and the tissue type involved.
It has been shown that stimulation of the efferent vagus nerve can release the important neurotransmitter ACh, which acts through the α-7 subunit of the nicotinic ACh receptor (α-7 nAChR) expressed in macrophages and in regulatory T cells. ACh, in a concentration-dependent manner, can decrease the release of cytokines in endotoxin-stimulated macrophage cultures via a posttranscriptional mechanism. Stimulation of α-7 nAChR by nicotine increases the suppressive capacity of naturally occurring regulatory CD4+CD25+ cells in mice in vitro (24, 28, 29, 37). Efferent vagus nerve signaling may facilitate the release of lymphocytes from the thymus through a nicotinic-receptor response (3) and can stimulate sets of splenic T lymphocytes that play a role in the propagation of nerve signals (29).
Postmyocardial wound healing consists of a number of complex inflammatory events that are both time- and cell-type-dependent (4, 37, 40). Macrophages are critical infiltrating cells in the infarcted myocardium, and these cells show a functional and phenotypic plasticity during the resolution phase of inflammation. M1 macrophages express high levels of proinflammatory mediators, such as IL-6, IL-1β, and NOS 2, and dominate up to 3 days after MI. However, M2 macrophages that express high levels of anti-inflammatory cytokines involved in repairing functions, such as IL-10 and TGF-β, gradually appear and remain predominant after 5 days (3, 25).
We found that PY increases the M2 macrophage number, altering the M1/M2 ratio in the infarcted and peri-infarcted zones, as soon as 3 days after MI. A previous study that used direct vagal stimulation to increase the cholinergic pathway verified (in a myocardial ischemia and reperfusion model) a reduction in the number of infiltrated macrophages and neutrophils in the MI area. The macrophages expressed immune reactivity for the nicotinic receptor α-7 nAChR, but were not classified in M1 or M2 (24). The difference in these observations may be related to the type of myocardial challenge (intermittent vs. permanent ischemia) and observation time (24 h after reperfusion vs. 3 days after coronary artery occlusion).
The concept of manipulating immune cells, such as macrophage polarization and Treg cells, to control the duration and extent of the inflammatory phase following MI has been recently proposed (11, 38). It was demonstrated that, following phosphatidylserine presenting liposome (PS-liposome) uptake by macrophages in vitro, the cells secreted high levels of anti-inflammatory cytokines concomitant with downregulation of proinflammatory markers. Furthermore, an intramyocardial injection of PS-presenting liposomes after MI had already induced the M2 phenotype on site on the third day after treatment, accompanied by elevated secretions of IL-10, TGF-β, and vascular endothelial growth factor and by decreased secretion of TNF-α, preventing ventricular dilation and remodeling (11).
In our study, administration of PY did not alter CD4+ and CD8+-staining cells at the infarcted and peri-infarcted myocardium but increased both the number of CD4+CD25+FOXP3+ lymphocytes in the MI area and the frequency of CD4+CD25+FOXP3+ T lymphocytes (Treg cells) in the peripheral blood. Moreover, the presence of peripheral Treg cells was inversely correlated with CD4+ and CD8+ T-cell activation in the peripheral blood.
CD4+ and CD8+ T-lymphocyte activation after ischemic injury of the heart has been detected in both MI and non-MI areas in experimental (13, 31, 34–36) and clinical studies (1). These cells have also been recovered from the peripheral blood and spleen tissue days after coronary artery ligation in rats and mice (13, 36). Furthermore, conventional (FOXP3−) and regulatory CD4+ (FOXP3+) T lymphocytes improve wound healing and survival after experimental MI in mice (13, 31). The fundamental importance of the regulatory T cells in preventing cell-mediated damage to tissues is known to be mediated through direct interaction with target cells and/or secretion of soluble factors, such as IL-10 and TGF-α (13, 31, 32, 40). A model of genetic Treg-cell ablation (FOXP3-DTR-mice) established that the depletion of Treg-cell compartments before MI induction resulted in aggravated cardiac inflammation and deteriorated clinical outcome associated with a M1-like macrophage polarization. In contrast, therapeutic Treg-cell activation by superagonist anti-CD28 monoclonal antibody administration induced an M2 macrophage differentiation within the healing myocardium associated with myofibroblast activation and increased expression of monocyte-/macrophage-derived proteins, thus fostering wound healing (21).
We can hypothesize that the increase in FOXP3+ T cells at the MI site may similarly have influenced the M1/M2 polarization observed in our study. Treg cells can profoundly affect macrophage function. Recent experimental studies have demonstrated that therapeutic Treg-cell activation triggers M2 macrophage differentiation in wound healing post-MI in mice (37). Furthermore, the syngeneic Treg adoptive transfer into the peritoneal cavity of immunodeficient mice polarizes the resident macrophages into a M2-phenotype. A similar phenomenon was observed in human cells. Human monocytes cultured in the presence of Treg cells differentiate into M2-like macrophages (34). Among the soluble factors that mediate this cross-talk, IL-10, IL-4, and IL-17 are the most likely candidates to play a role in this process (34, 37).
Controlling the nicotinic effect accurately to restore the balance is the most crucial challenge. The ultimate goal of Treg immunotherapy is to tip the balance between Treg and effector cells.
Previous studies showed that a 2-wk cholinergic stimulation with PY after MI increases angiogenesis and blood flow in the ischemic myocardium of sinoaortic, denervated rats (parasympathetic-deprived rats) (41). Moreover, administration of PY after MI ameliorates cardiac fibrosis and attenuates cardiac structural changes via TGF-β1-activated kinase pathway inhibition (21). Considering that cytokines and other molecules involved in signaling of the mentioned pathways are released by different cells, mainly immune cells, it would be desirable to perform further studies focusing on PY's effect on these mechanisms.
In conclusion, our study revealed that cholinergic stimulation with PY induces greater anti-inflammatory cell recruitment in the heart and in the peripheral blood soon after MI in rats. We can speculate that the prompt increase in M2 macrophages and FOXP3+ T cells at the MI site, as induced by PY, is a key event in the process of myocardial protection, as previous studies have detected.
Perspectives and Significance
How to control the immune system accurately to restore the balance between inflammatory and anti-inflammatory profile after an ischemic myocardial insult is a central challenge. The concept of treating an exacerbated immune response by increasing the endogenous cholinergic anti-inflammatory pathway has been used in different experimental scenarios, including after myocardial ischemia. Treg cells play a critical role in reparative phase after myocardial infarction by suppressing the immune-activation of self-aggressive effector T cells and by triggering the polarization of the macrophages with inflammatory profile to the macrophages with anti-inflammatory phenotype. Therefore, the therapeutic Treg cell activation represents an eligible strategy to accelerate and improve healing after myocardial infarction. Our study revealed that cholinergic stimulation with PY induces greater anti-inflammatory cell recruitment in the heart and in the peripheral blood soon after MY in rats. We can speculate that the increase in FOXP3+ cells and the decrease in the M1/M2 macrophage ratio in the myocardium induced by PY after MI is a key event interrelated to the process of the myocardial protection mediated by the cholinergic anti-inflammatory pathway. The electric stimulation of the vagus nerve has been used in patients with chronic inflammatory disease to decrease inflammatory milieu and to improve symptoms. Whether the immune modulation with anticholinesterase drugs improves the myocardial injury and alleviates other inflammatory disorders in humans deserves further evaluation. Moreover, studies addressing the cellular and molecular mechanisms involved in the cholinergic anti-inflammatory modulation in animal models and in humans are demanded.
GRANTS
This study was supported by São Paulo Research Foundation, Fundação Zerbini, and National Council for Scientific and Technological Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.A.R., E.G.K., and F.M.C.-C. conception and design of research; J.A.R., S.P.R., C.M.F., O.C., G.A., and S.L. performed experiments; J.A.R., S.P.R., C.M.F., E.G.K., M.C.I., and F.M.C.-C. analyzed data; J.A.R., S.P.R., C.M.F., S.L., and F.M.C.-C. interpreted results of experiments; J.A.R. and C.M.F. prepared figures; J.A.R., E.G.K., and F.M.C.-C. drafted manuscript; S.P.R., C.M.F., M.C.I., and F.M.C.-C. approved final version of manuscript; C.M.F., M.C.I., and F.M.C.-C. edited and revised manuscript.
REFERENCES
- 1.Abbate A, Bonanno E, Mauriello A, Bussani R, Biondi-Zoccai GG, Liuzzo G, Leone AM, Silvestri F, Dobrina A, Baldi F, Pandolfi F, Biasucci LM, Baldi A, Spagnoli LG, Crea F. Widespread myocardial inflammation and infarct-related artery patency. Circulation 110: 46–50, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Akinci SB, Ulu N, Yondem OZ, Firat P, Guc MO, Kanbak M, Aypar U. Effect of neostigmine on organ injury in murine endotoxemia: missing facts about the cholinergic antiinflammatory pathway. World J Surg 2005: 29: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 3.Antonica A, Magni E, Mearini L, Paolocci N. Vagal control of lymphocyte release from thymus. J Auton Nerv Syst 48: 187–197, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Anzai T. Post-infarction inflammation and left ventricular remodeling. Circ J 77: 580–587, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Azevedo PS, Polegato BF, Minicucci MF, Pio SM, Silva IA, Santos PP, Okoshi K, Paiva SA, Zornoff LA. Early echocardiographic predictors of increased left ventricular end-diastolic pressure three months after myocardial infarction in rats. Med Sci Monit 18: BR253–BR258, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Calvillo L, Vanoli E, Andreoli E, Besana A, Omodeo E, Gnecchi M, Zerbi P, Vago G, Busca G, Schwartz PJ. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J Cardiovasc Pharmacol 58: 500–507, 2011. [DOI] [PubMed] [Google Scholar]
- 8.de La Fuente RN, Rodrigues B, Moraes-Silva IC, Souza LE, Sirvente R, Mostarda C, De Angelis K, Soares PP, Lacchini S, Consolim-Colombo F, Irigoyen MC. Cholinergic stimulation with pyridostigmine improves autonomic function in infarcted rats. Clin Exp Pharmacol Physiol 40: 610–616, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Durand MT, Becari C, de Oliveira M, do Carmo JM, Silva CA, Prado CM, Fazan R Jr, Salgado HC. Pyridostigmine restores cardiac autonomic balance after small myocardial infarction in mice. PLoS One 9: e104476, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA 108: 1827–1832, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann Martina PD, Mautner S, Wente MN, Encke J, Werner J, Dalpke AH, Stremmel W, Nawroth PP, Martin E, Krammer PH, Bierhaus A, Weigand MA. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med 36: 404–408, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125: 1652–1663, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Li D, Hu Y, Yang K. Changes of CD4+CD25+ regulatory T cells in patients with acute coronary syndrome and the effects of atorvastatin. J Huazhong Univ Sci Technolog Med Sci 27: 524–7, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versusresolution of inflammation following myocardial infarction. Basic Res Cardiol 109: 444–461, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Kox M, Pompe JC, Peters E, Vaneker M, van der Laak JW, van der Hoeven JG, Scheffer GJ, Hoedemaekers CW, Pickkers P. α7 nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced tumour necrosis factor-α production and lung injury. Br J Anaesth 107: 559–566, 2011. [DOI] [PubMed] [Google Scholar]
- 17.La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351: 478–484, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Lataro RM, Silva CA, Fazan R Jr, Rossi MA, Prado CM, Godinho RO, Salgado HC. Increase in parasympathetic tone by pyridostigmine prevents ventricular dysfunction during the onset of heart failure. Am J Physiol Regul Integr Comp Physiol 305: R908–R916, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Leib C, Katus HA, Kaya Z. Cholinergic control of inflammation in cardiovascular diseases. Trends Cardiovasc Med 23: 46–51, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold Lewis EF JM, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA; Study CARE. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol 42: 1446–1453, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Liu JJ, Bi XY, Yu XJ, Kong SS, Qin FF, Zhou J, Zang WJ. Pyridostigmine ameliorates cardiac remodeling induced by myocardial infarction via inhibition of the transforming growth factor-β1/TGF-β1-activated kinase pathway. J Cardiovasc Pharmacol 63: 412–420, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Mostarda C, Moraes-Silva IC, Moreira ED, Medeiros A, Piratello AC, Consolim-Colombo FM, Caldini EG, Brum PC, Krieger EM, Irigoyen MC. Baroreflex sensitivity impairment is associated with cardiac diastolic dysfunction in rats. J Card Fail 17: 519–25, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 248: 188–204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med 5: 661–674, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro SP, Milush JM, Cunha-Neto E, Kallas EG, Kalil J, Somsouk M, Hunt PW, Deeks SG, Nixon DF, SenGupta D. The CD8+ memory stem T cell (TSCM) subset is associated with improved prognosis in chronic HIV-1 infection. J Virol 88: 13,836–13,844, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues B, Lira FS, Consolim-Colombo FM, Rocha JA, Caperuto EC, De Angelis K, Irigoyen MC. Role of exercise training on autonomic changes and inflammatory profile induced by myocardial infarction. Mediators Inflamm 2014: 702473, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med 265: 663–679, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soares PPS, Nóbrega ACL, Ushizima MR, Irigoyen MC. Cholinergic stimulation with pyridostigmine increases heart rate variability and baroreflex sensitivity in rats. Auton Neurosci 113: 24–31, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Li JJ, Yao R, Liao MY, Tu X, Liao YH, Cheng X. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol 107: 232, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Tang TT, Ding YJ, Liao YH, Yu X, Xiao H, Xie JJ, Yuan J, Zhou ZH, Liao MY, Yao R, Cheng Y, Cheng X. Defective circulating CD25+CD4+ Foxp3+CD127 (low) regulator T-cells in patients with chronic heart failure. Cell Physiol Biochem 25: 451–458, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Thomas CJ. Improving cardiac autonomic function following myocardial infarction: The case for anticholinesterase drugs. Clin Exp Pharmacol Physiol 40: 597–599, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA 104: 19,446–19,451, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Laan AM, Nahrendorf M, Piek JJ. Healing and adverse remodeling after acute myocardial infarction: role of the cellular immune response. Heart 98: 1384–1390, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Varda-Bloom N, Leor J, Ohad DG, Hasin Y, Amar M, Fixler R, Battler A, Eldar M, Hasin D. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J Mol Cell Cardiol 32: 2141–2149, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Wang DW, Zhou RB, Yao YM, Zhu XM, Yin YM, Zhao G, Dong NJ, Sheng ZY. Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther 335: 553–561, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115: 55–67, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Xiong J, Xue FS, Yuan YJ, Wang Q, Liao X, Wang WL. Cholinergic anti-inflammatory pathway: a possible approach to protect against myocardial ischemia reperfusion injury. Chin Med J (Engl) 123: 2720–2726, 2010. [PubMed] [Google Scholar]
- 40.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62: 24–35, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Yu JG, Song SW, Shu H, Fan SJ, Liu AJ, Liu C, Guo W, Guo JM, Miao CY, Su DF. Baroreflex deficiency hampers angiogenesis after myocardial infarction via acetylcholine-α7-nicotinic ACh receptor in rats. Eur Heart J 34: 2412–20, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Zhang XJ, Yang L, Zhao Q, Caen JP, He HY, Jin QH, Guo LH, Alemany M, Zhang LY, Shi YF. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ 9: 790–800, 2009. [DOI] [PubMed] [Google Scholar]